Synthesizing and Characterizing a Mesoporous Silica Adsorbent for Post-Combustion CO2 Capture in a Fixed-Bed System
Abstract
:1. Introduction
2. Results and Discussion
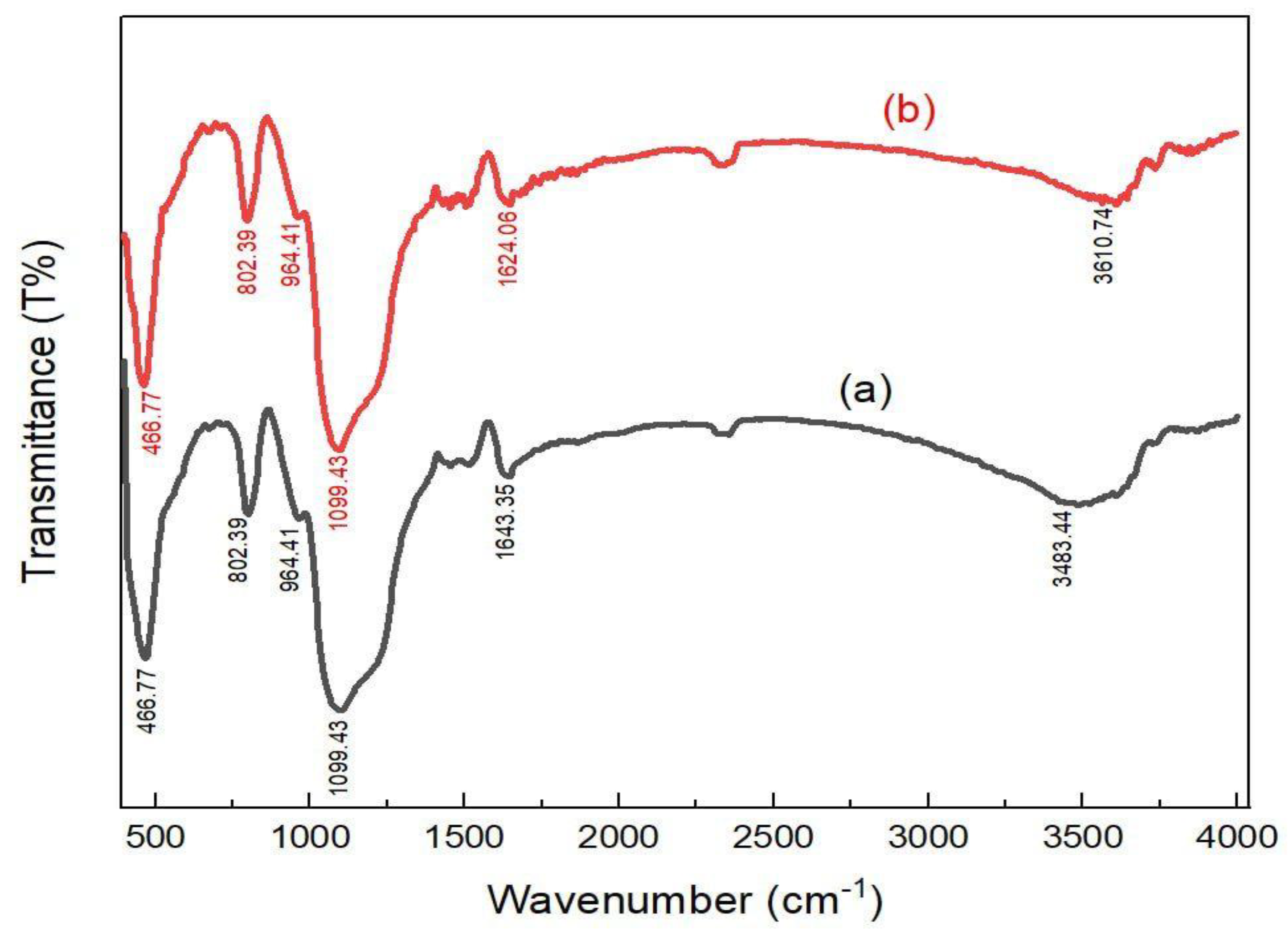
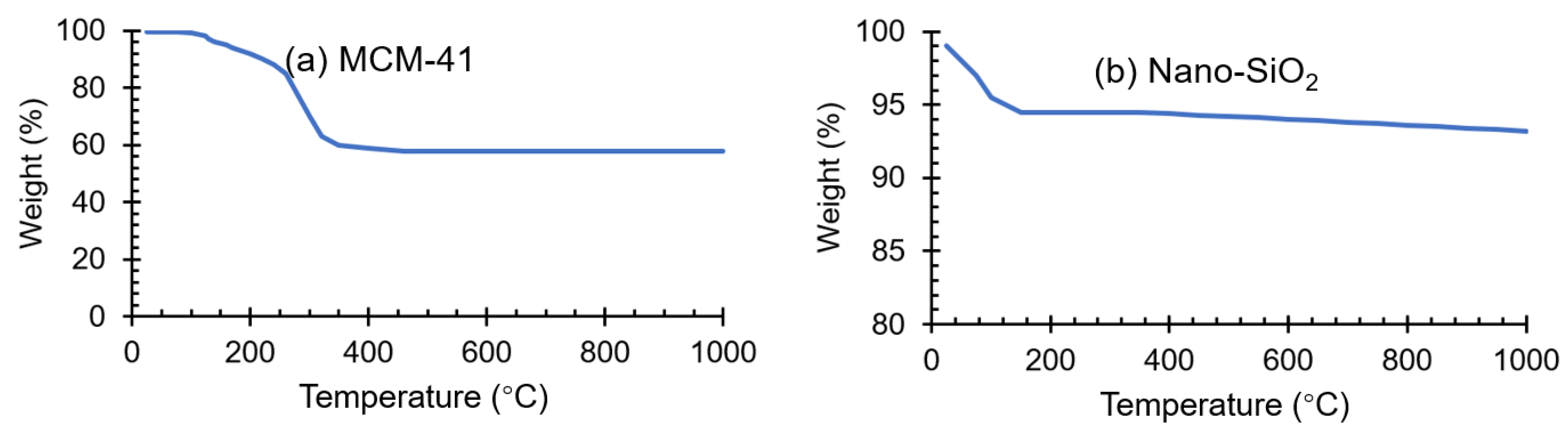
2.1. Adsorbents Characterization
2.2. CO2 Adsorption Behavior
2.2.1. Effect of Adsorbent Type on the CO2 Adsorption
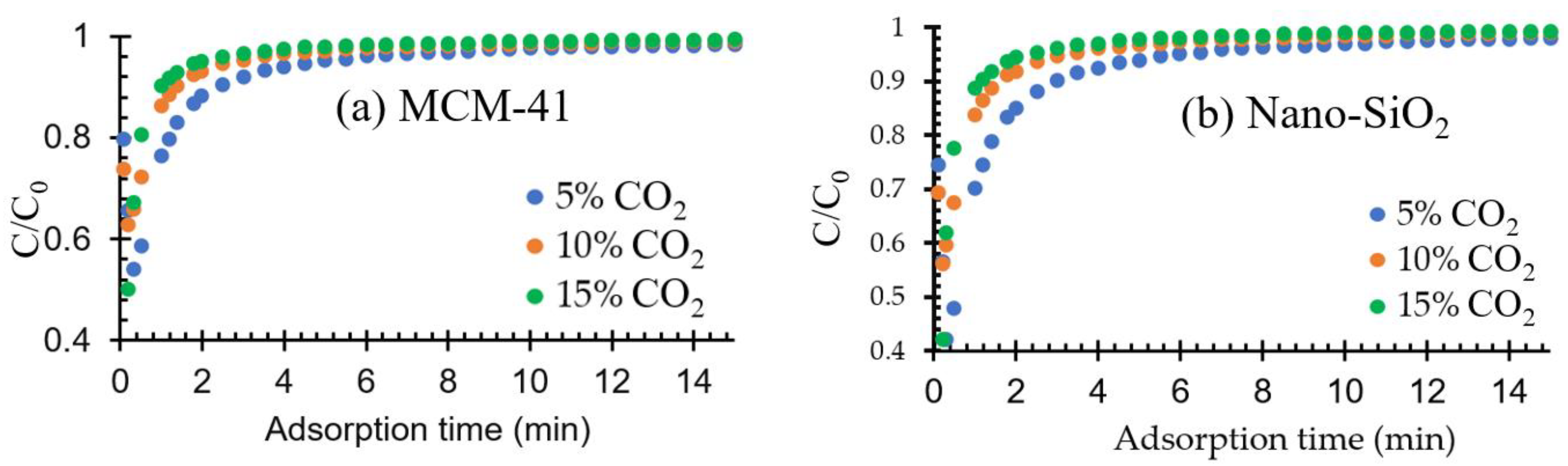
2.2.2. Effect of CO2 Composition on Adsorption Performance
2.2.3. Effect of the Bed Sorption Temperature on the CO2 Adsorption
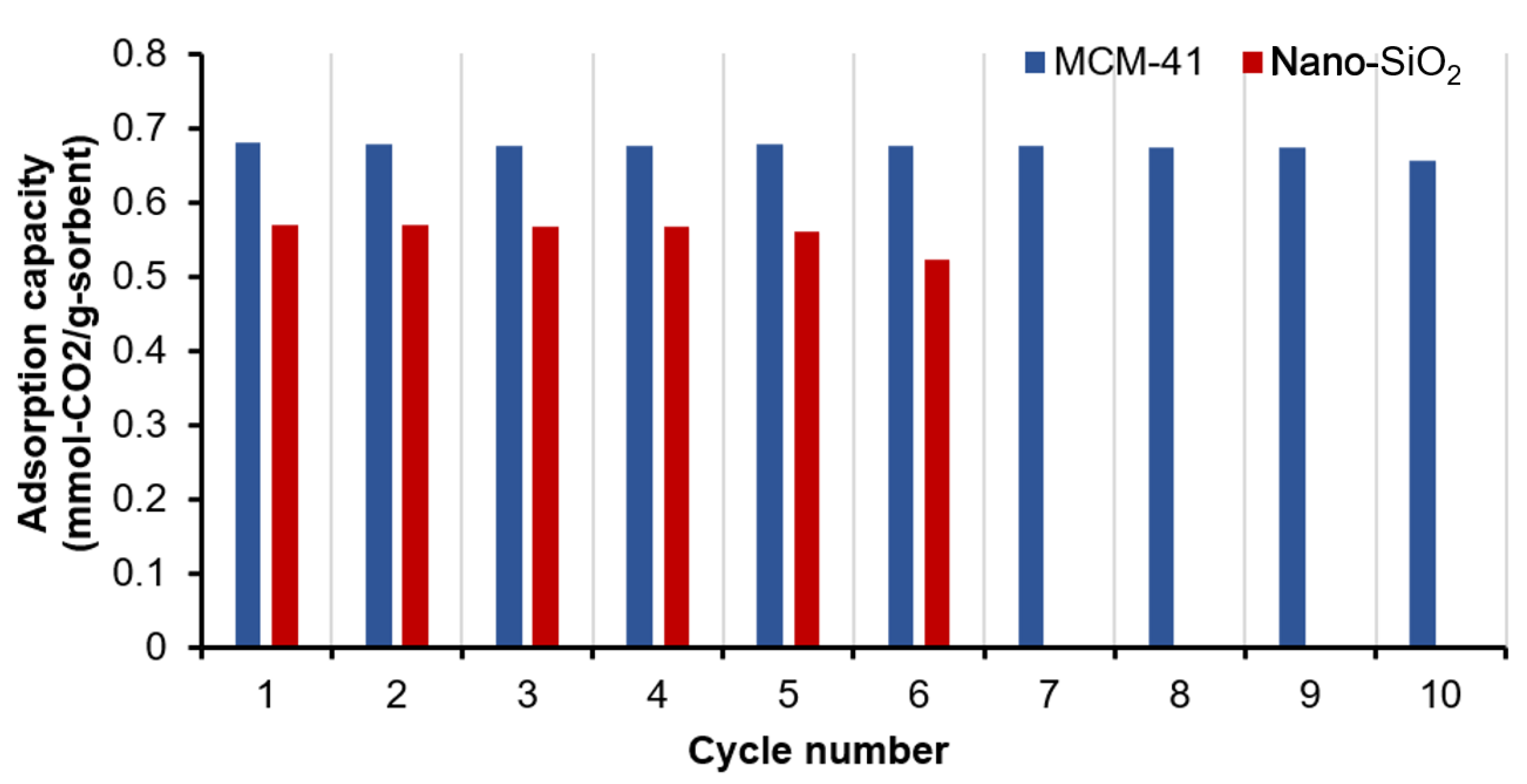
2.2.4. Cyclic CO2 Adsorption
3. Experimental Work
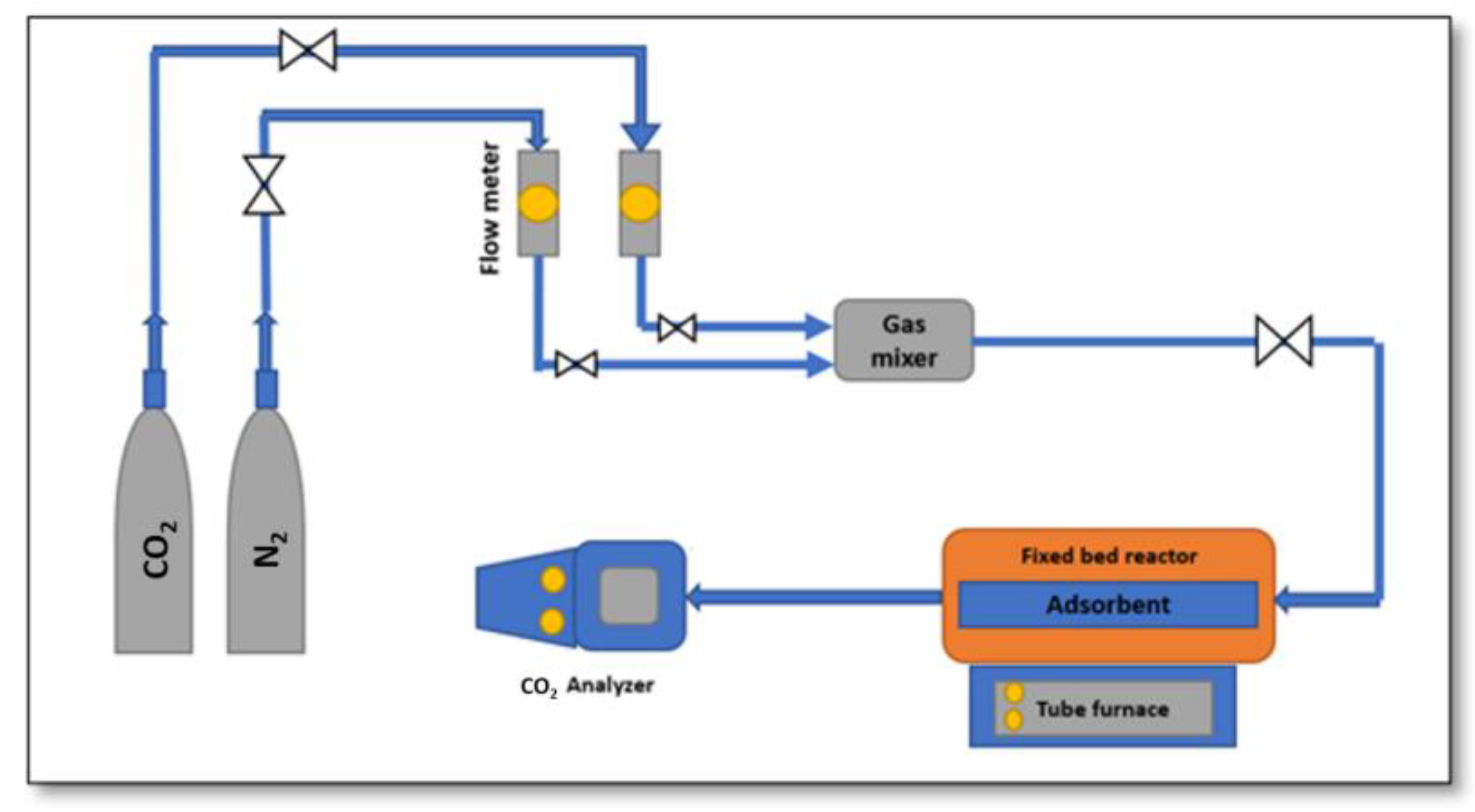
3.1. Materials and Apparatus
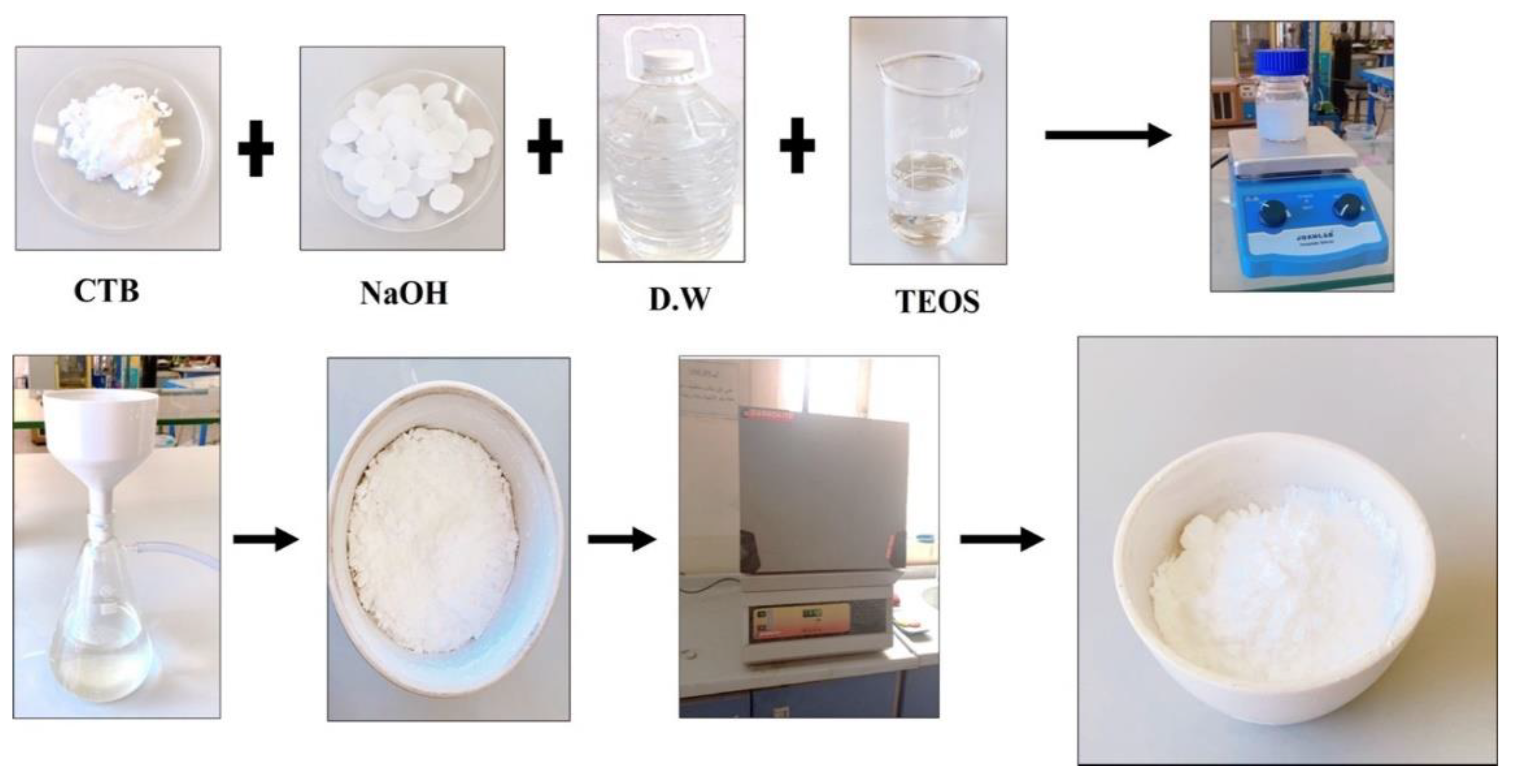
3.2. Preparation of MCM-41
3.3. Adsorbent Morphology and Surface Properties
3.4. CO2 Adsorption and Desorption Performance Measurement
3.4.1. CO2 Adsorption Experiments
3.4.2. CO2 Desorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muchan, P.; Saiwan, C.; Nithitanakul, M. Carbon dioxide adsorption/desorption performance of single- and blended-amines-impregnated MCM-41 mesoporous silica in post-combustion carbon capture. Clean Energy 2022, 6, 424–437. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, L.; Li, L.; Xiao, F.; Zhao, N.; Wei, Z.; Chen, Y.; Wu, F. Synthesis of Micro-Mesoporous Composites MCM-41/13X and Their Application on CO2 Adsorption: Experiment and Modeling. Ind. Eng. Chem. Res. 2016, 55, 7853–7859. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Khan, A.; Carlisle, S.; Leonenko, Y. Ex Situ Dissolution of CO2: A New Engineering Methodology Based on Mass-Transfer Perspective for Enhancement of CO2 Sequestration. Energy Fuels 2011, 25, 3323–3333. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; Zhao, J.; Chen, L. Mixed amine-modified MCM-41 sorbents for CO2 capture. Int. J. Greenh. Gas Control 2015, 37, 90–98. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Einbu, A.; Pettersen, T.; Morud, J.; Tobiesen, A.; Jayarathna, C.K.; Skagestad, R.; Nysæther, G. Energy assessments of onboard CO2 capture from ship engines by MEA-based post combustion capture system with flue gas heat integration. Int. J. Greenh. Gas Control 2022, 113, 103526. [Google Scholar] [CrossRef]
- Dashti, A.; Raji, M.; Razmi, A.; Rezaei, N.; Zendehboudi, S.; Asghari, M. Efficient hybrid modeling of CO2 absorption in aqueous solution of piperazine: Applications to energy and environment. Chem. Eng. Res. Des. 2019, 144, 405–417. [Google Scholar] [CrossRef]
- Scholes, C.A.; Ho, M.T.; Wiley, D.E.; Stevens, G.W.; Kentish, S.E. Cost competitive membrane—Cryogenic post-combustion carbon capture. Int. J. Greenh. Gas Control 2013, 17, 341–348. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Purif. Technol. 2022, 299, 121734. [Google Scholar] [CrossRef]
- Damartzis, T.; Asimakopoulou, A.; Koutsonikolas, D.; Skevis, G.; Georgopoulou, C.; Dimopoulos, G.; Nikolopoulos, L.; Bougiouris, K.; Richter, H.; Lubenau, U.; et al. Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment. Appl. Sci. 2022, 12, 6100. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Younas, M.; Rezakazemi, M. Application of Membrane Contactor Technology for Post-combustion Carbon Dioxide (CO2) Capture. In Membrane Contactor Technology: Water Treatment, Food Processing, Gas Separation, and Carbon Capture; Wiley: Hoboken, NJ, USA, 2022; pp. 281–304. [Google Scholar]
- Boonchuay, A.; Worathanakul, P. The Diffusion Behavior of CO2 Adsorption from a CO2/N2 Gas Mixture on Zeolite 5A in a Fixed-Bed Column. Atmosphere 2022, 13, 513. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Ye, B.; Jiang, J.; Zhou, Y.; Liu, J.; Wang, K. Technical and economic analysis of amine-based carbon capture and sequestration at coal-fired power plants. J. Clean. Prod. 2019, 222, 476–487. [Google Scholar] [CrossRef]
- Knoope, M.M.J.; Ramírez, A.; Faaij, A.P.C. A state-of-the-art review of techno-economic models predicting the costs of CO2 pipeline transport. Int. J. Greenh. Gas Control 2013, 16, 241–270. [Google Scholar] [CrossRef]
- Mazari, S.A.; Si Ali, B.; Jan, B.M.; Saeed, I.M.; Nizamuddin, S. An overview of solvent management and emissions of amine-based CO2 capture technology. Int. J. Greenh. Gas Control 2015, 34, 129–140. [Google Scholar] [CrossRef]
- Jha, A.; Ravuru, V.; Yadav, M.; Mandal, S.; Das, A. A Critical Analysis of CO2 Capture Technologies. Hydrocarb. Process. 2021, 6, 59–65. [Google Scholar]
- Babamohammadi, S.; Yusoff, R.; Aroua, M.; N.Borhani, T. Mass Transfer Coefficients of Carbon Dioxide in Aqueous Blends of Monoethanolamine and Glycerol Using Wetted-Wall Column. J. Environ. Chem. Eng. 2021, 9, 106618. [Google Scholar] [CrossRef]
- Lu, A.-H.; Liu, R.-S.; Shi, X.-D.; Wang, C.-T.; Gao, Y.-Z.; Xu, S.; Hao, G.-P.; Chen, S. The advances in post-combustion CO2 capture by physical adsorption: From materials innovation to separation practice. ChemSusChem 2021, 14, 1428–1471. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Meng, Y.; Ju, T.; Han, S.; Meng, F.; Li, J.; Du, Y.; Song, M.; Lan, T.; Jiang, J. Characteristics, application and modeling of solid amine adsorbents for CO2 capture: A review. J. Environ. Manag. 2023, 325, 116438. [Google Scholar] [CrossRef]
- Sani, S.; Liu, X.; Li, M.; Stevens, L.; Sun, C. Synthesis and characterization of three-dimensional interconnected large-pore mesoporous cellular lignin carbon materials and their potential for CO2 capture. Microporous Mesoporous Mater. 2023, 347, 112334. [Google Scholar] [CrossRef]
- Sai Bhargava Reddy, M.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Lelong, G.; Saboungi, M.L. Recent progress in the synthesis and selected applications of MCM-41: A short review. J. Exp. Nanosci. 2006, 1, 375–395. [Google Scholar] [CrossRef]
- Roik, N.V.; Belyakova, L.A. Sol–gel synthesis of MCM-41 silicas and selective vapor-phase modification of their surface. J. Solid State Chem. 2013, 207, 194–202. [Google Scholar] [CrossRef]
- Garcés Polo, S.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.A.; Gil, A. A comparative study of CO2 diffusion from adsorption kinetic measurements on microporous materials at low pressures and temperatures. Chem. Eng. J. 2016, 302, 278–286. [Google Scholar] [CrossRef]
- Ali, N.S.; Harharah, H.N.; Salih, I.K.; Cata Saady, N.M.; Zendehboudi, S.; Albayati, T.M. Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 2023, 13, 9837. [Google Scholar] [CrossRef]
- Dubey, D.R.S.; Rajesh, Y.B.R.D.; More, M. Synthesis and Characterization of SiO2 Nanoparticles via Sol-gel Method for Industrial Applications. Mater. Today Proc. 2015, 2, 3575–3579. [Google Scholar] [CrossRef]
- Farjadian, F.; Ahmadpour, P.; Mohammadi-Samani, S.; Hosseini, M. Controlled size synthesis and application of nanosphere MCM-41 as potent adsorber of drugs: A novel approach to new antidote agent for intoxication. Microporous Mesoporous Mater. 2015, 213, 30–39. [Google Scholar] [CrossRef]
- Choolaei, M.; Rashidi, A.; Ardjmand, M.; Yadegari, A.; Soltanian, H. The effect of nanosilica on the physical properties of oil well cement. Mater. Sci. Eng. A 2012, 538, 288–294. [Google Scholar] [CrossRef]
- Chen, H.; Fu, S.; Fu, L.; Yang, H.; Chen, D. Simple Synthesis and Characterization of Hexagonal and Ordered Al-MCM-41 from Natural Perlite. Minerals 2019, 9, 264. [Google Scholar] [CrossRef]
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Cata Saady, N.M.; Albayati, T.M. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef]
- Ebrahimi-Gatkash, M.; Younesi, H.; Shahbazi, A.; Heidari, A. Amino-functionalized mesoporous MCM-41 silica as an efficient adsorbent for water treatment: Batch and fixed-bed column adsorption of the nitrate anion. Appl. Water Sci. 2015, 7, 1887–1901. [Google Scholar] [CrossRef]
- Barbosa, T.; Barros, T.; Lins Almeida Barbosa, T.; Rodrigues, M. Green Synthesis for MCM-41 and SBA-15 Silica Using the Waste Mother Liquor. Silicon 2022, 14, 6233–6243. [Google Scholar] [CrossRef]
- Salaimi, F.; Tahmasobi, K.; Karami, C.; Jahangiri, A. Preparation of Modified nano-SiO2 by Bismuth and Iron as a novel Remover of Methylene Blue from Water Solution. J. Mex. Chem. Soc. 2017, 61, 250–259. [Google Scholar] [CrossRef]
- Ali, N.S.; Salih, I.K.; Harharah, H.N.; Majdi, H.S.; Salih, H.G.; Kalash, K.R.; Al-Shathr, A.; Al-Sudani, F.T.; Abdulrahman, M.A.; Alrubaye, J.M.; et al. Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane. Catalysts 2023, 13, 1138. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Cata Saady, N.M. Removal of sulfur compounds from real diesel fuel employing the encapsulated mesoporous material adsorbent Co/MCM-41 in a fixed-bed column. Microporous Mesoporous Mater. 2022, 341, 112020. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Isa, Y.M. Effect of operating variables on CO2 adsorption capacity of activated carbon, kaolinite, and activated carbon—Kaolinite composite adsorbent. Water-Energy Nexus 2022, 5, 21–28. [Google Scholar] [CrossRef]
- Selvaraj, M.; Pandurangan, A.; Seshadri, K.S.; Sinha, P.K.; Lal, K.B. Synthesis, characterization and catalytic application of MCM-41 mesoporous molecular sieves containing Zn and Al. Appl. Catal. A Gen. 2003, 242, 347–364. [Google Scholar] [CrossRef]
- Guo, W.; Huang, L.; Deng, P.; Xue, Z.; Li, Q. Characterization of Beta/MCM-41 composite molecular sieve compared with the mechanical mixture. Microporous Mesoporous Mater. 2001, 44–45, 427–434. [Google Scholar] [CrossRef]
- Gao, F.; Ji, C.; Wang, S.; Wang, W.; Dong, J.; Guo, C.; Gao, Y.; Chen, G. Sterically hindered amine-functionalized MCM-41 composite for efficient carbon dioxide capture. Korean J. Chem. Eng. 2022, 39, 1981–1988. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Vakili, R.; Kalumpasut, P.; Gorgojo, P.; Siperstein, F.; Fan, X.; McCloskey, P. Velocity Variation Effect in Fixed Bed Columns: A Case Study of CO2 Capture using Porous Solid Adsorbents. AIChE J. 2018, 64, 2189–2197. [Google Scholar] [CrossRef]
- Monazam, E.R.; Spenik, J.; Shadle, L.J. Fluid bed adsorption of carbon dioxide on immobilized polyethylenimine (PEI): Kinetic analysis and breakthrough behavior. Chem. Eng. J. 2013, 223, 795–805. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture. Chem. Eng. J. 2014, 260, 573–581. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.; Shamiri, A.; Aghamohammadi, N. Modeling of Carbon Dioxide Adsorption onto Ammonia-Modified Activated Carbon: Kinetic Analysis and Breakthrough Behavior. Energy Fuels 2015, 29, 6565–6577. [Google Scholar] [CrossRef]
- Al Mesfer, M.; Danish, M.; Khan, M.; Ali, I.; Hasan, M.; Jery, A. Continuous Fixed Bed CO2 Adsorption: Breakthrough, Column Efficiency, Mass Transfer Zone. Processes 2020, 8, 1233. [Google Scholar] [CrossRef]
- Rege, S.; Yang, R. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina. Chem. Eng. Sci. 2001, 56, 3781–3796. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar]
- Ghorbani, B.; Miansari, M.; Zendehboudi, S.; Hamedi, M.H. Exergetic and economic evaluation of carbon dioxide liquefaction process in a hybridized system of water desalination, power generation, and liquefied natural gas regasification. Energy Convers. Manag. 2020, 205, 112374. [Google Scholar]

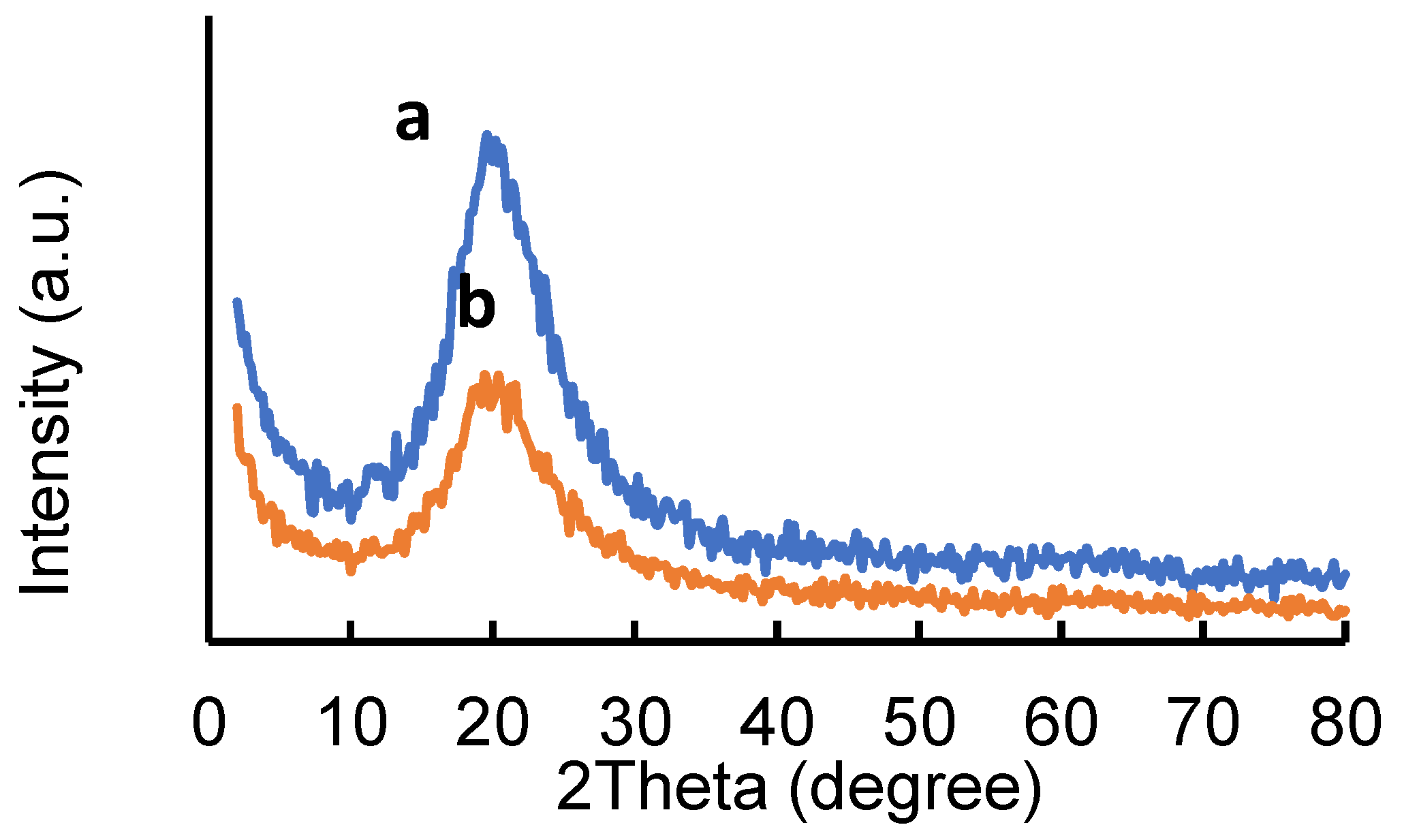
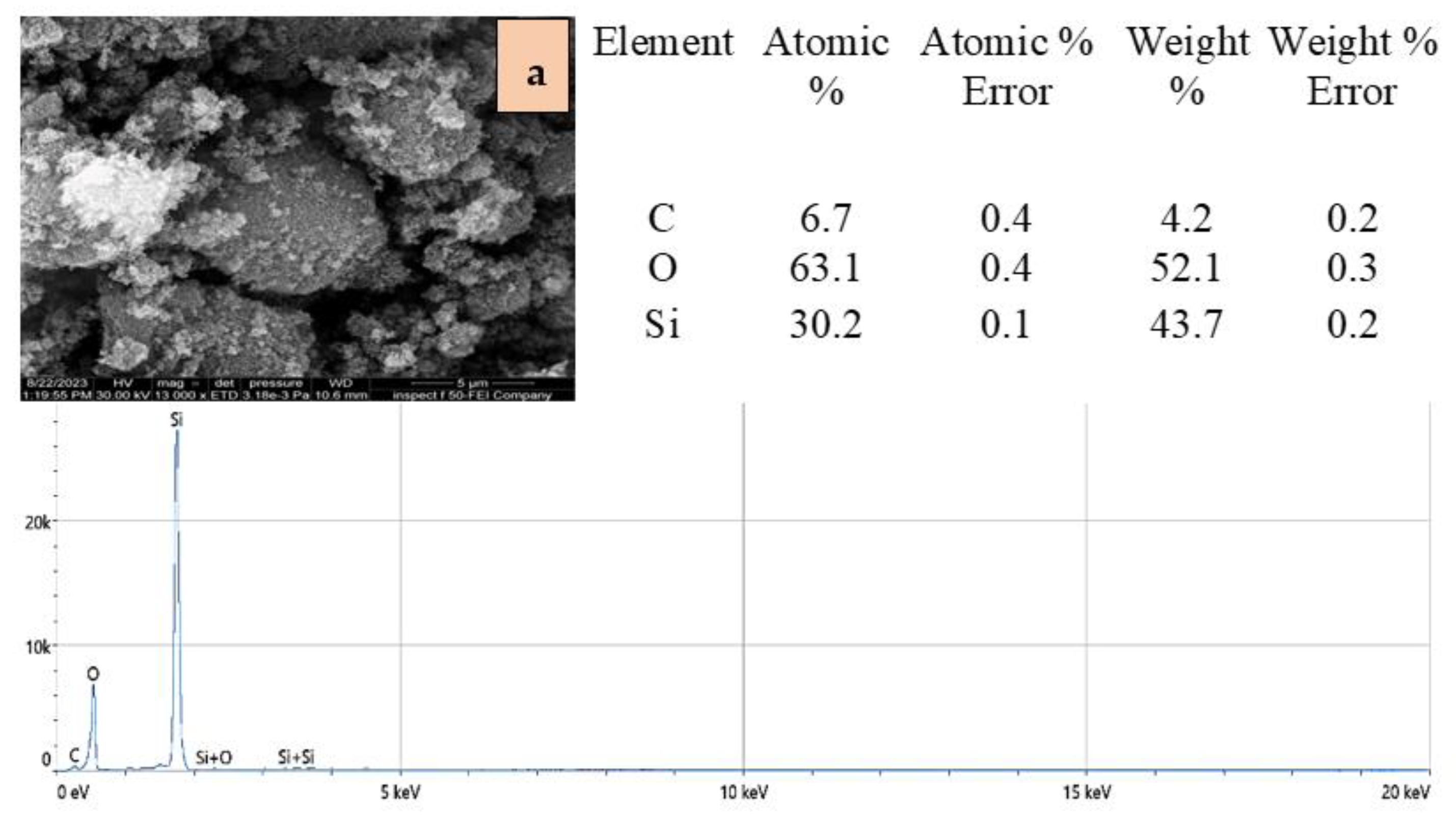
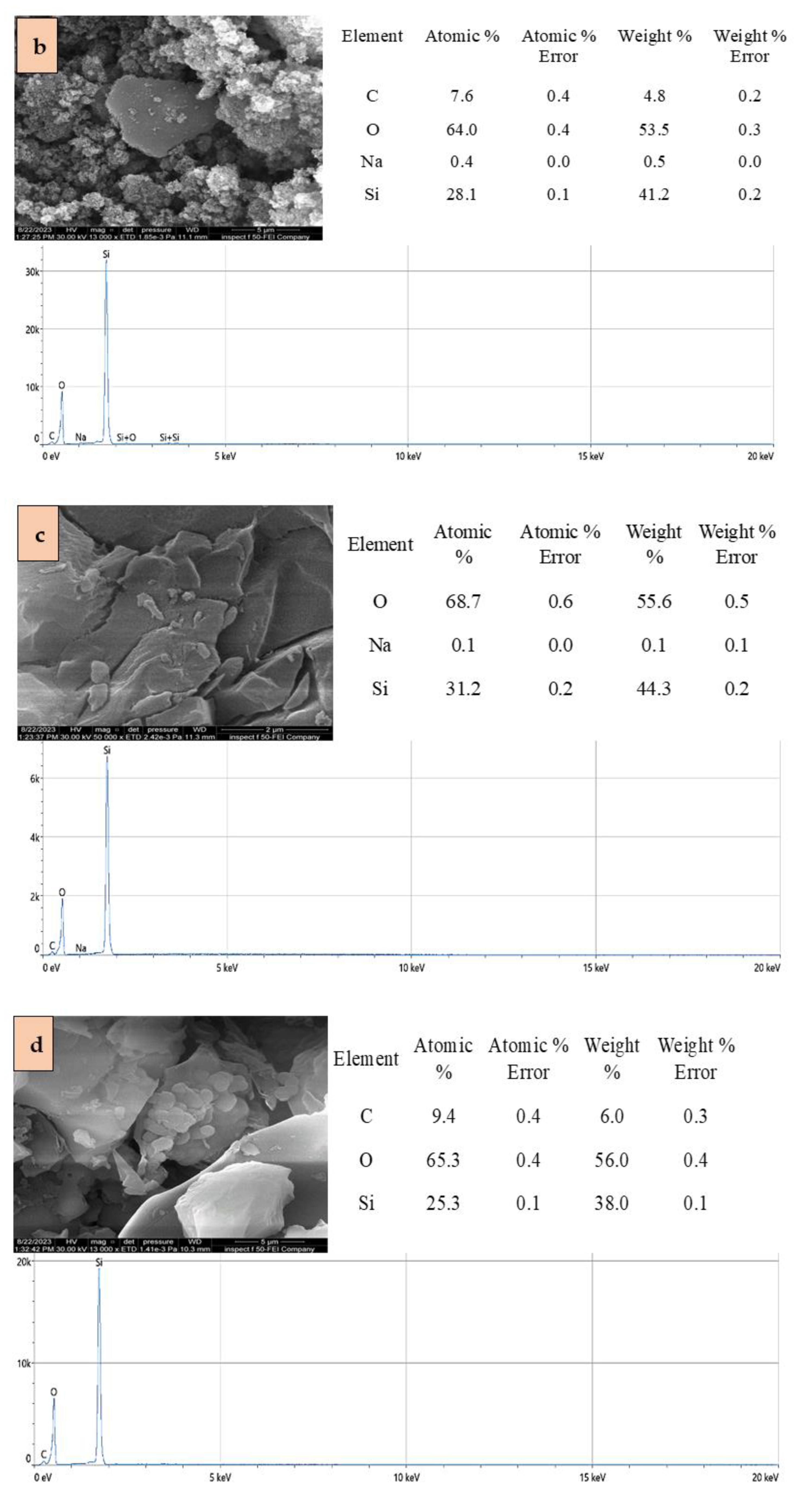
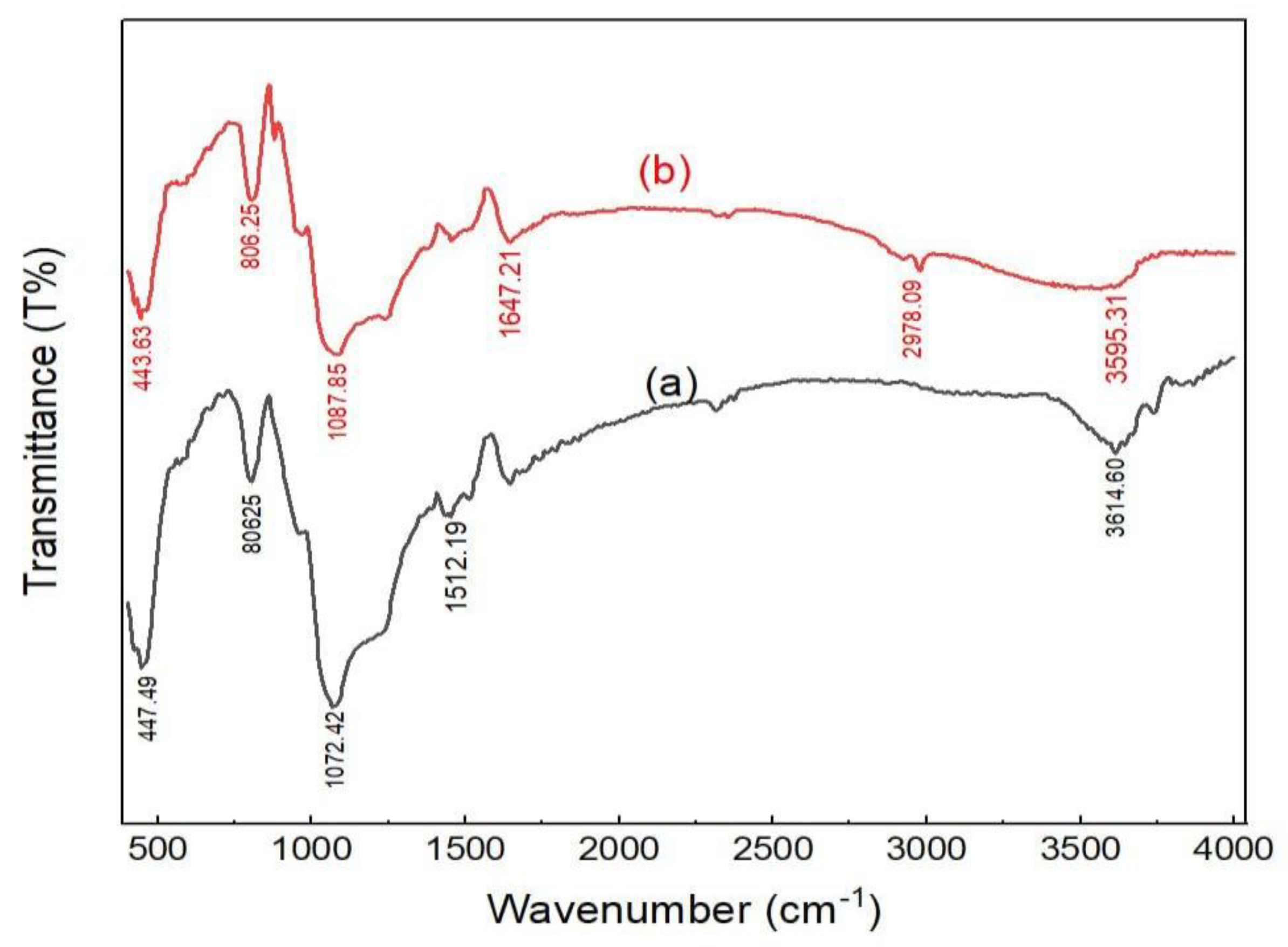

| Adsorbent | Specific Surface Area (BET) (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| MCM-41 | 996 | 0.91 |
| Nano-SiO2 | 136.36 | 0.59 |
| Bed Temp. (°C) | Adsorption Capacity (mmol-CO2/g-Sorbent) | Saturation Time (min) | ||||
|---|---|---|---|---|---|---|
| MCM-41 | ||||||
| 5% | 10% | 15% | 5% | 10% | 15% | |
| 20 | 0.48 | 0.56 | 0.61 | 4.5 | 2.5 | 1.8 |
| 40 | 0.55 | 0.62 | 0.71 | 5 | 2.7 | 2.3 |
| 60 | 0.62 | 0.68 | 0.73 | 6 | 3 | 2.3 |
| 80 | 0.5 | 0.62 | 0.72 | 4.8 | 2.8 | 2.3 |
| Nano-SiO2 | ||||||
| 5% | 10% | 15% | 5% | 10% | 15% | |
| 20 | 0.6 | 0.66 | 0.71 | 4.5 | 2.5 | 2.5 |
| 40 | 0.53 | 0.60 | 0.67 | 5 | 2.5 | 2.5 |
| 60 | 0.45 | 0.57 | 0.61 | 6 | 3.5 | 2.5 |
| 80 | 0.31 | 0.43 | 0.52 | 6.3 | 3.8 | 2.9 |
| Chemical Compound | Chemical Formula | Molecular Weight (g/mole) | Origin | Purity (wt.%) |
|---|---|---|---|---|
| Tetraethyl orthosilicate (TEOS) | Si (OC2H5)4 | 208.33 | Hubei Bluesky New Material Inc., Xiantao, China | 98% |
| CetyltrimethylammoniumBromide (CTAB) | C19H42BrN | 364.45 | Interchiniques SA, France | 98% |
| Sodium hydroxide | NaOH | 39.99 | Didactic, Barcelona, Spain | 99% |
| Nano-Silicon dioxide | SiO2 | 60.08 | Hubei Bluesky New MaterialInc., Xiantao, China | >98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, H.F.; Al-Sudani, F.T.; Albayati, T.M.; Salih, I.K.; Harharah, H.N.; Majdi, H.S.; Saady, N.M.C.; Zendehboudi, S.; Amari, A. Synthesizing and Characterizing a Mesoporous Silica Adsorbent for Post-Combustion CO2 Capture in a Fixed-Bed System. Catalysts 2023, 13, 1267. https://doi.org/10.3390/catal13091267
Hasan HF, Al-Sudani FT, Albayati TM, Salih IK, Harharah HN, Majdi HS, Saady NMC, Zendehboudi S, Amari A. Synthesizing and Characterizing a Mesoporous Silica Adsorbent for Post-Combustion CO2 Capture in a Fixed-Bed System. Catalysts. 2023; 13(9):1267. https://doi.org/10.3390/catal13091267
Chicago/Turabian StyleHasan, Hind F., Farah T. Al-Sudani, Talib M. Albayati, Issam K. Salih, Hamed N. Harharah, Hasan Sh. Majdi, Noori M. Cata Saady, Sohrab Zendehboudi, and Abdelfattah Amari. 2023. "Synthesizing and Characterizing a Mesoporous Silica Adsorbent for Post-Combustion CO2 Capture in a Fixed-Bed System" Catalysts 13, no. 9: 1267. https://doi.org/10.3390/catal13091267
APA StyleHasan, H. F., Al-Sudani, F. T., Albayati, T. M., Salih, I. K., Harharah, H. N., Majdi, H. S., Saady, N. M. C., Zendehboudi, S., & Amari, A. (2023). Synthesizing and Characterizing a Mesoporous Silica Adsorbent for Post-Combustion CO2 Capture in a Fixed-Bed System. Catalysts, 13(9), 1267. https://doi.org/10.3390/catal13091267










