Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals
Abstract
1. Introduction
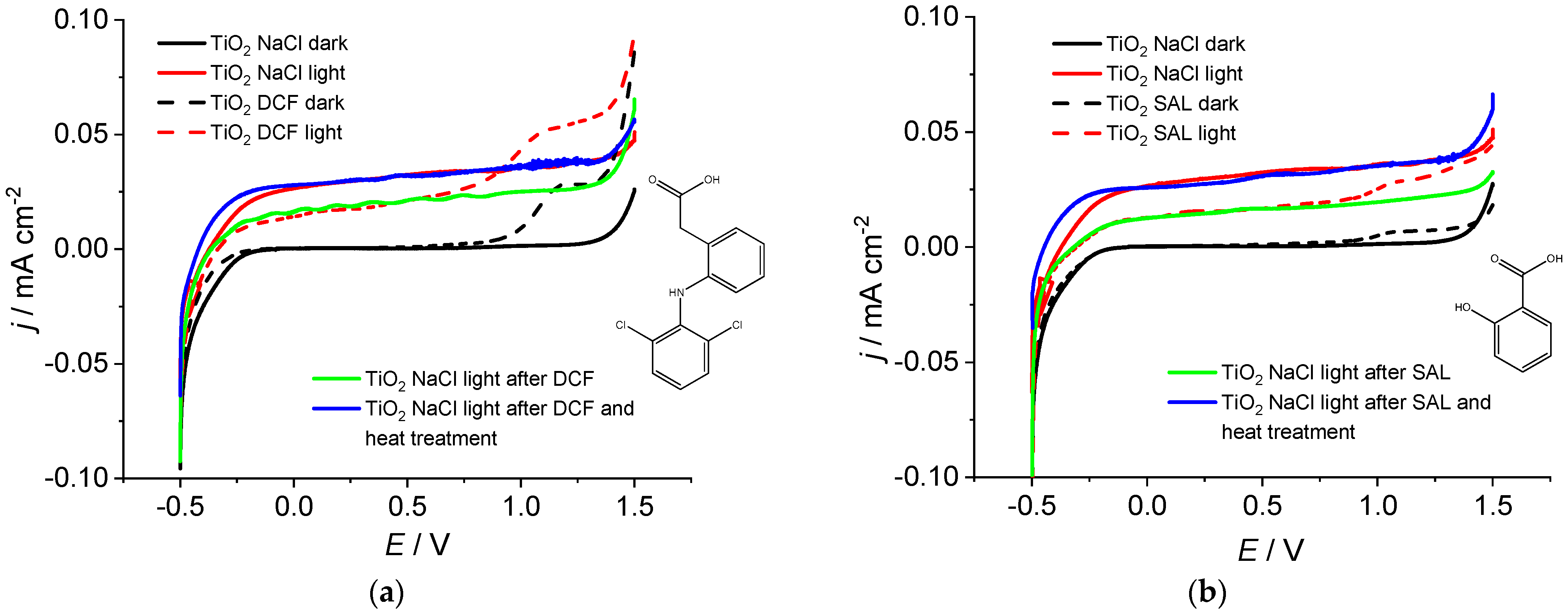
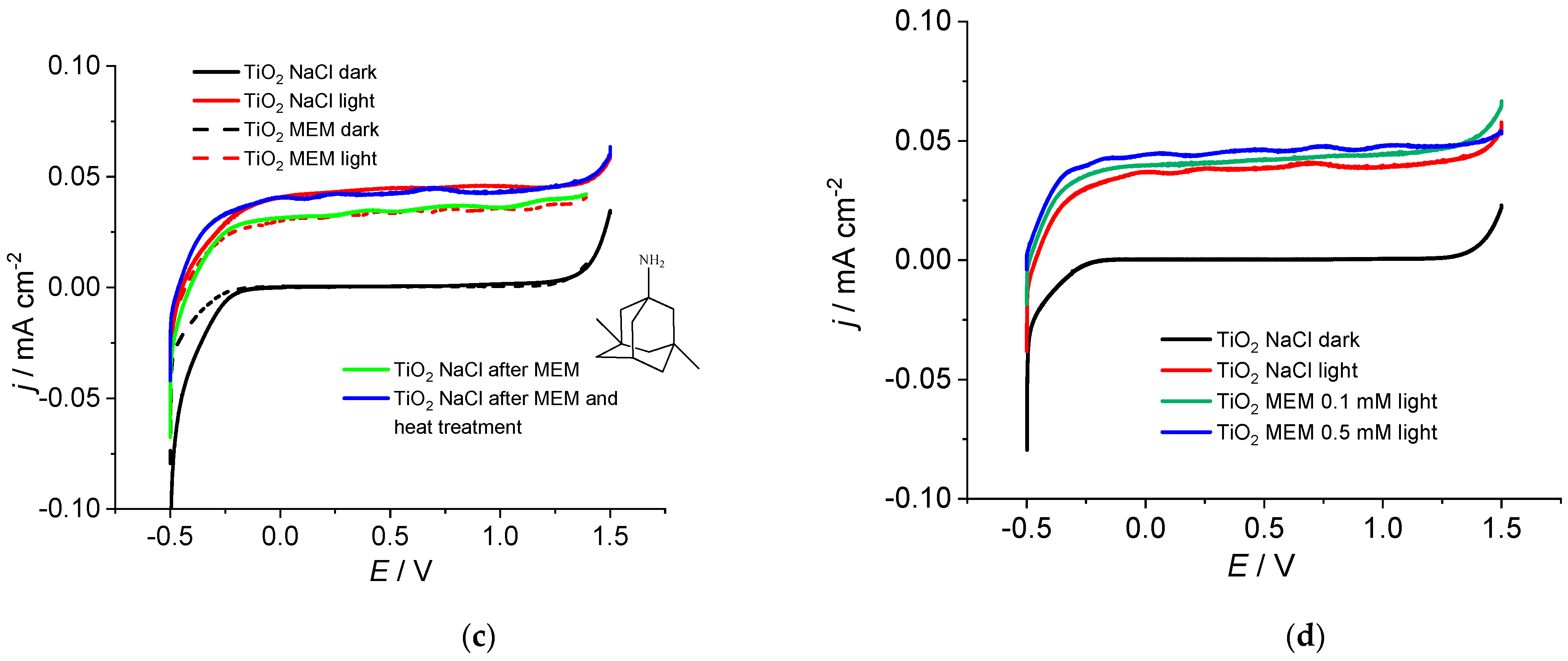
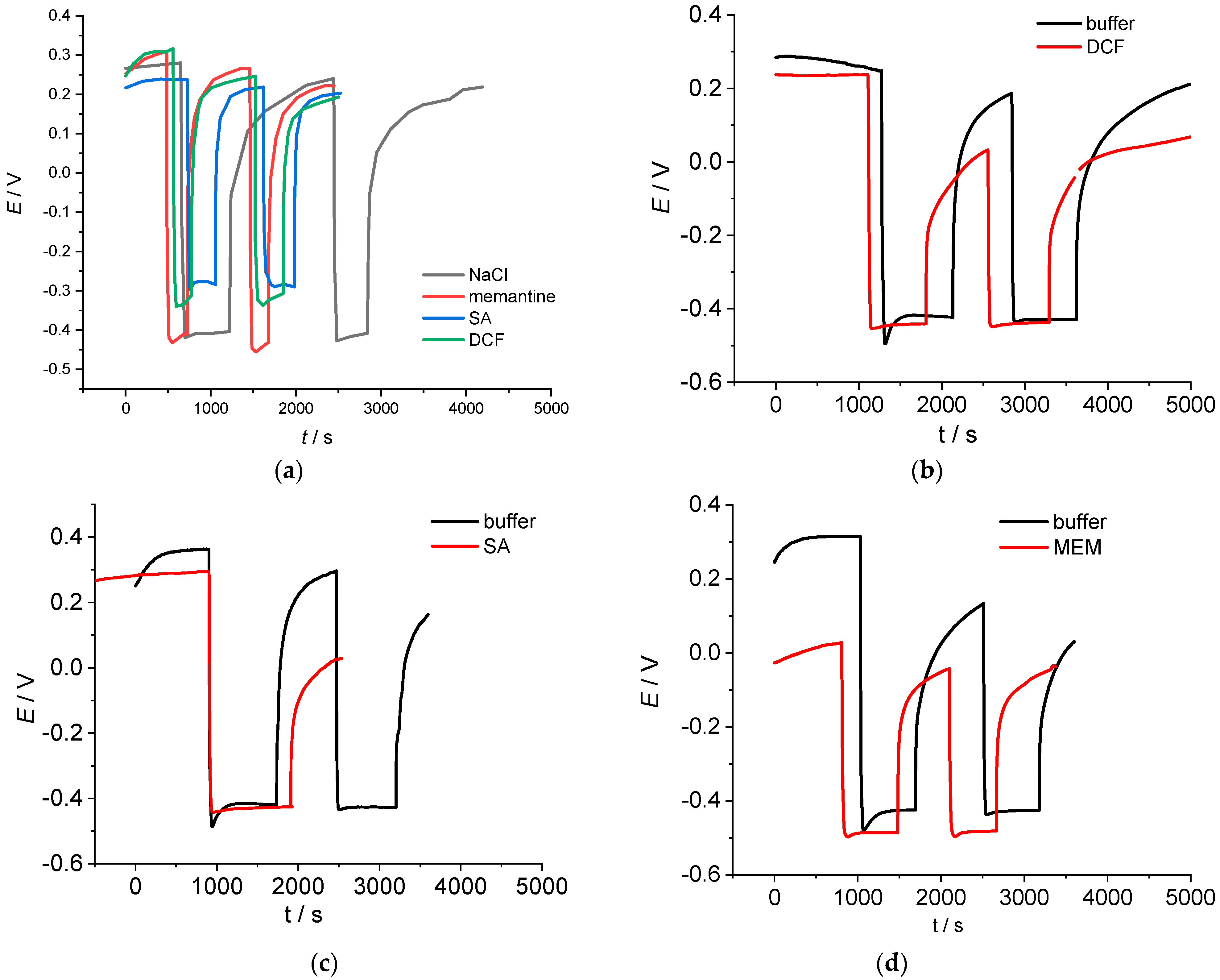
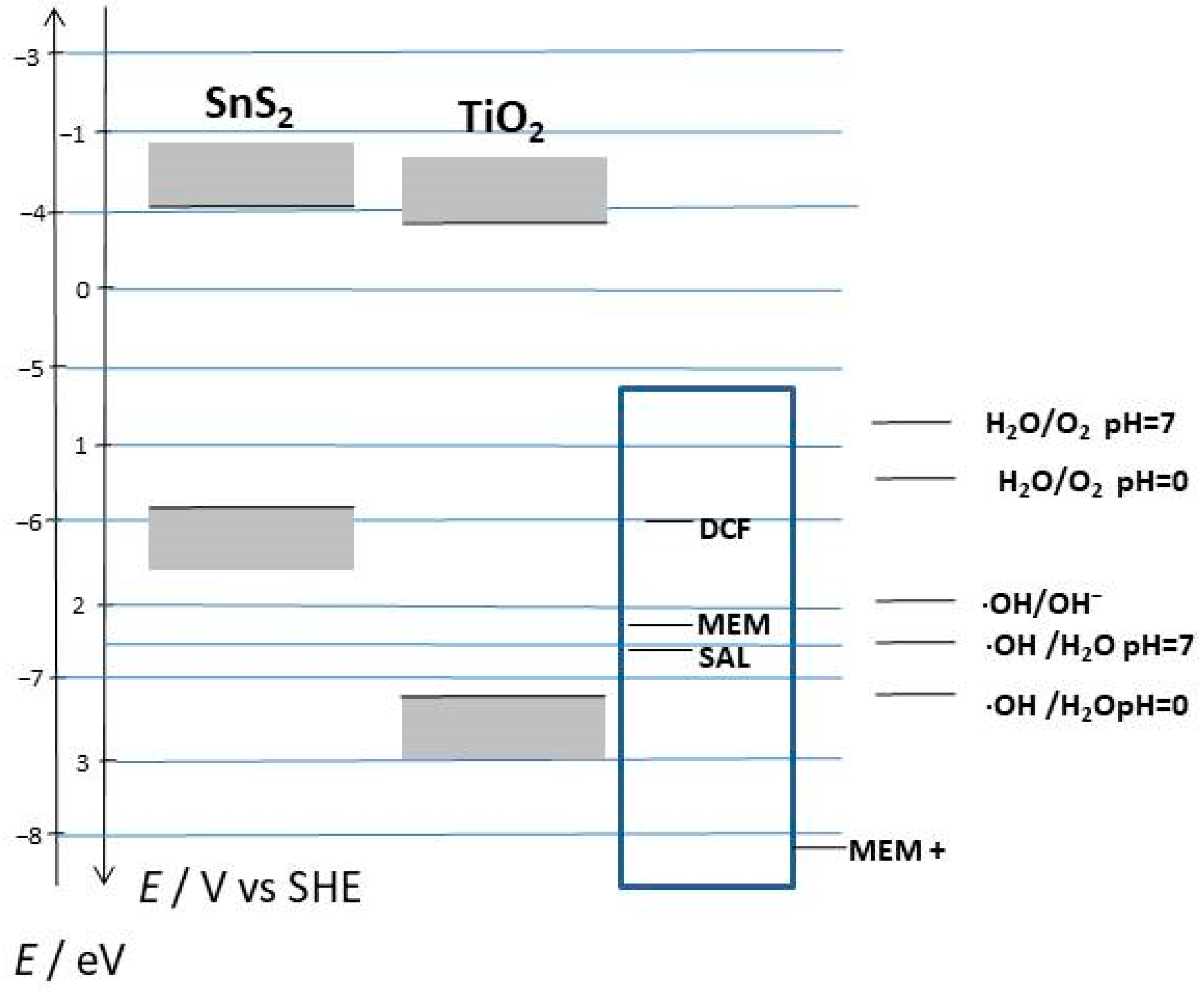
2. Results and Discussion
3. Materials and Methods
3.1. Photocatalyst Synthesis and Immobilization
3.2. Photoelectrochemical Characterisation
3.3. Photocatalytic Degradation Experiments
3.4. Computational Details
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albistur, A.; Rivero, P.J.; Esparza, J.; Rodríguez, R. Evaluation of the Photocatalytic Activity and Anticorrosion Performance of Electrospun Fibers Doped with Metallic Oxides. Polymers 2021, 13, 2011. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, K.; Leung, M.K.H.; Zhang, Y.; Li, L.; Li, G.; Xuan, J.; Li, J. Photocatalytic fuel cell—A review. Chem. Eng. J. 2022, 428, 131074. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Su, D.; Wang, G.; Zhong, Y. Application of Photocatalytic Materials in Sensors. Adv. Mater. Technol. 2020, 5, 1900993. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Kovacic, M.; Katic, J.; Kusic, H.; Loncaric Bozic, A.; Metikos Hukovic, M. Elucidating the photocatalytic behavior of TiO2-SnS2 composites based on their energy band structure. Materials 2018, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Perovic, K.; dela Rosa, F.; Kovacic, M.; Kusic, H.; Lavrencic Stangar, U.; Fresno, F.; Dionysiou, D.D.; Loncaric Bozic, A. Recent achievements in development of TiO2-based composite photocatalytic materials for solar driven water purification and splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- Sambur, J.B.; Chen, P. Distinguishing Direct and Indirect Photoelectrocatalytic Oxidation Mechanisms Using Quantitative Single-Molecule Reaction Imaging and Photocurrent Measurements. J. Phys. Chem. C 2016, 120, 20668–20676. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Beltrán, F.J. TiO2 photocatalytic oxidation of a mixture of emerging contaminants: A kinetic study independent of radiation absorption based on the direct-indirect model. Chem. Eng. J. 2018, 339, 369–380. [Google Scholar] [CrossRef]
- Villarreal, T.L.; Gὁmez, R.; Gonzalez, M.; Salvador, P. A Kinetic Model for Distinguishing between Direct and Indirect Interfacial Hole Transfer in the Heterogeneous Photooxidation of Dissolved Organics on TiO2 Nanoparticle Suspensions. J. Phys. Chem. B 2004, 108, 20278–20290. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Villarreal, T.L.; Gὁmez, R.; Neumann-Spallart, M.; Alonso-Vante, N.; Salvador, P. Semiconductor Photooxidation of Pollutants Dissolved in Water: A Kinetic Model for Distinguishing between Direct and Indirect Interfacial Hole Transfer. I. Photoelectrochemical Experiments with Polycrystalline Anatase Electrodes under Current Doubling and Absence of Recombination. J. Phys. Chem. B 2004, 108, 15172–15181. [Google Scholar] [CrossRef]
- Yalavarthi, R.; Zboril, R.; Schmuki, P.; Naldoni, A.; Kment, S. Elucidating the role of surface states of BiVO4 with Mo doping and a CoOOH co-catalyst for photoelectrochemical water splitting. J. Power Sources 2021, 483, 229080. [Google Scholar] [CrossRef]
- Levchuk, I.; Sillanpää, M. Titanium dioxide—Based nanomaterials for photocatalytic water treatment. In Advanced Water Treatment; Sillanpää, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–56. ISBN 978-0-12-819225-2. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Loncaric Bozic, A. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2020, 382, 122826. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Bonete, P.; Djellabi, R.; Cerrato, G.; Operti, L.; Gómez, R.; Bianchi, C.L. Comparative Photo-Electrochemical and Photocatalytic Studies with Nanosized TiO2 Photocatalysts towards Organic Pollutants Oxidation. Catalysts 2021, 11, 349. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, X.; Ma, Q.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q.; Li, B. Kinetics of photoelectrocatalytic degradation of diclofenac using n, s Co-doped TiO2 nano-crystallite decorated TiO2 nanotube arrays photoelectrode. Environ. Prot. Eng. 2018, 44, 117–130. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhou, J.; Xi, Q.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Fabricating I doped TiO2 photoelectrode for the degradation of diclofenac: Performance and mechanism study. Chem. Eng. J. 2019, 396, 968–978. [Google Scholar] [CrossRef]
- Hey, G.; Vega, S.R.; Fick, J.; Tysklind, M.; Ledin, A.; la Cour Jansen, J.; Andersen, H.R. Removal of pharmaceuticals in WWTP effluents by ozone and hydrogen peroxide. Water SA 2014, 40, 165–174. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Q.; Li, B.; Deng, X.; Li, J.; Wangc, P.; Zhang, B.; Liuc, H.; Wang, X. One-step construction of N/Ti3+ codoped TiO2 nanotubes photoelectrode with high photoelectrochemical and photoelectrocatalytic performance. Electrochim. Acta 2015, 186, 442–448. [Google Scholar] [CrossRef]
- Oliveros, A.N.; Pimentel, J.A.I.; de Luna, M.D.G.; Garcia-Segura, S.; Abarca, R.R.M.; Doong, R. Visible-light photocatalytic diclofenac removal by tunable vanadium pentoxide/boron-doped graphitic carbon nitride composite. Chem. Eng. J. 2021, 403, 126213. [Google Scholar] [CrossRef]
- Macías-Tamez, R.; Villanueva-Rodríguez, M.; Ramos-Delgado, N.A.; Maya-Treviño, L.; Hernández-Ramírez, A. Comparative Study of the Photocatalytic Degradation of the Herbicide 2,4-D Using WO3/TiO2 and Fe2O3/TiO2 as Catalysts. Water Air Soil Pollut. 2017, 228, 379. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts. Environ. Nanotechnol. Monit. Manag. 2018, 10, 322–330. [Google Scholar] [CrossRef]
- Cordero-García, A.; Turnes Palomino, G.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Maya-Teviño, L.; Hernández-Ramírez, A. Photocatalytic behaviour of WO3/TiO2-N for diclofenac degradation using simulated solar radiation as an activation source. Environ. Sci. Pollut. Res. Int. 2017, 24, 4613–4624. [Google Scholar] [CrossRef]
- Cordero-García, A.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A. Effect of carbon doping on WO3/TiO2 coupled oxide and its photocatalytic activity on diclofenac degradation. Ceram. Int. 2016, 42, 9796–9803. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontas, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic degradation of diclofenac sodium salt: Adsorption and reaction kinetic studies. Environ. Earth Sci. 2020, 79, 277. [Google Scholar] [CrossRef]
- John, D.; Rajalakshmi, A.S.; Lopez, R.M.; Achari, V.S. TiO2 reduced graphene oxide nanocomposites for the trace removal of diclofenac. SN Appl. Sci. 2018, 2, 840. [Google Scholar] [CrossRef]
- Švagelj, Z.; Mandić, V.; Ćurković, L.; Biošić, M.; Žmak, I.; Gaborardi, M. Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials 2020, 13, 227. [Google Scholar] [CrossRef]
- Papac, J.; Garcia Ballesteros, S.; Tonkovic, S.; Kovacic, M.; Tomic, A.; Cvetnić, M.; Kušic, H.; Senta, I.; Terzić, S.; Ahel, M.; et al. Degradation of pharmaceutical memantine by photo-based advanced oxidation processes: Kinetics, pathways and environmental aspects. J. Environ. Chem. Eng. 2023, 11, 109334. [Google Scholar] [CrossRef]
- Li, M.C.; Shen, J.N. Photoelectrochemical oxidation behavior of organic substances on TiO2 thin-film electrodes. J. Solid State Electrochem. 2006, 10, 980–986. [Google Scholar] [CrossRef]
- Kratochvilová, K.; Hoskovcová, I.; Jirkovský, J.; Klíma, J.; Ludvík, J. A spectroelectrochemical study of chemisorption, anodic polymerization and degradation of salicylic acid on conductor and TiO2 surfaces. Electrochim. Acta 1995, 40, 2603–2609. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, J.; Wang, Y.; Feng, J.; Yan, W.; Xu, H. Electrochemical assisted photocatalytic degradation of salicylic acid with highly ordered TiO2 nanotube electrodes. Appl. Surf. Sci. 2014, 308, 161–169. [Google Scholar] [CrossRef]
- Loukopoulos, S.; Toumazatou, A.; Sakellis, E.; Xenogiannopoulou, V.; Boukos, N.; Dimoulas, A.; Likodimos, V. Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis. Materials 2020, 13, 4305. [Google Scholar] [CrossRef]
- Tian, M.; Adams, B.; Wen, J.; Asmussen, R.M.; Chen, A. Photoelectrochemical oxidation of salicylic acid and salicylaldehyde on titanium dioxide nanotube arrays. Electrochim. Acta 2009, 54, 3799–3805. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, D.; Kumari, P.; Pal, B. Influence of photodeposition time and loading amount of Ag co-catalyst on growth, distribution and photocatalytic properties of Ag@TiO2 nanocatalysts. Opt. Mater. 2020, 106, 109975. [Google Scholar] [CrossRef]
- Bracco, E.; Butler, M.; Carnelli, P.; Candal, R. TiO2 and N-TiO2-photocatalytic degradation of salicylic acid in water: Characterization of transformation products by mass spectrometry. Environ. Sci. Pollut. Res. 2020, 27, 28469–28479. [Google Scholar] [CrossRef]
- Apostolaki, M.-A.; Toumazatou, A.; Antoniadou, M.; Sakellis, E.; Xenogiannopoulou, E.; Gardelis, S.; Boukos, N.; Falaras, P.; Dimoulas, A.; Likodimos, V. Graphene Quantum Dot-TiO2 Photonic Crystal Films for Photocatalytic Applications. Nanomaterials 2020, 10, 2566. [Google Scholar] [CrossRef]
- Chachvalvutikul, A.; Luangwanta, T.; Pattisson, S.; Hutchings, G.J.; Kaowphong, S. Enhanced photocatalytic degradation of organic pollutants and hydrogen production by a visible light–responsive Bi2WO6/ZnIn2S4 heterojunction. Appl. Surf. Sci. 2021, 544, 148885. [Google Scholar] [CrossRef]
- Božanić, D.K.; Garcia, G.A.; Nahon, L.; Sredojević, D.; Lazić, V.; Vukoje, I.; Ahrenkiel, S.P.; Djoković, V.; Šljivančanin, Ž.; Nedeljkovic, J.M. Interfacial Charge Transfer Transitions in Colloidal TiO2 Nanoparticles Functionalized with Salicylic acid and 5-Aminosalicylic acid: A Comparative Photoelectron Spectroscopy and DFT Study. J. Phys. Chem. C 2019, 123, 29057–29066. [Google Scholar] [CrossRef]
- Roncaroliz, F.; Blesa, M.A. Kinetics of adsorption of carboxylic acids onto titanium dioxide. Phys. Chem. Chem. Phys. 2010, 12, 9938–9944. [Google Scholar] [CrossRef]
- Tang, W.-R.; Wu, T.; Lin, Y.-W. Salicylic acid-sensitised titanium dioxide for photocatalytic degradation of fast green FCF under visible light irradiation. Micro Nano Lett. 2019, 14, 359–362. [Google Scholar] [CrossRef]
- Zarei, E. Electrochemically assisted photocatalytic removal of m-cresol using TiO2 thin film-modified carbon sheet photoelectrode. Int. J. Ind. Chem. 2018, 9, 285–294. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Papaderakis, A.; Karanasios, N.; Georgieva, J.; Pavlidou, E.; Litsardakis, G.; Poulios, I.; Sotiropoulos, S. Comparison of the photoelectrochemical performance of particulate and nanotube TiO2 photoanodes. Catal. Today 2017, 280, 14–20. [Google Scholar] [CrossRef]
- Pablos, C.; Marugán, J.; van Grieken, R.; Adán, C.; Riquelme, A.; Palma, J. Correlation between photoelectrochemical behaviour and photoelectrocatalytic activity and scaling-up of P25-TiO2 electrodes. Electrochim. Acta 2014, 130, 261–270. [Google Scholar] [CrossRef]
- Teixeira, S.; Martins, P.M.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Reusability of photocatalytic TiO2 and ZnO nanoparticles immobilized in poly(vinylidene difluoride)-co-trifluoroethylene. Appl. Surf. Sci. 2016, 384, 497–504. [Google Scholar] [CrossRef]
- Gerischer, H. The impact of semiconductors on the concepts of electrochemistry. Electrochim. Acta 1990, 35, 1677–1699. [Google Scholar] [CrossRef]
- Matarrese, R.; Mascia, M.; Vacca, A.; Mais, L.; Usai, E.M.; Ghidelli, M.; Mascaretti, L.; Bricchi, B.R.; Russo, V.; Casari, C.S.; et al. Integrated Au/TiO2 Nanostructured Photoanodes for Photoelectrochemical Organics Degradation. Catalysts 2019, 9, 340. [Google Scholar] [CrossRef]
- Colucci, J.; Montalvo, V.; Hernandez, R.; Poullet, C. Electrochemical oxidation potential of photocatalyst reducing agents. Electrochim. Acta 1990, 44, 2507–2514. [Google Scholar] [CrossRef]
- Zrinski, I.; Martinez, S.; Gospić, E. A catalytic and photocatalytic effects of TiO2 nanoparticles on electrooxidation of common antioxidants on carbon past. J. Solid State Electrochem. 2021, 25, 1591–1600. [Google Scholar] [CrossRef]
- Fischer, K.; Sydow, S.; Griebel, J.; Naumov, S.; Elsner, C.; Thomas, I.; Abdul Latif, A.; Schulze, A. Enhanced Removal and Toxicity Decline of Diclofenac by Combining UVA Treatment and Adsorption of Photoproducts to Polyvinylidene Difluoride. Polymers 2020, 12, 2340. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Fang, Y.; Liang, L.; Ding, H.; Shi, G.; Jin, L. Photoelectrochemical oxidation behavior of methanol on highly ordered TiO2 nanotube array electrodes. J. Electroanal. Chem. 2007, 610, 179–185. [Google Scholar] [CrossRef]
- Radić, G.; Šajnović, I.; Petrović, Ž.; Kraljić Roković, M. Reduced Graphene Oxide/α-Fe2O3 Fibres as Active Material for Supercapacitor Application. Croat. Chem. Acta 2018, 91, 481–490. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Kovacic, M.; Kusic, H.; Fanetti, M.; Lavrencic Stangar, U.; Valant, M.; Dionysios, D.D.; Loncaric Bozic, A. TiO2-SnS2 nanocomposites: Solaractive photocatalytic materials for water. Environ. Sci. Pollut. Res. 2017, 24, 19965–19979. [Google Scholar] [CrossRef]
- Sharifi, T.; Crmaric, D.; Kovacic, M.; Kraljic Rokovic, M.; Kusic, H.; Jozic, D.; Ambrozic, G.; Kralj, D.; Kontrec, J.; Zener, B.; et al. Tailored BiVO4 for enhanced visible-light photocatalytic performance. J. Environ. Chem. Eng. 2021, 9, 106025. [Google Scholar] [CrossRef]
- Mu, J.; Miao, H.; Liu, E.; Feng, J.; Teng, F.; Zhang, D.; Kou, Y.; Jin, Y.; Fan, J.; Hu, X. Enhanced light trapping and high charge transmission capacities of novel structures for efficient photoelectrochemical water splitting. Nanoscale 2018, 10, 11881–11893. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, J.; Xu, H.Y. One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI). Appl. Catal. B 2012, 123–124, 18–26. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for the local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force field. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Lipparini, F.; Scalamani, G.; Mennucci, B.; Cances, E.; Caricato, M.; Frisch, M.J. A variational formulation of the polarizable continuum model. J. Chem. Phys. 2010, 133, 014106. [Google Scholar] [CrossRef] [PubMed]
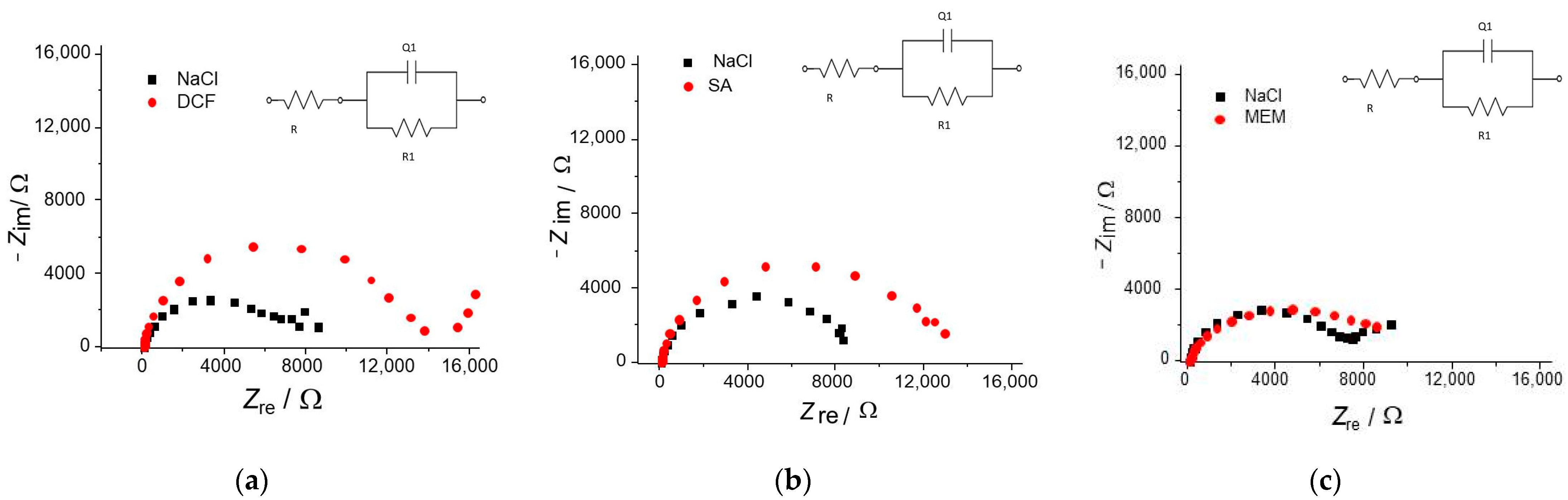


| (a) for DCF | Rel/Ω | R1 × 10−5/Ω | Q1 × 105/S sn | n1 |
| NaCl OCP | 54.94 | 0.069 | 4.24 | 0.85 |
| NaCl 50 mV | 55.73 | 1.10 | 1.48 | 0.93 |
| NaCl 500 mV | 55.34 | 0.92 | 1.14 | 0.94 |
| NaCl 1000 mV | 54.42 | 0.50 | 1.09 | 0.92 |
| DCF OCP | 57.59 | 0.13 | 2.44 | 0.92 |
| DCF 50 mV | 56.82 | 1.08 | 1.65 | 0.92 |
| DCF 500 mV | 57.12 | 0.77 | 1.23 | 0.93 |
| DCF 1000 mV | 56.69 | 1.02 | 1.17 | 0.92 |
| (b) for SA | Rel/Ω | R1 × 10−5/Ω | Q1 × 105/S sn | n1 |
| NaCl OCP | 54.44 | 0.083 | 2.95 | 0.91 |
| NaCl 50 mV | 56.76 | 1.13 | 1.44 | 0.95 |
| NaCl 500 mV | 57.53 | 1.41 | 1.14 | 0.95 |
| NaCl 1000 mV | 56.88 | 2.19 | 0.967 | 0.95 |
| SA OCP | 53.75 | 1.22 | 2.65 | 0.92 |
| SA 50 mV | 57.44 | 0.731 | 1.57 | 0.95 |
| SA 500 mV | 54.28 | 1.17 | 1.18 | 0.95 |
| SA 1000 mV | 53.90 | 1.18 | 1.21 | 0.95 |
| (c) for MEM | Rel/Ω | R1 × 10−5/Ω | Q1 × 105/S sn | n1 |
| NaCl OCP | 56.18 | 0.068 | 2.02 | 0.92 |
| NaCl 50 mV | 55.63 | 0.973 | 0.724 | 0.94 |
| NaCl 500 mV | 54.41 | 2.545 | 1.29 | 0.94 |
| NaCl 1000 mV | 53.98 | 2.275 | 0.516 | 0.96 |
| MEM OCP | 51.22 | 0.070 | 2.20 | 0.92 |
| MEM 50 mV | 51.54 | 1.250 | 2.11 | 0.92 |
| MEM 500 mV | 51.22 | 1.208 | 0.84 | 0.94 |
| MEM 1000 mV | 51.30 | 1.628 | 0.38 | 0.95 |
| DCF | DCF− | SA | SA− | MEM | MEM+ | |
|---|---|---|---|---|---|---|
| EHOMO, eV | −6.044 | −5.615 | −6.772 | −6.073 | −6.684 | −8.102 |
| ELUMO, eV | −1.084 | −0.980 | −1.790 | −0.896 | −0.083 | −0.448 |
| ΔEHOMO-LUMO, eV | −4.960 | −4.636 | −4.982 | −5.177 | −6.601 | −7.654 |
| Catalyst | PEC j0.5V/μA | jTiO2,0.5V/jSnS2,0.5V | Degradation Efficency/% | TiO2 PC Efficiency/SnS2 PC Efficiency | |
|---|---|---|---|---|---|
| DCF | TiO2 | 17 | 2.70 | 79.86 | 1.34 |
| SnS2 | 6.3 | 58.75 | |||
| MEM | TiO2 | 43 | 9.77 | 80.70 | 4.15 |
| SnS2 | 4.4 | 19.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, G.; Perović, K.; Sharifi, T.; Kušić, H.; Kovačić, M.; Kraljić Roković, M. Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals. Catalysts 2023, 13, 909. https://doi.org/10.3390/catal13050909
Radić G, Perović K, Sharifi T, Kušić H, Kovačić M, Kraljić Roković M. Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals. Catalysts. 2023; 13(5):909. https://doi.org/10.3390/catal13050909
Chicago/Turabian StyleRadić, Gabrijela, Klara Perović, Tayebeh Sharifi, Hrvoje Kušić, Marin Kovačić, and Marijana Kraljić Roković. 2023. "Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals" Catalysts 13, no. 5: 909. https://doi.org/10.3390/catal13050909
APA StyleRadić, G., Perović, K., Sharifi, T., Kušić, H., Kovačić, M., & Kraljić Roković, M. (2023). Electrochemical Characterisation of the Photoanode Containing TiO2 and SnS2 in the Presence of Various Pharmaceuticals. Catalysts, 13(5), 909. https://doi.org/10.3390/catal13050909











