Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Subsection
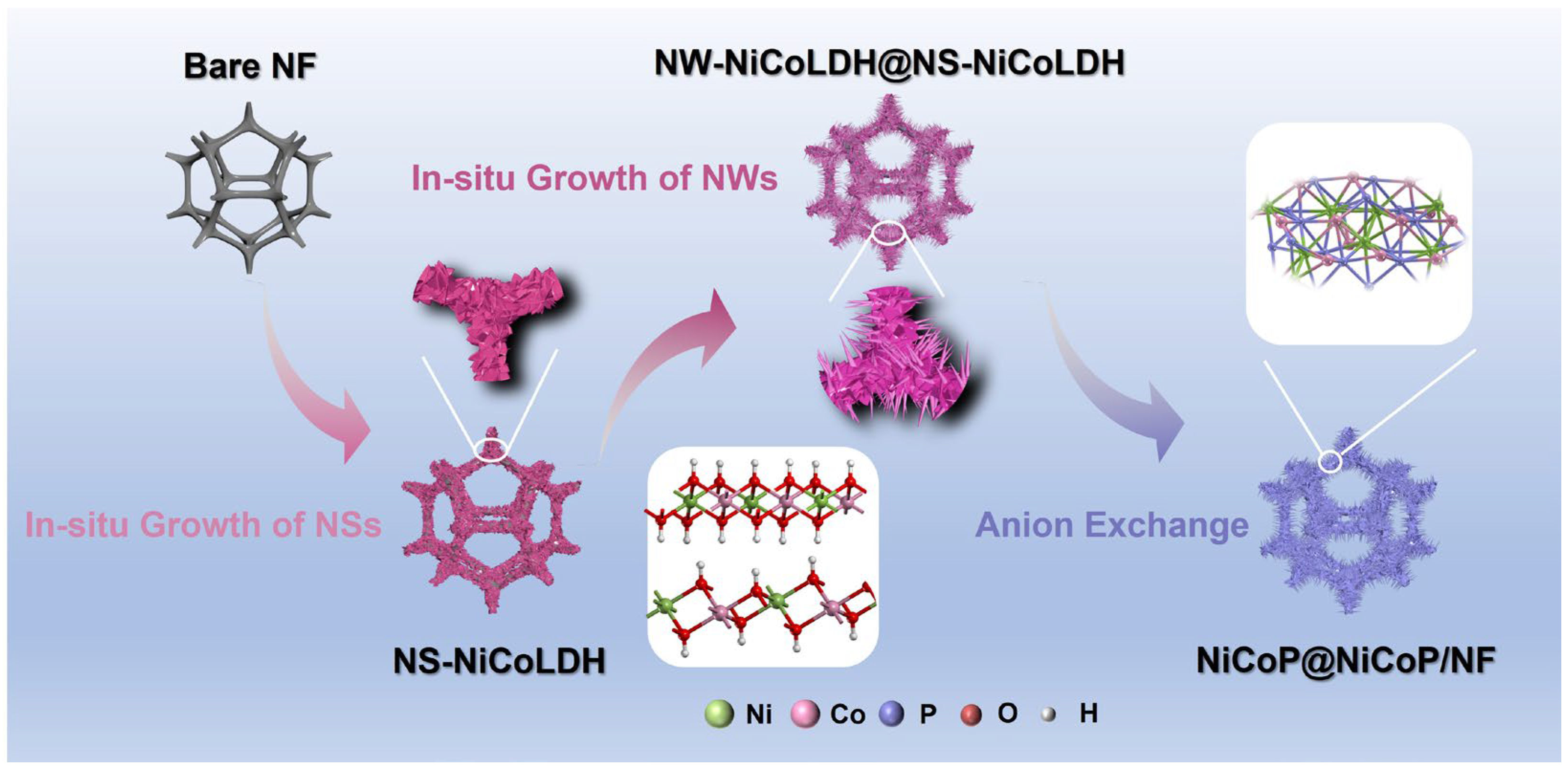
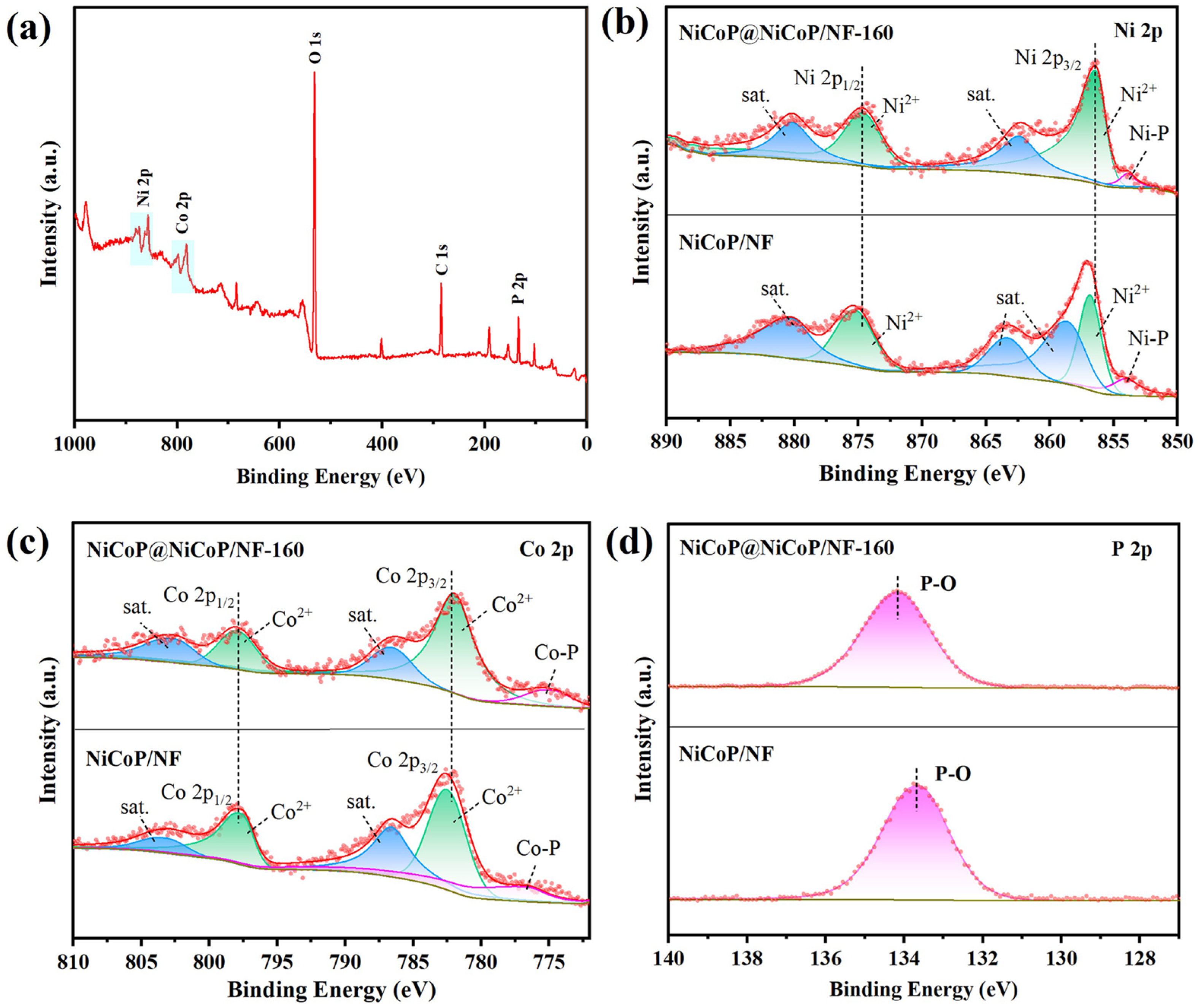
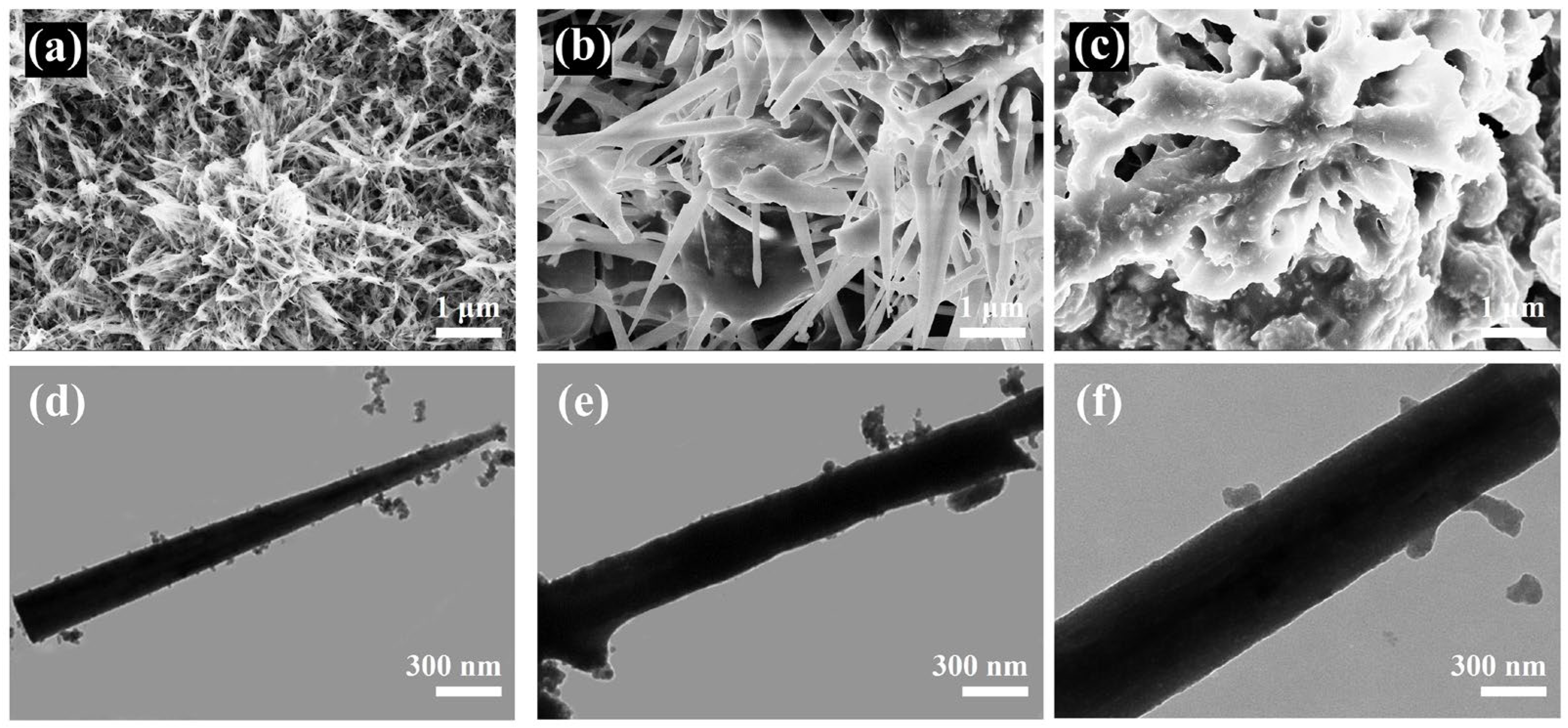
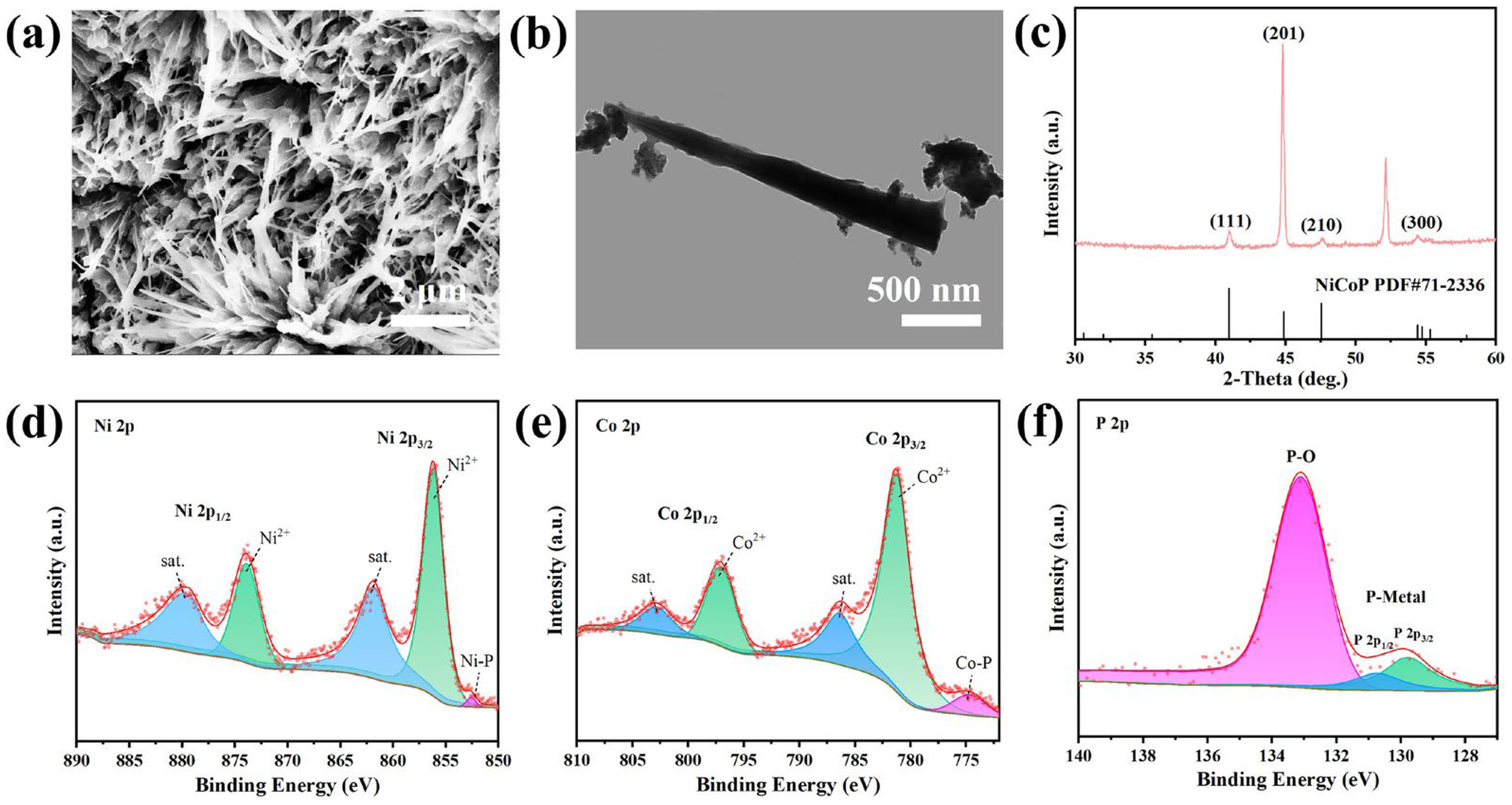
2.1.1. Structural Characterization of the Catalyst
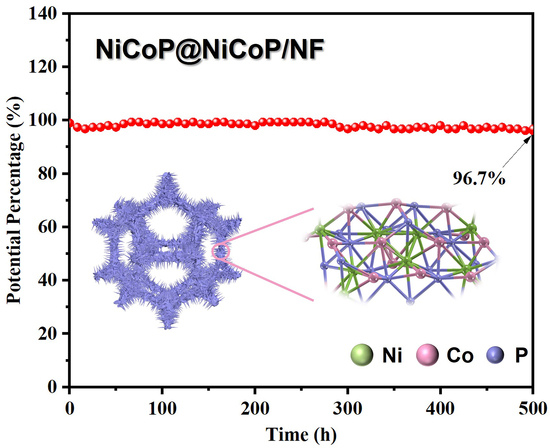
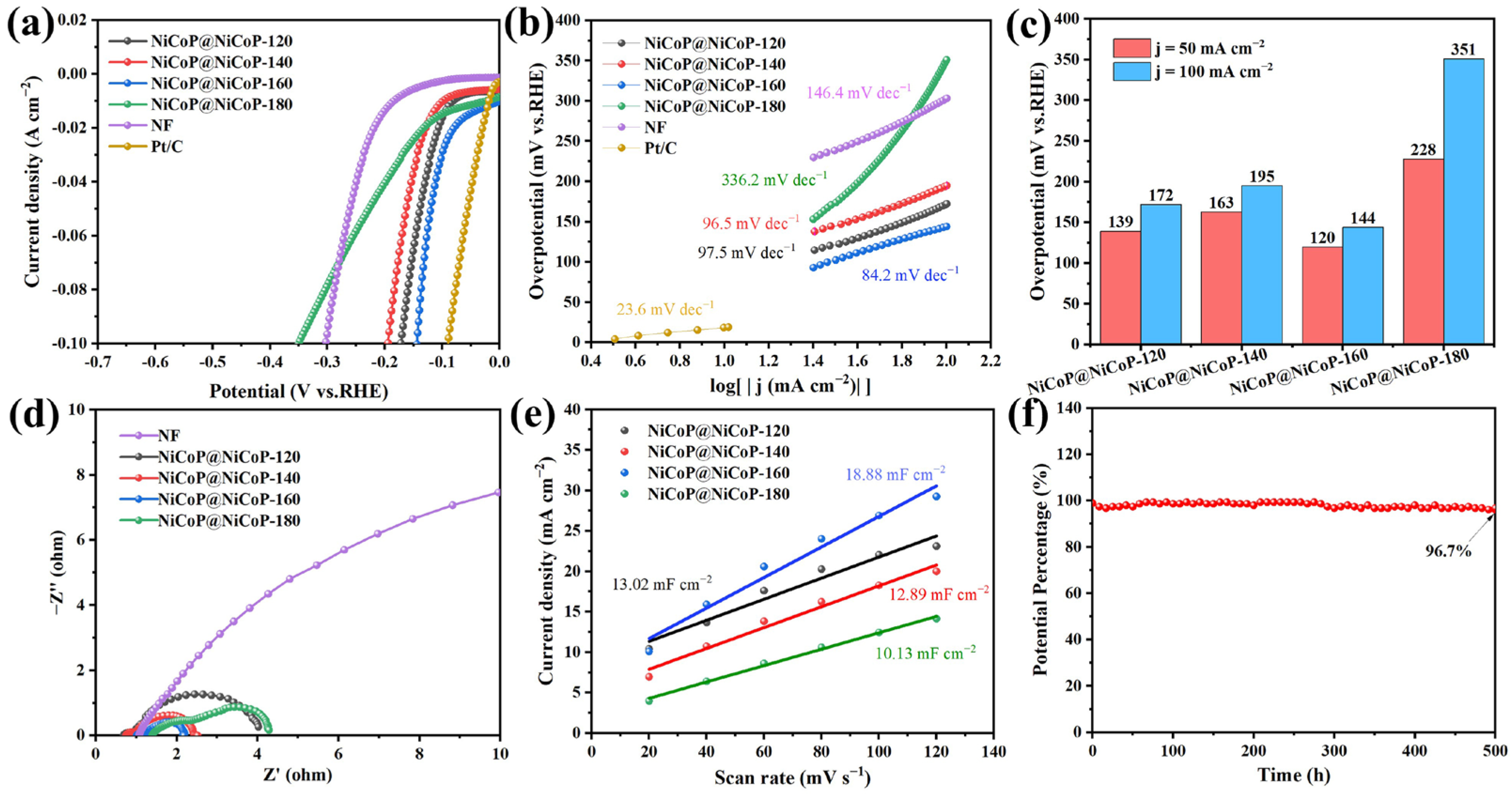
2.1.2. Characterization of Electrochemical Performance
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation
3.2.1. Synthesis of NiCo-LDH/NF
3.2.2. Synthesis of NiCo-LDH@NiCo-LDH/NF
3.2.3. Synthesis of NiCoP@NiCoP/NF
3.3. Materials Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Suliman, M.H.; Siddiqui, M.N.; Yamani, Z.H.; Merzougui, B.; Qamar, M. Interconnected Hollow Cobalt Phosphide Grown on Carbon Nanotubes for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 29407. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xie, Y.; Wang, H.; Xiao, F.; Wu, A.; Li, L.; Xu, Z.; Xiong, N.; Pan, K. Flower-like CoP microballs assembled with (002) facet nanowires via precursor route: Efficient electrocatalysts for hydrogen and oxygen evolution. Electrochim. Acta 2018, 259, 830. [Google Scholar] [CrossRef]
- Zhan, Z.; Gao, P.; Lei, T.; Zhang, S.; Du, Y. N-, Se-, and S-Doped Bimetallic NiCoP Nanosheet Arrays as Efficient Hydrogen Evolution Electrocatalysts. ACS Sustain. Chem. Eng. 2023, 11, 4980. [Google Scholar] [CrossRef]
- Wei, D.; Chen, L.; Tian, L.; Ramakrishna, S.; Ji, D. Hierarchically Structured CoNiP/CoNi Nanoparticle/Graphene/Carbon Foams as Effective Bifunctional Electrocatalysts for HER and OER. Ind. Eng. Chem. Res. 2023, 62, 4987. [Google Scholar] [CrossRef]
- Yang, L.; Yang, T.; Wang, E.; Yu, X.; Wang, K.; Du, Z.; Cao, S.; Chou, K.-C.; Hou, X. Bifunctional hierarchical NiCoP@FeNi LDH nanosheet array electrocatalyst for industrial-scale high-current-density water splitting. J. Mater. Sci. Technol. 2023, 159, 33. [Google Scholar] [CrossRef]
- Deng, Y.; Cao, Y.; Xia, Y.; Xi, X.; Wang, Y.; Jiang, W.; Yang, D.; Dong, A.; Li, T. Self-Templated Synthesis of CoFeP@C Cage-In-Cage Superlattices for Enhanced Electrocatalytic Water Splitting. Adv. Energy Mater. 2022, 12, 2202394. [Google Scholar] [CrossRef]
- Li, A.; Zhang, L.; Wang, F.; Zhang, L.; Li, L.; Chen, H.; Wei, Z. Rational design of porous Ni-Co-Fe ternary metal phosphides nanobricks as bifunctional electrocatalysts for efficient overall water splitting. Appl. Catal. B Environ. 2022, 310, 121353. [Google Scholar] [CrossRef]
- Lin, J.; Yan, Y.; Li, C.; Si, X.; Wang, H.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Bifunctional Electrocatalysts Based on Mo-Doped NiCoP Nanosheet Arrays for Overall Water Splitting. Nano Micro Lett. 2019, 11, 55. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Huang, X.; Zhu, Y.; Wang, D. Bimetallic Phosphide Heterostructure Coupled with Ultrathin Carbon Layer Boosting Overall Alkaline Water and Seawater Splitting. Small 2023, 19, e2206533. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Zhang, G.; Wu, M.; Chen, Z.; Sun, S.; Shi, Z. MnOx-Decorated Nickel-Iron Phosphides Nanosheets: Interface Modifications for Robust Overall Water Splitting at Ultra-High Current Densities. Small 2022, 18, e2105803. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, B.; De Luna, P.; Liang, Y.; Comin, R.; Voznyy, O.; Han, L.; Garcia de Arquer, F.P.; Liu, M.; Dinh, C.T.; et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 2018, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fan, J.; Wang, R.; Li, M.; Sun, S.; Xu, C.; Pan, F. Vacancies and interfaces engineering of core-shell heterostuctured NiCoP/NiO as trifunctional electrocatalysts for overall water splitting and zinc-air batteries. Green Energy Environ. 2023, 8, 601. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, L.; Sun, L.; Chen, G.; Luo, Q.; Xin, H.; Peng, J.; Li, Y.; Ma, F. Sulfur doping enhanced desorption of intermediates on NiCoP for efficient alkaline hydrogen evolution. Nanoscale 2020, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Fei, T.; Mao, C.; Song, Y.; Zhou, Y.; Dong, G. NiCoP/NF 1D/2D Biomimetic Architecture for Markedly Enhanced Overall Water Splitting. ChemElectroChem 2021, 8, 3064. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wu, Z.; Sheng, H.; Hu, Y.; Li, C.; Li, H.; Cao, L.; Dong, B. Crystalline/amorphous composite interface induced by NaBH4 hydrolysis reaction: A new interfacial electrocatalyst for efficient oxygen evolution reaction. Mater. Today Energy 2022, 26, 100987. [Google Scholar] [CrossRef]
- Chen, D.; Lu, R.; Pu, Z.; Zhu, J.; Li, H.-W.; Liu, F.; Hu, S.; Luo, X.; Wu, J.; Zhao, Y.; et al. Ru-doped 3D flower-like bimetallic phosphide with a climbing effect on overall water splitting. Appl. Catal. B Environ. 2020, 279, 119396. [Google Scholar] [CrossRef]
- Ding, X.; Pei, L.; Huang, Y.; Chen, D.; Xie, Z. Hollow NiCoP Nanoprisms Derived from Prussian Blue Analogues as Bifunctional Electrocatalysts for Urea-Assisted Hydrogen Production in Alkaline Media. Small 2022, 18, e2205547. [Google Scholar] [CrossRef]
- Jiang, N.; Shi, S.J.; Cui, Y.Y.; Jiang, B.L. The effect of calcination temperature on the hydrogen evolution reaction performance of Co/NiCoP nano-heterojunction. J. Alloys Compd. 2022, 929, 167229. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Lee, K.; Kim, K.; Kim, J.; Novak, T.G.; Jeon, S. Conformally Coated Nickel Phosphide on 3D, Ordered Nanoporous Nickel for Highly Active and Durable Hydrogen Evolution. ACS Sustain. Chem. Eng. 2020, 8, 17116. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Bae, G.; Yoon, Y.; Kim, K.; Kim, J.; Lee, Y.-L.; An, K.-S.; Jeon, S. Chemical Strain Engineering of Copper Atoms on Continuous Three-Dimensional-Nanopatterned Nickel Nitride to Accelerate Alkaline Hydrogen Evolution. ACS Sustain. Chem. Eng. 2023, 11, 5229. [Google Scholar] [CrossRef]
- Li, W.; Cheng, G.; Peng, S.; Sun, M.; Wang, S.; Han, S.; Liu, Y.; Zhai, T.; Yu, L. Tuning hydrogen binding energy by interfacial charge transfer enables pH-universal hydrogen evolution catalysis of metal phosphides. Chem. Eng. J. 2022, 430, 132699. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Q.; Liu, W.; Li, Q.; Wang, Y.; Liu, B.; Zheng, L.; Wang, W.; Huang, L.; Chen, L.; et al. Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification. Nano Energy 2021, 81, 105641. [Google Scholar] [CrossRef]
- Shi, J.; Peng, W.; Yang, Y.F.; Li, B.; Nie, J.; Wan, H.; Li, Y.; Huang, G.F.; Hu, W.; Huang, W.Q. A General Strategy for Synthesis of Binary Transition Metal Phosphides Hollow Sandwich Heterostructures. Small 2023, 19, 2302906. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.J.; Yan, Y.; Jiang, S.; Liu, T.; Sun, T.; Zhou, W.; Guo, L.; Li, J. Interfaces Decrease the Alkaline Hydrogen-Evolution Kinetics Energy Barrier on NiCoP/Ti3C2Tx MXene. ACS Nano 2022, 16, 11049. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, A.; Li, J.; Su, K.; Tang, Y.; Qiu, X. Top-down and facet-selective phase-segregation to construct concave nanocages with strongly coupled hetero-interface for oxygen evolution reaction. Appl. Catal. B Environ. 2022, 300, 120727. [Google Scholar] [CrossRef]
- Yang, H.; Guo, P.; Wang, R.; Chen, Z.; Xu, H.; Pan, H.; Sun, D.; Fang, F.; Wu, R. Sequential Phase Conversion-Induced Phosphides Heteronanorod Arrays for Superior Hydrogen Evolution Performance to Pt in Wide pH Media. Adv. Mater. 2022, 34, e2107548. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ge, S.; Tan, Y.; Wang, Z.; Li, J.; Li, X.; Hu, G.; Zhu, X.; Huang, M.; Zhu, Y.; et al. High Capacity and Energy Density of Zn-Ni-Co-P Nanowire Arrays as an Advanced Electrode for Aqueous Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2020, 12, 9158. [Google Scholar] [CrossRef]
- Li, L.; Zou, W.; Ye, Q.; Li, Q.; Feng, Q.; Wei, J.; Xu, X.; Wang, F. Quasi-parallel nickel cobalt phosphide nanosheet arrays as highly efficient electrocatalyst for hydrogen evolution and overall water splitting at large current densities. J. Power Sources 2021, 516, 230657. [Google Scholar] [CrossRef]
- Tong, X.; Liao, W.; Zhai, Y.; Liu, P.; Yang, Q.; Chen, T. NiO/Co2P Nanosheets with Heterogeneous Structure as a Multifunctional Catalyst for Overall Water Splitting. J. Electrochem. Soc. 2023, 170, 063509. [Google Scholar] [CrossRef]
- Yu, W.; Sun, L.; Huo, L.; Zhao, H. Synergistic Coupling of CoFe-LDH Nanosheets with Cu3P Nanoarrays for Efficient Water Splitting. ACS Appl. Energy Mater. 2023, 6, 5548. [Google Scholar] [CrossRef]
- Ding, Z.; Yu, H.; Liu, X.; He, N.; Chen, X.; Li, H.; Wang, M.; Yamauchi, Y.; Xu, X.; Amin, M.A.; et al. Prussian blue analogue derived cobalt-nickel phosphide/carbon nanotube composite as electrocatalyst for efficient and stable hydrogen evolution reaction in wide-pH environment. J. Colloid Interface Sci. 2022, 616, 210. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel-Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Zhou, X.; Fei, F.; Li, M.; Cao, X.; Dong, X.; Li, L.; Yuan, N.; Ding, J. Electrochemically Oxidized Aligned Carbon Nanotube Arrays Embedding Foam Substrates for Water Splitting. ACS Appl. Nano Mater. 2023, 6, 8028. [Google Scholar] [CrossRef]
- Yue, Z.; Yao, S.; Li, Y.; Zhu, W.; Zhang, W.; Wang, R.; Wang, J.; Huang, L.; Zhao, D.; Wang, J. Surface engineering of hierarchical Ni(OH)2 nanosheet@nanowire configuration toward superior urea electrolysis. Electrochim. Acta 2018, 268, 211. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, Y.; Xu, S.; Huang, L.; Liu, Y.; Li, D.; Zhu, J.; Jiang, D. NiCoP-nanocubes-decorated CoSe2 nanowire arrays as high-performance electrocatalysts toward oxygen evolution reaction. J. Colloid Interface Sci. 2023, 648, 141. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Chen, H.; Zhang, H.; Sun, Z.; Du, P. Ternary metal phosphide nanosheets as a highly efficient electrocatalyst for water reduction to hydrogen over a wide pH range from 0 to 14. J. Mater. Chem. A 2016, 4, 10195. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Yang, R.; Li, D.; Meng, S.; Chen, M. CoP3/CoMoP Heterogeneous Nanosheet Arrays as Robust Electrocatalyst for pH-Universal Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 9309. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Bai, J.; Yuan, G.; He, P.; Zhu, Y.; Wu, S.; Qin, F.; Ren, L. Interface engineering assisted Fe-Ni3S2/Ni2P heterostructure as a high-performance bifunctional electrocatalyst for OER and HER. Electrochim. Acta 2023, 458, 142524. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Peng, S.; Zhang, L.; Al-Enizi, A.M.; Zhang, H.; Sun, X.; Zheng, G. Co-Ni-Based Nanotubes/Nanosheets as Efficient Water Splitting Electrocatalysts. Adv. Energy Mater. 2016, 6, 1501661. [Google Scholar] [CrossRef]
- Bai, L.; Song, A.; Lei, X.; Zhang, T.; Song, S.; Tian, H.; Liu, H.; Qin, X.; Wang, G.; Shao, G. Hierarchical construction of hollow NiCo2S4 nanotube@NiCo2S4 nanosheet arrays on Ni foam as an efficient and durable electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 38524. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Liu, Y.; Xu, L.; Qin, J.; Lei, Y.; Tang, Y. NiCoP nanoleaves array for electrocatalytic alkaline H2 evolution and overall water splitting. J. Energy Chem. 2020, 50, 395. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, L.; Qian, Y.; Zhang, X.; Xie, Y.; Cha, J.J.; Yu, G. Dual Tuning of Ni-Co-A (A = P, Se, O) Nanosheets by Anion Substitution and Holey Engineering for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 5241. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, X.; Yang, C.; Ding, X.; Zhang, Y.; Zheng, Y.Z.; Li, S.; Sun, X.; Tao, X. Large-Size, Porous, Ultrathin NiCoP Nanosheets for Efficient Electro/Photocatalytic Water Splitting. Adv. Funct. Mater. 2020, 30, 1910830. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Xu, S.; Liu, Y.; Jiang, D.; Zhu, J. Facile synthesis of hierarchical NiCoP nanosheets/NiCoP nanocubes homojunction electrocatalyst for highly efficient and stable hydrogen evolution reaction. Appl. Surf. Sci. 2021, 565, 150537. [Google Scholar] [CrossRef]
- Guo, J.; Zhan, Z.; Lei, T.; Yin, P. Facile synthesis of self-supported intertwined columnar NiCoP as a high efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 5974. [Google Scholar] [CrossRef]
- Wu, H.; Liu, P.; Yin, M.; Hou, Z.; Hu, L.; Dang, J. Surface modification engineering on three-dimensional self-supported NiCoP to construct NiCoOx/NiCoP for highly efficient alkaline hydrogen evolution reaction. J. Alloys Compd. 2020, 835, 155364. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Song, E.; Wang, N.; Dong, J.; Zhang, X.; Ajayan, P.M.; Yao, W.; Wang, C.; Liu, J.; et al. Manipulation on active electronic states of metastable phase beta-NiMoO4 for large current density hydrogen evolution. Nat. Commun. 2021, 12, 5960. [Google Scholar] [CrossRef]
- Geng, L.; Liu, Q.; Chen, J.; Jia, P.; Ye, H.; Yan, J.; Zhang, L.; Tang, Y.; Huang, J. In situ observation of electrochemical Ostwald ripening during sodium deposition. Nano Res. 2021, 15, 2650. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, e1807134. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Song, A.; Bai, L.; Duanmu, M.; Wang, L.; Dong, H.; Qin, X.; Shao, G. Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution. Catalysts 2023, 13, 1232. https://doi.org/10.3390/catal13091232
Song S, Song A, Bai L, Duanmu M, Wang L, Dong H, Qin X, Shao G. Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution. Catalysts. 2023; 13(9):1232. https://doi.org/10.3390/catal13091232
Chicago/Turabian StyleSong, Shenglu, Ailing Song, Lei Bai, Manman Duanmu, Lixin Wang, Haifeng Dong, Xiujuan Qin, and Guangjie Shao. 2023. "Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution" Catalysts 13, no. 9: 1232. https://doi.org/10.3390/catal13091232
APA StyleSong, S., Song, A., Bai, L., Duanmu, M., Wang, L., Dong, H., Qin, X., & Shao, G. (2023). Hierarchical Design of Homologous NiCoP/NF from Layered Double Hydroxides as a Long-Term Stable Electrocatalyst for Hydrogen Evolution. Catalysts, 13(9), 1232. https://doi.org/10.3390/catal13091232