Synthesis and Analysis of In2CdO4/Y2SmSbO7 Nanocomposite for the Photocatalytic Degradation of Rhodamine B within Dye Wastewater under Visible Light Irradiation
Abstract
1. Introduction
2. Result and Discussion
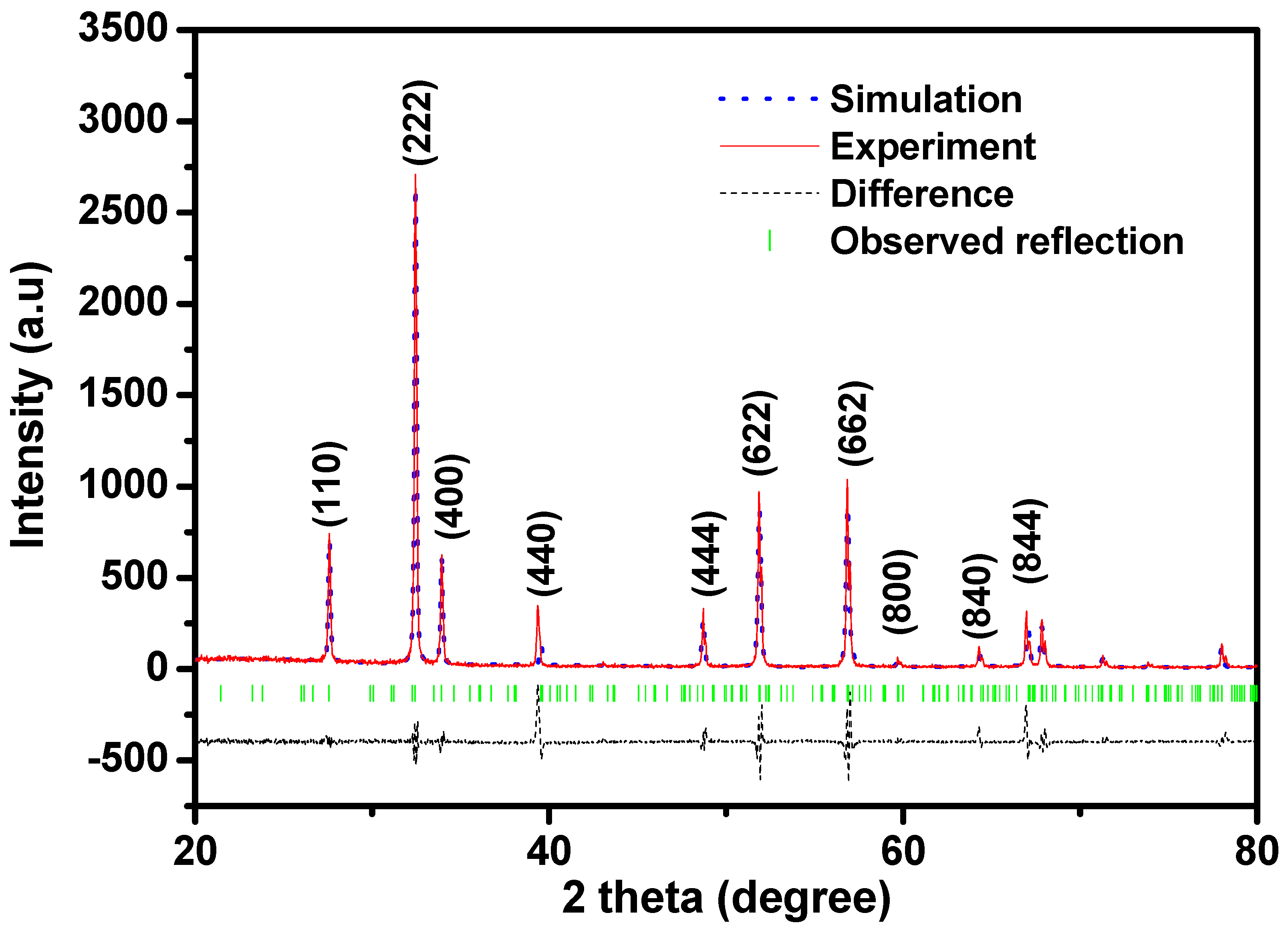
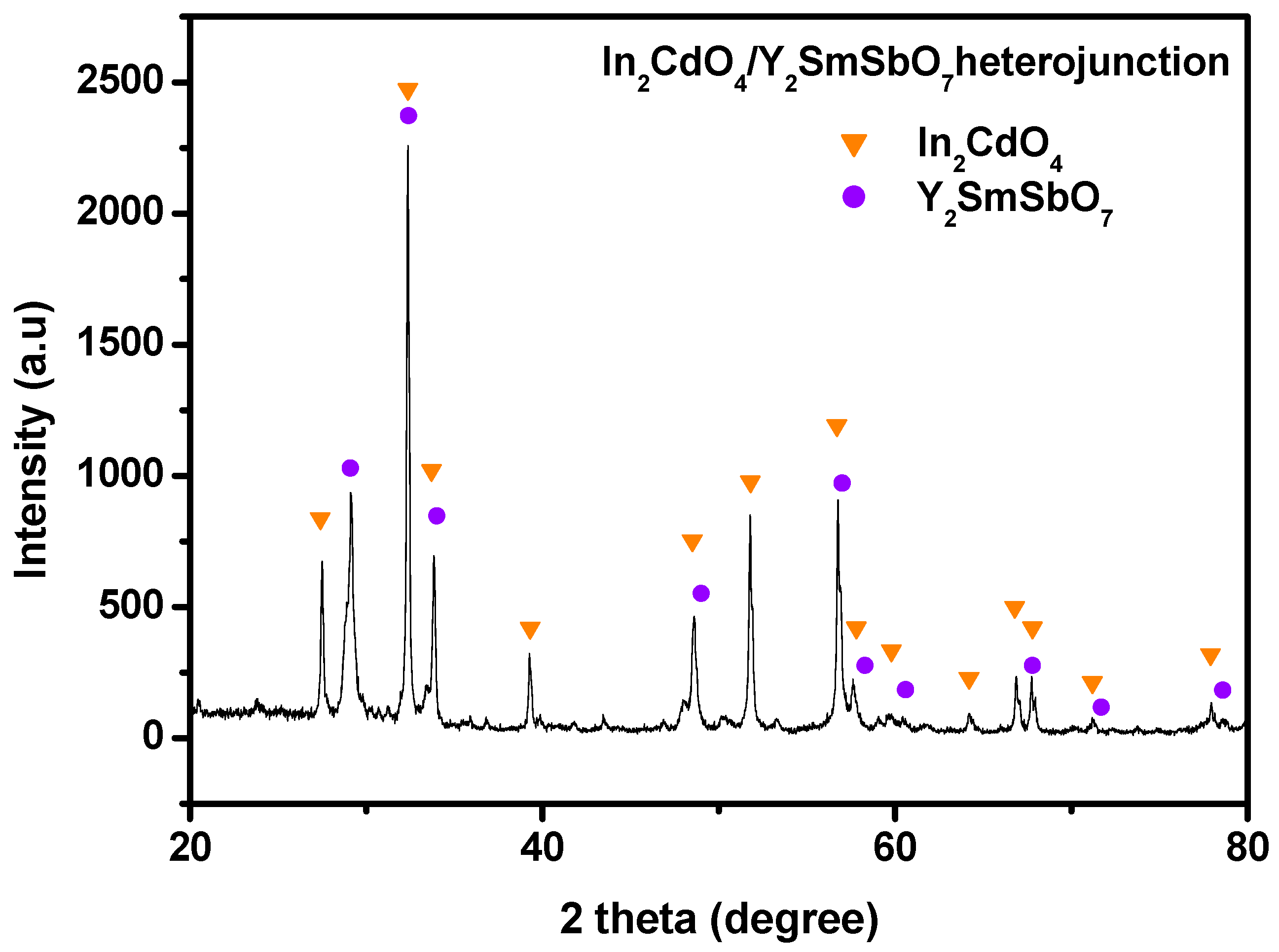
2.1. X-Ray Diffractometer (XRD) Analysis
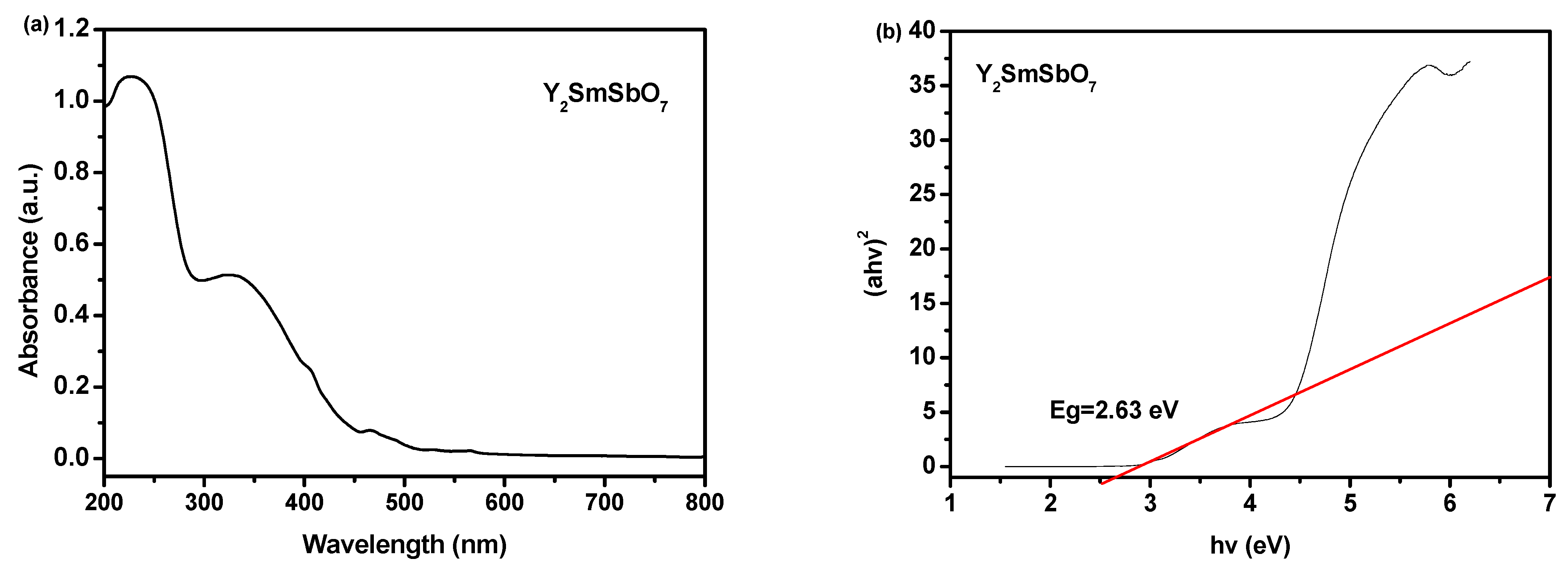
2.2. UV-Vis Diffuse Reflectance Spectra
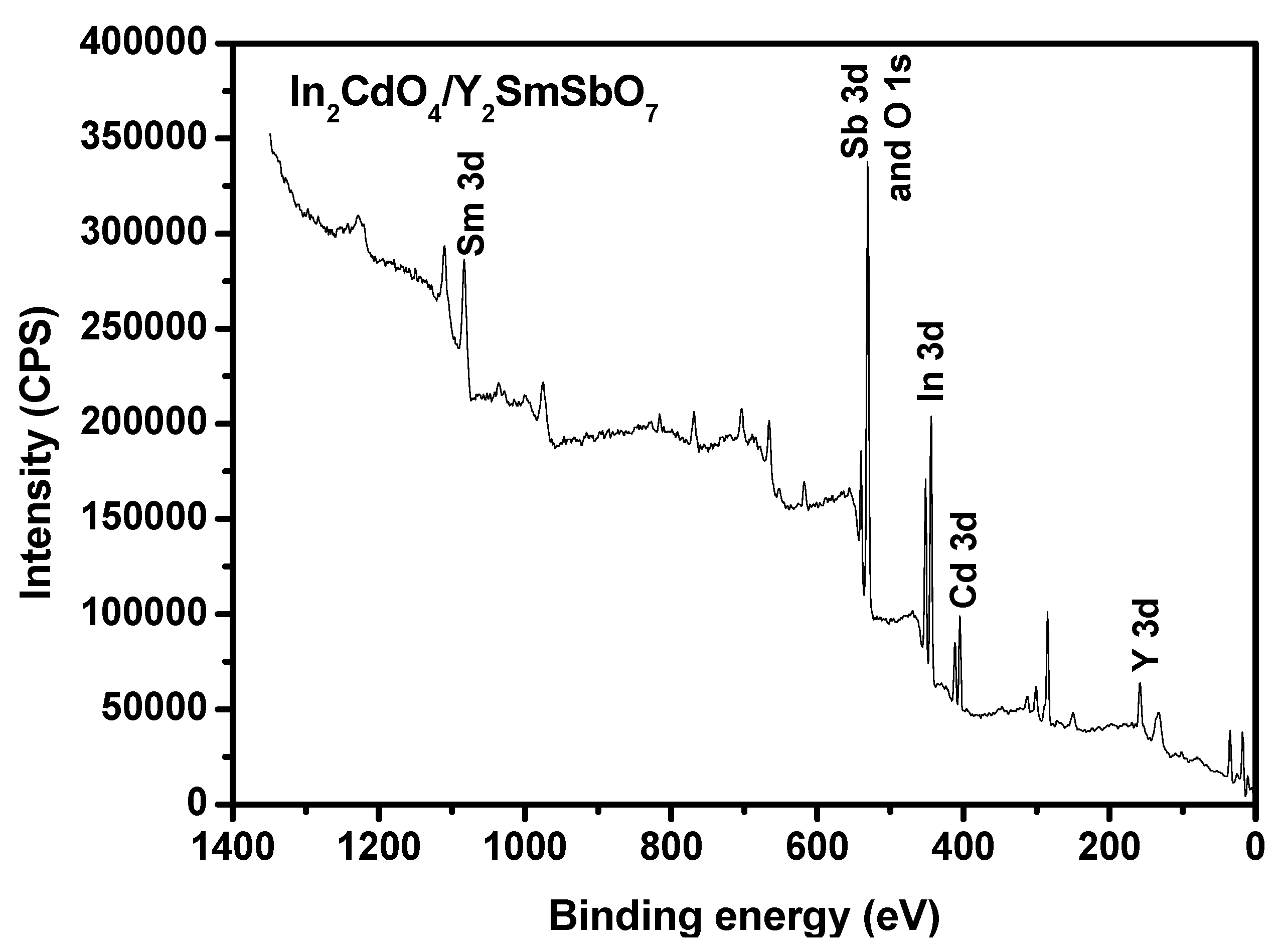
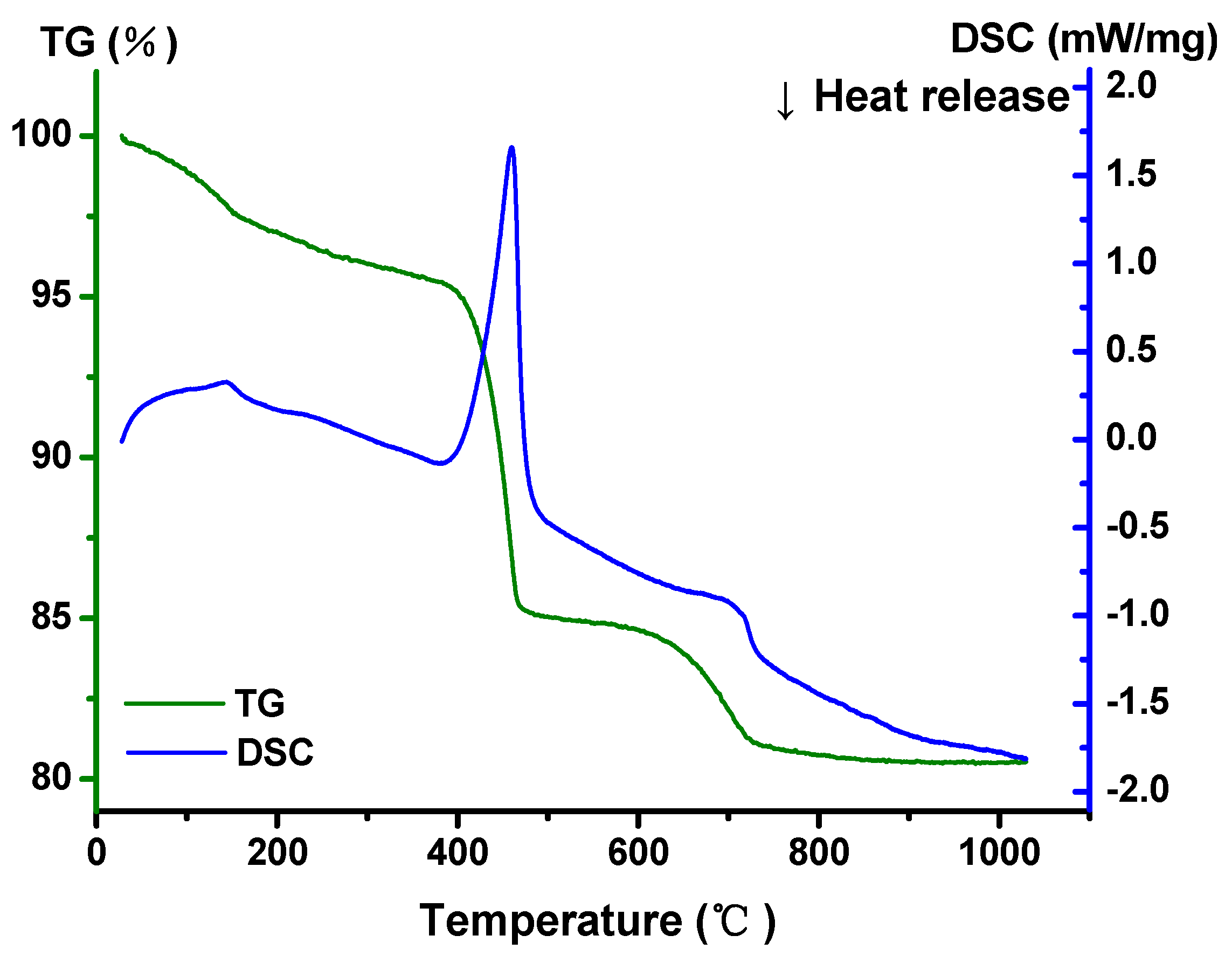
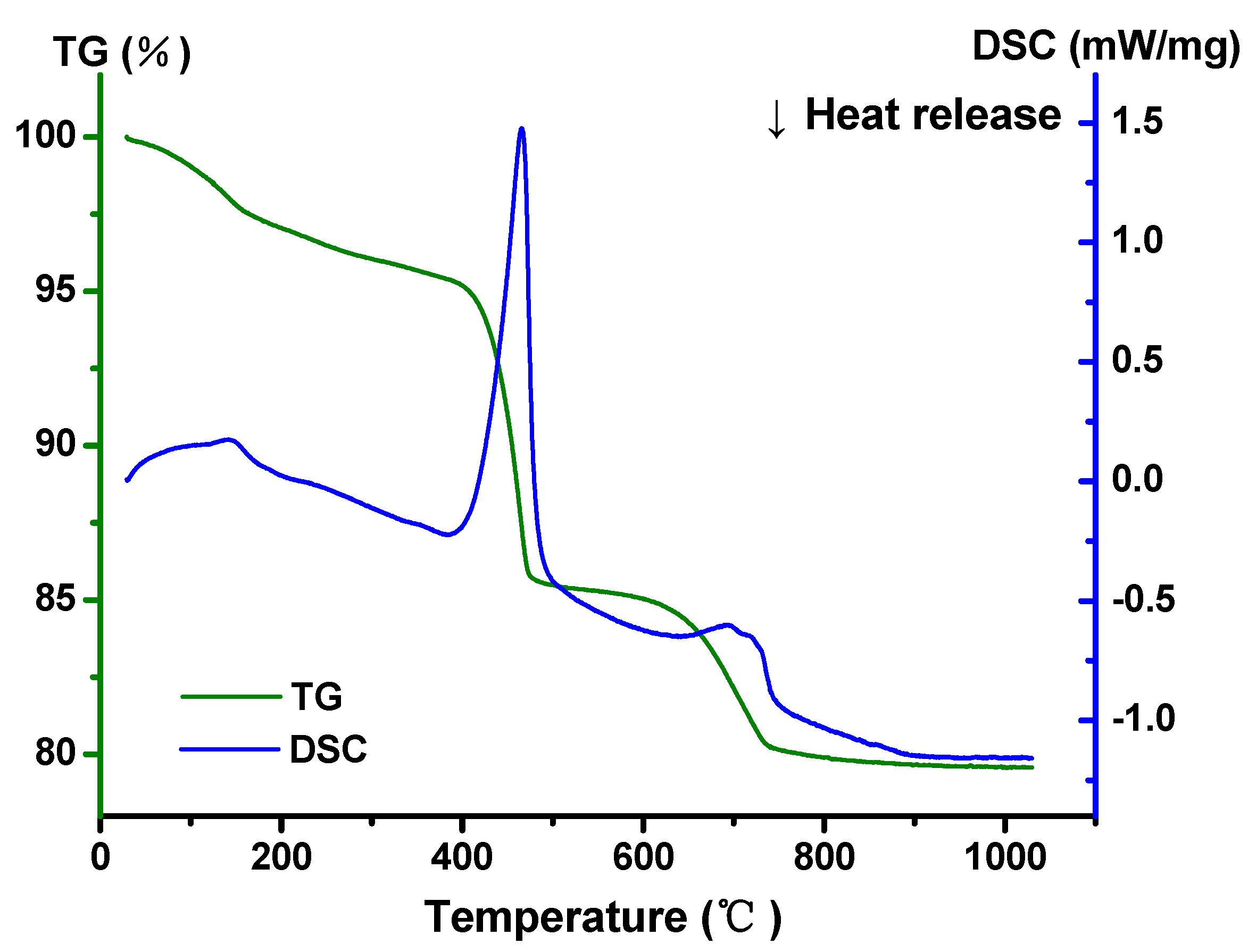
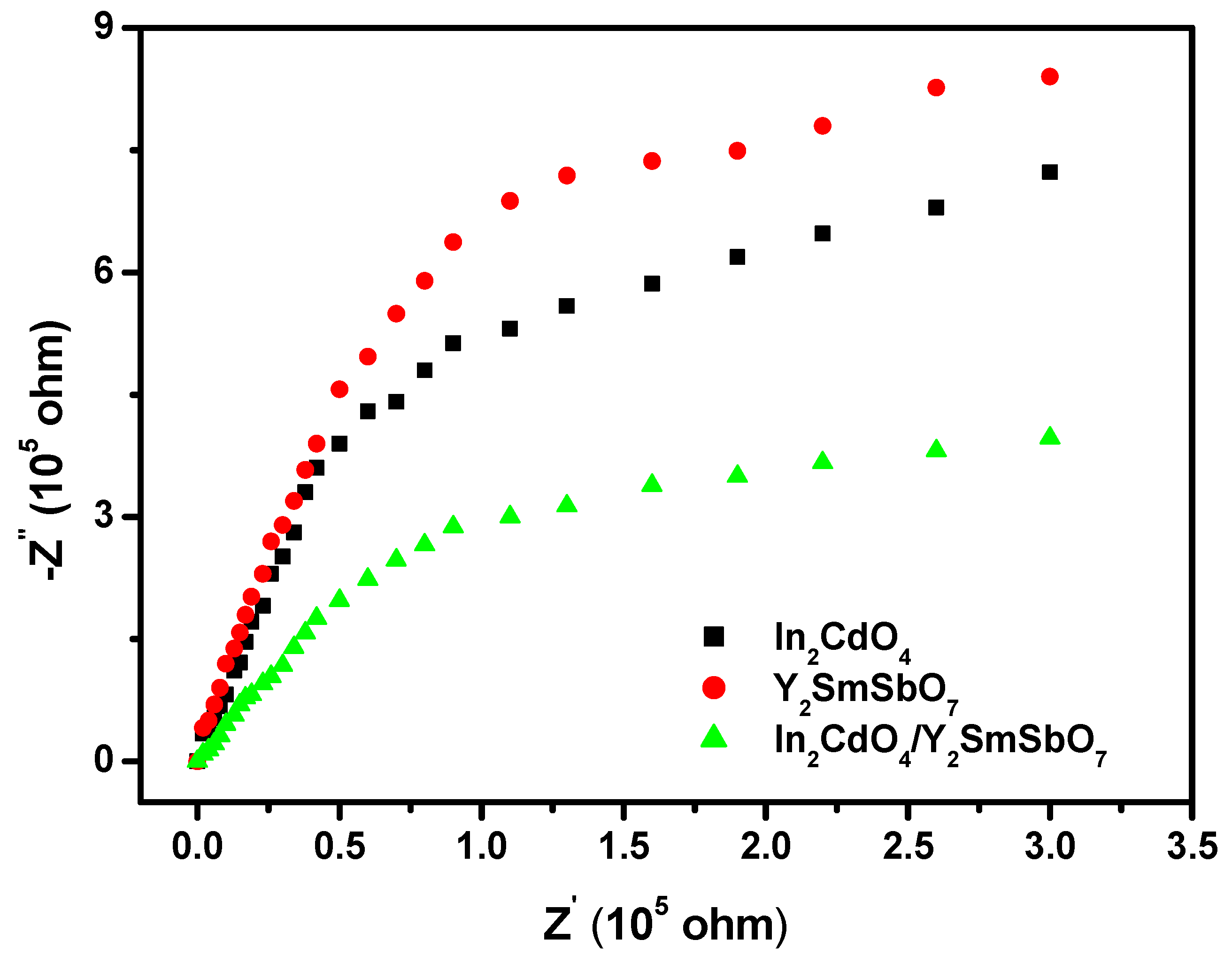
2.3. Property Characterization of In2CdO4/Y2SmSbO7 Heterojunction Photocatalyst
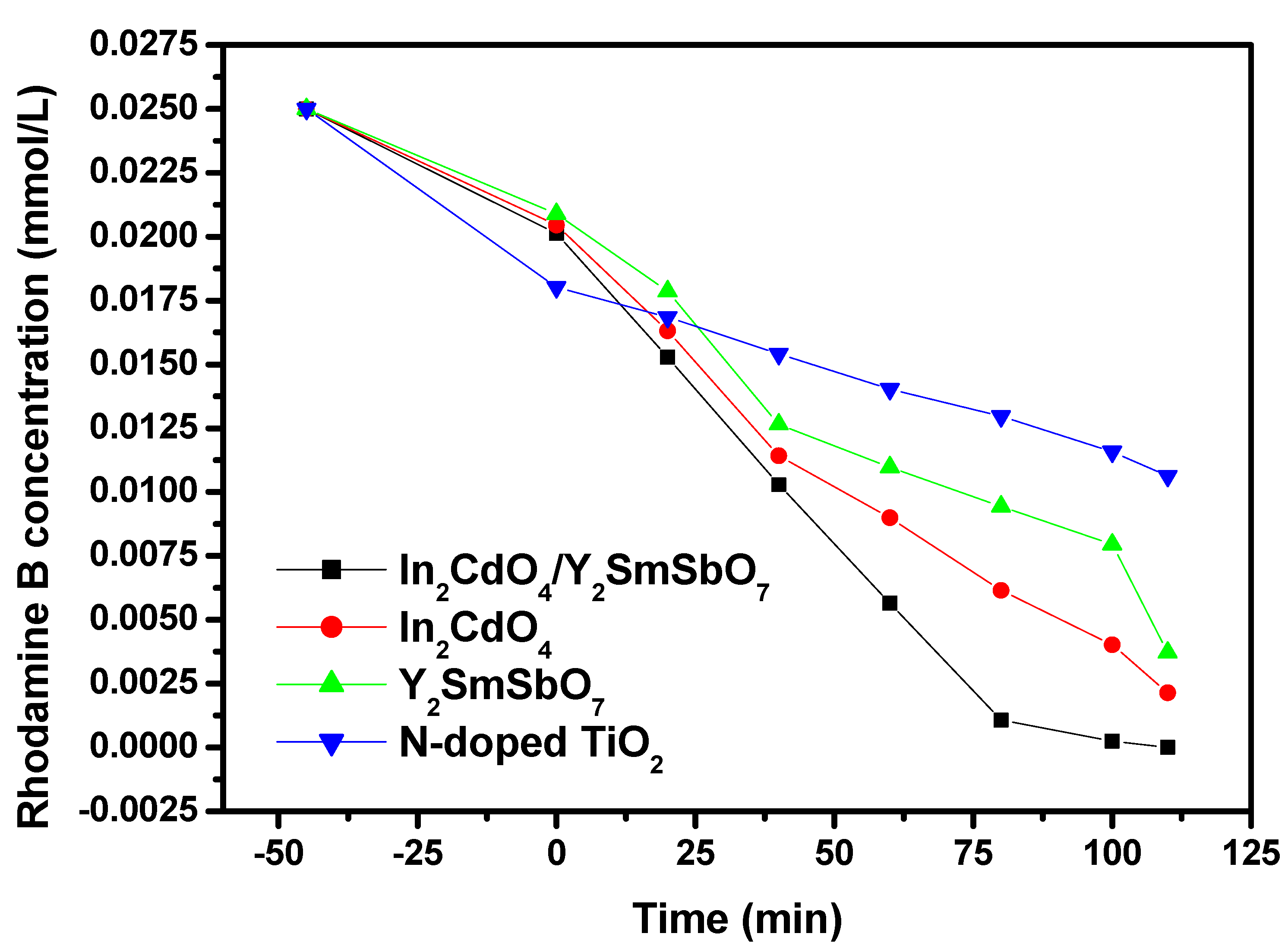
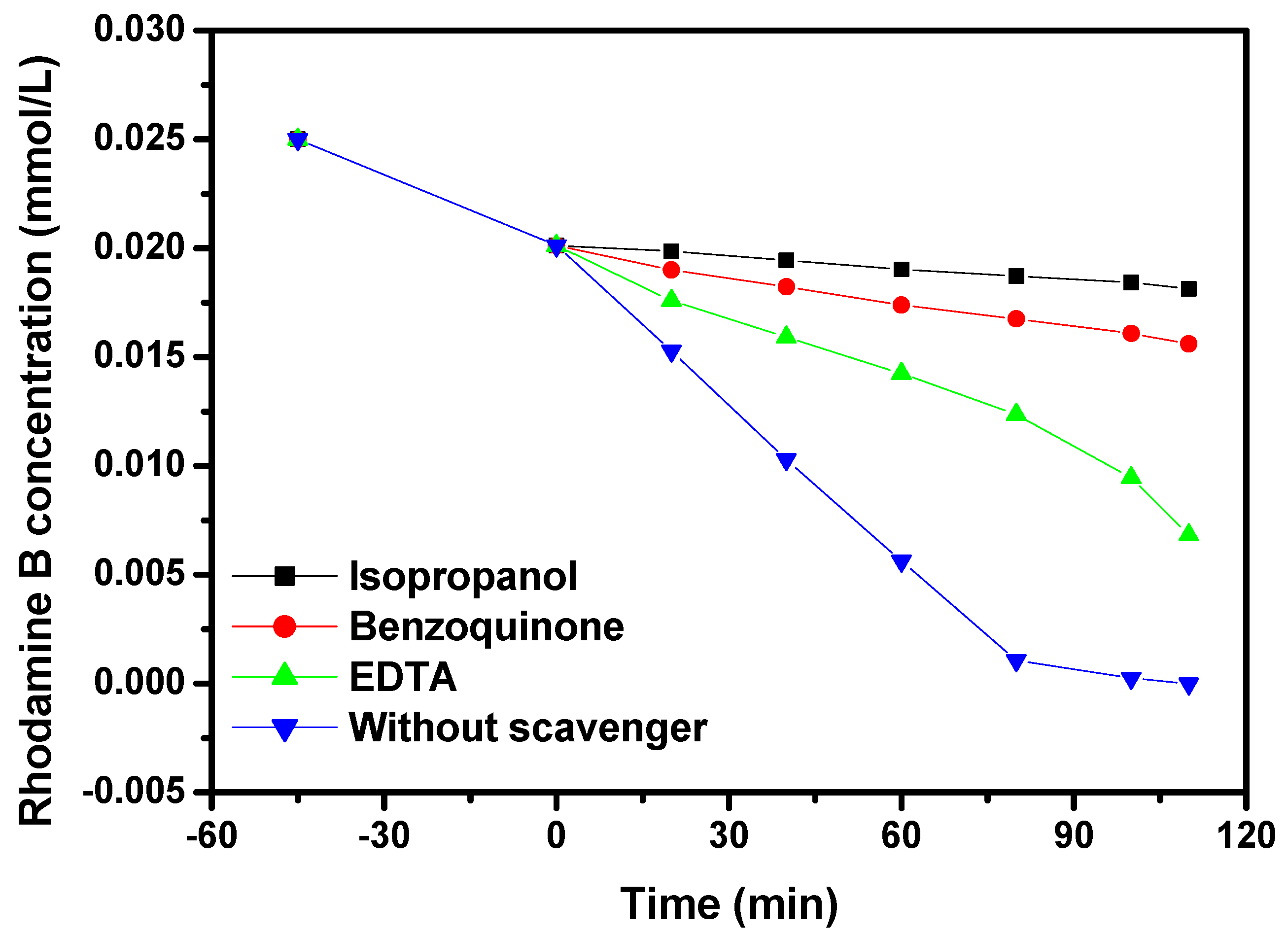
2.4. Photocatalytic Activity
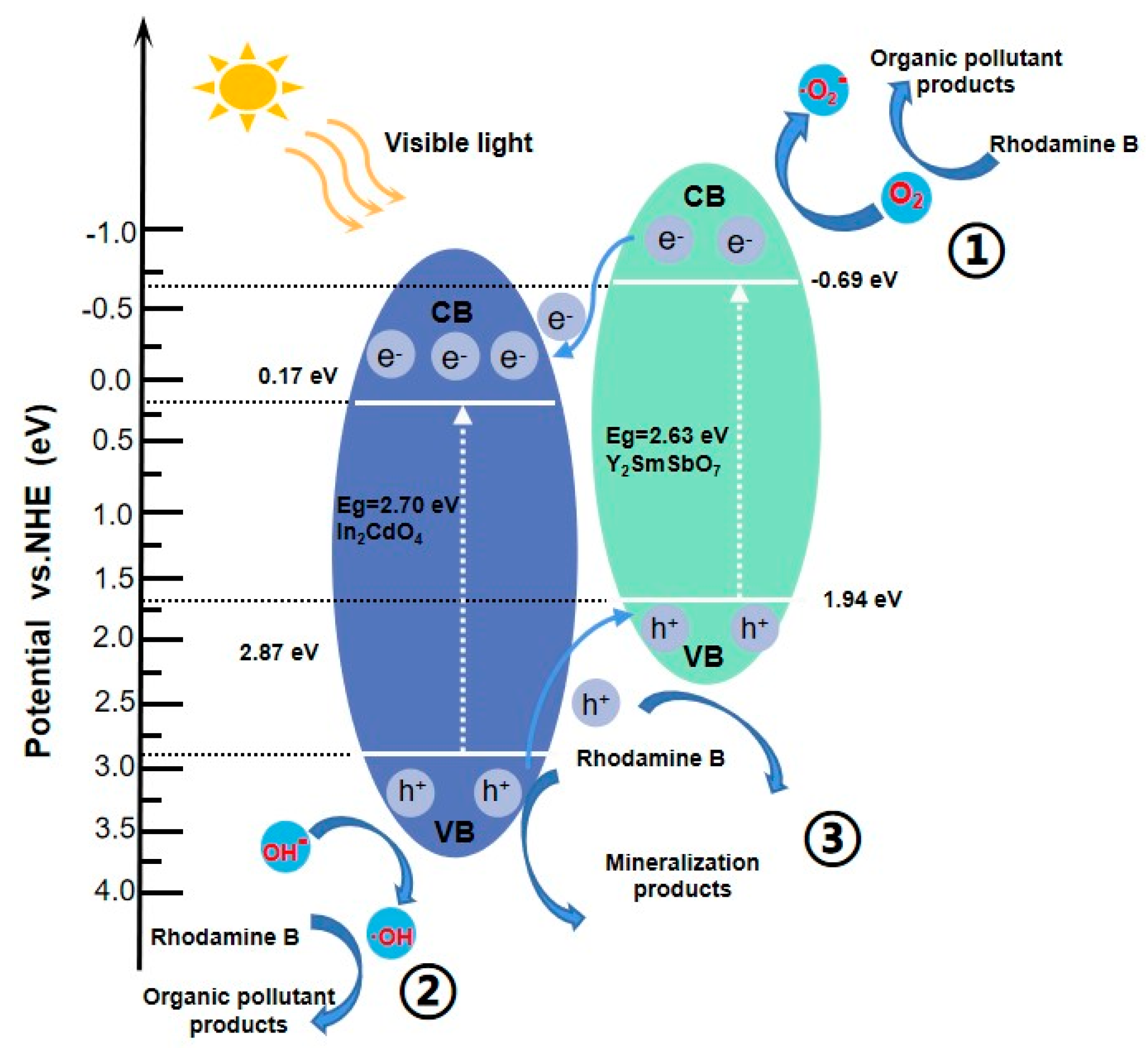
2.5. Analysis of Possible Degradation Mechanisms
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation Method of In2CdO4
3.3. Preparation Method of Y2SmSbO7
3.4. Synthesis of N-Doping TiO2
3.5. Synthesis of In2CdO4/Y2SmSbO7 Heterojunction Photocatalyst
3.6. Characterization
3.7. Photoelectrochemical Experiments
3.8. Experimental Setup and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aziz, K.H.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling fifilm reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process. Saf. Environ. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A. Degradation of Remazol Black B dye and its simulated dye bath wastewater by advanced oxidation processes of heterogeneous and homogeneous media. Color Technol. 2001, 117, 38–42. [Google Scholar] [CrossRef]
- Young, L.; Yu, J. Ligninase-catalysed decolorization of synthetic dyes. Water Res. 1997, 31, 1187–1193. [Google Scholar] [CrossRef]
- Daneshvar, N.; Ashassi-Sorkhabi, H.; Tizpar, A. Decolorization of orange II by electro-coagulation method. Sep. Purif. Technol. 2003, 31, 153–162. [Google Scholar] [CrossRef]
- He, X.H.; Kai, T.H.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Ma, Y.S.; Chang, C.N.; Chao, C.R. Decolorization of Rhodamine B by a Photo-Fenton process: Effect of System Parameters and Kinetic Study. Int. J. Environ. Res. 2012, 1, 73–80. [Google Scholar]
- Soares, E.T.; Lansarin, M.A.; Moro, C.C. A Study of Process Variables for the Photocatalytic Degradation of Rhodamine B. Braz. J. Chem. Eng. 2007, 24, 29–36. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zheng, L.; Xian, Y.; Jin, L. Photoelectrocatalytic Degradation of Rhodamine B using Ti/TiO2 Electrode Prepared by Laser Calcination Method. Electrochim. Acta 2006, 51, 4942–4949. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, Y.; Zhu, T.; Wang, Q.; Song, H. The Promoted Photoelectrocatalytic Degradation of Rhodamine B over TiO2 Thin Film under the Half-wave Pulsed Direct Current. Appl. Catal. B Environ. 2011, 102, 464–469. [Google Scholar] [CrossRef]
- Niu, J.F.; Maharana, D.; Xu, J.L.; Chai, Z.; Bao, Y.P. A High Activity of Ti/Sn02-Sb Electrode in the Electrochemical Degradation of 2, 4-Dichlorophenol in Aqueous Solution. Environ. Sci. 2013, 25, 1424–1430. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Wang, D. Design of hollow nanostructures for energy storage, conversion and production. Adv. Mater. 2019, 31, 1801993. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef]
- Bouyarmane, H.; El Bekkali, C.; Labrag, J.; Es-saidi, I.; Bouhnik, O.; Abdelmoumen, H.; Laghzizil, A.; Nunzi, J.M.; Robert, D. Photocatalytic degradation of emerging antibiotic pollutants in waters by TiO2/Hydroxyapatite nanocomposite materials. Surf. Interfaces. 2021, 24, 101155. [Google Scholar] [CrossRef]
- Huang, P.Q.; Luan, J.F. Synthesis of a GaOOH/ZnBiTaO5 heterojunction photocatalyst with enhanced photocatalytic performance toward enrofloxacin. Rsc. Adv. 2020, 10, 4286–4292. [Google Scholar] [CrossRef]
- Saeed, M.; ul Haq, A.; Muneer, M.; Ahmad, A.; Bokhari, T.H.; Sadiq, Q. Synthesis and characterization of Bi2O3 and Ag-Bi2O3 and evaluation of their photocatalytic activities towards photodegradation of crystal violet dye. Phys. Scr. 2021, 96, 125707. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, T.Y.; Sun, J.; Ma, X.L.; Wang, Y.J.; Wang, J.H.; Xie, Z.L. Preparation of TiO2-modified Biochar and its Characteristics of Photo-catalysis Degradation for enrofloxacin. Sci. Rep. 2020, 10, 6588. [Google Scholar] [CrossRef]
- Fink, L.; Dror, I.; Berkowitz, B. Enrofloxacin oxidative degradation facilitated by metal oxide nanoparticles. Chemosphere 2012, 86, 144–149. [Google Scholar] [CrossRef]
- Raees, A.; Jamal, M.A.; Ahmed, I.; Silanpaa, M.; Algarni, T.S. Synthesis and Characterization of CeO2/CuO Nanocomposites for Photocatalytic Degradation of Methylene Blue in Visible Light. Coatings 2021, 11, 305. [Google Scholar] [CrossRef]
- Davenport, C.L.M.; Boston, R.C.; Richardson, D.W. Effects of enrofloxacin and magnesium deficiency on matrix metabolism in equine articular cartilage. Am. J. Vet. Res. 2001, 62, 160–166. [Google Scholar] [CrossRef]
- Chen, Z.G.; Chen, X.L.; Di, J.; Liu, Y.L.; Yin, S.; Xia, J.X.; Li, H.M. Graphene-like boron nitride modified bismuth phosphate materials for boosting photocatalytic degradation of enrofloxacin. J. Colloid Interf. Sci. 2017, 492, 51–60. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X.M. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, Y.G.; Li, H.M.; Xia, J.X.; Xiong, J.; Yin, S.; Huang, C.J.; Wan, H.L. Synthesis, characterization and photocatalytic property of AgBr/BiPO4 heterojunction photocatalyst. Dalton T 2012, 41, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yuto, S.; Hasegawa, T.; Shiroishi, W.; Honghao, H.; Nakabayashi, Y.; Nosaka, Y.; Saito, N. Compositing effects of CuBi2O4 on visible-light responsive photocatalysts. Mater. Sci. Semicond. Process. 2017, 57, 12–17. [Google Scholar] [CrossRef]
- Su, Y.H.; Chen, P.; Wang, F.L.; Zhang, Q.X.; Chen, T.S.; Wang, Y.F.; Yao, K.; Lv, W.Y.; Liu, G.G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. Rsc. Adv. 2017, 7, 34096–34103. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, J.; Zhang, Y.M.; Tang, Y.W. Construction of a novel ZnCo2O4/Bi2O3 heterojunction photocatalyst with enhanced visible light photocatalytic activity. Chin. Chem. Lett. 2019, 30, 735–738. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.Q.; Peng, H.P.; Wang, Z.W.; Huang, W.Q. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. NanomaterIals 2018, 8, 105. [Google Scholar] [CrossRef]
- Luan, J.F.; Shen, Y.; Li, Y.Y.; Paz, Y. The Structural, Photocatalytic Property Characterization and Enhanced Photocatalytic Activities of Novel Photocatalysts Bi2GaSbO7 and Bi2InSbO7 during Visible Light Irradiation. Materials 2016, 9, 801. [Google Scholar] [CrossRef]
- Kahng, S.; Yoo, H.; Kim, J.H. Recent advances in earth-abundant photocatalyst materials for solar H-2 production. Adv. Powder Technol. 2020, 31, 11–28. [Google Scholar] [CrossRef]
- Hebeish, A.A.; Abdelhady, M.M.; Youssef, A.M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohyd. Polym. 2013, 91, 549–559. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. ChemInt. Ed. Engl. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, A.; Cao, S.W.; Bosman, M.; Li, S.; Xue, C. Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO2 nanofibers. Nanoscale 2014, 6, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an alpha-beta phase junction on Ga2O3. Angew. Chem. Int. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Zhao, X.G.; Zheng, M.Y.; Li, S.; Wang, Y.; Liu, X.J. Preparation of N-doped TiO2 by oxidizing TiN and its application on phenol degradation. Water Sci. Technol. 2013, 68, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shi, J.; Mao, S.S.; Guo, L. Co3O4 quantum dots: Reverse micelle synthesis and visible-light-driven photocatalytic overall water splitting. Chem. Commun. 2014, 50, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, S.; Zou, J.J.; Huang, Z.F.; Wang, L.; Zhang, X. Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41. Chem. Commun. 2014, 50, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, D.; Ning, C. P-Type hydrogen sensing with Al-and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huo, Y.; Zhu, J. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410–4414. [Google Scholar] [CrossRef]
- Zhang, W.J.; Ma, Z.; Du, L.; Li, H. Role of PEG4000 in sol-gel synthesis of Sm2Ti2O7 photocatalyst for enhanced activity. J. Alloys Compd. 2017, 704, 26–31. [Google Scholar] [CrossRef]
- Kanhere, P.; Shenai, P.; Chakraborty, S.; Ahuja, R.; Zheng, J.; Chen, Z. Mono- and co-doped NaTaO3 for visible light photocatalysis. Phys. Chem. Chem. Phys. 2014, 16, 16085–16094. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, W.; Wang, S.; Jiang, J.M.; Chen, Z.H. Synthesis of well-defined hierarchical porous La2Zr2O7 monoliths via non-alkoxidesolegel process accompanied by phase separation. Micropor. Mesopor. Mat. 2016, 221, 32–39. [Google Scholar] [CrossRef]
- Xu, X.X.; Lu, T.T.; Liu, X.X.; Wang, X.L. Chemical-Bond-Mediated p-n Heterojunction Photocatalyst Constructed from Coordination Polymer Nanoparticles and a Conducting Copolymer: Visible-Light Active and Highly Efficient. Chem.-Eur. J. 2015, 21, 17430–17436. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.; Sultana, S.; Parida, K. One-Pot-Architectured Au-Nanodot-Promoted MoS2/ZnIn2S4: A Novel p−n Heterojunction Photocatalyst for Enhanced Hydrogen Production and Phenol Degradation. Inorg. Chem. 2019, 58, 9941–9955. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.L.; Li, T.T.; Ren, H.T.; Mao, Z.Y.; Zhang, X.F.; Lin, J.H.; Lou, C.W. Enhanced photocatalytic performance through the ferroelectric synergistic effect of p-n heterojunction BiFeO3/TiO2 under visible-light irradiation. Ceram. Int. 2021, 47, 10786–10795. [Google Scholar] [CrossRef]
- Luan, J.F.; Zhao, W.; Feng, J.W.; Cai, H.L.; Zheng, Z.; Pan, B.C.; Wu, X.S.; Zou, Z.G.; Li, Y.M. Structural, photophysical and photocatalytic properties of novel Bi2AlVO7. J. Hazard. Mater. 2009, 164, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, C.M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: Photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Saravanakumar, k.; Muthuraj, V.; Jeyaraj, M. The design of novel visible light driven Ag/CdO as smart nanocomposite for photodegradation of different dye contaminants. Spectrochim. Acta. A 2018, 188, 291–300. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Dewasurendra, S.; Zhang, F.X. Blue and red up-conversion light emission in TM-doped A(2)B(2)O(7) oxides. Mater. Lett. 2016, 170, 53–57. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Camardello, S.J.; Comanzo, H.A.; Trevisani, M.; Bettinelli, M. Optical spectroscopy of Pr3+ in the weberite, NaGdSb2O7: High covalence of Pr3+-O2- bonding. J. Lumin. 2014, 148, 262–266. [Google Scholar] [CrossRef]
- Garza-Tovar, L.L.; Torres-Martinez, L.M.; Rodriguez, D.B.; Gomez, R.; del Angel, G. Photocatalytic degradation of methylene blue on Bi2MNbO7 (M = Al, Fe, In, Sm) sol–gel catalysts. J. Mol. Catal. A-Chem. 2006, 247, 283–290. [Google Scholar] [CrossRef]
- Luan, J.F.; Cai, H.L.; Zheng, S.R.; Hao, X.P.; Luan, G.Y.; Wu, X.S.; Zou, Z.G. Structural and photocatalytic properties of novel Bi2GaVO7. Mater. Chem. Phys. 2007, 104, 119–124. [Google Scholar] [CrossRef]
- Luan, J.F.; Pan, B.C.; Paz, Y.; Li, Y.M.; Wu, X.S.; Zou, Z.G. Structural, photophysical and photocatalytic properties of new Bi2SbVO7 under visible light irradiation. Phys. Chem. Chem. Phys. 2009, 11, 6289–6298. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.G.; Ye, J.H.; Arakawa, H. Photophysical and photocatalytic properties of InMO4 (M = Nb5+, Ta5+) under visible light irradiation. Mater. Res. Bull. 2001, 36, 1185–1193. [Google Scholar] [CrossRef]
- Luan, J.F.; Tan, W.C. Preparation, Photophysical and Photocatalytic Property Characterization of Sm2FeSbO7 during Visible Light Irradiation. Chinese. J. Inorg. Chem. 2018, 34, 1950–1965. [Google Scholar]
- Li, K.; Ji, M.X.; Chen, R.; Jiang, Q.; Xia, J.X.; Li, H.M. Construction of nitrogen and phosphorus co-doped graphene quantum dots/Bi5O7I composites for accelerated charge separation and enhanced photocatalytic degradation performance. Chinese J. Catal. 2020, 41, 1230–1239. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef]
- Wang, J.H.; Zou, Z.G.; Ye, J.H. Synthesis, Structure and Photocatalytic Property of a New Hydrogen Evolving Photocatalyst Bi2InTaO7. Mater. Sci. Forum 2003, 423–425, 485–490. [Google Scholar] [CrossRef]
- Kohno, M.; Ogura, S.; Sato, K.; Inoue, Y. Properties of photocatalysts with tunnel structures: Formation of a surface lattice O− radical by the UV irradiation of BaTi4O9 with a pentagonal-prism tunnel structure. Chem. Phys. Lett. 1997, 267, 72–76. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water Splitting into H2 and O2 on New Sr2M2O7 (M = Nb and Ta) Photocatalysts with Layered Perovskite Structures: Factors Affecting the Photocatalytic Activity. J. Phys. Chem. B 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Guo, N.; Liu, H.Y.; Fu, Y.; Hu, J.S. Preparation of Fe2O3 nanoparticles doped with In2O3 and photocatalytic degradation property for rhodamine B. Optik 2020, 201, 163537. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis with YFeO3 electrodes. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Cui, B.Y.; Cui, H.T.; Li, Z.R.; Dong, H.Y.; Li, X.; Zhao, L.F.; Wang, J.W. Novel Bi3O5I2 hollow microsphere and its enhanced photocatalytic activity. Catalysts 2019, 9, 709. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 2020, 10, 528. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Patil, S.S.; Tamboli, M.S.; Ambekar, J.D.; Kulkarni, M.V.; Panmand, R.P.; Umarji, G.G.; Shinde, M.D.; Rane, S.B.; Munirathnam, N.R.; et al. Growth study of hierarchical Ag3PO4/LaCO3OH heterostructures. Phys. Chem. Chem. Phys. 2017, 19, 20541–20550. [Google Scholar] [CrossRef]
- Patil, S.S.; Tamboli, M.S.; Deonikar, V.G.; Umarji, G.G.; Ambekar, J.D.; Kulkarni, M.V.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Magnetically separable Ag3PO4/NiFe2O4 composites with enhanced photocatalytic activity. Dalton T 2015, 44, 20426–20434. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.Y.; Chen, C.; Zhou, H.F.; Wang, Y.P. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhou, M.; Gu, J.; Li, X. Spectrophotometric and high performance liquid chromatographic methods for sensitive determination of bisphenol A. Spectrochim. Acta A 2013, 122, 153–157. [Google Scholar] [CrossRef] [PubMed]






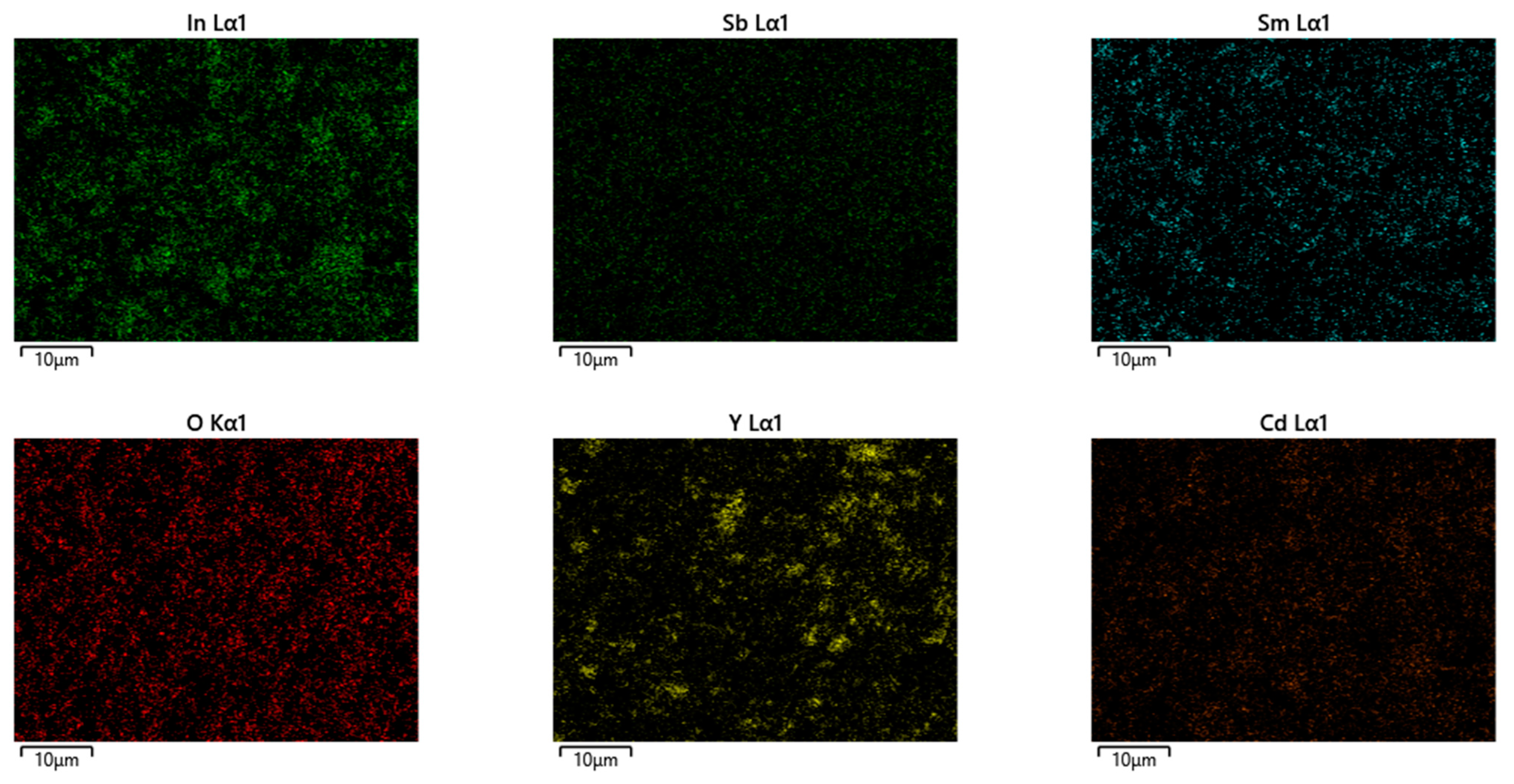


| Atom | x | y | z | Occupation Factor |
|---|---|---|---|---|
| Y | 0 | 0 | 0 | 1 |
| Sm | 0.5 | 0.5 | 0.5 | 0.5 |
| Sb | 0.5 | 0.5 | 0.5 | 0.5 |
| O (1) | −0.185 | 0.125 | 0.125 | 1 |
| O (2) | 0.125 | 0.125 | 0.125 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Liu, W.; Yang, G.; Niu, B.; Ma, B. Synthesis and Analysis of In2CdO4/Y2SmSbO7 Nanocomposite for the Photocatalytic Degradation of Rhodamine B within Dye Wastewater under Visible Light Irradiation. Catalysts 2023, 13, 608. https://doi.org/10.3390/catal13030608
Luan J, Liu W, Yang G, Niu B, Ma B. Synthesis and Analysis of In2CdO4/Y2SmSbO7 Nanocomposite for the Photocatalytic Degradation of Rhodamine B within Dye Wastewater under Visible Light Irradiation. Catalysts. 2023; 13(3):608. https://doi.org/10.3390/catal13030608
Chicago/Turabian StyleLuan, Jingfei, Wenlu Liu, Guangmin Yang, Bowen Niu, and Bingbing Ma. 2023. "Synthesis and Analysis of In2CdO4/Y2SmSbO7 Nanocomposite for the Photocatalytic Degradation of Rhodamine B within Dye Wastewater under Visible Light Irradiation" Catalysts 13, no. 3: 608. https://doi.org/10.3390/catal13030608
APA StyleLuan, J., Liu, W., Yang, G., Niu, B., & Ma, B. (2023). Synthesis and Analysis of In2CdO4/Y2SmSbO7 Nanocomposite for the Photocatalytic Degradation of Rhodamine B within Dye Wastewater under Visible Light Irradiation. Catalysts, 13(3), 608. https://doi.org/10.3390/catal13030608










