Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystallographic Phase Properties
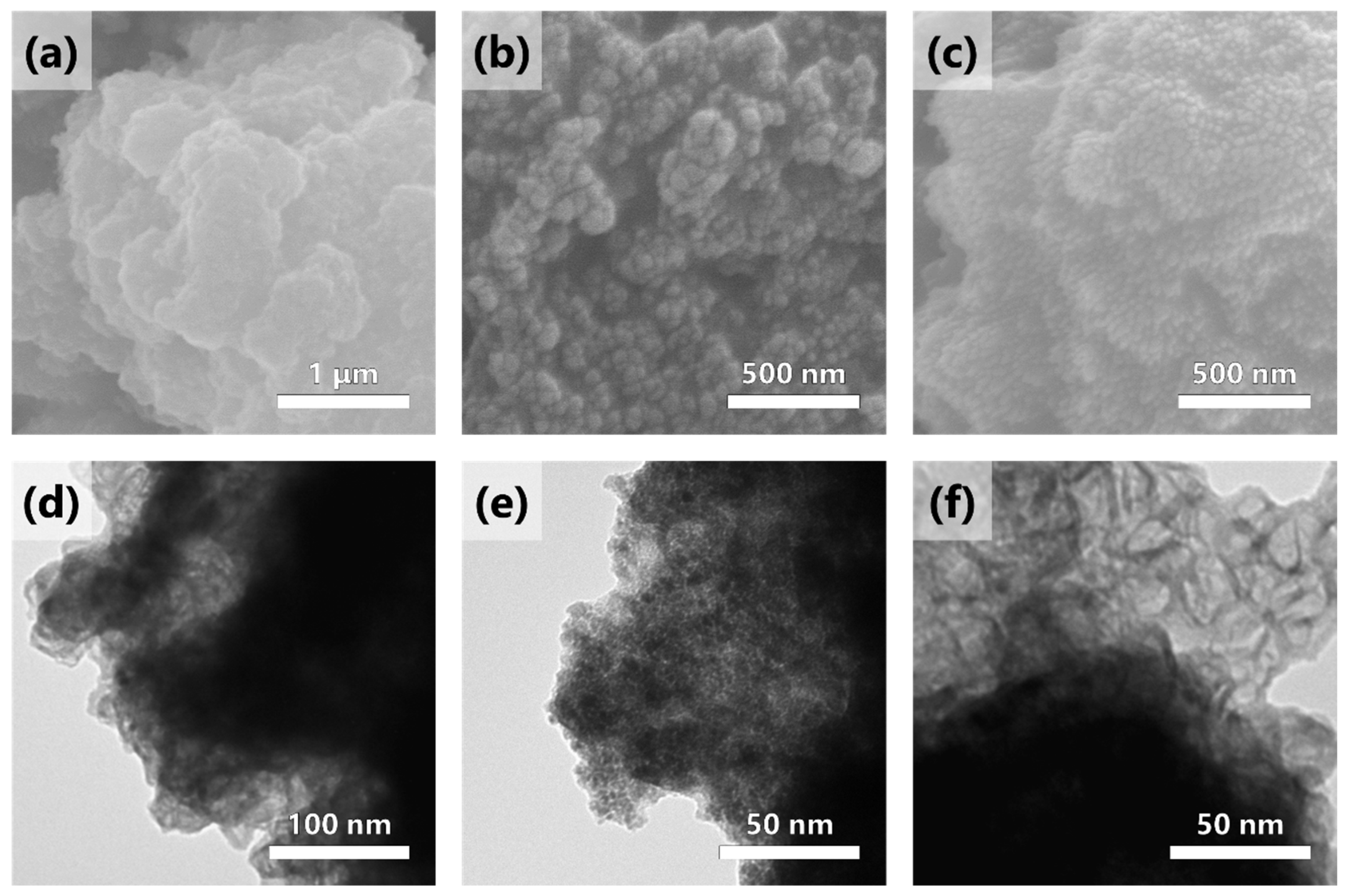
2.2. Surface Morphology
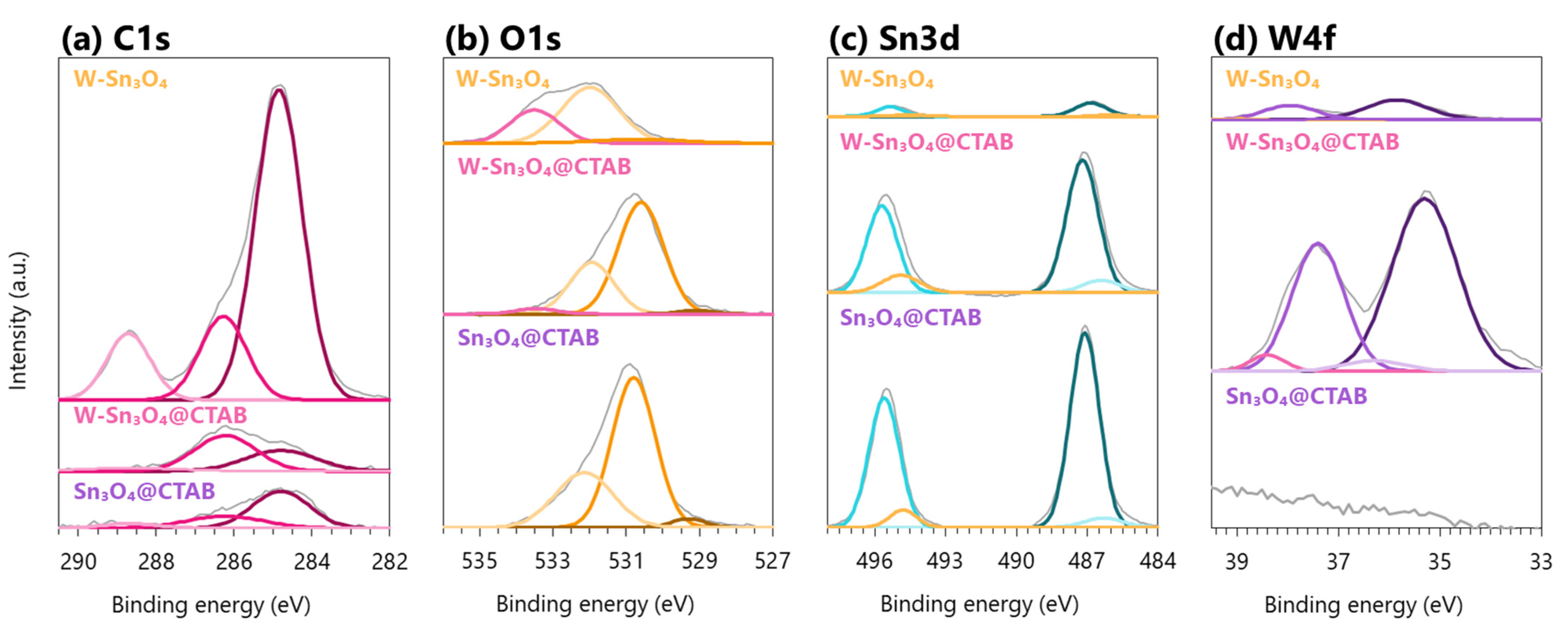
2.3. Chemical Structure
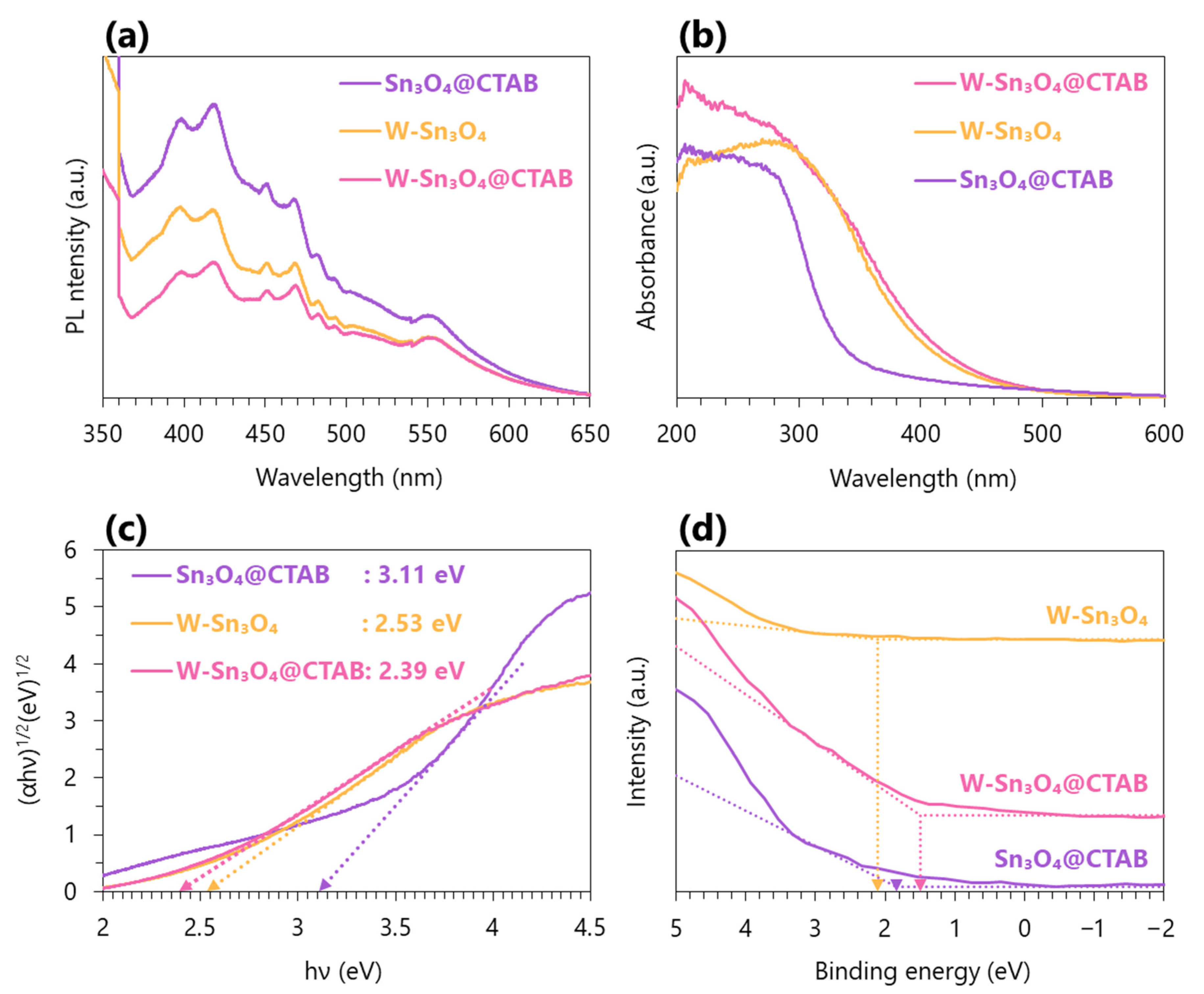
2.4. Optical Property
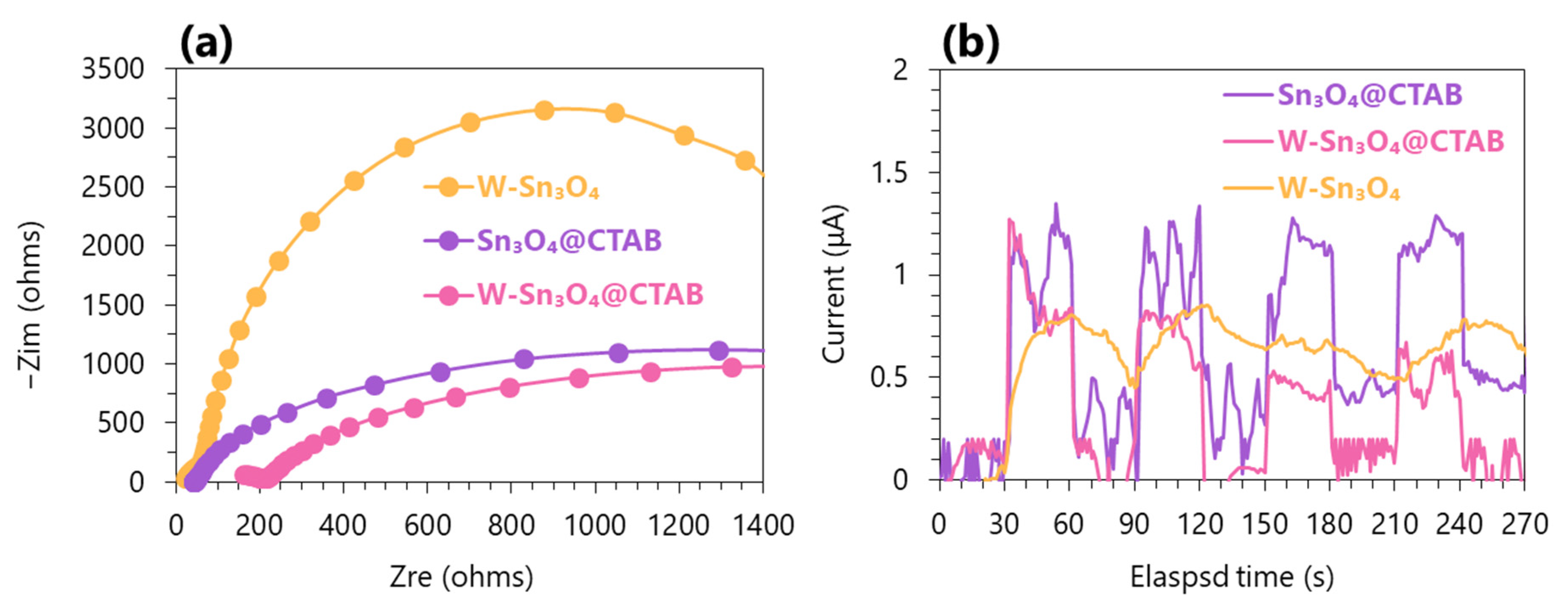
2.5. Electrochemical Characterization
2.6. Photocatalytic Dye Degradation
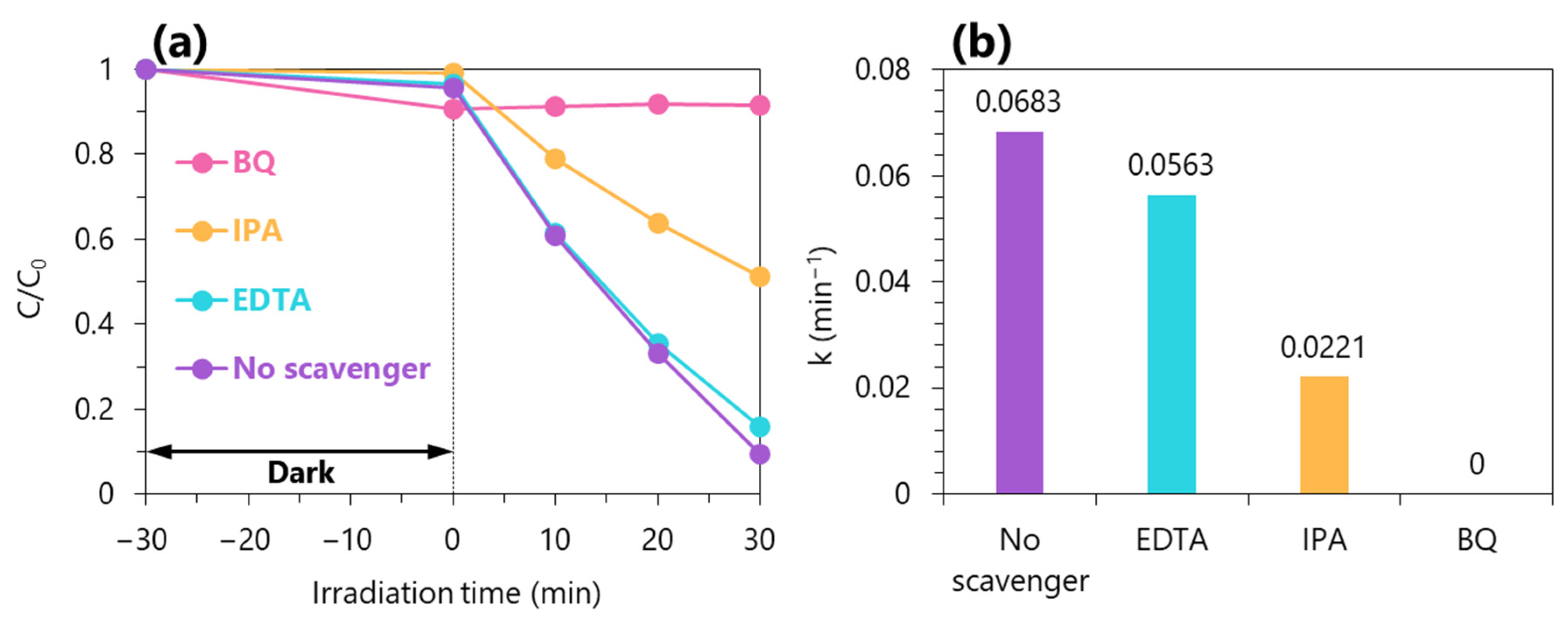
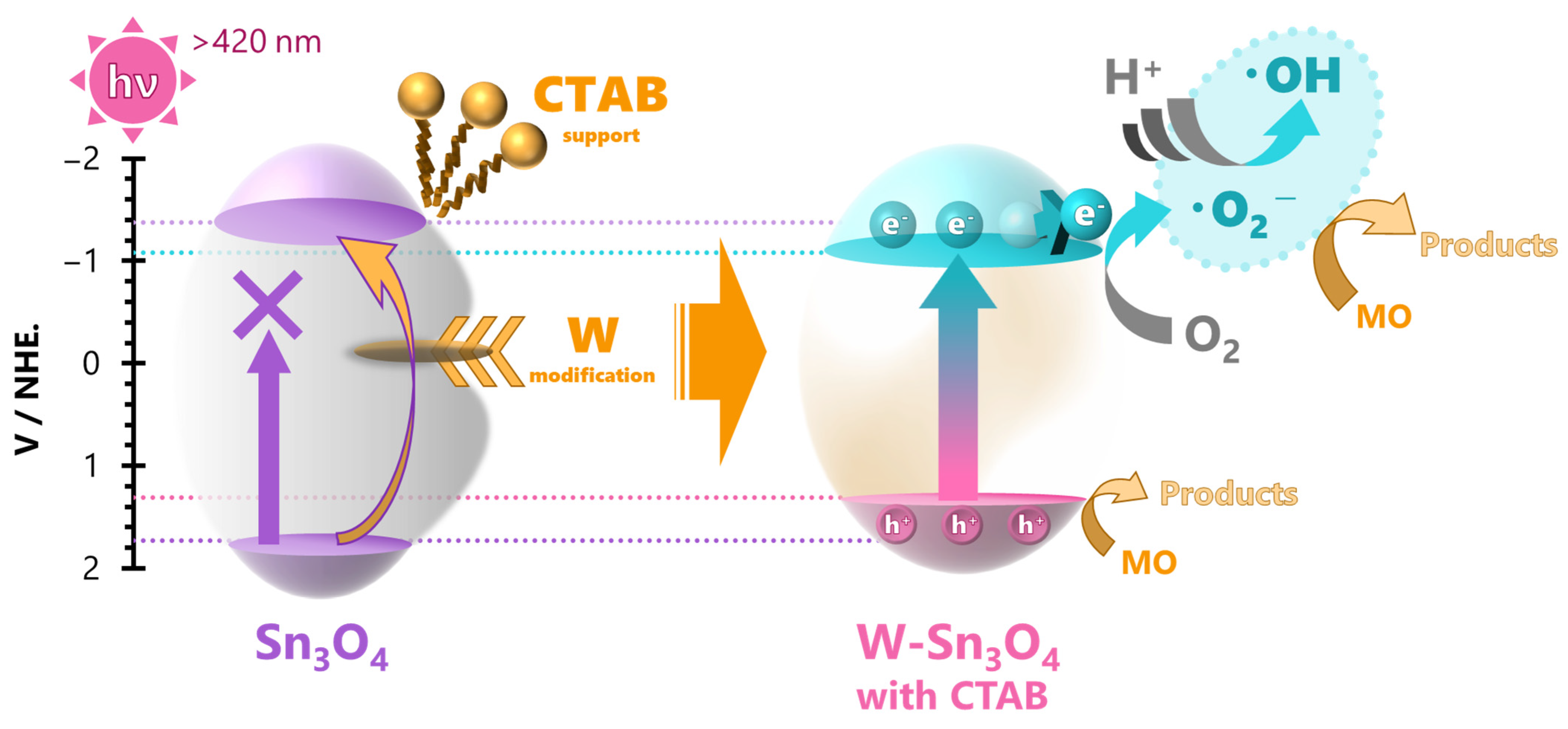
2.7. Reaction Mechanisms
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Characterization
3.4. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Yang, C.; Wu, S.; Li, X.; Chen, Y.; Yang, Y.C. Construction of built-in electric field within silver phosphate photocatalyst for enhanced removal of recalcitrant organic pollutants. Adv. Funct. Mater. 2020, 30, 2002918. [Google Scholar] [CrossRef]
- Anjaneya, O.; Shrishailnath, S.S.; Guruprasad, K.; Anand, S.N.l.; Mashetty, S.B.l.; Karegoudar, T.B. Decolourization of Amaranth dye by bacterial biofilm in batch and continuous packed bed bioreactor. Int. Biodeterior. Biodegrad. 2013, 79, 64–72. [Google Scholar] [CrossRef]
- Sun, L.; Mo, Y.; Zhang, L. A mini review on bio-electrochemical systems for the treatment of azo dye wastewater: State-of-the-art and future prospects. Chemosphere 2022, 294, 133801. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Zeng, G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 622, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Zhang, G.; Cui, W.; Cen, W.; Wu, G.; Lee, S.C.; Dong, F. Probing ring-opening pathways for efficient photocatalytic toluene decomposition. J. Mater. Chem. 2019, 7, 3366–3374. [Google Scholar] [CrossRef]
- Abdul, H.; Jina-Ming, P.; Afzal, S.; Hazrat, H.; Wei-dong, H. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Abdul, H.; Anum, S.; Sheng-Qi, C.; Mudasir, N. A comprehensive review on adsorption, photocatalytic and chemical degradation of dyes and nitro-compounds over different kinds of porous and composite materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi, Y.A.; Pouranb, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Poll. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Yue, X.; Li, J.; Zhang, T.; Qiu, F.; Yang, D.; Xue, M. In situ one-step fabrication of durable superhydrophobic-superoleophilic cellulose/LDH membrane with hierarchical structure for efficiency oil/water separation. Chem. Eng. J. 2017, 328, 117–123. [Google Scholar] [CrossRef]
- Prasad, A.S.A.; Kumar, G.; Thomas, D.M. Microbial decolorization of azo dys-A mini review. Bull. Chem. Pharma Res. 2017, 1, 30–39. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Guo, Y.; Hu, C.; Wang, E. Efficient degradation of dye pollutants on nanoporous polyoxotungstate–anatase composite under visible-light irradiation. J. Mol. Catal. A Chem. 2005, 225, 203. [Google Scholar] [CrossRef]
- Ge, L. Novel Pd/BiVO4 composite photocatalysts for efficient degradation of methyl orange under visible light irradiation. Mater. Chem. Phys. 2008, 107, 465–470. [Google Scholar] [CrossRef]
- Attar, A.S. Binary Zn-doped SnO2/Al2O3 nanotube composites for visible-light-driven photocatalytic degradation of basic blue 41. ACS Appl. Nano Mater. 2020, 3, 9931–9942. [Google Scholar] [CrossRef]
- Mei, L.; Wu, W.; Yang, S.; Xing, X.Z.; Ren, F.; Xiao, X.; Jiang, C. Design of enhanced catalysts by coupling of noble metals (Au,Ag) with semiconductor SnO2 for catalytic reduction of 4-nitrophenol. Part. Part. Syst. Char. 2016, 33, 212–220. [Google Scholar] [CrossRef]
- Prakash, K.; Senthil Kumar, P.; Pandiaraj, S.; Saravanakumar, K.; Karuthapandian, S. Controllable synthesis of SnO2 photocatalyst with superior photocatalytic activity for the degradation of methylene blue dye solution. J. Exp. Nanosci. 2016, 11, 1138–1155. [Google Scholar] [CrossRef]
- Seko, A.; Togo, A.; Oba, F.; Tanaka, I. Structure and stability of a homologous series of tin oxides. Phys. Rev. Lett. 2008, 100, 045702. [Google Scholar] [CrossRef] [PubMed]
- Berengue, O.M.; Simon, R.A.; Chiquito, A.J.; Dalmaschio, C.J.; Leite, E.R.; Guerreiro, H.A.; Guimarñaes, F.E.G. Semiconducting Sn3O4 nanobelts: Growth and electronic structure. J. Appl. Phys. 2010, 107, 033717. [Google Scholar] [CrossRef]
- Mone, P.; Mardikar, S.P.; Balgude, S. Morphology-controlled synthesis of Sn3O4 nanowires for enhanced solar-light driven photocatalytic H2 production. Nano-Struct. Nano-Objects 2020, 24, 100615. [Google Scholar] [CrossRef]
- Sun, M.; Yan, T.; Qu, T.; He, Y.; Shao, Y.; Wei, D.; Du, B. Self-assembled hierarchical Sn3O4-multi-wall carbon nanotubes: Facile fabrication, promoted charge separation, and enhanced photocatalytic performances. Mater. Res. Bull. 2018, 103, 104–113. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.A.; Hidalgo, M.C.; Macías, M. High UV-photocatalytic activity of ZnO and Ag/ZnO synthesized by a facile method. Catal. Today 2017, 284, 121–128. [Google Scholar] [CrossRef]
- Han, Y.; Wi, M.; Qu, S.; Zhong, M. Ag@AgCl quantum dots embedded on Sn3O4 nanosheets towards synergistic 3D flower-like heterostructured microspheres for efficient visible-light photocatalysis. Ceram. Int. 2020, 46, 24060–24070. [Google Scholar] [CrossRef]
- Ki, S.J.; Park, Y.K.; Kim, J.S.; Lee, W.J.; Lee, H.; Jung, S.C. Facile preparation of tungsten oxide doped TiO2 photocatalysts using liquid phase plasma process for enhanced degradation of diethyl phthalate. Chem. Eng. J. 2019, 377, 120087. [Google Scholar] [CrossRef]
- Ma, J.S.; Lin, L.Y.; Chen, Y.S. Facile solid-state synthesis for producing molybdenum and tungsten co-doped monoclinic BiVO4 as the photocatalyst for photoelectrochemical water oxidation. Int. J. Hydrog. Energy 2019, 44, 7905–7914. [Google Scholar] [CrossRef]
- Liu, X.; Liang, B.; Yang, J.; Li, Q. Solvent effect on morphological evolution and photocatalytic property of α-SnWO4. J. Taiwan Inst. Chem. Eng. 2019, 95, 575–582. [Google Scholar] [CrossRef]
- Wang, Q.L.; Li, H.B.; Jiang, H.Y.; Ding, S.T.; Song, Z.W.; Shi, J.S. Effect of solvent on α-SnWO4 photocatalyst for degradation of methyl orange under visible light irradiation. Mater. Technol. 2015, 30, 288–293. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Watcharakitti, J.; Nanan, S. PVP-assisted synthesis of rod-like ZnO photocatalyst for photodegradation of reactive red (RR141) and Congo red (CR) azo dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 17804–17819. [Google Scholar] [CrossRef]
- Sánchez-Martínez, D.; Gomez-Solis, C.; Torrs-Martinez, M. CTAB-assisted ultrasonic synthesis, characterization and photocatalytic properties of WO3. Mater. Res. Bull. 2015, 61, 165–172. [Google Scholar] [CrossRef]
- Rajeev, Y.N.; Magdalane, C.M.; Ramalingam, G.; Kumar, L.B.; Alwadai, N.; Al-Buriahi, M.S. Photocatalytic activity of hierarchical CTAB-assisted TiO2 nanoparticles for polluted water treatment using solar light illumination. Appl. Phys. A 2022, 128, 299. [Google Scholar] [CrossRef]
- Stefan, M.; Lostean, C.; Pana, O.; Popa, A.; Toloman, D.; Macavei, S.; Perhaita, I.; Barbu-Tudoran, L.; Silipas, D. Interface tailoring of SnO2–TiO2 photocatalysts modified with anionic/cationic surfactants. J. Mater. Sci. 2020, 55, 3279–3298. [Google Scholar] [CrossRef]
- Shen, A.; Zhao, L.; Guo, L. Crystallite, optical and photocatalytic properties of visible-light-driven ZnIn2S4 photocatalysts synthesized via a surfactant-assisted hydrothermal method. Mater. Res. Bull. 2009, 44, 100–105. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, S.; Liu, Z.; Wang, C.; Mao, Z. Studies on the enhanced photocatalytic hydrogen evolution over Pt/PEG-modified TiO2 photocatalysts. Int. J. Hydrog. Energy 2023, 33, 1112–1117. [Google Scholar] [CrossRef]
- Balgude, S.D.; Sethi, Y.A.; Kale, B.B.; Munirathnam, N.R.; Amalnerkar, D.P.; Adhyapak, P.V. Nanostructured layered Sn3O4 for hydrogen production and dye degradation under sunlight. RSC Adv. 2016, 6, 95663–95669. [Google Scholar] [CrossRef]
- Balgude, S.D.; Sethi, Y.A.; Kale, B.B.; Amalnerkar, D.P.; Adhyapak, P.V. ZnO decorated Sn3O4 nanosheet nano-heterostructure: A stable photocatalyst for water splitting and dye degradation under natural sunlight. RSC Adv. 2019, 9, 10289–10296. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwaran, P.; Sivarajan, A.; Raja, G.; Madhan, D.; Rajkumar, P. Effect of tungsten (W6+) metal ion dopant on structural, optical and photocatalytic activity of SnO2 nanoparticles by a novel microwave method. J. Mater. Sci.-Mater. Electron. 2016, 27, 2419–2425. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Gao, Z.; Ye, B.C. Effect of different activated carbon as carrier on the photocatalytic activity of Ag-N-ZnO photocatalyst for methyl orange degradation under visible light irradiation. Nanomaterials 2017, 7, 258. [Google Scholar] [CrossRef]
- Ai, F.; Zhao, G.; Lv, W.; Lin, J. Facile synthesis of cetyltrimethylammonium bromide-loaded mesoporous silica nanoparticles for efficient inhibition of hepatocellular carcinoma cell proliferation. Mater. Res. Express 2020, 7, 085008. [Google Scholar] [CrossRef]
- Yang, L.Q.; Lv, M.F.; Song, Y.; Yin, K.Y.; Wang, X.L.; Cheng, X.L.; Cao, K.S.; Li, S.T.; Wang, C.; Yao, Y.F.; et al. Porous Sn3O4 nanosheets on PPy hollow rod with photo-induced electrons oriented migration for enhanced visible-light hydrogen production. Appl. Catal. B 2020, 279, 119341. [Google Scholar] [CrossRef]
- Fukumoto, M.; Hirose, Y.; Benjamin, W.; Nakao, S.; Kimura, K.; Hayashi, K.; Sugisawa, Y.; Sekiba, D.; Scanlon, D.; Hasegawa, T. Ligand field-induced exotic dopant for infrared transparent electrode: W in rutile SnO2. Adv. Funct. Mater. 2022, 32, 210832. [Google Scholar] [CrossRef]
- Uemura, Y.; Kido, D.; Wakisaka, Y.; Uehara, H.; Ohba, T.; Niwa, Y.; Nozawa, S.; Sato, T.; Ichiyanagi, K.; Fukaya, R.l.; et al. Dynamics of photoelectrons and structural changes of tungsten trioxide observed by femtosecond transient XAFS. Angew. Chem. Int. Ed. 2016, 55, 1364–1367. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhu, G.Q.; Hojamberdiev, M.; Gao, J.Z.; Zhu, R.L.; Wang, C.H.; Wei, X.M.; Liu, P. Three-dimensional Ag2O/Bi5O7I p–n heterojunction photocatalyst harnessing UV–vis–NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54. [Google Scholar] [CrossRef]
- Cho, I.S.; Kwak, C.H.; Kim, D.W.; Lee, S.; Hong, K.S. Photophysical, Photoelectrochemical, and Photocatalytic Properties of novel SnWO4 oxide semiconductors with narrow band gaps. J. Phys. Chem. C. 2009, 113, 10647–10653. [Google Scholar] [CrossRef]
- Manikandan, M.; Tanabe, T.; Li, P.; Ueda, S.; Ramesh, G.V.; Kodiyath, R.; Wang, J.; Hara, T.; Dakshanamoorthy, A.; Ishihara, S.; et al. Photocatalytic water splitting under visible light by mixed-valence Sn3O4. ACS Appl. Mater. Interfaces 2016, 6, 3790. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.l.; Miao, C.; Jiang, W.; Li, H.; Liu, C.; Che, G. An S-scheme photocatalyst constructed by modifying Ni-doped Sn3O4 micro-flowers on g-C3N4 nanosheets for enhanced visible-light-driven hydrogen evolution. J. Ind. Eng. Chem. 2022, 113, 380–388. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Alkali-assisted synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Huda, A.; Suman, P.H.; Torquato, L.D.M.; Silva, B.F.; Handoko, C.T.; Gulo, F.; Zanoni, M.V.B.; Orlandi, M.O. Visible light-driven photoelectrocatalytic degradation of acid yellow 17 using Sn3O4 flower-like thin films supported on Ti substrate (Sn3O4/ TiO2/Ti). J. Photochem. Photobiol. A 2019, 376, 196–205. [Google Scholar] [CrossRef]
- Hong, Y.; Zengwei, L.; Weifeng, L. Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property. Colloids Surf. A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Qinglan, C.; Shiyun, L.; Yongqiang, W.; Shaomin, Z. Red blood cell-like hollow TiO2@WO3 microspheres as highly efficient photocatalysts for degradation of organic pollutants. Inorg. Chem. Commun. 2023, 148, 110307. [Google Scholar] [CrossRef]
- Feng, Q.; Yaya, M.; Shizheng, Z.; Changyuan, H.; Lin, W.; Ruofan, Y.; Yanting, M.; Cuiqing, Z. Construction of novel Z-scheme N-CQDs/Sn3O4 heterojunction for excellent photocatalytic degradation of organic pollutant. J. Clust. Sci. 2022, 33, 913. [Google Scholar] [CrossRef]
- Ruiqi, Y.; Yanchen, J.; Longwei, W.; Guoxin, S.; Aizhu, W.; Longhua, D.; Na, R.; Yawei, L.; Jian, Z.; Xin, Y. Crystalline Ni-doped Sn3O4 nanosheets for photocatalytic H2 production. ACS Appl. Nano Mater. 2020, 3, 9268–9275. [Google Scholar] [CrossRef]
- Zhang, L.; Xinyu, L.; Xing, Z.; Wei, Z.; Jinwen, M.; Qiushi, W.; Shi, S. Sulfur-doped Sn3O4 nanosheets for improved photocatalytic performance. J. Alloys Compd. 2023, 961, 170904. [Google Scholar] [CrossRef]
- Saison, T.; Gras, P.; Chemin, N.; Chanéac, C.; Durupthy, O.; Brezová, V.; Colbeau-Justin, C.; Jolivet, J.P. New insights into Bi2WO6 properties as a visible-light photocatalyst. J. Phys. Chem. C 2013, 117, 22656–22666. [Google Scholar] [CrossRef]
- Naik, B.; Manoratne, C.H.; Chandrashekhar, A.; Iyer, A.; Prasad, V.S.; Ghosh, N.N. Preparation of TiO2, Ag-doped TiO2 nanoparticle and TiO2–SBA-15 nanocomposites using simple aqueous solution-based chemical method and study of their photocatalytical activity. J. Exp. Nanosci. 2013, 8, 462–479. [Google Scholar] [CrossRef]
- Li, G.T.; Wong, K.H.; Zhang, X.W.; Hu, C.; Yu, J.C.; Chan, R.C.Y.; Wong, P.K. Degradation of Acid Orange 7 using magnetic AgBr under visible light: The roles of oxidizing species. Chemosphere 2009, 76, 1185–1191. [Google Scholar] [CrossRef] [PubMed]




| Sample | CTAB Amount | Vm (cm3(STP)/g) | asBET (m2/g) | Vp (p/p0 = 0.990)(cm3/g) | dp (nm) |
|---|---|---|---|---|---|
| Sn3O4 | 0.03 g | 28.5 | 124.0 | 0.20 | 6.36 |
| W-Sn3O4 | 0.03 g | 42.8 | 186.0 | 0.17 | 3.54 |
| W-Sn3O4 | 0 g | 64.6 | 281.4 | 0.22 | 3.14 |
| Photocatalyst | Preparation Method | Light Source | Photocatalyst Concentration (mg/mL) | MO Concentration (mg/L) | Efficiency (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| WO3/g-C3N4 | Thermal polymerization | 300 W xenon lamp | 1 | 10 | 93 | 120 | [47] |
| TiO2@ WO3 | Etching + hydrolysis processes | 300 W xenon lamp | 1 | 10 | 95 | 25 | [48] |
| N-CQDs/Sn3O4 | CQDs process + hydrothermal | 500 W xenon lamp | 0.6 | 10 | 78 | 80 | [49] |
| Ni-Sn3O4 | One step hydrothermal | 300 W xenon lamp | 1 | 10 | 72 | 60 | [50] |
| S-Sn3O4 | One step hydrothermal | 500 W xenon lamp | 1 | 20 | 99 | 80 | [51] |
| W-Sn3O4@CTAB | One step solvothermal | 500 W xenon lamp | 0.6 | 10 | 99 | 40 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, M.; Iwamoto, D.; Inamori, K.; Tateishi, I.; Katsumata, H.; Kaneco, S. Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation. Catalysts 2023, 13, 1179. https://doi.org/10.3390/catal13081179
Furukawa M, Iwamoto D, Inamori K, Tateishi I, Katsumata H, Kaneco S. Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation. Catalysts. 2023; 13(8):1179. https://doi.org/10.3390/catal13081179
Chicago/Turabian StyleFurukawa, Mai, Daichi Iwamoto, Koki Inamori, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2023. "Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation" Catalysts 13, no. 8: 1179. https://doi.org/10.3390/catal13081179
APA StyleFurukawa, M., Iwamoto, D., Inamori, K., Tateishi, I., Katsumata, H., & Kaneco, S. (2023). Synthesis of Tungsten-Modified Sn3O4 through the Cetyltrimethylammonium Bromide-Assisted Solvothermal Method for Dye Decolorization under Visible Light Irradiation. Catalysts, 13(8), 1179. https://doi.org/10.3390/catal13081179






