Triboelectrification Catalytic Degradation of Organic Pollutants in Water Environment
Abstract
1. Introduction
2. Results and Discussion
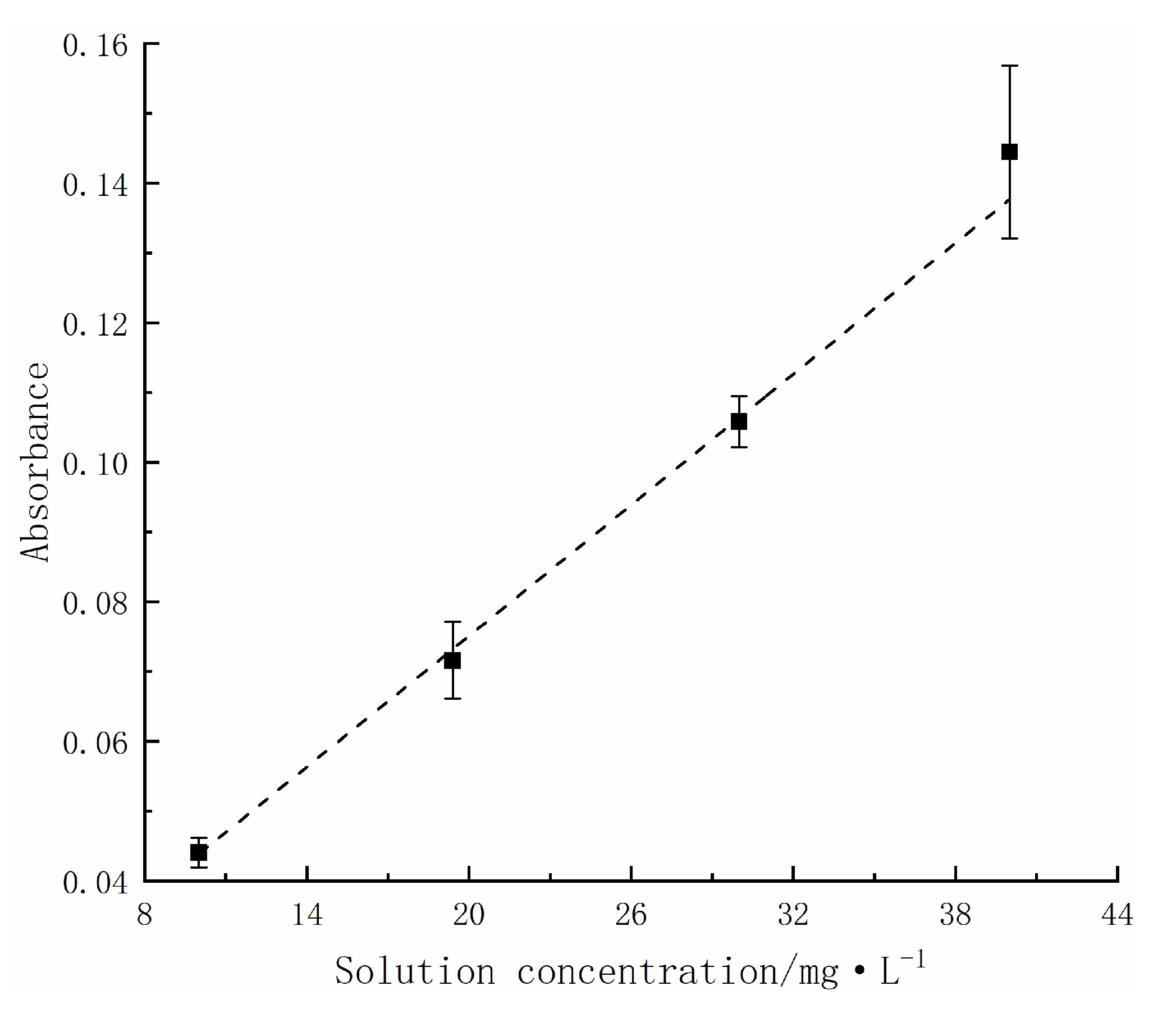
2.1. Relationship between Solution Absorbance and Concentration
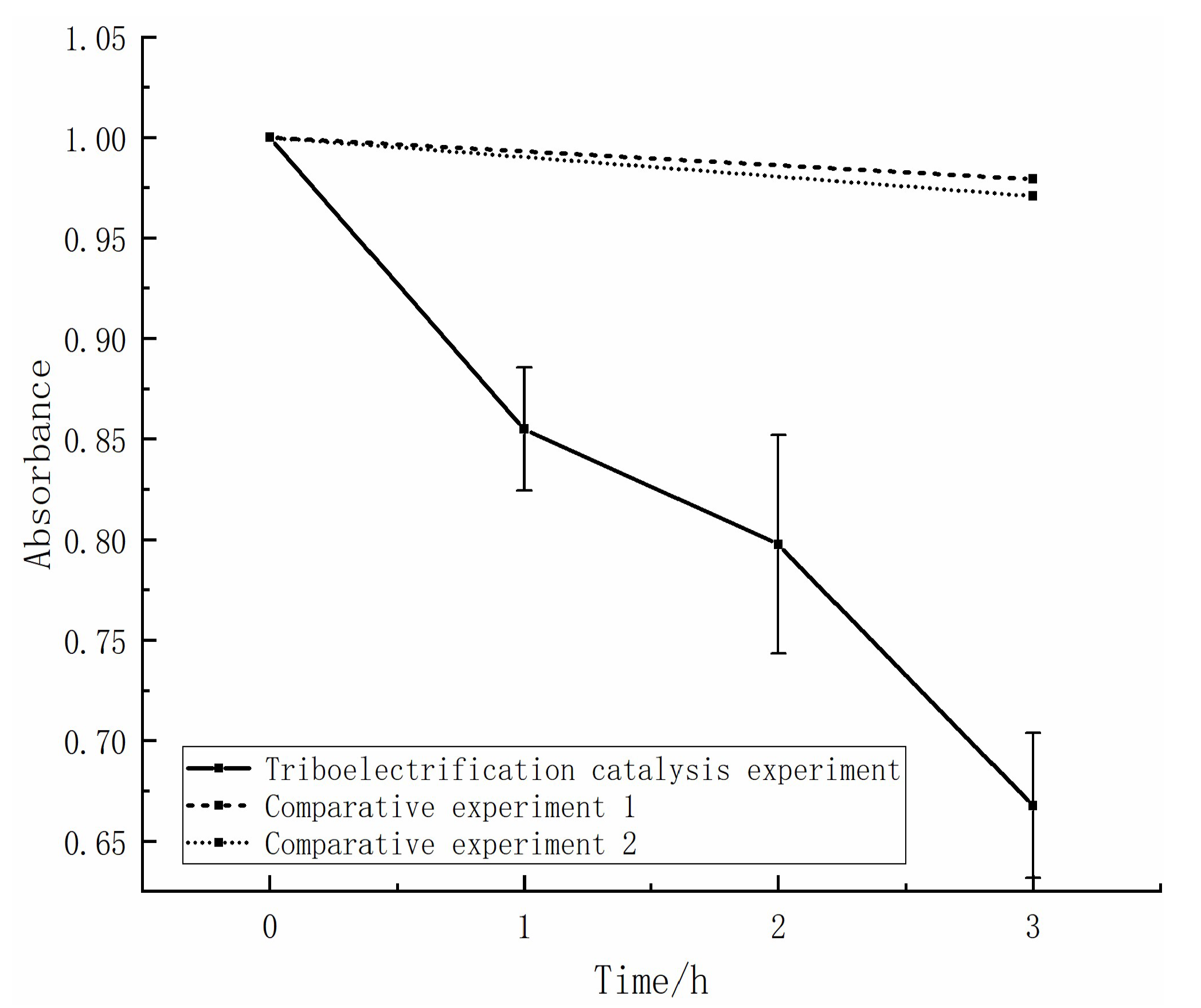
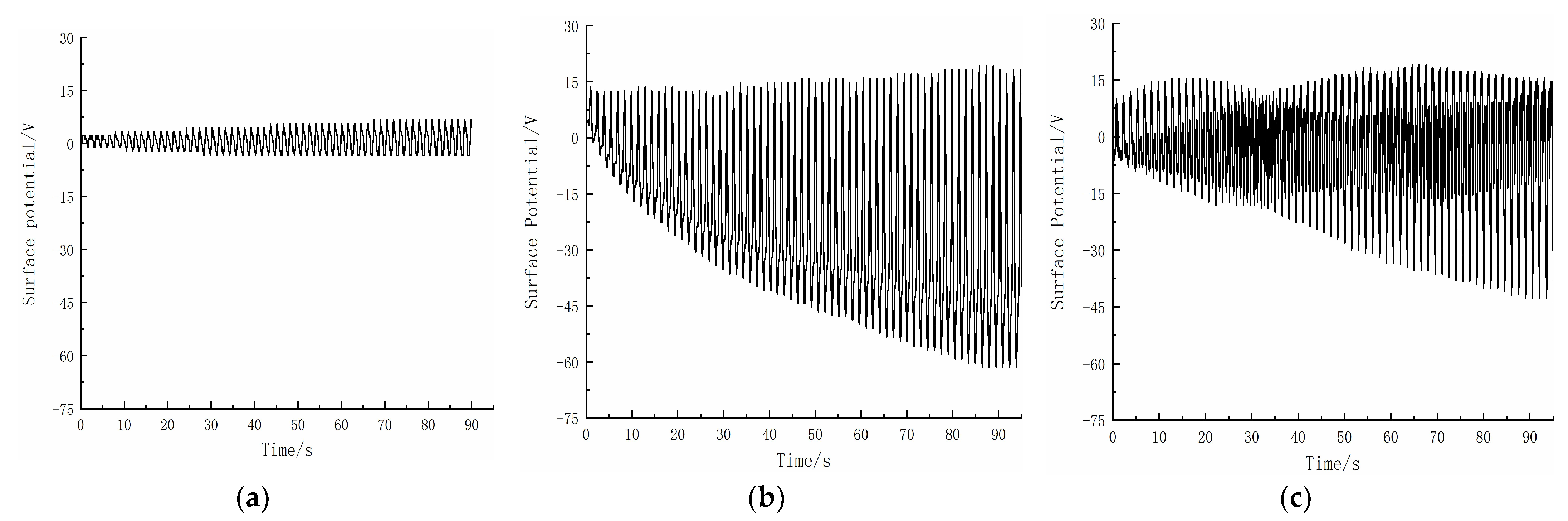
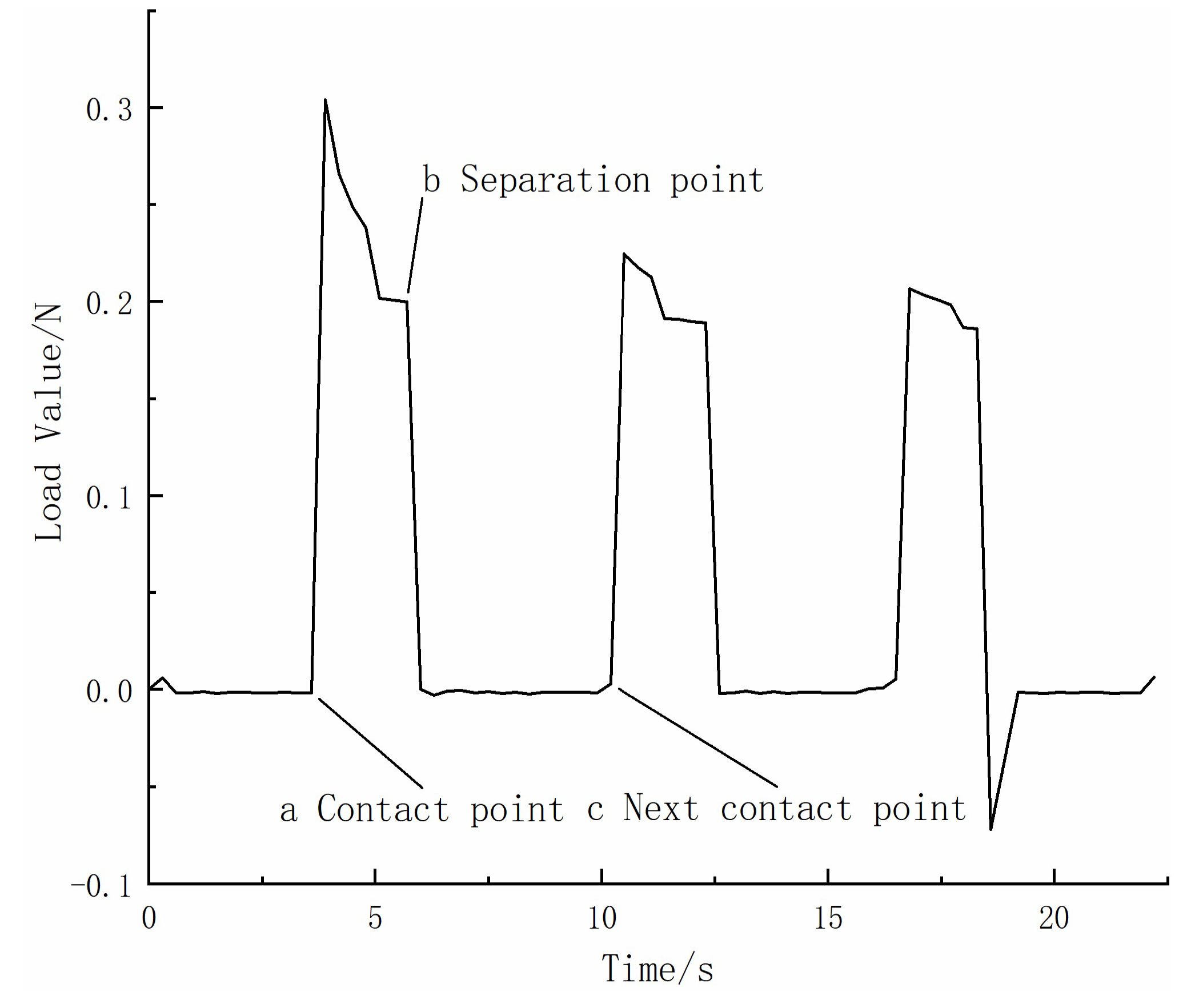
2.2. Degradation Rate Variation during the Contact Separation Process
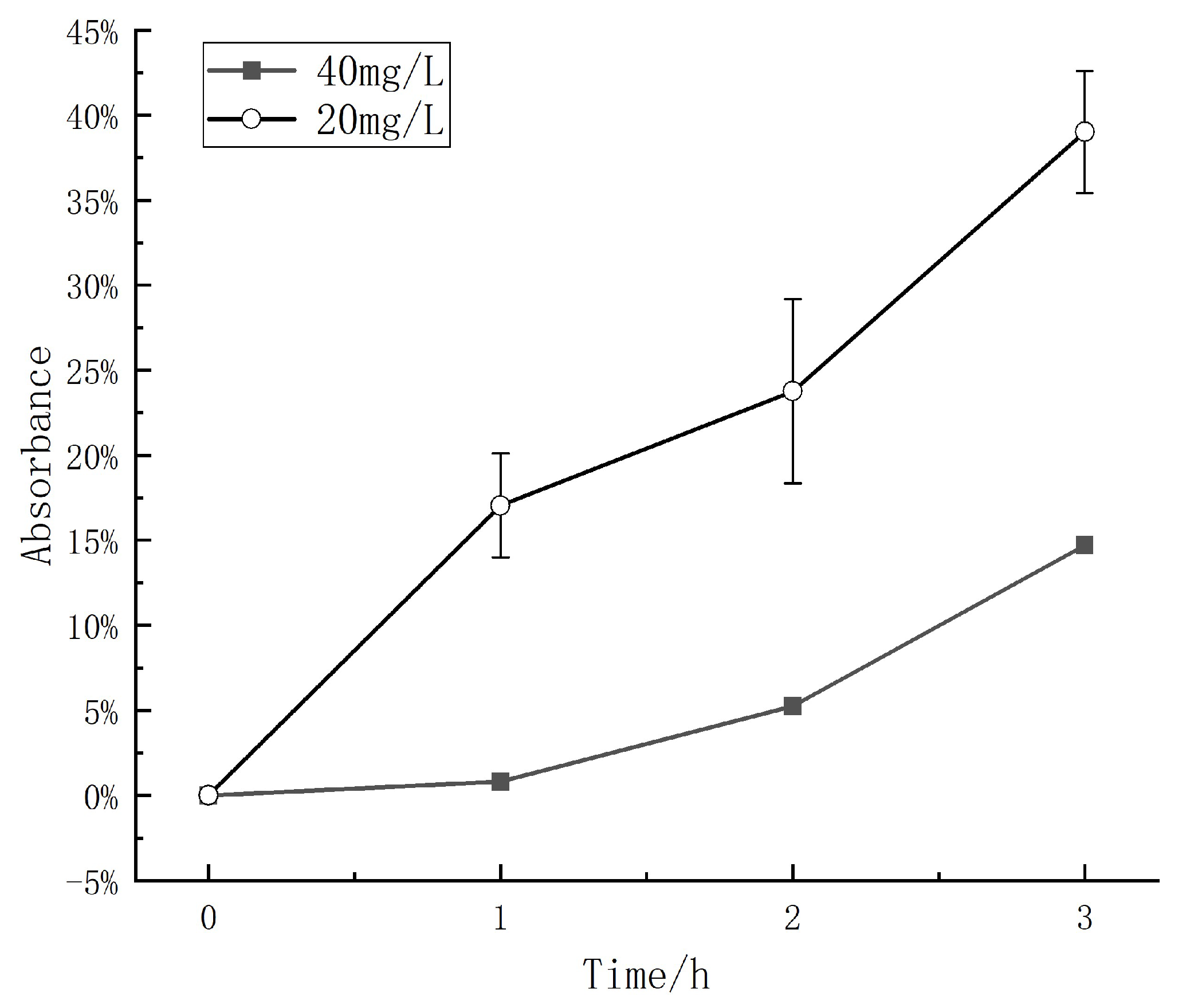
2.3. Degradation Rate at Different Methyl Orange Concentrations
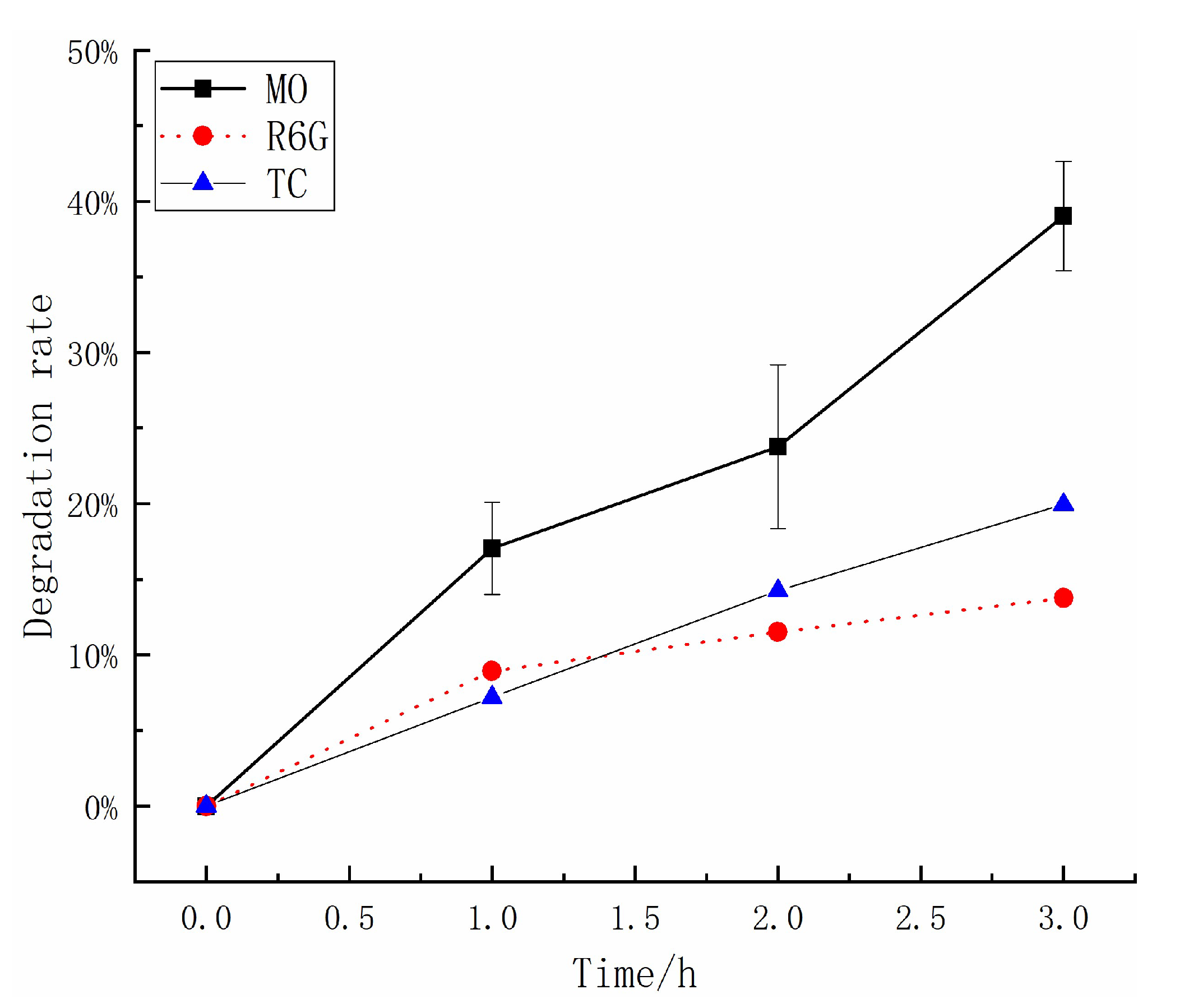
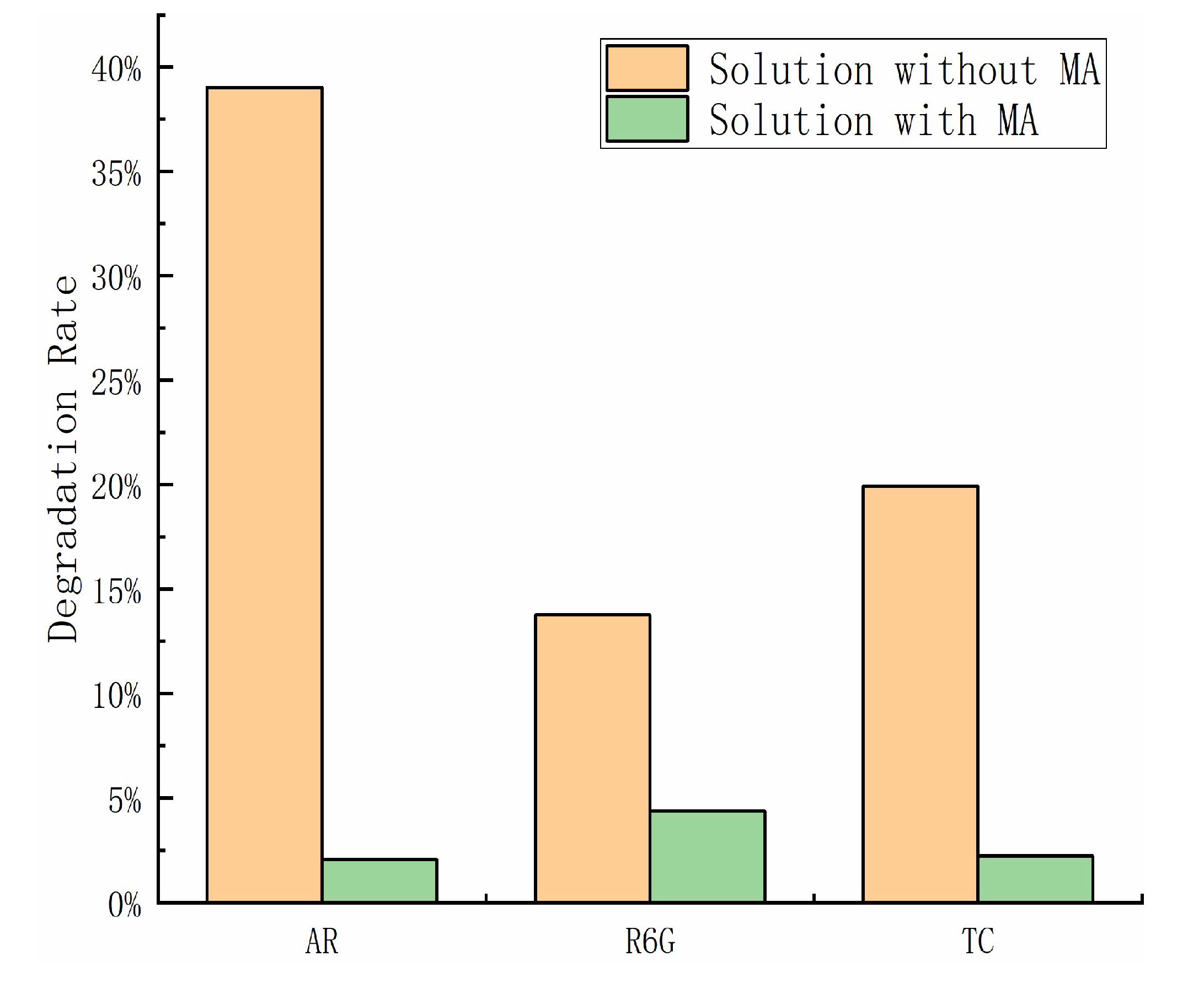
2.4. Triboelectrification Catalytic Degradation Effect of Other Organic Pollutants
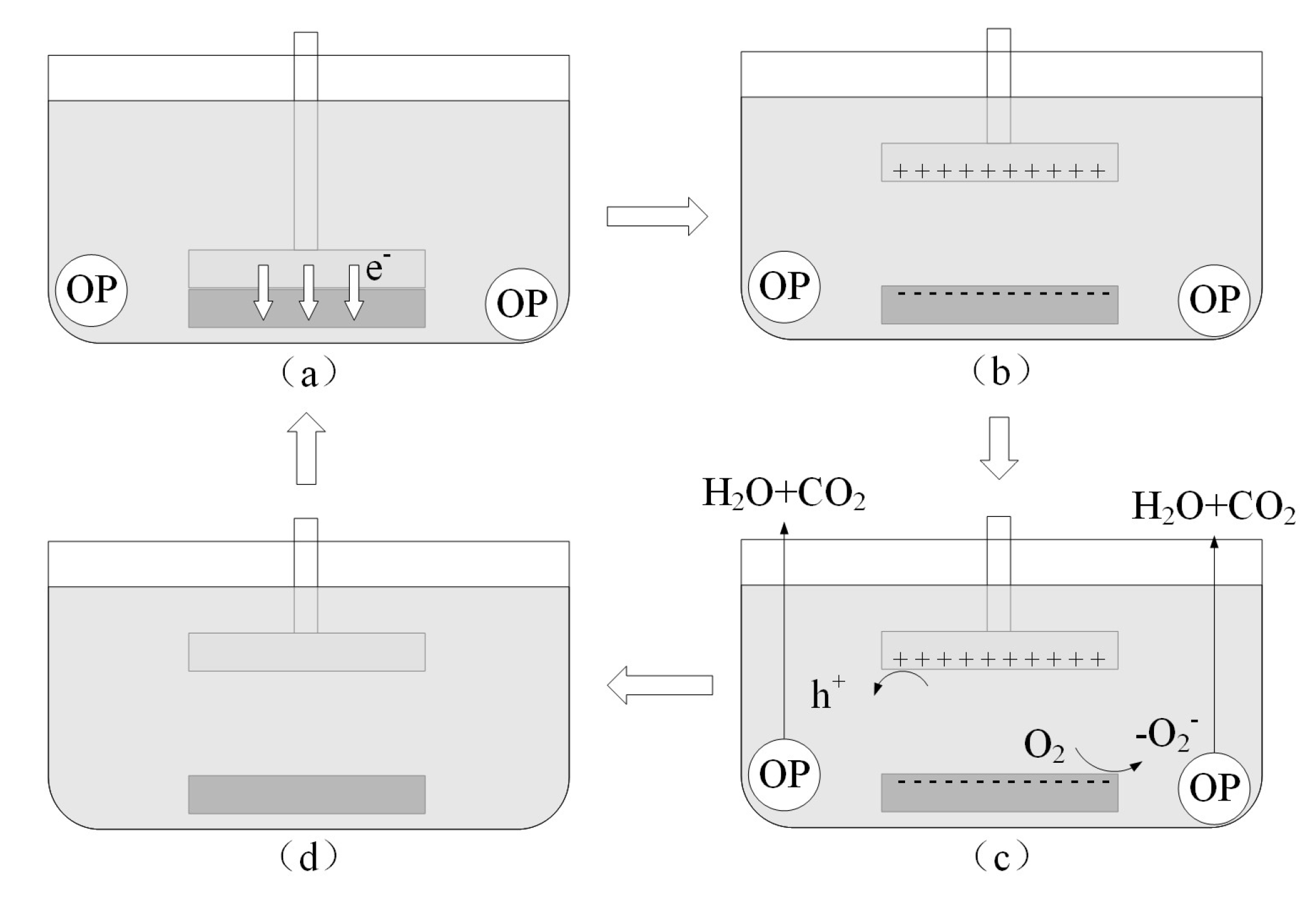
2.5. Triboelectrification Catalytic Mechanism
3. Development of the Experimental Device
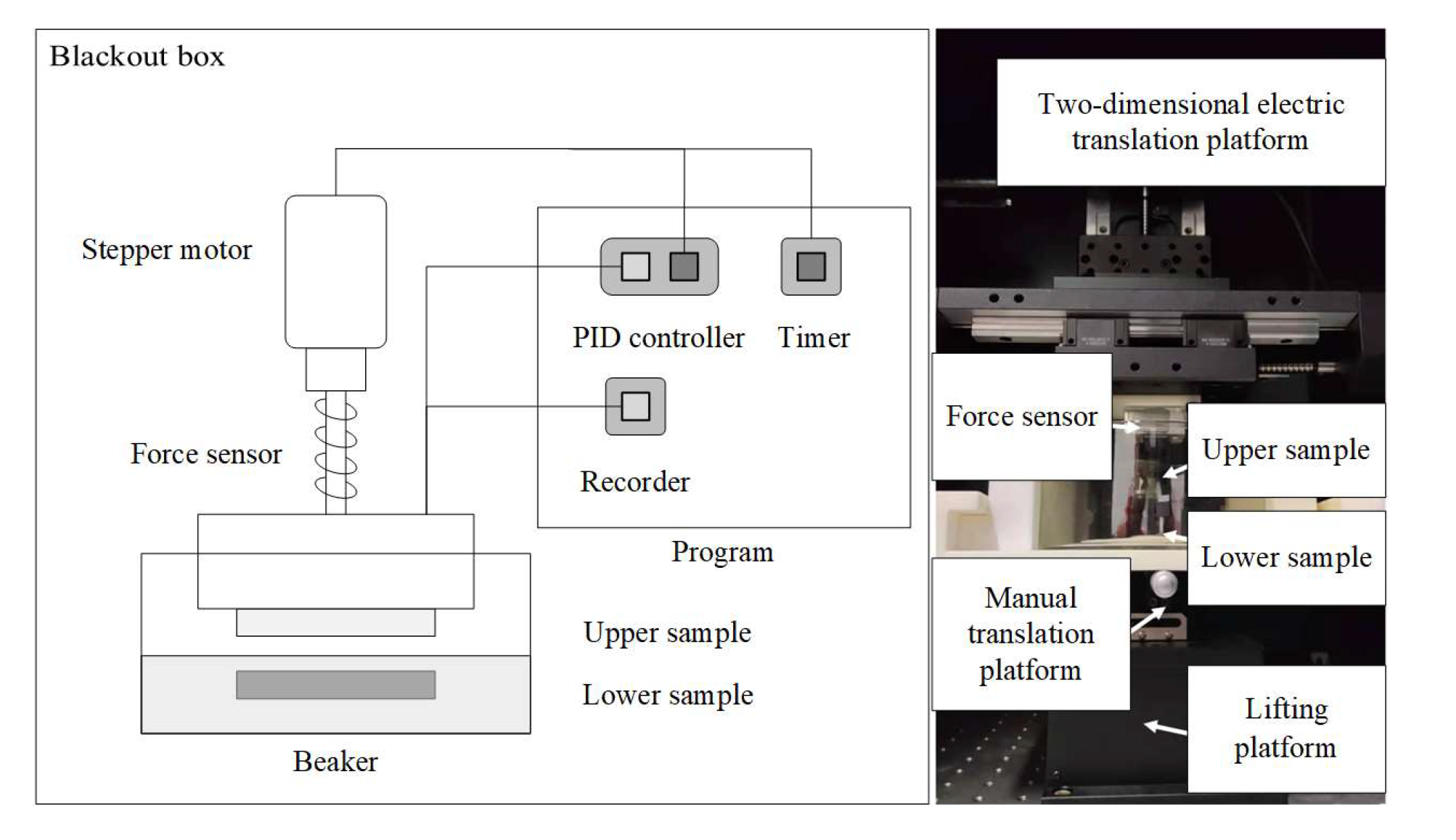
3.1. Overall Design of the Friction-Starting Electrocatalytic Device
- (1)
- Mechanical system: A stepper motor and screw are used to control the contact and separation between the upper and lower samples.
- (2)
- Control system: According to the experimental settings, the load size, contact time, separation time, contact frequency, and other parameters are adjusted in real-time during the experiment.
- (3)
- Measurement system: A tri-axial force sensor is used to convert the force signal into an electrical signal, which is then transmitted to a computer through an amplifier and an acquisition card. The measurement system collects and records the load size and other parameters between the samples in real time.
3.2. Mechanical System
3.3. Control System
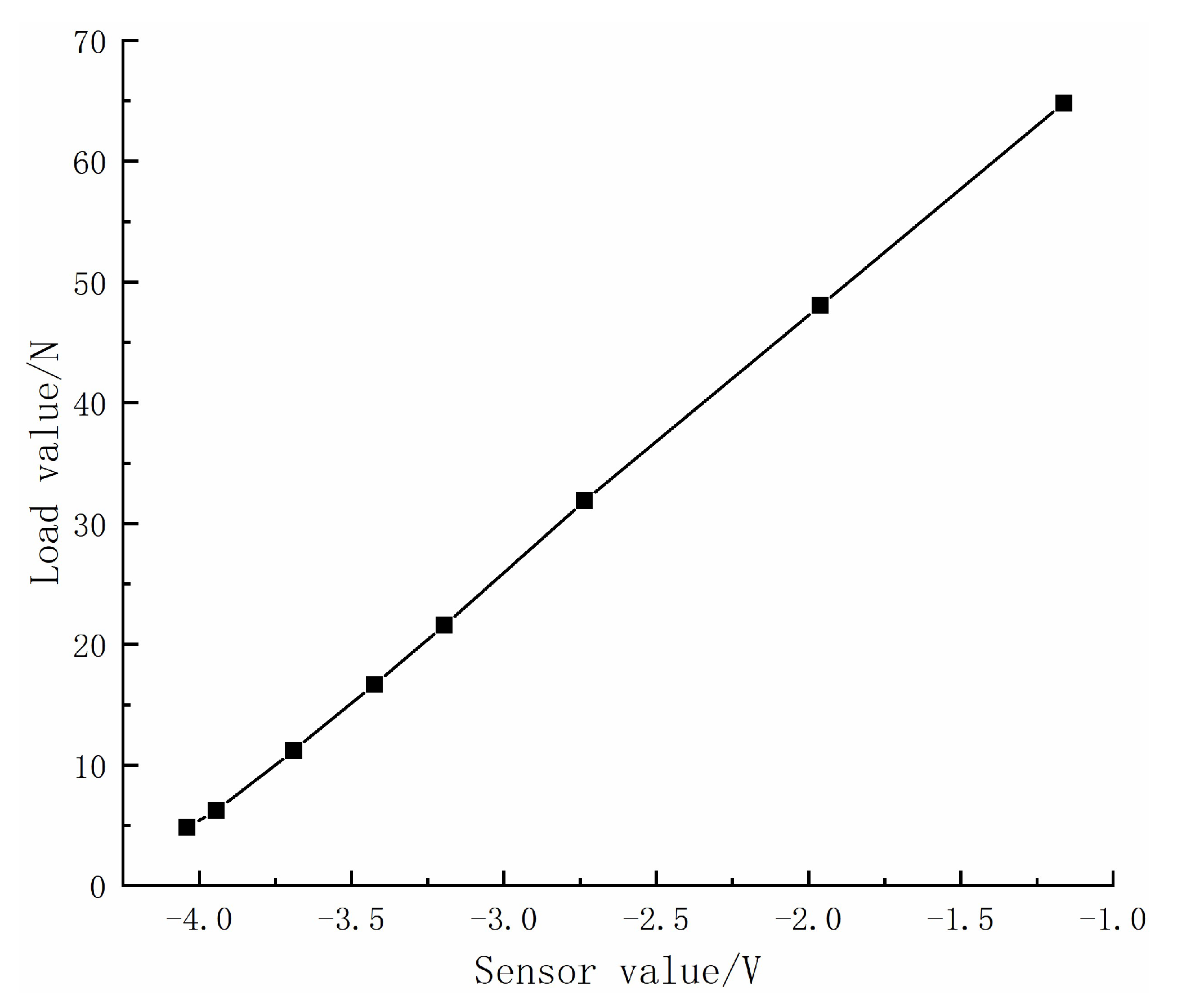
3.4. Measurement System
4. Materials and Methods
4.1. Experimental Materials and Parameters
4.2. The Calculation of the Degradation Rate of Organic Pollutants
4.3. Triboelectrification Catalysis Experiment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, F.; Cheng, P.; Zhang, L.; Wang, Y.; Xue, H. Organic Pollutant Types and Concentration Changes of the Water from Songhua River, China, in 1975–2013. Water Air Soil Pollut. 2016, 227, 214. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, H.; Liu, Z.; Gao, Z.; Lu, T.; Feng, J.; Sun, H. Health Risk Assessment of Groundwater in the Dawu Water Source Area, Zibo City. J. Arid Land Resour. Environ. 2021, 35, 106–113. [Google Scholar]
- Han, F. Environmental Monitoring Status of Persistent Organic Pollutants in China. Clean. World 2022, 38, 75–77. [Google Scholar]
- Liu, Y.; Wang, L.; Liu, W.; Liu, Q. Research of Organic Pollution’s Character in Songhua River. Environ. Sci. Manag. 2006, 31, 73–75. [Google Scholar]
- Song, X. Study on Degradation Methods of Organic Pollutants. Shanxi Chem. Ind. 2022, 42, 356–357. [Google Scholar]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2-Pd and TiO2-Ag on the Photocatalytic Oxidation of Diclofenac, Isoproturon and Phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Dai, B.; Zhao, W.; Huang, H.; Li, S.; Yang, G.; Wu, H.; Sun, C.; Dennis, Y.C.L. Constructing an Ohmic Junction of Copper@ Cuprous Oxide Nanocomposite with Plasmonic Enhancement for Photocatalysis. J. Colloid Interface Sci. 2022, 616, 163–176. [Google Scholar] [CrossRef]
- Lou, W.; Wang, L.; Dong, S.; Cao, Z.; Sun, J.; Zhang, Y. A Facile Synthesis of Bismuth-Iron Bimetal MOF Composite Silver Vanadate Applied to Visible Light Photocatalysis. Opt. Mater. 2022, 126, 112168. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, D.; Kondamareddy, K.K.; Wang, H.; Wu, Q.; Fan, H.; Wang, Q.; Zhang, B.; Xie, L.; Zhang, Y. Enhanced Visible Light Photocatalysis and Mechanism Insight for Novel Z-Scheme MoS2/Ag2S/AgVOx Ternary Heterostructure with Fast Interfacial Charges Transfer. J. Alloys Compd. 2022, 908, 164642. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Zhong, Y.; Chen, D.; Hao, Q. Preparation of BiPO4/Graphene Photoelectrode and Its Photoelectrocatalytic Performance. Chin. J. Catal. 2020, 41, 302–311. [Google Scholar] [CrossRef]
- Ai, L.; Luo, Y.; Huang, W.; Tian, Y.; Jiang, J. Cobalt/Cerium-Based Metal-Organic Framework Composites for Enhanced Oxygen Evolution Electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 12893–12902. [Google Scholar] [CrossRef]
- Zeng, L.; He, Z.; Luo, Y.; Xu, J.; Chen, J.; Wu, L.; Huang, P.; Xu, S. A Simple g-C3N4/TNTs Heterojunction for Improving the Photoelectrocatalytic Degradation of Methyl Orange. J. Electrochem. Soc. 2021, 168, 116520. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene co-catalyst assembled with mesoporous TiO2 for boosting photocatalytic activity of methyl orange degradation and hydrogen production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Pathania, D.; Gupta, D.; Al-Muhtaseb, A.H.; Sharma, G.; Kumar, A.; Naushad, M.; Ahamad, T.; Alshehri, S.M. Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J. Photochem. Photobiol. A Chem. 2016, 329, 61–68. [Google Scholar] [CrossRef]
- Saeed, M.; Al-Saeed, F.A.; Altaf, M.; Alahmari, S.D.; Bokhari, T.H.; Bajaber, M.A.; Khan, I.; Ahmed, A.E. Synthesis of a visiblelight-driven Ag2O-Co3O4 Z-scheme photocatalyst for enhanced photodegradation of a reactive yellow dye. New J. Chem. 2022, 46, 23297–23304. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, I.; Adeel, M.; Akram, N.; Muneer, M. Synthesis of a CoO–ZnO photocatalyst for enhanced visible-light assisted photodegradation of methylene blue. New J. Chem. 2022, 46, 2224–2231. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; Ben Farhat, L.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 heterojunction: An effective photocatalyst for photodegradation of rhodamine B dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, X.; Zou, S.; Cai, W. Recent advances in glycerol electrooxidation on Pt and Pd: From reaction mechanisms to catalytic materials. J. Electrochem. 2021, 27, 233–256. [Google Scholar]
- Zhang, X.; Song, X.; Lu, Z.; Chen, A.; Li, C. Anode modified by BiFeO3 and application in resourceful treatment of salty organic wastewater. Micro Nano Lett. 2019, 14, 683–687. [Google Scholar] [CrossRef]
- Bai, H.; He, P.; Chen, J.; Liu, K.; Lei, H.; Dong, F.; Zhang, X.; Li, H. Fabrication of Sc2O3-magneli phase titanium composite electrode and its application in efficient electrocatalytic degradation of methyl orange. Appl. Surf. Sci. 2017, 401, 218–224. [Google Scholar] [CrossRef]
- Hong, Y.; Ma, J.; Wu, Z.; Ying, J.; You, H.; Jia, Y. Piezo-electrochemical coupling of AgNbO3 Piezoelectric Nanomaterials. Acta Phys. Sin. 2018, 67, 202–208. [Google Scholar]
- Li, G.; Yang, S.; Xing, P.; Liu, T.; Gao, H.; Song, Y.; Zhang, H. Experimental Investigation of Triboelectrification Behaviour in the Friction Process. Lubricants 2022, 10, 180. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Manoharan, S.; Kim, S. Decoupling Contact and Rotary Triboelectrification vs Materials Property: Toward Understanding the Origin of Direct-Current Generation in TENG. ACS Appl. Mater. Interfaces 2022, 14, 34593–34602. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, Z.; Wang, J.; Liu, Y.; Chen, R.; An, H.; Park, J.H.; Suk, C.H.; Wu, C.; Lin, J.; et al. Effect of Relative Humidity on the Enhancement of the Triboelectrification Efficiency Utilizing Water Bridges between Triboelectric Materials. Nano Energy 2022, 93, 106880. [Google Scholar] [CrossRef]
- Xu, C.; Zi, Y.; Wang, A.C.; Zou, H.; Dai, Y.; He, X.; Wang, P.; Wang, Y.; Feng, P.; Li, D.; et al. On the Electron-Transfer Mechanism in the Contact-Electrification Effect. Adv. Mater. 2018, 30, 1706790. [Google Scholar] [CrossRef]
- Niu, S.; Wang, S.; Lin, L.; Liu, Y.; Zhou, Y.; Hu, Y.; Wang, Z. Theoretical Study of Contact-Mode Triboelectric Nanogenerators as an Effective Power Source. Energy Environ. Sci. 2013, 6, 3576–3583. [Google Scholar] [CrossRef]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric Charging of Powders: A Review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef]
- Matsusyama, T.; Yamamoto, H. Impact charging of particulate materials. Chem. Eng. Sci. 2005, 61, 2230–2238. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Zi, Y.; Zhang, G.; Wang, Z. Excluding contact electrification in surface potential measurement using Kelvin probeforce microscopy. ACS Nano 2016, 10, 2528–2535. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Y.; Zhao, J.; Ma, J.; Wu, Z.; Yuan, G.; Cui, X. Strong piezocatalysis in barium titanate/carbon hybrid nanocomposites for dye wastewater decomposition. J. Colloid Interface Sci. 2021, 586, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Guo, X.; Ji, R.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Strong Tribocatalytic Dye Degradation by Tungsten Bronze Ba4Nd2Fe2Nb8O30. Ceram. Int. 2021, 47, 5038–5043. [Google Scholar] [CrossRef]
- You, H.; Ma, X.; Wu, Z.; Fei, L.; Chen, X.; Yang, J.; Liu, Y.; Jia, Y.; Li, H.; Wang, F.; et al. Piezoelectrically/Pyroelectrically- Driven Vibration/Cold-Hot Energy Harvesting for Mechano-/Pyro-bi-catalytic Dye Decomposition of NaNbO3 Nanofibers. Nano Energy 2018, 52, 351–359. [Google Scholar] [CrossRef]
- Ma, J.; Ren, J.; Jia, Y.; Wu, Z.; Chen, L.; Haugen, N.O.; Huang, H.; Liu, Y. High efficiency bi-harvesting light/vibration energy using piezoelectric zinc oxide nanorods for dye decomposition. Nano Energy 2019, 62, 376–383. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic degradation of organic pollutants through bismuth oxyiodate triboelectrically harvesting mechanical energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Y.; Feng, J. Study of TiO2 photocatalytic degradation process of methyl orange using two-dimensional correlation spectroscopy. In 18th National Conference on Molecular Spectroscopy; Chinese Optical Society, Chinese Chemical Society; Peking University Press: Beijing, China, 2014; p. 2. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, X. Triboelectrification Catalytic Degradation of Organic Pollutants in Water Environment. Catalysts 2023, 13, 936. https://doi.org/10.3390/catal13060936
Zhang H, Xu X. Triboelectrification Catalytic Degradation of Organic Pollutants in Water Environment. Catalysts. 2023; 13(6):936. https://doi.org/10.3390/catal13060936
Chicago/Turabian StyleZhang, Haocheng, and Xuefeng Xu. 2023. "Triboelectrification Catalytic Degradation of Organic Pollutants in Water Environment" Catalysts 13, no. 6: 936. https://doi.org/10.3390/catal13060936
APA StyleZhang, H., & Xu, X. (2023). Triboelectrification Catalytic Degradation of Organic Pollutants in Water Environment. Catalysts, 13(6), 936. https://doi.org/10.3390/catal13060936




