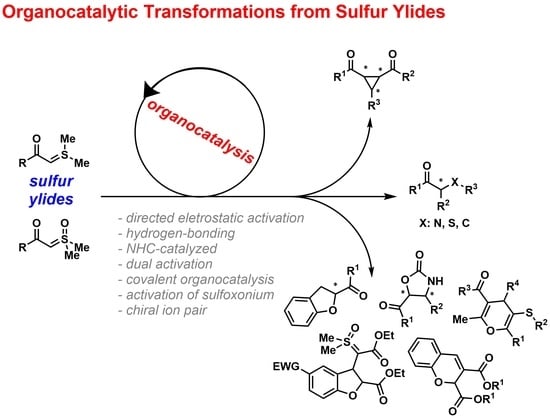
Organocatalytic Transformations from Sulfur Ylides
Abstract
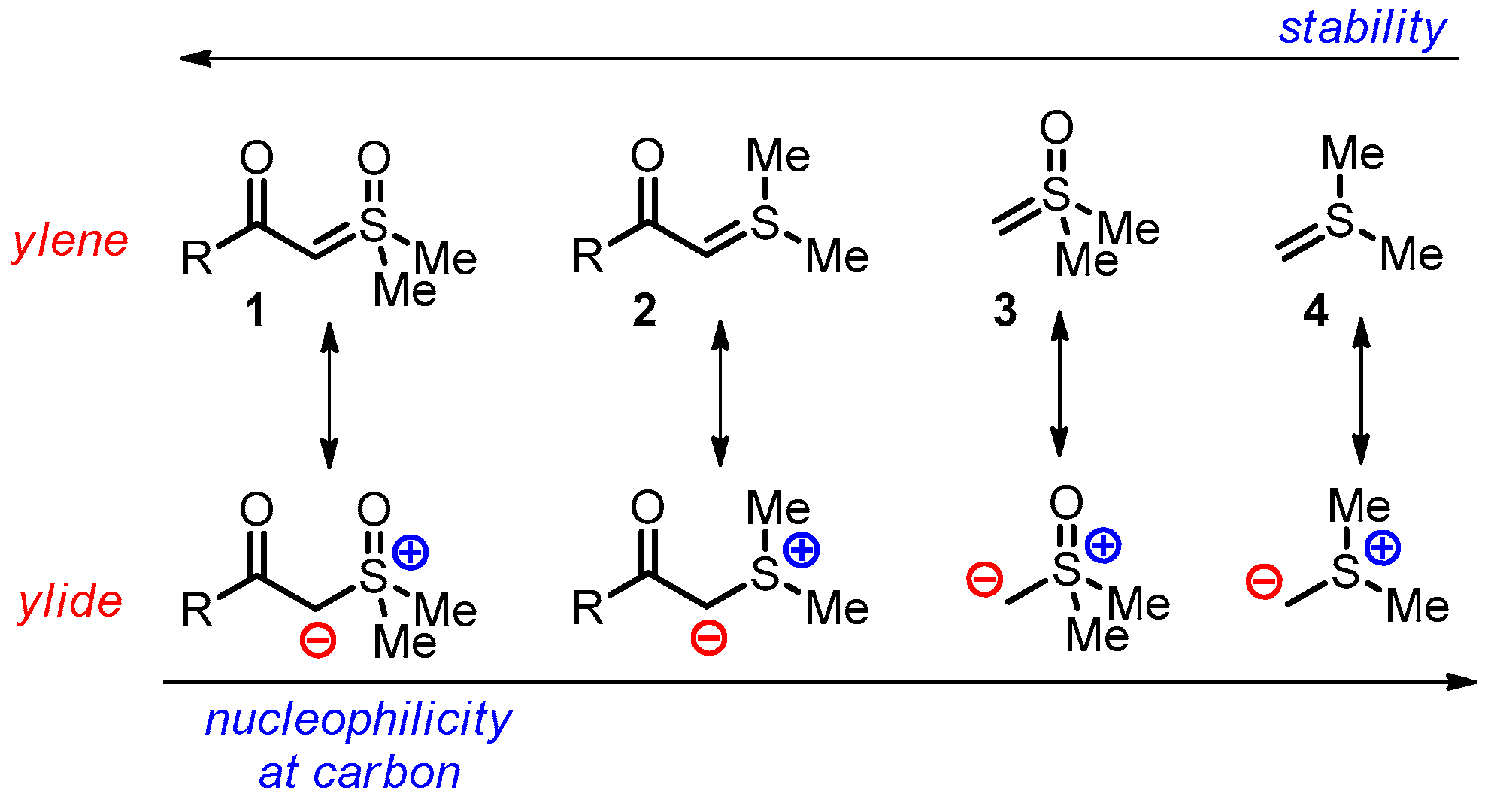
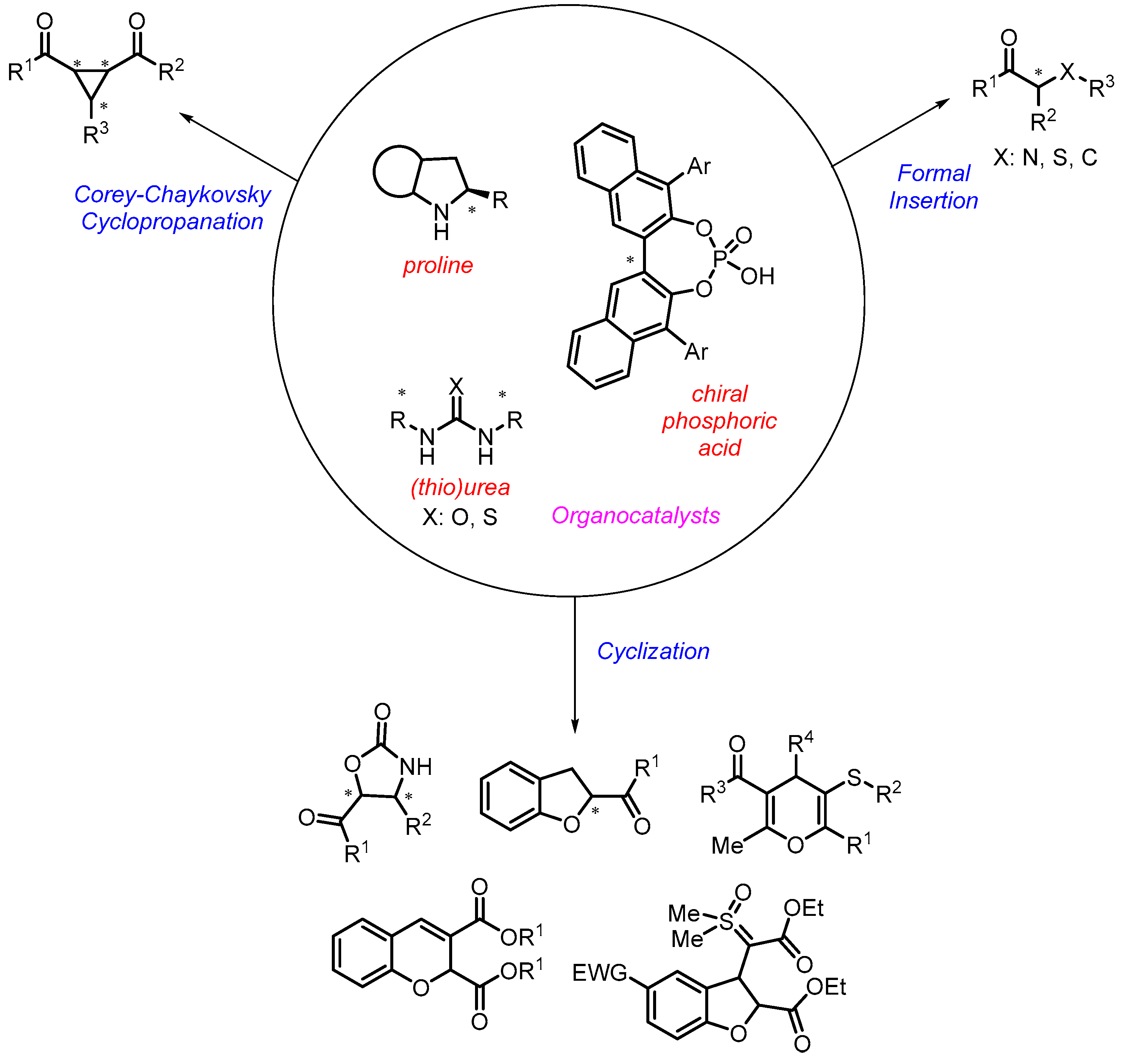
1. Introduction
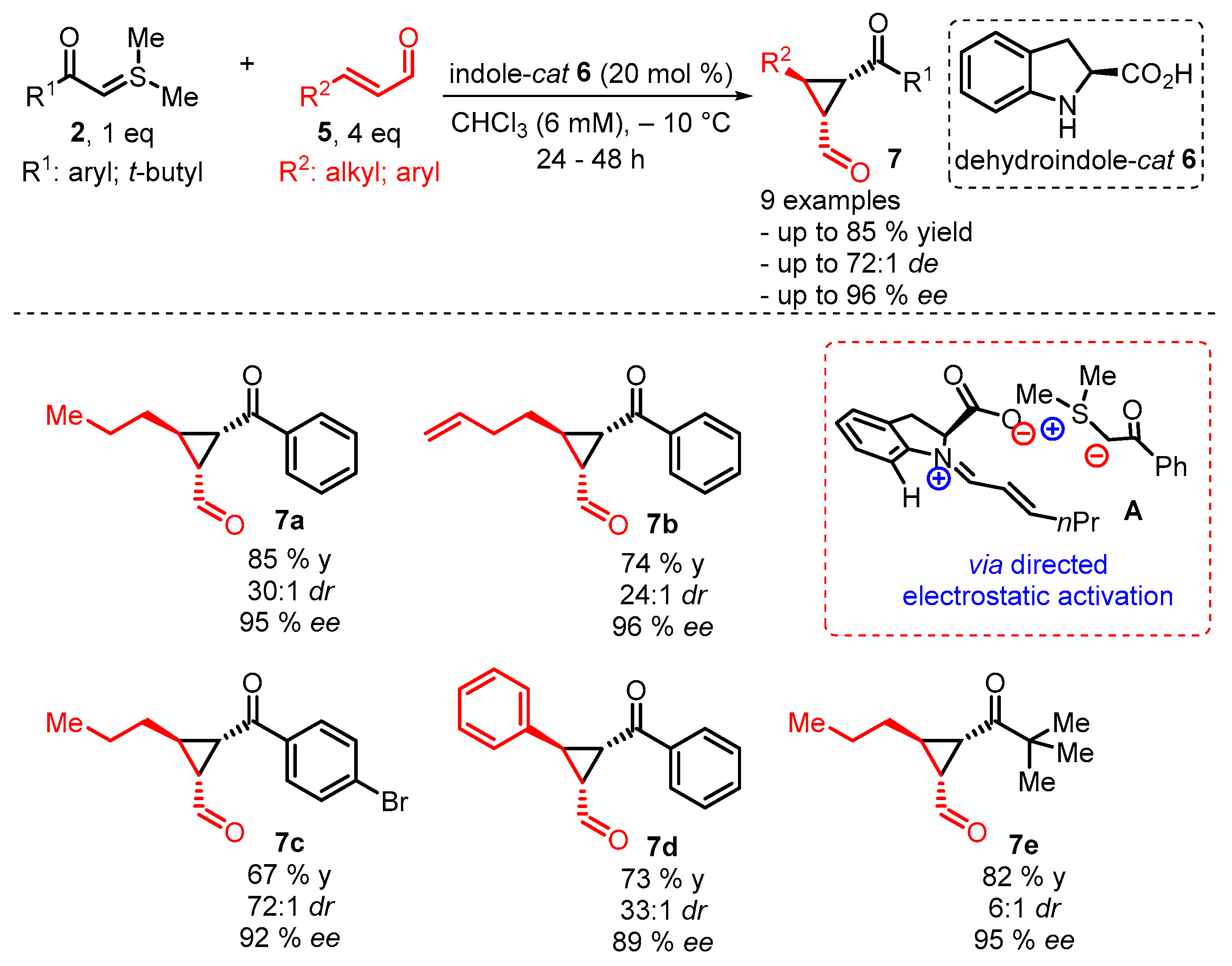
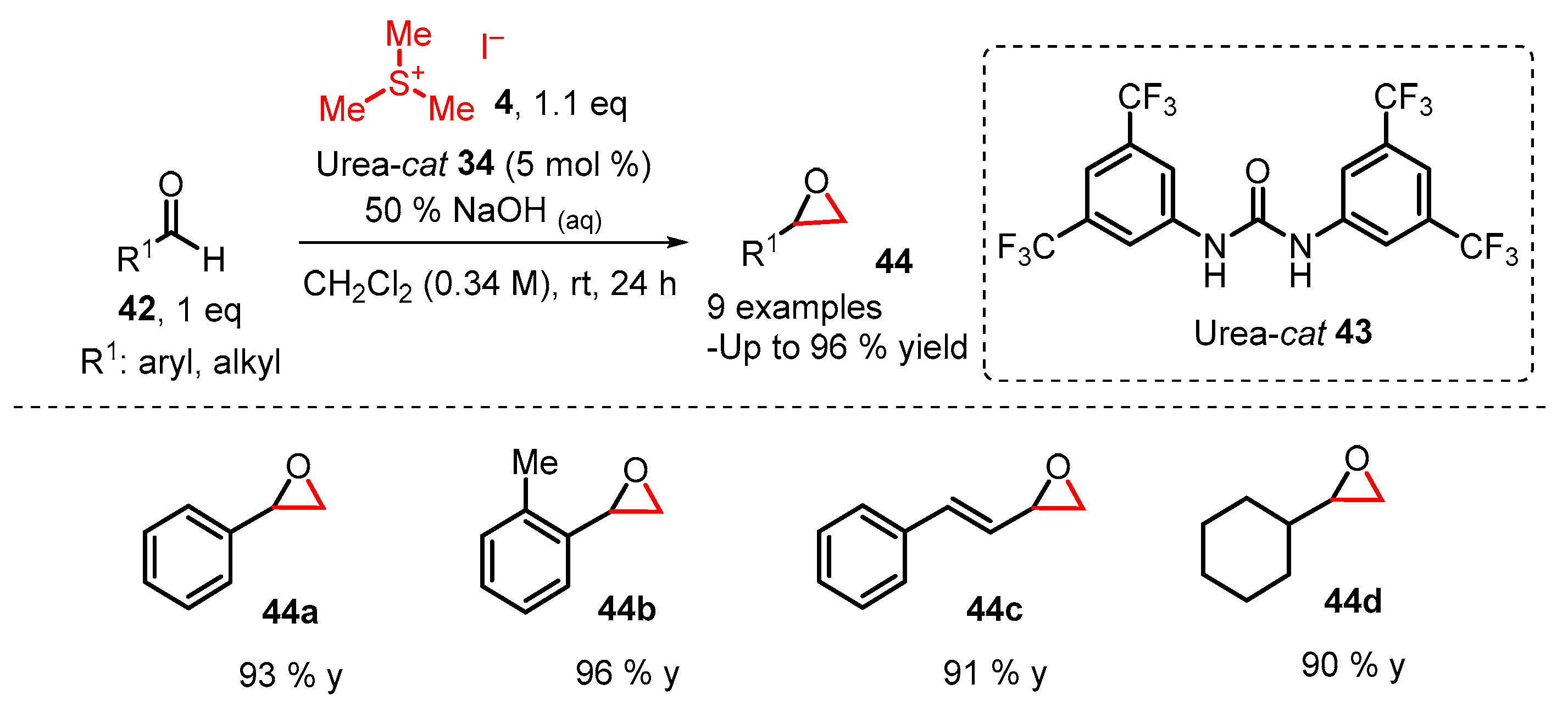
2. Organocatalytic Corey–Chaykovsky Reaction
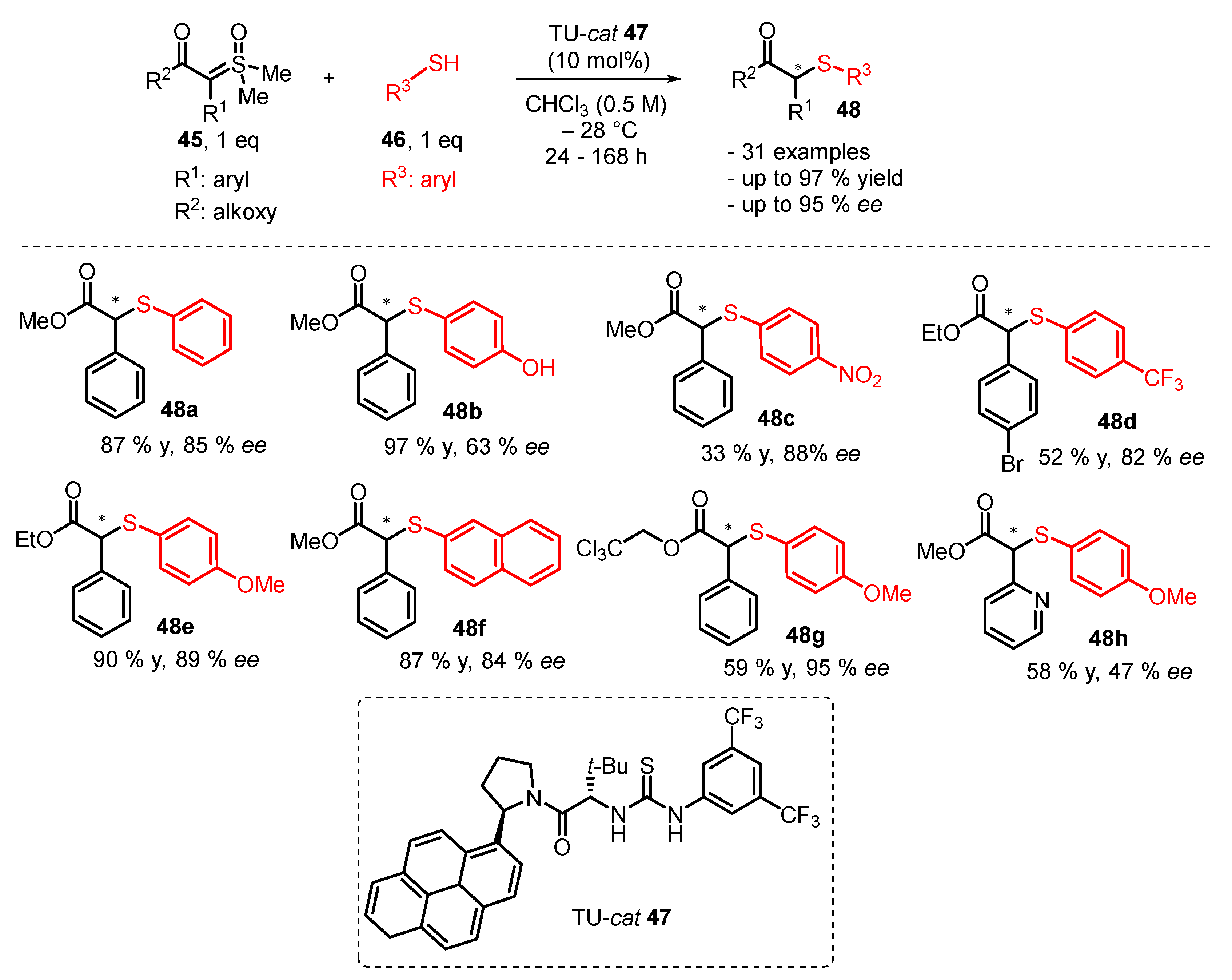
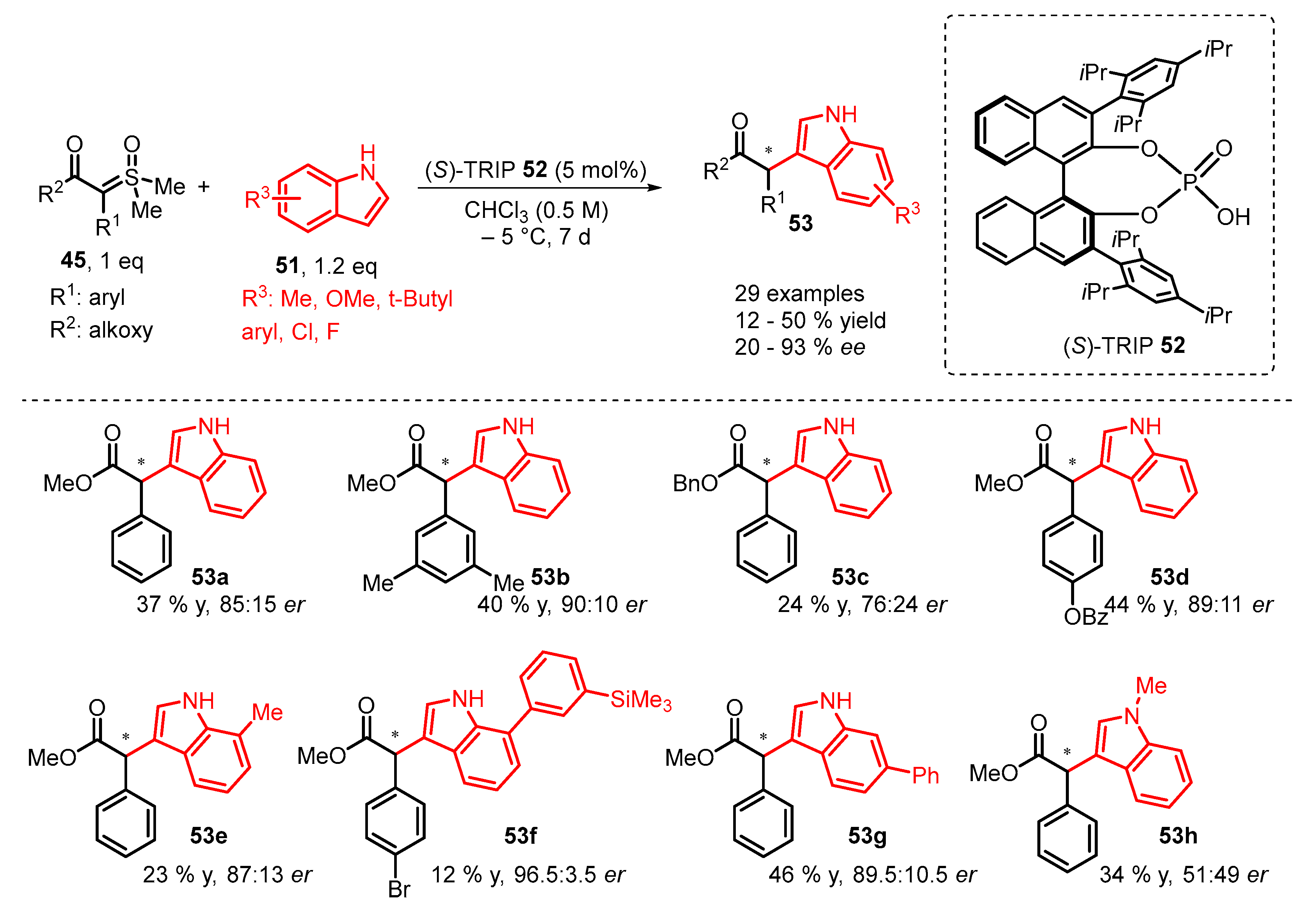
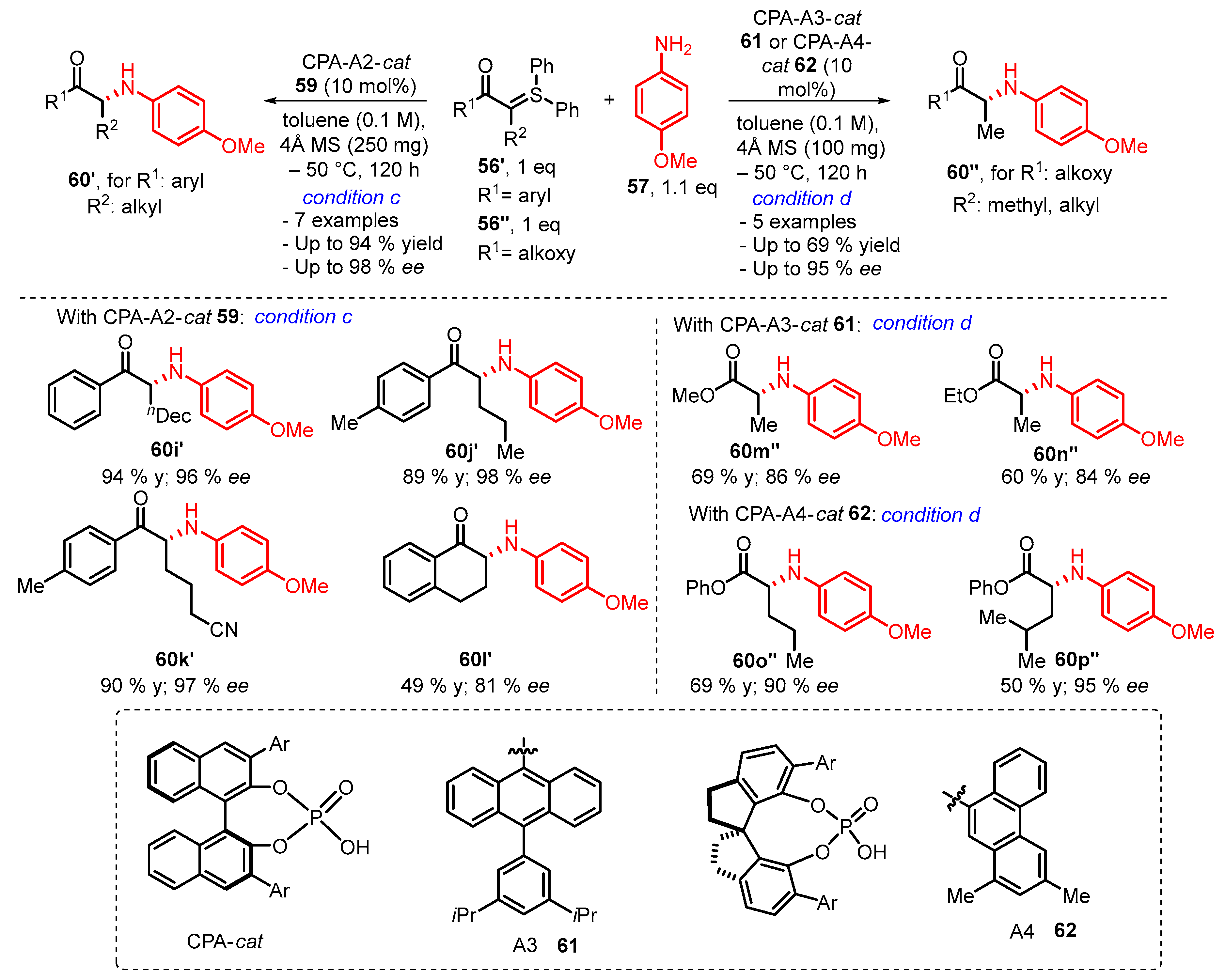
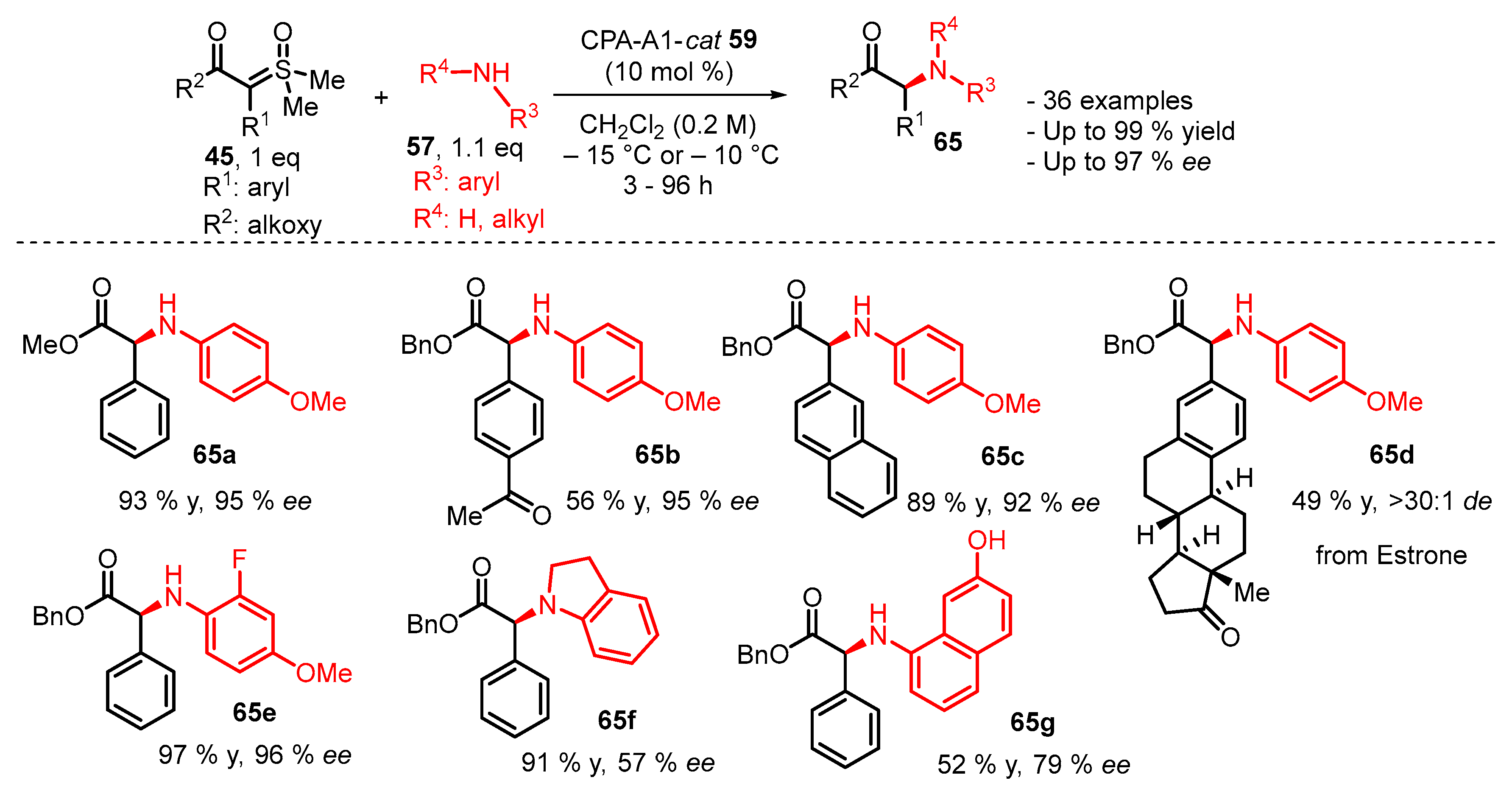
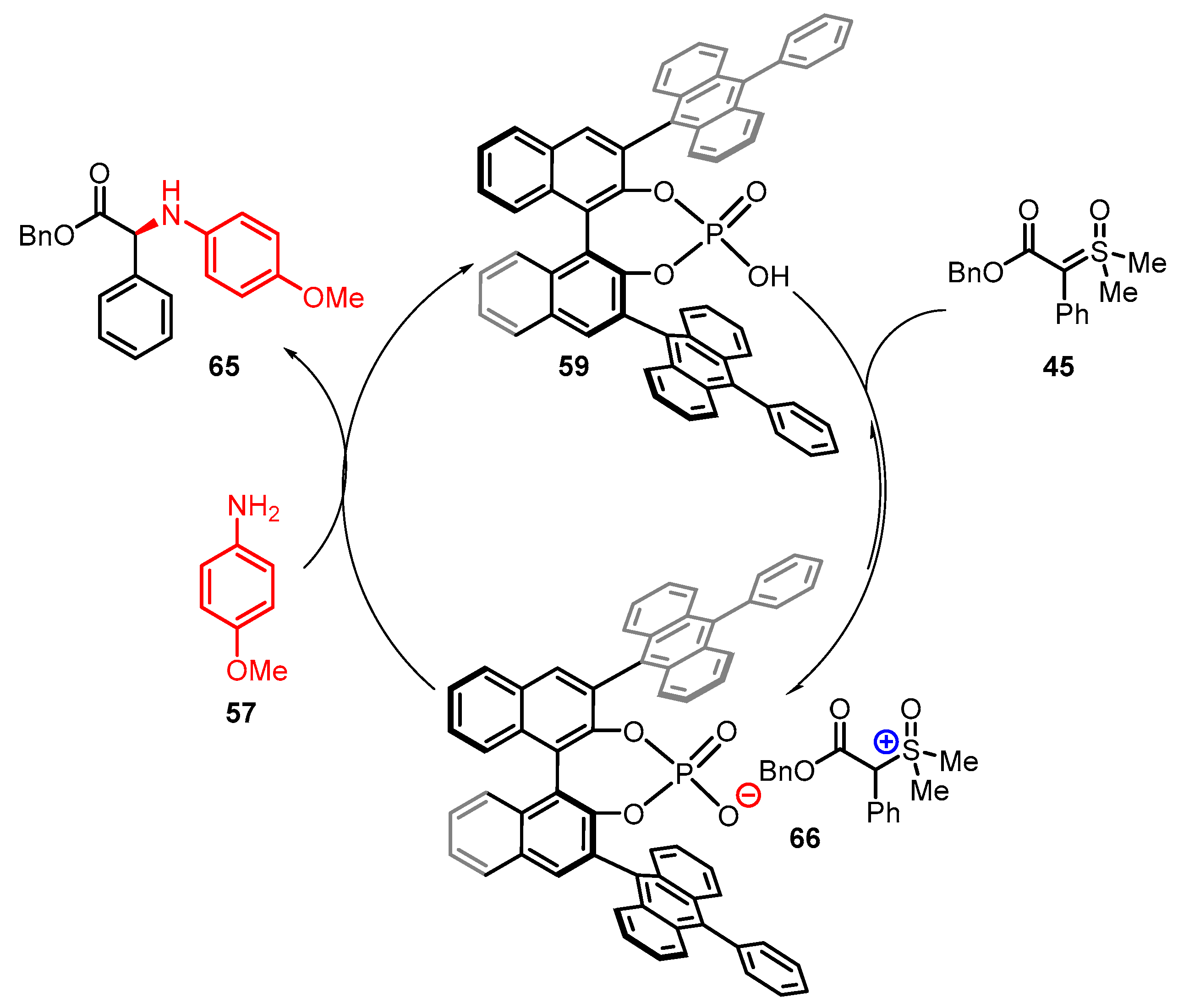
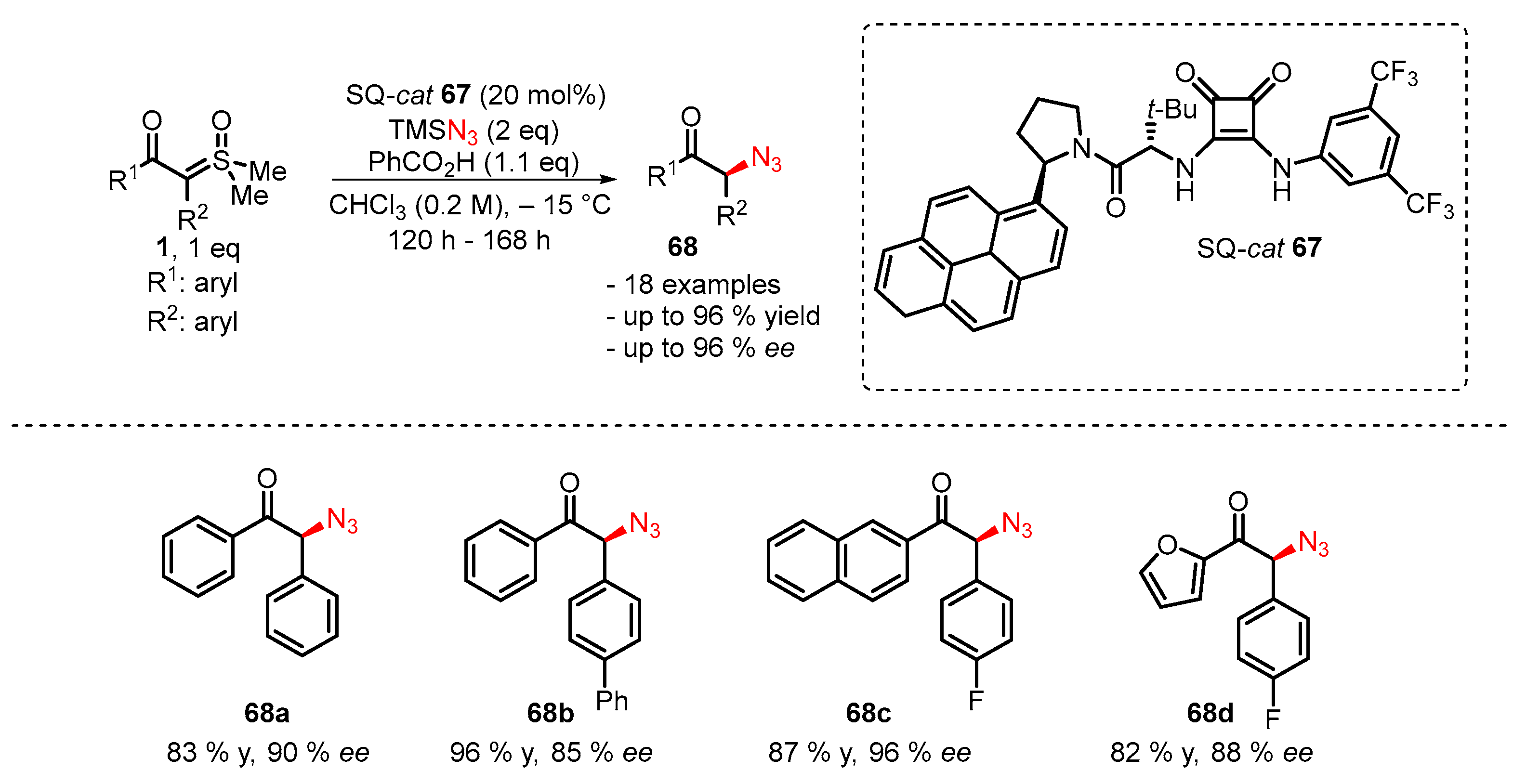
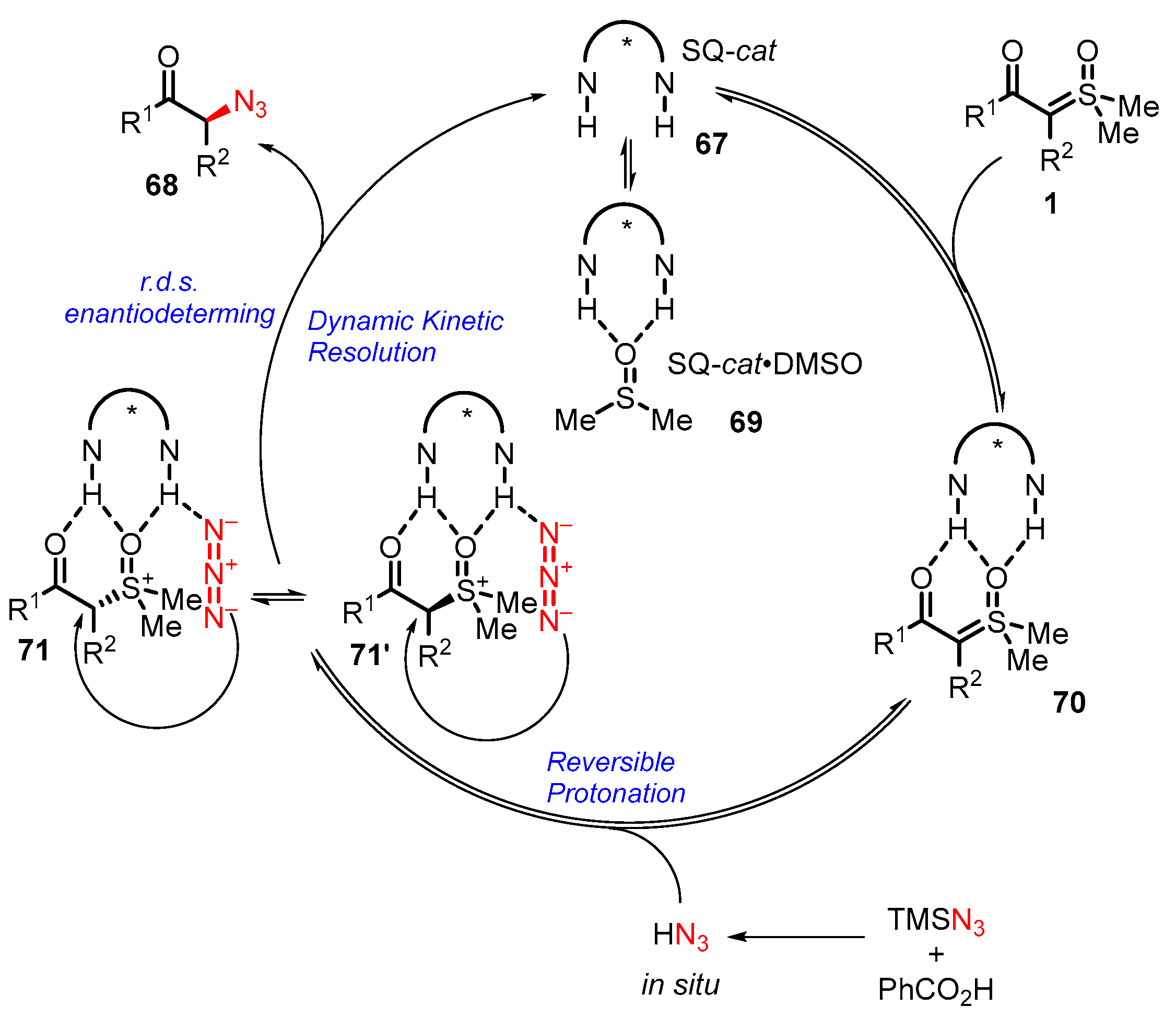
3. Organocatalytic Formal C–H, N–H, and S–H Insertion
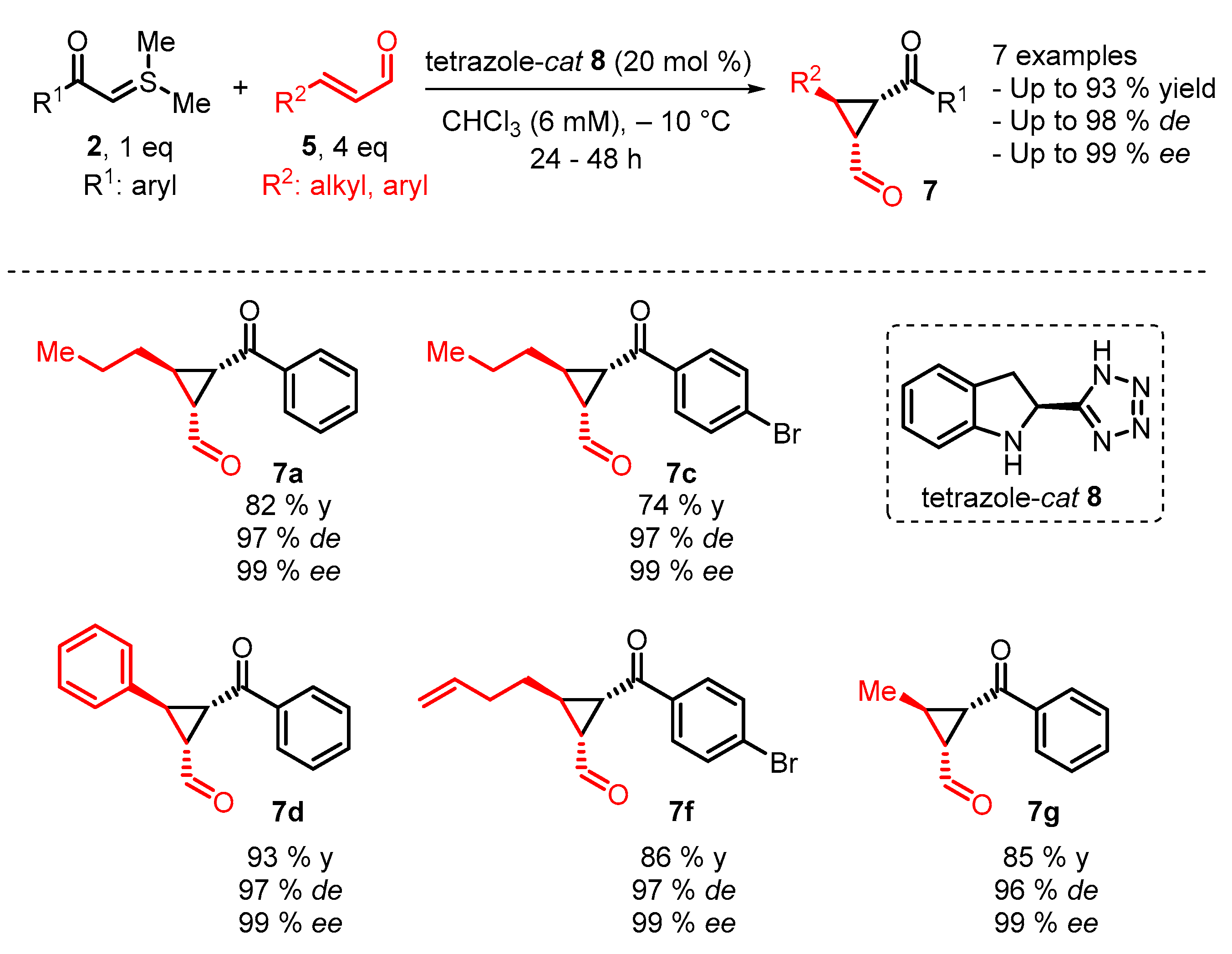
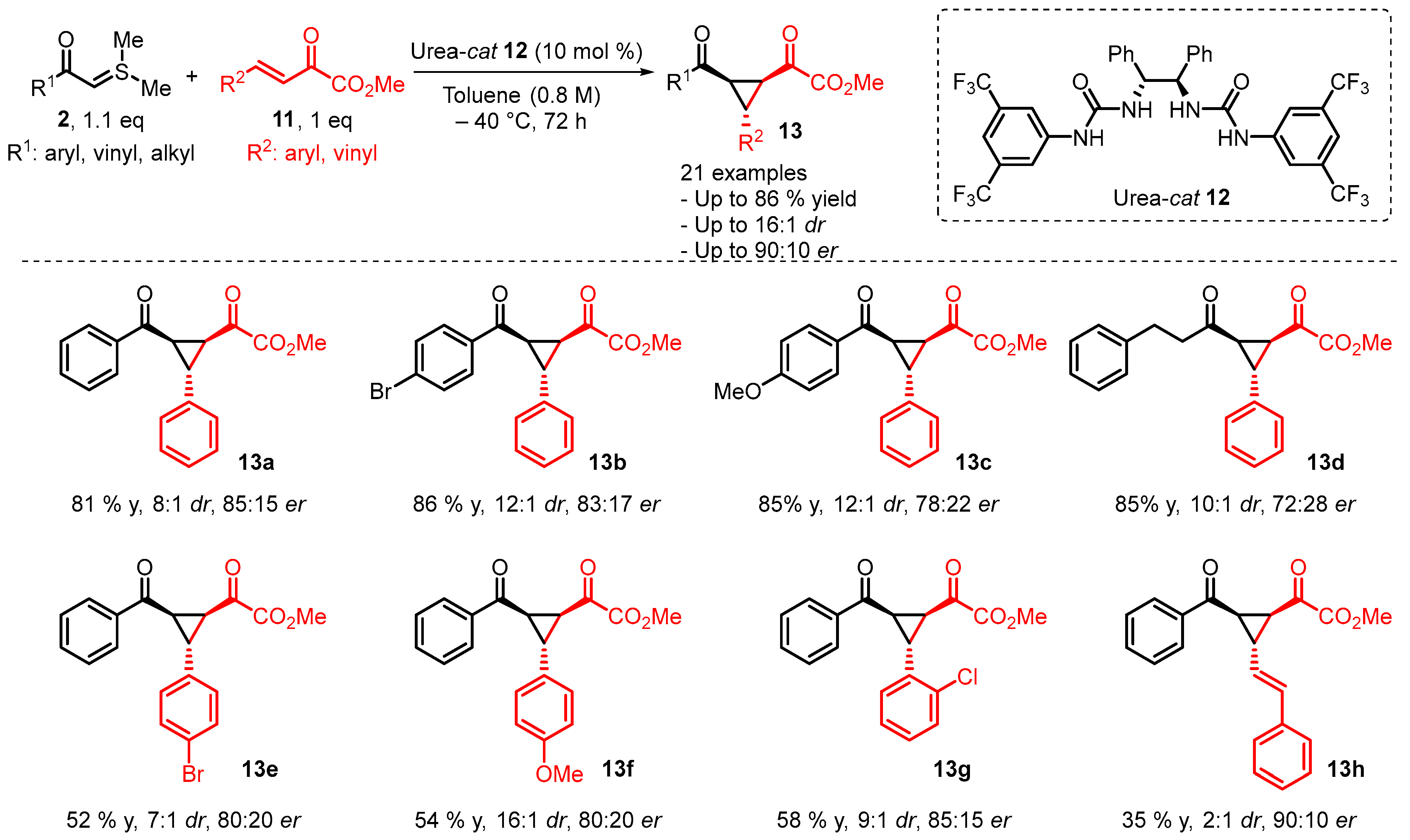
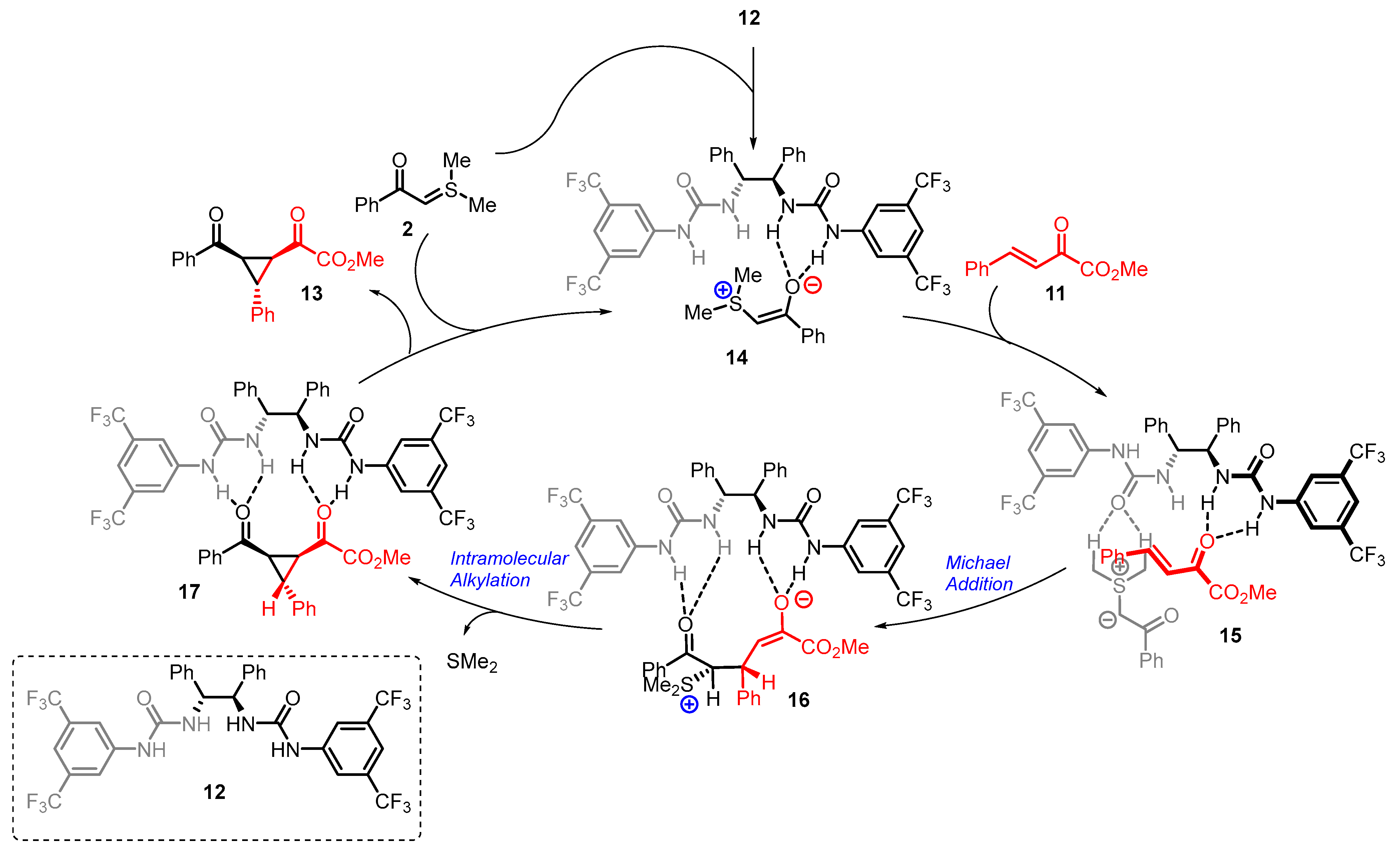
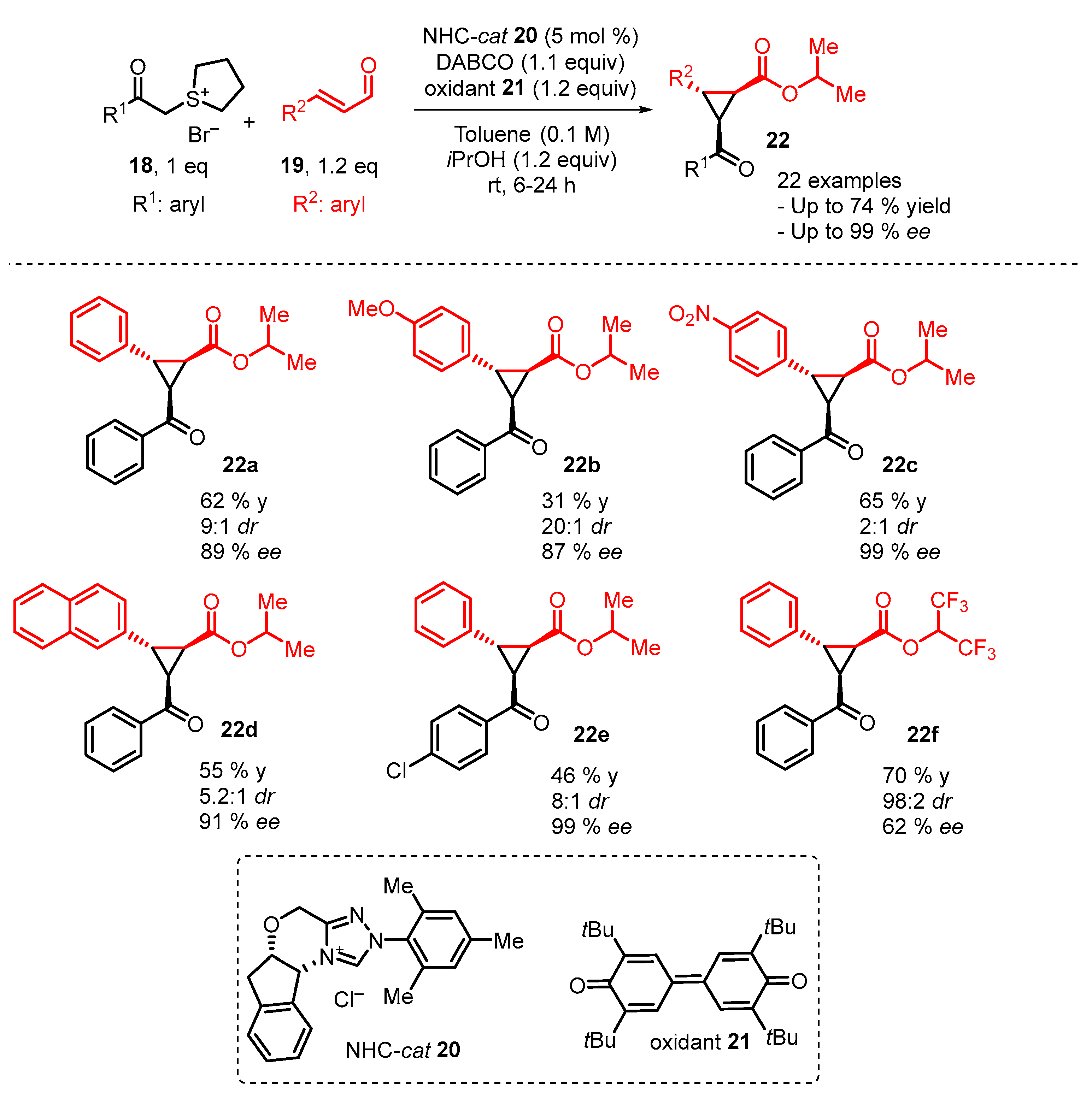
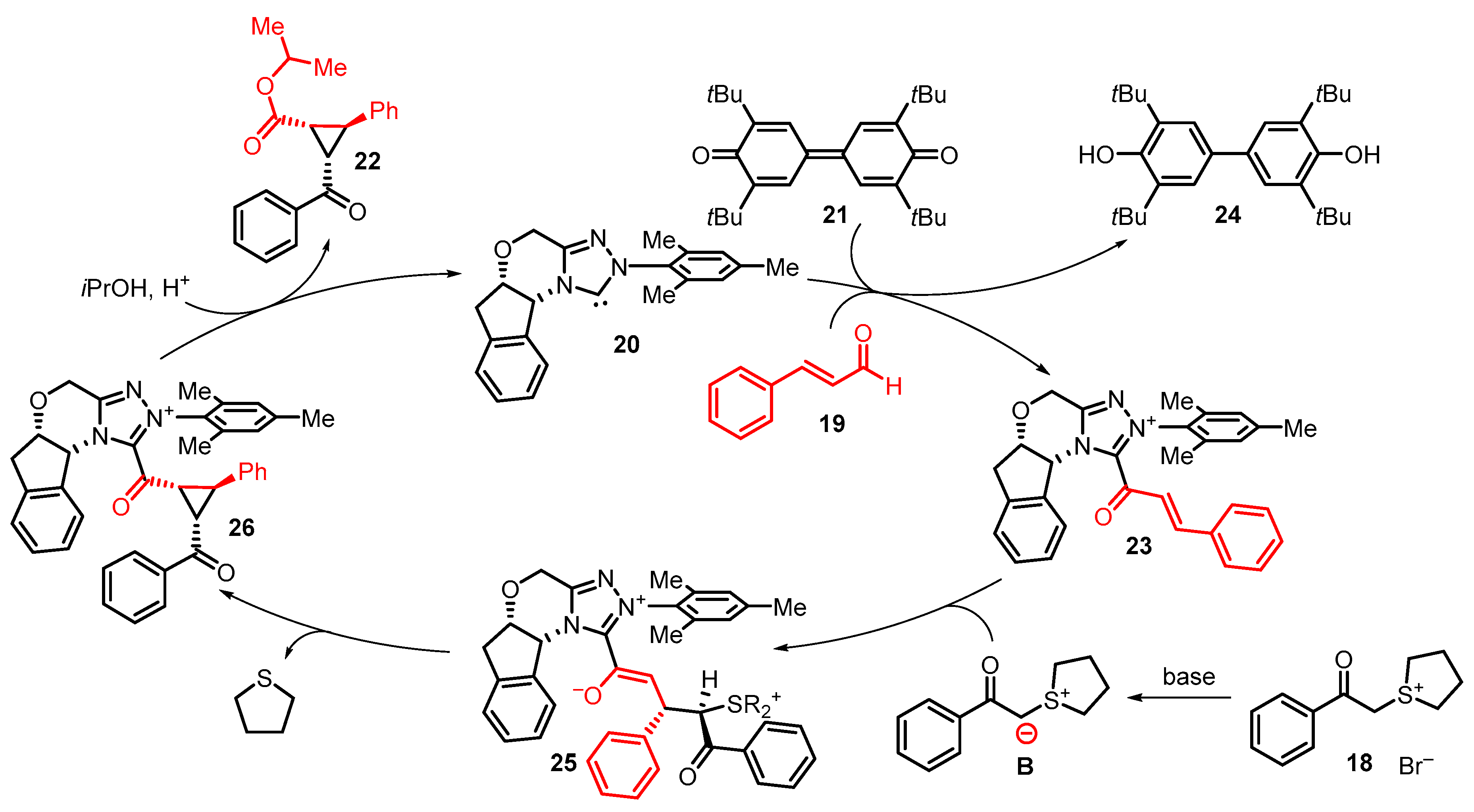
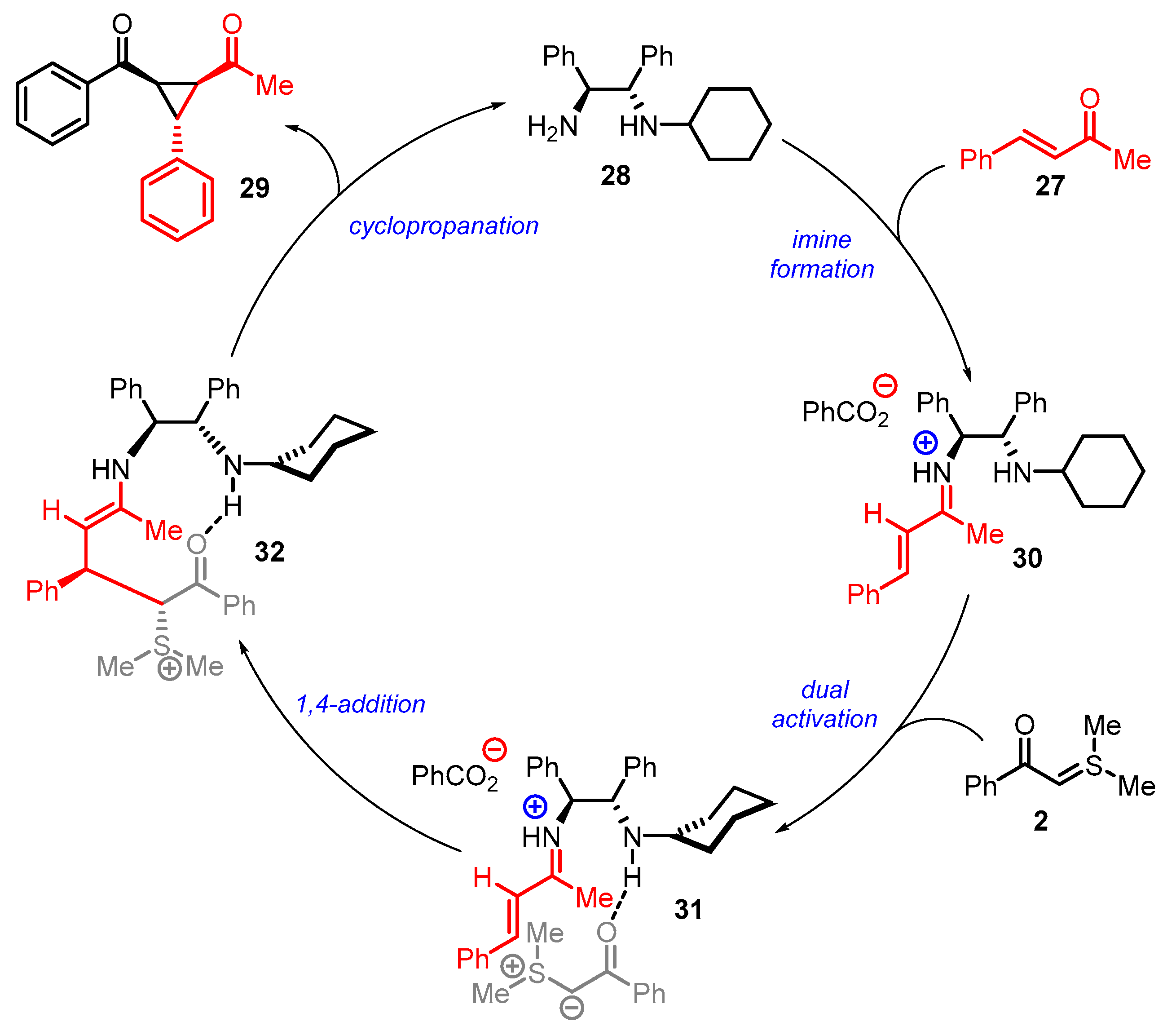
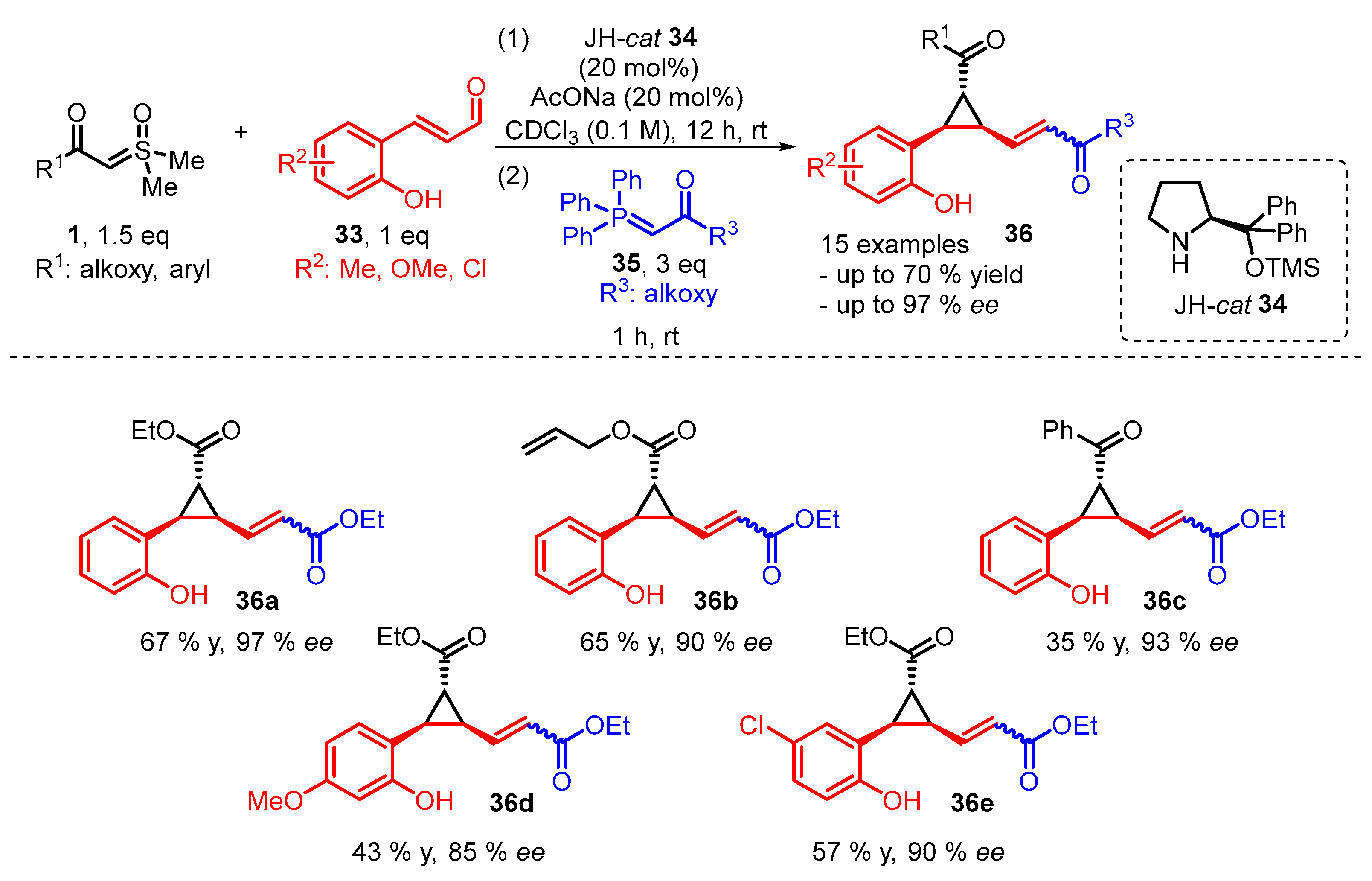
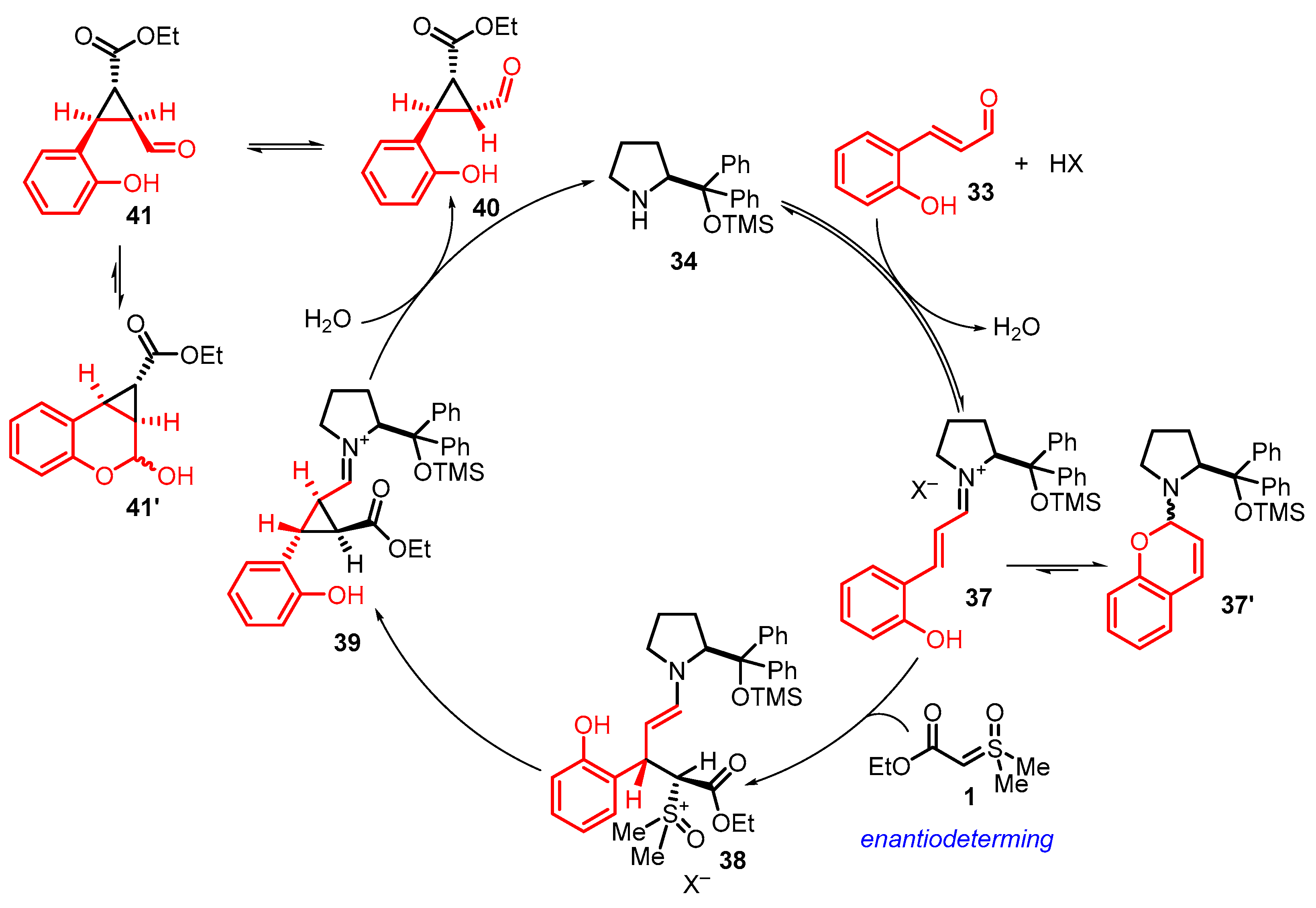
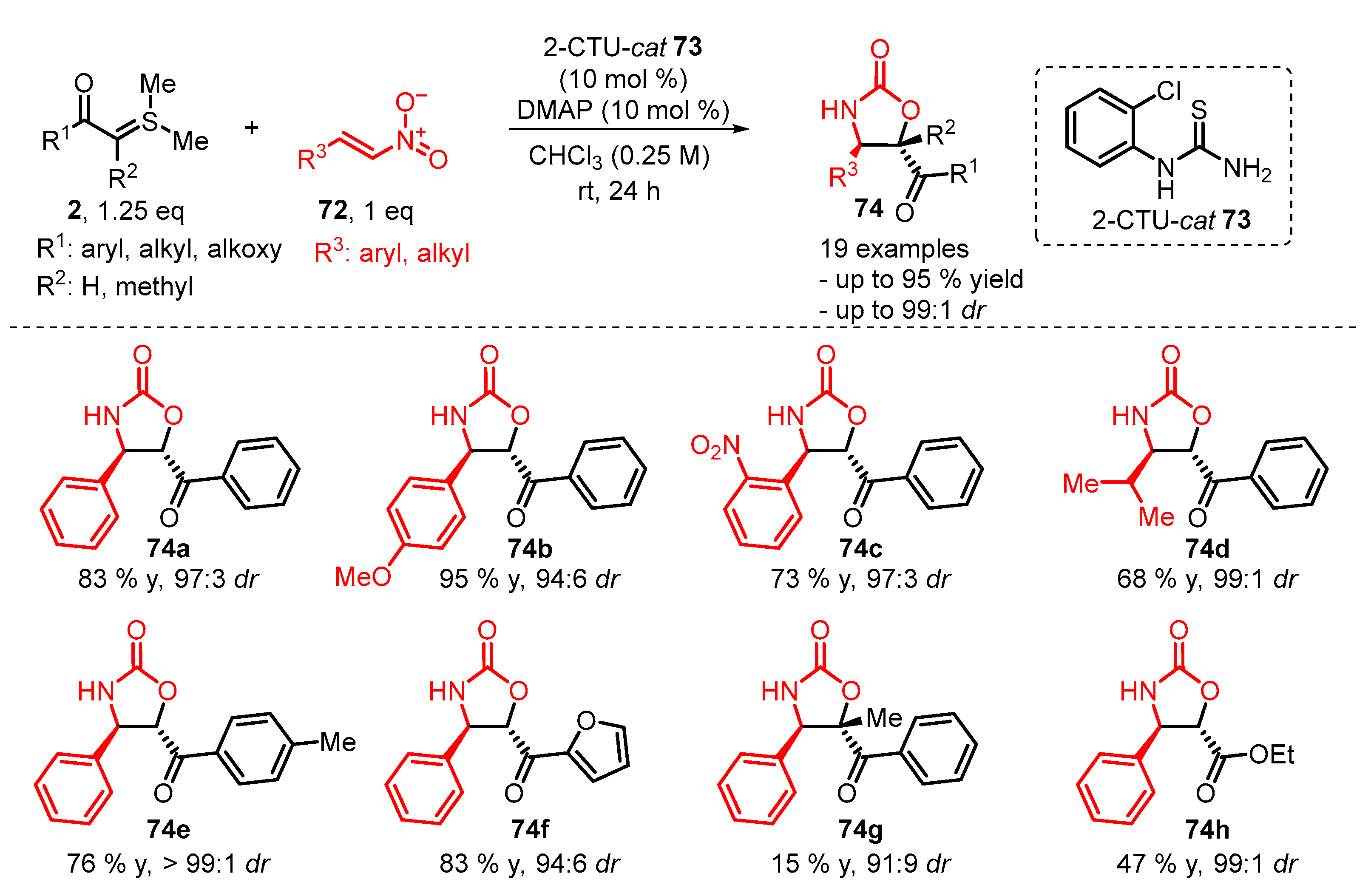
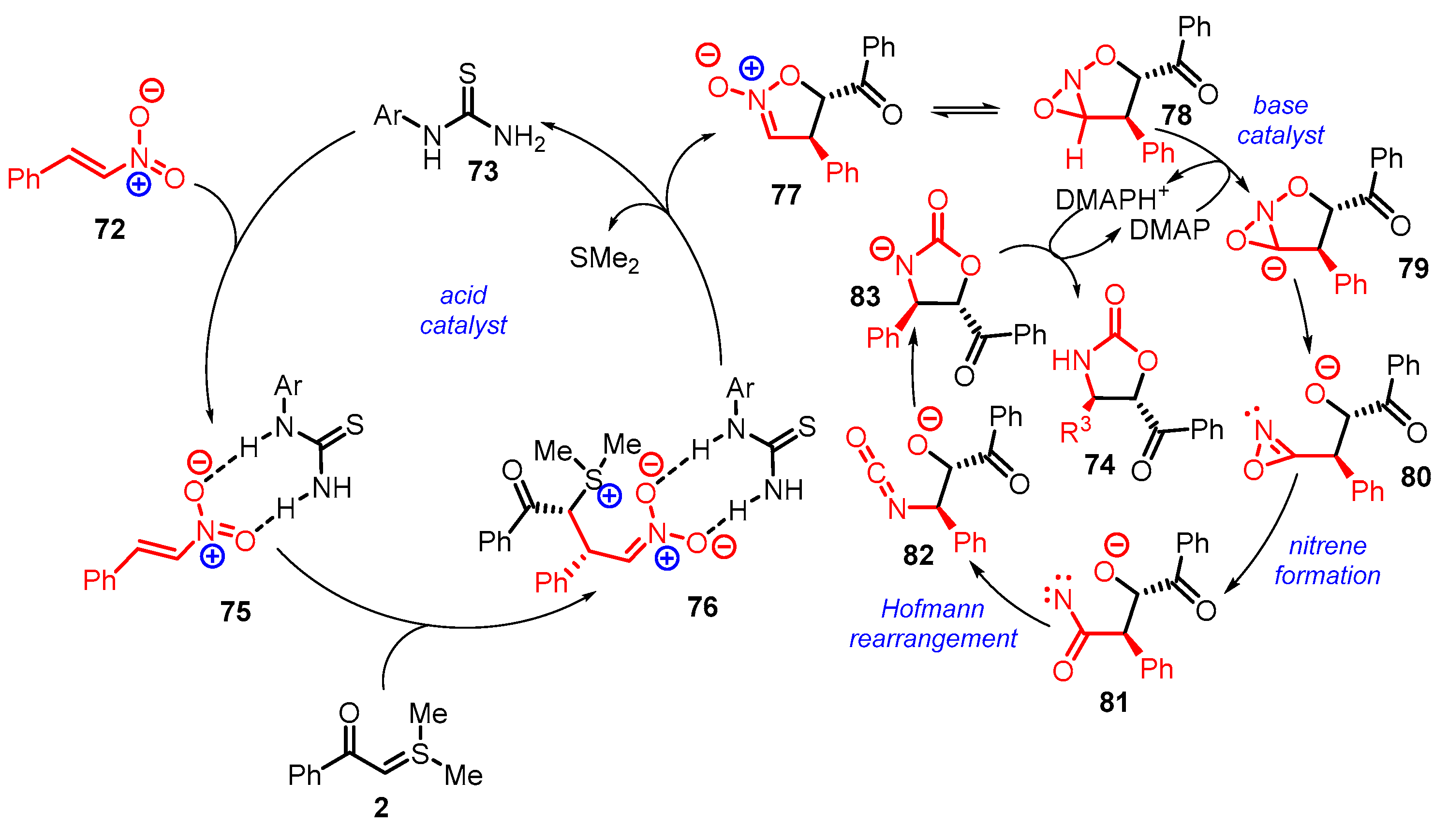
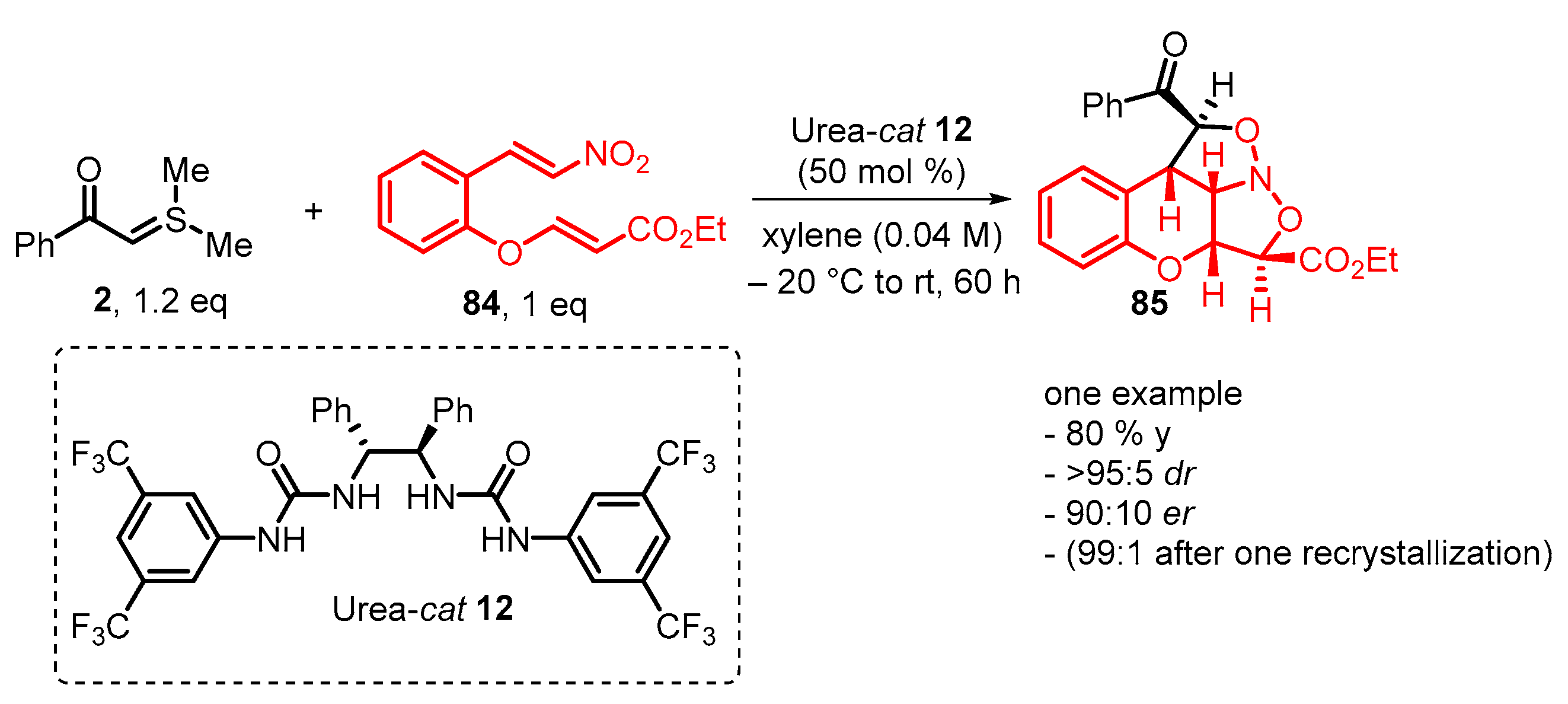
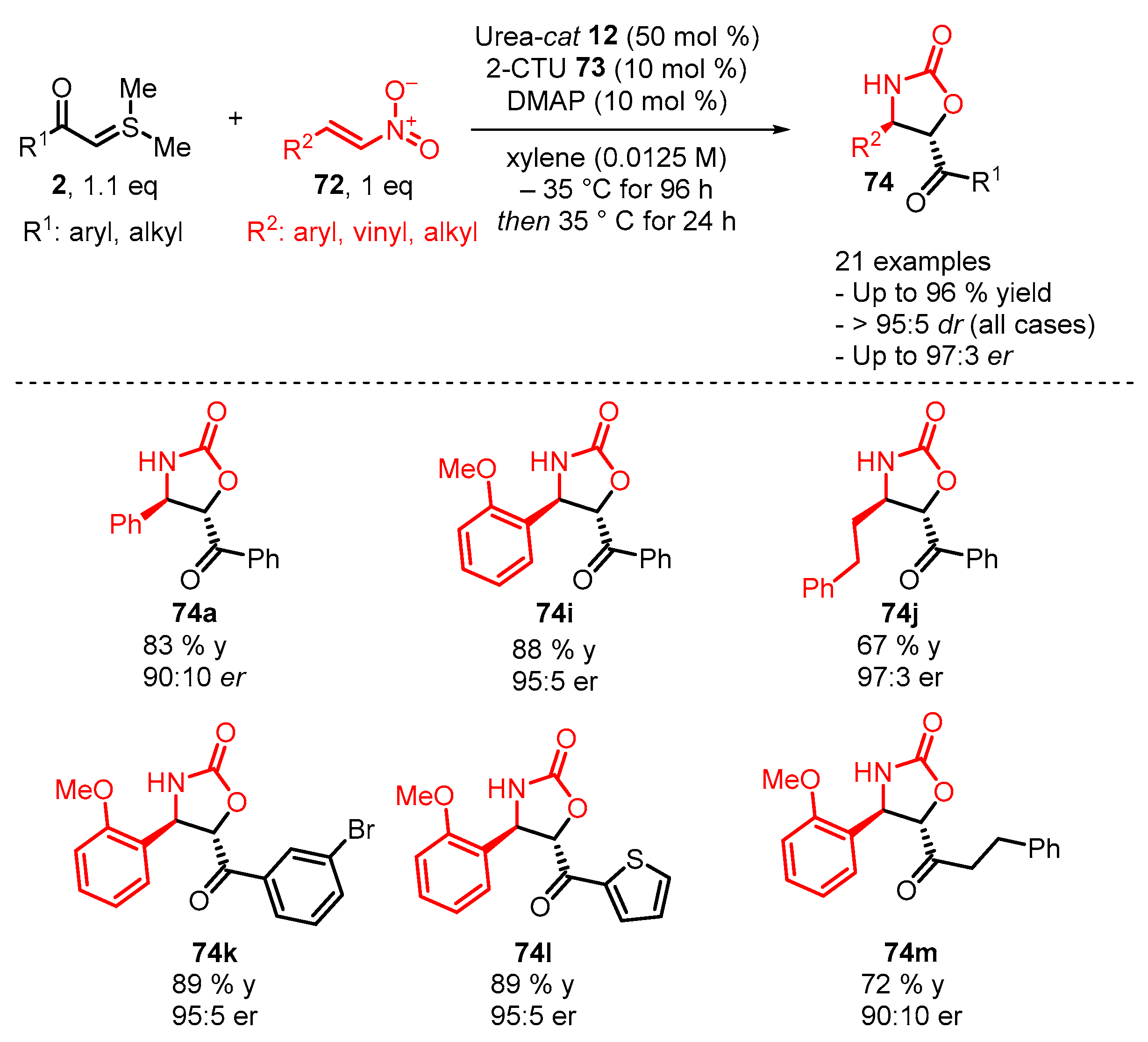
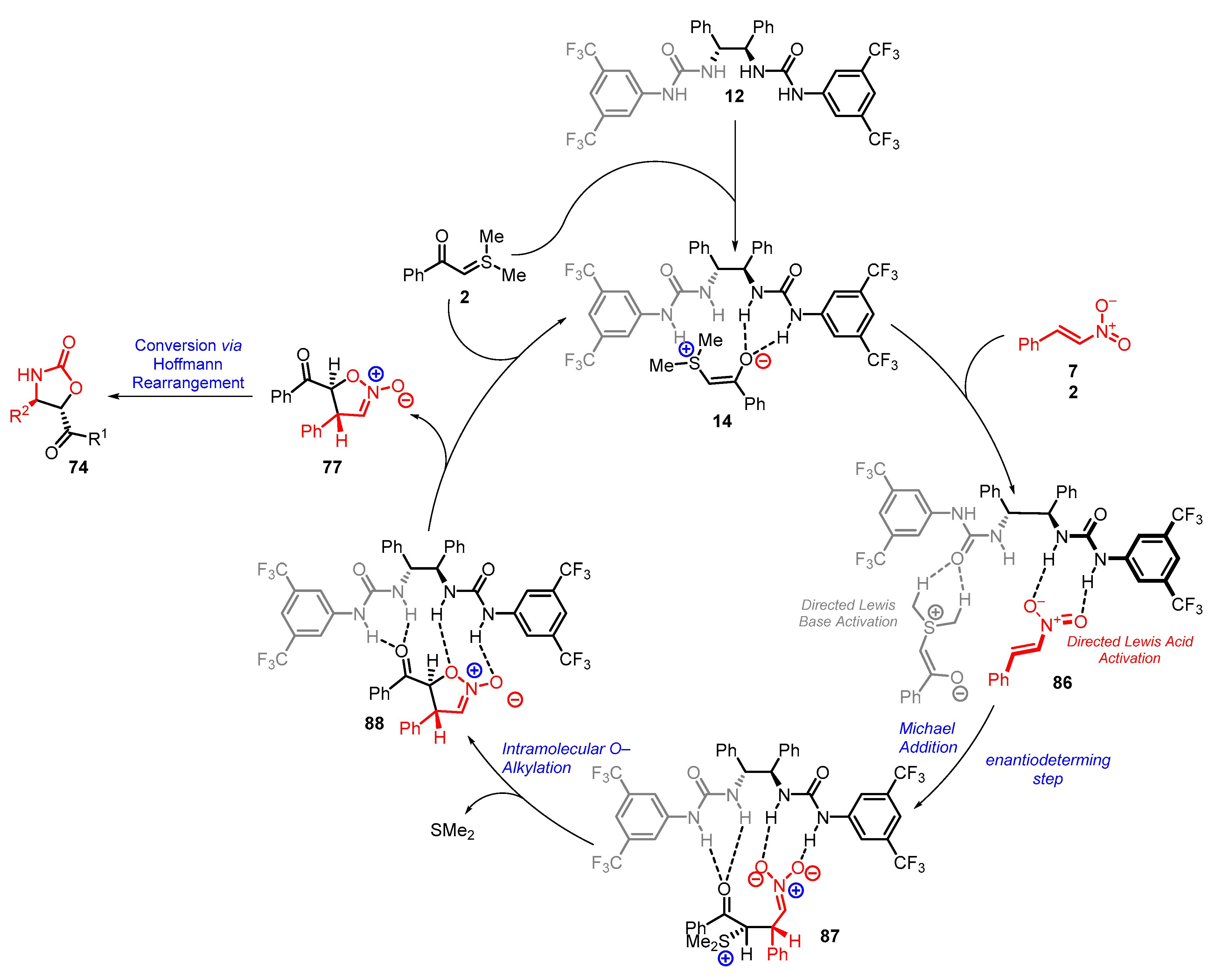
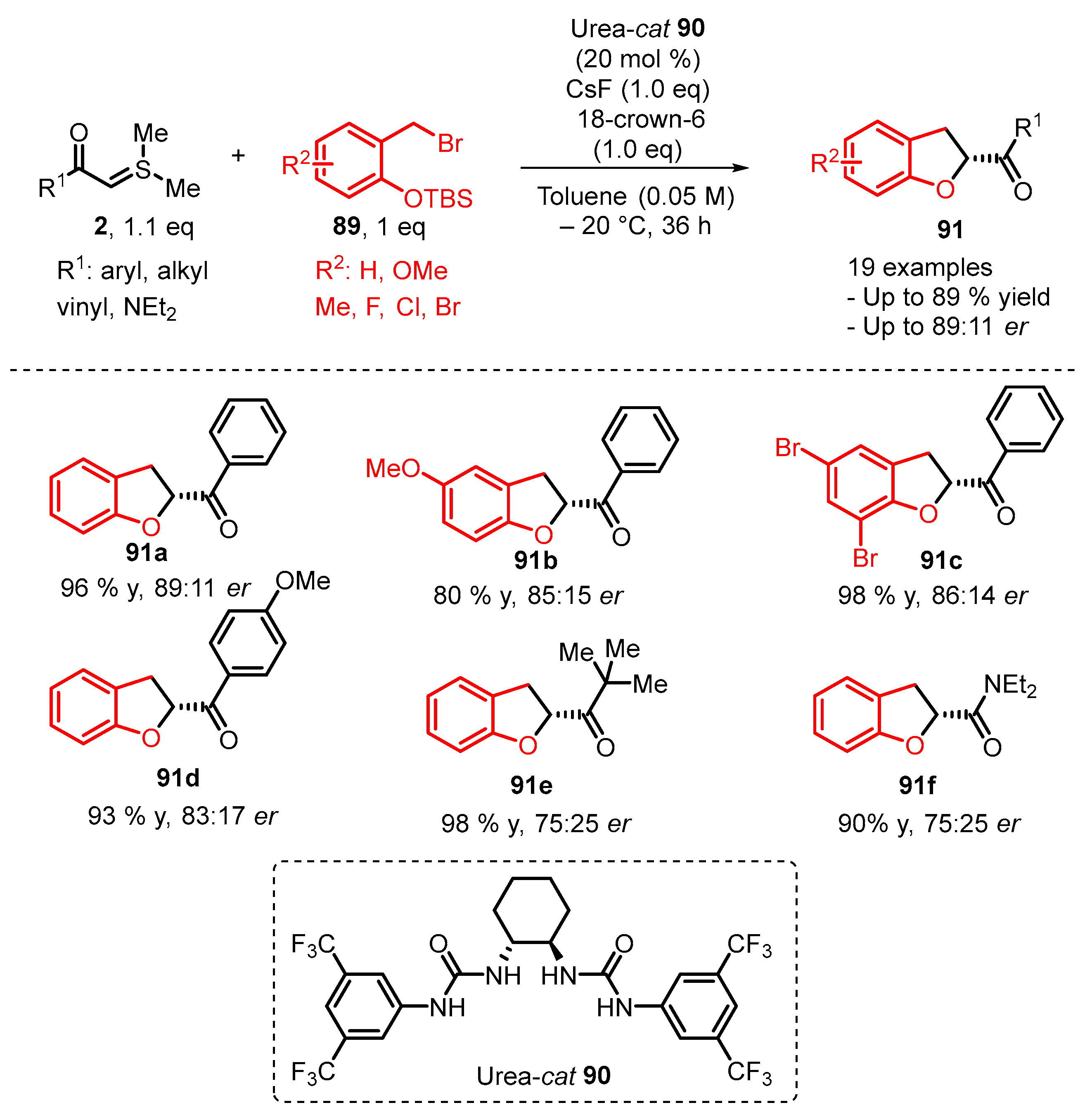
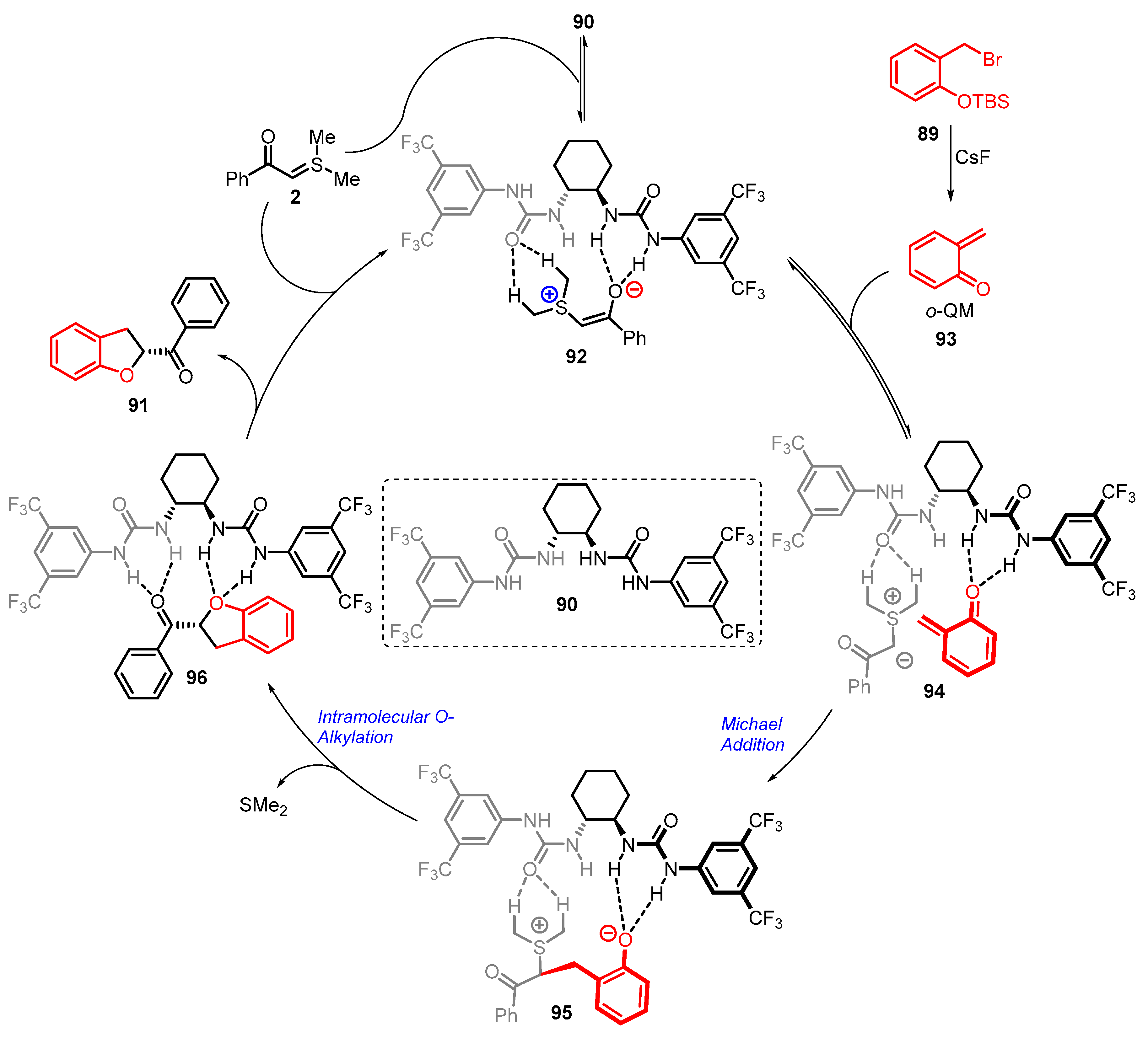
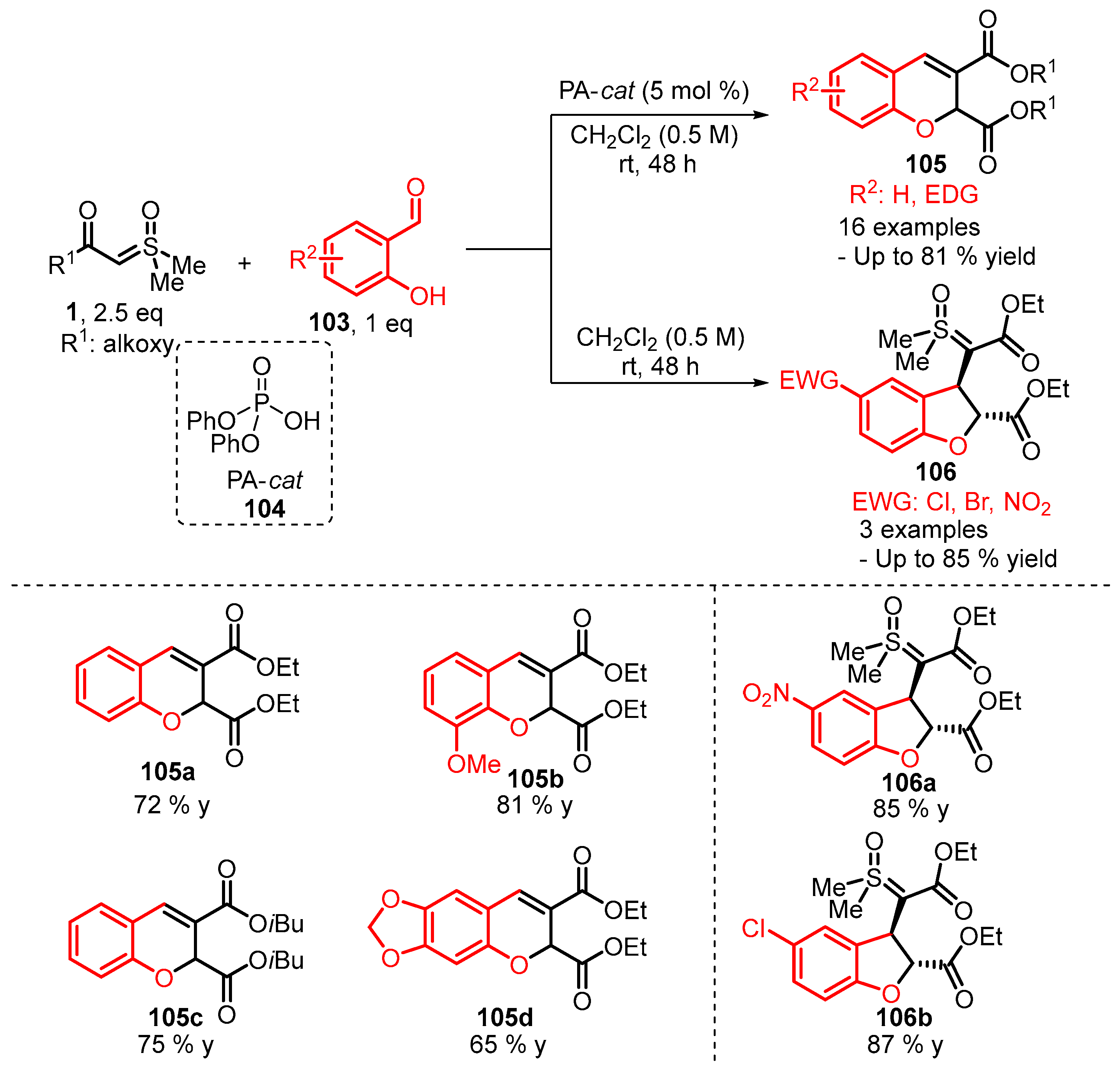
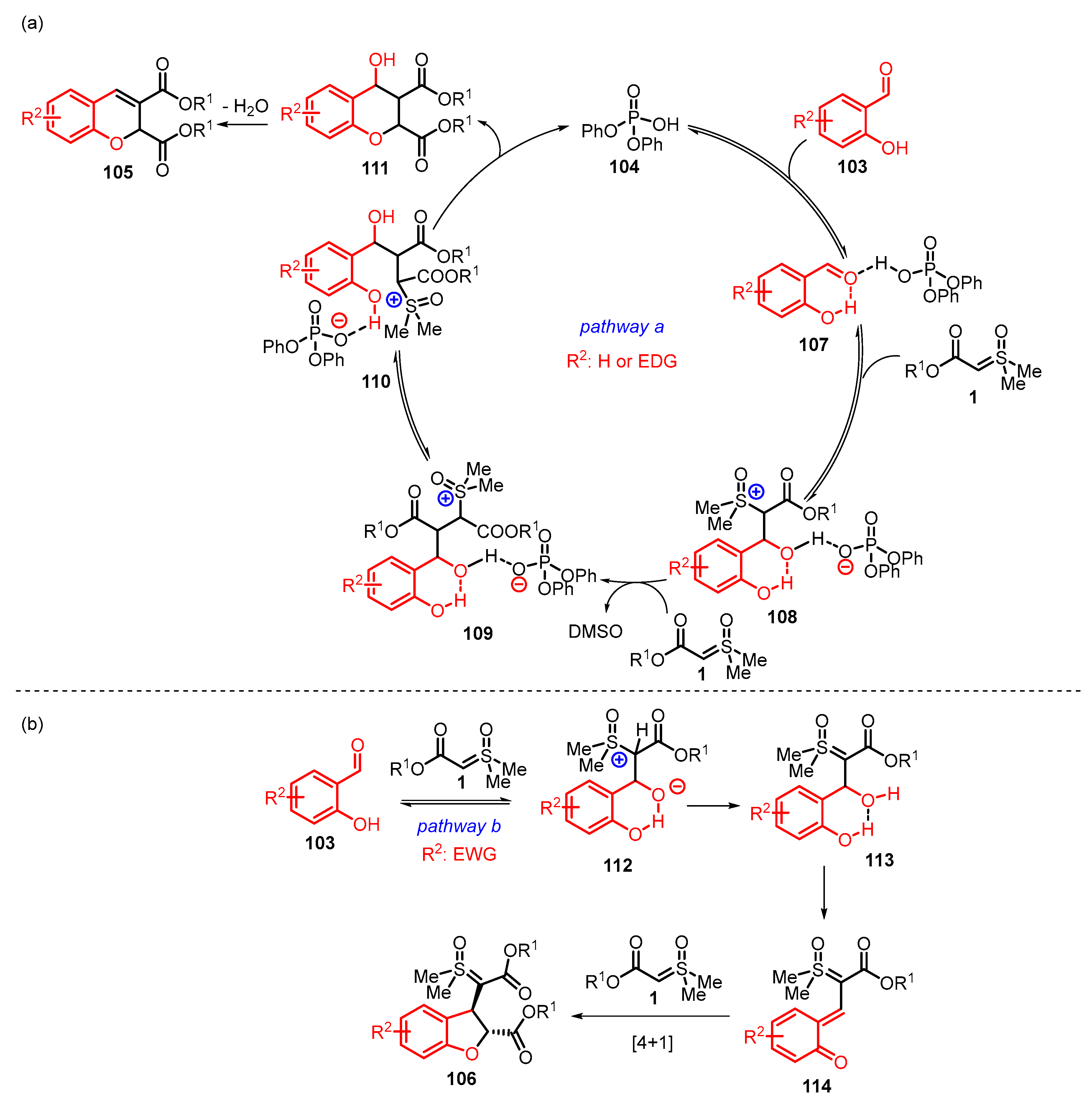
4. Cyclization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18-crown-6 | 1,4,7,10,13,16-hexaoxacyclooctadecane |
| AcO | acetyl |
| Å | Angstrom |
| aq | aqueous |
| Ar | aryl |
| Bn | benzyl |
| Bu | butyl |
| cat | catalyst |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| Dec | decanyl |
| DMAP | N,N-4-dimethylaminopyridine |
| DMSO | dimethylsulfoxide |
| de | diastereomeric excess |
| dr | diastereomeric ratio |
| EDG | electron directing group |
| ee | enantiomeric excess |
| eq | equivalent |
| er | enatiomeric ratio |
| Et | ethyl |
| EWG | electron withdrawing group |
| i | iso |
| m | mili |
| M | molar (mol·L−1) |
| Me | methyl |
| MS | molecular sieves |
| n | normal |
| NHC | N-heterocyclic carbene |
| o | ortho |
| Ph | phenyl |
| Pr | propyl |
| QM | quinone methide |
| rt | room temperature |
| t | tert |
| TBS | tert-butyldimethylsilyl |
| TMS | trimethylsilyl |
| y | yield |
References and Notes
- The Nobel Prize in Chemistry 2021. Available online: https://www.nobelprize.org/prizes/chemistry/2021/summary/ (accessed on 8 December 2022).
- Dondoni, A.; Massi, A. Asymmetric Organocatalysis: From Infancy to Adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P.I.; Moisan, L. In the Golden Age of Organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Marqués-López, E.; Herrera, R.P.; Christmann, M. Asymmetric Organocatalysis in Total Synthesis—A Trial by Fire. Nat. Prod. Rep. 2010, 27, 1138–1167. [Google Scholar] [CrossRef] [PubMed]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic Cascade Reactions as a New Tool in Total Synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric Organocatalysis: An Enabling Technology for Medicinal Chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef]
- García Mancheño, O.; Waser, M. Recent Developments and Trends in Asymmetric Organocatalysis. Eur. J. Org. Chem. 2022, 26, e202200950. [Google Scholar] [CrossRef]
- Dalko, P.I. Enantioselective Organocatalysis: Reactions and Experimental Procedures; Wiley-VCH; John Wiley: Weinheim, Germany; Chichester, UK, 2007. [Google Scholar]
- Ingold, C.K.; Jessop, J.A. XCV.—Influence of Poles and Polar Linkings on the Course Pursued by Elimination Reactions. Part IX. Isolation of a Substance Believed to Contain a Semipolar Double Linking with Participating Carbon. J. Chem. Soc. 1930, 713–718. [Google Scholar] [CrossRef]
- Johnson, A.W.; LaCount, R.B. The Chemistry of Ylids. VI. Dimethylsulfonium Fluorenylide—A Synthesis of Epoxides. J. Am. Chem. Soc. 1961, 83, 417–423. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethylsulfoxonium Methylide. J. Am. Chem. Soc. 1962, 84, 867–868. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethylsulfonium Methylide, a Reagent for Selective Oxirane Synthesis from Aldehydes and Ketones. J. Am. Chem. Soc. 1962, 84, 3782–3783. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethyloxosulfonium Methylide ((CH3)2SOCH2) and Dimethylsulfonium Methylide ((CH3)2SCH2). Formation and Application to Organic Synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. [Google Scholar] [CrossRef]
- Lu, L.-Q.; Li, T.-R.; Wang, Q.; Xiao, W.-J. Beyond Sulfide-Centric Catalysis: Recent Advances in the Catalytic Cyclization Reactions of Sulfur Ylides. Chem. Soc. Rev. 2017, 46, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Burtoloso, A.C.B.; Dias, R.M.P.; Leonarczyk, I.A. Sulfoxonium and Sulfonium Ylides as Diazocarbonyl Equivalents in Metal-Catalyzed Insertion Reactions: Sulfoxonium and Sulfonium Ylides in Insertion Reactions. Eur. J. Org. Chem. 2013, 2013, 5005–5016. [Google Scholar] [CrossRef]
- Belkin, Y.V.; Polezhaeva, N.A. The Chemistry of Stabilised Sulphonium Ylides. Russ. Chem. Rev. 1981, 50, 481–497. [Google Scholar] [CrossRef]
- Bisag, G.D.; Ruggieri, S.; Fochi, M.; Bernardi, L. Sulfoxonium Ylides: Simple Compounds with Chameleonic Reactivity. Org. Biomol. Chem. 2020, 18, 8793–8809. [Google Scholar] [CrossRef] [PubMed]
- Caiuby, C.A.D.; Furniel, L.G.; Burtoloso, A.C.B. Asymmetric Transformations from Sulfoxonium Ylides. Chem. Sci. 2022, 13, 1192–1209. [Google Scholar] [CrossRef]
- Neuhaus, J.D.; Oost, R.; Merad, J.; Lady, N. Sulfur-Based Ylides in Transition-Metal-Catalysed Processes. Top. Curr. Chem. 2018, 376, 15. [Google Scholar] [CrossRef]
- Li, A.-H.; Dai, L.-X.; Aggarwal, V.K. Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement. Chem. Rev. 1997, 97, 2341–2372. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Winn, C.L. Catalytic, Asymmetric Sulfur Ylide-Mediated Epoxidation of Carbonyl Compounds: Scope, Selectivity, and Applications in Synthesis. Acc. Chem. Res. 2004, 37, 611–620. [Google Scholar] [CrossRef]
- McGarrigle, E.M.; Myers, E.L.; Illa, O.; Shaw, M.A.; Riches, S.L.; Aggarwal, V.K. Chalcogenides as Organocatalysts. Chem. Rev. 2007, 107, 5841–5883. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Gao, X.-H.; Yue, J.-M. Attractive Natural Products with Strained Cyclopropane and/or Cyclobutane Ring Systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Z.; Jiang, H. Recent Advances in the Synthesis of Cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Charette, A.B.; Beauchemin, A. Simmons-Smith Cyclopropanation Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 1–415. [Google Scholar]
- Lebel, H.; Marcoux, J.-F.; Molinaro, C.; Charette, A.B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.K.; MacMillan, D.W.C. Enantioselective Organocatalytic Cyclopropanations. The Identification of a New Class of Iminium Catalyst Based upon Directed Electrostatic Activation. J. Am. Chem. Soc. 2005, 127, 3240–3241. [Google Scholar] [CrossRef]
- Hartikka, A.; Arvidsson, P.I. Tetrazolic Acid Functionalized Dihydroindol: Rational Design of a Highly Selective Cyclopropanation Organocatalyst. J. Org. Chem. 2007, 72, 5874–5877. [Google Scholar] [CrossRef]
- Hartikka, A.; Ślósarczyk, A.T.; Arvidsson, P.I. Application of Novel Sulfonamides in Enantioselective Organocatalyzed Cyclopropanation. Tetrahedron Asymmetry 2007, 18, 1403–1409. [Google Scholar] [CrossRef]
- Cheng, Y.; An, J.; Lu, L.-Q.; Luo, L.; Wang, Z.-Y.; Chen, J.-R.; Xiao, W.-J. Asymmetric Cyclopropanation of β,γ-Unsaturated α-Ketoesters with Stabilized Sulfur Ylides Catalyzed by C2-Symmetric Ureas. J. Org. Chem. 2011, 76, 281–284. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Biswas, A.; De Sarkar, S.; Tebben, L.; Studer, A. Enantioselective Cyclopropanation of Enals by Oxidative N-Heterocyclic Carbene Catalysis. Chem. Commun. 2012, 48, 5190–5192. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- De Risi, C.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A.; Bortolini, O. Oxidative N-Heterocyclic Carbene Catalysis. Chem. Eur. J. 2023, 29, e202202467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Dong, S.; Lin, L.; Feng, X. Asymmetric Organocatalytic Cyclopropanation of Cinnamone Derivatives with Stabilized Sulfonium Ylides. J. Org. Chem. 2013, 78, 6322–6327. [Google Scholar] [CrossRef]
- Bisag, G.D.; Pecchini, P.; Mancinelli, M.; Fochi, M.; Bernardi, L. Sulfoxonium Ylides in Aminocatalysis: An Enantioselective Entry to Cyclopropane-Fused Chromanol Structures. Org. Lett. 2022, 24, 5468–5473. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.K. (Ed.) Aziridines and Epoxides in Organic Synthesis, 1st ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kavanagh, S.A.; Piccinini, A.; Fleming, E.M.; Connon, S.J. Urea Derivatives Are Highly Active Catalysts for the Base-Mediated Generation of Terminal Epoxides from Aldehydes and Trimethylsulfonium Iodide. Org. Biomol. Chem. 2008, 6, 1339–1343. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Adlington, R.M.; Godfrey, C.R.A.; Gollins, D.W.; Smith, M.L.; Russel, A.T. A New Approach to the Synthesis of γ-Keto-α-Amino Acids: Synthesis of Optically Pure 5-Hydroxy-4-Oxo-L-Norvaline, L-HON. Synlett 1993, 1993, 51–53. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Adlington, R.M.; Godfrey, C.R.A.; Gollins, D.W.; Vaughan, J.G. A Novel Entry to Carbenoid Species via β-Ketosulfoxonium Ylides. J. Chem. Soc. Chem. Commun. 1993, 18, 1434–1435. [Google Scholar] [CrossRef]
- Mangion, I.K.; Ruck, R.T.; Rivera, N.; Huffman, M.A.; Shevlin, M. A Concise Synthesis of a β-Lactamase Inhibitor. Org. Lett. 2011, 13, 5480–5483. [Google Scholar] [CrossRef]
- Molinaro, C.; Bulger, P.G.; Lee, E.E.; Kosjek, B.; Lau, S.; Gauvreau, D.; Howard, M.E.; Wallace, D.J.; O’Shea, P.D. CRTH2 Antagonist MK-7246: A Synthetic Evolution from Discovery through Development. J. Org. Chem. 2012, 77, 2299–2309. [Google Scholar] [CrossRef]
- Ruck, R.T.; Pan, J.; Vaswani, R.G.; Kosjek, B.; Strotman, N.A.; Cai, C.; Humphrey, G.R. Harnessing the Power of Catalysis for the Synthesis of CRTH2 Antagonist MK-1029. Org. Process Res. Dev. 2022, 26, 648–656. [Google Scholar] [CrossRef]
- Talero, A.G.; Martins, B.S.; Burtoloso, A.C.B. Coupling of Sulfoxonium Ylides with Arynes: A Direct Synthesis of Pro-Chiral Aryl Ketosulfoxonium Ylides and Its Application in the Preparation of α-Aryl Ketones. Org. Lett. 2018, 20, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Janot, C.; Palamini, P.; Dobson, B.C.; Muir, J.; Aïssa, C. Palladium-Catalyzed Synthesis of Bis-Substituted Sulfoxonium Ylides. Org. Lett. 2019, 21, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Janot, C.; Chagnoleau, J.-B.; Halcovitch, N.R.; Muir, J.; Aïssa, C. Palladium-Catalyzed Synthesis of α-Carbonyl-A′-(Hetero)Aryl Sulfoxonium Ylides: Scope and Insight into the Mechanism. J. Org. Chem. 2020, 85, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Momo, P.B.; Leveille, A.N.; Farrar, E.H.E.; Grayson, M.N.; Mattson, A.E.; Burtoloso, A.C.B. Enantioselective S–H Insertion Reactions of A-Carbonyl Sulfoxonium Ylides. Angew. Chem. Int. Ed. 2020, 59, 15554–15559. [Google Scholar] [CrossRef]
- Fang, X.; Wang, C.-J. Recent Advances in Asymmetric Organocatalysis Mediated by Bifunctional Amine–Thioureas Bearing Multiple Hydrogen-Bonding Donors. Chem. Commun. 2015, 51, 1185–1197. [Google Scholar] [CrossRef]
- Leveille, A.N.; Echemendía, R.; Mattson, A.E.; Burtoloso, A.C.B. Enantioselective Indole Insertion Reactions of α-Carbonyl Sulfoxonium Ylides. Org. Lett. 2021, 23, 9446–9450. [Google Scholar] [CrossRef]
- Guo, W.; Luo, Y.; Sung, H.H.-Y.; Williams, I.D.; Li, P.; Sun, J. Chiral Phosphoric Acid Catalyzed Enantioselective Synthesis of α-Tertiary Amino Ketones from Sulfonium Ylides. J. Am. Chem. Soc. 2020, 142, 14384–14390. [Google Scholar] [CrossRef]
- Furniel, L.G.; Burtoloso, A.C.B. Copper-Catalyzed N–H Insertion Reactions from Sulfoxonium Ylides. Tetrahedron 2020, 76, 131313. [Google Scholar] [CrossRef]
- Furniel, L.G.; Echemendía, R.; Burtoloso, A.C.B. Cooperative Copper-Squaramide Catalysis for the Enantioselective N–H Insertion Reaction with Sulfoxonium Ylides. Chem. Sci. 2021, 12, 7453–7459. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Han, Z.; Huang, H.; Sun, J. Organocatalytic Asymmetric Synthesis of α-Amino Esters from Sulfoxonium Ylides. Chem. Sci. 2021, 12, 11191–11196. [Google Scholar] [CrossRef]
- Guo, W.; Jiang, F.; Li, S.; Sun, J. Organocatalytic Asymmetric Azidation of Sulfoxonium Ylides: Mild Synthesis of Enantioenriched α-Azido Ketones Bearing a Labile Tertiary Stereocenter. Chem. Sci. 2022, 13, 11648–11655. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Cao, Y.-J.; Liu, X.-P.; An, J.; Yao, C.-J.; Ming, Z.-H.; Xiao, W.-J. A New Entry to Cascade Organocatalysis: Reactions of Stable Sulfur Ylides and Nitroolefins Sequentially Catalyzed by Thiourea and DMAP. J. Am. Chem. Soc. 2008, 130, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Li, F.; An, J.; Zhang, J.-J.; An, X.-L.; Hua, Q.-L.; Xiao, W.-J. Construction of Fused Heterocyclic Architectures by Formal [4 + 1]/[3 + 2] Cycloaddition Cascade of Sulfur Ylides and Nitroolefins. Angew. Chem. Int. Ed. 2009, 48, 9542–9545. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Li, F.; An, J.; Cheng, Y.; Chen, J.-R.; Xiao, W.-J. Hydrogen-Bond-Mediated Asymmetric Cascade Reaction of Stable Sulfur Ylides with Nitroolefins: Scope, Application and Mechanism. Chem. Eur. J. 2012, 18, 4073–4079. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Xiao, W.-J. Catalytic Asymmetric Synthesis of Chiral Dihydrobenzofurans through a Formal [4 + 1] Annulation Reaction of Sulfur Ylides and In Situ Generated Ortho-Quinone Methides. Eur. J. Org. Chem. 2017, 2017, 233–236. [Google Scholar] [CrossRef]
- Li, K.; Hu, J.; Liu, H.; Tong, X. Amine-Catalyzed Formal (3 + 3) Annulations of 2-(Acetoxymethyl)Buta-2,3-Dienoate with Sulfur Ylides: Synthesis of 4H-Pyrans Bearing a Vinyl Sulfide Group. Chem. Commun. 2012, 48, 2900. [Google Scholar] [CrossRef]
- Denisa Bisag, G.; Ruggieri, S.; Fochi, M.; Bernardi, L. Catalyst- and Substrate-Dependent Chemodivergent Reactivity of Stabilised Sulfur Ylides with Salicylaldehydes. Adv. Synth. Catal. 2021, 363, 3053–3059. [Google Scholar] [CrossRef]
- Day, D.P.; Vargas, J.A.M.; Burtoloso, A.C.B. Synthetic Routes Towards the Synthesis of Geminal A-Difunctionalized Ketones. Chem. Rec. 2021, 21, 2837–2854. [Google Scholar] [CrossRef]
- After we have submitted our manuscript, a review about applications of sulfonium and sulfoxonium ylides in enantioselective organocatalyzed reactions was also published in the literature. Wang, Z.-H.; Sun, T.-J.; Zhang, Y.-P.; You, Y.; Zhao, J.-Q.; Yin, J.-Q.; Yuan, W.-C. Application of Sulfonium and Sulfoxonium Ylides in Organocatalyzed Asymmetric Reaction. Chem. Synth. 2023, 3, 12. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Burtoloso, A.C.B. Organocatalytic Transformations from Sulfur Ylides. Catalysts 2023, 13, 689. https://doi.org/10.3390/catal13040689
Hayashi M, Burtoloso ACB. Organocatalytic Transformations from Sulfur Ylides. Catalysts. 2023; 13(4):689. https://doi.org/10.3390/catal13040689
Chicago/Turabian StyleHayashi, Marcio, and Antonio C. B. Burtoloso. 2023. "Organocatalytic Transformations from Sulfur Ylides" Catalysts 13, no. 4: 689. https://doi.org/10.3390/catal13040689
APA StyleHayashi, M., & Burtoloso, A. C. B. (2023). Organocatalytic Transformations from Sulfur Ylides. Catalysts, 13(4), 689. https://doi.org/10.3390/catal13040689