Abstract
In this study, nickel cobaltate (NiCo2O4) powders are employed as a catalyst in conjunction with persulfate for the development of a catalytic oxidation system to enhance fuel desulfurization. The hydrothermal synthesis conditions of NiCo2O4 powders, which significantly influenced the desulfurization efficiency, were optimized using a response surface methodology with a Box–Behnken design. These conditions were ranked in the following order: calcination temperature > hydrothermal temperature > calcination time > hydrothermal time. Through the optimization process, the ideal preparation conditions were determined as follows: a hydrothermal temperature of 143 °C, hydrothermal time of 6.1 h, calcination temperature of 330 °C, and calcination time of 3.7 h. Under these optimized conditions, the predicted desulfurization rate was approximately 85.8%. The experimental results closely matched the prediction, yielding a desulfurization rate of around 84%, with a minimal error of only 2.1%. To characterize the NiCo2O4 powders prepared under the optimal conditions, XRD, SEM, and TEM analyses were conducted. The analysis revealed that the microscopic morphology of NiCo2O4 exhibited a rectangular sheet structure, with an average particle size of 20 nm. Additionally, fan-shaped NiCo2O4 particles were observed as a result of linear and bundle agglomerations. Thus, this work is innovative in its ability to synthesize nano-catalysts using hydrothermal synthesis in a controllable manner and establishing a correlation between the hydrothermal synthesis conditions and catalytic activity.
1. Introduction
Hydrothermal synthesis is widely utilized in the preparation of nano-powders due to its distinct advantages. It offers high particle dispersion, uniform and easily controllable particle size distribution, as well as good particle purity. This method operates under subcritical conditions with temperatures ranging from 100–240 °C and pressures from 0.3–4 MPa [1]. In 1996, Qian successfully synthesized GaN nanocrystals using benzene thermal synthesis, which marked a significant milestone in the field of the hydrothermal (solvent heat) synthesis of nanomaterials [2]. Since then, the hydrothermal synthetic method has gained popularity in various fields, including crystalline materials [3], ceramic materials, molecular sieve materials [4], nanomaterials, electrode materials [5], catalytic materials, and more.
Nickel cobaltate (NiCo2O4) exhibits an excellent performance as a material in supercapacitors [6], direct catalytic oxidation [7], photocatalysis [8], electrocatalysis [9], and other applications. Particularly, it is often employed as a catalyst coupled with oxidants, such as hydrogen peroxide, ozone, or persulfate, forming Fenton-like oxidation systems for the degradation of organic pollutants [10]. Zhou et al. utilized a co-precipitation hydrothermal method to synthesize NiCo2O4, which acted as a catalyst for peroxymonosulfate (PMS) in the degradation of citric acid in water [11]. It is indeed challenging to achieve the precise control of particle morphology using the co-precipitation method. The sol-gel method is a commonly used approach for synthesizing nanostructured NiCo2O4 materials. However, this method also faces challenges in controlling the morphology and size of the resulting particles. Additionally, the sol-gel method often leads to significant product non-uniformity, which hinders large-scale industrial production [12]. Similarly, Xu et al. employed a simple hydrothermal method to load NiCo2O4 onto expanded graphite, creating a catalyst that was coupled with PMS to decompose sulfamethoxazole, focusing on the oxidizing agent properties of the species involved [13]. Tian et al. also used a straightforward hydrothermal method to synthesize dandelion-shaped NiCo2O4 microspheres, which were coupled with PMS to form a Fenton-like system for the degradation of humic acids in water, exhibiting an excellent performance [14]. In a previous study, we successfully synthesized sea urchin-shaped NiCo2O4 using a simple hydrothermal method, and coupled it with PMS to catalyze the oxidative removal of dibenzothiophene (DBT) from octane, demonstrating excellent activity [15]. The NiCo2O4 as a catalyst can also be applied in various aspects, such as methanol conversion and combustion [16,17], propane oxidation [18], electrode materials [19], and so on.
Based on the literature analysis, it is evident that previous studies focused primarily on determining specific conditions for hydrothermal synthesis to achieve desired microstructures of NiCo2O4. The hydrothermal synthesis of NiCo2O4 in an aqueous solution offers the advantage of efficient mixing and heat transfer, leading to the uniform dispersion of reactants and precise control over the reaction process. This results in the generation of high-quality materials with consistent properties. Moreover, this approach enables the manipulation of product morphology, size, and structure, thereby influencing the catalytic activity of NiCo2O4. The hydrothermal synthesis method can also be utilized for synthesizing various oxides, such as ZnMoO4 [20], NiMoO4 [21], Sn3O4 [22], and SAPO-18 [23], and can be applied in specific catalytic processes.
To address the scarcity of studies focusing on optimizing hydrothermal synthesis conditions, this study employs a response surface methodology (RSM). RSM is a statistical method widely utilized in experimental optimization processes across various fields, including chemistry, food, environment, and materials [24]. In the context of desulfurization, dibenzothiophene (DBT) is used as a representative sulfur-containing compound, octane is used as a simulated oil, peroxymonosulfate (PMS) serves as the oxidant, and acetonitrile acts as the extractant. The four hydrothermal synthesis conditions, hydrothermal temperature, hydrothermal time, calcination temperature, and calcination time are optimized using RSM to determine the optimal preparation conditions for achieving the highest desulfurization activity with the NiCo2O4 catalyst. The goal of this study is to establish the relationship between the powder preparation conditions and the resulting catalytic activity.
2. Results and Discussion
2.1. Desulfurization Experimental Results and Modeling
Table 1 shows 29 sets of experiments arranged by the Box–Behnken design principle on Design Expert 8.0.6. The desulfurization experiments were performed according to the experimental conditions arranged by the design to obtain the corresponding desulfurization rates. A second-order model of the desulfurization rate (Y) versus the four conditions of hydrothermal temperature (A), hydrothermal time (B), calcination temperature (C), and calcination time (D) for the preparation of NiCo2O4 powders was established as shown in Equation (1):
Y = 83.80 + 1.78A + 0.80B−4.68C − 1.59D + 0.075AB + 0.52AC + 1.05AD + 1.3BC − 2.52BD − 0.5CD − 4.50A2 − 6.85B2 − 5.54C2 − 5.09D2

Table 1.
Box–Behnken experimental arrangement and desulfurization rates [25,26].
Figure 1 shows a comparison between the predicted desulfurization values (regression values) calculated using the second-order model, Equation (1), and the corresponding experimental values. The plot demonstrates that the regression and experimental values are primarily distributed along the diagonal of the coordinate system. There is a lack of data points that significantly deviate from the diagonal line, indicating that the residuals of the model are small and the model itself is reasonable. This observation suggests that the second-order model adequately represents the relationship between the desulfurization rate and the four experimental conditions for the preparation of NiCo2O4 powders. The close alignment of the regression values with the experimental values supports the accuracy and validity of the model in predicting the desulfurization rates based on the chosen hydrothermal synthesis conditions.
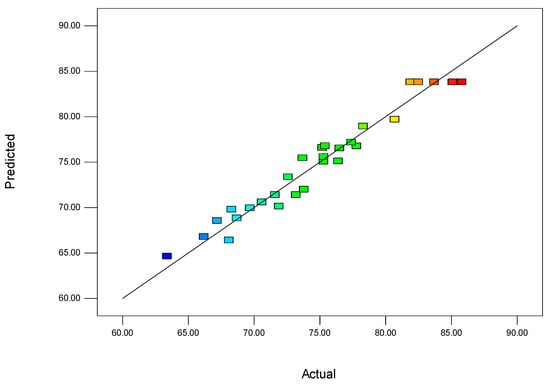
Figure 1.
Comparison of predicted and experimental values based on the second-order model.
Figure 2 displays the internally studentized residuals corresponding to the 29 experimental runs. The plot reveals that the residuals exhibited irregular characteristics, indicating that the experimental errors stemmed from random fluctuations rather than systematic errors. Notably, the range of the internally studentized residuals remained within −2 to 2, suggesting that there were no significant outliers or faulty measurements in the experimental results. No residuals exceed −3 and 3, indicating the correctness of the fitting formula and the absence of significant residuals. This further supports the reliability and validity of the obtained data. The absence of residuals outside the −2 to 2 range indicates that the experimental results are consistent with the predicted values from the second-order model, confirming the overall robustness of the experimental setup and the accuracy of the desulfurization rate measurements.
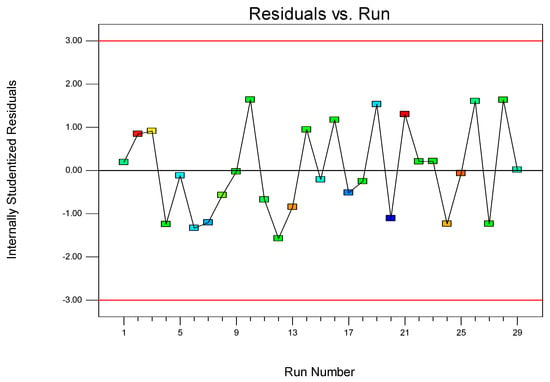
Figure 2.
The internally studentized residuals at run number from 1 to 29.
2.2. Analysis of Variance (ANOVA)
Table 2 provides the analysis of variance (ANOVA) for the response surface second-order model. The F-value was 21.8, with a p-value (<0.0001) significantly less than 0.01, indicating that the model obtained through the response surface method was highly significant. Analyzing Table 2 reveals that the desulfurization rate (Y) is primarily influenced by hydrothermal temperature (A), calcination temperature (C), and calcination time (D), as evidenced by their low p-values (<0.05). In contrast, hydrothermal time (B) had a relatively lower impact, as its p-value exceeded 0.05. This observation was further supported by the coefficient values in Equation (1), where the coefficients associated with hydrothermal temperature (A), calcination temperature (C), and calcination time (D) were significantly greater than the coefficient for hydrothermal time (B). The interaction between hydrothermal time (B) and calcination time (D), represented by the BD term, was found to be significant (p-value = 0.0108), while other interactions, such as AB, AC, AD, BC, and CD, could be considered negligible. This suggested that the interaction between the four conditions was not substantial. Based on the mean square data and F-values, it was observed that calcination temperature (C) had the highest impact on the desulfurization rate, followed by hydrothermal temperature (A), calcination time (D), and hydrothermal time (B). Additionally, the squared terms A2, B2, C2, and D2 were all extremely significant, indicating a non-linear relationship between the desulfurization rate (Y) and the respective factors A, B, C, and D. The residual term’s sum of squares and mean square values were relatively low at 41.37 and 2.96, respectively, indicating that the fitted desulfurization rate was not significantly different from the experimental desulfurization rate. The misfit term’s F-value was 1.104 with a p-value of 0.5034, suggesting no significant correlation between the model misfit and the pure difference, indicating a good fit for the model. The mean value of the desulfurization rate was reported as 74.7. The precision of the model measurement was 15.5 (>4), and the coefficient of variation was 2.3% (<4%), both indicating the high accuracy of the experiments. The fitted correlation coefficient R22 = 0.95 indicated the correctness of the obtained model. The difference between Adj R2 and Pred R2 was 0.12, which was less than 0.2, implying the reasonableness of the obtained second-order model. Overall, the ANOVA results established the correctness and significance of the second-order model derived using the response surface method.

Table 2.
ANOVA table for the response surface second-order model of the desulfurization rate.
2.3. Response Surface Analysis
Figure 3 illustrates the response surface plots depicting the effects of hydrothermal temperature (A), hydrothermal time (B), calcination temperature (C), and calcination time (D) on the desulfurization rate. From the figure, it is evident that these factors exhibit a trend of an increasing and then decreasing impact on the desulfurization rate. This suggests the presence of optimal experimental conditions within the selected range. The color gradient from the edge to the center of the surface signifies a gradual increase in the response value (desulfurization rate). The highest point of the response surface, denoted by the center of the minimum ellipse, represents the optimized preparation conditions achieved through the response surface method.
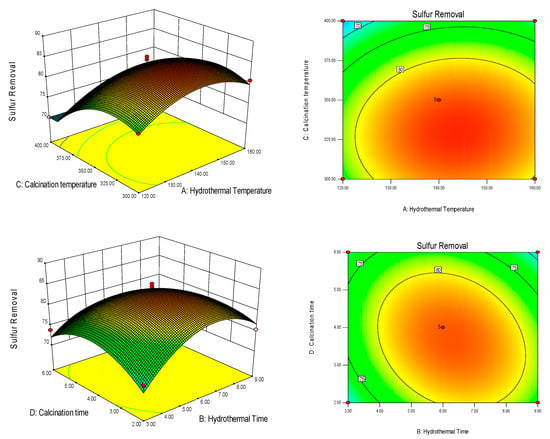
Figure 3.
Response surface plot of the effect of hydrothermal temperature (A), hydrothermal time (B), calcination temperature (C), and calcination time (D) on the desulfurization rate.
Analyzing Figure 3 further reveals that the contours formed between the hydrothermal temperature (A) and calcination temperature (C) are elliptical, but with small curvature. On the other hand, the contours between hydrothermal time (B) and calcination time (D) also appear elliptical, but with a larger curvature. This indicates that the interaction between hydrothermal temperature (A) and calcination temperature (C) is not significant, while the interaction between hydrothermal time (B) and calcination time (D) is pronounced. Hydrothermal temperature (A) and calcination temperature (C) can be considered independent of each other, without needing to account for their mutual influence. However, when dealing with the time conditions, attention should be given to the interaction between hydrothermal time (B) and calcination time (D), as it has a noticeable impact. Since the influence of hydrothermal time (B) on the desulfurization rate is relatively low, greater emphasis should be placed on considering the influence of calcination time (D) when determining the optimal preparation time conditions.
The reasons behind these observations can be analyzed as follows: during the hydrothermal synthesis of NiCo2O4 powders, they undergo a nucleation–growth–agglomeration process. This formation process occurs rapidly, requiring only a relatively short time to complete the hydrothermal process. Consequently, the influence of hydrothermal time (B) on the desulfurization rate is relatively minor. Hydrothermal temperature (A) exerts a greater impact on particle morphology. Increasing the temperature enhances the molecular movement, facilitating particle nucleation but hindering growth and agglomeration, thereby significantly affecting the desulfurization effectiveness of the powders. Calcination temperature (C) plays a crucial role in removing inorganic and organic substances from the powder surface during the hydrothermal process. It directly affects the formation and transformation process of NiCo2O4 crystalline structures, as well as the particle size. Consequently, it has a significant influence on the desulfurization rate. The pronounced effect of calcination time (D) can be attributed to inadequate removal rates of inorganic and organic matter from the powder surface in shorter durations. Additionally, slower NiCo2O4 crystalline formation and the time required for temperature conduction to the powder in the muffle furnace contribute to the notable influence of calcination time (D) on the desulfurization process.
2.4. Optimal Experimental Conditions from RSM and NiCo2O4 Characterization
Figure 4 presents the optimized NiCo2O4 preparation conditions obtained through the response surface method. These conditions included a hydrothermal temperature of 143 °C, hydrothermal time of 6.1 h, calcination temperature of 330 °C, and calcination time of 3.7 h. The predicted desulfurization rate corresponding to these conditions was 85.8%. The synthesis results achieved using the optimized NiCo2O4 preparation conditions are displayed in Figure 5. The XRD characterization results of the synthesized NiCo2O4, as shown in Figure 5, precisely match the JCPDS card (20–0781). The characteristic peaks of 2θ are located at 18.90°, 31.15°, 36.70°, 38.40°, 44.62°, 55.43°, 59.09°, 64.98°, and 77.54°, corresponding to the (111), (220), (311), (222), (400), (422), (511), (440), and (533) crystal planes of cubic NiCo2O4. This indicates the successful synthesis of pure NiCo2O4 powders. Furthermore, the excellent magnetic separation properties of the material can be observed in Figure 5, where the powders can be separated from acetonitrile using a magnet. This suggests that the material possesses strong magnetic separation capabilities. The SEM image presented in Figure 4 illustrates that the particles of NiCo2O4 are at the nano-level and arranged in a linear agglomeration pattern.
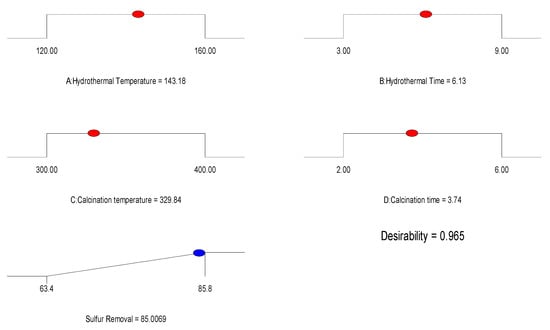
Figure 4.
Optimal experimental conditions from RSM.
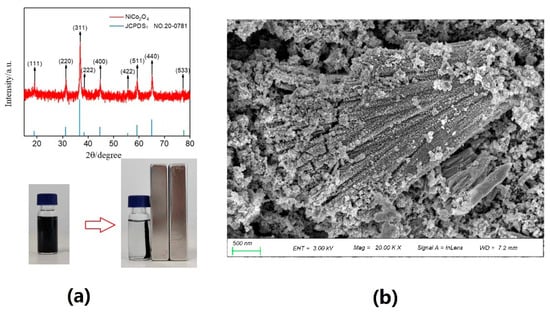
Figure 5.
XRD (a) and SEM (b) images of NiCo2O4 powders.
Figure 6 displays the TEM analysis of NiCo2O4 powders, providing a further insight into the agglomeration mechanism of the particles. The analysis revealed a clear progression in the formation process of NiCo2O4 powders. Initially, NiCo2O4 nuclei were observed to nucleate, resulting in the formation of rectangular-shaped particles with an average particle size of approximately 20 nm. These nuclei served as the initial building blocks for further agglomeration. Subsequently, the nuclei underwent primary linear agglomeration, leading to the formation of nanowires. This primary agglomeration process involved the alignment and connection of the individual rectangular-shaped particles, resulting in the elongated structure of the nanowires. Following primary agglomeration, the nanowires underwent a secondary bundled agglomeration. This process involved the bundling or clustering of multiple nanowires together, leading to the formation of larger structures. Finally, the agglomeration process culminated in the formation of fan-shaped NiCo2O4 powders. These powders exhibited a distinctive fan-like morphology, which was the result of the secondary bundled agglomeration of the nanowires. Overall, the TEM analysis provided a comprehensive view of the agglomeration mechanism of NiCo2O4 powders, illustrating the sequential progression from nuclei formation to primary linear agglomeration, followed by secondary bundled agglomeration, and ultimately resulting in the formation of fan-shaped powders.
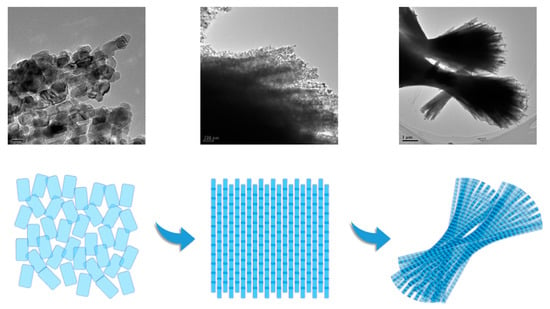
Figure 6.
TEM of NiCo2O4 and the formation mechanism of the powders.
2.5. Desulfurization Cycle Experiment of NiCo2O4 Powders
Figure 7 presents the experimental results of the desulfurization cycle using the prepared NiCo2O4 powders. The figure demonstrates that the desulfurization rate achieved with the NiCo2O4 powders is approximately 84%. Additionally, it can be observed that the activity of the powder remains consistent, even after six cycles, indicating its excellent recycling performance. Comparing the experimental desulfurization rate of 84% with the predicted value of 85.8% (red dash line), it can be deduced that the error between the predicted and actual values is only around 2.1%. This suggests that the predicted desulfurization rate obtained through the response surface method is highly accurate. The close agreement between the predicted and experimental results further validates the effectiveness and reliability of the response surface model in optimizing the preparation conditions for NiCo2O4 powders. The minimal error indicates the success of the optimization process and reinforces the suitability of the obtained conditions for achieving high desulfurization rates.
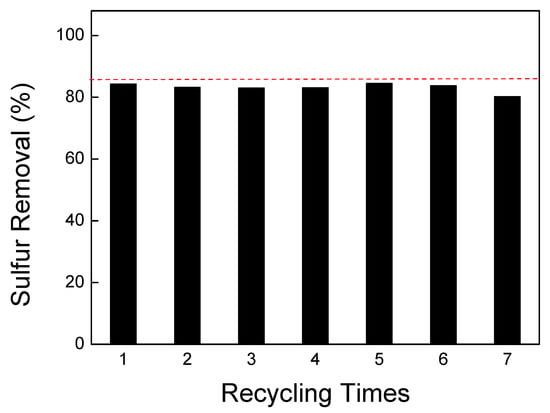
Figure 7.
Cyclic desulfurization experiment of NiCo2O4 powders.
3. Experimental
3.1. Preparation of NiCo2O4 Powders by Hydrothermal Synthesis
Dissolve 1.1897 g of CoCl2, 0.5942 g of NiCl2, 0.9109 g of cetyltrimethyl ammonium bromide, and 1.35 g of urea in 37.5 mL of deionized water. Stir the mixture magnetically for 20 min. Transfer the solution to a hydrothermal kettle with a PTFE liner. Subject the solution to different hydrothermal times and temperatures. Collect the resulting powders by magnetic separation. Purify the collected powders by washing with ethanol. Dry the purified material under vacuum at 70 °C for 4 h. Calcine the dried material at different times and temperatures to obtain NiCo2O4 powder. It is worth mentioning that the reagents used for the experiments were purchased from Shanghai Aladdin Reagent Company (Shanghai, China).
3.2. Designing Experiments by Response Surface Method
The experimental ranges for the four hydrothermal synthesis conditions were determined based on pre-experiments in the previous section. These ranges were as follows: hydrothermal temperature (A) of 120–160 °C, hydrothermal time (B) of 3–9 h, calcination temperature (C) of 300–400 °C, and calcination time (D) of 2–6 h. To design the experiments, a Box–Behnken design (BBD) was implemented using Design-Expert 8.0.6 software. The BBD design involved setting up four experimental levels and codes, which are shown in Table 3. For each factor, three levels were designed, including a cubic point with coded values of 1 and −1, and a central point with a coded value of 0. Additionally, five replicate experiments were conducted at the center point (coded 0) to test repeatability, rationality, random error, and reduce systematic errors. In total, 29 desulfurization experiments were performed using the response surface method based on the experimental levels arranged by the Box–Behnken design. This approach aimed to establish the nonlinear relationship between the experimental results (desulfurization rates) and the four experimental conditions. The accuracy of this relationship was tested using analysis of variance (ANOVA).

Table 3.
Level setting table of experimental factors for response surface method.
3.3. Characterization of NiCo2O4 Powders
The characterization of the NiCo2O4 powders was conducted using three different instruments: X-ray diffractometer (XRD): XRD is used to study the crystal structure of materials by analyzing the diffraction pattern produced when X-rays interact with the sample. This technique provides information about the crystalline phases present in the NiCo2O4 powders. When performing tests using the PANalytical X’Pert Powder X-ray diffractometer (Almelo, Netherlands), we utilized the following conditions: CuKα/Graphite monochromator as the X-ray source, with a tube voltage of 40 KV and a tube current of 40 mA. The step angle was set to 0.1°, and a scan rate of 5°/min was employed within the range of 10° to 80°. Scanning electron microscope (SEM): the SEM analysis was conducted using a Hitachi Regulus 8100 instrument from Japan (Tokyo). SEM allows for the high-resolution imaging of the surface morphology and topography of materials. It provides detailed information about the particle size, shape, and distribution of the NiCo2O4 powders. Transmission electron microscope (TEM): TEM analysis was conducted using a Thermo Fisher FEI Tecnai G2 F20 instrument from the USA (Hillsboro, OH). TEM enables the observation of materials at a very high resolution, allowing for the examination of their atomic-level structure. It provides valuable information about the internal structure, lattice defects, and individual particle characteristics of the NiCo2O4 powders. These characterization techniques were employed to gain insights into the structural and morphological properties of the synthesized NiCo2O4 powders.
3.4. Desulfurization Experiment
To perform the catalytic oxidative desulfurization, the following procedure was followed: diphenylthiophene (DBT) was dissolved in n-octane to create a simulated oil with an initial sulfur content of 600 ppm. A total of 0.5 g of NiCo2O4 powders was added to 6 mL of simulated oil. To this mixture, 2 mL of acetonitrile was added. The mixture was vigorously stirred for 30 min to allow for the equilibrium of adsorption and extraction to be reached. The catalytic oxidative desulfurization was initiated by adding 0.5 mL of PMS (20 wt. %) to the mixture. The reaction was performed at 40 °C for a duration of 60 min. After this reaction time, the desulfurization process was considered complete. The supernatants from the reaction were then measured for sulfur content in n-octane using a UV-Vis spectrophotometer (Shimadzu UV-2700, Kyoto, Japan). By measuring the sulfur content in the supernatants, the extent of desulfurization achieved by the catalytic process could be determined.
4. Conclusions
In this study, response surface methodology was utilized to optimize the hydrothermal preparation process of NiCo2O4 powders. The desulfurization activity of the synthesized powders was also investigated. Among the factors considered, hydrothermal temperature (A), calcination temperature (C), and calcination time (D) had a significant impact on the desulfurization rate. However, hydrothermal time (B) did not exhibit a substantial effect. The order of significance for the factors was determined as follows: calcination temperature (C) > hydrothermal temperature (A) > calcination time (D) > hydrothermal time (B). Based on the response surface method, the optimal preparation conditions were determined as follows: hydrothermal temperature of 143 °C, hydrothermal time of 6.1 h, calcination temperature of 330 °C, and calcination time of 3.7 h. The predicted desulfurization rate for the NiCo2O4 powders prepared under these conditions was 85.8%. In the experimental evaluation, the desulfurization rate achieved was approximately 84%, with an error of only 2.1% compared to the predicted value. The NiCo2O4 powders obtained exhibited a rectangular particle shape with an average size of 20 nm. During the agglomeration process, the particles underwent linear and bundled agglomerations, resulting in the formation of fan-shaped NiCo2O4 powders. Furthermore, the desulfurization rate of the NiCo2O4 powders remained stable throughout six cycles of catalytic oxidation and magnetic separation experiments. This demonstrated the excellent recycling performance of the prepared powders. Overall, the utilization of response surface methodology allowed for the optimization of the hydrothermal preparation process of NiCo2O4 powders, leading to high desulfurization rates. The synthesized powders exhibited desirable morphological characteristics and demonstrated an effective desulfurization performance over multiple cycles of operation.
Author Contributions
Experiment, analysis, calculation, writing, Y.Z.; calculation, writing, L.L.; picture, Z.S.; conceptualization, writing and editing, reagents, equipment and supervision, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Nature Science Foundation of Henan Province (No: 202300410155).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Hang Xu, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Qian, Y.T.; Wang, W.Z.; Xie, Y.; Qian, Y.; Wang, W.; Zhang, S.; Zhang, Y. A benzene-thermal synthetic route to nanocrystalline GaN. Science 1996, 272, 1926–1927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y. Selected-control hydrothermal synthesis of alpha- and beta MnO2 single crystal nanowires. J. Am. Chem. Soc. 2002, 124, 2880–2881. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.S.; Vedovello, P.; Paranhos, C.M. Use of Ionic Liquid as Template for Hydrothermal Synthesis of the MCM-41 Mesoporous Material. Silicon 2020, 12, 289–294. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Guang, T.; Fan, B.; Zhu, K.; Wang, X. Controlled Hydrothermal/Solvothermal Synthesis of High-Performance LiFePO4 for Li-Ion Batteries. Small Methods 2021, 5, 2100193. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Shi, B.; Huang, F.; Rong, F.; Que, R. Three-dimensional NiCo2O4@NiCo2O4 core-shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Zhang, D.; Zhang, G.; Zhang, L. Superior performance of 3D Co-Ni bimetallic oxides for catalytic degradation of organic dye: Investigation on the effect of catalyst morphology and catalytic mechanism. Appl. Catal. B-Environ. 2016, 186, 193–203. [Google Scholar] [CrossRef]
- Cheng, C.; Mao, L.; Shi, J.; Xue, F.; Zong, S.; Zheng, B.; Guo, L. NiCo2O4 nanosheets as a novel oxygen-evolution-reaction cocatalyst in situ bonded on the g-C3N4 photocatalyst for excellent overall water splitting. J. Mater. Chem. A 2021, 9, 12299–12306. [Google Scholar] [CrossRef]
- Ge, R.; Ma, M.; Ren, X.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Chen, L.; Zheng, B.; Sun, X. A NiCo2O4@Ni-Co-Ci core-shell nanowire array as an efficient electrocatalyst for water oxidation at near-neutral pH. Chem. Commun. 2017, 53, 7812–7815. [Google Scholar] [CrossRef]
- Qian, H.; Hou, Q.; Yu, G.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Enhanced removal of dye from wastewater by Fenton process activated by core-shell NiCo2O4@FePc catalyst. J. Clean. Prod. 2020, 273, 123028. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Xu, C.; Yang, L.; Yang, G.; Guo, L. A persulfate oxidation system for removing acid orange from aqueous solution: Evaluation and degradation mechanism. J. Environ. Manag. 2022, 322, 116054. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.H.; Sabzyan, H.; Bayranvand, M. Sol-gel preparation, structural, thermal and spectroscopic analyses, and opto-electronic and photocatalytic activities of normal spinel nickel cobaltite nano-powder for photo-degradation of Reactive Red RB. J. Mater. Sci.-Mater. Electron. 2017, 28, 8546–8553. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Wu, Z.; Li, N.; Xiong, Z.; Yao, G.; Lai, B. Efficient degradation of sulfamethoxazole by NiCo2O4 modified expanded graphite activated peroxymonosulfate: Characterization, mechanism and degradation intermediates. J. Hazard. Mater. 2020, 399, 123103. [Google Scholar] [CrossRef]
- Tian, X.; Tian, C.; Nie, Y.; Dai, C.; Yang, C.; Tian, N.; Zhou, Z.; Li, Y.; Wang, Y. Controlled synthesis of dandelion-like NiCo2O4 microspheres and their catalytic performance for peroxymonosulfate activation in humic acid degradation. Chem. Eng. J. 2018, 331, 144–151. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, F.; Xu, H.; Wang, X.; Luo, J.; Song, Y.; Guo, Y.; Wei, X. Preparation of magnetic urchin-like NiCo2O4 powders by hydrothermal synthesis for catalytic oxidative desulfurization. RSC Adv. 2022, 12, 32659–32666. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.W.; Wang, H.W.; Lv, M.G. A Novel Preparation of Mn/NiCo2O4 Catalyst with High Catalytic Activity on Methane. J. Nanoelectron. Optoelectron. 2021, 16, 926–932. [Google Scholar] [CrossRef]
- Wang, T.; Qiu, L.; Li, H.; Zhang, C.; Sun, Y.; Xi, S.; Ge, J.; Xu, Z.J.; Wang, C. Facile synthesis of palladium incorporated NiCo2O4 spinel for low temperature methane combustion: Activate lattice oxygen to promote activity. J. Catal. 2021, 404, 400–410. [Google Scholar] [CrossRef]
- Zhang, M.; Sui, X.; Zhang, X.; Niu, M.; Li, C.; Wan, H.; Qiao, Z.-A.; Xie, H.; Li, X. Multi-defects engineering of NiCo2O4 for catalytic propane oxidation. Appl. Surf. Sci. 2022, 600, 154040. [Google Scholar] [CrossRef]
- Zhu, Y.; Zuo, Y.; Ye, F.; Zhou, J.; Tang, Y.; Chen, Y. Dual-regulation strategy to enhance electrochemical catalysis ability of NiCo2O4-x for polysulfides conversion in Li-S batteries. Chem. Eng. J. 2022, 428, 131109. [Google Scholar] [CrossRef]
- Xing, Y.; Tian, F.; Wu, D.; Yong, X.; Jin, X.; Ni, G. Facile synthesis of Z-scheme ZnMoO4/Bi2O4 heterojunction photocatalyst for effective removal of levofloxacin. Inorg. Chem. Commun. 2022, 143, 109763. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, J.; Wu, X.; Feng, L. Microwave-assisted hydrothermal synthesis of NiMoO4 nanorods for high-performance urea electrooxidation. Chin. Chem. Lett. 2022, 33, 1105–1109. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Das, H.T.; Kumar, Y.A.; Naushad, M.; Sambasivam, S.; Jung, J.H.; Joo, S.W. Marigold flower-like Sn3O4 nanostructures as efficient battery-type electrode material for high-performing asymmetric supercapacitors. J. Electroanal. Chem. 2022, 920, 116641. [Google Scholar] [CrossRef]
- Simancas, R.; Takemura, M.; Yonezawa, Y.; Sukenaga, S.; Ando, M.; Shibata, H.; Chokkalingam, A.; Iyoki, K.; Okubo, T.; Wakihara, T. Exploring Hydrothermal Synthesis of SAPO-18 under High Hydrostatic Pressure. Nanomaterials 2022, 12, 396. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Vo, D.V.N.; Nguyen, T.T.; Nguyen, T.T.T.; Nguyen, L.T.; Van Tran, T. Optimization of tetracycline adsorption onto zeolitic-imidazolate framework-based carbon using response surface methodology. Surf. Interfaces 2022, 28, 101549. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Chen, S.-Y.; Zhang, H.-Q.; Feng, J.-H.; Sun, J.-B.; Shi, W.-D. Optimization of magnetization and mechanical properties of Fe-Ni-based alloys by Response Surface Methodology. Mater. Today Commun. 2022, 30, 103203. [Google Scholar] [CrossRef]
- Boublia, A.; Lebouachera, S.E.; Haddaoui, N.; Guezzout, Z.; Ghriga, M.A.; Hasanzadeh, M.; Drouiche, Y.B.N. State-of-the-art review on recent advances in polymer engineering: Modeling and optimization through response surface methodology approach. Polym. Bull. 2022, 80, 5999–6031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).