Copper-Catalyzed N-Arylation of Pyranoquinolinones with Boronic Acids at Room Temperature without Ligand
Abstract
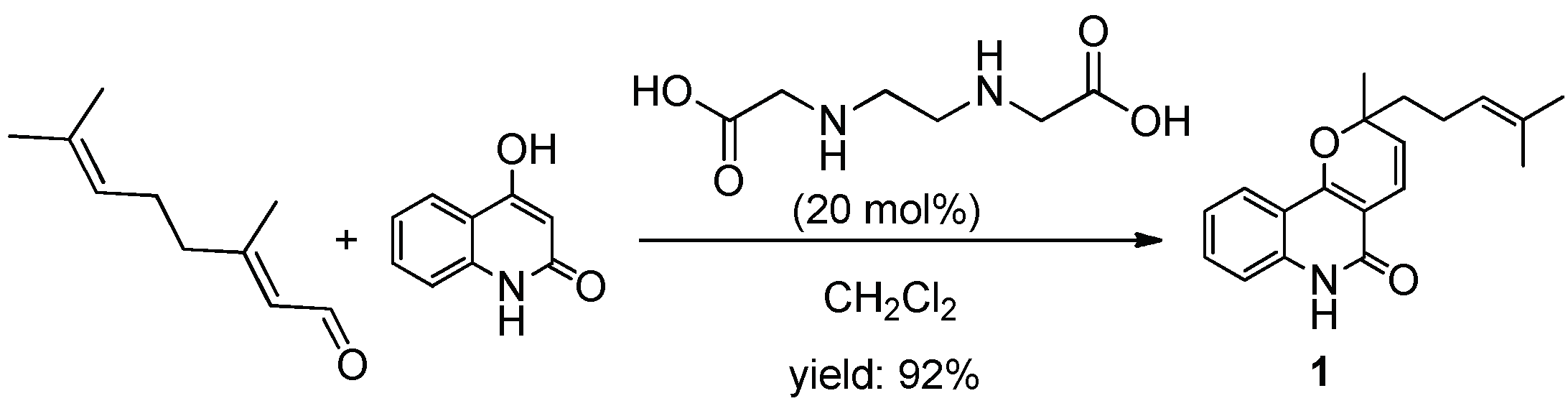
1. Introduction
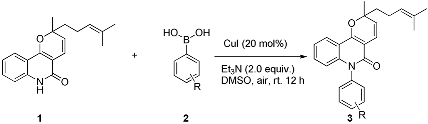
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the N-Arylation of Pyranoquinolinones with Boronic Acids
3.3. Characterization Data for Products 3a–3y
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamle, M.; Mahato, D.K.M.; Lee, K.E.; Bajpai, V.K.; Gajurel, P.R.; Gu, K.S.; Kumar, P. Ethnopharmacological properties and medicinal uses of litsea cubeba. Plants 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.H.; Zhao, Y.; Lu, Z.J.; Xing, R.L.; Yao, X.M.; Jin, Z.X.; Wang, Y.Y.; Yu, F. Citral-loaded chitosan/carboxymethyl cellulose copolymer hydrogel microspheres with improved antimicrobial effects for plant protection. Int. J. Biol. Macromol. 2020, 164, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Jing, G.X.; Wang, X.; Ouyang, Q.L.; Jia, L.; Tao, N.G. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K. Anticancer effect of lemograss oil and citral on cervical cancer cell lines. Pharmacogn. Commun. 2013, 3, 41–48. [Google Scholar]
- Yun, H.; Xiu, W.; Wengui, D.; Guishan, L.; Bo, C.; Fuhou, L. Synthesis and antifungal activity of citral-based 1,2,3-triazole compounds. Fine Chem. 2021, 38, 640–648. [Google Scholar]
- Jin, X.X.; Wang, J.T.; Bai, J. Synthesis and antibacterial activity of schiff base from chitosan and citral. Chem. Ind. Eng. Prog. 2009, 28, 2014–2018. [Google Scholar]
- Narasimhan, S.; Ravi, S.; Vijayakumar, B. Synthesis and antibacterial activities of citral derivatives. Int. J. Chem. Appl. 2011, 3, 229–235. [Google Scholar]
- Grundon, M.F. The Alkaloids: Quinoline Alkaloids Related to Anthranilic Acid; Academic Press: London, UK, 1988; Volume 32, p. 341. [Google Scholar]
- Ulubelen, A.; Mericli, A.H.; Mericli, F.; Kaya, Ü. An alkaloid and lignans from Haplophyllum telephioides. Phytochemistry 1994, 35, 1600–1601. [Google Scholar] [CrossRef]
- Campbell, W.E.; Davidowitz, B.; Jackson, G.E. Quinolinone alkaloids from an agathosma species. Phytochemistry 1990, 29, 1303–1306. [Google Scholar] [CrossRef]
- Khalid, S.A.; Waterman, P.G. Alkaloids from stem barks of Oricia renieri and Oricia gabonensis. Phytochemistry 1981, 20, 2761–2763. [Google Scholar] [CrossRef]
- Hifnawy, M.S.; Vaquette, J.; Sévenet, T.; Pousset, J.-L.; Cavé, A. Produits neutres et alcaloides de Myrtopsis macrocarpa, M. myrtoidea, M. novae-caledoniae et M. sellingii. Phytochemistry 1977, 16, 1035–1039. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Sharifi, I.A. Alkaloids of Zanthoxylum monophyllum and Z. punctatum. Phytochemistry 1977, 16, 2003–2006. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1995, 12, 77–89. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1999, 16, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Wu, S.-J.; Tsai, I.J.; Wu, T.-S.; Pezzuto, J.M.; Lu, M.C.; Chai, H.; Suh, N.; Teng, C.-M. Chemical and bioactive constituents from Zanthoxylum simulans. J. Nat. Prod. 1994, 57, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.A.; Neville, C.F.; Grundon, M.F.; Boyd, D.R.; Malone, J.F.; Evans, T.A. ChemInform abstract: Quinolinone cycloaddition as a potential synthetic route to dimeric quinoline alkaloids. J. Chem. Soc. Perkin Trans. 1 1995, 445–452. [Google Scholar] [CrossRef]
- Asherson, J.L.; Young, D.W. Cheminform abstract: A general and practicable synthesis of polycyclic heteroaromatic compounds. part 1. use of a putative quinolone-quinone-methide in the synthesis of polycyclic heteroaromatic compounds. J. Chem. Soc. Perkin Trans. 1 1980, 512–521. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Ayafor, J.F.; Bilon, A.E.N.; Sondengam, B.L.; Connolly, J.D.; Rycroft, D.S. Synthesis of veprisine dimers and the formation of a novel cyclic tetramer from precocene I. Tetrahedron 1992, 48, 8711–8724. [Google Scholar] [CrossRef]
- Clark, E.A.; Grundon, M.F. The synthesis of lunasia alkaloids. part I. (±)-Lunacridine and (±)-lunacrine. J. Chem. Soc. 1964, 438–445. [Google Scholar] [CrossRef]
- Grundon, M.F. The biosynthesis of aromatic hemiterpenes. Tetrahedron 1978, 34, 143–161. [Google Scholar] [CrossRef]
- Ramesh, M.; Mohan, P.S.; Shanmugam, P. A convenient synthesis of flindersine, atanine and their analogues. Tetrahedron 1984, 40, 4041–4049. [Google Scholar] [CrossRef]
- Reisch, J.; Bathe, A.; Rosenthal, B.H.W.; Salehi-Artimani, R.A. Acetylenchemie. 4 mitt. synthese von haplophyllin und N-methylflindersin, zwei wege zu N-funktio-nalisierten pyrano[3,2-c]chinolin-alkaloiden. J. Heterocycl. Chem. 1987, 24, 869–872. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Choudhury, P.K. Regioselective synthesis of flindersine analogues. Synth. Commun. 1993, 23, 1087–1100. [Google Scholar] [CrossRef]
- Huffman, J.W.; Hsu, T.M. A one step synthesis of flindersine. Tetrahedron Lett. 1972, 13, 141–143. [Google Scholar] [CrossRef]
- Ye, J.-H.; Ling, K.-Q.; Zhang, Y.; Li, N.; Xu, J.-H. Syntheses of 2-hydroxypyrano[3,2-c]quinolin-5-ones from 4-hydroxyquinolin-2-ones by tandem knoevenagel condensation with aldehyde and Michael addition of enamine with the quinone methide-thermo- and photochemical approaches. J. Chem. Soc. Perkin Trans. 1 1999, 2017–2024. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kweon, H.I.; Koh, W.S.; Min, K.R.; Kim, Y.; Lee, S.H. One-pot preparation of pyranoquinolinones by Ytterbium(III) trifluoromethanesulfonate-catalyzed reactions: Efficient synthesis of flindersine, N-methylflindersine, and zanthosimuline natural products. Synthesis 2001, 2001, 1851–1855. [Google Scholar] [CrossRef]
- Wang, X.; Lee, Y.R. Efficient synthesis of substituted pyranoquinolinones from 2,4-dihydroxyquinoline: Total synthesis of zanthosimuline, cis-3′,4′-Dihydroxy-3′,4′-dihydroflindersine, and orixalone D. Synthesis 2007, 19, 3044–3050. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. The complementary competitors: Palladium and copper in C-N cross-coupling reactions. Organometallics 2012, 31, 7753–7808. [Google Scholar] [CrossRef]
- Bariwal, J.; Eycken, E.V. C-N bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Fisher, C.; Koenig, B. Palladium- and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds. Beilstein J. Org. Chem. 2011, 7, 59–74. [Google Scholar] [CrossRef]
- Sadig, J.E.R.; Willis, M.C. Palladium- and copper-catalyzed aryl halide amination, etherification and thioetherification reactions in the synthesis of aromatic heterocycles. Synthesis 2011, 2011, 1–22. [Google Scholar] [CrossRef]
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef] [PubMed]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Sambiago, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 2008, 41, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Surry, D.S.; Buchwald, S.L. Dialkylbiaryl phosphines in Pd-catalyzed amination: A user’s guide. Chem. Sci. 2011, 2, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.M.T.; Monaco, K.L.; Wang, R.-P.; Winters, M.P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. [Google Scholar] [CrossRef]
- Evans, D.A.; Katz, J.L.; West, T.R. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. an expedient synthesis of thyroxine. Tetrahedron Lett. 1998, 39, 2937–2940. [Google Scholar] [CrossRef]
- Lam, P.Y.S.; Clark, C.G.; Saubern, S.; Adams, J.; Winters, M.P.; Chan, D.M.T.; Combs, A. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2944. [Google Scholar] [CrossRef]
- Qiao, J.X.; Lam, P.Y.S. Copper-promoted carbon-heteroatom bond cross-coupling with boronic acids and derivatives. Synthesis 2011, 2011, 829–856. [Google Scholar] [CrossRef]
- Sanjeeva Rao, K.; Wu, T.-S. Chan-Lam coupling reactions: Synthesis of heterocycle. Tetrahedron 2012, 68, 7735–7754. [Google Scholar] [CrossRef]
- Yoo, W.-J.; Tsukamoto, T.; Kobayashi, S. Visible-light-mediated Chan-Lam coupling reactions of aryl boronic acids and aniline derivatives. Angew. Chem. Int. Ed. 2015, 54, 6587–6590. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Kim, U.B.; Sung, D.-B.; Kim, W.-S. A synthetic approach to N-aryl carbamates via copper-catalyzed Chan-Lam coupling at room temperature. J. Org. Chem. 2015, 80, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Umemoto, H.; Miki, Y.; Nakamura, A.; Maegawa, T. [Cu(OH)(TMEDA)]2Cl2-catalyzed regioselective 2-arylation of 5-substituted tetrazoles with boronic acids under mild conditions. J. Org. Chem. 2014, 79, 6073–6707. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Li, Y.; Ding, J.-Y.; Dong, D.-W.; Han, F.-S. The development of copper-catalyzed aerobic oxidative coupling of H-tetrazoles with boronic acids and an insight into the reaction mechanism. Chem. Eur. J. 2014, 20, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.; Molander, G.A. Copper(II)-mediated O-arylation of protected serines and threonines. Org. Lett. 2014, 16, 4944–4947. [Google Scholar] [CrossRef]
- Huang, F.; Quach, T.D.; Batey, R.A. Copper-catalyzed nondecarboxylative cross coupling of alkenyltrifluoroborate salts with carboxylic acids or carboxylates: Synthesis of enol esters. Org. Lett. 2013, 15, 3150–3153. [Google Scholar] [CrossRef] [PubMed]
- Racine, E.; Monnier, F.; Vors, J.-P.; Taillefer, M. Direct N-cyclopropylation of secondary acyclic amides promoted by copper. Chem. Commun. 2013, 49, 7412–7414. [Google Scholar] [CrossRef]
- Li, J.; Neuville, L. Copper-catalyzed oxidative three-component synthesis of N, N′,N″-trisubstituted guanidines. Org. Lett. 2013, 15, 6124–6127. [Google Scholar] [CrossRef]
- Raghuvanshi, D.S.; Gupta, A.K.; Singh, K.N. Nickel-mediated N-arylation with arylboronic acids: An avenue to Chan-Lam coupling. Org. Lett. 2012, 14, 4326–4329. [Google Scholar] [CrossRef]
- Rossi, S.A.; Shimkin, K.W.; Xu, Q.; Mori-Quiroz, L.M.; Watson, D.A. Selective formation of secondary amides via the copper-catalyzed cross-coupling of alkylboronic acids with primary amides. Org. Lett. 2013, 15, 2314–2317. [Google Scholar] [CrossRef] [PubMed]
- Sueki, S.; Kuninobu, Y. Copper-catalyzed N- and O-alkylation of amines and phenols using alkylborane reagents. Org. Lett. 2013, 15, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Che, L.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. A mild copper-catalyzed aerobic oxidative thiocyanation of arylboronic acids with TMSNCS. Org. Biomol. Chem. 2015, 13, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Rao, D.N.; Das, P. Copper-catalyzed inter- and intramolecular C-N bond formation: Synthesis of benzimidazole-fused heterocycles. J. Org. Chem. 2015, 80, 9321–9327. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.N.; Rasheed, S.; Vishwakarma, R.A.; Das, P. Copper-catalyzed sequential N-arylation of C-amino-NH-azoles. Chem. Commun. 2014, 50, 12911–12914. [Google Scholar]
- Anil Kumar, K.; Kannaboina, P.; Dhaked, D.K.; Vishwakarma, R.A.; Bharatam, P.V.; Das, P. Cu-catalyzed arylation of the amino group in the indazole ring: Regioselective synthesis of pyrazolo-carbazoles. Org. Biomol. Chem. 2015, 13, 1481–1491. [Google Scholar] [CrossRef]
- Rasheed, S.; Rao, D.N.; Ranjith Kumar, K.; Aravinda, S.; Vishwakarma, R.A.; Das, P. C-N bond formation via Cu-catalyzed cross-coupling with boronic acids leading to methyl carbazole-3-carboxylate: Synthesis of carbazole alkaloids. RSC Adv. 2014, 4, 4960–4969. [Google Scholar] [CrossRef]
- Rao, D.N.; Rasheed, S.; Aravinda, S.; Vishwakarma, R.A.; Das, P. Base and ligand free copper-catalyzed N-arylation of 2-amino-N-heterocycles with boronic acids in air. RSC Adv. 2013, 3, 11472–11475. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kannaboina, P.; Jaladanki, C.K.; Bharatam, P.V.; Das, P. Copper-catalyzed N-arylation of tautomerizable heterocycles with boronic acids and its application to synthesis of oxygenated carbazoles. ChemistrySelect 2016, 3, 601–607. [Google Scholar] [CrossRef]
- Mederski, W.W.K.R.; Lefort, M.; Germann, M.; Kux, D. N-aryl heterocycles via coupling reactions with arylboronic acids. Tetrahedron 1999, 55, 12757–12770. [Google Scholar] [CrossRef]
 | ||||
| Entry | [Cu] | Base | Solvent | Yield b |
|---|---|---|---|---|
| 1 | Cu(OTf)2 | 1,10-Phen | DMSO | trace |
| 2 c | Cu(OAc)2 | 1,10-Phen | DMSO | 5% |
| 3 | Cu(OAc)2 | 1,10-Phen | DMSO | 10% |
| 4 | Cu(OAc)2 | Pyridine | DMSO | 16% |
| 5 | Cu(OAc)2 | K2CO3 | DMSO | 19% |
| 6 | Cu(OAc)2 | Et3N | DMSO | 25% |
| 7 | Cu(OAc)2 | Et3N | DCM | 11% |
| 8 | Cu(OAc)2 | Et3N and 1,10-Phen | DCM | 10% |
| 9 d | Cu(OAc)2 | Et3N and 1,10-Phen | DCM | trace |
| 10 | Cu(OAc)2 | Et3N | DCE | 13% |
| 11 | Cu(OAc)2 | Et3N | DMF | 18% |
| 12 | Cu(OAc)2 | Et3N | MeCN | trace |
| 13 | Cu(OAc)2 | Et3N | DMAc | 15% |
| 14 | Cu(OAc)2 | Et3N | MeOH | trace |
| 15 | Cu(OAc)2 | Et3N | EtOAc | trace |
| 16 | CuCl | Et3N | DMSO | 36% |
| 17 | CuBr | Et3N | DMSO | 42% |
| 18 | CuI | Et3N | DMSO | 76% |
| 19 | CuO | Et3N | DMSO | 18% |
| 20 | CuSO4 | Et3N | DMSO | trace |
| 21 | Cu(acac)2 | Et3N | DMSO | trace |
| 22 | CuF2 | Et3N | DMSO | 20% |
| 23 | CuI | Et3N | DCM | trace |
| 24 | CuI | Et3N | MeCN | trace |
| 25 e | CuI | Et3N | DMSO | 19% |
| 26 f | CuI | Et3N | DMSO | 9% |
| 27 g | CuI | Et3N | DMSO | 19% |
| 28 h | CuI | Et3N | DMSO | trace |
| 29 i | CuI | Et3N | DMSO | trace |
 |
 |
| Compound | IC50/μM | Compound | IC50/μM |
|---|---|---|---|
| 3a | 22.7 | 3n | 26.3 |
| 3b | 17.1 | 3o | 30.4 |
| 3c | 18.2 | 3p | 4.6 |
| 3d | 20.3 | 3q | 24.5 |
| 3e | 29.8 | 3r | 10.2 |
| 3f | 16.3 | 3s | 23.3 |
| 3g | 26.2 | 3t | 29.3 |
| 3h | 14.2 | 3u | 18.9 |
| 3i | 28.7 | 3v | 23.2 |
| 3j | 14.3 | 3w | 6.4 |
| 3k | 10.3 | 3x | 18.7 |
| 3l | 40.5 | 3y | 14.6 |
| 3m | 21.1 | cisplatin | 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Gao, F.; Yang, H.; Wang, Z.; Huang, Y. Copper-Catalyzed N-Arylation of Pyranoquinolinones with Boronic Acids at Room Temperature without Ligand. Catalysts 2023, 13, 1060. https://doi.org/10.3390/catal13071060
Gao W, Gao F, Yang H, Wang Z, Huang Y. Copper-Catalyzed N-Arylation of Pyranoquinolinones with Boronic Acids at Room Temperature without Ligand. Catalysts. 2023; 13(7):1060. https://doi.org/10.3390/catal13071060
Chicago/Turabian StyleGao, Wei, Fang Gao, Haikuan Yang, Zongde Wang, and Yaru Huang. 2023. "Copper-Catalyzed N-Arylation of Pyranoquinolinones with Boronic Acids at Room Temperature without Ligand" Catalysts 13, no. 7: 1060. https://doi.org/10.3390/catal13071060
APA StyleGao, W., Gao, F., Yang, H., Wang, Z., & Huang, Y. (2023). Copper-Catalyzed N-Arylation of Pyranoquinolinones with Boronic Acids at Room Temperature without Ligand. Catalysts, 13(7), 1060. https://doi.org/10.3390/catal13071060






