Abstract
Carbon materials are widely used in catalysis as effective catalyst supports. Carbon supports can be produced from coal, organic precursors, biomass, and polymer wastes. Biomass is one of the promising sources used to produce carbon-based materials with a high surface area and a hierarchical structure. In this review, we briefly discuss the methods of biomass-derived carbon supported catalyst preparation and their application in biodiesel production, organic synthesis reactions, and electrocatalysis.
1. Introduction
Carbon materials, such as graphite, graphene, activated carbon, carbon nanotubes (CNT), nanosheets (CNS), and nanofibers (CNF), are widely used in catalysis. Such materials are considered to be promising catalytic supports because of their high specific surface area, resistance to acidic or basic media, amphoteric character, high-temperature stability, tailored pore size distribution, ability for modification, and cheapness [1,2,3]. Carbon supports can be produced from coal, organic precursors, biomass, and polymer waste through different techniques, which are mainly based on thermochemical degradation/decomposition methods. In recent years, biomass-derived carbonaceous materials have attracted great attention due to their cheapness, simple preparation, and wide range of biomass resources.
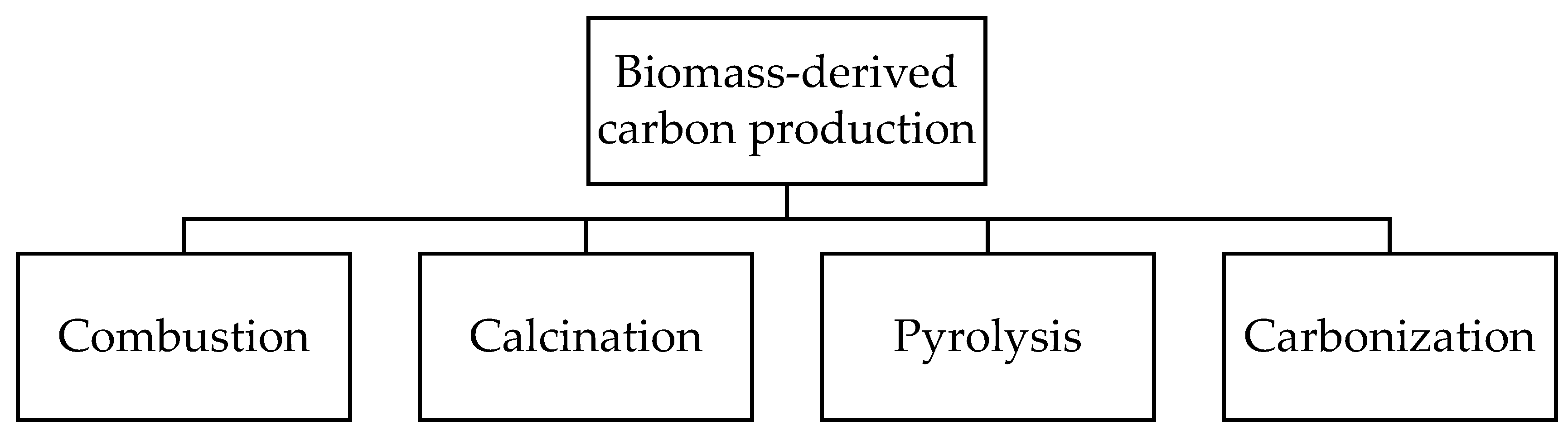
To produce carbon materials from biomass, different thermochemical methods can be used. Among them, four main techniques are highlighted (Figure 1). Combustion of biomass is widely used to obtain ashes that are characterized by the contents of alkali and alkali-earth metal compounds with a low content of carbon (1–15 wt.%). Depending on the biomass type, the resulting ashes have different elemental compositions, varying from 10–50 wt.% of alkali metals, 5–20 wt.% of alkali-earth metals, 0.5–2 wt.% of Al, 2–80 wt.% of Si, and 0.5–10 wt.% of Cl, P, Mn, Fe, Zn, etc. [4]. The calcination method is carried out in a wide temperature range from 300 to 1000 °C [5]. Calcination can be performed either in the air or in an inert atmosphere, resulting in the degradation of biomass components to form carbon and inorganic compounds, such as alkali and alkali-earth metal carbonates and carbides. During calcination, temperature and biomass retention time play a critical role in the formation of the resulting carbon material morphology and structure. It is reported that a high reaction temperature and a short retention time lead to the aggregation of particles of Ca, Na, Mg, and Si oxides and, thus, reduce the specific surface area and porosity of the resulting catalysts [6,7]. Despite the simplicity of the combustion and calcination methods, the resulting materials have a low specific surface area, poor porosity, and low carbon content, leading to their limited application in catalysis.
Figure 1.
Main methods for biomass-derived carbon production.
Another two methods are used to prepare biochar characterized by a high porosity with a hierarchical structure. Pyrolysis is a widely used method to obtain biochar from organogenic feedstock. Depending on the temperature and the feedstock residence time, pyrolysis can be divided into slow pyrolysis occurring at 300–700 °C for several hours [8,9] and fast pyrolysis performed at 500–1000 °C at a residence time < 10 s [10]. Typically, slow pyrolysis results in a biochar yield ranging from 30 to 70 wt.%, while the fast process provides a biochar yield of up to 15 wt.% [9]. Besides conventional non-catalytic pyrolysis to provide biochar production, biomass feedstock can be preliminarily treated with a metal salt solution (catalytic pyrolysis), which leads to the formation of metal-containing composites. The carbonization method seems to be close to pyrolysis and consists of heating biomass in an inert atmosphere (N2 or Ar) at relatively low temperatures. This method can be divided into two types depending on the media used. Dry carbonization is carried out at 300–600 °C using dried feedstock [11]. Wet or hydrothermal carbonization (HTC) is performed in superheated water using either dried or wet feedstock at a temperature of 180–800 °C [12]. The latter can be classified into high-temperature (300–800 °C) and low-temperature (180–250 °C) HTC [13,14,15]. The high-temperature process results in the formation of carbon materials with a high surface area and a high porosity, with a hierarchical structure, while the low-temperature HTC is used for the production of colloidal carbonaceous spheres [15].
Moreover, techniques such as gasification and torrefaction can be mentioned for biochar production. Gasification is the process mainly used for synthesis gas production from biomass occurring at a temperature of 750–900 °C. Meanwhile, about 10 wt.% of biochar can be obtained by gasification [16]. Torrefaction is direct carbonization of biomass at low temperatures (up to 250–300 °C), resulting in over 80 wt.% of biochar formation [17]. The composition, structure, morphology, and textural properties of carbon materials strongly depend on the biomass type, the preparation method used, the temperature, the size of the particle, the heating rate, the residence time, etc.
Despite the different production methods, biomass-derived carbon materials find a wide range of application in catalysis. Four main directions can be highlighted: biodiesel production, organic reactions, electrocatalysis, and photocatalysis. In this review, we briefly discuss the first three directions for the use of biomass-derived carbon-based catalysts.
2. Biomass-Derived Carbon-Based Catalysts for Biodiesel Production
In recent years, biomass-carbon-based catalysts are widely used in esterification/transesterification processes to produce fatty acid methyl esters (FAMEs). Carbon produced via pyrolysis, calcination, or carbonization tends to be functionalized by sulfonic groups or alkali to form acidic or basic active sites (Figure 2). Two directions for the functionalization are used: (i) direct functionalization through the introduction of functional groups into the raw material, and (ii) post-synthesis modification through the treatment of carbonaceous materials [15].
2.1. Acid-Functionalized Catalysts
Acidic carbon-based catalysts are efficiently applied in esterification/transesterification processes. The most widely used ones are sulfonated carbons characterized by a high acid density. These catalysts can substitute concentrated sulfuric acid in many reactions, including biodiesel production [18]. Sulfonated catalysts are synthesized by either direct sulfonation with H2SO4 or by sulfonation via reductive alkylation. In contrast to concentrated sulfuric acid, carbon-based catalysts tend to cause less corrosion and can be easily separated from the reaction mixture.
A review of recent works on sulfonated carbon catalysts shows that these catalysts, regardless of the method of preparation, are consisted of amorphous or graphite-like carbon with covalently bonded –SO3H groups. The last ones provide the Brønsted acid sites with a high density. The surface area of the resulting composites mainly depends on the carbon preparation method. These catalysts have a high activity in the production of biodiesel from feedstock with high free fatty acid (HFFA) content [19,20]. Incomplete carbonization of the biomass raw material during the catalyst synthesis leads to the formation of a polycyclic carbon-ring structure with –COOH or –OH groups on the surface, which increases the catalyst acidity and facilitates the covalent bonding of sulfonic groups [21,22].
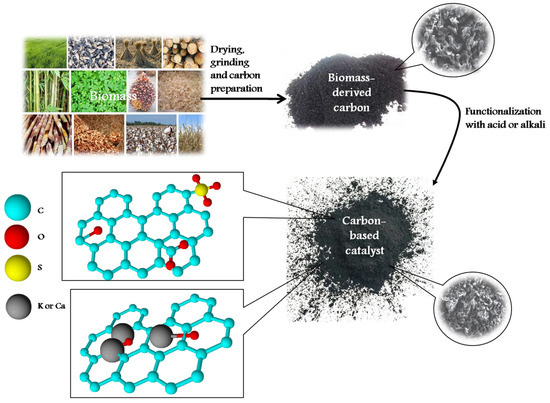
Figure 2.
Biomass-derived carbons for the application in biodiesel production. Summarized from [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Figure 2.
Biomass-derived carbons for the application in biodiesel production. Summarized from [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
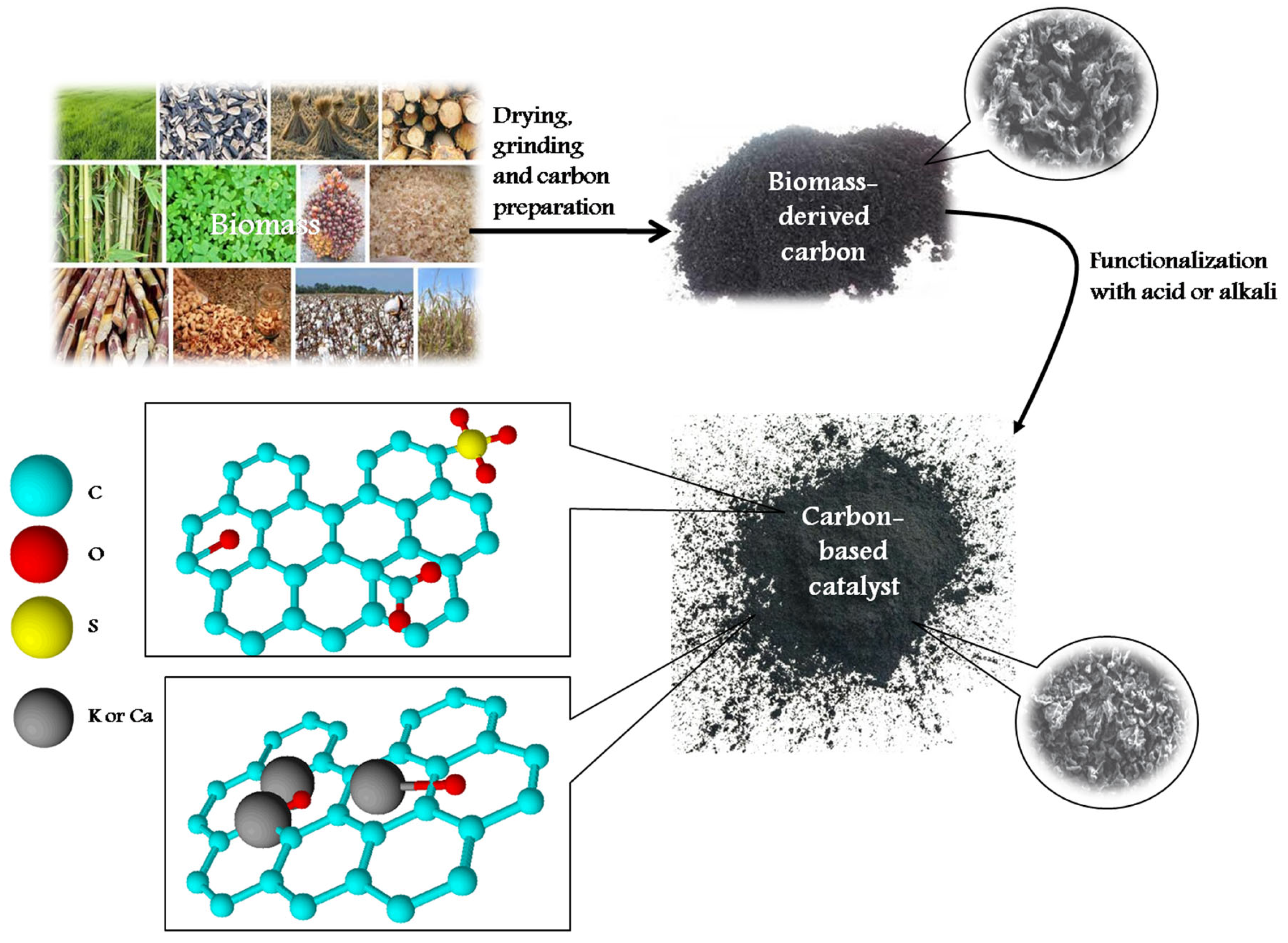
Table 1 presents the literature data on sulfonated carbon-based catalysts and their activity during the esterification/transesterification process.

Table 1.
Sulfonated carbon-based catalysts for biodiesel production.
Carbonization and pyrolysis of biomass with sulfonation result in a relatively low specific surface area of the catalyst. Meanwhile, pretreatment of biomass with phosphorous acid leads to a significant increase in the surface area three times that of non-pretreated ones [33]. Sulfonated biocarbon-based catalysts mainly show a FAME yield over 80 wt.%. It is observed that the catalyst activity slightly depends on the specific surface area of the catalysts, but the acid sites’ density on the carbon surface plays an important role. However, a high surface area can be crucial for the reactions with large molecules (i.e., triglycerides). Thus, the catalyst surface area, acid site density, and carbon preparation method play important roles in the acid-catalyzed esterification/transesterification process.
However, one of the main problems for sulfonated carbon-based catalysts is the loss in activity after 5–7 cycles. Moreover, sulfonated catalysts tend to be deactivated at high temperatures because of the loss of sulfonic groups from the surface. Thus, future works should focus on the improvement of catalyst stability. Another challenge associated with oil transesterification in the presence of biomass-derived catalysts is the investigation of reaction kinetics and mechanisms.
2.2. Base-Functionalized Catalysts
Basic carbon-based catalysts are less used in the transesterification process in comparison to acidic ones. This is due to the high saponification activity of bases, especially for HFFA oils [33]. Moreover, a critical disadvantage of a basic catalyst is its sensitivity to water [34]. Meanwhile, several studies on the application of base-functionalized carbon-based catalysts can be mentioned. Two directions in the preparation of base-functionalized carbon-based catalysts can be highlighted. The first one is calcination of biomass feedstock. As it is known, plant biomass contains a high amount of alkali and alkali-earth metals [4]. Calcination leads to a decrease in the concentration of H, O, and C atoms, producing alkali or alkali-metal oxides or hydroxides along with alumina or silicon oxides. The second method for base-functionalized catalyst preparation is preliminary treatment of raw biomass with solutions of KOH, potassium, or calcium salts (i.e., KF, Ca(Ac)2), followed by hydrothermal or thermal carbonization. This method results in a biochar containing metal oxides on the surface. The last ones catalyze the transesterification process and show high effectiveness. Table 2 presents the results of recent studies on transesterification of oils over a base-functionalized catalyst.

Table 2.
Basic carbon-based catalysts for biodiesel production.
The activity of base-functionalized catalysts is determined by both the base concentration and catalyst surface area. In this case, the surface area seems to be a significant factor, providing good triglyceride adsorption on the catalyst as well as the distribution and availability of basic active sites. For this reason, the carbonization method (in particular, HTC) can be one of the most promising methods. Additionally, the use of additional alkali during the catalyst preparation process provides higher activity and biodiesel yield in comparison with catalysts obtained without impregnation.
Generally, base-functionalized carbon catalysts prepared through the calcination method are less stable and lose their activity after 3–5 consecutive cycles because of the leaching of alkali via water or methanol or because the interaction of alkali with free fatty acids. Meanwhile, catalysts prepared by the carbonization method, followed by activation by a base, maintain their effectiveness in terms of oil conversion of FAME yield for a minimum of five cycles. In spite of the lower stability, alkali catalysts allow transesterification to be performed at lower temperatures (even at room temperature) with a high biodiesel yield. This can be significant from an economical point of view.
In summary, basic biocarbon catalysts have proven to have a higher conversion of oil into biodiesel at a lower reaction temperature in comparison with sulfonated ones. However, the use of these composites in the transesterification of waste cooking oil is limited because of the high free fatty acid content in the oil.
3. Biomass-Derived Carbon-Based Catalysts for Biomass Conversion and Organic Synthesis
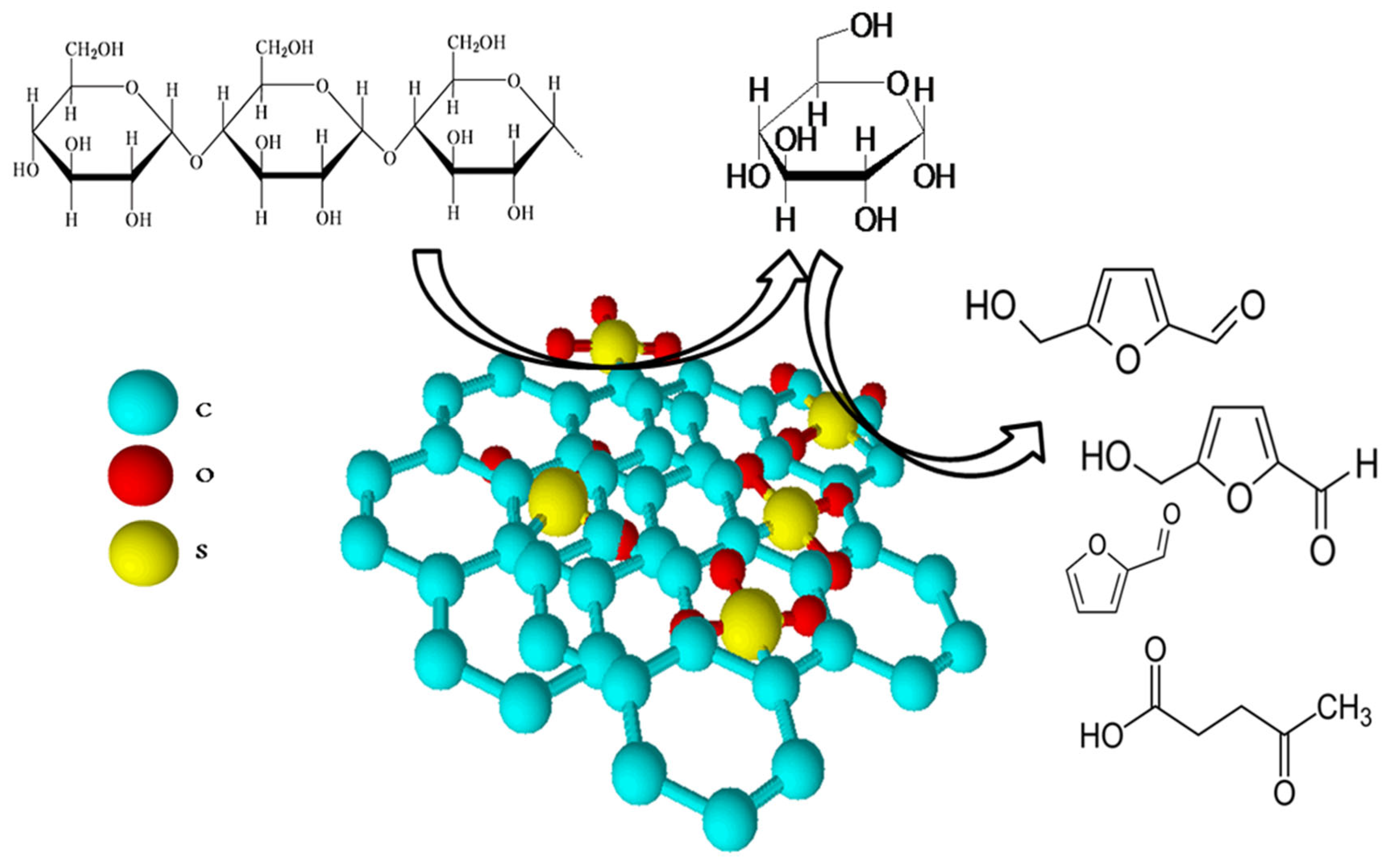
Sulfonated biomass-derived carbons have been effectively used for biomass conversion into valuable chemicals, such as furfural, 5-hydroxymethylfurfural, 5-ethoxymethylfurfural, and hexitols. The presence of –SO3H groups on the carbon surface effectively catalyzes the hydrolysis and dehydration of polysaccharides of biomass (cellulose and hemicelluloses) (Figure 3) [15]. These catalysts are mainly produced by biomass pyrolysis with or without templates, followed by sulfonation by concentrated sulfuric acid. As for biodiesel synthesis, the resulting composites have the same structure and are characterized by high acidity. The summarized results are presented in Table 3.
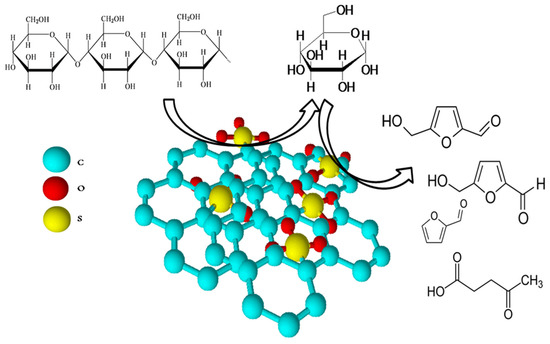
Figure 3.
Application of acidic carbon-based catalysts for biomass conversion. Summarized from [46,47,48,49,50,51,52,53,54,55].

Table 3.
Sulfonated carbon-based catalysts for biomass conversion.
High catalytic activity of sulfonated carbons in biomass hydrolysis and dehydration reactions can be attributed to the high ability of SO3H-groups to adsorb carbohydrates, as well as their tolerance to hydration. Moreover, the catalyst effect in hydrolysis depends significantly on the water concentration and reaches a maximum when the water content is equal to the catalyst acidity [15,56]. In comparison with traditionally used catalysts containing Lewis and Brønsted acid sites (e.g., zeolites, Amberlyst, and Nafion), sulfonated carbons show higher effectiveness in biomass hydrolysis and dehydration and are considered promising for obtaining platform molecules from biomass.
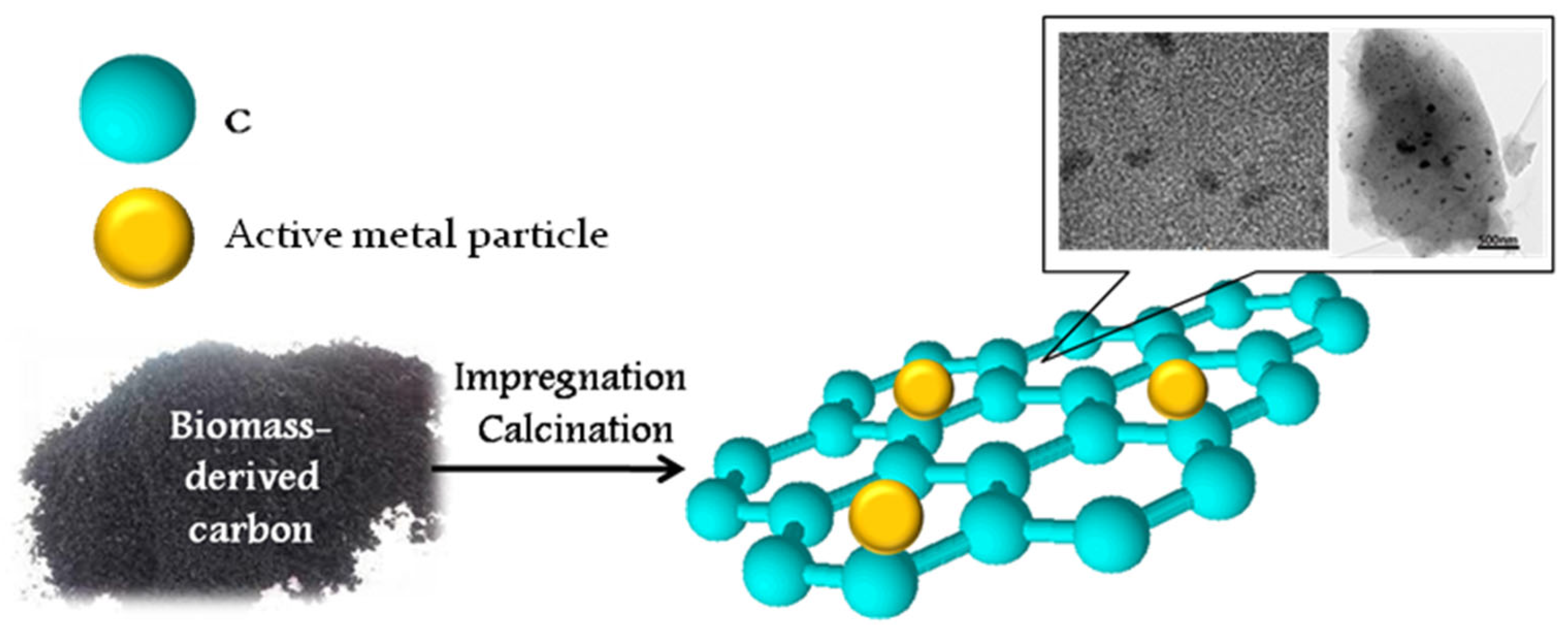
Besides the functionalization of biocarbon with acid or basic sites, the production of carbon-based composites containing different metal nanoparticles is widely used. Carbon-supported catalysts have great potential in many organic reactions, such as hydrogenation, oxidation, alkylation, and dehydrohalogenation. Biomass-derived carbonaceous supports for metal-containing catalysts are effectively prepared through hydrothermal or dry carbonization, thus providing a high surface area for the resulting materials (200–1000 m2/g). The active metal-containing phase is traditionally formed by impregnation of the carbon supports with solutions of metal salts. Acetate and nitrates are widely used as metal precursors because of their easy decomposition by heating or calcination (Figure 4). A summary of the reactions using biomass-derived carbon-based catalysts is shown in Table 4.
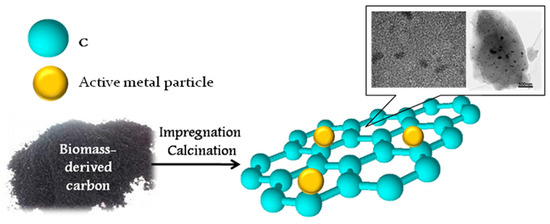
Figure 4.
Preparation of biochar-based metal-containing catalysts. Summarized from [57,58].

Table 4.
Summary of biomass-derived carbon-based catalysts for organic reactions.
Along with traditional carbon-based catalysts, biomass-derived ones exhibit high activity in different reactions. Moreover, the “green” nature of these systems makes them preferable for use in sustainable technologies. Carbon materials derived from biomass are considered to provide a high dispersion of metals deposited on their surface, as well as a narrow metal particle size distribution, leading to a higher reducibility of the active phase. Moreover, the presence of dopped atoms (i.e., N and O) on the carbon surface increases the catalyst activity in comparison with traditional carbon-based catalysts. The higher surface area of the biocarbon-derived catalysts compared to those supported on inorganic oxides (such as SiO2 or Al2O3) also provides a good availability of active sites and higher hydrogen adsorption, resulting in their higher activity [15].
4. Biomass-Derived Carbon-Based Catalysts for Electrocatalysis
Carbon-based materials are widely used as catalyst supports in electrocatalysis. Biomass-based carbon is a cheap and structured material that can be easily synthesized for large-scale applications. These supports can effectively replace existing electrocatalysts in the electrooxidation processes, hydrogen or oxygen revolution reactions (HRR and ORR), and oxygen reduction reactions [15]. Electrocatalysts based on biomass-derived carbons have shown high activity and stable onset potential.
The structure of carbonaceous supports plays a great role in electrocatalysis. A higher activity has been shown by catalysts with a graphite- or grapheme-like structure. Biomass-based carbon catalysts as electrocatalysts are usually synthesized through pyrolysis or carbonization of biomass feedstock. The control over the synthesis process temperature, time of carbonization, and pyrolysis atmosphere are the key factors that allow the required structure of carbon-based catalysts to be obtained [15]. Moreover, the introduction of heteroatoms in the crystal lattice of carbon is a key factor for these electrocatalysts since it forms the active sites [69]. Doping usually serves as a charge transfer and modification of the electronic structure of carbon atoms (Figure 5). Among the doping heteroatoms, nitrogen, phosphorus, sulfur, and oxygen should be mentioned.
An overview of carbon-based electrocatalysts, their synthesis methods, structure, and onset potential is presented in Table 5.

Table 5.
Summary of biomass-derived carbon-based electrocatalysts.
Table 5.
Summary of biomass-derived carbon-based electrocatalysts.
| Catalyst | Carbon Source | Synthesis Conditions | Crystal Structure | Porosity | Onset Potential, V | Reaction | Catalyst Stability | Reference |
|---|---|---|---|---|---|---|---|---|
| Pd/N-C | Mildew-starting orange waste | Juice extraction, mixing with K2PdCl4, hydrothermal carbonization at 180 °C for 4 h, and pyrolysis in nitrogen atmosphere at 800 °C for 2 h. | Graphite-like structure with pyridinic and graphite N | Microporous, SA * = 26 m2/g | 0.5 | Methanol oxidation | 90% in 50 cycles | [70] |
| N-C | N-rich yuba | Carbonization at 850 °C, followed by impregnation. | Graphite-like structure with pyridinic and pyrrolic N | Mesoporous, SA * = 740–1000 m2/g | 0.97 | Fuel cells | 85% after 5000 s | [71] |
| Fe3C/C | Cellulose fiber | Impregnation with an iron salt, addition of dicyandiamide, carbonization, and etching. | Fe3C crystals over a graphite-like structure | - | 0.98 | Oxygen reduction | 85% after 10,000 s | [72] |
| FeOx/N-C | Acorn shell | Impregnation with melanin, (MgOH)2CO3, and NaCl, carbonization at 900 °C, treatment with hemin, and calcination in nitrogen atmosphere. | Graphite-like structure with pyridinic and pyrrolic N, Fe3O4, and Fe2O3 coated with C | SA * = 819 m2/g | 0.88 in ORR 0.72 in HRR | ORR and HRR | 73% after 36,000 s | [73] |
| N-C | Wood char | Activation with alkali and doping with nitrogen. | Graphite-like structure with pyridinic and pyrrolic N | Microporous, SA * = 1924 m2/g | 0.2 | ORR | 95% after 1200 min | [74] |
| Co/C | Cotton carbon fiber | Impregnation with CoCl2, ultrasonification, and carbonization at 900 °C for 3 h. | Graphite-like structure with amorphous carbon | Mesoporous, SA * = 358 m2/g | - | Lie-SeS2 cells | 70% after 45 cycles | [75] |
| Ru/N-C | Yeasts | Impregnation with RuCl3, calcinations at 950 °C for 5 h, and doping with nitrogen. | Graphite-like structure | - | 0.2 | HRR | 85% after 60 h | [76] |
| Fe3O4/N-C | Yeasts | Impregnation with Fe(NO3)3, calcinations at 950 °C for 5 h, and doping with nitrogen. | Graphite-like structure | - | 0.3 | ORR | 85% after 60 h | [76] |
| Mo/C | Sugar beet, corn stover, pine, and miscanthus | Impregnation with Mo salt, pyrolysis at 600 °C for 6 h, and NaCl/NaF salt flux. | Graphite-like structure | - | 0.3–0.5 V | HRR | - | [77] |
| N-C | Cocoon silk | Carbonization followed by activation by KOH. | Graphite-like structure with pyridinic and pyrrolic N | - | 0.6 | ORR | - | [78] |
| N-C | Cocoon silk | Pyrolysis followed by activation by ZnCl2. | 1D structure | - | 0.85 | ORR | 95% after 12,000 s | [79] |
| N-C | Keratin | Precarbonization, activation with KOH, and treatment in ammonia at 1000 °C. | Grapheme-like 2D structure | - | 0.8 | ORR | 92% after 300 s | [80] |
| N-C | Coconut shell | Carbonization followed by activation by H3PO4. | Graphite-like structure | Mesoporous, SA * = 1260 m2/g | 90% after 12 h | [81] | ||
| N-C | Ophiopogon japonicus | Hydrothermal carbonization at 180 °C. | Carbon nanodot/nanosheet aggregates | - | 0.88 | ORR | - | [82] |
| N-C | Basswood | Pyrolysis at 600 °C and activation in ammonia atmosphere. | Graphite-like structure | SA * = 1438 m2/g | 0.98 | ORR | 95% after 20 h | [83] |
| N-C | Pinecone | Precarbonization at 800 °C and activation in ammonia. | Graphite-like structure | - | 0.95 | ORR | - | [84] |
| N-C | Spent coffee grounds | Pyrolysis followed by activation by KOH. | Graphite-like structure | SA * = 1018 m2/g | 0.94 | ORR | 95% after 10,000 s | [85] |
| N-C | Banana peels | Carbonization followed by activation by KOH, and treatment with ammonia | Graphite-like structure | SA * = 1756 m2/g | 0.98 | ORR | 95% after 10,000 s | [86] |
* SA—surface area.
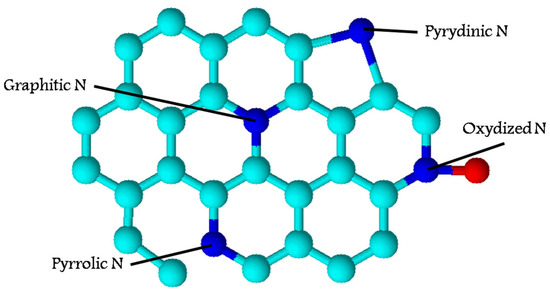
Figure 5.
Overall structure of N-doped carbon-based electrocatalyst. Summarized from [69,70,73,77,78,79,80,81,82,83,84,85].
Figure 5.
Overall structure of N-doped carbon-based electrocatalyst. Summarized from [69,70,73,77,78,79,80,81,82,83,84,85].
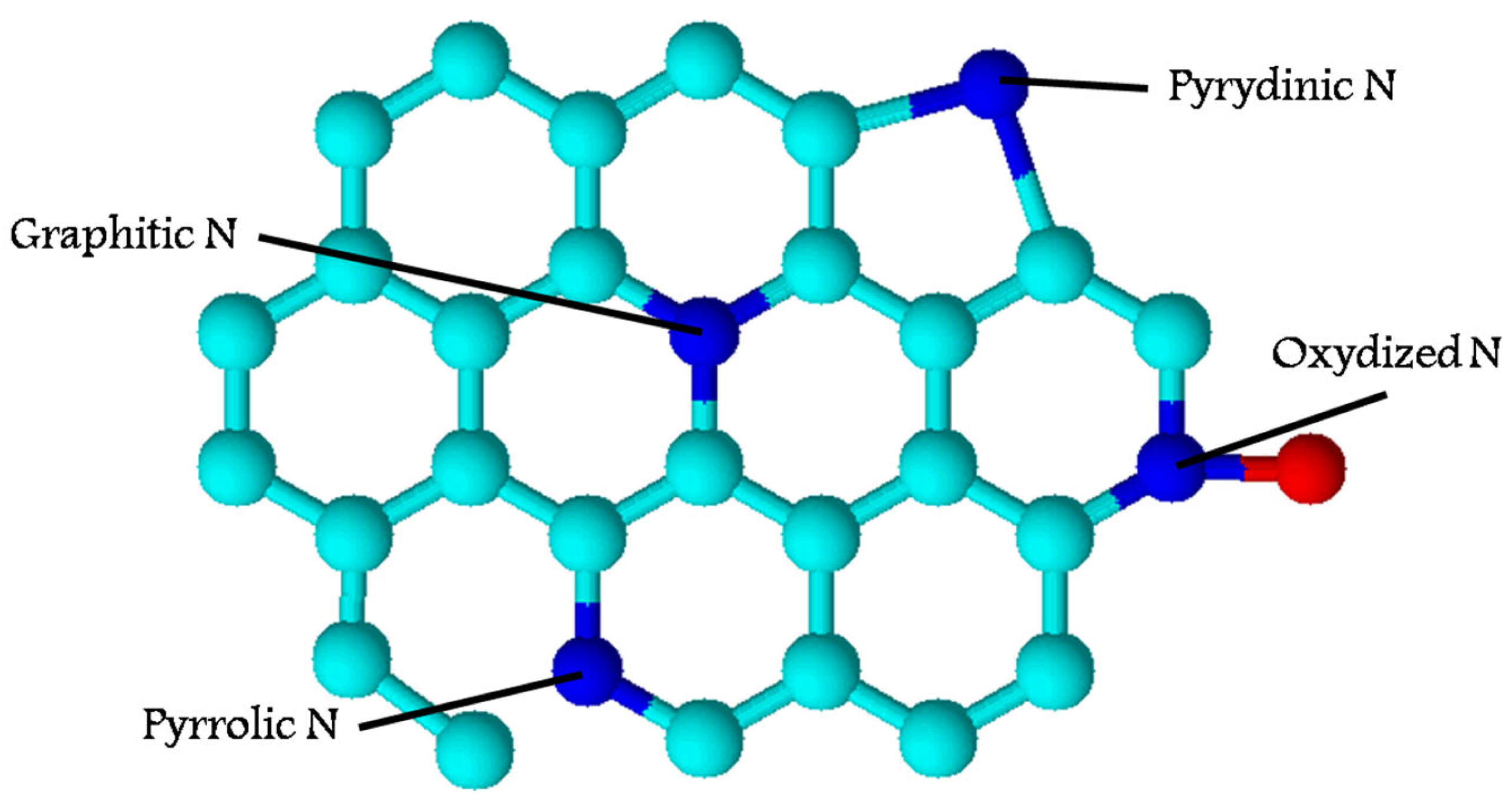
For electrocatalysts, the distribution of metal-containing particles and heteroatoms, the crystal structure of carbonaceous support, and its porosity play a great role. It has been shown that the presence of heteroatoms exhibits a higher catalytic activity than that of non-doped carbons, as well as a higher potential stability. The hierarchical porous structure can also improve the activity of these electrocatalysts in most reactions because of the facilitation of the diffusion of reactants and products [69,87]. A high surface area of the electrodes obtained from biocarbons is crucial for the energy storage and ion/electron transport. Thus, biomass-derived carbonaceous materials can be effectively applied as supercapacitors. Biomass-derived carbon electrodes can be considered a promising alternative to traditional electrodes [87].
5. Conclusions and Future Prospective
The key requirements that catalysts should meet are high activity and selectivity toward the target products, stability in the reaction medium, mechanical effects, and temperature. In terms of structure, heterogeneous catalysts should have a uniform distribution of the active phase, thus avoiding its aggregation; high porosity to allow reagent molecules to easily reach the active sites; and a proper structure of the active sites to catalyze the target reactions. Therefore, the catalyst design is of great interest.
Carbon-based catalysts have attracted much attention in recent years. Their low cost, simple preparation methods, and “green” nature make carbon materials a promising support for catalysts. Biomass can be successfully used for the preparation of carbon-based catalysts through different methods, mainly based on thermochemical decomposition. Different types of biomasses, e.g., starch, lignin, cellulose, chitosan, wood, agricultural or food waste, and marine waste, can be promising carbon feedstock. Expanding biomass sources, development of new methods for direct catalyst synthesis, expanding the choice of synthesis conditions, and development of “green” preparation methods are the main directions for future research for biomass-derived catalyst synthesis. Moreover, wider applications of biomass-derived catalysts, including electrocatalysis, fine and organic synthesis, and flow processes, should be developed to provide sustainable and environmentally friendly processes.
Author Contributions
Conceptualization, A.A.S. and M.G.S.; data collection, Y.V.L. and Y.Y.K.; writing—original draft preparation, A.A.S. and V.G.M.; writing—review and editing, M.E.M.; project administration, A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support for this work was provided by the Russian Science Foundation (grant 22-79-10096).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elliott, D.C.; Hart, T.R. Catalytic hydroprocessing of chemical models for bio-oil. Energy Fuels 2008, 23, 631–637. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and. DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Maroa, S.; Inambao, F. A review of sustainable biodiesel production using biomass-derived heterogeneous catalysts. Eng. Life Sci. 2021, 21, 790–824. [Google Scholar] [CrossRef] [PubMed]
- Alrobaian, A.; Rajasekar, V.; Alagumalai, A. Critical insight into biowaste-derived biocatalyst for biodiesel production. Environ. Prog. Sustain. Energy 2020, 39, e13391. [Google Scholar] [CrossRef]
- Smith, S.M.; Oopathum, C.; Weeramongkhonlert, V.; Smith, C.B. Transesterification of soybean oil using bovine bone waste as new catalyst. Bioresour. Technol. 2013, 143, 686–690. [Google Scholar] [CrossRef]
- Roschat, W.; Kacha, M.; Yoosuk, B.; Sudyoadsuk, T. Biodiesel production based on heterogeneous process catalyzed by solid waste coral fragment. Fuel 2012, 98, 194–202. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Nunoura, T.; Wade, S.R.; Bourke, J.P.; Antal, M.J. Studies of the flash carbonization process. 1. Propagation of the flaming pyrolysis reaction and performance of a catalytic afterburner. Ind. Eng. Chem. Res. 2006, 45, 585–599. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; van der Waal, J.C.; Lique, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 2015, 157, 37–47. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations; Report No. ECNC05013 Energy Research Centre of The Netherlands (ECN); Energy Research Centre of The Netherlands: Petten, The Netherlands, 2005; p. 71. [Google Scholar]
- Shokrolahi, A.; Zali, A.; Keshavarz, M.H. Wet carbon-based solid acid/NaNO3 as a mild and efficient reagent for nitration of aromatic compound under solvent free conditions. Chin. Chem. Lett. 2007, 18, 1064–1066. [Google Scholar] [CrossRef]
- Zong, M.H.; Duan, Z.Q.; Lou, W.Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 5, 434–437. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-derived Solid Acid Catalysts for Biodiesel Production from Low-Cost Feedstocks: A Review. Catal. Rev. Sci. Eng. 2014, 56, 187–219. [Google Scholar] [CrossRef]
- Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J.N.; Domen, K.; Hayashi, S. A carbon material as a strong protonic acid. Angew. Chem. Int. Ed. 2004, 43, 2955–2958. [Google Scholar] [CrossRef]
- Shu, Q.; Zhang, Q.; Xu, G.; Nawaz, Z.; Wang, D.; Wang, J. Synthesis of biodiesel from cottonseed oil and methanol using a carbon-based solid acid catalyst. Fuel Process. Technol. 2009, 90, 1002–1008. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Xina, J.; Zhanga, S. Application of solid acid catalyst derived from low value biomass for a cheaper biodiesel production. J. Chem. Technol. Biotechnol. 2014, 89, 1898–1909. [Google Scholar] [CrossRef]
- Ayodele, O.O.; Dawodu, F.A. Conversion of Calophyllum inophyllum Oil with a High Free Fatty Acid Content to Biodiesel using a Starch-Derived Catalyst. Energy Technol. 2014, 2, 912–920. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Guo, F.; Xiu, Z.L.; Liang, Z.X. Synthesis of biodiesel from acidified soybean soapstock using a lignin-derived carbonaceous catalyst. Appl. Energy 2012, 98, 47–52. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Biodiesel-Like Biofuels from Simultaneous Transesterification/Esterification of Waste Oils with a Biomass-Derived Solid Acid Catalyst. ChemCatChem. 2011, 3, 594–597. [Google Scholar] [CrossRef]
- Choksi, H.; Pandian, S.; Gandhi, Y.H.; Deepalakshmi, S. Studies on production of biodiesel from Madhuca indica oil using a catalyst derived from cotton stalk. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 43, 3424–3433. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Lim, S.; Pang, Y.-L.; Chen, W.-H.; Lam, M.-K.; Tan, I.-S. Synthesis of glycerol-free fatty acid methyl ester using interesterification reaction based on solid acid carbon catalyst derived from low-cost biomass wastes. Int. J. Energy Res. 2020, 46, 147–162. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Akinfalabi, S.-I.; Rashid, U.; Ngamcharussrivichai, C.; Nehdi, I.A. Synthesis of reusable biobased nano-catalyst from waste sugarcane bagasse for biodiesel production. Environ. Technol. Innov. 2020, 18, 100788–100801. [Google Scholar] [CrossRef]
- Meher, L.; Churamani, C.; Arif, M.; Ahmed, Z.; Naik, S. Jatropha curcas as a renewable source for bio-fuels—A review. Renew. Sustain. Energy Rev. 2013, 26, 397–407. [Google Scholar] [CrossRef]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. A novel biobased heterogeneous catalyst derived from Musa acuminata peduncle for biodiesel production—Process optimization using central composite design. Energy Convers. Manag. 2019, 189, 118–131. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using Napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Vadery, V.; Narayanan, B.N.; Ramakrishnan, R.M.; Cherikkallinmel, S.K.; Sugunan, S.; Narayanan, D.P.; Sasidharan, S. Room temperature production of jatropha biodiesel over coconut husk ash. Energy 2014, 70, 588–594. [Google Scholar] [CrossRef]
- Niju, S.; Kanna, S.K.A.; Ramalingam, V.; Kumar, M.S.; Balajii, M. Sugarcane bagasse derived biochar—A potential heterogeneous catalyst for transesterification process. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Inayat, A.; Jamil, F.; Raza, F.; Khurram, S.; Ghenai, C.; Al-Muhatseb, A.H. Upgradation of waste cooking oil to biodiesel in the presence of green catalyst derived from date seeds. Biofuels 2021, 12, 1245–1250. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Hazmi, B.; Ibrahim, M.L.; Tsubota, T.; Alharthi, F.A. Potential heterogeneous nano-catalyst via integrating hydrothermal carbonization for biodiesel production using waste cooking oil. Chemosphere 2022, 286, 131913. [Google Scholar] [CrossRef]
- Atelge, M. Production of biodiesel and hydrogen by using a double-function heterogeneous catalyst derived from spent coffee grounds and its thermodynamic analysis. Renew. Energy 2022, 198, 1–15. [Google Scholar] [CrossRef]
- Fernández, J.V.; Faria, D.N.; Santoro, M.C.; Mantovaneli, R.; Cipriano, D.F.; Brito, G.M.; Carneiro, M.T.W.; Schettino, M.A.; Gonzalez, J.L.; Freitas, J.C. Use of Unmodified Coffee Husk Biochar and Ashes as Heterogeneous Catalysts in Biodiesel Synthesis. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- Yani, F.; Ulhaqi, R.; Pratiwi, W.; Pontas, K.; Husin, H. Utilization of water hyacinth-based biomass as a potential heterogeneous catalyst for biodiesel production. J. Phys. Conf. Ser. 2019, 1402, 055005. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Singh, K.; Sinuraya, J.S.; Supeno, M.; Sembiring, H.; Tarigan, K.; Rambe, S.M.; Karo-Karo, J.A.; Sitepu, E.K. Waste Passion Fruit Peel as a Heterogeneous Catalyst for Room-Temperature Biodiesel Production. ACS Omega 2022, 7, 7885–7892. [Google Scholar] [CrossRef]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of Cellulose by Amorphous Carbon Bearing SO3H, COOH, and OH Groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Li, L.; Smith, R.L., Jr. Hydrolysis of cellulose over functionalized glucose-derived carbon catalyst in ionic liquid. Bioresour. Technol. 2012, 116, 355–359. [Google Scholar] [CrossRef]
- Liu, M.; Jia, S.; Gong, Y.; Song, C.; Guo, X. Effective hydrolysis of cellulose into glucose over sulfonated sugar-derived carbon in an ionic liquid. Ind. Eng. Chem. Res. 2013, 52, 8167–8173. [Google Scholar] [CrossRef]
- Guo, H.; Lian, Y.; Yan, L.; Qi, X.; Smith, R.L., Jr. Cellulose-derived superparamagnetic carbonaceous solid acid catalyst. Green Chem. 2013, 15, 2167–2174. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, W.J.; Ren, J.W.; Liu, X.H.; Lu, G.Z.; Wang, Y.Q. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Qi, X.H.; Guo, H.X.; Li, L.Y.; Smith, R.L., Jr. Acid-Catalyzed Dehydration of Fructose into 5-Hydroxymethylfurfural by Cellulose-Derived Amorphous Carbon. ChemSusChem 2012, 5, 2215–2220. [Google Scholar] [CrossRef]
- Ji, L.; Tang, Z.; Yang, D.; Ma, C.; He, Y.-C. Improved one-pot synthesis of furfural from corn stalk with heterogeneous catalysis using corn stalk as biobased carrier in deep eutectic solvent–water system. Bioresour. Technol. 2021, 340, 125691–125699. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, Z.; Li, K.; Guo, H.; Dai, Y.; Yan, L. Fabrication of biobased heterogeneous solid Brønsted acid catalysts and their application on the synthesis of liquid biofuel 5-ethoxymethylfurfural from fructose. Green Energy Environ. 2018, 3, 384–391. [Google Scholar] [CrossRef]
- Liang, J.; Zha, J.; Zhao, N.; Tang, Z.; He, Y.; Ma, C. Valorization of Waste Lignocellulose to Furfural by Sulfonated Biobased Heterogeneous Catalyst Using Ultrasonic-Treated Chestnut Shell Waste as Carrier. Processes 2021, 9, 2269. [Google Scholar] [CrossRef]
- Kang, S.; Ye, J.; Chang, J. Recent Advances in Carbon-Based Sulfonated Catalyst: Preparation and Application. Int. Rev. Chem. Eng. 2013, 5, 133–144. [Google Scholar]
- Sahoo, B.; Formenti, D.; Topf, C.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Sustainable Biomass-Derived Catalysts for Selective Hydrogenation of Nitroarenes. ChemSusChem 2017, 10, 3035–3039. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y. Metal Nanoparticles Supported on Biomass-Derived Hierarchical Porous Heteroatom-Doped Carbon from Bamboo Shoots Design, Synthesis and Applications. Chem. Rec. 2018, 19, 1283–1301. [Google Scholar] [CrossRef]
- Zhang, F.; Li, G.-D.; Chen, J.-S. Preparation and gas storage of high surface area microporous carbon derived from biomass source cornstalks. J. Colloid Interface Sci. 2008, 327, 108–114. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Water-tolerant Ru-Starbon (R) materials for the hydrogenation of organic acids in aqueous ethanol. Catal. Commun. 2010, 11, 928–931. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, J.; Fellinger, T.-P.; Antonietti, M.; Li, H.; Wang, Y. Improving hydrothermal carbonization by using poly(ionic liquid)s. Angew. Chem. Int. Ed. 2013, 52, 6028–6032. [Google Scholar] [CrossRef]
- Ojeda, M.; Balu, A.M.; Romero, A.A.; Esquinazi, P.; Ruokolainen, J.; Sixta, H.; Luque, R. MAGBONS: Novel Magnetically Separable Carbonaceous Nanohybrids from Porous Polysaccharides. ChemCatChem 2014, 6, 2847–2853. [Google Scholar] [CrossRef]
- Varila, T.; Makela, E.; Kupila, R.; Romar, H.; Hu, T.; Karinen, R.; Puurunen, R.L.; Lassi, U. Conversion of furfural to 2-methylfuran over CuNi catalysts supported on biobased carbon foams. Catal. Today 2021, 367, 16–27. [Google Scholar] [CrossRef]
- Liang, Y.; Han, J.; Wang, H.; Zhao, B.; Qin, L.; Wang, Y. Synthesis of Ni-Mg@HC catalyst derived from sugarcane bagasse and its application for producing syngas via CO2 dry reforming. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Sahoo, B.; Surkus, A.-E.; Pohl, M.-M.; Radnik, J.; Schneider, M.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A Biomass-Derived Non-Noble Cobalt Catalyst for Selective Hydrodehalogenation of Alkyl and (Hetero)Aryl Halides. Angew. Chem. 2017, 129, 11394–11399. [Google Scholar] [CrossRef]
- Gronnow, M.J.; Luque, R.; Macquarrie, D.J.; Clark, H. A novel highly active biomaterial supported palladium catalyst. Green Chem. 2005, 7, 552–557. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; White, R.J. Versatile mesoporous carbonaceous materials for acid catalysis. Green Chem. 2008, 10, 382–387. [Google Scholar] [CrossRef]
- Sk, M.P.; Jana, C.K.; Chattopadhyay, A. A gold–carbon nanoparticle composite as an efficient catalyst for homocoupling reaction. Chem. Commun. 2013, 49, 8235–8237. [Google Scholar]
- Zhang, Z.; Yang, S.; Li, H.; Zan, Y.; Li, X.; Zhu, Y.; Dou, M.; Wang, F. Sustainable Carbonaceous Materials Derived from Biomass as Metal-Free Electrocatalysts. Adw. Mater. 2019, 31, 1805718. [Google Scholar] [CrossRef]
- Da, Z.; Niu, X.; Li, X.; Zhang, W.; He, Y.; Pan, J.; Qui, F.; Yan, Y. From mildewy orange waste to natural reductant and catalyst support: Active palladium/biomass-derived carbonaceous hybrids for promoted methanol electro-oxidation. ChemElectroChem 2017, 4, 1372–1377. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sun, Y.; Guo, L.K.; Sun, X.N.; Huang, N.B. Ball-Milling Effect on Biomass-Derived Nanocarbon Catalysts for the Oxygen Reduction Reaction. ChemistrySelect 2021, 6, 6019–6028. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H. A Versatile Iron–Tannin-Framework Ink Coating Strategy to Fabricate Biomass-Derived Iron Carbide/Fe-N-Carbon Catalysts for Efficient Oxygen Reduction. Agnew. Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, J.; Wang, W.L.; Li, W.; Sun, M.; Wang, Y.; Wang, X.; Ye, J.; Rao, H. Electrocatalytic, Kinetic, and Mechanism Insights into the Oxygen-Reduction Catalyzed Based on the Biomass-Derived FeOx@N-Doped Porous Carbon Composites. Small 2021, 17, 2007326. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Käämbre, T.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; TamasauskaiteTamasiunaite, L.; Norkus, E.; Kruusenberg, I. Biomass-derived Graphene-like Catalyst Material for Oxygen Reduction Reaction. ChemNanoMat 2021, 7, 307–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jing, P.; Guo, Y.; Zhang, Y.; Wei, Y.; Wang, Q.; Zhang, Y.; Wu, H. Bioderived carbon fiber conductive networks with inlaid electrocatalysts as an ultralight freestanding interlayer for working Lie-SeS2 pouch cells. Carbon 2022, 189, 10–20. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Dang, N.K.; Sultan, S.; Thangavel, P.; Jeong, H.Y.; Kim, K.S. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nature Sust. 2020, 3, 556–563. [Google Scholar] [CrossRef]
- Harris, D.; Budhi, S.; She, Y.; Henry, J.; Leonard, B.M. Biomass derived metal carbide catalysts formed using a salt flux synthesis. Mater. Res. Express 2019, 6, 115519. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Wang, H. Astridia velutina-like S, N-codoped hierarchical porous carbon from silk cocoon for superior oxygen reduction reaction. RSC Adv. 2016, 6, 73560–73565. [Google Scholar] [CrossRef]
- Fu, P.; Zhou, L.; Sun, L.; Huang, B.; Yuan, Y. Nitrogen-doped porous activated carbon derived from cocoon silk as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2017, 7, 13383–13389. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, X.; Zhang, J.; Peng, T.; Yang, J.; Huang, Y.; Mu, S. Keratin-derived S/N co-doped graphene-like nanobubble and nanosheet hybrids for highly efficient oxygen reduction. J. Mater. Chem. A 2016, 4, 15870–15879. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction. Appl. Catal. B 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.; Jang, D.; Shin, Y.; Lim, D.; Park, S. Metal-free N-doped carbon blacks as excellent electrocatalysts for oxygen reduction reactions. Carbon 2019, 145, 481–487. [Google Scholar] [CrossRef]
- Tang, Z.; Pei, Z.; Wang, Z.; Li, H.; Zeng, J.; Ruan, Z.; Huang, Y.; Zhu, M.; Xue, Q.; Yu, J.; et al. Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 2018, 130, 532–538. [Google Scholar] [CrossRef]
- Lei, X.; Wang, M.; Lai, Y.; Hu, L.; Wang, H.; Fang, Z.; Li, J.; Fang, J. Nitrogen-doped micropore-dominant carbon derived from waste pine cone as a promising metal-free electrocatalyst for aqueous zinc/air batteries. J. Power Sources 2017, 365, 76–82. [Google Scholar] [CrossRef]
- Srinu, A.; Peera, S.G.; Parthiban, V.; Bhuvaneshwari, B.; Sahu, A.K. Heteroatom Engineering and Co-Doping of N and P to Porous Carbon Derived from Spent Coffee Grounds as an Efficient Electrocatalyst for Oxygen Reduction Reactions in Alkaline Medium. ChemistrySelect 2018, 3, 690–702. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhao, Y.; Amiinu, I.S.; Zhou, H.; Liu, X.; Tang, Y.; Mu, S. Three dimensional few-layer porous carbon nanosheets towards oxygen reduction. Appl. Catal. B 2017, 211, 148–156. [Google Scholar] [CrossRef]
- Manasa, P.; Sambasivam, S.; Ean, F. Recent progress on biomass waste derived activated carbon electrode materials for supercapacitors applications—A review. J. Energy Storage 2022, 54, 105290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).