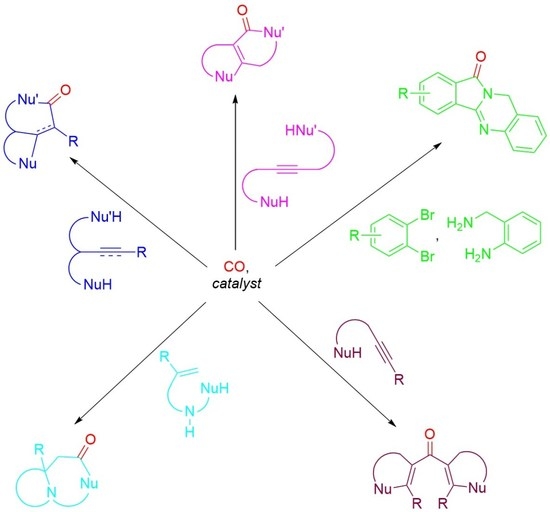
An Overview of Catalytic Carbonylative Double Cyclization Reactions
Abstract
1. Introduction
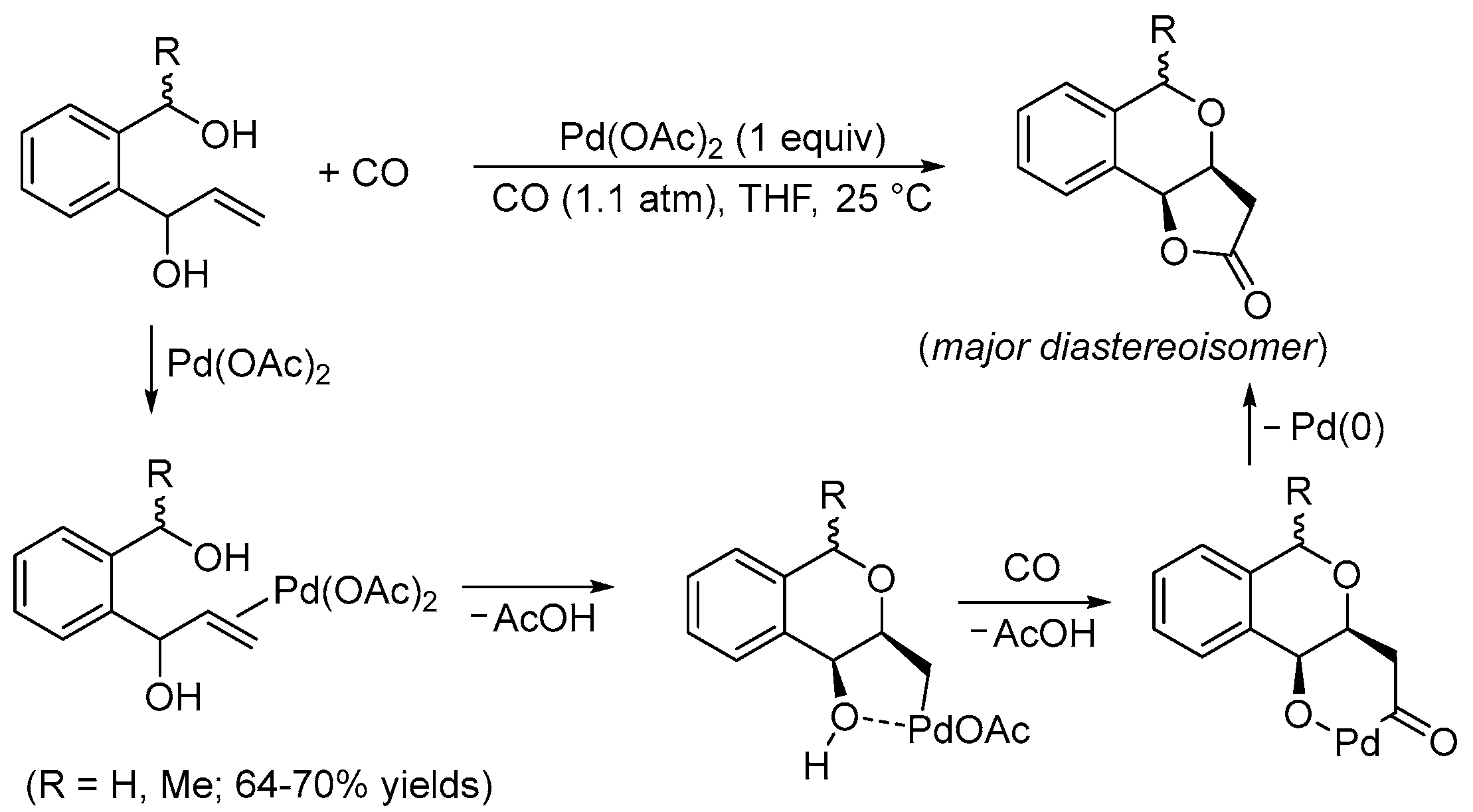
2. Functionalized Olefinic Substrates
3. Functionalized Acetylenic Substrates
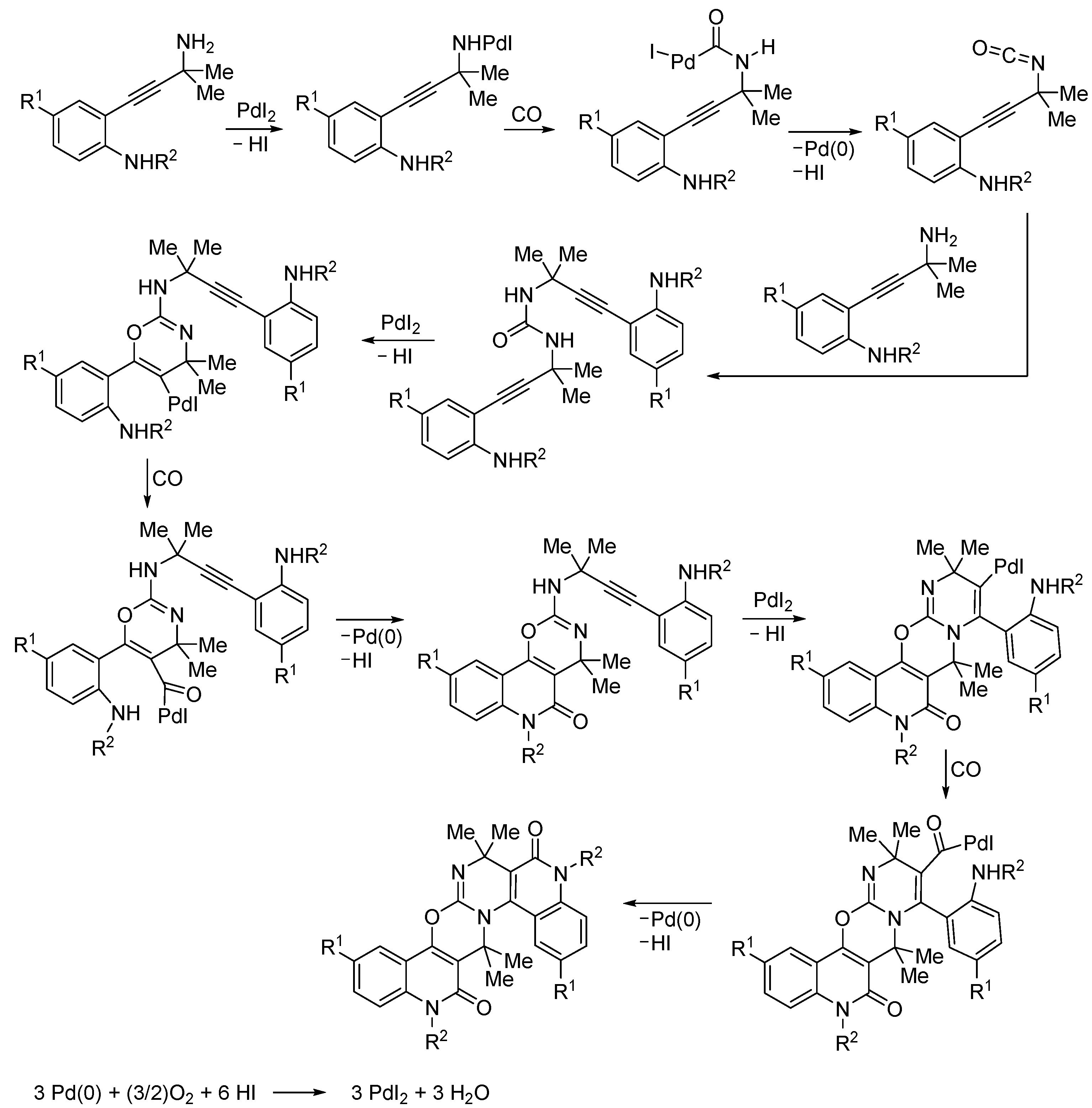
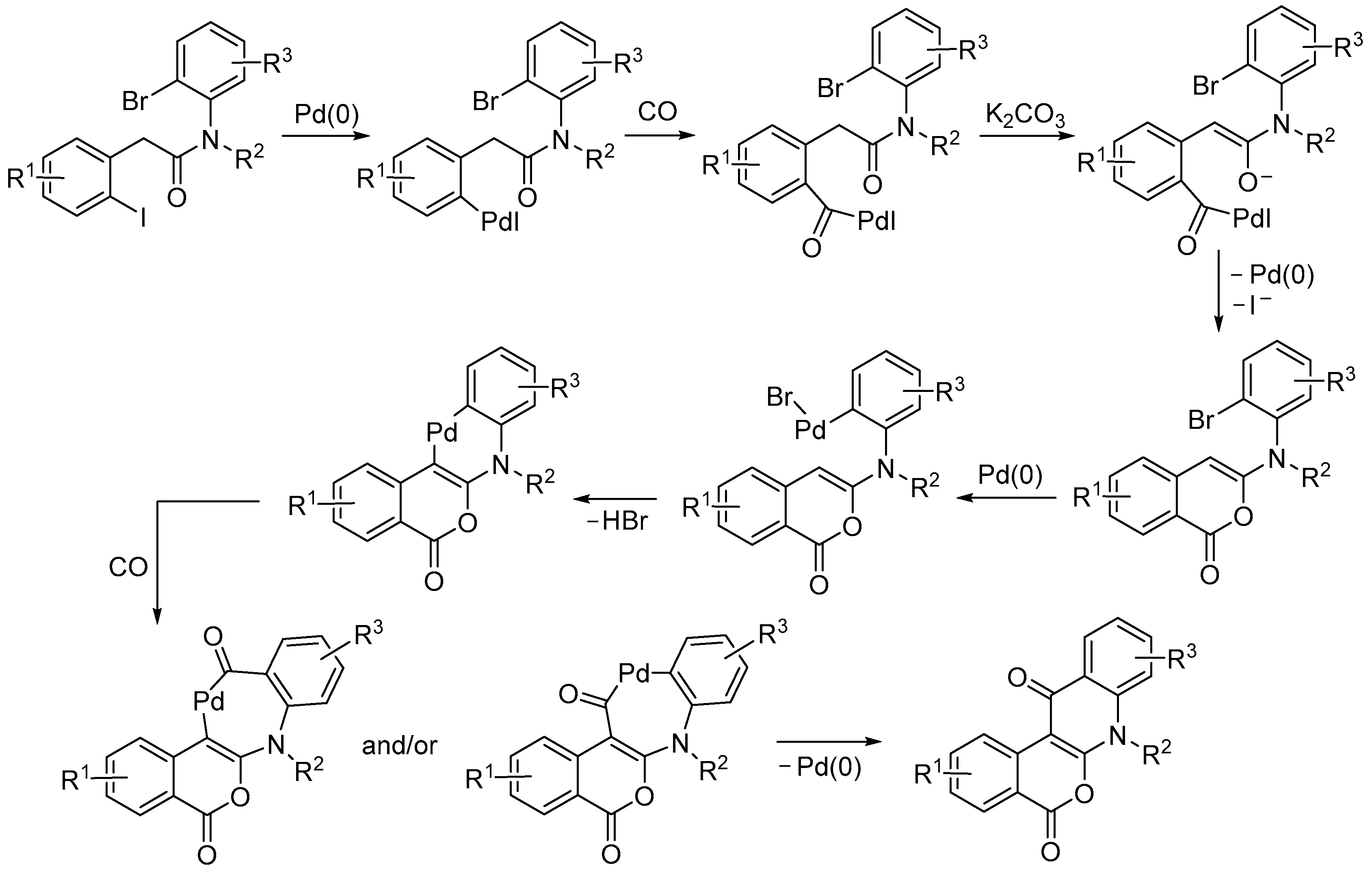
4. Functionalized Halides
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gabriele, B. (Ed.) Carbon Monoxide in Organic Synthesis—Carbonylation Chemistry; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; Schaub, G. Gas production, 2. In Ullmann’s Encyclopedia of Industrial Chemistry; Baltes, H., Göpel, W., Hesse, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 423–479. [Google Scholar]
- Karl, J.; Pröll, T. Steam Gasification of Biomass in Dual Fluidized Bed Gasifiers: A Review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Figueres, C.; Le Quéré, C.; Mahindra, A.; Bäte, O.; Whiteman, G.; Peters, G.; Guan, D. Emissions Are Still Rising: Ramp up the Cuts. Nature 2018, 564, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Gribble, G. (Eds.) Palladium in Heterocyclic Chemistry—A Guide for the Synthetic Chemist, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Gabriele, B.; Della Ca’, N.; Mancuso, R.; Veltri, L.; Ziccarelli, I. Palladium(II)-Catalyzed Carbonylations. In Carbon Monoxide in Organic Synthesis—Carbonylation Chemistry; Gabriele, B., Ed.; Wiley-VCH: Weinheim, Germany, 2022; Chapter 7. [Google Scholar]
- Semmelhack, M.F.; Bodurow, C.; Baum, M. Direct Synthesis of Pyran-Lactones Related to Nafhthoquinone Antibiotics. Tetrahadron Lett. 1984, 25, 3171–3174. [Google Scholar] [CrossRef]
- Tamaru, H.; Higashimura, K.; Hojo, M.; Yoshida, Z. PdII-Catalyzed Stereoselective Bis-Lactonization. Angew. Chem. Int. Ed. 1985, 24, 1045–1046. [Google Scholar] [CrossRef]
- Tamaru, H.; Kobayashi, T.; Kawamura, S.; Hojo, M.; Yoshida, Z. Palladium Catalyzed Oxycarbonylation of 4-Penten-1,3-diols: Efficient Stereoselective Synthesis of cis 3-Hydroxytetrahydrofuran 2-Acetic Acid Lactones. Tetrahedron Lett. 1985, 26, 3207–3210. [Google Scholar] [CrossRef]
- Gracza, T.; Hasenöhrl, T.; Stahl, T.; Jäger, V. Synthesis of 3,5-Anhydro-2-deoxy-1,4-glyconolactones by Palladium(II)-Catalyzed, Regioselective Oxycarbonylation of C5- and C6-enitols. ω-Homologation of Aldoses to Produce Intermediates for C-Glycoside/C-Nucleoside Synthesis. Synthesis 1991, 1991, 1108–1118. [Google Scholar] [CrossRef]
- Kraus, G.A.; Li, J. Regiocontrol by Remote Substituents. An Enantioselective Total Synthesis of Frenolicin B via a Highly Regioselective Diels-Alder Reaction. J. Am. Chem. Soc. 1993, 115, 5859–5860. [Google Scholar] [CrossRef]
- Gracza, T.; Jäger, V. Synthesis of Natural and Unnatural Enantiomers of Goniofufurone and Its 7-Epimers from D-Glucose. Application of Palladium(II)—Catalyzed Oxycarbonylation of Unsaturated Polyols. Synthesis 1994, 1994, 1359–1368. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Fortier, G.; Radu, I.-I. Efficient Synthesis of (−)-trans-Kumausyne via Tandem Intramolecular Alkoxycarbonylation-Lactonization. J. Org. Chem. 1998, 63, 916–917. [Google Scholar] [CrossRef]
- Dixon, D.J.; Ley, S.V.; Gracza, T.; Szolcsanyi, P. Total Synthesis of the Polyenoyltetramic acid Mycotoxin Erythroskyrine. J. Chem. Soc. Perkin Trans. 1 1999, 1999, 831–841. [Google Scholar] [CrossRef]
- Paddon-Jones, G.C.; Hungerford, N.L.; Haynes, P.; Kitching, W. Efficient Palladium(II)-Mediated Construction of Functionalized Plakortone Cores. Org. Lett. 1999, 1, 1905–1908. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Shanmugam, P. Development of an Approach to the Synthesis of the Plakortones. Tetrahedron Lett. 2000, 41, 3567–3571. [Google Scholar] [CrossRef]
- Paddon-Jones, G.C.; McErlean, C.S.P.; Haynes, P.; Moore, C.J.; Konig, W.A.; Kitching, W. Synthesis and Stereochemistry of Some Bicyclic γ-Lactones from Parasitic Wasps (Hymenoptera: Braconidae). Utility of Hydrolytic Kinetic Resolution of Epoxides and Palladium(II)-Catalyzed Hydroxycyclization-Carbonylation-Lactonization of Ene-diols. J. Org. Chem. 2001, 66, 7487–7495. [Google Scholar] [CrossRef] [PubMed]
- Haynes, P.Y.; Kitching, W. Total Synthesis and Absolute Stereochemistry of Plakortone D. J. Am. Chem. Soc. 2002, 124, 9718–9719. [Google Scholar]
- Haynes, P.Y.; Kitching, W. Synthesis of the Plakortone Series: Plakortone E. Heterocycles 2004, 62, 173–177. [Google Scholar]
- Babjak, M.; Kapitán, P.; Gracza, T. Synthesis of (+)-Goniothalesdiol and (+)-7-epi-Goniothalesdiol. Tetrahedron 2005, 61, 2471–2479. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Hooley, R.J.; Kraml, C.M. Synthesis of Plakortone B and Analogs. Org. Lett. 2006, 8, 5203–5206. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Pouliot, M.; Robichaud, J.; MacNeil, S.; Snieckus, V. Asymmetric Total Synthesis of (−)-Panacene and Correction of Its Relative Configuration. Org. Lett. 2006, 8, 3597–3599. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Stereocontrolled Oxycarbonylation of 4-Benzyloxyhepta-1,6-diene-3,5-diols Promoted by Chiral Palladium(II) Complexes. Tetrahedron Asymm. 2008, 19, 38–44. [Google Scholar] [CrossRef]
- Nesbitt, C.L.; McErlean, C.S.P. An Expedient Synthesis of 2,5-Disubstituted-3-oxygenated Tetrahydrofurans. Tetrahedron Lett. 2009, 50, 6318–6320. [Google Scholar] [CrossRef]
- Nesbitt, C.L.; McErlean, C.S.P. Total Synthesis of C19 Lipid Diols Containing a 2,5-Disubstituted-3-Oxygenated Tetrahydrofuran. Org. Biomol. Chem. 2011, 9, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Haynes, P.Y.; Chow, S.; Rahm, F.; Bernhardt, P.V.; De Voss, J.J.; Kitching, W. Synthesis of the Sponge-Derived Plakortone Series of Bioactive Compounds. J. Org. Chem. 2010, 75, 6489–6501. [Google Scholar]
- Werness, J.B.; Tang, W. Stereoselective Total Synthesis of (−)-Kumausallene. Org. Lett. 2011, 13, 3664–3666. [Google Scholar] [CrossRef] [PubMed]
- Markovič, M.; Ďuranová, M.; Koóš, P.; Szolcsányi, P.; Gracza, T. Synthesis of bis-Tetrahydrofuran Subunit of (−)-Neopallavicinin. Tetrahedron 2013, 69, 4185–4189. [Google Scholar] [CrossRef]
- Markovič, M.; Koóš, P.; Čarný, T.; Sokoliová, S.; Bohačiková, N.; Moncol’, J.; Gracza, T. Total Synthesis, Configuration Assignment, and Cytotoxic Activity Evaluation of Protulactone A. J. Nat. Prod. 2017, 80, 1631–1638. [Google Scholar] [CrossRef]
- Markovič, M.; Koóš, P.; Gracza, T. A Short Asymmetric Synthesis of Sauropunols A–D. Synthesis 2017, 49, 2939–2942. [Google Scholar]
- Lopatka, P.; Gavenda, M.; Markovič, M.; Koóš, P.; Gracza, T. Flow Pd(II)-Catalyzed Cyclisation in the Total Synthesis of Jaspine B. Catalysts 2021, 11, 1513. [Google Scholar] [CrossRef]
- Kapitán, P.; Gracza, T. Asymmetric Intramolecular Pd(II)-catalyzed Oxycarbonylation of Alkene-1,3-diols. Arkivoc 2008, viii, 8–17. [Google Scholar]
- Doháňošová, J.; Lásikivá, A.; Toffano, M.; Gracza, T.; Vo-Thanh, G. Kinetic Resolution of Pent-4-ene-1,3-diol by Pd(II)-Catalysed Oxycarbonylation in Ionic Liquids. New J. Chem. 2012, 36, 1744–1750. [Google Scholar] [CrossRef]
- Babjak, M.; Markovič, K.; Kandríkova, B.; Gracza, T. Homogeneous Cyclocarbonylation of Alkenols with Iron Pentacarbonyl. Synthesis 2014, 46, 809–816. [Google Scholar] [CrossRef]
- Markovič, K.; Lopatka, P.; Koóš, P.; Gracza, T. Asymmetric Formal Synthesis of (+)-Pyrenolide D. Synthesis 2014, 46, 817–821. [Google Scholar]
- Lopatka, P.; Markovič, K.; Koóš, P.; Ley, S.V.; Gracza, T. Continuous Pd-Catalyzed Carbonylative Cyclization Using Iron Pentacarbonyl as a CO Source. J. Org. Chem. 2019, 84, 14394–14406. [Google Scholar] [CrossRef] [PubMed]
- Babjak, M.; Zálupský, P.; Gracza, T. Regiocontrol in the Palladium(II)-Catalysed Oxycarbonylation of Unsaturated Polyols. Arkivoc 2005, 45, 57. [Google Scholar] [CrossRef]
- Tamaru, Y.; Kobayashi, T.; Kawamura, S.; Ochiai, H.; Yoshida, Z. Stereoselective Intramolecular Aminocarbonylation of 3-Hydroxypent-4-enylamides Catalyzed by Palladium. Tetrahedron Lett. 1985, 26, 4479–4482. [Google Scholar] [CrossRef]
- Tamaru, Y.; Hojo, M.; Yoshida, Z. Palladium(2+)-Catalyzed Intramolecular Aminocarbonylation of 3-Hydroxy-4-pentenylamines and 4-Hydroxy-5-hexenylamines. J. Org. Chem. 1988, 53, 5731–5741. [Google Scholar] [CrossRef]
- Hümmer, W.; Dubois, E.; Gracza, T.; Jäger, V. Halocyclization and Palladium(II)-Catalyzed Amidocarbonylation of Unsaturated Aminopolyols. Synthesis of 1,4-Iminoglycitols as Potential Glycosidase Inhibitors. Synthesis 1997, 1997, 634–642. [Google Scholar] [CrossRef]
- Caletková, O.; Ďurišová, N.; Gracza, T. Aminohydroxylation of Divinylcarbinol and its Application to the Synthesis of Bicyclic hydroxypyrrolidine and Aminotetrahydrofuran Building Blocks. Chem. Pap. 2013, 67, 66–75. [Google Scholar] [CrossRef]
- Koóš, P.; Špánik, I.; Gracza, T. Asymmetric Intramolecular Pd(II)-Catalysed Amidocarbonylation of Unsaturated Amino Alcohols. Tetrahedron Asymm. 2009, 20, 2720–2723. [Google Scholar] [CrossRef]
- Shi, L.; Weng, M.; Li, F. Palladium-Catalyzed Tandem Carbonylative Aza-Wacker-Type Cyclization of Nucleophile Tethered Alkene to Access Fused N-Heterocycles. Chin. J. Chem. 2021, 39, 317–322. [Google Scholar] [CrossRef]
- Lee, H.-W.; Kwong, F.-Y. A Decade of Advancements in Pauson-Khand-Type Reactions. Eur. J. Org. Chem. 2010, 2010, 789–811. [Google Scholar] [CrossRef]
- Shibata, T. Recent Advances in the Catalytic Pauson-Khand-type Reaction. Adv. Synth. Catal. 2006, 348, 2328–2336. [Google Scholar] [CrossRef]
- Blanco-Urgoiti, J.; Añorbe, L.; Pérez-Serrano, L.; Domínguez, G.; Pérez-Castells, J. The Pauson–Khand Reaction, a Powerful Synthetic Tool for the Synthesis of Complex Molecules. Chem. Soc. Rev. 2004, 33, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Mohammadi, L. Application of Pauson-Khand Reaction in the Total Synthesis of Terpenes. RSC Adv. 2021, 11, 38325–38373. [Google Scholar] [CrossRef]
- Yang, Z. Navigating the Pauson-Khand Reaction in Total Syntheses of Complex Natural Products. Acc. Chem. Res. 2021, 54, 556–568. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, C.; Zheng, N.; Yang, Z.; Shi, L. Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020). Catalysts 2020, 10, 1199. [Google Scholar] [CrossRef]
- Keese, F.; Guidetti-Grept, R.; Herzog, B. Synthesis of [5.5.5.5]Fenestranes by Pd-Catalyzed Carbonylation-Cyclisation. Tetrahedron Lett. 1992, 33, 1207–1210. [Google Scholar] [CrossRef]
- Yasuhara, S.; Sasa, M.; Kusakabe, T.; Takayama, H.; Kimura, M.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reactions of Propargyl Acetates and Amides with Palladium(II)–Bisoxazoline Catalysts. Angew. Chem. Int. Ed. 2011, 50, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Kusakabe, T.; Yatsu, T.; Kanno, Y.; Takahashi, K.; Nemoto, K.; Kato, K. Palladium(II) Catalyzed Cyclization-Carbonylation-Cyclization Coupling Reaction of (ortho-Alkynyl Phenyl) (Methoxymethyl) Sulfides Using Molecular Oxygen as the Terminal Oxidant. Molecules 2016, 21, 1177. [Google Scholar] [CrossRef]
- Bartish, C.M.; Drissel, G.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Grayson, M., Eckroth, D., Bushey, G.J., Campbell, L., Klingsberg, A., van Nes, L., Eds.; John Wiley & Sons: New York, NY, USA, 1978; Volume 4, p. 774. [Google Scholar]
- Kusakabe, T.; Kawaguchi, K.; Kawamura, M.; Niimura, N.; Shen, R.; Takayama, H.; Kato, K. Cyclization-Carbonylation-Cyclization Coupling Reaction of Propargyl Ureas with Palladium(II)-bisoxazoline Catalyst. Molecules 2012, 17, 9220–9230. [Google Scholar] [CrossRef]
- Kusakabe, T.; Kawai, Y.; Shen, R.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reaction of γ-Propynyl-1,3-diketones with Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2012, 10, 3192–3194. [Google Scholar] [CrossRef]
- Kusakabe, T.; Sekiyama, E.; Ishino, Y.; Motodate, S.; Kato, S.; Mochida, T.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling Reactions of N-Propargylanilines and o-Alkynylphenols with Palladium(II)–bisoxazoline Catalysts. Synthesis 2012, 44, 1825–1832. [Google Scholar] [CrossRef]
- Kusakabe, T.; Sagae, H.; Kato, K. Cyclization–Carbonylation–Cyclization Coupling reaction of α,β-Alkynic Hydrazones with Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2013, 11, 4943–4948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kusakabe, T.; Takahashi, K.; Kato, K. A Cyclization–Carbonylation–Cyclization Coupling Reaction of (ortho-Alkynyl phenyl) (Methoxymethyl) Sulfides with the Palladium(II)-bisoxazoline Catalyst. Org. Biomol. Chem. 2014, 12, 3380–3385. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Kusakabe, T.; Takahashi, K.; Kato, K. Pd(II)-Catalyzed Ligand Controlled Synthesis of Methyl 1-benzyl-1H-indole-3-carboxylates and Bis(1-benzyl-1H-indol-3-yl)methanones. Org. Biomol. Chem. 2014, 12, 4602–4609. [Google Scholar] [CrossRef]
- Ariyama, T.; Kusakabe, T.; Sato, K.; Funatogawa, M.; Lee, D.; Takahashi, K.; Kato, K. Pd(II)-Catalyzed Ligand-Controlled Synthesis of 2,3-Dihydroisoxazole-4-carboxylates and Bis(2,3-dihydroisoxazol-4-yl)methanones. Heterocycles 2016, 93, 512–518. [Google Scholar]
- Kubasabe, T.; Mochida, T.; Ariyama, T.; Lee, D.; Ohkubo, S.; Takahashi, K.; Kato, K. PdII Catalyzed Ligand Controlled Synthesis of Bis(3-furanyl)methanones and Methyl 3-furancarboxylates. Org. Biomol. Chem. 2019, 17, 6860–6865. [Google Scholar]
- Gabriele, B.; Chimento, A.; Mancuso, R.; Pezzi, V.; Ziccarelli, I.; Sirianni, R. Derivati 6,6a-diidrofuro[3,2-b]furan-2-(5H)onici, loro Preparazione e Uso nel Trattamento dei Tumori. Italian Patent 102017000078586, 8 October 2019. [Google Scholar]
- Gabriele, B.; Chimento, A.; Mancuso, R.; Pezzi, V.; Ziccarelli, I.; Sirianni, R. 6,6a-Dihydrofuro[3,2-b]furan-2-(5H)one Derivatives, their Preparation and Use for Treating Tumors. European Patent EP3428169, 20 January 2021. [Google Scholar]
- Mancuso, R.; Ziccarelli, I.; Chimento, A.; Marino, N.; Della Ca’, N.; Sirianni, R.; Pezzi, V.; Gabriele, B. Catalytic Double Cyclization Process for Antitumor Agents against Breast Cancer Cell Lines. iScience 2018, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, A.; Carfagna, C.; Costa, M.; Mancuso, R.; Gabriele, B.; Della Ca’, N. An Unprecedented Pd-Catyalyzed Carbonylative Route to Fused Furo[3,4-b]indol-1-ones. Chem. Eur. J. 2018, 24, 4835–4840. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2–Based Catalysis for Carbonylation Reactions: A Personal Account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef]
- Gabriele, B. Recent Advances in the PdI2-Catalyzed Carbonylative Synthesis of Heterocycles from Acetylenic Substrates: A Personal Account. Targets Heterocycl. Syst. 2018, 22, 41–55. [Google Scholar]
- Gabriele, B.; Salerno, G. PdI2. In e-EROS (Electronic Encyclopedia of Reagents for Organic Synthesis); Crich, D., Ed.; Wiley–Interscience: New York, NY, USA, 2006. [Google Scholar]
- Gabriele, B.; Salerno, G.; Costa, M. PdI2-Catalyzed Synthesis of Heterocycles. Synlett 2004, 2004, 2468–2483. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G.P. Recent Developments in the Synthesis of Heterocyclic Derivatives by PdI2-Catalyzed Oxidative Carbonylation Reactions. J. Organomet. Chem. 2003, 687, 219–228. [Google Scholar] [CrossRef]
- Gabriele, B.; Costa, M.; Salerno, G.; Chiusoli, G.P. An Efficient and Selective Palladium-Catalysed Oxidative Sicarbonylation of Alkynes to Alkyl- or Aryl-Maleic Esters. J. Chem. Soc. Perkin Trans. 1 1994, 1994, 83–87. [Google Scholar] [CrossRef]
- Mancuso, R.; Miliè, R.; Palumbo Piccionello, A.; Olivieri, D.; Della Ca’, N.; Carfagna, C.; Gabriele, B. Catalytic Carbonylative Double Cyclization of 2-(3-Hydroxy-1-yn-1-yl)phenols in Ionic Liquids Leading to Furobenzofuranone Derivatives. J. Org. Chem. 2019, 84, 7303–7311. [Google Scholar] [CrossRef]
- Pancrazzi, F.; Sarti, N.; Mazzeo, P.P.; Bacchi, A.; Carfagna, C.; Mancuso, R.; Gabriele, B.; Stirling, A.; Della Ca’, N. Site-Selective Double and Tetracyclization Routes to Fused Polyheterocyclic Structures by Pd-Catalyzed Carbonylation Reactions. Org. Lett. 2020, 22, 1569–1574. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Della Ca’, N.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-Catalyzed Synthesis of Symmetrical Urea Derivatives by Oxidative Carbonylation of Primary Amines in Carbon Dioxide Medium. J. Catal. 2011, 282, 120–127. [Google Scholar]
- Mancuso, R.; Russo, P.; Lettieri, M.; Santandrea, M.; Cuocci, C.; Gabriele, B. Disclosing Polycyclic Heterocycles: Synthesis of Furothienopyran and Pyranothienopyran Derivatives by Palladium Iodide Catalyzed Carbonylative Double Cyclization. Adv. Synth. Catal. 2022, 364, 3917–3926. [Google Scholar] [CrossRef]
- Mancuso, R.; Strangis, R.; Ziccarelli, I.; Della Ca’, N.; Gabriele, B. Palladium Catalysis with Sulfurated Substrates under Aerobic Conditions: A Direct Oxidative Carbonylation Approach to Thiophene-3-carboxylic Esters. J. Catal. 2021, 393, 335–343. [Google Scholar] [CrossRef]
- Mancuso, R.; Cuglietta, S.; Strangis, R.; Gabriele, B. Synthesis of Benzothiophene-3-carboxylic Esters by Palladium Iodide-Catalyzed Oxidative Cyclization−Deprotection−Alkoxycarbonylation Sequence under Aerobic Conditions. J. Org. Chem. 2023, 88, 5180–5186. [Google Scholar] [CrossRef]
- Mancuso, R.; Russo, P.; Miliè, R.; Dell’Aera, M.; Grande, F.; Della Ca’, N.; Gabriele, B. Palladium Iodide Catalyzed Carbonylative Double Cyclization to a New Class of S,O-Bicyclic Heterocycles. Catal. Today 2022, 397–399, 631–638. [Google Scholar] [CrossRef]
- Chen, J.; Neumann, H.; Beller, M.; Wu, X.-F. Palladium-Catalyzed Synthesis of Isoindoloquinazolinones via Dicarbonylation of 1,2-Dibromoarenes. Org. Biomol. Chem. 2014, 12, 5835–5838. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Man, N.Y.T.; Stewart, S.; Wu, X.-F. Palladium-Catalyzed Dicarbonylative Synthesis of Tetracycle Quinazolinones. Org. Biomol. Chem. 2015, 13, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Natte, K.; Chen, J.; Li, H.; Neumann, H.; Beller, M.; Wu, X.-F. Palladium-Catalyzed Carbonylation of 2-Bromoanilines with 2-Formylbenzoic Acid and 2-Halobenzaldehydes: Efficient Synthesis of Functionalized Isoindolinones. Chem. Eur. J. 2014, 20, 14184–14188. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Spannenberg, A.; Baumann, W.; Neumann, H.; Beller, M.; Wu, X.-F. A Novel Domino Synthesis of Quinazolinediones by Palladium-Catalyzed Double Carbonylation. Chem. Eur. J. 2014, 20, 8541–8544. [Google Scholar] [CrossRef]
- Frutos-Pedreño, R.; García-López, J.-A. 2-Arylacetamides as Versatile Precursors for 3-Aminoisocoumarin and Homophthalimide Derivatives: Palladium-Catalyzed Cascade Double Carbonylation Reactions. Adv. Synth. Catal. 2016, 358, 2692–2700. [Google Scholar] [CrossRef]









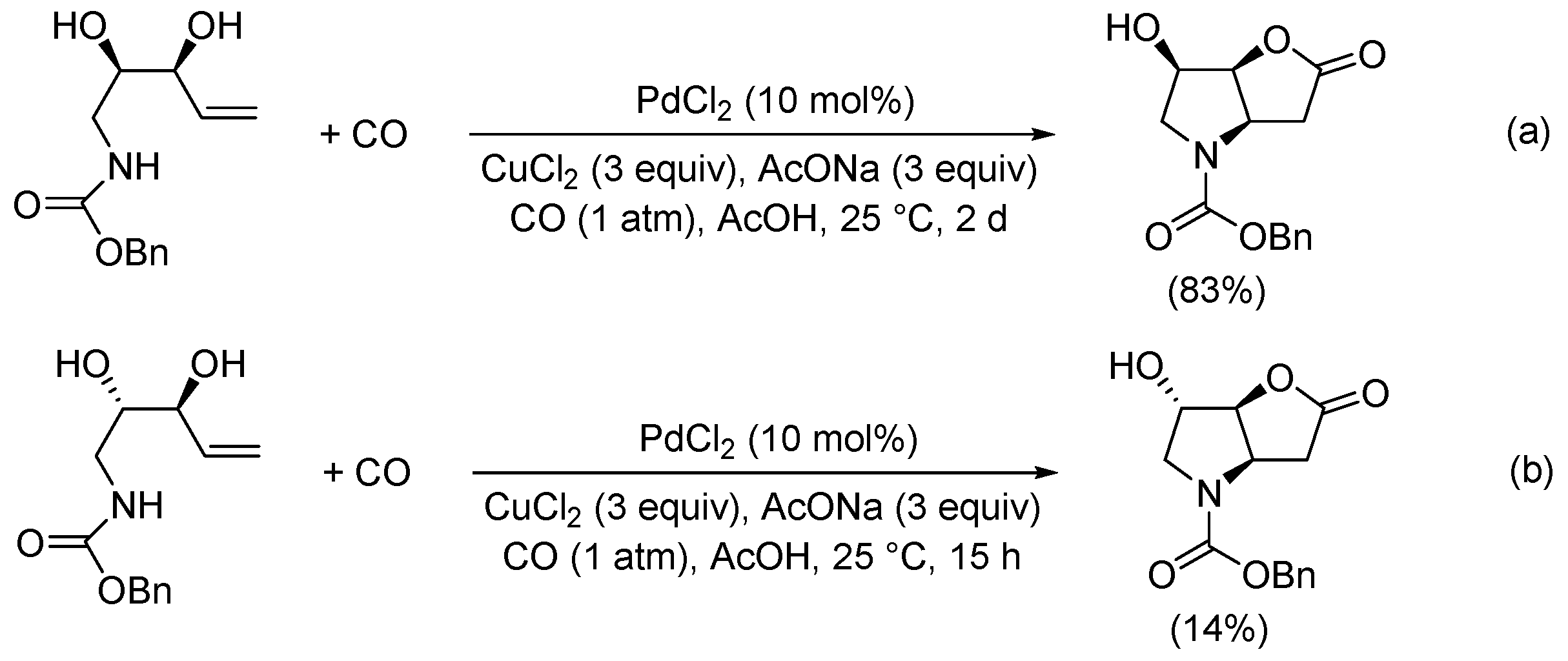
















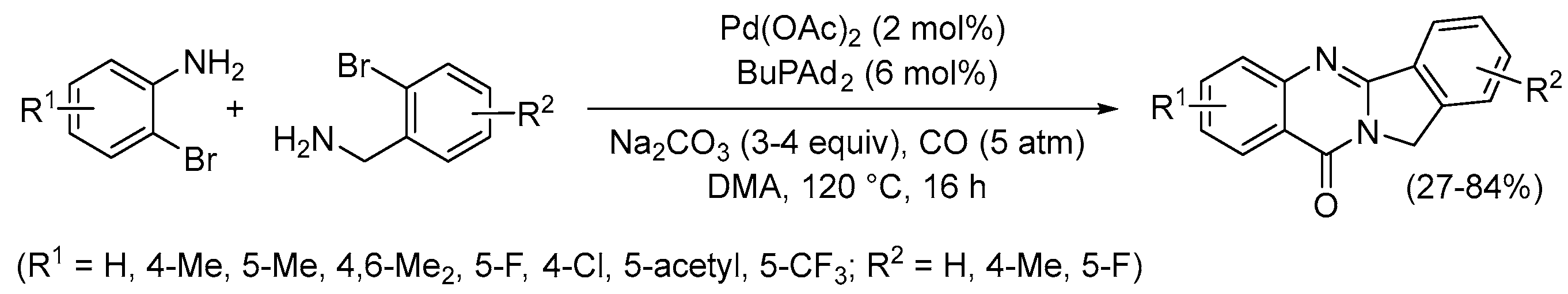





| Entry | Conditions | Substrate | Product | Yield (%) | Refs. |
|---|---|---|---|---|---|
| 1 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 41 h | 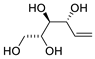 |  | 63 | [10] |
| 2 | PdCl2(MeCN)2, (10 mol%), CuCl2 (2.4 equiv), CO (1 atm), THF, 25 °C, 24 h | 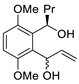 |  | 65 | [11] |
| 3 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 8 h |  |  | 85 | [12] |
| 4 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (4 equiv), CO (1 atm), AcOH, 25 °C, 24 h |  |  | 93 | [13] |
| 5 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 33 h |  |  | 38 | [14] |
| 6 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 15 h | 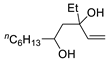 |  | >80 | [15] |
| 7 | Pd(OAc)2 (1.5 equiv), CO (1.1 atm), THF, 23 °C, 4 h |  |  | 87 | [16] |
| 8 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 15 h |  |  | 81 | [17] |
| 9 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C |  |  | 63 | [18] |
| 10 | PdCl2, CuCl, AcONa, CO, AcOH |  |  | 33 | [19] |
| 11 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 10 h |  | 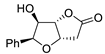 | 85 | [20] |
| 12 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 23 °C, 24 h | 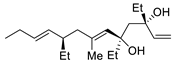 |  | 75 | [21] |
| 13 | Pd(OAc)2 (1.5 equiv), N-methylmorpholine (3 equiv), CO, THF, 25 °C, 15 h |  |  | 58 | [22] |
| 14 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 20 h |  |  | 65 | [23] |
| 15 | Pd(OAc)2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 15 h | 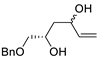 |  | 63, 70 | [24,25] |
| 16 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 24 h |  |  | 33 | [26] |
| 17 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 24 h |  |  | 87 | [27] |
| 18 | PdCl2 (10 mol%), CuCl2 (3 equiv), AcONa (3 equiv), CO (1 atm), AcOH, 25 °C, 12 h | 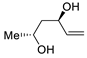 |  | 61 | [28] |
| 19 | PdCl2(MeCN)2 (10 mol%), CuCl2 (5 equiv), AcOLi (5 equiv), [Fe(CO)5] (0.5 equiv), AcOH, 60 °C, 1 h |  | 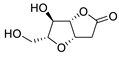 | 47 | [29] |
| 20 | PdCl2(MeCN)2 (10 mol%), Cu(OAc)2 (4 equiv), LiCl (4 equiv), [Fe(CO)5] (0.25 equiv), AcOH, 60 °C, 15 min | 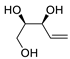 |  | 67 | [30] |
| 21 | PdCl2(MeCN)2 (10 mol%), CuCl2 (4 equiv), AcOLi (4 equiv), [Fe(CO)5] (0.3 equiv), AcOH, 60 °C, 30 min | 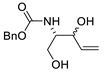 |  | 75 | [31] |
| Entry | Conditions | Substrate | Product | Yields (%) | Refs. |
|---|---|---|---|---|---|
| 1 | Pd(tfa)2 (5 mol%),  (10 mol%), p-benzoquinone (10 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 0 °C, 5–12 h |  |  | 90–92 | [51] |
| 2 | Pd(L)(tfa)2 (5 mol%), L = 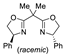 p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, 7 to 25 °C, 18–48 h |  |  | 24–89 | [54] |
| 3 | Pd(tfa)2 (5–10 mol%), 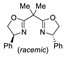 (7.5–12 mol%), (7.5–12 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, −30 to 0 °C, 2–53 h | 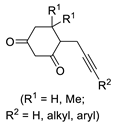 | 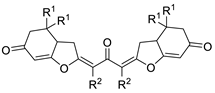 | 71–99 | [55] |
| 4 | Pd(tfa)2 (5 mol%), 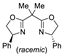 (7.5 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 25 °C, 1–63 h (7.5 mol%), p-benzoquinone (2 equiv), CO (1 atm), MeOH, 25 °C, 1–63 h |  |  | 10–89 | [56] |
| 5 | Pd(L)(tfa)2 (5 mol%), L = 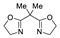 p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, −5 to 25 °C, 1–46 h | 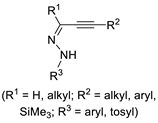 | 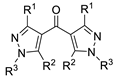 | 70–94 | [57] |
| 6 | Pd(L)(tfa)2 (5 mol%), L = 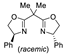 p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, −30 to 25 °C, 24–144 h |  | 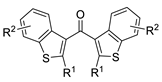 | 75–100 | [52,58] |
| 7 | Pd(L)(tfa)2 (5 mol%), L =  p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), iPrOH, −5 to 15 °C, 47–72 h |  |  | 73–92 | [59] |
| 8 | Pd(L)(tfa)2 (5 mol%), L =  p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, −20 to 0 °C, 24–76 h |  | 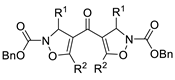 | 70–94 | [60] |
| 9 | Pd(L)(tfa)2 (5 mol%), L =  p-benzoquinone p-benzoquinone (1.5 equiv), CO (1 atm), MeOH, −5 to 25 °C, 24–55 h | 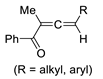 | 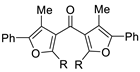 | 12–86 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriele, B.; Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I. An Overview of Catalytic Carbonylative Double Cyclization Reactions. Catalysts 2023, 13, 1025. https://doi.org/10.3390/catal13061025
Gabriele B, Mancuso R, Della Ca’ N, Veltri L, Ziccarelli I. An Overview of Catalytic Carbonylative Double Cyclization Reactions. Catalysts. 2023; 13(6):1025. https://doi.org/10.3390/catal13061025
Chicago/Turabian StyleGabriele, Bartolo, Raffaella Mancuso, Nicola Della Ca’, Lucia Veltri, and Ida Ziccarelli. 2023. "An Overview of Catalytic Carbonylative Double Cyclization Reactions" Catalysts 13, no. 6: 1025. https://doi.org/10.3390/catal13061025
APA StyleGabriele, B., Mancuso, R., Della Ca’, N., Veltri, L., & Ziccarelli, I. (2023). An Overview of Catalytic Carbonylative Double Cyclization Reactions. Catalysts, 13(6), 1025. https://doi.org/10.3390/catal13061025