Abstract
Methacrolein (MAL) is an important intermediate extensively used in the manufacture of methyl methacrylate and other materials (polymers and resins). In this study, a series of secondary amines/acids were explored as catalysts for the condensation of formaldehyde and propionaldehyde to prepare MAL. It was found that the structure of the amines and acids directly affected the yield of MAL. The effect of the catalyst was closely related to the nucleophilicity of the amines as well as the steric hindrance effect, while acids also played a role as co-catalysts. Dibutylamine acetate was selected as the catalyst after investigation. The catalytic performance of the system was systematically studied by a series of single-factor experiments, including stirring rate, temperature, reaction time, acid/amine ratio, and the solvent, and the optimized reaction conditions were obtained. In the optimum condition, the yield of MAL was up to 97.3%. Kinetic experiments were performed for the condensation of formaldehyde and propionaldehyde to MAL, and the activation energies, reaction orders, and rate-limiting step of the reaction were determined. The results indicate that the decomposition of the Mannich base is a rate-limiting step.
1. Introduction
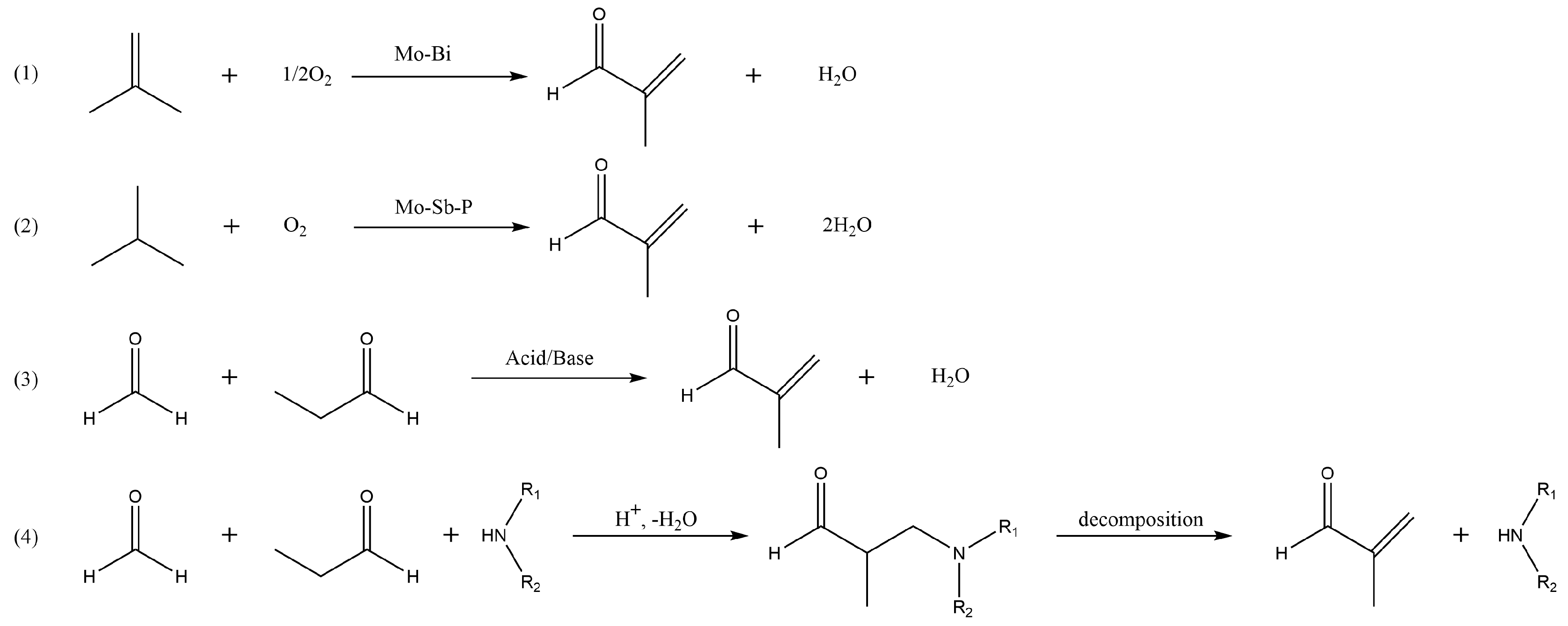
Methacrolein (MAL) is a key chemical extensively used in the production of polymers and resins [1,2]. It is also applied as a raw material for the synthesis of diacetates and unsaturated esters [3,4,5,6], which are monomers of thermoplastics [7] and also intermediates of various chemical reactions. In the production of methyl methacrylate (MMA), MAL is an important upstream raw material and can be selectively oxidized to produce methacrylic acid (MA) and then esterified with methanol to prepare MMA [8,9,10]. MAL continues to increase in demand with high margins and use value. The production of MAL is mainly achieved by three routes (Scheme 1): direct oxidation of isobutylene, selective oxidation of isobutane, and aldol condensation of formaldehyde (FA) with propionaldehyde (PA) [11]. Reaction processes involving isobutylene and isobutane as raw materials are generally catalyzed using Mo-Bi composites. However, the yield of MAL is low [12]. Conversely, the aldol condensation process can be performed under mild conditions with relatively high yields and is a clean process route with good industrial prospects. Therefore, it requires the development of highly efficient catalysts and technologies.
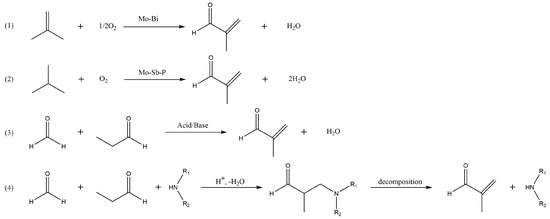
Scheme 1.
MAL synthesis routes: (1) isobutene selective oxidation, (2) indirect oxidation of isobutane, (3) direct aldol condensation of formaldehyde with propionaldehyde, and (4) Mannich pathway.
Acidic or alkaline substances can be used to catalyze the aldol condensation reaction between FA and PA. Li et al. [13,14] investigated this pathway using different acids and alkalis including hydrochloric acid, sodium hydroxide, diethylamine, and triethylamine as catalysts. Although the conversion of PA is up to 97%, the selectivity of MAL is only 10.1% due to the self-condensation of PA and the polymerization of the unsaturated aldehydes. Therefore, the need for a method with high efficiency is urgent. The Mannich reaction catalyzed by an amine was regarded as a promising method. In the 1950s, aldol condensation over amines to synthesize MAL was developed. In this process, the methylamine hydrochloride was introduced to catalyze the Mannich reaction between FA and PA. Since then, several attempts have been made to achieve the commercial production of MAL via the Mannich reaction with amines. Mironov et al. [15] proposed that the pH of the reaction system directly affected its catalytic activity, and they obtained a yield of 97% in the pH range of 6–7 at 50 °C. Dashko et al. [16] reported that increasing the basicity of the solvent and the catalyst can promote the cross-condensation reactions in the system. Moreover, Wang et al. [17] immobilized an amine on cation-exchange resin and applied it to the MAL synthesis process, but the conversion of aldehyde was low. Pyo et al. [18] observed that aldehydes were more readily converted into a β-hydroxy aldehyde, and the selectivity of MAL was not ideal. Erkkila et al. [19] found that the acidities of co-catalysts were closely related with the catalytic performance. Subsequently, amines/acids as catalysts have attracted the interest of researchers. Erkkila et al. [20] investigated the catalytic ability of diverse secondary amine/carboxylic acid combinations for the condensation of aldehydes and FA, drawing the conclusion that MAL could be obtained in 90% yield under optimal reaction conditions using pyrrolidine/propionic acid as the catalyst. Li et al. [21] tested a series of catalysts composed of secondary amines/acetic acids, and diethylamine/acetic acid was the best catalyst for the synthesis of MAL from FA and PA with 97% conversion and 94% yield.
Compared with the direct aldol condensation pathway, the preparation of MAL via the Mannich reaction exhibits obvious superiority in catalytic efficiency and relative environmental hazards. Secondary amine/acid combinations show desirable catalytic performance as catalysts [22,23,24,25]. However, the currently optimal amine/acid catalyst shows an obvious decline in catalytic activity after several cycles. Moreover, the differences in the catalytic activity of different kinds of secondary amines/acids have not been systematically investigated. Therefore, further investigation of the Mannich reaction catalyzed by a secondary amine/acid to prepare MAL is desirable.
In this study, the differences in the activity of different kinds of secondary amines/acids as catalysts were explored, and dibutylamine acetate was selected as the optimal catalyst for the synthesis of MAL from the aldol condensation of FA and PA. Then, a series of single-factor experiments were performed to study the optimal reaction parameters of the system. In addition, a kinetic model was established based on the experimental results, and the rate-controlling steps of the reaction were determined. The condensation reaction catalyzed by dibutylamine acetate is an effective method for the synthesis of MAL.
2. Results and Discussion
MAL was prepared by condensation of FA and PA with various catalysts. The effects of temperature, reaction time, and interactions among the components of amine catalysts were investigated systematically. A variety of secondary amine acetates were tested (Table 1). Aliphatic amines with straight-chain structures have better catalytic effects than aromatic amines and aliphatic amines with branched-chain structures. Aromatic amines linked to the phenyl group have much lower catalytic activities than those not linked to the phenyl group.

Table 1.
Catalytic effect of different secondary amines.
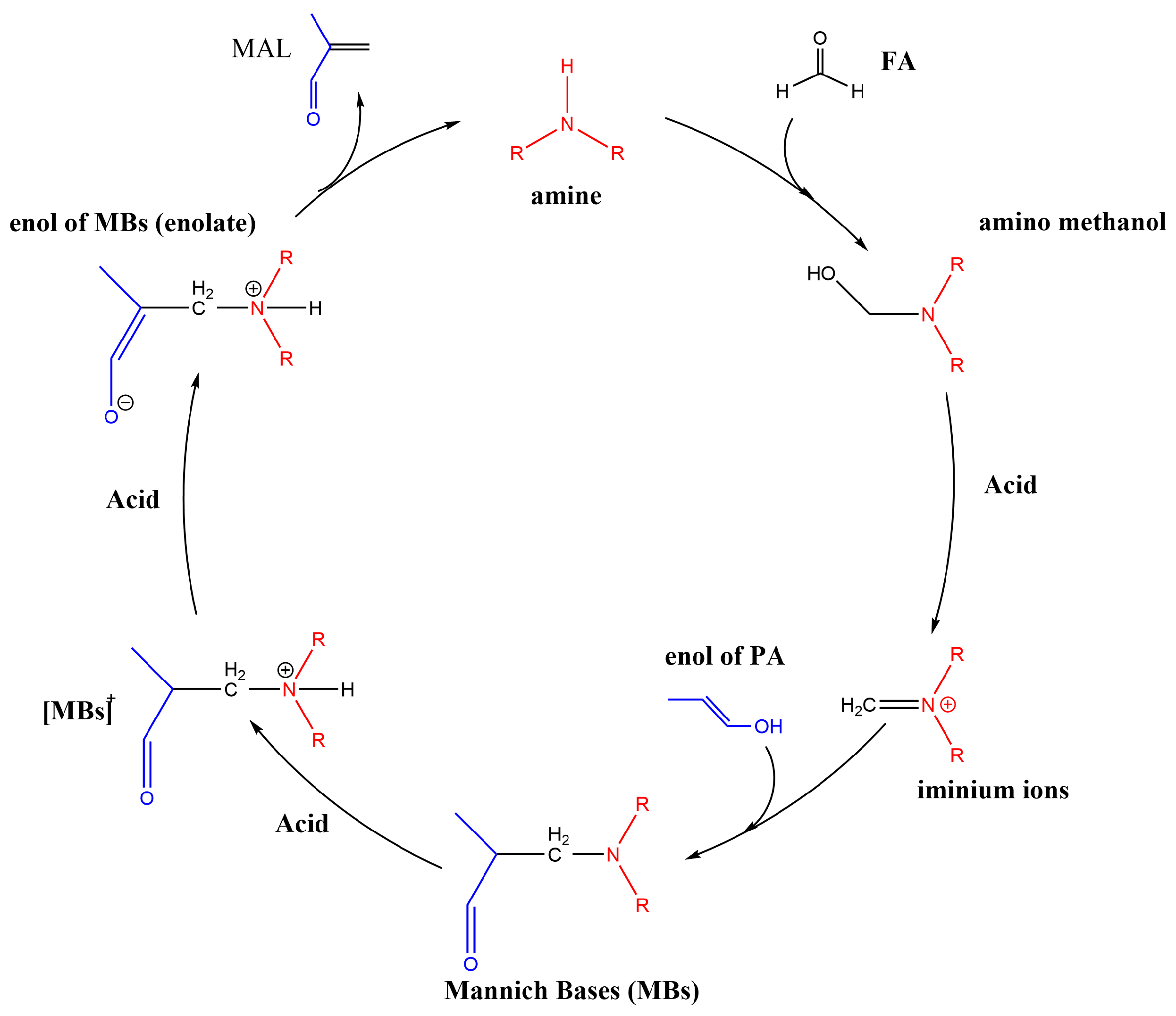
The mechanism of the Mannich reaction has been reported in many articles [26,27,28,29,30], and the reaction path of the Mannich reaction is shown in Scheme 2. In these reactions, the amines form iminium ions as key intermediates [31]. Thus, the stabilization of the iminium ions affects the catalytic activity. When the transition state leading to the product involves a iminium ion intermediate, the concentration of the iminium ions generated in situ may affect the reaction rate [31].
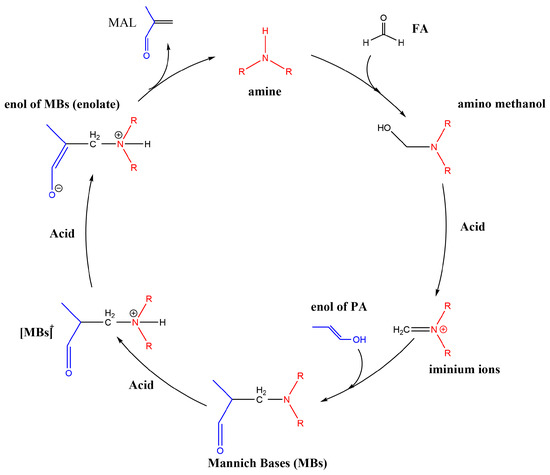
Scheme 2.
The reaction path of the Mannich reaction.
The synthesis of MAL is divided into six steps. Firstly, FA and the secondary amine undergo a nucleophilic addition reaction to form amino methanol, and then H2O is removed under the promotion of an acid or base to form iminium ions. Next, PA is enolated under the influence of acid, and iminium ions as electrophilic reagents attack the enol of PA to form the Mannich base. Finally, the Mannich base is enolated and then decomposed to form MAL and the secondary amine to complete a cycle under the catalysis with acid. The step that determines the reaction rate is the nucleophilic attack of the amino group on the carbonyl group to form an intermediate, and amines with strong nucleophilicity are more likely to undergo a nucleophilic addition reaction [32,33].
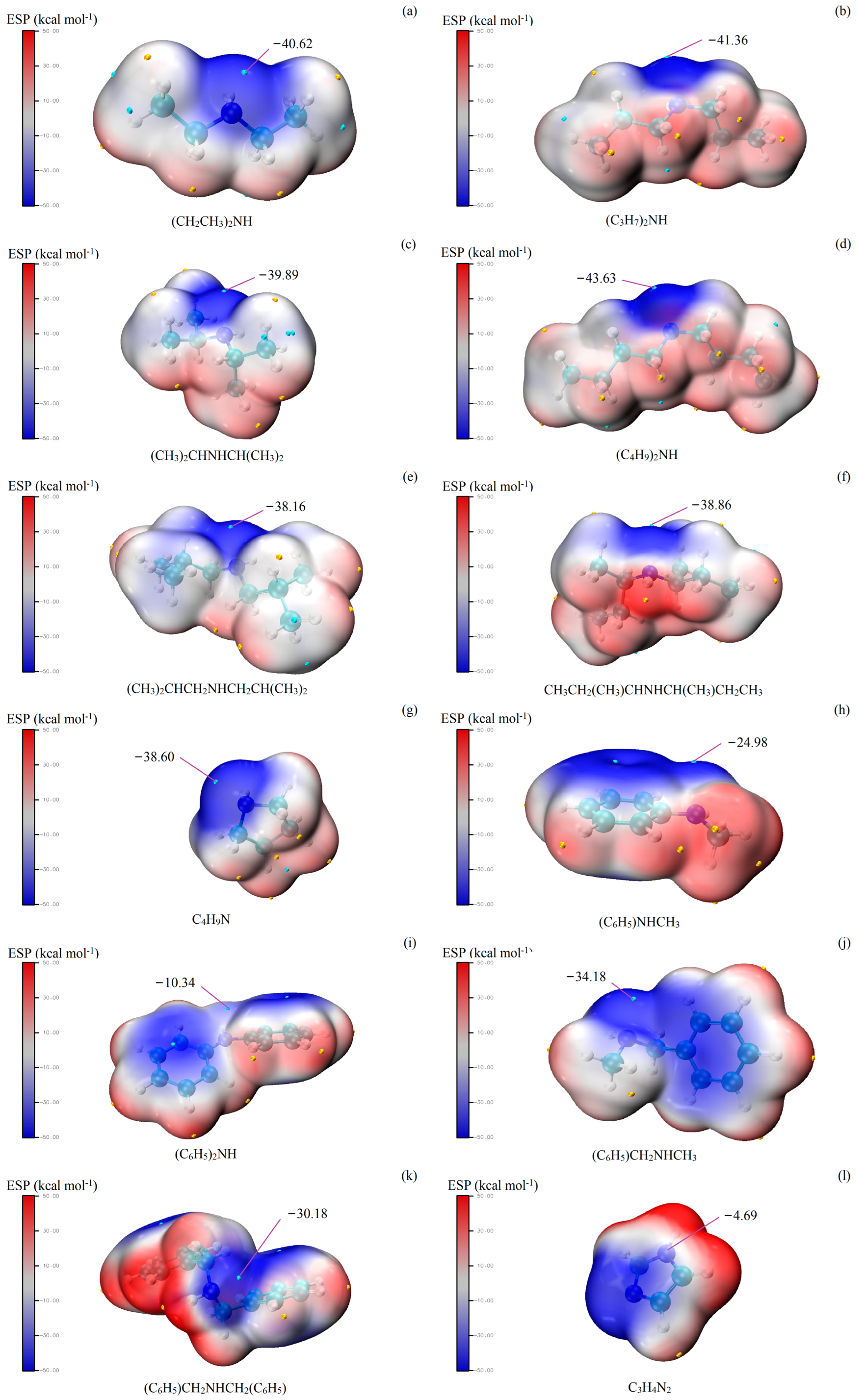
In order to study the reasons for the different catalytic effects of different amines, multiwfn and visual molecular dynamics were applied to analyze the synthesis of MAL catalyzed by amines/acids. The electrostatic potential (ESP) distribution on the van der Waals surface is usually considered to predict electrophilic and nucleophilic reactions [34]. The lower the ESP, the stronger the nucleophilicity. Thus, the N atom being closer to the global ESP minimum on the van der Waals surface indicates that it may have stronger nucleophilicity. The ESPs of different amines are shown in Figure 1. Overall, it can be seen that the catalytic effect is negatively correlated with ESP. Imidazole has the highest ESP and the worst selection and yield. For diethylamine, di-n-propylamine, di-n-butylamine, and pyrrolidine (Figure 1a,b,d,g), ESP is low, and their catalytic effect is excellent. However, diisopropylamine and di-sec-butylamine have low ESP but not a satisfactory catalytic effect. As shown in Figure 1c,f, the van der Waals surface near the N atom of diisopropylamine and di-sec-butylamine is not in a prominent position. It may be that the steric hindrance affects the electron pair of the N atom to attack FA [35]. The steric hindrance effect may also affect the catalytic effect of aromatic amines [36,37,38]. The ESP of n-methylbenzylamine was close to that of pyrrolidine; however, the catalytic effect differs significantly.
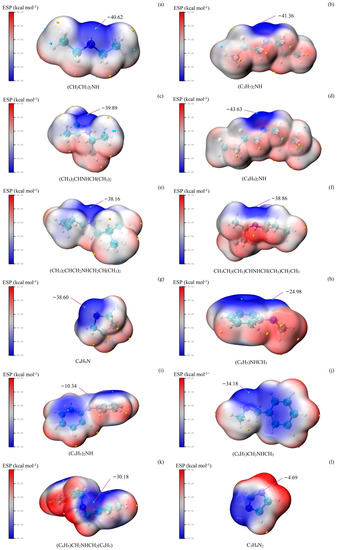
Figure 1.
ESP—mapped molecular van der Waals surfaces of amines. The surface local maxima and minima of ESP are marked in red and blue, respectively.
As for aromatic amines, the catalytic activity of n-methylaniline and diphenylamine was bad. In general, the lone-pair electron on the N atom of aniline interacts with the electrons of the phenyl group to form a conjugated system that becomes stable [39,40,41]. In a similar pattern, diphenylamine and n-methylaniline also form a stable conjugated system [42]. The reaction mechanism shows that the nucleophilic attack can only occur if there is a lone-pair electron on the N atom. The results support this reaction mechanism, and the ESP (Figure 1h,i) also shows that the electron pairs on the N atom of n-methylaniline and diphenylamine form a conjugated system with phenyl group electrons.
If the conjugated system is absent and the phenyl group has less influence on the N atom, the aromatic amine should have a catalytic effect. The results of n-benzylmethylamine and dibenzylamine verify this conjecture (Table 1, entry 10–11). By comparing the ESP of these aromatic amines (Figure 1j,k), we can see that the N atom electron pair in n-benzylmethylamine and dibenzylamine remains unbound and does not form a conjugated system with the electrons of benzene. However, phenyl groups have a large steric hindrance effect and electronic interaction [43,44], which may lead to the catalytic effect of dibenzylamine being weaker than that of n-benzylmethylamine.
Imidazole with both acidic and alkaline properties has a conjugated structure [45,46]. The extreme ESP value near the N atom of imidazole is close to a positive value, which means the N atom has almost no nucleophilic property. Figure 1l also shows that the lone-pair electron of the N atom of imidazole participates in the conjugated system. These effects caused imidazole to have no catalytic activity.
In order to further verify this conjecture, n-methylaniline, diphenylamine, imidazole, and dibutylamine were separately reacted with FA under the same reaction conditions. After the reaction, the concentration of FA was measured by acetylacetone spectrophotometry. The results show that the concentration of FA was not significantly reduced except for dibutylamine, indicating that N-methylaniline, diphenylamine, and imidazole were difficult to react with FA.
Yan’s [34] study showed that acids play two roles: one is to promote dehydration to form iminium ions after the secondary amine attacks FA to form amino methanol, and the other is to significantly reduce the energy barrier of the decomposition of the Mannich base so as to promote its decomposition. These effects require that acids can provide protons to promote the formation of iminium ions but cannot provide excessive protons to protonate amines. Once the amine combines with protons to form ammonium ions, the amine cannot conduct a nucleophilic attack on FA due to lacking lone-pair electrons.
Table 2 shows the catalytic activities of different acids. When hydrochloric acid and sulfuric acid (Table 2, entry 1–2) are used as acid components, the catalyst shows poor catalytic activity. Since hydrochloric acid and sulfuric acid are completely ionized in solution, protons will combine with the amine to form ammonium ions after mixing the amine and acid in an equal molar ratio. The Mannich reaction cannot be catalyzed by ammonium ions. In order to verify this interpretation, the molar ratio of the amine/acid was adjusted to control the pH of the catalyst within the range of 6~6.5. The experimental results show that the catalytic effect of the adjusted catalyst has been improved compared with that of the catalyst with equal proportion. Short-chain aliphatic acid can maintain a balance in the provision of protons. Thus, acetic acid, propionic acid, and butyric acid (Table 2, entry 3–5) have good catalytic effects. The simulation results also show that these acids have a catalytic effect on the formation of iminium ions and the reduction in the energy barrier of Mannich base decomposition [34]. The reason why formic acid has not been investigated is that the Eschweiler–Clarke reaction will occur when formic acid, FA, and amine coexist, which will lead to irreversible loss of amine [47].

Table 2.
Effects of different acid components.
2.1. Optimization of MAL Synthesis
After twelve amines and five acids were investigated, dibutylamine acetate showed the best catalytic effect. The catalyst composition was determined, and a series of reaction conditions were optimized (stirring rate, temperature, time, and molar ratio of acid and amine).
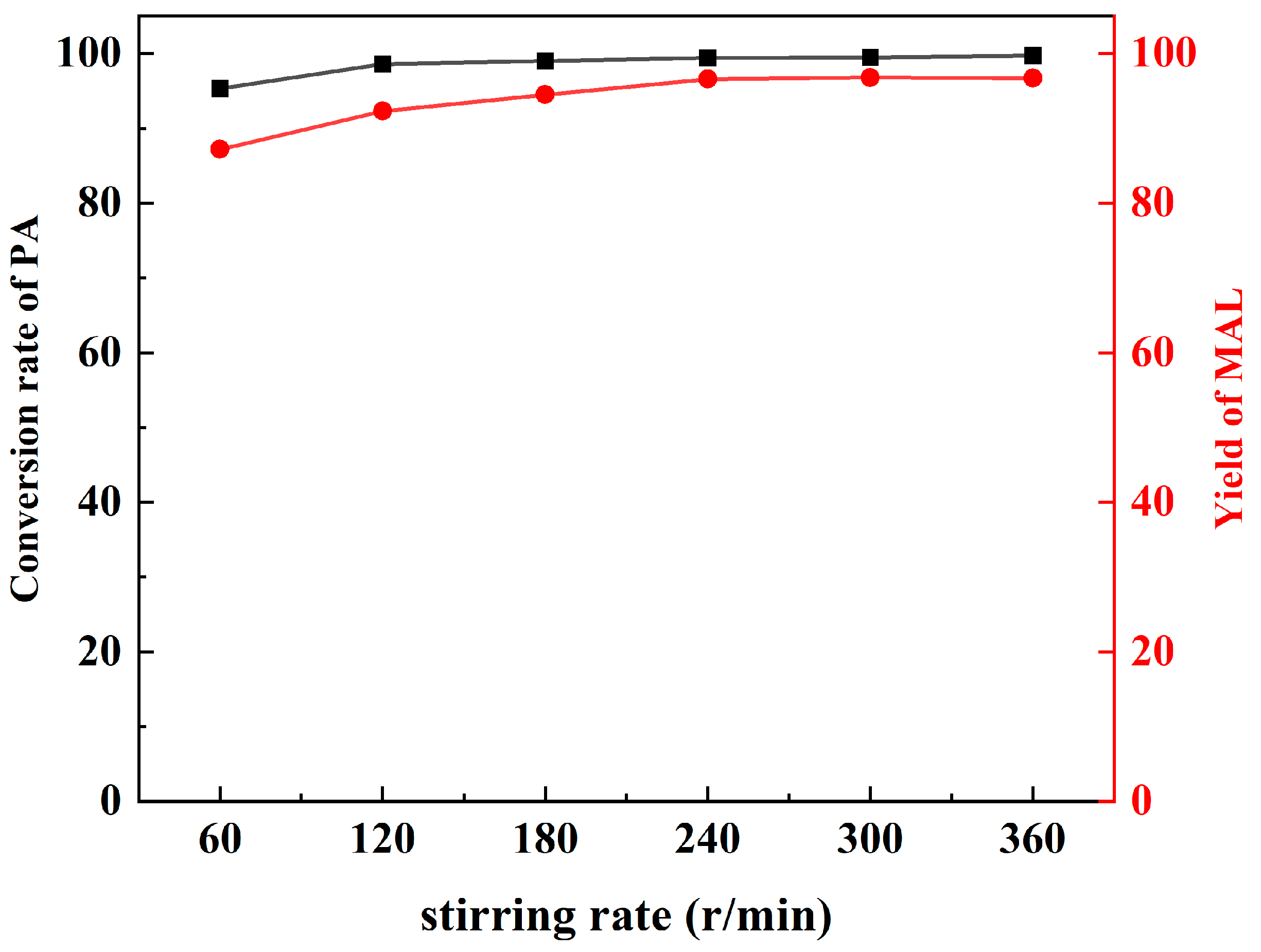
For the purpose of excluding the influence of external and internal diffusion, preliminary experiments were conducted for the synthesis of the MAL reaction at variable stirring rates. According to the results (Figure 2), when the stirring rate is higher than 240 r/min, the selectivity and yield of MAL remain stable, which indicates that the influence of diffusion has been eliminated.
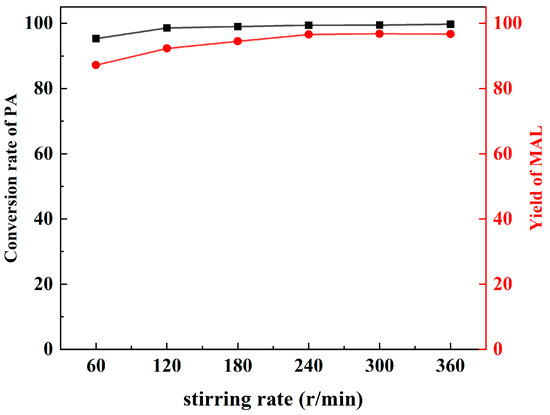
Figure 2.
The influence of stirring rate.
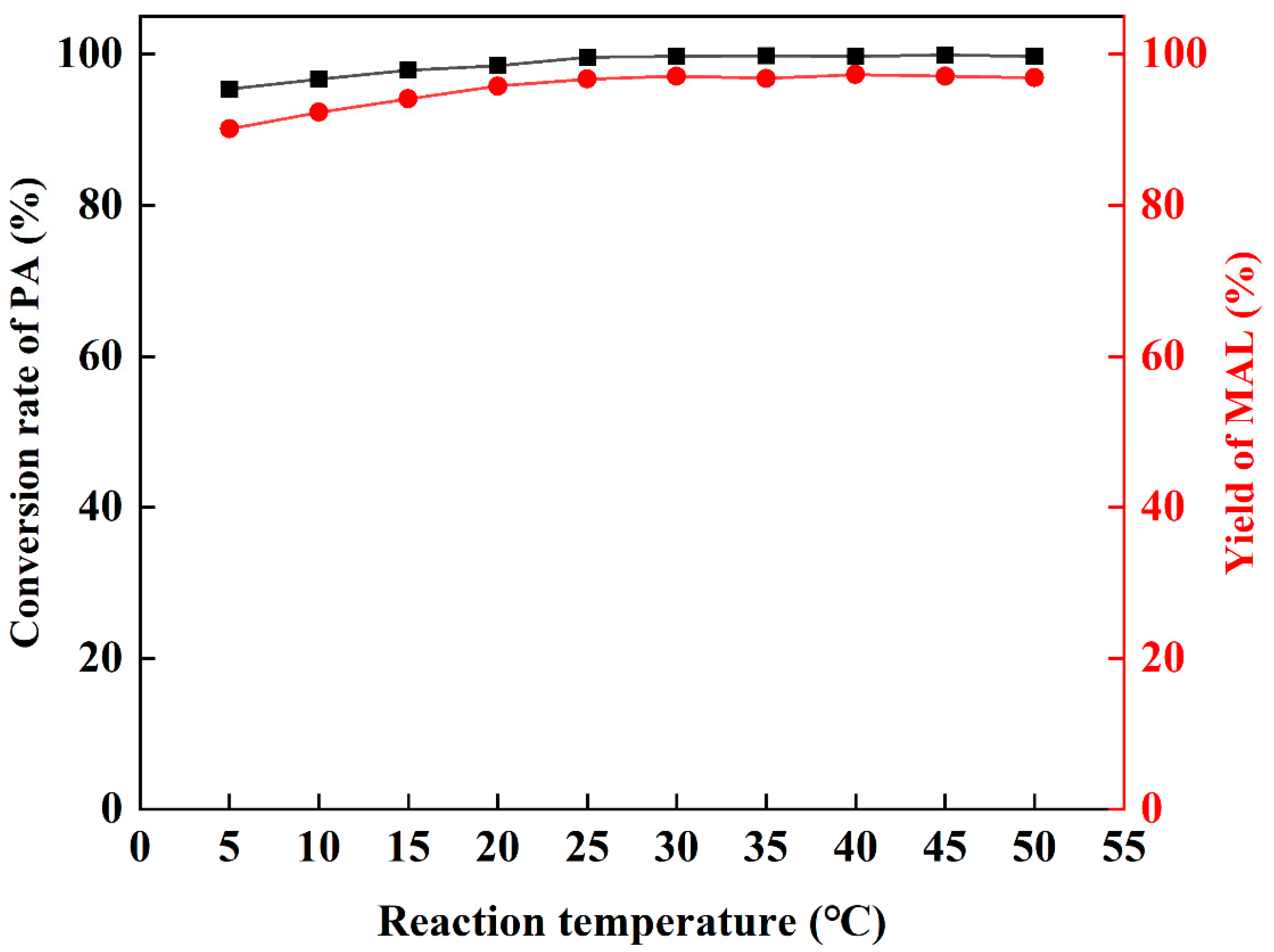
The influence of different temperatures on the reaction was studied. Dibutylamine acetate shows good catalytic ability in the temperature range of 5–50 °C (Figure 3), and the conversion of PA and yield of MAL are higher than 92%. With the increase in temperature, the conversion and yield also increase. When the reaction temperature is higher than 25 °C, the yield of MAL remains above 96%. Thus, the optimal reaction was 25 °C.
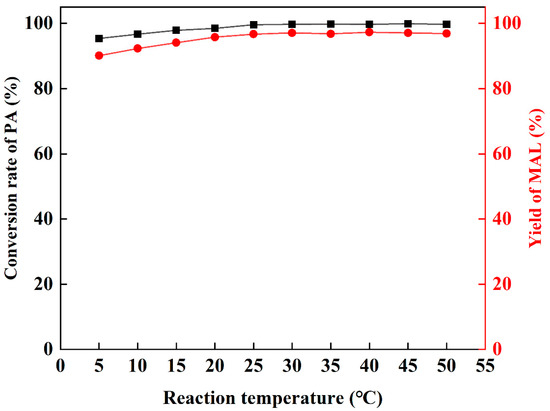
Figure 3.
The influence of temperature.
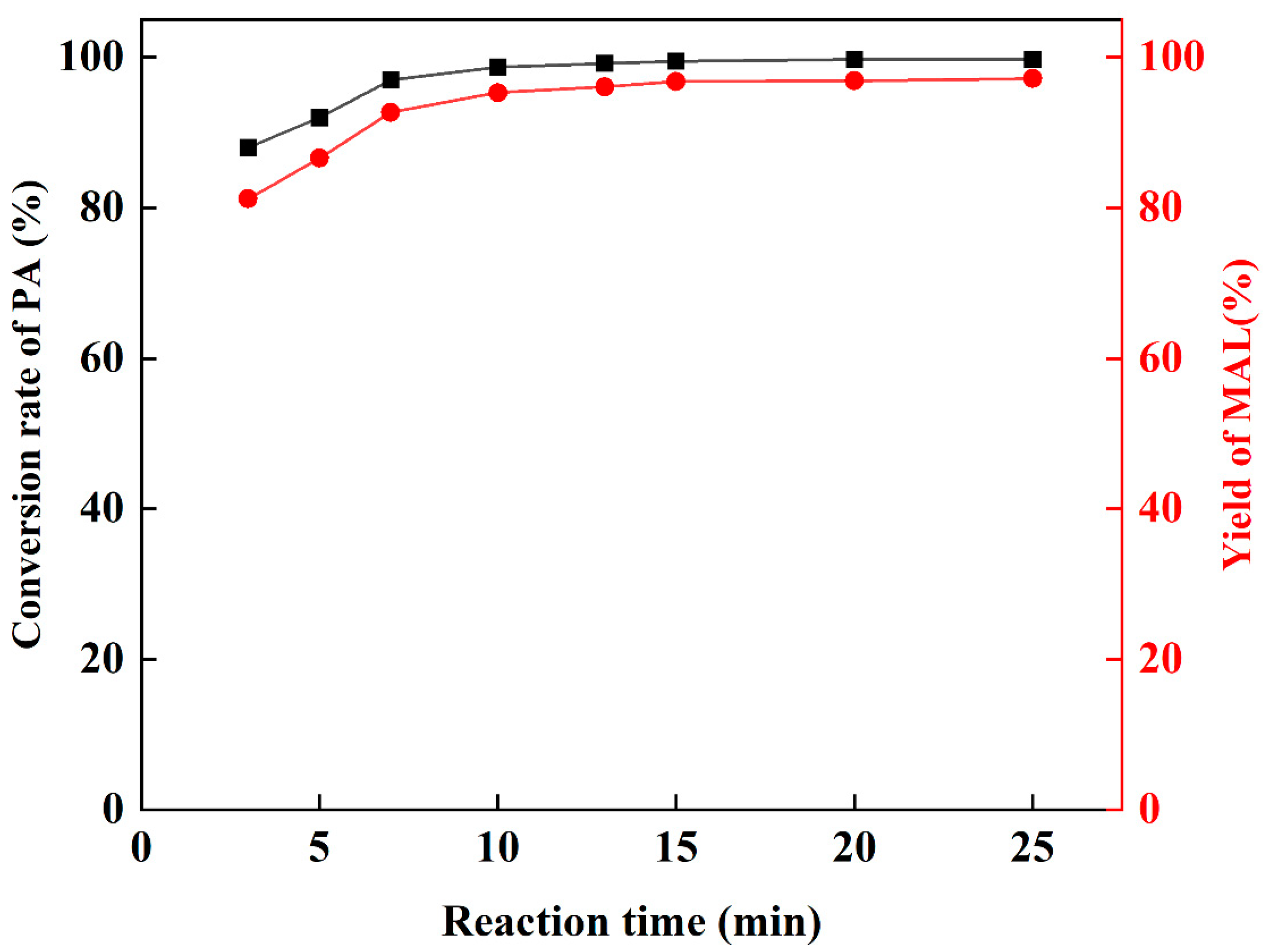
Then, keeping the reaction temperature at 25 °C, the conversion of PA and yield of MAL under different reaction times were measured (Figure 4). It can be observed that the yield of MAL does not fluctuate significantly after 15 min, which indicates that the reaction rate is fast at 25 °C, and the dibutylamine acetate has a more efficient catalytic performance. Yu’s [48] study showed that there is an optimal interval of reaction time for the preparation of MAL by the amine-catalyzed condensation of FA and PA, and extending the reaction time after reaching the reaction equilibrium will lead to a decrease in MAL yield. Therefore, the reaction time was set to 15 min.
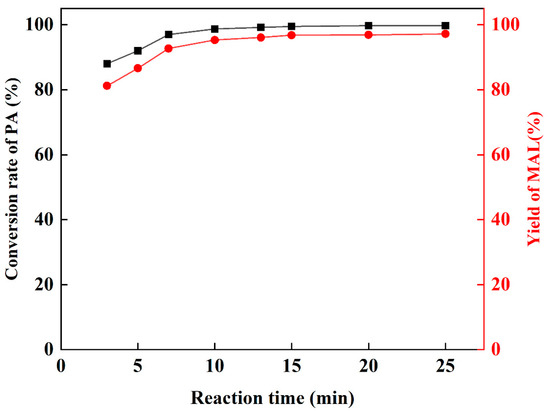
Figure 4.
The influence of reaction time.
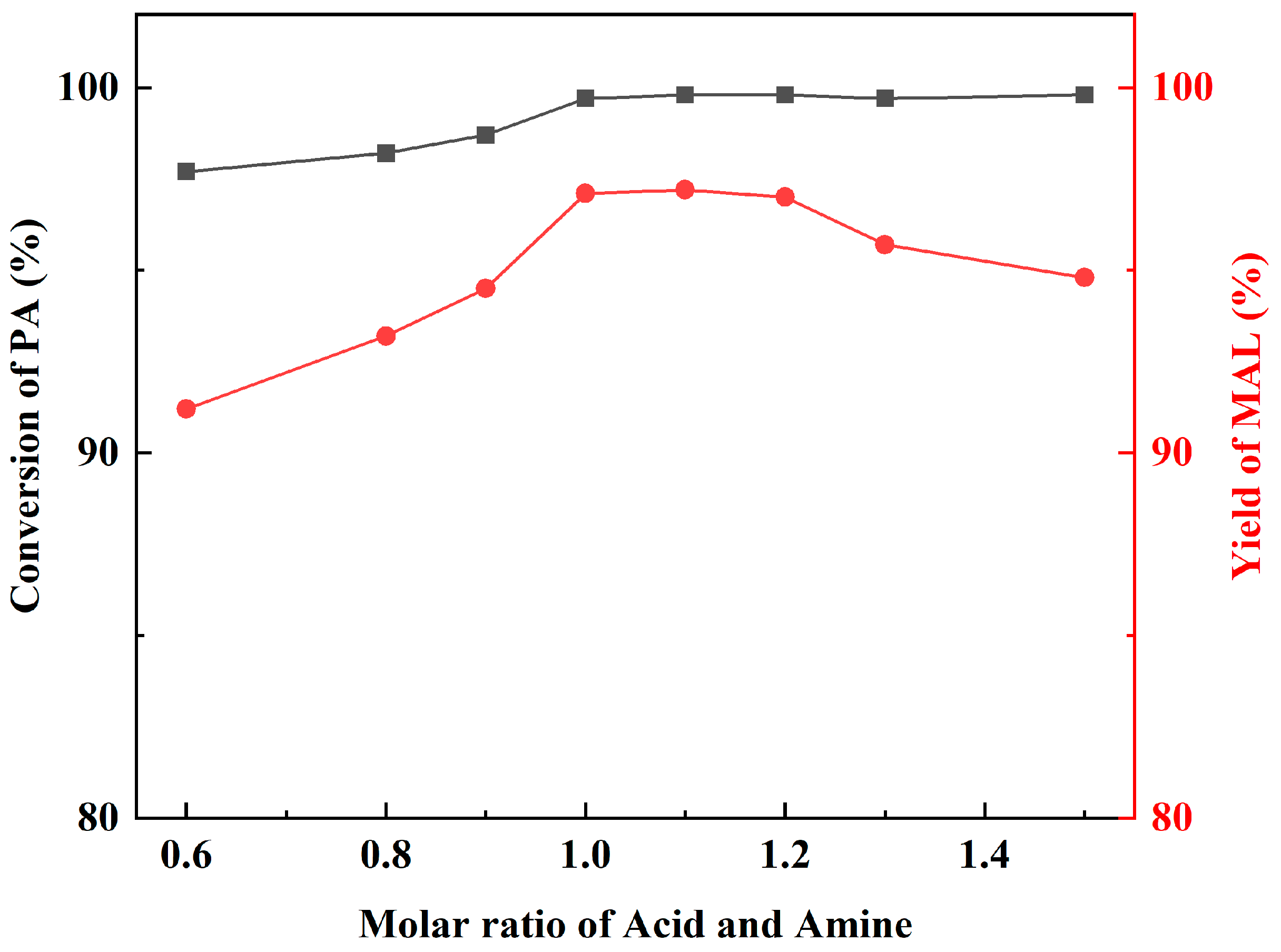
The pH of the reaction system can be controlled by changing the molar ratio of the amine and acid, which in turn affects the catalytic effect. Dibutylamine was kept at 0.1 mol, and acetic acid was added at 0.15, 0.13, 0.12, 0.11, 0.1, 0.09, and 0.08 mol. The reaction was performed under the same reaction conditions. Figure 5 shows that catalytic activity rises with increasing acetic acid addition, reaching the optimum at acid: amine molar ratios in the range of 1.0–1.1, and then gradually decreases with increasing acetic acid. According to the reaction mechanism, acids can provide protons to promote the dehydration of amino methanol to form iminium ions, which are important intermediates in the reaction, and the lack of acids would disfavor the generation of iminium ions. Meanwhile, the dibutylamine molecule plays a catalytic role, and excess acetic acid binds to dibutylamine so that the lone pair of electrons on the N atom of dibutylamine interacts with the proton. That would result in the inability of dibutylamine to react with FA to produce amino methanol. Thus, the optimized molar ratio of dibutylamine and acetic acid was 1:1.1.
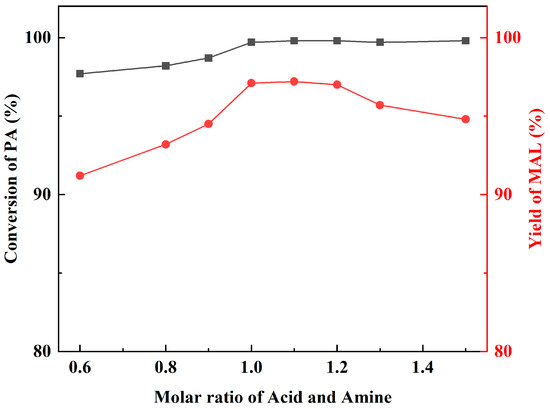
Figure 5.
The influence of molar ratio of amine and acid.
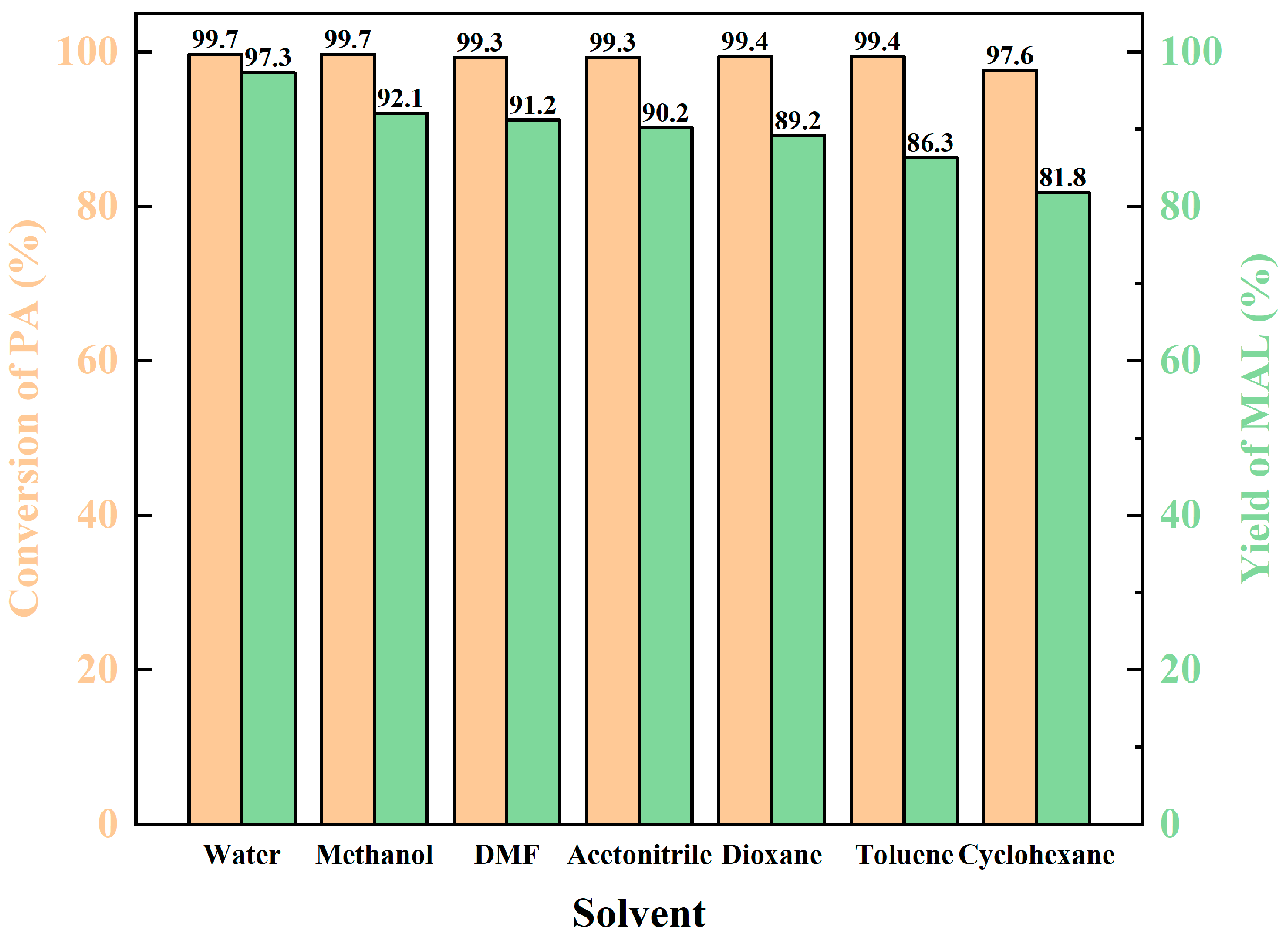
The solvent plays a crucial role in the chemical reaction. Several solvents were examined. From Figure 6, it can be seen that with the enhancement of solvent polarity, the yield of MAL also rises continuously. The highest yield was obtained when water was used as the solvent due to having the strongest polarity. This may result from the polar solvent contributing to proton transfer between the reactants. Notably, the requirement of dibutylamine acetate for the solvent was not strict, and the yield of MAL remained above 89% under the polar solvents. When non-polar solvents were used, the yield of MAL was reduced to less than 85%.
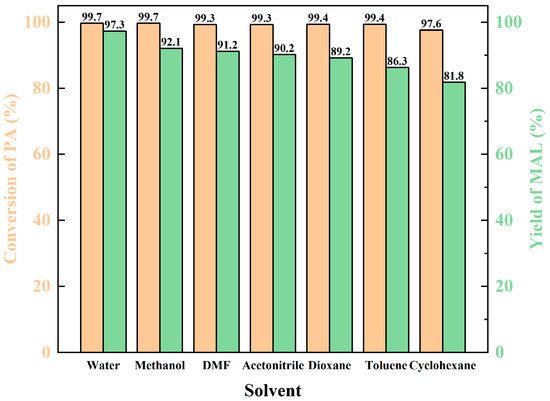
Figure 6.
The influence of solvent.
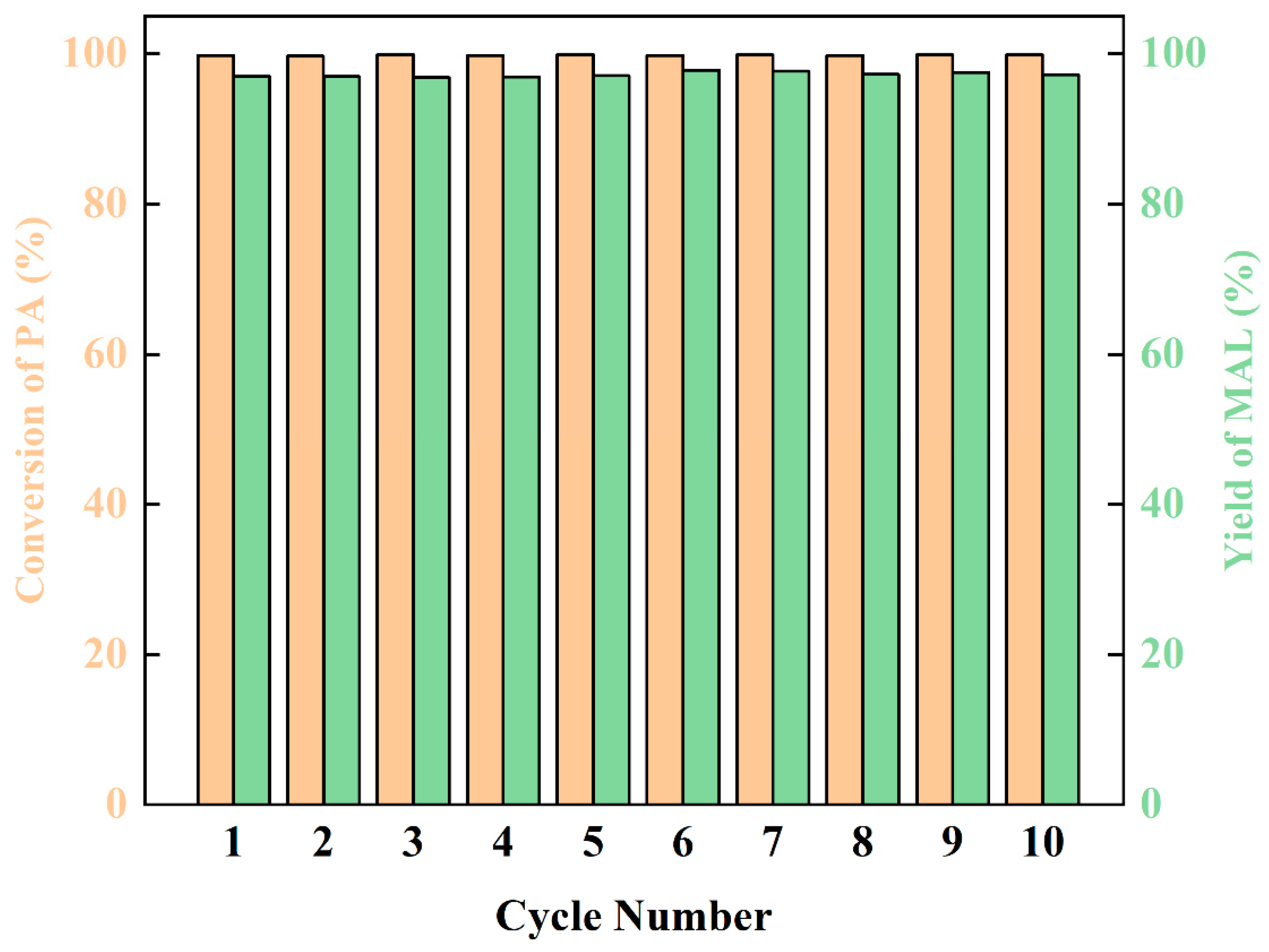
The stability of the catalyst is an important factor to evaluate the catalytic performance. After the reaction finished, the product and catalyst were separated by distillation, and the reaction was circulated 10 times. The yield of MAL remained stable at about 97% (Figure 7). This discovery reveals that dibutylamine acetate has good stability and has potential for commercialization.
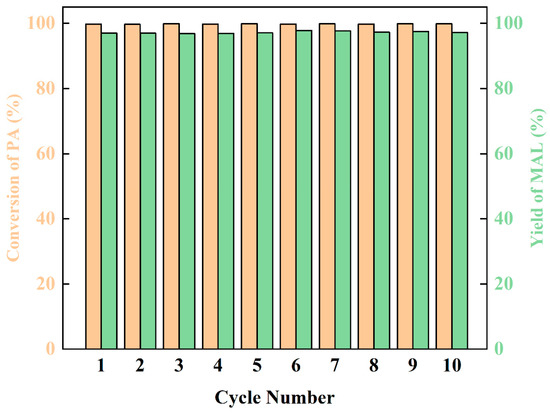
Figure 7.
The life cycle and stability of the catalyst.
2.2. Kinetics of Synthesis of MAL from FA and PA
The kinetic study of MAL synthesis was carried out in a 250 mL 3-necked glass-jacketed reactor and equipped with a magnetic stirrer. The reaction temperature was controlled by the water temperature injected into the jacket. If the reaction was too fast, the amount of catalyst used was reduced. The molar ratio of reactant to catalyst was controlled to 5:1. In order to ensure that the reaction could be fully carried out, the preliminary experiment was carried out at the reaction temperature of 25 °C. The expected selectivity and yield were achieved at 40 min, which shows that the current amount of catalyst will not affect the balance and effect of the reaction. The samples were taken from the reaction system regularly to analyze them by GC. The weights of the samples and the sampling times were recorded accurately.
The product was analyzed by GC, and it was detected that side products were generated from the reaction. GC-MS analysis shows that the main component is 2-methyl-2-pentenal, which is the product of the aldol condensation reaction of two molecules of PA. Although the electron pair of secondary amines mainly attack the carbonyl group of FA, a small part of them attack the carbonyl group of PA, the intermediate formed by PA, and the secondary amine further reacts with PA to generate the Mannich base, which is decomposed to obtain 2-methyl-2-pentenal. This mechanism has been confirmed by Yu [48], who detected the existence of the intermediate by ESI-MS and proved that this is the path of 2-methyl-2-pentenal generation. In the experiment, the yield of by-products is lower than 0.5%, which indicates that the utilization rate of reactants is very high, and the interference of by-products can be ignored.
The influence of internal diffusion and external diffusion has been reported above. The diffusion effect was eliminated at 300 r/min, and the stirring rate of all kinetic experiments was set at 360 r/min.
The kinetic equation of the aldol condensation reaction of FA and PA to generate MAL and water can be expressed as:
where r is the reaction rate, and its unit is mol L−1 min−1; k1 and k2 represent the forward and reverse reaction rate constants, respectively; and a, b, c, and d are the reaction orders of FA, PA, MAL, and water, respectively.
After mixing MAL and dibutylamine acetate under the same reaction conditions, no PA was detected by GC analysis, and the yield of MAL was higher than 96%. Thus, at this time, the reverse reaction can be ignored, and Equation (1) can be simplified as:
where C1 and C2 represent the real-time concentrations of FA and PA.
In the kinetic reaction, the molar amounts of the added FA and PA were equivalent, and the reaction coefficient ratio of FA and PA was 1:1. Thus, the real-time concentrations of FA and PA were equivalent. Therefore, the real-time concentration of FA could be replaced by the concentration of PA, and the kinetic Equation (2) can be further simplified as:
where f is the total reaction order of FA and PA.
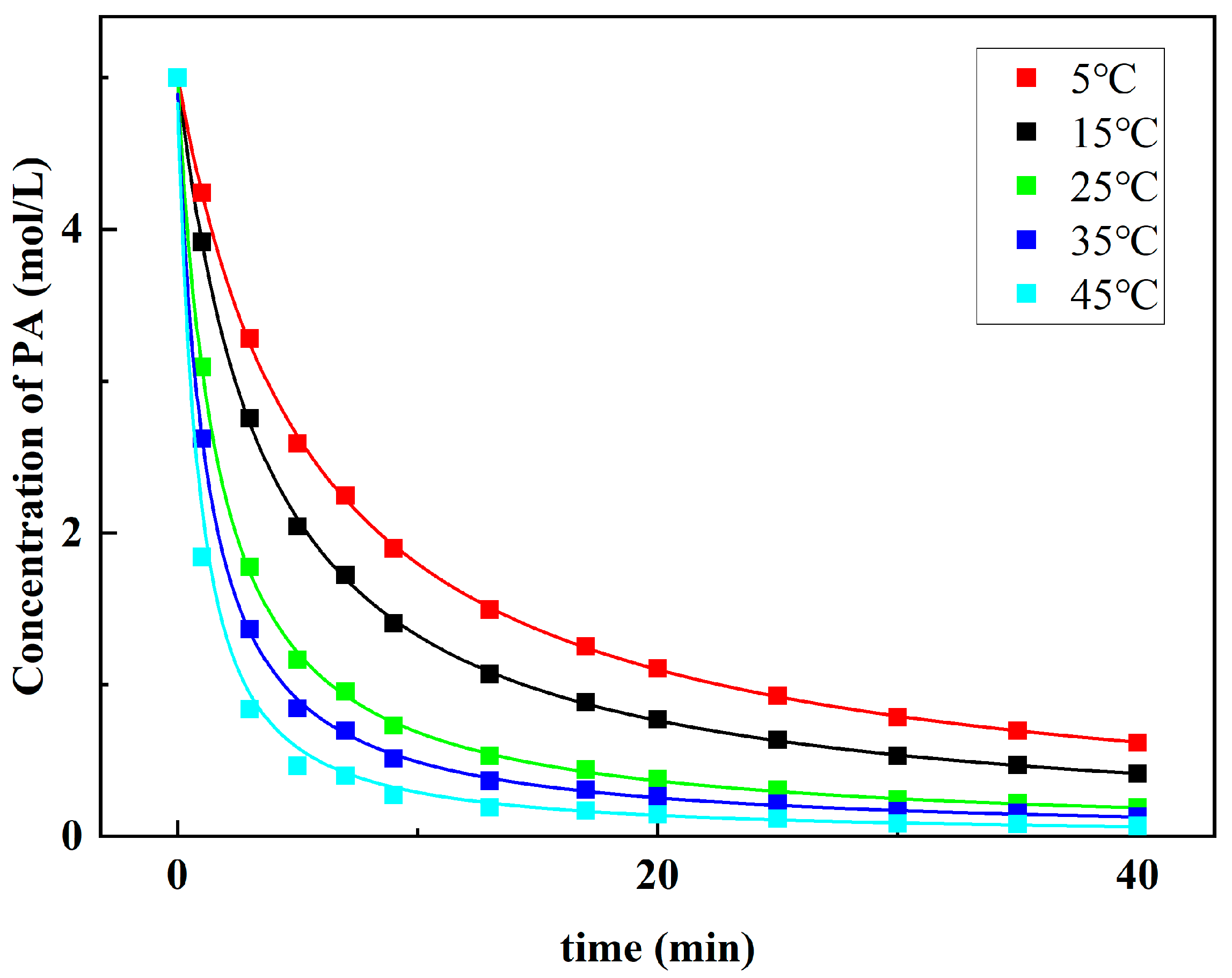
The kinetic results at 5–45 °C are shown in Figure 8. It can be seen that the concentration of PA decreases rapidly in a short time and then gradually slows down as the reaction proceeds. The curve of PA concentration drawn at a high reaction temperature is steeper. Equation (3) can be transformed into Equation (4) by logarithmic operation:
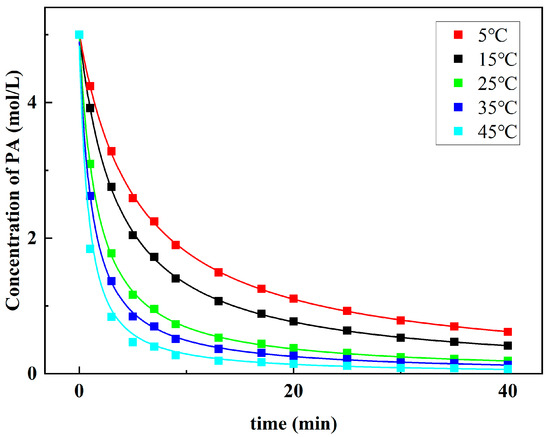
Figure 8.
Kinetic profiles for the dibutylamine acetate-catalyzed aldol reaction at different temperatures.
Linear fitting with the collected data shows that this is an approximate second-order reaction, and the total reaction order f is 2.15.
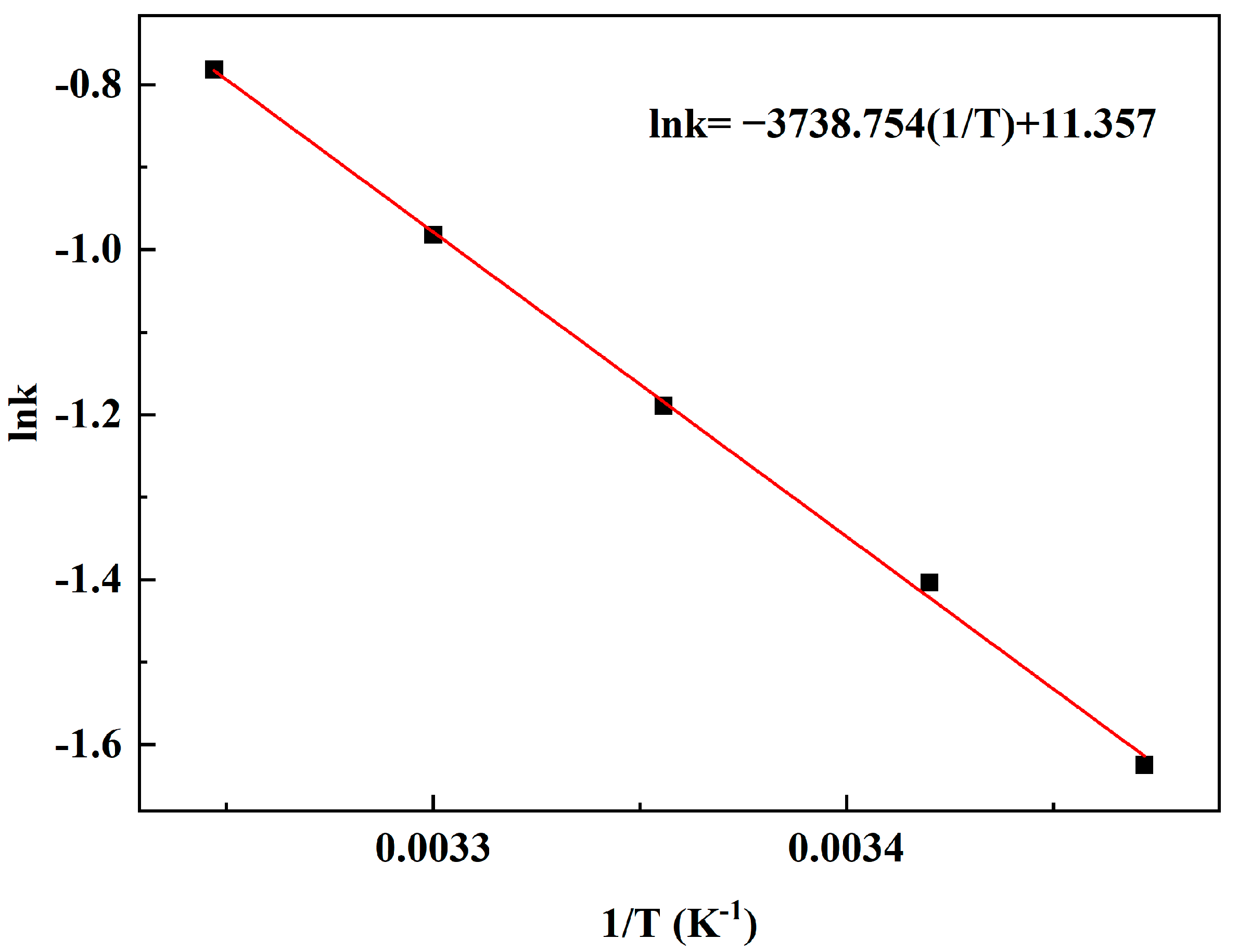
The relationship between the reaction constant k1 and reaction temperature is shown in Figure 9. The activation energy and pre-exponential factor of the reaction are calculated by Arrhenius formula. The slope and intercept of the line represent Ea and A, which are 31.084 kJ/mol and 8.7 × 105 (mol/L) −1 s−1, respectively.
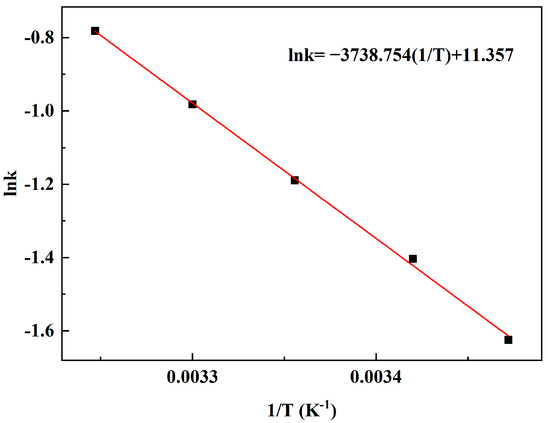
Figure 9.
Arrhenius plot for the reaction rate constants of MAL synthesis.
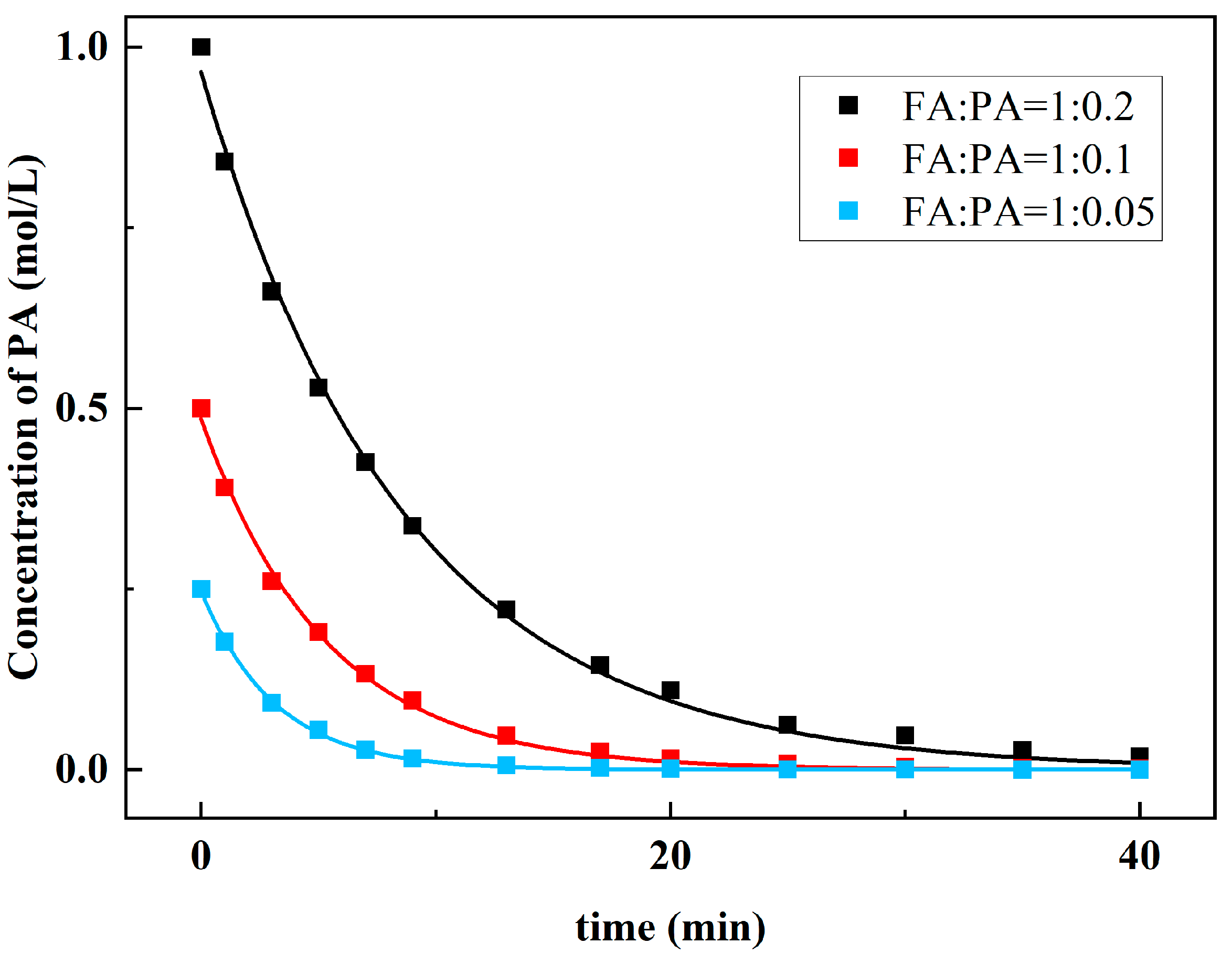
Then, the FA was fully overdosed, and three groups of kinetic experiments under different proportions were measured. The molar ratios of FA and PA in the 3 groups were 1:0.2, 1:0.1, and 1:0.05, respectively. In this case, it can be approximately considered that the FA concentration remains constant.
The kinetic Equation (2) for solving the reaction order of PA can be simplified as:
The collected data are fitted nonlinearly. Figure 10 is the fitting diagram of C2-t, and the rate of decrease in PA concentration under different molar ratios was quite different. Using Equation (5), the reaction order of PA is 0.9. Therefore, the reaction order of FA can be obtained:
a = f − b = 1.25
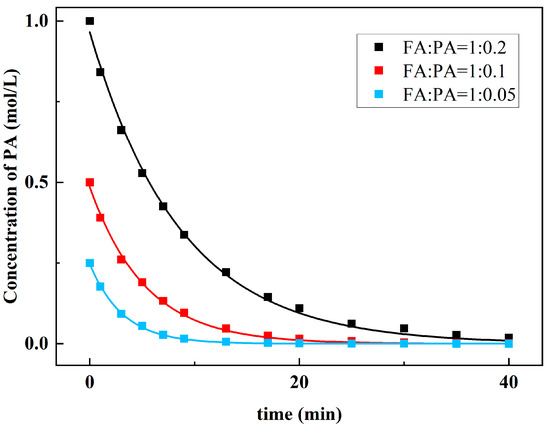
Figure 10.
Relationship between concentration of PA and reaction time.
Finally, the reaction kinetics equation of MAL synthesis can be expressed as Equation (7). The result also explains why the catalytic effect of dibutylamine acetate is insensitive to temperature changes, and the reaction can be activated at room temperature (25 °C).
To determine whether the rate-limiting step of MAL synthesis has a certain contribution to the reaction mechanism, there are two rate-limiting step theories: one is the generation of iminium ions, and the other is the decomposition of mannich bases [32,34]. In order to verify which theory is more consistent with the actual situation, the reaction was carried out at −15 °C. Samples were taken for analysis at 1, 3, and 5 min of the reaction. By GC analysis, it was found that the selectivity of MAL shows a slow upward trend, while the conversion of PA is maintained in the range of 95–97%. For comparison, the experiments conducted at 25 °C were also sampled and analyzed at 1, 3, and 5 min. It was found that the selectivity of MAL was not sensitive to the reaction time. As the reaction progressed, the selectivity of MAL ought to remain stable if the formation of iminium ions is the rate-limiting step. However, this hypothesis does not match the experimental results. Assuming the decomposition of the Mannich base is used as the rate-limiting step, the excess Mannich base would continue to decompose to produce MAL as the reaction time was extended because the Mannich base was not able to decompose. Therefore, the selectivity of MAL improved with a longer reaction time. The experimental results support the decomposition of the Mannich base as the rate-limiting step.
It is worth noting that the selectivity of MAL for 1, 3, and 5 min at −15 °C was 90.63%, 92.41%, and 93.88%, respectively, which remained above 90%, indicating that the decomposition energy barrier of the Mannich base was slightly higher than the formation of iminium ions. At a reaction temperature higher than room temperature, the difference in the rate between the two reaction steps can hardly be observed.
These kinetic data provide a meaningful reference for reactor design and scale-up experiments.
3. Experimental Section
Materials: FA aqueous solution (37.0 wt%), FA standard solution (100 mg/L, in 5% ethanol), di-n-butylamine (purity ≥ 99.0%), PA (purity of 97.0%), MAL (purity ≥ 95.0%), n-methyldibutylamine (purity ≥ 99.0%), THF (purity ≥ 99.0%), DMF (purity ≥ 99.0%), diethylamine (purity ≥ 99.0%), di-n-propylamine (purity of 99.0%), diisopropylamine (purity ≥ 99.0%), diisobutylamine (purity of 99.0%), di-sec-butylamine (purity of 99.0%), pyrrolidine (purity of 99.0%), imidazole (purity of 99.0%), n-methylaniline (purity of 98.0%), diphenylamine (purity ≥ 99.0%), n-benzylmethylamine (purity ≥ 99.0%), dibenzylamine (purity of 98.0%), acetic acid (purity ≥ 99.5%), propionic acid (purity ≥ 99.5%), butyric acid (purity of 99.5%), sulfuric acid (purity of 98.0%), hydrochloric acid (purity of 37%), acetylacetone (purity ≥ 99.0%), and methanol (purity ≥ 99.9%) were supplied by Aladdin chemical company (Shanghai, China).
3.1. Preparation of Catalyst
The synthesis of the catalyst was conducted in 15 °C water baths equipped with magnetic stirrers. We dropped 0.1 mol of amine into a flask, and then 7.20 g of distilled water and 0.2 mol of acetic acid were mixed and dropped into the flask. After mixing and stirring for 1 h, the preparation of the catalyst was complete.
3.2. Synthesis of MAL
We added 0.1 mol of the catalyst into a flat-bottomed flask in 40 °C water bath equipped with magnetic stirrers. Then, a mixture of 8.12 g of FA (37.0% wt) and 5.99 g of PA was dropped into the flat-bottomed flask. After mixing and stirring for 10 min, the synthesis of MAL was complete.
3.3. Recovery of Catalyst
After completing the reaction, the solution was distilled by rotary evaporator at 70 °C under 13 kPa for 30 min. The remaining liquid was the recovered catalyst, and the complete cycle was finished.
3.4. Computational Details
All of the calculations were carried out with the Gaussian 09 program package [49]. This study optimized the geometric structure of all substances at the calculation level of B3LYP/6-311 + G(d,p) [50,51] and recalculated the single-point energy at the level of M06-2X/def2-TZVP [52,53,54]. The computational data of all substances were processed with multiwfn to obtain the data of electrostatic potential (ESP) distribution on van der Waals surfaces [55,56,57]. Then, visual molecular dynamics (VMD) [58] was used to plot the electrostatic potential (ESP) distribution on van der Waals surfaces.
3.5. Analysis Methods
We determined the concentration of FA aqueous solution by acetylacetone spectrophotometry. We diluted 0.2 mL of FA aqueous solution to 100 mL with distilled water and then prepared it as 0.2–10.6 mg/L solution A. Solution B was obtained by dissolving 0.75 mL of acetylacetone, 9 mL of acetic acid, and 75 g of ammonium acetate in 150 mL of water. A mixture of 25 mL of solution A and 2.5 mL of solution B was heated in a 60 °C water bath for 15 min and cooled to room temperature, and then we measured the concentration of solution A at 414 nm with an ultraviolet spectrophotometer [48].
Qualitative analysis of the product samples was performed by gas chromatography−mass spectrometer (GC−MS) (7890B, Agilent Technologies) using an HP-5 column (30 m, 0.32 mm, 0.25 μm); He carrier gas; temperature of 40 to 260 °C (hold for 4 min) at 20 °C/min; detector temperature of 280 °C; and injector temperature of 260 °C.
The product and the reactants were quantified by gas chromatography (GC) (7890B, Agilent Technologies) using a DB-624 column (60 m, 0.25 mm, 1.4 μm); N2 carrier gas; temperature of 40 to 260 °C (hold for 13 min) at 20 °C/min; detector temperature of 280 °C; and injector temperature of 260 °C.
In this study, the conversion of PA and the yield of MAL were selected to evaluate the catalyst performance. The corresponding expressions of these parameters were as follows:
Conversion of PA:
Yield of MAL:
In these equations, nPA, in and nPA,out are the moles of PA in the system before and after the reaction, respectively, and nMAL,out is the moles of MAL synthesized in this reaction.
4. Conclusions
We studied the effects of different secondary amines and acids on the catalytic synthesis of MAL from FA and PA and the mechanism causing the difference. The nucleophilicity of the amine and the steric hindrance effect of the functional group are closely related to the catalytic effect. The conjugation effect and steric hindrance of aromatic amines lead to a poor catalytic effect. The strong nucleophilic property of the N atom on the amine is conducive to the reaction with FA. At the same time, different acids were evaluated and analyzed, and weak acids were found to be more suitable as acid components. After screening, it was found that dibutylamine acetate as the catalyst had the best catalytic effect, and the reaction conditions were optimized to determine the best reaction conditions. Moreover, kinetic studies were performed to obtain the reaction kinetic equations and rate-limiting steps. The results showed that the activation energy of the reaction was lower than that of the catalyst previously reported, and the decomposition of the Mannich base is the slowest step in the reaction. These data have certain guiding significance for further industrialization.
Author Contributions
Writing—original draft, T.W. Writing—review and editing, J.L., H.Y., G.Z. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Fund for Distinguished Young Scholars [22025803] and Hebei Natural Science Foundation [B2021103012].
Data Availability Statement
The data presented in this study are available in this paper.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Smith, W.E.; Ham, G.E.; Anspon, H.D.; Gebura, S.E.; Alwani, D.W. Polylactones Derived from Polymethacrolein and StyreneMethacrolein Copolymerizations. J. Polym. Sci. Part A Polym. Chem. 1968, 6, 2001–2012. [Google Scholar] [CrossRef]
- Andreeva, I.V.; Koton, M.M.; Getmanchuk, Y.P.; Madorsikaya, L.Y.; Pokrovskii, E.I.; Koltsov, A.I. Polymerization of α-methacrolein and the structure of the polymers. J. Polym. Sci. Part C Polym. Symp. 2007, 16, 1409–1421. [Google Scholar] [CrossRef]
- Kumar, P.; Hegde, V.R.; Kumar, T.P. An Efficient Synthesis of Diacetates from Aldehydes Using Beta Zeolite. Tetrahedron Lett. 1995, 36, 601–602. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Jing, X.; Zhu, J. Solvent free catalytic synthesis of 2-methylallylidene diacetate using cation-exchange resin. Catal. Commun. 2010, 11, 753–757. [Google Scholar] [CrossRef]
- Diao, Y.; Yang, P.; Yan, R.; Jiang, L.; Wang, L.; Zhang, H.; Li, C.; Li, Z.; Zhang, S. Deactivation and regeneration of the supported bimetallic Pd–Pb catalyst in direct oxidative esterification of methacrolein with methanol. Appl. Catal. B Environ. 2013, 142–143, 329–336. [Google Scholar] [CrossRef]
- Yan, R.; Li, Z.; Diao, Y.; Fu, C.; Wang, H.; Li, C.; Chen, Q.; Zhang, X.; Zhang, S. Green process for methacrolein separation with ionic liquids in the production of methyl methacrylate. AlChE J. 2011, 57, 2388–2396. [Google Scholar] [CrossRef]
- Muller, A.H.E.; Roos, S.G.; Maryjaszewski, K. Copolymerization of N-butyl acrylate with methyl methacrylate and PMMA macromonomers: Comparison of reactivity ratios in conventional and atom transfer radical copolymerization. Polym. Prepr. 1999, 40, 352–353. [Google Scholar]
- Qi, M.; Wu, X.; Wang, L.; Song, Y.; Diao, Y. The effect of the bimetallic Pd-Pb structures on direct oxidative esterification of methacrolein with methanol. Mol. Catal. 2021, 510, 111714. [Google Scholar] [CrossRef]
- Gao, J.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Li, F. Oxidative Esterification of Methacrolein to Methyl Methacrylate over Gold Nanoparticles on Hydroxyapatite. ChemCatChem 2017, 9, 1230–1241. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Zhu, J.; Sun, W.; Chen, S. Preparation and characterization of mono-/multi-metallic hydrophobic catalysts for the oxidative esterification of methacrolein to methyl methacrylate. J. Mol. Catal. A Chem. 2013, 379, 322–326. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Wang, L.; Fu, Z. Oxidative Esterification of Methacrolein to Methyl Methacrylate over Supported Gold Catalysts Prepared by Colloid Deposition. ChemCatChem 2017, 9, 1960–1968. [Google Scholar] [CrossRef]
- Song, N.; Rhodes, C.; Bartley, J.; Taylor, S.; Chadwick, D.; Hutchings, G. Oxidation of isobutene to methacrolein using bismuth molybdate catalysts: Comparison of operation in periodic and continuous feed mode. J. Catal. 2005, 236, 282–291. [Google Scholar] [CrossRef]
- Mascarenhas, C.M.; Miller, S.P.; White, P.S.; Morken, J.P. First Catalytic Asymmetric Aldol-Tishchenko Reaction-Insight into the Catalyst Structure and Reaction Mechanism. Angew. Chem. Int. Ed. 2001, 40, 601–603. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, H. Reaction kinetics for preparation of methacrolein by condensation of formaldehyde with propionaldehyde. Acc. Chem. Res. 2004, 37, 570–579. [Google Scholar] [CrossRef]
- Mironov, G.S.; Farberov, M.I. Commercial methods of synthesis of α, β-unsaturated aldehydes and ketones. Russ. Chem. Rev. 1964, 33, 649–663. [Google Scholar] [CrossRef]
- Dashko, L.V.; Dmitriev, D.V.; Pestov, S.M.; Flid, V.R. Cross aldol condensation of acetaldehyde and formaldehyde in the presence of bifunctional systems. Russ. J. Org. Chem. 2015, 50, 1732–1737. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Fan, L.; Li, C.; Zhang, S. Sec-amine grafted D301 resin catalyzed fixed-bed process for continuous preparation of methacrolein via mannich reaction. Chem. Eng. J. 2019, 370, 625–636. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Hedström, M.; Lundmark, S.; Rehnberg, N.; Hatti-Kaul, R. Self- and Cross-Aldol Condensation of Propanal Catalyzed by Anion-Exchange Resins in Aqueous Media. Org. Process Res. Dev. 2011, 15, 631–637. [Google Scholar] [CrossRef]
- Erkkilä, A.; Pihko, P.M. Rapid Organocatalytic Aldehyde-Aldehyde Condensation Reactions. Eur. J. Org. Chem. 2007, 2007, 4205–4216. [Google Scholar] [CrossRef]
- Erkkilä, A.; Pihko, P.M. Mild Organocatalytic r-Methylenation of Aldehydes. J. Org. Chem. 2006, 71, 2538–2541. [Google Scholar] [CrossRef]
- Li, Y.C.; Yan, R.Y.; Wang, L.; Liu, L.; Liu, D.; Zhang, H.; Diao, Y.Y.; Li, Z.X.; Zhang, S.J. Synthesis of Methacrolein by Condensation of Propionaldehyde with Formaldehyde. Adv. Mater. Res. 2011, 396–398, 1094–1097. [Google Scholar]
- He, J.; Xue, Y.; Han, B.; Zhang, C.; Wang, Y.; Zhu, S. Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem. Int. Ed. Engl. 2020, 59, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.T.; Boulton, L.T.; Sneddon, H.F.; Sheppard, T.D. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2019, 2, 10–17. [Google Scholar] [CrossRef]
- Sun, B.; Pluta, R.; Kumagai, N.; Shibasaki, M. Direct Catalytic Asymmetric mannich-Type Reaction en Route to alpha-Hydroxy-beta-amino Acid Derivatives. Org. Lett. 2018, 20, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, Y.; Zhang, S.; Li, Z.; Li, C. An ionic liquid catalyzed probase method for one-pot synthesis of α,β-unsaturated esters from esters and aldehydes under mild conditions. Green Chem. 2017, 19, 4838–4848. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.; Gan, L.-H. Theoretical study on the mechanisms of Proline-catalyzed mannich reaction between acetaldehyde and N-Boc imines. J. Phys. Org. Chem. 2012, 25, 667–673. [Google Scholar] [CrossRef]
- Li, Y.M.; Xiao, H.M. Studies on the Mechanism of mannich Reaction Involving Iminium Salt as Potential mannich Reagent. 111. Furan as Pseudo Acid Component. Int. J. Quantum Chem. 1995, 54, 293–297. [Google Scholar] [CrossRef]
- Pierce, M.D.; Johnston, R.C.; Mahapatra, S.; Yang, H.; Carter, R.G.; Ha-Yeon Cheong, P. Mechanism and stereoselectivity of a dual amino-catalyzed robinson annulation: Rare duumvirate stereocontrol. J. Am. Chem. Soc. 2012, 134, 13624–13631. [Google Scholar] [CrossRef]
- Yamashita, Y.; Suzuki, H.; Kobayashi, S. Development of strong Bronsted base catalysis: Catalytic direct-type mannich reactions of non-activated esters via a product-base mechanism. Org. Biomol. Chem. 2012, 10, 5750–5752. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Gu, Y. Polymerization mechanism of 1,3-benzoxazine catalyzed by PCl5 and rearrangement of chemical structures. Eur. Polym. J. 2021, 142, 110133. [Google Scholar] [CrossRef]
- Tanaka, F. Amines as Catalysts: Dynamic Features and Kinetic Control of Catalytic Asymmetric Chemical Transformations to Form C-C Bonds and Complex Molecules. Chem. Rec. 2022. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.P.; Simijonovic, D.; Milovanovic, V.M.; Petrovic, Z.D. Acetophenone mannich bases: Study of ionic liquid catalysed synthesis and antioxidative potential of products. R. Soc. Open Sci. 2018, 5, 181232. [Google Scholar] [CrossRef] [PubMed]
- Zuvev, V.V.; Shibayev, L.; Stepanov, N.G.; Solovskaya, N.A.; Buryko, A.V. Electron cause of the evenness effect and its manifestation in the thermostability of a series of poly-4-n-alkylstyrenes. Polym. Sci. U.S.S.R. 1990, 32, 1780–1784. [Google Scholar] [CrossRef]
- Yan, H.; Huo, F.; Li, P.; Li, C. Mild Catalytic Mechanism of the mannich Reaction for Synthesizing Methylacrolein by sec-Amine Short-Chain Aliphatic Acid Ionic Liquid Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 6687–6698. [Google Scholar] [CrossRef]
- Niu, H.; Lu, L.; Shi, R.; Chiang, C.W.; Lei, A. Catalyst-free N-methylation of amines using CO2. Chem. Commun. 2017, 53, 1148–1151. [Google Scholar] [CrossRef]
- Zou, Q.; Long, G.; Zhao, T.; Hu, X. Catalyst-free selective N-formylation and N-methylation of amines using CO2 as a sustainable C1 source. Green Chem. 2020, 22, 1134–1138. [Google Scholar] [CrossRef]
- Majerz, I.; Dziembowska, T. Geometric Aspects of Aromaticity: Interrelations between Intramolecular Hydrogen Bonds, Steric Effects and π-Electron Delocalisation in Nitroanilines. Eur. J. Org. Chem. 2011, 2011, 280–286. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, H.; Liu, F.; Huo, F.; He, Y.; Chen, Z.; Liu, X.; Bo, S.; Qiu, L.; Zhen, Z. Synthesis of novel nonlinear optical chromophores: Achieving enhanced electro-optic activity and thermal stability by introducing rigid steric hindrance groups into the julolidine donor. J. Mater. Chem. C 2017, 5, 1675–1684. [Google Scholar] [CrossRef]
- Ohashi, K.; Inokuchi, Y.; Izutsu, H.; Hino, K.; Yamamoto, N.; Nishi, N. Electronic and vibrational spectra of aniline–benzene hetero-dimer and aniline homo-dimer ions. Chem. Phys. Lett. 2000, 323, 43–48. [Google Scholar] [CrossRef]
- Arasi, A.Y.; Jeyakumari, J.J.; Sundaresan, B.; Dhanalakshmi, V.; Anbarasan, R. The structural properties of poly(aniline)—Analysis via FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 1229–1234. [Google Scholar] [CrossRef]
- Honkawa, Y.; Inokuchi, Y.; Ohashi, K.; Nishi, N.; Sekiya, H. Infrared spectra and structures of aniline+–furan and aniline+–phenol. Preference between π-type and σ-type hydrogen-bonded structures. Chem. Phys. Lett. 2003, 376, 244–250. [Google Scholar] [CrossRef]
- Seliger, J.; Zaga, V. 14 N NQR Study of Diphenylamine. Verl. Der Z. Für Nat. A 2008, 63, 88–92. [Google Scholar]
- Hao, W.J.; Jiang, B.; Tu, S.J.; Cao, X.D.; Wu, S.S.; Yan, S.; Zhang, X.H.; Han, Z.G.; Shi, F. A new mild base-catalyzed mannich reaction of hetero-arylamines in water: Highly efficient stereoselective synthesis of beta-aminoketones under microwave heating. Org. Biomol. Chem. 2009, 7, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Meng, X.; Luo, Y.; Cao, S.; Zhao, G. A novel quaternary ammonium salts derived from α-amino acids with large steric hindrance group and its application in asymmetric mannich reaction. Tetrahedron 2020, 76, 131484. [Google Scholar] [CrossRef]
- Rogalski, M.; Domannska, U.; Czyrny, D.; Dyczko, D. Surface and conductivity properties of imidazoles solutions. Chem. Phys. 2002, 285, 355–370. [Google Scholar] [CrossRef]
- Thomason, M.J.; Seabourne, C.R.; Sattelle, B.M.; Hembury, G.A.; Stevens, J.S.; Scott, A.J.; Aziz, E.F.; Schroeder, S.L. Self-association of organic solutes in solution: A NEXAFS study of aqueous imidazole. Faraday Discuss. 2015, 179, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.; Burrows, W. Clarke—Eschweiler Cyclization. Scope and Mechanism. J. Org. Chem. 1966, 31, 3099–3103. [Google Scholar] [CrossRef]
- Yu, J.; Jensen, A.D.; Wang, L.; Li, C.; Zhang, S. Catalytic synthesis of methacrolein via the condensation of formaldehyde and propionaldehyde with l-proline. Green Chem. 2020, 22, 4222–4230. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev C.; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Johnson, B.G.; Gill, P.M.; Weoplee, J.A. The performance of a family of density functional methods. J. Chem. Phys. 1993, 98, 5612–5626. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first row transition metals Sc−Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. van der Waals Potential: An Important Complement to Molecular Electrostatic Potential in Studying Intermolecular Interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).