Abstract
Ultraviolet radiation (UVR) has been scientifically proven to cause skin disorders such as sunburn, skin cancer and the symptoms of chronic exposure. Natural sun screening compounds have recently gained tremendous attention from the cosmetic and cosmeceutical sectors for treating skin disorders such as hyperpigmentation and aging. A wide range of natural UV-absorbing compounds have been used to replace or reduce the number of synthetic sunscreen molecules. One of the primary causes of photoaging is DNA damage, mainly caused by UVR. Photoprotection provided by traditional sunscreens is purely preventative and has no efficacy after DNA damage has been initiated. As a result, the quest for DNA-repair mechanisms that block, reverse, or postpone pathologic processes in UV-exposed skin has stimulated anti-photoaging research and methods to increase the effectiveness of traditional sunscreens. This review summarizes many natural compounds from microalgae, lichens, and plants that have demonstrated potential photoprotection effects against UV radiation-induced skin damage. Furthermore, it offers an overview of current breakthroughs in DNA-repair enzymes utilized in sunscreens and their influence on photoaging.
1. Introduction
Prolonged sun exposure has long been recognized to induce photoaging [1], a process in which the skin suffers changes in epidermal thickness, significantly increasing pigment heterogeneity and dermal collagen deterioration, dermal elastosis and keratinocyte and melanocyte mutagenesis. [2]. Regular UV exposure has a variety of biological impacts on mammals, including the start of early aging, hyperpigmentation (dark spots), erythema, immune system suppression and DNA damage. Recent qualitative research further distinguished between hypertrophic and atrophic skin aging, with the latest characterized by increasing skin thickness and sallowness and the latter by erythema and an increased risk of skin cancer [3]. The multibillion-dollar anti-aging goods market [4] is a reflection of the priority today’s society places on looking young. Ultraviolet (UV) radiation has been shown to be responsible for 80% of skin aging on the face [5]. Based on this, the strongest defence against cutaneous age-related abnormalities is prevention with strict photoprotection [4], despite the market’s emphasis on skin-aging reversal. A wide-brimmed hat, photoprotective apparel, sunglasses, and the use of sun protection factor (SPF) 30 broad-spectrum hued sunscreen to exposed areas are all necessary for adequate photoprotection.
In order to defend themselves, microalgae produce useful compounds that attract the attention of the beauty industry and make them prospective candidates for cosmetic or cosmeceutical products, particularly those that protect skin from UV damage. Solar energy is converted by microalgal cells into chemical energy, some of which is then stored as bioactive molecules [6]. These secondary metabolites are referred to as “bioactive molecules” since they exhibit biological activity [6]. Researchers concluded that microalgal extracts or bioactive compounds derived from microalgae have enormous potential for innovative new bio-based products such as cosmetics, medications, and nutraceuticals after several studies revealed that microalgae have high antioxidant capacities [6]. Several skin care products for humans include microalgae. For instance, the company Soliance employs an entire Arthrospira species, and personal skin care products include the Chlorella species-derived peptide sequence LVMH. Alguronic acid is another ingredient in the anti-aging skin care products produced by the company Solazyme [7]. The production of more reliable and secure formulations at a cheaper cost is made possible by the use of Spirulina extracts in dermo-cosmetic formulations. Long-term research is necessary to confirm its anti-aging efficacy. Dunaliella salina is an esteemed microalga that is abundant in β-carotene, which has anti-aging potencial [8]. Similar to other organisms, exposure to UV light in humans can have negative effects, including short-term erythema or “sunburn” and long-term premature aging of the skin [9]. In this review, we discussed the value of cosmetics made from promising microalgae and their prospective applications in sunscreen [10,11,12,13,14,15], anti-aging [16], and skin-whitening products.
Furthermore, DNA damage is one of the primary causes of photoaging and is mostly caused by ultraviolet radiation (UV-R) [17]. In an attempt to identify the biological targets of UV-R and the subsequent cascade of impaired cell functioning and tissue deterioration, recent research examined the harmful effects of UV-R at the cellular and molecular levels [18]. Although endogenous DNA repair mechanisms, persistent DNA damage from prolonged UV exposure can build up and result in photoaging and the development of skin cancer [19,20]. Conventional sunscreen protection is preventative and useless once DNA damage has already occurred. While the majority of the literature to date has focused on how DNA-repair enzymes affect the development of cancers linked to UV exposure [20], the goal of the current review is to examine recent research on the biological process of photoaging and the function of DNA-repair enzymes. The top sunscreens with DNA-repair enzymes that are currently on the market are mentioned below [21].
2. UV-Induced Skin Damage
The prevalence of several diseases and conditions linked to solar UV radiation has dramatically risen and is still rising. Mammalian skin exposed to UV radiation on a regular basis has a variety of biological effects, such as the onset of early aging, hyperpigmentation (dark patches), erythema, DNA damage, and immune system suppression [2,18]. These changes can directly or indirectly contribute to the growth of skin cancer [22,23]. The least prevalent but most active component of solar radiation is UV-B (280-315 nm). As the “burning ray”, UV-B radiation comprises up to 4–5% of total solar radiation, and sunburn brought on by UV-B is 1000 times more intensive than UV-A. Moreover, UV-B seems to be more genotoxic than UV-A. Ionizing radiation may directly damage atomic structures in live cells, resulting in alterations in chemical and biological processes. It can also work in an indirect manner by radiolyzing water, which leads to endogenous bursts of reactive oxygen species (ROS) in the intercellular matrix as well as in and around the radiation track that could harm lipids, proteins, and nucleic acids [24]. Proteins and genes involved in oxidative metabolism are susceptible to direct or indirect effects from bursts of ROS. It mostly affects the basal cell layer of the skin’s epidermis. The formation of the isomerization of trans- to cis-urocanic acid, pyrimidine photoproducts, the upregulation of ornithine decarboxylase activity, the production of free radicals in the skin, photoaging, cell cycle growth arrest, and photo carcinogenesis are just a few of the harmful biological effects it causes. UV-B exposure most likely initiates the production of free radicals, which depletes the skin’s antioxidant reserves, making the skin less able to defend itself against free radicals produced by exposure to daylight. DNA damage is thought to be the cause of squamous and basal cell carcinoma in the skin. Moreover, it may also weaken the skin’s immunological defences [25,26]. Even very brief exposures to UV-C are extremely harmful to all forms of life. The skin suffers severe damage as a result. Thankfully, ozone in the Earth’s atmosphere entirely absorbs UV-C from the sun, and no solar radiation with wavelengths below 290 nm reaches the planet’s surface [25,26]. In this review, we will briefly touch on how UV radiation causes the development of ROS [27] and how these ROS can cause skin ailments [28], including hyperpigmentation (dark patches), skin aging, and photoaging [29].
2.1. Hyperpigmentation
Hyperpigmentation is a process in which patches of the skin become darker in color than the surrounding skin. The pigment melanin was overproduced and accumulated, changing the hue of the skin. Lentigines, liver spots, and age spots are other names for these hyperpigmented lesions. They appear on sun-exposed areas of the body, especially the arms and face, and are thus most likely caused by long-term UV exposure [30]. The dermal melanophages that have been seen histologically to lay beneath the lentigines may also contribute, at least in part, to their appearance of darkness. These findings probably point to a difference in the genetic expression of the keratinocytes (KC) and melanocyte (MC) within the spot as compared to the MC in the non-spot skin around it. The epidermal architecture is frequently drastically altered, which may be partially due to the damage caused by persistent UV exposure that is linked to spot formation. Tyrosinase, a glycoprotein [30] found in the melanosome membrane that catalyzes the conversion of L-tyrosine to melanin, is one enzyme that plays a major role in regulating melanogenesis [31].
Melanogenesis is controlled at the level of tyrosinase maturation and translocation. The presence of certain carbohydrate moieties regulates the translocation of tyrosinases [32]. Eumelanin and pheomelanin, two different kinds of melanin, are produced by melanosomes. Tyrosinase and associated proteins (TRP-1 and TRP-2) are created by MITF’s phosphorylation, which is triggered by a number of signaling pathways, including the ERK, cAMP, and Wnt pathways. Upstream of the receptor molecules such as KIT (ligand SCF) and MC1R (ligands α-MSH, ASP, and ACTH) upregulate these signaling pathways, and the cAMP pathway is activated by the KIT receptor, as well as the MC1R which stimulates the ERK and cAMP pathways simultaneously. This further phosphorylates the MITF (Figure 1). Finally, tyrosinase-related enzymes are expressed as a result, which further facilitates the formation of melanin [33,34,35]. Likewise, when exposed to UV, the skin produces ROS, which activate the MC1R and α-MSH receptors and increase the synthesis of tyrosinase, resulting in the excess production of melanin [36] (Figure 1).
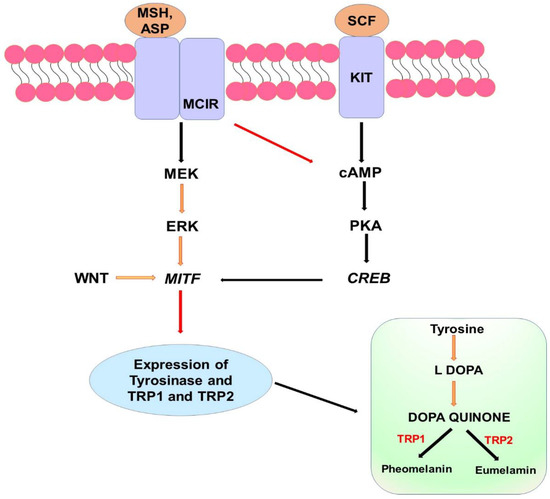
Figure 1.
Signaling channels that are involved in melanin production. Adenosine 3′,5′-cyclic AMP (cAMP), cAMP response element-binding (CREB), tyrosinase-related protein (TRP), microphthalmia-associated transcription factor (MITF), melanocortin 1 receptor (MC1R), wingless-related integration site (Wnt), -melanocyte-stimulating hormone (MSH), agonist stimulating protein (ASP), and stem cell factor (SC).
However, actinic lentigos exhibit a rise in the mRNA levels of melanogenesis-related genes, such as tyrosinase, TYRP1 (tyrosinase-related protein 1), POMC (proopiomelanocortin), DCT (dopachrome tautomerase), and MITF (microphthalmia-associated transcription factor). Moreover, the epidermal endothelin cascade (which includes endothelin-1, endothelin converting enzyme 1-a, and endothelin-B receptor) is accentuated, and a role for stem cell factors in hyperpigmentation (particularly solar lentigos) has been discovered [37]. Changes in the factor XIII1 melanophages and epidermal–melanin axis have also been noted. As many of these alterations continue even after avoiding more UV exposure, they appear to be irreversible [38]. The specifics of these apparent alterations in genomic expression have not yet been established.
2.2. Skin Aging
Higher mammals go through the intricate process of skin aging, which is brought on by two things. One is intrinsic, in which genetics, epigenetics, and hormonal status have a role in aging [39]. The latter is extrinsic, where skin aging results from exposure to UV, visible, and IR radiations, climate, air pollution, chemicals, smoking, drugs, lifestyle, diet, emotional factors and stress, etc. [40]. Premature aging or photoaging are terms used to describe this process [41]. All skin regions are impacted by intrinsic aging. Aged skin is thin, translucent, dry, prone to fine lines and wavy hair growth, unable to produce enough sweat, and loses subcutaneous fat tissue, which results in hollowed cheeks and eye sockets, and inadequate sweating [42]. Each bodily site’s symptoms could be different. According to Davis et al., the degree of pigmentation and maybe other, as of yet unidentified, contributing variables cause the intrinsic aging process, which is found to vary by ethnic group [43]. Indeed, there is no such thing as skin that ages only due to intrinsic reasons. Those who have lived solely indoors their entire lives may develop skin conditions that are quite similar to that. Individuals often have skin that displays distinct extrinsic aging phases overlaid on the degree of intrinsic aging.
The aging of the skin is significantly influenced by ROS. Around 1.5–5% of the oxygen that is ingested by the skin is converted into ROS by radiolyzing water [44]. Inherent aging is thought to be mostly caused by ROS, which is continually created as a byproduct of aerobic metabolism in the mitochondria’s electron transport chain [45]. In the skin, keratinocytes (KC) and fibroblasts (FB) are the primary sources of “mitochondrial” ROS. The reactive superoxide anion radical (•O2−) is a ROS that is primarily created in mitochondria from oxygen by adding an electron to each oxygen (O2) molecule. Superoxide anions, which are ROS particles, can damage cellular structures when produced in large quantities [44].
The numerous growth factor and cytokine receptors are activated by ROS, and this further stimulates the P13/AKT pathway and mitogen-activated protein kinase (MAPK) signal transmission. However, the FoxO is rendered inactive by the AKT pathway, which prevents the cell’s production of antioxidant enzymes. Activator protein-1 (AP-1) and NF-κB are both nuclear proteins that MAPK regulates, and the expression of MMP is caused by AP-1 stimulation [46]. MMPs are a group of extracellular proteinases that include zinc that breaks down extracellular substances, including collagen and elastic fibers, which results in the development of wrinkles [46,47]. Moreover, the hyaluronidase enzyme, which breaks down hyaluronic acid, is activated by ROS. The extracellular matrix contains hyaluronic acid, which helps to maintain the skin smooth, wet, and lubricated by absorbing and holding onto water molecules [48,49]. Yet, recent experimental data demonstrate that low amounts of ROS might serve as a beneficial signaling agent, particularly when superoxide anions are transformed into hydrogen peroxide. The principal mitochondrial neutralizer of constantly generated superoxide anions is manganese superoxide dismutase (MnSOD). Outside of the mitochondria, there are other types of SOD, but only MnSOD has been proven to be crucial for the survival of aerobic life [50]. Although, the extracellular matrix degrades more quickly, and cells have a decreased capacity for cell replication, which are two additional key phenomena linked to intrinsic skin aging. All dividing cells lose some of their capacity to replicate over time. This particularly impacts MC, FB, and KC in the skin. Cellular senescence is the name of this process. In older skin, senescent, non-dividing cells are more prevalent. [51].
Extrinsic aging is brought on by oxidative environmental variables such as sunlight [52,53], cigarette smoke, or other pollutants [54]. According to epidemiological research, prolonged exposure to cigarette smoke and UV-A radiation both hasten the aging process of the skin [55]. The main cause of extrinsic skin aging, also known as photoaging, is exposure to UV light [53]. The rate of skin aging caused by UV radiation relies on the frequency, length, and intensity of sun exposure as well as the natural defense provided by skin pigmentation [56]. Deep wrinkles, loss of elasticity, dryness, laxity, an appearance of having a rough texture, telangiectasias, and pigmentation disorders are characteristics of photo-aged skin, which is considerably different from the skin that is mostly intrinsically old [57]. According to the Fitzpatrick skin phototype, photoaging is more pronounced in people with fair skin (skin types I and II) and less pronounced in those with skin types III or above [58]. As a result, the degree of photoaging is mostly determined by the total UV radiation received as well as by the skin’s level of pigmentation. Damage to the structural elements of the connective tissue of the dermis is the main factor responsible for the aged look of photodamaged skin [59]. The three main groups of biomolecules that make up connective tissue structural proteins (collagen and elastin), glycosaminoglycans (GAGs), proteoglycans, and special macromolecules are created by FB (laminin fibrillin, hyaluronan, and fibronectin) [59]. In recent years, a great deal of work has been carried out to understand the molecular alterations in photoaged skin [60]. The direct and indirect effects of UV radiation on epidermal and dermal structures in connection to aging and the function of oxidative processes herein are discussed in the next portion of this review.
Principles of Sun-Induced Photoaging
The word “photoaging” refers to certain functional, clinical, and histological characteristics of skin that has had repeated sun exposure [61]. It originated from a number of words, including rapid skin aging and heliodermatosis. Nonetheless, several characteristics of photoaged skin are unique, making it a distinct process with its own pathophysiology. Particularly among the Western population, longer lifespans, more free time, and excessive exposure to UV radiation from natural sunshine or tanning beds have led to an increase in the need for skin protection against the harmful effects of UV exposure. Photoaging will thus be a growing problem in the future. The clinical and histological signs of photoaged skin have been recognized for some years, but the underlying molecular processes causing the precise macro- and microscopic abnormalities have only recently been identified [61].
“Telomere shortening and damage are recognized causes of cellular senescence and aging” [62,63]. A cell’s age may be determined by the length of its telomeres. Many age-related diseases, including benign and malignant neoplasms and photoaged skin, have been linked to genomic instability brought on by telomere shortening [64,65,66,67]. Accelerated telomere shortening has been associated with DNA-damaging substances such as ROS in vitro investigations [68]. This contributes to UV-induced damage to important regulatory genes, which results in the classic signs of photoaging [69]. Reactive oxygen species (ROS), which are produced by UV irradiation, activate a variety of cell surface receptors, including those for keratinocyte growth factor, insulin, tumor necrosis factor (TNF-α), and interleukin-1. Protein-tyrosine phosphatase-j, an enzyme that keeps receptors such as the EGF receptor inactive (hypophosphorylated), is inhibited by ROS, which mediates receptor activation in part. By stimulation of the stress-related mitogen-activated protein (MAP) kinases p38 and c-Jun amino-terminal kinase, receptor activation causes intracellular signaling (JNK). The nuclear transcription complex AP-1, which is made up of the proteins c-Jun and c-Fos, is transcriptionally induced by kinase activation [70].
In addition to activating receptors, UV also produces ROS that damage membrane lipids, causing ceramide to be released and AP-1 to be activated (Figure 2) [71]. Cyclooxygenase enzymes convert arachidonic acid into prostaglandins, which attract inflammatory cells to the location. Since it inhibits the effects of transforming growth factor-β (TGF-β), a cytokine that stimulates collagen gene transcription [70,72] and negatively controls keratinocyte proliferation, increased AP-1 transcription and activity prevent the production of the main dermal collagens I and III (Figure 2) [70,73]. Activated receptors drive the transcription of the matrix metalloproteinase (MMP) gene, which in turn encourages signaling cascades that lead to the production of transcription factor AP-1. Furthermore, procollagen gene expression in fibroblasts is suppressed by AP-1. Matrix metalloproteinases, which are produced by KC and FB, degrade collagen and other proteins that make up the dermal extracellular matrix. The structural and functional integrity of the extracellular matrix is threatened by dermal damage that is not effectively healed. Repeated sun exposure causes a build-up of dermal damage, which eventually results in the recognizable wrinkles of photodamaged skin (Figure 2).
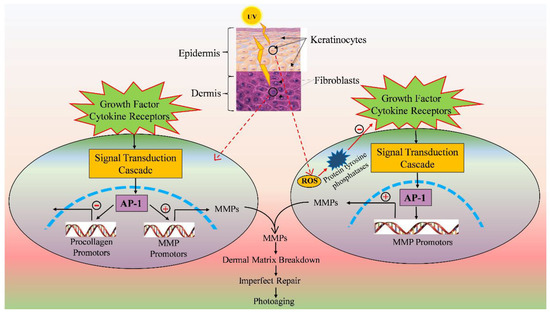
Figure 2.
Model showing the effects of solar UV rays on connective skin tissue. The keratinocytes (KC) and fibroblasts (FB) growth factor and cytokine receptors are activated by ultraviolet rays (jagged arrow). Matrix metalloproteinase (MMP) gene transcription is stimulated by activated receptors, which in turn promote signal transduction cascades that result in transcription factor AP-1. AP-1 also suppresses procollagen gene expression in FB. Collagen and other proteins that make up the dermal extracellular matrix are broken down by matrix metalloproteinases, which are released by KC and FB dermal injury that is not properly repaired; this compromises the extracellular matrix’s structural and functional integrity. Frequent sun exposure leads to dermal damage build-up, which finally produces the distinctive wrinkles of photodamaged skin (Modified from 70). Dashed lines represent the nucleus.
The intracellular signaling proteins SMAD2, SMAD3, SMAD3, and SMAD7 are activated by TGF-β to mediate its actions, and SMAD7 inhibits these effects [74]. In fact, UV causes the human skin’s SMAD7 protein to be produced, which disrupts the TGF-β SMAD2-3 signaling [75,76]. This causes keratinocyte proliferation, epidermal hyperplasia, and a reduction in type I procollagen synthesis, which results in collagen loss. Further reducing collagen transcription, AP-1 lowers the number of TGF-β receptors in the body. This also counteracts the intrinsic retinoic acid stimulatory action on collagen synthesis. Collagen production is therefore decreased in the skin that has been photodamaged by UV radiation on a regular basis [75]. Moreover, UV-induced PTEN phosphatase inhibition and Akt kinase activation, which both work by stimulating the phosphoinositide 3-kinase signaling pathway, boost AP-1 activity [77]. The cysteine-rich 61 protein (CYR61), a new regulator of collagen production that is triggered by UV irradiation in FB, has recently been demonstrated also to stimulate AP-1. Matrix metalloproteinase (MMP)-1 and other enzymes that break down extracellular matrix components are produced as a result of CYR61 (collagenase). Moreover, CYR61 lowers the amount of TGF-β receptors and reduces the formation of type I procollagen [78]. The levels and activity of MMPs, notably MMP-1, MMP-3 (stromelysin-1), and MMP-9, are increased by CYR61′s induction of AP-1 (92-kDa gelatinase) [70,79]. Furthermore, UV irradiation enhances the development of MMPs and activates the nuclear factor (NF)-κB transcription factor, which in turn causes the expression of proinflammatory cytokines such as vascular endothelial growth factor (VEGF), IL-1, IL-6, and TNF-β [80,81]. After neutrophil infiltration into UV-irradiated skin, MMP-8, a collagenase of neutrophil origin, further exacerbates matrix deterioration. In fact, one study contends that infiltrating cutaneous neutrophils is the primary cause of UV-induced MMP and elastase release. Tissue inhibitors of metalloproteinases (TIMPs), which slow down matrix breakdown, cannot completely counteract the negative effects of MMPs. Superoxide and peroxynitrite, a byproduct of the nitric oxide process generated by UV-irradiated KC, can activate NF-κB [82]. The dermis accumulates partially damaged collagen fragments as a result of UV-induced collagen degradation, which is usually incomplete and is thought to lessen the structural integrity of the skin [70]. Large collagen degradation products also prevent the synthesis of new collagen [83], which means that collagen degradation itself adversely controls the production of new collagen.
3. Application of Microalgae in Cosmetics
Microalgae produce a number of secondary metabolites with anti-inflammatory, anti-blemish and antimicrobial activities [5]. Certain microalgal extracts such as Arthrospira platensis, Chlorella vulgaris, and Dunaliella salina can be used for repairing skin aging, healing and preventing wrinkle formation [84,85,86,87]. The microalgae or its components’ activity is the basis for the creation of several commercially available cosmetics and cosmeceuticals. Anti-aging creams, refreshing/regenerating care items, emollients and anti-irritants in peelers, sunscreen cream, and hair care items are a few examples of commercialized microalgae sources in the skin care industry. These cosmetics and cosmeceuticals contain bioactive microalgae components or algal extract (Table 1 and Figure 3). The products could offer promising and innovative alternatives to existing cosmetics and drive the development of new functions for cosmetic products.
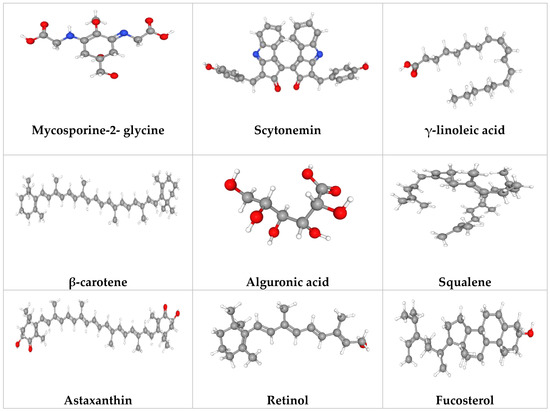
Figure 3.
Potential microalgal compounds used in cosmetics such as sunscreen and anti-aging.
Cosmetic and cosmeceutical products must safeguard the natural dermal qualities and improve its appearance of health. They are typically applied when skin becomes dry due to a change in the filaggrin gene, which produces the skin’s natural moisturizing component. Additionally, by protecting against both external and internal influences, they could be used to lessen the signs and symptoms of aging [6]. In addition to offering these advantages, they should have anti-bacterial and anti-fungal activities against organisms such as Mucor ramaniannus and Candida albicans to protect the equilibrium of the skin flora. Moreover, extracellular matrix stability, acne management, cell regeneration, skin whitening, inflammation control, stimulation of angiogenesis, and oxidative stress management are all areas where microalgal antimicrobial peptides play distinctive roles. Cosmetics may contain chemicals that have unintended adverse effects, such as triggering hypersensitivity reactions, anaphylactic shock, or fatal poisoning [88]. To select compounds as safe cosmetics, some crucial studies such as genetic toxicity, phototoxicity, photo genotoxicity, toxicokinetics, and carcinogenicity should be conducted. Due to the growing popularity of cosmetics, there is a greater demand for natural and sustainable resources for the manufacture of cosmetics [87]. Cosmetics made from microalgae can replace current goods and are safe for the environment; the FDA has authorized Arthrospira extract as a “safe” food ingredient.

Table 1.
Potential application of microalgae as natural cosmetics.
Table 1.
Potential application of microalgae as natural cosmetics.
| Species | Ingredient | Activity/Uses | References |
|---|---|---|---|
| Anabaena virabilis, Nostoc punctiforme, Gloeocapsa sp. | Mycosporine-like amino acids (MAAs) | Photoprotection | [89,90] |
| Arthrospira platensis, Dunaliella salina, Chlorella vulgaris, and Nannochloropsis oculata | Sporopollenin, scytonemin, mycosporine-like amino acids (MAAs) | Photoprotection | [91] |
| Odontella aurita | Polyunsaturated fatty acids (PUFAs) | Prevents oxidative stress on skin | [92] |
| Porphyra sp., Porphyridium sp. | Phycoerythrin | Cosmetics (face powder, eye shadow) | [93] |
| Arthrospira sp. | Phycocyanin | Cosmetics (eye shadow) | [94] |
| Dunaliella, Nannochloropsis | Carotenoids | Wrinkle reduction, collagen formation and tissue regeneration | [95] |
| Arthrospira sp. | Phycobiliprotein | Improves moisture balance of the skin, skin complexion, strengthens skin’s immunity and reduces wrinkle | [96] |
| Dunaliella salina | β-carotene | Stimulates cell proliferation | [95] |
| Arthrospiraplatensis | γ-linoleic acid | Prevents early skin aging and wrinkle formation | [96] |
| Asterocapsa nidulans | Alguronic acid | Strengthens skin immunity | [97] |
| Porphyridium sp. | Sulfated polysaccharide | Strengthens skin immunity | [97] |
| Botryococcus braunii | Squalene | Improves skin elasticity and moisture retention, prevents age spots and hyperpigmentation | [98] |
| Haematococcus lacustris | Astaxanthin | Photoprotection | [99,100] |
| Nostoc commune, Anabaena variabilis, Aphanothece halophytica | Mycosporine-2- glycine | Inhibition of advanced glycation end products | [97] |
| Olisthodiscus luteus, Microchloropsis salina | Fucosterol | Decrease matrix metalloproteinases expression and increase collagen production | [91] |
| Dunaliella bardawil | β-carotene | Improves bioavailability and antioxidant properties on skin | [101] |
Researchers have discovered that compounds derived from microalgae can be utilized as the primary active ingredient in cosmetics, but some of these compounds also have characteristics that allow for them to be employed as excipients, such as stabilizers, dyes, or thickening agents [87,102]. Typically, personal care items, including face lotion, cream, shampoo, body soap, and colorants for cosmetics, are formulated using their extracts or bioactive compounds [6,103,104]. Their sterols can also be utilized in moisturizing lotions [105]. Moreover, their pigments, like the keto carotenoid astaxanthin and β-carotene, are utilized in skin care, anti-aging, and anti-irritant treatments [105]. In addition, β-carotene with non-modified β-ionone rings such as β-carotenes are the precursor molecules for vitamin A [104]. Fucoxanthin protects against UV-B-induced skin damage by reducing intracellular ROS, like astaxanthin, an orange-colored pigment with antioxidant and provitamin A properties. Fucoxanthin has anti-pigmentary action in UV-B-induced melanogenesis in addition to its sunscreen properties [105,106]. Fucoxanthin could be an active component of cosmeceuticals and nutraceuticals utilized in the defense of the skin from photoaging [16,105,107]. Microalgae are also a source of phenolic compounds which are valuable antioxidants and photoprotective compounds [108,109]. The green microalga Lobosphaera incisa accumulates triacylglycerols (TAGs) with exceptionally high levels of long-chain polyunsaturated fatty acids (LC-PUFA) and arachidonic acids (ARA) under nitrogen (N)-deprived conditions [110]. The cosmetic industry uses extracts from microalgae which are high in pigments, PUFAs, phycobiliproteins and carbohydrates which can be used to make lotions, creams and ointments. Mycosporine-like amino acids (MAAs) are small <400 Da, water-soluble, colorless UV-absorbing compounds synthesized by marine microbes as an adaptation for their high sunlight environments. They have a unique absorption spectrum between 309 and 362 nm. Structurally, MAAs are divided into two groups: (i) the mycosporines, which have a single modified amino acid residue connected to a cyclohexenone core, and (ii) MAAs, which have two such amino acid substituents [9,14]. MAAs have good antioxidant properties. MAAs produced from Dunaliella, Arthrospira, and Chlorella have the potential to act as sunscreens to reduce the damage induced by ultraviolet rays. Microalgae Odontella aurita also showed potential free radical scavenging activity to maintain youthful skin.
Adequate research is currently lacking in order to apply the findings of in vitro experiments on model organisms and the application of efficient compounds to human skin. Additionally, patients and researchers must understand that compliance is crucial when using natural cosmetics since they work more slowly than traditional cosmetics made of synthetic substances. Important research on genetic toxicity, photo genotoxicity, phototoxicity, toxicokinetics, and carcinogenicity should be carried out in order to choose substances as safe cosmetics. To specify compounds as a harmless cosmetic, certain crucial studies such genetic toxicity, photogenotoxicity, phototoxicity, toxicokinetics, and carcinogenicity should be performed [111]. Using genetic toxicity studies, such as the Ames Salmonella test, one may determine the carcinogenic potential of bio compounds produced from microalgae [112]. Moreover, the 3T3 neutral red uptake (3T3 NRU) experiment, the 3-dimensional (3D) epidermis model, and the erythrocyte photohemolysis test can be used to determine the phototoxicity of microalgal bioactive compounds. Nevertheless, as natural products have long been used to promote health and wellbeing, they may have promised nutritional and therapeutic benefits with minimal-to-no negative effects.
3.1. Photoprotection Prospects of Microalgal Products
Organic carbon molecules and oxygen, which are necessary for life on Earth, are produced by cyanobacteria, algae, and plants, which also turn solar energy into chemical energy. UV rays hit the Earth’s surface more intensely as a result of ozone layer depletion and the rising emission of atmospheric contaminants. The ozone layer absorbs UV-C radiation, which ranges in wavelength from 100 to 280 nm. The ozone layer blocks most of the ultraviolet-B (UV-B) radiation (280–315 nm) that reaches the Earth’s surface, but some do get through. As the ozone layer thins, UV-B radiation intensity rises. However, the ozone layer has little effect on ultraviolet-A (UV-A) light, which ranges in wavelength from 315 to 400 nm (320 to 400 nm). The primary kind of solar radiation that enters our atmosphere is UV-B, which harms living things exposed to the sun by causing DNA strand breaks, membrane rupture, enzyme deactivation, the production of cytotoxic DNA lesions, and other extremely hazardous effects. Because native DNA molecules cannot absorb UV-A radiation, ROS are created, which cause indirect DNA damage [113]. As a result of these consequences, UV-A and UV-B have mutagenic and carcinogenic effects on humans, speed up the skin’s aging process, and cause photodermatitis. In order to defend themselves from UV radiation, microalgae have developed a number of defenses, including (i) the expression of DNA-repair enzymes; (ii) the creation of antioxidant enzymes; (iii) the avoidance of the UV; and (iv) the production and accumulation of UV filter metabolites [114]. These mechanisms, along with the manufacture of UV filter metabolites, commonly known as “microbial sunscreens”, make microalgae potential candidates for the cosmetic sector to be employed in sunscreens produced from natural sources [113,115].
To defend themselves from sun radiation, microalgae produce a variety of UV filter substances, including sporopollenin, scytonemin, mycosporine-like amino acids, β-carotene, and other substances such as biopterin glucoside, lycopene, and ectoine for UV protection and photoaging [91]. These bioactive substances shield the body from skin cancer, sunburn, and other diseases by inhibiting the manufacture of melanin, among other things.
The skin is shielded from UV damage by lutein, which is generated by Chlorella protothecoides, Scenedesmus almeriensis, Muriellopsis sp., Neospongiococcus gelatinosum, Chlorococcum citriforme, Chlorella zofingiensis, D. salina, and Galdieria sulphuraria [15,87,102]. The chemicals in microalgal extracts or extracts from microalgae help shield the skin from UV damage [87]. The most significant and extensively researched compounds that are utilized in sunscreens made by microalgae are scytonemin and mycosporine-like amino acids. Cyanobacteria produce scytonemin—a lipophilic, extracellular yellow-brown pigment—in their sheath when exposed to intense sun radiation in order to shield themselves from UV-A radiation with an absorption of up to 90% [10,15,114]. Scytonemin has a maximal absorption range of 252–386 nm [114,116,117]. Coelastrin A and Coelastrin B, two novel MAAs from Coelastrella rubescens, exhibit photoprotective properties [118]. Klebsormidin A and klebsormidin B, the newly identified MAAs from Klebsormidium, showed that their biosynthesis and intracellular enrichment is strongly induced by UVR exposure, supporting the function of these compounds as natural UV-sunscreens [119].
Scytonemin is produced when the gene responsible for its production is activated by UV-A, and it then builds up in the body. Scytonemin and derivatives of scytonemin can be produced by a number of cyanobacterial species such as Anabaena, Calothrix, Chlorogloeopsis, Diplocolon, Gloeocapsa, Hapalosiphon, Lyngbya, Nostoc, Phormidium, Pleurocapsa, Rivularia, Schizothrix, Scytonema, and Tolypothrix [113].
The hydrophilic and colorless mycosporine-like amino acids (MAAs) are produced by marine organisms such as cyanobacteria [11,12], microalgae, macroalgae, fungi, etc., that act as an antioxidant by preventing ROS-induced DNA damage as well as a photoprotectant by shielding cells from UV-B and UV-A radiation by absorbing radiation and dissipating excess heat energy into the cell and surroundings [120,121,122]. Only a small percentage of the physical and chemical filters on the market, referred to as “broad-spectrum sunscreen”, can effectively block both UV-A and UV-B rays [123]. Therefore, it is crucial to include MAAs as a UV filter agent in sunscreens since they have a high capacity to absorb UV between 309 and 362 nm, making them a broad-spectrum sunscreen [115]. They can be a very stable cosmetic product because they are also very photostable and very resistant to heat, pH fluctuations, and different solvents [124]. The first sunscreen product, named Helioguard 365, was created by the Swiss company Mibelle AG Biotechnology using a natural UV screening substance called an MAA containing a certain proportion of Porphyra-334 and shinorine obtained from red algae Porphyra umbilicalis [125,126].
3.2. Microalgal Compounds as Anti-Aging Therapies
The formation of AGE (advanced glycation end products), the impact of ROS, and matrix metalloproteinases (MMPs) are the most important processes among theories about the aging process. Pharmaceutical companies have recently become interested in substances that are preventing AGE. Recently described as an inhibitor of AGE formation, mycosporine-2-glycine (M2G), a very uncommon mycosporine-like amino acid, has been proposed as a key component in anti-aging therapies [127]. Nostoc commune, Anabaena variabilis, and Aphanothece halophytica have all been found to be capable of producing mycosporine-2-glycine. The key strategy to delay the aging of the skin is moisturization. It can support skin elasticity and beauty maintenance and increase environmental harm prevention. Hydroxy acid (HA) benefits the skin and has been utilized in cosmetic goods to moisturize skin. Plants are capable of producing hydroxy acids, but because plant output is restricted, interest in algal polysaccharides is growing. According to studies, Pediastrum duplex extract has a significant number of polysaccharides and can be used to preserve and moisturize skin [87]. Salicylic acid, α-HA, and β-HA are different types of HA. Due to the hydroxyl group connected to the carbon atom adjacent to the carboxyl group, α-HA is also known as 2-hydroxy acid. Lactic and glycolic acids are cosmetics’ two most widely used 2-hydroxy acids. As a result of the hydroxyl group linked to the carbon atom that comes in second place when counting, starting from the carboxyl group, β-HA is also known as a 3-hydroxy acid. Citric acid is the most well-known 3-hydroxy acid utilized in the cosmetic formulation [128]. A. variabilis, Anacystis nidulans, Chlorella pyrenoidosa, Chlamydomonas reinhardtii, Cyanidium caldarium, Phormidium foveolarum, and Oscillatoria species have also been shown to create 2-hydroxy acids and 3-hydroxy acids, and their extracts can act as antioxidants [129,130].
3.3. Microalgal Product’s Potential as Skin Whitening Agents
The pigment melanin is what gives hair, skin, and eyes their pigmentation, and it is created to protect the skin from UV damage. Nevertheless, melanin overproduction gives the skin a distinct color [87,131]. Tyrosinase is a crucial enzyme that starts the manufacture of melanin, and tyrosinase inhibitors can stop pigmentation in the skin [132]. Tyrosinase inhibitors contain phenolic structures or metal chelating groups, which result in two mechanisms: inhibiting interactions between the substrate and the enzyme and chelating copper within the active site of the enzyme. Finding novel, naturally derived alternatives to these synthetic tyrosinase inhibitors is important due to their high toxicity, low stability, insufficient action, and poor skin penetration, and microalgae can serve as a promising possibility [133]. S. plantensis extracts can be employed as tyrosinase inhibitors [133]. According to Oh et al. (2015), Pavlova lutheri inhibits melanogenesis [134]. Tyrosinase inhibitory activity was also demonstrated by Oscillapeptin G from Oscillatoria agardhii, asthaxanthin from Haematococcus pluvialis, and zeaxanthin from N. oculata [135]. In addition to inhibiting the tyrosinase enzyme, vitamins C and E can inhibit the skin’s synthesis of melanosomes. A well-known NADH (Nicotinamide Adenine Dinucleotide Hydrate)-based process that protects mammalian skin from UV radiation damage is the self-acting, synergistic combination of vitamins E and C. Pediastrum cruentum’s high concentration of vitamins E and C make it a potential candidate as a cosmetic to prevent melanoma [136].
4. Excipients in Sunscreen
Despite the increasing use of cosmeceuticals and sunscreen additives, assessing their safety and efficacy is critical. While the specific mechanism of UV-R and VL-induced photoaging is unknown, the downstream consequences of increased ROS, MMPs, and DNA damage have already been widely described [137]. Sunscreen additives have been used or suggested in sunscreens to improve photoprotection and assist in preventing photoaging by battling the negative effects of sunshine on the skin. Antioxidants are crucial in avoiding, alleviating, and damping free radicals and oxidative stress. Despite the fact that our cells manufacture natural antioxidants, UV-R and other stressors frequently outnumber our indigenous supply [138]. To restore depleted antioxidant reserves and reduce oxidative stress on the skin, topical antioxidants have been included in sunscreens. Wang et al. [139] examined the radical skin protection factor (RSF) and antioxidant power (AP) of 12 sunscreen lotions containing vitamin C, vitamin E, or other antioxidant compounds against simulated UVA- and UVB-induced ROS in an ex vivo investigation conducted in 2011. Recent reviews and research have shown that adding antioxidants to sunscreen formulas has a favorable effect. In one study, sunscreens containing SPF 25 plus a combination of caffeine, vitamin E, vitamin C, Gorgonian extract, Echinacea pallida extract, and chamomile essential oil revealed a lower MMP-1 expression than those with simply SPF 25. [140]. The effectiveness of antioxidants in sunscreens may vary depending on the formulation of the sunscreen. It has been argued that in order for antioxidants to be effective, they must have strong antioxidative capabilities, be present in high concentrations, be viable in the final formulation, and be able to permeate the stratum corneum while being active in the epidermis and dermis [138]. With regard to antioxidants studied in topical formulations, vitamin C (l-ascorbic acid) is the most abundant antioxidant in the skin and, due to its water solubility, plays a key role in the skin’s aqueous compartments [138]. It also aids in the replenishment of vitamin E, functions as a cofactor in collagen formation, and lowers elastin build-up. Thankfully, it is possible to create a stable formulation by combining it with other antioxidants, such as vitamin E (α-tocopherol) and ferulic acid [138,141]. Murray et al. [142] revealed that once compared to untreated skin, skin pre-treated with sunlight-simulated UV-R after application of a topical formulation of 15% l-ascorbic acid, 0.5% ferulic acid (CEFer), and 1% α-tocopherol for 4 days significantly reduced UV-induced erythema, thymine dimers, sunburn cells, and p53 induction. Additionally, vitamin E has been proven in several animal and human trials to be helpful in reducing lipid peroxidation, photoaging, and photocarcinogenesis [138]. This shows that topical CEFer may play a function in photoaging and skin cancer prevention [141,142]. Vitamin A and its analogues, namely, retinoids and β-carotene, have indeed been extensively researched in the field of anti-aging and have demonstrated efficacy in the prevention and restoration of photoaging [143]. To decrease protein-1 and MMP-1 production, they bind to cytoplasmic receptors, including cellular retinoic acid-binding protein types I and II, in addition to nuclear receptors such as nuclear retinoic acid receptors and retinoid X receptors [138]. This causes enhanced epidermal proliferation, epidermal thickness, stratum corneum compression, glycosaminoglycan biosynthesis and deposition, and increased collagen formation [138]. Additionally, there is scientific proof that topical retinoids might play a role in non-melanoma skin cancer prevention by beginning tumor cell growth arrest and normal cellular differentiation [144]. Nevertheless, because retinol and retinoids are somewhat unstable when exposed to UV and visible light, their usage as a sunscreen component is primarily for anti-aging benefits rather than improved photoprotection. Several botanicals contain polyphenols, such as tea leaves, almond seeds, grape seeds (Vitis vinifera), and pomegranate extract [145]. Through one study, sunscreen incorporating polyphenols such as epigallocatechin-3-gallate from tea extracts outperformed sunscreen alone in terms of protecting human skin versus solar-simulated UV-R [140]. Moreover, green tea extract combined with resveratrol, another polyphenol, gave SPF protection irrespective of chemical and physical UV filters.
Melatonin functions as an antioxidant in three distinct but complementary ways. It can serve as a free radical scavenger, reduce free radical production, and increase the activity of antioxidant enzymes [146]. It has shown potential in the treatment of both UV-B and UVA-induced oxidative damage. Melatonin treatment reduced p53 expression, enhanced DNA repair, and lowered CPD production in human MC and KC [147]. Melatonin also inhibited UV-induced erythema and stimulated endogenous enzymes to combat oxidative stress [9]. According to this study, melatonin may be an additive in protecting KC, FB, and MC against UV-induced photoaging. Genistein, a soybean isoflavone, has been shown to block tyrosine kinase—the enzyme that begins epithelial receptor-mediated signaling [148]. It has been shown that applying genistein to human skin prior to delivering an erythemogenic UV irradiation dosage inhibits JNK and MMP-1 overexpression without causing cutaneous erythema, suggesting that genistein does not serve as a sunscreen but rather impacts UV-mediated signaling [149].
Photolyases, in addition to antioxidants, are useful sunscreen ingredients. Photolyases, especially CPDs, are enzymes with the unique capacity to repair DNA damage. These are flavoproteins that require flavonoids as cofactors to absorb UV. In both in vivo and in vitro experiments, UV-absorbed energy is subsequently transferred to damaged DNA to break CPD links [150]. When combined with SPF 50 sunscreen and antioxidants, it dramatically decreases photoaging indicators compared to sunscreen exclusively or sunscreen with antioxidants [151].
5. DNA-Repair Enzymes with Sunscreens: Recent Concepts
Sunscreens are essential for combating photoaging because they block the transmission of UV-R [152]. Numerous studies have shown that using traditional sunscreens on a regular basis can help prevent the development of skin cancer [153,154]. They do not heal skin cells that have already been harmed by sun exposure [155]. Sunscreens that incorporate DNA-repair enzymes, as well as antioxidants in combination with the sun protection factor (SPF), provide “active photoprotection” [155]. Using a dual mechanism of prevention and repair, these chemicals may overcome the existing insufficiency of sun radiation damage management [155]. The two most important examples, photolyase and T4 endonuclease V, are explored further below.
5.1. Photolyase
The genome of 81 strains of the genera Synechococcus, Cyanobium, and Prochlorococcus, obtained from various marine and brackish habitats, were compared. It was observed that these strains together had eight distinct photolyase/cryptochrome protein members [156]. Photolyase, a flavoenzyme containing the flavin adenine dinucleotide molecule, functions as a catalytic cofactor in restoring UV-induced DNA damage in CPD and 6-4PPs [157]. Photolyase identifies damaged thymine dimers and repairs them by directly absorbing blue light, even by flavin adenine dinucleotide molecules or by transferring energy from an activated antenna chromophore to second chromophore [157]. This eventually divides into separate pyrimidines, returning the electron towards the enzyme’s redox cofactor [158]. Photolyase use is also related to a decrease in MMP-1 in the skin’s dermal and epidermal compartments. MMP-1 overexpression in human skin cells causes collagen breakdown, which is important in photoaging [159]. Because CPD has a far higher mutagenesis potential than 6-4PPs or other lesions and is responsible for most UV-induced mutations, current research has focused exclusively on CPD photolyase as a repair enzyme [160]. CPD photolyase has been shown to be useful in reducing photodamage in both in vitro and in vivo experiments [161]. Numerous clinical trials regarding the use of a topical medication comprising liposome-encapsulated CPD photolyase have now been reported. It has been utilized either in people with no skin lesions or as an adjuvant treatment in patients with actinic keratoses (AK), which are in situ squamous cell carcinomas caused by persistent sun exposure [162]. Sunscreens with chemical UV filters mixed with liposome-encapsulated CPD photolyase were used in all human investigations [161,163]. The encapsulation of liposomes transports enzymes through the human stratum corneum and delivers biologically active proteins into the living epidermis. [164]. This method may open up a new avenue for photoprotection against some types of UV-induced skin damage [163].
5.2. T4-bacteriophage Endonuclease V (T4 Endonuclease V)
T4 endonuclease V is an enzyme discovered in Escherichia coli infected with the T4 bacteriophage. It has been demonstrated to repair UV-induced cyclobutane pyrimidine dimers in DNA, which, if left unrepaired, lead to mutations that cause actinic keratoses and non-melanoma skin malignancies (NMSC) [165]. When UV-damaged DNA is identified, it is cleaved by two coupled activities: apurinic-apyrimidinic endonuclease and pyrimidine dimer-DNA glycosylase. This enzyme improves UV-damaged DNA repair and has additional favorable effects on UV-damaged cells. The effect of T4 endonuclease V increases the efficacy and speed of naturally occurring DNA repair by around four-fold. [166]. Moreover, the enzyme promotes skin regeneration and repair while preventing the breakdown of extracellular matrix components, which aids in the prevention of photoaging [167]. T4 endonuclease V encapsulation into liposomes as delivery vehicles, dubbed “T4N5”, is essential for sufficient penetration into the stratum corneum. Mouse research found that applying T4N5 to the skin might be a beneficial adjuvant to sunscreens for preventing and decreasing UV-R-related local effects such as sunburn cell development [168].
5.3. Comparison of Sunscreens with and without DNA-Repair Enzymes
Improvements in the study of skin biology have resulted in the creation of a wide range of therapies to prevent aging and enable skin regeneration. The following characteristics should be included in a perfect sunscreen: 1) UV-B and UV-A radiation protection; 2) ROS scavenging capabilities; preferably, 3) filter stability and safety; 4) and the incorporation of enzymes that aid in cellular DNA repair [34]. Recent irradiation experiments have shown that adding DNA-repair enzymes to traditional sunscreens may prevent UV-R-induced molecular damage to exposed skin more than traditional sunscreens alone [169]. For example, in clinical research conducted by Carducci et al. [169], 28 AK patients were randomly assigned to either topically apply sunscreens containing DNA-repair enzymes (n = 14) or sunscreens alone (n = 14) for 6 months. The primary end measures were hyperkeratosis, field cancerization, and changes in CPD levels in skin biopsies. CPD levels were found to be 61% lower in patients who used sunscreens containing DNA-repair enzymes versus 35% lower in patients who used traditional sunscreens (p < 0.001), showing their effectiveness in lowering CPD production [169]. The combination of CPD photolyase and topical antioxidants greatly reduced CPD and free radical-induced protein degradation. The scientists found that sunscreens combining antioxidants and photolyase outperform traditional sunscreens to prevent skin aging, most likely due to a synergistic effect [151]. So far, this has been the only clinical study examining the effect of sunscreens containing DNA-repair enzymes on photoaging.
The current study focuses on creating novel sunscreens and their increased protective impact based on previous evidence from comparable investigations. A sunscreen containing CPD photolyase (Eryfotona® AK-NMSC, Isdin SA) has recently been examined as an example. Clinical and histological investigations revealed good effects on field cancerization in AK patients, such as an increase in the number of AK lesions and a reduction in the degree of cancerization in the field [170]. Because DNA photodamage and ROS are both early events in the formation of AK, the impact might also be applied to photoaging. Another new product (Ateia® Kwizda Pharma, Vienna, Austria) combines a traditional sunscreen with a proprietary component combination, Nopasome®. Nopasome® is a liposome-encapsulated CPD photolyase (Photosome®), T4 endonuclease V (Ultrasome®), and nopal cactus extract. Ladival® med (STADA Arzneimittel, Bad Vilbel, Germany) is another branded photolyase-containing sunscreen with SPF 15 or 20 [171]. The following is a list of presently available sunscreens that contain DNA-repair enzymes and have adequate scientific backing (Table 2).

Table 2.
A description of the existing range of sunscreens with DNA-repair enzymes. a American SPF (Currently, SPF is exclusively calculated for US goods based on UV-B protection).
In conclusion, recent research indicates that including DNA-repair enzymes in traditional sunscreens gives a more effective alternative for avoiding UV-R-generated damage that causes carcinogenesis and photoaging. Combining them with topically applied antioxidants is a good way to enhance this impact further. Unfortunately, there is minimal evidence for these effects in people, notably in the prevention of skin aging. So far, only one clinical trial has shown the effect of DNA-repair enzymes in sunscreens on photoaging [151].
5.4. Limitation of DNA-Repair Enzymes in Sunscreens
This research’s limitations include topical liposomal DNA-repair enzymes that were reported to be protective against UV-induced skin cancer in humans, which does not necessarily justify their preventative impact on photoaging. Most of the included studies failed to discriminate between the effects of DNA-repair enzymes on carcinogenesis and photoaging. As a result, it can now not clearly translate improved carcinogenesis outcomes to photoaging treatment or prevention. The tiny size of study cohorts further restricts the significance of their findings. Clinical investigations with a significant number of individuals, concentrating exclusively on anti-photoaging effects, are required.
6. Conclusions and Future Perspectives
The overexposure of human skin to external stressors such as UV and pollution causes an increase in ROS generation, which causes various skin-related issues such as hyperpigmentation, early aging, etc. The cosmeceutical and cosmetic industries have taken notice of the numerous distinctive and significant bioactive metabolites that microalgae synthesize through photosynthesis and other mechanisms. Microalgae are natural and sustainable sources of bioactive compounds and are thus thought to be great possibilities for cosmetics. It is necessary to assess all potential microalgal metabolites for use in cosmetics/cosmeceuticals and optimize production technology for each. Although microalgal bioactive metabolites can be produced using biotechnologically advantageous and environmentally friendly processes, they are also considered “safe”. This study explores a number of microalgae species and bioactive compounds obtained from microalgae for use in sunscreen, skin-whitening cosmetics, and anti-aging products. Microalgae are regarded to be attractive prospects for cosmetic products since they are natural and sustainable sources of bioactive compounds. Furthermore, this study presents an outline of contemporary advancements in DNA-repair enzymes employed in sunscreens and their impact on photoaging. In Table 1 and Table 2, we have included some of these bioactive compounds together with their producer and the current range of sunscreens that contain DNA-repair enzymes. Since UV-R-induced DNA damage plays a significant role in both processes, it is possible to hypothesize that DNA-repair enzyme’s positive effects can also be applied to photoaging. Controlled trials that support this effect and show that DNA- repair enzyme-infused sunscreens are better than regular sunscreens are still missing. Antioxidants, photolyases, and other sunscreen compounds have made it possible to reverse skin aging in addition to providing better photoprotection.
Nevertheless, before clinical guidelines are released, larger-scale, repeatable investigations must be conducted. Although the majority of microalgae are investigated for their cosmetic properties, several species remain unexplored. As a result, there is a need to standardize low-cost, effective, and more productive extraction techniques. In addition to effectiveness, the molecular basis of these compound’s actions and safety issues are particularly important for forthcoming difficulties in the cosmetics sector.
Author Contributions
Study concept and design, A.G., V.U. and R.P.S.; manuscript writing, A.G., A.P.S., V.K.S. and P.R.S.; manuscript editing, A.G., A.P.S., J.J., N.K. and S.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Not applicable.
Acknowledgments
Amit Gupta (09/013 (0912)/2019-EMR-I), Prashant R. Singh (09/013 (0795)/2018-EMR-I), Neha Kumari (09/013 (0819)/2018-EMR-I) and Varsha K. Singh (09/0013 (12862)/2021-EMR-1) are thankful to the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for the Senior Research Fellowship (SRF) and Junior Research Fellowship (JRF). Ashish P. Singh (NTA Ref. No. 191620014505) and Jyoti Jaiswal (926/CSIR-UGC-JRF DEC, 2018) are thankful to the University Grants Commission (UGC), New Delhi, India, for the financial assistance in the form of Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF). The incentive grant received from IoE (Scheme no. 6031), Banaras Hindu University, Varanasi, India, to Rajeshwar P. Sinha is highly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.L.; Varani, J.; Chubb, H.; Fligiel, S.E.G.; Cui, Y.; Calderone, K.; Helfrich, Y.; Fisher, G.J.; Voorhees, J. Atrophic and hypertrophic photoaging: Clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J. Am. Acad. Dermatol. 2019, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal proteins and bioactives for food, feed, and other applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology applied to cosmetics and aesthetic medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Santiesteban-Romero, B.; Martínez-Ruiz, M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M. Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology. Mar. Drugs 2022, 20, 487. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalized sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Mallick, N. Mycosporine-like amino acids: Algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res. 2021, 58, 102425. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Milito, A.; Castellano, I.; Damiani, E. From sea to skin: Is there a future for natural photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- Emanuele, E. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: Clues to skin cancer prevention. Mol. Med. Rep. 2012, 5, 570–574. [Google Scholar] [CrossRef]
- Rai, S.; Rai, G.; Kumar, A. Eco-evolutionary impact of ultraviolet radiation (UVR) exposure on microorganisms, with a special focus on our skin microbiome. Microbiol. Res. 2022, 260, 127044. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investg. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Müllegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing-A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, B. Relevance of oral supplementation with antioxidants for prevention and treatment of skin disorders. Skin Pharmacol. Physiol. 2001, 14, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B Biol. 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Physiol. 2002, 15, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Schalka, S.; Silva, M.S.; Lopes, L.F.; de Freitas, L.M.; Baptista, M.S. The skin redoxome. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 181–195. [Google Scholar] [CrossRef]
- Roy, A.; Sahu, R.; Matlam, M.; Deshmukh, V.; Dwivedi, J.; Jha, A. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97. [Google Scholar]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Imokawa, G.; Mishima, Y. Importance of glycoproteins in the initiation of melanogenesis: An electron microscopic study of B-16 melanoma cells after release from inhibition of glycosylation. J. Investig. Dermatol. 1986, 87, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Shin, H.; Hong, S.D.; Roh, E.; Jung, S.H.; Cho, W.J.; Hong Park, S.; Yoon, D.Y.; Ko, S.M.; Hwang, B.Y.; Hong, J.T.; et al. cAMP-dependent activation of protein kinase A as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide. Br. J. Pharmacol. 2015, 172, 3434–3445. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Ünver, N.; Freyschmidt-Paul, P.; Hörster, S.; Wenck, H.; Stäb, F.; Blatt, T.; Elsässer, H.P. Alterations in the epidermal–dermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br. J. Dermatol. 2006, 155, 119–128. [Google Scholar] [CrossRef]
- Motokawa, T.; Kato, T.; Katagiri, T.; Matsunaga, J.; Takeuchi, I.; Tomita, Y.; Suzuki, I. Messenger RNA levels of melanogenesis-associated genes in lentigo senilis lesions. Br. J. Dermatol. 2005, 37, 120–123. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Adjaye, J.; Herwig, R.; Brink, T.C.; Groth, D.; Hultschig, C.; Lehrach, H.; Zouboulis, C.C. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging cell. 2006, 5, 331–344. [Google Scholar] [CrossRef]
- Fussell, J.C.; Kelly, F.J. Oxidative contribution of air pollution to extrinsic skin ageing. Free Radic Biol Med. 2020, 151, 111–122. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for antiaging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Sjerobabski-Masnec, I.; Šitum, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar] [PubMed]
- Davis, E.C.; Callender, V.D. Aesthetic dermatology for aging ethnic skin. Dermatol. Surg. 2011, 37, 901–917. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Panon. Adriat. 2012, 21, 33–36. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Leem, K.H. Effects of Olibanum extracts on the collagenase activity and procollagen synthesis in Hs68 human fibroblasts and tyrosinase activity. Adv. Sci. Technol. Lett. 2015, 88, 172–175. [Google Scholar]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the antiaging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304–311. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- St Clair, D.; Kasarskis, E. Genetic polymorphism of the human manganese superoxide dismutase: What difference does it make? Pharmacogenet. Genom. 2003, 13, 129–130. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Skin aging and photoaging: An overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Moser, C.; Backovic, A.; Wck, G. Cigarette smoke–an aging accelerator? Exp. Gerontol. 2007, 42, 160–165. [Google Scholar] [CrossRef]
- Yin, L.; Morita, A.; Tsuji, T. Skin aging induced by ultraviolet exposure and tobacco smoking: Evidence from epidemiological and molecular studies. Photodermatol. Photoimmunol. Photomed. 2001, 17, 178–183. [Google Scholar] [CrossRef]
- Ortonne, J.P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002, 146, 7–10. [Google Scholar] [CrossRef]
- Kligman, L.M. The nature of photoaging: Its prevention and repair. Photodermatology 1986, 3, 215–227. [Google Scholar]
- Šitum, M.; Buljan, M.; Čavka, V.; Bulat, V.; Krolo, I.; Lugović Mihić, L. Skin changes in the elderly people–how strong is the influence of the UV radiation on skin aging? Collegium antropologicum. 2010, 34, 9–13. [Google Scholar]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Lim, H.W. Clinical Photomedicine; CRC Press: Boca Raton, FL, USA, 1993; Volume 6. [Google Scholar]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 35–147. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2005, 26, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Press, W.; Compton, C.C. Telomere shortening in cultured autografts of patients with burns. Lancet 2003, 361, 1345–1346. [Google Scholar] [CrossRef]
- Nakamura, K.I.; Izumiyama-Shimomura, N.; Takubo, K.; Sawabe, M.; Arai, T.; Aoyagi, Y.; Fujiwara, M.; Tsuchiya, E.; Kobayashi, Y.; Kato, M.; et al. Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J. Investig. Dermatol. 2002, 119, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, U.; Griese, E.U.; Schwab, M.; Fritz, P.; Thon, K.P.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef]
- Yin, B.; Jiang, X. Telomere shortening in cultured human dermal fibroblasts is associated with acute photodamage induced by UVA irradiation. Postepy. Dermatol. Alergol. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Adelfalk, C.; Lorenz, M.; Serra, V.; von Zglinicki, T.; Hirsch-Kauffmann, M.; Schweiger, M. Accelerated telomere shortening in Fanconi anemia fibroblasts–a longitudinal study. FEBS lett. 2001, 506, 22–26. [Google Scholar] [CrossRef]
- Li, G.Z.; Eller, M.S.; Firoozabadi, R.; Gilchrest, B.A. Evidence that exposure of the telomere 3′ overhang sequence induces senescence. Proc. Natl. Acad. Sci. USA 2003, 100, 527–531. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Garmyn, M.; Yarosh, D.B. The molecular and genetic effects of ultraviolet radiation exposure on skin cells. In Photodermatology; CRC Press: Boca Raton, FL, USA, 2007; pp. 41–54. [Google Scholar]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Abdollah, S.; Qiu, Y.; Cai, J.; Xu, Y.Y.; Grinnell, B.W.; Richardson, M.A.; Topper, J.N.; Gimbrone, M.A.; Wrana, J.L.; et al. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 1997, 89, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J. Biol. Chem. 2001, 276, 26349–26356. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J. Biol. Chem. 2005, 280, 8079–8085. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, A.; Park, J.M.; Kim, S.H.; Chung, A.S. Ultraviolet B-induced matrix metalloproteinase-1 and-3 secretions are mediated via PTEN/Akt pathway in human dermal fibroblasts. J. Cell. Physiol. 2006, 209, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Shao, Y.; Lin, L.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am. J. Pathol. 2006, 169, 482–490. [Google Scholar] [CrossRef]
- Angel, P.; Szabowski, A.; Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 2001, 20, 2413–2423. [Google Scholar] [CrossRef]
- Ruland, J.; Mak, T.W. Transducing signals from antigen receptors to nuclear factor κB. Immunol. Rev. 2003, 193, 93–100. [Google Scholar] [CrossRef]
- Kang, S.; Fisher, G.J.; Voorhees, J.J. Photoaging: Pathogenesis, prevention, and treatment. Clin. Geriatr. Med. 2001, 17, 643–659. [Google Scholar] [CrossRef]
- Cooke, C.L.M.; Davidge, S.T. Peroxynitrite increases iNOS through NF-κB and decreases prostacyclin synthase in endothelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, C395–C402. [Google Scholar] [CrossRef]
- Varani, J.; Spearman, D.; Perone, P.; Fligiel, S.E.; Datta, S.C.; Wang, Z.Q.; Shao, Y.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am. J. Clin. Pathol. 2001, 158, 931–942. [Google Scholar] [CrossRef]
- Kumar, M.; Enamala, S.; Chavali, M.; Donepudi, J. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications-A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Wang, H.D.; Chen, C.; Huynh, P.; Chang, J. Exploring the Potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Alencar-Silva, T.; Braga, M.C.; Santana, G.O.S.; Saldanha-Araujo, F.; Pogue, R.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018, 36, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Garciapichel, F.; Wingard, C.E.; Castenholz, R.W. evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Arad, S.M.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Tech. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. potential applications of blue green algae. J. Sci. Ind. Res. 2012, 71, 13–20. [Google Scholar]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Achitouv, E.; Metzger, P.; Rager, M.N.; Largeau, C. C-31-C-34 methylated squalenes from a bolivian strain of Botryococcus braunii. Phytochemistry 2004, 65, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Walker, T.L.; Purton, S.; Becker, D.K.; Collet, C. Microalgae as bioreactors. Plant Cell Rep. 2005, 24, 629–664. [Google Scholar] [CrossRef]
- Levasseur, W.; Perr’e, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mujtaba, G.; Ahmed, S.; Lee, K.; Rashid, N. Exploring the Potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Gayen, K.; Kumar, T. Food and bioproducts processing downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar]
- Balić, A.; Mokos, M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, 183. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Kokabi, K.; Gorelova, O.; Zorin, B.; Didi-Cohen, S.; Itkin, M.; Malitsky, S.; Solovchenko, A.; Boussiba, S.; Khozin-Goldberg, I. Lipidome Remodeling and Autophagic Respose in the Arachidonic-Acid-Rich Microalga Lobosphaera incisa Under Nitrogen and Phosphorous Deprivation. Front. Plant Sci. 2020, 11, 614846. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and methods for in vitro toxicity. In In Vitro Toxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Rajneesh; Singh, S.P.; Hader, D.P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Rajneesh; Pathak, J.; Richa; Hader, D.P.; Sinha, R.P. Impacts of ultraviolet radiation on certain physiological and biochemical processes in cyanobacteria inhabiting diverse habitats. Environ. Exp. Bot. 2019, 161, 375–387. [Google Scholar] [CrossRef]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal Interfilum and Klebsormidium species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499. [Google Scholar] [CrossRef]
- Fernanda, P.M. Algae and aquatic macrophytes responses to cope to ultraviolet radiation—A Review. Emir. J. Food Agric. 2012, 24, 527–545. [Google Scholar] [CrossRef]
- Singh, A.; Tyagi, M.B.; Kumar, A. Cyanobacteria growing on tree barks possess high amount of sunscreen compound mycosporine-like amino acids (MAAs). Plant Physiol. Biochem. 2017, 119, 110–120. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2- glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)- stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Pandey, A.; Gupta, A.; Mishra, S.; Sinha, R.P. Characterization of UV-screening pigment scytonemin from cyanobacteria inhabiting diverse habitats of Varanasi, India. Biologia 2023, 78, 319–330. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Phcog. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J. Microbial sunscreens. Microb. Biotechnol. 2011, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Cosmetics 2006, 9, 1–4. [Google Scholar]
- Tarasuntisuk, S.; Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett. Appl. Microbiol. 2018, 67, 314–320. [Google Scholar] [CrossRef]
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Effects of cosmetic formulations containing hydroxyacids on sun-exposed skin: Current applications and future developments. Dermatol. Res. Pract. 2012, 2012, 710893. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G.I.; Nagashima, H. Occurrence of 3-hydroxy acids in microalgae and cyanobacteria and their geochemical significance. Geochem. Cosmochim. Acta 1984, 48, 1683–1687. [Google Scholar] [CrossRef]
- Matsumoto, G.I.; Shioya, M.; Nagashima, H. Occurrence of 2-hydroxy acids in microalgae. Phytochemistry 1984, 23, 1421–1423. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.C. The Potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. South Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Oh, G.; Ko, S.; Heo, S.; Nguyen, V.; Jung, W. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process Biochem. 2015, 50, 1318–1326. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Wang, S.Q.; Osterwalder, U.; Jung, K. Ex vivo evaluation of radical sun protection factor in popular sunscreens with antioxidants. J. Am. Acad. Dermatol. 2011, 65, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S.; Hsia, A.; Miller, J.D.; Hanneman, K.; Scull, H.; Cooper, K.D.; Baron, E. Non-sunscreen photoprotection: Antioxidants add value to a sunscreen. J. Investig. Dermatol. Symp.Proc. 2009, 14, 56–59. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Grove, G.L.; Hirose, R.; Leyden, J.J. Topical tretinoin for photoaged skin. J. Am. Acad. Dermatol. 1986, 15, 836–859. [Google Scholar] [CrossRef]
- Rosenthal, A.; Stoddard, M.; Chipps, L.; Herrmann, J. Skin cancer prevention: A review of current topical options complementary to sunscreens. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Rodella, L.F.; Favero, G.; Damiani, G.; Paganelli, C.; Reiter, R.J. Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by melatonin. Br. J. Dermatol. 2014, 170, 382–391. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 2017, 7, 1274. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cai, Q.; Rahn, R.O. Inhibition of UV light-and Fenton Reaction-induced oxidative DNA damage by the soybcan isoflavone genistein. Carcinogenesis 1996, 17, 73–77. [Google Scholar] [CrossRef]
- Kang, S.; Chung, J.H.; Lee, J.H.; Fisher, G.J.; Wan, Y.S.; Duell, E.A.; Voorhees, J.J. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J. Investig. Dermatol. 2003, 120, 835–841. [Google Scholar] [CrossRef]
- Yeager, D.G.; Lim, H.W. What’s new in photoprotection: A review of new concepts and controversies. Dermatol. Clin. 2019, 37, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Spencer, J.M.; Braun, M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: Implications for preventing skin aging and cancer. J. Drugs Dermatol. 2014, 13, 309–314. [Google Scholar]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Photoaging: Prevention and topical treatments. Am. J. Clin. Dermatol. 2010, 11, 95–102. [Google Scholar] [CrossRef]
- Green, A.; Williams, G.; Nèale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistutta, D.; Frost, C.; et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomized controlled trial. Lancet 1999, 354, 723–729. [Google Scholar] [CrossRef]
- Darlington, S.; Williams, G.; Neale, R.; Frost, C.; Green, A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch. Dermatol. 2003, 139, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “ Active” photoprotection: Sunscreens with DNA repair enzymes. G. Ital. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Haney, A.M.; Sanfilippo, J.E.; Garczarek, L.; Partensky, F.; Kehoe, D.M. Multiple photolyases protect the marine cyanobacterium Synechococcus from ultraviolet radiation. Mbio 2022, 13, 1511–1522. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zhong, D. Photolyase: Dynamics and electron-transfer mechanisms of DNA repair. Arch. Biochem. Biophys. 2017, 632, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Singh, P.R.; Häder, D.P.; Sinha, R.P. UV-induced DNA damage and repair: A cyanobacterial perspective. Plant Gene 2019, 19, 100194. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Zhong, D. Dynamics and mechanisms of DNA repair by photolyase. Phys. Chem. Chem. Phys. 2015, 17, 11933–11949. [Google Scholar] [CrossRef]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar] [CrossRef]
- Wlaschek, M.; Briviba, K.; Stricklin, G.P.; Sies, H.; Scharffetter-Kochanek, K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J. Investig. Dermatol. 1995, 104, 194–198. [Google Scholar] [CrossRef]
- Wolf, P.; Müllegger, R.R.; Soyer, H.P.; Hofer, A.; Smolle, J.; Horn, M.; Cerroni, L.; Hofmann-Wellenhof, R.; Kerl, H.; Maier, H.; et al. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-α. J. Investig. Dermatol. 2000, 114, 149–156. [Google Scholar] [CrossRef]
- Yarosh, D.; Klein, J.; O’Connor, A.; Hawk, J.; Rafal, E.; Wolf, P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: A randomized study. Lancet 2001, 357, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Cafardi, J.A.; Elmets, C.A. T4 endonuclease V: Review and application to dermatology. Expert Opin. Biol. Ther. 2008, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zattra, E.; Coleman, C.; Arad, S.; Helms, E.; Levine, D.; Bord, E.; Guillaume, A.; El-Hajahmad, M.; Zwart, E.; van Steeg, H.; et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am. J. Pathol. 2009, 175, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Nakayama, H. UV endonuclease of Micrococcus luteus, a cyclobutane pyrimidine dimer–DNA glycosylase/abasic lyase: Cloning and characterization of the gene. Proc. Natl. Acad. Sci. USA 1997, 94, 593–598. [Google Scholar] [CrossRef]
- Wolf, P.; Cox, P.; Yarosh, D.B.; Kripke, M.L. Sunscreens and T4N5 liposomes differ in their ability to protect against ultraviolet-induced sunburn cell formation, alterations of dendritic epidermal cells, and local suppression of contact hypersensitivity. J. Investig. Dermatol. 1995, 104, 287–292. [Google Scholar] [CrossRef]
- Carducci, M.; Pavone, P.S.; De Marco, G.; Lovati, S.; Altabas, V.; Altabas, K.; Emanuele, E. Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: Clinical and molecular findings from a 6-month, randomized, clinical study. J. Drugs Dermatol. 2015, 14, 986–990. [Google Scholar]
- Navarrete-Dechent, C.; Molgó, M. The use of a sunscreen containing DNA-photolyase in the treatment of patients with field cancerization and multiple actinic keratoses: A case-series. Dermatol. Online J. 2017, 23. [Google Scholar] [CrossRef]
- Krutmann, J.; Hansen, P.M. Algenenzym Photolyase verbessert Schutz vor UVB-Schäden. Pharm. Ztg. 2004, 149, 50–53. [Google Scholar]
- Puviani, M.; Barcella, A.; Milani, M. Efficacy of a photolyase-based device in the treatment of cancerization field in patients with actinic keratosis and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2013, 148, 693–698. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).