DFT Studies of the Adsorption of Propane and Propene on Metallic Surfaces in Ag/ZrO2 Catalysts as a Model for Catalytic Combustion Reactions of Light Hydrocarbons
Abstract
1. Introduction
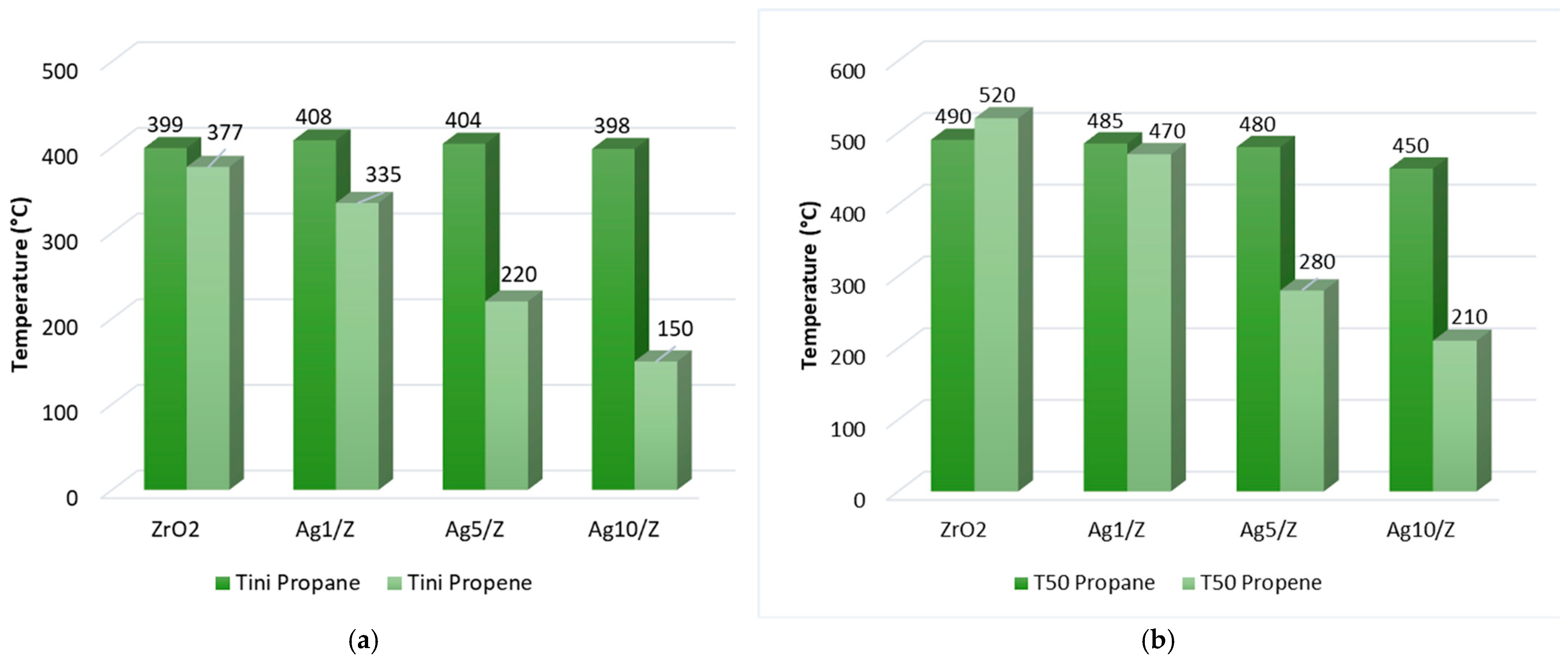
2. Results and Discussion
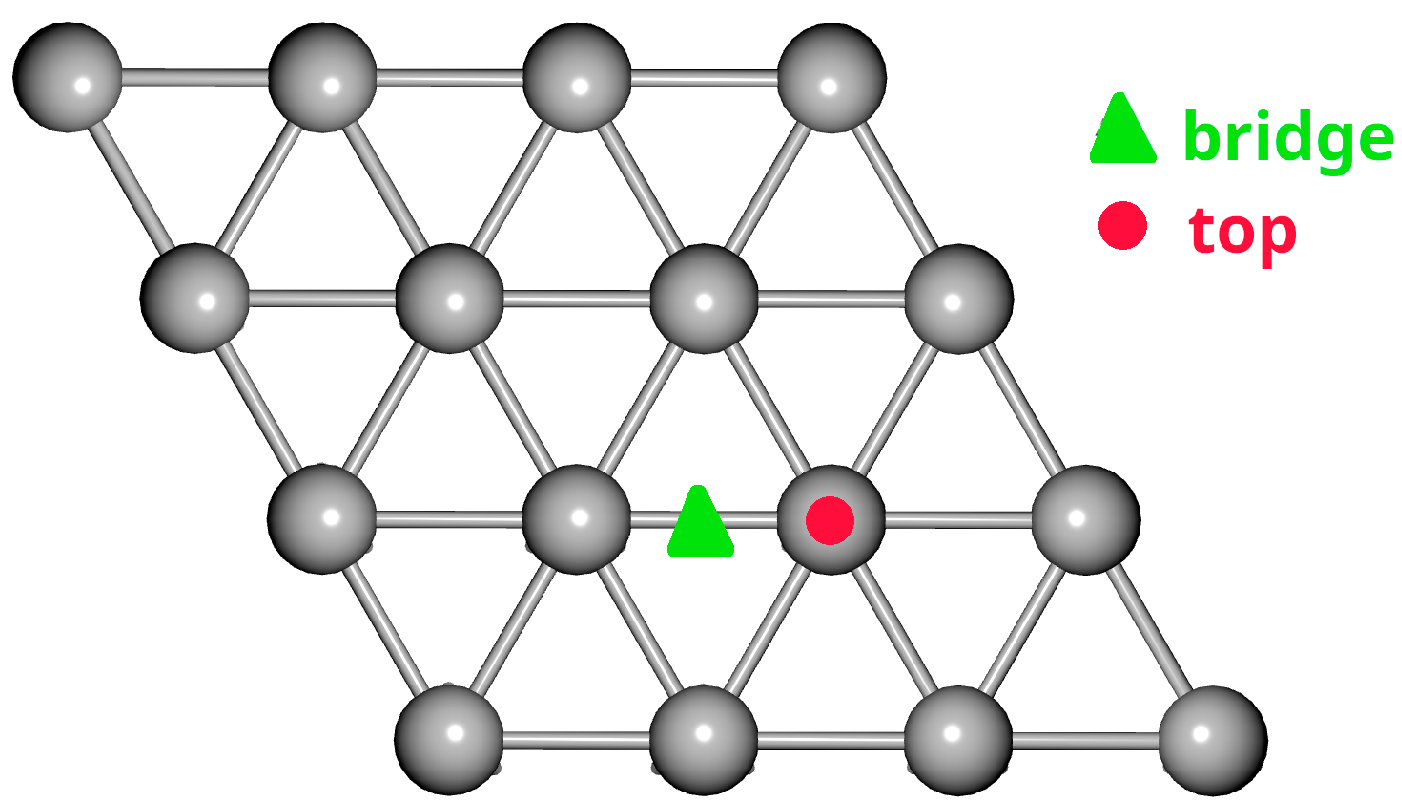
2.1. Adsorption on Ag(111)
2.2. Adsorption on Ag (111) in the Presence of Oxygen
2.3. Electronic Structure Studies
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V. Progress and Future Challenges in Controlling Automotive Exhaust Gas Emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Yang, A.-C.; Streibel, V.; Choksi, T.S.; Aljama, H.; Werghi, B.; Bare, S.R.; Sánchez-Carrera, R.S.; Schäfer, A.; Li, Y.; Abild-Pedersen, F.; et al. Insights and Comparison of Structure–Property Relationships in Propane and Propene Catalytic Combustion on Pd- and Pt-Based Catalysts. J. Catal. 2021, 401, 89–101. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for Combustion of Methane and Lower Alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Shelef, M.; McCabe, R.W. Twenty-Five Years after Introduction of Automotive Catalysts: What Next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Stratakis, G.A.; Stamatelos, A.M. Thermogravimetric Analysis of Soot Emitted by a Modern Diesel Engine Run on Catalyst-Doped Fuel. Combust. Flame 2003, 132, 157–169. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic Oxidation of Volatile Organic Compounds on Supported Noble Metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. Intrinsic Reactivity Analysis of Soot Removal in LNT-Catalysts. Appl. Catal. B Environ. 2016, 193, 110–120. [Google Scholar] [CrossRef]
- Demoulin, O.; Le Clef, B.; Navez, M.; Ruiz, P. Combustion of Methane, Ethane and Propane and of Mixtures of Methane with Ethane or Propane on Pd/γ-Al2O3 Catalysts. Appl. Catal. A Gen. 2008, 344, 1–9. [Google Scholar] [CrossRef]
- Montaña, M.; Leguizamón Aparicio, M.S.; Ocsachoque, M.A.; Navas, M.B.; de CL Barros, I.; Rodriguez-Castellón, E.; Casella, M.L.; Lick, I.D. Zirconia-Supported Silver Nanoparticles for the Catalytic Combustion of Pollutants Originating from Mobile Sources. Catalysts 2019, 9, 297. [Google Scholar] [CrossRef]
- Avila, M.S.; Vignatti, C.I.; Apesteguía, C.R.; Garetto, T.F. Effect of Support on the Deep Oxidation of Propane and Propylene on Pt-Based Catalysts. Chem. Eng. J. 2014, 241, 52–59. [Google Scholar] [CrossRef]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at Well-Defined Surfaces. Surf. Sci. 1994, 299–300, 742–754. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-Temperature Catalysis for VOCs Removal in Technology and Application: A State-of-the-Art Review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Langmuir, I. Part II.—“Heterogeneous Reactions”. Chemical Reactions on Surfaces. Trans. Faraday Soc. 1922, 17, 607–620. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations Carried out by Means of Vanadium Oxide Catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A Comparative Study of Cu, Ag and Au Doped CeO2 in the Total Oxidation of Volatile Organic Compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Hutchings, G.J.; Taylor, S.H.; Makkee, M. TAP Reactor Study of the Deep Oxidation of Propane Using Cobalt Oxide and Gold-Containing Cobalt Oxide Catalysts. Appl. Catal. A Gen. 2009, 365, 222–230. [Google Scholar] [CrossRef]
- Leguizamón Aparicio, M.S.; Ocsachoque, M.A.; Rodríguez-Castellón, E.; Gazzoli, D.; Casella, M.L.; Lick, I.D. Promoting Effect of Rhodium on Co/ZnAl2O4 Catalysts for the Catalytic Combustion of Hydrocarbons. Catal. Today 2021, 372, 2–10. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 Composite Oxides for Methane Emissions Abatement: Relationship between Co3O4–CeO2 Interaction and Catalytic Activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Feng, C.; Chen, C.; Wang, J.; Xiong, G.; Wang, Z.; Pan, Y.; Fei, Z.; Lu, Y.; Liu, Y.; Zhang, R.; et al. Total Oxidation of Propane in Ag-Doped MnCeOx Catalysts: The Role of Ag Species. Fuel 2023, 332, 126208. [Google Scholar] [CrossRef]
- Heynderickx, P.; Thybaut, J.; Poelman, H.; Marin, G. Kinetic Model Discrimination for Propane Total Oxidation over CuO-CeO2/g-Al2O3. In Proceedings of the Catalysis, 14th International Congress, Abstracts, Seoul, Republic of Korea, 13–18 July 2008. [Google Scholar]
- Yao, Y.-F.Y. The Oxidation of CO and Hydrocarbons over Noble Metal Catalysts. J. Catal. 1984, 87, 152–162. [Google Scholar] [CrossRef]
- Diehl, F.; Barbier, J.; Duprez, D.; Guibard, I.; Mabilon, G. Catalytic Oxidation of Heavy Hydrocarbons over Pt/Al2O3. Influence of the Structure of the Molecule on Its Reactivity. Appl. Catal. B Environ. 2010, 95, 217–227. [Google Scholar] [CrossRef]
- Zhou, Z.; Harold, M.P.; Luss, D. Enhanced NO, CO and C3H6 Conversion on Pt/Pd Catalysts: Impact of Oxygen Storage Material and Catalyst Architecture. Catal. Today 2021, 360, 375–387. [Google Scholar] [CrossRef]
- Ocsachoque, M.A.; Leguizamón-Aparicio, M.S.; Casella, M.L.; Lick, I.D. Promoting Effect of Palladium on ZnAl2O4-Supported Catalysts Based on Cobalt or Copper Oxide on the Activity for the Total Propene Oxidation. Materials 2021, 14, 4814. [Google Scholar] [CrossRef] [PubMed]
- Leguizamón Aparicio, M.S.; Ruiz, M.L.; Ocsachoque, M.A.; Ponzi, M.I.; Rodríguez-Castellón, E.; Lick, I.D. Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia. Catalysts 2020, 10, 387. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Y.; Deng, J.; Jing, L.; Wang, J.; Pei, W.; Dai, H. Bimetallic CoNi Single Atoms Supported on Three-Dimensionally Ordered Mesoporous Chromia: Highly Active Catalysts for n-Hexane Combustion. Green Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Shang, Z.; Cao, X.; Guo, Y.; Li, J.; Gu, Q.; Li, K.; Li, X. Regulating Potassium State to Enable the High Performance of Co3O4 for Catalytic Oxidation. Fuel 2023, 335, 126968. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, H.; Pan, T.; Wang, L.; Zhang, C.; He, H. Interaction between Noble Metals (Pt, Pd, Rh, Ir, Ag) and Defect-Enriched TiO2 and Its Application in Toluene and Propene Catalytic Oxidation. Appl. Surf. Sci. 2022, 606, 154834. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Jiang, G.; Zhang, Z.; Hao, Z. Heterogeneous Selective Oxidation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Appl. Catal. B Environ. 2023, 325, 122384. [Google Scholar] [CrossRef]
- Long, R.; Huang, H.; Li, Y.; Song, L.; Xiong, Y. Palladium-Based Nanomaterials: A Platform to Produce Reactive Oxygen Species for Catalyzing Oxidation Reactions. Adv. Mater. 2015, 27, 7025–7042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Ren, Z.; Qu, Y.; Zhang, Y.; Li, J. Promotional Effect of Pt-Doping on the Catalytic Performance of Pt−CeO2 Catalyst for CO Oxidation. Catalysts 2022, 12, 529. [Google Scholar] [CrossRef]
- Wang, X.; Rui, Z.; Ji, H. DFT Study of Formaldehyde Oxidation on Silver Cluster by Active Oxygen and Hydroxyl Groups: Mechanism Comparison and Synergistic Effect. Catal. Today 2020, 347, 124–133. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Farooq, A.; Yang, L.; Mebel, A.M. Combustion Chemistry of Alkenes and Alkadienes. Prog. Energy Combust. Sci. 2022, 90, 100983. [Google Scholar] [CrossRef]
- Yang, A.-C.; Choksi, T.; Streibel, V.; Aljama, H.; Wrasman, C.J.; Roling, L.T.; Goodman, E.D.; Thomas, D.; Bare, S.R.; Sánchez-Carrera, R.S.; et al. Revealing the Structure of a Catalytic Combustion Active-Site Ensemble Combining Uniform Nanocrystal Catalysts and Theory Insights. Proc. Natl. Acad. Sci. USA 2020, 117, 14721–14729. [Google Scholar] [CrossRef]
- Wu, S.; Tatarchuk, B.J.; Adamczyk, A.J. Ethylene Oxidation on Unpromoted Silver Catalysts: Reaction Pathway and Selectivity Analysis Using DFT Calculations. Surf. Sci. 2021, 708, 121834. [Google Scholar] [CrossRef]
- Lindgren, E.B.; Monteiro, J.G.S.; dos Santos, A.R.; Fleming, F.P.; Barbosa, A.G.H. Initiation Mechanisms and Kinetics of the Combustion of Cyclopentane and Cyclopentene from ReaxFF Molecular Dynamics. Fuel 2021, 303, 121205. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, Z.; White, J.M. Distinct Oxidation Behaviors of π-Bonded and Di-σ-Bonded Propylene on Ag(111). Catal. Today 2008, 131, 360–366. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Yang, M.-L.; Zhu, J.; Zhu, Y.-A.; Sui, Z.-J.; Yu, Y.-D.; Zhou, X.-G.; Chen, D. Tuning Selectivity and Stability in Propane Dehydrogenation by Shaping Pt Particles: A Combined Experimental and DFT Study. J. Mol. Catal. A Chem. 2014, 395, 329–336. [Google Scholar] [CrossRef]
- Arya, M.; Mirzaei, A.A.; Davarpanah, A.M.; Barakati, S.M.; Atashi, H.; Mohsenzadeh, A.; Bolton, K. DFT Studies of Hydrocarbon Combustion on Metal Surfaces. J. Mol. Model. 2018, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.N.; Lin, Z.; Chen, J.G. Experimental and Theoretical Studies of Reaction Pathways of Direct Propylene Epoxidation on Model Catalyst Surfaces. Surf. Sci. Rep. 2021, 76, 100524. [Google Scholar] [CrossRef]
- Burch, R.; Hayes, M.J. CH Bond Activation in Hydrocarbon Oxidation on Solid Catalysts. J. Mol. Catal. A Chem. 1995, 100, 13–33. [Google Scholar] [CrossRef]
- Tomita, A.; Miki, T.; Tai, Y. Effect of Water Treatment and Fe Doping on Pt Sintering and the Propane Oxidation Activity of Pt/Al2O3. Appl. Catal. A Gen. 2016, 522, 138–144. [Google Scholar] [CrossRef]
- Lian, Z.; Ali, S.; Liu, T.; Si, C.; Li, B.; Su, D.S. Revealing the Janus Character of the Coke Precursor in the Propane Direct Dehydrogenation on Pt Catalysts from a KMC Simulation. ACS Catal. 2018, 8, 4694–4704. [Google Scholar] [CrossRef]
- Yang, M.-L.; Zhu, Y.-A.; Fan, C.; Sui, Z.-J.; Chen, D.; Zhou, X.-G. DFT Study of Propane Dehydrogenation on Pt Catalyst: Effects of Step Sites. Phys. Chem. Chem. Phys. 2011, 13, 3257–3267. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; He, G.; Wang, F.; Xu, G.; Li, Y.; Zhang, C.; He, H. Specific Role of Potassium in Promoting Ag/Al2O3 for Catalytic Oxidation of Formaldehyde at Low Temperature. J. Phys. Chem. C 2018, 122, 27331–27339. [Google Scholar] [CrossRef]
- Hinsch, J.J.; Liu, J.; Wang, Y. Reinvestigating Oxygen Adsorption on Ag(111) by Using Strongly Constrained and Appropriately Normed Semi-Local Density Functional with the Revised Vydrov van Voorhis van Der Waals Force Correction. J. Chem. Phys. 2021, 155, 234704. [Google Scholar] [CrossRef]
- Garetto, T.F.; Rincón, E.; Apesteguía, C.R. Deep Oxidation of Propane on Pt-Supported Catalysts: Drastic Turnover Rate Enhancement Using Zeolite Supports. Appl. Catal. B Environ. 2004, 48, 167–174. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Hu, C.; Ting, S.-W.; Chan, K.-Y.; Huang, W. Reaction Pathways Derived from DFT for Understanding Catalytic Decomposition of Formic Acid into Hydrogen on Noble Metals. Int. J. Hydrog. Energy 2012, 37, 15956–15965. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density Functional Theory in Surface Chemistry and Catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Pascucci, B.; Otero, G.S.; Belelli, P.G.; Illas, F.; Branda, M.M. Comparative Density Functional Theory Based Study of the Reactivity of Cu, Ag, and Au Nanoparticles and of (111) Surfaces toward CO Oxidation and NO2 Reduction. J. Mol. Model. 2014, 20, 2448. [Google Scholar] [CrossRef]
- Gao, W.; Keith, J.A.; Anton, J.; Jacob, T. Oxidation of Formic Acid on the Pt(111) Surface in the Gas Phase. Dalton Trans. 2010, 39, 8450–8456. [Google Scholar] [CrossRef]
- Yang, M.-L.; Zhu, Y.-A.; Fan, C.; Sui, Z.-J.; Chen, D.; Zhou, X.-G. Density Functional Study of the Chemisorption of C1, C2 and C3 Intermediates in Propane Dissociation on Pt(111). J. Mol. Catal. A Chem. 2010, 321, 42–49. [Google Scholar] [CrossRef]
- Maurer, R.J.; Ruiz, V.G.; Camarillo-Cisneros, J.; Liu, W.; Ferri, N.; Reuter, K.; Tkatchenko, A. Adsorption Structures and Energetics of Molecules on Metal Surfaces: Bridging Experiment and Theory. Prog. Surf. Sci. 2016, 91, 72–100. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
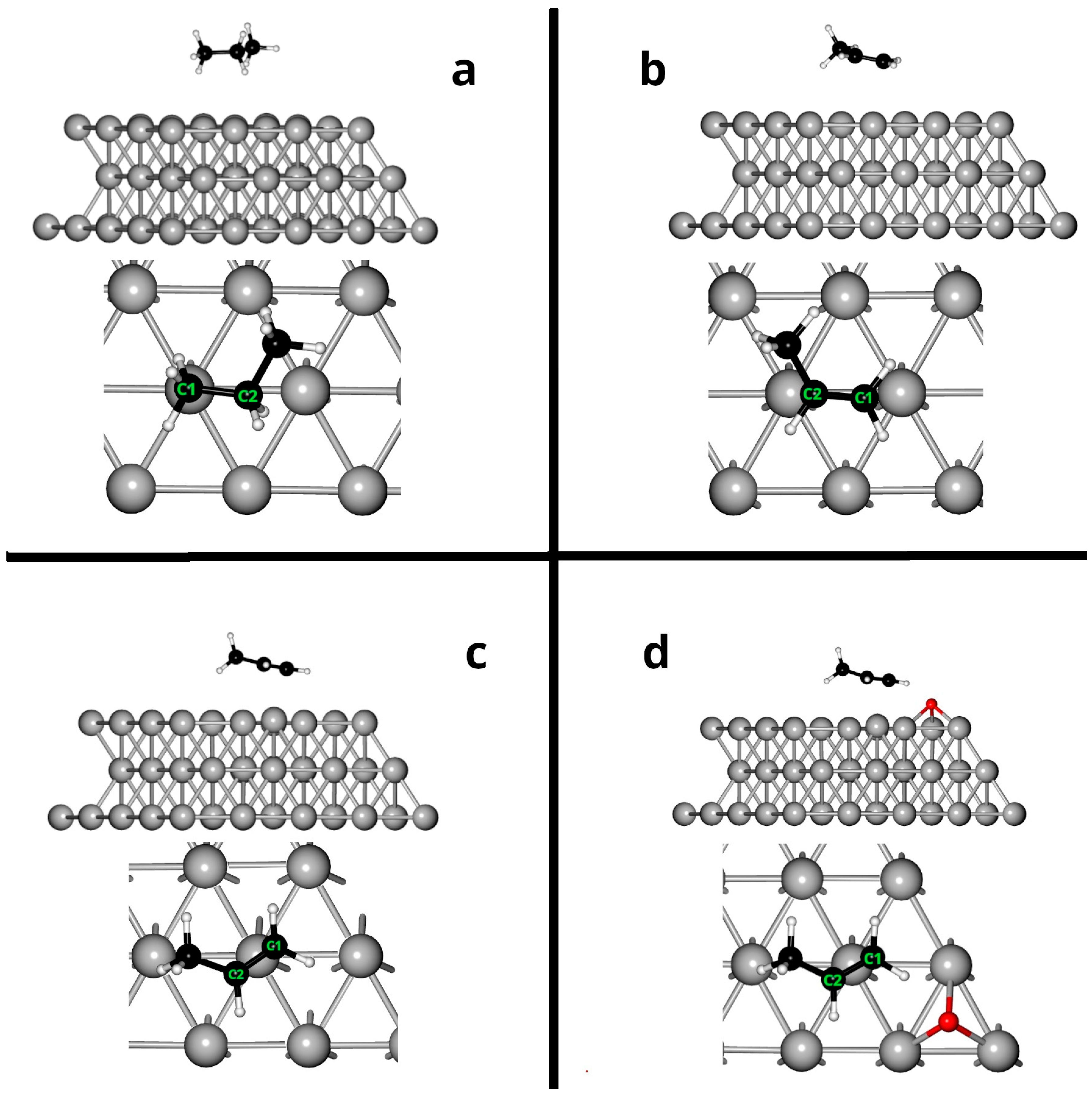

| Distances C1–Ag (Å) | Distances C2–Ag (Å) | Bond Length C1–C2 Free (Å) | Bond Length C1–Adsorbed C2 (Å) | Eads (eV) | |
|---|---|---|---|---|---|
| Propane | 3.57 | 3.87 | 1.52 | 1.52 | −0.14 |
| Propene bridge | 3.10 | 3.25 | 1.34 | 1.34 | −0.21 |
| Propene top | 2.58 | 2.74 | 1.34 | 1.35 | −0.77 |
| Propene O | 2.50 | 2.66 | 1.34 | 1.36 | −0.87 |
| Ag1/Z | Ag5/Z | Ag10/Z | |
|---|---|---|---|
| KE AgM4N45N45 | - | 357.5 | 357.9 |
| KE AgM5N45N45 | - | 352.5 (97) * | 352.8 (94) |
| 349.5 (3) | 349.4 (6) | ||
| Ag/Zr atomic ratio | 0.009 | 0.154 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggera, J.F.; Ocsachoque, M.A.; Montaña, M.; Casella, M.L.; Lick, I.D. DFT Studies of the Adsorption of Propane and Propene on Metallic Surfaces in Ag/ZrO2 Catalysts as a Model for Catalytic Combustion Reactions of Light Hydrocarbons. Catalysts 2023, 13, 1068. https://doi.org/10.3390/catal13071068
Ruggera JF, Ocsachoque MA, Montaña M, Casella ML, Lick ID. DFT Studies of the Adsorption of Propane and Propene on Metallic Surfaces in Ag/ZrO2 Catalysts as a Model for Catalytic Combustion Reactions of Light Hydrocarbons. Catalysts. 2023; 13(7):1068. https://doi.org/10.3390/catal13071068
Chicago/Turabian StyleRuggera, José F., Marco A. Ocsachoque, Maia Montaña, Mónica L. Casella, and Ileana D. Lick. 2023. "DFT Studies of the Adsorption of Propane and Propene on Metallic Surfaces in Ag/ZrO2 Catalysts as a Model for Catalytic Combustion Reactions of Light Hydrocarbons" Catalysts 13, no. 7: 1068. https://doi.org/10.3390/catal13071068
APA StyleRuggera, J. F., Ocsachoque, M. A., Montaña, M., Casella, M. L., & Lick, I. D. (2023). DFT Studies of the Adsorption of Propane and Propene on Metallic Surfaces in Ag/ZrO2 Catalysts as a Model for Catalytic Combustion Reactions of Light Hydrocarbons. Catalysts, 13(7), 1068. https://doi.org/10.3390/catal13071068







