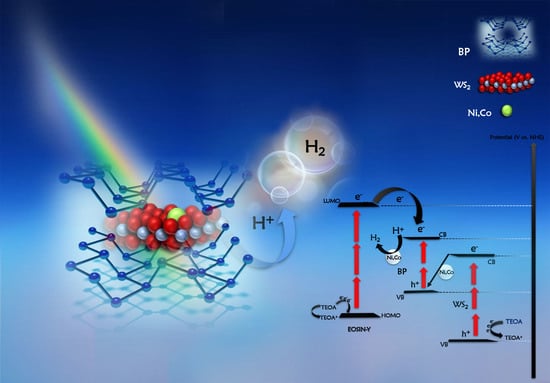
Black Phosphorus/WS2-TM (TM: Ni, Co) Heterojunctions for Photocatalytic Hydrogen Evolution under Visible Light Illumination
Abstract
1. Introduction
2. Results and Discussion
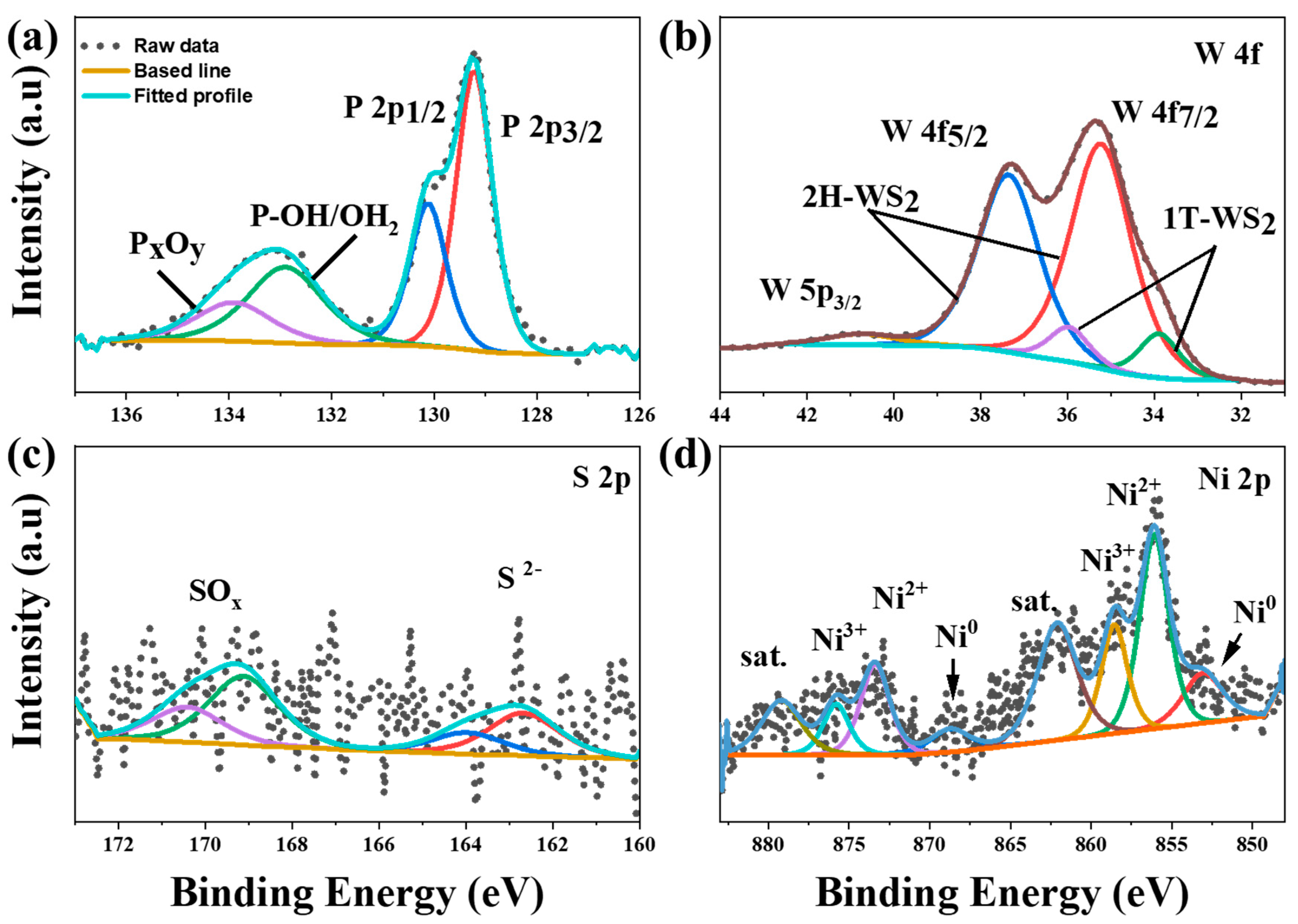
2.1. Materials Characterization
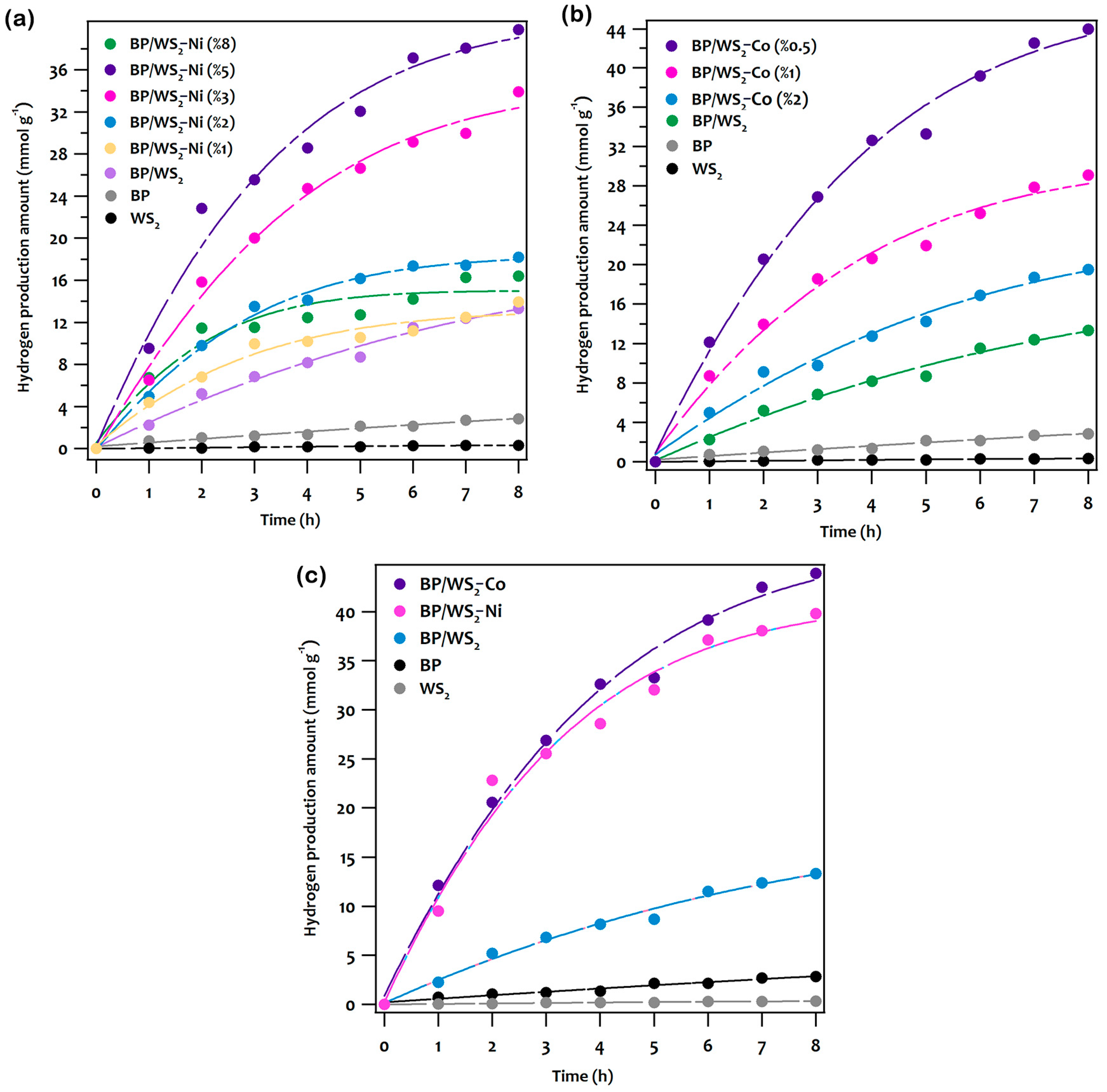
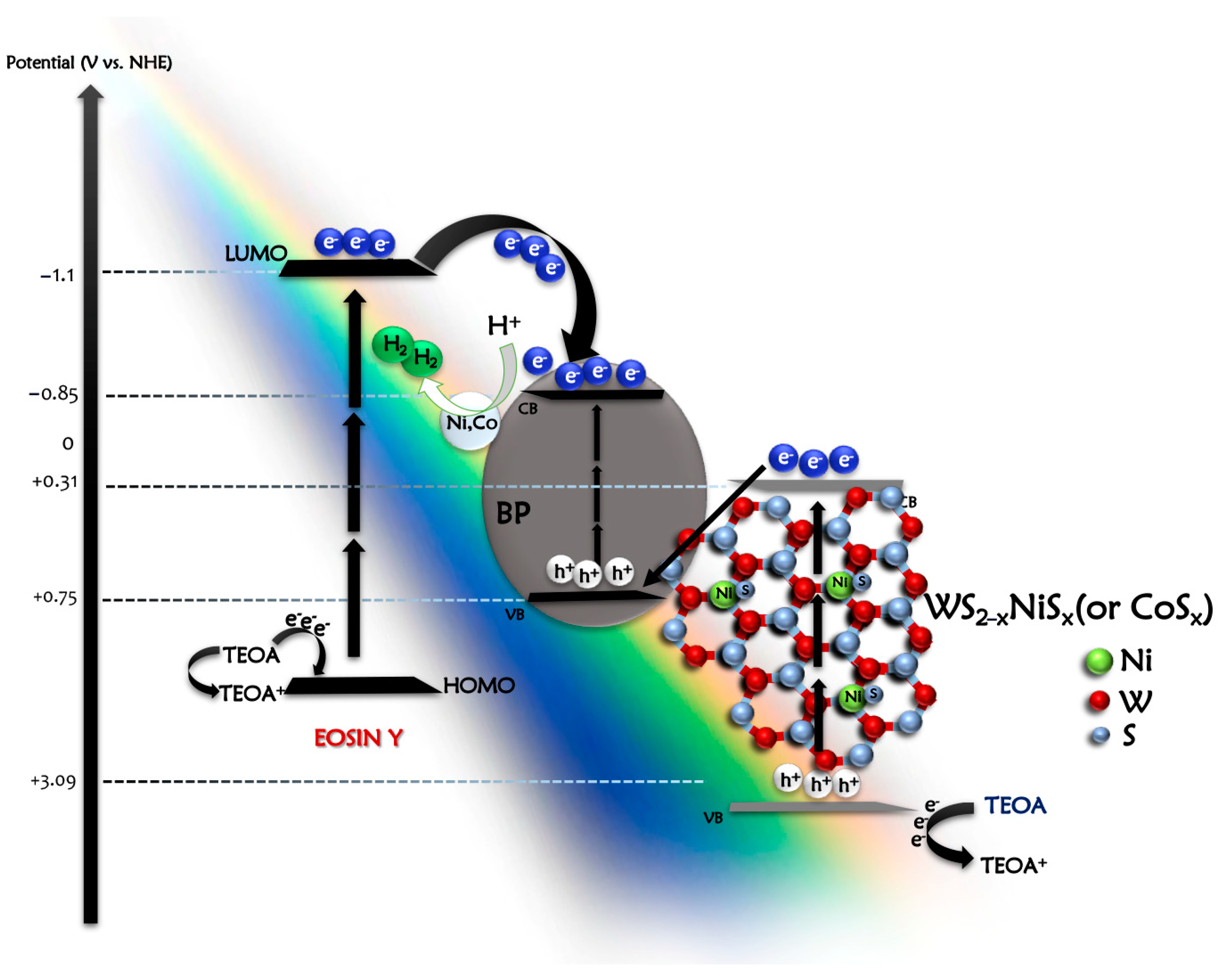
2.2. The Photocatalytic Hydrogen Evolution Studies
3. Experimental Section
3.1. Materials and Chemicals
3.2. The Material Synthesis
3.2.1. The Synthesis of Crystalline BP and Few-Layer BP
3.2.2. The Synthesis of WS2
3.2.3. The Fabrication of BP/WS2 Binary Nanocomposites
3.2.4. The Fabrication of BP/WS2-TM (TM: Ni, Co) Ternary Nanocomposites
3.3. The Photocatalytic Hydrogen Evolution Reaction (HER)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Darkwah, W.K.; Oswald, K.A. Photocatalytic applications of heterostructure graphitic carbon nitride: Pollutant degradation, hydrogen gas production (water splitting), and CO2 reduction. Nanoscale Res. Lett. 2019, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, J.K.; Ma, M.; Yim, S.Y.; Lee, C.L.; Shin, H.; Park, J.H. Delocalized electron accumulation at nanorod tips: Origin of efficient H2 generation. Adv. Funct. Mater. 2016, 26, 4527–4534. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, X.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an α–β phase junction on Ga2O3. Angew. Chem. 2012, 124, 13266–13269. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-embedded p-type copper (I) oxide nanocrystals for broad-spectrum photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1501865. [Google Scholar] [CrossRef]
- Núñez, J.; Fresno, F.; Platero-Prats, A.E.; Jana, P.; Fierro, J.L.; Coronado, J.M.; Serrano, D.P.; de la Peña O’Shea, V.A. Ga-promoted photocatalytic H2 production over Pt/ZnO nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 23729–23738. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Jia, Z.; Lyu, F.; Zhang, L.; Zeng, S.; Liang, S.; Li, Y.; Lu, J. Pt nanoparticles decorated heterostructured g-C3N4/Bi2MoO6 microplates with highly enhanced photocatalytic activities under visible light. Sci. Rep. 2019, 9, 7636. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Y.; Gao, B.; Lin, B.; Wang, X. Black phosphorus and carbon nitride hybrid photocatalysts for photoredox reactions. Adv. Funct. Mater. 2020, 30, 2002021. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Bashir, S.; Iqbal, N.; Jamil, A.; Alazmi, A.; Shahid, M. Facile synthesis of a novel photocatalyst with integrated features for industrial effluents treatment. Ceram. Int. 2022, 48, 3172–3184. [Google Scholar] [CrossRef]
- Liu, F.; Huang, C.; Liu, C.X.; Shi, R.; Chen, Y. Black Phosphorus-Based Semiconductor Heterojunctions for Photocatalytic Water Splitting. Chem. Eur. J. 2020, 26, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Wang, P.; Li, Z.; Wu, Y.; Bai, W.; Su, Y.; Guan, J.; Wu, S.; Zhong, J.; Yu, Z.-T. The role of bandgap and interface in enhancing photocatalytic H2 generation activity of 2D-2D black phosphorus/MoS2 photocatalyst. Appl. Catal. B Environ. 2019, 242, 1–8. [Google Scholar] [CrossRef]
- Ozer, M.S.; Eroglu, Z.; Yalin, A.S.; Kılıç, M.; Rothlisberger, U.; Metin, O. Bismuthene as a versatile photocatalyst operating under variable conditions for the photoredox CH bond functionalization. Appl. Catal. B Environ. 2022, 304, 120957. [Google Scholar] [CrossRef]
- Altan, O.; Eroğlu, Z.; Küçükkeçeci, H.; Metin, Ö. Black phosphorus-based photocatalysts for energy and environmental applications. In Nanostructured Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2021; pp. 421–449. [Google Scholar]
- Xue, Y.; Min, S.; Wang, F. Dye-sensitized black phosphorus nanosheets decorated with Pt cocatalyst for highly efficient photocatalytic hydrogen evolution under visible light. Int. J. Hydrogen Energy 2019, 44, 21873–21881. [Google Scholar] [CrossRef]
- Lv, W.; Yang, B.; Wang, B.; Wan, W.; Ge, Y.; Yang, R.; Hao, C.; Xiang, J.; Zhang, B.; Zeng, Z. Sulfur-doped black phosphorus field-effect transistors with enhanced stability. ACS Appl. Mater. Interfaces 2018, 10, 9663–9668. [Google Scholar] [CrossRef]
- Kim, D.-K.; Hong, S.-B.; Jeong, K.; Lee, C.; Kim, H.; Cho, M.-H. p–n junction diode using plasma boron-doped black phosphorus for high-performance photovoltaic devices. ACS Nano 2019, 13, 1683–1693. [Google Scholar] [CrossRef]
- Liang, Q.; Shi, F.; Xiao, X.; Wu, X.; Huang, K.; Feng, S. In situ growth of CoP nanoparticles anchored on black phosphorus nanosheets for enhanced photocatalytic hydrogen production. ChemCatChem 2018, 10, 2179–2183. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.; Hua, R.; Lei, Q.; Tian, Y. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353 K. Nat. Commun. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Tang, S.; Kang, Y.; Wang, J.; Yu, Y.; Zhou, Z.K.; Ma, C.; Zhang, X.; Jiang, J. Black phosphorus/platinum heterostructure: A highly efficient photocatalyst for solar-driven chemical reactions. Adv. Mater. 2018, 30, 1803641. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, T.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M. Bandgap-and local field-dependent photoactivity of Ag/black phosphorus nanohybrids. ACS Catal. 2016, 6, 8009–8020. [Google Scholar] [CrossRef]
- Yan, J.; Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Recent progress on black phosphorus-based materials for photocatalytic water splitting. Small Methods 2018, 2, 1800212. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zheng, J.; Peng, Y.-K.; Prabhakaran, D.; Taylor, R.A.; Tsang, S.C.E. 2D photocatalysts with tuneable supports for enhanced photocatalytic water splitting. Mater. Today 2020, 41, 34–43. [Google Scholar] [CrossRef]
- Wang, Y.; Di, Y.; Antonietti, M.; Li, H.; Chen, X.; Wang, X. Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chem. Mater. 2010, 22, 5119–5121. [Google Scholar] [CrossRef]
- Siddiqui, I.; Mittal, H.; Kohli, V.K.; Gautam, P.; Ali, M.; Khanuja, M. Hydrothermally synthesized micron sized, broom-shaped MoSe2 nanostructures for superior photocatalytic water purification. Mater. Res. Express 2018, 5, 125020. [Google Scholar] [CrossRef]
- Singhal, C.; Khanuja, M.; Chaudhary, N.; Pundir, C.; Narang, J. Detection of chikungunya virus DNA using two-dimensional MoS2 nanosheets based disposable biosensor. Sci. Rep. 2018, 8, 7734. [Google Scholar] [CrossRef]
- Narang, J.; Mishra, A.; Pilloton, R.; Vv, A.; Wadhwa, S.; Pundir, C.S.; Khanuja, M. Development of MoSe2 nano-urchins as a sensing platform for a selective bio-capturing of Escherichia coli shiga toxin DNA. Biosensors 2018, 8, 77. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khanuja, M.; Islam, S. Hydrothermal synthesis of MoS2 nanosheets for multiple wavelength optical sensing applications. Sens. Actuators A Phys. 2018, 277, 190–198. [Google Scholar] [CrossRef]
- Zhu, M.; Zhai, C.; Fujitsuka, M.; Majima, T. Noble metal-free near-infrared-driven photocatalyst for hydrogen production based on 2D hybrid of black Phosphorus/WS2. Appl. Catal. B Environ. 2018, 221, 645–651. [Google Scholar] [CrossRef]
- Ashraf, W.; Fatima, T.; Srivastava, K.; Khanuja, M. Superior photocatalytic activity of tungsten disulfide nanostructures: Role of morphology and defects. Appl. Nanosci. 2019, 9, 1515–1529. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, R.; Yang, Y.; Han, Q.; Liu, X.; Zhang, C.; Zhou, H.; Han, J.; Gou, J.; Wang, J. High-Performance Photodetector based on a 3D Dirac Semimetal Cd3As2/Tungsten Disulfide (WS2) van der Waals Heterojunction. Adv. Photonics Res. 2021, 2, 2000194. [Google Scholar] [CrossRef]
- Reddy, D.A.; Kim, E.H.; Gopannagari, M.; Kim, Y.; Kumar, D.P.; Kim, T.K. Few layered black phosphorus/MoS2 nanohybrid: A promising co-catalyst for solar driven hydrogen evolution. Appl. Catal. B Environ. 2019, 241, 491–498. [Google Scholar] [CrossRef]
- Yanalak, G.; Eroglu, Z.; Yılmaz, S.; Bas, S.Z.; Metin, O.; Patir, I.H. Metal doped black phosphorus/molybdenum disulfide (BP/MoS2–Y (Y: Ni, Co)) heterojunctions for the photocatalytic hydrogen evolution and electrochemical nitrite sensing applications. Int. J. Hydrogen Energy 2023, 48, 14238–14254. [Google Scholar] [CrossRef]
- Boppella, R.; Yang, W.; Tan, J.; Kwon, H.-C.; Park, J.; Moon, J. Black phosphorus supported Ni2P co-catalyst on graphitic carbon nitride enabling simultaneous boosting charge separation and surface reaction. Appl. Catal. B Environ. 2019, 242, 422–430. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Liu, D.; Zhang, J.; Peng, T. Layered WS2/WO3 Z-scheme photocatalyst constructed via an in situ sulfurization of hydrous WO3 nanoplates for efficient H2 generation. Appl. Surf. Sci. 2020, 529, 147013. [Google Scholar] [CrossRef]
- Liu, W.; Benson, J.; Dawson, C.; Strudwick, A.; Raju, A.P.A.; Han, Y.; Li, M.; Papakonstantinou, P. The effects of exfoliation, organic solvents and anodic activation on the catalytic hydrogen evolution reaction of tungsten disulfide. Nanoscale 2017, 9, 13515–13526. [Google Scholar] [CrossRef]
- Mahler, B.; Hoepfner, V.; Liao, K.; Ozin, G.A. Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: Applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xu, Q.; Li, H.; Wang, Y.; Yan, B.; Zhou, Y.; Chen, J.; Zhang, J.; Wang, K. Fabrication of Two-Dimensional Lateral Heterostructures of WS2/WO3⋅H2O Through Selective Oxidation of Monolayer WS2. Angew. Chem. 2015, 127, 15441–15445. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ozer, M.S.; Kubanaliev, T.; Kilic, H.; Metin, Ö. Synergism between few-layer black phosphorus and graphitic carbon nitride enhances the photoredox C–H arylation under visible light irradiation. Catal. Sci. Technol. 2022, 12, 5379–5389. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Liu, F.; Shi, R.; Wang, Z.; Weng, Y.; Che, C.M.; Chen, Y. Direct Z-scheme hetero-phase junction of black/red phosphorus for photocatalytic water splitting. Angew. Chem. 2019, 131, 11917–11921. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Ou, H.; Asiri, A.M.; Chen, Y.; Wang, X. Black phosphorus and polymeric carbon nitride heterostructure for photoinduced molecular oxygen activation. Adv. Funct. Mater. 2018, 28, 1705407. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Yin, S.; Liang, Z.; Xue, Y.; Wang, X.; Cui, H.; Tian, J. Photocatalytic H2 evolution on TiO2 assembled with Ti3C2 MXene and metallic 1T-WS2 as co-catalysts. Nano-Micro Lett. 2020, 12, 6. [Google Scholar] [CrossRef]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to near-infrared, WS2 nanosheet: A novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y. Construction of C@ WS2/g-C3N4 Z-scheme photocatalyst with C film as an effective electron mediator and its enhanced degradation of 2, 4-dichlorophenol under visible light. Chemosphere 2021, 273, 129746. [Google Scholar] [CrossRef]
- Lai, Z.; He, Q.; Tran, T.H.; Repaka, D.; Zhou, D.-D.; Sun, Y.; Xi, S.; Li, Y.; Chaturvedi, A.; Tan, C. Metastable 1T′-phase group VIB transition metal dichalcogenide crystals. Nat. Mater. 2021, 20, 1113–1120. [Google Scholar] [CrossRef]
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS2-edge functionalized multi-channel carbon nanofibers: Effect of WS2 edge-abundant structure on room temperature NO2 sensing. J. Mater. Chem. A 2017, 5, 8725–8732. [Google Scholar] [CrossRef]
- Kotta, A.; Kim, E.-B.; Ameen, S.; Shin, H.-S.; Seo, H.K. Communication—Ultra-small NiO nanoparticles grown by low-temperature process for electrochemical application. J. Electrochem. Soc. 2020, 167, 167517. [Google Scholar] [CrossRef]
- Cai, L.; Qiu, B.; Lin, Z.; Wang, Y.; Ma, S.; Wang, M.; Tsang, Y.H.; Chai, Y. Active site engineering of Fe-and Ni-sites for highly efficient electrochemical overall water splitting. J. Mater. Chem. A 2018, 6, 21445–21451. [Google Scholar] [CrossRef]
- Lin, R.; Salehi, M.; Guo, J.; Seifitokaldani, A. High oxidation state enabled by plated Ni-P achieves superior electrocatalytic performance for 5-hydroxymethylfurfural oxidation reaction. iScience 2022, 25, 104744. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Xia, C.; Hedhili, M.N.; Anjum, D.H.; Schwingenschlogl, U.; Alshareef, H.N. Amorphous NiFe-OH/NiFeP electrocatalyst fabricated at low temperature for water oxidation applications. ACS Energy Lett. 2017, 2, 1035–1042. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Li, G.; Xia, H.; Wang, P.; Sun, P.; Li, X.; Cai, H.; Xiong, J. One-step sulfuration synthesis of hierarchical NiCo2S4@ NiCo2S4 nanotube/nanosheet arrays on carbon cloth as advanced electrodes for high-performance flexible solid-state hybrid supercapacitors. RSC Adv. 2019, 9, 3041–3049. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Ren, X.; Ji, X.; Liu, Y.; Guo, X.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X. Co(OH)2 nanoparticle-encapsulating conductive nanowires array: Room-temperature electrochemical preparation for high-performance water oxidation electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef]
- Li, X.-C.; She, F.-S.; Shen, D.; Liu, C.-P.; Chen, L.-H.; Li, Y.; Deng, Z.; Chen, Z.-H.; Wang, H.-E. Coherent nanoscale cobalt/cobalt oxide heterostructures embedded in porous carbon for the oxygen reduction reaction. RSC Adv. 2018, 8, 28625–28631. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhou, Y.; Zhang, X.; Cui, H.; Cheng, G.; Tian, J. Co doped MoS2 as cocatalyst considerably improved photocatalytic hydrogen evolution of g-C3N4 in an alkalescent environment. Chem. Eng. J. 2021, 421, 130016. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhang, X.-H.; Wu, J.; Li, N.; Zheng, Y.-Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Jin, X.; Wang, X.; Zhang, F.; Xue, J.; Li, Y.; Li, J.; Zhang, G. General surface grafting strategy-derived carbon-modified graphitic carbon nitride with largely enhanced visible light photocatalytic H2 evolution coupled with benzyl alcohol oxidation. J. Mater. Chem. A 2021, 9, 7143–7149. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Nickel-based cocatalysts for photocatalysis: Hydrogen evolution, overall water splitting and CO2 reduction. Mater. Today Phys. 2020, 15, 100279. [Google Scholar] [CrossRef]
- Gonce, M.K.; Dogru, M.; Aslan, E.; Ozel, F.; Patir, I.H.; Kus, M.; Ersoz, M. Photocatalytic hydrogen evolution based on Cu2ZnSnS4, Cu2ZnSnSe4 and Cu2ZnSnSe4−xSx nanofibers. RSC Adv. 2015, 5, 94025–94028. [Google Scholar] [CrossRef]
- Abe, R.; Hara, K.; Sayama, K.; Domen, K.; Arakawa, H. Steady hydrogen evolution from water on Eosin Y-fixed TiO2 photocatalyst using a silane-coupling reagent under visible light irradiation. J. Photochem. Photobiol. A Chem. 2000, 137, 63–69. [Google Scholar] [CrossRef]
- Kimura, K.; Miwa, T.; Imamura, M. The radiolysis and photolysis of methanolic solutions of eosin. I. The γ-radiolysis of neutral and alkaline solutions. Bull. Chem. Soc. Jpn. 1970, 43, 1329–1336. [Google Scholar] [CrossRef]
- Eken Korkut, S.; Kucukkececi, H.; Metin, O. Mesoporous graphitic carbon nitride/black phosphorus/AgPd alloy nanoparticles ternary nanocomposite: A highly efficient catalyst for the methanolysis of ammonia borane. ACS Appl. Mater. Interfaces 2020, 12, 8130–8139. [Google Scholar] [CrossRef]
- Hari, D.P.; König, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.P. Eosin Y catalysed photoredox synthesis: A review. RSC Adv. 2017, 7, 31377–31392. [Google Scholar] [CrossRef]
- Acar, E.G.; Yılmaz, S.; Eroglu, Z.; Aslan, E.; Metin, Ö.; Patır, İ.H. Solar-light-driven Photocatalytic Hydrogen Evolution Activity of gCN/WS2 Heterojunctions Incorporated with the First-row Transition Metals. J. Alloys Compd. 2023, 950, 169753. [Google Scholar] [CrossRef]
- Yılmaz, S.; Acar, E.G.; Yanalak, G.; Aslan, E.; Kılıç, M.; Patır, İ.H.; Metin, Ö. Enhanced hydrogen evolution by using ternary nanocomposites of mesoporous carbon nitride/black phosphorous/transition metal nanoparticles (m-gCN/BP-M.; M = Co, Ni, and Cu) as photocatalysts under visible light: A comparative experimental and theoretical study. Appl. Surf. Sci. 2022, 593, 153398. [Google Scholar]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, S.; Ryu, J.; Lim, S.K.; Park, H. Titania nanofibers as a photo-antenna for dye-sensitized solar hydrogen. Photochem. Photobiol. Sci. 2012, 11, 1437–1444. [Google Scholar] [CrossRef]
- Peimyoo, N.; Shang, J.; Cong, C.; Shen, X.; Wu, X.; Yeow, E.K.; Yu, T. Nonblinking, intense two-dimensional light emitter: Monolayer WS2 triangles. ACS Nano 2013, 7, 10985–10994. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Yüzer, A.C.; Genc, E.; Kurtay, G.; Yanalak, G.; Aslan, E.; Harputlu, E.; Ocakoglu, K.; Patir, I.H.; Ince, M. Imidazole substituted Zinc (ii) phthalocyanines for co-catalyst-free photoelectrochemical and photocatalytic hydrogen evolution: Influence of the anchoring group. Chem. Commun. 2021, 57, 9196–9199. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.C.; von Zamory, J.; Passerini, S.; Nilges, T.; Winter, M. Puzzling out the origin of the electrochemical activity of black P as a negative electrode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 5293–5300. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, T.; Shao, Y.; Wu, Y.; Huang, B.; Hao, X. A Novel Mild Phase-Transition to Prepare Black Phosphorus Nanosheets with Excellent Energy Applications. Small 2017, 13, 1602243. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhu, B.; Qiao, S.-Z. Phosphorene Co-catalyst Advancing Highly Efficient Visible-Light Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2017, 56, 10373–10377. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Fujitsuka, M.; Majima, T. Z-Scheme Photocatalytic Water Splitting on a 2D Heterostructure of Black Phosphorus/Bismuth Vanadate Using Visible Light. Angew. Chem. Int. Ed. 2018, 57, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fujitsuka, M.; Zeng, L.; Liu, M.; Majima, T. Dual function of graphene oxide for assisted exfoliation of black phosphorus and electron shuttle in promoting visible and near-infrared photocatalytic H2 evolution. Appl. Catal. B 2019, 256, 117864. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, H.; Wang, B.; He, W.; Dong, H.; Sui, L.; Gan, Z.; Ma, S.; Pang, B.; Dong, L.; et al. Synthesis and photocatalytic performance of MoS2/Polycrystalline black phosphorus heterojunction composite. Int. J. Hydrogen Energy 2021, 46, 3530–3538. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Yan, Y.; Liu, F.; Huang, Z.; Ren, X.; Zhang, H.; Li, Y.; Ye, J. Synergy of heterojunction and interfacial strain for boosting photocatalytic H2 evolution of black phosphorus nanosheets. J. Colloid Interface Sci. 2022, 627, 969–977. [Google Scholar] [CrossRef] [PubMed]
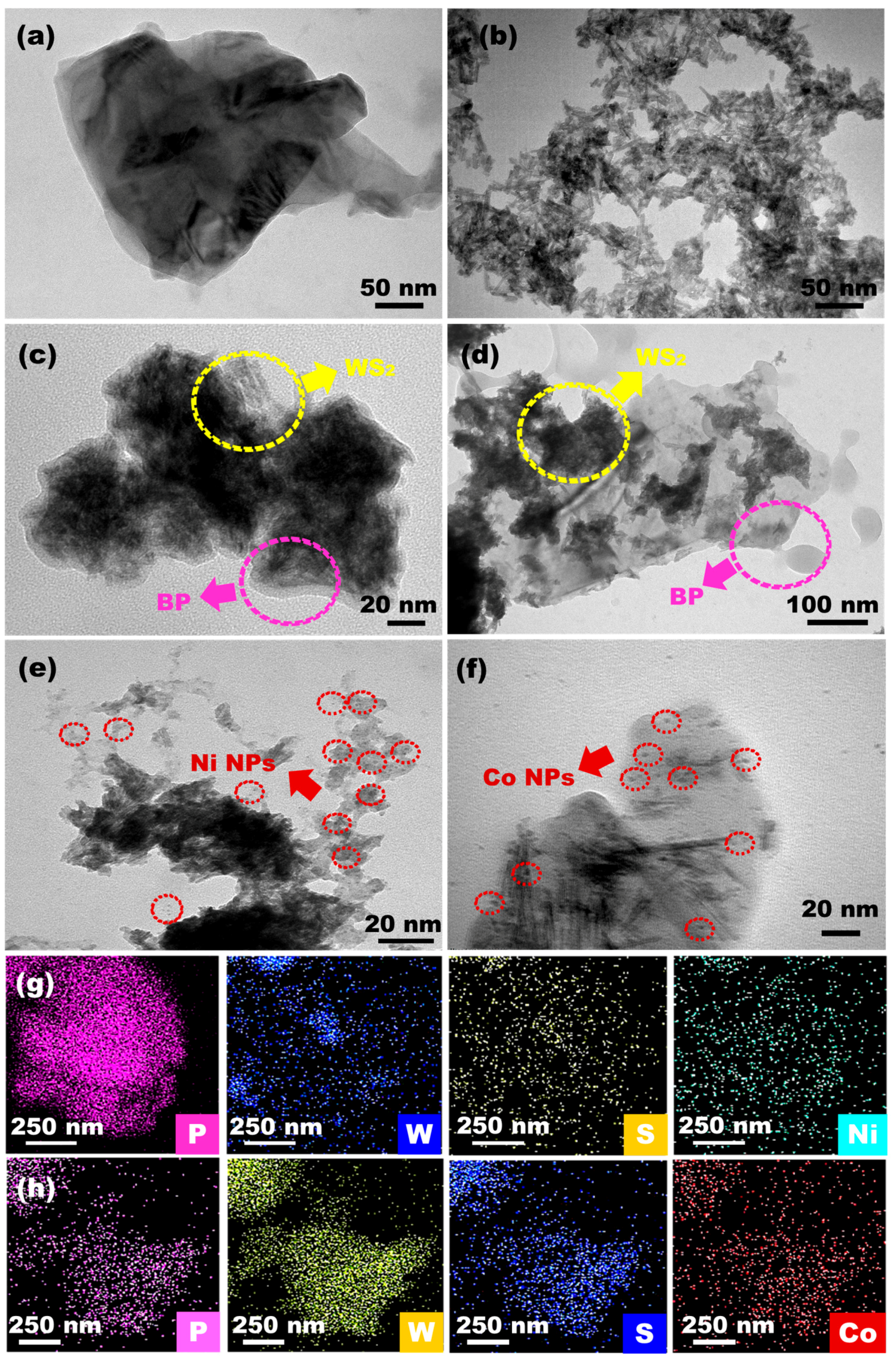
 indicates the BP planes
while
indicates the BP planes
while  indicates the planes of
WS2).
indicates the planes of
WS2).
 indicates the BP planes
while
indicates the BP planes
while  indicates the planes of
WS2).
indicates the planes of
WS2).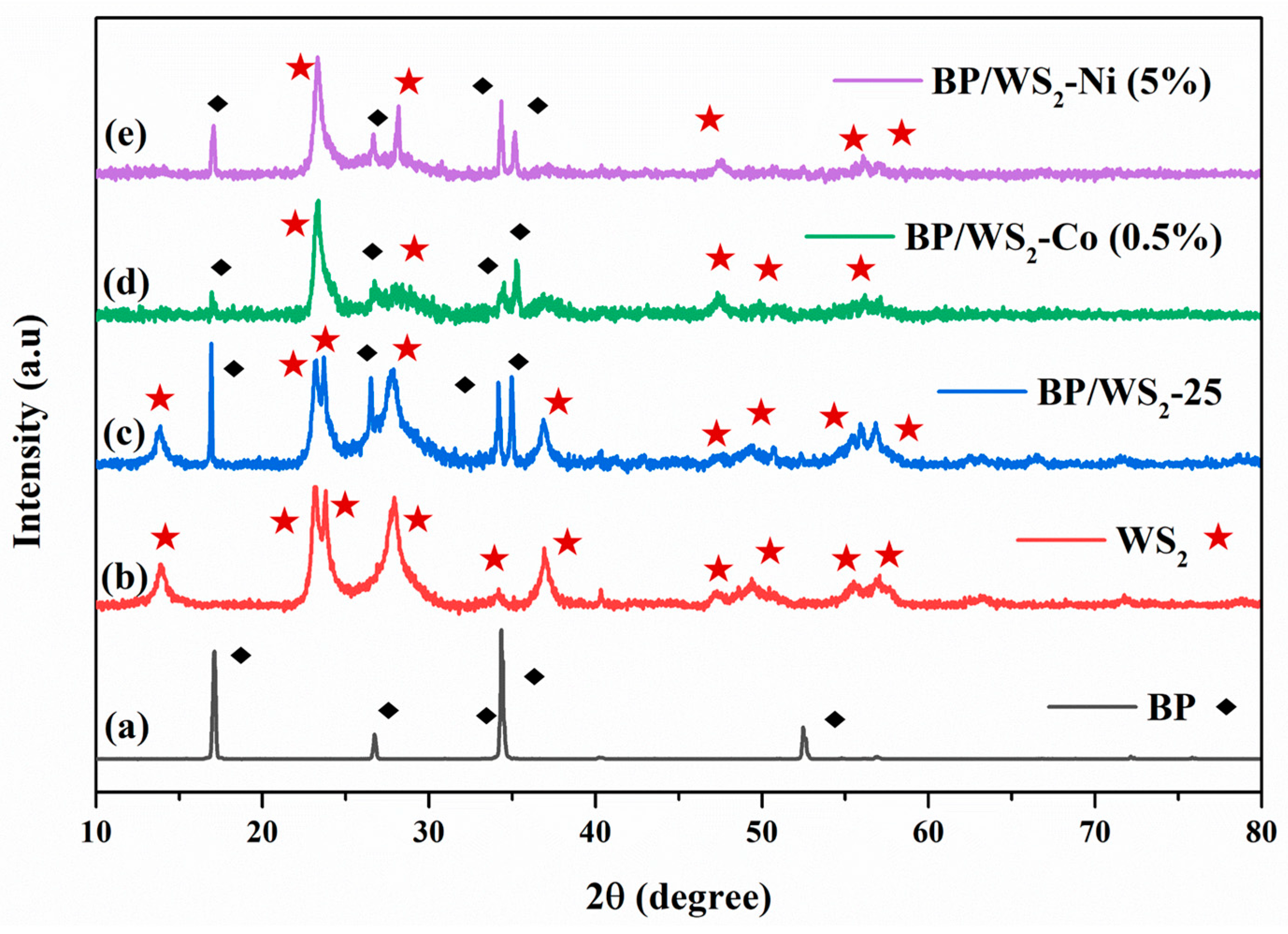

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acar, E.G.; Yılmaz, S.; Eroglu, Z.; Çekceoğlu, İ.A.; Aslan, E.; Patır, İ.H.; Metin, O. Black Phosphorus/WS2-TM (TM: Ni, Co) Heterojunctions for Photocatalytic Hydrogen Evolution under Visible Light Illumination. Catalysts 2023, 13, 1006. https://doi.org/10.3390/catal13061006
Acar EG, Yılmaz S, Eroglu Z, Çekceoğlu İA, Aslan E, Patır İH, Metin O. Black Phosphorus/WS2-TM (TM: Ni, Co) Heterojunctions for Photocatalytic Hydrogen Evolution under Visible Light Illumination. Catalysts. 2023; 13(6):1006. https://doi.org/10.3390/catal13061006
Chicago/Turabian StyleAcar, Eminegül Genc, Seda Yılmaz, Zafer Eroglu, İlknur Aksoy Çekceoğlu, Emre Aslan, İmren Hatay Patır, and Onder Metin. 2023. "Black Phosphorus/WS2-TM (TM: Ni, Co) Heterojunctions for Photocatalytic Hydrogen Evolution under Visible Light Illumination" Catalysts 13, no. 6: 1006. https://doi.org/10.3390/catal13061006
APA StyleAcar, E. G., Yılmaz, S., Eroglu, Z., Çekceoğlu, İ. A., Aslan, E., Patır, İ. H., & Metin, O. (2023). Black Phosphorus/WS2-TM (TM: Ni, Co) Heterojunctions for Photocatalytic Hydrogen Evolution under Visible Light Illumination. Catalysts, 13(6), 1006. https://doi.org/10.3390/catal13061006