Ferrous Salt-Catalyzed Oxidative Alkenylation of Indoles: Facile Access to 3-Alkylideneindolin-2-Ones
Abstract
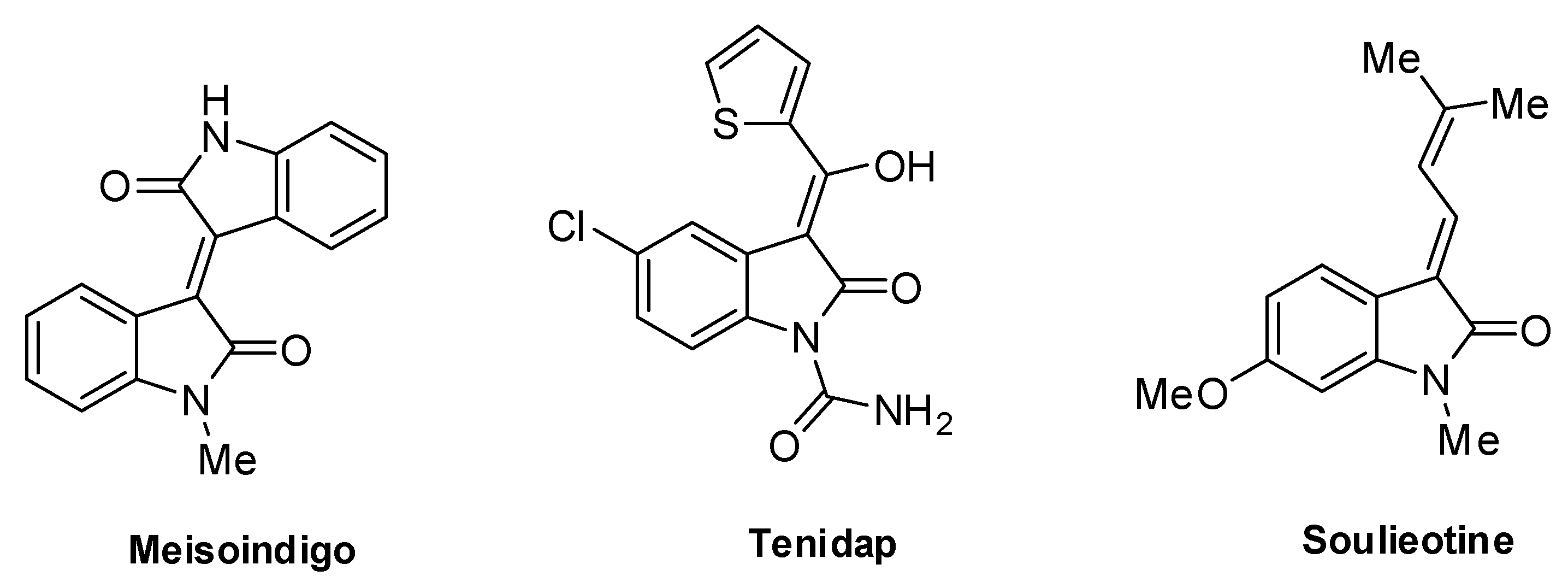
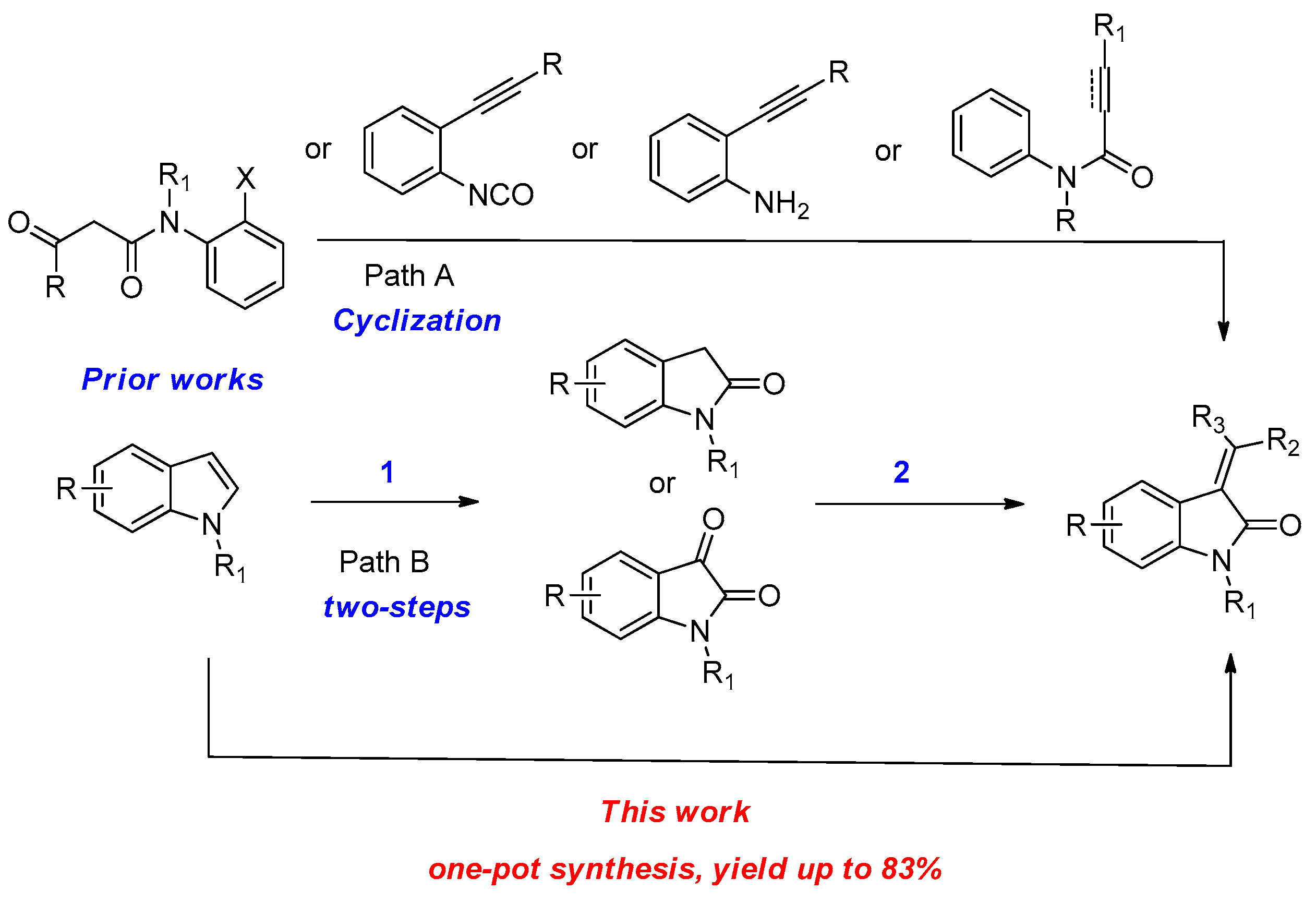
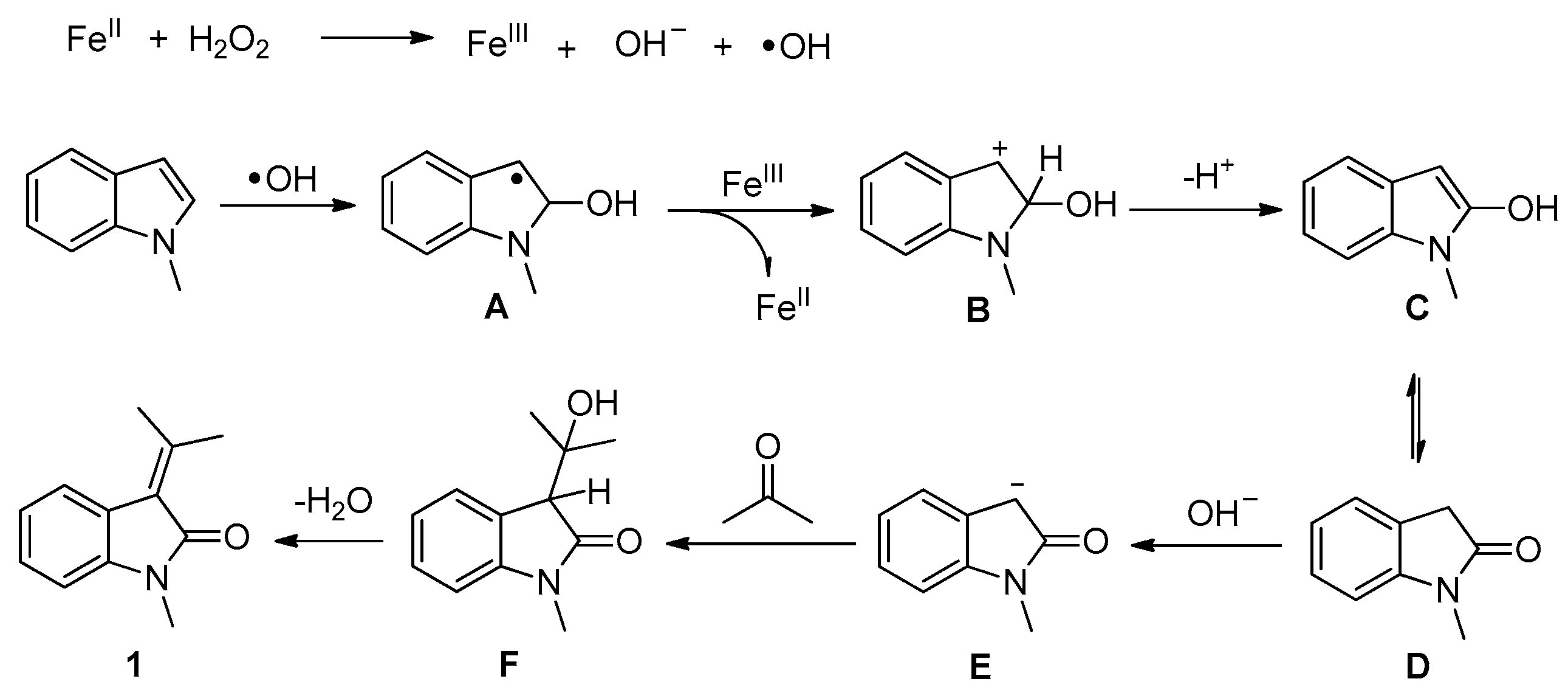
1. Introduction
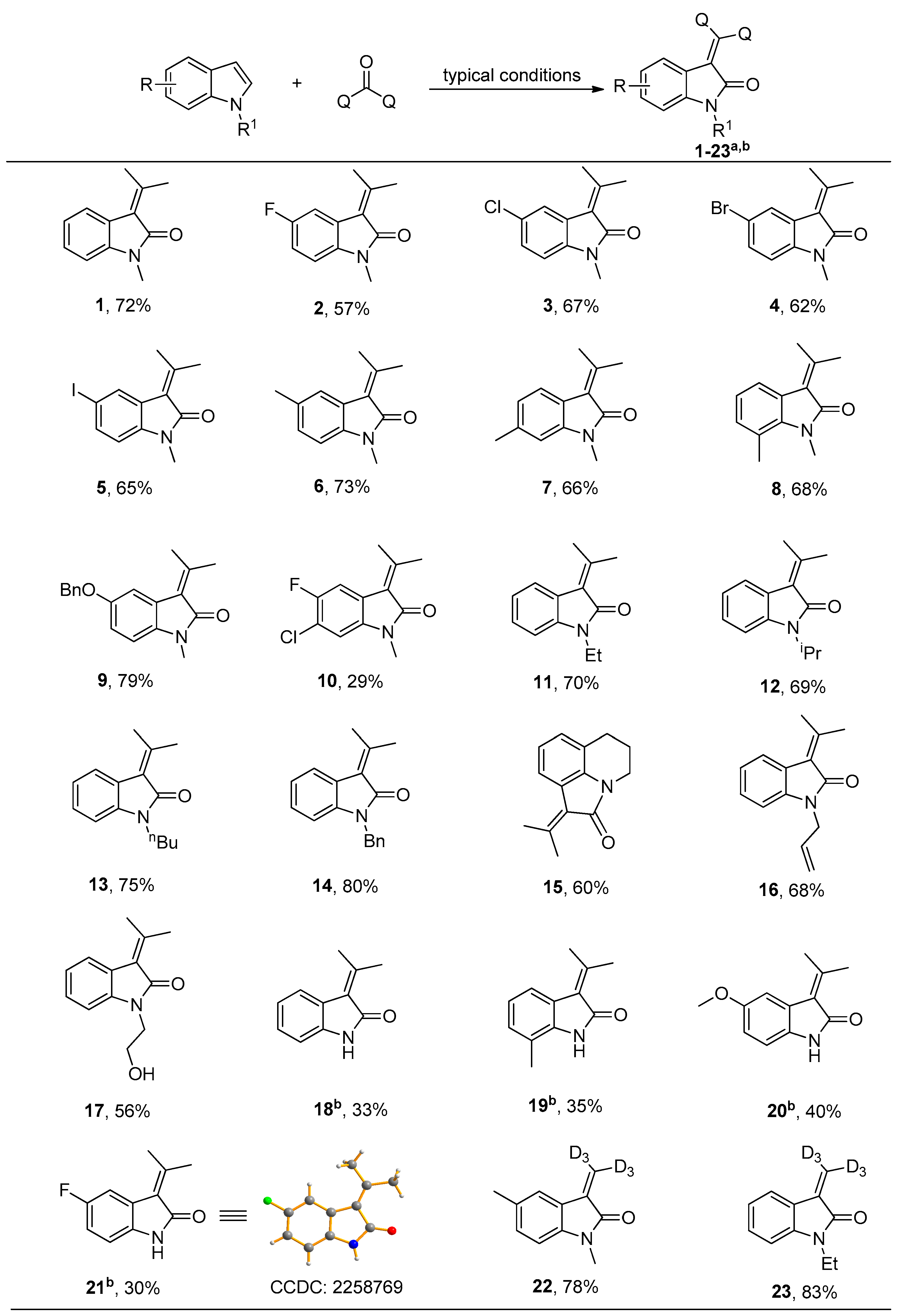
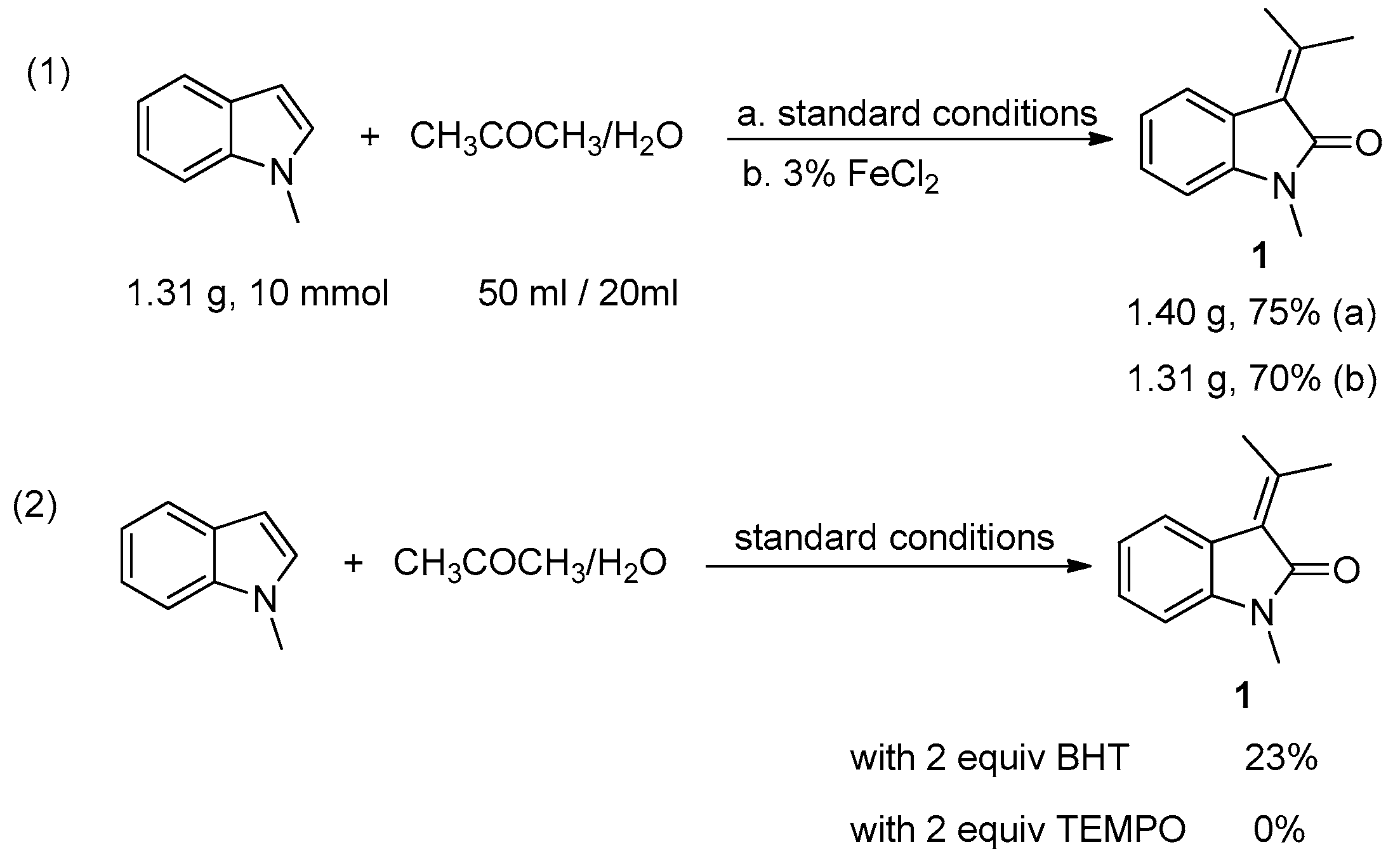
2. Results
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, A.; Nagaseshadri, B.; Nayak, V.L.; Srinivasulu, V.; Sathish, M.; Kapure, J.S.; Reddy, S.C. Synthesis and biological evaluation of benzimidazole-oxindole conjugates as microtubule-targeting agents. Bioorg. Chem. 2015, 63, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Prencipe, F.; Oliva, P.; Baraldi, S.; Salvador, M.K.; Cara, L.C.L.; Bortolozzi, R.; Mattiuzzo, E.; Basso, G.; et al. Design, synthesis and biological evaluation of 3-substituted-2-oxindole hybrid derivatives as novel anticancer agents. Eur. J. Med. Chem. 2017, 134, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Chai, Y.-F.; Wang, W.V.; Shi, Z.-F.; Xu, Z.-G.; Zhang, H.-L. Pyrazine-fused isoindigo: A new building block for polymer solar cells with high open circuit voltage. Chem. Commun. 2017, 53, 5882–5885. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem. Soc. Rev. 2012, 41, 7247–7290. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Huang, H.; Li, S.; Wu, H.; Hu, C.; Tang, J.; Zhang, Q. (Z)-(Thienylmethylene)oxindole-Based Polymers for High-Performance Solar Cells. Macromolecules 2016, 49, 2145–2152. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Y.; Hu, W.; Liu, Y. Design and effective synthesis methods for high-performance polymer semiconductors in organic field-effect transistors. Mater. Chem. Front. 2017, 1, 2423–2456. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, H.R.; Lee, J.; Kima, J.N. Palladium-Catalyzed Construction of Spirooxindoles by Arylative Cyclization of 3-(γ,δ-Disubstituted)allylidene-2-Oxindoles. Adv. Synth. Catal. 2015, 357, 701–708. [Google Scholar] [CrossRef]
- Trost, B.M.; Cramer, N.; Bernsmann, H. Concise Total Synthesis of (±)-Marcfortine B. J. Am. Chem. Soc. 2007, 129, 3086–3087. [Google Scholar] [CrossRef]
- Du, T.-P.; Zhu, G.-G.; Zhou, J. A Facile Method for the Synthesis of 3-Alkyloxindole. Lett. Org. Chem. 2012, 9, 225–232. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, J.W.; Yu, J.; Kim, J.N. An expedient synthesis of 3-alkylideneoxindoles by Ti(OiPr)4/pyridine-mediated Knoevenagel condensation. Tetrahedron Lett. 2014, 55, 1183–1187. [Google Scholar] [CrossRef]
- Kise, N.; Sasaki, K.; Sakurai, T. Reductive coupling of isatins with ketones and aldehydes by low-valent titanium. Tetrahedron Lett. 2014, 70, 9668–9675. [Google Scholar] [CrossRef]
- Lu, B.; Ma, D. Assembly of 3-Acyloxindoles via CuI/l-Proline-Catalyzed Intramolecular Arylation of β-Keto Amides. Org. Lett. 2006, 8, 6115–6118. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Okada, T.; Nagasawa, H. Oxindole synthesis by palladium-catalysed aromatic C–H alkenylation. Chem. Commun. 2010, 46, 2462–2464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-S.; Tang, R.-Y.; Zhang, X.-G.; Li, X.-H.; Li, J.-H. Palladium-Catalyzed Intramolecular 5-exo-dig Hydroarylations of N-Arylpropiolamides: Thermodynamics-Controlled Stereoselective Synthesis of 3-Methyleneoxindoles. J. Org. Chem. 2009, 74, 8834–8837. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Sekar, G. Palladium Nanoparticle-Catalyzed Stereoselective Domino Synthesis of All-Carbon Tetrasubstituted Olefin Containing Oxindoles via Carbopalladation/C–H Activation. J. Org. Chem. 2020, 85, 10514–10524. [Google Scholar] [CrossRef]
- Yu, Y.; Shin, J.K.; Seo, J.H. Stereoselective Synthesis of 3-(1,3-Diarylallylidene)oxindoles via a Palladium-Catalyzed Tandem Reaction. J. Org. Chem. 2017, 82, 1864–1871. [Google Scholar] [CrossRef]
- Tang, S.; Yu, Q.-F.; Peng, P.; Li, J.-H.; Zhong, P.; Tang, R.-Y. Palladium-Catalyzed Carbonylative Annulation Reaction of 2-(1-Alkynyl)benzenamines: Selective Synthesis of 3-(Halomethylene)indolin-2-ones. Org. Lett. 2007, 9, 3413–3416. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, E.; Chung, Y.K. Heterobimetallic Cobalt/Rhodium Nanoparticle-Catalyzed Carbonylative Cycloaddition of 2-Alkynylanilines to Oxindoles. Org. Lett. 2008, 10, 4719–4721. [Google Scholar] [CrossRef]
- Miao, B.; Zheng, Y.; Wu, P.; Li, S.; Ma, S. Bis(cycloocta-1,5-diene)nickel-Catalyzed Carbon Dioxide Fixation for the Stereoselective Synthesis of 3-Alkylidene-2-indolinones. Adv. Synth. Catal. 2017, 359, 1691–1707. [Google Scholar] [CrossRef]
- Kamijo, S.; Sasaki, Y.; Kanazawa, C.; Schüβeler, T.; Yamamoto, Y. Oxindole Synthesis through Intramolecular Nucleophilic Addition of Vinylpalladiums to Aryl Isocyanates. Angew. Chem. Int. Ed. 2005, 44, 7718–7721. [Google Scholar] [CrossRef]
- Miura, T.; Toyoshima, T.; Takahashi, Y.; Murakami, M. Stereoselective Synthesis of 3-Alkylideneoxindoles by Palladium-Catalyzed Cyclization Reaction of 2-(Alkynyl)aryl Isocyanates with Organoboron Reagents. Org. Lett. 2008, 10, 4887–4889. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Takahashi, Y.; Murakami, M. Stereoselective Synthesis of 3-Alkylideneoxindoles by Rhodium-Catalyzed Cyclization Reaction of 2-Alkynylaryl Isocyanates with Aryl- and Alkenylboronic Acids. Org. Lett. 2007, 9, 5075–5077. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Z.; Peng, L.; Wang, Q.; Xu, X.; Wang, L. A New Cyclization/Decarboxylation Reaction of Isatins with Acyl Chlorides for the Facile Synthesis of 3-Alkenyl-Oxindoles. Chin. J. Chem. 2014, 32, 844–852. [Google Scholar] [CrossRef]
- Rashed, M.; Touchy, A.; Chaudhari, C.; Jeon, J.; Siddiki, S.; Toyao, T.; Shimizu, K. Selective C3-alkenylation of oxindole with aldehydes using heterogeneous CeO2 catalyst. Chin. J. Catal. 2020, 41, 970–976. [Google Scholar] [CrossRef]
- Bisht, G.; Pandey, A.M.; Chaudhari, M.B.; Agalave, S.G.; Kanyal, A.; Karmodiya, K.K.; Gnanaprakasam, B. Ru-Catalyzed dehydrogenative synthesis of antimalarial arylidene oxindoles. Org. Biomol. Chem. 2018, 16, 7223–7229. [Google Scholar] [CrossRef]
- Gopalaiah, K.; Tiwari, A. Synthesis of (E)-3-Alkylideneindolin-2-ones by an Iron-Catalyzed Aerobic Oxidative Condensation of Csp3–H Bonds of Oxindoles and Benzylamines. Eur. J. Org. Chem. 2020, 2020, 7229–7237. [Google Scholar] [CrossRef]
- Zhao, B.; Shang, R.; Wang, G.-Z.; Wang, S.; Chen, H.; Fu, Y. Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Lu, G.-P.; Yuan, T.; Xu, Z.-B.; Wan, L.; Cai, C. Oxidative trifluoromethylation and fluoroolefination of unactivated olefins. Chem. Commun. 2016, 52, 13668–13670. [Google Scholar] [CrossRef]
- Cao, H.; Ma, S.; Feng, Y.; Guo, Y.; Jiao, P. Synthesis of β-nitro ketones from geminal bromonitroalkanes and silyl enol ethers by visible light photoredox catalysis. Chem. Commun. 2022, 58, 1780–1783. [Google Scholar] [CrossRef]
- Li, Z.; Cui, X.; Niu, L.; Ren, Y.; Bian, M.; Yang, X.; Yang, B.; Yan, Q.; Zhao, J. An Iron(II) Chloride-Promoted Radical Cascade Methylation or α-Chloro-β-methylation of N-Arylacrylamides with Dimethyl Sulfoxide. Adv. Synth. Catal. 2017, 359, 246–249. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Xiao, Z.; Zheng, L.; Zhou, X.; Guo, Y.; Chen, Q.-Y.; Zheng, C.; Liu, C. Intermolecular oxidative radical fluoroalkylfluorosulfonylation of unactivated alkenes with (fluoroalkyl)trimethylsilane, silver fluoride, sulfur dioxide and N-fluorobenzenesulfonimide. Org. Chem. Front. 2019, 6, 447–450. [Google Scholar] [CrossRef]
- Luo, X.; Fan, Z.; Zhang, B.; Chen, C.; Xi, C. Visible-light-triggered direct keto-difluoroacetylation of styrenes with (fluorosulfonyl)difluoroacetate and dimethyl sulfoxide leads to α-difluoroacetylated ketones. Chem. Commun. 2019, 55, 10980–10983. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, J.; Ouyang, X.; Qin, J.; Song, R.; Li, J.H. Radical-mediated alkoxypolyhaloalkylation of styrenes with polyhaloalkanes and alcohols via C(sp3)–H bond cleavage. Chem. Commun. 2021, 57, 3684–3687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, H.; Li, Z.; Yan, Q.; Li, P.; Wu, J.; Qi, J.; Jiang, M.; Sun, L. Iron-Mediated Azidomethylation or Azidotrideuteromethylation of Active Alkenes with Azidotrimethylsilane and Dimethyl Sulfoxide. Adv. Synth. Catal. 2018, 360, 1384–1388. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, Y.; Zheng, L.; Yan, Q.; Ren, Y.; Wang, Z.; Zhang, K.; Wang, Z.; Zhao, J.; Li, Z. A Free Radical Cascade Difunctionalization of o-Vinylanilides with Simple Ketones and Esters. Asian J. Org. Chem. 2019, 8, 2188–2191. [Google Scholar] [CrossRef]
- Ge, Y.; Tian, Y.; Wu, J.; Yan, Q.; Zheng, L.; Ren, Y.; Zhao, J.; Li, Z.-J. Iron-promoted free radical cascade difunctionalization of unsaturated benzamides with silanes. Chem. Commun. 2020, 56, 12656–12659. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, L.; Wang, Z.; Li, Z.-J.; Fu, W. Metal-Free Electrochemical Oxidative Difluoroethylation/Cyclization of Olefinic Amides To Construct Difluoroethylated Azaheterocycles. J. Org. Chem. 2023, 88, 1875–1883. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
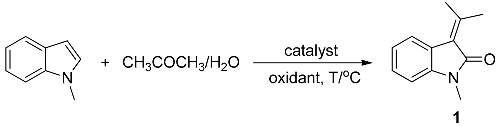 | |||||
|---|---|---|---|---|---|
| Entry | Solvent (mL) | Catalyst (mol %) | Oxidant (equiv.) | T/°C | Yield b (%) |
| 1 | 5/1 (3.5) | FeCl2 (5) | H2O2 (3) | 25 | 26 |
| 2 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (3) | 25 | 45 |
| 3 | 1/1 (3.5) | FeCl2 (5) | H2O2 (3) | 25 | 19 |
| 4 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (1) | 25 | 28 |
| 5 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (2) | 25 | 51 |
| 6 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (5) | 25 | 39 |
| 7 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (7) | 25 | 22 |
| 8 | 2.5/1 (3.5) | FeCl2 (5) | DTBP (2) | 25 | 7 |
| 9 | 2.5/1 (3.5) | FeCl2 (5) | TBPA (2) | 25 | 8 |
| 10 | 2.5/1 (3.5) | FeCl2 (5) | TBHP(in H2O) (2) | 25 | 12 |
| 11 | 2.5/1 (3.5) | Fe(SO4)2·7H2O (5) | H2O2 (2) | 25 | 37 |
| 12 | 2.5/1 (3.5) | FeCl3 (5) | H2O2 (2) | 25 | 17 |
| 13 | 2.5/1 (3.5) | CuCl2 (5) | H2O2 (2) | 25 | 24 |
| 14 | 2.5/1 (3.5) | ZnBr2 (5) | H2O2 (2) | 25 | NR |
| 15 | 2.5/1 (3.5) | FeCl2 (0) | H2O2 (2) | 25 | trace |
| 16 | 2.5/1 (3.5) | FeCl2 (2) | H2O2 (2) | 25 | 29 |
| 17 | 2.5/1 (3.5) | FeCl2 (10) | H2O2 (2) | 25 | 45 |
| 18 | 2.5/1 (3.5) | FeCl2 (20) | H2O2 (2) | 25 | 37 |
| 19 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (2) | 35 | 65 |
| 20 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (2) | 45 | 72 |
| 21 | 2.5/1 (3.5) | FeCl2 (5) | H2O2 (2) | 55 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zheng, L.; Chen, Y.; Li, Y.; Wang, M.; Fu, W.; Li, Z. Ferrous Salt-Catalyzed Oxidative Alkenylation of Indoles: Facile Access to 3-Alkylideneindolin-2-Ones. Catalysts 2023, 13, 930. https://doi.org/10.3390/catal13060930
Tian Y, Zheng L, Chen Y, Li Y, Wang M, Fu W, Li Z. Ferrous Salt-Catalyzed Oxidative Alkenylation of Indoles: Facile Access to 3-Alkylideneindolin-2-Ones. Catalysts. 2023; 13(6):930. https://doi.org/10.3390/catal13060930
Chicago/Turabian StyleTian, Yunfei, Luping Zheng, Ying Chen, Yufei Li, Mengna Wang, Weijun Fu, and Zejiang Li. 2023. "Ferrous Salt-Catalyzed Oxidative Alkenylation of Indoles: Facile Access to 3-Alkylideneindolin-2-Ones" Catalysts 13, no. 6: 930. https://doi.org/10.3390/catal13060930
APA StyleTian, Y., Zheng, L., Chen, Y., Li, Y., Wang, M., Fu, W., & Li, Z. (2023). Ferrous Salt-Catalyzed Oxidative Alkenylation of Indoles: Facile Access to 3-Alkylideneindolin-2-Ones. Catalysts, 13(6), 930. https://doi.org/10.3390/catal13060930






