Synthesis of Dual Z-Scheme CuBi2O4/Bi2Sn2O7/Sn3O4 Photocatalysts with Enhanced Photocatalytic Performance for the Degradation of Tetracycline under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
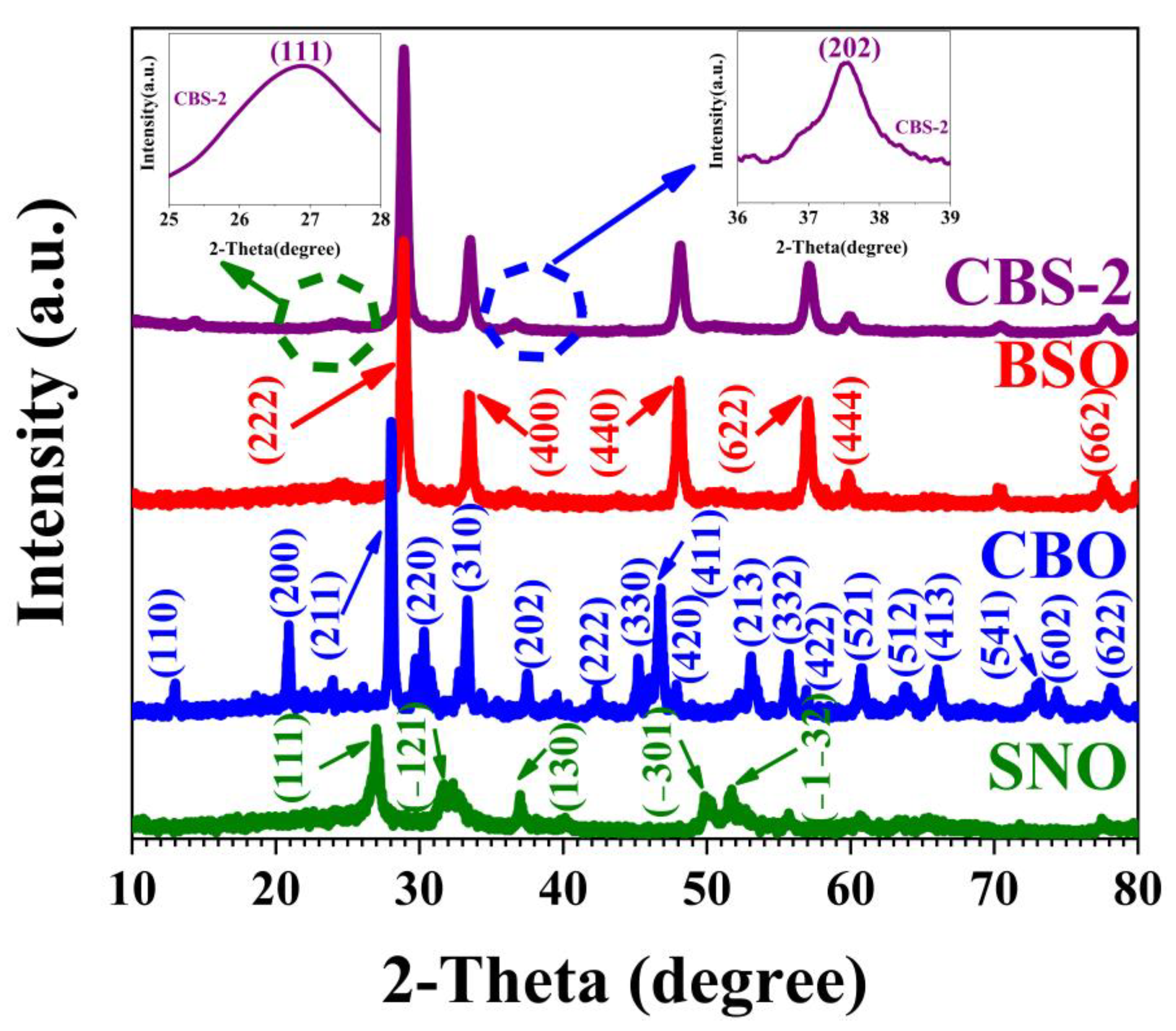
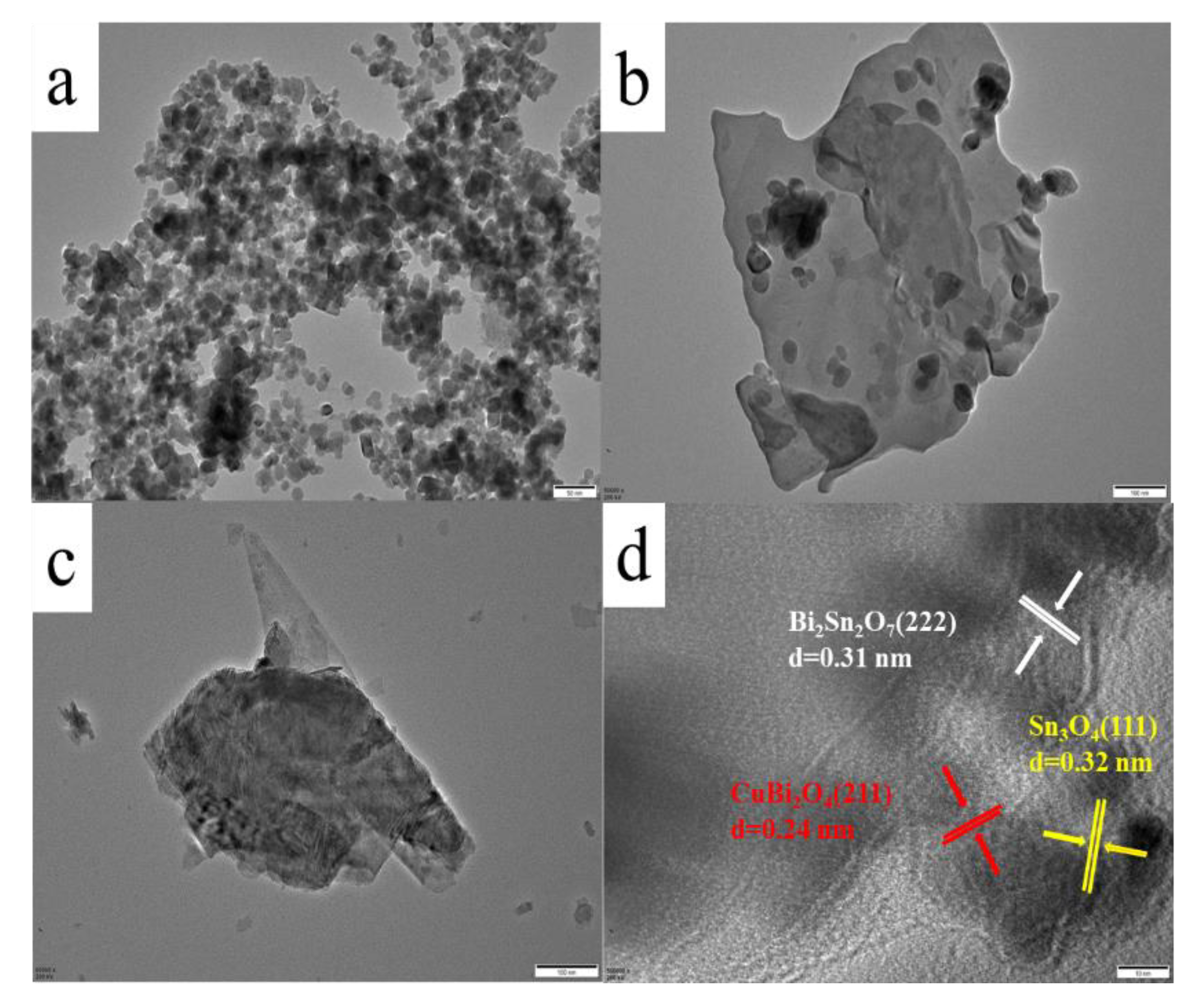
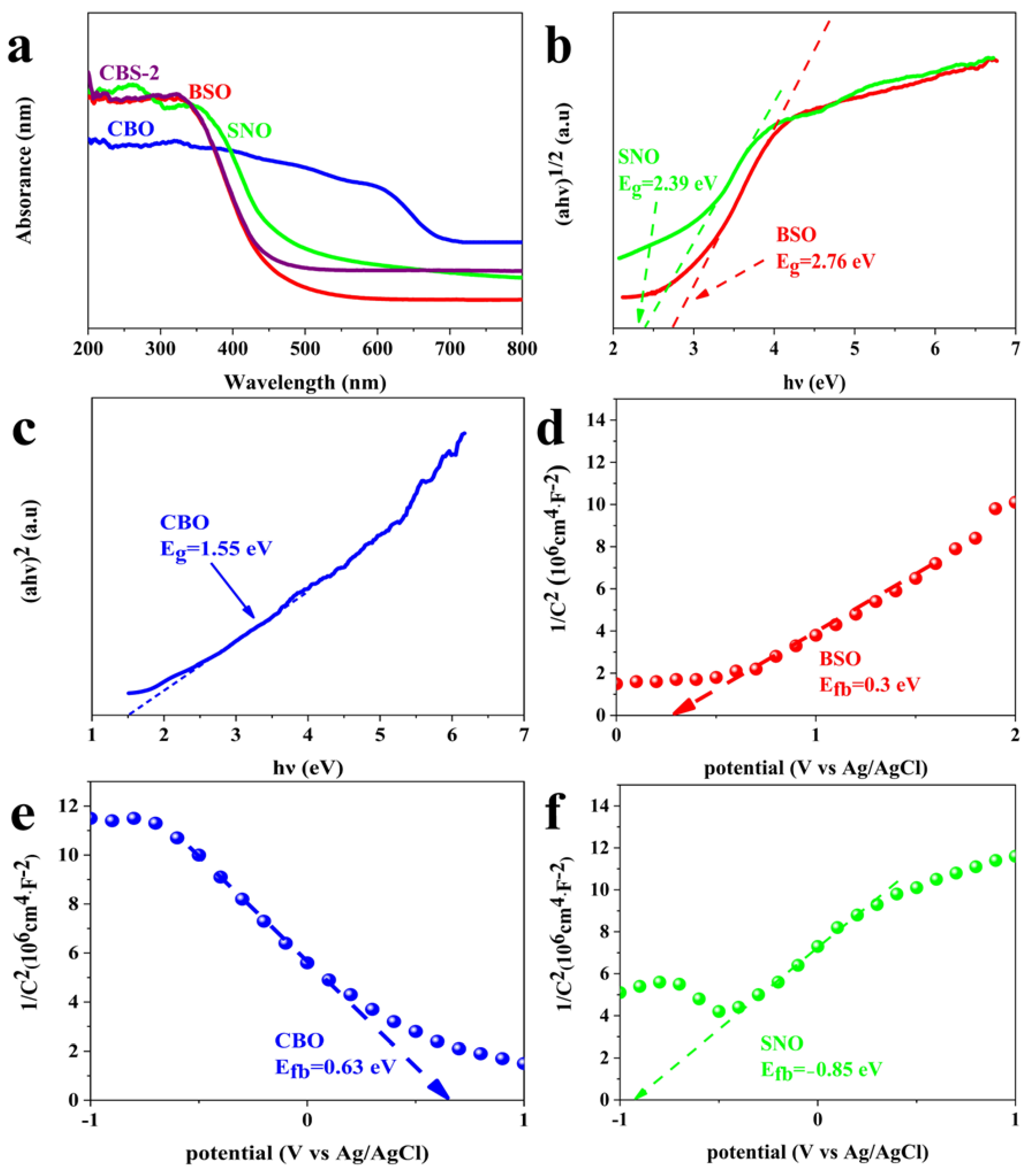
2.1. Characterization
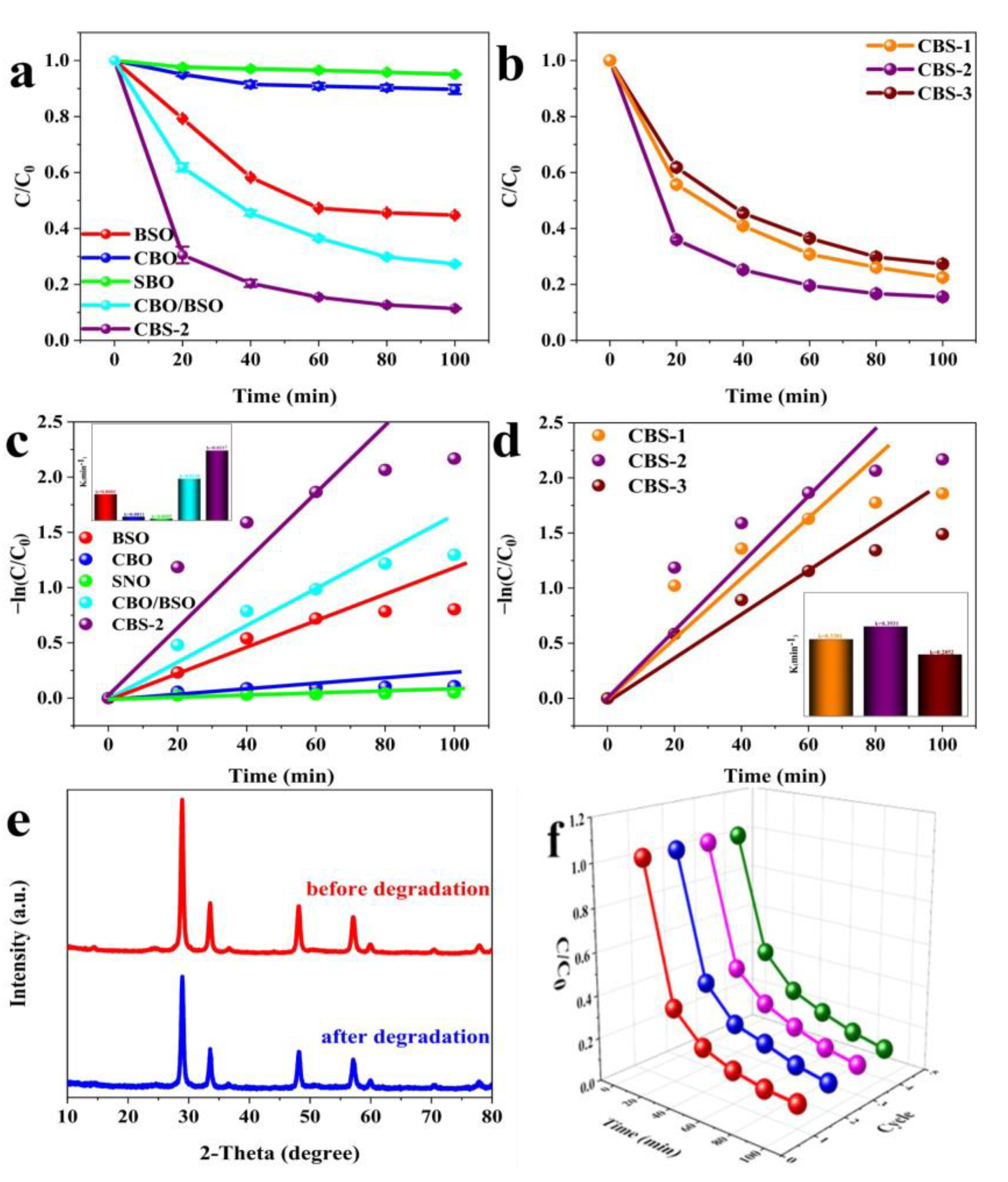
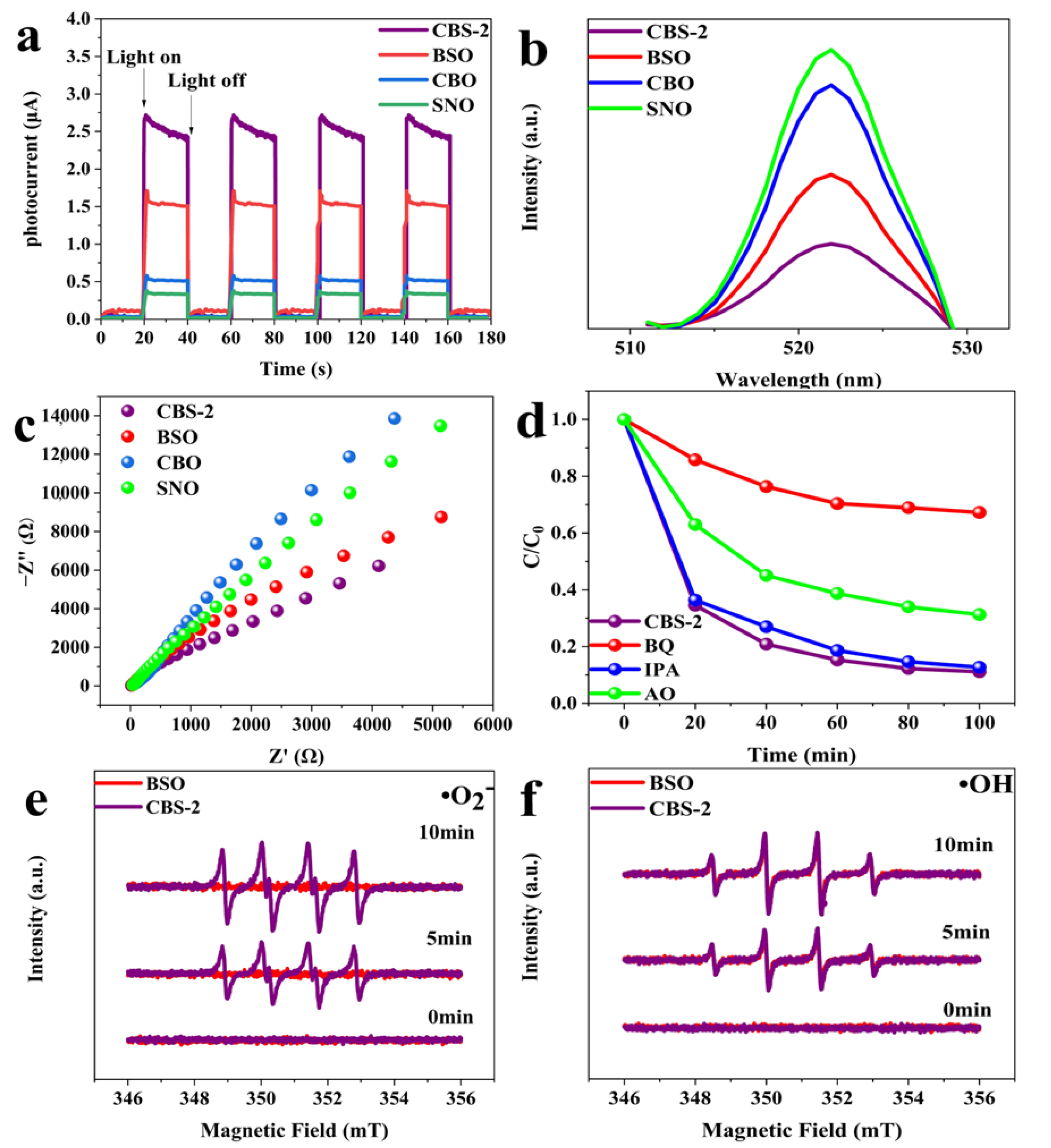
2.2. Photocatalytic Activity
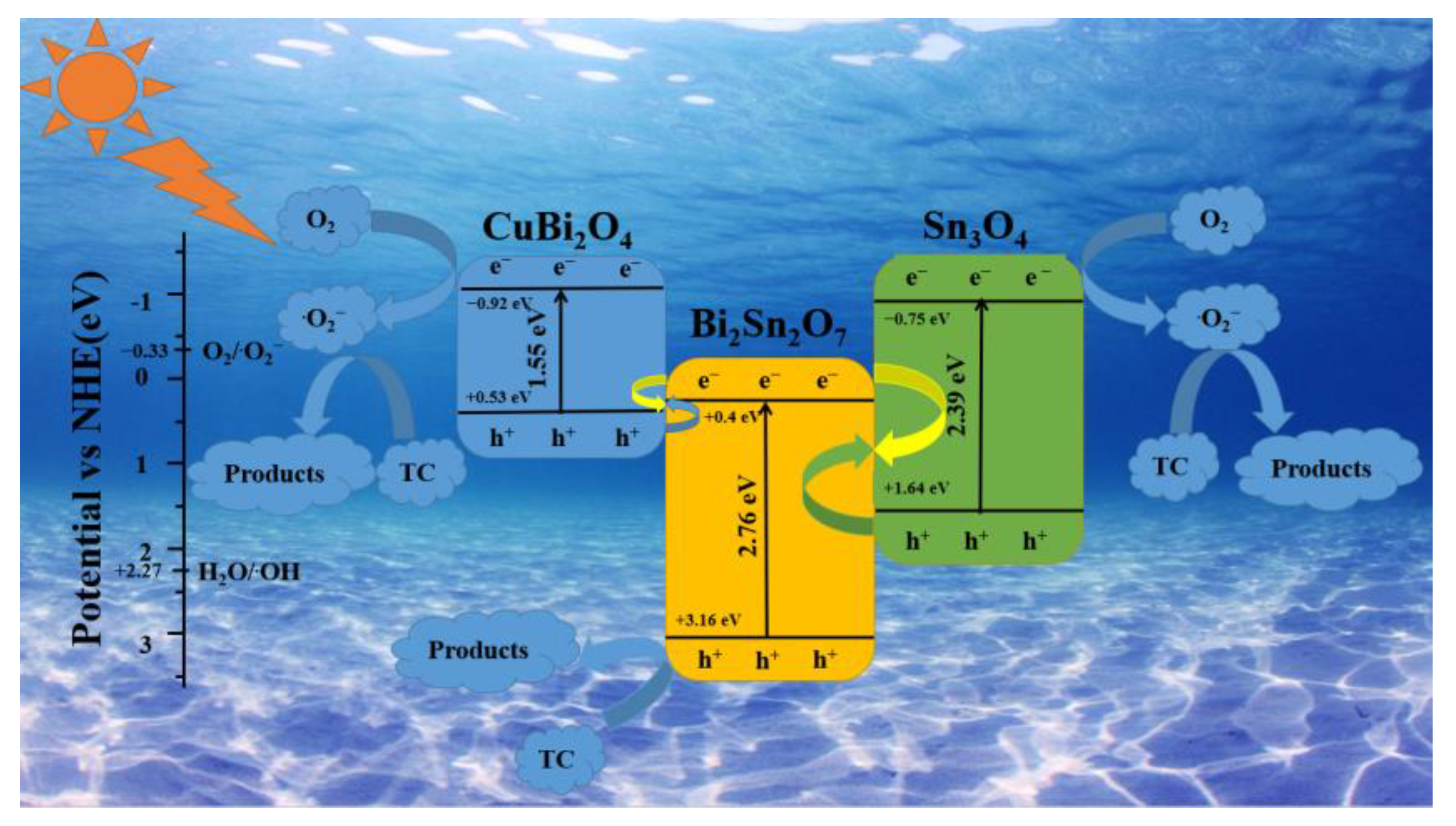
2.3. Photocatalytic Mechanism
3. Experimental
3.1. Preparation of Photocatalysts
3.1.1. Synthesis of BSO
3.1.2. Synthesis of CBO/BSO
3.1.3. Synthesis of CBS
3.2. Characterization Methods
3.3. Photocatalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Gao, M.; Zhou, Y.; Zhou, Y. Efficient photocatalytic remediation of typical antibiotics in water via Mn3O4 decorated carbon nitride nanotube. Chemosphere 2023, 311, 136925. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.; Ma, H.; Zhang, J.; Jiao, L.; Hao, H.; Liu, E.; Xu, L.; Wang, C.; Zhou, B. Dynamic construction of self-assembled supramolecular H12SubPcB-OPhCOOH/Ag3PO4 S-scheme arrays for visible photocatalytic oxidation of antibiotics. Appl. Catal. B Environ. 2022, 318, 121882. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, P.; Zhang, Y.; Sun, K.; Shi, X.; Li, L. The preparation of conjugated microporous polymer composite materials with montmorillonite template and its improvement in photocatalytic degradation for multiple antibiotics. Appl. Clay Sci. 2023, 231, 106752. [Google Scholar] [CrossRef]
- Chin, J.Y.; Ahmad, A.L.; Low, S.C. Evolution of photocatalytic membrane for antibiotics degradation: Perspectives and insights for sustainable environmental remediation. J. Water Process Eng. 2023, 51, 103342. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Chen, Z.; Gao, Y.; Teppen, B.; Boyd, S.A.; Zhang, W.; Tiedje, J.M.; Li, H. Tetracycline accumulation in biofilms enhances the selection pressure on Escherichia coli for expression of antibiotic resistance. Sci. Total Environ. 2023, 857, 159441. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, W.; Cui, M.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Li, L.; Du, Q. Filtration and adsorption of tetracycline in aqueous solution by copper alginate-carbon nanotubes membrane which has the muscle-skeleton structure. Chem. Eng. Res. Des. 2022, 183, 424–438. [Google Scholar] [CrossRef]
- Varadharajan, V.; Senthilkumar, D.S.; Senthilkumar, K.; Sundramurthy, V.P.; Manikandan, R.; Senthilarasan, H.; Ganesan, H.; Kesavamoorthy, I.; Ramasamy, A. Process modeling and toxicological evaluation of adsorption of tetracycline onto the magnetized cotton dust biochar. J. Water Process Eng. 2022, 49, 103046. [Google Scholar] [CrossRef]
- Han, K.; Liu, Y.; Hu, J.; Jia, J.; Sun, S. Effect of live and inactivated Chlamydomonas reinhardtii on the removal of tetracycline in aquatic environments. Chemosphere 2022, 309, 136666. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Shao, J.; Qian, G.; Adeleye, A.S.; Hao, T. Removal of tetracycline by aerobic granular sludge from marine aquaculture wastewater: A molecular dynamics investigation. Bioresour. Technol. 2022, 355, 127286. [Google Scholar] [CrossRef]
- Das, S.; Ahn, Y.-H. Synthesis and application of CdS nanorods for LED-based photocatalytic degradation of tetracycline antibiotic. Chemosphere 2022, 291, 132870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Liu, J.; Zhai, Z.; Yang, Z.; Xia, J.; Deng, S.; Qu, X.; Zhang, H.; Wu, D. Mo-modified band structure and enhanced photocatalytic properties of tin oxide quantum dots for visible-light driven degradation of antibiotic contaminants. J. Environ. Chem. Eng. 2022, 10, 107091. [Google Scholar] [CrossRef]
- Pei, Z.; Guo, H.; Zhu, L.; Li, C.; Fu, Z.; Xu, J. Photocatalytic degradation of various antibiotics under visible light irradiation by CdS-doped SiO2@ BiOX (X= Br, Cl) prepared by mixed solvothermal method. Mater. Sci. Eng. B 2023, 287, 116134. [Google Scholar] [CrossRef]
- Pan, J.; Wang, L.; Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. Construction of nanodiamonds/UiO-66-NH2 heterojunction for boosted visible-light photocatalytic degradation of antibiotics. Sep. Purif. Technol. 2022, 284, 120270. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Manzoor, S.; Ashiq, M.N.; Batool, S.; Hasan, M.; Iqbal, F. Multifunctional dual Z-scheme heterostructured Sm2O3-WO3-La2O3 nanocomposite: Enhanced electrochemical, photocatalytic, and antibacterial properties. Adv. Powder Technol. 2023, 34, 104061. [Google Scholar] [CrossRef]
- Yin, N.; Chen, H.; Yuan, X.; Zhang, Y.; Zhang, M.; Guo, J.; Zhang, Y.; Qiao, L.; Liu, M.; Song, K. Highly efficient photocatalytic degradation of norfloxacin via Bi2Sn2O7/PDIH Z-scheme heterojunction: Influence and mechanism. J. Hazard. Mater. 2022, 436, 129317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Di, J.; Zhu, X.; Ji, M.; Chen, C.; Liu, Y.; Li, L.; Wei, T.; Li, H.; Xia, J. Chemical Bonding Interface in Bi2Sn2O7/BiOBr S-Scheme Heterojunction Triggering Efficient N2 Photofixation. Appl. Catal. B Environ. 2022, 323, 122148. [Google Scholar] [CrossRef]
- Zhou, J.; Fan, G.; Ruan, F.; Li, Y.; Fan, D.; Chen, Q. Construction of Bi2Sn2O7/Ag/Ag3PO4 heterojunction and its photocatalytic degradation properties. J. Taiwan Inst. Chem. Eng. 2022, 138, 104443. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Hao, J.; Zhang, T.; Sun, Q.; Wang, Y. Boosted charge transfer in dual Z-scheme BiVO4@ ZnIn2S4/Bi2Sn2O7 heterojunctions: Towards superior photocatalytic properties for organic pollutant degradation. Chemosphere 2021, 276, 130226. [Google Scholar] [CrossRef]
- Wu, C.; Shen, Q.; Liu, J.; Jiang, L.; Sheng, J.; Li, Y.; Yang, H. Regulation of charge transfer in ZnO/Bi2Sn2O7 heterojunction for enhanced photocatalytic performance towards antibiotic degradation. J. Photochem. Photobiol. A Chem. 2022, 433, 114142. [Google Scholar] [CrossRef]
- Wu, C.; Shen, Q.; Zheng, S.; Zhang, X.; Sheng, J.; Yang, H. Fabrication of Bi2Sn2O7@ MIL-100 (Fe) composite photocatalyst with enhanced superoxide-radical-dominated photocatalytic activity for ciprofloxacin degradation. J. Mol. Struct. 2022, 1258, 132657. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, Y.; Wei, J.; Li, Z.; Liang, T.; Yu, Z.; Zhu, H.; Wang, S.; Hou, Y. Flower-like microspheres Z-scheme Bi2Sn2O7/NiAl-LDH heterojunction for boosting photocatalytic CO2 reduction under visible light. J. Colloid Interface Sci. 2023, 629, 604–615. [Google Scholar] [CrossRef]
- Zhu, P.; Lin, J.; Liu, M.; Duan, M.; Luo, D.; Wu, X.; Zhang, S. Nd2Sn2O7/Bi2Sn2O7/Ag3PO4 double Z-type heterojunction for antibiotic photodegradation under visible light irradiation: Mechanism, optimization and pathways. Sep. Purif. Technol. 2022, 300, 121897. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, D.; Pu, X.; Li, Y.; Yin, J.; Ma, H.; Dou, J. Facile synthesis of BiOBr/Bi2Sn2O7 heterojunction photocatalysts with improved photocatalytic activities. J. Am. Ceram. Soc. 2016, 99, 3973–3979. [Google Scholar] [CrossRef]
- Wu, X.-F.; Sun, Y.; Li, H.; Wang, Y.-J.; Zhang, C.-X.; Zhang, J.-R.; Su, J.-Z.; Wang, Y.-W.; Zhang, Y.; Wang, C. In-situ synthesis of novel pn heterojunction of Ag2CrO4-Bi2Sn2O7 hybrids for visible-light-driven photocatalysis. J. Alloys Compd. 2018, 740, 1197–1203. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, H.; Li, H.; Han, S. Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin. Inorganics 2022, 10, 131. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Hao, J.; Zheng, S.; Yang, Y.; Yao, T.; Wang, Y. Construction of Z-scheme heterojunction by coupling Bi2Sn2O7 and BiOBr with abundant oxygen vacancies: Enhanced photodegradation performance and mechanism insight. J. Colloid Interface Sci. 2022, 612, 550–561. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chai, J.; Xue, S.; Wang, R.; Jiang, L.; Wang, J.; Zhang, Z.; Dionysiou, D.D. Construction of novel symmetric double Z-scheme BiFeO3/CuBi2O4/BaTiO3 photocatalyst with enhanced solar-light-driven photocatalytic performance for degradation of norfloxacin. Appl. Catal. B Environ. 2020, 272, 119017. [Google Scholar] [CrossRef]
- Chen, J.; Yao, L.; Chen, X.; Li, N.; Yu, C.; Zhang, Y.; Lai, Y. Hydrothermal synthesis of dendritic CuBi2O4 and its photocatalytic performance towards tetracycline degradation under different light conditions. Mater. Sci. Semicond. Process. 2022, 142, 106503. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Yuan, S. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, M.; Luo, B.; Yang, Z.; Li, W.; Zhang, D.; Pu, X.; Cai, P. Magnetically recoverable flower-like Sn3O4/SnFe2O4 as a type-II heterojunction photocatalyst for efficient degradation of ciprofloxacin. J. Alloys Compd. 2022, 926, 166878. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Qu, Z.; Piao, C.; Liu, J.; Xu, D.; Li, X.; Wang, J.; Song, Y. An all-solid-state Z-scheme NaNbO3-Au-Sn3O4 photocatalyst for effective degradation of carbofuran under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2020, 389, 112246. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Wang, Y.; Zhang, Y.; Yuan, H. The g-C3N4/Bi2Sn2O7@ PAN nanofibes: Enhanced photocatalytic activity in H2 evolution by the formation of heterojunction and in-situ growth. Appl. Surf. Sci. 2023, 608, 155228. [Google Scholar] [CrossRef]
- Wang, J.-C.; Wang, B.; Shi, W.; Qiao, X.; Yang, X.; Zhang, L.; Zhang, W.; Li, R.; Hou, Y. Natural-sunlight-driven synchronous degradation of 4-nitrphenol and rhodamine B over S-scheme heterojunction of α-Fe2O3 nanoparticles decorated CuBi2O4 rods. J. Environ. Chem. Eng. 2022, 10, 108565. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Cai, M.; Wang, Y.; Zhang, H.; Guo, Y.; Zhao, W.; Wang, Z.; Chen, X. Photocatalytic degradation of tetracycline antibiotic by a novel Bi2Sn2O7/Bi2MoO6 S-scheme heterojunction: Performance, mechanism insight and toxicity assessment. Chem. Eng. J. 2022, 429, 132519. [Google Scholar] [CrossRef]
- Jin, C.-Z.; Yang, X.-A.; Zhai, X.-M.; Wang, S.-B.; Zhang, W.-B. ZnO/Sn3O4 amorphous-crystalline heterojunctions for Cr (VI) visible photocatalysis: Simple synthesis with excellent performance. Appl. Surf. Sci. 2023, 608, 155263. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Ghosh, S. Novel ZnO/CuBi2O4 heterostructures for persulfate-assisted photocatalytic degradation of dye contaminants under visible light. J. Photochem. Photobiol. A Chem. 2020, 391, 112397. [Google Scholar] [CrossRef]
- Shen, J.-C.; Zeng, H.-Y.; Chen, C.-R.; Xu, S. Novel plasmonic pn heterojunction Ag-Ag2CO3/Bi2Sn2O7 photocatalyst for Cr (VI) reduction. J. Taiwan Inst. Chem. Eng. 2021, 120, 106–115. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Yang, M.-J.; Yang, S.-Y.; Xu, Y.-H. Enhanced photocatalytic degradation of glyphosate over 2D CoS/BiOBr heterojunctions under visible light irradiation. J. Hazard. Mater. 2021, 407, 124798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Yamaguchi, A.; Yang, Y.; El Aisnada, A.N.; Uchida, S.; Abe, H.; Ueda, S.; Yamaguchi, K.; Tanabe, T.; Miyauchi, M. Synthesis and Characterization of the Orthorhombic Sn3O4 Polymorph. Angew. Chem. Int. Ed. 2023, 62, e202300640. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, W.; Tan, P.; Zhu, A.; Liu, Y.; Xiong, X.; Pan, J. Insights into the synergy effect of anisotropic {001} and {230} facets of BaTiO3 nanocubes sensitized with CdSe quantum dots for photocatalytic water reduction. Appl. Catal. B Environ. 2018, 227, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, Y.; Xiao, B.; Ou-yang, J.; Li, H.; Chen, Z. Z-scheme 2D/2D g-C3N4/Sn3O4 heterojunction for enhanced visible-light photocatalytic H2 evolution and degradation of ciprofloxacin. Mater. Sci. Semicond. Process. 2021, 129, 105767. [Google Scholar] [CrossRef]
- Feng, S.; Chen, T.; Liu, Z.; Shi, J.; Yue, X.; Li, Y. Z-scheme CdS/CQDs/g-C3N4 composites with visible-near-infrared light response for efficient photocatalytic organic pollutant degradation. Sci. Total Environ. 2020, 704, 135404. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, M.; Jiang, W.; Gao, B.; Li, Y.; Zhou, S.; Wang, D.; Liu, C.; Che, G. Self-photoreduced Ag 0-doped Ag (i)–organic frameworks with efficient visible-light-driven photocatalytic performance. CrystEngComm 2021, 23, 7496–7501. [Google Scholar] [CrossRef]
- Yang, C.; Feng, S.; Ma, C.; Zhou, Y.; Dai, X.; Ye, Z.; Wang, Y. Bi2Sn2O7/UiO-66-NH2 heterojunction photocatalyst simultaneously adsorbed and photodegraded tetracycline. J. Environ. Chem. Eng. 2023, 11, 109664. [Google Scholar] [CrossRef]
- Fan, G.; Zhou, J.; Ruan, F.; Li, Y.; Tian, H.; Fan, D.; Chen, Q.; Li, N. The Z-scheme photocatalyst S-BiOBr/Bi2Sn2O7 with 3D/0D interfacial structure for the efficient degradation of organic pollutants. Sep. Purif. Technol. 2023, 309, 123099. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Ultrasound assisted dispersion of Bi2Sn2O7-C3N4 nanophotocatalyst over various amount of zeolite Y for enhanced solar-light photocatalytic degradation of tetracycline in aqueous solution. Ultrason. Sonochem. 2018, 43, 61–72. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Qin, Y.; Song, F.; Du, C.; Su, Y. Direct Z-scheme SnO2/Bi2Sn2O7 photocatalyst for antibiotics removal: Insight on the enhanced photocatalytic performance and promoted charge separation mechanism. J. Photochem. Photobiol. A Chem. 2021, 404, 112947. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Sono-photodeposition of Ag over sono-fabricated mesoporous Bi2Sn2O7-two dimensional carbon nitride: Type-II plasmonic nano-heterojunction with simulated sunlight-driven elimination of drug. Chem. Eng. J. 2020, 389, 123418. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, A.; Xiong, M.; Macharia, D.K.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. TiO2/BiOI pn junction-decorated carbon fibers as weavable photocatalyst with UV–vis photoresponsive for efficiently degrading various pollutants. Chem. Eng. J. 2021, 415, 129019. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Ghosh, S. Anchoring Bi4O5I2 and AgI nanoparticles over g-C3N4 nanosheets: Impressive visible-light-induced photocatalysts in elimination of hazardous contaminates by a cascade mechanism. Adv. Powder Technol. 2020, 31, 2618–2628. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Shehzad, N.; Maafa, I.M.; Akhter, P.; Shafique, S.; Razzaq, A.; Yang, W.; Tahir, M.; Russo, N. The effect of crystal facets and induced porosity on the performance of monoclinic BiVO4 for the enhanced visible-light driven photocatalytic abatement of methylene blue. J. Environ. Chem. Eng. 2019, 7, 103265. [Google Scholar] [CrossRef]
- Feng, Z.; Zeng, L.; Zhang, Q.; Ge, S.; Zhao, X.; Lin, H.; He, Y. In situ preparation of g-C3N4/Bi4O5I2 complex and its elevated photoactivity in Methyl Orange degradation under visible light. J. Environ. Sci. 2020, 87, 149–162. [Google Scholar] [CrossRef]

| Photocatalysts | Quality | TC Content | Light Source | Efficiency | Ref |
|---|---|---|---|---|---|
| Bi2Sn2O7/UiO-66-NH2 | 10 mg | 50 mL 25 mg/L | 400 W Xe lamp λ > 400 nm | 86% (120 min) | [47] |
| Bi2Sn2O7/Bi2MoO6 | 35 mg | 100 mL 20 mg/L | 300 W Xe lamp λ > 400 nm | 99.4% (100 min) | [37] |
| BiOBr/Bi2Sn2O7 | 20 mg | 20 mL 20 mg/L | 500 W Xe lamp λ > 400 nm | 51.3% (80 min) | [48] |
| C3N4/Bi2Sn2O7 | 200 mg | 100 mL 20 mg/L | 300 W Tungsten Halogen lamp | 80.4% (90 min) | [49] |
| SnO2/Bi2Sn2O7 | 25 mg | 50 mL 20 mg/L | 300 W Xe lamp λ > 400 nm | 88.4% (180 min) | [50] |
| Ag/Bi2Sn2O7-C3N4 | 200 mg | 200 mL 20 mg/L | 400 W Tungsten Halogen lamp | 89.1% (90 min) | [51] |
| BiOBr/Bi2Sn2O7 | 10 mg | 30 mL 10 mg/L | 300 W Xe lamp λ > 400 nm | 50.4% (60 min) | [27] |
| Ag@ZnO/Bi2Sn2O7 | 50 mg | 100 mL 10 mg/L | 300 W Xe lamp λ > 400 nm | 74% (60 min) | [20] |
| Nd2Sn2O7/Bi2Sn2O7/Ag3PO4 | 20 mg | 50 mL 15 mg/L | 45 W lamp λ > 400 nm | 97.1% (120 min) | [23] |
| CuBi2O4/Bi2Sn2O7/Sn3O4 | 50 mg | 100 mL 5 mg/L | 300 W Xe lamp λ > 400 nm | 89.7% (100 min) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhu, Y.; Liu, Z.; Teng, X.; Gao, H.; Zhao, Y.; Chen, M. Synthesis of Dual Z-Scheme CuBi2O4/Bi2Sn2O7/Sn3O4 Photocatalysts with Enhanced Photocatalytic Performance for the Degradation of Tetracycline under Visible Light Irradiation. Catalysts 2023, 13, 1028. https://doi.org/10.3390/catal13071028
Xu J, Zhu Y, Liu Z, Teng X, Gao H, Zhao Y, Chen M. Synthesis of Dual Z-Scheme CuBi2O4/Bi2Sn2O7/Sn3O4 Photocatalysts with Enhanced Photocatalytic Performance for the Degradation of Tetracycline under Visible Light Irradiation. Catalysts. 2023; 13(7):1028. https://doi.org/10.3390/catal13071028
Chicago/Turabian StyleXu, Jingjing, Yanlin Zhu, Zeyu Liu, Xueyu Teng, Haiqing Gao, Yaxin Zhao, and Mindong Chen. 2023. "Synthesis of Dual Z-Scheme CuBi2O4/Bi2Sn2O7/Sn3O4 Photocatalysts with Enhanced Photocatalytic Performance for the Degradation of Tetracycline under Visible Light Irradiation" Catalysts 13, no. 7: 1028. https://doi.org/10.3390/catal13071028
APA StyleXu, J., Zhu, Y., Liu, Z., Teng, X., Gao, H., Zhao, Y., & Chen, M. (2023). Synthesis of Dual Z-Scheme CuBi2O4/Bi2Sn2O7/Sn3O4 Photocatalysts with Enhanced Photocatalytic Performance for the Degradation of Tetracycline under Visible Light Irradiation. Catalysts, 13(7), 1028. https://doi.org/10.3390/catal13071028






