Abstract
A transition metal-free protocol has been developed for the synthesis of 3-acyl quinolines through aza-Michael addition and intramolecular annulation of enaminones with anthranils. Both methanesulfonic acid (MSA) and NaI play an important role in the reaction. This ring-opening/reconstruction strategy features easy operation, high yields, broad substrate scope and excellent efficiency.
1. Introduction
The quinoline skeleton, as one of the most prevalent heterocycles, widely exist in natural products and synthetic compounds [1,2,3,4]. In the past decades, significant attention has been devoted to the synthesis of quinolines because of their valuable biological and chemical properties in the application of medicals [5,6,7,8,9], ligands [10,11], and materials [12,13,14,15]. Therefore, the development of novel, practical, and efficient synthetic methods is highly desirable for the synthesis of quinoline derivatives [16,17].
Traditional strategies for the synthesis of quinolines, such as Friedlander synthesis [18] and Doebner–von Miller synthesis [19], could date back to a long time ago. The establishment of quinoline reservoirs nowadays has been broadly prepared by using aromatic amines, isoxazoles, or amino acids as nitrogen sources [20,21,22,23]. Transition metal catalysts were normally required for such multi-component coupling and cascade cyclization reactions [24,25,26,27,28,29,30,31,32]. Most of these methods suffered from some drawbacks such as a complex reaction system, harsh reaction conditions, and complex by-products. Consequently, the development of a transition metal-free protocol for the synthesis of quinoline derivatives appears desirable and synthetically attractive [33,34,35,36,37,38,39].
In particular, the synthesis of 3-acylquinolines has attracted significant attention due to their valuable application. Representative methods for the preparation of such compounds include (i) Fe powder-catalyzed reaction of 2-nitrobenzaldehyde with enaminones [40]; (ii) copper-catalyzed one-pot domino reactions starting from 2-aminobenzylalcohols and propiophenones [41]; and (iii) copper-catalyzed annulation of anthranils and saturated ketones [42]. Besides, several transition metal-free protocols have also been developed. In recent years, the application of enaminones [43,44,45] and anthranils [46,47,48,49,50,51,52,53] as useful building blocks have attracted significant attention in transition metal-catalyzed organic synthesis and construction of a variety of frameworks. Based on our previous work involving sulfoxonium ylides, aldehydes, and anthranils in constructing quinoline derivatives [54,55,56], we were intrigued to investigate the reactivity of enaminones in the annulation reactions for the construction of heterocycles [57,58]. Herein, we report a transition metal-free protocol for the synthesis of 3-acylquinolines under simple and easy-to-operate conditions.
2. Results
The initial screening and optimization of the reaction conditions were conducted with compounds 1a and 2a as substrates. Using 1a and 2a in a 2:1 ratio (on a 0.2 mmol scale), EtOH (2 mL) as a solvent and methanesulfonic acid (MSA) (0.3 mmol) as an additive, product 3aa was obtained in 31% yield by heating at 110 °C (Table 1, entry 1). Next, the use of other solvents, such as HFIP, DME, n-octanol, dioxane, THF, and DMSO, was investigated but no better results were achieved (Table 1, entries 2–7). No product was obtained in the absence of MSA (Table 1, entry 8). An attempt to employ a mixture of additives MSA and KBr (each 0.3 mmol) in EtOH or THF led to higher yields of 58% and 37%, respectively, indicating that EtOH was more suitable for this reaction (Table 1, entries 9 and 10). Next, we tried to use other salts instead of KBr as an additive, such as KI and NaI, affording product 3aa in 89% and 90% yields, respectively (Table 1, entries 11 and 12). When the reaction was performed at lower temperatures, the yields dropped significantly, while a higher temperature did not improve the reaction efficiency (Table 1, entries 13–16).

Table 1.
Optimization of reaction conditions for the synthesis of 3aa a.
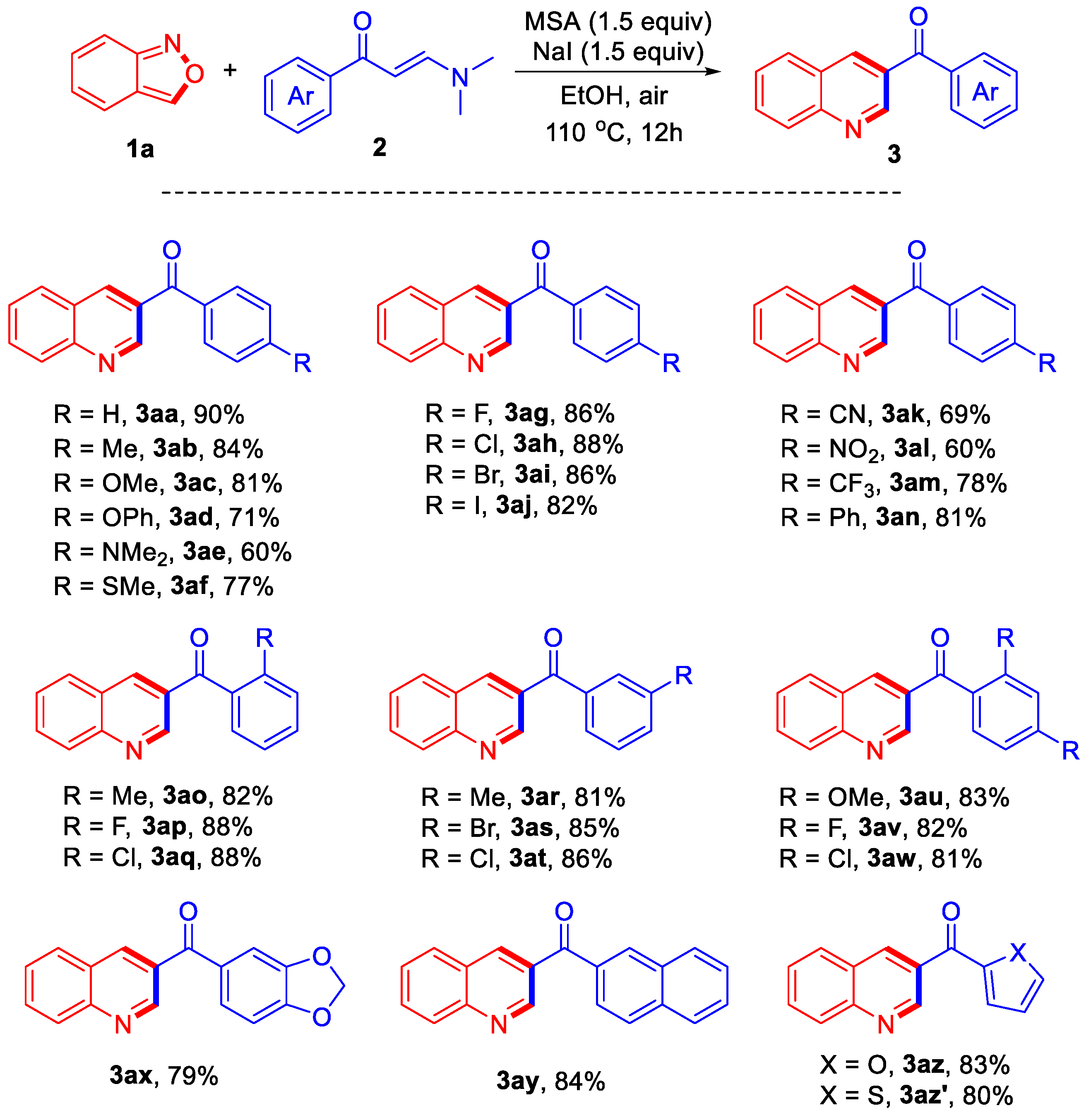
With the optimized reaction conditions in hand (Table 1, entry 10), the scope of the reaction of anthranil 1a with a variety of enaminones 2 was investigated and the results are summarized in Scheme 1. Substrates with various electron-donating or electron-withdrawing substituents were applied to the reaction system to provide a series of 3-acylquinolines. For example, the reaction of substrates bearing electron-donating substituents such as -Me, -OMe, -OPh, -NMe2, and -SMe afforded the corresponding products 3ab–3af in good yields ranging from 60% to 84%. For the substrates with -F, -Cl, -Br, and -I groups at the para-position, the reaction also proceeded well to give the corresponding products 3ag–3ai in high yields. Substrates containing substituents -CN, -NO2, and -CF3 were also successfully employed in the reaction, yielding products 3ak–3am in moderate yields. The reaction of one substrate bearing a phenyl group afforded the corresponding product 3an in 81% yield. It appeared that the steric factors did not influence the efficiency significantly. For example, various substituents at the ortho- or meta-position were well tolerated, providing the corresponding products 3ao–3at in similarly good yields ranging from 81% to 88%. Substrates bearing two substituents at the ortho- and meta-positions were also investigated, affording products 3au–3aw in 83%, 82%, and 81% yields, respectively. Furthermore, one substrate bearing electron-donating dioxolo-group was also successfully employed in the reaction, providing the desired product 3ax in 79% yield. The reaction of the substrate bearing naphthyl group afforded product 3ay in 84% yield. At last, substrates containing oxygen- or sulfur-heterocycle also reacted well with 1a, giving products 3az and 3az’ in 83% and 80% yields, respectively.
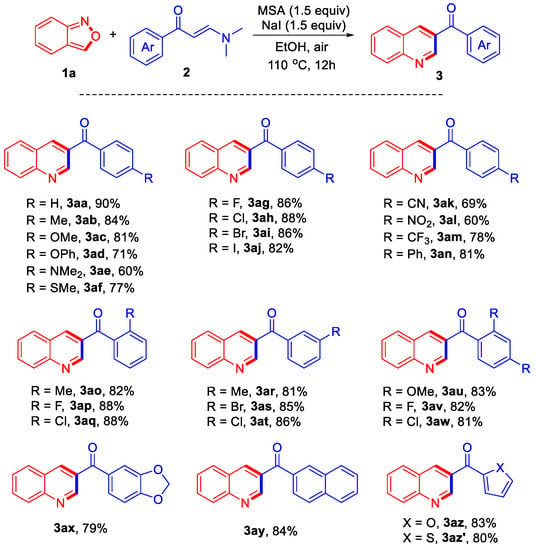
Scheme 1.
Reaction conditions: 1a (0.4 mmol), 2 (0.2 mmol), methanesulfonic acid (MSA) (0.3 mmol), NaI (0.3 mmol), EtOH (2 mL), 110 °C, air, 12 h.
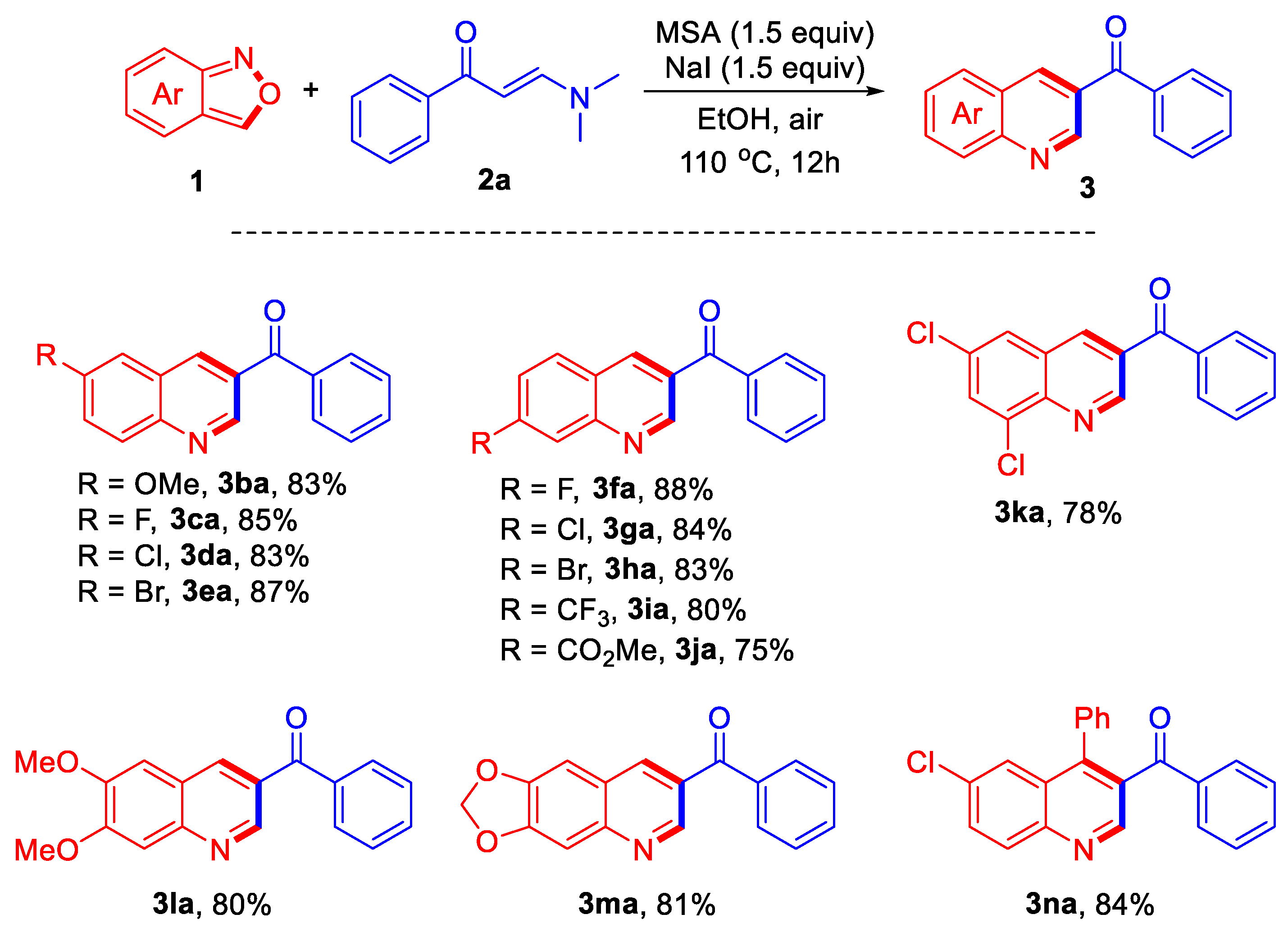
In order to further explore the scope of the reaction, a series of anthranil 1 were introduced to react with compound 2a under the optimized reaction conditions, and the results are summarized in Scheme 2. The reactions of various anthranil-bearing electron-donating substituent -OMe proceeded well, affording product 3ba in 83% yield. The substrate bearing halogen substituents -F, -Cl, and -Br could also be employed in the reaction, providing the corresponding products 3ca–3ea in high yields. Under the conditions, products 3fa–3ha with halogen substituents -F, -Cl, and -Br at 7-position were also successfully obtained in yields ranging from 83% to 88%. The reactions of anthranils bearing electron-withdrawing substituents -CF3 and -CO2Me provided products 3ia and 3ja in 80% and 75% yields, respectively. In addition, bisubstitued substrates were also investigated, affording the corresponding products 3ka–3ma in satisfying yields. Finally, one C3-aryl substituted example was investigated in the reaction, giving product 3na in 84% yield.
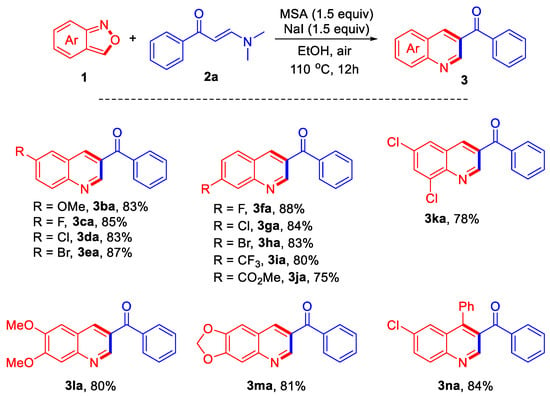
Scheme 2.
Reaction conditions: 1 (0.4 mmol), 2a (0.2 mmol), methanesulfonic acid (MSA) (0.3 mmol), NaI (0.3 mmol), EtOH (2 mL), 110 °C, air, 12 h.
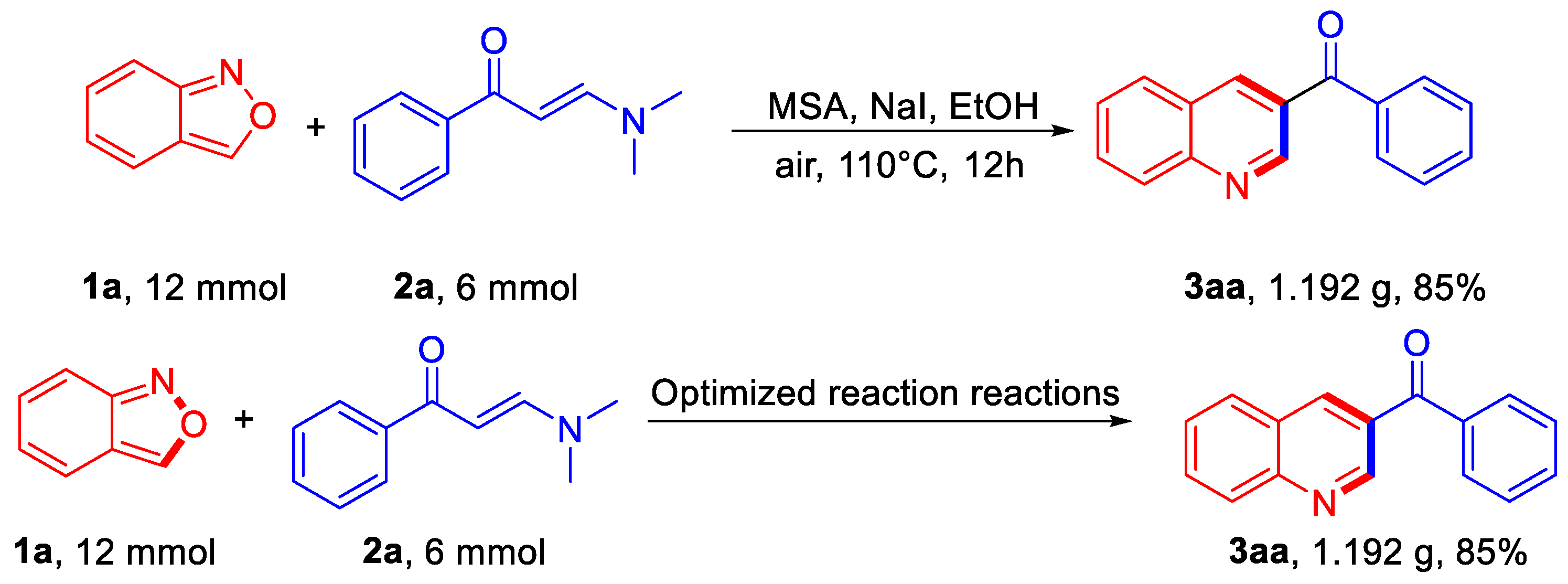
To test the scalability of the new protocol, gram-scale preparation was carried out under the standard conditions with 12 mmol of 1a and 6 mmol of 2a, and product 3aa was successfully obtained in 85% yield (1.192 g) (Scheme 3).
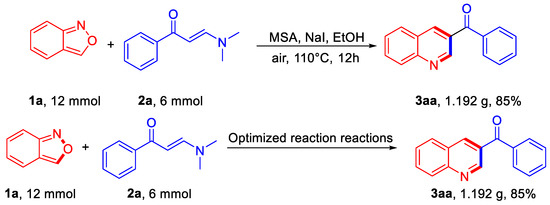
Scheme 3.
Gram-scale reaction.
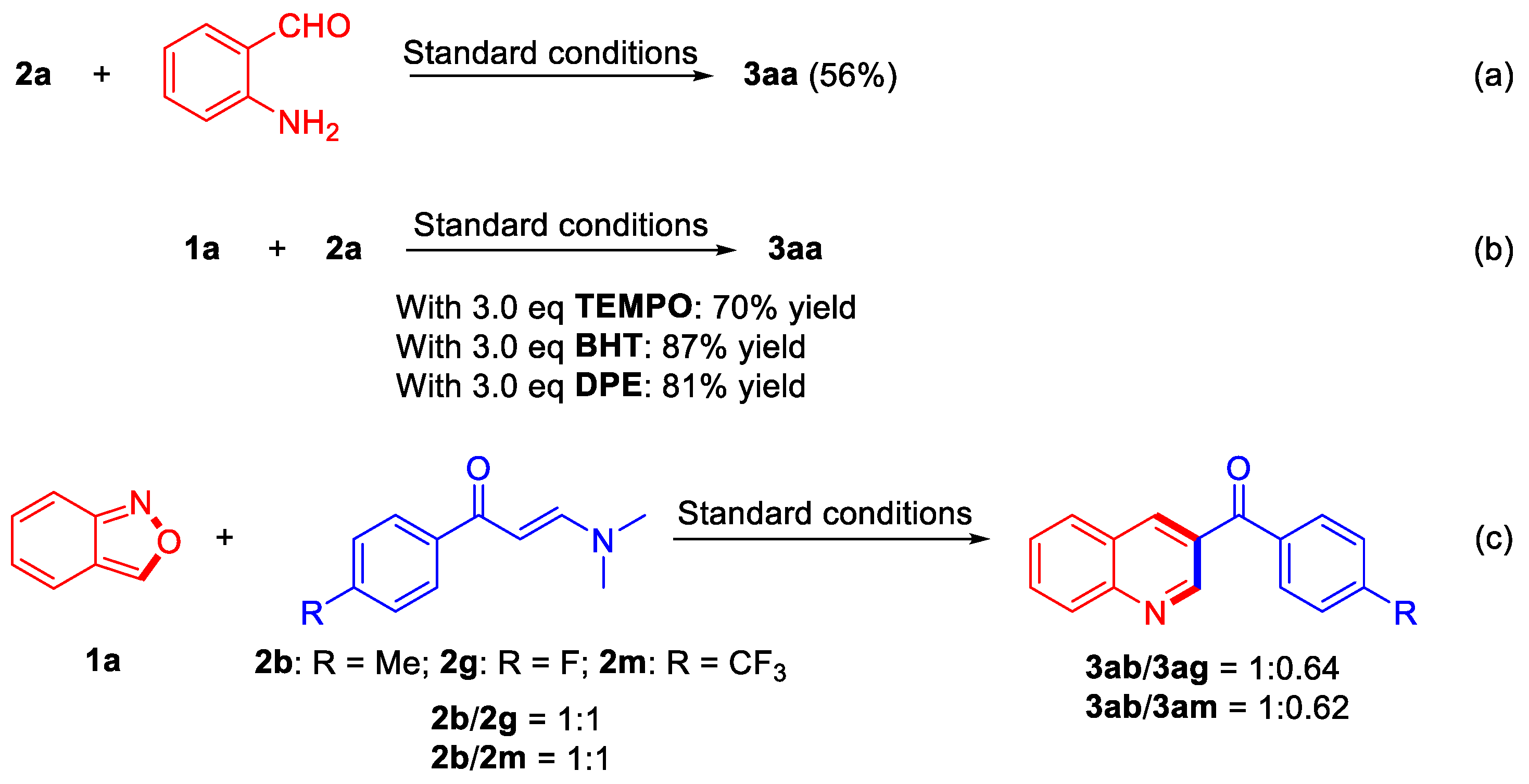
To elucidate the possible mechanism, several preliminary experiments were carried out. At first, 2-aminobenzaldehyde was employed instead of anthranil 2a to investigate whether it was an intermediate in the process, affording product 3aa in 56% yield (Scheme 4, Equation (a)). The results showed that 2-aminobenzaldehyde might be an intermediate for the transformation of anthranil 2a. Furthermore, radical scavengers, such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), 2,6-bis(1,1-dimethylethyl)-4-methylphenol (BHT), and 1,1-diphenyltheylene, were tested in the reaction, affording the desired product 3aa in 70%, 87%, and 81% yields, respectively, indicating that there might be no radical step in the process (Scheme 4, Equation (b)). The 1H NMR spectrum of the intermolecular competition experiment between (E)-3-(dimethylamino)-1-(p-tolyl)prop-2-en-1-one (2b) and (E)-3-(dimethylamino)-1-(4-fluorophenyl)prop-2-en-1-one (2g) showed that the ratio of products 3ab and 3ag was 1:0.64. When the electron-deficient substrate was changed to (E)-3-(dimethylamino)-1-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (2m), the ratio of products 3ab and 3am in the intermolecular competition experiment was 1:0.62. Both results indicated that substrates bearing electron-donating substituents were more active than that with electron-withdrawing substituents (Scheme 4, Equation (c)).
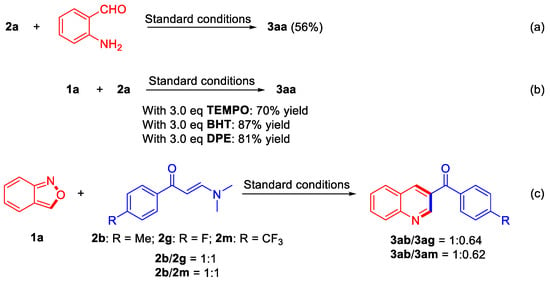
Scheme 4.
Control reactions.
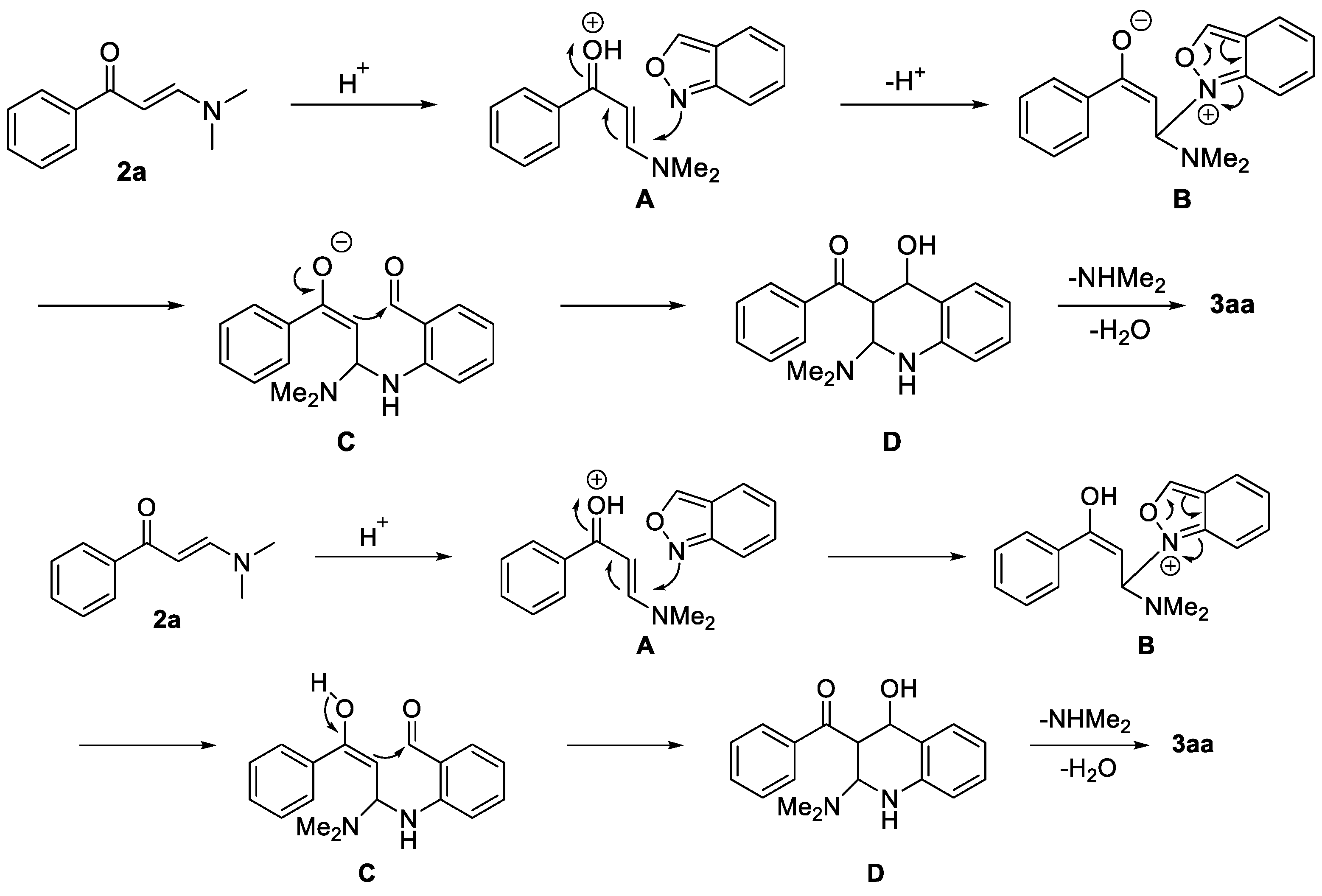
On the basis of our results and previous reports on enaminone 2a [59], a possible mechanism on the synthesis of 3aa is proposed as shown in Scheme 5. First, 2a is activated by methanesulfonic acid (MSA) to form intermediate A, which is attacked by 1a to give intermediate B. NaI is crucial for the success of this reaction, probably due to the fact that it can promote the stability of intermediate B. Then, intermediate C is produced, affording D through an intramolecular nucleophilic addition. Finally, D is converted into the desired product 3aa.
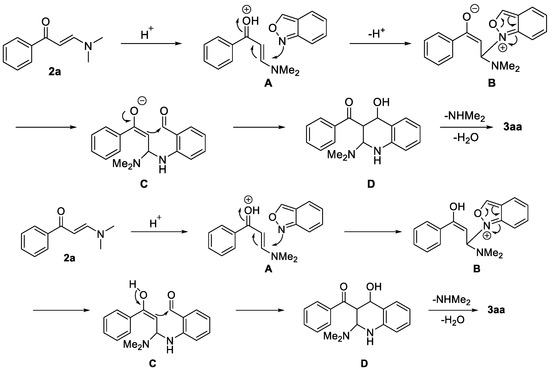
Scheme 5.
Proposed mechanism for the synthesis of 3aa.
3. Conclusions
In conclusion, a mild and efficient protocol has been developed for the synthesis of a broad range of 3-acyl quinolines under transition metal-free conditions. Various enaminones and anthranils were well employed in the reaction, providing a series of 3-acyl quinolines through aza-Michael addition and intramolecular annulation. Mechanism studies showed that no radical step was involved in the process. It was noteworthy that MSA and NaI played a significant role for the success of the transformation.
4. Experimental Section
General Information. All solvents were dried over molecular sieves. Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. Anthranil 1n was purchased and other anthranils were all synthesized according to our previous work [55]. All the substrates enaminones were prepared according to the reference [60]. The products were isolated using column chromatography on silica gel (200–300 mesh) by using petroleum ether (PE, 30–60 °C) and ethyl acetate (EA) as eluents. All yields described herein were the isolated yields after column chromatography. Reaction progress and product mixtures were routinely monitored by using TLC using TLC SiO2 sheets, and compounds were visualized under ultraviolet light. 1H NMR, 13C NMR, and 19F NMR spectra were recorded on a Bruker AVANCE III 400 spectrometer. The spectra were recorded using CDCl3 as a solvent. 1H NMR chemical shifts are referenced to tetramethylsilane (TMS, 0 ppm). 19F NMR chemical shifts are referenced to p-fluorotoluene (p-fluorotoluene, 0 ppm). Abbreviations are as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). High-resolution mass spectra (HRMS) were recorded on Agilent 6540 or FTICR-MS bruker7 T. Melting points were measured with a melting point instrument (Shanghai Yidian Physical Optical Instrument Co., Ltd., Shanghai, China, SGW, X-4A) and were uncorrected.
General procedure for the synthesis of 3aa: A mixture of 1a (0.4 mmol, 2.0 equiv), 2a (0.2 mmol, 1.0 equiv) and NaI (0.3 mmol, 1.5 equiv) were added in a Schlenk tube equipped with a stirring bar. Dry EtOH (2 mL) and MSA (0.3 mmol, 1.5 equiv) were added and the mixture was stirred at 110 °C in a pre-heated oil bath for 12 h under air atmosphere. Then, the mixture was cooled to room temperature and concentrated in vacuo, and the resulting residue was purified by using column chromatography on silica gel with EtOAc/petroleum ether, affording product 3aa as a white solid in 90% yield (42.1 mg).
4.1. Phenyl(quinolin-3-yl)methanone (3aa)
Compound 3aa was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3aa (42.1 mg, 90%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.3 Hz, 1H), 8.54 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.91 (d, J = 8.3 Hz, 1H), 7.84 (td, J = 8.2, 2.1 Hz, 3H), 7.63 (q, J = 7.3 Hz, 2H), 7.53 (t, J = 7.7 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.8, 150.3, 149.4, 138.8, 137.0, 133.0, 131.8, 130.04, 130.0, 129.4, 129.1, 128.6, 127.6, 126.6. HRMS m/z (ESI) calcd for C16H12NO (M+H)+ 234.0913, found 234.0920. The NMR spectra are consistent with the reported literature [31].
4.2. Quinolin-3-yl(p-tolyl)methanone (3ab)
Compound 3ab was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ab (41.4 mg, 84%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.30 (d, J = 2.1 Hz, 1H), 8.54 (d, J = 2.1 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 9.5 Hz, 1H), 7.84 (t, J = 7.7 Hz, 1H), 7.78 (d, J = 8.1 Hz, 2H), 7.63 (t, J = 7.0 Hz, 1H), 7.33 (d, J = 7.9 Hz, 2H), 2.47 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 150.3, 149.3, 144.0, 138.6, 134.3, 131.7, 130.4, 130.2, 129.4, 129.3, 129.1, 127.5, 126.6, 21.7. The NMR spectra are consistent with the reported literature [31].
4.3. (4-Methoxyphenyl)(quinolin-3-yl)methanone (3ac)
Compound 3ac was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ac (42.6 mg, 81%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.26 (d, J = 2.2 Hz, 1H), 8.50 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.87 (td, J = 13.7, 8.6 Hz, 4H), 7.65–7.59 (m, 1H), 7.00 (d, J = 8.8 Hz, 2H), 3.89 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.4, 163.7, 150.2, 149.2, 138.1, 132.5, 131.5, 130.8, 129.7, 129.4, 129.0, 127.4, 126.6, 113.9, 55.5. The NMR spectra are consistent with the reported literature [31].
4.4. (4-Phenoxyphenyl)(quinolin-3-yl)methanone (3ad)
Compound 3ad was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ad (46.0 mg, 71%) as a white solid, m.p. 78–80 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.29 (d, J = 2.1 Hz, 1H), 8.53 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.93–7.87 (m, 2H), 7.87–7.81 (m, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.41 (t, J = 7.9 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H), 7.09 (dd, J = 18.3, 8.7 Hz, 4H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.4, 162.2, 155.2, 150.2, 149.3, 138.3, 132.4, 131.6, 131.3, 130.4, 130.1, 129.4, 129.0, 127.5, 126.6, 124.8, 120.3, 117.3. HRMS m/z (ESI) calcd for C22H15NO2(M+H)+ 326.1176, found 326.1183.
4.5. (4-(Dimethylamino)phenyl)(quinolin-3-yl)methanone (3ae)
Compound 3ae was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 5:1) afforded product 3ae (33.1 mg, 60%) as a yellow solid, m.p. 118–120 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.25 (d, J = 2.2 Hz, 1H), 8.49 (d, J = 3.1 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.85–7.79 (m, 3H), 7.62 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 6.73–6.69 (m, 2H), 3.10 (s, 6H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 192.7, 153.6, 150.4, 148.9, 137.6, 132.7, 131.8, 131.1, 129.3, 128.9, 127.3, 126.8, 124.2, 110.7, 40.0. HRMS m/z (ESI) calcd for C18H16N2O (M+H)+ 277.1335, found 277.1327.
4.6. (Methylthio)phenyl)(quinolin-3-yl)methanone (3af)
Compound 3af was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3af (43.1 mg, 77%) as a white solid, m.p. 110–112 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.27 (d, J = 2.1 Hz, 1H), 8.50 (d, J = 2.2 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.84–7.76 (m, 3H), 7.61 (t, J = 7.5 Hz, 1H), 7.31 (d, J = 8.2 Hz, 2H), 2.53 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.7, 150.2, 149.3, 146.3, 138.3, 133.0, 131.6, 130.5, 130.3, 129.4, 129.0, 127.5, 126.5, 124.9, 14.7. HRMS m/z (ESI) calcd for C17H13NOS (M+H)+ 280.0791, found 280.0784.
4.7. (4-Fluorophenyl)(quinolin-3-yl)methanone (3ag)
Compound 3ag was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ag (43.0 mg, 86%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.28 (d, J = 2.2 Hz, 1H), 8.52 (d, J = 2.8 Hz, 1H), 8.18 (d, J = 8.6 Hz, 1H), 7.93–7.88 (m, 3H), 7.85 (ddd, J = 8.5, 6.9, 1.5 Hz, 1H), 7.66–7.62 (m, 1H), 7.21 (t, J = 8.6 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.4, 165.7 (d, J = 255.4 Hz), 150.1, 149.4, 138.6, 133.2 (d, J = 2.8 Hz), 132.6 (d, J = 9.4 Hz), 131.9, 129.3, 127.7, 126.5, 115.9 (d, J = 21.9 Hz). 19F NMR (376 MHz, CDCl3) δ ppm: −104.4. The NMR spectra are consistent with the reported literature [31].
4.8. (4-Chlorophenyl)(quinolin-3-yl)methanone (3ah)
Compound 3ah was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ah (47.1 mg, 88%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.28 (d, J = 2.2 Hz, 1H), 8.52 (d, J = 2.3 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.92–7.83 (m, 2H), 7.82–7.79 (m, 2H), 7.64 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.53–7.46 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.6, 150.0, 149.5, 139.6, 138.7, 135.2, 132.0, 131.4, 129.6, 129.4, 129.1, 129.0, 127.7, 126.5. The NMR spectra are consistent with the reported literature [31].
4.9. (4-Bromophenyl)(quinolin-3-yl)methanone (3ai)
Compound 3ai was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ai (53.5 mg, 86%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.29 (d, J = 2.2 Hz, 1H), 8.53 (d, J = 1.3 Hz, 1H), 8.19 (d, J = 9.5 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.86 (ddd, J = 8.5, 6.9, 1.4 Hz, 1H), 7.76–7.72 (m, 2H), 7.70–7.63 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.8, 150.1, 149.5, 138.8, 135.7, 132.0, 132.0, 131.5, 129.6, 129.5, 129.1, 128.3, 127.7, 126.5. The NMR spectra are consistent with the reported literature [31].
4.10. (4-Iodophenyl)(quinolin-3-yl)methanone (3aj)
Compound 3aj was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3aj (66.3 mg, 82%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.28 (d, J = 2.2 Hz, 1H), 8.51 (d, J = 1.5 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.92–7.83 (m, 4H), 7.66–7.60 (m, 1H), 7.59–7.53 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.0, 150.0, 149.5, 138.7, 137.9, 136.2, 132.0, 131.3, 129.5, 129.4, 127.7, 126.4, 101.0. The NMR spectra are consistent with the reported literature [22].
4.11. 4-(Quinoline-3-carbonyl)benzonitrile (3ak)
Compound 3ak was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 5:1) afforded product 3ak (35.5 mg, 69%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.28 (d, J = 2.2 Hz, 1H), 8.51 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.4 Hz, 1H), 7.92 (t, J = 7.4 Hz, 3H), 7.88 (d, J = 7.5 Hz, 1H), 7.83 (d, J = 8.1 Hz, 2H), 7.65 (t, J = 7.5 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.3, 149.8, 149.7, 140.4, 139.0, 132.4, 132.4, 130.1, 129.5, 129.2, 128.8, 127.9, 126.4, 117.7, 116.2. The NMR spectra are consistent with the reported literature [31].
4.12. (4-Nitrophenyl)(quinolin-3-yl)methanone (3al)
Compound 3al was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 5:1) afforded product 3al (35.4 mg, 60%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.32 (d, J = 2.2 Hz, 1H), 8.54 (d, J = 2.2 Hz, 1H), 8.39 (d, J = 8.7 Hz, 2H), 8.20 (d, J = 8.5 Hz, 1H), 8.01 (d, J = 8.8 Hz, 2H), 7.93 (d, J = 8.3 Hz, 1H), 7.89 (t, J = 7.6 Hz, 1H), 7.70–7.64 (m, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.1, 150.2, 149.8, 142.1, 139.1, 132.5, 130.7, 129.6, 129.3, 128.8, 128.0, 126.4, 123.8. The NMR spectra are consistent with the reported literature [31].
4.13. Quinolin-3-yl(4-(trifluoromethyl)phenyl)methanone (3am)
Compound 3am was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3am (47.6 mg, 78%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.32 (d, J = 2.2 Hz, 1H), 8.54 (d, J = 2.3 Hz, 1H), 8.20 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 8.2 Hz, 3H), 7.88 (ddd, J = 8.5, 6.9, 1.5 Hz, 1H), 7.81 (d, J = 7.9 Hz, 2H), 7.66 (ddd, J = 8.1, 6.9, 1.1 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.8, 150.0, 149.7, 140.0, 139.1, 134.3 (q, J = 32.7 Hz), 132.3, 130.1, 129.5, 129.2, 129.1, 127.8, 126.4, 125.7 (q, J = 3.5 Hz), 123.5 (q, J = 272.5 Hz). 19F NMR (376 MHz, CDCl3) δ ppm: −62.9. The NMR spectra are consistent with the reported literature [31].
4.14. [1,1′-Biphenyl]-4-yl(quinolin-3-yl)methanone (3an)
Compound 3an was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3an (50.2 mg, 81%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.35 (d, J = 2.2 Hz, 1H), 8.59 (d, J = 2.1 Hz, 1H), 8.21 (d, J = 8.5 Hz, 1H), 7.94 (t, J = 8.3 Hz, 3H), 7.85 (t, J = 8.4 Hz, 1H), 7.75 (d, J = 8.1 Hz, 2H), 7.65 (t, J = 6.6 Hz, 3H), 7.45 (dt, J = 29.3, 7.4 Hz, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.3, 150.2, 149.4, 145.8, 139.6, 138.6, 135.6, 131.7, 130.6, 130.2, 129.4, 129.1, 129.0, 128.3, 127.5, 127.2, 127.2, 126.6. The NMR spectra are consistent with the reported literature [22].
4.15. Quinolin-3-yl(o-tolyl)methanone (3ao)
Compound 3ao was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ao (40.4 mg, 82%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.36 (d, J = 2.2 Hz, 1H), 8.47 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 7.4 Hz, 1H), 7.89–7.82 (m, 2H), 7.64–7.59 (m, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.40–7.34 (m, 2H), 7.32–7.28 (m, 1H), 2.40 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 197.0, 150.3, 149.7, 139.4, 137.5, 137.3, 132.1, 131.4, 131.0, 130.1, 129.4, 129.3, 128.9, 127.5, 126.7, 125.4, 20.1. The NMR spectra are consistent with the reported literature [31].
4.16. (2-Fluorophenyl)(quinolin-3-yl)methanone (3ap)
Compound 3ap was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ap (44.1 mg, 88%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.33 (s, 1H), 8.54 (s, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.92–7.82 (m, 2H), 7.69–7.58 (m, 3H), 7.33 (td, J = 7.5, 1.1 Hz, 1H), 7.21 (t, J = 9.2 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 191.8, 160.2 (d, J = 253.0 Hz), 158.9, 149.8, 134.0 (d, J = 8.4 Hz), 134.0, 132.2, 131.0 (d, J = 2.6 Hz), 129.9, 129.4, 127.5, 126.1, 126.0, 124.7 (d, J = 3.4 Hz), 116.5 (d, J = 21.8 Hz). 19F NMR (376 MHz, CDCl3) δ ppm: −109.7. The NMR spectra are consistent with the reported literature [31].
4.17. (2-chlorophenyl)(quinolin-3-yl)methanone (3aq)
Compound 3aq was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3aq (47.2 mg, 88%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.34 (d, J = 2.2 Hz, 1H), 8.47 (d, J = 3.0 Hz, 1H), 8.17 (d, J = 8.4 Hz, 1H), 7.90–7.83 (m, 2H), 7.63–7.59 (m, 1H), 7.51 (d, J = 3.0 Hz, 2H), 7.49–7.41 (m, 2H). 13C{1H}NMR (101 MHz, CDCl3) δ ppm: 193.9, 149.9, 139.5, 137.6, 132.4, 131.8, 131.4, 130.3, 129.5, 129.4, 128.9, 127.6, 127.0, 126.7. The NMR spectra are consistent with the reported literature [31].
4.18. Quinolin-3-yl(m-tolyl)methanone (3ar)
Compound 3ar was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ar (40.1 mg, 81%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.2 Hz, 1H), 8.55 (d, J = 2.2 Hz, 1H), 8.19 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.85 (ddd, J = 8.5, 6.9, 1.5 Hz, 1H), 7.69–7.61 (m, 3H), 7.48–7.39 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 195.1, 150.3, 149.4, 138.8, 138.6, 137.0, 133.8, 131.8, 130.4, 130.2, 129.4, 129.1, 128.4, 127.5, 127.3, 126.6, 21.3. The NMR spectra are consistent with the reported literature [31].
4.19. (3-Bromophenyl)(quinolin-3-yl)methanone (3as)
Compound 3as was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3as (54.1 mg, 86%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.28 (d, J = 2.2 Hz, 1H), 8.53 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.4 Hz, 1H), 7.99 (s, 1H), 7.92 (d, J = 9.5 Hz, 1H), 7.87–7.83 (m, 1H), 7.78–7.72 (m, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.40 (t, J = 7.8 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.3, 150.0, 149.6, 138.8, 135.8, 132.7, 132.1, 130.1, 129.5, 129.4, 129.2, 128.4, 127.7, 126.5, 122.9. The NMR spectra are consistent with the reported literature [35].
4.20. (3-Chlorophenyl)(quinolin-3-yl)methanone (3at)
Compound 3at was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3at (43.2 mg, 85%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.29 (d, J = 2.2 Hz, 1H), 8.53 (d, J = 2.2 Hz, 1H), 8.18 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 9.6 Hz, 1H), 7.87–7.82 (m, 2H), 7.70 (d, J = 7.6 Hz, 1H), 7.66–7.59 (m, 2H), 7.46 (t, J = 7.8 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.4, 150.0, 149.6, 138.8, 138.6, 135.0, 132.9, 132.0, 129.9, 129.8, 129.5, 129.4, 129.2, 128.0, 127.7, 126.5. The NMR spectra are consistent with the reported literature [31].
4.21. (2,4-Dimethoxyphenyl)(quinolin-3-yl)methanone (3au)
Compound 3au was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3au (48.6 mg, 83%) as a yellow solid, m.p. 82–84 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.18 (d, J = 2.2 Hz, 1H), 8.53 (d, J = 2.2 Hz, 1H), 8.14 (d, J = 9.3 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.80 (t, J = 7.7 Hz, 1H), 7.58 (d, J = 8.4 Hz, 2H), 6.62 (dd, J = 8.5, 2.2 Hz, 1H), 6.52 (d, J = 2.2 Hz, 1H), 3.89 (s, 3H), 3.65 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.7, 164.4, 159.9, 150.7, 149.3, 138.1, 133.0, 131.8, 131.6, 129.4, 127.3, 127.1, 120.7, 105.4, 98.8, 55.7, 55.6. HRMS m/z (ESI) calcd for C18H15NO3 (M+H)+ 294.1125, found 294.1133.
4.22. (2,4-Difluorophenyl)(quinolin-3-yl)methanone (3av)
Compound 3av was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3av (44.1 mg, 82%) as a white solid, m.p. 94–96 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.29 (s, 1H), 8.52 (s, 1H), 8.17 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 8.2 Hz, 1H), 7.87–7.82 (m, 1H), 7.76–7.69 (m, 1H), 7.62 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.09–7.04 (m, 1H), 6.95 (ddd, J = 10.1, 8.7, 2.4 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 190.3, 163.2 (ddd, J = 438.1, 256.3, 12.1 Hz), 149.7 (d, J = 13.8 Hz), 138.8 (d, J = 2.0 Hz), 132.9 (dd, J = 10.4, 4.0 Hz), 132.2, 129.4 (d, J = 11.5 Hz), 127.6, 126.6, 122.8 (dd, J = 14.0, 3.7 Hz), 112.4 (dd, J = 21.7, 3.6 Hz), 104.9 (t, J = 25.6 Hz) 19F NMR (376 MHz, CDCl3) δ ppm: −101.8, −104.5. HRMS m/z (ESI) calcd for C16H9F2NO (M+H)+ 270.0725, found 270.0723.
4.23. (2,4-Dichlorophenyl)(quinolin-3-yl)methanone (3aw)
Compound 3aw was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3aw (48.6 mg, 81%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.30 (d, J = 2.2 Hz, 1H), 8.45 (d, J = 2.2 Hz, 1H), 8.16 (d, J = 8.5 Hz, 1H), 7.87 (t, J = 8.3 Hz, 2H), 7.61 (t, J = 7.6 Hz, 1H), 7.52 (s, 1H), 7.42 (s, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 192.8, 149.9, 149.7, 139.4, 137.4, 135.9, 132.5, 130.5, 130.2, 129.5, 128.7, 127.7, 127.5, 126.6. The NMR spectra are consistent with the reported literature [31].
4.24. Benzo[d][1,3]dioxol-5-yl(quinolin-3-yl)methanone (3ax)
Compound 3ax was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ax (43.6 mg, 79%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.24 (d, J = 2.2 Hz, 1H), 8.49 (d, J = 2.2 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.90 (d, J = 8.1 Hz, 1H), 7.82 (t, J = 7.7 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 1.8 Hz, 2H), 6.88 (d, J = 8.5 Hz, 1H), 6.08 (s, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 193.0, 152.0, 150.1, 149.2, 148.3, 138.1, 131.6, 131.4, 130.6, 129.4, 129.0, 127.5, 127.0, 126.5, 109.6, 107.9, 102.0. The NMR spectra are consistent with the reported literature [22].
4.25. Naphthalen-2-yl(quinolin-3-yl)methanone (3ay)
Compound 3ay was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ay (48.7 mg, 84%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.38 (d, J = 2.2 Hz, 1H), 8.61 (d, J = 2.8 Hz, 1H), 8.31 (s, 1H), 8.22 (d, J = 9.5 Hz, 1H), 7.99 (d, J = 1.3 Hz, 2H), 7.95–7.82 (m, 4H), 7.68–7.54 (m, 3H).13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.8, 150.3, 149.4, 138.8, 135.4, 134.2, 132.2, 132.1, 131.8, 130.3, 129.4, 129.1, 128.7, 128.7, 127.8, 127.6, 127.0, 126.6, 125.4. The NMR spectra are consistent with the reported literature [31].
4.26. Quinolin-3-yl(thiophen-2-yl)methanone (3az)
Compound 3az was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3az (38.6 mg, 80%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.43 (d, J = 2.2 Hz, 1H), 8.80 (d, J = 2.2 Hz, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.81 (t, J = 7.1 Hz, 1H), 7.74 (s, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.36 (d, J = 3.6 Hz, 1H), 6.64 (d, J = 3.7 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 180.3, 152.3, 149.7, 149.4, 147.4, 138.2, 131.8, 129.6, 129.4, 129.2, 127.5, 126.7, 120.7, 112.6. The NMR spectra are consistent with the reported literature [31].
4.27. Furan-2-yl(quinolin-3-yl)methanone (3az′)
Compound 3az′ was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3az′ (36.8 mg, 83%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.33 (d, J = 2.2 Hz, 1H), 8.63 (d, J = 2.2 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.93 (d, J = 8.2 Hz, 1H), 7.83 (t, J = 8.4 Hz, 1H), 7.78 (d, J = 4.9 Hz, 1H), 7.71 (s, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.20 (t, J = 4.4 Hz, 1H).13C{1H} NMR (101 MHz, CDCl3) δ ppm: 186.1, 149.5, 149.4, 143.1, 137.6, 135.0 (2C), 131.7, 130.6, 129.4, 129.0, 128.3, 127.6, 126.6. The NMR spectra are consistent with the reported literature [31].
4.28. (6-Methoxyquinolin-3-yl)(phenyl)methanone (3ba)
Compound 3ba was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ba (46.1 mg, 83%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.14 (d, J = 2.1 Hz, 1H), 8.44 (d, J = 2.2 Hz, 1H), 8.07 (d, J = 9.8 Hz, 1H), 7.87–7.84 (m, 2H), 7.67–7.62 (m, 1H), 7.55–7.46 (m, 3H), 7.14 (d, J = 2.8 Hz, 1H), 3.93 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 195.1, 158.4, 147.9, 145.6, 137.4, 137.1, 133.0, 130.7, 130.3, 130.0, 128.6, 127.8, 124.8, 106.0, 55.6. The NMR spectra are consistent with the reported literature [31].
4.29. (6-Fluoroquinolin-3-yl)(phenyl)methanone (3ca)
Compound 3ca was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ca (42.5 mg, 85%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.26 (d, J = 2.1 Hz, 1H), 8.49 (d, J = 2.2 Hz, 1H), 8.19 (dd, J = 9.2, 5.2 Hz, 1H), 7.85 (dd, J = 8.3, 1.3 Hz, 2H), 7.68–7.59 (m, 2H), 7.56–7.51 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.6, 160.9 (d, J = 250.3 Hz), 149.6 (d, J = 2.8 Hz), 137.9 (d, J = 5.6 Hz), 136.7, 133.2, 132.0 (d, J = 9.2 Hz), 130.7, 130.0, 128.7, 127.3 (d, J = 10.2 Hz), 122.0 (d, J = 25.8 Hz), 112.0 (d, J = 21.8 Hz). 19F NMR (376 MHz, CDCl3) δ ppm: −111.2. The NMR spectra are consistent with the reported literature [31].
4.30. (6-Chloroquinolin-3-yl)(phenyl)methanone (3da)
Compound 3da was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3da (43.3 mg, 83%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.1 Hz, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.10–8.04 (m, 2H), 7.92–7.84 (m, 3H), 7.69–7.65 (m, 1H), 7.55 (t, J = 7.7 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 150.4, 147.7, 137.7, 136.7, 133.4, 133.3, 132.7, 131.0, 130.8, 130.0, 128.7, 127.6, 127.2. The NMR spectra are consistent with the reported literature [31].
4.31. (6-Bromoquinolin-3-yl)(phenyl)methanone (3ea)
Compound 3ea was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ea (54.2 mg, 87%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.1 Hz, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.10–8.04 (m, 2H), 7.91 (dd, J = 8.9, 2.2 Hz, 1H), 7.87–7.83 (m, 2H), 7.69–7.65 (m, 1H), 7.55 (t, J = 7.7 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 150.6, 148.0, 137.6, 136.7, 135.2, 133.3, 131.1, 131.0, 130.7, 130.0, 128.7, 127.7, 121.5. The NMR spectra are consistent with the reported literature [31].
4.32. (7-Fluoroquinolin-3-yl)(phenyl)methanone (3fa)
Compound 3fa was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3fa (44.1 mg, 88%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.2 Hz, 1H), 8.55 (d, J = 2.4 Hz, 1H), 7.93 (dd, J = 9.0, 6.0 Hz, 1H), 7.86–7.79 (m, 3H), 7.68–7.63 (m, 1H), 7.54 (t, J = 7.7 Hz, 2H), 7.42 (td, J = 8.5, 2.5 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 164.4 (d, J = 254.1 Hz), 151.4, 150.6 (d, J = 13.0 Hz), 138.6, 136.8, 133.1, 131.4 (d, J = 10.2 Hz), 123.0, 129.5, 128.7, 123.6, 118.3 (d, J = 25.6 Hz), 113.4 (d, J = 20.6 Hz) 19F NMR (376 MHz, CDCl3) δ ppm: −104.8. The NMR spectra are consistent with the reported literature [31].
4.33. (7-Chloroquinolin-3-yl)(phenyl)methanone (3ga)
Compound 3ga was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ga (44.8 mg, 84%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.32 (d, J = 2.2 Hz, 1H), 8.54 (d, J = 2.2 Hz, 1H), 8.19 (d, J = 2.0 Hz, 1H), 7.88–7.84 (m, 3H), 7.69–7.64 (m, 1H), 7.61–7.52 (m, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 151.3, 149.7, 138.5, 137.9, 136.7, 133.2, 130.3, 130.1, 130.0, 128.8, 128.7, 128.5, 125.0. The NMR spectra are consistent with the reported literature [31].
4.34. (7-Bromoquinolin-3-yl)(phenyl)methanone (3ha)
Compound 3ha was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ha (52.1 mg, 83%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.31 (d, J = 2.1 Hz, 1H), 8.53 (dd, J = 2.1, 0.8 Hz, 1H), 8.38 (d, J = 2.0 Hz, 1H), 7.85 (dd, J = 8.3, 1.3 Hz, 2H), 7.79 (d, J = 8.6 Hz, 1H), 7.73 (dd, J = 8.7, 1.9 Hz, 1H), 7.69–7.65 (m, 1H), 7.57–7.53 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 151.3, 149.9, 138.6, 136.7, 133.2, 131.9, 131.3, 130.3, 130.2, 130.0, 128.7, 126.3, 125.2. The NMR spectra are consistent with the reported literature [31].
4.35. Phenyl(7-(trifluoromethyl)quinolin-3-yl)methanone (3ia)
Compound 3ia was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ia (47.1 mg, 80%) as a white solid, m.p. 88–92 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.39 (d, J = 2.2 Hz, 1H), 8.60 (d, J = 2.2 Hz, 1H), 8.50 (s, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.88–7.85 (m, 2H), 7.81 (dd, J = 8.5, 1.8 Hz, 1H), 7.68 (t, J = 7.5 Hz, 1H), 7.56 (t, J = 7.6 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.4, 151.5, 148.4, 138.2, 136.5, 133.4, 133.2 (q, J = 33.0 Hz), 131.7, 130.3, 130.0, 128.8, 128.1, 127.3 (q, J = 4.3 Hz), 123.6 (q, J = 271.5 Hz), 123.2 (d, J = 3.0 Hz). 19F NMR (376 MHz, CDCl3) δ ppm: −62.8. HRMS m/z (ESI) calcd for C17H10F3NO (M+H)+ 302.0787, found 302.0779.
4.36. Methyl 3-benzoylquinoline-7-carboxylate (3ja)
Compound 3ja was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ja (43.6 mg, 75%) as a white solid, m.p. 142–145 °C; 1H NMR (400 MHz, CDCl3) δ ppm: 9.36 (d, J = 2.2 Hz, 1H), 8.87 (s, 1H), 8.56 (d, J = 3.1 Hz, 1H), 8.21 (dd, J = 8.5, 1.7 Hz, 1H), 7.97 (d, J = 8.9 Hz, 1H), 7.88–7.85 (m, 2H), 7.69–7.64 (m, 1H), 7.57–7.52 (m, 2H), 4.02 (s, 3H).13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.5, 166.3, 151.1, 148.8, 138.2, 136.6, 133.3, 132.8, 131.8, 131.4, 130.0, 129.3, 129.0, 128.7, 127.0, 52.7. HRMS m/z (ESI) calcd for C18H13NO3 (M+H)+ 292.0968, found 292.0961.
4.37. (6,8-Dichloroquinolin-3-yl)(phenyl)methanone (3ka)
Compound 3ka was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ka (46.5 mg, 78%) as a white solid.
1H NMR (400 MHz, CDCl3) δ ppm: 9.37 (d, J = 2.1 Hz, 1H), 8.49 (d, J = 2.1 Hz, 1H), 7.94 (d, J = 2.2 Hz, 1H), 7.87–7.83 (m, 3H), 7.68 (t, J = 7.4 Hz, 1H), 7.55 (t, J = 7.8 Hz, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.0, 150.9, 144.1, 138.1, 137.8, 136.4, 135.0, 133.6, 132.9, 132.2, 131.7, 130.1, 128.8, 128.3, 126.7, 126.5. The NMR spectra are consistent with the reported literature [16].
4.38. (6,7-Dimethoxyquinolin-3-yl)(phenyl)methanone (3la)
Compound 3la was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3la (46.8 mg, 80%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.11 (d, J = 2.1 Hz, 1H), 8.41 (d, J = 2.9 Hz, 1H), 7.85–7.82 (m, 2H), 7.64–7.59 (m, 1H), 7.54–7.47 (m, 3H), 7.10 (s, 1H), 4.06 (s, 3H), 4.00 (s, 3H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 195.1, 154.4, 150.4, 148.5, 147.0, 137.3, 136.8, 132.7, 129.9, 128.5, 122.3, 107.8, 106.0, 56.3, 56.1. The NMR spectra are consistent with the reported literature [31].
4.39. [1,3]Dioxolo [4,5-g]quinolin-7-yl(phenyl)methanone (3ma)
Compound 3ma was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3ma (45.1 mg, 81%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 9.08 (d, J = 2.2 Hz, 1H), 8.37 (d, J = 2.1 Hz, 1H), 7.85–7.81 (m, 2H), 7.63 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.6 Hz, 2H), 7.43 (s, 1H), 7.11 (s, 1H), 6.15 (s, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 194.9, 152.7, 148.6, 148.5, 148.4, 137.4, 137.3, 137.2, 132.8, 128.6, 128.5, 123.8, 105.8, 103.6, 102.2. The NMR spectra are consistent with the reported literature [31].
4.40. (6-Chloro-4-phenylquinolin-3-yl)(phenyl)methanone (3na)
Compound 3na was synthesized in accordance with the typical procedure. Purification using column chromatography on silica gel (PE:EA = 10:1) afforded product 3na (57.0 mg, 84%) as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm: 8.96 (s, 1H), 8.17 (d, J = 9.7 Hz, 1H), 7.73 (dd, J = 6.9, 2.4 Hz, 2H), 7.59 (dd, J = 8.3, 1.4 Hz, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.34–7.27 (m, 5H), 7.26–7.23 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3) δ ppm: 196.4, 148.6, 147.1, 146.1, 137.0, 134.2, 133.6, 133.4, 132.5, 131.4, 129.9, 129.7, 128.8, 128.4, 128.3, 127.2, 125.4. The NMR spectra are consistent with the reported literature [61].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal13040778/s1.
Author Contributions
Methodology, K.-L.Z.; writing—review and editing, K.-L.Z. and J.-C.Y.; review and editing, Q.G.; supervision, L.-H.Z.; project administration, L.-H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2022B01) is greatly appreciated.
Data Availability Statement
Data supporting reported results can be found in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Behenna, D.C.; Stockdill, J.L.; Stoltz, B.M. The Biology and Chemistry of the Zoanthamine Alkaloids. Angew. Chem. Int. Ed. 2008, 47, 2365–2386. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.K.; Alumasa, J.N.; Yearick, K.; Ekoue-Kovi, K.A.; Casabianca, L.B.; de Dios, A.C.; Wolf, C.; Roepe, P.D. 4-N-, 4-S-, and 4-O-Chloroquine Analogues: Influence of Side Chain length and Quinolyl Nitrogen pK(a) on Activity vs Chloroquine Resistant Malaria. J. Med. Chem. 2008, 51, 3466–3479. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Quinoline, Quinazoline and Acridone Alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Rouffet, M.; de Oliveira, C.A.F.; Udi, Y.; Agrawal, A.; Sagi, I.; McCammon, J.A.; Cohen, S.M. From Sensors to Silencers: Quinoline- and Benzimidazole-sulfonamides as Inhibitors for Zinc Proteases. J. Am. Chem. Soc. 2010, 132, 8232–8233. [Google Scholar] [CrossRef]
- Andrews, S.; Burgess, S.J.; Skaalrud, D.; Kelly, J.X.; Peyton, D.H. Reversal Agent and Linker Variants of Reversed Chloroquines: Activities against Plasmodium Falciparum. J. Med. Chem. 2010, 53, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.M.; Mahon, M.F.; Lloyd, M.D.; Threadgill, M.D. Design, Synthesis, and Evaluation in vitro of Quinoline-8-carboxamides, a New Class of Poly(adenosine-diphosphate-ribose)polymerase-1 (PARP-1) Inhibitor. J. Med. Chem. 2009, 52, 868–877. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-based Antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a Privileged Scaffold in cancer Drug Discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Gohlmann, H.W.H.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium Tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Liu, C.; Zhang, D.; Lu, Z.; Geng, H.; Shuai, Z.; Zhu, D. Coordination Complexes of 2-(4-quinolyl)nitronyl Nitroxide with M(hfac)(2) [M = Mn(II), Co(II), and Cu(II)]: Syntheses, Crystal Structures, and Magnetic Characterization. Inorg. Chem. 2004, 43, 4091–4098. [Google Scholar] [CrossRef]
- Hu, Y.-Z.; Zhang, D.; Thummel, R.P. Friedlander Approach for the Incorporation of 6-Bromoquinoline into Novel Chelating Ligands. Org. Lett. 2003, 5, 2251–2253. [Google Scholar] [CrossRef]
- Nakatani, K.; Sando, S.; Saito, I. Recognition of a Single Guanine Bulge by 2-Acylamino-1,8-naphthyridine. J. Am. Chem. Soc. 2000, 122, 2172–2177. [Google Scholar] [CrossRef]
- Nakatani, K.; Sando, S.; Saito, I. Improved Selectivity for the Binding of Naphthyridine Dimer to Guanine-guanine Mismatch. Bioorg. Med. Chem. 2001, 9, 2381–2385. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Marchand, C.; Delage, S.; Sun, J.-S.; Garestier, T.; Helene, C.; Bisagni, E. Synthesis of 13H-benzo[6,7]-and 13H-benzo[4,5]indolo[3,2-c]quinolines: A New Series of Potent Specific Ligands for Triplex DNA. J. Am. Chem. Soc. 1998, 120, 2501–2507. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Shu, C.-F. Synthesis and Characterization of New Polyquinolines Containing 9,9′-Spirobifluorene Units. Chem. Mater. 2002, 14, 682–687. [Google Scholar] [CrossRef]
- Dong, J.; Wang, L.; Li, H.; Leng, X.; Guo, X.; Hu, Z.; Xu, X. Self-cyclization vs. Dimerization of O-alkenyl Arylisocyanides: Chemodivergent Synthesis of Quinolines and Pyrrolo-fused Diindoles. Org. Chem. Front. 2021, 8, 2595–2600. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Yang, J.; Xie, X.; Huang, H.; Li, Y. Iron-catalyzed Cascade Reaction of Ynone with O-aminoaryl Compounds: A Michael Addition–Cyclization Approach to 3-Carbonyl Quinolines. Tetrahedron Lett. 2011, 52, 530–533. [Google Scholar] [CrossRef]
- Marco-Contelles, J. Perez-Mayoral, E.; Samadi, A.; Carreiras, M.D.; Soriano, E. Recent Advances in the Friedlander Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef]
- Denmark, S.E.; Venkatraman, S. On the Mechanism of the Skraup–Doebner–Von Miller Quinoline Synthesis. J. Org. Chem. 2006, 71, 1668–1676. [Google Scholar] [CrossRef]
- Manske, R.H.F. The Chemistry of Quinolines. Chem. Rev. 1942, 30, 113–144. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Chen, X.; Zhang, X.; Yi, W. The One-pot Synthesis of Quinolines via Co(III)-catalyzed C-H Activation/Carbonylation/Cyclization of Anilines. Org. Biomol. Chem. 2017, 15, 9061–9065. [Google Scholar] [CrossRef] [PubMed]
- Wakade, S.B.; Tiwari, D.K.; Ganesh, P.; Phanindrudu, M.; Likhar, P.R.; Tiwari, D.K. Transition-metal-free Quinoline Synthesis from Acetophenones and Anthranils via Sequential One-carbon Homologation/Conjugate Addition/Annulation Cascade. Org. Lett. 2017, 19, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yan, B.; Tao, H.; Zhang, Y.; Li, Y. Metal-free Photocatalyzed Aerobic Oxidative C(sp3)-H Functionalization of Glycine Derivatives: One-step Generation of Quinoline-fused Lactones. Org. Biomol. Chem. 2018, 16, 3816–3823. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Li, Y.Z.; Stepnicka, P.; Kitamura, M.; Liu, Y.J.; Nakajima, K.; Kotora, M. Coupling Reaction of Zirconacyclopentadienes with Dihalonaphthalenes and Dihalopyridines: A New Procedure for the Preparation of Substituted Anthracenes, Quinolines, and Isoquinolines. J. Am. Chem. Soc. 2002, 124, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Peng, J.; Li, M. Copper(II)-Catalyzed Three-Component Cascade Annulation of Diaryliodoniums, Nitriles, and Alkynes: A Regioselective Synthesis of Multiply Substituted Quinolines. Angew. Chem. Int. Ed. 2013, 52, 5323–5327. [Google Scholar] [CrossRef]
- Yan, R.L.; Liu, X.X.; Pan, C.M.; Zhou, X.Q.; Li, X.N.; Kang, X.; Huang, G.S. Aerobic Synthesis of Substituted Quinoline from Aldehyde and Aniline: Copper-Catalyzed Intermolecular C-H Active and C-C Formative Cyclization. Org. Lett. 2013, 15, 4876–4879. [Google Scholar] [CrossRef]
- Wang, F.; Xu, P.; Wang, S.Y.; Ji, S.J. Cu(II)/Ag(I)-Catalyzed Cascade Reaction of Sulfonylhydrazone with Anthranils: Synthesis of 2-Aryl-3-sulfonyl Substituted Quinoline Derivatives. Org. Lett. 2018, 20, 2204–2207. [Google Scholar] [CrossRef]
- McNaughton, B.R.; Miller, B.L. A Mild and Efficient One-step Synthesis of Quinolines. Org. Lett. 2003, 5, 4257–4259. [Google Scholar] [CrossRef]
- Wu, J.L.; Cui, X.L.; Chen, L.M.; Jiang, G.J.; Wu, Y.J. Palladium-Catalyzed Alkenylation of Quinoline-N-oxides via C-H Activation under External-Oxidant-Free Conditions. J. Am. Chem. Soc. 2009, 131, 13888–13889. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef]
- Yan, K.; Liu, M.; Wen, J.; Liu, X.; Wang, X.; Sui, X.; Shang, W.; Wang, X. Synthesis of 3-Substituted Quinolines by Ruthenium-catalyzed aza-Michael Addition and Intramolecular Annulation of Enaminones with Anthranils. New J. Org. Chem. 2022, 46, 7329–7333. [Google Scholar] [CrossRef]
- Kaewmee, B.; Rukachaisirikul, V.; Kaeobamrung, J. Synthesis of quinolines via copper-catalyzed domino reactions of enaminones. Org. Biomol. Chem. 2017, 15, 7387–7739. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-P.; Jing, Y.; Wei, L. Branched C=C and C-N Bond Cleavage on Enaminones toward the Synthesis of 3-Acyl Quinolines. Asian J. Org. Chem. 2017, 6, 666–668. [Google Scholar] [CrossRef]
- Wakade, S.B.; Tiwari, D.K.; Phanindrudu, M.; Pushpendra; Tiwari, D.K. Synthesis of 3-Keto-quinolines from Enaminones, Anilines and DMSO: Transition Metal Free One Pot Cascade. Tetrahedron 2019, 75, 4024–4030. [Google Scholar] [CrossRef]
- Gao, Y.; Hider, R.C.; Ma, Y. An Efficient 3-Acylquinoline Synthesis from Acetophenones and Anthranil via Csp3-H Bond Activation Mediated by Selectfluor. RSC Adv. 2019, 9, 10340–10344. [Google Scholar] [CrossRef]
- Rahul, P.; Veena, S.; John, J. Inverse Electron Demand Diels Alder Reaction of Aza-o-Quinone Methides and Enaminones: Accessing 3-Aroyl Quinolines and Indeno 1,2-b Quinolinones. J. Org. Chem. 2022, 87, 13708–13714. [Google Scholar] [CrossRef]
- Jiang, P.; Shan, Z.; Chen, S.; Wang, Q.; Jiang, S.; Zheng, H.; Deng, G.-J. Metal-Free Synthesis of Benzo a phenanthridines from Aromatic Aldehydes, Cyclohexanones, and Aromatic Amines. Chin. J. Chem. 2022, 40, 365–370. [Google Scholar] [CrossRef]
- Cai, Z.-N.; Feng, X.-X.; Zhang, Y.; Lu, C.-C.; Han, Y.-P.; Zhao, J. Transition-Metal-Free Catalyzed Dehydrative Coupling of Quinoline and Isoquinoline N-Oxides with Propargylic Alcohols. Chin. J. Chem. 2022, 40, 71–78. [Google Scholar] [CrossRef]
- Liu, S.-J.; Chen, Z.-H.; Chen, J.-Y.; Ni, S.-F.; Zhang, Y.-C.; Shi, F. Rational Design of Axially Chiral Styrene-Based Organocatalysts and Their Application in Catalytic Asymmetric (2+4) Cyclizations. Angew. Chem. Int. Ed. 2022, 61, e202112226. [Google Scholar] [CrossRef]
- Medishetti, N.; Kale, A.; Nanubolu, J.B.; Atmakur, K. Iron(III)chloride Induced Synthesis of Pyrazolopyridines & Quinolines. Synth. Commun. 2020, 50, 3642–3651. [Google Scholar] [CrossRef]
- Ha, V.D.; Hoang, T.B.L.; Loan, T.B.T.; Hiep, Q.H.; Ha, V.L.; Nam, T.S.P. Copper-catalyzed One-pot Domino Reactions via C-H Bond Activation: Synthesis of 3-Aroylquinolines from 2-Aminobenzylalcohols and Propiophenones under Metal-organic Framework Catalysis. RSC Adv. 2018, 8, 31455–31464. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Phanindrudu, M.; Wakade, S.B.; Nanubolu, J.B.; Tiwari, D.K. alpha, beta-Functionalization of Saturated Ketones with Anthranils via Cu-catalyzed Sequential Dehydrogenation/aza-Michael Addition/Annulation Cascade Reactions in One-pot. Chem. Commun. 2017, 53, 5302–5305. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.P.; Gao, Y. Domino Reactions Based on Combinatorial Bond Transformations in Electron-Deficient Tertiary Enamines. Chem. Rec. 2016, 16, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.G.; Wang, J.H.; Wang, L.L.; Song, C.; Chen, K.H.; Zhu, J. Enaminones as Synthons for a Directed C-H Functionalization: Rh-III-Catalyzed Synthesis of Naphthalenes. Angew. Chem. Int. Ed. 2016, 55, 9384–9388. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, H. Rhodium-catalyzed C-H Activation/Cyclization of Enaminones with Sulfoxonium Ylides toward Polysubstituted Naphthalenes. Tetrahedron Lett. 2019, 60, 664–667. [Google Scholar] [CrossRef]
- Zou, M.C.; Liu, J.Z.; Tang, C.H.; Jiao, N. Rh-Catalyzed N-O Bond Cleavage of Anthranil: A C-H Amination Reagent for Simultaneous Incorporation of Amine and A Functional Group. Org. Lett. 2016, 18, 3030–3033. [Google Scholar] [CrossRef]
- Jin, H.M.; Huang, L.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed C-H Annulation of Anthranils with Alkynes: A Facile, Flexible, and Atom-Economical Synthesis of Unprotected 7-Acylindoles. Angew. Chem. Int. Ed. 2016, 55, 794–797. [Google Scholar] [CrossRef]
- Yu, S.J.; Tang, G.D.; Li, Y.Z.; Zhou, X.K.; Lan, Y.; Li, X.W. Anthranil: An Aminating Reagent Leading to Bifunctionality for Both C(sp(3))-H and Csp2-H under Rhodium(III) Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8696–8700. [Google Scholar] [CrossRef]
- Jin, H.M.; Tian, B.; Song, X.L.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Synthesis of Quinolines from Propargyl Silyl Ethers and Anthranils through the Umpolung of a Gold Carbene Carbon. Angew. Chem. Int. Ed. 2016, 55, 12688–12692. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Yu, S.J.; Yang, X.F.; Li, X.W. Cooperative Co(III)/Cu(II)-Catalyzed C-N/N-N Coupling of Imidates with Anthranils: Access to 1H-Indazoles via C-H Activation. Org. Lett. 2016, 18, 3662–3665. [Google Scholar] [CrossRef]
- Biswas, A.; Karmakar, U.; Nandi, S.; Samanta, R. Copper-Catalyzed Direct, Regioselective Arylamination of N-Oxides: Studies to Access Conjugated pi-Systems. J. Org. Chem. 2017, 82, 8933–8942. [Google Scholar] [CrossRef]
- Xie, F.; Shen, B.X.; Li, X.W. Enantioselective Copper-Catalyzed Hydroamination of Vinylarenes with Anthranils. Org. Lett. 2018, 20, 7154–7157. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Sun, H.M.; Wang, Y.; Xie, F. Cu/Ag-Catalyzed Reaction of Azirines with Anthranils: Synthesis of (Quinazolin-2-yl)methanone Derivatives. Org. Lett. 2020, 22, 6756–6759. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.H.; Zhu, H.; Zhu, S.; Shi, K.; Yan, C.; Li, P.G. Copper-Catalyzed Ring-Opening/Reconstruction of Anthranils with Oxo-Compounds: Synthesis of Quinoline Derivatives. J. Org. Chem. 2019, 84, 12301–12313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, K.; Zhu, H.; Jia, Z.K.; Xia, X.F.; Wang, D.; Zou, L.H. Copper-Catalyzed Annulation or Homocoupling of Sulfoxonium Ylides: Synthesis of 2,3-Diaroylquinolines or alpha, alpha, beta-Tricarbonyl Sulfoxonium Ylides. Org. Lett. 2020, 22, 1504–1509. [Google Scholar] [CrossRef]
- Shi, K.; Zhu, H.; Ren, F.; Liu, S.; Song, Y.; Li, W.; Zou, L.-H. Copper-catalyzed [3+2+1] Annulation of Anthranils with Phenylacetaldehydes: Synthesis of 8-Acylquinolines. Eur. J. Org. Chem. 2021, 2021, 1003–1006. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, R.; Xiao, F.; Li, T.; Mao, G.; Deng, G.-J. Four-Component Synthesis of 9H-Pyrimido 4,5-b indoles Using Ammonium Iodide as the Nitrogen Source. Catalysts 2023, 13, 623. [Google Scholar] [CrossRef]
- Gong, Z.-Y.; Yang, C.-L.; Wang, D.; Huang, L.; Dong, Z.-B. One-Pot Synthesis of Benzoxazole/Benzothiazole-Substituted Esters by Michael Addition: A Selective Construction of C-N/C-S Bonds. Catalysts 2023, 13, 658. [Google Scholar] [CrossRef]
- Duan, L.; Zhou, H.; Gu, Y.; Gong, P.; Qin, M. The Use of Enaminones and Enamines as Effective Synthons for MSA-catalyzed Regioselective Synthesis of 1,3,4-Tri- and 1,3,4,5-Tetrasubstituted Pyrazoles. New J. Chem. 2019, 43, 16131–16137. [Google Scholar] [CrossRef]
- Borah, A.; Goswami, L.; Neog, K.; Gogoi, P. DMF Dimethyl Acetal as Carbon Source for alfa-Methylation of Ketones: A Hydrogenation-Hydrogenolysis Strategy of Enaminones. J. Org. Chem. 2015, 80, 4722–4728. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Zhang, X.; Fan, X. Synthesis of 3-Acylquinolines through Cu-catalyzed Double C(sp3)-H Bond Functionalization of Saturated Ketones. Org. Chem. Front. 2017, 4, 612–616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).