Effect of Molecular Structure of C10 Hydrocarbons on Production of Light Olefins in Catalytic Cracking
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Catalyst
2.1.1. Textural Properties of Catalyst
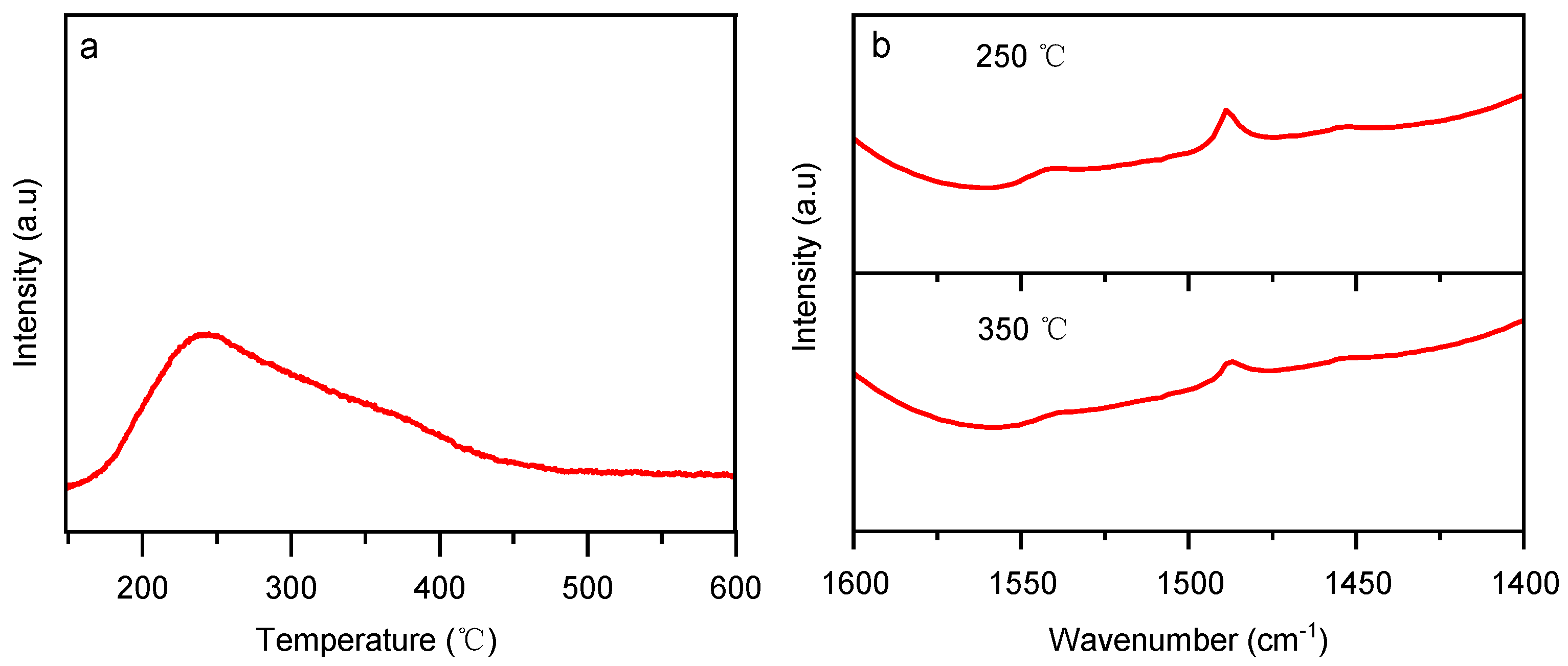
2.1.2. Catalysis Acidity
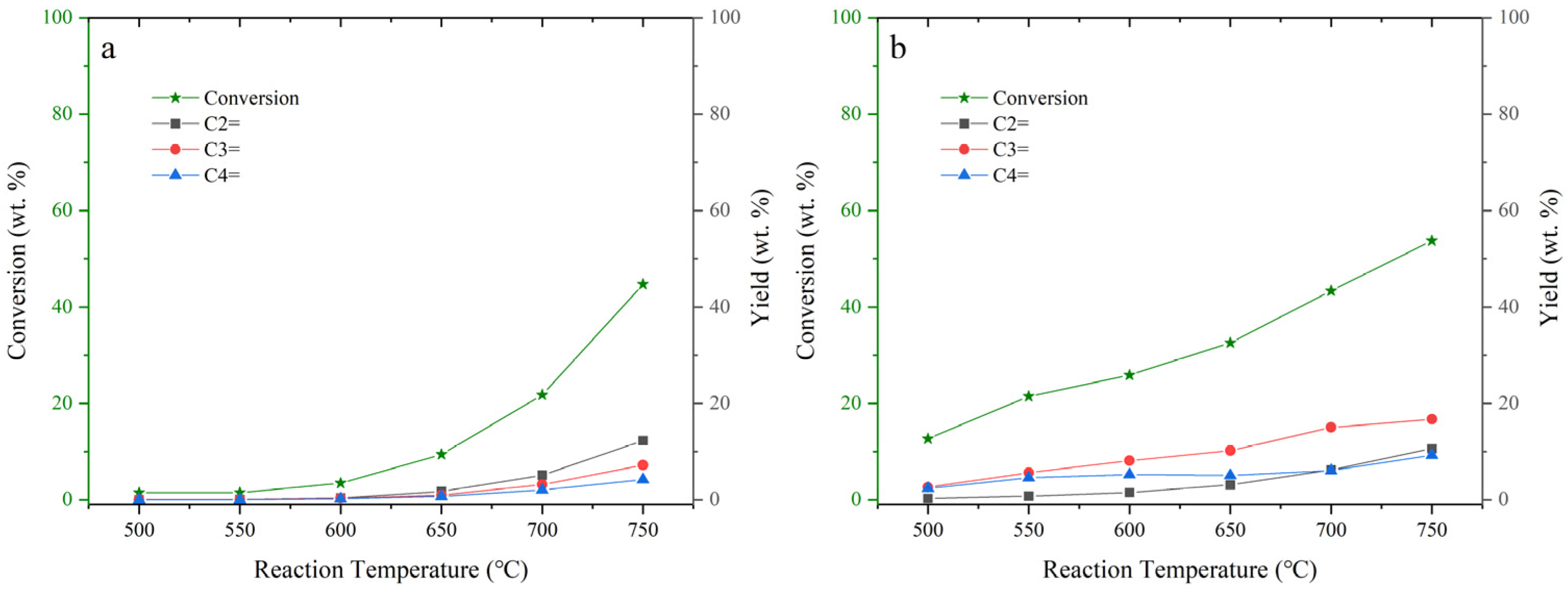
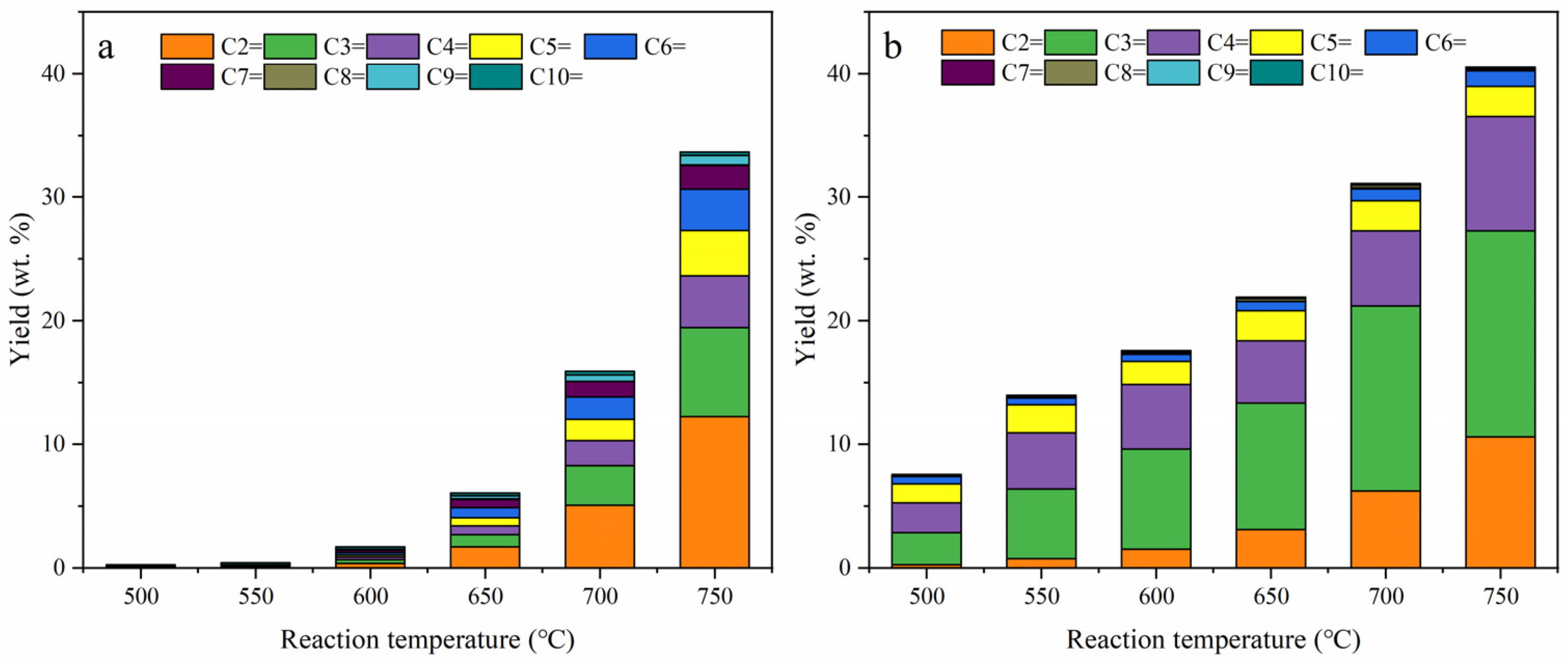
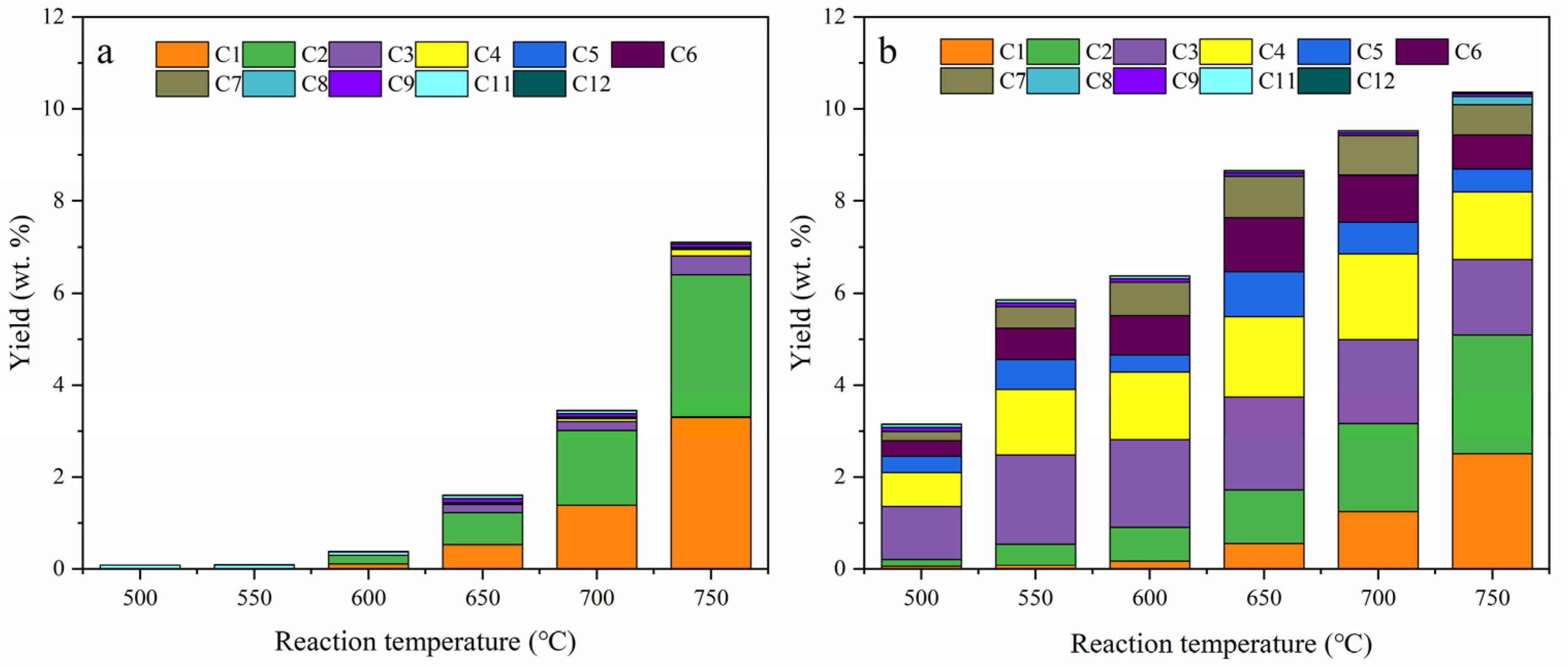
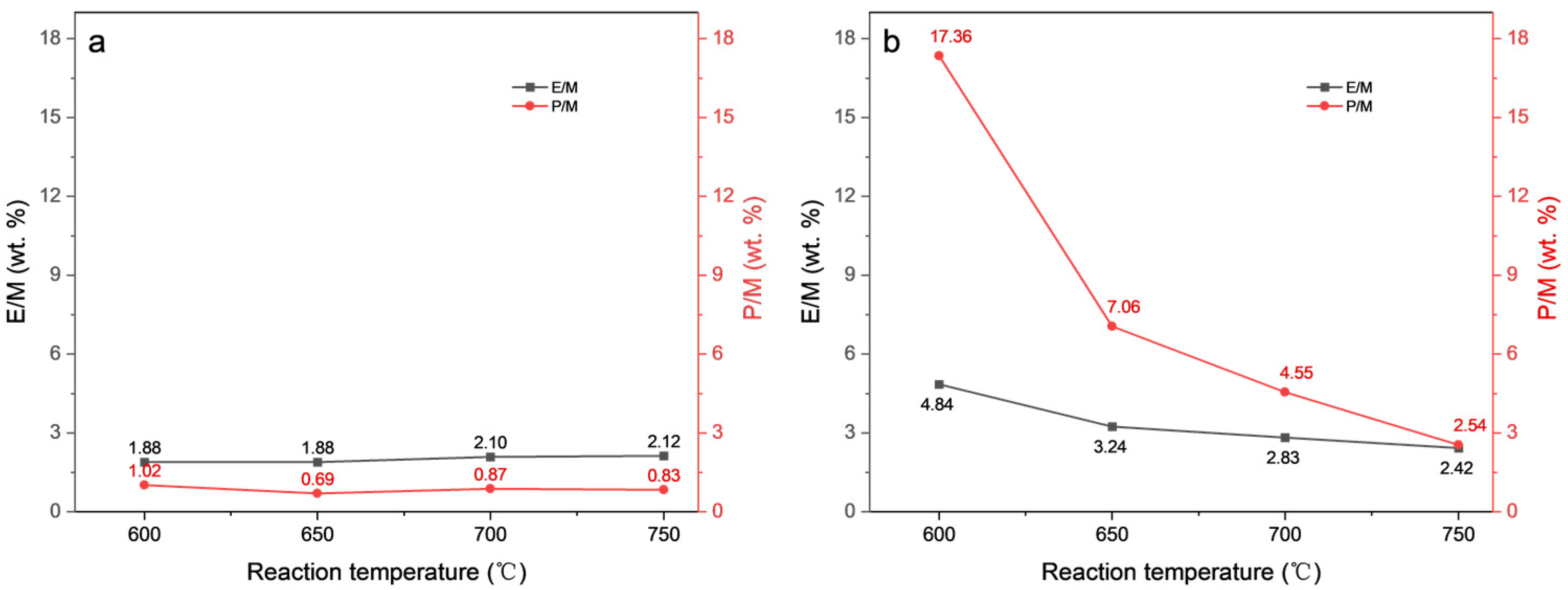
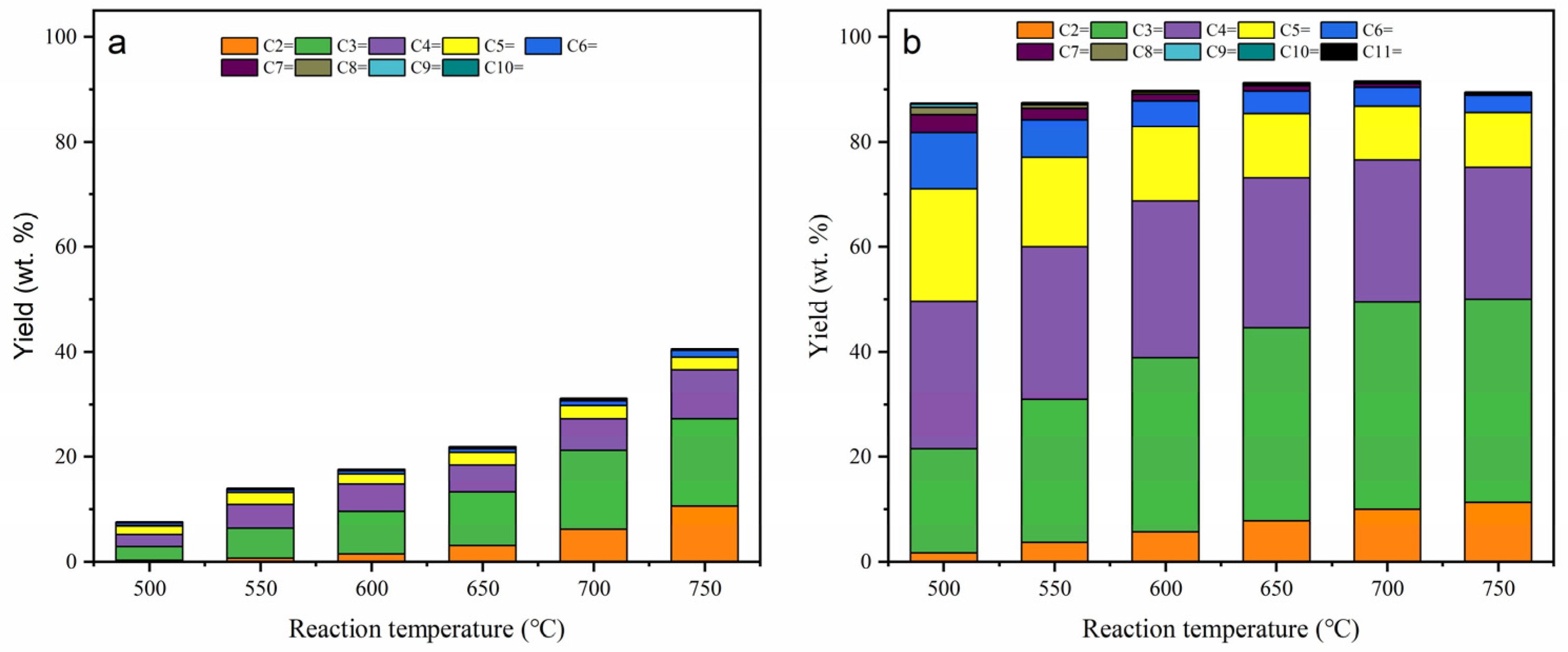
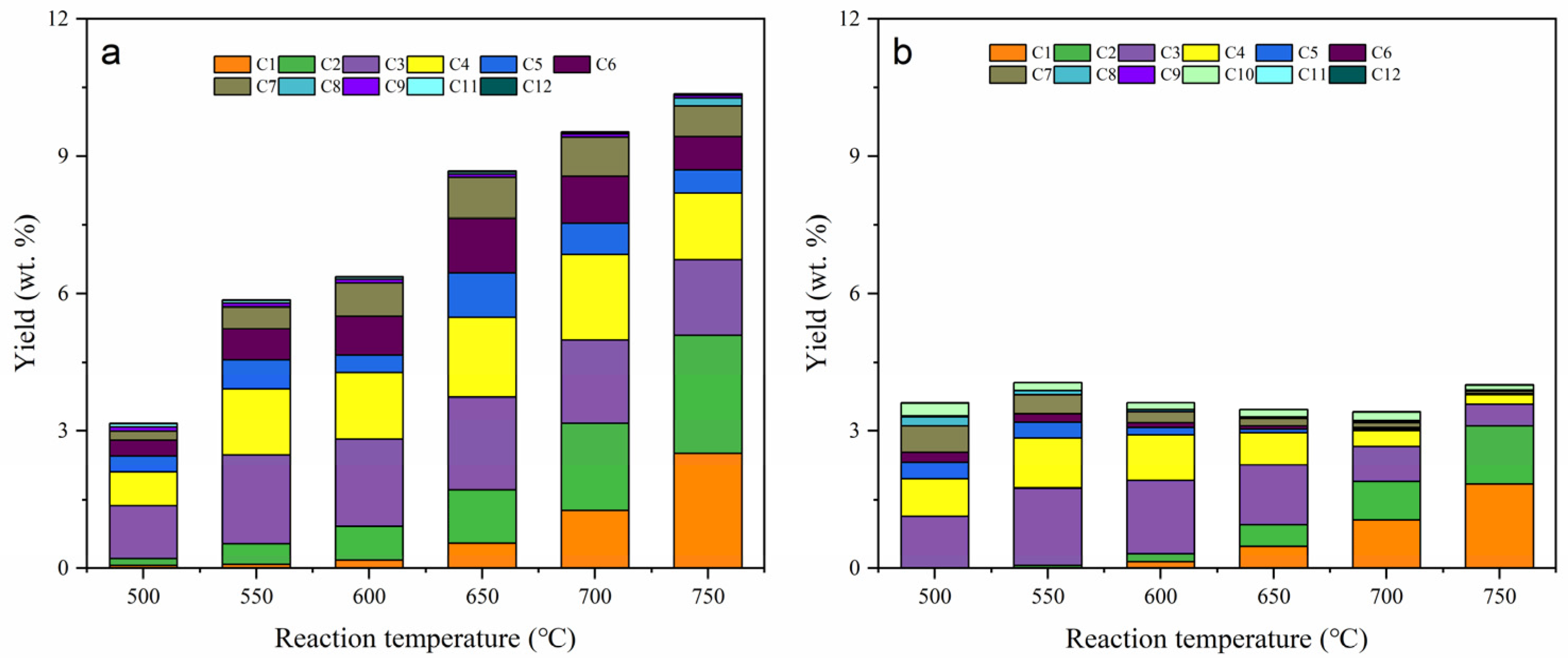
2.2. Thermal Cracking and Catalytic Cracking of Decane
2.3. Catalytic Cracking of Decane and Decene
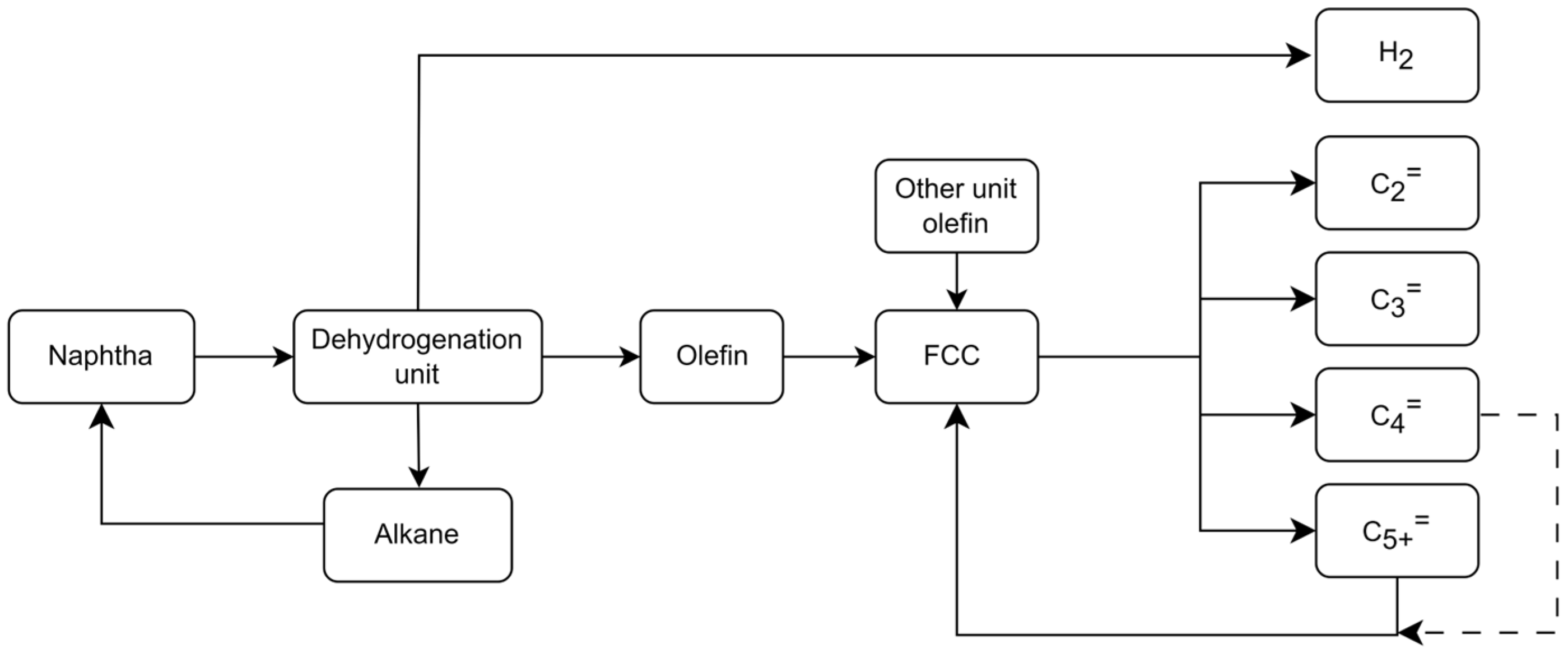
2.4. Light Olefin Production Scheme
3. Experimental Section
3.1. Materials
3.2. Catalytic Cracking Experiment
3.3. Catalysts Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blay, V.; Epelde, E.; Miravalles, R.; Perea, L.A. Converting olefins to propene: Ethene to propene and olefin cracking. Catal. Rev. 2018, 60, 278–335. [Google Scholar] [CrossRef]
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Corda, M.; Dath, J.-P.; Ordomsky, V.V.; Khodakov, A.Y. Light olefin synthesis from a diversity of renewable and fossil feedstocks: State-of the-art and outlook. Chem. Soc. Rev. 2022, 51, 7994–8044. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Neal, L.; Ding, D.; Wu, W.; Baroi, C.; Gaffney, A.M.; Li, F. Recent Advances in Intensified Ethylene Production—A Review. ACS Catal. 2019, 9, 8592–8621. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Young, B.; Hawkins, T.R.; Chiquelin, C.; Sun, P.; Gracida-Alvarez, U.R.; Elgowainy, A. Environmental life cycle assessment of olefins and by-product hydrogen from steam cracking of natural gas liquids, naphtha, and gas oil. J. Clean. Prod. 2022, 359, 131884. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Ma, H.; Sun, Q.; Li, C.; Ying, W.; Fang, D. Kinetics of coupling cracking of butene and pentene on modified HZSM-5 catalyst. Chem. Eng. J. 2018, 346, 397–405. [Google Scholar] [CrossRef]
- Guo, Y.-H.; Pu, M.; Wu, J.-Y.; Zhang, J.-Y.; Chen, B.-H. Theoretical study of the cracking mechanisms of linear α-olefins catalyzed by zeolites. Appl. Surf. Sci. 2007, 254, 604–609. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, J.; Liu, C.; Wang, L. Mechanistic insight into the catalytic cracking mechanism of α-olefin on H-Y zeolite: A DFT study. Comput. Theor. Chem. 2021, 1198, 113183. [Google Scholar] [CrossRef]
- Hao, J.; Cheng, D.-g.; Chen, F.; Zhan, X. n-Heptane catalytic cracking on ZSM-5 zeolite nanosheets: Effect of nanosheet thickness. Microporous Mesoporous Mater. 2021, 310, 110647. [Google Scholar] [CrossRef]
- Corma, A.; Mengual, J.; Miguel, P.J. Stabilization of ZSM-5 zeolite catalysts for steam catalytic cracking of naphtha for production of propene and ethene. Appl. Catal. A Gen. 2012, 421–422, 121–134. [Google Scholar] [CrossRef]
- Hou, X.; Ni, N.; Wang, Y.; Zhu, W.; Qiu, Y.; Diao, Z.; Liu, G.; Zhang, X. Roles of the free radical and carbenium ion mechanisms in pentane cracking to produce light olefins. J. Anal. Appl. Pyrolysis 2019, 138, 270–280. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Xu, Y.; Zuo, Y.; Bai, X.; Du, L.; Han, Y. Development Background, Development Idea and Conceptual Design of FCC Process for Targeted Cracking to Light Olefins. Pet. Process. Petrochem. 2021, 52, 1–11. [Google Scholar]
- Kissin, Y.V. Chemical Mechanisms of Catalytic Cracking over Solid Acidic Catalysts: Alkanes and Alkenes. Catal. Rev. 2001, 43, 85–146. [Google Scholar] [CrossRef]
- Chen, C.-J.; Rangarajan, S.; Hill, I.M.; Bhan, A. Kinetics and Thermochemistry of C4–C6 Olefin Cracking on H-ZSM-5. ACS Catal. 2014, 4, 2319–2327. [Google Scholar] [CrossRef]
- Standl, S.; Kühlewind, T.; Tonigold, M.; Hinrichsen, O. On Reaction Pathways and Intermediates During Catalytic Olefin Cracking over ZSM-5. Ind. Eng. Chem. Res. 2019, 58, 18107–18124. [Google Scholar] [CrossRef]
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering Zeolites for Catalytic Cracking to Light Olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- del Campo, P.; Navarro, M.T.; Shaikh, S.K.; Khokhar, M.D.; Aljumah, F.; Martínez, C.; Corma, A. Propene Production by Butene Cracking. Descriptors for Zeolite Catalysts. ACS Catal. 2020, 10, 11878–11891. [Google Scholar] [CrossRef]
- Zhao, R.; Haller, G.L.; Lercher, J.A. Alkene adsorption and cracking on acidic zeolites—A gradual process of understanding. Microporous Mesoporous Mater. 2022, 358, 112390. [Google Scholar] [CrossRef]
- He, M.; Ali, M.-F.; Song, Y.-Q.; Zhou, X.-L.; Wang, J.A.; Nie, X.-Y.; Wang, Z. Study on the deactivation mechanism of HZSM-5 in the process of catalytic cracking of n-hexane. Chem. Eng. J. 2023, 451, 138793. [Google Scholar] [CrossRef]
- Corma, A.; Miguel, P.J.; Orchillés, A.V. Product selectivity effects during cracking of alkanes at very short and longer times on stream. Appl. Catal. A: Gen. 1996, 138, 57–73. [Google Scholar] [CrossRef]
- Ishihara, A.; Ninomiya, M.; Hashimoto, T.; Nasu, H. Catalytic cracking of C12-C32 hydrocarbons by hierarchical β- and Y-zeolite-containing mesoporous silica and silica-alumina using Curie point pyrolyzer. J. Anal. Appl. Pyrolysis 2020, 150, 104876. [Google Scholar] [CrossRef]
- Bastiani, R.; Lam, Y.L.; Henriques, C.A.; Teixeira da Silva, V. Application of ferrierite zeolite in high-olefin catalytic cracking. Fuel 2013, 107, 680–687. [Google Scholar] [CrossRef]
- Lin, L.F.; Zhao, S.F.; Zhang, D.W.; Fan, H.; Liu, Y.M.; He, M.Y. Acid Strength Controlled Reaction Pathways for the Catalytic Cracking of 1-Pentene to Propene over ZSM-5. ACS Catal. 2015, 5, 4048–4059. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Arudra, P.; Bhuiyan, T.I.; Akhtar, M.N.; Aitani, A.M.; Al-Khattaf, S.S.; Hattori, H. Silicalite-1 As Efficient Catalyst for Production of Propene from 1-Butene. ACS Catal. 2014, 4, 4205–4214. [Google Scholar] [CrossRef]
- Lv, J.; Hua, Z.; Ge, T.; Zhou, J.; Zhou, J.; Liu, Z.; Guo, H.; Shi, J. Phosphorus modified hierarchically structured ZSM-5 zeolites for enhanced hydrothermal stability and intensified propylene production from 1-butene cracking. Microporous Mesoporous Mater. 2017, 247, 31–37. [Google Scholar] [CrossRef]
- Fan, J.; Xiao, C.; Mei, J.; Liu, C.; Duan, A.; Li, J.; Liu, J.; Zhang, M. A hierarchical ZSM-22/PHTS composite material and its hydro-isomerization performance in hydro-upgrading of gasoline. Catal. Sci. Technol. 2021, 11, 5448–5459. [Google Scholar] [CrossRef]
- Vitale, G.; Molero, H.; Hernandez, E.; Aquino, S.; Birss, V.; Pereira-Almao, P. One-pot preparation and characterization of bifunctional Ni-containing ZSM-5 catalysts. Appl. Catal. A Gen. 2013, 452, 75–87. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Zhang, Y.; Zhao, L.; Gao, J.; Xu, C. Effect of Catalyst Acidity and Reaction Temperature on Hexene Cracking Reaction to Produce Propylene. Energy Fuels 2021, 35, 3295–3306. [Google Scholar] [CrossRef]
- Jermy, B.R.; Tanimu, A.; Siddiqui, M.A.; Qureshi, Z.S.; Aitani, A.; Akah, A.; Xu, Q.; AlHerz, M. Crude oil conversion to chemicals over green synthesized ZSM-5 zeolite. Fuel Process. Technol. 2023, 241, 107610. [Google Scholar] [CrossRef]
- Alabdullah, M.; Rodriguez-Gomez, A.; Shoinkhorova, T.; Dikhtiarenko, A.; Chowdhury, A.D.; Hita, I.; Kulkarni, S.R.; Vittenet, J.; Sarathy, S.M.; Castaño, P. One-step conversion of crude oil to light olefins using a multi-zone reactor. Nat. Catal. 2021, 4, 233–241. [Google Scholar] [CrossRef]



| Sample | Surface Area (m2g−1) | Pore Volume (cm3g−1) | ||||
|---|---|---|---|---|---|---|
| SBET | Smicro | Sext | Vtotal | Vmicro | Vmeso | |
| H-ZSM-5 | 379.74 | 370.35 | 9.39 | 0.18 | 0.16 | 0.02 |
| Sample | B Acidity (μmol g−1) | L Acidity (μmol g−1) | Total Acidity (μmol g−1) | B/L | ||||
|---|---|---|---|---|---|---|---|---|
| 250 °C | 350 °C | 250 °C | 350 °C | 250 °C | 350 °C | 250 °C | 350 °C | |
| H-ZSM-5 | 18.4 | 7.2 | 6.2 | 4.8 | 24.6 | 12.0 | 2.96 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Han, Y.; Xu, Y. Effect of Molecular Structure of C10 Hydrocarbons on Production of Light Olefins in Catalytic Cracking. Catalysts 2023, 13, 1013. https://doi.org/10.3390/catal13061013
Du L, Han Y, Xu Y. Effect of Molecular Structure of C10 Hydrocarbons on Production of Light Olefins in Catalytic Cracking. Catalysts. 2023; 13(6):1013. https://doi.org/10.3390/catal13061013
Chicago/Turabian StyleDu, Lingyin, Yueyang Han, and Youhao Xu. 2023. "Effect of Molecular Structure of C10 Hydrocarbons on Production of Light Olefins in Catalytic Cracking" Catalysts 13, no. 6: 1013. https://doi.org/10.3390/catal13061013
APA StyleDu, L., Han, Y., & Xu, Y. (2023). Effect of Molecular Structure of C10 Hydrocarbons on Production of Light Olefins in Catalytic Cracking. Catalysts, 13(6), 1013. https://doi.org/10.3390/catal13061013




