Nickel Foam-Supported Hierarchical NiCo2S4 Nanostructures as Efficient Electrocatalysts for the Methanol Oxidation Reaction
Abstract
1. Introduction
2. Results and Discussion
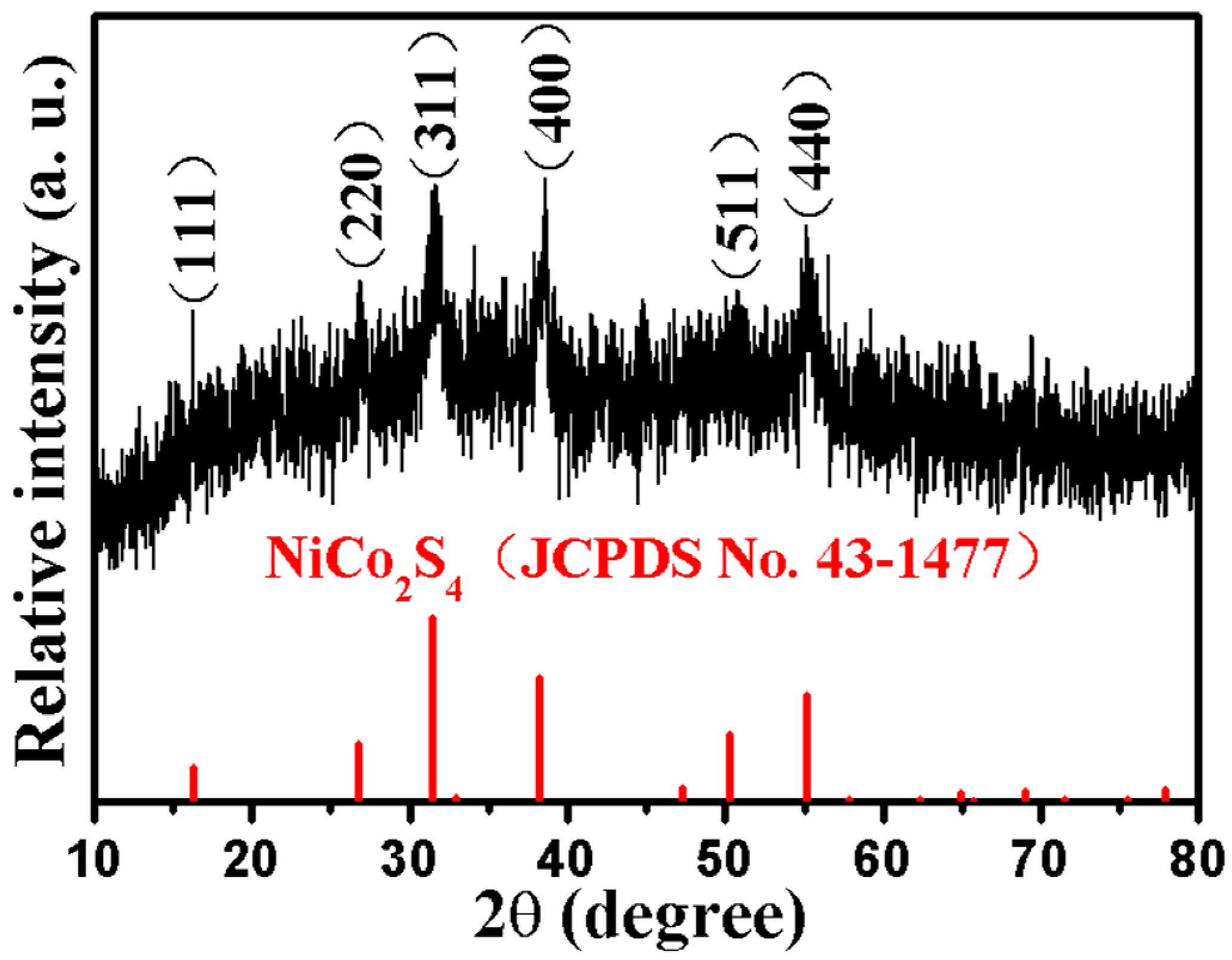
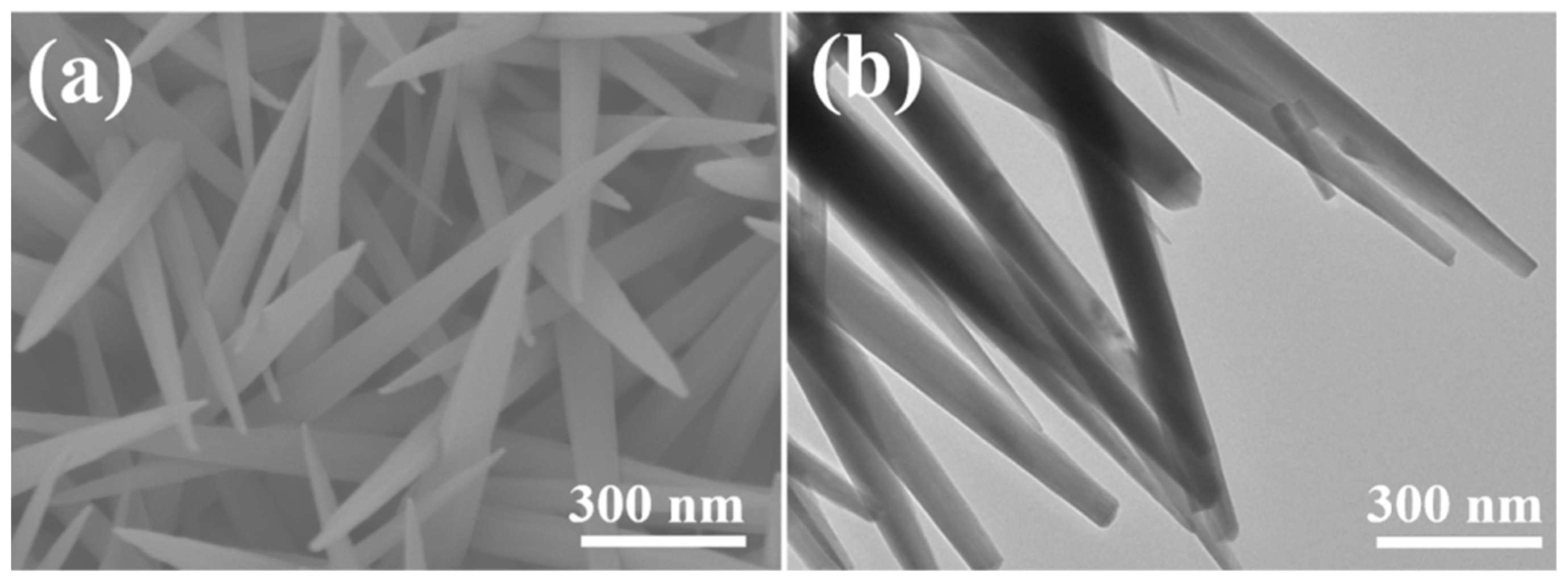
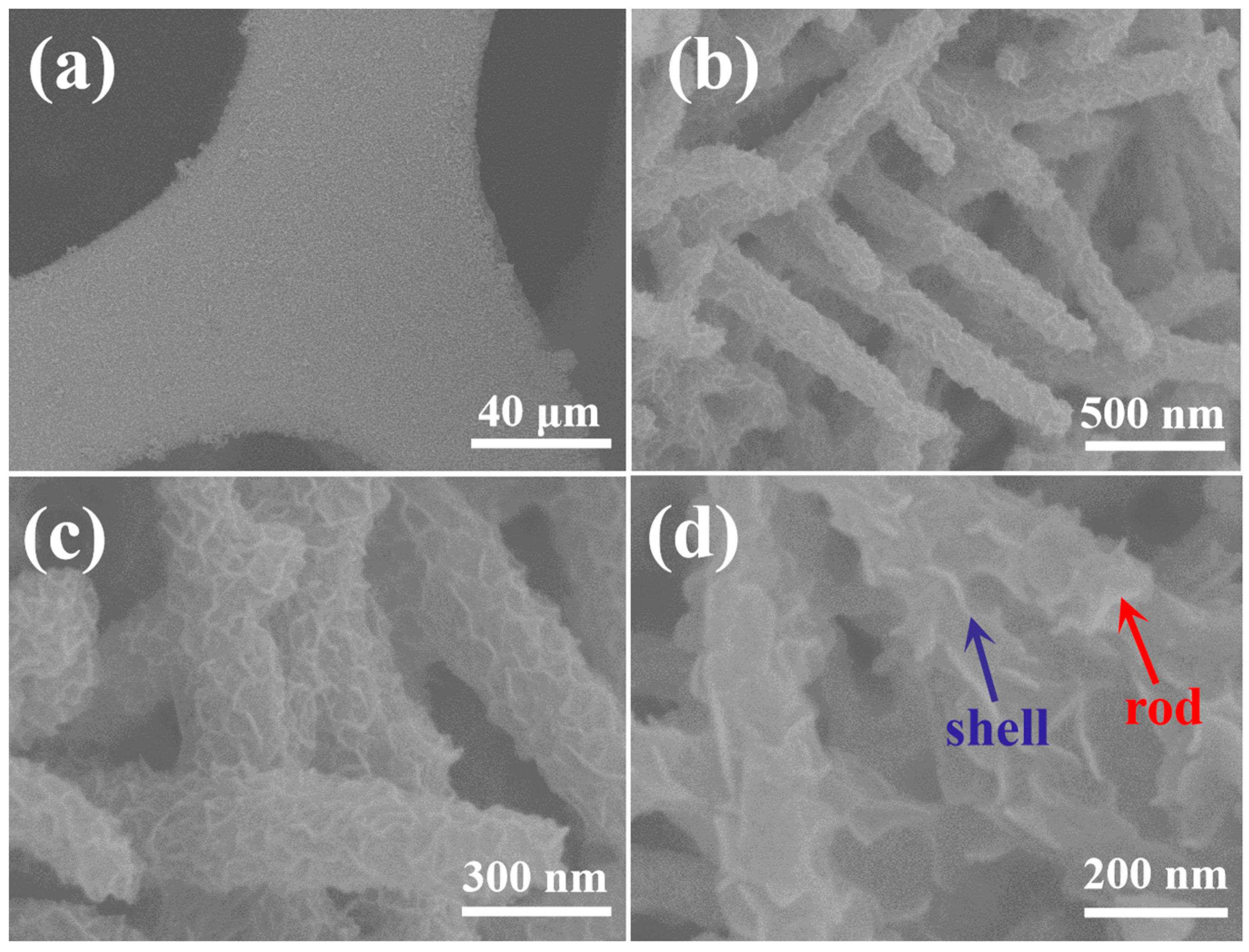
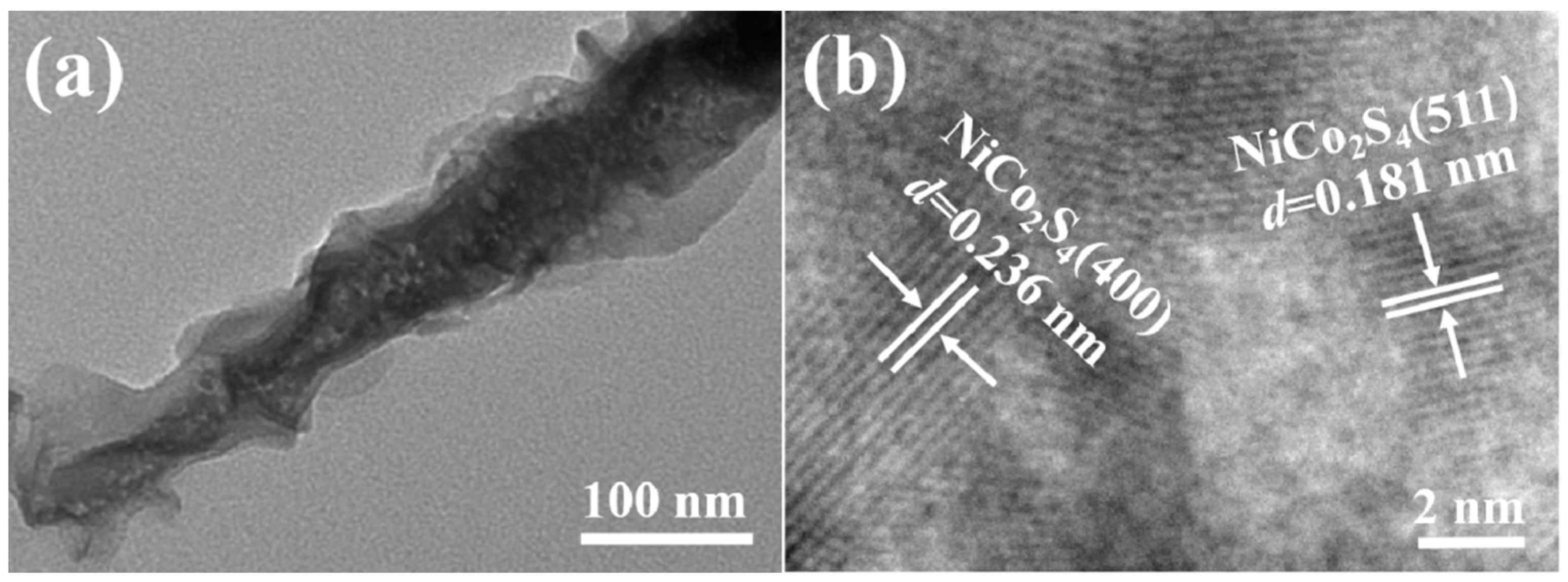
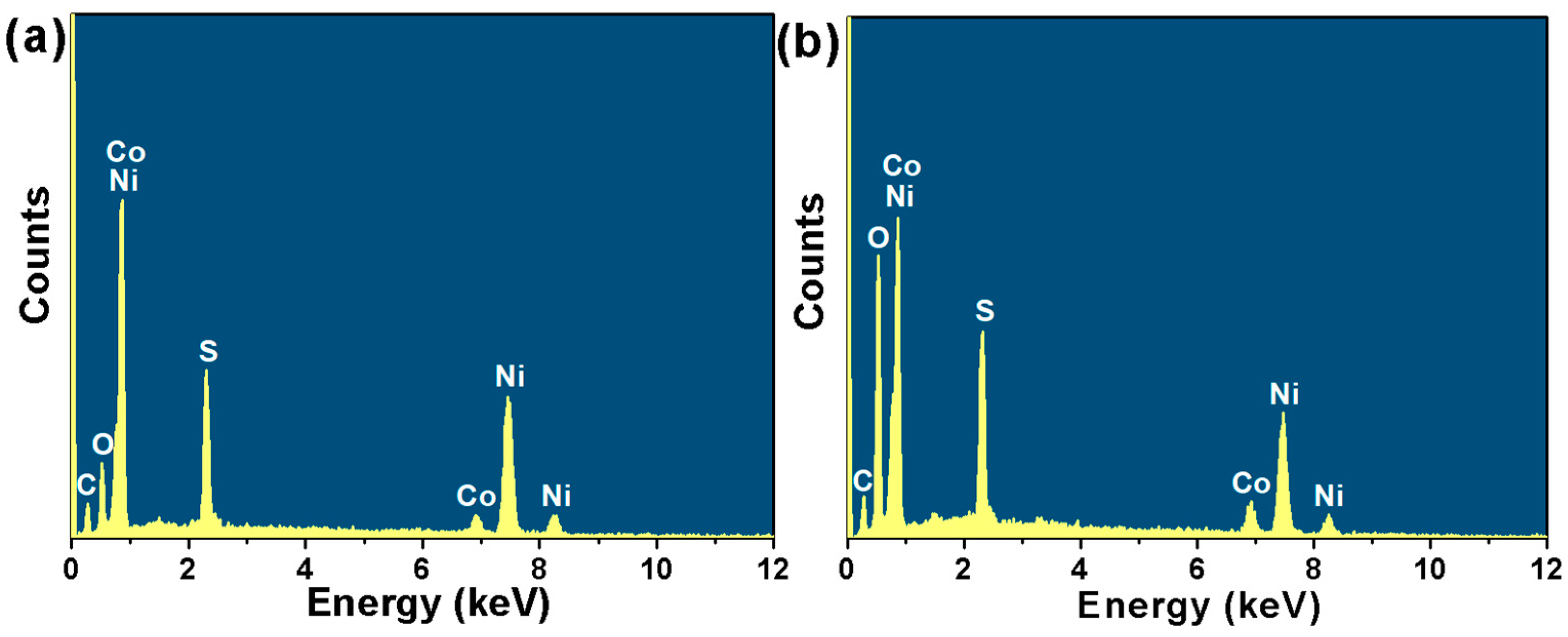
2.1. Phase and Microscopic Morphology Analysis
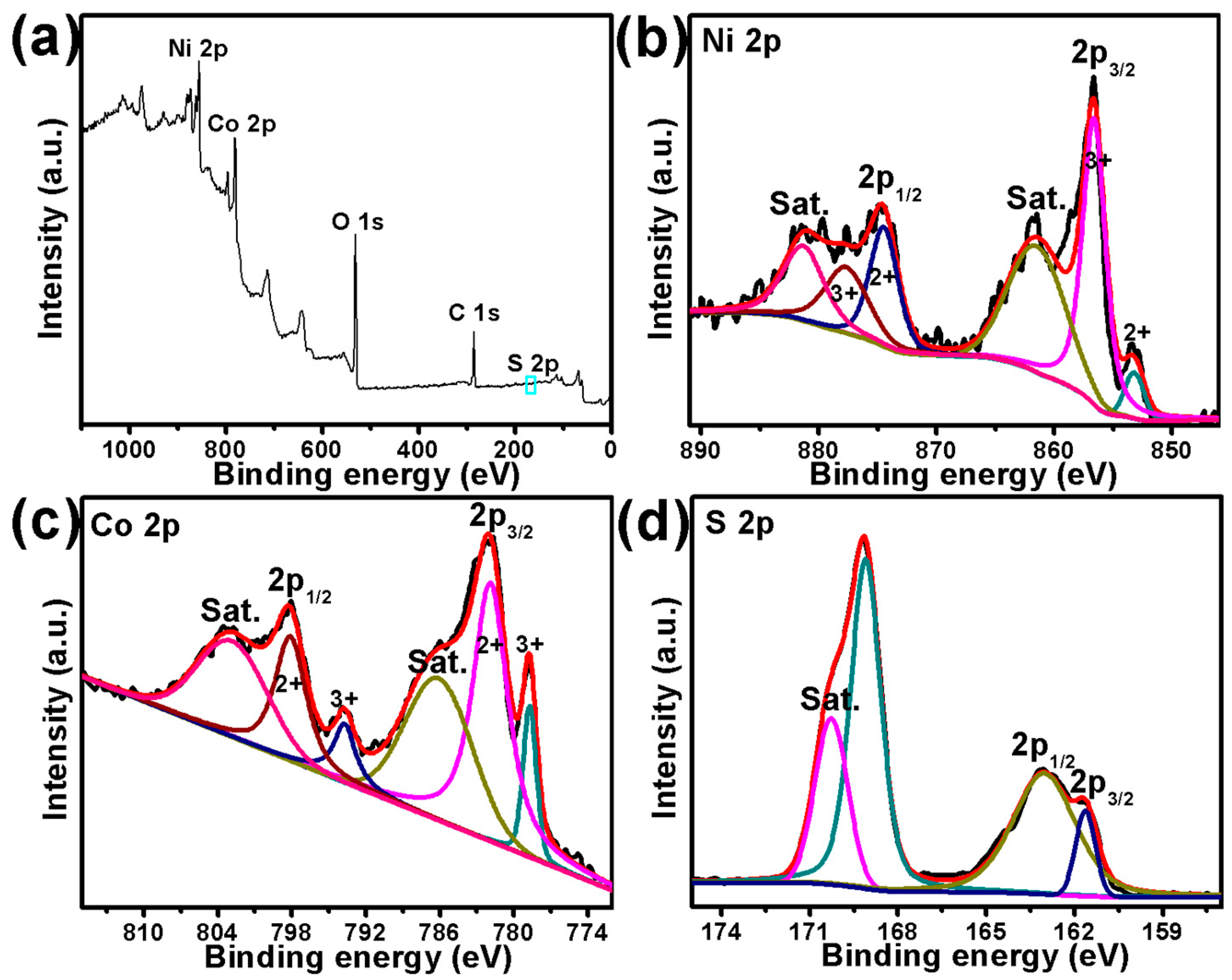
2.2. XPS Analysis
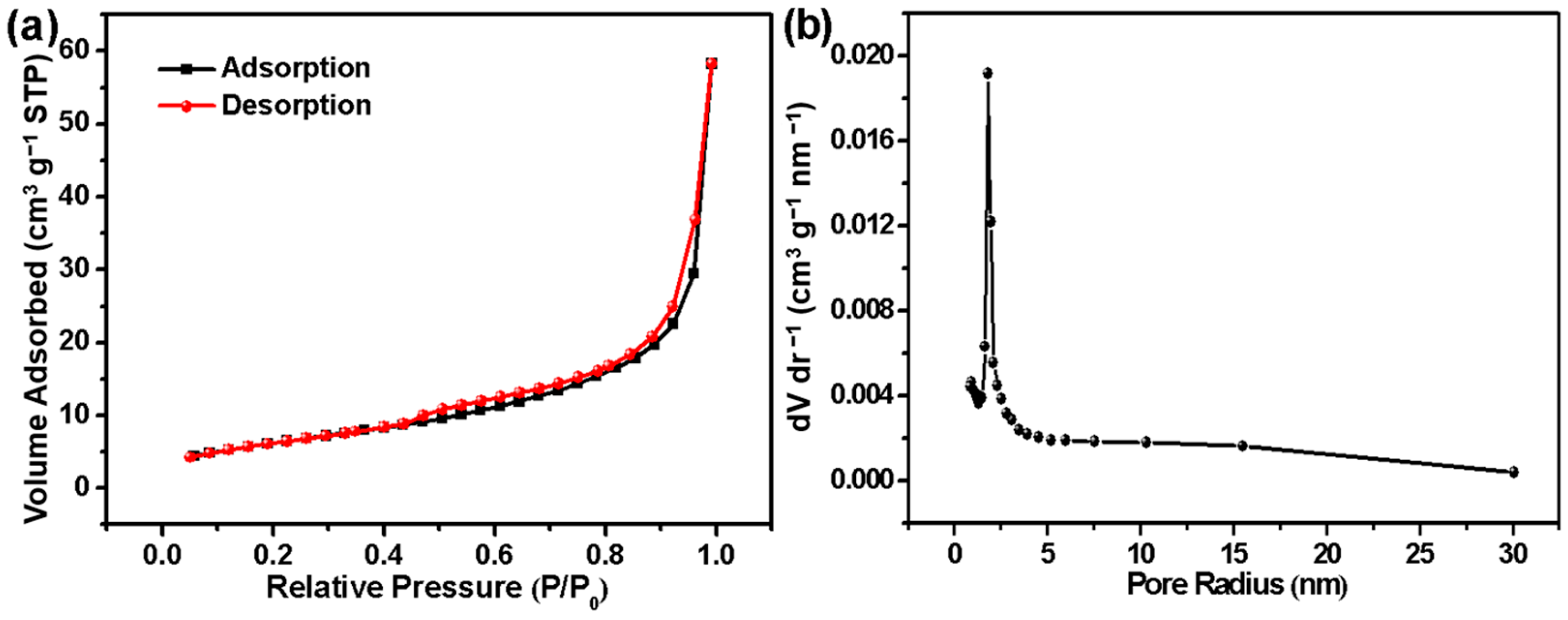
2.3. Specific Surface Area
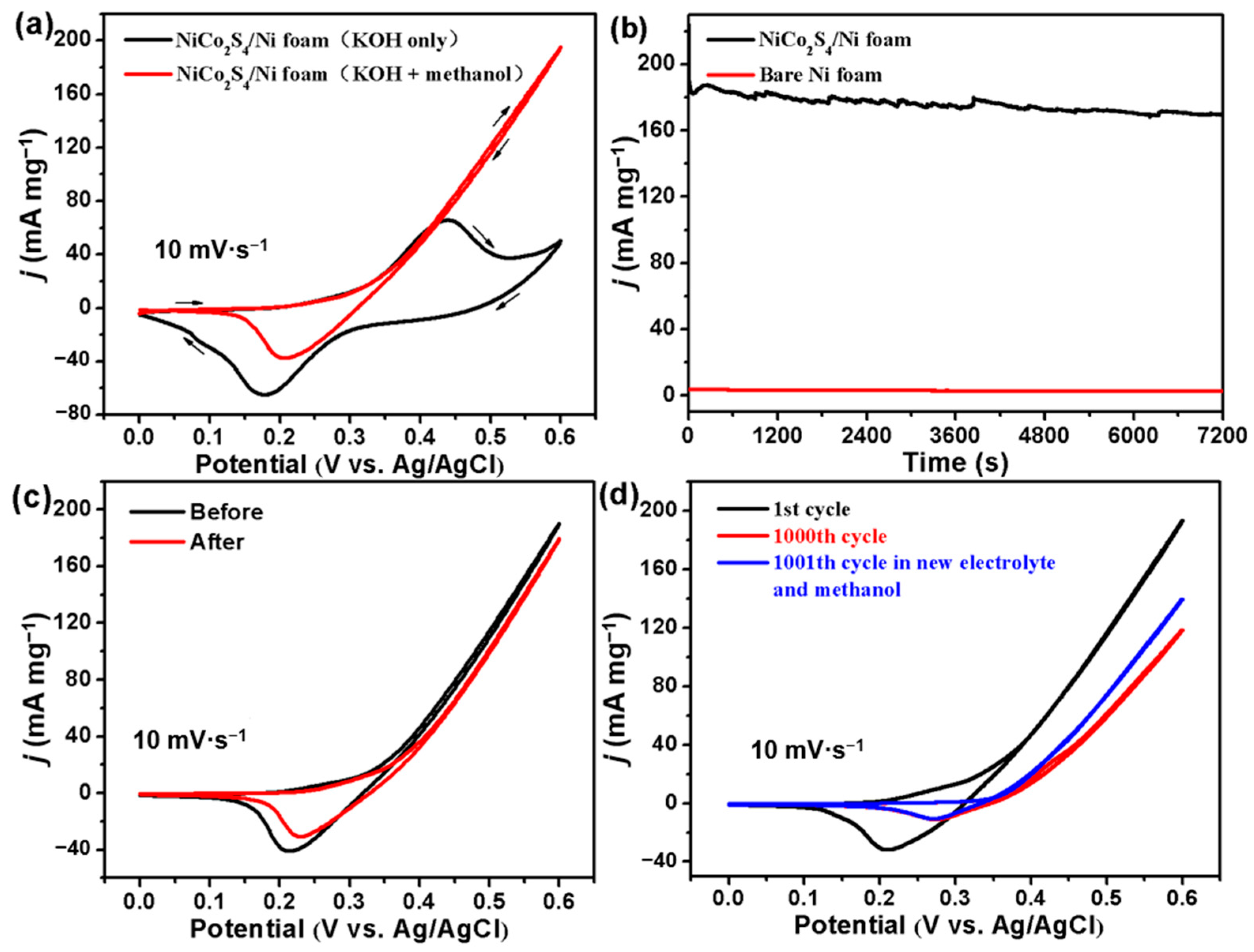
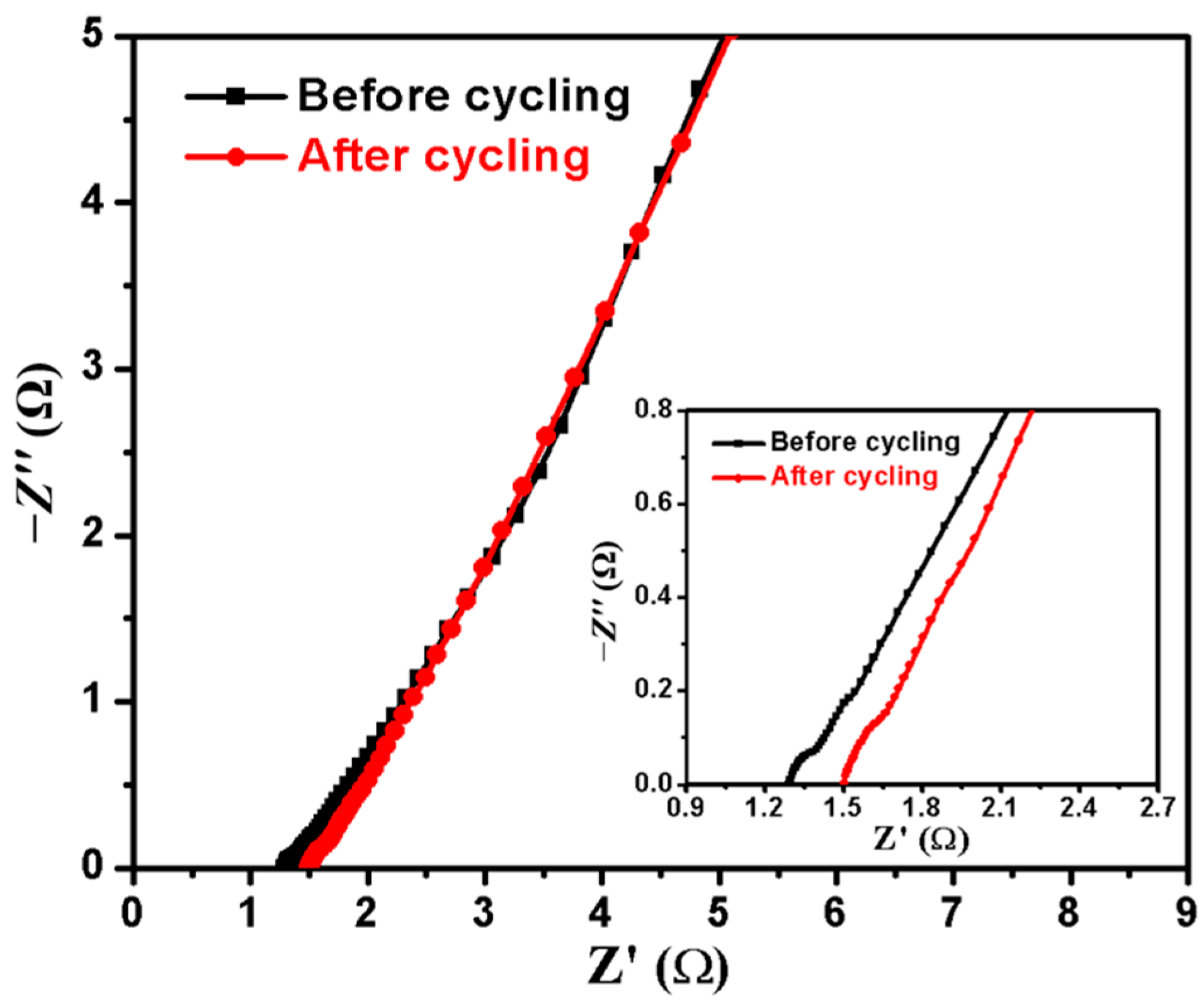
2.4. Electrocatalytic Performances for MOR
3. Experimental
3.1. Materials
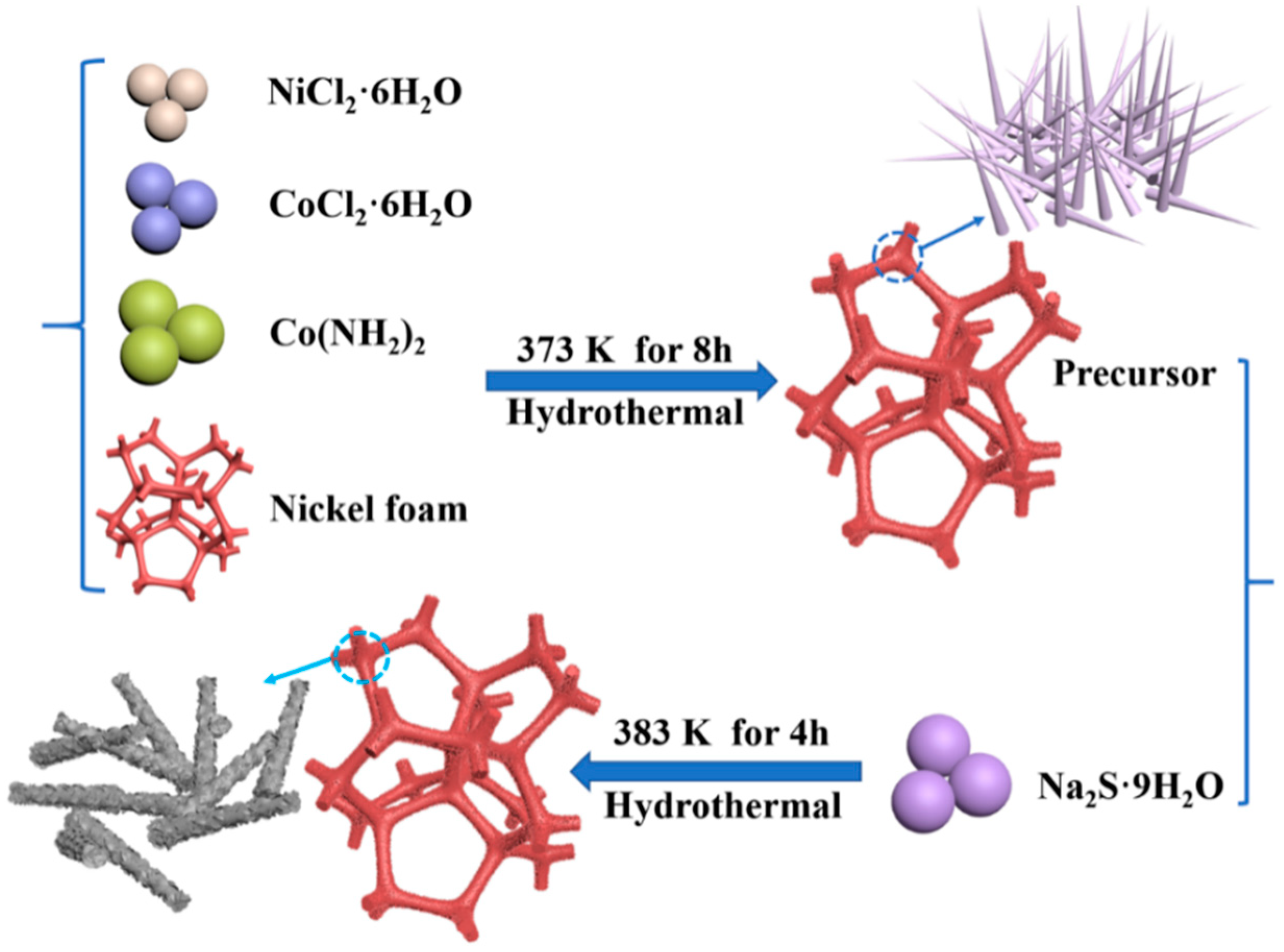
3.2. Synthesis Process of the NiCo2S4, NiS, and Co9S8 Samples
3.3. Characterizations
3.4. Electrochemical Measures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.B.; Yin, G.P.; Shi, P.F. Effects of ozone treatment of carbon support on Pt-Ru/C catalysts performance for direct methanol fuel cell. Carbon 2006, 44, 133–140. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Gao, M.R.; Gao, Q.; Jiang, J.; Cui, C.H.; Yao, W.T.; Yu, S.H. A methanol-tolerant Pt/CoSe2 nanobelt cathode catalyst for direct methanol fuel cells. Angew. Chem. Int. Ed. 2011, 50, 4905–4908. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [PubMed]
- Xia, Z.; Xu, X.; Zhang, X.; Li, H.; Wang, S.; Sun, G. Anodic engineering towards high performance direct methanol fuel cells with non-precious-metal cathode catalyst. J. Mater. Chem. A 2020, 8, 1113–1119. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Deng, X.; Fu, X.Z.; Luo, J.L. Decoration of NiO hollow spheres composed of stacked nanosheets with CeO2 nanoparticles: Enhancement effect of CeO2 for electrocatalytic methanol oxidation. Electrochim. Acta 2020, 337, 135684. [Google Scholar] [CrossRef]
- Xu, X.; Xia, Z.; Zhang, X.; Sun, R.; Sun, X.; Li, H.; Wu, C.; Wang, J.; Wang, S.; Sun, G. Atomically dispersed Fe-N-C derived from dual metal-organic frameworks as efficient oxygen reduction electrocatalysts in direct methanol fuel cells. Appl. Catal. B Environ. 2019, 259, 118042. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Gong, X.; Geng, P.; Gao, Z.; Wang, Y. Preparation of sulfonated polysulfone/sulfonated titanium dioxide hybrid membranes for DMFC applications. J. Appl. Polym. Sci. 2020, 137, 48938. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Li, D.; Shen, C.; Li, M.; Zhang, X.; Jiang, Q.; Zhang, J.; Wu, Y. Pt nanoparticles grown on 3D RuO2-modified graphene architectures for highly efficient methanol oxidation. J. Mater. Chem. A 2017, 5, 4560–4567. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Jin, L.; Chen, C.; Du, Y. Nanoscale engineering of porous Fe-doped Pd nanosheet assemblies for efficient methanol and ethanol electrocatalyses. Nanoscale 2020, 12, 2126–2132. [Google Scholar] [CrossRef]
- Lou, W.; Ali, A.; Shen, P.K. Recent development of Au arched Pt nanomaterials as promising electrocatalysts for methanol oxidation reaction. Nano Res. 2022, 15, 18–37. [Google Scholar]
- Li, S.; Tian, Z.Q.; Liu, Y.; Jang, Z.; Hasan, S.W.; Chen, X.; Tsiakaras, P.; Shen, P.K. Hierarchically skeletal multi-layered Pt-Ni nanocrystals for highly efficient oxygen reduction and methanol oxidation reactions. Chin. J. Catal. 2021, 42, 648–657. [Google Scholar] [CrossRef]
- Zhang, P.; Bin, D.; Wei, J.S.; Niu, X.Q.; Chen, X.B.; Xia, Y.Y.; Xiong, H.M. Efficient oxygen electrocatalyst for Zn-air batteries: Carbon dots and Co9S8 nanoparticles in a N, S-codoped carbon matrix. ACS Appl. Mater. Interfaces 2019, 11, 14085–14094. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a highly efficient electrocatalyst for overall water splitting in a wide pH range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Zhao, Y.; Xu, C. 3D Ni3S2 nanosheet arrays supported on Ni foam for high-performance supercapacitor and non-enzymatic glucose detection. J. Mater. Chem. A 2014, 2, 15111–15117. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, S.; Wang, X.; Han, H.; Bai, Z.; Yang, L. Hierarchical Co9S8 hollow microspheres as multifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2017, 246, 380–390. [Google Scholar] [CrossRef]
- Qian, L.; Chen, W.; Huang, R.; Xiao, D. Direct growth of NiCo2Sx nanostructures on stainless steel with enhanced electrocatalytic activity for methanol oxidation. RSC Adv. 2015, 5, 4092–4098. [Google Scholar] [CrossRef]
- Jin, D.; Li, Z.; Wang, Z.H. Hierarchical NiCo2O4 and NiCo2S4 nanomaterials as electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 32069–32080. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Yang, X.; Song, W.; Ji, X. Mesoporous NiCo2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 2015, 273, 584–590. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Cui, H.; Yin, M.; Li, Q.; Zhao, Y.; Li, H.; Zhou, W.; Feng, C. One-pot synthesis of NiCo2S4 hollow spheres via sequential ion exchange as an enhanced oxygen bifunctional electrocatalyst in alkaline solution. ACS Appl. Mater. Interfaces 2018, 10, 29521–29531. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.X.; Gong, Q.J.; Sun, X.L.; Xu, N.N.; Gong, P.N.; Qiao, J.L. Rational fabrication of thin-layered NiCo2S4 loaded graphene as bifunctional non-oxide catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2020, 342, 136108. [Google Scholar] [CrossRef]
- Dong, M.X.; Wang, Z.X.; Yan, G.C.; Wang, J.X.; Guo, H.J.; Li, X.H. Confine growth of NiCo2S4 nanoneedles in graphene framework toward high-performance asymmetric capacitor. J. Alloys Compd. 2020, 822, 153645. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, B.; Hei, J.; Gao, D.; Xu, X.; Wei, Z.; Wu, H. Self-assembled synthesis of waxberry-like open hollow NiCo2S4 with enhanced capacitance for high-performance hybrid asymmetric supercapacitors. Electrochim. Acta 2020, 347, 136314. [Google Scholar] [CrossRef]
- Jin, D.; Li, Z.; Ma, T.T.; Wang, Z.H. Three-dimensional flower-like Mn-Ni-Co-O microstructure as a high-performance electrocatalyst for methanol oxidation reaction. New J. Chem. 2022, 46, 7657–7662. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhang, J.; Wu, S.; Zhang, D.; Shui, J. Interface engineering of NiCo2O4/BCN nanotube for performance enhancement of lithium-oxygen battery. Chem. Eng. J. 2021, 411, 128403. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Singh, B.S.; Ibomcha, I.T.; Dai, J.Y.; Kshetri, T.; Kim, N.H.; Lee, J.H. Flexible transparent supercapacitor with core-shell Cu@Ni@NiCoS nanofibers network electrode. Chem. Eng. J. 2020, 395, 125019. [Google Scholar]
- Ao, D.; Shi, Y.; Li, S.; Chang, Y.; Xu, A.; Jia, J.; Jia, M. 3D Co-Ni-C network from milk as competitive bifunctional catalysts for methanol and urea electrochemical oxidation. Catalysts 2021, 11, 844. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, J.; Qin, G.; Ni, Y. Carbon cloth supported hierarchical core-shell NiCo2S4@CoNi-LDH nanoarrays as catalysts for efficient oxygen evolution reaction in alkaline solution. J. Alloys Compd. 2020, 830, 154658. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Qi, T.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.F.; Li, Y.M.; Wu, J.Z.; Xie, Y.; Luo, S. Hierarchical mesoporous flower-like ZnCo2O4@NiO nanoflakes grown on nickel foam as high-performance electrodes for supercapacitors. Electrochim. Acta 2018, 284, 128–141. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, T.; Jiang, S.; Cheng, S.; Tao, X.; Zhong, Y.; Liao, G.; Tang, Z. Enhanced cycling stability of NiCo2S4@NiO core-shell nanowire arrays for all-solid-state asymmetric supercapacitors. Sci. Rep. 2016, 6, 38620. [Google Scholar] [CrossRef]
- He, G.; Qiao, M.; Li, W.; Lu, Y.; Zhao, T.; Zou, R.; Li, B.; Darr, J.A.; Hu, J.; Titirici, M.M.; et al. S, N-Co-doped graphene-nickel cobalt sulfide aerogel: Improved energy storage and electrocatalytic performance. Adv. Sci. 2017, 4, 1600214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.; Cheng, W.; Wu, S.; Li, Z.; Wang, Z. Nickel Foam-Supported Hierarchical NiCo2S4 Nanostructures as Efficient Electrocatalysts for the Methanol Oxidation Reaction. Catalysts 2023, 13, 1099. https://doi.org/10.3390/catal13071099
Jin D, Cheng W, Wu S, Li Z, Wang Z. Nickel Foam-Supported Hierarchical NiCo2S4 Nanostructures as Efficient Electrocatalysts for the Methanol Oxidation Reaction. Catalysts. 2023; 13(7):1099. https://doi.org/10.3390/catal13071099
Chicago/Turabian StyleJin, Dan, Wenting Cheng, Shaoyun Wu, Zhen Li, and Zhenghua Wang. 2023. "Nickel Foam-Supported Hierarchical NiCo2S4 Nanostructures as Efficient Electrocatalysts for the Methanol Oxidation Reaction" Catalysts 13, no. 7: 1099. https://doi.org/10.3390/catal13071099
APA StyleJin, D., Cheng, W., Wu, S., Li, Z., & Wang, Z. (2023). Nickel Foam-Supported Hierarchical NiCo2S4 Nanostructures as Efficient Electrocatalysts for the Methanol Oxidation Reaction. Catalysts, 13(7), 1099. https://doi.org/10.3390/catal13071099




