CO2 Hydrogenation to Methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2-SiO2 Catalysts Synthesized with β-Cyclodextrin Template
Abstract
:1. Introduction
2. Results
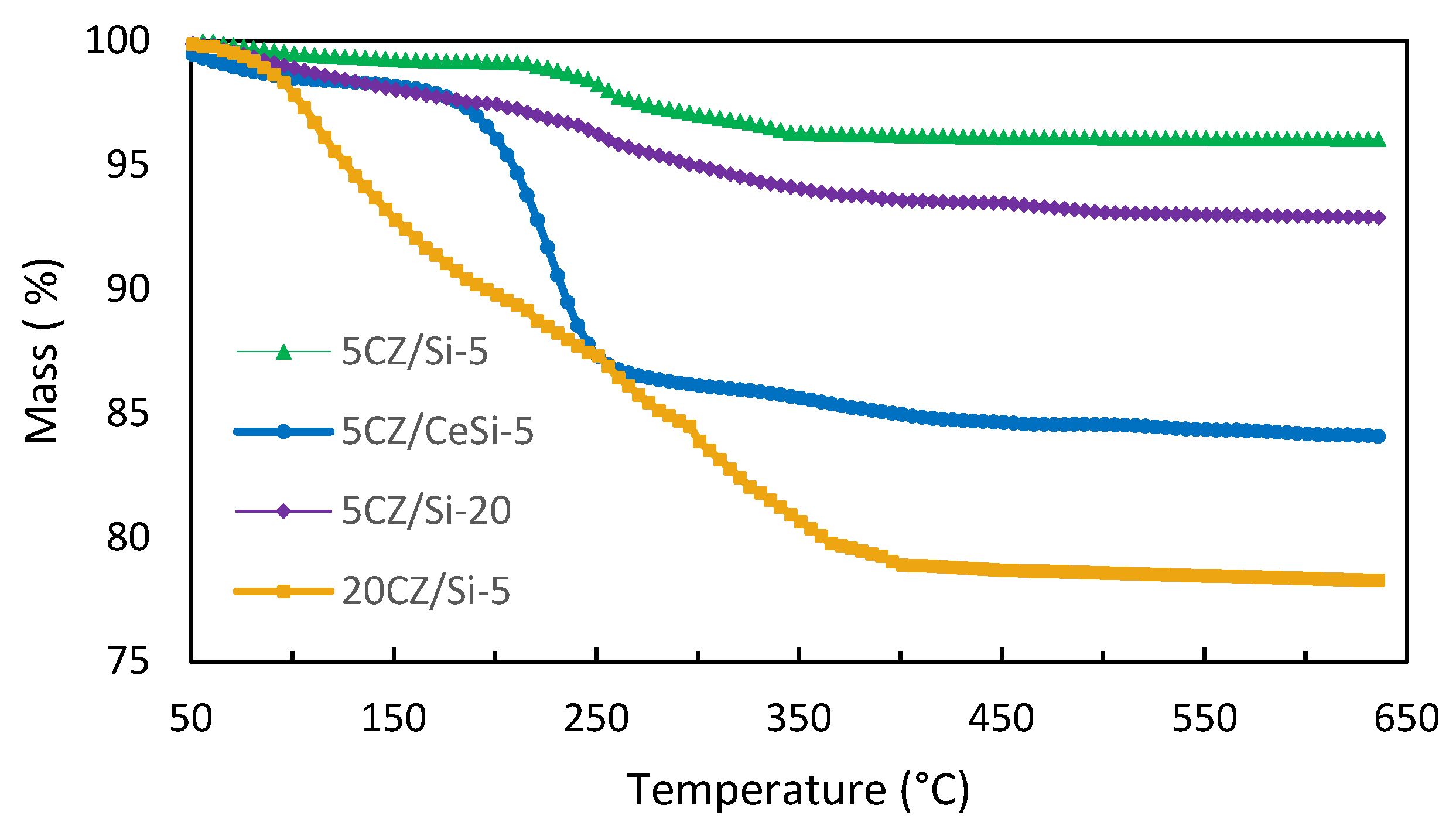
2.1. Thermal Analysis
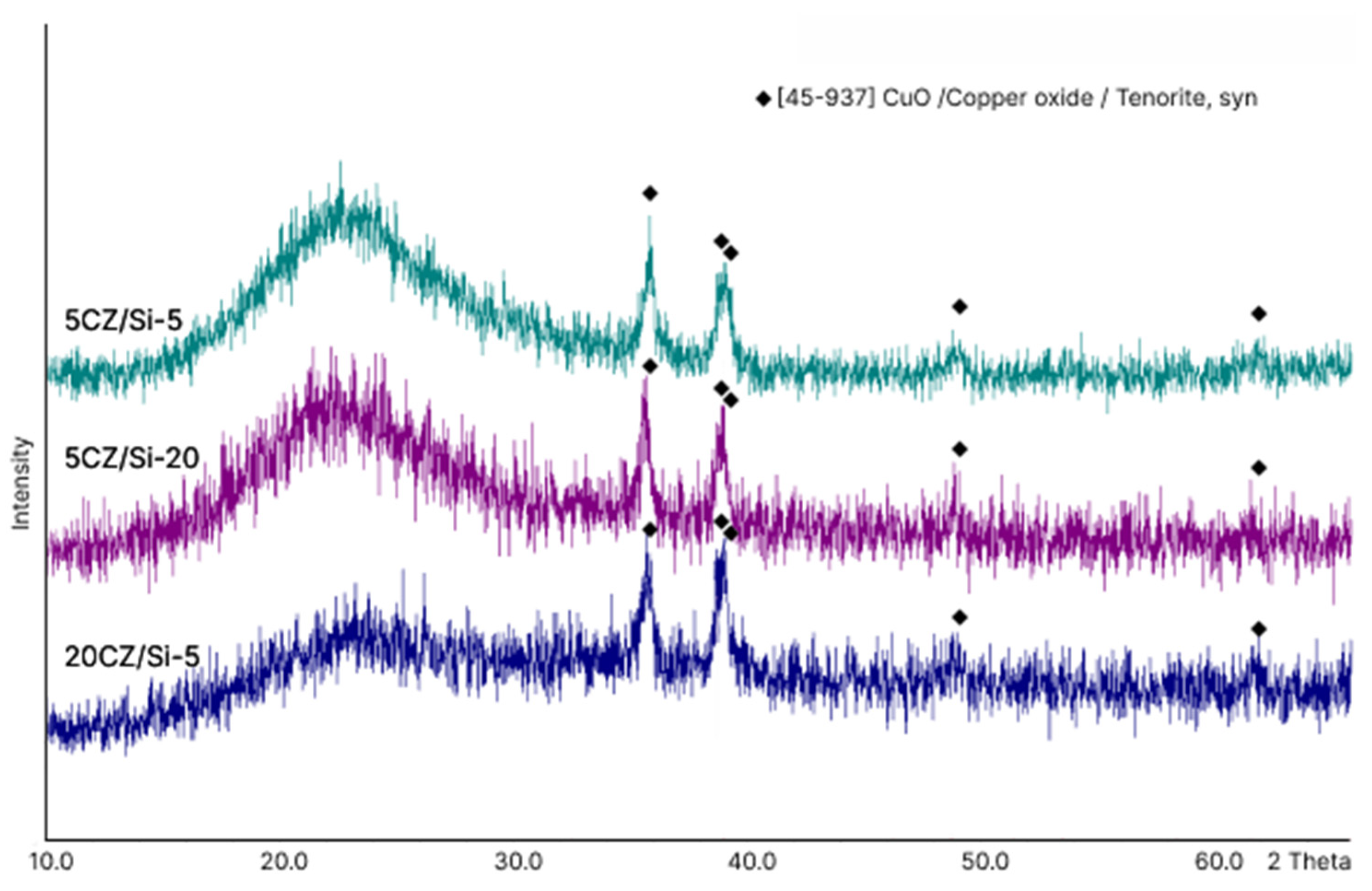
2.2. X-ray Diffraction Analysis
2.3. Textural Properties
2.4. Energy-Dispersive X-ray Spectroscopy
2.5. X-ray Photoelectron Spectroscopy
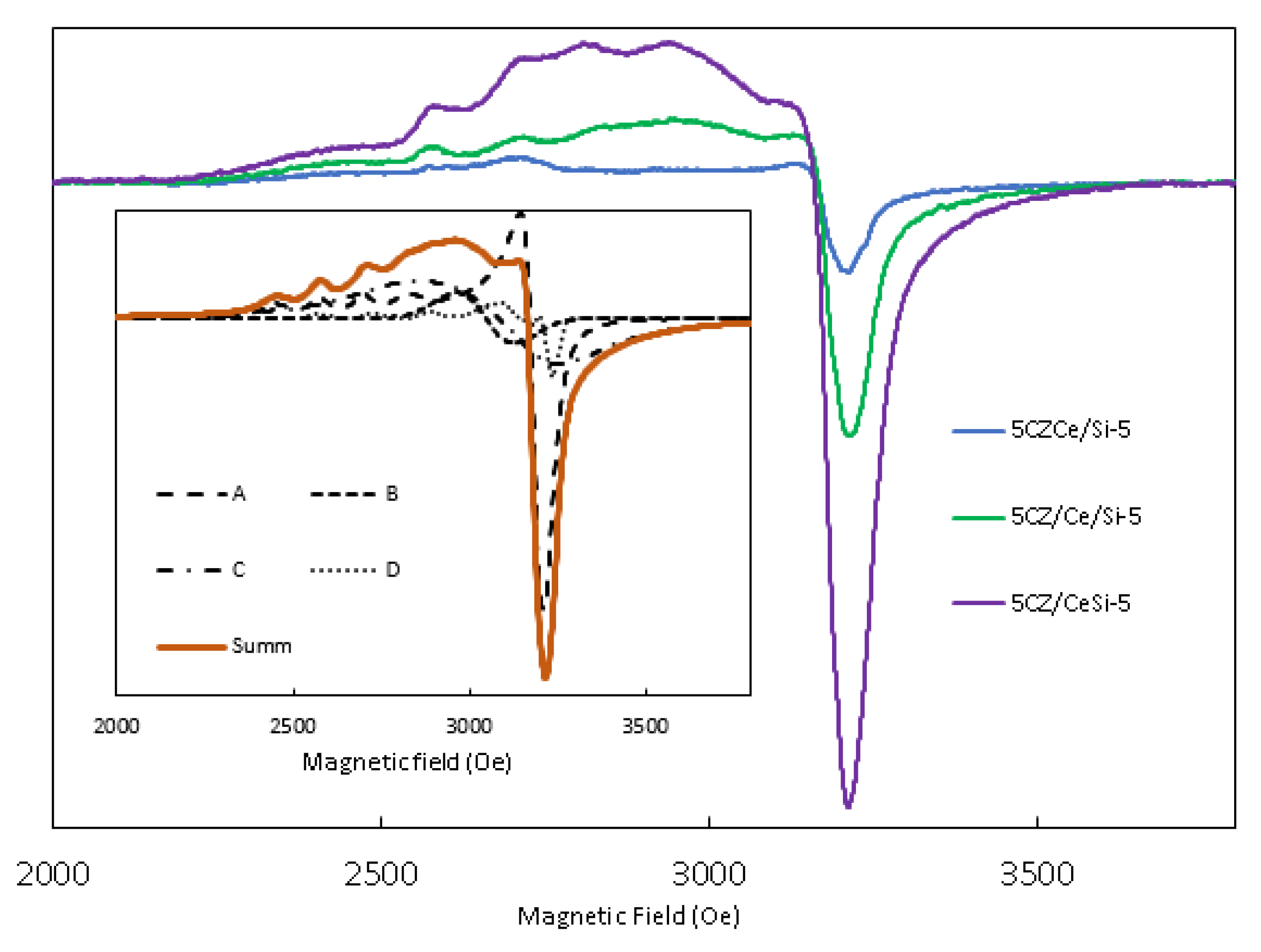
2.6. Magnetic Properties
2.7. Electron Paramagnetic Resonance Spectroscopy
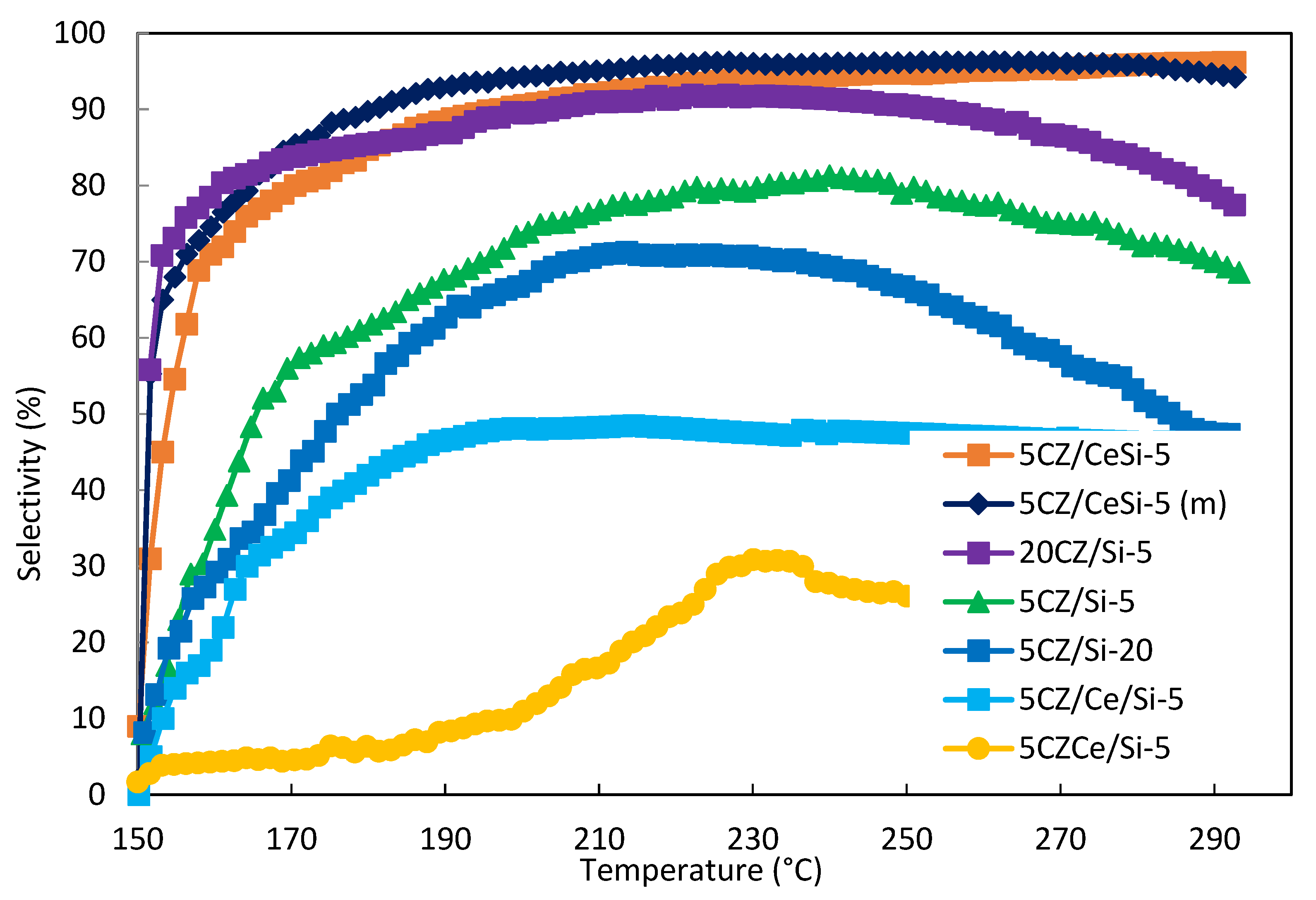
2.8. Catalytic Activity
3. Materials and Methods
3.1. Synthesis of the Supports
3.2. Synthesis of the Catalysts
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irish, J.L.; Sleath, A.; Cialone, M.A.; Knutson, T.R.; Jensen, R.E. Simulations of Hurricane Katrina (2005) under sea level and climate conditions for 1900. Clim. Chang. 2014, 122, 635–649. [Google Scholar] [CrossRef]
- Li, M.M.-J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. Catalytic conversion of CO2 to chemicals and fuels. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Synthesis, Characterization, and Activity Pattern of Ni–Al Hydrotalcite Catalysts in CO2 Methanation. Ind. Eng. Chem. Res. 2016, 55, 8299–8308. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, H.; Wang, Y.; Gao, J.; Tang, Y.; Wan, P.; Hu, Q.; Lv, J.; Zhang, T.; Yang, X.J. In situ hydrogenation of CO2 by Al/Fe and Zn/Cu alloy catalysts under mild conditions. Chem. Eng. Technol. 2019, 42, 1223–1231. [Google Scholar] [CrossRef]
- Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 2019, 371, 368–375. [Google Scholar] [CrossRef]
- Schittkowski, J.; Ruland, H. Methanol Synthesis from Steel Mill Exhaust Gases: Challenges for the Industrial Cu/ZnO/Al2O3 Catalyst. Chem. Ing. Tech. 2018, 90, 1419–1429. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Deka, T.J.; Osman, A.I.; Baruah, D.C. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Dubois, J.L.; Sayama, K.; Arakawa, H. Conversion of CO2 to Dimethylether and Methanol over Hybrid Catalysts. Chem. Lett. 1992, 21, 1115–1118. [Google Scholar] [CrossRef]
- Yang, Y.; Evans, J.; Rodriguez, J.A.; White, M.G.; Liu, P. Fundamental studies of methanol synthesis from CO2 hydrogenation on Cu(111), Cu clusters, and Cu/ZnO(000). Phys. Chem. Chem. Phys. 2010, 12, 9909–9917. [Google Scholar] [CrossRef] [PubMed]
- Grabow, L.C.; Mavrikakis, M. Mechanism of Methanol Synthesis on Cu through CO2 and CO Hydrogenation. J. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Yang, Y.; Mims, C.; Peden, C.H.F.; Li, J.; Mei, D. Insight into methanol synthesis from CO2 hydrogenation on Cu(111): Complex reaction network and the effects of H2O. J. Catal. 2011, 281, 199–211. [Google Scholar] [CrossRef]
- Nabi, A.G.; Aman-ur-Rehman; Hussain, A.; Chass, A.G.; Tommaso, D.D. Optimal icosahedral copper-based bimetallic clusters for the selective electrocatalytic CO2 conversion to one carbon products. Nanomaterials 2023, 13, 87. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, F.; Geng, S.; Yao, M.; Ma, J.; Cao, J. Tuning the content of S vacancies in MoS2 by Cu doping for enhancing catalytic hydrogenation of CO2 to methanol. J. Mol. Catal. 2023, 547, 113288. [Google Scholar] [CrossRef]
- Latsiou, A.I.; Charisiou, N.D.; Frontistis, Z.; Bansode, A.; Goula, M.A. CO2 hydrogenation for the production of higher alcohols: Trends in catalyst developments, challenges and opportunities. Catal. Today 2023, 420, 114179. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. J. Nanomater. 2022, 12, 2379. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable Ni/La-M (M = Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation. J. CO2 Util. 2021, 51, 101618. [Google Scholar] [CrossRef]
- Evdokimenko, N.; Yermekova, Z.; Roslyakov, S.; Tkachenko, O.; Kapustin, G.; Bindiug, D.; Kustov, A.; Mukasyan, A.S. Sponge-like CoNi Catalysts Synthesized by Combustion of Reactive Solutions: Stability and Performance for CO2 Hydrogenation. Materials 2022, 15, 5129. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Kustov, A.L.; Pokusaeva, Y.A.; Bogdan, T.V.; Kustov, L.M. Carbon Dioxide Reduction with Hydrogen on Fe, Co Supported Alumina and Carbon Catalysts under Supercritical Conditions. Molecules 2021, 26, 2883. [Google Scholar] [CrossRef]
- Grosse, P.; Yoon, A.; Rettenmaier, C. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 2021, 5, 6736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Huang, X.B.; Wang, X.X.; Wang, X.K. Progress in Catalyst Exploration for Heterogeneous CO2 Reduction and Utilization: A Critical Review. J. Mater. Chem. 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Wang, J.; Funk, S.; Burghaus, U. Indications for Metal-Support Interactions: The Case of CO2 Adsorption on Cu/ZnO(0001). Catal. Lett. 2005, 103, 219–223. [Google Scholar] [CrossRef]
- Kuld, S.; Conradsen, C.; Moses, P.G.; Chorkendorff, I.; Sehested, J. Quantification of Zinc Atoms in a Surface Alloy on Copper in an Industrial-Type Methanol Synthesis Catalyst. Angew. Chem. Int. Edit. 2014, 53, 5941–5945. [Google Scholar] [CrossRef]
- Kim, K.O.; Evdokimenko, N.D.; Pribytkov, P.V.; Tedeeva, M.A.; Borkov, S.A.; Kustov, A.L. Synthesis of Methanol from CO2 on Cu–Zn/xAl2O3–(1—X)SiO2 Catalysts. Effect of Support Composition. Russ. J. Phys. Chem. 2021, 95, 2422–2425. [Google Scholar] [CrossRef]
- Tursunov, O.; Kustov, A.; Kustov, L. A brief review of carbon dioxide hydrogenation to methanol over copper and iron based catalysts. Oil Gas Sci. Technol. 2017, 72, 30. [Google Scholar] [CrossRef]
- Evdokimenko, N.D.; Kapustin, G.I.; Tkachenko, O.P.; Kalmykov, K.B.; Kustov, A.L. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules 2022, 27, 1065. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, S.Q.; Wang, W.W. Partially sintered copper–ceria as excellent catalyst for the high-temperature reverse water gas shift reaction. Nat. Commun. 2022, 13, 867. [Google Scholar] [CrossRef]
- Wang, W.-W.; Du, P.-P.; Zou, S.-H.; He, H.-Y.; Wang, R.-X.; Jin, Z.; Shi, S.; Huang, Y.-Y.; Si, R.; Song, Q.-S.; et al. Highly Dispersed Copper Oxide Clusters as Active Species in Copper–Ceria Catalyst for Preferential Oxidation of Carbon Monoxide. ACS Catal. 2015, 5, 2088–2099. [Google Scholar] [CrossRef]
- Wang, W.-W.; Yu, W.-Z.; Du, P.-P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.-J.; Yan, C.-H. Crystal Plane Effect of Ceria on Supported Copper Oxide Cluster Catalyst for CO Oxidation: Importance of Metal-Support Interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Sripada, P.; Kimpton, J.; Barlow, A.; Williams, T.; Kandasamy, S.; Bhattacharya, S. Investigating the dynamic structural changes on Cu/CeO2 catalysts observed during CO2 hydrogenation. J. Catal. 2020, 381, 415–426. [Google Scholar] [CrossRef]
- Evdokimenko, N.D.; Kustov, A.L.; Kim, K.O.; Mishin, I.V.; Nissenbaum, V.D.; Kapustin, G.I.; Aymaletdinov, T.R.; Kustov, L.M. Ce–Zr materials with a high surface area as catalyst supports for hydrogenation of CO2. Funct. Mat. Lett. 2020, 13, 2040004. [Google Scholar] [CrossRef]
- Cui, S.; Cheng, W.; Shen, X.; Fan, M.; Russell, A.T.; Wu, Z.; Yi, X. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energ. Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.H.; Chen, J.G.G.; Liu, P. CO2 Hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of Synergy between Pt and Oxide Support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef]
- Kusama, H.; Bando, K.K.; Okabe, K.; Arakawa, H. CO2 Hydrogenation Reactivity and Structure of Rh/SiO2 Catalysts Prepared from Acetate, Chloride and Nitrate Precursors. Appl. Catal. A Gen. 2001, 205, 285–294. [Google Scholar] [CrossRef]
- Feng, Y.; Panwar, N.; Tng, D.J.H.; Tjin, S.C.; Wang, K.; Yong, K.T. The application of mesoporous silica nanoparticle family in cancer theranostics. Coord. Chem. Rev. 2016, 319, 86–109. [Google Scholar] [CrossRef]
- Gurav, I.L.; In-Keun, J.; Hyung-Ho, P.; Eul Son, K.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Milea, C.; Bogatu, C.; Duta, A. The influence of parameters in silica sol-gel process. Bull. Transilv. Univ. Brasov. Eng. Sci. 2011, 4, 5. [Google Scholar]
- Davis, M.; Brewster, M. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Mashkin, M.; Tedeeva, M.; Fedorova, A.; Vasiliev, A.; Egorov, A.; Pribytkov, P.; Kalmykov, K.; Kapustin, G.; Morozov, I.; Kustov, L.; et al. CrOx/SiO2 mesoporous catalysts prepared using beta-cyclodextrin as a template and their catalytic properties in propane oxidative dehydrogenation in the presence of carbon dioxide. Microporous and Mesoporous. Materials 2022, 38, 111967. [Google Scholar] [CrossRef]
- Fedorova, A.A.; Morozov, I.V.; Kotovshchikov, Y.N.; Romanovsky, B.V.; Sirotin, S.V.; Knyazeva, E.E.; Lermontov, A.S.; Shaporev, A.S. Preparation and characterization of copper- and iron-containing mesoporous silica using β-cyclodextrin as a structure-directing agent. Mendeleev Comm. 2011, 21, 171–172. [Google Scholar] [CrossRef]
- Solms, J.; Egli, R.H. Harze mit Einschlusshohlraumen von cyclodextrin-struktur. Helv. Chim. Acta 1965, 48, 1225–1228. [Google Scholar] [CrossRef]
- Mosher, G.; Thompson, D.O. Complexation and cyclodextrins. In Encyclopedia of Pharmaceutical Technology, 2nd ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 531–558. [Google Scholar]
- Kaplin, I.Y.; Lokteva, E.S.; Maslakov, K.I.; Tikhonov, A.V.; Kharlanov, A.N.; Fionov, A.V.; Kamaev, A.O.; Isaikina, O.Y.; Maksimov, S.V.; Golubina, E.V. Ceria-silica mesoporous catalysts for CO preferential oxidation in H2-rich stream: The effect of Ce:Si ratio and copper modification. Appl. Surf. Sci. 2022, 594, 153473. [Google Scholar] [CrossRef]
- Kappis, K.; Papadopoulos, C.; Papavasiliou, J.; Vakros, J.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Tuning the Catalytic Properties of Copper-Promoted Nanoceria via a Hydrothermal Method. Catalysts 2019, 9, 138. [Google Scholar] [CrossRef]
- Punnoose, A.; Mathew, J.; Mayura, B.P.; Umar, M.; Singh, R.J. ESR Study of CuO Bulk Powder and Thin Films. Mod. Phys. Lett. 1992, 6, 1043–1047. [Google Scholar] [CrossRef]
- Andreev, M.N.; Ratnikov, D.S.; Dolzhenko, V.D.; Belousov, Y.A.; Drozdov, A.A. Influence of macro- and microcomponent content on the color of M2O-PbO-SiO2 (M = Li, Na, K, Rb, Cs) glasses doped with copper. Russ. Chem. Bull. 2020, 69, 697–703. [Google Scholar] [CrossRef]
- Vasenin, N.T.; Fedorova, A.A.; Anifrienko, V.F.; Larina, T.V.; Morozov, I.V.; Paukshtis, E.A.; Ismagilov, Z.R. The special features of the electronic state of copper and the structure of copper-containing fragments in CuO-ZrO2 catalysts synthesized in molten ammonium nitrate. Rus. J. Phys. Chem. 2005, 79, 1249–1255. [Google Scholar]
- Gonella, F.; Caccavale, F.; Bogomolova, L. Application of electron paramagnetic resonance to the study of Cu2+ ions in Cu-Na ion-exchanged glasses. Appl. Phys. A 1999, 68, 539–546. [Google Scholar] [CrossRef]
- Komova, O.V.; Simaov, A.V.; Rogov, V.A.; Kochunei, D.I.; Odegova, G.V.; Kriventsov, V.V.; Paukshtis, E.A.; Ushakov, V.A.; Sazonova, N.N.; Nikoro, T.A. Investigation of the state of copper in supported copper-titanium oxide catalysts. J. Mol. Cat. A Chem. 2000, 161, 191–204. [Google Scholar] [CrossRef]
- Reddy, K.P.; Kim, D.; Hong, S.; Kim, K.J.; Ryoo, R.; Park, J.Y. Tuning CO2 Hydrogenation Selectivity through Reaction-Driven Restructuring on Cu–Ni Bimetal Catalysts. ACS Appl. Mater. Interfaces 2023, 15, 9373–9381. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Chen, S. Effect of promoter SiO2, TiO2 or SiO2-TiO2 on the performance of CuO-ZnO-Al2O3 catalyst for methanol synthesis from CO2 hydrogenation. Appl. Catal. A Gen. 2012, 415–416, 118–123. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Li, L.; Chang, X.; Wu, S.; Yang, C.; Song, X.; Zhao, Z.J.; Gong, J. Nature of catalytic behavior of cobalt oxides for CO2 hydrogenation. JACS Au 2023, 3, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, E.; Wang, Y. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1033. [Google Scholar] [CrossRef]

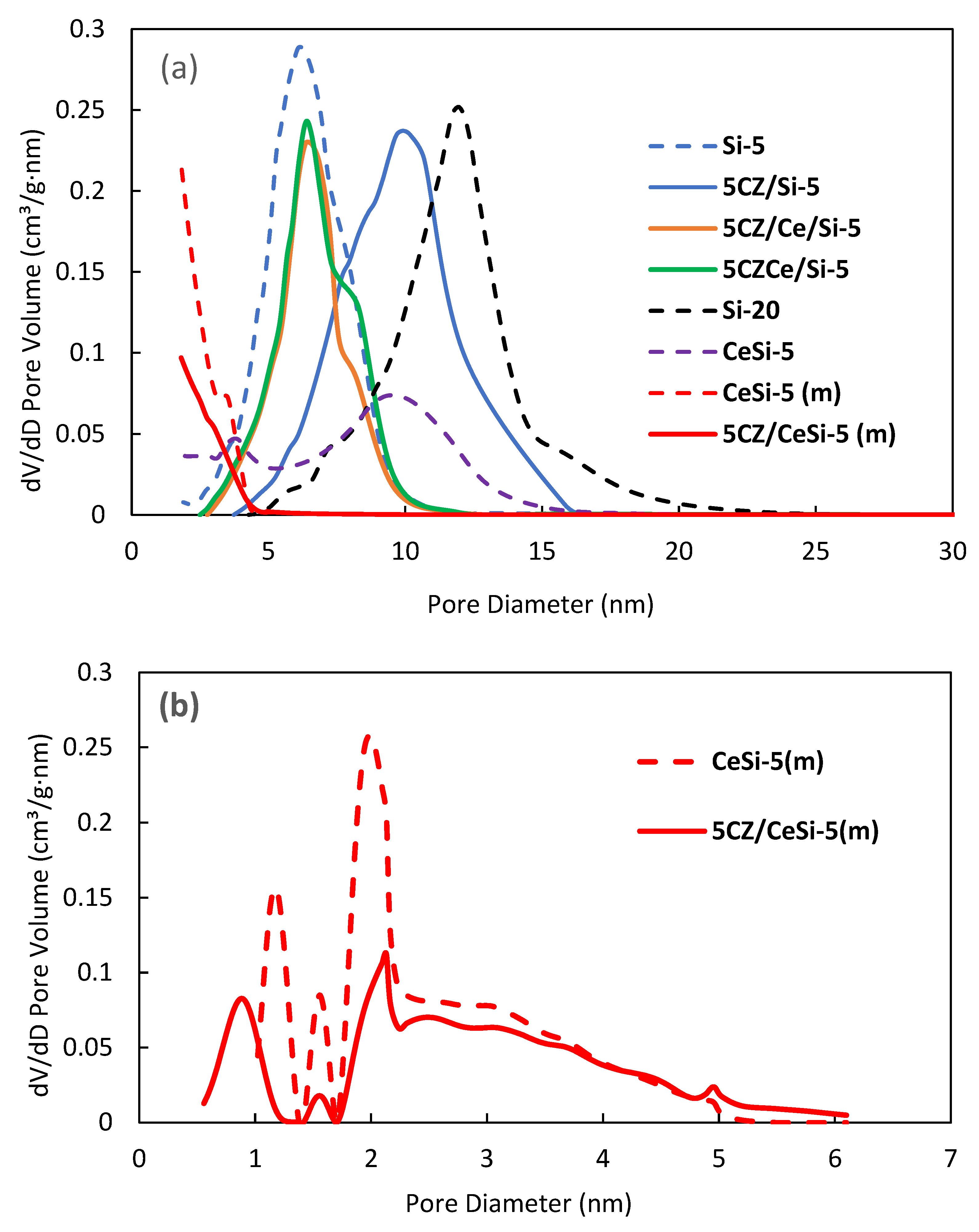


| Sample | S, m2/g | Dmax, nm | Vtot, cm3/g | Vmeso, cm3/g | Vmicro, cm3/g |
|---|---|---|---|---|---|
| Si-5 | 542 | 6.4 | 0.89 | 0.89 | - |
| 5CZ/Si-5 | 363 | 10.2 | 0.90 | 0.90 | - |
| 5CZCe/Si-5 | 376 | 6.4 | 0.66 | 0.66 | - |
| 5CZ/Ce/Si-5 | 325 | 6.4 | 0.59 | 0.59 | - |
| Si-20 | 354 | 12.2 | 1.06 | 1.06 | - |
| CeSi-5 | 247 | 3.9, 9.5 | 0.46 | 0.46 | - |
| CeSi-5 (m) | 554 (273 *) | 1.2, 1.6, 2.0 | 0.29 | 0.17 | 0.12 |
| 5CZ/CeSi-5 (m) | 380 (80 *) | 0.7, 2.1 | 0.22 | 0.17 | 0.05 |
| Sample | ν (Ce), % | ν (Cu), % | ||
|---|---|---|---|---|
| Ce4+ | Ce3+ | Cu2+ | Cu+ | |
| 5CZ/Ce/Si-5 | ||||
| fast acquisition (start *) | 97 | 3 | 77 | 23 |
| long-term acquisition | 90 | 10 | 53 | 47 |
| fast acquisition (end **) | 88 | 12 | 37 | 63 |
| 5CZCe/Si-5 | ||||
| fast acquisition (start) | 97 | 3 | 87 | 13 |
| long-term acquisition | 93 | 7 | 63 | 37 |
| fast acquisition (end) | 91 | 9 | 58 | 42 |
| 5CZ/CeSi-5 | ||||
| fast acquisition (start) | 92 | 8 | 97 | 3 |
| long-term acquisition | 71 | 29 | 62 | 38 |
| fast acquisition (end) | 65 | 35 | 54 | 46 |
| Sample | Magnetic Susceptibility, emu/g | Magnetic Susceptibility Taking into Account Pascal Constants, emu/g | Magnetic Susceptibility, emu/mol Cu | Cu+2 Content, % |
|---|---|---|---|---|
| 5CZ/Ce/Si-5 | 4.26 × 10−7 | 4.34 × 10−7 | 6.90 × 10−4 | 54 |
| 5CZ/CeSi-5 | 6.76 × 10−7 | 6.76 × 10−7 | 1.07 × 10−3 | 84 |
| Catalyst | Support | CD Type | m(H2O)/m(SiO2) | wt.% CuO |
|---|---|---|---|---|
| 5CZ/Si-5 | Si-5 | β-CD | 5:1 | 5 |
| 5CZ/Si-20 | Si-20 | β-CD | 20:1 | 5 |
| 20CZ/Si-5 | Si-5 | β-CD | 5:1 | 20 |
| 5CZ/CeSi-5 | CeSi-5 | β-CD | 5:1 | 5 |
| 5CZ/CeSi-5(m) | CeSi-5 (m) | m-β-CD | 5:1 | 5 |
| 5CZCe/Si-5 * | Si-5 | β-CD | 5:1 | 5 |
| 5CZ/Ce/Si-5 ** | Si-5 | β-CD | 5:1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vertepov, A.E.; Fedorova, A.A.; Batkin, A.M.; Knotko, A.V.; Maslakov, K.I.; Doljenko, V.D.; Vasiliev, A.V.; Kapustin, G.I.; Shatalova, T.B.; Sorokina, N.M.; et al. CO2 Hydrogenation to Methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2-SiO2 Catalysts Synthesized with β-Cyclodextrin Template. Catalysts 2023, 13, 1231. https://doi.org/10.3390/catal13091231
Vertepov AE, Fedorova AA, Batkin AM, Knotko AV, Maslakov KI, Doljenko VD, Vasiliev AV, Kapustin GI, Shatalova TB, Sorokina NM, et al. CO2 Hydrogenation to Methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2-SiO2 Catalysts Synthesized with β-Cyclodextrin Template. Catalysts. 2023; 13(9):1231. https://doi.org/10.3390/catal13091231
Chicago/Turabian StyleVertepov, Andrey E., Anna A. Fedorova, Alexander M. Batkin, Alexander V. Knotko, Konstantin I. Maslakov, Vladimir D. Doljenko, Alexander V. Vasiliev, Gennadiy I. Kapustin, Tatyana B. Shatalova, Nadezhda M. Sorokina, and et al. 2023. "CO2 Hydrogenation to Methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2-SiO2 Catalysts Synthesized with β-Cyclodextrin Template" Catalysts 13, no. 9: 1231. https://doi.org/10.3390/catal13091231
APA StyleVertepov, A. E., Fedorova, A. A., Batkin, A. M., Knotko, A. V., Maslakov, K. I., Doljenko, V. D., Vasiliev, A. V., Kapustin, G. I., Shatalova, T. B., Sorokina, N. M., Kustov, L. M., Morozov, I. V., & Kustov, A. L. (2023). CO2 Hydrogenation to Methanol on CuO-ZnO/SiO2 and CuO-ZnO/CeO2-SiO2 Catalysts Synthesized with β-Cyclodextrin Template. Catalysts, 13(9), 1231. https://doi.org/10.3390/catal13091231







