Exploring Simultaneous Upgrading and Purification of Biomass−Gasified Gases Using Plasma Catalysis
Abstract
1. Introduction
2. Results
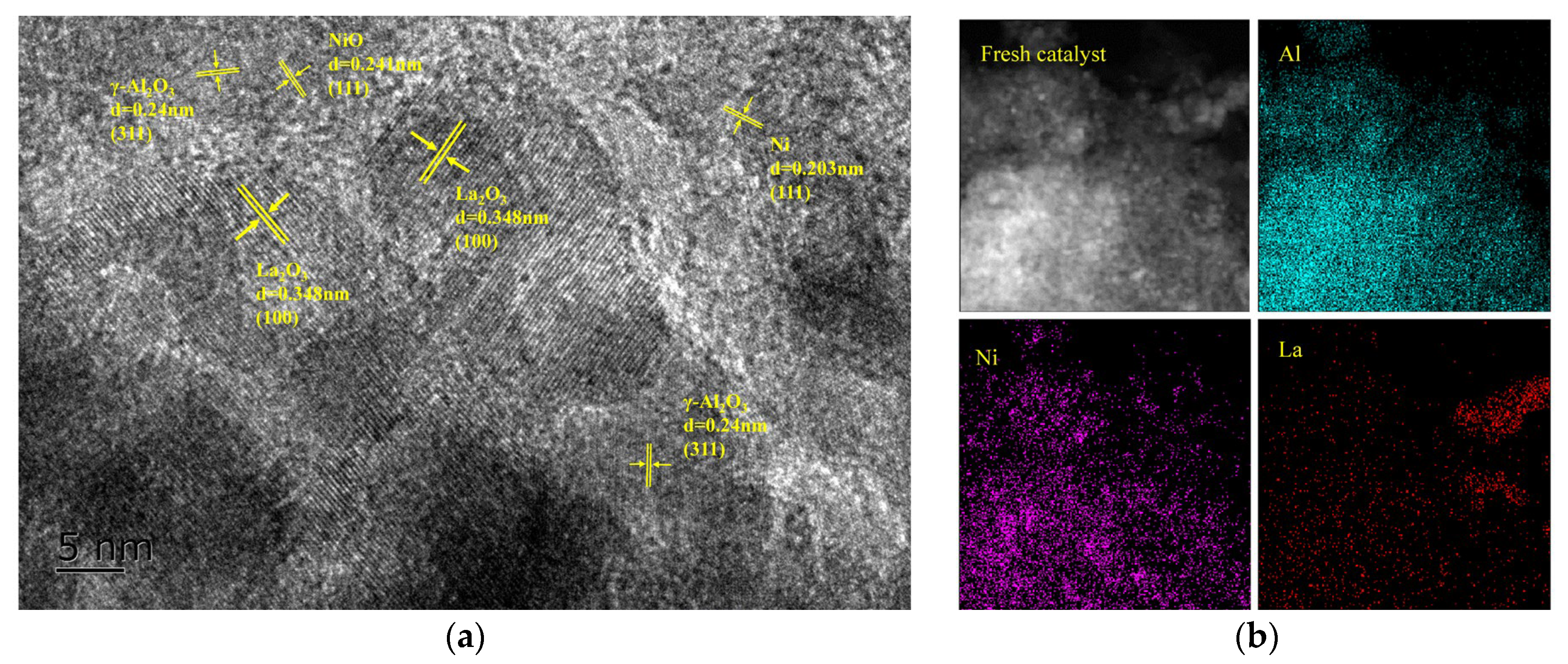
2.1. Characterization of the Fresh Catalysts
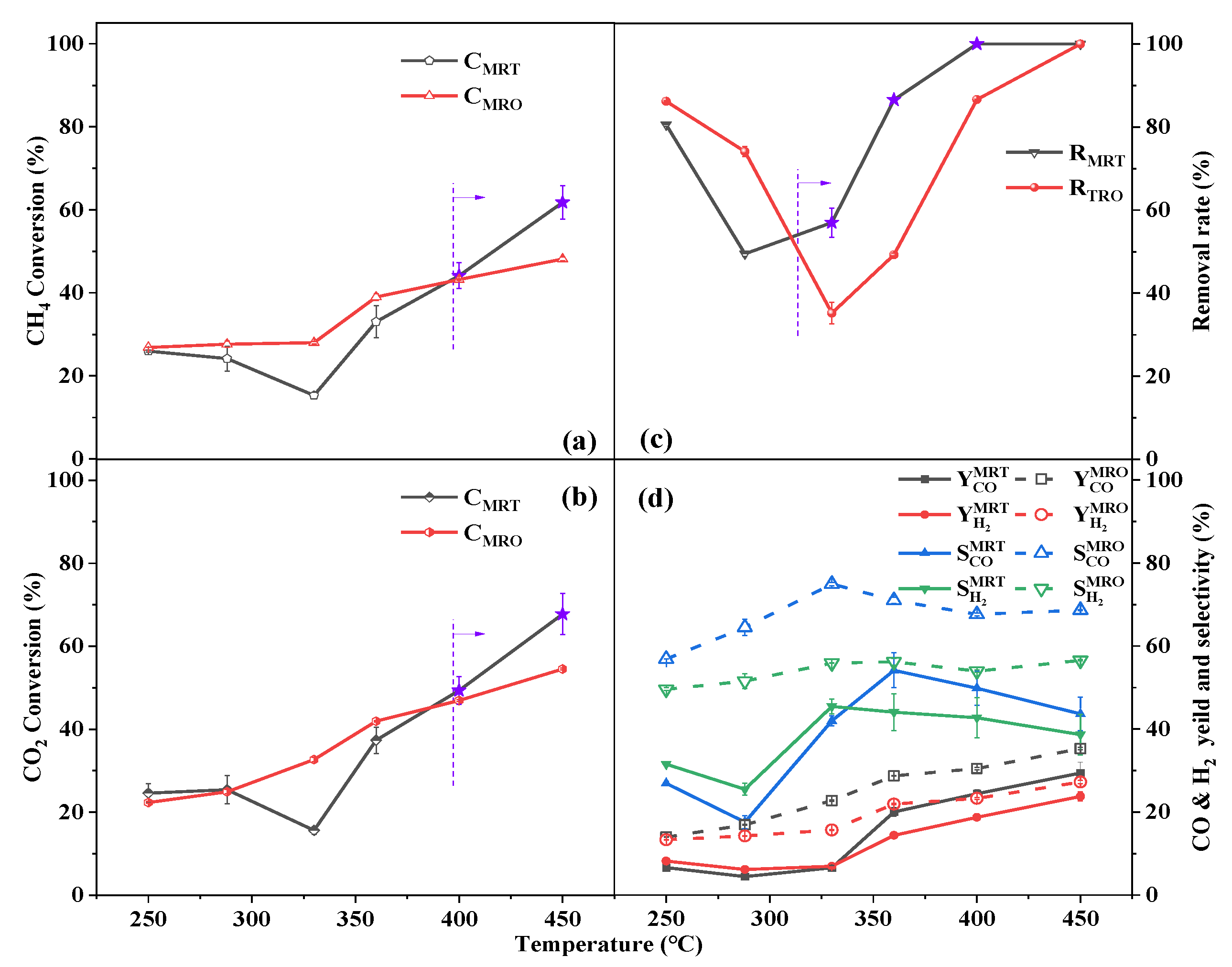
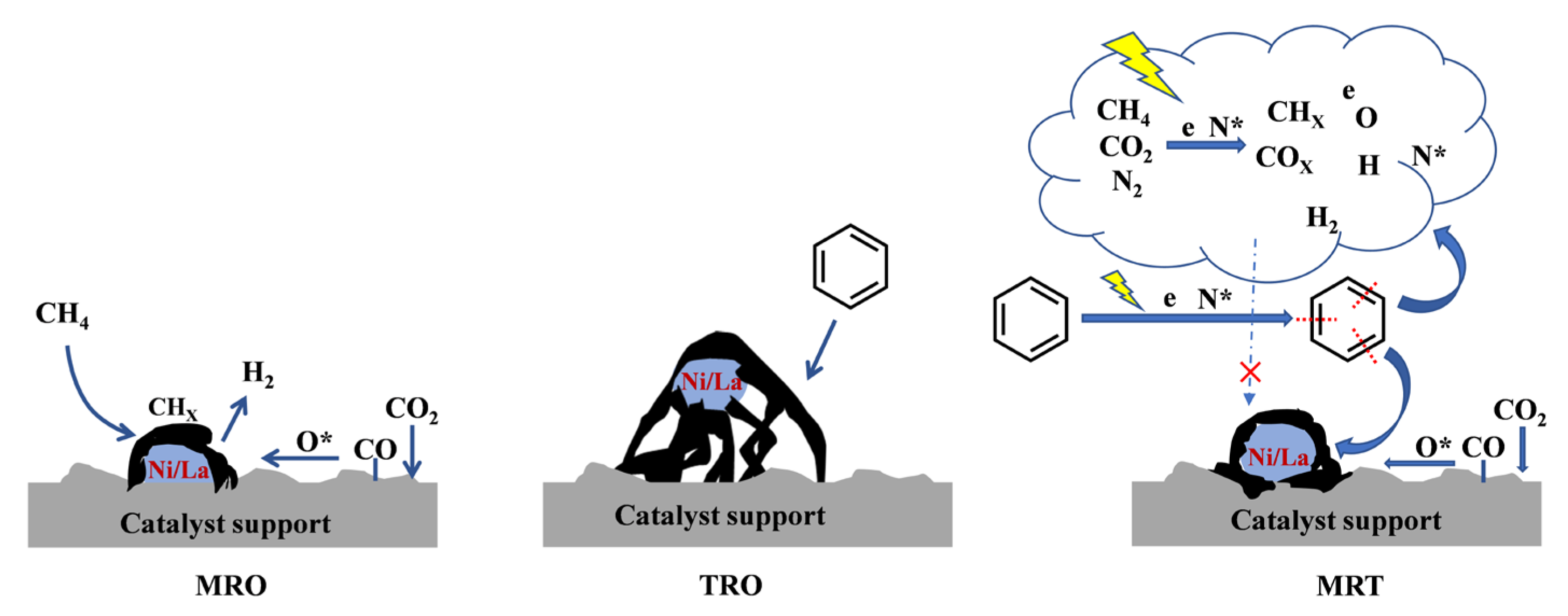
2.2. Interaction between Dry Reforming of CH4 and Benzene Removal
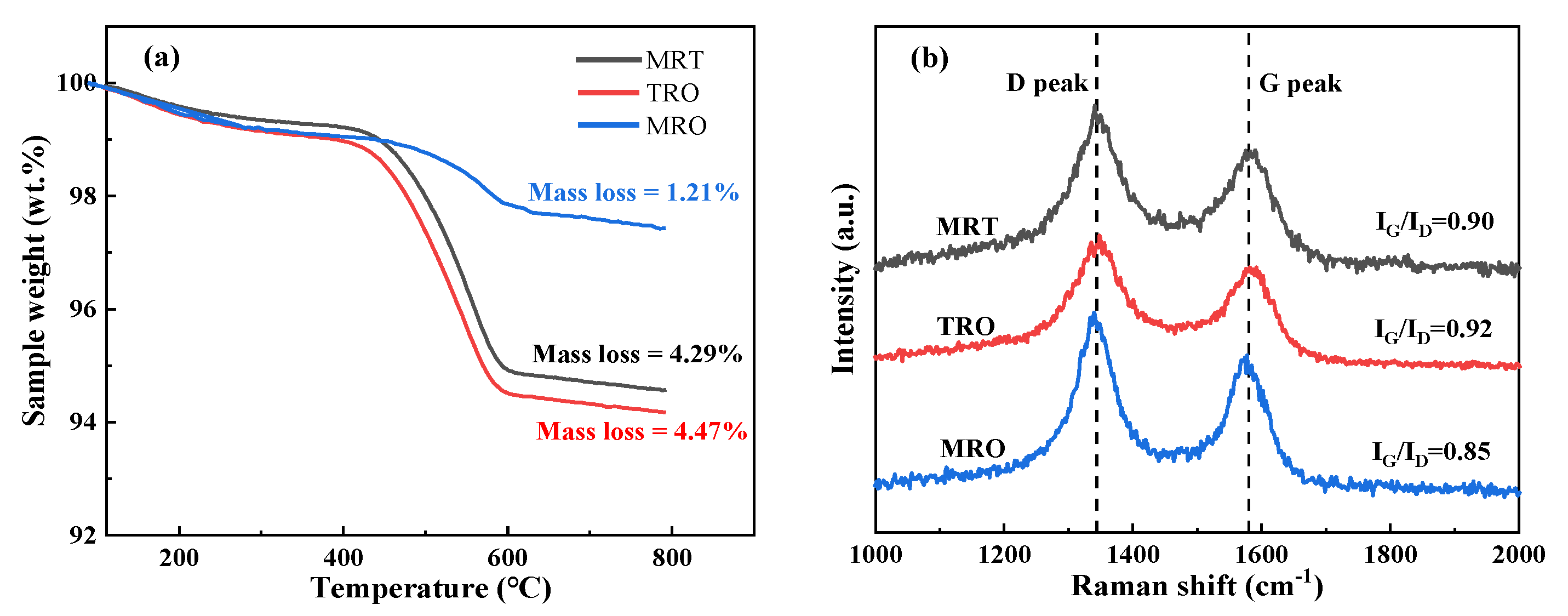
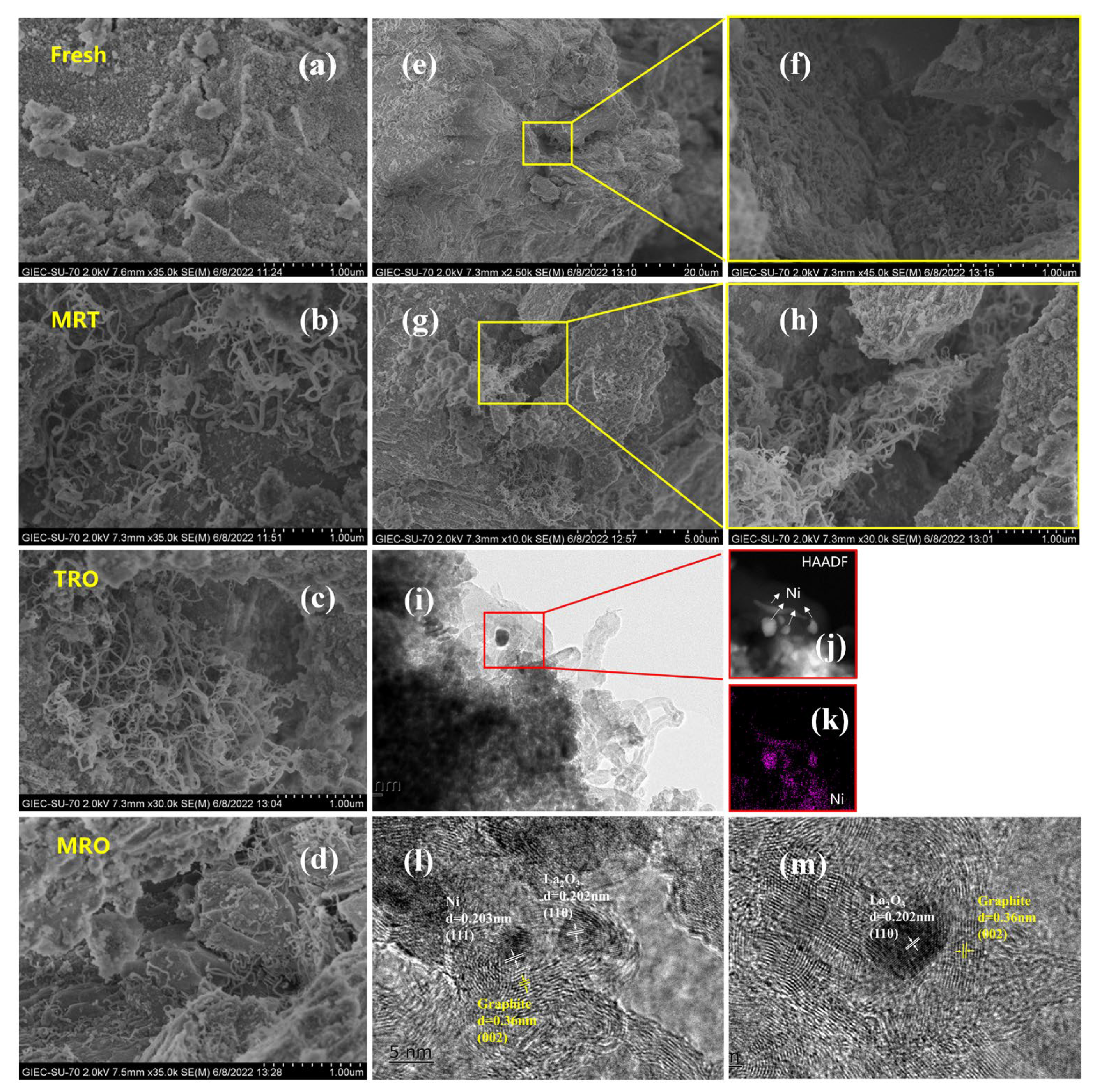
2.3. Reaction Mechanism Analysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Experimental Setup
3.3. Method of Analysis and Definition of Parameters
4. Conclusions
- For CH4–CO2 reforming, the addition of benzene affected the physicochemical properties of the catalyst surface. The excessive graphitic carbon caused encapsulation of the active sites or coverage of the defective sites, which weakened the adsorption of CO2 and CH4. In addition, the addition of benzene increased the gas−phase reaction density by generating ring−opening products and benzene radicals via the plasma action, which reacted or recombined with the active components (CHX, H, H2, CO, O) that originated from CH4 and CO2.
- For tar removal, the introduction of CH4 and CO2 improved the catalyst surface activity by increasing the concentration of reactive oxygen species on the catalyst surface, which in turn increased the resistance of the catalyst to graphitization and carbon deposits. This further enhanced the oxidation of carbonaceous species on the catalyst surface and facilitated the cracking of benzene to gas phase products (CO, CO2) via indirect or direct reaction pathways. On the other hand, the introduction of CH4 and CO2 may increase the amount of H, O, H2 and COX in the gas phase by plasma action, facilitating benzene reforming and ring−opening reactions; for example, benzene radicals recombine with CHX to form other aromatic compounds (e.g., toluene, ethylbenzene and styrene).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Wang, W.T.; Ma, Y.; Chen, G.X.; Quan, C.; Yanik, J.; Gao, N.B.; Tu, X. Enhanced hydrogen production using a tandem biomass pyrolysis and plasma reforming process. Fuel Process. Technol. 2022, 234, 107333. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sust. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Hussain, I.; Hambali, H.U.; Nabgan, W. Enhancing resistance of carbon deposition and reaction stability over nickel loaded fibrous silica-alumina (Ni/FSA) for dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 42250–42265. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew Sust Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Liu, Y.L. Fundamentals and applications of char in biomass tar reforming. Fuel Process. Technol. 2021, 216, 106782. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.H.; Mao, D.H.; Hu, C.W. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Nielsen, N.D.; Thrane, J.; Jensen, A.D.; Christensen, J.M. Bifunctional synergy in CO hydrogenation to methanol with supported Cu. Catal. Lett. 2020, 150, 1427–1433. [Google Scholar] [CrossRef]
- Diao, Y.; Zhang, X.; Liu, Y.; Chen, B.; Wu, G.; Shi, C. Plasma-assisted dry reforming of methane over Mo2C-Ni/Al2O3 catalysts: Effects of β-Mo2C promoter. Appl. Catal. B 2022, 301, 120779. [Google Scholar] [CrossRef]
- Abiev, R.S.; Sladkovskiy, D.A.; Semikin, K.V.; Murzin, D.Y.; Rebrov, E.V. Non-Thermal Plasma for Process and Energy Intensification in Dry Reforming of Methane. Catalysts 2020, 10, 1358. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.; Diao, X.; Liu, L.; Sun, Y. Removal of tar derived from biomass gasification via synergy of non-thermal plasma and catalysis. Sci. Total Environ. 2020, 721, 137671. [Google Scholar] [CrossRef] [PubMed]
- Cimerman, R.; Cibikova, M.; Satrapinskyy, L.; Hensel, K. The Effect of Packing Material Properties on Tars Removal by Plasma Catalysis. Catalysts 2020, 10, 1476. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, H.; Li, X.; Xu, R.; Mubeen, I.; Li, L.; Yan, J. Destruction of Toluene, Naphthalene and Phenanthrene as Model Tar Compounds in a Modified Rotating Gliding Arc Discharge Reactor. Catalysts 2019, 9, 19. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Dry reforming of methane by combined spark discharge with a ferroelectric. Energy Convers. Manage. 2016, 124, 305–314. [Google Scholar] [CrossRef]
- Li, S.; Dang, X.; Yu, X.; Abbas, G.; Zhang, Q.; Cao, L. The application of dielectric barrier discharge non-thermal plasma in VOCs abatement: A review. Chem. Eng. J. 2020, 388, 124275. [Google Scholar] [CrossRef]
- Eliasson, B.; Liu, C.-j.; Kogelschatz, U. Direct conversion of methane and carbon dioxide to higher hydrocarbons using catalytic dielectric-barrier discharges with zeolites. Ind. Eng. Chem. Res. 2000, 39, 1221–1227. [Google Scholar] [CrossRef]
- Tang, Y.; Zhuo, J.K.; Cui, W.; Li, S.Q.; Yao, Q. Enhancing ignition and inhibiting extinction of methane diffusion flame by in situ fuel processing using dielectric-barrier-discharge plasma. Fuel Process. Technol. 2019, 194, 106128. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Wu, C.; Guo, H.; Tu, X. One-step reforming of CO2 and CH4 into high-value liquid chemicals and fuels at room temperature by plasma-driven catalysis. Angew. Chem. Int. Ed. 2017, 56, 13679–13683. [Google Scholar] [CrossRef] [PubMed]
- Goujard, V.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Use of a non-thermal plasma for the production of synthesis gas from biogas. Appl. Catal. A 2009, 353, 228–235. [Google Scholar] [CrossRef]
- Ray, D.; Manoj Kumar Reddy, P.; Challapalli, S. Glass beads packed DBD-plasma assisted dry reforming of methane. Top. Catal. 2017, 60, 869–878. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew Sust Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Mei, D.H.; Liu, S.Y.; Tu, X. CO2 reforming with methane for syngas production using a dielectric barrier discharge plasma coupled with Ni/γ-Al2O3 catalysts: Process optimization through response surface methodology. J. CO2 Util. 2017, 21, 314–326. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.-L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2014, 24, 015011. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Jafari, M.F.; Estifaee, P. Plasma assisted synthesis and physicochemical characterizations of Ni–Co/Al2O3 nanocatalyst used in dry reforming of methane. Plasma Chem. Plasma Process. 2013, 33, 663–680. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, X.; Mei, D.; Ashford, B.; Tu, X. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts. Catal. Today 2015, 256, 80–87. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/(sic)-Al2O3-MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Saleem, F.; Rehman, A.; Abbas, A.; Hussain Khoja, A.; Ahmad, F.; Liu, L.; Zhang, K.; Harvey, A. A comparison of the decomposition of biomass gasification tar compound in CO, CO2, H2 and N2 carrier gases using non-thermal plasma. J. Energy Inst. 2021, 97, 161–168. [Google Scholar] [CrossRef]
- Liu, J.-L.; Li, X.-S.; Zhu, X.; Li, K.; Shi, C.; Zhu, A.-M. Renewable and high-concentration syngas production from oxidative reforming of simulated biogas with low energy cost in a plasma shade. Chem. Eng. J. 2013, 234, 240–246. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Ahmad, S.; Yang, X.; Ji, M.; Sun, Y. Steam reforming of toluene as model biomass tar to H2-rich syngas in a DBD plasma-catalytic system. J. Energy Inst. 2018, 91, 927–939. [Google Scholar] [CrossRef]
- Liu, S.Y.; Mei, D.H.; Nahil, M.A.; Gadkari, S.; Gu, S.; Williams, P.T.; Tu, X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts. Fuel Process. Technol. 2017, 166, 269–275. [Google Scholar] [CrossRef]
- Xu, B.; Xie, J.; Yin, X.; Liu, H.; Sun, C.; Wu, C. Mechanisms of Toluene Removal in Relation to the Main Components of Biosyngas in a Catalytic Nonthermal Plasma Process. Energy Fuels 2019, 33, 4287–4301. [Google Scholar] [CrossRef]
- Nair, S.A.; Pemen, A.J.M.; Yan, K.; Van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Chemical processes in tar removal from biomass derived fuel gas by pulsed corona discharges. Plasma Chem. Plasma Process. 2003, 23, 665–680. [Google Scholar] [CrossRef]
- Wnukowski, M.; Jamróz, P. Microwave plasma treatment of simulated biomass syngas: Interactions between the permanent syngas compounds and their influence on the model tar compound conversion. Fuel Process. Technol. 2018, 173, 229–242. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Smith, P.J.; Lu, L.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Kelly, G.J.; Park, C.W.; Kiely, C.J.; Hutchings, G.J. Preparation of a highly active ternary Cu-Zn-Al oxide methanol synthesis catalyst by supercritical CO2 anti-solvent precipitation. Catal. Today 2018, 317, 12–20. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, B.H.; Jin, Y.; Cheng, Y. Dry Reforming of Methane in a Dielectric Barrier Discharge Reactor with Ni/Al2O3 Catalyst: Interaction of Catalyst and Plasma. Energy Fuels 2009, 23, 4196–4201. [Google Scholar] [CrossRef]
- Xu, B.; Xie, J.; Yuan, H.; Yin, X.; Wu, C. Experimental study on benzene removal of fuel gas in a packed-bed dielectric barrier discharge reactor. J. Fuel Chem. Technol. 2019, 47, 493–503. [Google Scholar]
- Blackbeard, T.; Demidyuk, V.; Hill, S.L.; Whitehead, J.C. The effect of temperature on the plasma-catalytic destruction of propane and propene: A comparison with thermal catalysis. Plasma Chem. Plasma Process. 2009, 29, 411–419. [Google Scholar] [CrossRef]
- Pan, W.; Meng, J.; Gu, T.; Zhang, Q.; Zhang, J.; Wang, X.; Bu, C.; Liu, C.; Xie, H.; Piao, G. Plasma-catalytic steam reforming of benzene as a tar model compound over Ni-HAP and Ni-gamma Al2O3 catalysts: Insights into the importance of steam and catalyst support. Fuel 2023, 339, 127327. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Li, G.; Liu, J.; Wang, Y.; Xu, Y.; Lyu, Y. Understanding structure-activity relationships of the highly active and stable La promoted Co/WC-AC catalyst for methane dry reforming. Int. J. Hydrogen Energy 2022, 47, 7823–7835. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Smith, M.; Scudiero, L.; Espinal, J.; McEwen, J.-S.; Garcia-Perez, M. Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon 2016, 110, 155–171. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Norskov, J.K. Hydrogen and synthesis gas by steam- and CO2 reforming. In Advances in Catalysis; Gates, B.C., Knozinger, H., Eds.; 2002; Volume 47, pp. 65–139. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Chen, W.; Tang, P.; Li, W.; Shi, Z.; Su, D.; Wang, J.; Ma, D. Catalytic epoxidation reaction over N-containing sp2 carbon catalysts. Acs Catal. 2014, 4, 1261–1266. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, G.; Wu, C.; Liu, J.; Lv, Y. Nitrogen-doped porous carbons derived from sustainable biomass via a facile post-treatment nitrogen doping strategy: Efficient CO2 capture and DRM. Int. J. Hydrogen Energy 2022, 47, 24388–24397. [Google Scholar] [CrossRef]
- Moussa, S.O.; Panchakarla, L.S.; Ho, M.Q.; El-Shall, M.S. Graphene-supported, iron-based nanoparticles for catalytic production of liquid hydrocarbons from synthesis gas: The role of the graphene support in comparison with carbon nanotubes. ACS Catal. 2014, 4, 535–545. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Arami-Niya, A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation. Appl. Surf. Sci. 2011, 257, 3936–3942. [Google Scholar] [CrossRef]
- Zhang, K.; He, Y.; Wang, Z.; Huang, T.; Li, Q.; Kumar, S.; Cen, K. Multi-stage semi-coke activation for the removal of SO2 and NO. Fuel 2017, 210, 738–747. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Z.; Zhao, L.; Li, Z.; Yi, W.; Kang, K.; Jia, J. Synthesis and characterization of different activated biochar catalysts for removal of biomass pyrolysis tar. Energy 2021, 232, 120927. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Z.; Tang, N.; Chen, S.; Wang, W.; Yuan, J.; Cao, Z.; He, H.; Cong, Y. Styrene hydrogenation over Ni–La/Al2O3 catalysts: The impact of added La on active metal dispersion. Chem. Phys. Lett. 2021, 775, 138604. [Google Scholar] [CrossRef]
- Cichy, M.; Pańczyk, M.; Słowik, G.; Zawadzki, W.; Borowiecki, T. Ni–Re alloy catalysts on Al2O3 for methane dry reforming. Int. J. Hydrogen Energy 2022, 47, 16528–16543. [Google Scholar] [CrossRef]
- Zhang, M.M.; Gao, Y.B.; Mao, Y.P.; Wang, W.L.; Sun, J.; Song, Z.L.; Sun, J.; Zhao, X.Q. Enhanced dry reforming of methane by microwave-mediated confined catalysis over Ni-La/AC catalyst. Chem. Eng. J. 2023, 451, 138616. [Google Scholar] [CrossRef]
- Zhang, F.S.; Song, Z.L.; Zhu, J.Z.; Liu, L.; Sun, J.; Zhao, X.Q.; Mao, Y.P.; Wang, W.L. Process of CH4-CO2 reforming over Fe/SiC catalyst under microwave irradiation. Sci. Total Environ. 2018, 639, 1148–1155. [Google Scholar] [CrossRef]
- Gao, N.B.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sustain. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castano, P. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]
- Snoeck, J.W.; Froment, G.F.; Fowles, M. Filamentous carbon formation and gasification: Thermodynamics, driving force, nucleation, and steady-state growth. J. Catal. 1997, 169, 240–249. [Google Scholar] [CrossRef]
- Chatla, A.; Abu-Rub, F.; Prakash, A.V.; Ibrahim, G.; Elbashir, N.O. Highly stable and coke-resistant Zn-modified Ni-Mg-Al hydrotalcite derived catalyst for dry reforming of methane: Synergistic effect of Ni and Zn. Fuel 2022, 308, 122042. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Choudhury, H.A.; Wang, D.; El-Halwagi, M.M.; Weitz, E.; Elbashir, N.O. A novel CO2 utilization technology for the synergistic co-production of multi-walled carbon nanotubes and syngas. Sci Rep-UK 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.J.; Liu, J.W.; Xu, Y.; Sun, Y.H. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010-2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Ren, H.; Gao, X. A short review on nickel-based catalysts in dry reforming of methane: Influences of oxygen defects on anti-coking property. Mater. Today Proc. 2021, 42, 153–160. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Zhang, X.; Tao, L.; Xia, L.; Xu, X.; Song, J.; Zhou, W.; Fang, X.; Wang, X. Ni/La2O3 Catalysts for Dry Reforming of Methane: Insights into the Factors Improving the Catalytic Performance. ChemCatChem 2019, 11, 2887–2899. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: Kinetic and mechanistic studies. Int. J. Hydrogen Energy 2016, 41, 4603–4615. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-ray photoelectron spectroscopy of rare-earth compounds. J. Electron. Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Z.; Wu, J.; Li, X.; Wang, L. Hydrotreating performance of La-modified Ni/Al2O3 catalysts prepared by hydrothermal impregnation method. Kinet. Catal. 2015, 56, 222–225. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Shalvoy, R.B.; Reucroft, P.J.; Davis, B.H. Characterization of coprecipitated nickel on silica methanation catalysts by X-ray photoelectron spectroscopy. J. Catal. 1979, 56, 336–348. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Song, J.; Ahmad, S.; Yang, X.; Sun, Y. Plasma-assisted catalytic reforming of toluene to hydrogen rich syngas. Catal. Sci. Technol. 2017, 7, 4216–4231. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Song, J.; Yang, X.; Sun, Y. Dry reforming of model biomass pyrolysis products to syngas by dielectric barrier discharge plasma. Int. J. Hydrogen Energy 2018, 43, 10281–10293. [Google Scholar] [CrossRef]
- Darwent, B.d. Bond dissociation energies in simple molecules. In National Standard Reference Data System; National Institute of Standards: Gaithersburg, MA, USA, 1970. [Google Scholar]
- Zhang, K.; Harvey, A.P. CO2 decomposition to CO in the presence of up to 50% O-2 using a non-thermal plasma at atmospheric temperature and pressure. Chem. Eng. J. 2021, 405, 126625. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Xu, B.; Lang, L.; Yang, W.; Liu, H.; Zhan, H.; Xie, J.; Yin, X.; Wu, C. Exploring Simultaneous Upgrading and Purification of Biomass−Gasified Gases Using Plasma Catalysis. Catalysts 2023, 13, 686. https://doi.org/10.3390/catal13040686
He W, Xu B, Lang L, Yang W, Liu H, Zhan H, Xie J, Yin X, Wu C. Exploring Simultaneous Upgrading and Purification of Biomass−Gasified Gases Using Plasma Catalysis. Catalysts. 2023; 13(4):686. https://doi.org/10.3390/catal13040686
Chicago/Turabian StyleHe, Wenyu, Bin Xu, Lin Lang, Wenshen Yang, Huacai Liu, Hao Zhan, Jianjun Xie, Xiuli Yin, and Chuangzhi Wu. 2023. "Exploring Simultaneous Upgrading and Purification of Biomass−Gasified Gases Using Plasma Catalysis" Catalysts 13, no. 4: 686. https://doi.org/10.3390/catal13040686
APA StyleHe, W., Xu, B., Lang, L., Yang, W., Liu, H., Zhan, H., Xie, J., Yin, X., & Wu, C. (2023). Exploring Simultaneous Upgrading and Purification of Biomass−Gasified Gases Using Plasma Catalysis. Catalysts, 13(4), 686. https://doi.org/10.3390/catal13040686





