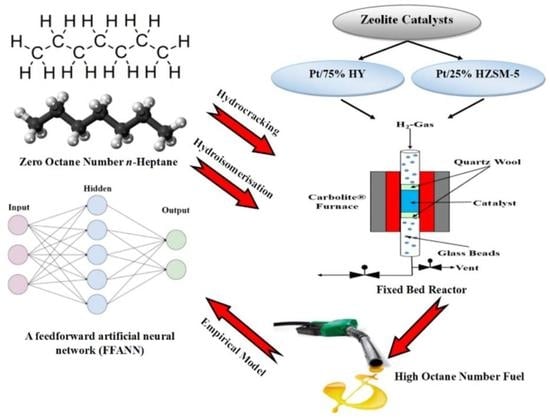
Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA)
Highlights
- Developing a new empirical model and ultra-accurate simulation of product selectivity of n-heptane catalytic reactions.
- High precision determination of optimal operating conditions for a complex hydroisomerisation and hydrocracking process to increase the octane number value using the hybrid ANN-GA approach.
- An effective reaction variable represented by the amount of Pt metal loaded on the surface of the zeolite catalysts was added to the other operating conditions using multilayer feedforward (FFANN).
- The highest percentage of experimentally produced isomers on the catalyst surface reaches a superior degree of agreement with the highest theoretical outcomes according to the ANN model.
Abstract
1. Introduction
2. Results and Discussion
2.1. Discussion of the Experimental Results
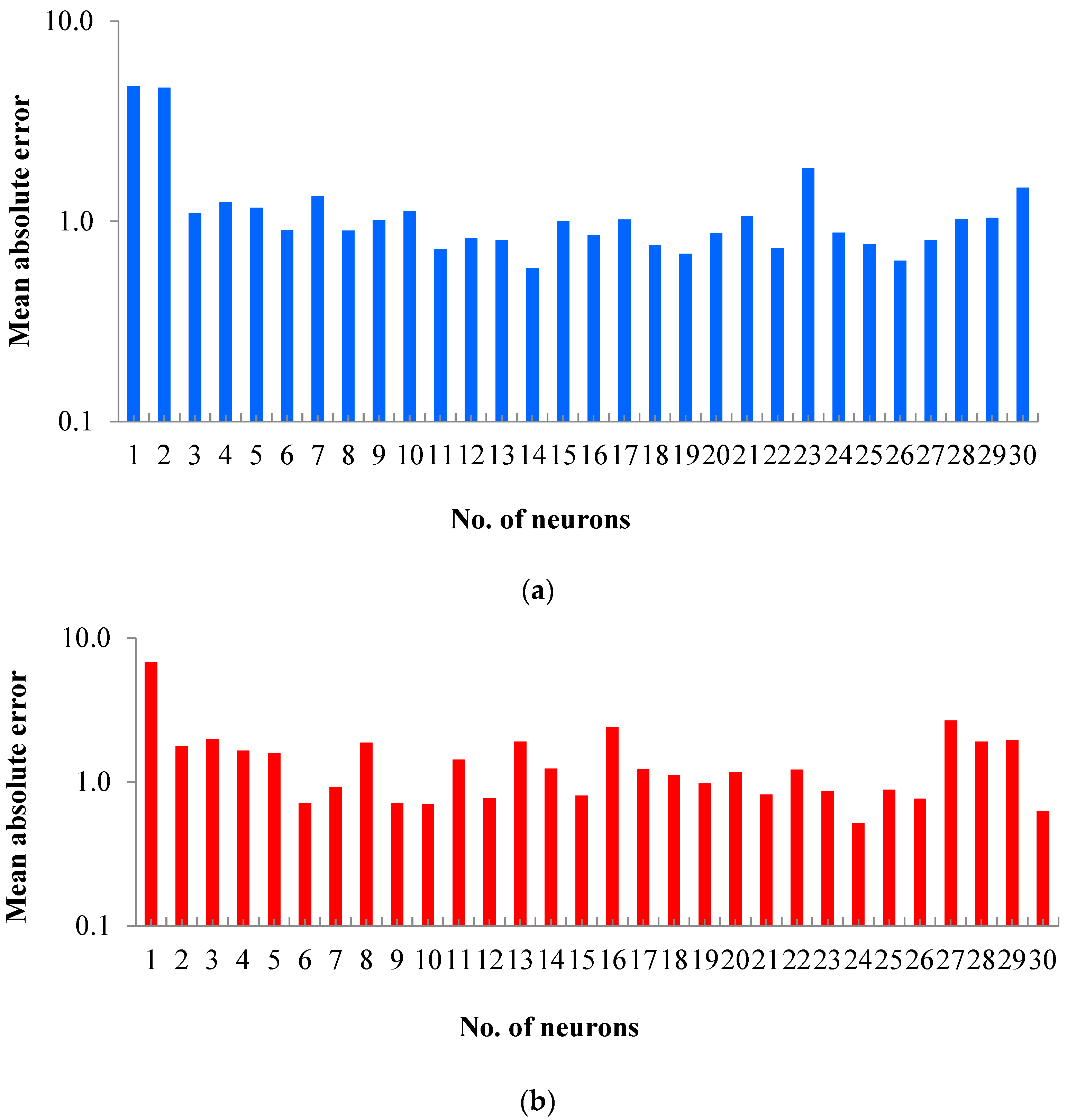
2.2. ANN Model Training
2.2.1. Training of Artificial Neural Networks (ANNs)
2.2.2. Comparison of ANN Predictions
2.2.3. Optimisation of Research Octane Number
3. Experimental Work
4. Theoretical Work
4.1. Artificial Neural Network Modelling
Designing an Artificial Neural Network
4.2. Statistical Analysis
4.3. Optimisation Method
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANN–GA | Hybrid artificial neural network–genetic algorithm |
| ANNs | Artificial neural networks |
| bj | User bias |
| exp. | Superscripts letters indicate experimental results |
| FF–ANN | Feedforward artificial neural network |
| Sigmoid function, which is the activation function of the jth neuron | |
| GA | Genetic algorithm |
| jth and kth | Hidden nodes |
| M | Number of experiments |
| MAE | Mean absolute error |
| MFF–ANN | Multilayer feedforward artificial neural network |
| MRE | Mean relative error |
| MSE | Mean square error |
| N | Number of components |
| pred. | Superscripts letters indicate predicted results |
| R2 | Correlation coefficient |
| RSM | Response surface methodology. |
| RON | Research octane number of all hydrocarbons produced |
| RONi | Octane number of only the pure component of each i-molecule within that product |
| SSE | Sum of square error |
| W/F | Weight of the catalyst divided by the molar flow rate of n-C7 in the feed |
| WHSV | Weight-hourly space velocity |
| Wi,j | Weight from ith neuron in the jth layer |
| xi | Input variable |
| Mole fraction of ith component in jth experiments | |
| Output variables | |
| Volumetric fractions of the reaction products | |
| Ø | Maximum free pore diameter |
References
- Al-Shafei, E.N.; Albahar, M.Z.; Aljishi, M.F.; Akah, A.; Aljishi, A.N.; Alasseel, A. Catalytic conversion of heavy naphtha to reformate over the phosphorus-ZSM-5 catalyst at a lower reforming temperature. RSC Adv. 2022, 12, 25465–25477. [Google Scholar] [CrossRef]
- Fedyna, M.; Śliwa, M.; Jaroszewska, K.; Trawczyński, J. Effect of zeolite amount on the properties of Pt/(AlSBA-15 + Beta zeolite) micro-mesoporous catalysts for the hydroisomerization of n-heptane. Fuel 2020, 280, 118607. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. [Google Scholar] [CrossRef]
- Shakor, Z.M.; Ramos, M.J.; AbdulRazak, A.A. A detailed reaction kinetic model of light naphtha isomerization on Pt/zeolite catalyst. J. King Saud Univ. Eng. Sci. 2022, 34, 303–308. [Google Scholar] [CrossRef]
- Agarwal, U.; Rigutto, M.S.; Zuidema, E.; Jansen, A.; Poursaeidesfahani, A.; Sharma, S.; Dubbeldam, D.; Vlugt, T.J. Kinetics of zeolite-catalyzed heptane hydroisomerization and hydrocracking with CBMC-modeled adsorption terms: Zeolite Beta as a large pore base case. J. Catal. 2022, 415, 37–50. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Fedyna, M.; Trawczyński, J. Hydroisomerization of long-chain n-alkanes over Pt/AlSBA-15+zeolite bimodal catalysts. Appl. Catal. B Environ. 2019, 255, 117756. [Google Scholar] [CrossRef]
- Melián-Cabrera, I. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18559. [Google Scholar] [CrossRef]
- Hamied, R.S.; Sukkar, K.A.; Majdi, H.S.; Shnain, Z.Y.; Graish, M.S.; Mahmood, L.H. Catalytic-Level Identification of Prepared Pt/HY, Pt-Zn/HY, and Pt-Rh/HY Nanocatalysts on the Reforming Reactions of N-Heptane. Processes 2023, 11, 270. [Google Scholar] [CrossRef]
- Gomez, L.Q.; Shehab, A.K.; Al-Shathr, A.; Ingram, W.; Konstantinova, M. H2-free Synthesis of Aromatic, Cyclic and Linear Oxygenates from CO2. ChemSusChem 2020, 13, 647–658. [Google Scholar] [CrossRef]
- Quintana-Gómez, L.; Connolly, M.; Shehab, A.K.; Al-Shathr, A.; McGregor, J. “Reverse combustion” of carbon dioxide in water: The influence of reaction conditions. Front. Energy Res. 2022, 10, 917943. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, Y.; Si, M.; Wei, Q.; Zhou, Y. Hydroisomerization of n-Hexadecane Over Nickel-Modified SAPO-11 Molecular Sieve-Supported NiWS Catalysts: Effects of Modification Methods. Front. Chem. 2022, 10, 857473. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.S.; Lapisardi, G.; Bouchy, C.; Rivallan, M.; Silva, J.M.; Ribeiro, M.F. Hydrogenating activity of Pt/zeolite catalysts focusing acid support and metal dispersion influence. Appl. Catal. A Gen. 2015, 504, 17–28. [Google Scholar] [CrossRef]
- Gao, S.-B.; Zhao, Z.; Lu, X.-F.; Chi, K.-B.; Duan, A.-J.; Liu, Y.-F.; Meng, X.-B.; Tan, M.-W.; Yu, H.-Y.; Shen, Y.-G.; et al. Hydrocracking diversity in n-dodecane isomerization on Pt/ZSM-22 and Pt/ZSM-23 catalysts and their catalytic performance for hydrodewaxing of lube base oil. Pet. Sci. 2020, 17, 1752–1763. [Google Scholar] [CrossRef]
- Yilmaz, B.; Müller, U. Catalytic Applications of Zeolites in Chemical Industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Zhang, H.; bin Samsudin, I.; Jaenicke, S.; Chuah, G.-K. Zeolites in catalysis: Sustainable synthesis and its impact on properties and applications. Catal. Sci. Technol. 2022, 12, 6024–6039. [Google Scholar] [CrossRef]
- Busca, G. Heterogeneous Catalytic Materials; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ibarra, Á.; Hita, I.; Arandes, J.M.; Bilbao, J. A Hybrid FCC/HZSM-5 Catalyst for the Catalytic Cracking of a VGO/Bio-Oil Blend in FCC Conditions. Catalysts 2020, 10, 1157. [Google Scholar] [CrossRef]
- Schmutzler, F.; Zschiesche, C.; Titus, J.; Poppitz, D.; Freiding, J.; Rakoczy, R.; Reitzmann, A.; Gläser, R. Hydroisomerization of Renewable and Fossil n-Alkanes over Bifunctional Dealuminated ZSM-5 Catalysts. Chem. Ing. Tech. 2021, 93, 981–989. [Google Scholar] [CrossRef]
- Kazakov, M.O.; Smirnova, M.Y.; Dubinin, M.E.; Bogomolova, T.S.; Dik, P.P.; Golubev, I.S.; Revyakin, M.E.; Klimov, O.V.; Noskov, A.S. Combining USY and ZSM-23 in Pt/zeolite hydrocracking catalyst to produce diesel and lube base oil with improved cold flow properties. Fuel 2023, 344, 128085. [Google Scholar] [CrossRef]
- Schweitzer, J.-M.; Rey, J.; Bignaud, C.; Bučko, T.; Raybaud, P.; Moscovici-Mirande, M.; Portejoie, F.; James, C.; Bouchy, C.; Chizallet, C. Multiscale Modeling as a Tool for the Prediction of Catalytic Performances: The Case of n-Heptane Hydroconversion in a Large-Pore Zeolite. ACS Catal. 2022, 12, 1068–1081. [Google Scholar] [CrossRef]
- Song, W.; Mahalec, V.; Long, J.; Yang, M.-L.; Qian, F. Modeling the Hydrocracking Process with Deep Neural Networks. Ind. Eng. Chem. Res. 2020, 59, 3077–3090. [Google Scholar] [CrossRef]
- Vandegehuchte, B.; Thybaut, J.; Martínez, A.; Arribas, M.; Marin, G. n-Hexadecane hydrocracking Single-Event MicroKinetics on Pt/H-beta. Appl. Catal. A Gen. 2012, 441–442, 10–20. [Google Scholar] [CrossRef]
- Choudhury, I.R.; Hayasaka, K.; Thybaut, J.W.; Narasimhan, C.L.; Denayer, J.F.; Martens, J.A.; Marin, G.B. Pt/H-ZSM-22 hydroisomerization catalysts optimization guided by Single-Event MicroKinetic modeling. J. Catal. 2012, 290, 165–176. [Google Scholar] [CrossRef]
- Martens, G.; Marin, G.; Martens, J.; Jacobs, P.; Baron, G. A Fundamental Kinetic Model for Hydrocracking of C8 to C12 Alkanes on Pt/US–Y Zeolites. J. Catal. 2000, 195, 253–267. [Google Scholar] [CrossRef]
- Bricker, M.; Thakkar, V.; Petri, J. Hydrocracking in Petroleum Processing. In Handbook of Petroleum Processing; Treese, S.A., Pujadó, P.R., Jones, D.S.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 317–359. [Google Scholar]
- Hamied, R.S.; Shakor, Z.M.; Sadeiq, A.H.; Razak, A.A.A.; Khadim, A.T. Kinetic Modeling of Light Naphtha Hydroisomerization in an Industrial Universal Oil Products Penex™ Unit. Energy Eng. 2023, 120, 1371–1386. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Al-Zaidi, B.Y.; Majdi, H.S.; AbdulRazak, A.A.; Aal-Kaeb, S.; Shohib, A.A.; McGregor, J. Reaction Kinetics of Cinnamaldehyde Hydrogenation over Pt/SiO2: Comparison between Bulk and Intraparticle Diffusion Models. Int. J. Chem. Eng. 2022, 2022, 8303874. [Google Scholar] [CrossRef]
- Maghami, S.; Mehrabani-Zeinabad, A.; Sadeghi, M.; Sánchez-Laínez, J.; Zornoza, B.; Téllez, C.; Coronas, J. Mathematical modeling of temperature and pressure effects on permeability, diffusivity and solubility in polymeric and mixed matrix membranes. Chem. Eng. Sci. 2019, 205, 58–73. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; Albayati, T.M. Comparison between Artificial Neural Network and Rigorous Mathematical Model in Simulation of Industrial Heavy Naphtha Reforming Process. Catalysts 2021, 11, 1034. [Google Scholar] [CrossRef]
- Shakor, Z.M.; Abdulrazak, A.A.; Sukkar, K. A Detailed Reaction Kinetic Model of Heavy Naphtha Reforming. Arab. J. Sci. Eng. 2020, 45, 7361–7370. [Google Scholar] [CrossRef]
- Galvan, D.; Cremasco, H.; Mantovani, A.C.G.; Bona, E.; Killner, M.; Borsato, D. Kinetic study of the transesterification reaction by artificial neural networks and parametric particle swarm optimization. Fuel 2020, 267, 117221. [Google Scholar] [CrossRef]
- Tai, X.Y.; Ocone, R.; Christie, S.D.; Xuan, J. Multi-objective optimisation with hybrid machine learning strategy for complex catalytic processes. Energy AI 2022, 7, 100134. [Google Scholar] [CrossRef]
- Baş, D.; Dudak, F.C.; Boyacı, İ.H. Modeling and optimization III: Reaction rate estimation using artificial neural network (ANN) without a kinetic model. J. Food Eng. 2007, 79, 622–628. [Google Scholar] [CrossRef]
- Gusmão, G.S.; Retnanto, A.P.; da Cunha, S.C.; Medford, A.J. Kinetics-informed neural networks. Catal. Today 2023, 417, 113701. [Google Scholar] [CrossRef]
- Gomes, L.C.; Rosas, D.D.O.; Chistone, R.C.; Zotin, F.M.Z.; de Araujo, L.R.R.; Zotin, J.L. Hydroisomerization of n-hexadecane using Pt/alumina-Beta zeolite catalysts for producing renewable diesel with low pour point. Fuel 2017, 209, 521–528. [Google Scholar] [CrossRef]
- Jiménez, C.; Romero, F.J.; Roldán, R.; Marinas, J.M.; Gómez, J.P. Hydroisomerization of a hydrocarbon feed containing n-hexane, n-heptane and cyclohexane on zeolite-supported platinum catalysts. Appl. Catal. A Gen. 2003, 249, 175–185. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Khel, T.A.K.; Azkaar, M.; Engblom, S.; Murzin, D.Y. Catalytic Hydroisomerization of Long-Chain Hydrocarbons for the Production of Fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, hydrogenation and hydro-deoxygenation of fatty acids, esters and glycerides: Mechanisms, kinetics and transport phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.-J.; Wu, W. Bifunctional catalysts for the hydroisomerization of n-alkanes: The effects of metal–acid balance and textural structure. Catal. Sci. Technol. 2019, 9, 4162–4187. [Google Scholar] [CrossRef]
- Du, H.; Liu, D.; Li, M.; Wu, P.; Yang, Y. Effects of the Temperature and Initial Hydrogen Pressure on the Isomerization Reaction in Heavy Oil Slurry-Phase Hydrocracking. Energy Fuels 2015, 29, 626–633. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Al-Zaidi, B.Y.; Shehab, A.K.; Shakoor, Z.M.; Aal-Kaeb, S.; Gomez, L.Q.; Majdi, H.S.; Al-Shafei, E.N.; AbdulRazak, A.A.; McGregor, J. Experimental and kinetic studies of the advantages of coke accumulation over Beta and Mordenite catalysts according to the pore mouth catalysis hypothesis. Catal. Commun. 2023, 181, 106718. [Google Scholar] [CrossRef]
- Al-Iessa, M.S.; Al-Zaidi, B.Y.; Almukhtar, R.S.; Shakor, Z.M.; Hamawand, I. Optimization of Polypropylene Waste Recycling Products as Alternative Fuels through Non-Catalytic Thermal and Catalytic Hydrocracking Using Fresh and Spent Pt/Al2O3 and NiMo/Al2O3 Catalysts. Energies 2023, 16, 4871. [Google Scholar] [CrossRef]
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Saady, N.M.C.; Albayati, T.M. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef] [PubMed]
- Calemma, V.; Peratello, S.; Perego, C. Modeling and Simulation of the Isomerization of n-Heptane over a Molybdenum Oxycarbide Catalyst for Elucidation of the Bifunctional Mechanism. Industrial & Engineering Chemistry Research. Appl. Catal. A Gen. 2023, 62, 9607–9618. [Google Scholar]
- Calemma, V.; Peratello, S.; Perego, C. Hydroisomerization and hydrocracking of long chain n-alkanes on Pt/amorphous SiO2–Al2O3 catalyst. Appl. Catal. A Gen. 2000, 190, 207–218. [Google Scholar] [CrossRef]
- Raseev, S. Thermal and Catalytic Processes in Petroleum Refining; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Khalaf, Y.H.; Al-Zaidi, B.Y.S.; Shakour, Z.M. Experimental and Kinetic Study of the Effect of using Zr- and Pt-loaded Metals on Y-zeolite-based Catalyst to Improve the Products of n-heptane Hydroisomerization Reactions. Orbital Electron. J. Chem. 2022, 14, 153–167. [Google Scholar] [CrossRef]
- Alemán-Vázquez, L.O.; Cano-Domínguez, J.L.; Torres-García, E.; Villagómez-Ibarra, J.R. Industrial application of catalytic systems for n-heptane isomerization. Molecules 2011, 16, 5916–5927. [Google Scholar] [CrossRef]
- Malayeri, M.; Nasiri, F.; Haghighat, F.; Lee, C.-S. Optimization of photocatalytic oxidation reactor for air purifier design: Application of artificial neural network and genetic algorithm. Chem. Eng. J. 2023, 462, 142186. [Google Scholar] [CrossRef]
- Kirilova, E.G. Artificial Neural Networks: Applications in Chemical Engineering. In Modeling and Simulation in Chemical Engineering: Project Reports on Process Simulation; Boyadjiev, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 127–146. [Google Scholar]
- Elçiçek, H.; Akdoğan, E.; Karagöz, S. The Use of Artificial Neural Network for Prediction of Dissolution Kinetics. Sci. World J. 2014, 2014, 194874. [Google Scholar] [CrossRef]
- Vogl, T.P.; Mangis, J.K.; Rigler, A.K.; Zink, W.T.; Alkon, D.L. Accelerating the convergence of the back-propagation method. Biol. Cybern. 1988, 59, 257–263. [Google Scholar] [CrossRef]
- Govindan, B.; Jakka, S.C.B.; Radhakrishnan, T.K.; Tiwari, A.K.; Sudhakar, T.M.; Shanmugavelu, P.; Kalburgi, A.K.; Sanyal, A.; Sarkar, S. Investigation on Kinetic Parameters of Combustion and Oxy-Combustion of Calcined Pet Coke Employing Thermogravimetric Analysis Coupled to Artificial Neural Network Modeling. Energy Fuels 2018, 32, 3995–4007. [Google Scholar] [CrossRef]
- Demir, B.; Eski, I.; Gürbüz, F.; Kuş, Z.A.; Sesli, Y.; Ercişli, S. Prediction of Walnut Mass Based on Physical Attributes by Artificial Neural Network (ANN). Erwerbs-Obstbau 2020, 62, 47–56. [Google Scholar] [CrossRef]
- Abdul Rahman, M.B.; Chaibakhsh, N.; Basri, M.; Salleh, A.B.; Rahman, R.N.Z.R.A. Application of Artificial Neural Network for Yield Prediction of Lipase-Catalyzed Synthesis of Dioctyl Adipate. Appl. Biochem. Biotechnol. 2009, 158, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Ajala, S.O. Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology. Ind. Crop. Prod. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Hafizi, A.; Ahmadpour, A.; Koolivand-Salooki, M.; Heravi, M.M.; Bamoharram, F.F. Comparison of RSM and ANN for the investigation of linear alkylbenzene synthesis over H14[NaP5W30O110]/SiO2 catalyst. J. Ind. Eng. Chem. 2013, 19, 1981–1989. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Aleboyeh, H. Modeling and Optimization of Heterogeneous Photo-Fenton Process with Response Surface Methodology and Artificial Neural Networks. Environ. Sci. Technol. 2008, 42, 7970–7975. [Google Scholar] [CrossRef] [PubMed]
- Marchitan, N.; Cojocaru, C.; Mereuta, A.; Duca, G.; Cretescu, I.; Gonta, M. Modeling and optimization of tartaric acid reactive extraction from aqueous solutions: A comparison between response surface methodology and artificial neural network. Sep. Purif. Technol. 2010, 75, 273–285. [Google Scholar] [CrossRef]
- Ofoefule, A.U.; Esonye, C.; Onukwuli, O.D.; Nwaeze, E.; Ume, C.S. Modeling and optimization of African pear seed oil esterification and transesterification using artificial neural network and response surface methodology comparative analysis. Ind. Crop. Prod. 2019, 140, 111707. [Google Scholar] [CrossRef]
- Günay, M.E.; Yıldırım, R. Neural network aided design of Pt-Co-Ce/Al2O3 catalyst for selective CO oxidation in hydrogen-rich streams. Chem. Eng. J. 2008, 140, 324–331. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Kumar, H. Process parameter assessment of biodiesel production from a Jatropha–algae oil blend by response surface methodology and artificial neural network. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 2119–2125. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Comparison of response surface methodology and artificial neural network approach towards efficient ultrasound-assisted biodiesel production from muskmelon oil. Ultrason. Sonochem. 2015, 23, 192–200. [Google Scholar] [CrossRef]
- Aksu, G.; Güzeller, C.O.; Eser, M.T. The Effect of the Normalization Method Used in Different Sample Sizes on the Success of Artificial Neural Network Model. Int. J. Assess. Tools Educ. 2019, 6, 170–192. [Google Scholar] [CrossRef]
- Pham, B.T.; Nguyen, M.D.; Ly, H.-B.; Pham, T.A.; Hoang, V.; Van Le, H.; Le, T.-T.; Nguyen, H.Q.; Bui, G.L. Development of Artificial Neural Networks for Prediction of Compression Coefficient of Soft Soil. In CIGOS 2019, Innovation for Sustainable Infrastructure; Ha-Minh, C., Dao, D.V., Benboudjema, F., Derrible, S., Huynh, D.V.K., Tang, A.M., Eds.; Springer: Singapore, 2020. [Google Scholar]

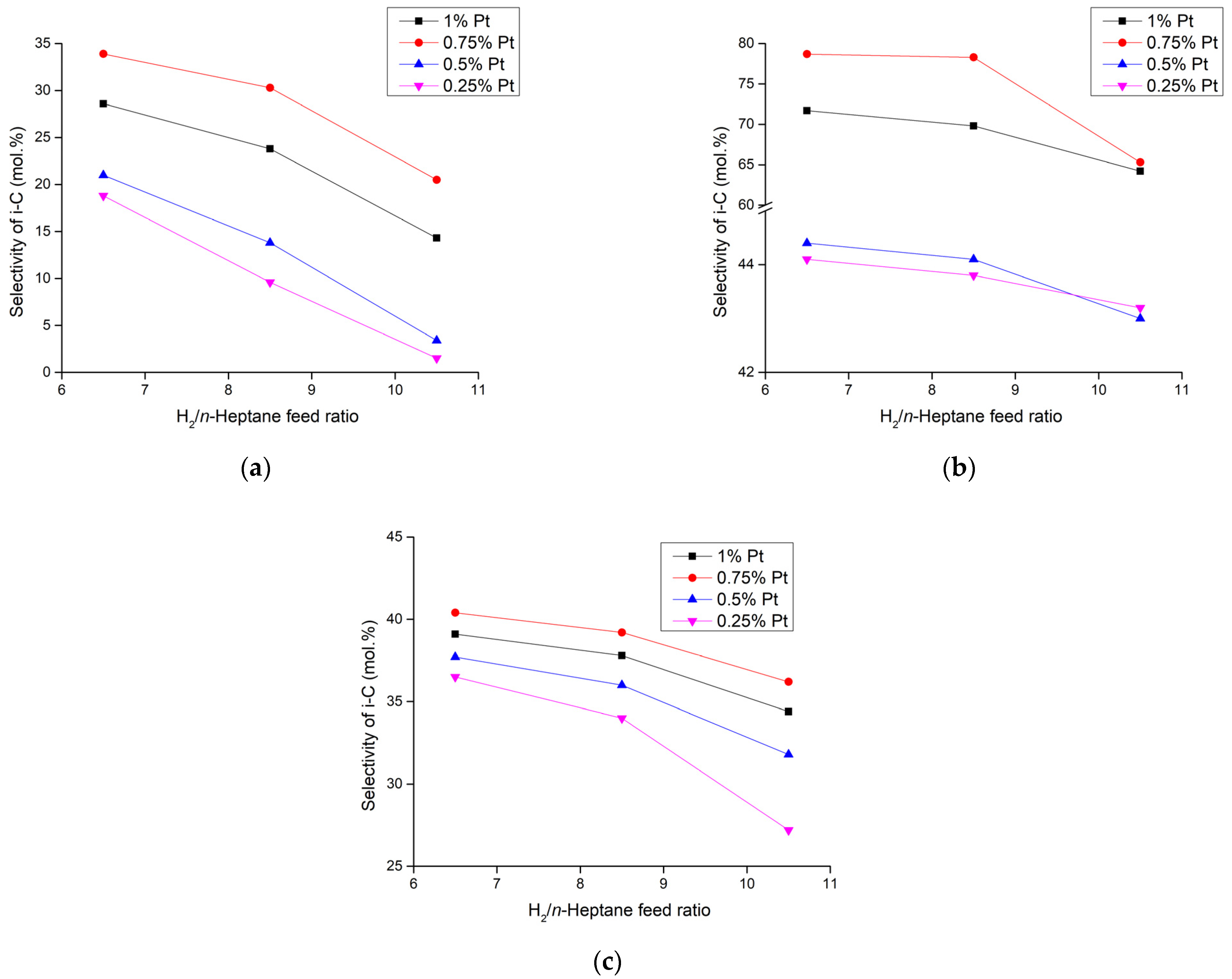
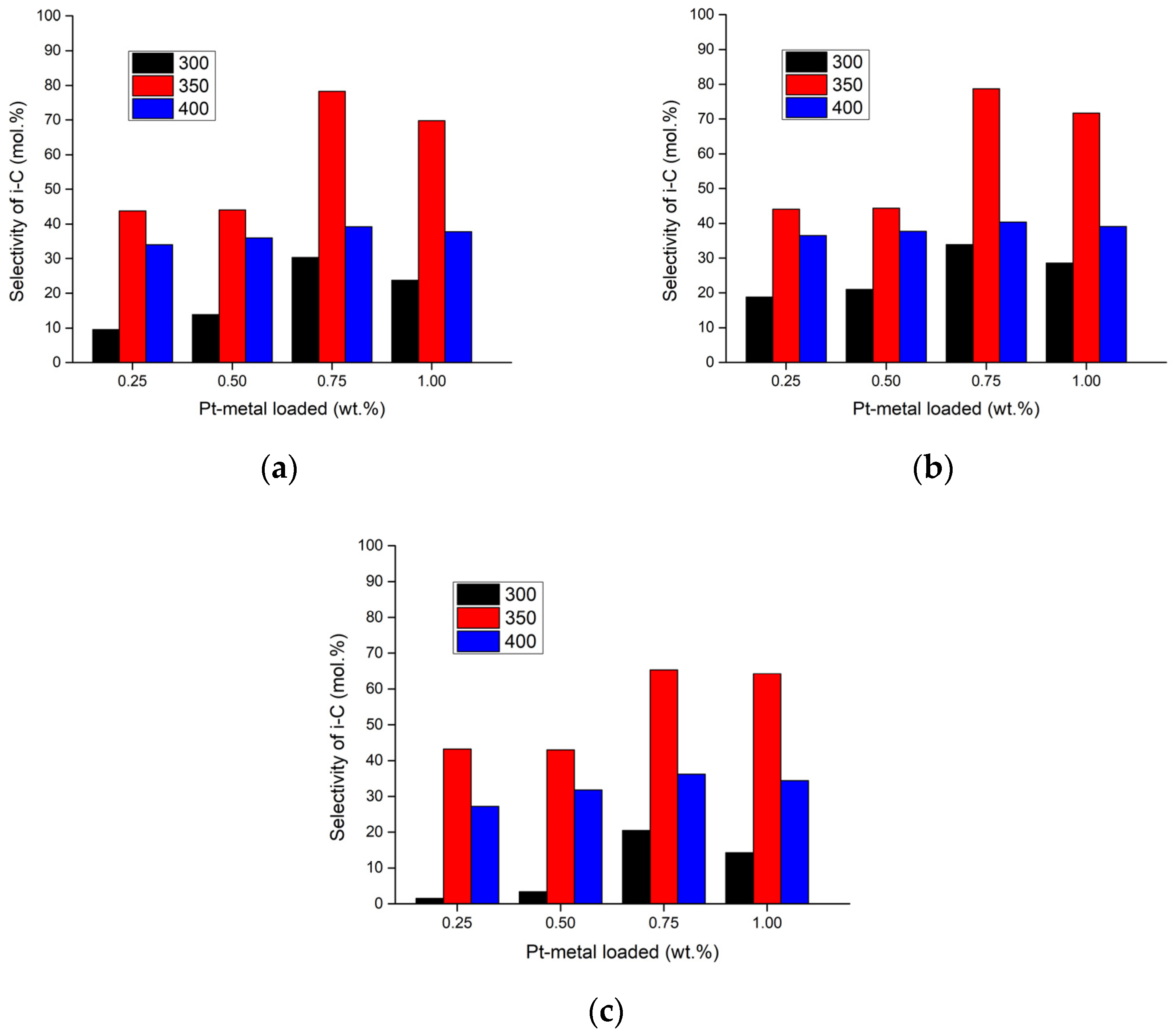
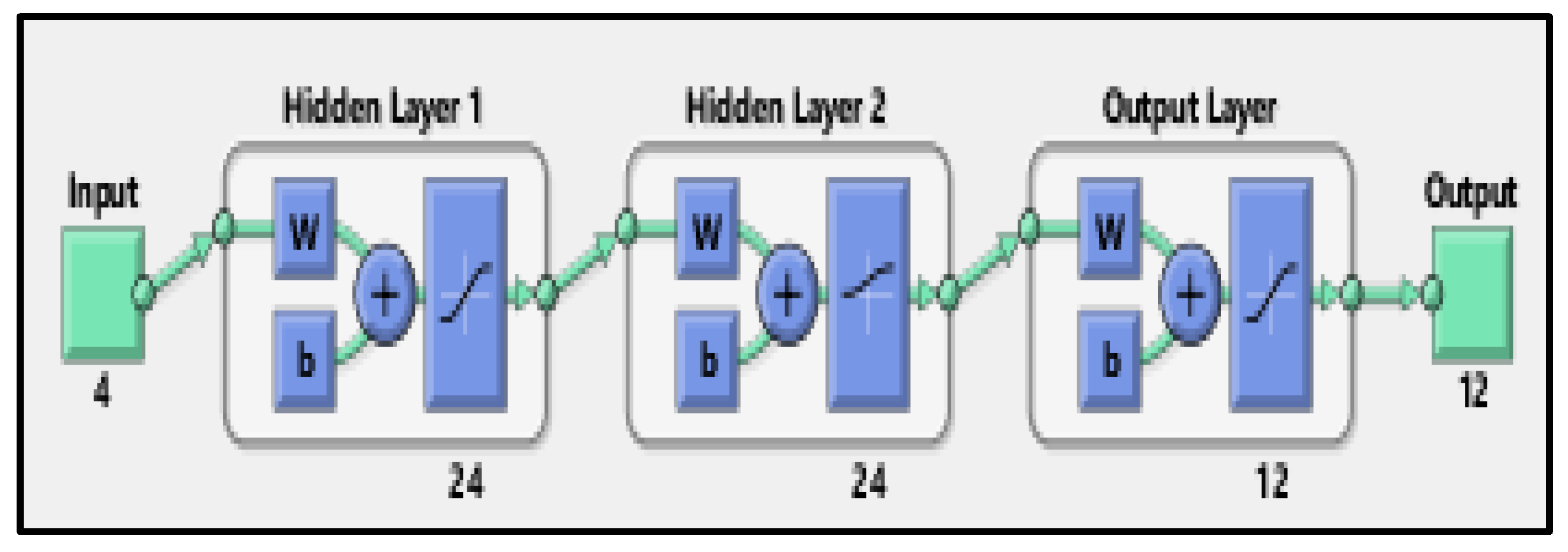
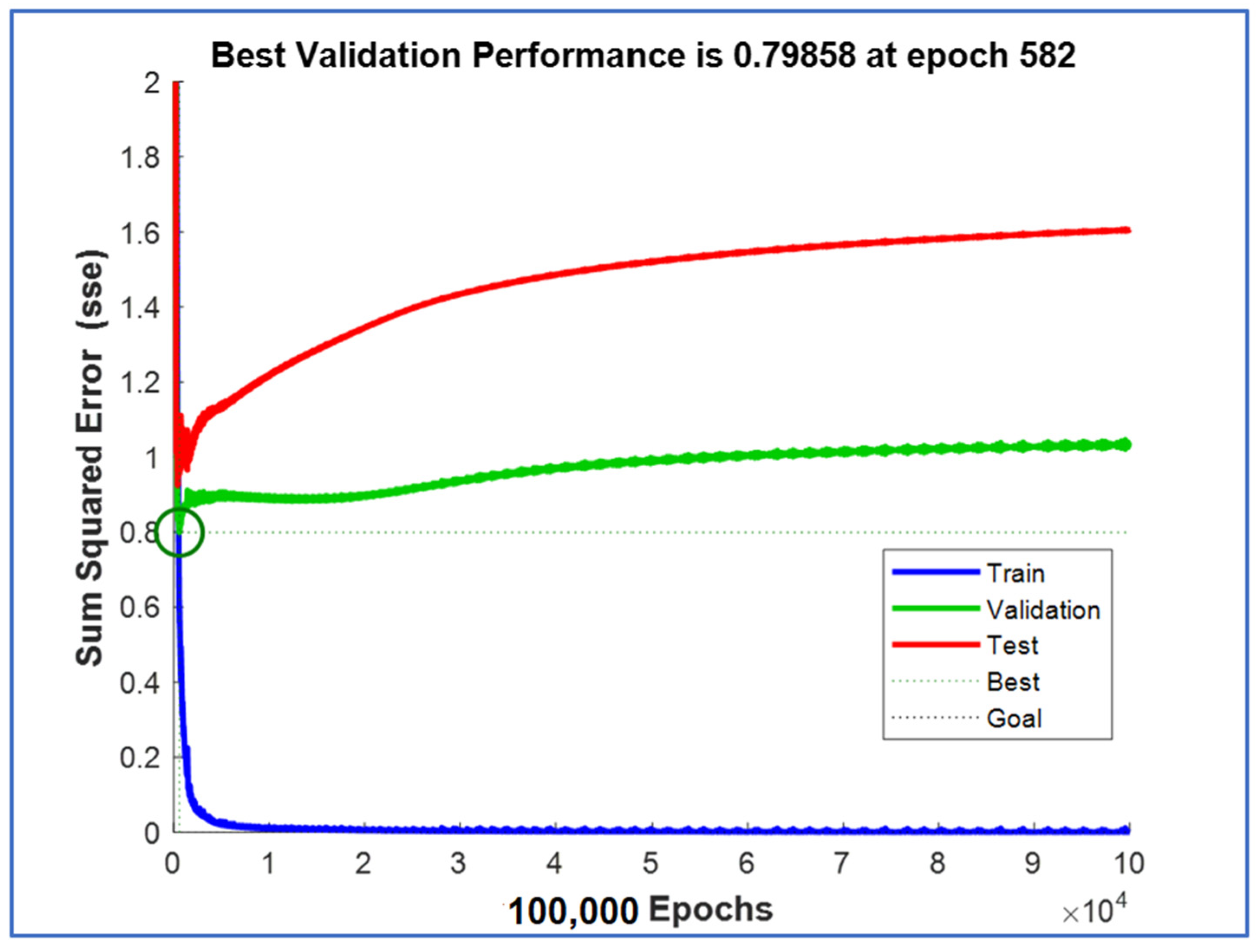

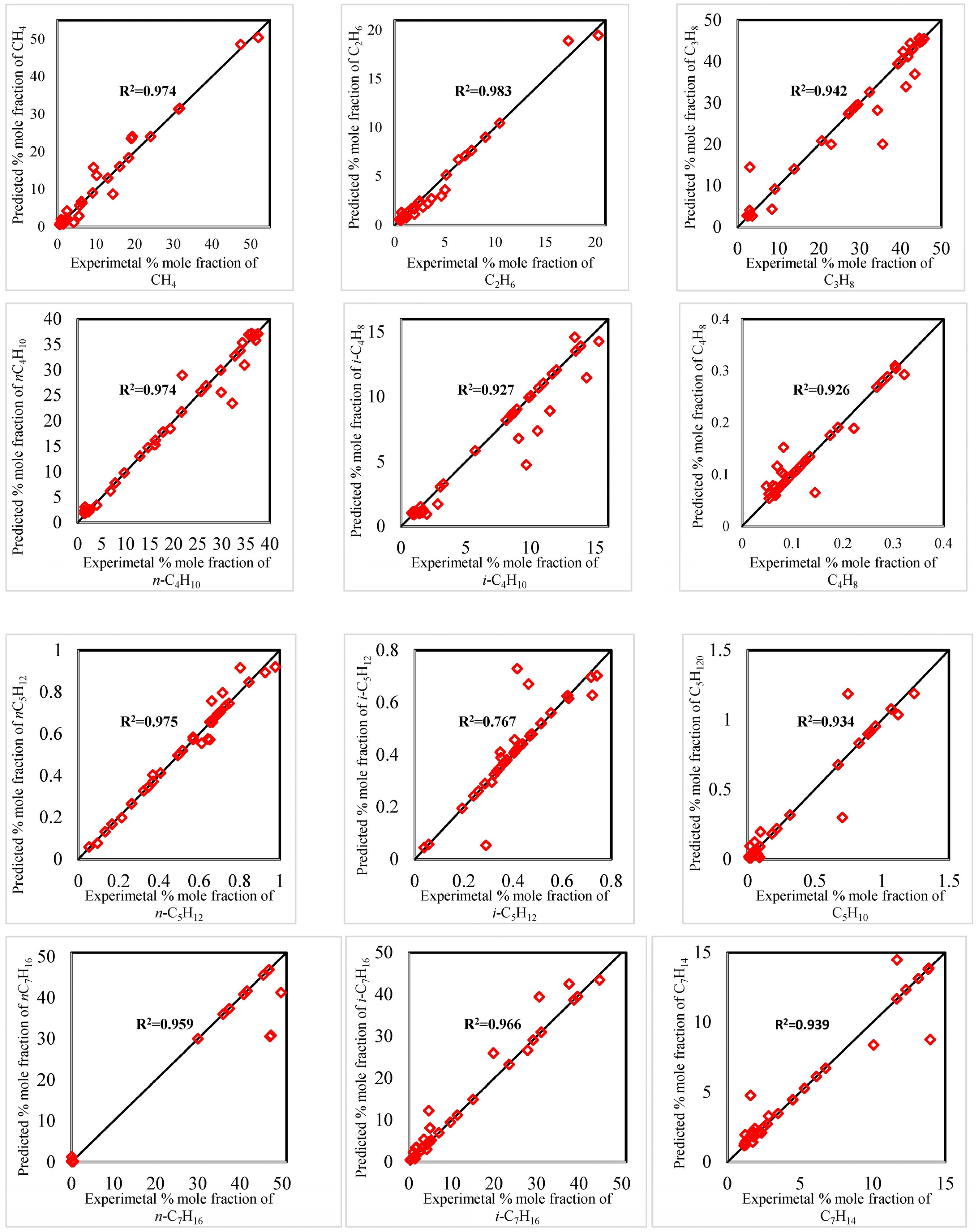
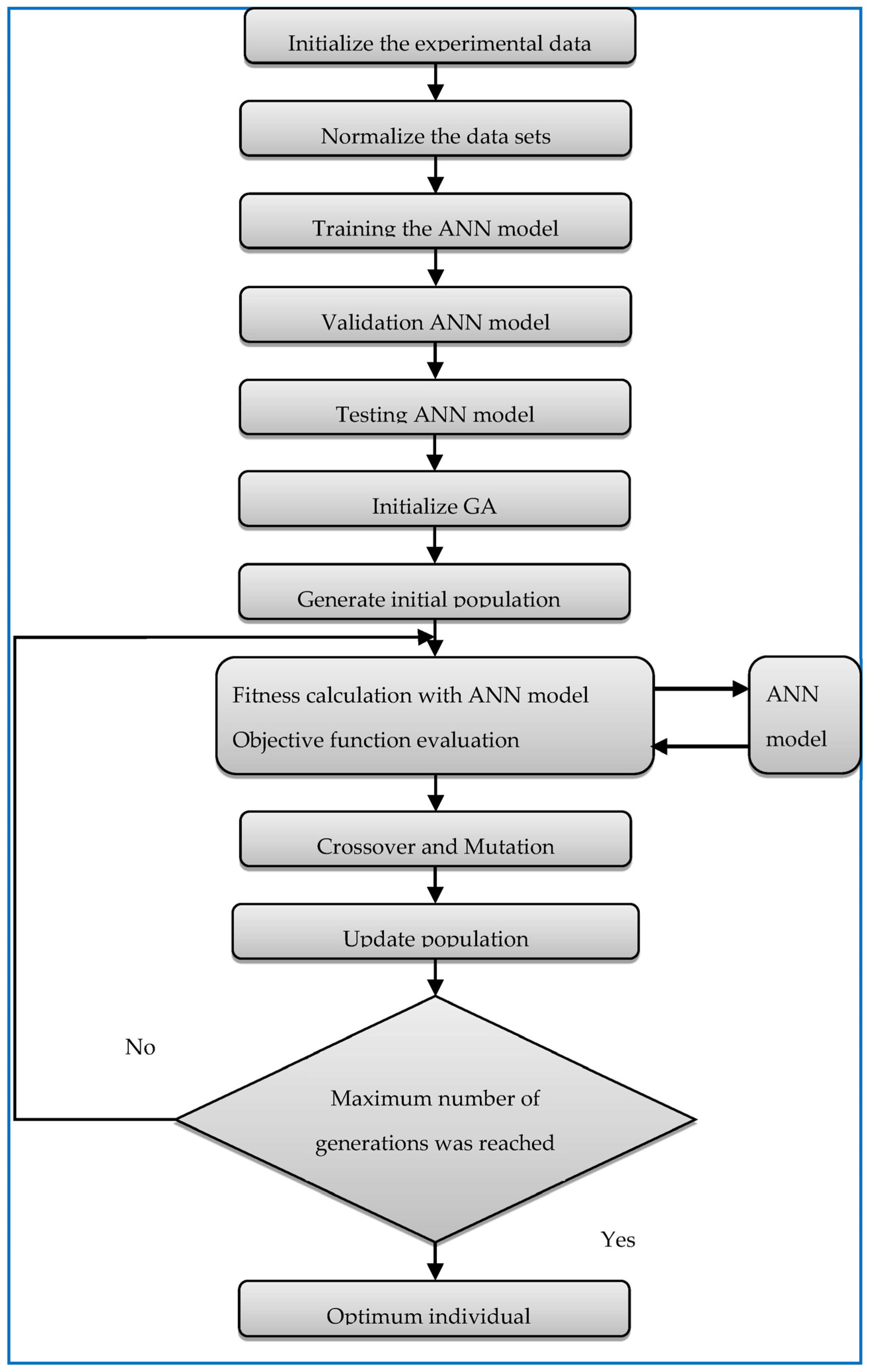



| Parameters | Selectivity towards Isomer Production (mol.%) | |||||
|---|---|---|---|---|---|---|
| Temperature (°C) | H2/n-C7 Feed Ratio | WHSV ( h−1) | 0.25 wt.% Pt/HY-HZSM-5 Zeolite Catalyst | 0.5 wt.% Pt/HY-HZSM-5 Zeolite Catalyst | 0.75 wt.% Pt/HY-HZSM-5 Zeolite Catalyst | 1 wt.% Pt/HY-HZSM-5 Zeolite Catalyst |
| 400 | 6.5 | 2.98 | 36.53 | 37.74 | 40.44 | 39.13 |
| 8.5 | 5.03 | 34.04 | 36.01 | 39.18 | 37.79 | |
| 10.5 | 7.61 | 27.16 | 31.81 | 36.14 | 34.37 | |
| 350 | 6.5 | 2.98 | 44.07 | 44.42 | 78.69 | 71.68 |
| 8.5 | 5.03 | 43.75 | 44.11 | 78.3 | 69.85 | |
| 10.5 | 7.61 | 43.18 | 43.03 | 65.29 | 64.23 | |
| 300 | 6.5 | 2.98 | 18.85 | 20.97 | 33.88 | 28.6 |
| 8.5 | 5.03 | 9.63 | 13.82 | 30.26 | 23.81 | |
| 10.5 | 7.61 | 1.49 | 3.39 | 20.5 | 14.32 | |
| Input layer | Input data (4 variables) |
| Output layer | Composition of the products (12 variables) |
| Number of hidden layers | 2 |
| Number of neurons for each hidden layer | 24 |
| Performance function | Mean absolute error (MAE) |
| Activation function | Sigmoid |
| Type of activation sigmoid | Tan-sigmoid transfer function was used in first hidden layer Log-sigmoid transfer function was used in second hidden layer |
| Algorithm used for training | Levenberg–Marquardt |
| Learning rate | 0.0001 |
| Max number of iterations | 1000 |
| Gradient | 0.00001 |
| Maximum | Minimum | Variables |
|---|---|---|
| Temperature (°C) | 300 | 400 |
| Pt-metal loaded (wt.%) | 0.25 | 1 |
| H2/n-C7 feed ratio | 6.5 | 10.5 |
| WHSV (h−1) | 2.98 | 7.61 |
| Mole fraction (%) | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zaidi, B.Y.; Al-Shathr, A.; Shehab, A.K.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; McGregor, J. Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA). Catalysts 2023, 13, 1125. https://doi.org/10.3390/catal13071125
Al-Zaidi BY, Al-Shathr A, Shehab AK, Shakor ZM, Majdi HS, AbdulRazak AA, McGregor J. Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA). Catalysts. 2023; 13(7):1125. https://doi.org/10.3390/catal13071125
Chicago/Turabian StyleAl-Zaidi, Bashir Y., Ali Al-Shathr, Amal K. Shehab, Zaidoon M. Shakor, Hasan Sh. Majdi, Adnan A. AbdulRazak, and James McGregor. 2023. "Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA)" Catalysts 13, no. 7: 1125. https://doi.org/10.3390/catal13071125
APA StyleAl-Zaidi, B. Y., Al-Shathr, A., Shehab, A. K., Shakor, Z. M., Majdi, H. S., AbdulRazak, A. A., & McGregor, J. (2023). Hydroisomerisation and Hydrocracking of n-Heptane: Modelling and Optimisation Using a Hybrid Artificial Neural Network–Genetic Algorithm (ANN–GA). Catalysts, 13(7), 1125. https://doi.org/10.3390/catal13071125