The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation
Abstract
1. Introduction
2. Results and Discussion
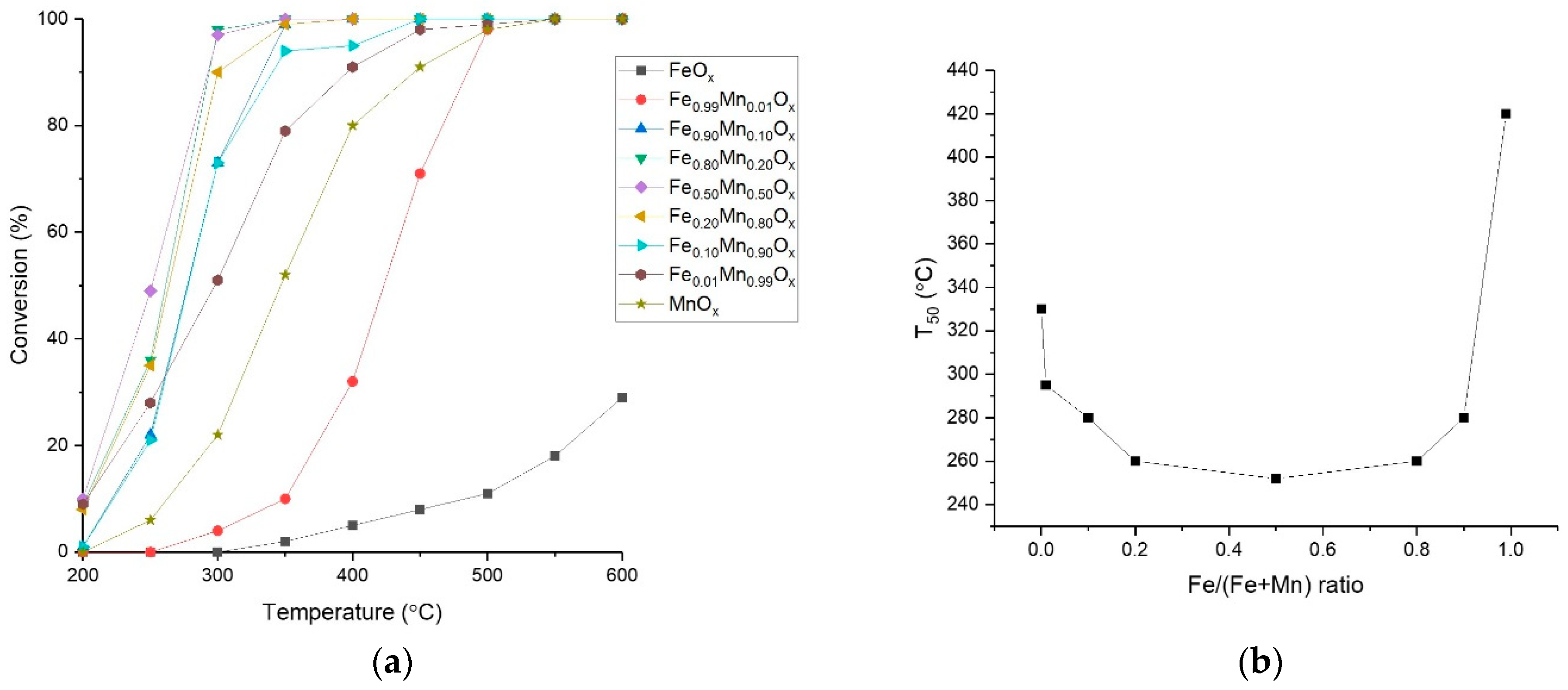
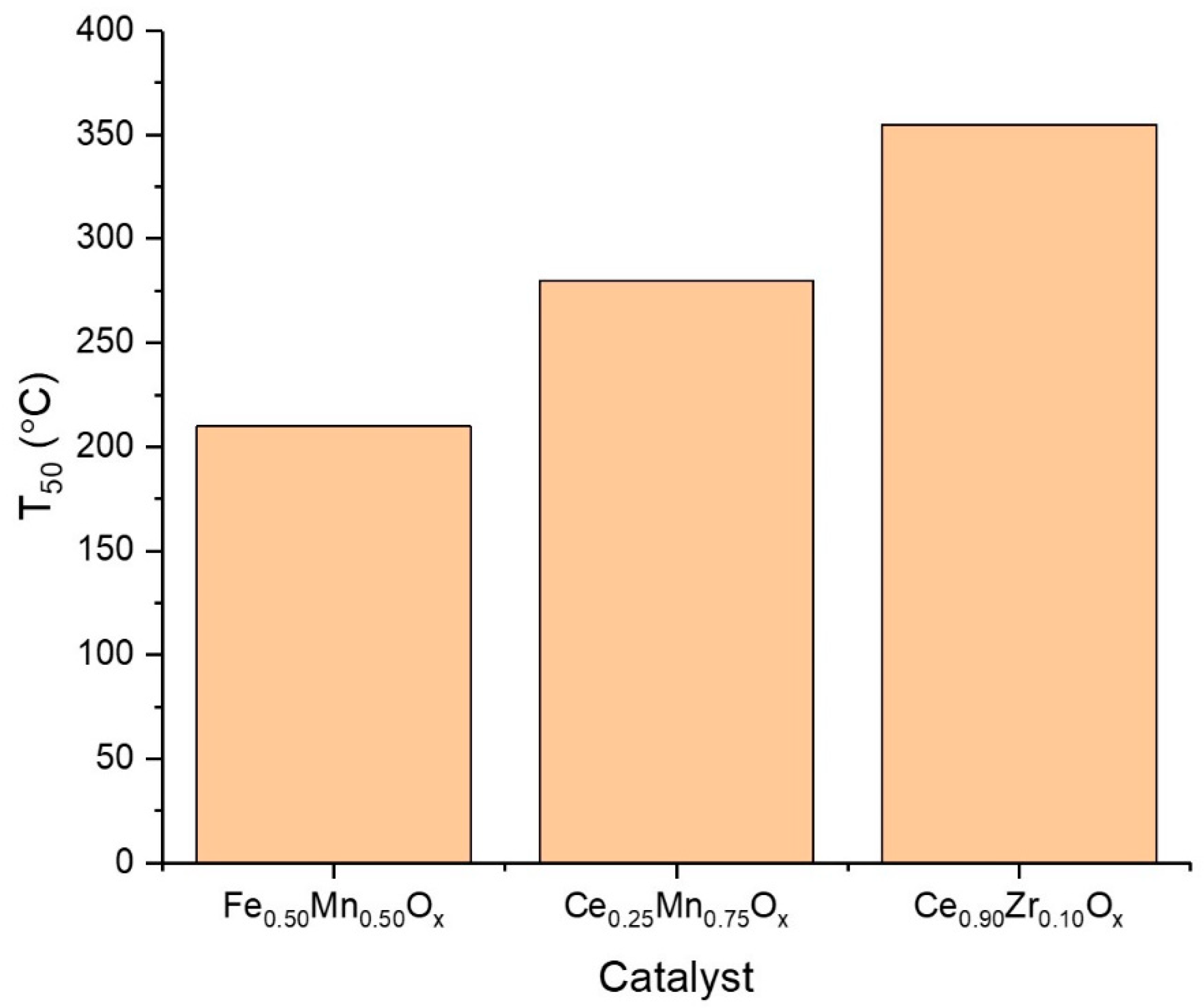
2.1. Performance of Sodium Carbonate Coprecipitated Catalysts
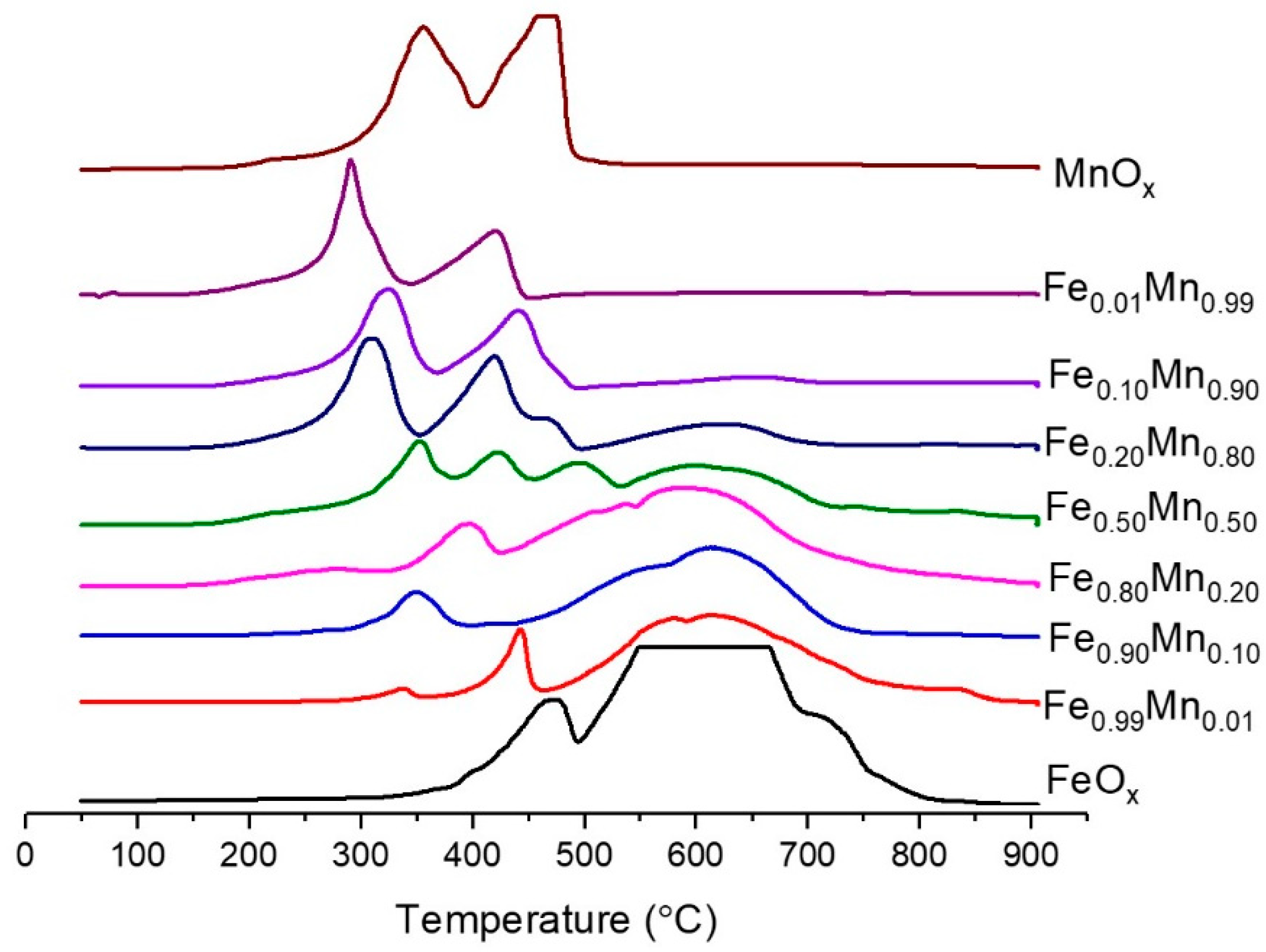
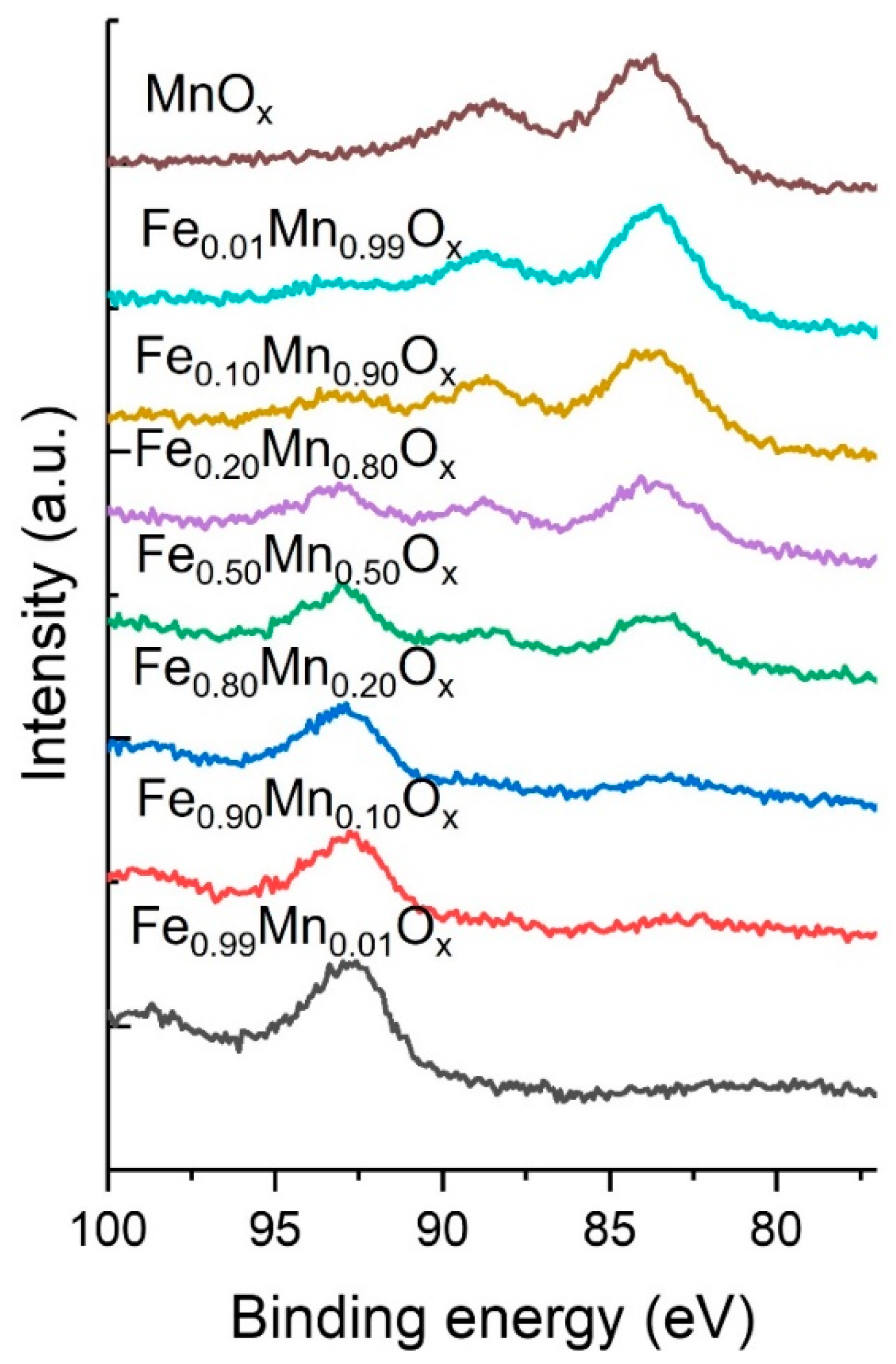
2.2. Characterisation of Sodium Carbonate Coprecipitated Catalysts
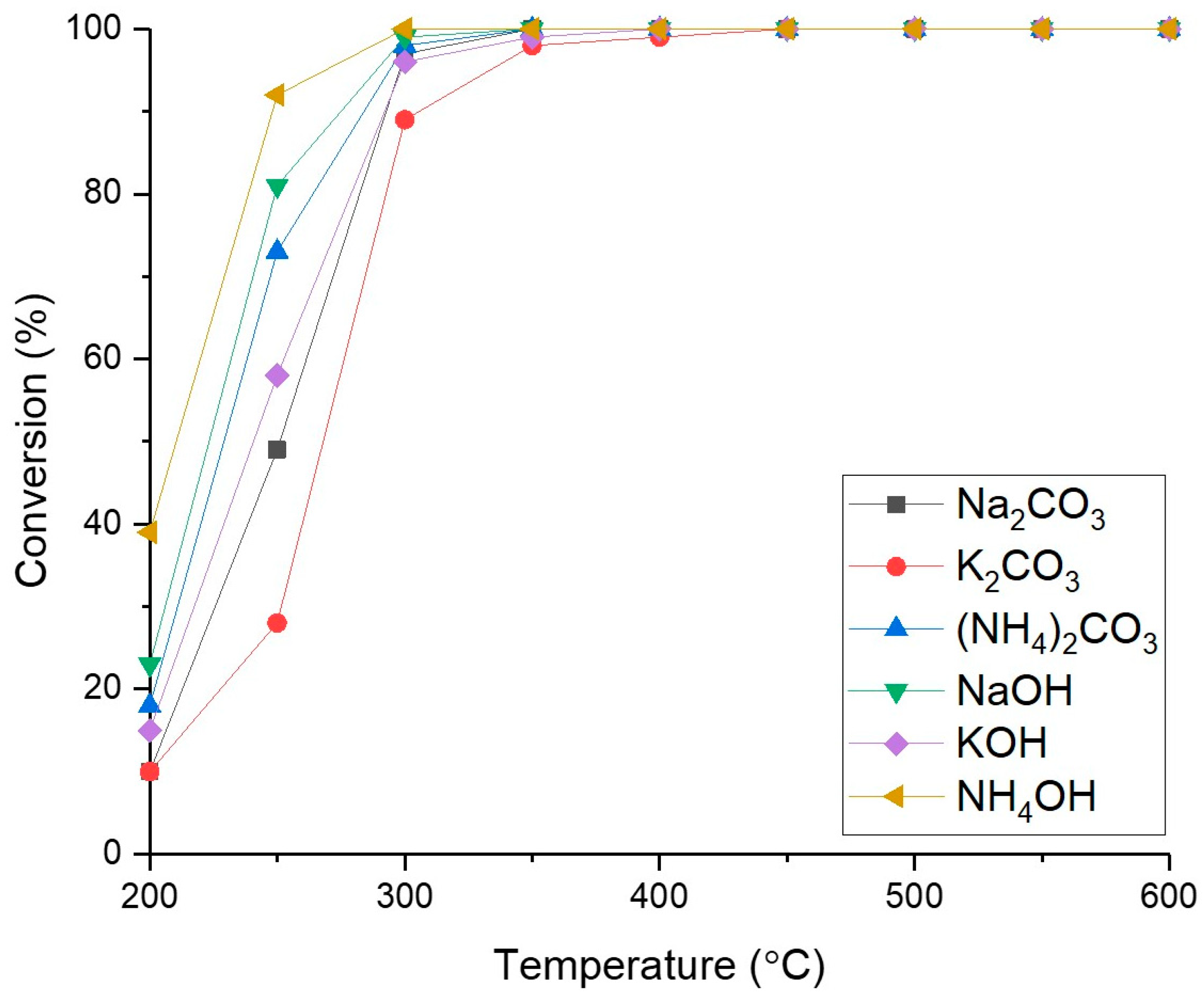
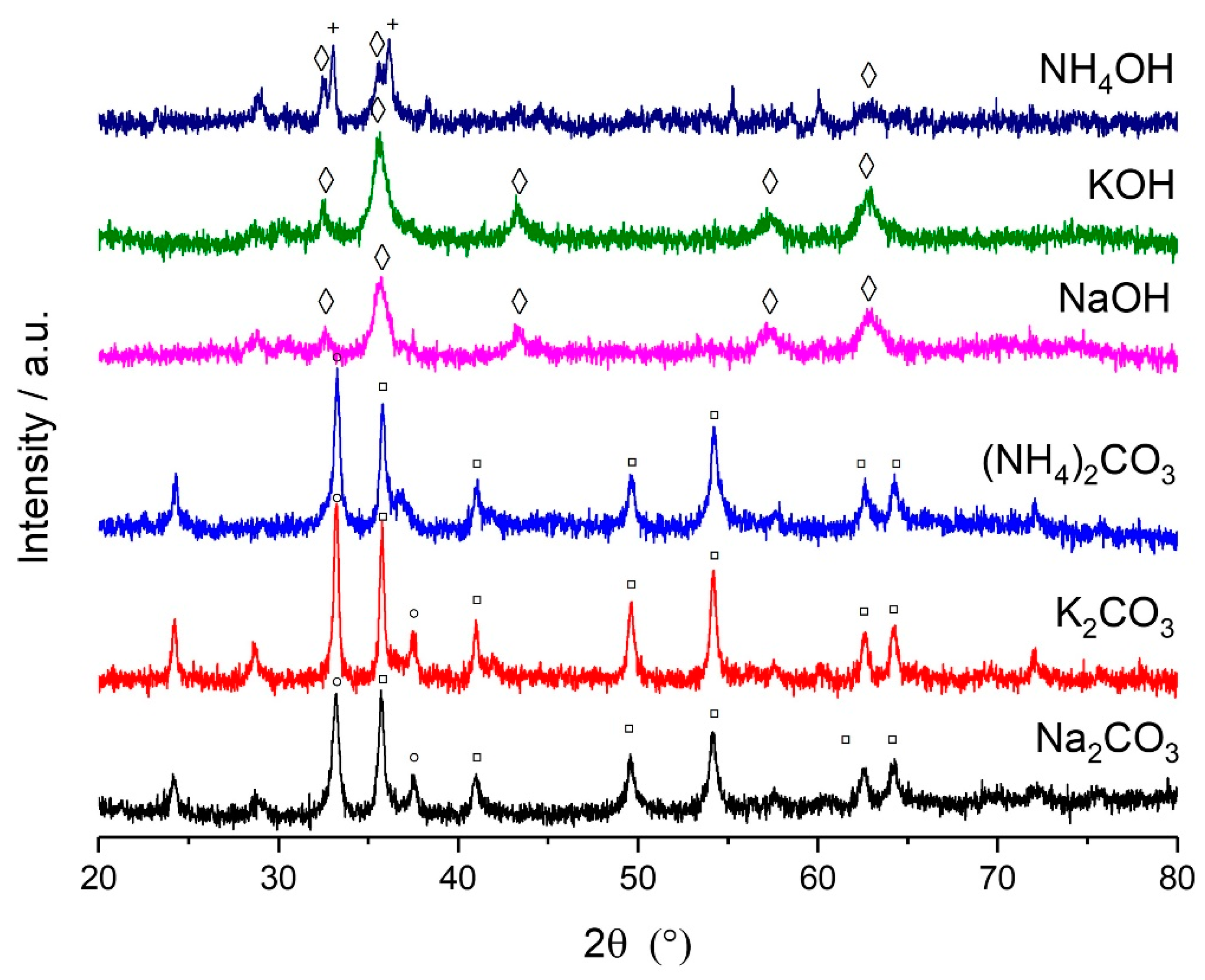
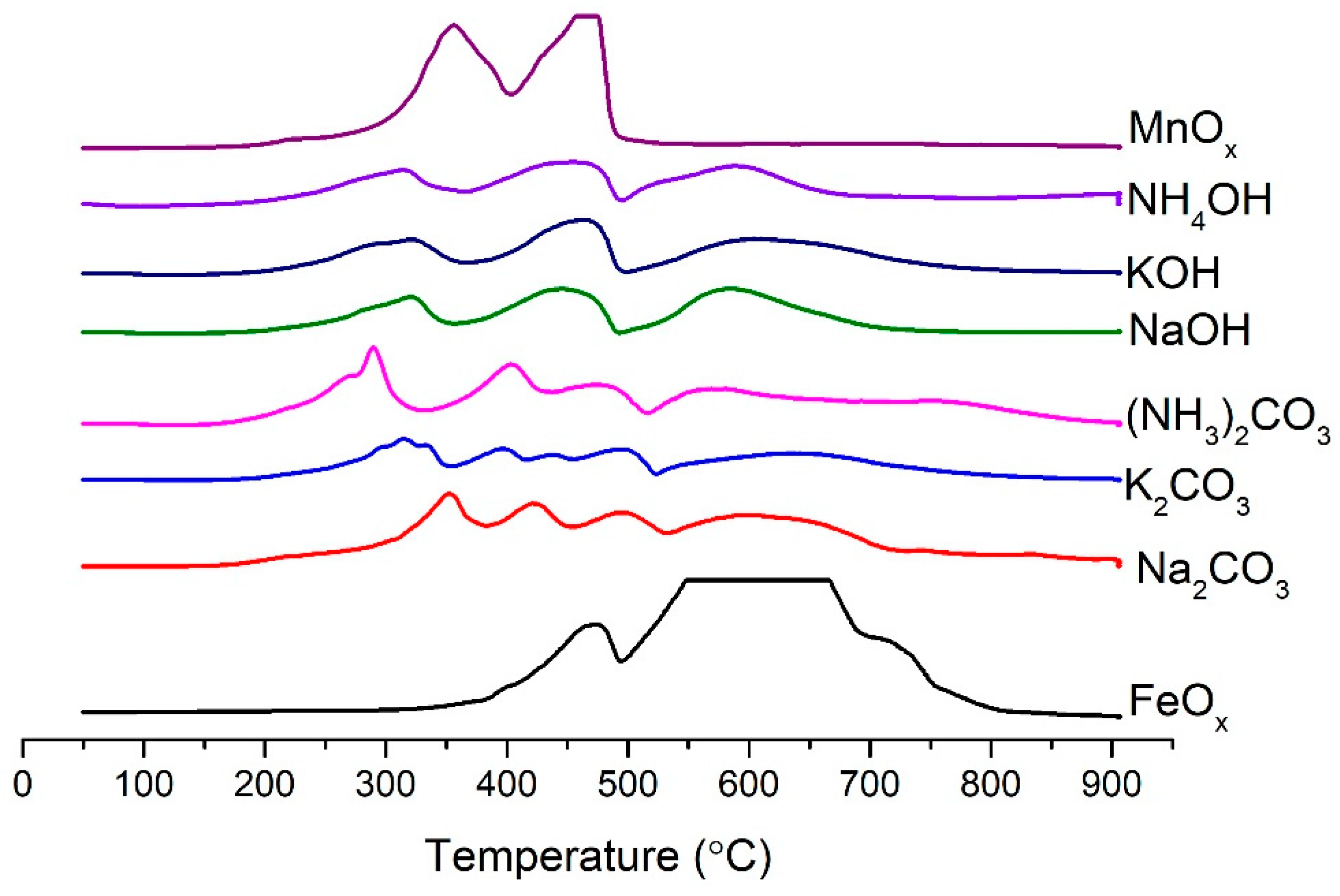
2.3. Influence of Precipitation Agent
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Testing
3.3. Catalyst Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Evuti, A. A Synopsis on Biogenic and Anthropogenic Volatile Organic Compounds Emissions: Hazards and Control. Int. J. Eng. Sci. 2013, 2, 145–153. [Google Scholar]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, Sources and Ozone Formation Potential of Volatile Organic Compounds (VOCs) during Ozone Episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Ryerson, T.B.; Trainer, M.; Holloway, J.S.; Parrish, D.D.; Huey, L.G.; Sueper, D.T.; Frost, G.J.; Donnelly, S.G.; Schauffler, S.; Atlas, E.L.; et al. Observations of Ozone Formation in Power Plant Plumes and Implications for Ozone Control Strategies. Science 2001, 292, 719–723. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on Pollution and Health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported Gold Catalysts for the Total Oxidation of Volatile Organic Compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent Advances in VOC Elimination by Catalytic Oxidation Technology onto Various Nanoparticles Catalysts: A Critical Review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Adebayo, B.; Gelles, T.; Rownaghi, A.; Rezaei, F. Abatement of Gaseous Volatile Organic Compounds: A Process Perspective. Catal. Today 2020, 350, 100–119. [Google Scholar] [CrossRef]
- Taylor, M.N.; Zhou, W.; Garcia, T.; Solsona, B.; Carley, A.F.; Kiely, C.J.; Taylor, S.H. Synergy between Tungsten and Palladium Supported on Titania for the Catalytic Total Oxidation of Propane. J. Catal. 2012, 285, 103–114. [Google Scholar] [CrossRef]
- Tichenor, B.A.; Palazzolo, M.A. Destruction of Volatile Organic Compounds via Catalytic Incineration. Environ. Prog. 1987, 6, 172–176. [Google Scholar] [CrossRef]
- Enterkin, J.A.; Setthapun, W.; Elam, J.W.; Christensen, S.T.; Rabuffetti, F.A.; Marks, L.D.; Stair, P.C.; Poeppelmeier, K.R.; Marshall, C.L. Propane Oxidation over Pt/SrTiO3 Nanocuboids. ACS Catal. 2011, 1, 629–635. [Google Scholar] [CrossRef]
- Aggett, K.; Davies, T.E.; Morgan, D.J.; Hewes, D.; Taylor, S.H. The Influence of Precursor on the Preparation of CeO2 Catalysts for the Total Oxidation of the Volatile Organic Compound Propane. Catalysts 2021, 11, 1461. [Google Scholar] [CrossRef]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [Google Scholar] [CrossRef]
- Huang, N.; Qu, Z.; Dong, C.; Qin, Y.; Duan, X. Superior Performance of α@β-MnO2 for the Toluene Oxidation: Active Interface and Oxygen Vacancy. Appl. Catal. A Gen. 2018, 560, 195–205. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Song, Z.; Liu, W.; Zhao, J.; Ma, Z.; Zhao, M.; Xing, Y. Insight into the Effect of Oxygen Species and Mn Chemical Valence over MnOx on the Catalytic Oxidation of Toluene. Appl. Surf. Sci. 2019, 493, 9–17. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic Combustion of Volatile Organic Compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef]
- Puértolas, B.; Smith, A.; Vázquez, I.; Dejoz, A.; Moragues, A.; Garcia, T.; Solsona, B. The Different Catalytic Behaviour in the Propane Total Oxidation of Cobalt and Manganese Oxides Prepared by a Wet Combustion Procedure. Chem. Eng. J. 2013, 229, 547–558. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Gong, X.; Guo, Y.; Guo, Y.; Wang, Y.; Lu, G. Effect of the Crystal Plane Figure on the Catalytic Performance of MnO2 for the Total Oxidation of Propane. CrystEngComm 2015, 17, 3005–3014. [Google Scholar] [CrossRef]
- Castaño, M.H.; Molina, R.; Moreno, S. Catalytic Oxidation of VOCs on MnMgAlOx Mixed Oxides Obtained by Auto-Combustion. J. Mol. Catal. A Chem. 2015, 398, 358–367. [Google Scholar] [CrossRef]
- Finocchio, E.; Busca, G. Characterization and Hydrocarbon Oxidation Activity of Coprecipitated Mixed Oxides Mn3O4/Al2O3. Catal. Today 2001, 70, 213–225. [Google Scholar] [CrossRef]
- Baldi, M.; Finocchio, E.; Milella, F.; Busca, G. Catalytic Combustion of C3 Hydrocarbons and Oxygenates over Mn3O4. Appl. Catal. B Environ. 1998, 16, 43–51. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of γ-MnO2as a VOC Removal Catalyst: Comparison with a Noble Metal Catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Cheng, W.; Wu, F.; Lu, X.; Li, Z. Controlled Synthesis of Diverse Manganese Oxide-Based Catalysts for Complete Oxidation of Toluene and Carbon Monoxide. Chem. Eng. J. 2014, 244, 59–67. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Wu, F.; Zhang, L.; Liu, X. Dispersion–Precipitation Synthesis of Nanorod Mn3O4 with High Reducibility and the Catalytic Complete Oxidation of Air Pollutants. Catal. Commun. 2013, 31, 52–56. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic Oxidation of Formaldehyde over Manganese Oxides with Different Crystal Structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Liu, F.; Yang, Y. Engineering Crystal Facet of α-MnO2 Nanowire for Highly Efficient Catalytic Oxidation of Carcinogenic Airborne Formaldehyde. ACS Catal. 2018, 8, 3435–3446. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The Effect of Manganese Vacancy in Birnessite-Type MnO2 on Room-Temperature Oxidation of Formaldehyde in Air. Appl. Catal. B Environ. 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Bai, B.; Li, J.; Hao, J. 1D-MnO2, 2D-MnO2 and 3D-MnO2 for Low-Temperature Oxidation of Ethanol. Appl. Catal. B Environ. 2015, 164, 241–250. [Google Scholar] [CrossRef]
- Garcia, T.; Sellick, D.; Varela, F.; Vázquez, I.; Dejoz, A.; Agouram, S.; Taylor, S.H.; Solsona, B. Total Oxidation of Naphthalene Using Bulk Manganese Oxide Catalysts. Appl. Catal. A Gen. 2013, 450, 169–177. [Google Scholar] [CrossRef]
- García, T.; Solsona, B.; Taylor, S.H. Naphthalene Total Oxidation over Metal Oxide Catalysts. Appl. Catal. B Environ. 2006, 66, 92–99. [Google Scholar] [CrossRef]
- Pérez, H.; Navarro, P.; Torres, G.; Sanz, O.; Montes, M. Evaluation of Manganese OMS-like Cryptomelane Supported on SBA-15 in the Oxidation of Ethyl Acetate. Catal. Today 2013, 212, 149–156. [Google Scholar] [CrossRef]
- Pérez, H.; Navarro, P.; Delgado, J.J.; Montes, M. Mn-SBA15 Catalysts Prepared by Impregnation: Influence of the Manganese Precursor. Appl. Catal. A Gen. 2011, 400, 238–248. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Fang, X.; Cheng, Y. Simple Strategy for the Construction of Oxygen Vacancies on α-MnO2 Catalyst to Improve Toluene Catalytic Oxidation. J. Hazard. Mater. 2021, 409, 125020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, X.; Bi, F.; Lu, G.; Wang, Y. Highly Efficient Mn2O3 Catalysts Derived from Mn-MOFs for Toluene Oxidation: The Influence of MOFs Precursors. Mol. Catal. 2020, 482, 110701. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, C.; Du, X.; Zhu, Y.; Zhang, Y.; Li, S. Catalytic Oxidation of Toluene over MnO2 Catalysts with Different Mn (II) Precursors and the Study of Reaction Pathway. Fuel 2020, 262, 116610. [Google Scholar] [CrossRef]
- Li, H.; Qi, G.; Tana; Zhang, X.; Huang, X.; Li, W.; Shen, W. Low-Temperature Oxidation of Ethanol over a Mn0.6Ce0.4O2 Mixed Oxide. Appl. Catal. B Environ. 2011, 103, 54–61. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous Introduction of CeOy into MnOx-Based Catalyst for Oxidation of Aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Shah, P.M.; Bailey, L.A.; Taylor, S.H. The Influence of Cerium to Manganese Ratio and Preparation Method on the Activity of Ceria-Manganese Mixed Metal Oxide Catalysts for VOC Total Oxidation. Catalysts 2023, 13, 114. [Google Scholar] [CrossRef]
- Matějová, L.; Topka, P.; Jirátová, K.; Šolcová, O. Total Oxidation of Model Volatile Organic Compounds over Some Commercial Catalysts. Appl. Catal. A Gen. 2012, 443–444, 40–49. [Google Scholar] [CrossRef]
- Clarke, T.J.; Kondrat, S.A.; Taylor, S.H. Total Oxidation of Naphthalene Using Copper Manganese Oxide Catalysts. Catal. Today 2015, 258, 610–615. [Google Scholar] [CrossRef]
- Cao, H.; Li, X.; Chen, Y.; Gong, M.; Wang, J. Effect of Loading Content of Copper Oxides on Performance of Mn-Cu Mixed Oxide Catalysts for Catalytic Combustion of Benzene. J. Rare Earths 2012, 30, 871–877. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-Nanocasting Synthesis of Mesoporous Cu–Mn Composite Oxides and Their Promoted Catalytic Activities for Gaseous Benzene Removal. Appl. Catal. B Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Clarke, T.J.; Davies, T.E.; Kondrat, S.A.; Taylor, S.H. Mechanochemical Synthesis of Copper Manganese Oxide for the Ambient Temperature Oxidation of Carbon Monoxide. Appl. Catal. B Environ. 2015, 165, 222–231. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Fabris, J.D.; Pereira, M.C. Iron Oxides and Their Applications in Catalytic Processes: A Review. Quím. Nova 2013, 36, 123–130. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Combustion of Volatile Organic Compounds on Manganese Iron or Nickel Mixed Oxide Catalysts. Appl. Catal. B Environ. 2007, 74, 1–10. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Sanchis, R.; Soriano, M.D.; Moreno, M.; Rodríguez-Castellón, E.; Agouram, S.; Dejoz, A.; López Nieto, J.M. Total Oxidation of VOCs on Mesoporous Iron Oxide Catalysts: Soft Chemistry Route versus Hard Template Method. Chem. Eng. J. 2016, 290, 273–281. [Google Scholar] [CrossRef]
- Solsona, B.E.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.F.; Hutchings, G.J. Supported Gold Catalysts for the Total Oxidation of Alkanes and Carbon Monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Manova, E.; Tsoncheva, T.; Estournès, C.l.; Paneva, D.; Tenchev, K.; Mitov, I.; Petrov, L. Nanosized Iron and Iron–Cobalt Spinel Oxides as Catalysts for Methanol Decomposition. Appl. Catal. A Gen. 2006, 300, 170–180. [Google Scholar] [CrossRef]
- Hammiche-Bellal, Y.; Zouaoui-Mahzoul, N.; Lounas, I.; Benadda, A.; Benrabaa, R.; Auroux, A.; Meddour-Boukhobza, L.; Djadoun, A. Cobalt and Cobalt-Iron Spinel Oxides as Bulk and Silica Supported Catalysts in the Ethanol Combustion Reaction. J. Mol. Catal. A Chem. 2017, 426, 97–106. [Google Scholar] [CrossRef]
- Biabani-Ravandi, A.; Rezaei, M.; Fattah, Z. Study of Fe–Co Mixed Metal Oxide Nanoparticles in the Catalytic Low-Temperature CO Oxidation. Process Saf. Environ. Prot. 2013, 91, 489–494. [Google Scholar] [CrossRef]
- Kedesdy, H.H.; Tauber, A. Formation of Manganese Ferrite by Solid-State Reaction. J. Am. Ceram. Soc. 1956, 39, 425–431. [Google Scholar] [CrossRef]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and Iron Oxides as Combustion Catalysts of Volatile Organic Compounds. Appl. Catal. B Environ. 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. NO Oxidation to NO2 over Manganese-Cerium Mixed Oxides. Catal. Today 2015, 258, 205–213. [Google Scholar] [CrossRef]
- Stobbe, E.R.; de Boer, B.A.; Geus, J.W. The Reduction and Oxidation Behaviour of Manganese Oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Li, Y.; Shen, W. Polymorphous Transformation of Rod-Shaped Iron Oxides and Their Catalytic Properties in Selective Reduction of NO by NH3. RSC Adv. 2015, 5, 66141–66146. [Google Scholar] [CrossRef]
- Liang, M.; Kang, W.; Xie, K. Comparison of Reduction Behavior of Fe2O3, ZnO and ZnFe2O4 by TPR Technique. J. Nat. Gas Chem. 2009, 18, 110–113. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.-F.; Zhang, Y. The Effect of Pore Size or Iron Particle Size on the Formation of Light Olefins in Fischer–Tropsch Synthesis. RSC Adv. 2015, 5, 29002–29007. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Junta, J.L.; Hochella, M.F. Manganese (II) Oxidation at Mineral Surfaces: A Microscopic and Spectroscopic Study. Geochim. Cosmochim. Acta 1994, 58, 4985–4999. [Google Scholar] [CrossRef]
- Shah, P.M.; Day, A.N.; Davies, T.E.; Morgan, D.J.; Taylor, S.H. Mechanochemical Preparation of Ceria-Zirconia Catalysts for the Total Oxidation of Propane and Naphthalene Volatile Organic Compounds. Appl. Catal. B Environ. 2019, 253, 331–340. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vázquez, I.; Dejoz, A.; Taylor, S.H. Total Oxidation of Propane Using Nanocrystalline Cobalt Oxide and Supported Cobalt Oxide Catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep Oxidation of Volatile Organic Compounds Using Ordered Cobalt Oxides Prepared by a Nanocasting Route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Hutchings, G.J.; Taylor, S.H.; Makkee, M. TAP Reactor Study of the Deep Oxidation of Propane Using Cobalt Oxide and Gold-Containing Cobalt Oxide Catalysts. Appl. Catal. A Gen. 2009, 365, 222–230. [Google Scholar] [CrossRef]
- Solsona, B.; Vázquez, I.; Garcia, T.; Davies, T.E.; Taylor, S.H. Complete Oxidation of Short Chain Alkanes Using a Nanocrystalline Cobalt Oxide Catalyst. Catal. Lett. 2007, 116, 116–121. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Pinnell, R.K.; Davies, T.E.; Golunski, S.; Bartley, J.K.; Hutchings, G.J.; Taylor, S.H. Green Preparation of Transition Metal Oxide Catalysts Using Supercritical CO2 Anti-Solvent Precipitation for the Total Oxidation of Propane. Appl. Catal. B Environ. 2013, 140–141, 671–679. [Google Scholar] [CrossRef]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Metal Scarcity and Sustainability, Analyzing the Necessity to Reduce the Extraction of Scarce Metals. Resour. Conserv. Recycl. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Trendafilova, I.; Ojeda, M.; Andresen, J.M.; Ristić, A.; Dimitrov, M.; Tušar, N.N.; Atanasova, G.; Popova, M. Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials. Molecules 2023, 28, 204. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured Manganese Doped Ceria Solid Solutions for CO Oxidation at Lower Temperatures. Appl. Catal. B Environ. 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; De Rossi, S.; Faticanti, M.; Porta, P. Methane Combustion and CO Oxidation on LaAl1–xMnxO3 Perovskite-Type Oxide Solid Solutions. Appl. Catal. B Environ. 2003, 43, 397–406. [Google Scholar] [CrossRef]
- Leith, I.R.; Howden, M.G. Temperature-Programmed Reduction of Mixed Iron—Manganese Oxide Catalysts in Hydrogen and Carbon Monoxide. Appl. Catal. 1988, 37, 75–92. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, K.; Zhang, K.; Li, W.; Sun, Y. Carbon Dispersed Iron-Manganese Catalyst for Light Olefin Synthesis from CO Hydrogenation. Korean J. Chem. Eng. 2009, 26, 890–894. [Google Scholar] [CrossRef]
- Łojewska, J.; Kołodziej, A.; Dynarowicz-Łątka, P.; Wesełucha-Birczyńska, A. Engineering and Chemical Aspects of the Preparation of Microstructured Cobalt Catalyst for VOC Combustion. Catal. Today 2005, 101, 81–91. [Google Scholar] [CrossRef]
- Łojewska, J.; Kołodziej, A.; Łojewski, T.; Kapica, R.; Tyczkowski, J. Structured Cobalt Oxide Catalyst for VOC Combustion. Part I: Catalytic and Engineering Correlations. Appl. Catal. A Gen. 2009, 366, 206–211. [Google Scholar] [CrossRef]
- Łojewska, J.; Kołodziej, A.; Łojewski, T.; Kapica, R.; Tyczkowski, J. Cobalt Catalyst Deposited on Metallic Microstructures for VOC Combustion: Preparation by Non-Equilibrium Plasma. Catal. Commun. 2008, 10, 142–145. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, A.-P.; Luo, M.-F.; Lu, J.-Q. Highly Active Spinel Type CoCr2O4 Catalysts for Dichloromethane Oxidation. Appl. Catal. B Environ. 2015, 165, 477–486. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Li, C.; Liu, Z.; Liang, C. Manipulating Morphology and Surface Engineering of Spinel Cobalt Oxides to Attain High Catalytic Performance for Propane Oxidation. J. Catal. 2021, 396, 179–191. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Zhu, X.; Yang, Y.; Hu, W.; Zhao, H.; Qu, R.; Zheng, C.; Gao, X. Low Temperature Catalytic Oxidation of Propane over Cobalt-Cerium Spinel Oxides Catalysts. Appl. Surf. Sci. 2019, 479, 1132–1140. [Google Scholar] [CrossRef]
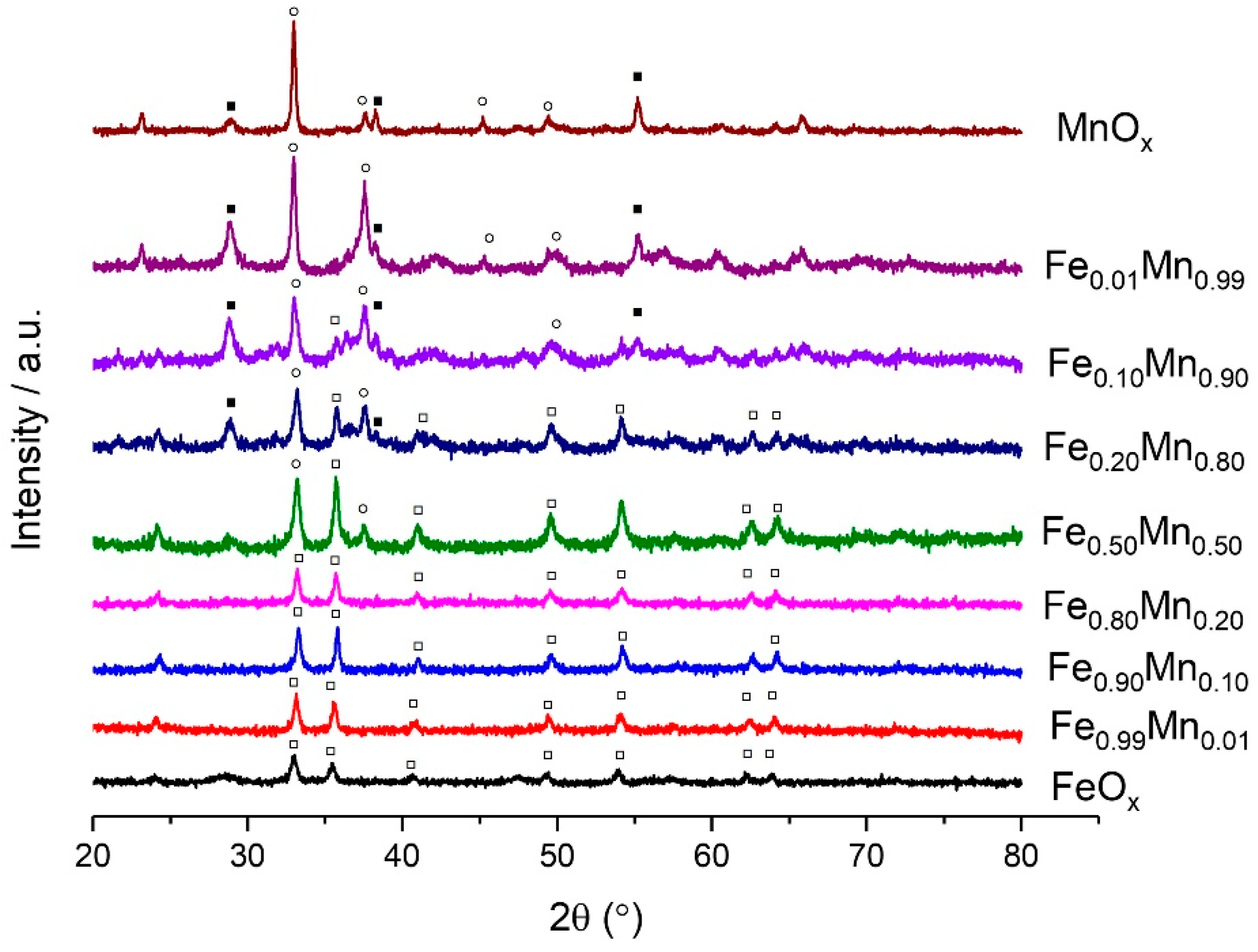


| Catalyst | Phases Present | Crystallite Size (Å) | Surface Area (m2 g−1) | |
|---|---|---|---|---|
| Fe2O3 | Mn2O3 | |||
| FeOx | Fe2O3 | 190 | - | 62 |
| Fe0.99Mn0.01Ox | Fe2O3 | 387 | - | 23 |
| Fe0.90Mn0.10Ox | Fe2O3 | 322 | - | 25 |
| Fe0.80Mn0.20Ox | Fe2O3 | 280 | - | 31 |
| Fe0.50Mn0.5Ox | Fe2O3, Mn2O3 | 291 | 143 | 42 |
| Fe0.20Mn0.80Ox | Fe2O3, MnO2, Mn2O3 | 366 | 188 | 33 |
| Fe0.10Mn0.90Ox | Fe2O3, MnO2, Mn2O3 | 225 | 160 | 31 |
| Fe0.01Mn0.99Ox | MnO2, Mn2O3 | 317 | 35 | |
| MnOx | MnO2, Mn2O3 | 361 | 18 | |
| Catalyst | Relative Surface Fe Concentration (%) | Relative Surface Mn Concentration (%) | Surface Na Concentration (Atomic %) |
|---|---|---|---|
| FeOx | 100 | 0 | 3.4 |
| Fe0.99Mn0.01Ox | 97.9 | 2.1 | 12.4 |
| Fe0.90Mn0.10Ox | 84.8 | 15.2 | 7.4 |
| Fe0.80Mn0.20Ox | 71.2 | 28.8 | 10.8 |
| Fe0.50Mn0.50Ox | 49.6 | 50.4 | 4.9 |
| Fe0.20Mn0.80Ox | 36.0 | 64.0 | 1.8 |
| Fe0.10Mn0.90Ox | 21.1 | 78.9 | 1.9 |
| Fe0.01Mn0.99Ox | 13.5 | 86.5 | 1.2 |
| MnOx | 0 | 100 | 6.7 |
| Catalyst | Surface Oxidation State of Fe | Magnitude of Mn 3s Peak Splitting (eV) | Surface Oxidation State of Mn |
|---|---|---|---|
| Fe0.99Mn0.01Ox | 3+ | - | - |
| Fe0.90Mn0.10Ox | 3+ | - | - |
| Fe0.80Mn0.20Ox | 3+ | - | - |
| Fe0.50Mn0.50Ox | 3+ | 5.4 | 3+ |
| Fe0.20Mn0.80Ox | 3+ | 5.1 | 4+ |
| Fe0.10Mn0.90Ox | 3+ | 4.9 | 4+ |
| Fe0.01Mn0.99Ox | 3+ | 4.8 | 4+ |
| MnOx | - | 4.8 | 4+ |
| Catalyst | FeOx Crystallite Size (Å) | MnOx Crystallite Size (Å) | BET Surface Area (m2g−1) |
|---|---|---|---|
| Na2CO3 | 291 | 143 | 42 |
| K2CO3 | 443 | 136 | 49 |
| (NH4)2CO3 | 274 | - | 88 |
| NaOH | 88 | - | 63 |
| KOH | 100 | - | 68 |
| NH4OH | 91 | 240 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, P.M.; Bailey, L.A.; Morgan, D.J.; Taylor, S.H. The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation. Catalysts 2023, 13, 794. https://doi.org/10.3390/catal13050794
Shah PM, Bailey LA, Morgan DJ, Taylor SH. The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation. Catalysts. 2023; 13(5):794. https://doi.org/10.3390/catal13050794
Chicago/Turabian StyleShah, Parag M., Liam A. Bailey, David J. Morgan, and Stuart H. Taylor. 2023. "The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation" Catalysts 13, no. 5: 794. https://doi.org/10.3390/catal13050794
APA StyleShah, P. M., Bailey, L. A., Morgan, D. J., & Taylor, S. H. (2023). The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation. Catalysts, 13(5), 794. https://doi.org/10.3390/catal13050794