Abstract
A general chemo-enzymatic approach to synthesize both enantioenriched trans-3-alkoxyamino-4-oxy-2-piperidones, which are important scaffold for various naturally occurring alkaloids, is reported. To this end, a selective transition-metal-free dual C−H oxidation of piperidines mediated by the TEMPO oxoammonium cation (TEMPO+) was used, followed by enzymatic resolution of the corresponding alkoxyamino-2-piperidones with Candida antarctica lipase (CAL-B), to yield the title compounds in high enantiomeric excess (ee). The absolute configuration of both enantioenriched compounds was determined using chemical correlation and circular dichroism (CD) spectroscopy. The former method highlights the oxidative ring contraction of the trans-alkoxyamine-2-piperidone ring into its corresponding 2-pyrrolidinone.
1. Introduction
Alkaloids are naturally occurring secondary metabolites containing a nitrogen atom in the form of amines or amides [1,2,3,4,5,6,7]. Either natural or synthetic alkaloids have gained much attention due to their biological activities with promising pharmacological applications [8].
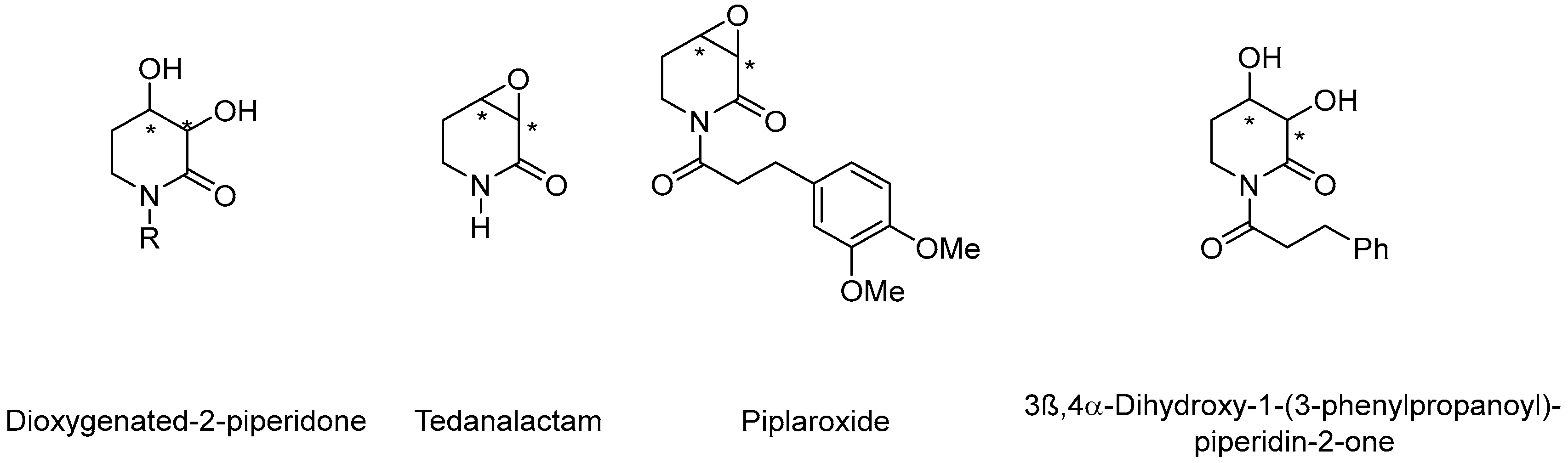
For example, 3,4-dioxygenated-2-pyperidone derived alkaloids from piper species, such as tedanalactam [9], piplaroxide [10], and 3β,4α-dihydroxy-1-(3-phenylpropanoyl)-piperidin-2-one [11], are piperidone alkaloids with enormous pharmaceutical potential (Figure 1).
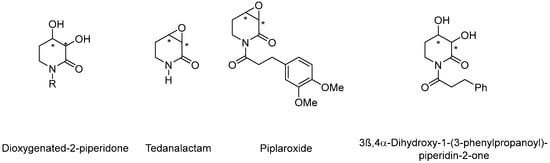
Figure 1.
Representative natural products containing the dioxygenated-2-piperidinone motif; where * represents chiral center.
Although the 3,4-dioxygenated-2-pyperidone system could be considered a non-complex molecular motif, there are only a few known approaches to accessing them in an asymmetric fashion [12].
In this regard, the dual C-H functionalization of piperidines to their 2-piperidones dioxidized at the C3 and C4 positions has proven to be a versatile chemo- and regioselective method to synthesize chiral alkaloid precursors [12,13]. However, given the fact that the sp3 C-H bond is barely reactive, their functionalization poses a great synthetic challenge [14,15]. For this reason, the use of metal catalysts is normally applied [16,17,18]. Unfortunately, these catalysts are generally quite expensive and toxic, causing economic and ecological drawbacks [19,20]. Most recently, a dual C-H oxidation of piperidines to their corresponding piperidin-2-one derivatives under transition-metal-free conditions has shown to be an efficient, accessible, and eco-friendly approach for accessing biologically important alkaloids [21,22,23].
On the other hand, chemo-enzymatic resolution of the racemic mixtures subjected to lipases-catalyzed hydrolysis reactions or esterases-catalyzed ester bond formation has been shown to be a convenient tool for accessing the desired alkaloid chiral products in high enantiomeric excess [23]. The most popular enzyme that carried out hydrolysis and transesterification, along with other reactions, because of its catalytic promiscuity capability [24,25], is probably Lipase B from Candida Antarctica (CAL-B). Another characteristic of CAL-B which is widely exploited by synthetic organic chemists is its capability to catalyze many kinds of organic reactions with a wide variety of substrates in different organic solvents [26,27,28,29,30,31,32,33,34]. Thus, the chemo-enzymatic resolution of the racemic mixtures catalyzed by CAL-B, through alcohol acylation or ester hydrolysis [35,36,37,38,39,40,41,42,43,44], would be a practical approach for accessing the desired chiral products. Since the resolution of secondary alcohols and amines of medium size is the main application of CAL-B, there is a great interest in methods of kinetic resolutions for bulky alcohols [44,45].
In this regard, we discuss the advances of a new approach to access trans-3-alkoxyamine-4-oxygenated-2-piperidones enantiomerically enriched taking advantage of the selective transition-metal-free dual C-H oxidation of piperidines followed by the enzymatic kinetic resolution (EKR) of racemic esters with CAL-B.
2. Results and Discussion
2.1. Synthesis of Rac-Trans-2
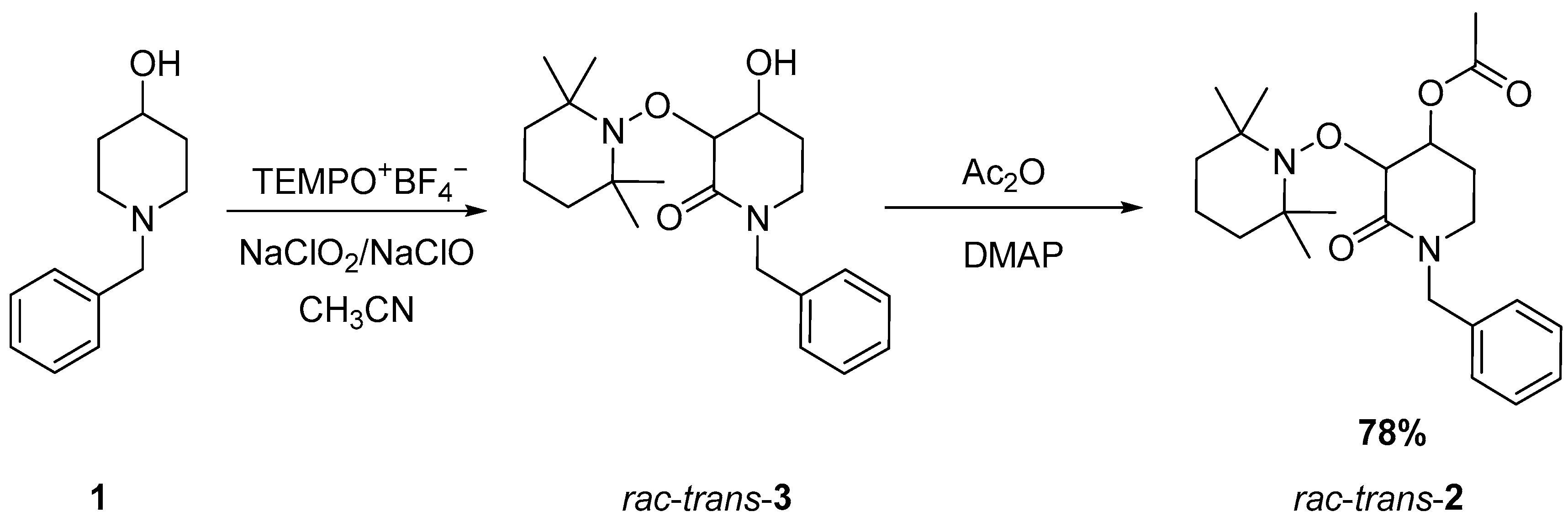
As a widely applied method in our research group [12,22,23], a tandem dual C−H oxidation was carried out using N-benzyl-4-hydroxy-piperidine 1 as starting material. The piperidine derivative was mixed with the TEMPO oxoammonium cation in the presence of sodium chlorite and sodium hypochlorite in acetonitrile to obtain the alkoxyamine lactam (rac-trans-3), which, after acylation, produces rac-trans-2 in good yield (Scheme 1).
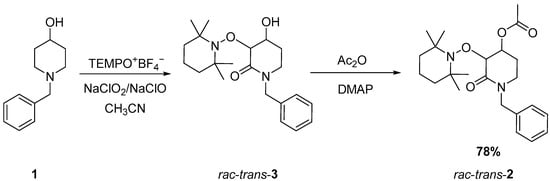
Scheme 1.
Synthesis of rac-trans-2 using dual C-H oxidation mediated by the TEMPO oxoammonium cation.
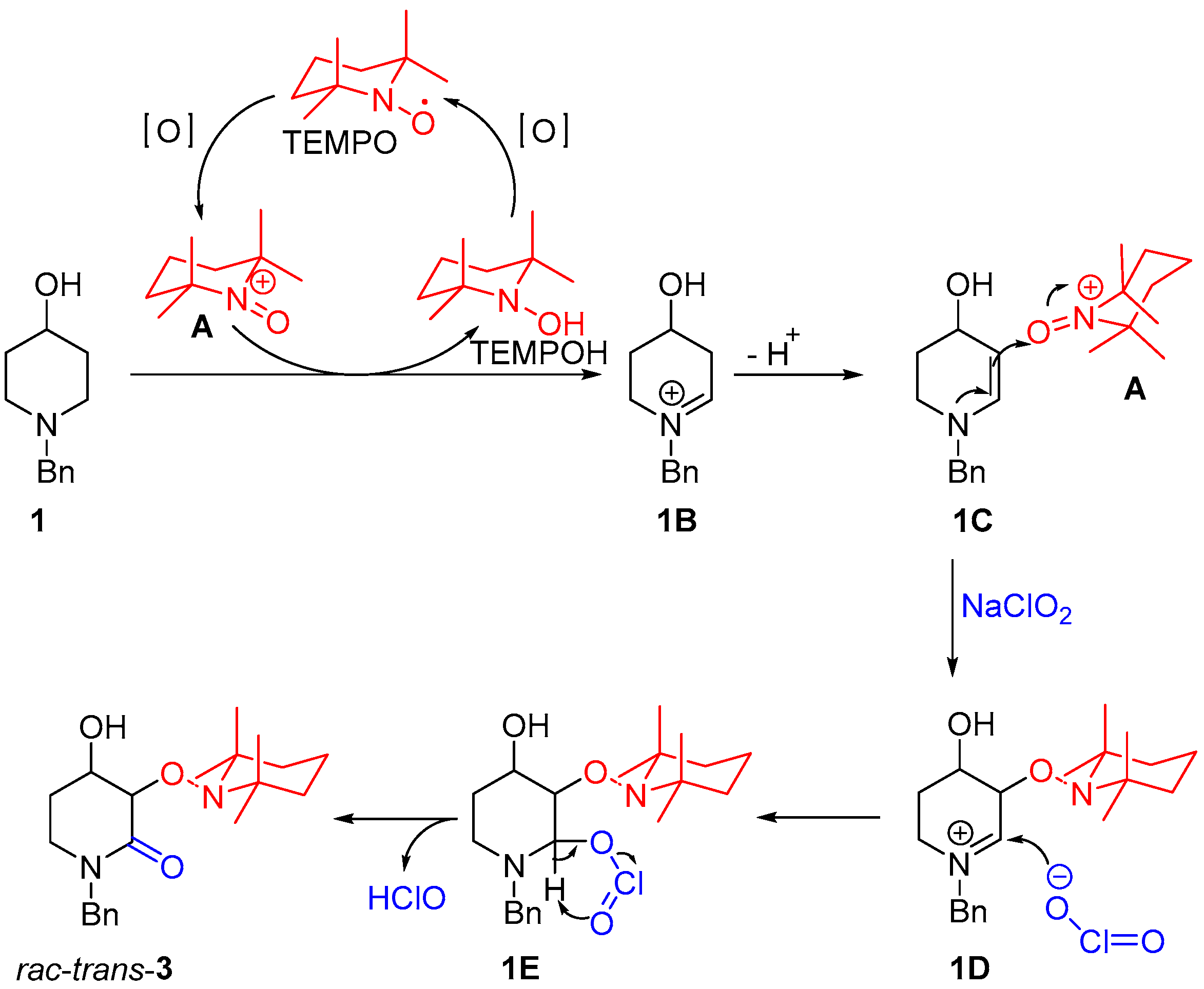
According to the proposed mechanism shown in Scheme 2, cation A promotes the formation of the iminium ion intermediate 1B, which, after the loss of a proton, leads to an enamine intermediate 1C. The addition of TEMPO comes after a nucleophilic attack from the enamine. Finally, a nucleophilic attack by the chlorite ion to the iminium ion intermediate 1D gives 1E, which, after HClO elimination, produces rac-trans-3.
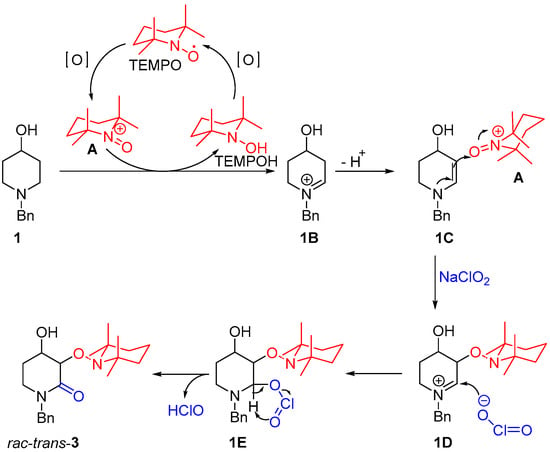
Scheme 2.
Reaction mechanism for the synthesis of rac-trans-3 from 1 (adapted from ref. [22]).
2.2. Enzymatic Resolution and Chiral-HPLC Enantiomeric Excess Evaluation
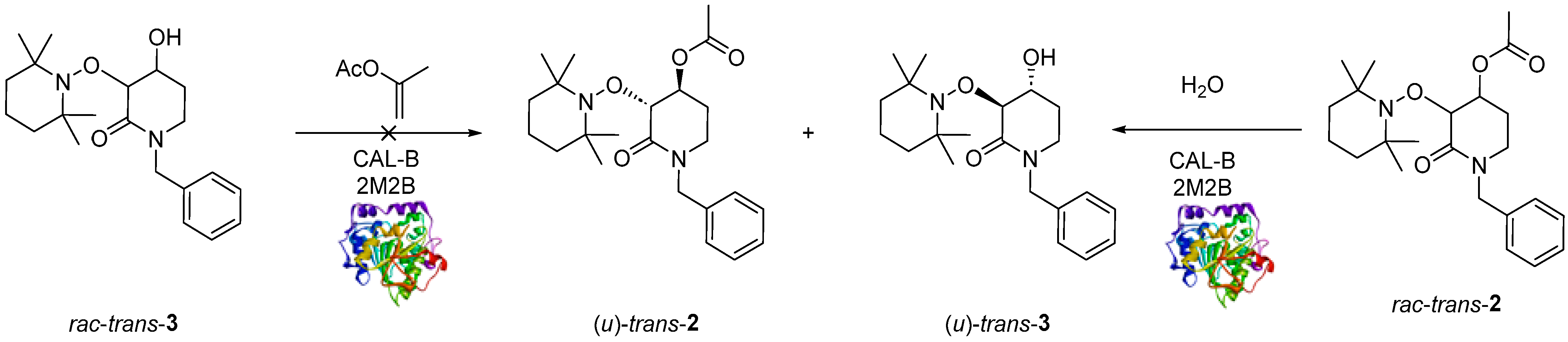
Once rac-trans-2 was obtained, two different approaches were considered for the enzymatic resolution: (a) enzymatic-catalyzed acylation of rac-trans-3 and (b) enzymatic-catalyzed hydrolysis of rac-trans-2 (Scheme 3).
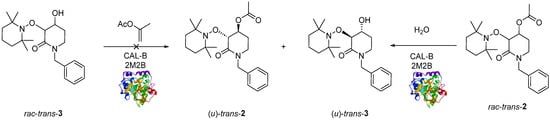
Scheme 3.
CAL-B catalyzed enzymatic resolution: rac-trans-3 acylation (left) and rac-trans-2 de-acylation (right).
First, rac-trans-3 was subjected to a typical enzymatic-catalyzed acylation using CAL-B as a biocatalyst and prop-1-en-2-yl acetate as an acetyl-transfer agent and 2-methyl-2-butanol (2M2B) as solvent [46]. The reaction was monitored with TLC; and after 22 h formation of rac-trans-2 was not detected by TLC plate (Scheme 3, left). One possible explanation is that the alcohol rac-trans-3 could not access the nucleophilic pocket due to its structural characteristics (See Supplementary Material, Figure S1).
Since it was not possible to achieve the enzyme-catalyzed acylation product mediated by CAL-B, we decided to explore the enzymatic de-acylation using rac-trans-2 as substrate [47], CAL-B as a biocatalyst, water as a reagent and 2M2B as the solvent (Scheme 3, right). Several experiments were carried out to determine the best conditions to promote the de-acylation reaction (Table 1).

Table 1.
Reaction conditions for the enzymatic resolution of the racemic mixture of rac-trans-2 to obtain (u)-trans-3.
The de-acylation reaction was carried out in a 0.2 M solution of rac-trans-2 in 2M2B. As can be seen, a very low quantity of the product rac-trans-3 was isolated, which represents a poor percentage of conversion (Entry 1, Table 1). In a second experiment, the equivalents of water were doubled and the reaction time was increased to 72 h (Entry 2, Table 1), which led to only a small improvement.
To enhance the conversion to alcohol (u)-trans-3, a third attempt was performed. We suspect that the low conversion yield can be attributed to the poor interaction of the substrate rac-trans-2 with the enzyme. Therefore, an assay where a more concentrated solution and a higher quantity of the enzyme were considered as well as a higher temperature (Table 1, Entry 3). A slight enhancement was measured. This last result supported the proposed hypothesis. Furthermore, to evaluate the selectivity of CAL-B, it was decided to use the recovered enantioenriched starting material trans-2. A lower conversion degree was obtained because there was less trans-2 enantiomer, which is selectively recognized by CAL-B.
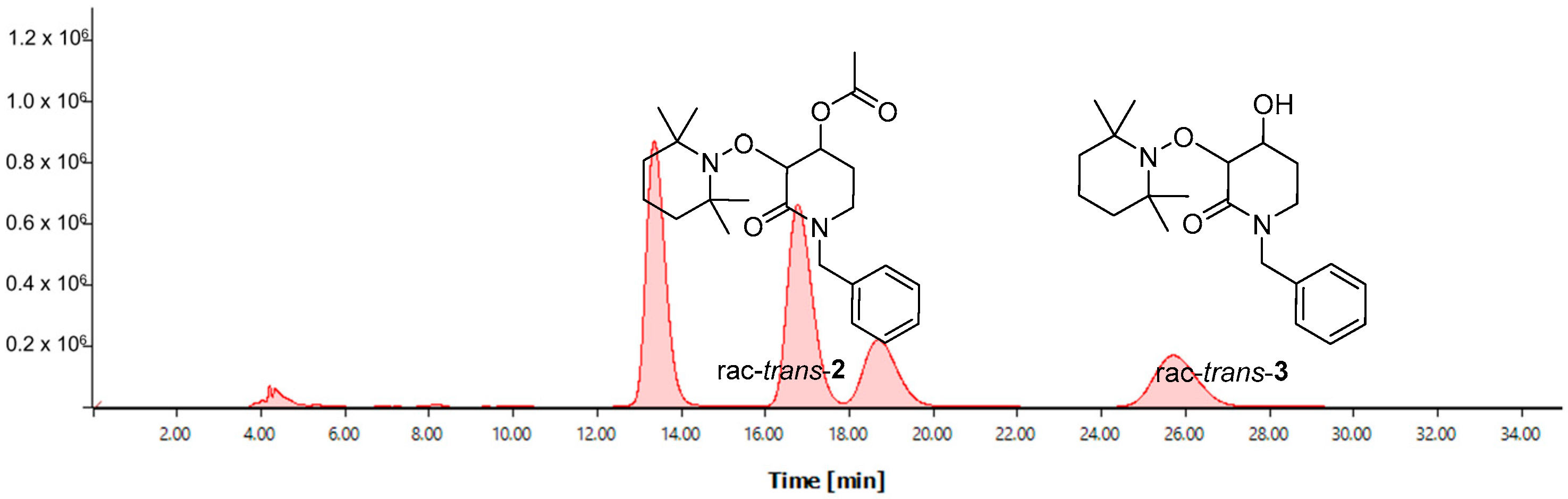
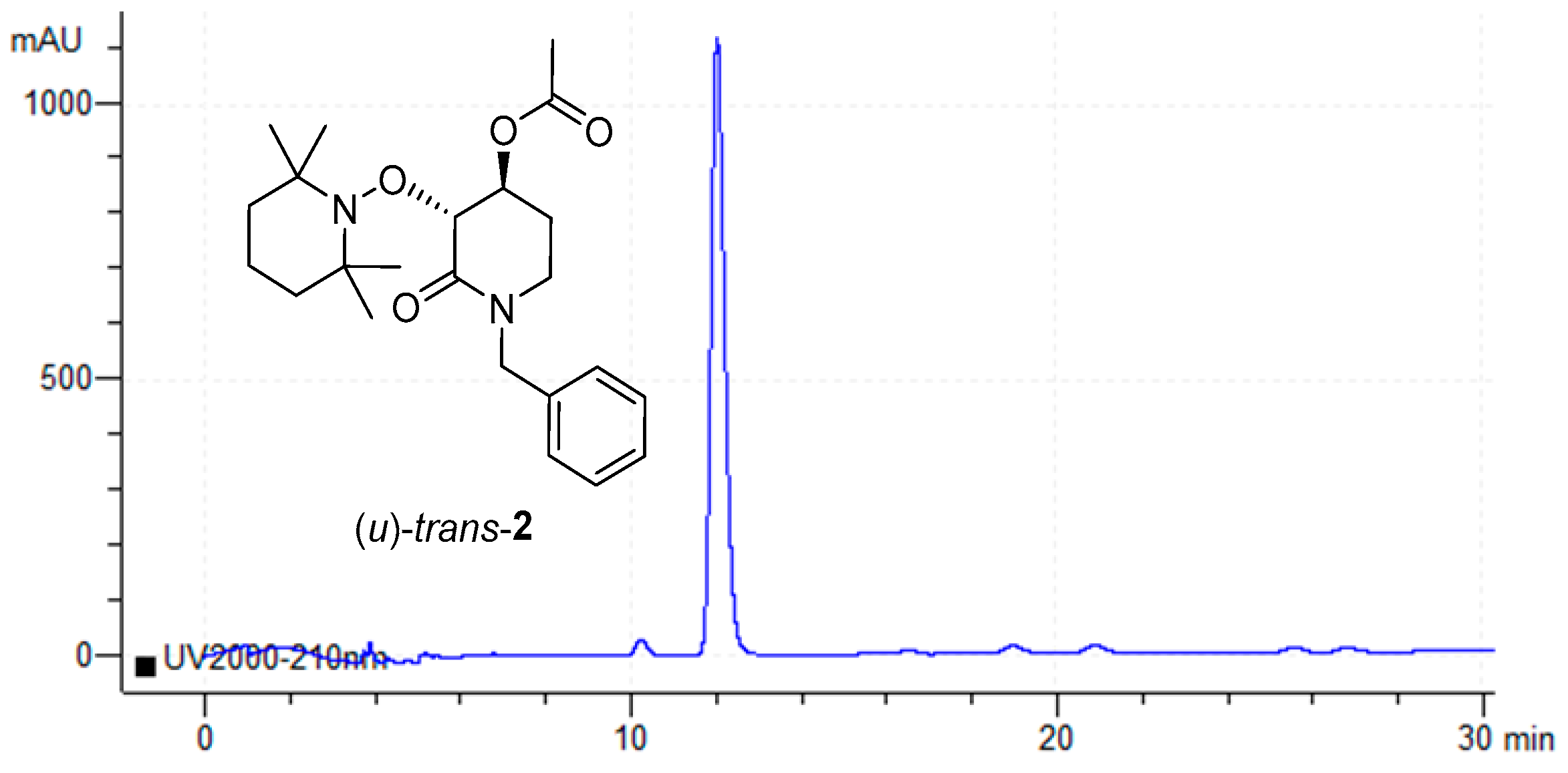
To achieve the enantiomerical quantification of the desired enantioenriched product (u)-trans-3, chiral-HPLC assays were developed. First, the rac-trans-2 substrate was injected into Chiral-HPLC using an OD-H chiral column in an elution system Hex:i-PrOH 98:02 with a flux of 0.8 mL min−1, using a UV-Vis detector at 210 nm, where an excellent chromatography resolution was observed. The corresponding retention times for both (u)-trans-2 enantiomers were 14.18 min and 18.02 min, respectively. Once the HPLC parameters were established, the next step was to demonstrate that these HPLC conditions were applicable for the correct differentiation of rac-trans-2 and rac-trans-3 with a high level of chromatography resolution. To this end, a 2:1 mixture of the corresponding rac-trans-2 and rac-trans-3 was injected into the chiral HPLC. Retention times for both rac-trans-2 enantiomers were 13.36 min and 16.45 min, respectively, while an increase in retention times was observed for both rac-trans-3 enantiomers with 18.69 min and 25.71 min, respectively (Figure 2).
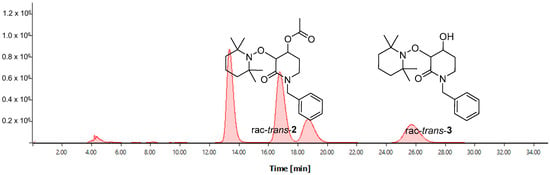
Figure 2.
Chiral-HPLC chromatogram of the 2:1 mixture of both rac-trans-2 and rac-trans-3.
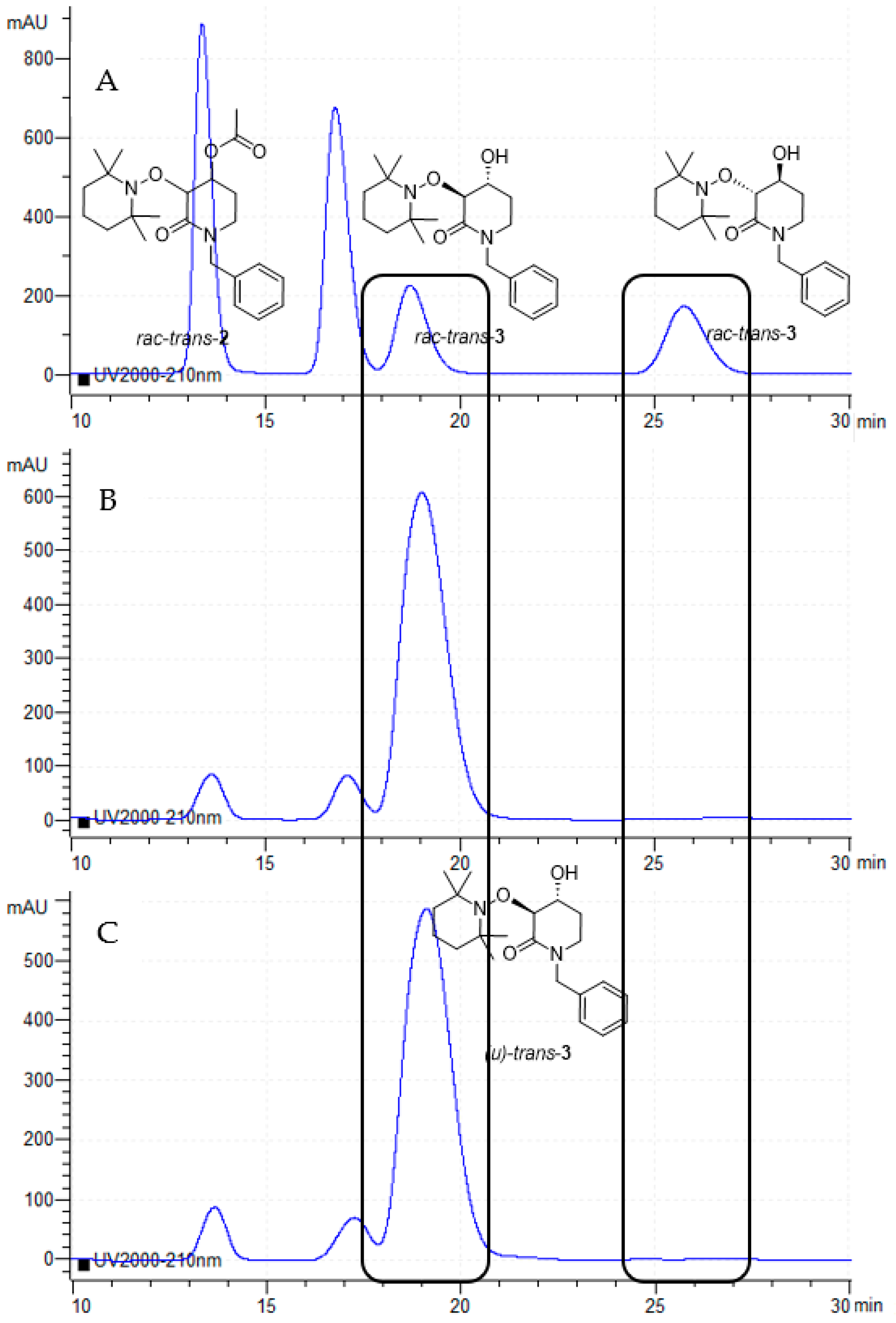
Percolated crude reactions, where rac-trans-3 de-acylated product predominates, were analyzed using the Chiral-Column HPLC technique. Although with low conversion, an excellent enantiomeric excess (99% ee, [α]D20 = +45.2, c 1.0, CHCl3) was obtained, which means that the enzymatic resolution of rac-trans-2 proceeds enantiospecifically (Figure 3), and, moreover, it is a worthwhile method because of the high value (380) obtained for the enantiomeric ratio (E).
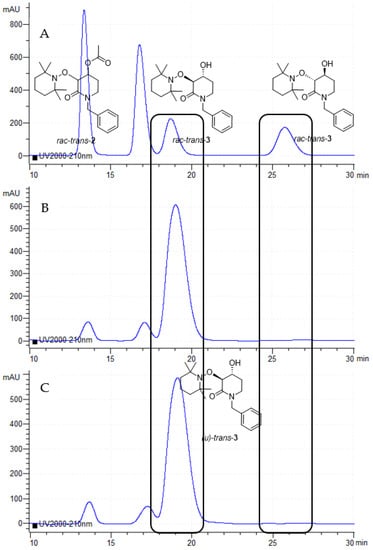
Figure 3.
(A) Chiral-HPLC chromatogram of the 2:1 mixture of both rac-trans-2 and rac-trans-3. (B) Chiral-HPLC chromatogram of the CALB-catalyzed de-acylation reaction at 24 h. (C) Chiral-HPLC chromatogram of the CALB-catalyzed de-acylation reaction at 72 h.
Along with the Chiral-Column HPLC Analysis, the NMR technique was used to determine the conversion percentage of the enzyme-catalyzed-mediated hydrolysis.
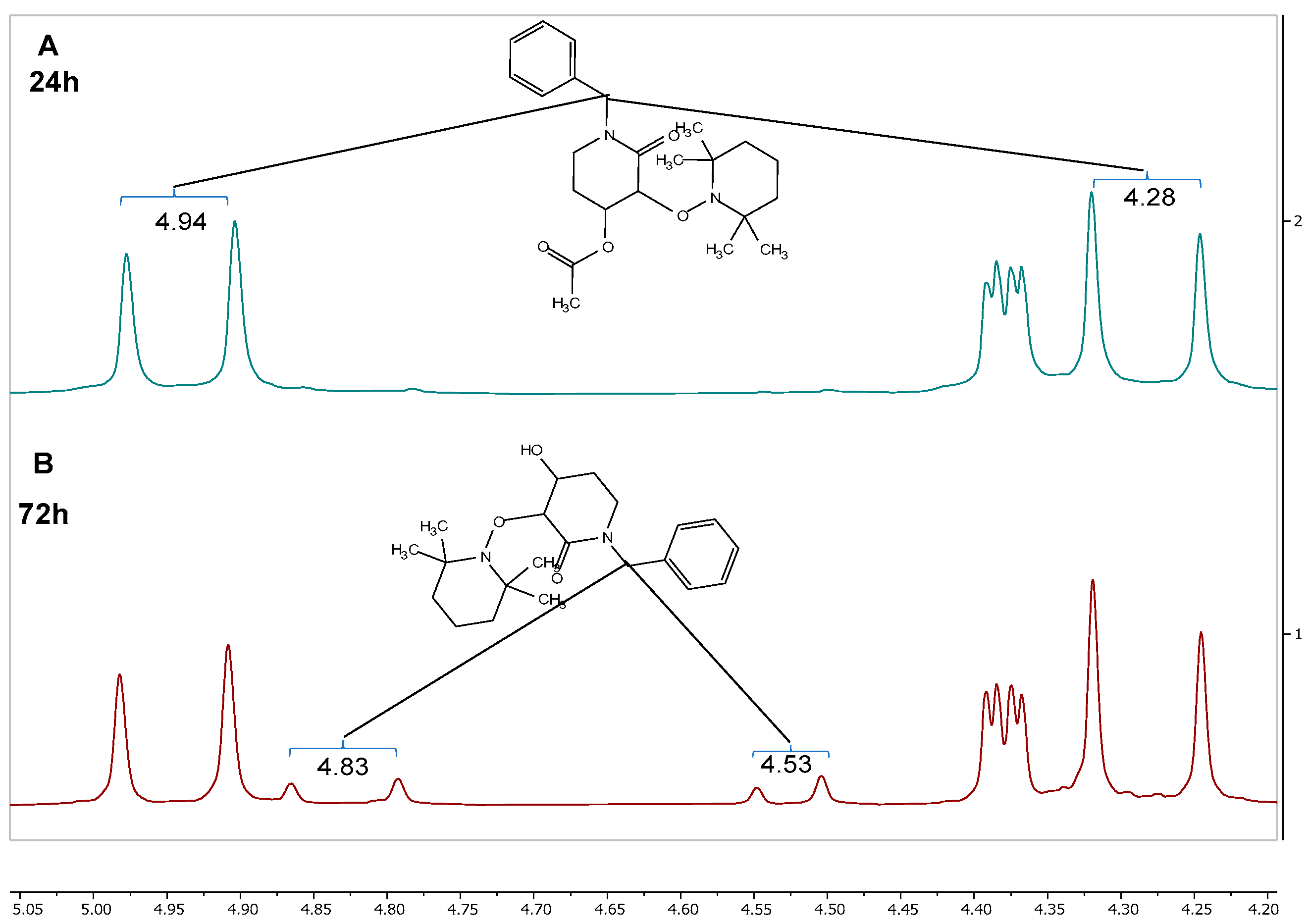
Both reactions previously mentioned were monitored with 1H-NMR using benzylic protons shifts as a reference. At 24 h, there were no notable changes in the 1H-NMR spectra compared with those for the starting material (rac-trans-2), as shown in Figure 4A. However, when the reaction time is increased to 72 h, new signals appeared at 4.83 and 4.53 ppm, which belong to the (u)-trans-3 product (Figure 4B).
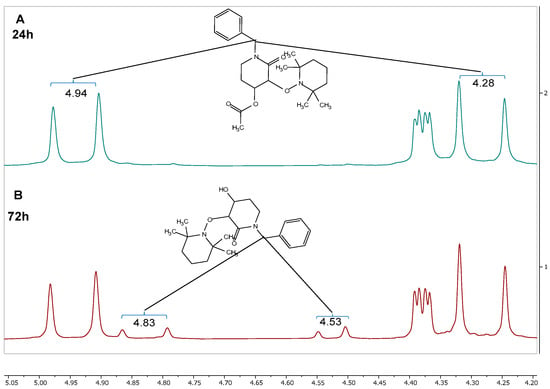
Figure 4.
1H-NMR spectra of the reaction crude after (A) 24 h and (B) 72 h.
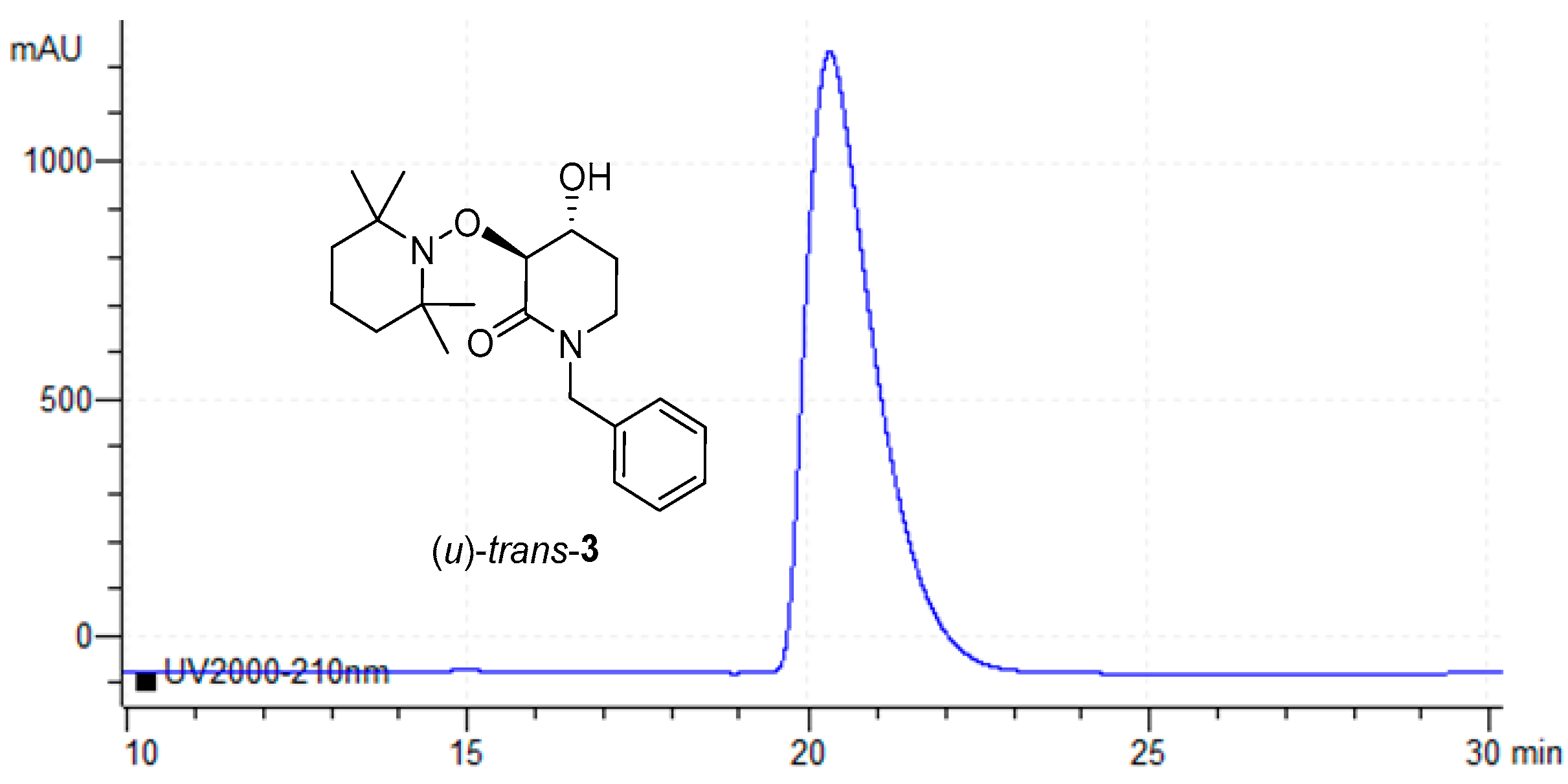
Finally, all isolated products (u)-trans-3 from each assay listed in Table 1 were collected as unique samples and their ee value was measured again using Chiral-HPLC. Identical values of ee as the individual measurements were obtained (≥99 ee). As can be seen, a peak around 20.3 min is detected with no other signals (Figure 5).
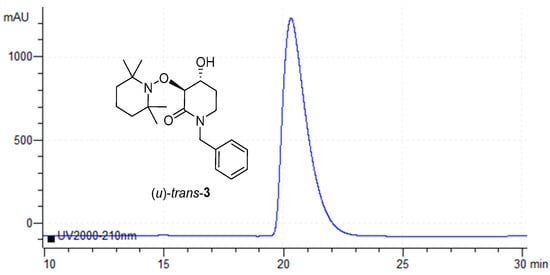
Figure 5.
Chiral-HPLC de-acylation product (u)-trans-3.
Since remanent was used for a second and third enzymatic resolution with the expectation to obtain a more enantiopure substrate (u)-trans-2, after analysis with Chiral-HPLC, a dominant peak was observed at 10.87 min, ≥97 ee (Figure 6).
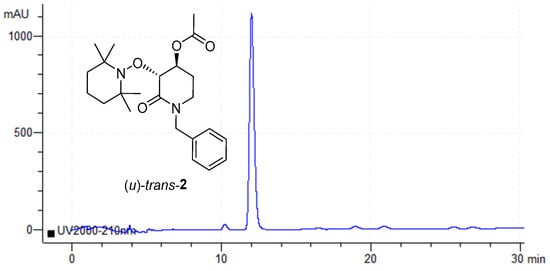
Figure 6.
Chiral-HPLC acetylated compound (u)-trans-2 after 3 enzymatic resolutions.
Although energetically expensive (long reaction times and amount of enzyme), the last method represents an excellent enantiospecific strategy. This result can be attributed to the behavior exhibited by one of the rac-trans-2 enantiomers as the first substrate, which effectively enters the acyl pocket (See Supplementary Material, Figure S1b).
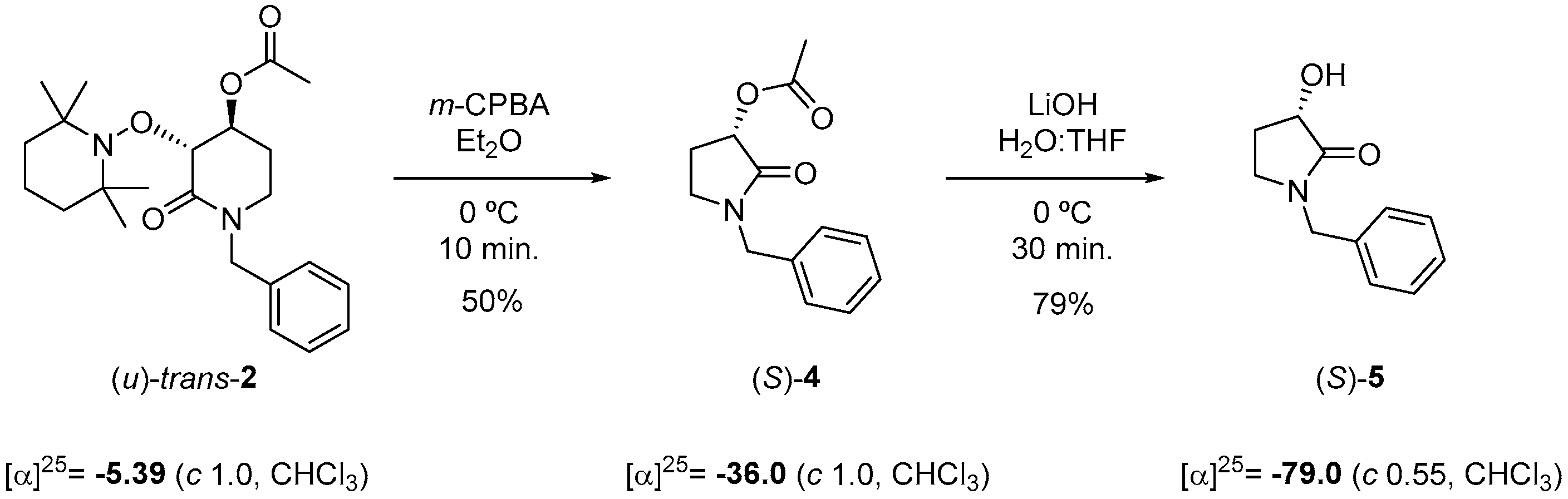
2.3. Chemical Correlation
To assign the absolute configuration, a chemical correlation was performed. Taking advantages that optically pure compound (S)-5 was prepared following Huang et al. [48] from (S)-malic acid, we transformed (u)-trans-2 to (S)-5 by applying deconstructive lactamization protocol [21] with m-CPBA followed by de-acylation in basic conditions of lactam (S)-4 (Scheme 4). The specific rotation for (S)-5 matched to reported [α]D20 = −76.2 (c 1.03, CHCl3).
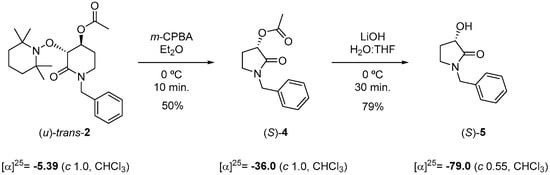
Scheme 4.
Chemical correlation for determining the absolute configuration of (u)-trans-2.
The last result allowed us to assign the absolute configuration of (u)-trans-2 to be (3R, 4S).
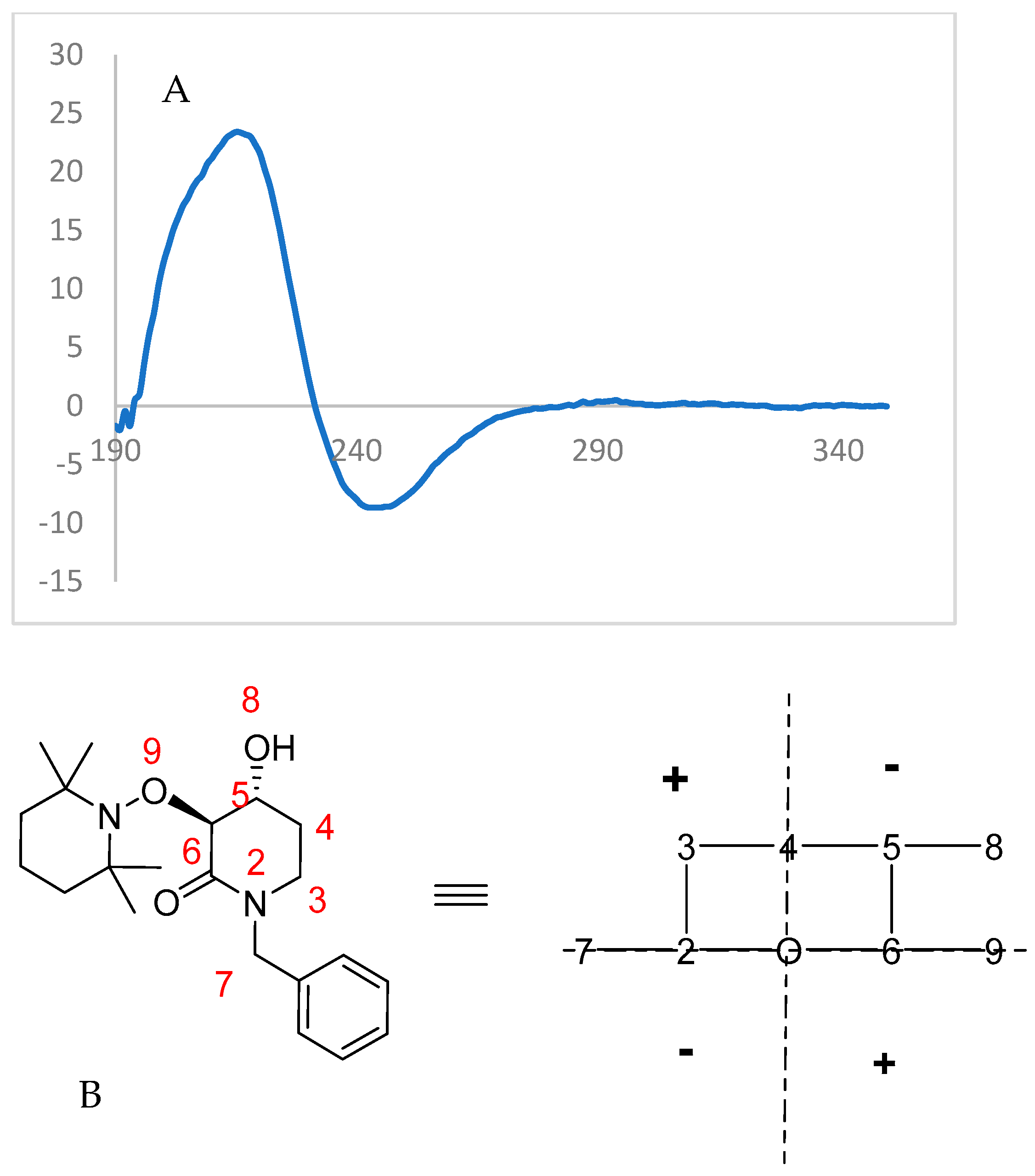
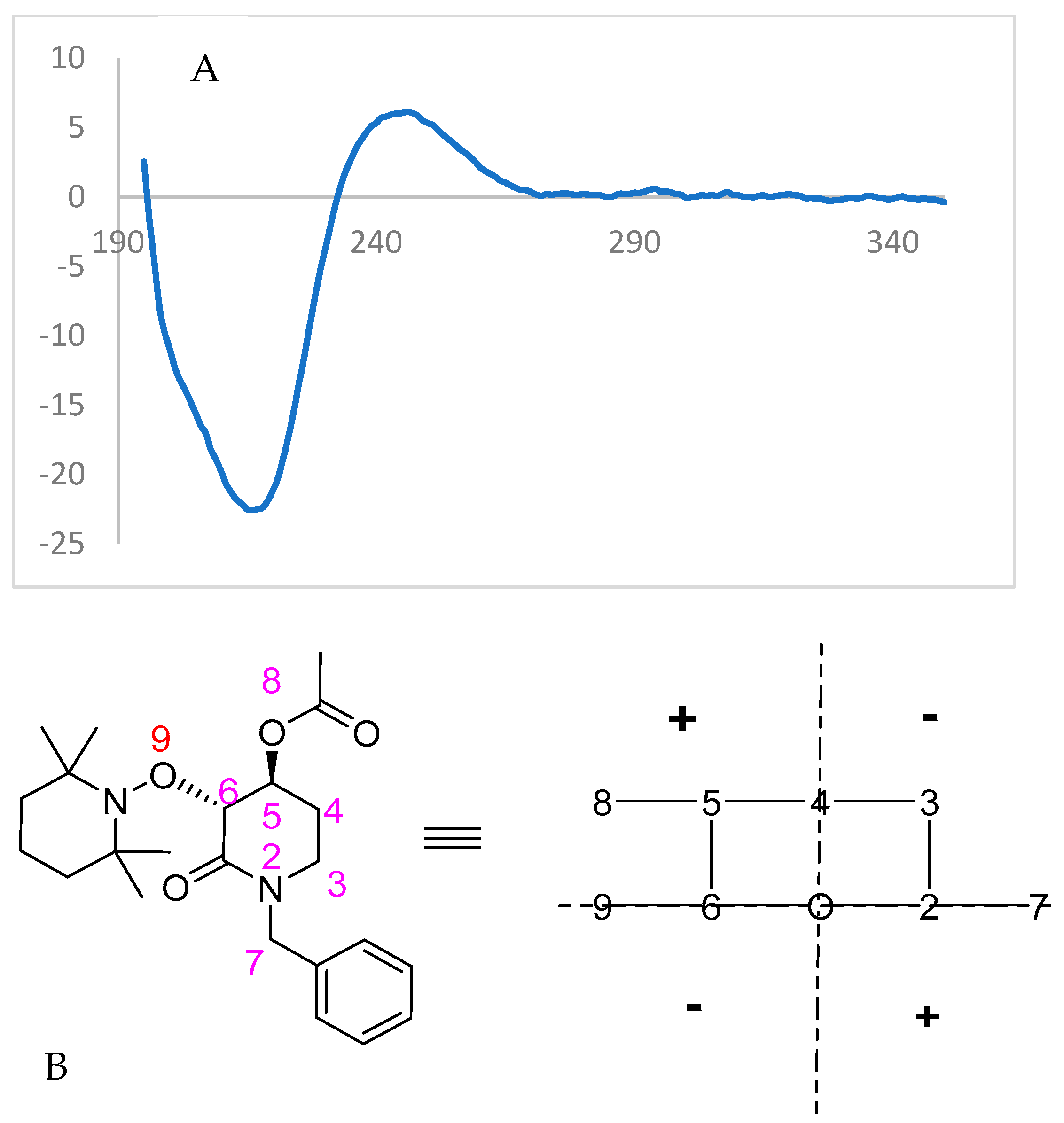
Finally, the highly enantioenriched isolated materials (u)-trans-2 and (u)-trans-3 were also subjected to Circular Dichroism (CD) spectroscopy (λ = 350–195 nm, Conc. = 8 × 10−5 mol/L, solvent = CH3CN). For the enzymatically solved alcohol (u)-trans-3, a negative Cotton effect was observed (Figure 7), while the acylated starting material (u)-trans-2 showed an opposite behavior to that expected from octant rule structures (Figure 8).
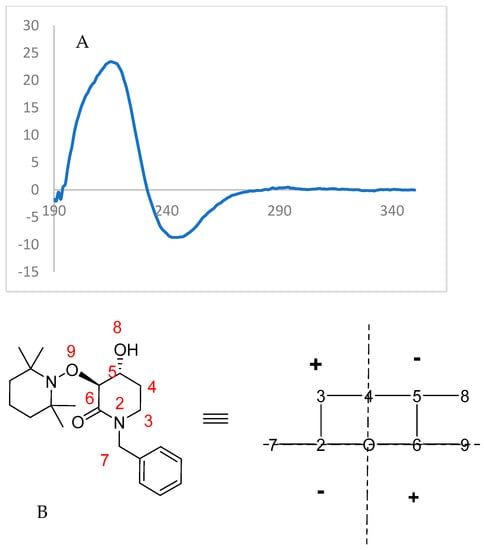
Figure 7.
(A) Experimental CD spectrum of (3S,4R)-(+)-3. (B) Structure transformed with octant rule; a negative cotton effect is expected.
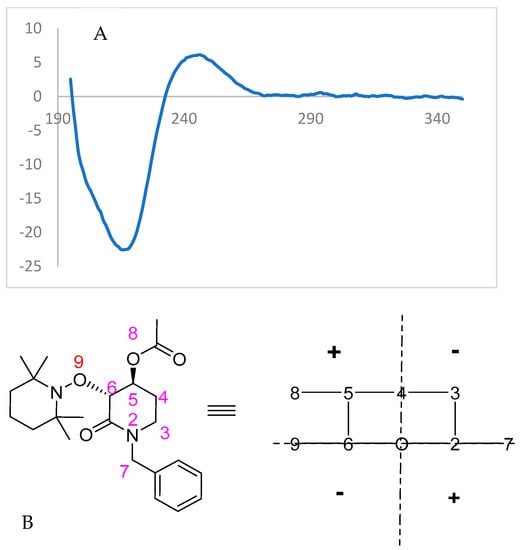
Figure 8.
(A) Experimental CD spectrum of (3R,4S)-(−)-3. (B) Structured transformed with octant rule; a positive cotton effect is expected.
3. Materials and Methods
Commercially available reagents were used without further purification. When inertness conditions were required, the reactions were carried out under an inert argon atmosphere with dry solvents under anhydrous conditions. Candida antarctica lipase B (CAL-B) was obtained as Novozym®435 (Novozymes Mexico). Solvents were used as technical grade and freshly distilled prior to use. Column chromatography (CC) was performed using silica gel (230–400 mesh) from Sigma-Aldrich, Toluca, Mexico with solvents indicated in the text. Analytical grade solvents were purchased from Tecsiquim Toluca, Mexico (i-PrOH), Caledon Laboratory Chemicals Georgetown, Ontario, Canada (n-hexane). NMR spectra were recorded with Bruker-500 (500 MHz) using as reference: TMS for 1H (0.0 ppm) and CDCl3 for 13C (77.16 ppm); chemical shifts (δ) are reported in parts per million (ppm) and Hz for the coupling constants (J). The following abbreviations (or combinations thereof) were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broadened. Melting points were not corrected and carried out using a Fisher-Scientific 12–144 melting point apparatus. Optical rotations were measured with an Autopol-III polarimeter using the sodium D line (589 nm).
Method for 1-Benzyl-2-oxo-3-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)piperidin-4-yl acetate (rac-trans-2): To a solution of 4-hydroxy-3-alkoxyaminolactam rac-trans-3 [16] (1.2 g, 3.33 mmol) and 4-DMAP from Sigma-Aldrich, Toluca, Mexico (0.4 g, 3.33 mmol) in 33.3 mL of anhydrous CH2Cl2 at 0 °C was added Et3N (0.5 g, 5.0 mmol) and acetic anhydride from Sigma-Aldrich, Toluca, Mexico (1.02 g, 10 mmol). After 5 min, the reaction mixture was left to warm and stirred for 1 h. Afterward, 20 mL of water was added, and both phases were separated. The aqueous phase was extracted with CH2Cl2 (25 mL × 3). The combined organic phases were dried (Na2SO4), and the solvent was removed under reduced pressure. The residue was purified using column chromatography [SiO2, hexanes:EtOAc, 5:1] to give 1.2 g (90%) of rac-trans-2 as a white solid. M.p.: 73–74 °C; 1H NMR (500 MHz, CDCl3) δ: 1.13 (br s, 6H), 1.25 (br s, 6H), 1.31–1.35 (m, 1H), 1.47–1.52 (br m, 5H), 1.90–1.95 (m, 1H), 2.01 (s, 3H), 2.28–2.35 (m, 1H), 3.21 (ddd, J = 12.5, 9.5, 6.0 Hz, 1H), 3.30 (ddd, J = 12.5, 7.5, 3.5 Hz, 1H), 4.29 (d, J = 15.0 Hz, 1H), 4.38 (d, J = 2.0 Hz, 1H), 4.94 (d, J = 15.0 Hz, 1H), 5.42 (q, J = 3.5 Hz, 1H), 7.25–7.33 (m, 5H); 13C NMR (125 MHz, CDCl3) δ: 17.2, 20.3, 20.7, 21.1, 23.0, 33.3, 33.9, 40.4(2C), 42.2, 50.1, 60.6(2C), 69.2, 80.7, 127.5, 128.2(2C), 128.6(2C), 136.9, 167.4, 169.9. HRMS (FAB+) m/z: [M+H]+ Calcd for C23H35N2O4+ m/z: 403.5350, Found 403.2599.
Method for (3S,4R)-1-Benzyl-4-hydroxy-3-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)piperidin-2-one ((u)-trans-3). Enzymatic de-acylation reactions were carried out in sealed glass vials. The reactions consisted of the preparation of a solution of rac-trans-2 0.2 M in 2M2B, 2 to 4 equivalents of water, and 20 to 100 mg mL−1 of CAL-B (Novozym®435). The reaction mixtures were stirred in a thermostated water bath at 45 °C or 60 °C. At the end of the reaction, the enzyme was filtered off and washed with DCM. The solvent was evaporated in vacuum. The residue was purified using column chromatography, Hex:EtOAc; 95:05 to 60:40] to give the product (u)-trans-3 as a white solid. Yield 39%; [α]D20 = +45.2 (c 1.0, CHCl3) for product obtained at 60 °C. Spectral data 1H and 13C NMR are consistent with the literature [16]. δ: 1.21–187 (m, 18H), 2.06 (dq, J = 13.5, 3.8 Hz, 1H), 3.14–3.24 (m, 2H), 4.28 (d, J = 14.6 Hz, 1H), 4.33 (m, 1H), 4.53 (d, J = 8.7 Hz, 1H), 4.83 (d, J = 14.6 Hz, 1H), 6.37 (br, 1H), 7.23–7.38 (m, 5H); 13C NMR (125 MHz, CDCl3) d: 17.2, 20.3, 20.7, 21.1, 23.0, 33.3, 33.9, 40.4(2C), 42.2, 50.1, 60.6(2C), 69.2, 80.7, 127.5, 128.2(2C), 128.6(2C), 136.9, 167.4, 169.9 HRMS (FAB+) m/z: [M+H]+ Calcd for C21H33N2O3+ m/z: 361.4680, Found 361.2516. Spectroscopyc data for (u)-trans-3 enantiomerically pure agree with those reported for racemic alcohol
Method for (S)-1-Benzyl-2-oxopyrrolidin-3-yl acetate (S-4): To an enantioenriched solution of (u)-trans-2 (0.1 g, 0.25 mmol) in 8.3 mL of Et2O at 0 °C was added m-CPBA (77%; 0.17 g, 0.75 mmol) slowly. After 10 min of stirring at this temperature, Et3N (0.23 g, 2.25 mmol) and 5 mL of H2O were added. The phases were separated, and the aqueous phase was extracted with Et2O (3 × 8 mL). The combined organic phases were dried with Na2SO4, and the solvent was removed under reduced pressure. The residue was purified using column chromatography [SiO2, hexanes/EtOAc; 4:1 until TEMPO came out of the column, then 1:1] to give 23.9 mg (50%) of S-4 [2] as a colorless oil. = −36.0 (ϲ = 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 1.93 (tdd, J = 13.5, 9.5, 8.0 Hz, 1H), 2.16 (s, 3H), 2.50–2.56 (m, 1H), 3.21 (dt, J = 10.0, 7.5 Hz, 1H), 3.29 (td, J = 9.5, 2.5 Hz, 1H), 4.45 (d, J = 14.5 Hz, 1H), 4.54 (d, J = 14.5 Hz, 1H), 5.35 (t, J = 8.0 Hz, 1H), 7.24–7.36 (m, 5H); 13C NMR (125 MHz, CDCl3) δ: 21.1, 26.1, 43.1, 47.2, 71.4, 128.0, 128.4(2C), 129.0(2C), 135.7, 170.2, 170.5.
Method for (S)-1-Benzyl-3-hydroxypyrrolidin-2-one (S-5): To a solution of S-4 (0.02 g, 0.086 mmol) in 2.9 mL of THF:H2O (2:1) at 0 °C was added LiOH (6.2 mg, 0.26 mmol). The reaction mixture was stirred and monitored with TLC. Upon completion, an aqueous solution of KHSO4 was added until a pH of 1–2 was reached. Then, 10 mL of Et2O was added, the phases were separated, and the aqueous phase was extracted with Et2O (3 × 10 mL). The combined organic phases were dried with Na2SO4, and the solvent was removed under reduced pressure. The residue was purified using column chromatography [SiO2, EtOAc:EtOH; 1:2] to give 13 mg (79%) of S-5 as a white solid. M.p.: 69–70 °C; = −79.0 (ϲ = 0.55, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 1.91–1.99 (m, 1H), 2.39–2.45 (m, 1H), 3.15–3.27 (m, 2H), 4.01 (br, 1H), 4.41–4.51 (m, 3H), 7.23–7.35 (m, 5H); 13C NMR (125 MHz, CDCl3) δ: 27.9, 43.2, 47.2, 70.2, 127.9, 128.3(2C), 128.9(2C), 135.8, 175.0.
4. Conclusions
An asymmetric synthesis approach to trans-3-alkoxyamine-4-oxy-2-piperidones from a simple 4-hydroxypiperidine is described. The synthetic goal was achieved by combining selective dual C−H oxidation of piperidines mediated by the TEMPO cation and CAL-B enzymatic resolution. Since the functionalization of sp3 C−H bonds is performed under transition-metal-free conditions and using cheap and innocuous reagents, this approach represents an eco-friendly alternative to access valuable alkaloid intermediates. This methodology also provides evidence for the capability to use the remaining material in order to achieve a good atom economy. Furthermore, the hydrolysis strategy seems to be a better alternative for bulky alcohol resolution. Deconstructive lactamization of an enantioenriched compound allowed for not only assigning the absolute configuration of the two chiral centers in the title compounds by chemical correlation but also enabled access to another valuable chiron with a high optical purity: (S)-4-hydroxy-2-pyrrolidinone [(S)-5]. Additionally, applications of this novel approach in the total synthesis of biologically important alkaloids will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13040703/s1, Figure S1. Schematic representation for: (a) resolution of the rac-trans-3 as the second substrate (for enantiomer (3S,4R)) and (b) resolution of the rac-trans-2 intermediate as the first substrate (for the fast-reacting enantiomer (3S,4R)-2).
Author Contributions
Conceptualization, J.E. and F.S.-P.; methodology, J.R.-I., M.A.O.-R. and J.R.V.-C.; validation, M.A.O.-R., J.R.V.-C. and J.R.-I.; formal analysis, L.G.H.-V.; investigation, M.A.O.-R., J.R.V.-C. and J.R.-I.; resources, J.E. and F.S.-P.; writing—original draft preparation, J.R.V.-C. and L.G.H.-V.; writing—review and editing, L.G.H.-V., J.E. and F.S.-P.; supervision, J.E. and F.S.-P.; project administration, J.E. and F.S.-P.; funding acquisition, J.E. and F.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT, project numbers CB2019/610262 and A1-S-21450 (FSP).
Data Availability Statement
There is no server where data can be saved for consultation but, if needed, data can be shared by contacting jaime@uaem.mx.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kukula-Koch, W.A.; Widelski, J. Alkaloids. In Pharmacognosy Fundamentals, Applications and Strategy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 163–198. ISBN 9780128021040. [Google Scholar]
- Funayama, S.; Cordell, G.A. Alkaloids: A Treasury of Poisons and Medicines; Academic Press: London, UK, 2015; ISBN 9780124173026. [Google Scholar]
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications, 2nd ed.; Elsevier: Boston, MA, USA, 2015; ISBN 9780444594334. [Google Scholar]
- Buckingham, J.; Baggaley, K.H.; Roberts, A.D.; Szábo, L.F. (Eds.) Dictionary of Alkaloids with CDROM, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420077698. [Google Scholar]
- Mahajan, M.; Kumar, V.; Yadav, S.K. Alkaloids: Properties, Applications, and Pharmacological Effects; Cassiano, N.M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 1–36. ISBN 13 9781612090962. [Google Scholar]
- von Nussbaum, F. In Alkaloids: Nature’s Curse or Blessing? By Manfred Hesse. Angew. Chem. Int. Ed. 2003, 42, 4852–4854. [Google Scholar] [CrossRef]
- Roberts, M.F.; Wink, M. (Eds.) Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Plenum Press: New York, NY, USA, 1998; ISBN 0-306-45465-3. [Google Scholar]
- Cigan, E.; Eggbauer, B.; Schrittwieser, J.-H.; Kroutil, W. The role of biocatalysis in the asymmetric synthesis of alkaloids—An update. RCS Adv. 2021, 11, 28223–28270. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Kato, M.J. 3α,4α-Epoxy-2-piperidone, a new minor derivative from leaves of Piper crassinervium Kunth (Piperaceae). Nat. Prod. Res. 2007, 21, 910–914. [Google Scholar] [CrossRef]
- Capron, M.A.; Wiemer, D.F. Piplaroxide, an Ant-Repellent Piperidine Epoxide from Piper tuberculatum. J. Nat. Prod. 1996, 59, 794. [Google Scholar] [CrossRef]
- Zhi-Yong, J.; Wen-Feng, L.; Chao-Guang, H.; Xiang-Zhong, H. New Amide Alkaloids from Piper longum. Fitoterapia 2013, 84, 222–226. [Google Scholar]
- Osorio-Nieto, U.; Chamorro-Arenas, D.; Quintero, L.; Höpfl, H.; Sartillo-Piscil, F. Transition Metal-Free Selective Double sp3 C-H Oxidation of Cyclic Amines to 3-Alkoxyamine Lactams. J. Org. Chem. 2016, 81, 8625–8632. [Google Scholar] [CrossRef]
- Griffiths, R.-J.; Burley, G.-A.; Talbot, E.-P.-A. Transition-Metal-Free Amine Oxidation: A Chemoselective Strategy for the Late-Stage Formation of Lactams. Org. Lett. 2017, 19, 870–873. [Google Scholar] [CrossRef]
- Xue, X.-S.; Ji, P.; Zhou, B.; Cheng, J.-P. The Essential Role of Bond Energetics in C–H Activation/Functionalization. Chem. Rev. 2017, 117, 8622–8648. [Google Scholar] [CrossRef] [PubMed]
- Blanksby, S.-J.; Ellison, G.-B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.-K.; Youn, S.W. C-H Activation: A Complementary Tool in the Total Synthesis of Complex Natural Products. Chem. Eur. J. 2012, 18, 9452–9474. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [Google Scholar] [CrossRef]
- Tan, P.-W.; Seayad, J. Advances in amide and thioamide assisted C(sp3)-H functionalization. Tetrahedron Lett. 2019, 60, 151338. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, U. Remote C-H bond activation/transformations: A continuous growing synthetic tool; Part II. Catal. Rev. 2018, 60, 497–565. [Google Scholar] [CrossRef]
- Li, B.; Ali, A.-I.-M.; Ge, H. Recent Advances in Using Transition-Metal-Catalyzed C–H Functionalization to Build Fluorescent Materials. Chem 2020, 6, 2591–2657. [Google Scholar] [CrossRef]
- Romero-Ibañez, J.; Cruz-Gregorio, S.; Sandoval-Lira, J.; Hernández-Pérez, J.M.; Quintero, L.; Sartillo-Piscil, F. Transition-metal-free deconstructive lactamization of piperidines. Angew. Chem. Int. Ed. 2019, 58, 8867–8871. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ibañez, J.; Fuentes, L.; Sartillo-Piscil, F. Transition-Metal-Free Functionalization of Saturated and Unsaturated Amines to Bioactive Alkaloids Mediated by Sodium Chlorite. Synlett 2021, 32, 1385–1396. [Google Scholar] [CrossRef]
- Recoba-Torres, A.; Cruz-Gregorio, A.; Quintero, L.; Sandoval-Lira, J.; Romero-Ibañez, J.; Sartillo-Piscil, F. Selective deconstructive lactamization of the indolo [2,3-α]quinolizine skeleton for the total synthesis of (+) and (−)-cuscutamine. Eur. J. Org. Chem. 2022, 2022, e202200292. [Google Scholar] [CrossRef]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef] [PubMed]
- Bordes, I.; Recatalá, J.; Świderek, K.; Moliner, V. Is Promiscuous CALB a Good Scaffold for Designing New Epoxidases? Molecules 2015, 20, 17789–17806. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. Enzyme-catalyzed processes in organic solvents. Proc. Nat. Acad. Sci. USA 1985, 82, 3192–3196. [Google Scholar] [CrossRef]
- Flores-Sánchez, P.; Escalante, J.; Castillo, E. Enzymatic Resolution of N-Protected-β3-Amino Methyl Esters, Using Lipase B from Candida Antarctica. Tetrahedron Assym. 2005, 16, 629–634. [Google Scholar] [CrossRef]
- Torres-Gavilán, A.; Escalante, J.; Regla, I.; López-Munguía, A.; Castillo, E. “Easy-On, Easy-Off” resolution of Chiral 1-Phenylethylamine Catalyzed by Candida Antarctica Lipase, B. Tetrahedron Asym. 2007, 18, 2621–2624. [Google Scholar] [CrossRef]
- Priego, J.; Ortíz-Nava, C.; Carrillo-Morales, M.; López-Munguía, A.; Escalante, J.; Castillo, E. Solvent Engineering: An Effective Tool to Direct Chemoselectivity in a Lipase-Catalyzed Michael Addition. Tetrahedron 2009, 65, 536–539. [Google Scholar] [CrossRef]
- Rivera-Ramírez, J.D.; Escalante, J.; López-Munguía, A.; Marty, A.; Castillo, E. Thermodynamically Controlled Chemoselectivity in Lipase-Catalyzed Aza-Michael Additions. J. Mol. Catal. B Enzym. 2015, 112, 76–82. [Google Scholar] [CrossRef]
- Pérez-Venegas, M.; Reyes-Rangel, G.; Neri, A.; Escalante, J.; Juaristi, E. Mechanochemical Enzymatic Resolution of N -Benzylated-β 3 -Amino Esters. Beilstein J. Org. Chem. 2017, 13, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Rojas, M.; Rivera-Ramírez, J.; Ávila-Ortiz, C.; Juaristi, E.; González-Muñoz, F.; Castillo, E.; Escalante, J. One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(Benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity. Molecules 2017, 22, 2189. [Google Scholar] [CrossRef]
- Ortega-Rojas, M.A.; Castillo, E.; Razo-Hernández, R.S.; Pastor, N.; Juaristi, E.; Escalante, J. Effect of the Substituent and Amino Group Position on the Lipase-Catalyzed Resolution of γ-Amino Esters: A Molecular Docking Study Shedding Light on Candida Antarctica Lipase B Enantioselectivity. Eur. J. Org. Chem. 2021, 2021, 4790–4802. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, G.K.; Ortega-Rojas, M.A.; Chavelas-Hernández, L.; Razo-Hernández, R.S.; Valdéz-Camacho, J.R.; Escalante, J. Solvent-Free Lipase-Catalyzed Transesterification of Alcohols with Methyl Esters Under Vacuum-Assisted Conditions. ChemistrySelect 2022, 7, e202202643. [Google Scholar] [CrossRef]
- Otera, J.; Nishikido, J. Kinetic Resolution. In Esterification- Methods, Reactions, and Applications; Wiley-VCH: Hoboken, NJ, USA, 2010; pp. 197–205. ISBN 978-3-527-32289-3. [Google Scholar]
- Ohtani, T.; Nakatsukasa, H.; Kamezaw, M.; Tachibana, H.; Naoshima, Y. Enantioselectivity of Candida antarctica lipase for some synthetic substrates including aliphatic secondary alcohols. J. Mol. Catal. B Enzym. 1998, 4, 53–60. [Google Scholar] [CrossRef]
- Rocha, L.-C.; Rosset, I.-G.; Luiz, R.-F.; Raminelli, C.; Porto, A.-L.-M. Kinetic resolution of iodophenylethanols by Candida antarctica lipase and their application for the synthesis of chiral biphenyl compounds. Tetrahedron Asym. 2010, 18, 926–929. [Google Scholar] [CrossRef]
- Ferraz, H.-M.-C.; Bianco, G.-G.; Teixeira, C.-C.; Andrade, L.-H.; Porto, A.-L.-M. Enzymatic resolution of α-tetralols by CALB-catalyzed acetylation. Tetrahedron Asym. 2007, 18, 1070–1076. [Google Scholar] [CrossRef]
- Raminelli, C.; Comasseto, J.-V.; Andrade, L.-H.; Porto, A.-L.-M. Kinetic resolution of propargylic and allylic alcohols by Candida antarctica lipase (Novozyme 435). Tetrahedron Asym. 2004, 15, 3117–3122. [Google Scholar] [CrossRef]
- Ferreira, H.-V.; Rocha, L.-C.; Severino, R.-P.; Viana, R.-B.; Da Silva, A.-B.-F.; Porto, A.-L.-M. Enzymatic Resolution of Racemic Sulcatol by Lipase from Candida antarctica in a Large Scale. J. Iran. Chem. Soc. 2010, 7, 883–889. [Google Scholar] [CrossRef]
- Ferreira, H.-V.; Rocha, L.-C.; Severino, R.-P.; Porto, A.-L.-M. Syntheses of Enantiopure Aliphatic Secondary Alcohols and Acetates by Bioresolution with Lipase B from Candida antarctica. Molecules 2012, 17, 8955–8967. [Google Scholar] [CrossRef] [PubMed]
- Henández, J.G.; Frings, M.; Bolm, C. Mechanochemical enzymatic resolution of secondary alcohols under ball-milling conditions. Chem. Cat. Chem. 2016, 8, 1769–1772. [Google Scholar] [CrossRef]
- Bocquin, L.; Egholm-Jacobsen, E. Chemoenzymatic Protocol for the Synthesis of Enantiopure β-Blocker (S)-Bisoprolol. Catalysts 2023, 13, 54. [Google Scholar] [CrossRef]
- Escorcia, A.-M.; Daza, M.-C.; Doerr, M. Computational study of the enantioselectivity of the O-acetylation of (R,S)-propranolol catalyzed by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2014, 108, 21–31. [Google Scholar] [CrossRef]
- Shen, J.-W.; Qi, J.-M.; Zhang, X.-J.; Liu, Z.-Q.; Zheng, Y.-G. Significantly increased catalytic activity of Candida antarctica lipase B for the resolution of cis-(±)-dimethyl 1-acetylpiperidine-2,3-dicarboxylate. Catal. Sci. Technol. 2018, 8, 4718–4725. [Google Scholar] [CrossRef]
- Husson, E.; Garcia-Matilla, V.; Humeau, C.; Chevalot, I.; Fournier, F.; Marc, I. Enzymatic acylation of a bifunctional molecule in 2-methyl-2-butanol: Kinetic modelling. Enzyme Microb. Technol. 2010, 46, 338–346. [Google Scholar] [CrossRef]
- Kamal, A.; Ramana, K.; Ramana, A.; Babu, A. Chemoenzymatic enantioselective synthesis of 3-hydroxy-2-pyrrolidinones and 3-hydroxy-2-piperidinones. Tetrahedron Asymm. 2003, 14, 2587–2594. [Google Scholar] [CrossRef]
- Huang., P.-Q.; Zheng, X.; Wei, H. Synthesis of (S)-Vasicol and (S)-3-hydroxy-2-pyrrolidinone. Heterocycles 2003, 60, 1833–1841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).