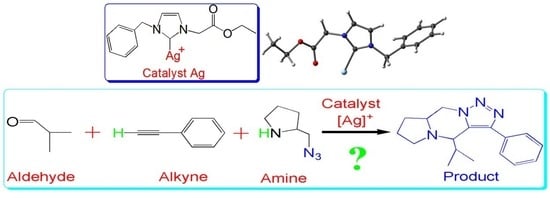
Insights into the Three-Component Coupling Reactions of Aldehydes, Alkynes, and Amines Catalyzed by N-heterocyclic Carbene Silver: A DFT Study
Abstract
1. Introduction
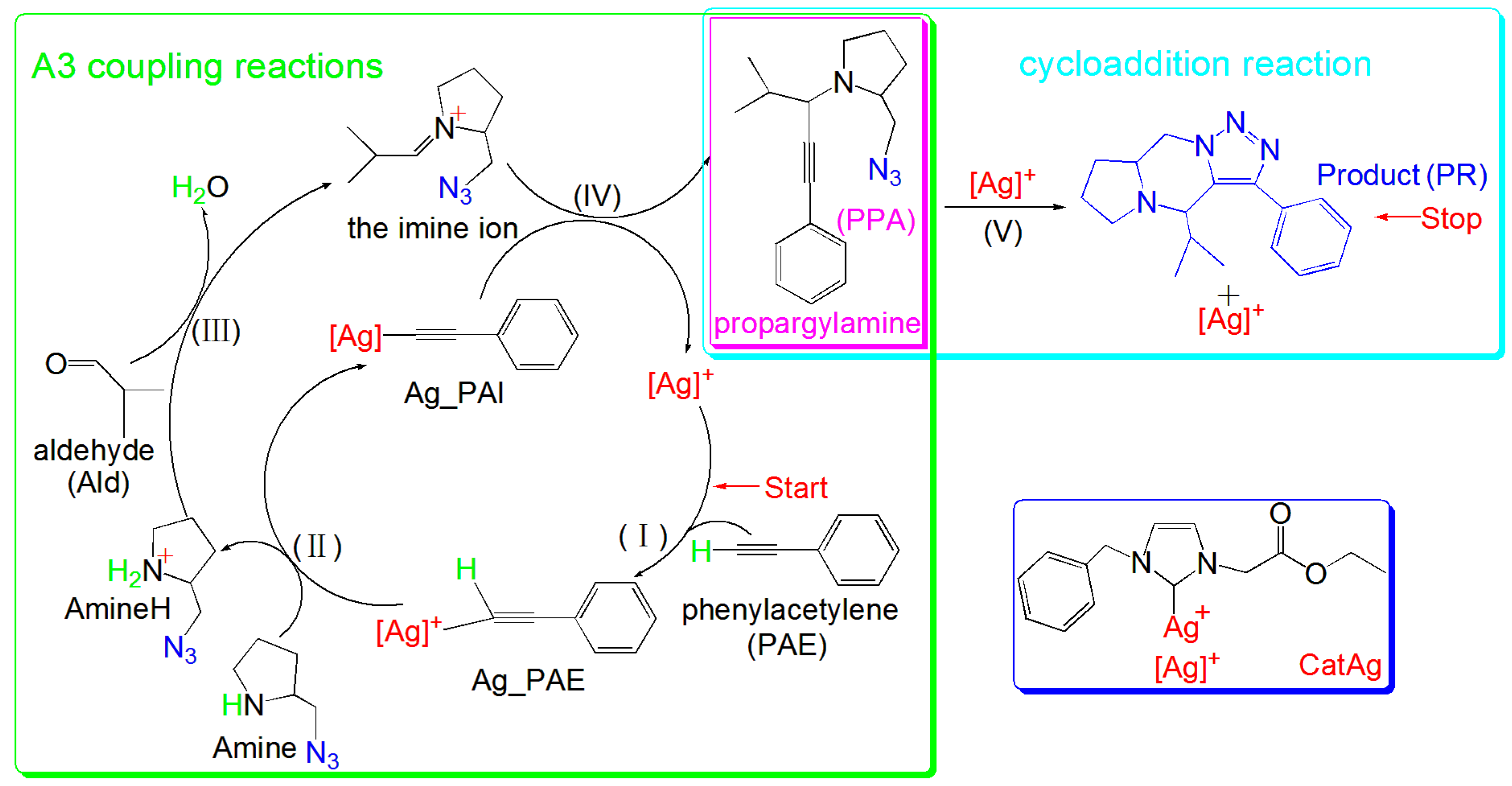
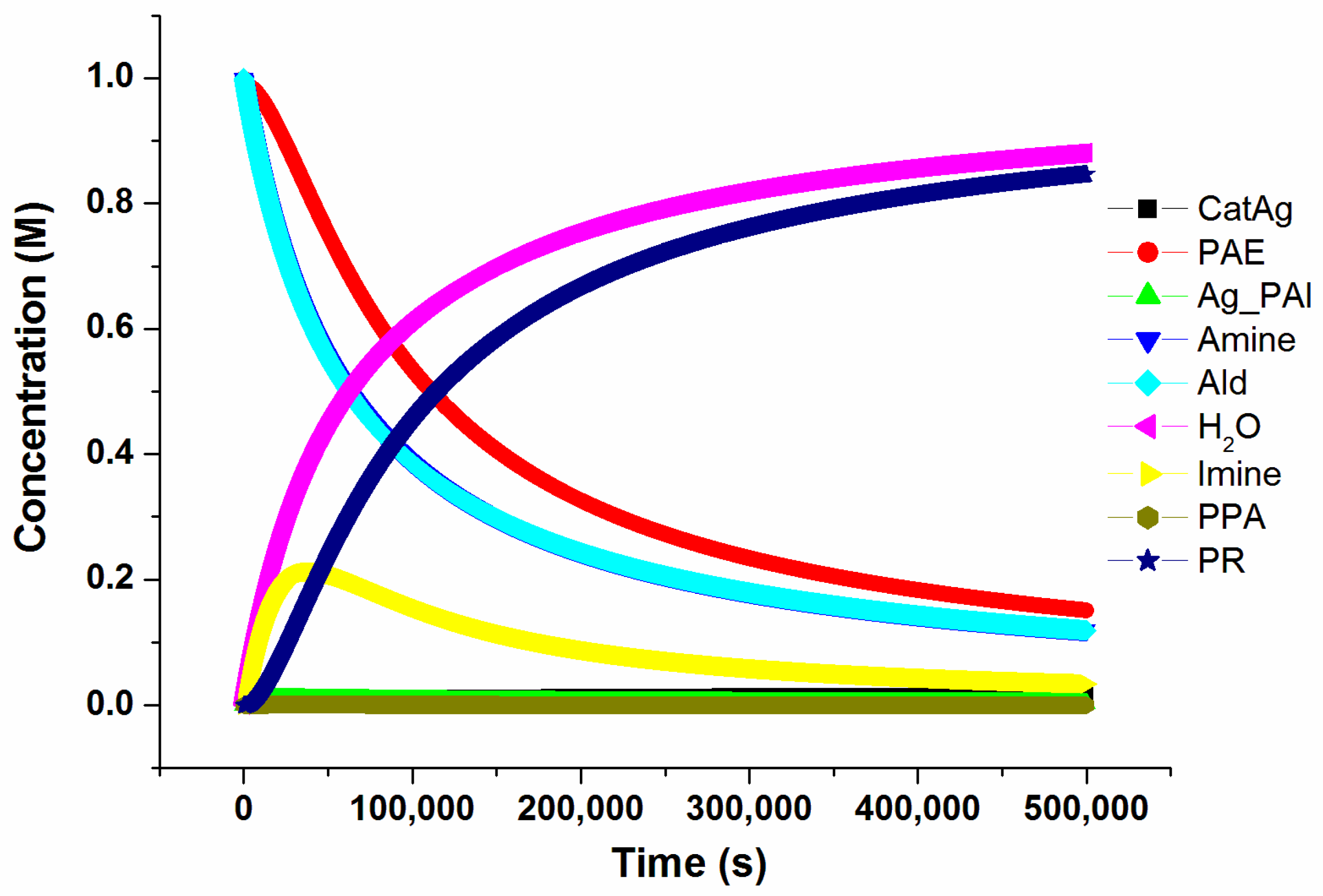
2. Results and Discussion
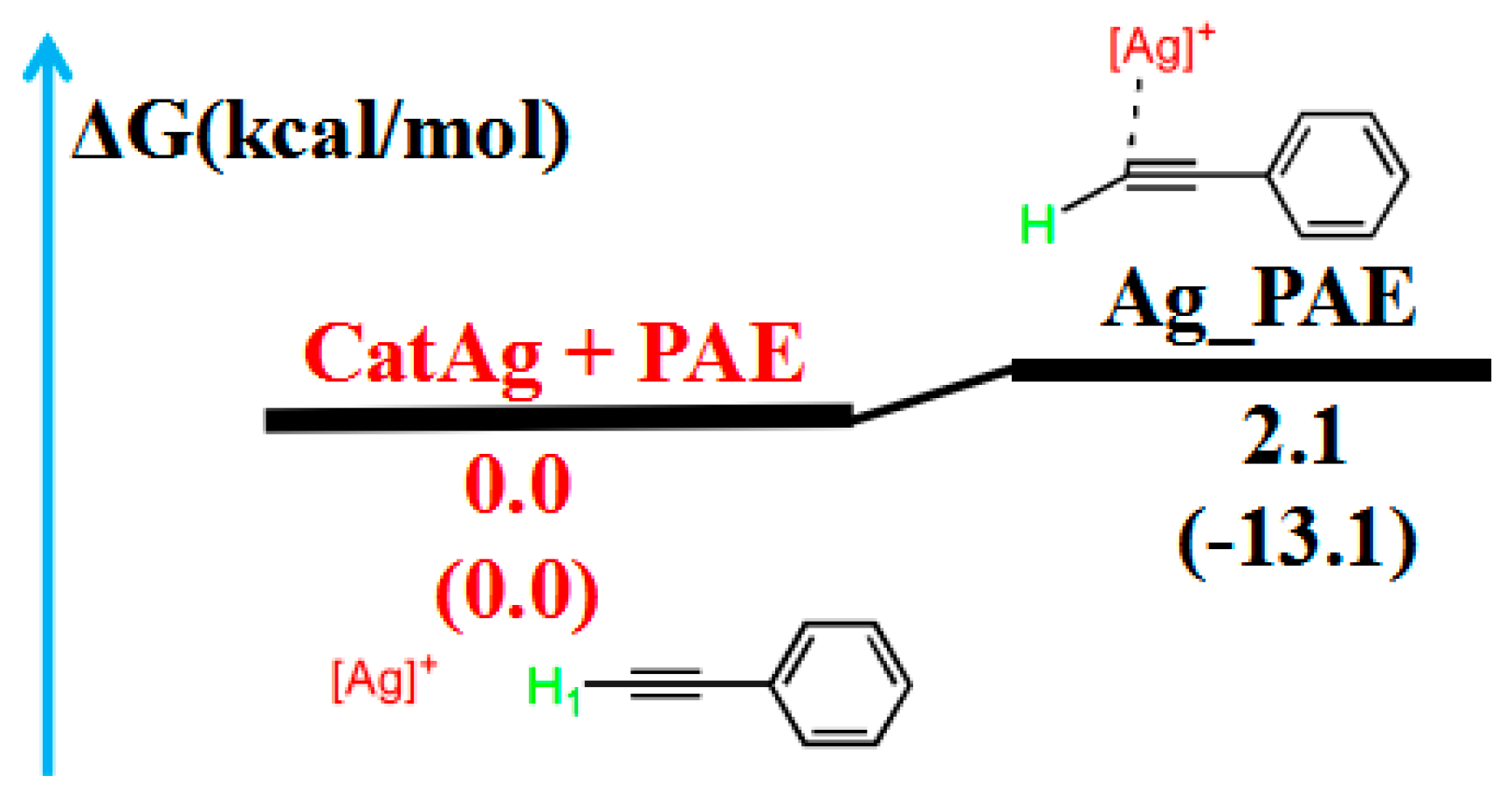
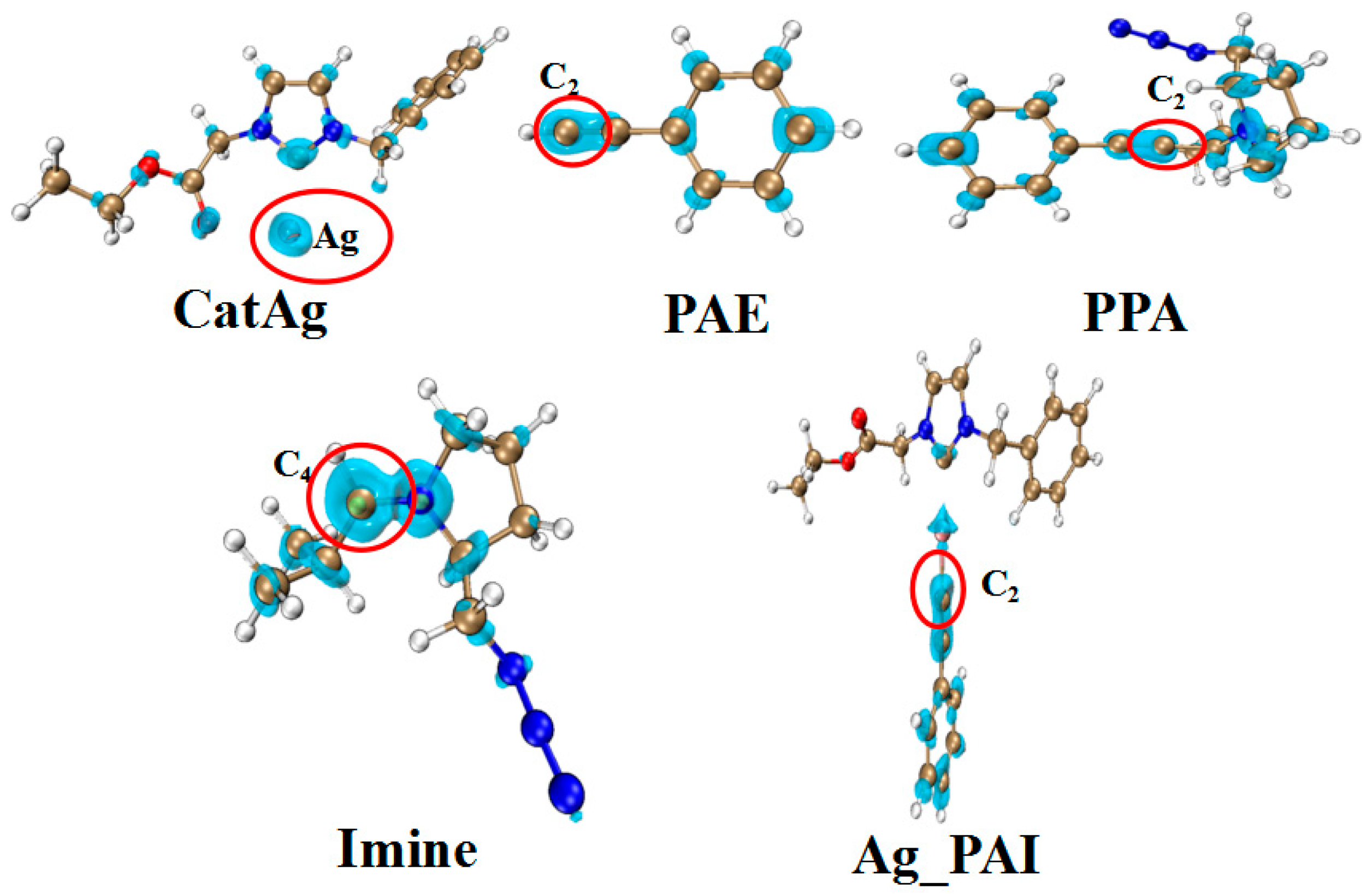
2.1. Addition Reaction between CatAg and PAE
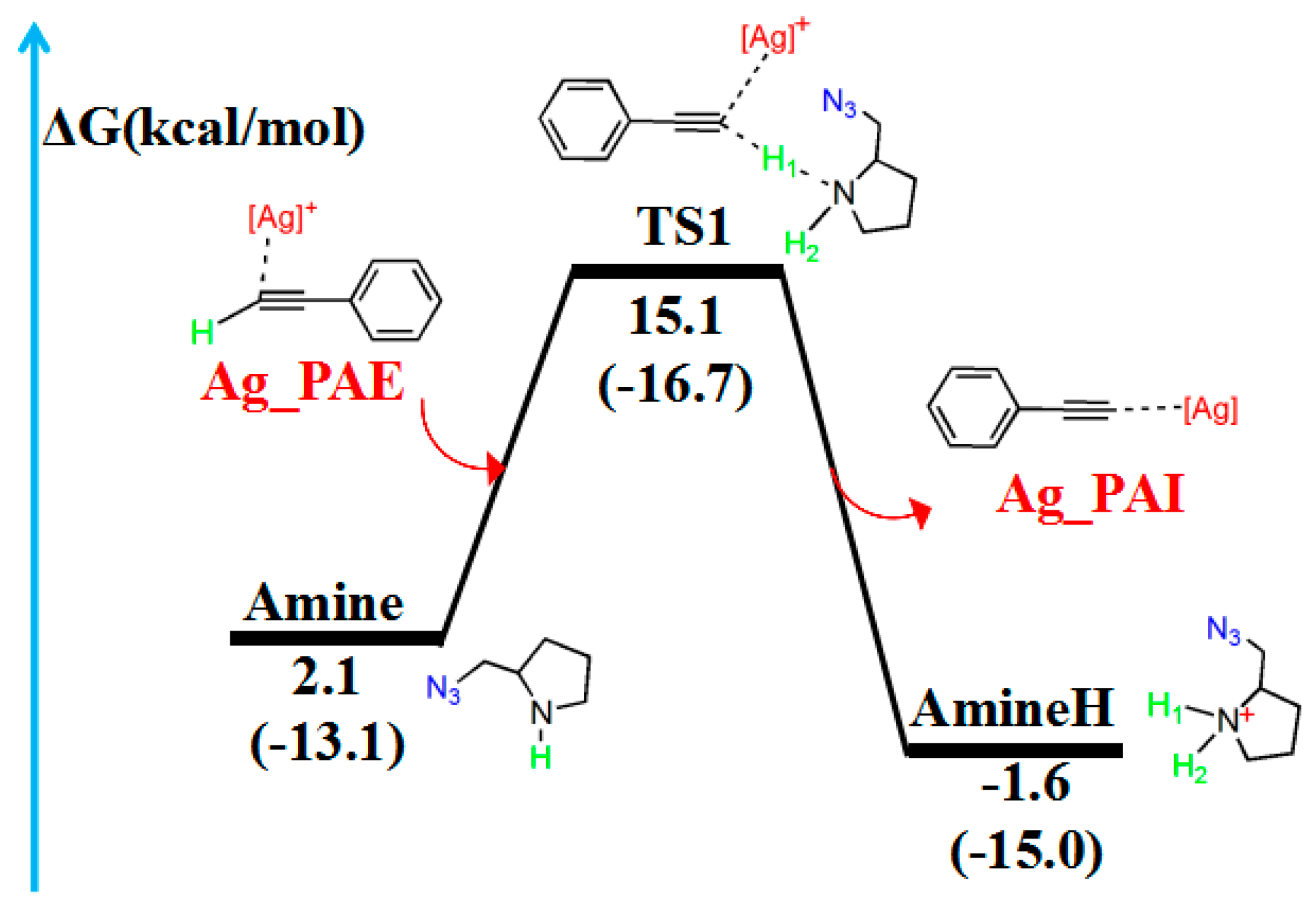
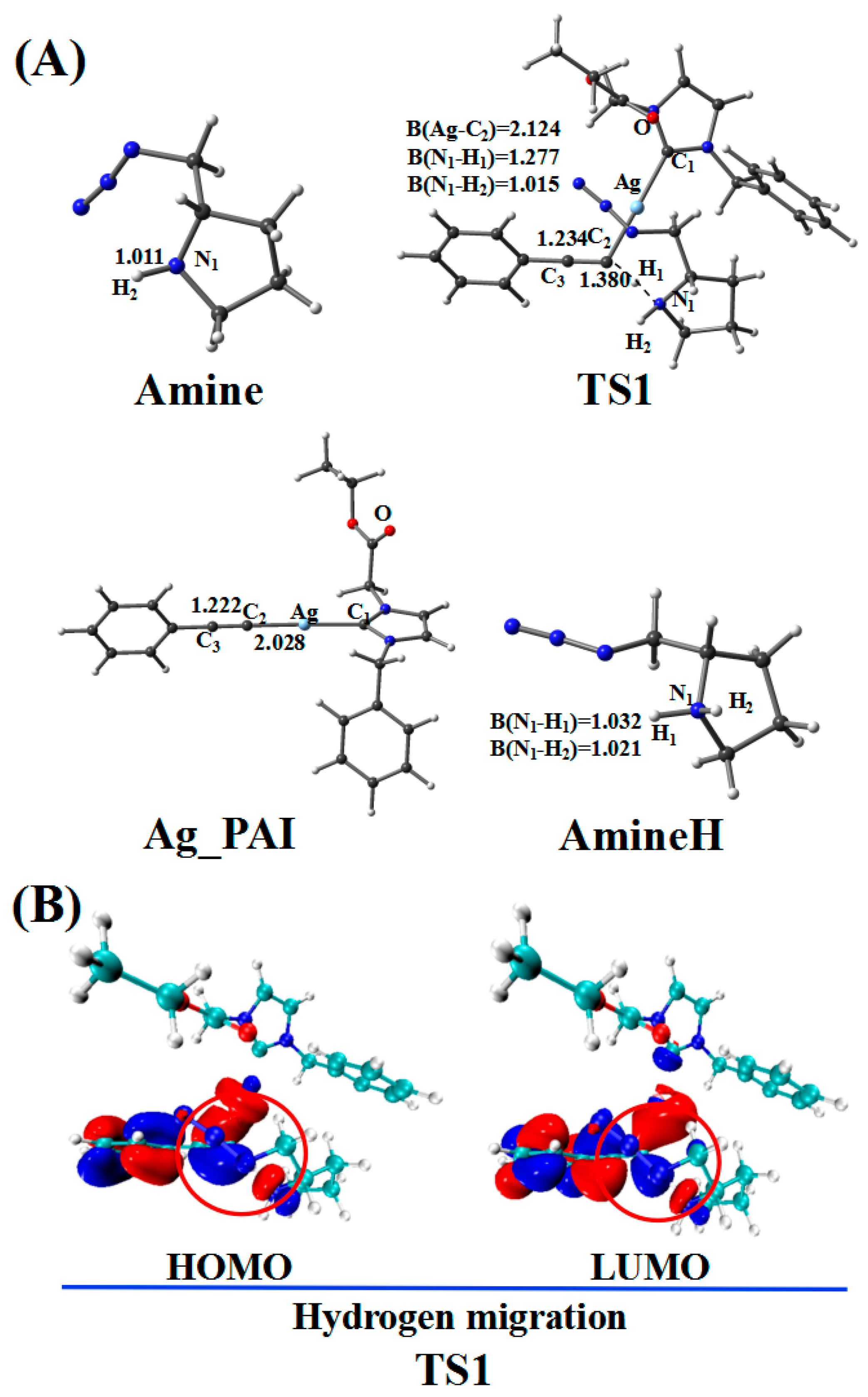
2.2. Hydrogen Migration Reaction Involving Amine
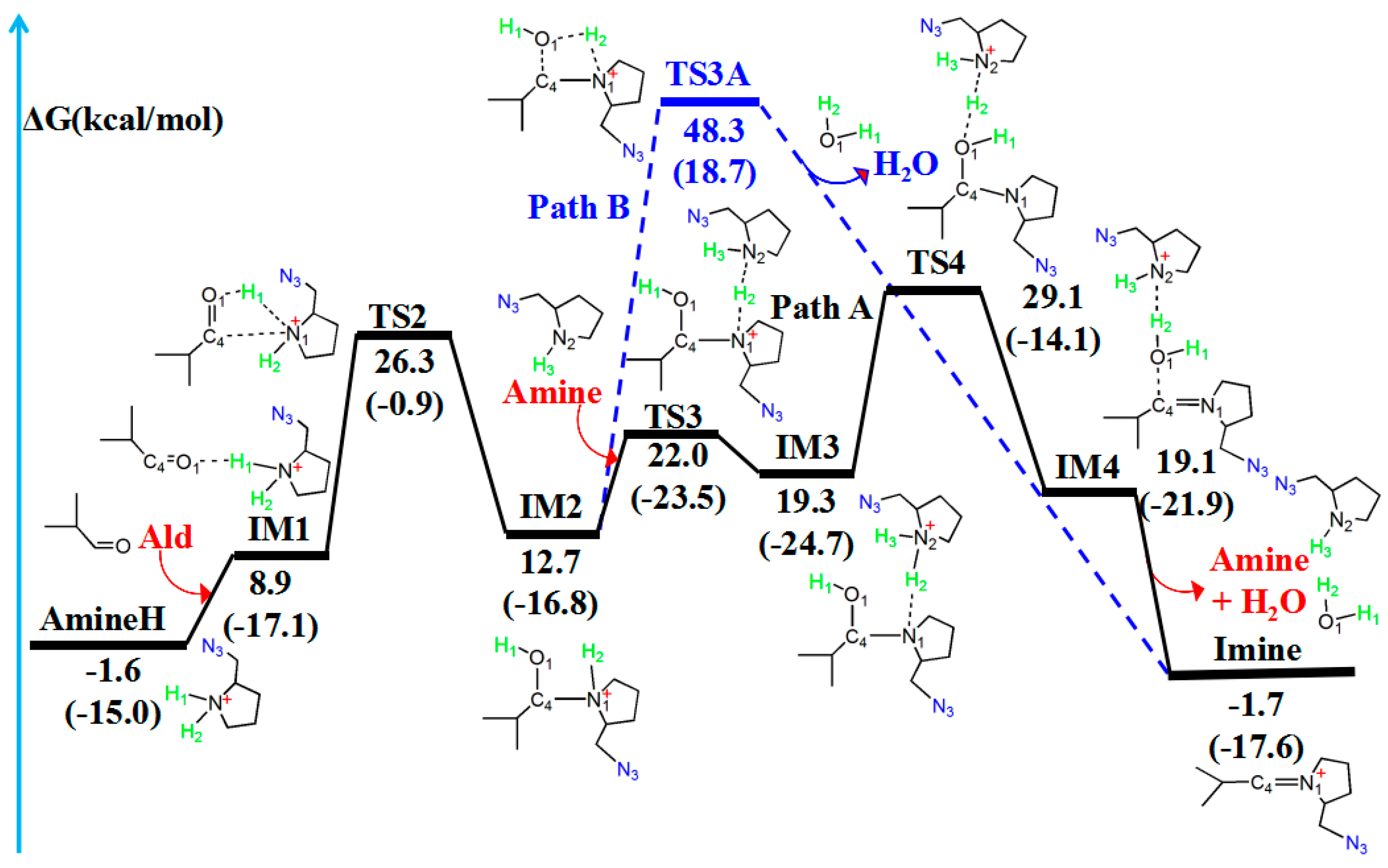
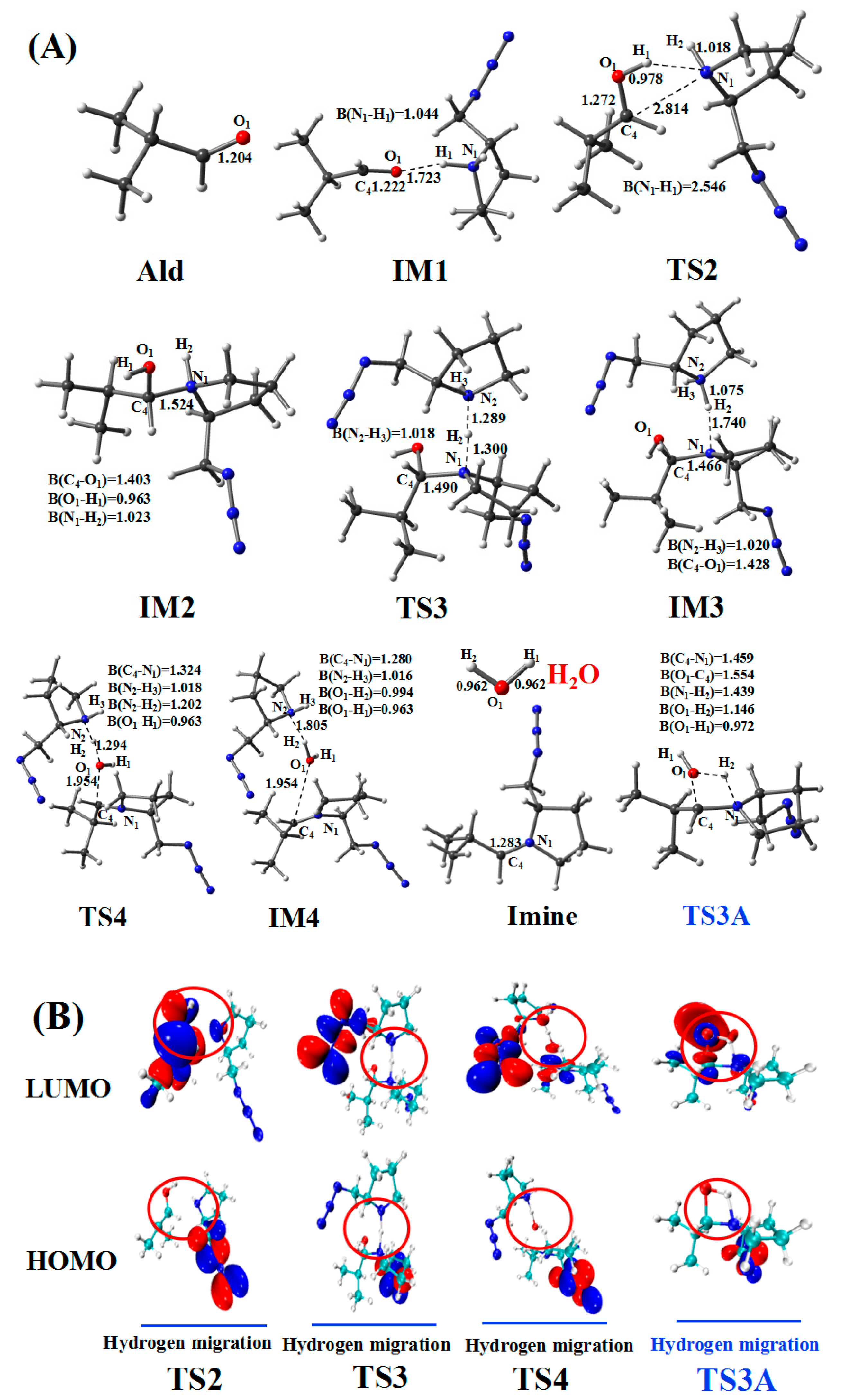
2.3. Amine–Aldehyde Condensation Reaction
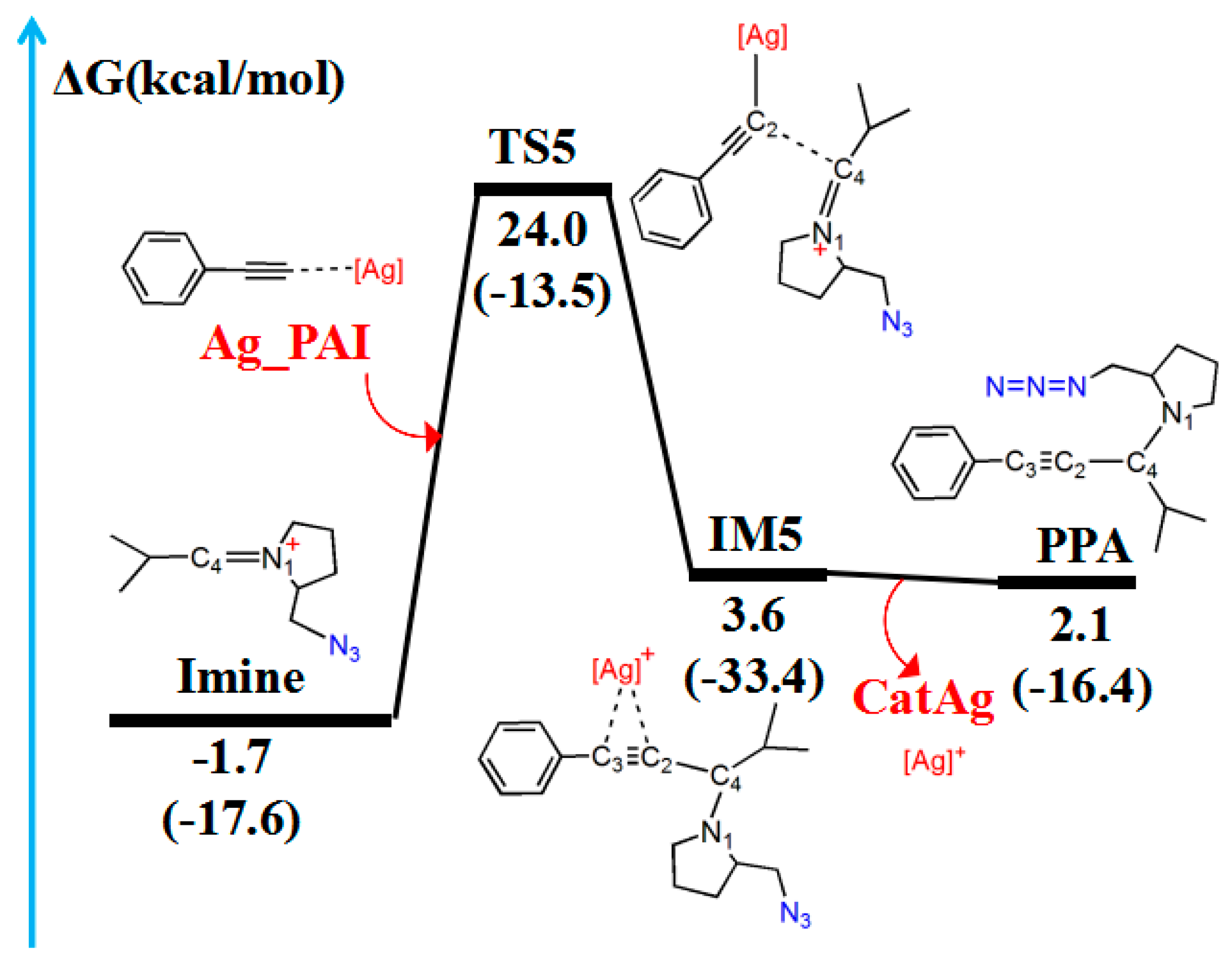
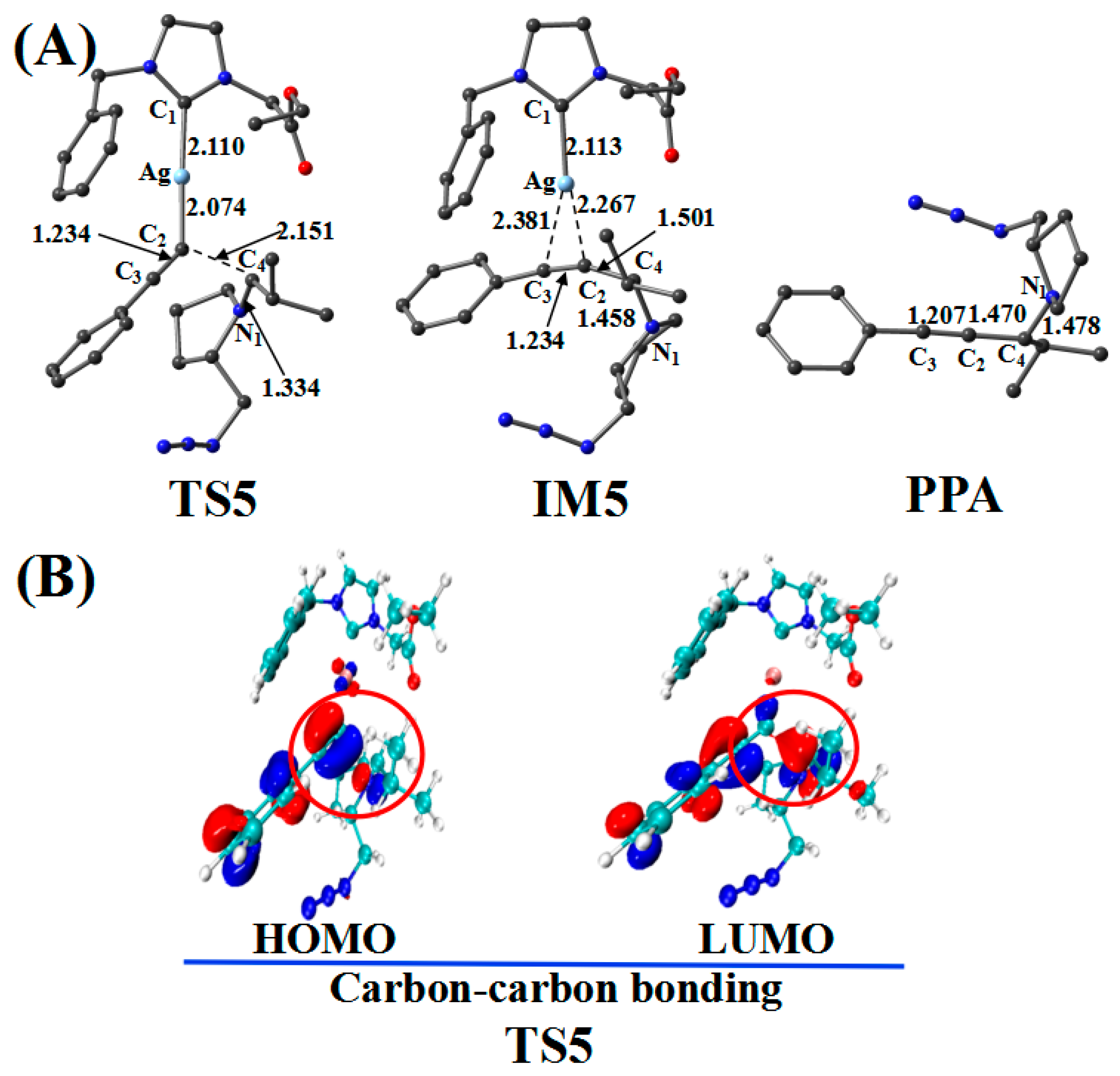
2.4. Imine Reaction with Ag_PAI
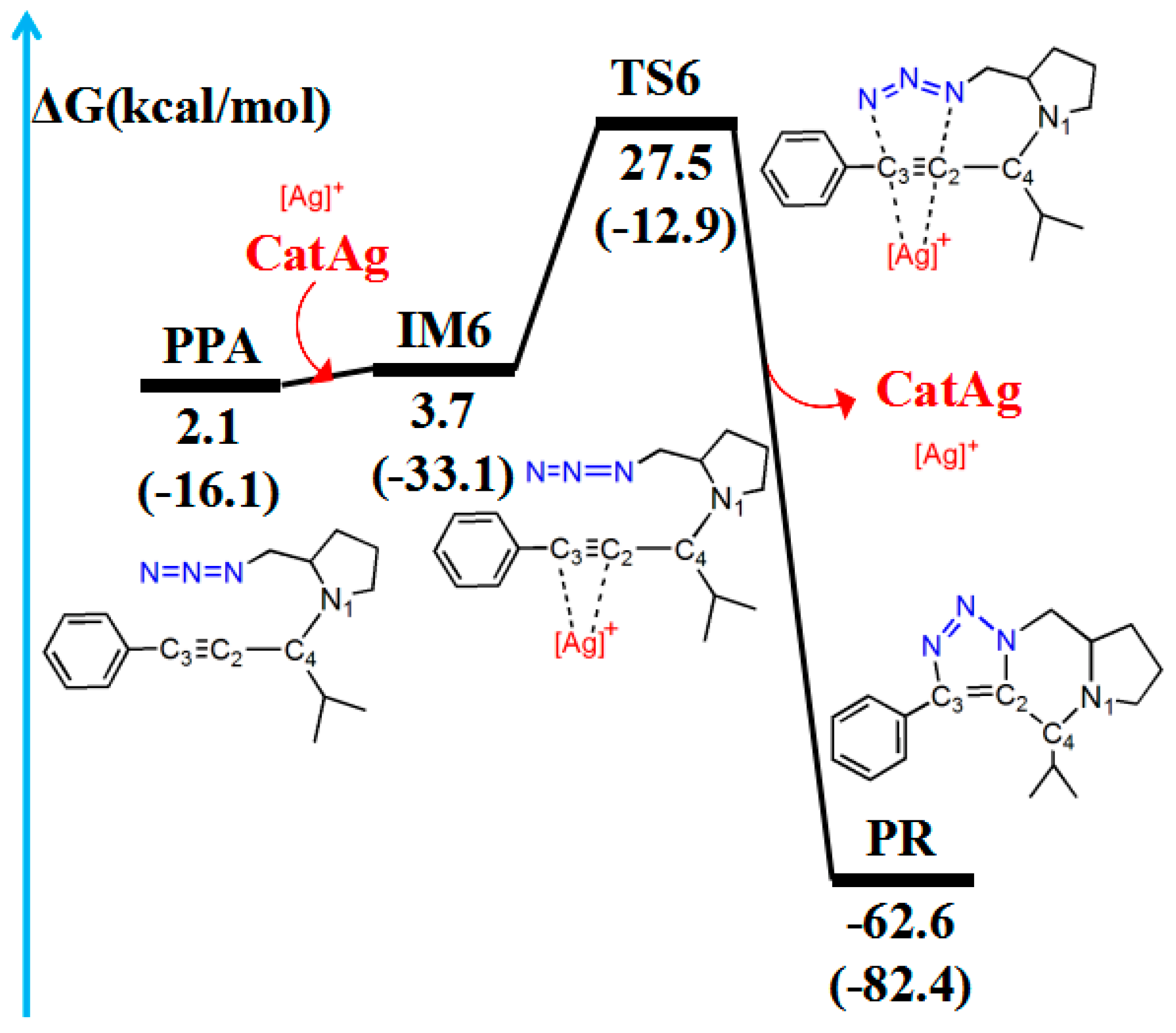
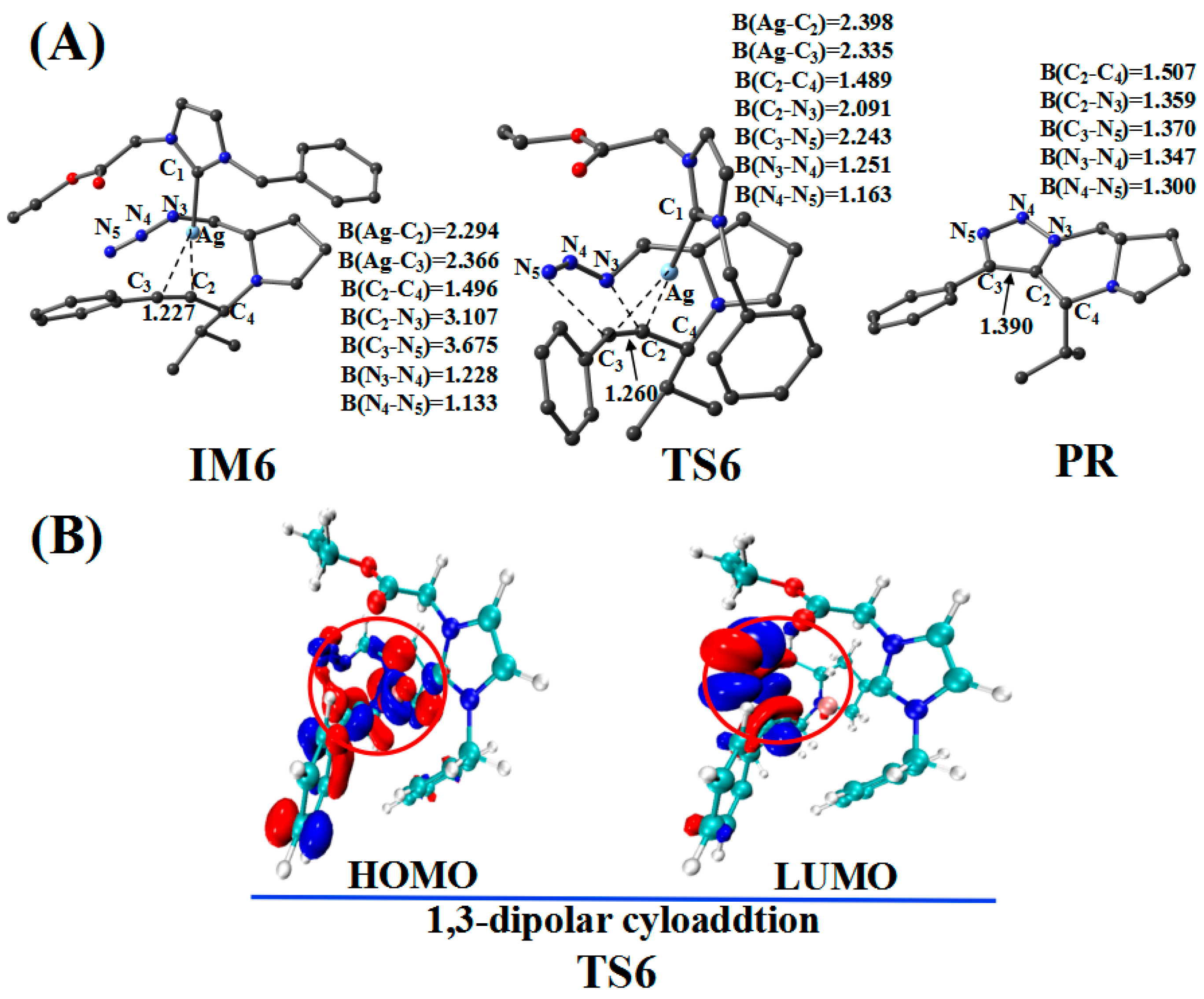
2.5. Cycloaddition Reaction
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiu, S.; Chen, W.; Li, D.; Chen, Y.; Niu, Y.; Wu, Y.; Lei, Y.; Wu, L.; He, W. The use of propargylamines to synthesize amino-1,2,3-triazoles via cycloaddition of azides with allenamines. Synthesis 2022, 54, 2175–2184. [Google Scholar]
- Jesin, I.; Nandi, G.C. Recent advances in the A3 coupling reactions and their applications. Eur. J. Org. Chem. 2019, 16, 2704–2720. [Google Scholar] [CrossRef]
- Ermolat’ev, D.S.; Bariwal, J.B.; Steenackers, H.P.L.; De Keersmaecker, S.C.J.; Van der Eycken, E.V. Concise and diversity-oriented route toward polysubstituted 2-aminoimidazole alkaloids and their analogues. Angew. Chem. Int. Ed. 2010, 49, 9465–9468. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zorba, L.P.; Vougioukalakis, G.C. The ketone-amine-alkyne (KA2) coupling reaction: Transition metal-catalyzed synthesis of quaternary propargylamines. Coord. Chem. Rev. 2021, 429, 213603. [Google Scholar] [CrossRef]
- Ramazani, A.; Ahankar, H.; Nafeh, T.Z.; Joo, W.S. Modern catalysts in A3—coupling reactions. Curr. Org. Chem. 2019, 23, 2783–2801. [Google Scholar] [CrossRef]
- McNally, J.J.; Youngman, M.A.; Dax, S.L. Mannich reactions of resin-bound substrates: 2. A versatile three-component solid-phase organic synthesis methodology. Tetrahedron Lett. 1998, 39, 967–970. [Google Scholar] [CrossRef]
- Dyatkin, A.B.; Rivero, R.A. The solid phase synthesis of complex propargylamines using the combination of sonogashira and mannich reactions. Tetrahedron Lett. 1998, 39, 3647–3650. [Google Scholar] [CrossRef]
- Li, C.-J.; Wei, C. Highly efficient grignard-type imine additions via C-H activation in water and under solvent-free conditions. Chem. Comm. 2002, 3, 268–269. [Google Scholar] [CrossRef]
- Shi, L.; Tu, Y.-Q.; Wang, M.; Zhang, F.-M.; Fan, C.-A. Microwave-promoted three-component coupling of aldehyde, alkyne, and amine via C-H activation catalyzed by copper in water. Org. Lett. 2004, 6, 1001–1003. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Naveenkumar, V.; Srinivasa Rao, R.; Nagaiah, K. [bmim]PF6/CuBr: A novel and recyclable catalytic system for the synthesis of propargyl amines. New J. Chem. 2004, 28, 335–337. [Google Scholar] [CrossRef]
- Bariwal, J.; Ermolat’ev, D.; Van der Eycken, E.V. Efficient microwave-assisted synthesis of secondary alkylpropargylamines by using A3-coupling with primary aliphatic amines. Chem. Eur. J. 2010, 16, 3281–3284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, M.; Maurya, S.; Khanna, R.S. Regioselective synthesis of fused imidazo[1,2-a]pyrimidines via intramolecular C-N bond formation/6-endo-dig cycloisomerization. J. Org. Chem. 2014, 79, 6905–6912. [Google Scholar] [CrossRef]
- Bisai, V.; Suneja, A.; Singh, V.K. Asymmetric alkynylation/lactamization cascade: An expeditious entry to enantiomerically enriched isoindolinones. Angew. Chem. Int. Ed. 2014, 53, 10737–10741. [Google Scholar] [CrossRef]
- Trose, M.; Dell’Acqua, M.; Pedrazzini, T.; Pirovano, V.; Gallo, E.; Rossi, E.; Caselli, A.; Abbiati, G. [Silver(I)(pyridine-containing ligand)] complexes as unusual catalysts for A3-coupling reactions. J. Org. Chem. 2014, 79, 7311–7320. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Joshi, H.; Kumar, U.; Sharma, A.K.; Singh, A.K. Acridine based (S,N,S) pincer ligand: Designing silver(I) complexes for the efficient activation of A3 (aldehyde, alkyne and amine) coupling. Dalton Trans. 2015, 44, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-heterocyclic carbenes: Emerging powerful catalysts. Trends Chem. 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Ghasemi, K.; Darroudi, M.; Rahimi, M.; Rouh, H.; Gupta, A.R.; Cheng, C.; Amini, A. Magnetic AgNPs/Fe3O4@chitosan/PVA nanocatalyst for fast one-pot green synthesis of propargylamine and triazole derivatives. New J. Chem. 2021, 45, 16119–16130. [Google Scholar] [CrossRef]
- Xin, N.; Jing, X.; Zhang, C.-G.; Peng, X.; Liu, J.; Wang, Q.; Wang, W.; Cao, J.; Tao, M. N-heterocyclic carbene silver complex modified polyacrylonitrile fiber/MIL-101(Cr) composite as efficient chiral catalyst for three-component coupling reaction. Nanomaterials 2022, 12, 4175. [Google Scholar] [CrossRef]
- Cao, J.; Li, P.; Xu, G.; Tao, M.; Ma, N.; Zhang, W. Cooperative N-heterocyclic carbene Au and amino catalysis for continuous synthesis of secondary propargylamines in a fiber supported hydrophilic microenvironment. Chem. Eng. J. 2018, 349, 456–465. [Google Scholar] [CrossRef]
- Srinivas, V.; Koketsu, M. Synthesis of indole-2-, 3-, or 5-substituted propargylamines via gold(III)-catalyzed three component reaction of aldehyde, alkyne, and amine in aqueous medium. Tetrahedron 2013, 69, 8025–8033. [Google Scholar] [CrossRef]
- Huang, J.-L.; Gray, D.G.; Li, C.-J. A3-coupling catalyzed by robust Au nanoparticles covalently bonded to HS-functionalized cellulose nanocrystalline films. Beilstein J. Org. Chem. 2013, 9, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-B.; Zhao, Y.; Liao, Y. Aldehyde-alkyne-amine (A3) coupling catalyzed by a highly efficient dicopper complex. RSC Adv. 2015, 5, 37737–37741. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Ermolat’ev, D.S.; Van der Eycken, E.V. Facile and diverse microwave-assisted synthesis of secondary propargylamines in water using CuCl/CuCl2. RSC Adv. 2015, 5, 28921–28924. [Google Scholar] [CrossRef]
- Neshat, A.; Gholinejad, M.; Afrasi, M.; Mastrorilli, P.; Todisco, S.; Gilanchi, S.; Osanlou, F. Heterocyclic thiolates and phosphine ligands in copper-catalyzed synthesis of propargylamines in water. Appl. Organomet. Chem. 2021, 35, e6180. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Hara Gopal, A.V.; Patil, K.S. InBr3-catalyzed three-component reaction: A facile synthesis of propargyl amines. Tetrahedron Lett. 2009, 50, 3493–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Wang, M.; Wang, L. Indium-catalyzed highly efficient three-component coupling of aldehyde, alkyne, and amine via C-H bond activation. J. Org. Chem. 2009, 74, 4364–4367. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Wang, L. Iron-catalyzed ligand-free three-component coupling reactions of aldehydes, terminal alkynes, and amines. Chem. Eur. J. 2009, 15, 2045–2049. [Google Scholar] [CrossRef]
- Chen, W.-W.; Nguyen, R.V.; Li, C.-J. Iron-catalyzed three-component coupling of aldehyde, alkyne, and amine under neat conditions in air. Tetrahedron Lett. 2009, 50, 2895–2898. [Google Scholar] [CrossRef]
- Huo, X.; Liu, J.; Wang, B.; Zhang, H.; Yang, Z.; She, X.; Xi, P. A one-step method to produce graphene-Fe3O4 composites and their excellent catalytic activities for three-component coupling of aldehyde, alkyne and amine. J. Mater. Chem. A 2013, 1, 651–656. [Google Scholar] [CrossRef]
- Samai, S.; Nandi, G.C.; Singh, M.S. An efficient and facile one-pot synthesis of propargylamines by three-component coupling of aldehydes, amines, and alkynes via C-H activation catalyzed by NiCl2. Tetrahedron Lett. 2010, 51, 5555–5558. [Google Scholar] [CrossRef]
- Shi, X.-L.; Sun, B.; Chen, Y.; Hu, Q.; Li, P.; Meng, Y.; Duan, P. Tuning anion species and chain length of ligands grafted on the fiber for an efficient polymer-supported Ni(II) complex catalyst in one-pot multicomponent A3-coupling. J. Catal. 2019, 372, 321–329. [Google Scholar] [CrossRef]
- Manikandan, R.; Anitha, P.; Viswanathamurthi, P.; Malecki, J.G. Palladium(II) pyridoxal thiosemicarbazone complexes as efficient and recyclable catalyst for the synthesis of propargylamines by a three-component coupling reactions in ionic liquids. Polyhedron 2016, 119, 300–306. [Google Scholar] [CrossRef]
- Nouruzi, N.; Dinari, M.; Mokhtari, N.; Gholipour, B.; Rostamnia, S.; Khaksar, S.; Boluki, R. Porous triazine polymer: A novel catalyst for the three-component reaction. Appl. Organomet. Chem. 2020, 34, e5677. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Hu, X.; Zhu, S.; Liu, L. Easy access to 2,4-disubstituted cyclopentenones by a gold(III)-catalyzed A3-coupling/cyclization cascade. Org. Lett. 2020, 22, 9478–9483. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Feng, H.; Van der Eycken, E.V. Microwave-assisted Cu(I)-catalyzed synthesis of unsymmetrical 1,4-diamino-2-butynes via cross-A3-coupling/decarboxylative A3-coupling. J. Org. Chem. 2021, 86, 14036–14043. [Google Scholar] [CrossRef]
- Cao, S.; Zou, B.; Yang, J.; Wang, J.; Feng, H. Hollow CuO-CeO2 nanospheres for an effectively catalytic annulation/A3-coupling reaction sequence. ACS Appl. Nano Mater. 2022, 5, 11689–11698. [Google Scholar] [CrossRef]
- Kumar, G.; Pandey, S.; Gupta, R. Ag-based coordination polymers based on metalloligands and their catalytic performance in multicomponent A3-coupling reactions. Cryst. Growth Des. 2022, 18, 5501–5511. [Google Scholar] [CrossRef]
- Costabile, C.; Mariconda, A.; Sirignano, M.; Crispini, A.; Scarpelli, F.; Longo, P. A green approach for A3-coupling reactions: An experimental and theoretical study on NHC silver and gold catalysts. New J. Chem. 2021, 45, 18509–18517. [Google Scholar] [CrossRef]
- Cao, J.; Xu, G.; Li, P.; Tao, M.; Zhang, W. Polyacrylonitrile fiber supported N-heterocyclic carbene Ag(I) as efficient catalysts for three-component coupling and intramolecular 1,3-dipolar cycloaddition reactions under flow conditions. ACS Sustain. Chem. Eng. 2017, 5, 3438–3447. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Pu, M.; Yang, Z.; Lei, M. Theoretical study on nitrogenous heterocyclic assisted aldimine condensation. Acta Chim. Sin. 2020, 78, 437–443. [Google Scholar] [CrossRef]
- Ciaccia, M.; Di Stefano, S. Mechanisms of imine exchange reactions in organic solvents. Org. Biomol. Chem. 2015, 13, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Boz, E.; Tüzün, N.Ş. Ag-catalyzed azide alkyne cycloaddition: A DFT approach. Dalton Trans. 2016, 45, 5752–5764. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Concvar: A computer program for simulating concentration variation of complex chemical reactions. ChemRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate Ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Smith, D.G.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised damping parameters for the D3 dispersion correction to density functional theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Benchmark energetic data in a model system for grubbs II metathesis catalysis and their use for the development, assessment, and validation of electronic structure methods. J. Chem. Theory Comput. 2009, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Hong, X.; Yu, J.-Q.; Houk, K.N. Experimental–computational synergy for selective Pd(II)-catalyzed C–H activation of aryl and alkyl groups. Acc. Chem. Res. 2017, 50, 2853–2860. [Google Scholar] [CrossRef]
- Deng, L.; Fu, Y.; Lee, S.Y.; Wang, C.; Liu, P.; Dong, G. Kinetic resolution via Rh-catalyzed C-C activation of cyclobutanones at room temperature. J. Am. Chem. Soc. 2019, 141, 16260–16265. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Qu, L.-B.; Houk, K.N.; Lan, Y. Origin of regiochemical control in Rh(III)/Rh(V)-catalyzed reactions of unsaturated oximes and alkenes to form pyrdines. ACS Catal. 2019, 9, 7154–7165. [Google Scholar] [CrossRef]
- Palani, V.; Hugelshofer, C.L.; Kevlishvili, I.; Liu, P.; Sarpong, R. A short synthesis of delavatine a unveils new insights into site-selective cross-coupling of 3,5-dibromo-2-pyrone. J. Am. Chem. Soc. 2019, 141, 2652–2660. [Google Scholar] [CrossRef]
- Qi, X.; Kohler, D.G.; Hull, K.L.; Liu, P. Energy decomposition analyses reveal the origins of catalyst and nucleophile effects on regioselectivity in nucleopalladation of alkenes. J. Am. Chem. Soc. 2019, 141, 11892–11904. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, S.; Wang, F.; Wang, F.; Cao, J.; Zheng, H.; Chen, X.; Ren, A. Density functional theory analysis of the copolymerization of cyclopropenone with ethylene using a palladium catalyst. Polymers 2022, 14, 5273. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Guo, J.; Hu, W.; Wang, Z.X. DFT mechanistic account for the site selectivity of electron-rich C(sp3)–H bond in the manganese-catalyzed aminations. Org. Lett. 2020, 22, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lu, Y.; Lu, G.; Wang, Z.X. Density functional theory mechanistic study of Ni-catalyzed reductive alkyne–alkyne cyclodimerization: Oxidative cyclization versus outer-sphere proton transfer. Org. Lett. 2020, 22, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Chen, G.; Hong, X.; Yu, J.-Q.; Houk, K.N. The origins of dramatic differences in five-membered vs six-membered chelation of Pd(II) on efficiency of C(sp3)-H bond activation. J. Am. Chem. Soc. 2017, 139, 8514–8521. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Peng, X.; Wang, F.; Cao, J.; Wang, F.; Zhang, C.-G. Density functional theory study of the regioselectivity in copolymerization of bis-styrenic molecules with propylene using zirconocene catalyst. Catalysts 2022, 12, 1039. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.X. Realization of conceptual density functional theory and information-theoretic approach in Multiwfn program. In Conceptual Density Functional Theory; WILEY-VCH GmbH: Weinheim, Germany, 2022; pp. 631–647. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness-companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Von Szentpaly, L.; Liu, S.B. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Fu, R.; Lu, T.; Chen, F.-W. Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys. Chim. Sin. 2014, 30, 628–639. [Google Scholar]
- Lu, T.; Chen, F.-W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. TSTcalculator. Available online: http://sobereva.com/310 (accessed on 10 March 2023).
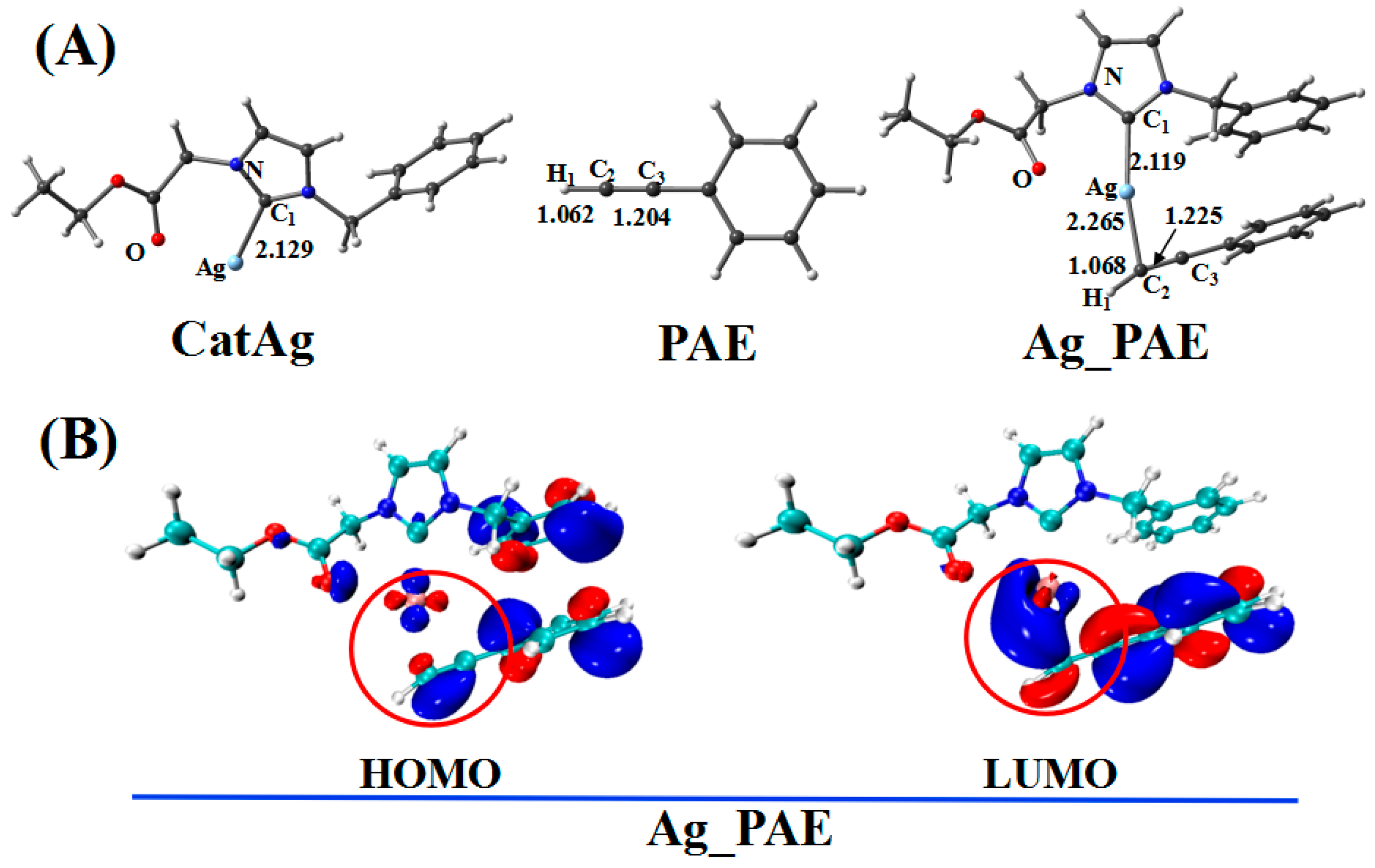

| WBI | QNBO (e) | |||||
|---|---|---|---|---|---|---|
| B (C2–H1) | B (C2–C3) | B (Ag–C1) | B (Ag–C2) | C2 | Ag | |
| CatAg + PAE | 0.934 | 2.825 | 0.482 | 0.000 | −0.201 | 0.701 |
| Ag_PAE | 0.899 | 2.623 | 0.491 | 0.199 | −0.187 | 0.469 |
| WBI | QNBO (e) | ||||||
|---|---|---|---|---|---|---|---|
| B (C2–H1) | B (N1–H1) | B (C2–C3) | B (N1–H2) | H1 | N1 | H2 | |
| Ag_PAE + Amine | 0.899 | 0.000 | 2.623 | 0.845 | 0.174 | −0.703 | 0.362 |
| TS1 | 0.464 | 0.347 | 2.617 | 0.810 | 0.362 | −0.438 | 0.263 |
| Ag_PAI + AmineH | 0.000 | 0.739 | 2.759 | 0.792 | 0.458 | −0.564 | 0.425 |
| WBI | QNBO (e) | ||||||
|---|---|---|---|---|---|---|---|
| B (N1–H1) | B (C4–O1) | B (O1–H1) | B (O1–H2) | O1 | H1 | H2 | |
| AmineH + Ald | 0.739 | 1.886 | −0.515 | 0.458 | 0.425 | ||
| IM1 | 0.693 | 1.734 | 0.071 | −0.639 | 0.459 | 0.423 | |
| TS2 | 0.008 | 1.405 | 0.697 | −0.526 | 0.521 | 0.360 | |
| IM2 | 0.009 | 0.950 | 0.745 | −0.740 | 0.491 | 0.417 | |
| TS3 | 0.917 | 0.750 | 0.004 | −0.766 | 0.482 | 0.484 | |
| IM3 | 0.893 | 0.756 | 0.004 | −0.785 | 0.478 | 0.485 | |
| TS4 | 0.636 | 0.783 | 0.299 | −0.963 | 0.463 | 0.471 | |
| IM4 | 0.002 | 0.795 | 0.670 | −0.984 | 0.456 | 0.493 | |
| Imine + H2O | 0.813 | 0.813 | −0.877 | 0.438 | 0.438 | ||
| TS3A | 0.790 | 0.674 | 0.411 | −0.695 | 0.526 | 0.524 | |
| WBI | QNBO (e) | |||||
|---|---|---|---|---|---|---|
| B (Ag–C2) | B (C4–N1) | B (C2–C4) | C2 | N1 | C4 | |
| Ag_PAI + Imine | 0.627 | 1.669 | 0.000 | −0.386 | −0.313 | 0.316 |
| TS5 | 0.399 | 1.332 | 0.378 | −0.449 | −0.412 | 0.223 |
| IM5 | 0.162 | 0.974 | 0.991 | −0.097 | −0.513 | −0.062 |
| CatAg + PPA | 0.000 | 0.946 | 1.034 | −0.386 | −0.534 | −0.082 |
| WBI | QNBO (e) | ||||||
|---|---|---|---|---|---|---|---|
| B (C2–C3) | B (C2–N3) | B (C3–N5) | B (N3–N4) | B (N4–N5) | C2 | C3 | |
| CatAg + PPA | 2.716 | 0.002 | 0.001 | 1.529 | 2.315 | −0.002 | −0.028 |
| IM6 | 2.550 | 0.004 | 0.003 | 1.504 | 2.334 | −0.084 | −0.013 |
| TS6 | 2.249 | 0.316 | 0.285 | 1.455 | 2.144 | −0.021 | −0.105 |
| PR + CatAg | 1.420 | 1.206 | 1.310 | 1.221 | 1.537 | 0.140 | 0.075 |
| η a | μ b | ω c | NNu d | f +e | f −f | ||
|---|---|---|---|---|---|---|---|
| Ag | C4 | C2 | |||||
| CatAg | 7.424 | −7.623 | 3.795 | −0.330 | 0.693 | ||
| PAE | 9.577 | −3.561 | 0.839 | 2.825 | 0.197 | ||
| PPA | 8.347 | −3.278 | 0.847 | 3.216 | 0.064 | ||
| Imine | 8.598 | −8.503 | 4.172 | −1.172 | 0.212 | ||
| Ag_PAI | 7.073 | −3.077 | 0.818 | 4.215 | 0.172 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yu, S.; Wang, F.; Cao, J.; Liang, X.; Wang, F.; Zheng, H.; Zhang, Y.; Yang, M.; Zhao, B. Insights into the Three-Component Coupling Reactions of Aldehydes, Alkynes, and Amines Catalyzed by N-heterocyclic Carbene Silver: A DFT Study. Catalysts 2023, 13, 646. https://doi.org/10.3390/catal13040646
Zhang C, Yu S, Wang F, Cao J, Liang X, Wang F, Zheng H, Zhang Y, Yang M, Zhao B. Insights into the Three-Component Coupling Reactions of Aldehydes, Alkynes, and Amines Catalyzed by N-heterocyclic Carbene Silver: A DFT Study. Catalysts. 2023; 13(4):646. https://doi.org/10.3390/catal13040646
Chicago/Turabian StyleZhang, Chenggen, Shuyuan Yu, Fei Wang, Jian Cao, Xinru Liang, Fuping Wang, Huimin Zheng, Yaning Zhang, Mengyao Yang, and Boyu Zhao. 2023. "Insights into the Three-Component Coupling Reactions of Aldehydes, Alkynes, and Amines Catalyzed by N-heterocyclic Carbene Silver: A DFT Study" Catalysts 13, no. 4: 646. https://doi.org/10.3390/catal13040646
APA StyleZhang, C., Yu, S., Wang, F., Cao, J., Liang, X., Wang, F., Zheng, H., Zhang, Y., Yang, M., & Zhao, B. (2023). Insights into the Three-Component Coupling Reactions of Aldehydes, Alkynes, and Amines Catalyzed by N-heterocyclic Carbene Silver: A DFT Study. Catalysts, 13(4), 646. https://doi.org/10.3390/catal13040646