Abstract
Microwave-induced oxidation and UV/TiO2 photocatalytic technologies are widely used for organic wastewater treatment. Furthermore, the combination of these technologies (MW/UV/TiO2) result in a new advanced oxidation process. As a green and efficient photocatalytic degradation technology, MW/UV/TiO2 is favored for its advantages of high removal rate, short time use, wide concentration range, low cost, good stability, and no secondary pollution. Herein, this paper has summarized insights into the removal process by unveiling the degradation mechanism of organic compounds with MW-assisted technology. Additionally, water quality factors and process parameters affect the photocatalytic efficiencies, consisting of initial concentration, initial volume, TiO2 dosage, UV intensity, microwave power, temperature, pH, and fluid velocity, which have been systematically analyzed. Finally, possible future research directions and guidelines are proposed. Our findings will provide a way forward for the development of effective microwave-assisted remediation technologies that are broadly applicable to various environmental contamination scenarios.
1. Introduction
Rapid industrialization and urbanization have aggravated the external inputs of organic contaminants to surface water, groundwater, and soil [1]. Various contaminants are toxins that can accumulate in the system and be transported over long distances. Unfortunately, organic contaminants can significantly affect human health (e.g., mutagenic, carcinogenic, and teratogenic) by entering food chains [2,3,4,5]. Traditional contaminants generally include simple hydrocarbons, halogenated hydrocarbons, benzene, phenol, and polycyclic aromatic hydrocarbons. Recent research has shown that emerging organic environmental pollutants are mainly from pharmaceuticals, personal care products (PCPs), endocrine disruptor chemicals (EDCs), surfactants, pesticides, flame retardants, industrial additives, etc. [6]. Specifically, pharmaceuticals mainly refer to analgesics, lipid-lowering drugs, antibiotics, diuretics, non-steroidal anti-inflammatory drugs, stimulants, antimicrobials, and analgesics. In addition, PCPs include cosmetics, sunscreens, spices, etc. Typical EDCs are steroid hormones, phthalates, bisphenol A, etc. [7,8,9]. Scientifically, there is a high probability that the organic contaminants will migrate through natural driving forces into environment media (such as soil, water, animals, and plants), and eventually threaten human health [10]. For example, the lipid-soluble estrogenic compounds, as typical emerging contaminants, are absorbable, and chemically stable, and they can accumulate in living organisms through the food chain and cannot be excreted [11]. After entering the human target cells, these compounds bind to steroid receptors and form hormone receptor complexes, further entering the cell nucleus and DNA to exert biological effects, and finally altering cell function and disrupting the normal metabolism of the human endocrine system [12,13,14]. Considering their environmental toxicity, it is necessary to find a friendly, efficient, and feasible treatment for organic pollution removal technology.
Recently, Tian and co-workers reviewed the research progress of microwave-induced oxidation technologies in the treatment of organic wastewater, volatile organic compounds, and hydrogen production [15]. It was noted that microwave-induced oxidation technologies combined with other advanced technologies were highly chosen by environment researchers due to their efficiency, rapidity, and minimal secondary pollution. Extensive technical and applied studies based on the novel MW/UV/TiO2 photocatalysis technology have been reported [15,16,17]. However, as far as we know, no review study based on the MW/UV/TiO2 technology has been published yet. As such, this review paper presents the degradation of environmental organic pollutants in aqueous solution by the MW/UV/TiO2 photocatalytic technology and summarizes the factors influencing the catalytic efficiency, catalyst materials, and catalysis mechanism. Furthermore, external conditions for optimizing the process and recently discovered technological features are explained. This review is designed to benefit future research and provides pertinent information on theoretical/technical grounds to support the remediation of organic pollution using the MW/UV/TiO2 technology.
2. Technology
2.1. Microwave Radiation Technology
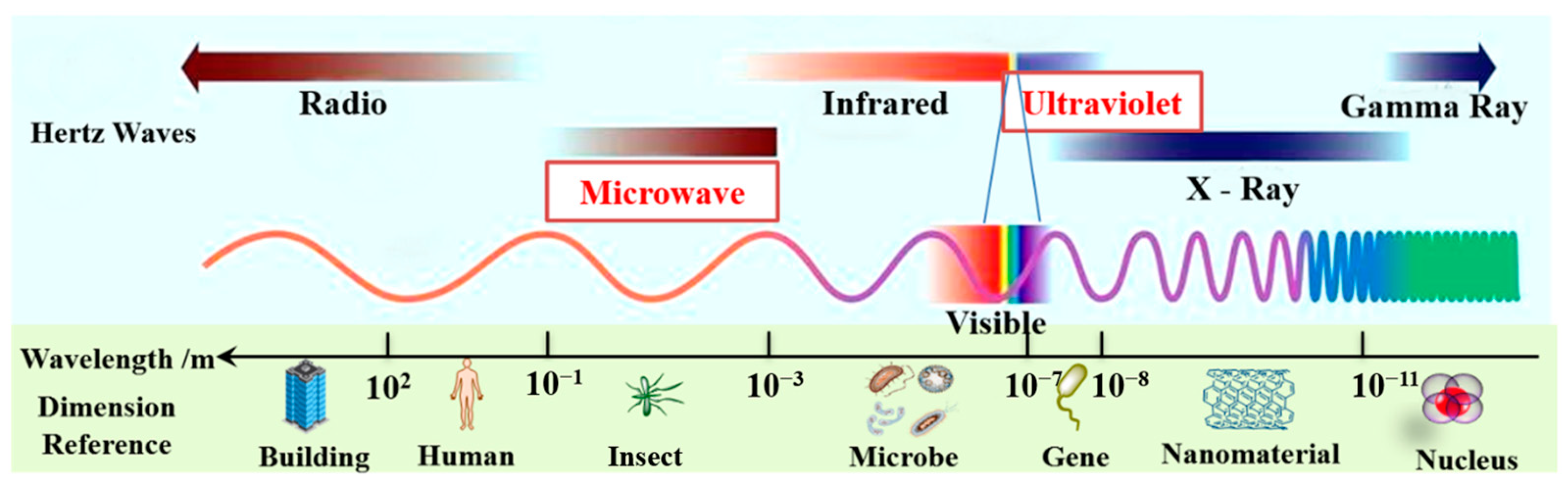
Microwave (MW) is a part of the electromagnetic spectrum that occurs in the frequency range of 300 MHz to 300 GHz and lies between infrared and radio frequencies, corresponding to wavelengths of 1 cm and 1 m (see Figure 1) [18,19]. The scientific consensus is that MW is barely absorbed when passing through non-polar compounds such as glass, plastic, and porcelain. However, MW can be rapidly absorbed by polar compounds such as polar organic solutes, water, and food, and these matters are then heated via polar molecules and ions [18]. Dielectric parameters are linked to the molecular structure that affects the parity of materials. As such, the change in the dielectric parameters of the processed materials significantly affects the efficiency and economy of microwave heating processes [20].
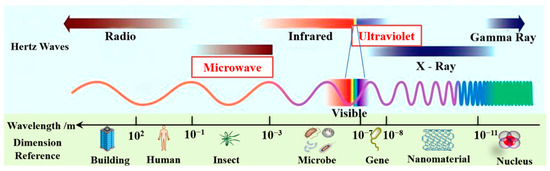
Figure 1.
The wavebands of microwave and ultraviolet in the electromagnetic waves.
Compared with traditional heating methods, MW-based technology offers several advantages, including high speed, high efficiency, mild conditions, high selectivity, easy controllability, and no contact with heating materials [21,22]. As a result, MW is widely applied in environmental pollution control, the food industry, plasma processing, organic synthesis, analytical chemistry, polymer treatment, metal sintering, disinfection and sterilization, regeneration and preparation of functional materials, and other physico-chemical and biological research fields [22,23,24,25,26,27,28].
It is scientifically known that MW irradiation can enhance the removal reaction rates and induce selective heating of contaminants through internal molecular vibrations in the treatment of organic wastewater/flue gas/landfill leaching [15,29,30], sludge recycling [31], and solid waste reduction [32,33]. For example, some substances (e.g., Cu/GAC) have minimal degradation efficiencies under conventional conditions and belong to the non-catalysts in the degradation of organic pollutants [34]. However, a hotspot effect radiated by MW can be on the surface of these substances, resulting in the easy degradation of organic pollutants [22,35,36,37]. This process would therefore transform the materials into highly efficient catalysts. Similarly, MW irradiation can be used to improve the catalytic efficiency of conventional catalysts [21].
In addition, MW heating can pyrolyze sewage sludge to produce activated carbon (AC) with high adsorption properties, and AC has been confirmed to effectively remove phenolic organic compounds in an aqueous solution [31]. Moreover, MW irradiation can treat solid waste to promote its solidification, reduce its volume, stabilize heavy metals, and reduce leaching [32,33]. More importantly, landfill leachate can be rapidly discolored, and total organic carbon (TOC) can be reduced by microwave treatment combined with strong oxidizers. Meanwhile, the elimination rates of chemical oxygen demand (COD) and NH3-N are significantly higher than that of the non-microwave treatment [30,38].
2.2. Ultraviolet Photodegradation Technology
As shown in Figure 1, the wavelength range of ultraviolet (UV) light (10~400 nm) falls between visible light and X-ray electromagnetic radiation [39]. The energy of the UV wave is high and UV can directly destroy the structure of organic matter in wastewater. Currently, UV radiation technology is primarily used to disinfect and sterilize water in conventional water treatment applications due to its low-toxic by-products, chemical-free process, and no overdose risk [40,41]. UV irradiation has a variety of applications in advanced oxidation processes (AOPs) for the degradation of organic wastewater contaminants. [42]. For example, two water pollutants, ranitidine and nizatidine, could be effectively degraded under UV irradiation [43]. In principle, some oxidants (e.g., H2O2, HClO, and S2O82−) radiated by UV could produce the corresponding higher oxidation of free radicals, which would accelerate the degradation of organic pollutants; then, these oxidants could be excited by UV to generate highly reactive radical species such as OH·, Cl·, and SO4−·, respectively [44,45,46]. The OH·, for example, could rapidly and non-selectively destroy most organic pollutants, degrading them to CO2, H2O, and mineral salts in water [47]. The previous literature showed that organic effluents, such as azathioprine [48], sulfamethoxazole [44], ibuprofen [49], thiamphenicol [50], cyclophosphamide, and 5-fluorouracil [51] have been investigated with UV photodegradation technology. Furthermore, ronidazole, metoprolol, ibuprofen, carbamazepine, chloramphenicol, and benzalkonium chloride were effectively decomposed using UV/chlorine as an advanced oxidation process [46,52,53,54,55,56].
2.3. UV/TiO2 Photocatalysis Technology
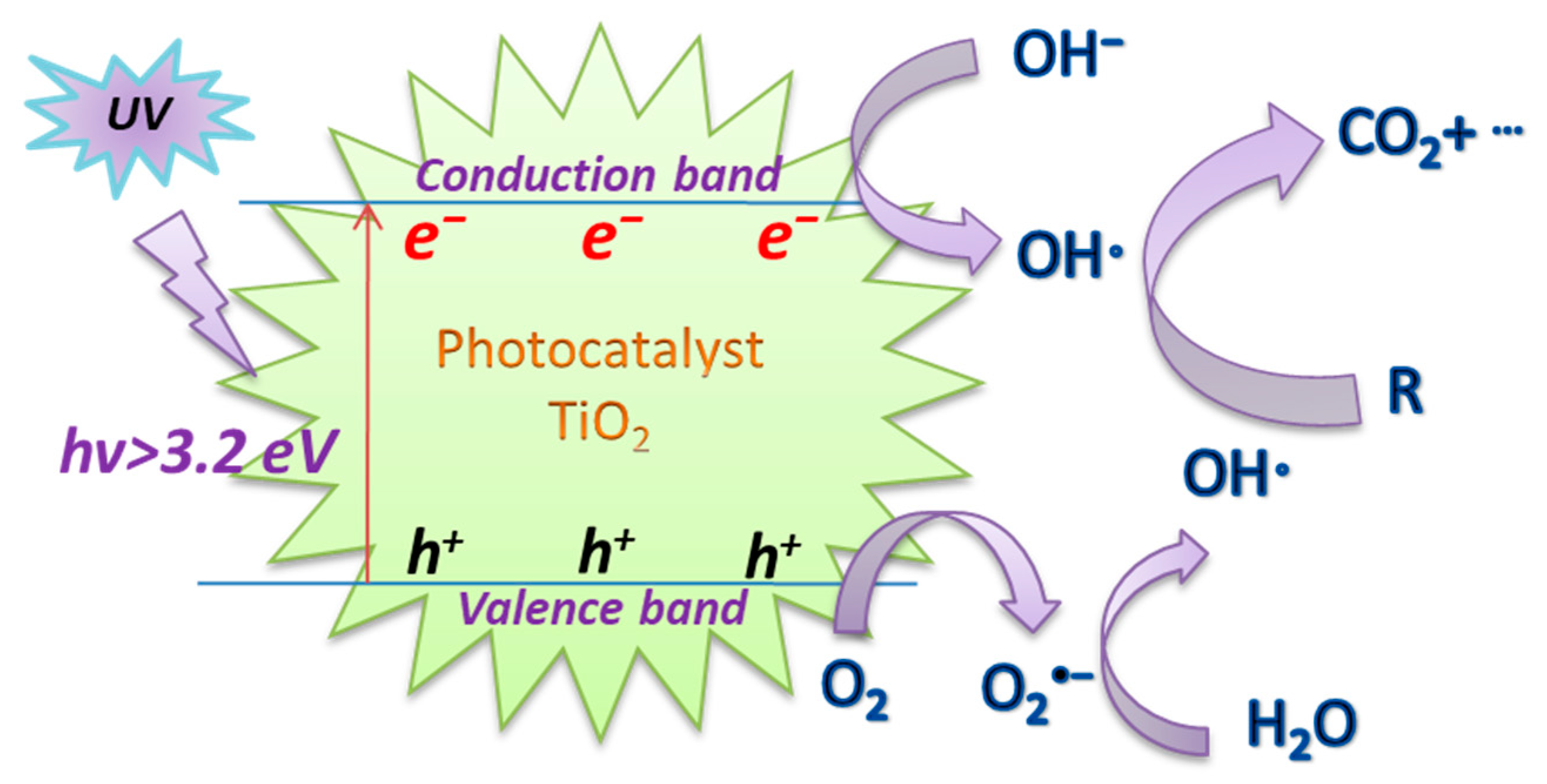
Titanium dioxide (TiO2), as a special type of semiconductor, has demonstrated great potential as an ideal photocatalyst material due to its advantages of high reactivity, non-toxicity, water insolubility, resistance to photocorrosion, relatively cheap price, and stability [57,58,59]. Thus, it has been applied to significant applications as the most widely used benchmark photocatalyst in the field of environmental protection [60,61]. Figure 2 depicts the UV/TiO2 photoactivation mechanism. Under the action of UV irradiation, the electron (e−)~hole (h+) pairs are formed on the surface of the TiO2 catalyst in the aqueous solution. Subsequently, O2 and OH− can trap the e−~h+ pairs and adsorb on the surface, and then generate the highly reactive O2−· and OH·, respectively, to degrade organic pollutants [62,63].
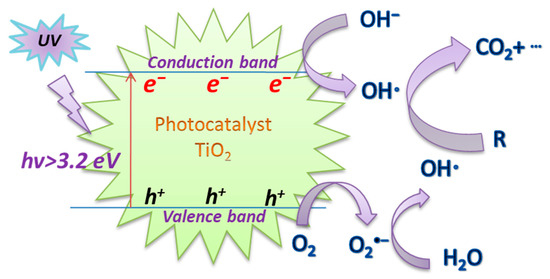
Figure 2.
The mechanism of UV/TiO2 photoactivation.
UV/TiO2 photocatalysis has been shown to have strong redox potential, high photocatalytic efficiency, low cost, and no secondary pollution [62,64,65]. Therefore, it is a promising technology for wastewater treatment. For example, the decolorization rate of acid blue 113 and acid red 114 treated with UV/TiO2 reached up to 96% and 99%, respectively, after 240 min [66]. In total, 97% of p-chlorophenol in the solution could be degraded by UV/TiO2 in combination with a Fenton reagent [67]. In addition, UV/TiO2 could be effective in treating dyes (e.g., rhodamine B, methyl orange, methyl blue, amaranth dye), antibiotic compounds, and endocrine disruptors in water [59,68,69].
2.4. MW/UV/TiO2 Photocatalysis Technology
MW/UV/TiO2 photocatalysis, a novel technology for environmental organic pollutant disposal, was developed by combining microwave irradiation with UV/TiO2 photocatalysis. In essence, it is a new advanced oxidation process for the improvement of photocatalytic technology. In addition, it congregates all the advantages of MW irradiation and UV/TiO2 photocatalysis. As a result, the degradation efficiency of organic pollutants is greatly improved, and the concentration range is wider, especially for refractory organics, with fast and efficient treatment effects through the synergistic actions of MW, UV, and TiO2 [70,71].
2.4.1. Technical Features
Table 1 shows the current state of research on the partial use of MW/UV/TiO2 photocatalytic technology for the treatment of organic wastewater. In detail, TiO2 catalysts, microwave electrodeless lamps (MEDLs), and oxidants are added to the reactor vessel containing wastewater. Then, organic pollutants can be degraded by UV emitted from MEDLs under MW irradiation.
It can be clearly seen from Table 1 that the types of organic pollutants removed by MW/UV/TiO2 are phenol, benzene, dyes, pesticides, carboxylic acids, endocrine substances, etc. Among them, dyes and pesticides are the main ones. Overall, all the removal efficiencies of pollutants are very high. Most of the removal efficiencies exceed 80%, with efficiencies approaching 100% in more than half of the studies. The initial concentration of the treated organic pollutants is higher, with a maximum value of 400 mg/L, and the degradation process is very rapid. For example, when methylene blue dye was degraded under the optimized conditions using MW/UV/TiO2, its removal rate could be as high as 96% within 15 min [72]. Notably, herbicide could be completely degraded in 5 min [73].
The majority of the catalysts in the MW/UV/TiO2 are newer and more effective commercial powders of P25 [74,75]. The P25 powders are a mixture of anatase and rutile crystallites, and the reported ratio is typically 70:30 or 80:20 [76]. As the co-presence of anatase and rutile crystallites increases the density defects in the TiO2 lattice, it can enhance the charge separation and improve the utilization efficiency of electron–hole pairs [74,75]. Scientifically, nanoscale TiO2 has a greater ability to trap solution components (e.g., water, oxygen, and organic matter) on its surface than conventional TiO2 [77,78,79]. More importantly, nano-sized TiO2 has excellent UV absorption capability [80]. Therefore, nano-P25 has a better effect on the degradation of organic pollutants and has become the most widely used catalyst in the MW/UV/TiO2 photocatalytic system.
The UV lamp sources for the MW/UV/TiO2 are microwave-powered electrodeless discharge lamps (MEDLs), which have many advantages including the absence of electrodes, low price, low energy cost, and simple reactor configuration [81]. The luminescence mechanism of MEDLs is that the UV light is generated by gas discharge through the excited emission of electrons from metal electrodes. More specifically, the production approach has generally been to fill a mixture of vaporizable metals and rare gases into a sealed body made of quartz, glass, etc., and then UV will be produced by irradiating the mixture with MW. [82,83,84]. Microwave irradiation can heat the metals to form a vapor state and stimulate rare gases to produce a low-pressure plasma, eventually leading to a high luminescence efficiency [85,86,87]. It has been reported that the MEDLs can emit photons in the UV as well as in the visible regions (254, 313, 365, 405, 436, 546, and 577–579 nm) [81]. This degradation method using MEDLs is a promising technology for the treatment of recalcitrant organic compounds based on economy and efficiency [81].
Generically, most of the MW reactors used in studies operated at 2.45 GHz [18]. The MW power in the process was predominantly in the range of 200–900 W, the wavelength of the effective UV was around 254 nm, and the initial volume was 10–1000 mL. In addition, the temperature, air flow, light intensity, pH, and solution circulation velocity were critical technological conditions for MW/UV/TiO2 photocatalysis to remove the organic pollutants.

Table 1.
List of studies on the removal of aqueous organic contamination with MW/UV/TiO2 photocatalysis 1.
Table 1.
List of studies on the removal of aqueous organic contamination with MW/UV/TiO2 photocatalysis 1.
| Year | Contaminant and Its Type | Initial Concentration | t | MW Power | Other Experimental Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|---|---|
| 2022 | Oxytetracycline (antibiotics) | 50 mg/L | 60 min | 200–800 W | Volume: 1000 mL; initial pH = 2~10; temperature: 288 K; circulating fluid velocity: 500 mL/min; TiO2 photocatalyst ball; H2O2 = 1~100 mM; lamp source: electrodeless mercury lamp | Max removal rate ≈ 95% | [88] |
| 2022 | 2,4-dichlorophenoxyacetic acid (herbicides) | 0.05 mM | 60 min | 65 W | Volume: 200 mL; circulating fluid velocity: 350 mL/min; initial pH = 6.7; lamp source: 14 pieces MDLED-TiO2 (D = 7 mm, L = 26–30 mm) | Removal rate 85% | [89] |
| 2022 | Methylene orange (dyes) | 20 mg/L | 60 min | 600 W | Volume: 40 mL; temperature: 333 K; nano-mixed TiO2/CNT = 40 mg; lamp source: mercury UV lamp | Max removal rate 98% | [90] |
| 2021 | Lignin (mill industry effluents) | 100 mg/L | 60 min | / | Volume: 400 mL; temperature: 308 K; initial pH = 8.5; TiO2 coated AC = 1 g/L; lamp source: EDLs | Removal rate 100% | [91] |
| 2020 | Cimetidine (pharmacologically active compounds) | 20 mg/L | 180 min | 200~600 W | Temperature: 298 K; initial pH = 2~10; dissolved oxygen = 20~60 mg/L; circulating fluid velocity: 500 mL/min; 300 TiO2 photocatalytic balls; lamp source: microwave electrodeless lamps (MEDLs) | Removal rate 87% | [92] |
| 2019 | p-chlorophenol (phenols) | 10 mg/L | 60 min | 300~600 W | Volume: 400 mL; 1.4 wt% Ag/TiO2 catalyst = 50 mg; lamp source: a UV lamp with 300W high pressure | Removal rate of TOC = 51% | [93] |
| Methyl orange (dyes) | Removal rate of TOC = 69% | ||||||
| 2018 | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (neurotoxin compounds) | 1 mg/L | 60 min | / | Volume: 100 mL; temperature: 298 K; dosage of TiO2 quantum-dot-decorated WO3 nanosheets: 0.1g/L; lamp source: 300 W Xe lamp equipped with a UV mirror module (λ < 400 nm) | Removal rate ≈ 88% | [94] |
| Tetanus toxin (neurotoxin compounds) | 20 mg/L | Removal rate ≈ 83% | |||||
| 2017 | 4-chlorophenol (phenols) | 0.15 mM | / | 400 W | Volume: 500 mL; light intensity: 5.56 W/cm2; temperature: 298 K; aeration rate: 0.03 m3/h; circulating fluid velocity: 400 mL/min; TiO2 coated onto Al2O3 balls; lamp source: MEDLs | / | [95] |
| 2017 | Nitrobenzene (benzenes) | 0.2 mM | 100 min | 500 W | Volume: 500 mL; light intensity: 5.56 W/cm2; temperature: 298 K; aeration rate: 0.03 m3/h; circulating fluid velocity: 400 mL/min; initial pH = 7, TiO2 coated onto Al2O3 balls; lamp source: MEDLs | Removal rate 99% | [96] |
| 2016 | 4-Chloro-2-nitrophenol (phenols) | 30 mg/L | 100 min | 150 W | Volume: 500 mL; temperature: 298 K; initial pH = 6; TiO2 is anatase type material; TiO2 dosage: 0.2 g/L | Removal rate 80.5% | [97] |
| 2015 | Alizarin Green (dyes) | 0.08 mM | 60 min | / | UV light intensity: 1.31 MW/cm2 (main wavelength of 254 nm); temperature: 338 ± 2 K; aeration rate: 70 L/h; initial pH = 6.85; TiO2 is nano-scale P25; lamp source: MEDLs | Decolorization rate 82% | [98] |
| 2014 | Active Black 5 (dyes) | 400 mg/L | 300 min | / | pH = 3; TiO2 is P25; TiO2 dosage: 2 g/L; vacuum pressure: −75 kPa; temperature: 338 K; lamp source: MEDLs | Decolorization rate 100% | [99] |
| 2012 | Cyromazine (insecticides) | 100 mg/L | 20 min | 800 W | Volume: 50 mL; TiO2 dosage: 1.0 g/L; lamp source: MEDLs | Removal rate 99.6% | [100] |
| 2011 | Crystal violet (dyes) | 20 mg/L | 3 min | 800 W | Volume: 30 mL; WM frequency: 2.450 GHz; TiO2 is P25; TiO2 dosage: 1.5 g/L; lamp source: MEDLs | Removal rate 94.4% | [101] |
| 2010 | Indole (dyes) | 50 mg/L | 60 min | 700 W | Volume: 1000 mL; UV light intensity: 1450 lx (main wavelength of 254 nm); pH = 7; aeration process is added in the system; TiO2 is prepared by sol-gel method; TiO2 dosage: 0.2 g/L; lamp source: MEDLs | Removal rate 81.8% | [102] |
| 2006 | Phenol (phenols) | 10 mg/L | 30 min | 900 W | Volume: 50 mL; circulating fluid velocity: 15 mL/min; TiO2 is prepared by sol-gel method; TiO2 dosage: 1~4 g/L; lamp source: MEDLs | Removal rate 92% | [103] |
| 2008 | Monochloroacetic acid (carboxylic acids) | 0.1 mol/L | 490 min | 900–1000 W | Volume: 150 mL; light intensity: 5.56 W/cm2; temperature: 373 K; aeration rate: 0.03 m3/h; TiO2 coated onto MEDLs; lamp source: MEDLs | Removal rate 100% | [104] |
| 2008 | Acid Orange 7 (dyes) | 100 mg/L | 120 min | 700 W | Volume: 750 mL; temperature: 311 ± 1 K; aeration rate: 0.15 m3/h; TiO2 is P25; TiO2 dosage: 0.5 g/L; lamp source: U-shaped MEDLs | Decolorization rate 100% | [105] |
| 2007 | 2,4-Dichlorophenoxyacetic acid (herbicides) | 0.05 mM | 10 min | 200 W | Volume: 30 mL; TiO2 is Degussa P25; TiO2 dosage: 1.67 g/L; lamp source: MEDLs | Removal rate ≈90% | [84] |
| 2007 | Atrazine (herbicides) | 20 mg/L | 5 min | 900 W | Volume: 50 mL; initial pH = 8.1; TiO2 is nanotube; TiO2 dosage: 1 g/L; lamp source; MEDLs (dominant wavelength is 254 nm) | Removal rate 100% | [73] |
| 2007 | Reactive Brilliant X-3B (dyes) | 400 mg/L | 180 min | 700 W | Volume: 1000 mL; temperature: 311 ± 1 K; aeration rate: 0.25 m3/h; TiO2 is bonded to alumina; TiO2 dosage: 4 g/L; lamp source: MEDLs | Decolorization rate 100% | [106] |
| 2006 | Methylene blue (dyes) | 100 mg/L | 15 min | 900 W | Volume: 50 mL; initial pH = 7; TiO2 is prepared by sol-gel method; TiO2 dosage: 0.1–0.4 g/L; lamp source: MEDLs | Removal rate 96% | [72] |
| 2004 | Reactive Brilliant X-3B (dyes) | 400 mg/L | 150 min | 750 W | Initial pH = 7; TiO2 is a powdery P25; TiO2 is nanoscale; prepared by sol-gel method and loaded on the glass plate; lamp source: MEDLs | Decolorization rate 56.35% | [107] |
| 2005 | 4-chlorophenol (phenols) | 30 mg/L | 120 min | 750 W | Volume: 1000 mL; TiO2 is a powdery P25; TiO2 dosage: 1 g/L; lamp source: 8 UV lamps | Removal rate82.85% | [108] |
| 2004 | Bisphenol A (endocrines) | 0.1 mM | 90 min | 300 W | Volume: 30 mL; light intensity: 0.9 mW/cm2; temperature: 423 K; pressure: 1 MPa; initial pH = 6.7; TiO2 is Degussa P25; TiO2 dosage: 2 g/L; lamp source: 250 W Mercury UV lamp | Mineralization rate 100% | [109] |
| 2004 | 2,4-Dichlorophenoxyacetic acid (herbicides) | 0.04 mM | 20 min | 700 W | Volume: 10 mL; initial pH = 3; UV: 310–400 nm; 0.3–0.4 MW/cm2; light intensity: 2 mW/cm2; TiO2 is Degussa P25; TiO2 dosage: 5 g/L; lamp source: Mercury-neon vacuum lamps | Removal rate 100% | [110] |
1 the symbol “/” in Table 1 stands for missing information in the literature.
2.4.2. Technical Advantages
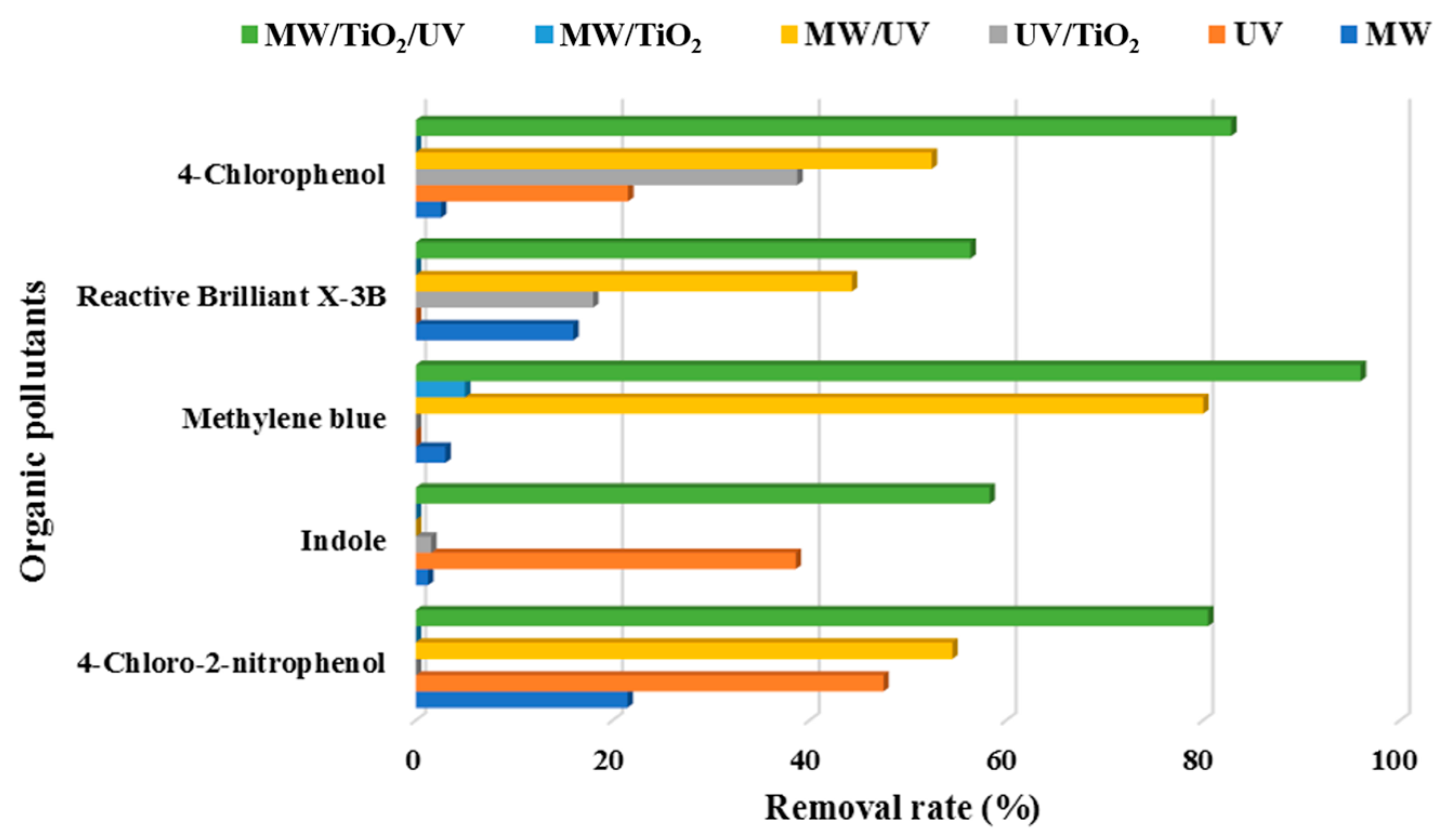
As shown in Table 2 and Figure 3, MW/UV/TiO2 provided the best photodegradation effect compared to other technologies (i.e., MW/UV, MW/TiO2, MW only, UV only, or TiO2 only). Essentially, MW was recommended to prevent the recombination between the generated positive holes and electrons on the surface of TiO2. Actually, MW could raise the pollutants to a higher electronic excited state. This would favor the formation of more OH· [19]. Even in the absence of the TiO2 catalyst, the effect of organic pollutant removal could therefore be achieved. However, the degradation efficiency of the pollutants was significantly reduced when UV light irradiation was not available, even with the presence of microwave and TiO2. The scientific foundation was that the catalytic system of MW/TiO2, MW, and TiO2 only could not effectively produce hydroxyl radicals if UV was absent [98,99]. The energy of MW only and the adsorption of TiO2 only were insufficient to break the chemical bonds of organic pollutants. Thus, it was clear that the microwave played an auxiliary role in the degradation of organic pollutants using the MW/UV/TiO2 technology. For example, the difference between the effects of MW/UV/TiO2 and heating/UV/TiO2 on the degradation of bisphenol A was insignificant, indicating that microwave radiation might play a role in heating [109]. It should also be noted that the addition of oxidizing agents (e.g., H2O2) would further improve the degradation efficiency of target organic pollutants in the MW/UV/TiO2 process [95].

Table 2.
Ranking of organic pollutants degradation efficiency under different conditions 1.
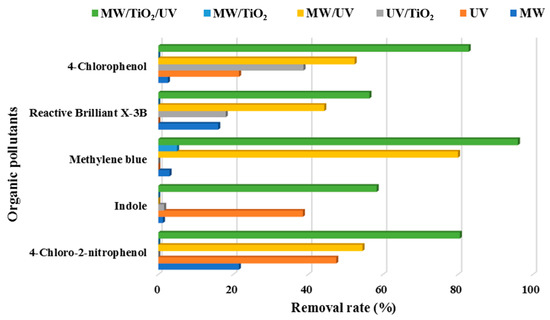
Figure 3.
Degradation of organic pollutants efficiency under different process conditions.
2.4.3. Photocatalytic Degradation Mechanism
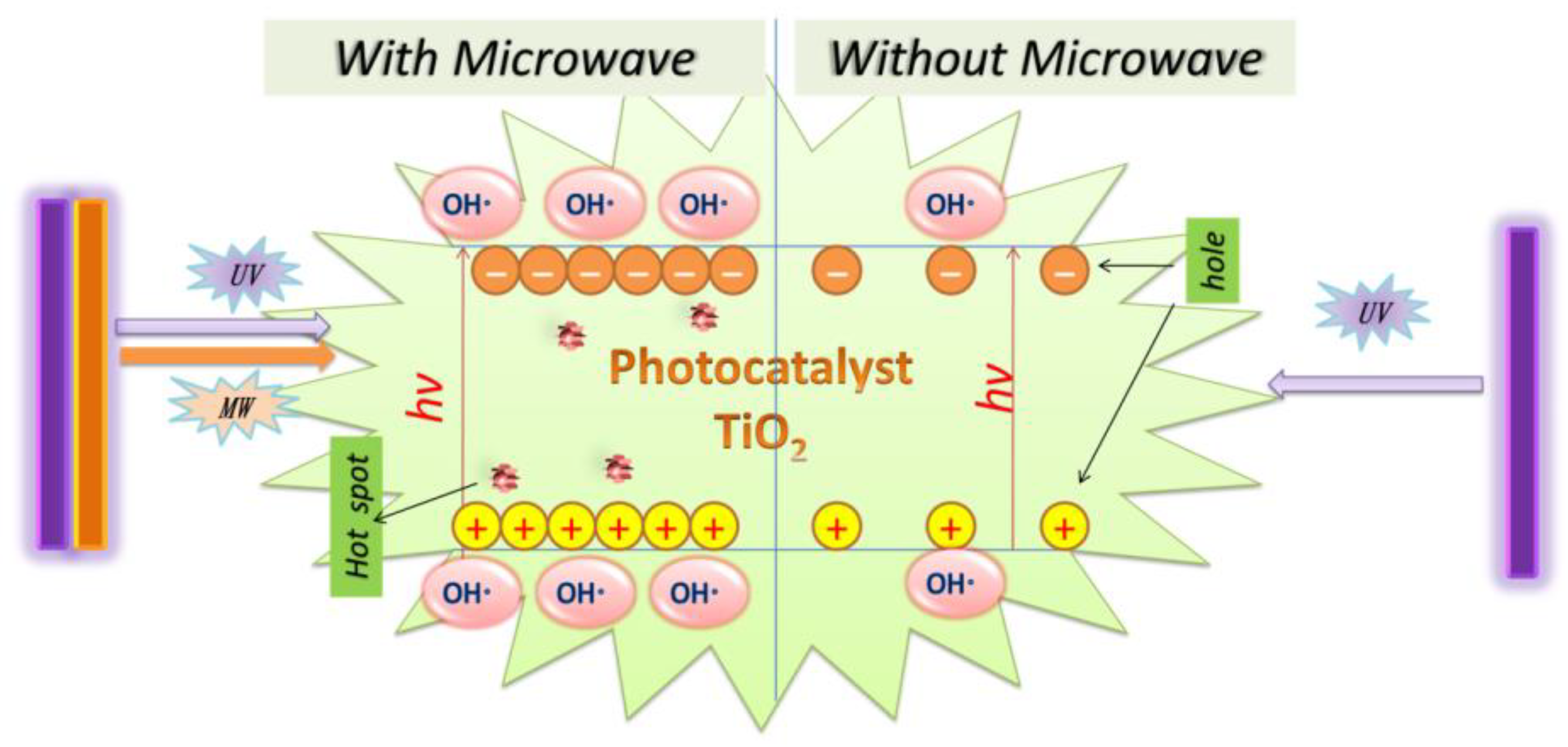
The MW/UV/TiO2 photocatalytic degradation technology is an advanced oxidation technology based on using hydroxyl radicals to degrade organic pollutants. The principle of OH· generation is the same as that of UV/TiO2. However, it is true that more OH· can be produced with MW radiation than without MW radiation, as shown in Figure 4. The OH·, with a redox potential of 2.8 V, is the second most reactive after the fluorine atom [112]. It is very highly oxidative and can attack organic contaminants non-selectively [113,114]. As a result, the photodegradation efficiency of MW/UV/TiO2 was better than that of UV/TiO2.
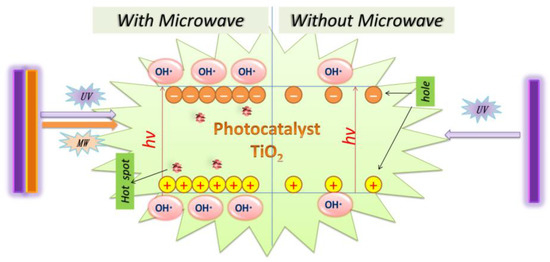
Figure 4.
The role of microwave in MW/UV/TiO2 photocatalytic technology.
Scientifically, the degradation process of organic pollutants in the MW/UV/TiO2 system is achieved via OH· generated by catalytic TiO2 absorption of UV with microwave assistance according to Equations (1)–(8), where the reaction formula (3) occurs in the alkaline condition, notably [11,60,61,73]. Environmental organic contaminants (EOCs) are eventually mineralized to CO2, H2O, and salts by OH· oxidation [73,105].
TiO2 + hv → e− + h+
h+ + H2O → OH· + H+
h+ + OH− → OH·
e− + O2 → O2−·
O2−· + H+ → OOH·
2OOH· → O2 + H2O2
H2O2 + O2−· → OH· + OH− + O2
EOCs + OH· → CO2 + H2O + salts
It can be explained that the microwave causes the TiO2 surface to produce the additional defect spot, increases the probability of h+ to e− transfer, and simultaneously reduces the reorganization of h+ to e−. Thus, the MW radiation can enhance the surface activity of the TiO2. The surface becomes more hydrophobic under the irradiation of both MW and UV, and it is beneficial to generate a large amount of OH− and O2, which can be easily converted to OH· by Equations (3)–(7) [115]. Horihoshi et al. demonstrated that the amount of OH· produced by microwave-assisted photocatalysis was 20% higher than that of conventional photocatalysis, and therefore MW increased the production rate of OH· [116]. Additionally, it was found that EOCs could be directly broken down by UV light under the MW/UV/TiO2 photocatalytic system [73].
2.4.4. Treatment Application of Wastewater Selection
It can safely be concluded from Table 1 that in the MW/UV/TiO2 system, TiO2 is the catalyst and UV is the basic requirement to excite the catalyst to produce high-energy hydroxyl radicals. Microwave radiation serves to enhance the degradation efficiency of the pollutants. Essentially, MW/UV/TiO2 technology belongs to an advanced oxidation process based on free-radical degradation reactions. Therefore, this technology has certain requirements for the selection of wastewater treatment. Technically, MW/UV/TiO2 is more suitable for refractory organic wastewater containing high concentrations of substances (e.g., phenol, benzene, dyes, pesticides, etc.). However, it is not capable of treating wastewater involving heavy metals due to the different removal mechanisms, as indicated in Table 1.
2.4.5. Potential Applications
Synthetically, the catalyst of TiO2 is green and economical, and the cost of the UV-producing MEDLs is low. Therefore, the MW/UV/TiO2 is an economically viable and promising technology for the treatment of refractory high-concentration organic wastewater. However, the current research was mostly carried out on a laboratory scale, as indicated in Table 1. The lack of integrated large-scale equipment is a basic constraint to its practical application on an industrial scale. There is thus an urgent need for manufacturers to develop specialized equipment tailored to the technical characteristics that meet the requirements for the wastewater treatment process.
2.5. Factors Affecting the Photocatalytic Efficiency of MW/UV/TiO2.
2.5.1. Pollutant Concentration
The initial concentration of pollutants is a parameter to be considered in the MW/UV/TiO2 photocatalytic treatment of organic wastewater. The target pollutants and their intermediates can be adsorbed on the surface of the TiO2 and occupy the position of reaction activity, thus affecting the removal effect [117]. Normally, the removal efficiency decreases as the concentration increases. Because the organic matter with high content can absorb UV, the UV intensity is reduced and the arrival of UV at the surface of the catalyst will be also delayed. Subsequently, this process limits the availability of OH· and UV photons, resulting in a decrease in the degradation efficiency of the organic compounds. Conversely, the proportion of organic matter adsorbed on the TiO2 surface is relatively higher at low initial concentrations, and the degradation efficiency is therefore better. This conclusion was further strengthened by the fact that the degradation rate was higher in a system with a lower initial concentration of organic pollutants, according to the studies conducted by Shokr [97], Xiong [98], and Wang [102]. Moreover, it was true that the MW/UV/TiO2 can effectively mitigate the negative effect of high concentrations [73,98,111].
2.5.2. TiO2 Dosage
It is clear that the TiO2 dosage significantly affects the treatment effect of the MW/UV/TiO2 [118]. When the TiO2 dosage is lower, the amount of the generated OH· is lower, and the photocatalytic oxidation ability to degrade pollutants is lower or even non-functional. Conversely, as the amount of TiO2 increases, the degradation efficiency will increase continuously. However, if the amount of TiO2 is more than a certain amount, the turbidity of the solution will increase, the transmittance of the system will decrease, the UV incidence will be obstructed, the photon yield will reduce, and the degradation efficiency will progressively decrease. For this reason, it is recommended to use a moderate amount of TiO2 [72,97,98,104].
It was worth noting that Crkva coated TiO2 powers to investigate the effect of the number of coating turns on the photocatalytic degradation efficiency, and found that multiple coatings did not significantly affect the degradation, suggesting that the active center of the reaction was at the surface of the TiO2 [104]. In addition, the other study showed that TiO2 nanotubes would enhance the catalytic activity and would adsorb more organic compounds due to their larger specific surface area, thereby promoting photodegradation efficiency [73].
2.5.3. UV Intensity
There is a consensus that the photocatalytic degradation effect of MW/UV/TiO2 improves significantly with increasing UV intensity. At higher UV intensities, more energy is available to the TiO2 catalyst, and more active centers are formed on the surface. The production rate of OH· will then be faster, and a higher photocatalytic efficiency can be achieved. In fact, there is a competitive relationship between the absorption of light energy by the catalyst and the water solvent. If the UV intensity is low, the pollutant degrading activity is insufficient, and the photocatalytic reaction is slow [72,103,104]. For example, Zhang pointed out that UV intensity can raise the temperature of the system, and then the high temperature can accelerate the degradation of target pollutants [106]. Moreover, Shokri was of the opinion that the effect of UV light on the degradation efficiency was related to the type of organic pollutants [97]. However, if the UV intensity is too high, the intermediates formed in the process will block the photon attacking the initial contaminant, ultimately reducing the photocatalytic efficiency [97].
2.5.4. Solution Volume
In MW/UV/TiO2, the initial volume of the solution influences the photocatalytic efficiency by indirectly absorbing MW and affecting the UV intensity. Since the H2O solvent is a polar substance, it can absorb MW and compete with MEDLs for microwave energy absorption [103]. This will cause the MW for excitation MEDLs luminescence to become less. Then, the effective UV intensity will be lower, resulting in a shorter effective irradiation time. Finally, it will be detrimental to the degradation of target organic contaminants [72].
2.5.5. MW Power and Temperature
Variations in MW power can affect the irradiance of MEDLs. Under normal circumstances, the UV intensity emitted by MEDLs will increase with the enhancement of MW radiation [98]. For example, in a study on microwave-assisted photocatalytic degradation of phenol, it was shown that the effective photocatalytic degradation deteriorated with decreasing microwave power from 900 W to 450 W. Furthermore, it was believed that the low MW power could reduce the luminescence time of MEDLs, and then the phenol exposure time would be shortened. However, it should be noted that the degradation rate per unit of MW power was higher at low MW power [103].
In the MW/UV/TiO2 photocatalytic system, the thermal effect also influences the catalytic reaction. Solvent water in the system absorbs microwave energy, and the temperature will rise. However, too high a temperature will affect the performance of the EDLs. Therefore, the influence of temperature generally has a tipping point. In the initial stage, the photocatalytic efficiency increases with the temperature increase. When the temperature turning point is reached, the efficiency starts to gradually decrease. For example, Xiong et al. found that the temperature turning point for degrading alizarin green was 65 °C [98]. In addition, the optimum temperature for the degradation of reactive brilliant red was 40–45 °C with the addition of iron filings [119].
2.5.6. pH
The pH is one of the most critical factors that affect the photocatalytic efficiency of MW/UV/TiO2. It is clear from Table 1 that all previous studies analyzed the influence of pH on the degradation of organic pollutants. It is interesting to note the different action mechanisms in both acidic and alkaline conditions and the optimum pH for removing organic substances varies significantly.
On the one hand, the pH can alter the surface properties of TiO2. The TiO2 is an amphoteric oxide with a zero charge (pHzpc) in 5.8–6.4. When the solution is in an acidic state, the pH value is less than pHzpc. To generate the electron (e−), it is advantageous that the TiO2 surface is positively charged TiO2+. The e− combines with adsorbed O2 to form H2O2 and OH·. This process inhibits the recombination of electrons and holes and thus improves the reaction rate [60,73,108,119,120,121,122]. When the system’s pH is greater than pHzpc, the surface is negatively charged with TiO2−. This facilitates the migration of h+ holes to the surface, and the h+ reacts with the H2O and OH− adsorbed on the surface to form OH· [73,108,119,120,121]. Therefore, MW/UV/TiO2 can effectively degrade the target organic pollutants under both acidic and alkaline conditions, reflecting its superiority [98,108,111]. For example, an experiment for removing alizarin green showed that the difference in degradation efficiency was small under different pH conditions [98]. Additionally, the degradation efficiency of cyromazine was excellent in acidic to alkaline conditions [100]. It was also explained why the degradation rate of 4-chlorophenol, which was used by MW/UV/TiO2, seemed to be slightly increased at both pH = 3 or 9 [108]. Theoretically, under alkaline conditions, the OH· is produced according to Equations (1)–(3).
On the other hand, the pH can also affect photocatalytic efficiency by changing the properties of the target organic pollutants. The organic compounds exhibit electronegativity under acidic conditions and will undergo electrostatic adsorption with positively charged catalysts (TiO2+). Because the adsorption of contaminants on the catalyst’s surface is a prerequisite for effective photocatalytic degradation, the degradation efficiency under acidic conditions was higher than that under alkaline conditions. For example, while the reactive black was under acidic conditions, they would be negatively charged sulfonic groups (R-SO3−) [99]. Ki’s research showed that the photodegradation efficiency of 4-chlorophenol did not increase significantly when the pH was reduced from four to two by adding hydrochloric acid.
In addition, the degradation rates of nitrobenzene at pH = 2, 4, and 7 were not shown to differ significantly [96]. Although it was easy to produce OH· under strong acid conditions, nitrobenzene was a less or non-polar compound that would be better adsorbed on an uncharged catalyst TiO2 surface near the ZPC [123,124]. As the effects of the two opposite aspects canceled each other out, the decomposition reaction rate was not drastically changed [95,123].
2.5.7. Adding an Oxidant
The oxidant H2O2 is added to the MW/UV/TiO2 photocatalytic system and can be excited by UV light to generate OH· according to Equations (3)–(9). Moreover, it can also directly react with O2−· to generate OH· via Equation (10). Consequently, this process will produce more OH· and accelerate the degradation of organic matter [95,104,107,125]. However, the excessive addition of H2O2 can reduce the surface activity of TiO2 and eliminate the OH·, which is not conducive to photocatalysis [95,126,127]. Similarly, the appropriate addition of oxygen via aeration contributes to the degradation of contaminants but excessive aeration is detrimental [95,104]. If S2O82− and Fe3+ are added to the MW/UV/TiO2 system, the degradation efficiency of reactive bed X-3B can be improved. The S2O82− can generate SO4−· (Eθ = 2.6 eV), which is another strong oxidant, according to Equation (11). Fe3+ can also cause OH· according to Equations (12) and (13) [97].
H2O2 + e− → OH· + OH−
H2O2 + O2−· → OH· + H2O
S2O82− + e− → SO4−· + SO42−
Fe3+ + e− → Fe2+
Fe(OH)2+ + hν → OH· + Fe2+
Furthermore, the effect of additional O3 is also investigated in the microwave-assisted photocatalytic system. The degradation rate of 2,4-D increases with increasing ozone dosage. O3 molecules can enhance OH· radical formation according to the following Equation (14) [126].
O3 + H2O → 2OH· + O2
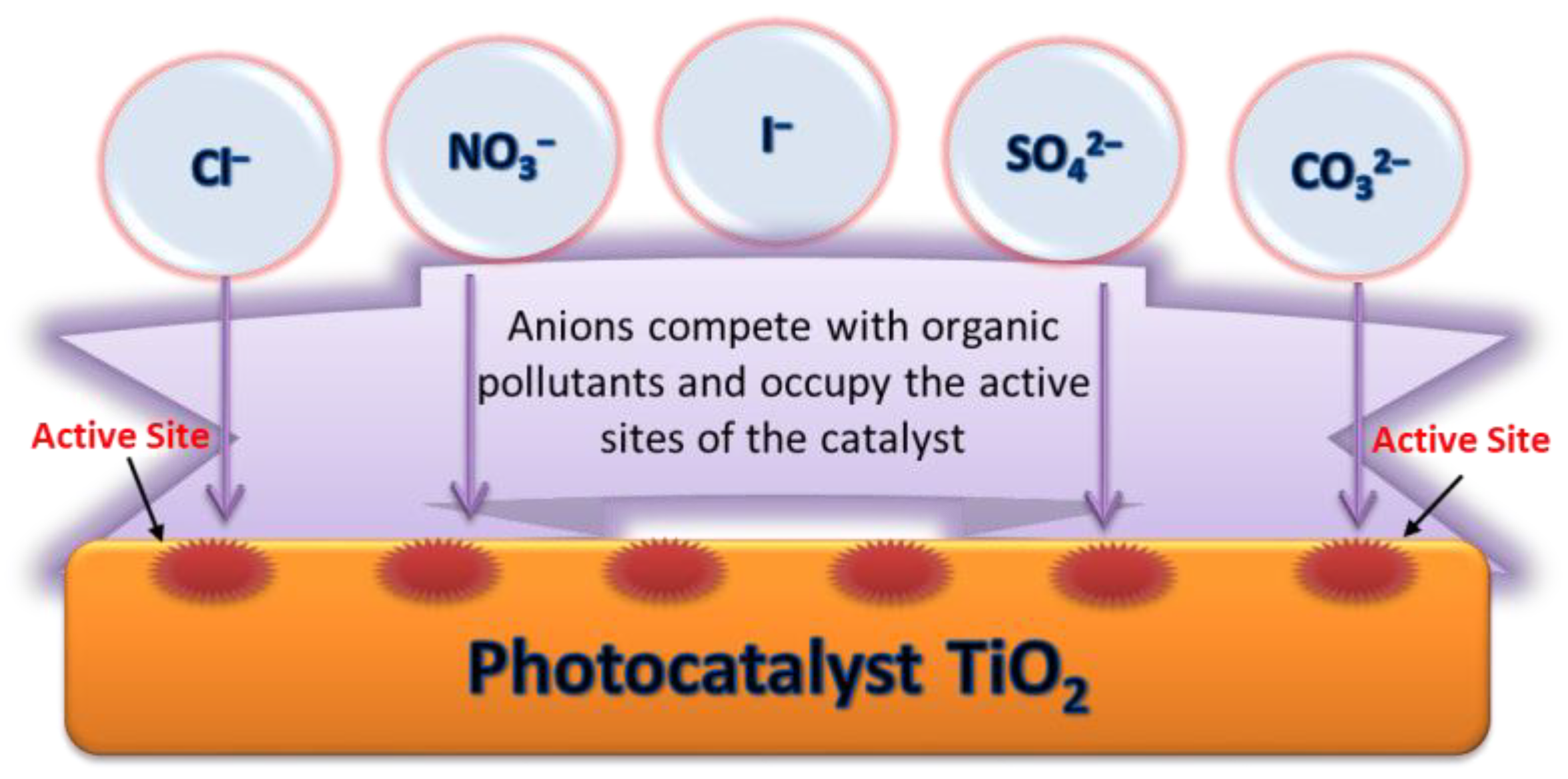
2.5.8. Anions
The degradation efficiency of the target organic pollutants will be reduced in the presence of anions (such as Cl−, CO32−, SO42−, NO3−, I−, etc.) in the MW/UV/TiO2 system. On the one hand, as shown in Figure 5, these anions have competitive adsorption with organic pollutants. They can be adsorbed on the surface of the catalysts to occupy reactive sites, resulting in a decrease in the activity of the catalyst. On the other hand, these anions can react with OH· and consume OH· according to Equations (15)–(17) [104,106]. For example, CO32− can consume OH· and change the pH of the reaction system, and records the most significant negative effect [128]. The NO3− can generate OH· according to Equation (18), which is beneficial to the occurrence of photocatalysis. The promotion will be counteracted by the side effects caused by the anion adsorption. Therefore, NO3− has little impact on the degradation rate [106]. Iodide ion is an excellent scavenger that can react with valence band holes and OH· [129]. When I- was used as a diagnostic tool to suppress the hole and OH· process, the photocatalytic degradation of the system was primarily inhibited [105].
Cl− + OH· → Cl· + OH−
CO32− + OH·→ CO32−· + OH−
SO42− + OH· → SO4−· + OH−
NO3− + hν + H2O → OH· + OH− + NO2·
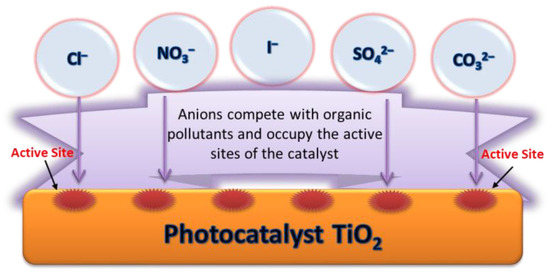
Figure 5.
The competitive adsorption of anions and organic pollutants.
2.5.9. Circulating Fluid Velocity
The fast circulating fluid velocity can improve the degradation rate up to certain threshold levels. For example, the degradation efficiency of 4-chlorophenol increased significantly when the flow rate was increased from 200 mL/min to 400 mL/min. However, the growth effect was not evident thereafter and even decreased when it was increased to 600 mL/min [95]. Moreover, Hong et al. also considered that the residence time of the solution in the reactor would be shortened at high flow rates. Explicably, the effective time of the photocatalytic reaction was shortened, which reduced the degradation efficiency [103].
3. Conclusions
- (1)
- The MW/UV/TiO2 photocatalytic technology is an efficient and new oxidation treatment technology with excellent application prospects in organic wastewater treatment. It has the advantages of a high removal rate, a short time, a wide concentration range, low cost, and higher stability;
- (2)
- The MW/UV/TiO2 photocatalytic technology can be used to treat a wide range of organic pollutants, including phenols, benzene, dyes, pesticides, carboxylic acids, endocrine substances, and so on. The processes have optimized the variables such as the wavelength of ultraviolet light (254 nm) and microwave power (700–900 W), and the catalyst TiO2 is nanomaterial P25. The photodegradation effect of the MW/UV/TiO2 process was preferable to that of MW/UV, MW/TiO2, UV, TiO2, and MW;
- (3)
- The strong oxidation OH· was rapidly produced on the surface of catalyst TiO2 under microwave and ultraviolet irradiation. The number and speed of OH· played decisive roles in the degradation efficiency of organic pollutants;
- (4)
- The main factors influencing the photocatalytic efficiency of MW/UV/TiO2 are initial pollutant concentration, initial solution volume, TiO2 dosage, UV intensity, microwave power, temperature, pH, oxidants, anions, and solution circulation fluid velocity. Overall, the high initial pollutant concentration, the large initial solution volume, and the addition of anions are unfavorable to the photocatalytic reaction. However, the strong UV and MV irradiation can promote photocatalytic efficiency. There was a turning point in the effect of TiO2 dosage, temperature, and solution circulation speed on the photocatalytic efficiency;
- (5)
- The addition of oxidants generally improved the degradation of the contaminants. The pH of the system is a critical factor due to the completely different reaction mechanisms under acidic and alkaline conditions.
4. Proposed Future Research Directions
- (1)
- The MW/UV/TiO2 photocatalysis technology was implemented on a laboratory scale with a single pollutant source presently. It is proposed to apply this technology to complex natural water bodies such as surface and groundwater, industrial effluents, etc. In addition, this technology should address emerging environmental pollutants and broaden the scope of applications
- (2)
- Currently, most of the MW-emission equipment used in the experiment is household microwave ovens, where the operation is unstable and the microwave transmitter is unreasonable. Therefore, efforts should be done to improve the professional MW transmitting device;
- (3)
- Modification of titanium dioxide catalyst can be opted for alteration in its structure to increase its active specific surface area. Metals with the catalytic activity carried on the surface of TiO2 (e.g., metal-doped TiO2) can also be further explored/optimized;
- (4)
- Current research rarely involves the study of the energy consumption of MW/UV/TiO2 photocatalytic systems. However, most of the microwave energy is absorbed by polaristic water and converted into thermal energy. The effective part utilized to excite MEDLs and TiO2 is less. Therefore, this shortcoming seriously restricts the technical application and development of high-efficiency wastewater treatment. Urgent efforts are needed to overcome this fundamental challenge in order to effectively utilize microwave energy on an industrial scale.
Author Contributions
H.X.: Conceptualization, Data curation, Writing—original draft. Formal analysis. M.A.A.: Conceptualization, Methodology, Software, Review an Editing, Formal analysis. Y.Y.: Supervision, Review and Editing. J.G.: Funding acquisition, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project Tackling of Key Scientific and Technical Problems of Henan Province, China (222102320200), and the Geological Survey Program of the CGS, China (DD20221782).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mian, M.M.; Alam, N.; Ahommed, M.S.; He, Z.; Ni, Y. Emerging applications of sludge biochar-based catalysts for environmental remediation and energy storage: A review. J. Clean. Prod. 2022, 360, 132131. [Google Scholar] [CrossRef]
- Taylor, K.W.; Novak, R.F.; Anderson, H.A.; Birnbaum, L.S.; Blystone, C.; Devito, M.; Jacobs, D.; Kohrle, J.; Lee, D.H.; Rylander, L.; et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: A national toxicology program workshop review. Environ. Health Perspect. 2013, 121, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Watson, A.; Forter, M.; Oliaei, F. Review Article: Persistent organic pollutants and landfills—A review of past experiences and future challenges. Waste Manag. Res. 2011, 29, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Higley, E.; Grund, S.; Jones, P.D.; Schulze, T.; Seiler, T.B.; Lubcke-von Varel, U.; Brack, W.; Wolz, J.; Zielke, H.; Giesy, J.P.; et al. Endocrine disrupting, mutagenic, and teratogenic effects of upper Danube River sediments using effect-directed analysis. Environ. Toxicol. Chem. 2012, 31, 1053–1062. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.; Li, X. Organic contamination and remediation in the agricultural soils of China: A critical review. Sci. Total Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Ferro-Garcia, M.A.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Xia, H.; Guo, J.; Yang, Y.; Wang, Y.; Wang, Z.; Wang, X.; Zhang, W. Remediation of PNP-contaminated groundwater using a modified CaO2/Fe(II) Fenton system: Reactive principles, degradation performance and potential pathways. J. Environ. Chem. Eng. 2022, 10, 107305. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Bustamante, M.; Byun, H.M.; Fernandez, M.F.; Santa Marina, L.; Basterrechea, M.; Ballester, F.; Murcia, M.; Tardon, A.; Fernandez-Somoano, A.; et al. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ. Int. 2014, 71, 81–87. [Google Scholar] [CrossRef]
- Yue, W.; Yager, J.D.; Wang, J.P.; Jupe, E.R.; Santen, R.J. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids 2013, 78, 161–170. [Google Scholar] [CrossRef]
- Savage, K.I.; Matchett, K.B.; Barros, E.M.; Cooper, K.M.; Irwin, G.W.; Gorski, J.J.; Orr, K.S.; Vohhodina, J.; Kavanagh, J.N.; Madden, A.F.; et al. BRCA1 deficiency exacerbates estrogen-induced DNA damage and genomic instability. Cancer Res. 2014, 74, 2773–2784. [Google Scholar] [CrossRef]
- Tian, L.; Lv, G.; Liu, M.; Lei, X.; Rao, W.; Liao, L. Reviews: Microwave-induced oxidation technology and its applications. Prog. Nat. Sci. Mater. 2022, 32, 665–673. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of pesticides using TiO2 based photocatalytic strategies: A review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sust. Energ. Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Remya, N.; Lin, J.-G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Kovacs, P.V.; Lemmer, B.; Keszthelyi-Szabo, G.; Hodur, C.; Beszedes, S. Application of dielectric constant measurement in microwave sludge disintegration and wastewater purification processes. Water Sci. Technol. 2018, 77, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, P. Study and application status of microwave in organic wastewater treatment—A review. Chem. Eng. J. 2016, 283, 193–214. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q.; Ma, C.; Zhang, J.; Zhao, X.; Song, Z. Review on microwave–metal discharges and their applications in energy and industrial processes. Appl. Energy 2016, 175, 141–157. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Martínez, E.J.; Gil, M.V.; Rosas, J.G.; Moreno, R.; Mateos, R.; Morán, A.; Gómez, X. Application of thermal analysis for evaluating the digestion of microwave pre-treated sewage sludge. J. Therm. Anal. Calorim. 2016, 127, 1209–1219. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors—A review. J. Power Sources 2014, 263, 338–360. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sust. Energ. Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Lee, D.-J.; Park, Y.-K.; Kim, S.-J.; Lee, H.; Jung, S.-C. Photo-catalytic destruction of ethylene using microwave discharge electrodeless lamp. Korean J. Chem. Eng. 2015, 32, 1188–1193. [Google Scholar] [CrossRef]
- Yeh, C.J.; Lo, S.L.; Kuo, J.; Chou, Y.C. Optimization of landfill leachate treatment by microwave oxidation using the Taguchi method. Int. J. Environ. Sci. Technol. 2017, 15, 2075–2086. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Adebayo, M.A.; Sampaio, C.H.; Lima, E.C.; Thue, P.S.; de Brum, I.A.S.; Dias, S.L.P.; Pavan, F.A. Removal of Phenolic Compounds from Aqueous Solutions Using Sludge-Based Activated Carbons Prepared by Conventional Heating and Microwave-Assisted Pyrolysis. Water Air Soil Pollut. 2016, 228, 33. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Domínguez, A.; Inguanzo, M.; Pis, J.J. Microwave-induced drying, pyrolysis and gasification (MWDPG) of sewage sludge: Vitrification of the solid residue. J. Anal. Appl. Pyrolysis 2005, 74, 406–412. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.L.; Zhang, Y.B.; Quan, X.; Zhao, B. Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst. J. Hazard. Mater. 2008, 153, 1201–1206. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, F.; Zhang, Y.; Song, Z. Degradation of Methyl Orange in Aqueous Solution by Microwave Irradiation in the Presence of Granular-Activated Carbon. Water Air Soil Pollut. 2014, 225, 1983. [Google Scholar] [CrossRef]
- Yuan, D.; Fu, D.Y.; Luo, Z.W. Study on Microwave Combined with Granular Active Carbon for Treatment of P-Nitrophenol Wastewater. Adv. Mater. Res. 2012, 602–604, 2287–2290. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, Y.; Wang, J.; Ling, H.; Zang, S.; Gao, W.; Zhao, Z.; Zhang, H. Investigation on the rapid degradation of congo red catalyzed by activated carbon powder under microwave irradiation. J. Hazard. Mater. 2007, 147, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Lee, L.K.; Hameed, B.H. Preparation of activated carbon from sugarcane bagasse by microwave assisted activation for the remediation of semi-aerobic landfill leachate. Bioresour. Technol. 2013, 134, 166–172. [Google Scholar] [CrossRef]
- Verhoeven, G.J.; Schmitt, K.D. An attempt to push back frontiers—Digital near-ultraviolet aerial archaeology. J. Archaeol. Sci. 2010, 37, 833–845. [Google Scholar] [CrossRef]
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019. [Google Scholar] [CrossRef]
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Development of a method for the characterization and operation of UV-LED for water treatment. Water Res. 2017, 122, 570–579. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Lian, J.; Qu, J. Degradation of nitro-based pharmaceuticals by UV photolysis: Kinetics and simultaneous reduction on halonitromethanes formation potential. Water Res. 2017, 119, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Masaki, S.; Kodamatani, H.; Ikehata, K. Degradation of N-Nitrosodimethylamine by UV-Based Advanced Oxidation Processes for Potable Reuse: A Short Review. Curr. Pollut. Rep. 2017, 3, 79–87. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Hu, J.; Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017, 121, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xiao, Y.; Chang, V.W.C.; Lim, T.-T. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate. Chem. Eng. J. 2016, 302, 526–534. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.-M.; Lee, J.; Kang, J.-W. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Yuan, S.; Wang, W.; Hu, Z.-H. Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution. J. Photoch. Photobio. A 2017, 348, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, J.; Chang, V.W.C.; Lim, T.-T. Degradation of cyclophosphamide and 5-fluorouracil in water using UV and UV/H2O2: Kinetics investigation, pathways and energetic analysis. J. Environ. Chem. Eng. 2017, 5, 1133–1139. [Google Scholar] [CrossRef]
- Huang, N.; Wang, T.; Wang, W.L.; Wu, Q.Y.; Li, A.; Hu, H.Y. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation. Water Res. 2017, 114, 246–253. [Google Scholar] [CrossRef]
- Qin, L.; Lin, Y.L.; Xu, B.; Hu, C.Y.; Tian, F.X.; Zhang, T.Y.; Zhu, W.Q.; Huang, H.; Gao, N.Y. Kinetic models and pathways of ronidazole degradation by chlorination, UV irradiation and UV/chlorine processes. Water Res. 2014, 65, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Yoon, Y.; Choi, D.J.; Zoh, K.D. Degradation characteristics of metoprolol during UV/chlorination reaction and a factorial design optimization. J. Hazard. Mater. 2015, 285, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Wu, Q.-Y.; Huang, N.; Wang, T.; Hu, H.-Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]
- Xiang, Y.; Fang, J.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Silver nanoparticles and defect-induced visible light photocatalytic and photoelectrochemical performance of Ag@m-TiO2 nanocomposite. Sol. Energy Mater. Sol. Cells 2015, 141, 162–170. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Tong, A.; Braund, R.; Warren, D.; Peake, B. TiO2-assisted photodegradation of pharmaceuticals—A review. Open Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sangal, V.K.; Bajpai, P.K. Photocatalytic Treatment of Binary Mixture of Dyes using UV/TiO2 Process: Calibration, Modeling, Optimization and Mineralization Study. Int. J. Chem. React. Eng. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Yousefi, M.; Ghanbari, F.; Zazouli, M.A.; Ahmadi, M.M.; Akbari, S. Investigation of the Efficiency of Electro-Fenton and UV/TiO2 Processes for Para-Chlorophenol Removal from Aqueous Solutions. J. Health 2016, 7, 600–610. [Google Scholar]
- Verma, P.; Samanta, S.K. Degradation kinetics of pollutants present in a simulated wastewater matrix using UV/TiO2 photocatalysis and its microbiological toxicity assessment. Res. Chem. Intermed. 2017, 43, 6317–6341. [Google Scholar] [CrossRef]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Chen, H.; Bramanti, E.; Longo, I.; Onor, M.; Ferrari, C. Oxidative decomposition of atrazine in water in the presence of hydrogen peroxide using an innovative microwave photochemical reactor. J. Hazard. Mater. 2011, 186, 1808–1815. [Google Scholar] [CrossRef]
- Církva, V.; Relich, S. Microwave Photochemistry and Photocatalysis. Part 1: Principles and Overview. Curr. Org. Chem. 2011, 15, 248–264. [Google Scholar] [CrossRef]
- Hong, J.; Sun, C.; Yang, S.G.; Liu, Y.Z. Photocatalytic degradation of methylene blue in TiO2 aqueous suspensions using microwave powered electrodeless discharge lamps. J. Hazard. Mater. 2006, 133, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yang, S.; Ta, N.; Sun, C. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J. Hazard. Mater. 2007, 145, 424–430. [Google Scholar] [CrossRef]
- Bessergenev, V.G.; Mateus, M.C.; do Rego, A.M.B.; Hantusch, M.; Burkel, E. An improvement of photocatalytic activity of TiO2 Degussa P25 powder. Appl. Cataly. A-Gen. 2015, 500, 40–50. [Google Scholar] [CrossRef]
- Wang, A.N.; Teng, Y.; Hu, X.F.; Wu, L.H.; Huang, Y.J.; Luo, Y.M.; Christie, P. Diphenylarsinic acid contaminated soil remediation by titanium dioxide (P25) photocatalysis: Degradation pathway, optimization of operating parameters and effects of soil properties. Sci. Total Environ. 2016, 541, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photoch. Photobio. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Haynes, V.N.; Ward, J.E.; Russell, B.J.; Agrios, A.G. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms-Current knowledge and suggestions for future research. Aquat. Toxicol. 2017, 185, 138–148. [Google Scholar] [CrossRef]
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of Organic Acids on TiO2 Nanoparticles: Effects of pH, Nanoparticle Size, and Nanoparticle Aggregation. Langmuir 2008, 24, 6659–6667. [Google Scholar] [CrossRef]
- Xiu, H.; Qi, X.; Bai, H.; Zhang, Q.; Fu, Q. Simultaneously improving toughness and UV-resistance of polylactide/titanium dioxide anocomposites by adding poly(ether)urethane. Polym. Degrad. Stab. 2017, 143, 136–144. [Google Scholar] [CrossRef]
- Hong, J.; Han, B.; Yuan, N.; Gu, J. The roles of active species in photo-decomposition of organic compounds by microwave powered electrodeless discharge lamps. J. Environ. Sci. 2015, 33, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zukawa, T.; Sasaki, Y.; Nagao, N. Ultraviolet Light Emitting Device. U.S. Patent 9691600 B2, 27 June 2017. [Google Scholar]
- Volkova, G.A.; Kirillova, N.N.; Pavlovskaya, E.N.; Yakovleva, A.V. Vacuum-ultraviolet lamps with a barrier discharge in inert gases. J. Appl. Spectrosc. 1984, 41, 1194–1197. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kajitani, M.; Sato, S.; Serpone, N. A novel environmental risk-free microwave discharge electrodeless lamp (MDEL) in advanced oxidation processes. J. Photoch. Photobio. A 2007, 189, 355–363. [Google Scholar] [CrossRef]
- Kuwano, K.; Nezu, A.; Matsuura, H.; Akatsuka, H. Dissociation degree of nitrogen molecule in low-pressure microwave-discharge nitrogen plasma with various rare-gas admixtures. Jpn. J. Appl. Phys. 2016, 55, 086101. [Google Scholar] [CrossRef]
- Ho, G.S.; Faizal, H.M.; Ani, F.N. Microwave induced plasma for solid fuels and waste processing: A review on affecting factors and performance criteria. Waste Manag. 2017, 69, 423–430. [Google Scholar] [CrossRef]
- Literák, J.ı.; Klán, P. The electrodeless discharge lamp: A prospective tool for photochemistry Part 2. Scope and limitation. J. Photoch. Photobio. A 2000, 137, 29–35. [Google Scholar] [CrossRef]
- Park, J.; Park, Y.-K.; Jung, S.-C. Destruction of oxytetracycline using a microwave-assisted fused TiO2 photocatalytic oxidation system. Korean J. Chem. Eng. 2022, 39, 3369–3376. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kimura, M.; Serpone, N. Development of a microwave-discharge light-emitting diode (MDLED): A novel UV source for the UV-driven microwave-assisted TiO2 photocatalytic treatment of contaminated wastewaters. Photochem. Photobiol. Sci. 2022, 21, 659–665. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Y.; Li, Q.; Li, H.; Bian, Z. Microwave-Assisted Photocatalytic Degradation of Organic Pollutants via CNTs/TiO2. Catalysts 2022, 12, 940. [Google Scholar] [CrossRef]
- Vedichi, M.; Mani, S. Enhanced microwave and photocatalytic decomposition of synthetic lignin wastewater by TiO2 nanoparticles coated on activated carbon. Mater. Today 2021, 47, 800–806. [Google Scholar] [CrossRef]
- Park, Y.K.; Ha, H.H.; Yu, Y.H.; Kim, B.J.; Bang, H.J.; Lee, H.; Jung, S.C. The photocatalytic destruction of cimetidine using microwave-assisted TiO2 photocatalysts hybrid system. J. Hazard. Mater. 2020, 391, 122568. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, Y.; Li, H.; Chen, Y.; Wen, J.; Zhu, J.; Bian, Z. Microwave induced surface enhanced pollutant adsorption and photocatalytic degradation on Ag/TiO2. Appl. Surf. Sci. 2019, 483, 772–778. [Google Scholar] [CrossRef]
- Fakhri, A.; Azad, M.; Fatolahi, L.; Tahami, S. Microwave-assisted photocatalysis of neurotoxin compounds using metal oxides quantum dots/nanosheets composites: Photocorrosion inhibition, reusability and antibacterial activity studies. J. Photochem. Photobiol. B 2018, 178, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.J.; Jeon, K.-J.; Park, Y.-K.; Jeong, S.; Lee, H.; Jung, S.-C. Improving removal of 4-chlorophenol using a TiO2 photocatalytic system with microwave and ultraviolet radiation. Catal. Today 2017, 293–294, 15–22. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, H.; Park, H.; Jeon, K.-J.; Park, Y.-K.; Jung, S.-C. Rapid photocatalytic degradation of nitrobenzene under the simultaneous illumination of UV and microwave radiation fields with a TiO2 ball catalyst. Catal. Today 2017, 307, 65–72. [Google Scholar] [CrossRef]
- Shokri, A.; Joshagani, A.H. Using microwave along with TiO2 for degradation of 4-chloro-2-nitrophenol in aqueous environment. Russ. J. Appl. Chem. 2016, 89, 1985–1990. [Google Scholar] [CrossRef]
- Xiong, Z.; Xu, A.; Li, H.; Ruan, X.; Xia, D.; Zeng, Q. Highly Efficient Photodegradation of Alizarin Green in TiO2 Suspensions Using a Microwave Powered Electrodeless Discharged Lamp. Ind. Eng. Chem. Res. 2012, 52, 362–369. [Google Scholar] [CrossRef]
- Qu, D.; Qiang, Z.; Xiao, S.; Liu, Q.; Lei, Y.; Zhou, T. Degradation of Reactive Black 5 in a submerged photocatalytic membrane distillation reactor with microwave electrodeless lamps as light source. Sep. Purif. Technol. 2014, 122, 54–59. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, R.; Feng, Y. Microwave assisted photocatalytic degradation of cyromazine in aqueous solutions. China Environ. Sci. 2012, 32, 603–608. [Google Scholar]
- Ju, Y.; Fang, J.; Liu, X.; Xu, Z.; Ren, X.; Sun, C.; Yang, S.; Ren, Q.; Ding, Y.; Yu, K.; et al. Photodegradation of crystal violet in TiO2 suspensions using UV-vis irradiation from two microwave-powered electrodeless discharge lamps (EDL(-2)): Products, mechanism and feasibility. J. Hazard. Mater. 2011, 185, 1489–1498. [Google Scholar] [CrossRef]
- Wang, J.; Hou, M.; Yu, W. Degradation of Indole by Microwave Assisted UV-catalyzed Oxidation. Ind. Water Wasterwater 2010, 41, 43–45. [Google Scholar]
- Hong, J.; Liu, Y.; Yang, S.; Sun, C. Microwave Assisted Photocatalytic Degradation of Phenol in Aqueous Solution. Environ. Sci. 2006, 27, 1808–1813. [Google Scholar]
- Církva, V.; Žabová, H.; Hájek, M. Microwave photocatalysis of mono-chloroacetic acid over nanoporous titanium(IV) oxide thin films using mercury electrodeless discharge lamps. J. Photoch. Photobio. A 2008, 198, 13–17. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.D.; Li, G.; Wang, Y. Investigation of the roles of active oxygen species in photodegradation of azo dye AO7 in TiO2 photocatalysis illuminated by microwave electrodeless lamp. J. Photoch. Photobio. A 2008, 199, 311–315. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wang, Y. Microwave assisted photocatalytic degradation of high concentration azo dye Reactive Brilliant Red X-3B with microwave electrodeless lamp as light source. Dyes Pigments 2007, 74, 536–544. [Google Scholar] [CrossRef]
- Yang, P.; Ai, Z.; Lu, X. Effect of Microwave Assisted UV Electrodeless Discharge Lamp Photocatalysis on Decolorization of Active Red X-3B. In Proceedings of the 2012 Second International Conference on Electric Technology and Civil Engineering (ICETCE ’12), Washington, DC, USA, 18–20 May 2012; pp. 1059–1061. [Google Scholar]
- Ai, Z.; Yang, P.; Lu, X. Degradation of 4-chlorophenol by a microwave assisted photocatalysis method. J. Hazard. Mater. 2005, 124, 147–152. [Google Scholar] [CrossRef]
- Horikoshi, S.; Tokunaga, A.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination method. J. Photochem. Photobiol. A Chem. 2004, 162, 33–40. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination technique. J. Photochem. Photobiol. A Chem. 2004, 161, 221–225. [Google Scholar] [CrossRef]
- tuantuan, Z.; Sida, W.; Libing, Z.; Dan, Q. Microwave assisted photocatalysis degradation of high concentration Active Black. Chin. J. Chem. Eng. 2013, 7, 2861–2866. [Google Scholar]
- Masten, S.J.; Davies, S.H. The use of ozonation to degrade organic contaminants in wastewaters. Environ. Sci. Technol. 1994, 28, 180A–185A. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, C.; Zhang, G.; Dionysiou, D.D.; Nadagouda, M.N. PEG-assisted synthesis of crystal TiO2 nanowires with high specific surface area for enhanced photocatalytic degradation of atrazine. Chem. Eng. J. 2015, 268, 170–179. [Google Scholar] [CrossRef]
- Kataoka, S.; Tompkins, D.T.; Zeltner, W.A.; Anderson, M.A. Photocatalytic oxidation in the presence of microwave irradiation:observations with ethylene and water. J. Photochem. Photobiol. A Chem. 2002, 148, 323–330. [Google Scholar] [CrossRef]
- Horikoshi, S.; Saitou, A.; Hidaka, H. Environmental remediation by all integrated microwave/UV-illumination method. 1. Microwave assisted degradation of Rhodamine-B dye in aqueous TiO2 dispersions. Environ. Sci. Technol. 2003, 37, 5813–5822. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photoch. Photobio. A 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Whang, T.-J.; Hsieh, M.-T.; Shi, T.-E.; Kuei, C.-H. UV-Irradiated Photocatalytic Degradation of Nitrobenzene by Titania Binding on Quartz Tube. Int. J. Photoenergy 2012, 2012, 681941. [Google Scholar] [CrossRef]
- Bhatkhande, D. Photocatalytic and photochemical degradation of nitrobenzene using artificial ultraviolet light. Chem. Eng. J. 2004, 102, 283–290. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Mohamed, A.R.; Lee, K.-T. Degrading Endocrine Disrupting Chemicals from Wastewater by Photocatalysis: A Review. Int. J. Photoenergy 2012, 2012, 185159. [Google Scholar] [CrossRef]
- Hassan, M.; Zhao, Y.; Xie, B. Employing TiO2 photocatalysis to deal with landfill leachate: Current status and development. Chem. Eng. J. 2016, 285, 264–275. [Google Scholar] [CrossRef]
- Nitoi, I.; Oancea, P.; Raileanu, M.; Crisan, M.; Constantin, L.; Cristea, I. UV–VIS photocatalytic degradation of nitrobenzene from water using heavy metal doped titania. J. Ind. Eng. Chem. 2015, 21, 677–682. [Google Scholar] [CrossRef]
- Safari, M.; Nikazar, M.; Dadvar, M. Photocatalytic degradation of methyl tert-butyl ether (MTBE) by Fe-TiO2 nanoparticles. J. Ind. Eng. Chem. 2013, 19, 1697–1702. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, G.; Qu, J. Oxidative decomposition of azo dye C.I. Acid Orange 7 (AO7) under microwave electrodeless lamp irradiation in the presence of H2O2. J. Hazard. Mater. 2006, 134, 183–189. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Park, Y.-K.; Kim, S.-J.; Seo, S.-G.; Ki, S.J.; Jung, S.-C. Photocatalytic reactions of 2,4-dichlorophenoxyacetic acid using a microwave-assisted photocatalysis system. Chem. Eng. J. 2015, 278, 259–264. [Google Scholar] [CrossRef]
- Liang, H.C.; Li, X.Z.; Yang, Y.H.; Sze, K.H. Effects of dissolved oxygen, pH, and anions on the 2,3-dichlorophenol degradation by photocatalytic reaction with anodic TiO2 nanotube films. Chemosphere 2008, 73, 805–812. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Malato, S.; Weber, J.-V. Solar photocatalytic degradation of humic acids as a model of organic compounds of landfill leachate in pilot-plant experiments: Influence of inorganic salts. Appl. Catal. B: Environ. 2004, 53, 127–137. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Lambropoulou, D.A. Cytarabine degradation by simulated solar assisted photocatalysis using TiO2. Chem. Eng. J. 2017, 316, 823–831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).