Abstract
To reduce vehicle exhaust gas pollution in transport-intensive roadways and tunnels, six types of photocatalytic asphalt binders were designed using graphite-phase carbon nitride (g-C3N4) and nanoscale titanium dioxide (nano-TiO2) particles. In this paper, the rheological behaviors and fatigue life of the nano-TiO2-modified asphalt binder (TiO2-MA) and g-C3N4-modified asphalt binder (C3N4-MA) were investigated. NO degradation capacity of six types of photocatalytic asphalt binders was characterized under visible light conditions. The results showed that TiO2-MA had more excellent rheological behaviors and rutting resistance than C3N4-MA. In addition, 4 wt% nano-TiO2 markedly improved the rheological behaviors and rutting resistance of MA compared to other dosages. TiO2-MA exhibited higher fatigue resistance. The fatigue life of TiO2-MA with 4 wt% nano-TiO2 was increased to 234.1% at 2.5% strain and 242.5% at 5% strain, respectively, compared to base asphalt binder (BA). C3N4-MA had better NO degradation capacity than TiO2-MA. Meanwhile, the NO degradation efficiency of C3N4-MA reached 17.8% with 5 wt% g-C3N4.
1. Introduction
In recent years, the Chinese economy has experienced tremendous development, leading to accelerated urbanization and a steadily increasing level of transport construction. As major highways are completed, the number of motor vehicles in China continues to rise, and the accompanying environmental pollution is becoming increasingly prominent. As of 2022, the number of motor vehicles in China had reached 408 million, with 312 million automobiles powered mainly by fossil fuels. The exhaust gas generated by the combustion of fossil fuels inevitably produces significant environmental problems, including acid rain, haze, photochemical smog, and the greenhouse effect [1,2,3]. These environmental problems pose a significant threat to human health. In particular, car exhaust contains a toxic and hazardous gas, NO, which can cause respiratory problems, such as asthma, lung cancer, and emphysema. Those can even result in instant death in humans [4,5]. Therefore, there is an urgent need for effective means to reduce the level of vehicle exhaust in the air.
Currently, scholars have proposed various strategies to solve vehicle exhaust issues, such as reforestation, improving energy efficiency, exploring green energy sources, conserving energy and reducing emissions, and improving motor vehicle power units. However, the growth in the number of vehicles continues to cause a large volume of exhaust to remain in the air [6,7,8,9,10,11]. With the advancement of technology, nano photocatalysis has emerged as an environmental technology with several advantages, such as high efficiency, mild reaction conditions, low energy consumption, and low secondary pollution [12,13,14]. It can convert sunlight into chemical energy and initiate or accelerate specific redox reactions, effectively decomposing car exhausts and purifying air and water through the excitation of light. At present, nano-TiO2 particles and g-C3N4 are primarily used as photocatalytic materials in air purification and sewage treatment due to their strong photocatalytic degradation, non-toxicity, and chemical and thermal stability [15,16,17]. Nano-TiO2 and g-C3N4 are semiconductor materials with a discontinuous energy band structure, where the valence band is filled with electrons and the conduction band is empty [18,19]. The band gap, also known as the forbidden bandwidth, refers to the energy difference between the bottom of the conduction band and the top of the valence band.
Based on the energy difference of the different band gap structures, nano photocatalysis is a complex chemical process. When the energy of the incident light is greater than or equal to the band gap energy of the semiconductor photocatalytic material, the electrons on the valence band of the semiconductor photocatalytic material will be excited and transit to the conduction band, forming photogenerated electrons, while at the same time, photogenerated holes will appear on the valence band. The photogenerated electrons and photogenerated holes are often referred to collectively as photogenerated electron–hole pairs. Photogenerated electron–hole pairs are generated and then migrate to the surface of the semiconductor photocatalytic material to interact with the substances in contact [20,21]. It can be seen that the utilization of nano-TiO2 and g-C3N4 for degrading pollutants has a favorable principle basis and feasibility.
Therefore, some scholars had tried to apply nano-TiO2 and g-C3N4 to the road sector to reduce the problem of vehicle exhaust gas degradation. For example, Zhang et al. [22] designed an innovative type of photocatalytic micro-surfacing mixture enhanced by nano-TiO2, confirming that vehicle exhaust gas degradation capacity was significantly strengthened with the increase of nano-TiO2 amount. Fan et al. [23,24] fabricated visible-light-activated photocatalytic asphalt by embedding carbon-modified TiO2 onto the asphalt surface under proper heat treatment, demonstrating the optimal sample can degrade 400 ppb of NOx within 1.5 h. Yu et al. [25] evaluated catalytic degradation using nano-TiO2 as asphalt pavement coating material for road environments and found it could efficiently degrade the automobile exhaust gas concentration in the air. Liu et al. [26] designed a degradation micro-unit by crosslinking nano-TiO2 to the surface of fine crumb rubber applied in asphalt binder, finding that the NO2 concentration in the asphalt pavement test section was 0.1–0.3 mg/m3 lower than the contrast section. Qian et al. [27] coated Fe2O3-TiO2 composite nanomaterial on the surface of the asphalt rutting plate specimens to simulate the field environment. The result showed that the photocatalytic asphalt specimens exhibited the best degradation efficiency under approximately 26.5 g/m2. Yang et al. [28] developed g-C3N4 nanosheets—recycled asphalt pavement aggregate composites, which displayed effective NOx removal under visible light irradiation. Huang et al. [29] explored the g-C3N4/TiO2 composite hydrosol to fabricate photocatalytic air-purifying pavement, and field studies showed that the NO removal rate of the hydrosol-coated pavement reached 70.7%. Hassan et al. [30] incorporated TiO2 into asphalt pavements, achieving an efficiency ranging from 31–55% for NOx pollutants. Obviously, existing studies have mainly used g-C3N4 and nano-TiO2 as coating materials. They both have promising photocatalytic properties to solve certain vehicle exhaust gas pollution problems.
However, when the coating materials with g-C3N4 and nano-TiO2 are applied to the road surface, they gradually abrade under the impact of traffic loads. Abrasion then lead to a further reduction or even loss of photocatalytic capacity. Thus, a completely homogeneous blend of g-C3N4 and nano-TiO2 into the asphalt binders will be an approach to reduce the degree of abrasion. This is because the asphalt binders are applied to the asphalt mixture to form a stable system with a certain thickness, which will provide a substantial resistance to the abrasion under traffic loads.
In this work, g-C3N4 and nano-TiO2 were applied as modifiers to design the photocatalytic asphalt binders. TiO2-MA and C3N4-MA underwent aging processing, then Fourier infrared was carried out to investigate the microscopic modification mechanism. The rheological behaviors and fatigue behaviors were tested by DSR. The NO degradation performance was investigated, and the variation in NO degradation efficiency of TiO2-MA and C3N4-MA were compared. The results of the study are expected to provide a reference for the application of photocatalysts to asphalt pavement materials.
2. Results and Discussion
2.1. FTIR Analysis
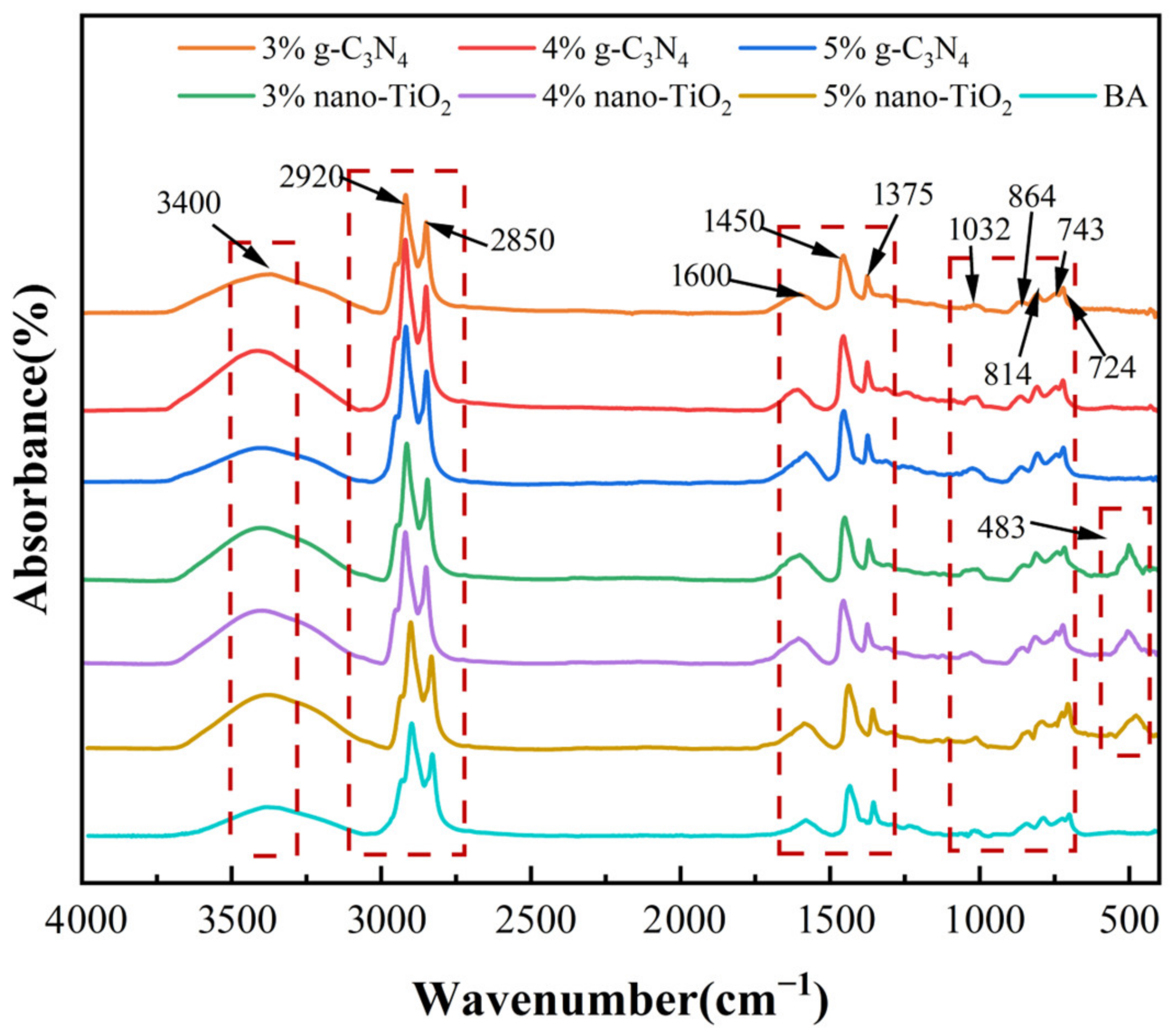
The Fourier transform infrared (FTIR) spectra results and characteristic absorption peaks of unaged photocatalytic asphalt binders are shown in Figure 1. It can be seen that the characteristic FTIR absorption peaks of the unaged asphalt binders were mainly distributed at wave numbers 3400 cm−1, 2920 cm−1, 2850 cm−1, 1600 cm−1, 1455 cm−1, 1375 cm−1, 1032 cm−1, etc. Based on the corresponding functional group information, most of these strong absorption peaks were the result of the C-H vibrations of cycloalkanes and alkanes in the asphalt molecules. The C-H vibrations of alkanes indicated the presence of hydrocarbons in the molecular composition of the BA. The conjugated double bond absorption peaks at 1600 cm−1 indicated the presence of aromatic compounds in the asphalt binders. In particular, for TiO2-MA, a peak at 483 cm−1 can be observed, which indicates the presence of a Ti-O bond [31]. In addition, the FITR images of the photocatalytic asphalt binders were in high agreement with BA, indicating that no new characteristic absorption peaks were generated. This proved that adding nano-TiO2 and g-C3N4 into BA was only a physical modification process [32].
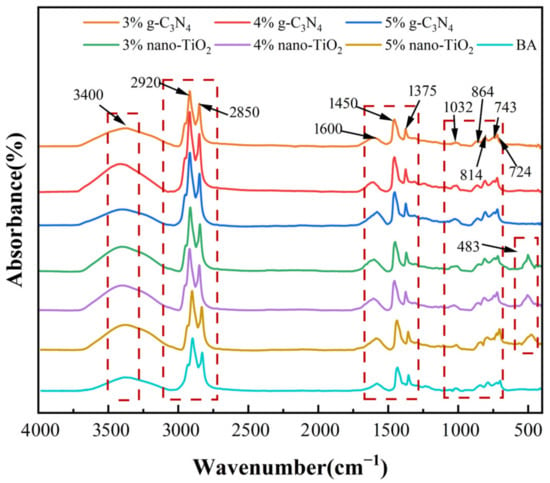
Figure 1.
FTIR spectra for all unaged asphalt binders.
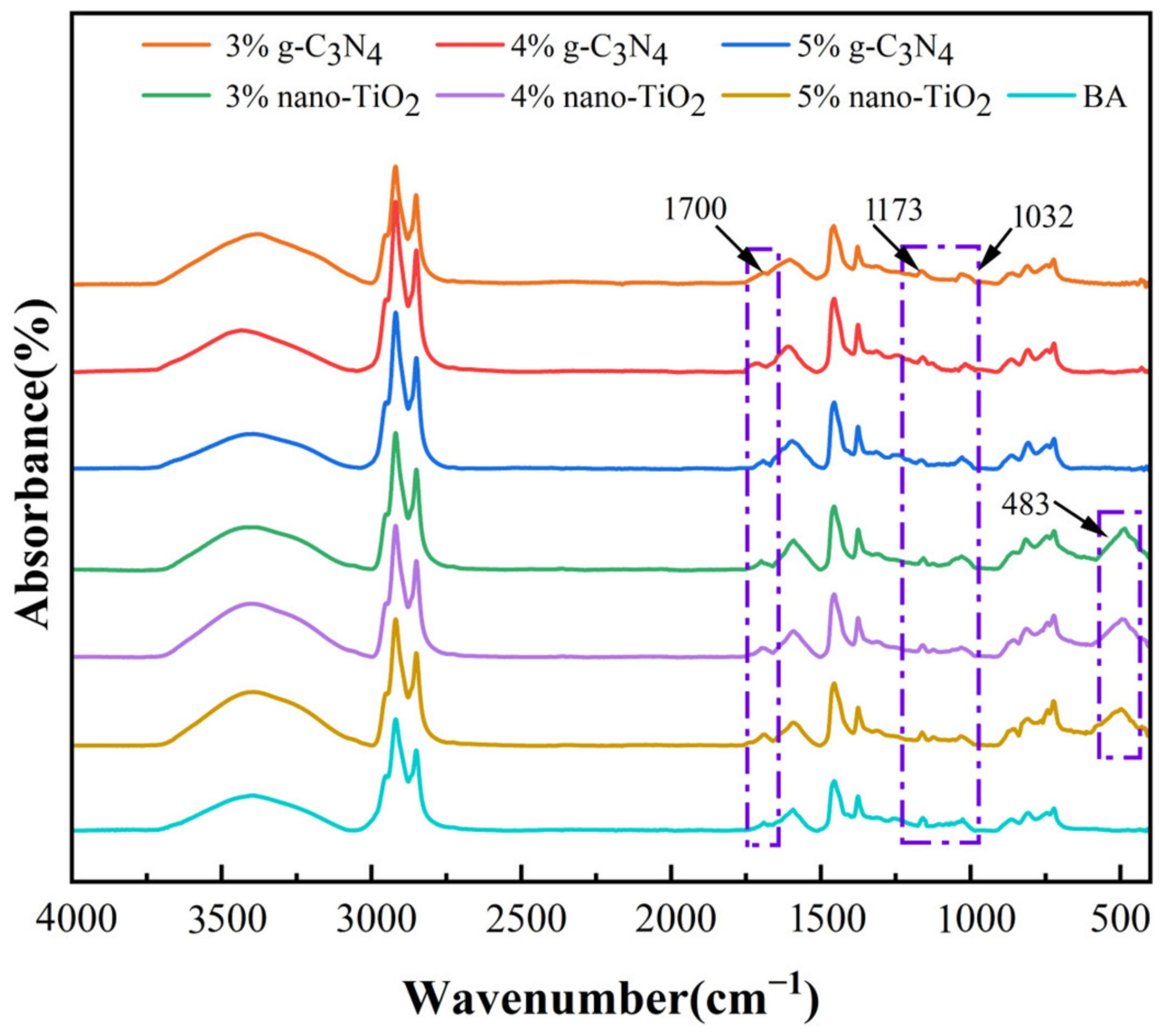
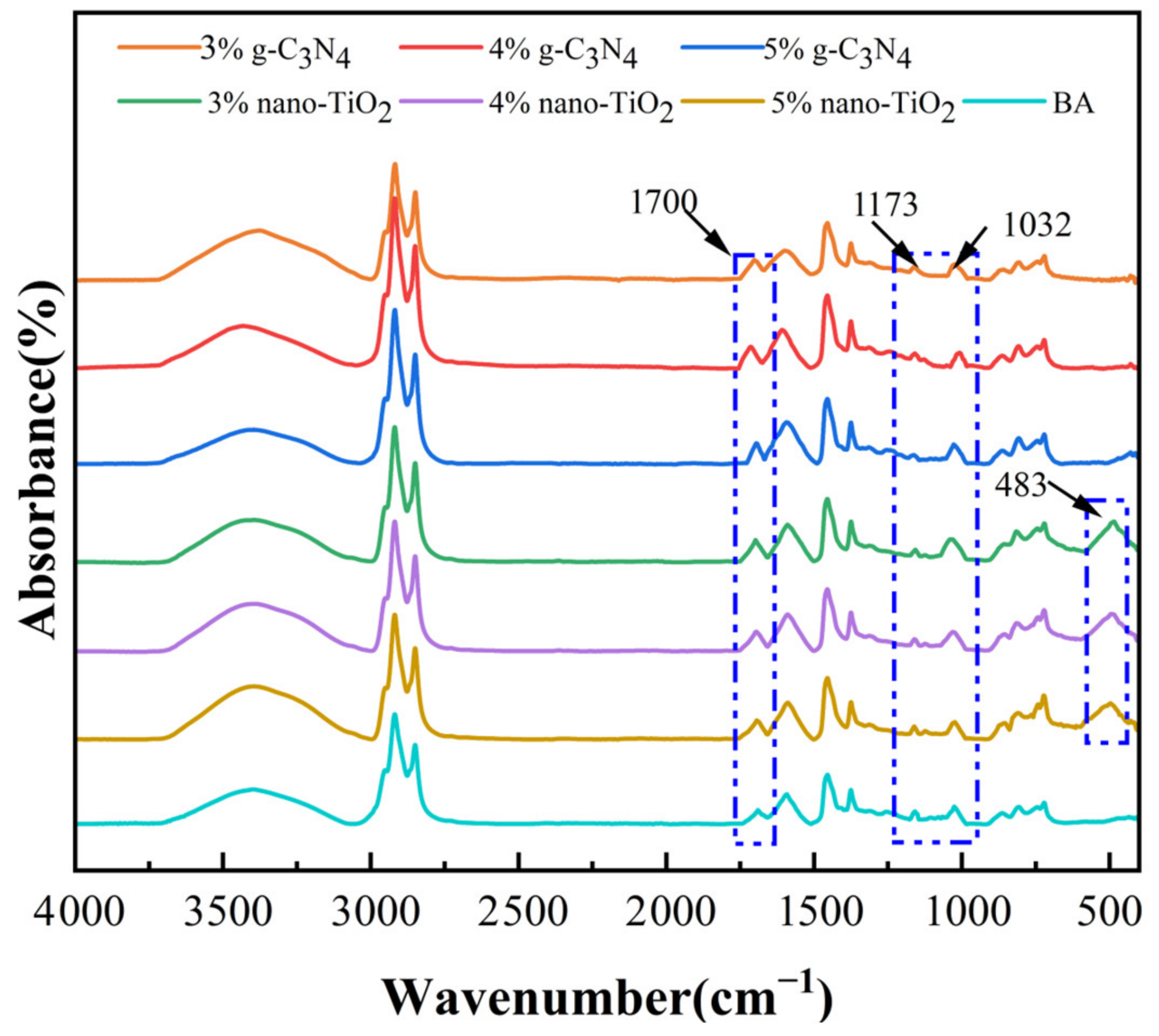
The FTIR spectra of the asphalt binders after rolling thin film oven test (RTFOT) aging and pressure aging vessel (PAV) aging are shown in Figure 2 and Figure 3. It can be seen from the results that a new peak was observed at 1700 cm−1, which can be attributed to the carbonyl group. In addition, the absorption peaks at 1173 cm−1 and 1032 cm−1 for BA, C3N4-MA, and TiO2-MA showed significant changes after PAV aging. These two functional groups represent ether and sulfoxide groups, respectively. The FTIR spectra of RTFOT aging and PAV aging were similar in peaks—they only showed differences in peaks at 1700 cm−1, 1173 cm−1, and 1032 cm−1, which were mainly because these were results for different aging levels. The changes in these oxygenated functional groups were the result of the oxidation of unsaturated light components in asphalt binders. This suggests that the oxidation of the lighter components to the heavier components was an important cause of the aging and hardening of the asphalt binders.
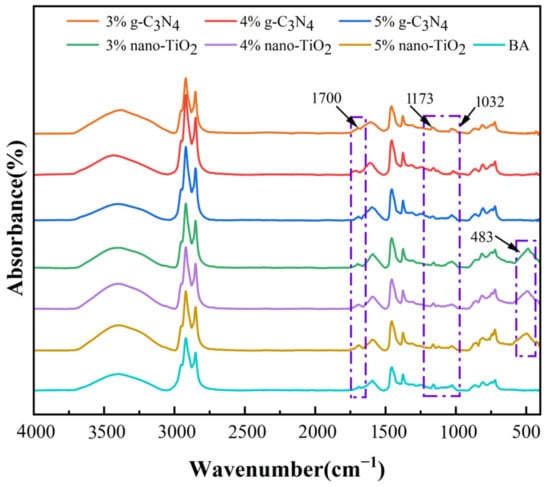
Figure 2.
FTIR spectra for asphalt binders after RTFOT aging.
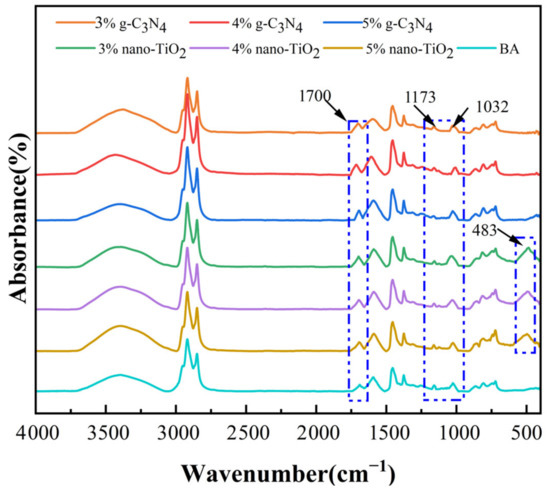
Figure 3.
FTIR spectra for asphalt binders after PAV aging.
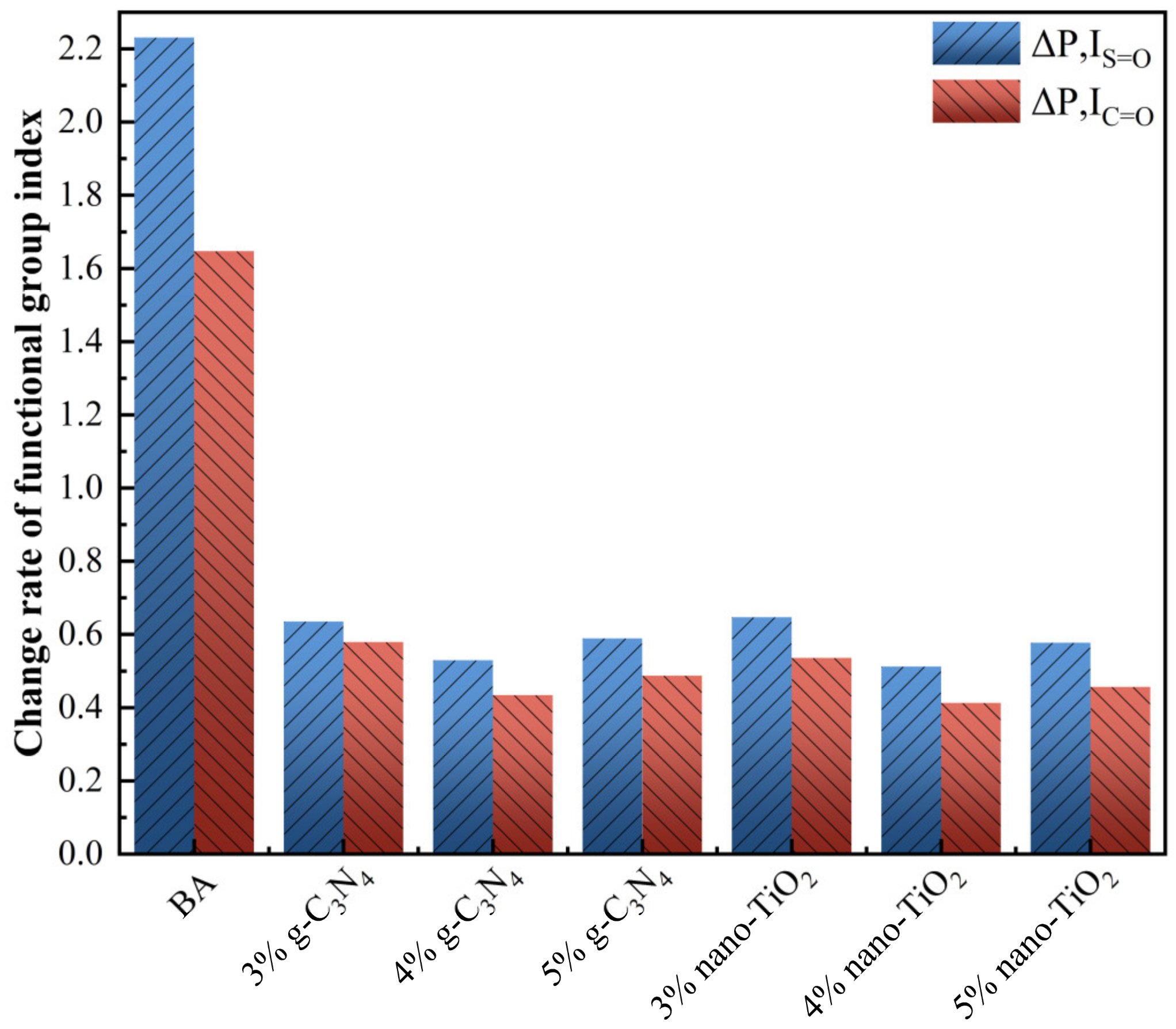
As the FTIR spectra of RTFOT aging and PAV aging were similar in peaks, Figure 4 only shows IS=O and IC=O index of asphalt binders after PAV aging stages. As can be seen from the graph, the BA had the highest value of IS=O and IC=O. While g-C3N4 and nano-TiO2 were added to modify the asphalt binders, the change rate of the functional group index became much lower compared to BA. With 4 wt% g-C3N4 and 4 wt% nano-TiO2, C3N4-MA reduced 77.1% IS=O and 74.9% IC=O, while TiO2-MA reduced 76.3% IS=O and 73.6% IC=O. They both presented the lowest change rate in three usage levels. This means that mixing photocatalysts into the BA could effectively enhance its anti-aging capability.
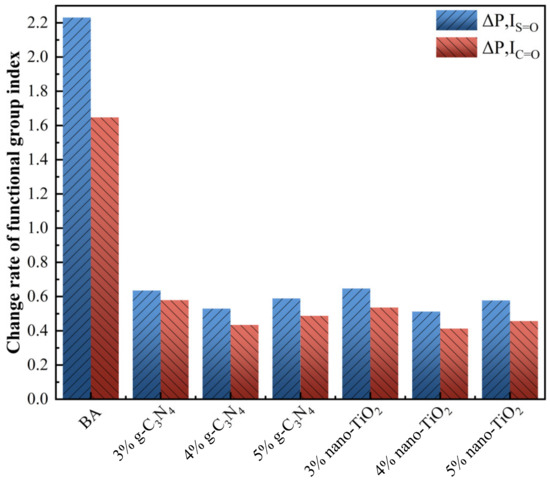
Figure 4.
The change rate of aging indexes of asphalt binders after PAV aging.
2.2. Rheological Behaviors Analysis
2.2.1. Frequency Sweep Analysis
In the analysis progress of frequency sweep, 40 °C was chosen as the reference temperature for the master curve. The corresponding phase angle and complex modulus master curves for each asphalt binder were plotted.
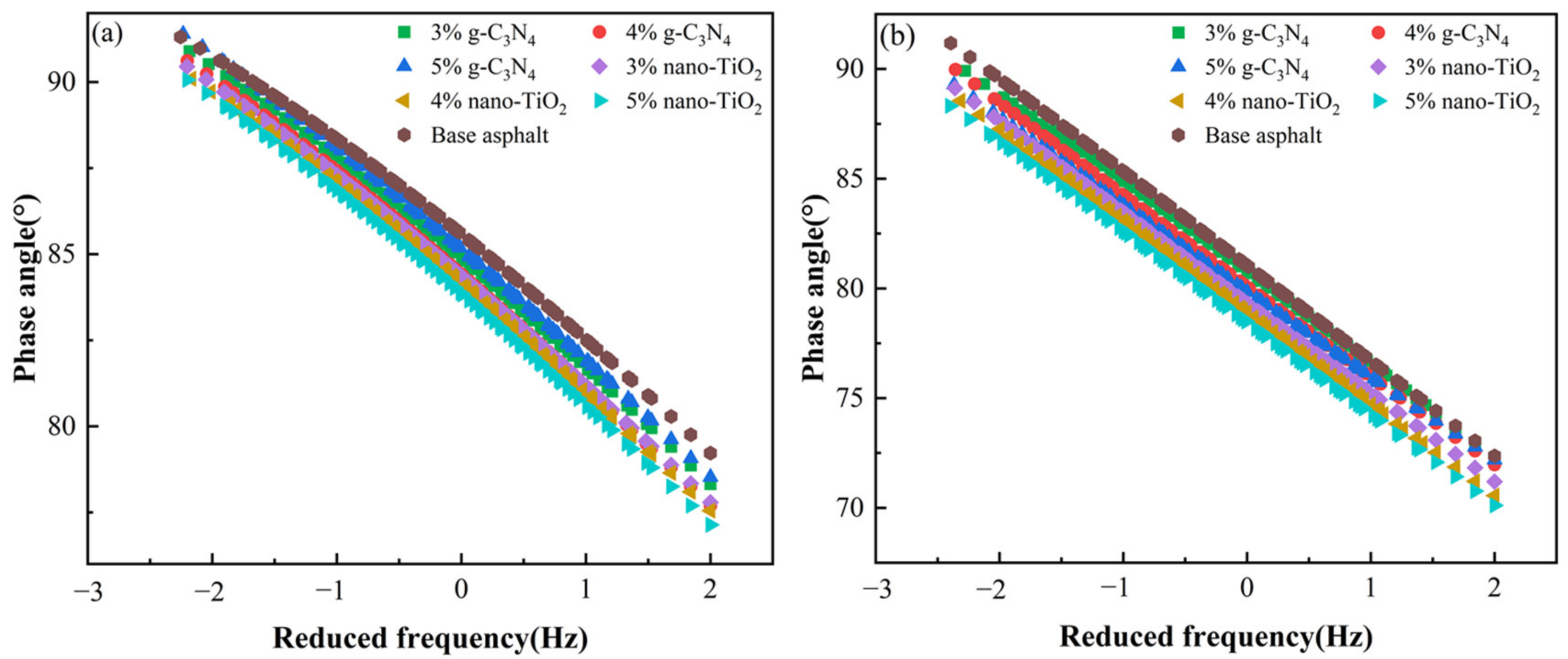
Combining Figure 5a,b, it can be seen that the PAV aging asphalt binders exhibited a lower phase angle at the same frequency compared to the unaged samples. This indicates that these samples demonstrated lower viscosity after PAV processing. The lighter components within them were converted into heavier components, which were less likely to flow at high temperatures. Under the same frequency level, the phase angles of the photocatalytic asphalt binders were all lower than those of the matrix asphalt. The phase angle was negatively correlated with the amount of photocatalyst used, which means that the more photocatalyst used, the lower the phase angle. This was mainly due to the fact that the addition of the photocatalysts changed the proportion of viscoelastic components in the asphalt binders so that they could be approximated as an elastomer.
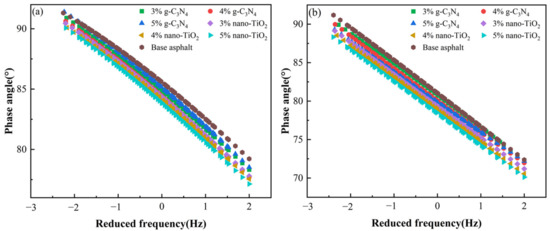
Figure 5.
Master curves of phase angle. (a) Master curves of unaged asphalt binders. (b) Master curves of asphalt binders after PAV aging.
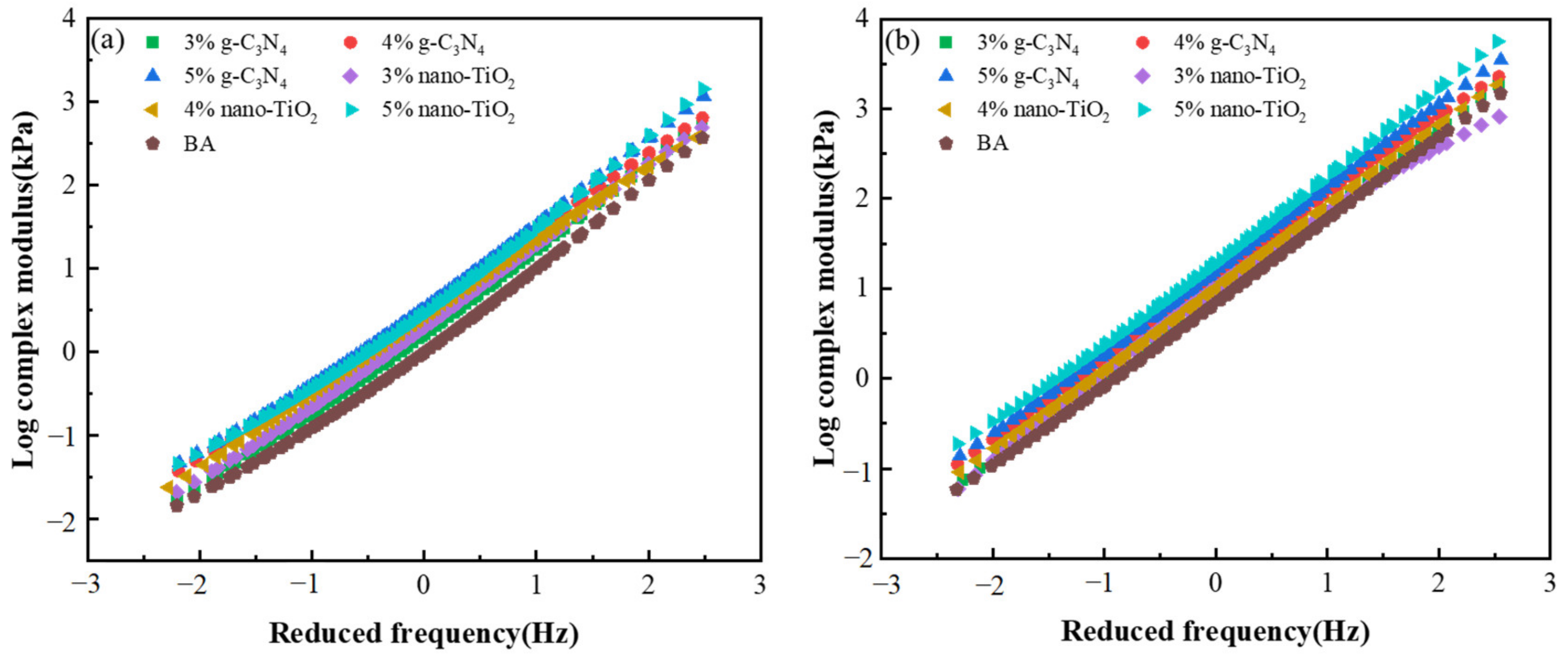
Figure 6a,b show the complex modulus master curves of the asphalt binders before and after the PAV test. The complex modulus of the BA was lower than TiO2-MA and C3N4-MA, indicating that the g-C3N4 and nano-TiO2 enhanced the modulus of the asphalt binders with the addition of both photocatalysts. Additionally, asphalt binders after PAV aging illustrated a higher complex modulus than those unaged binders; this phenomenon was consistent with the result of the master curve of phase angle. Although the hardness and modulus of the asphalt binders were improved, and their rates of resistance to rutting deformation at high temperatures were optimized, the viscous components were reduced and became less fluid. This led to the decrease in asphalt viscosity, decaying in low temperatures, and increases in fatigue cracks, which in turn deteriorated the durability of the pavement and affected the operational quality and service life of the asphalt pavement. The shear frequency increased, resulting in the rise of the complex shear modulus of asphalt binders after the PAV state. This was due to the fact that when the loading frequency reached a high level, the asphalt binders exhibited near pure fluid flow behavior and the elastic component was reduced to a minimum.
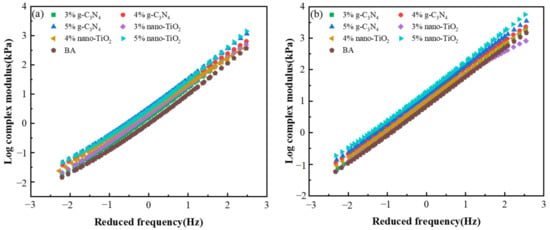
Figure 6.
Master curves of complex modulus. (a) Master curves of unaged asphalt binders. (b) Master curves of asphalt binders after PAV aging.
2.2.2. MSCR Analysis
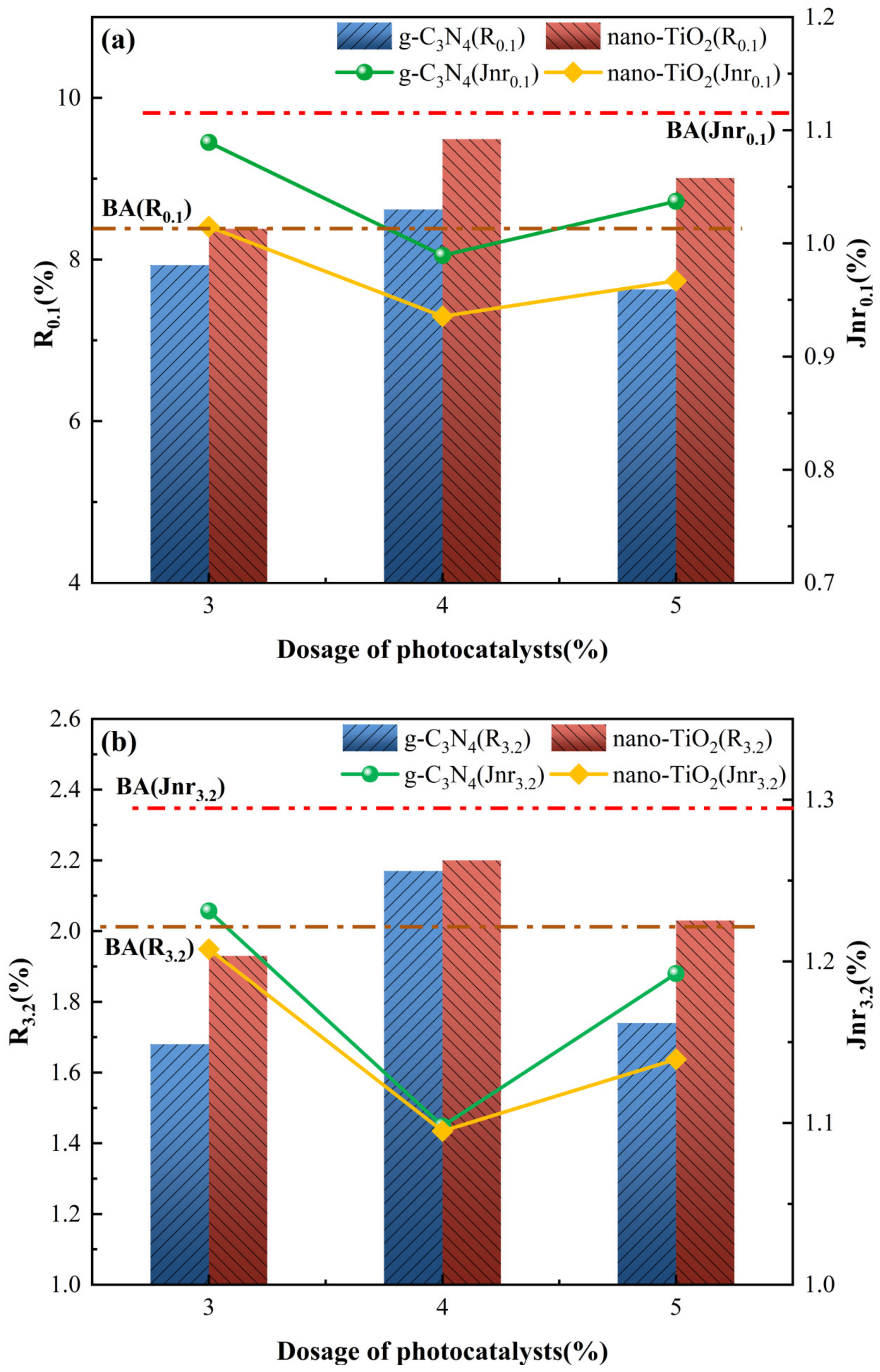
The average percentages for recoverable and non-recoverable creep compliance of the asphalt binders for 10 creep recovery cycles at 0.1 kPa and 3.2 kPa stress levels are expressed as R0.1, R3.2, Jnr0.1 and Jnr3.2, respectively. The results of the R0.1 and R3.2 for each photocatalytic asphalt binders are shown in Figure 7a,b. The magnitude of the parameters R0.1 and R3.2 can be used as indicators to evaluate the delayed elastic behavior and characterize the creep recovery capacity of the asphalt binders. The TiO2-MA exhibited the highest R0.1 and R3.2 of all the asphalt binders, indicating that TiO2-MA had the smallest percentage of elastic components and a better creep recovery capability. Compared to TiO2-MA, the C3N4-MA exhibited weaker creep recovery capability. This was related to the microscopic size of g-C3N4 and nano-TiO2; g-C3N4 had a larger size than nano-TiO2, thus affecting the creep response rate of the modified asphalt binders.
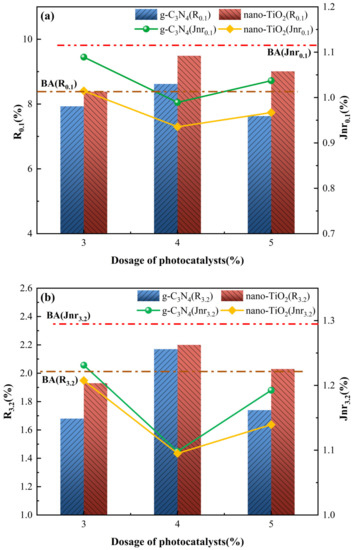
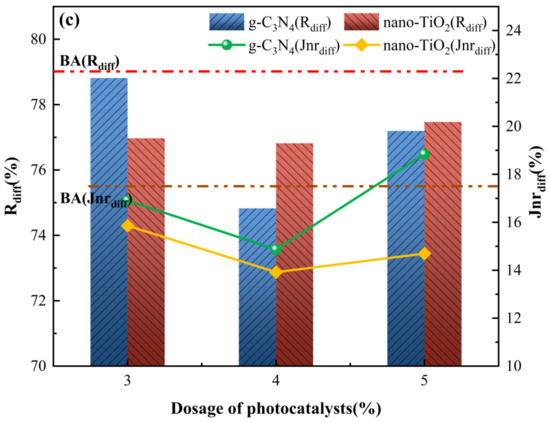
Figure 7.
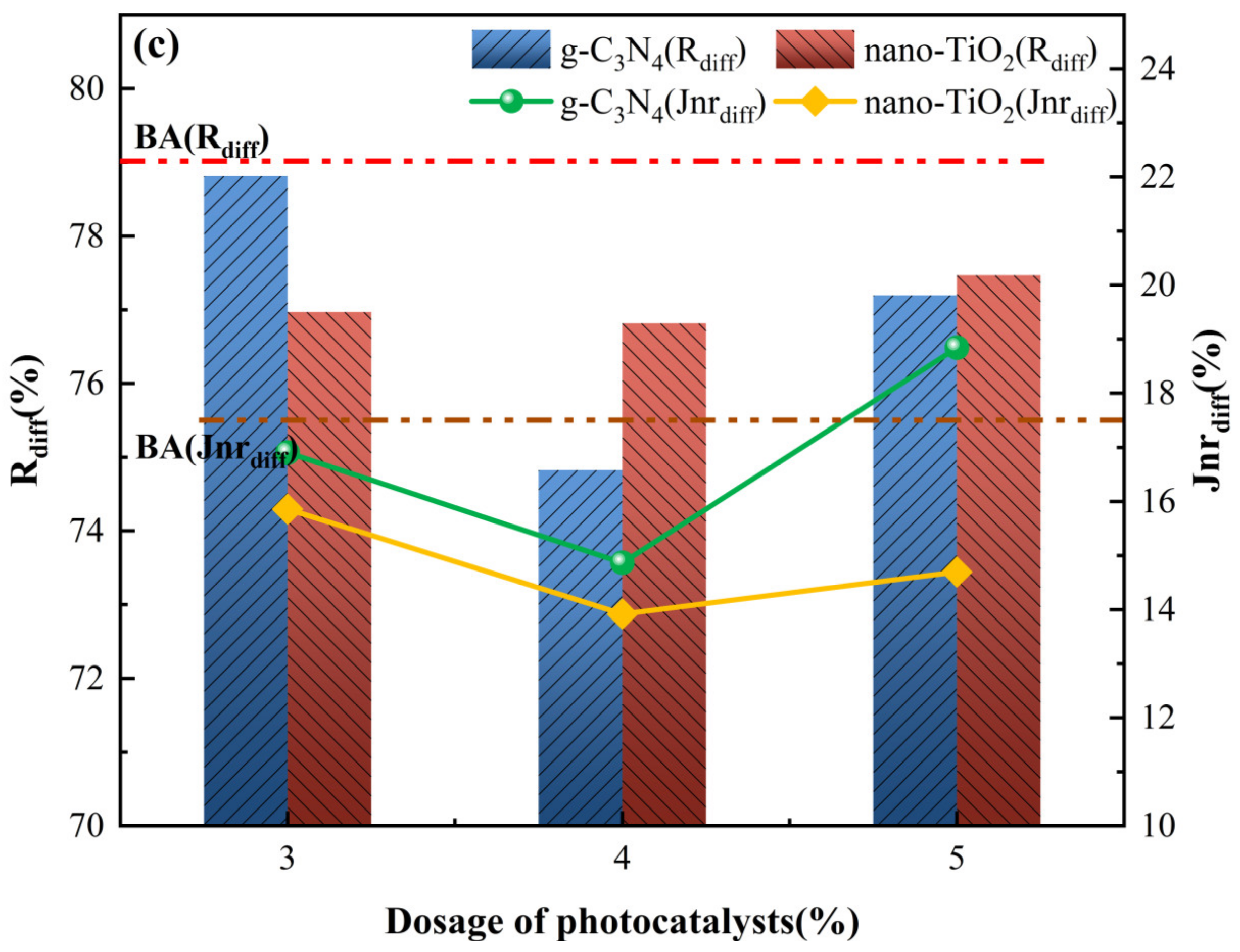
MSCR parameters for asphalt binders. (a) R and Jnr at 0.1 kPa load level; (b) R and Jnr at 3.2 kPa load level; (c) Rdiff and Jnrdiff.
Figure 7a,b also show the results of the non-recoverable creep compliance for each of the asphalt binders. Jnr can be used to characterize the viscosity of the asphalt, with smaller Jnr values indicating that the asphalt binders mainly exhibit more pronounced high-temperature flexibility. As can be seen from the graph, the relationship between the non-reversible creep flexibility of asphalt binders was the exact opposite of the creep reversion rate. C3N4-MA with 4 wt% g-C3N4 and TiO2-MA with 4 wt% nano-TiO2 both exhibited the smallest Jnr value and highest R value, under 0.1 or 3.2 kPa stress levels. This indicates that the 4 wt% g-C3N4 and 4 wt% nano-TiO2 can most efficiently inhibit the non-reversible deformation of the asphalt binders, which exhibited better rutting resistance.
To further characterize the sensitivity of asphalt binders to the magnitude of the load, stress sensitivity calculations were carried out for the non-reversible creep flexibility and creep recovery rates, with the results shown in Figure 7c. For the stress sensitivity index Rdiff, both C3N4-MA and TiO2-MA had the lowest value with 4 wt% g-C3N4 and 4 wt% nano-TiO2. Meanwhile, their Rdiff values were lower than the BA at all usage levels, indicating that g-C3N4 and nano-TiO2 reduced the stress sensitivity of the asphalt binders. As for stress sensitivity index Jnrdiff, its tendency fitted well with index Rdiff when photocatalyst dosage changed. Jnrdiff of C3N4-MA was slightly higher than BA only with 5 wt% g-C3N4. For TiO2-MA, the Jnrdiff values were all smaller than BA, indicating that nano-TiO2 in test amounts inhibited generation of cumulative deformation when asphalt binders were subjected to different traffic loads. With 4 wt% nano-TiO2, TiO2-MA displayed the lowest stress sensitivity values for Jnrdiff (14.1%) and Rdiff (74.8%), exhibiting superior creep recovery capability compared to C3N4-MA.
2.3. Fatigue Behaviors Analysis
2.3.1. Time Sweep Analysis
The 50% Modulus Reduction Criterion
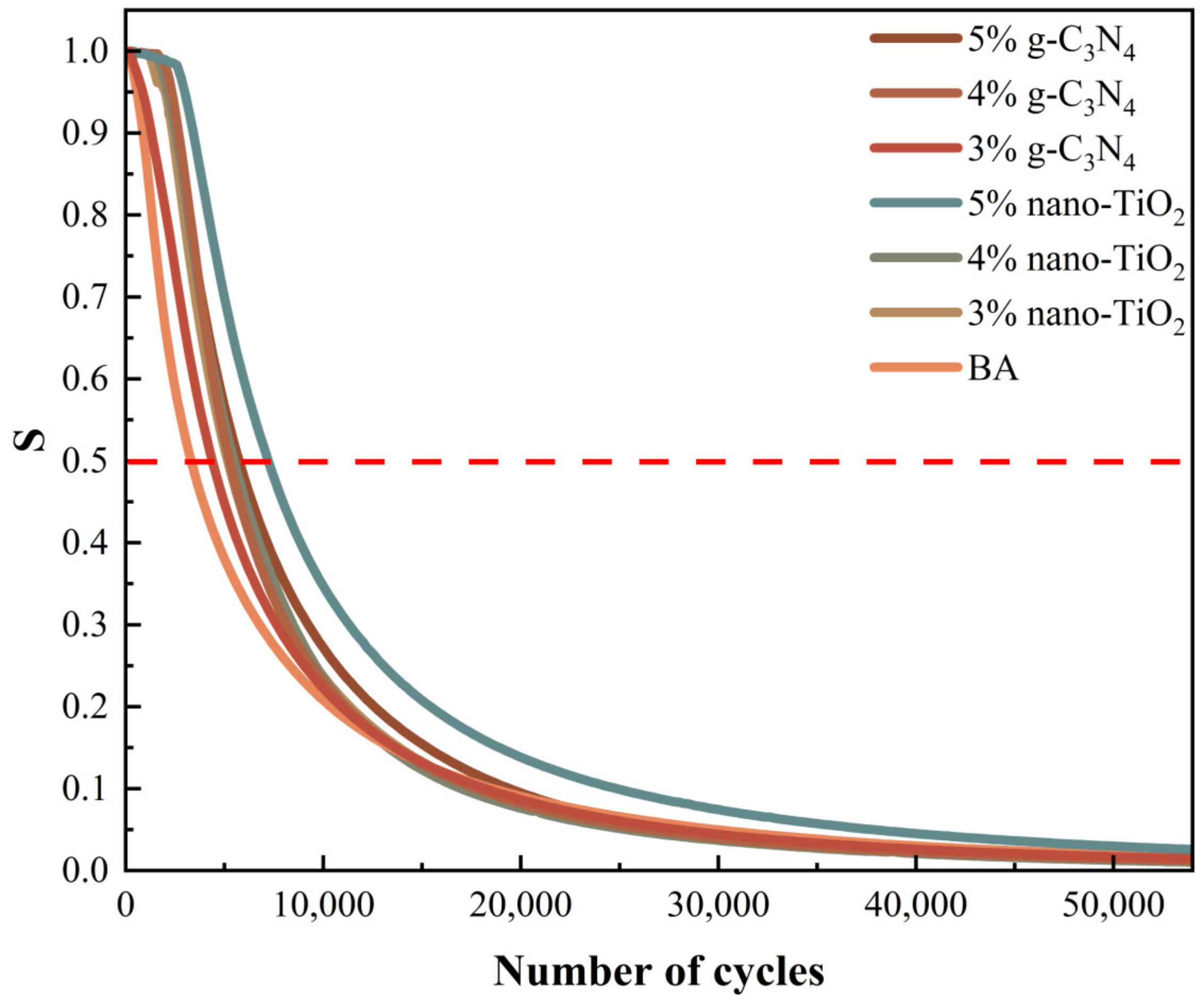
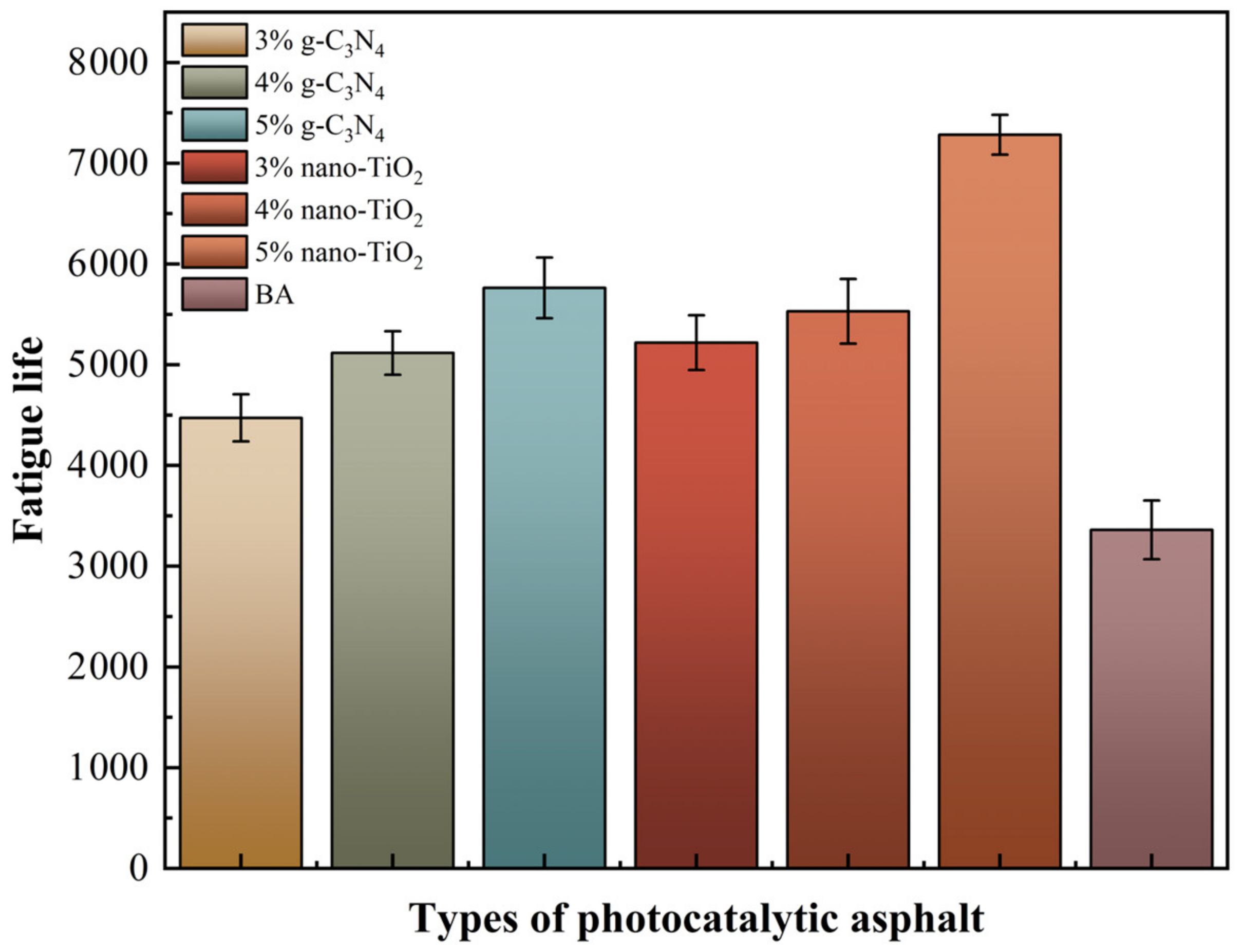
The 50% modulus reduction criterion uses the number of loading cycles at which the modulus of the asphalt binders is reduced by 50% as the fatigue life [33]. For visual analysis, the normalized modulus ratio (S) was used as the index. The value of S and the fatigue life of asphalt binders were tested, which are shown in Figure 8 and Figure 9 respectively.
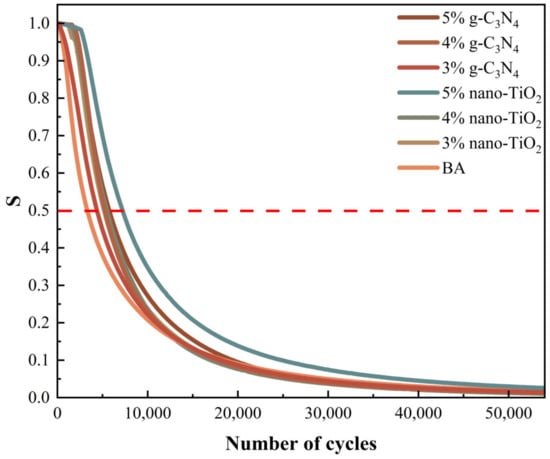
Figure 8.
Modulus of asphalt binders versus the number of loading cycles.
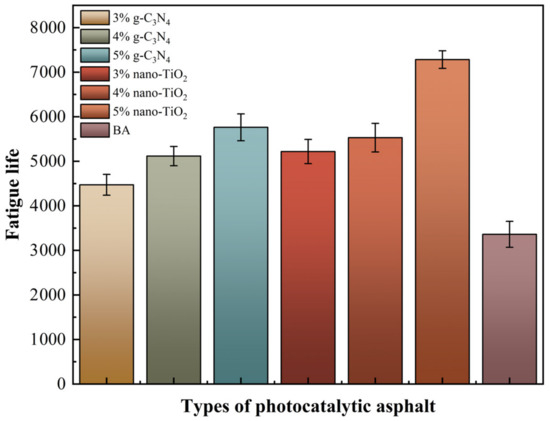
Figure 9.
Fatigue life of asphalt binders based on the complex modulus reduction criterion.
It was clear based on the analysis results of the 50% modulus reduction criterion that the 5% TiO2-MA had the highest fatigue life, followed by the 5% C3N4-MA. The reasons for this were that nano-TiO2 had a smaller average particle size than g-C3N4, and nano-TiO2 particles enhanced the binders’ susceptibility to time/temperature [34]. When the modulus of the specimen decayed to 50%, the TiO2-MA samples were still able to maintain a high fatigue life. All of the photocatalytic asphalt binders had a higher fatigue life than BA, indicating that nano-TiO2 and g-C3N4 had an enhancing effect on the fatigue damage resistance of the asphalt binders.
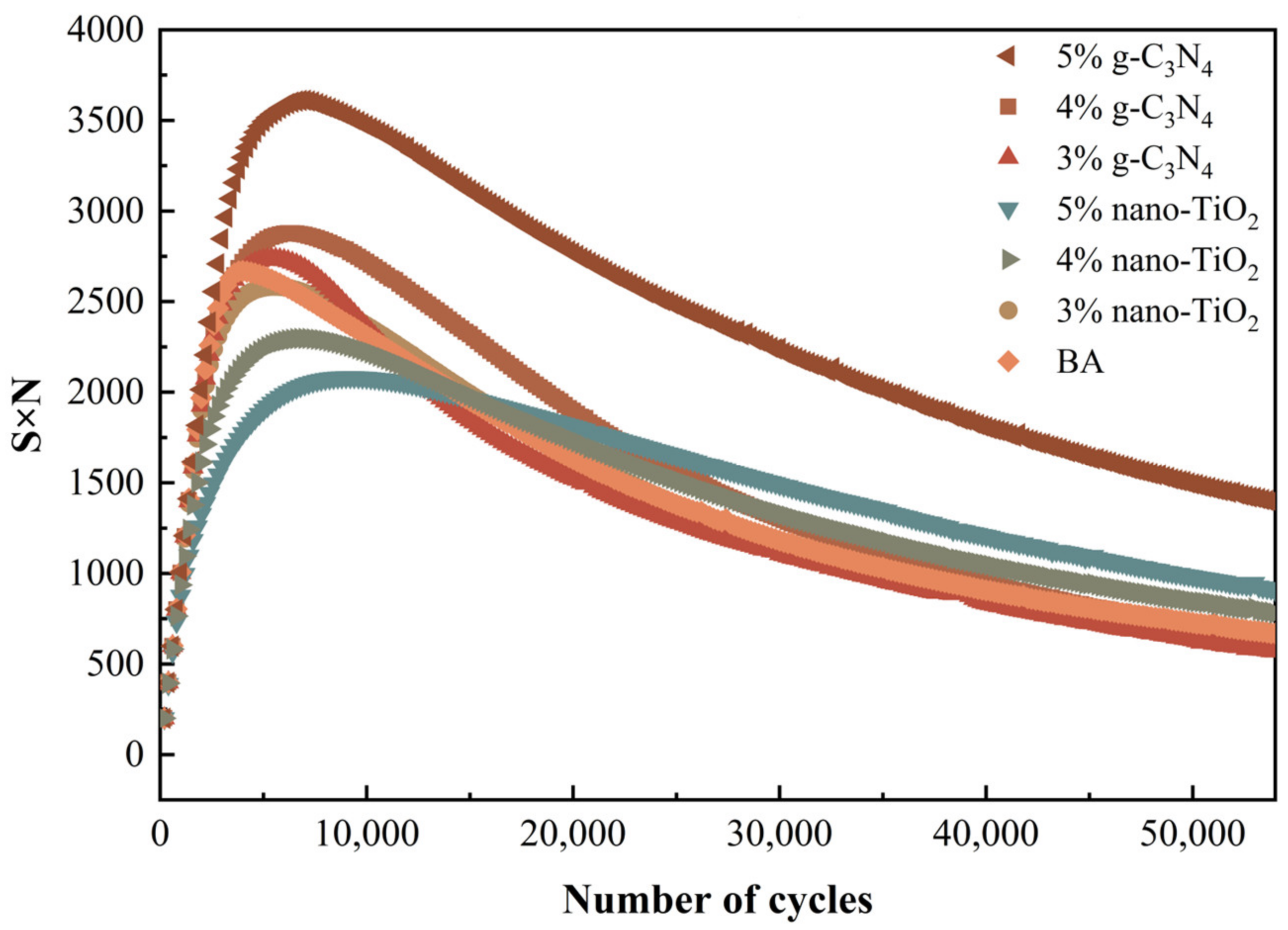
Peak in S×N Criterion
S is the normalized modulus ratio defined during the fatigue test and N is the number of loading cycles. Fatigue life is defined as the number of loading cycles at which the asphalt reaches its maximum S×N value. Figure 10 shows the S×N values of different asphalt binders as a function of the number of loading cycles. All curves showed peaks, while BA showed the earliest peak, indicating that BA had the lowest fatigue life. It can be seen that the fatigue life of the different doped photocatalytic asphalt was ranked as 5% nano-TiO2 > 5% g-C3N4 > 4% nano-TiO2 > 3% nano-TiO2 > 4% g-C3N4 > 3% g-C3N4 > BA. This result was consistent with the results of 50% modulus reduction criterion.
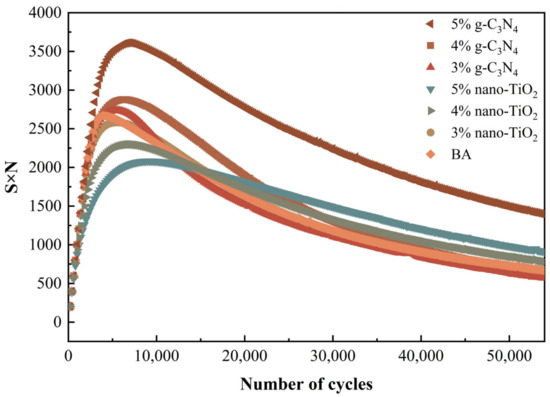
Figure 10.
Plot of S×N versus loading cycles.
2.3.2. LAS Analysis
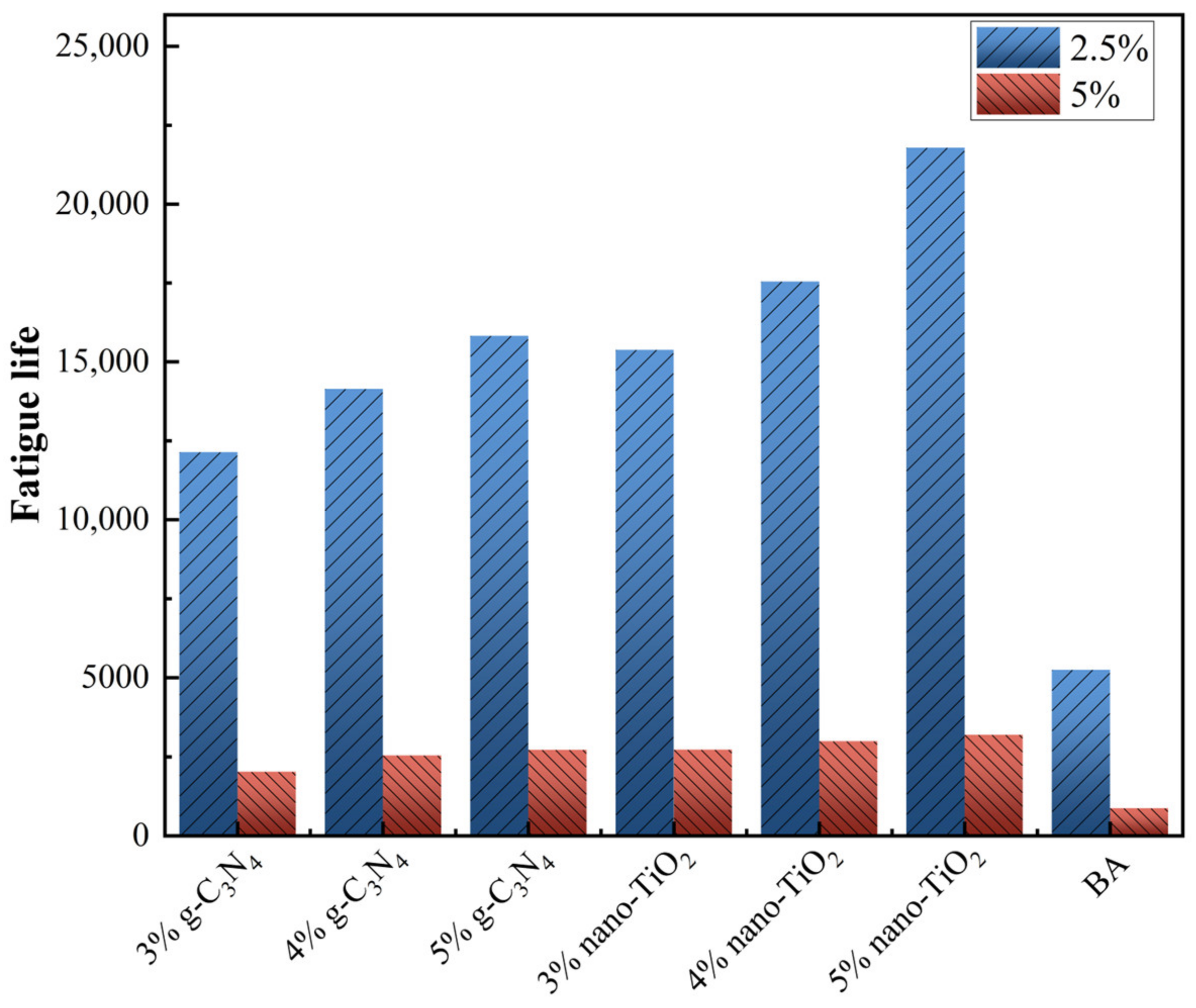
The fatigue life (Nf) of asphalt binders was predicted at 2.5% and 5% strain conditions, and the results are shown in Figure 11. At 2.5% strain, the fatigue life of the asphalt binders increased significantly with increasing photocatalysts usage. At 4% photocatalysts usage, g-C3N4 and nano-TiO2 improved Nf by 169.3% and 234.1%, respectively. At 5% strain, the Nf of all asphalt binders decreased significantly compared to the 2.5% strain, but the enhanced amplitude was approached. It can be seen that g-C3N4 and nano-TiO2 improved Nf of asphalt binders to 190.6% and 242.5%. This indicates that the photocatalyst can effectively improve the resistance of BA to fatigue damage at low strain levels.
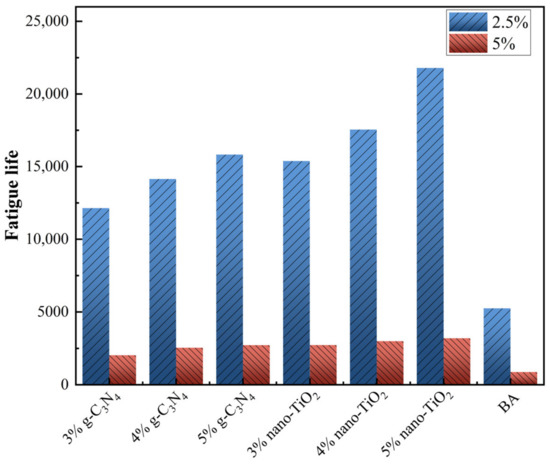
Figure 11.
LAS test results.
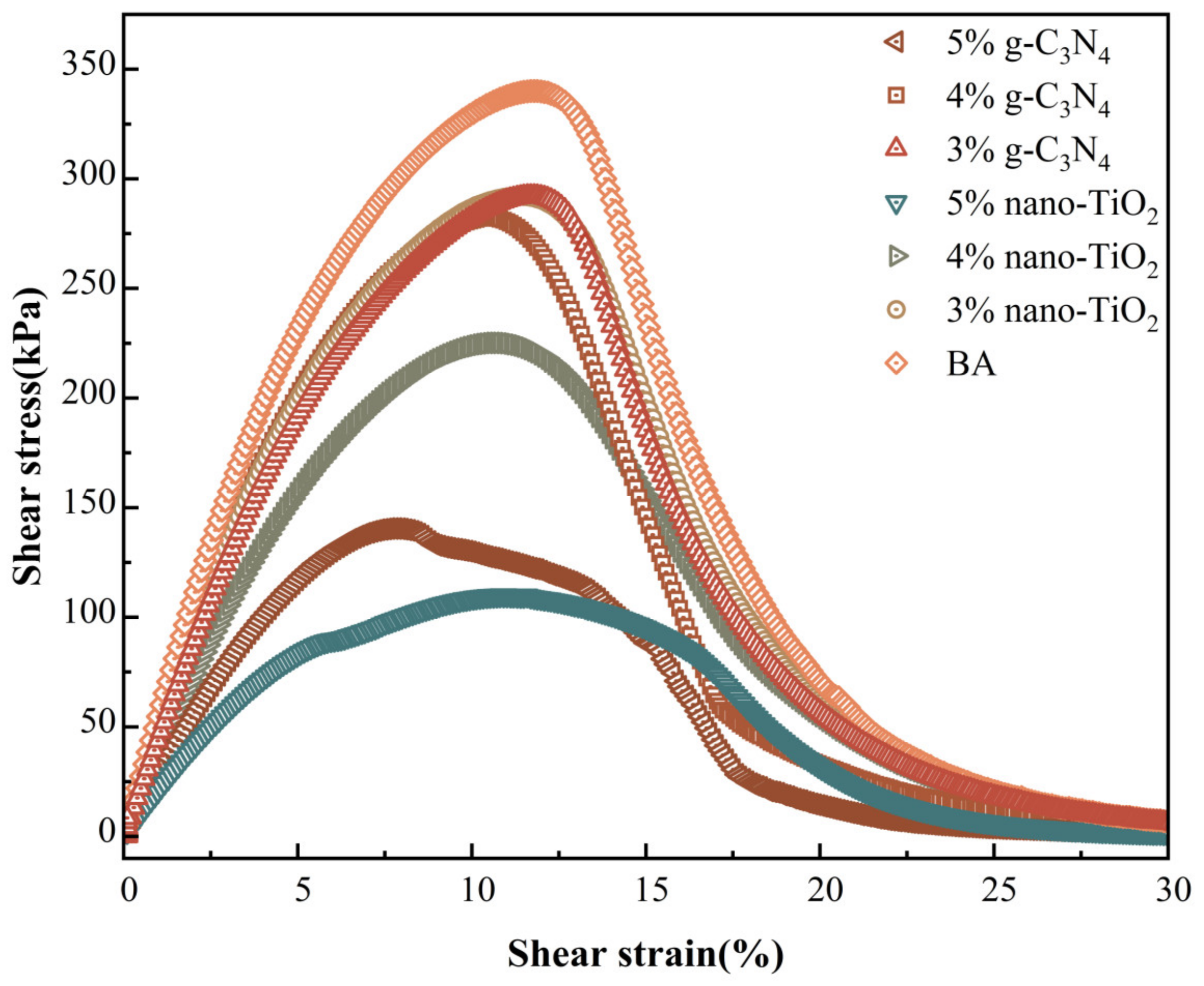
Figure 12 presents the results of the stress–strain curves for the different photocatalytic asphalts after PAV aging. It can be seen that as the shear strain increased, the shear stress response of the asphalt binders reached peaks at certain points. The point was the yield stress of the asphalt material, and its corresponding shear strain was the yield strain. The shear stress decreased sharply after the peak, which indicates that the material was severely damaged at this point. Different peaks and their corresponding widths can be noted for different photocatalytic asphalt binders during this process. BA exhibited the highest yield stress values, indicating that it was more susceptible to brittle fracture. Compared to BA, the stress peak of photocatalytic asphalt decreased with the addition of photocatalysts, indicating that both photocatalysts improved the fatigue resistance of the matrix asphalt.
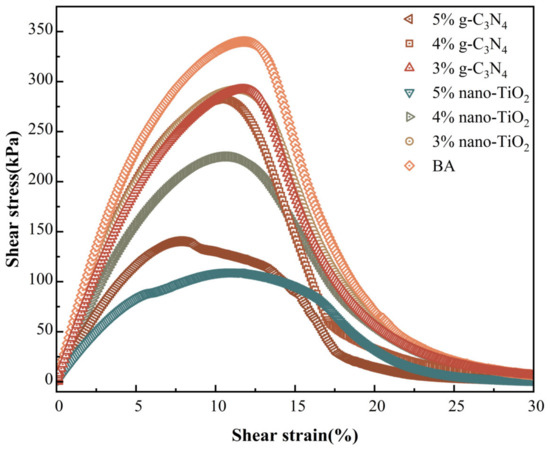
Figure 12.
Photocatalytic asphalt stress–strain curve.
2.4. NO Degradation Capacity Analysis
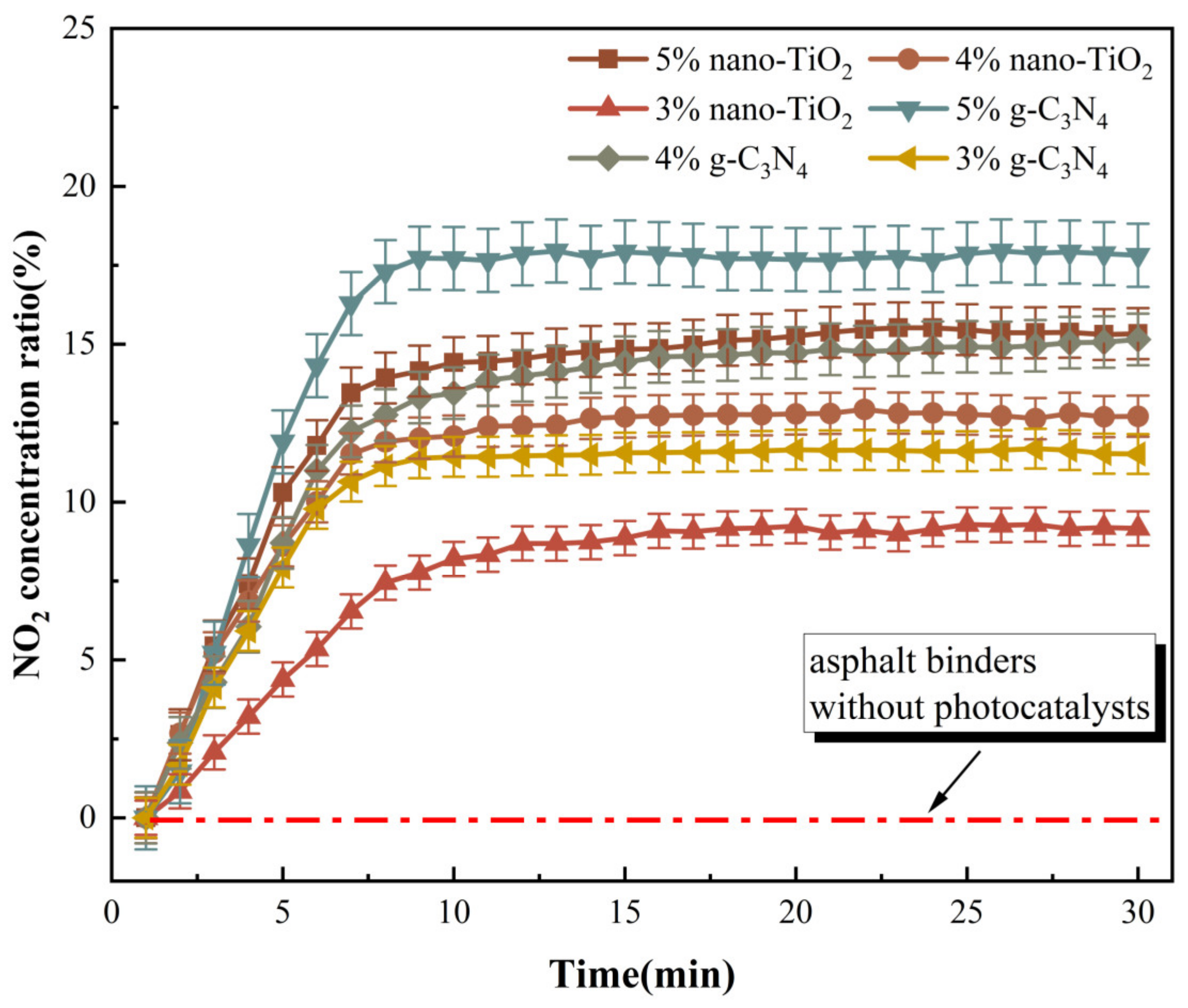
NO2 was generated during the photocatalytic degradation of NO by g-C3N4 and nano-TiO2. The change in NO2 concentration ratio can reflect the intensity of the NO degradation under visible light irradiation. Based on this, an NO degradation test was carried out and the results are shown in Figure 13.
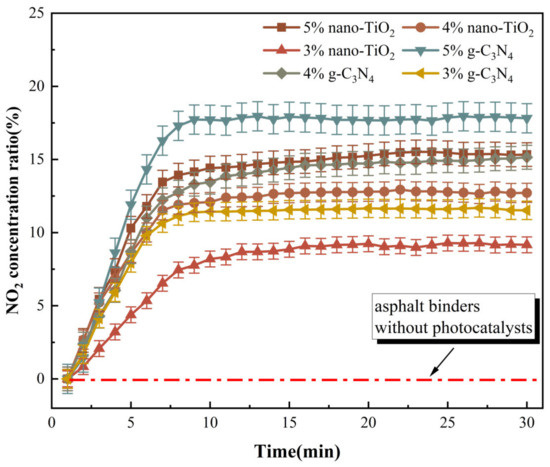
Figure 13.
NO degradation effect of photocatalytic asphalt binders.
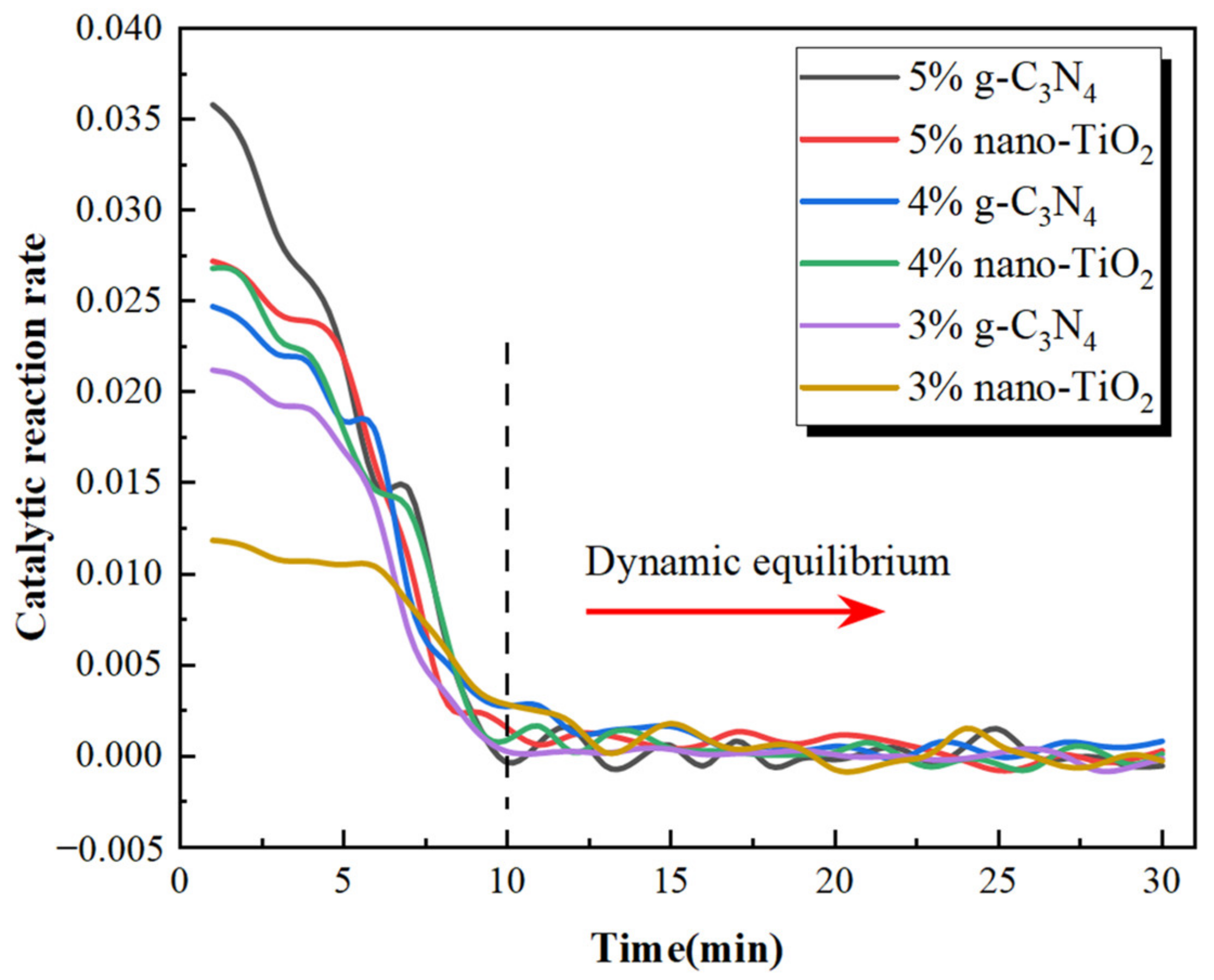
The results revealed that the proportion of NO2 concentration tended to increase after the NO degradation test started. One of the reasons for the increase in NO2 concentration was that NO2 was generated by the NO photocatalytic reaction. In addition, the total volume of NO2 and NO decreased during the whole NO degradation test, which also caused the proportion of NO2 concentration to increase. When usages of g-C3N4 and nano-TiO2 were the same, NO degradation efficiency of C3N4-MA was significantly higher than TiO2-MA. When the g-C3N4 usage was 5 wt%, its degradation rate of NO reached the maximum value of 17.8%. Asphalt binders modified with 4 wt% g-C3N4 and 5 wt% nano-TiO2 exhibited similar NO degradation efficiency. The catalytic reaction rates of six catalytic asphalt binders were calculated according to the slope of the NO2 concentration ratio curves, the results are shown in Figure 14.
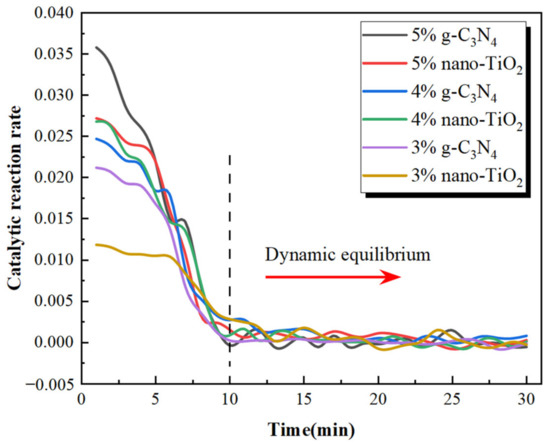
Figure 14.
NO degradation rate of photocatalytic asphalt binders.
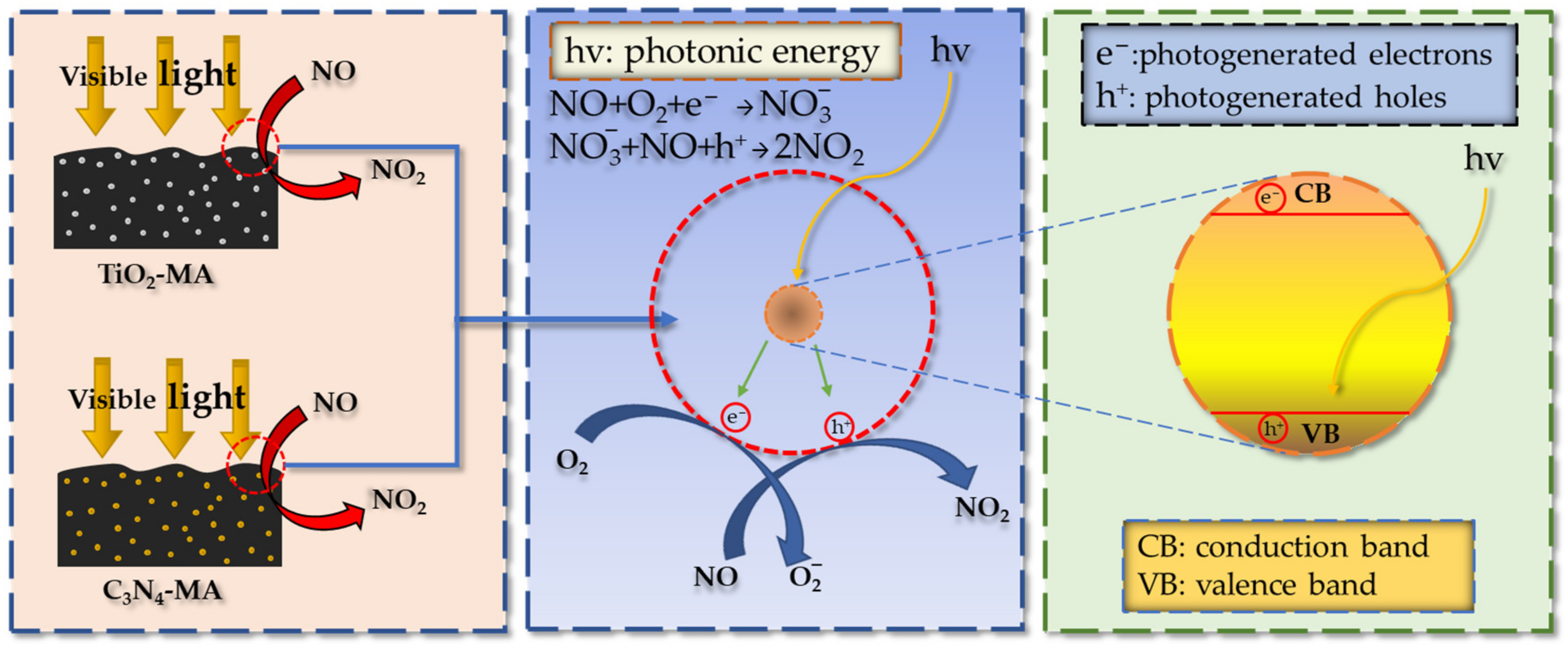
It can be seen that six asphalt binders had the fastest catalytic reaction rates during the first 10 min and kept decreasing until 10 min. The catalytic reaction rates plateaued after 10 min. This indicates that the NO degradation rate had reached dynamic equilibrium after 10 min. The reason for the occurrence of dynamic equilibrium consisted of two factors. Firstly, the six photocatalytic asphalt specimens had fixed reactive surface areas which limited the effective contact areas with NO gas. Secondly, the generating rates of photogenerated electrons and photogenerated holes in nano-TiO2 and g-C3N4 under visible light irradiation was in equilibrium with the rate of NO oxidation. The photocatalytic reaction mechanism of TiO2-MA and C3N4-MA is shown in Figure 15.
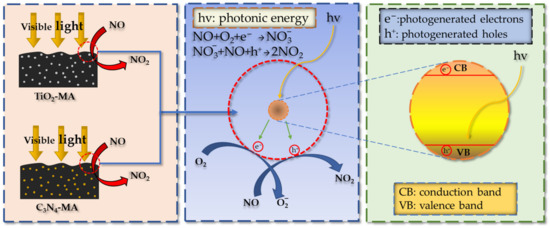
Figure 15.
NO degradation mechanism of photocatalytic asphalt binders.
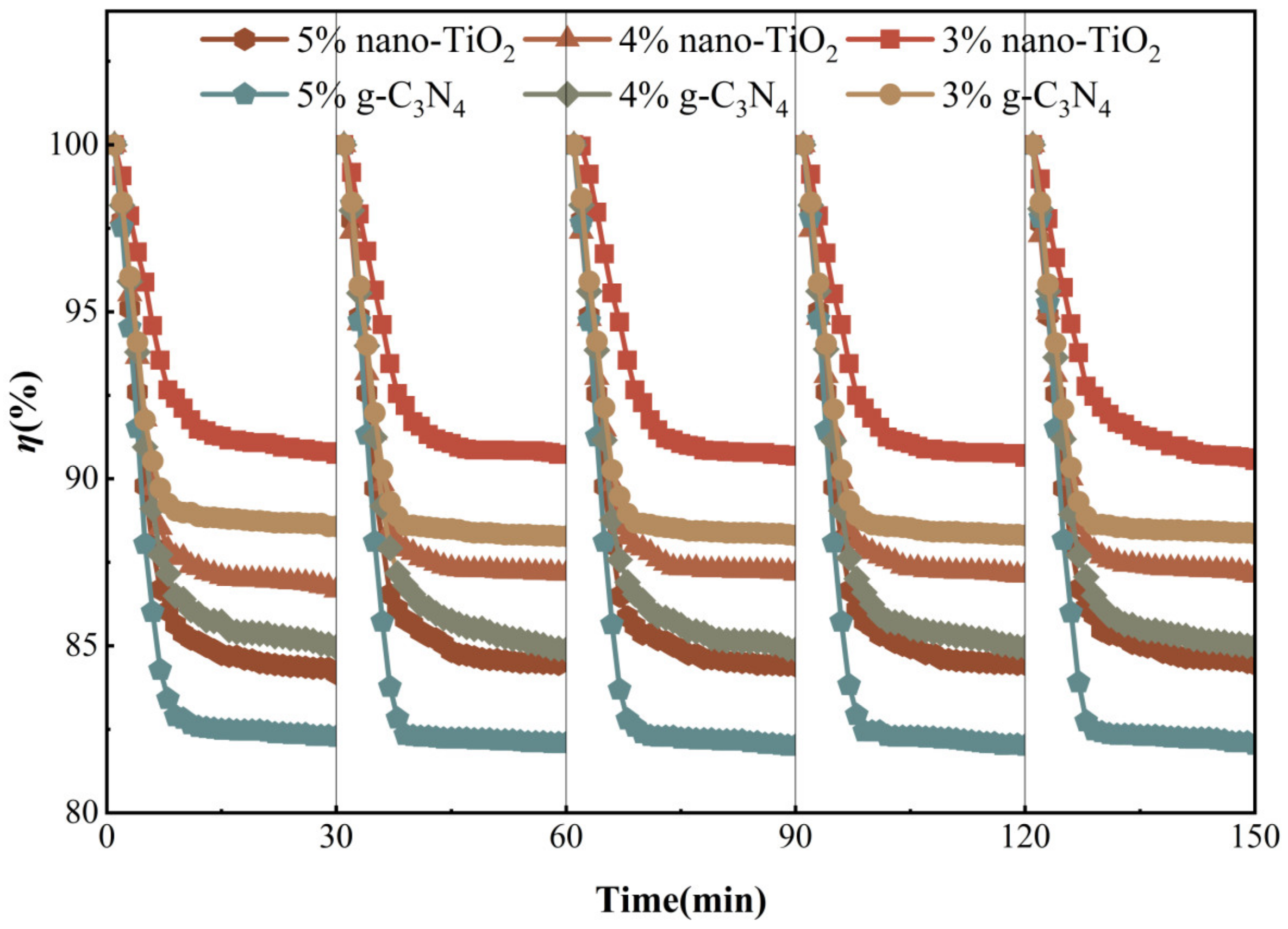
The use of photocatalytic materials in harsh environments makes it vital to ensure adequate chemical stability and reusability in practical applications. All asphalt binders were tested for five cycles of NO degradation under visible light irradiation, respectively. This was to evaluate the decay of photocatalytic activity of the photocatalytic asphalt binders when repeatedly used to degrade NO. As can be seen from Figure 16, all six samples still had photocatalytic activity for NO degradation after five repeated uses under visible light conditions. None of the photocatalytic activities showed a significant trend of decay. The test results indicate that these asphalt binders had chemical stability. This supports the potential for long-term stable reuse in actual road environments.
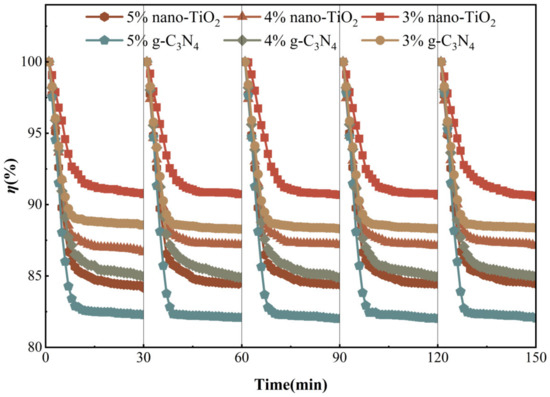
Figure 16.
Cycle degradation performance test.
3. Materials and Experiments
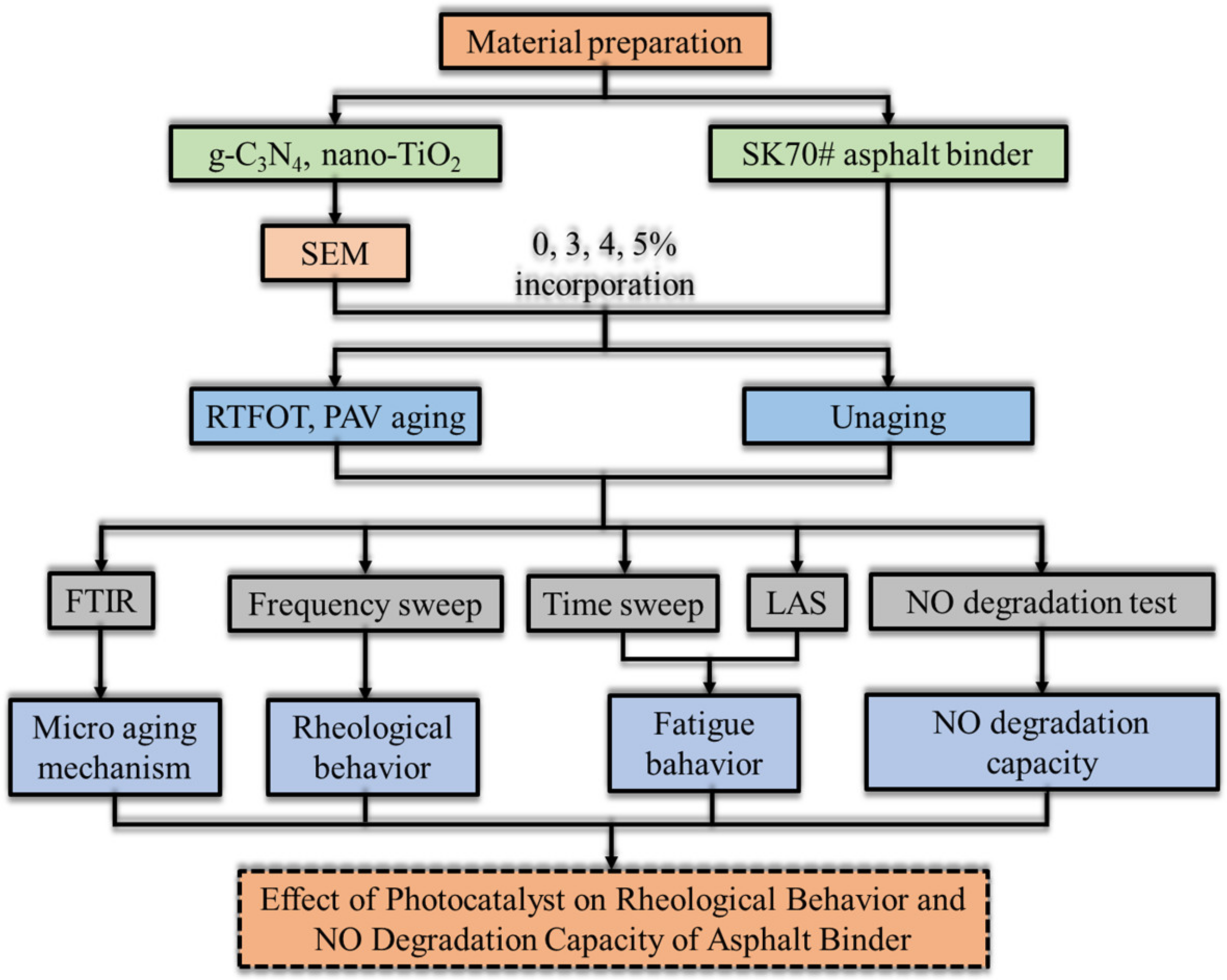
Figure 17 displays the framework of the steps developed in this research.
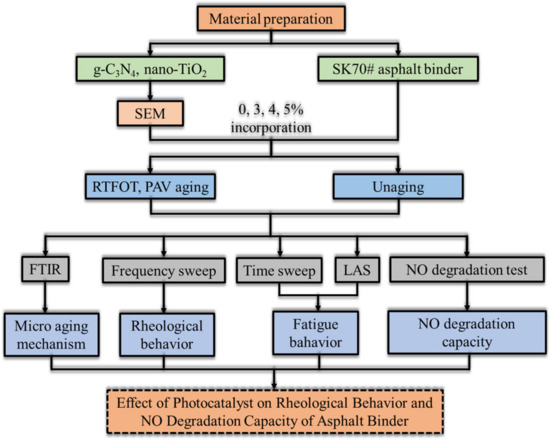
Figure 17.
The experimental flow diagram.
3.1. Raw Materials
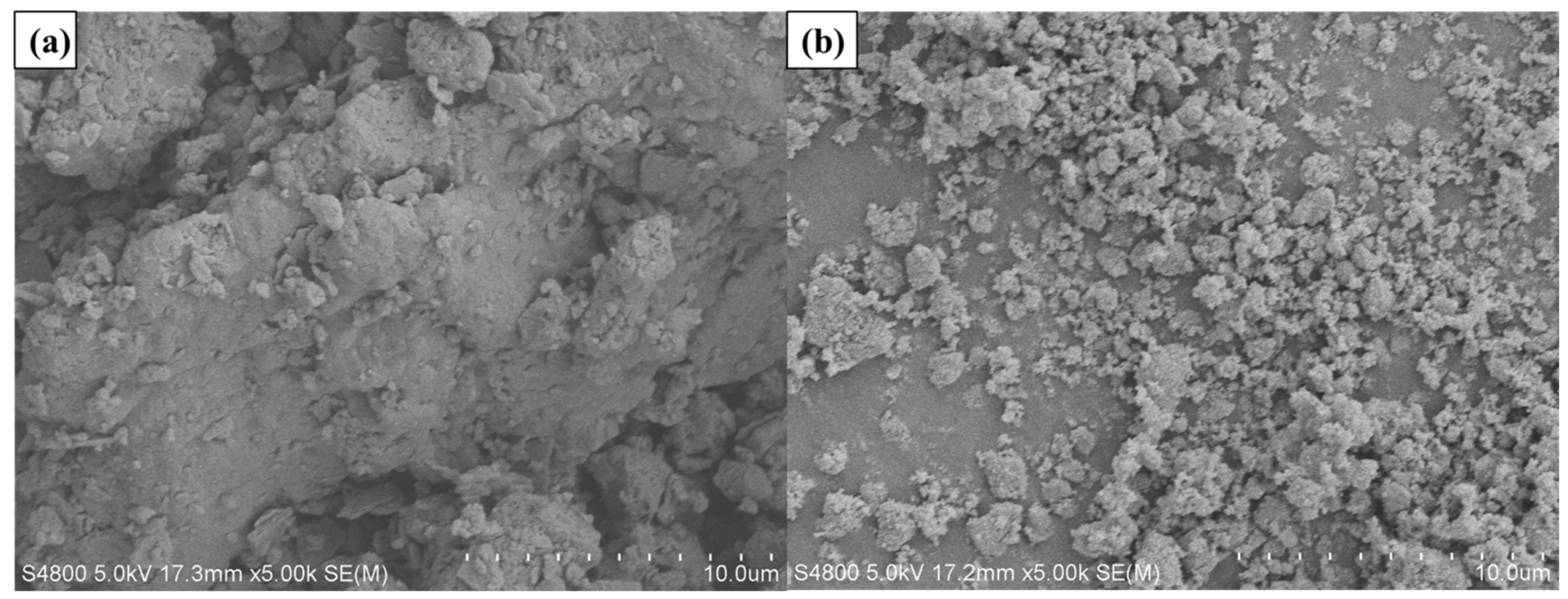
The present study used SK-70 asphalt binder as the BA, and its physical properties are presented in Table 1. In this research, two types of photocatalysts were selected, namely nano-TiO2 from Shanghai Jianghu Industrial Company, Shanghai Province, China, and g-C3N4 produced by Dongguan Bangte Surface Treatment Material Co., Ltd., Guangdong Province, China, as modifiers to enhance the properties of SK70# asphalt binder. The basic characteristics of nano-TiO2 and g-C3N4 are illustrated in Table 2 and Table 3, respectively. Additionally, the apparent morphology of the photocatalysts was observed using an S-4800 cold field emission scanning electron microscope (SEM), and the images are displayed in Figure 18. The SEM images revealed that the pure nano-TiO2 particles comprised a large number of irregular spherical nanoparticles that were densely clustered due to the agglomeration of fine particles. In contrast, the g-C3N4 sample exhibited a well-dispersed lamellar morphology, high surface area, mesoporous structure.

Table 1.
Technical parameters of BA.

Table 2.
Nano-TiO2 technical indicators.

Table 3.
Technical indexes of g-C3N4.
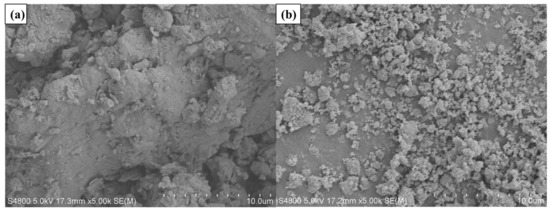
Figure 18.
The SEM images of (a) g-C3N4 and (b) nano-TiO2.
The basic properties of the two photocatalysts are as follows.
3.2. Preparation of Photocatalytic Asphalt
The photocatalyst was incorporated into the asphalt binders as a modifier, following the guidelines specified in the “Standard Test Methods of Asphalt and Bituminous Mixture for Highway Engineering” (JTG E20-2011). Firstly, the BA was heated to 180 °C and melted completely, followed by the addition of a specified amount of photocatalyst. The mixture was then subjected to shearing at a speed of 6000 rpm for 40 min to ensure uniform dispersion of the photocatalysts. The final contents of the photocatalyst in the asphalt mass were set at 3 wt%, 4 wt%, and 5 wt%, respectively.
3.2.1. Aging Methods
According to ASTM D1754 [35], all photocatalytic asphalt binders underwent RTFOT and PAV tests after preparation to simulate short-term and long-term aging, respectively. The 50 g ± 0.5 g RTFOT samples were placed in saucers to form a film of uniform thickness, and the saucers were placed on the turntable in an oven at 163 ± 1 °C for 5 h. When the time of RTFOT was over, each sample was placed in the PAV. An air pressure of 2.1 ± 0.1 MPa was applied and maintained for 20 h ± 10 min.
3.2.2. FTIR
In this study, the FTIR spectra were collected using a Bruker TENSOR II FTIR spectrometer (manufactured by Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with a reflection diamond ATR accessory. Scans within the wavenumber ranging from 400 cm−1 to 4000 cm−1 were obtained and averaged for each sample. The intensity of the FTIR can be plotted as the percentage of light transmittance or absorbance at each wavenumber. The spectrum baseline correction was performed using the OMNIC software.
To accurately determine the changes in the BA before and after aging, the absorption peaks of the sulfoxide (S=O), near 1032 cm−1 [36], and the absorption peak of the carbonyl (C=O) group [37], located near 1700 cm−1, were quantified. In the calculation, to exclude the calculation errors caused by the thickness of the sample, the C-H vibrational absorption peak [38] near 2800–3000 cm−1, which was more stable during the aging process, was selected as the calculation benchmark. The ratio of the peak areas of the two functional groups to the benchmark peak area was used as the evaluation index, as shown in Equations (1)–(4).
Sulfoxide index (SI):
Carbonyl index (CI):
where is the sum of the peak areas at 2800–3000 cm−1, A1032 is the sulfoxide absorption peak at 1032 cm−1, and A1700 is the carbonyl absorption peak at 1700 cm−1.
The change rate of aging indexes was calculated according to the following equations.
3.2.3. Frequency Sweep Test
Frequency sweep tests were conducted by using the 25 mm diameter, 1 mm gap, parallel plate on a dynamic shear rheometer (DSR). The frequencies in the tests were from 0.1 Hz to 100 Hz and temperatures were selected as 40 °C, 46 °C, 52 °C, 58 °C, and 64 °C. Based on the results, rheological master curves were constructed at a reference temperature of 46 °C. The shift factors for all samples were fitted with the WLF functions. The CAM model can be used to describe the complex modulus over a wide range of reduced frequencies at a reference temperature [39,40].
3.2.4. MSCR Test
The multiple stress creep recovery (MSCR) test has been used to characterize modified asphalt binders and it is considered to have good correlations to asphalt mixture rutting [41]. The MSCR test was also performed on a DSR to test the elastic recovery behavior and stress-dependent behavior of the asphalt binders under virgin, PAV-aged conditions at 64 °C under 0.1 kPa and 3.2 kPa creep stress following AASHTO TP 70-1. A total of 30 recovery and creep cycles were also tested. Twenty cycles were operated at 0.1 kPa stress level, and then 10 cycles at 3.2 kPa stress level, totaling 30 cycles. The first 10 cycles at 0.1 kPa stress level were used to adjust the samples. For the last 10 cycles of 0.1 kPa stress level and 10 cycles of 3.2 kPa stress level, the percentages of creep and restoring stresses were recorded and analyzed.
3.2.5. Time Sweep Test
The time sweep test is a strain-controlled fatigue test that is carried out at an ambient temperature. This test ensures that fatigue failure of the asphalt material occurs by applying cyclic loads to the asphalt binders at low strain levels [42]. The parallel loading plate used in this test was 8 mm in diameter (with a gap of 2 mm) and the sample was cyclically loaded 54,000 times at a temperature of 20 °C. A frequency of 10 Hz and a strain amplitude of 5% were applied to determine the fatigue parameters. On this basis, the fatigue life of asphalt binders was predicted and assessed using a range of fatigue damage criteria. There are several failure criteria available to define the fatigue life of time sweep tests. In this paper, the most representative fatigue criteria were selected, namely the 50% complex modulus decay criterion and the S×N peak criterion to evaluate the fatigue resistance of asphalt binders.
3.2.6. LAS Test
The linear amplitude sweep (LAS) test was carried out on asphalt binders to identify their fatigue damage characteristics [43,44]. The amplitude of the oscillatory shear strain was increased linearly from 0 to 30% within 5 min at a loading frequency of 10 Hz. The magnitude of the stress generated by the linearly increasing strain conditions for different asphalt binders were obtained. The parameters in the asphalt binders’ fatigue, shown in Equation (5), were calculated in conjunction with the VECD model, which in turn predicted fatigue life as follows:
where Nf is the fatigue life, γmax is the applied amplitude shear strain, and A and B are the model fitting coefficients.
3.2.7. NO Degradation Test
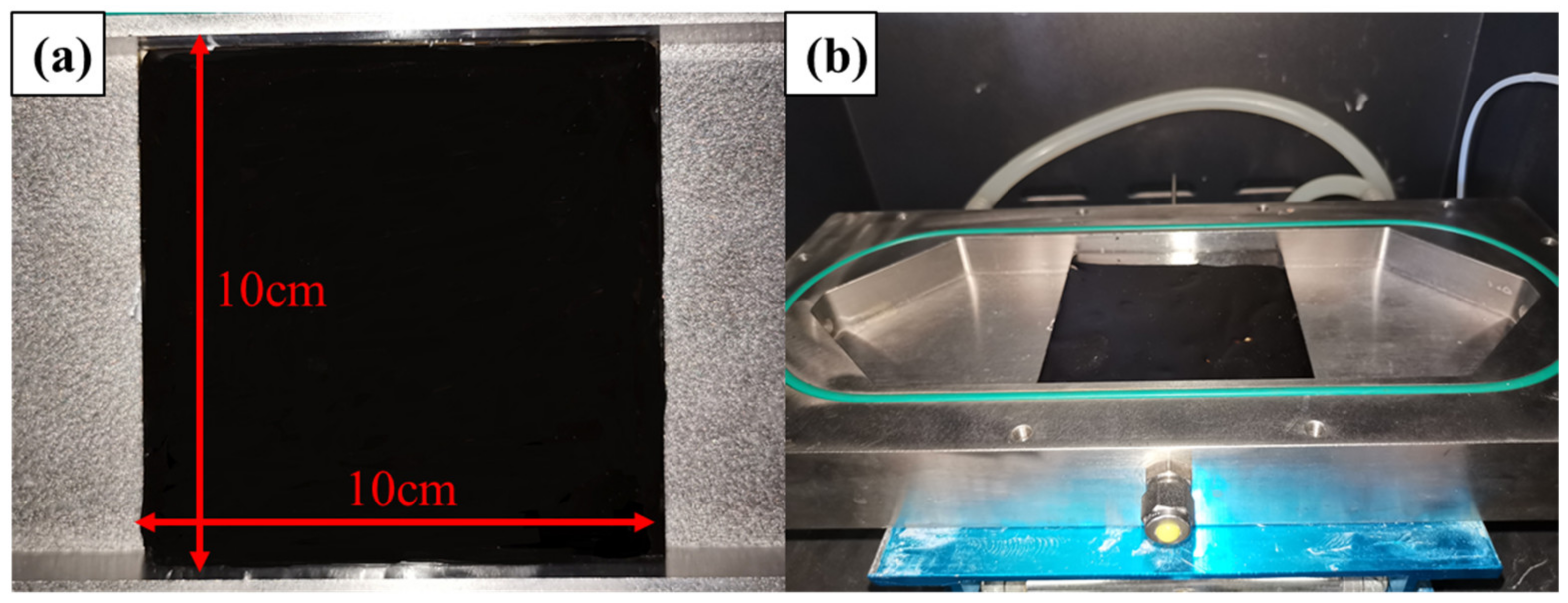
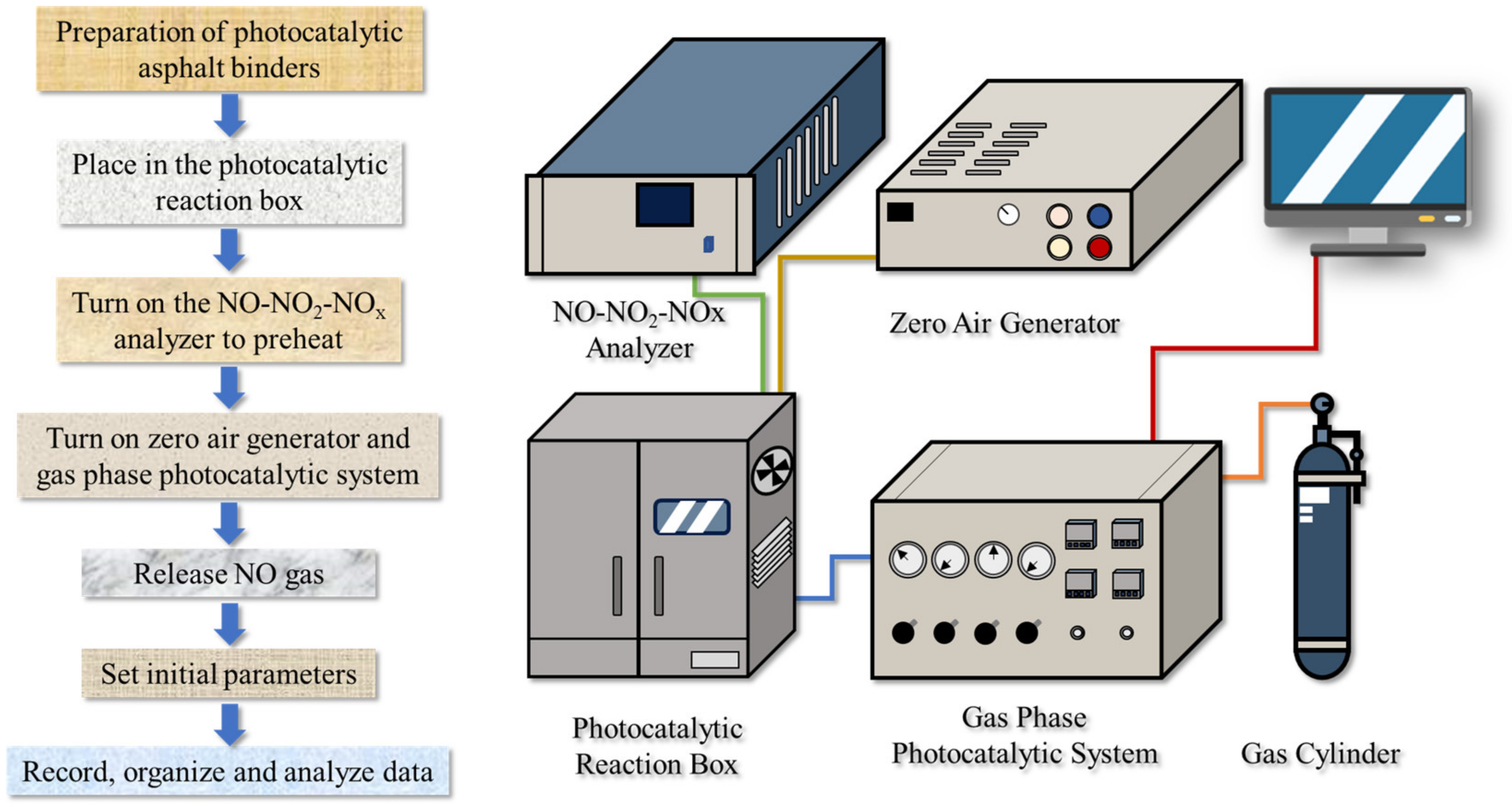
As NO is one of the main components of vehicle exhaust gas, it is considered as a target pollutant in photocatalytic degradation tests [45,46]. The NO concentration before and after the photocatalytic reaction was recorded to evaluate the photocatalytic activity. The test device consisted of a NO cylinder, photocatalytic reaction box containing a glass-covered metal reaction vessel, the gas phase photocatalytic system, zero air generator, and NO-NO2-NOx analyzer. Photocatalytic asphalt binders were loaded on glass sheets measuring 10 × 10 cm, as shown in Figure 19a. The specimens were placed in the center of the glass-covered metal reaction vessel, which is displayed in Figure 19b. The specific operation process of the experiment and the schematic of the NO photocatalytic degradation reaction system are displayed in Figure 20. The photocatalytic reaction time for each sample was 30 min.
Figure 19.
The photos of (a) asphalt specimens and (b) the reaction vessel.
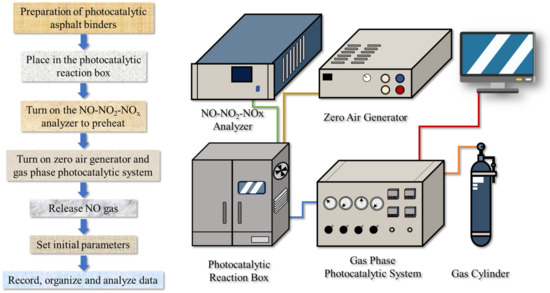
Figure 20.
NO degradation processes.
The NO concentration before and after the NO photocatalytic reaction was used to evaluate the photocatalytic activity of the photocatalyst, and the NO degradation rate was calculated as in Equation (6).
where η is the photocatalytic degradation rate, C0 is the initial concentration of NO, C1 is the NO concentration at NO degradation stage.
4. Conclusions
Photocatalysts g-C3N4 and nano-TiO2 were applied to modify the asphalt binders with 3 wt%, 4 wt%, and 5 wt% usages. The rheological behaviors, fatigue life, and NO degradation capacity of the photocatalytic asphalt binders were systematically evaluated. The findings can be drawn as follows:
- (1)
- Oxidation of the lighter components to the heavier components acted as an important cause of aging and hardening of the asphalt binders. With 4 wt% nano-TiO2, TiO2-MA reduced 77.1% IS=O and 74.9% IC=O, presenting better aging resistance than C3N4-MA.
- (2)
- The proportions of viscoelastic components in photocatalytic asphalt binders were changed by the addition of g-C3N4, and nano-TiO2. TiO2-MA had more excellent rheological behaviors and rutting resistance than C3N4-MA.
- (3)
- TiO2-MA with 4 wt% nano-TiO2 displayed 14.1% Jnrdiff and 74.8% Rdiff, showing the lowest stress sensitivity compared to other dosages. TiO2-MA had superior creep recovery capability, which inhibited more non-reversible deformation compared to C3N4-MA.
- (4)
- Fatigue life of TiO2-MA with 4 wt% nano-TiO2 increased to 234.1% at 2.5% strain and 242.5% at 5% strain, respectively. TiO2-MA exhibited more fatigue resistance than C3N4-MA.
- (5)
- The NO degradation efficiencies of TiO2-MA and C3N4-MA improved with the increasing photocatalyst dosages. C3N4-MA with 5 wt% g-C3N4 achieved 17.8% NO degradation efficiency, behaving better than TiO2-MA.
In future work, the road performance of a photocatalytic asphalt mixture and its durability under frequent traffic loads will be explored. Meanwhile, a trial road using the photocatalytic asphalt mixture will be constructed and its vehicle exhaust gas degradation capacity and environmental impacts will be studied.
Author Contributions
Writing—original draft preparation, Y.W. (Yan Wang); data curation, X.W.; review and editing, D.N.; project administration, Y.N.; conceptualization and supervision, H.X.; methodology and investigation, Y.W. (Yue Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 52278427), Shaanxi Housing and Urban-Rural Development Science and Technology Project (2020-K11), the Fundamental Research Funds for the Central Universities, CHD (Nos. 300102310301, 300102311404).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, X.R.; Fu, L.W.; Ji, M.S.; Lang, J.L.; Chen, D.S.; Cheng, S.Y. Scenario analysis to vehicular emission reduction in Beijing-Tianjin-Hebei (BTH) region, China. Environ. Pollut. 2016, 216, 470–479. [Google Scholar] [CrossRef]
- Yin, C.Y.; Li, H.Y.; Cha, Y.Y.; Zhang, S.J.; Du, J.E.; Li, Z.H.; Ye, W. Characterizing in-cabin air quality and vehicular air filtering performance for passenger cars in China. Environ. Pollut. 2023, 318, 120884–120892. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Zhao, J.C.; Man, H.Y.; Qi, L.J.; Yin, H.; Lv, Z.F.; Jiang, Y.H.; Dong, J.J.; Zeng, M.; Cai, Z.T.; et al. Updating emission inventories for vehicular organic gases: Indications from cold-start and temperature effects on advanced technology cars. Sci Total Environ. 2023, 882, 163544–163552. [Google Scholar] [CrossRef]
- Niu, X.; Chuang, H.C.; Wang, X.; Ho, S.S.H.; Li, L.; Qu, L.; Chow, J.C.; Watson, J.G.; Sun, J.; Lee, S.; et al. Cytotoxicity of PM2.5 vehicular emissions in the Shing Mun Tunnel, Hong Kong. Environ. Pollut. 2020, 263, 114386–114394. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Wu, Y.M.; Zhao, S.; Bai, X.X.; Liu, S.H.; Zhao, H.Y.; Hao, Y.; Tian, H.Z. Emissions of multiple metals from vehicular brake linings wear in China, 1980–2020. Sci. Total Environ. 2023, 889, 164380–164410. [Google Scholar] [CrossRef]
- Paster, M.D.; Ahluwalia, R.K.; Berry, G.; Elgowainy, A.; Lasher, S.; McKenney, K.; Gardiner, M. Hydrogen storage technology options for fuel cell vehicles: Well-to-wheel costs, energy efficiencies, and greenhouse gas emissions. Int. J. Hydrogen Energy 2011, 36, 14534–14551. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, T.; Feng, Q. Capital allocation efficiency, technological innovation and vehicle carbon emissions: Evidence from a panel threshold model of Chinese new energy vehicles enterprises. Sci. Total Environ. 2021, 784, 147104–147114. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.; Kumari, G.; Bisht, S.; Malik, A.; Kumar, N.; Singh, M.; Raturi, A.; Barthwal, S.; Thakur, A.; et al. Adaptive resilience of roadside trees to vehicular emissions via leaf enzymatic, physiological, and anatomical trait modulations. Environ. Pollut. 2022, 313, 120191–120200. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, D.; Kelly, J.; Stephens, T.; Wu, X.Y.; Zhou, Y. Mitigation of emissions and energy consumption due to light-duty vehicle size increases. Transp. Res. D Transp. Environ. 2023, 114, 103543–103554. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Wei, N.; Yin, J.W.; Zhao, X.Y.; Wu, L.; Zhang, Y.J.; Peng, J.F.; Wang, T.; Yang, Z.W.; Zhang, Q.J.; et al. Energy saving and emission reduction effects from the application of green light optimized speed advisory on plug-in hybrid vehicle. J. Clean. Prod. 2023, 412, 137452–137460. [Google Scholar] [CrossRef]
- Yuan, D.D.; Jiang, W.; Sha, A.M.; Xiao, J.J.; Wu, W.J.; Wang, T. Technology method and functional characteristics of road thermoelectric generator system based on Seebeck effect. Appl. Energy 2023, 331, 120459–120479. [Google Scholar] [CrossRef]
- Dubsok, A.; Khamdahsag, P.; Kittipongvises, S. Life cycle environmental impact assessment of cyanate removal in mine tailings wastewater by nano-TiO2/FeCl3 photocatalysis. J. Clean. Prod. 2022, 366, 132928–132936. [Google Scholar] [CrossRef]
- Feng, X.T.; Gu, L.F.; Wang, N.Y.; Pu, Q.S.; Liu, G.L. Fe/N co-doped nano-TiO2 wrapped mesoporous carbon spheres for synergetically enhanced adsorption and photocatalysis. J. Mater. Sci. Technol. 2023, 135, 54–64. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.J.; Li, J.M.; Liu, N.X.; Zhang, M.; Le, T. Advances in photocatalysis for mycotoxins elimination: Engineering strategies in photocatalyst designing, practical applications and future prospects. J. Alloys Compd. 2023, 955, 170234–170242. [Google Scholar] [CrossRef]
- He, H.Y.; Zhao, L.S.; Wei, L.G.; Wang, J.; Mao, W.; Liu, S.Y.; Deng, Y.H.; Zhan, Z.S.; Bai, H.H.; Liang, B.Y. Efficient degradation of Rhodamine B by coupling of Lys-CoFe-layered double hydroxide@graphitic-C3N4 nanohybrids via photocatalysis under visible light. Mater. Lett. 2023, 343, 134405–134408. [Google Scholar] [CrossRef]
- Lu, N.; Cai, J.Z.; Niu, B.L.; Zhou, Y.; Zhao, G.H. Preferential removal of phthalic esters by photocatalysis on selective TiO2. Chem. Eng. J. 2023, 460, 141735–141745. [Google Scholar] [CrossRef]
- Wang, H.H.; Yu, S.W.; Gao, T.; Tan, X.Y.; Meng, X.G.; Xiao, S.J. The efficient degradation of organic pollutants by Z-scheme MIL-88A@TiO2 heterojunction photo-Fenton catalyst: The synergistic effect of photocatalysis and Fenton catalysis. J. Alloys Compd. 2023, 960, 170688–170715. [Google Scholar] [CrossRef]
- Kim, Y.; Park, B. Photo-persistent effect-induced energy band bending at the TiO2/Ag nanoparticle surface plasmonic interface. Mater. Lett. 2022, 316, 132001–132004. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Z.Y.; Liu, Q.J. Regulating effect on photocatalytic water splitting performance of g-C3N4 via confinement of single atom Pt based on energy band engineering: A first principles investigation. Appl. Surf. Sci. 2022, 577, 151916–151926. [Google Scholar] [CrossRef]
- Bouteh, E.; Bentel, M.J.; Cates, E.L. Semiconductor-hydrophobic material interfaces as a new active site paradigm for photocatalytic degradation of perfluorocarboxylic acids. J. Hazard. Mater. 2023, 453, 131437–131443. [Google Scholar] [CrossRef]
- Ishak, N.; Jeyalakshmi, V.; Setka, M.; Grandcolas, M.; Devadas, B.; Šoóš, M. Upgrading of g-C3N4 semiconductor by a Nitrogen-doped carbon material: A photocatalytic degradation application. J. Environ. Chem. Eng. 2023, 11, 109381–109393. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Chong, D.; Niu, D.Y.; Lin, P.; Liu, X.Y.; Niu, Y.H.; Jing, R.X. Evaluation of photocatalytic micro-surfacing mixture: Road performance, vehicle exhaust gas degradation capacity and environmental impacts. Constr. Build. Mater. 2022, 345, 128367–128378. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.X.; Leung, M.K.H. Advanced Solar Photocatalytic Asphalt for Removal of lity of Vehilcular NOx. Energy Procedia 2017, 143, 811–816. [Google Scholar] [CrossRef]
- Fan, W.G.; Chan, K.Y.; Zhang, C.X.; Zhang, K.; Ning, Z.; Leung, M.K.H. Solar photocatalytic asphalt for removal of vehicular NOx: A feasibility study. Appl. Energy 2018, 225, 535–541. [Google Scholar] [CrossRef]
- Yu, H.N.; Dai, W.; Qian, G.P.; Gong, X.B.; Zhou, D.Y.; Li, X.; Zhou, X.L. The NOx Degradation Performance of Nano-TiO2 Coating for Asphalt Pavement. Nanomaterials 2020, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, S.Y.; Zhang, J.; Fan, J.F. Photocatalytic degradation of vehicle exhausts on asphalt pavement by TiO2/rubber composite structure. Constr. Build. Mater. 2015, 81, 224–232. [Google Scholar] [CrossRef]
- Qian, G.P.; Zhu, X.; Yu, H.N.; Shi, C.Y.; Yao, D. The oil pollution and nitric oxide photocatalytic degradation evaluation of composite nanomaterials for asphalt pavement. Constr. Build. Mater. 2022, 314, 125497–125511. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, T.; Su, W.Y.; Kang, Y.Q.; Wu, Y.H.; Zhang, Y.J. Enhanced washing resistance of photocatalytic exposed aggregate cementitious materials based on g-C3N4 nanosheets-recycled asphalt pavement aggregate composites. Constr. Build. Mater. 2019, 228, 116748–116758. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Wang, Z.Y.; Liu, Y.; Wang, P.G.; Cao, J.L.; Ho, W.K. g-C3N4/TiO2 Composite Film in the Fabrication of a Photocatalytic Air-Purifying Pavements. Sol. RRL 2020, 4, 2000170. [Google Scholar] [CrossRef]
- Hassan, M.; Mohammad, L.N.; Asadi, S.; Dylla, H.; Cooper, S. Sustainable Photocatalytic Asphalt Pavements for Mitigation of Nitrogen Oxide and Sulfur Dioxide Vehicle Emissions. J. Mater. Civ. Eng. 2013, 25, 365–371. [Google Scholar] [CrossRef]
- Da Costa Gomez, T.M.; Radtke, C.L.; Kalscheur, V.L.; Swain, C.A.; Scollay, M.C.; Edwards, R.B.; Santschi, E.M.; Markel, M.D.; Muir, P. Effect of focused and radial extracorporeal shock wave therapy on equine bone microdamage. J. Nano Electron. Phys. 2004, 33, 49–55. [Google Scholar] [CrossRef]
- Liao, M.J.; Liu, Z.H.; Gao, Y.L.; Liu, L.; Xiang, S.C. Study on UV aging resistance of nano-TiO2/montmorillonite/styrene-butadiene rubber composite modified asphalt based on rheological and microscopic properties. Constr. Build. Mater. 2021, 301, 124108–124117. [Google Scholar] [CrossRef]
- Yan, C.Q.; Yuan, L.X.; Yu, X.T.; Ji, S.Z.; Zhou, Z.F. Characterizing the fatigue resistance of multiple modified asphalts using time sweep test, LAS test and elastic recovery test. Constr. Build. Mater. 2022, 322, 125806–125814. [Google Scholar] [CrossRef]
- Neto, V.F.S.; Lucena, L.C.F.L.; de Barros, A.G.; Lucena, A.E.F.L.; Filho, P.G.T.M. Rheological evaluation of asphalt binder modified with zinc oxide nanoparticles. Case Stud. Constr. Mater. 2022, 17, e01224–e01234. [Google Scholar] [CrossRef]
- D1754/D1754M-20; Standard Test Method for Effects of Heat and Air on Asphaltic Materials (Thin-Film Oven Test). ASTM International: West Conshohocken, PA, USA, 2020.
- Rahimi, J.; Azizi, M.; Niksefat, M.; Heidari, M.; Naderi, M.; Maleki, A. An efficient superparamagnetic PEO-based nanocatalyst for selective oxidation of sulfides to sulfoxides. Inorg. Chem. Commun. 2023, 148, 110320–110327. [Google Scholar] [CrossRef]
- Mohamed, I.M.; Dao, V.D.; Yasin, A.S.; Mousa, H.M.; Mohamed, H.O.; Choi, H.S.; Hassan, K.M.; Barakat, N.A.M. Nitrogen-doped&SnO2-incoportaed TiO2 nanofibers as novel and effective photoanode for enhanced efficiency dye-sensitized solar cells. Chem. Eng. J. 2016, 304, 48–60. [Google Scholar]
- Saral, A.; Sudha, P.; Muthu, S.; Sevvanthi, S.; Irfan, A. Molecular structure spectroscopic Elucidation, IEFPCM solvation (UV–Vis, MEP, FMO, NBO, NLO), molecular docking and biological assessment studies of lepidine (4-Methylquinoline). J. Mol. Liq. 2022, 345, 118249. [Google Scholar] [CrossRef]
- Ling, M.; Luo, X.; Gu, F.; Lytton, R.L. Time-temperature-aging-depth shift functions for dynamic modulus master curves of asphalt mixtures. Constr. Build. Mater. 2017, 157, 943–951. [Google Scholar] [CrossRef]
- Vestena, P.M.; Schuster, S.L.; Almeida, P.O.B., Jr.; Faccin, C.; Specht, L.P.; Pereira, D.S. Dynamic modulus master curve construction of asphalt mixtures: Error analysis in different models and field scenarios. Constr. Build. Mater. 2021, 301, 124343–124356. [Google Scholar] [CrossRef]
- Gajewski, M.; Bańkowski, W.; Gajewska, B.; Sybilski, D.; Horodecka, R. Estimation of asphalt binders’ resistance to permanent deformation with application of the MSCR and multiple shear creep long recovery (MSCLR) tests. Constr. Build. Mater. 2021, 284, 122808–122820. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Castorena, C.; Zhang, J.; Kim, Y.R. Identifying fatigue failure in asphalt binder time sweep tests. Constr. Build. Mater. 2016, 121, 535–546. [Google Scholar] [CrossRef]
- Chen, H.; Bahia, H.U. Modelling effects of aging on asphalt binder fatigue using complex modulus and the LAS test. Int. J. Fatigue 2021, 146, 106150–106159. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.G.; Ren, Z.Y. Toward to a viscoelastic fatigue and fracture model for asphalt binder under cyclic loading. Int. J. Fatigue 2023, 168, 107479–107495. [Google Scholar] [CrossRef]
- Jin, J.; Xiao, T.; Tan, Y.Q.; Zheng, J.; Liu, R.H.; Qian, G.P.; Wei, H.; Zhang, J.H. Effects of TiO2 pillared montmorillonite nanocomposites on the properties of asphalt with exhaust catalytic capacity. J. Clean. Prod. 2018, 205, 339–349. [Google Scholar] [CrossRef]
- Lasne, J.; Lostier, A.; Salameh, T.; Athanasopoulou, E.; Karagiannis, D.; Kakouri, A.; Vassaux, S.; Lesueur, D.; Romanias, M.N. NOx emissions by real-world fresh and old asphalt mixtures: Impact of temperature, relative humidity, and UV-irradiation. Urban Clim. 2023, 49, 101457–101473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).