First Application of Nitrogen-Doped Carbon Nanosheets Derived from Lotus Leaves as the Electrode Catalyst for Li-CO2/O2 Battery
Abstract
1. Introduction
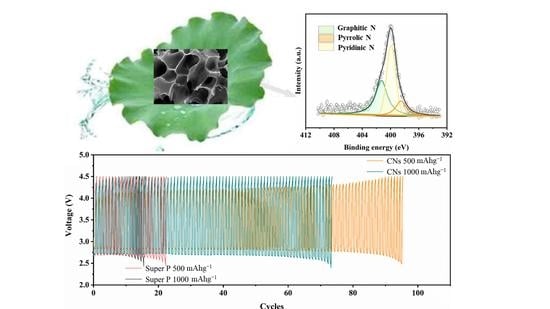
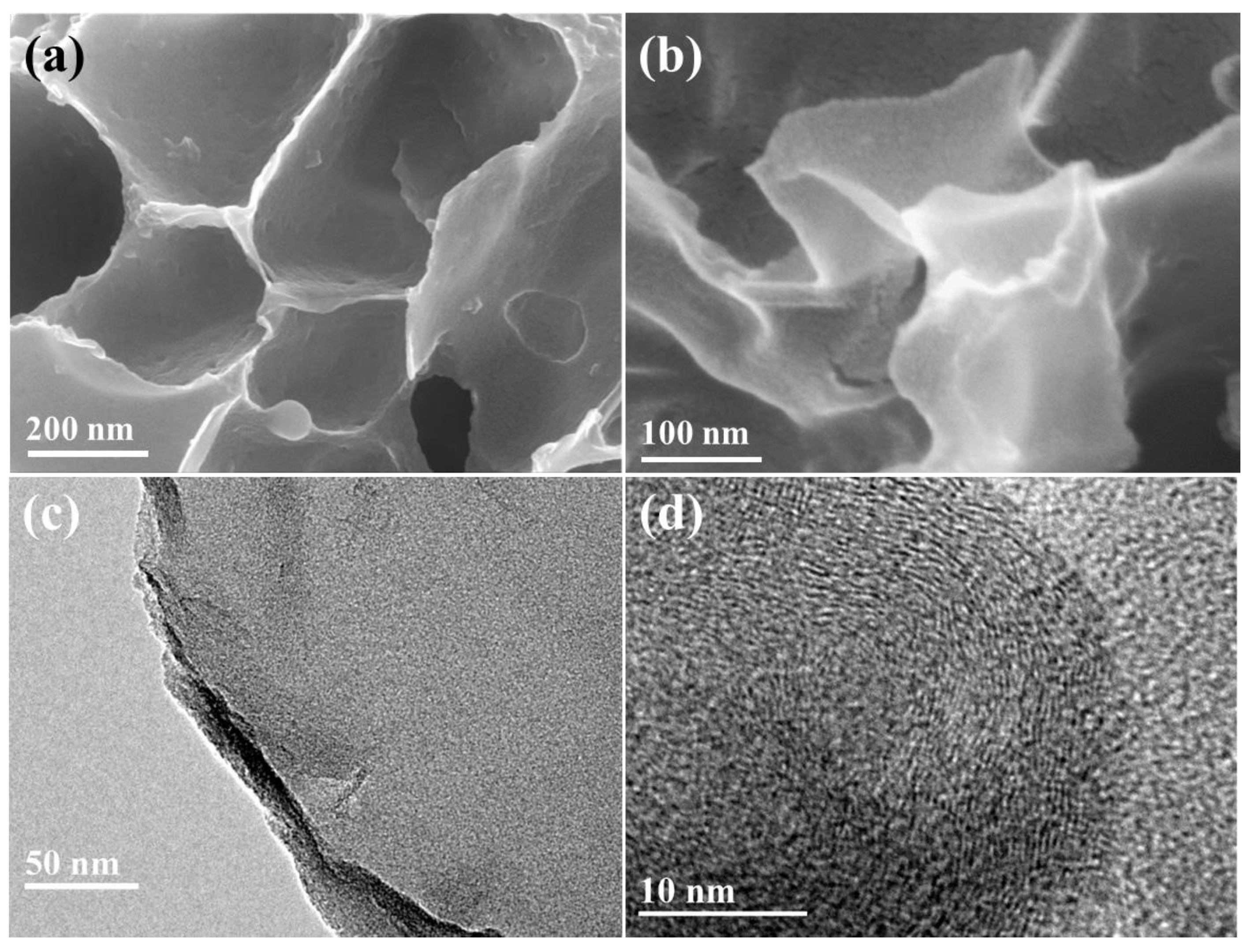
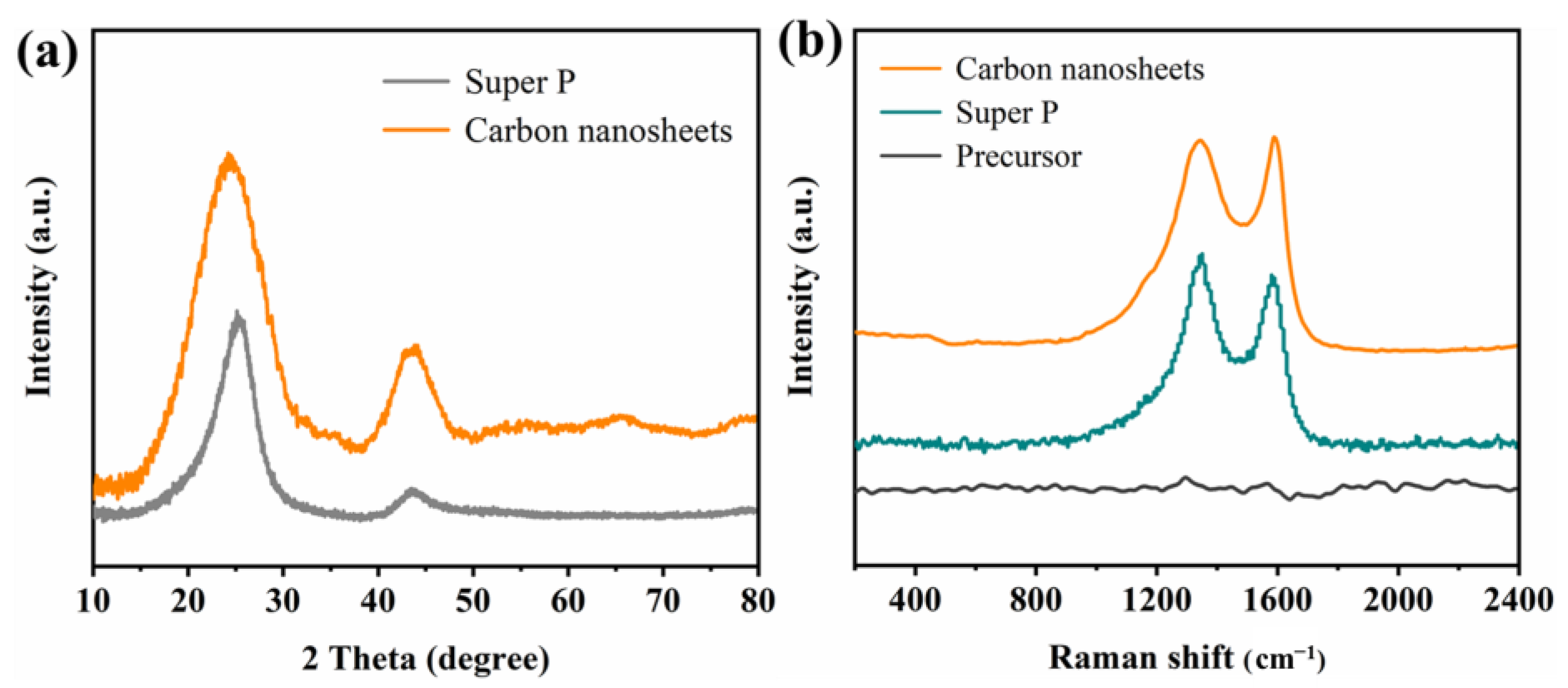
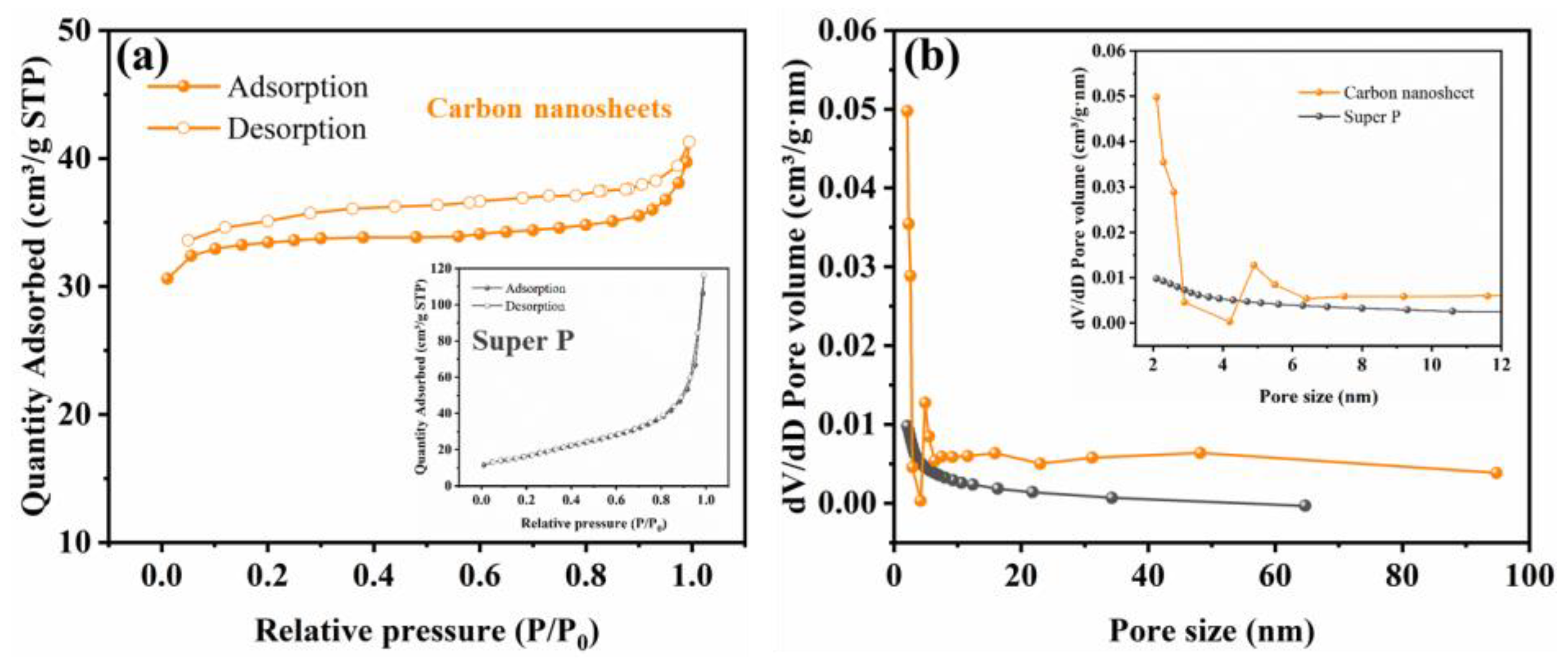
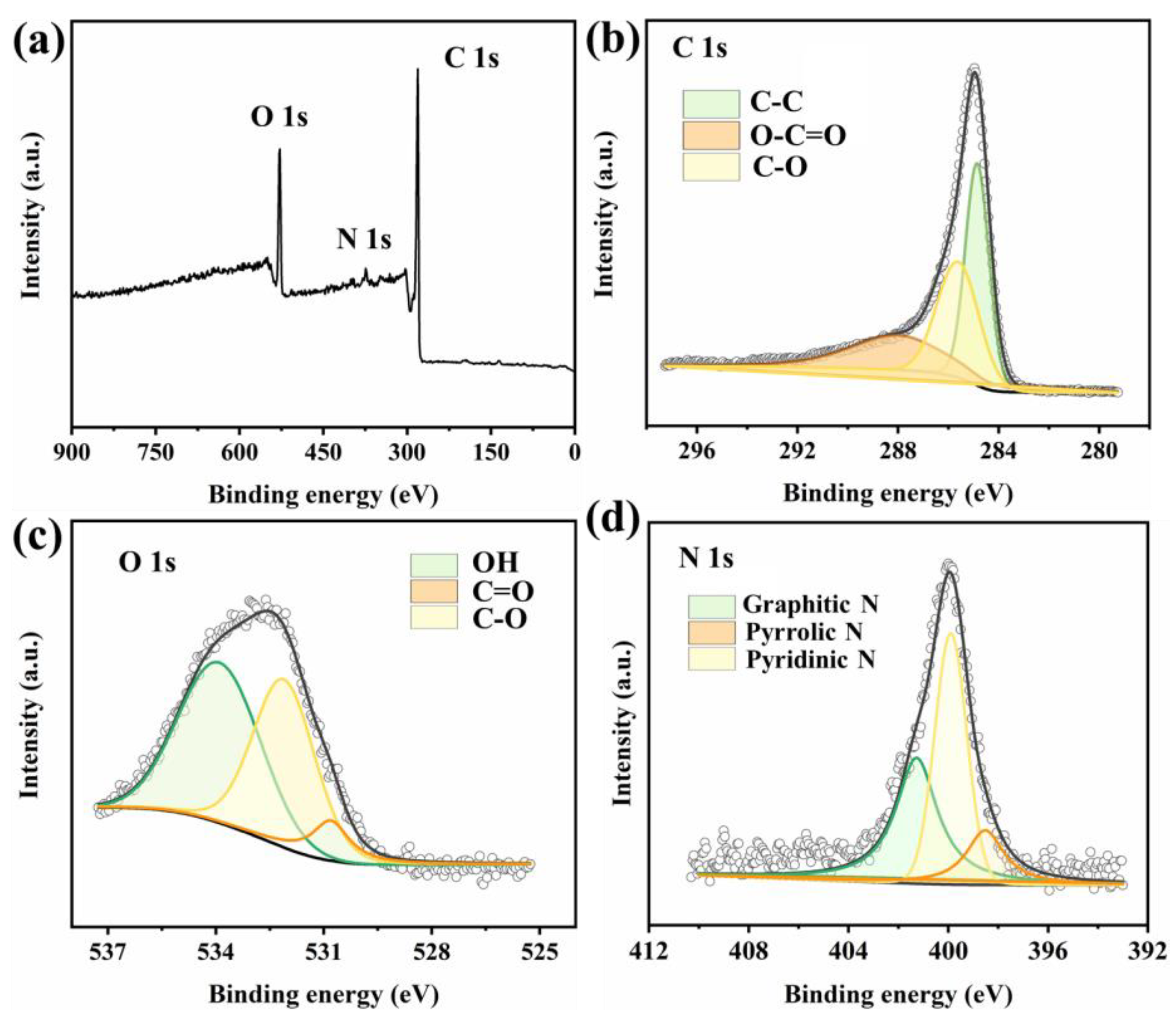
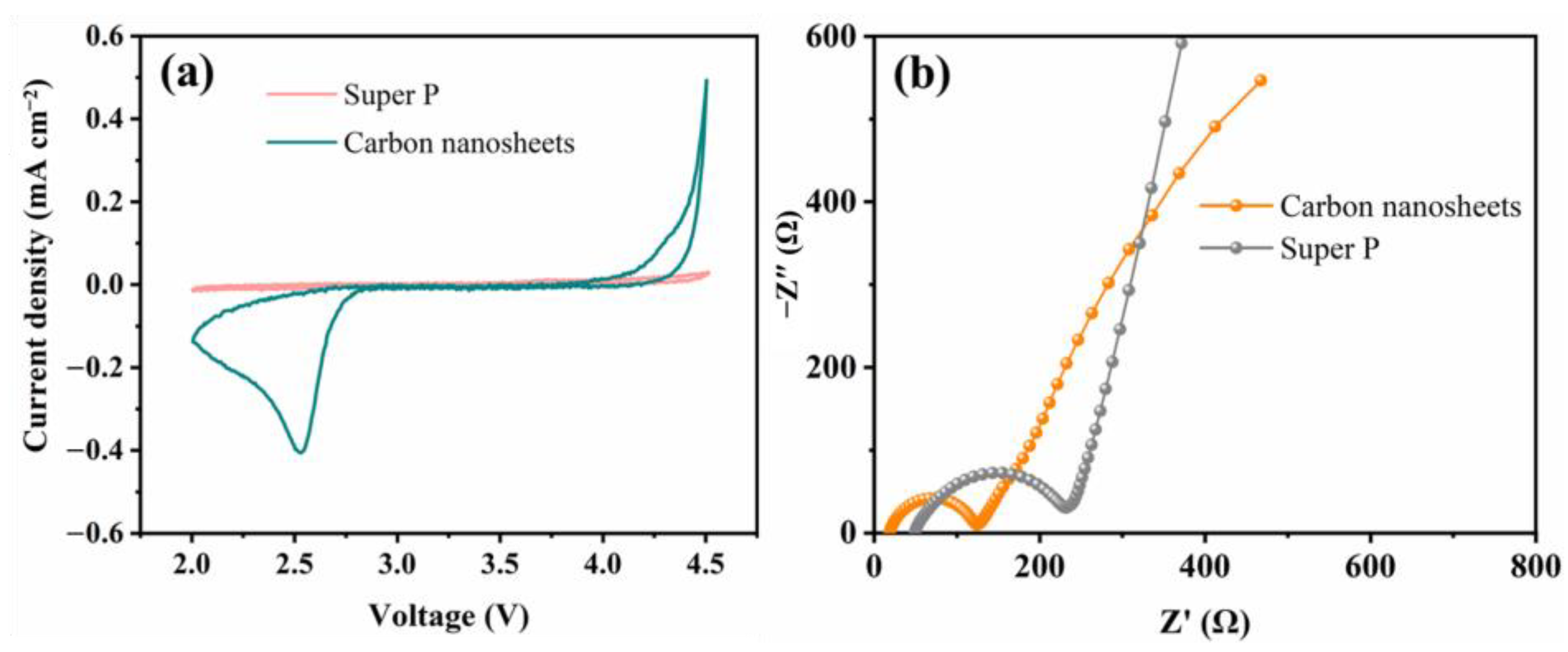
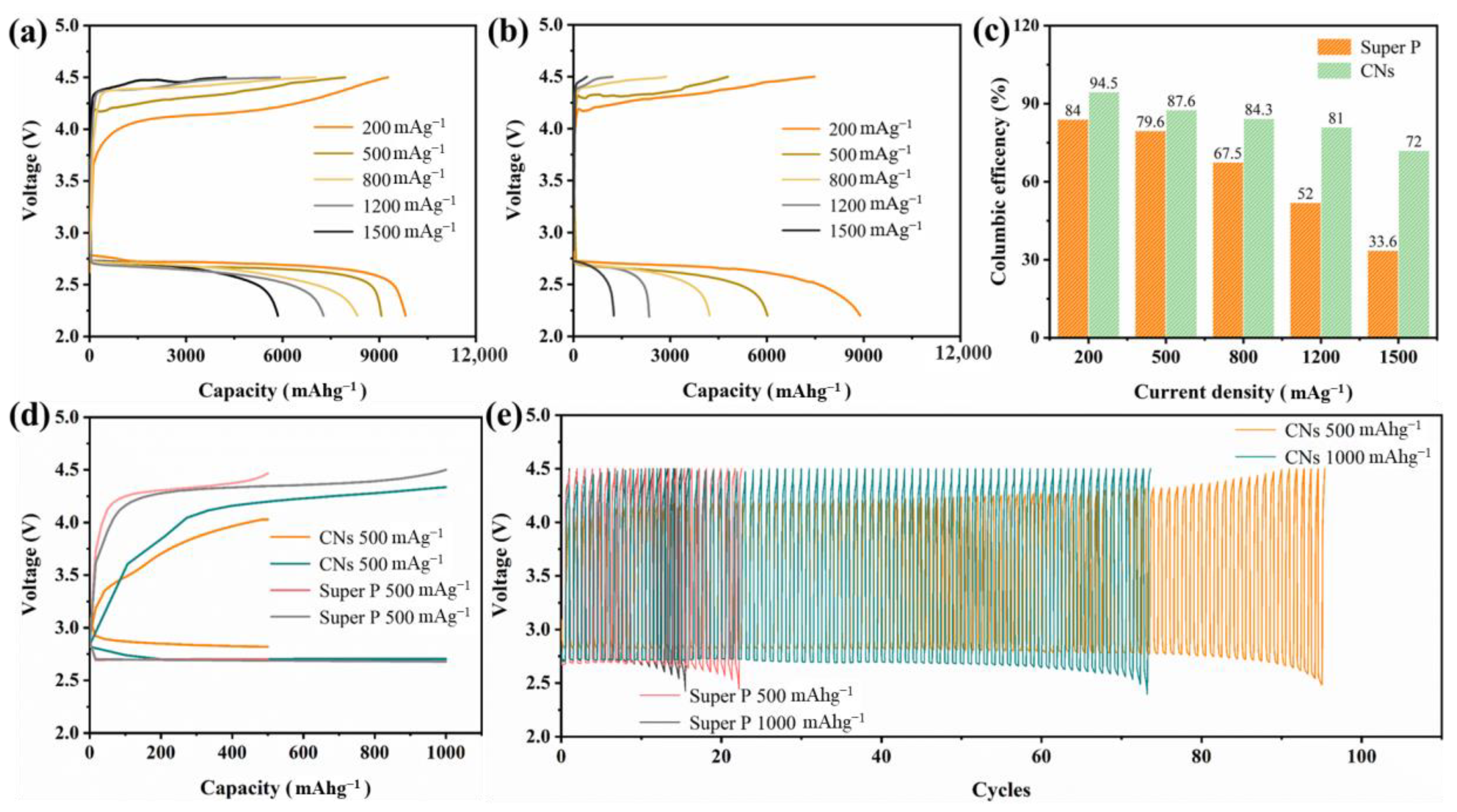
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Carbon Nanosheets
3.2. Characterization
3.3. Cathode Preparation and Battery Assembly
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, Z. The underground economy and carbon dioxide (CO2) emissions in China. Sustainability 2019, 11, 2802. [Google Scholar] [CrossRef]
- Qiao, Y.; Yi, J.; Wu, S.; Liu, Y.; Yang, S.; He, P.; Zhou, H. Li-CO2 electrochemistry: A new strategy for CO2 fixation and energy storage. Joule 2017, 1, 359–370. [Google Scholar] [CrossRef]
- Xu, S.; Das, S.K.; Archer, L.A. The Li–CO2 battery: A novel method for CO2 capture and utilization. RSC Adv. 2013, 3, 6656. [Google Scholar] [CrossRef]
- Takechi, K.; Shiga, T.; Asaoka, T. A Li-O2/CO2 battery. Chem. Commun. 2011, 47, 3463–3465. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Lim, H.D.; Park, K.Y.; Seo, D.H.; Gwon, H.; Hong, J.; Goddard, W.A.; Kim, H.; Kang, K. Toward a lithium-“air” battery: The effect of CO2 on the chemistry of a lithium-oxygen cell. J Am Chem Soc. 2013, 135, 9733–9742. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, Y.B.; Ma, J.L.; Chang, Z.W.; Sun, T.; Zhu, Y.H.; Zhang, X.-B. Designing a self-healing protective film on a lithium metal anode for long-cycle-life lithium-oxygen batteries. Energy Storage Mater. 2019, 18, 382–388. [Google Scholar] [CrossRef]
- Chen, K.; Yang, D.-Y.; Wang, J.; Huang, G.; Zhang, X.-B. Overcharge to remove cathode passivation layer for reviving failed Li-O2 batteries. Chin. Chem. Soc. 2022, 5, 641–653. [Google Scholar]
- Qiao, Y.; Yi, J.; Guo, S.; Sun, Y.; Wu, S.; Liu, X.; Yang, S.; He, P.; Zhou, H. Li2CO3-free Li–O2/CO2 battery with peroxide discharge product. Energy Environ. Sci. 2018, 11, 1211–1217. [Google Scholar] [CrossRef]
- Jiao, Y.; Qin, J.; Sari, H.M.K.; Li, D.; Li, X.; Sun, X. Recent progress and prospects of Li-CO2 batteries: Mechanisms, catalysts and electrolytes. Energy Storage Mater. 2021, 34, 148–170. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Qi, G.; Cheng, J.; Wang, B. Vertically aligned N-doped carbon nanotubes arrays as efficient binder-free catalysts for flexible Li-CO2 batteries. Energy Storage Mater. 2021, 35, 148–156. [Google Scholar] [CrossRef]
- Chen, K.; Huang, G.; Ma, J.; Wang, J.; Yang, D.; Yang, X.; Zhang, X.B. The stabilization effect of CO2 in lithium–oxygen/CO2 batteries. Angew. Chem. Int. Ed. 2020, 59, l6661–l16667. [Google Scholar]
- Wang, J.; Hao, Q.; Zhong, H.; Li, K.; Zhang, X.B. Ligand centered electrocatalytic efficient CO2 reduction reaction at low overpotential on single-atom Ni regulated molecular catalyst. Nano Res. 2022, 15, 5816–5823. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhuo, H.Y.; Dong, Y.Y.; Zhou, Y.; Li, J.T. Pt nanoparticles confined in a 3D porous FeNC matrix as efficient catalysts for rechargeable Li-CO2/O2 batteries. ACS Appl. Mater. Interfaces 2023, 15, 2940–2950. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Sheng, T.; Zhou, Y.; Wu, Y.-J.; Xiang, C.-C.; Lin, J.-X.; Li, Y.-Y.; Li, J.-T.; Sun, S.-G. Li-CO2/O2 battery operating at ultra-low overpotential and low O2 content on Pt/CNT catalyst. Chem. Eng. J. 2022, 448, 137541. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Zhang, J.-Y.; Sheng, T.; Lu, Y.-Q.; Yin, Z.-W.; Peng, Y.-Y.; Zhou, X.-X.; Wu, J.-T.; Lin, Y.-J.; Xu, J.-X.; et al. Synergetic effect of Ru and NiO in the electrocatalytic decomposition of Li2CO3 to enhance the performance of a Li-CO2/O2 battery. ACS Catal. 2020, 10, 1640–1651. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Lu, Y.-Q.; Wu, Y.-J.; Yin, Z.-W.; Li, J.-T.; Zhou, Y.; Hong, Y.-H.; Li, Y.-Y.; Huang, L.; Sun, S.-G. High-performance rechargeable Li-CO2/O2 battery with Ru/N-doped CNT catalyst. Chem. Eng. J. 2019, 363, 224–233. [Google Scholar] [CrossRef]
- Kim, C.H.J.; Varanasi, C.V.; Liu, J. Synergy of polypyrrole and carbon x-aerogel in lithium-oxygen batteries. Nanoscale 2018, 10, 3753–3758. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Yao, Y.; Wu, F.; Yu, Y.; Zhang, C. New application of waste citrus maxima peel-derived carbon as an oxygen electrode material for lithium oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 32058–32066. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, S.; Kweon, H.; Moon, S.; Lee, C.H.; Kim, H. Preparation of graphene hollow spheres from vacuum residue of ultra-heavy oil as an effective oxygen electrode for Li–O2 batteries. J. Mater. Chem. A 2018, 6, 4040–4047. [Google Scholar] [CrossRef]
- Liu, K.; Li, Z.; Xie, W.; Li, J.; Rao, D.; Shao, M.; Zhang, B.; Wei, M. Oxygen-rich carbon nanotube networks for enhanced lithium metal anode. Energy Storage Mater. 2018, 15, 308–314. [Google Scholar] [CrossRef]
- Luo, J.; Yao, X.; Yang, L.; Han, Y.; Chen, L.; Geng, X.; Vattipalli, V.; Dong, Q.; Fan, W.; Wang, D.; et al. Free-standing porous carbon electrodes derived from wood for high-performance Li-O2 battery applications. Nano Res. 2017, 10, 4318–4326. [Google Scholar] [CrossRef]
- Shen, J.; Wu, H.; Sun, W.; Qiao, J.; Cai, H.; Wang, Z.; Sun, K. In-situ nitrogen-doped hierarchical porous hollow carbon spheres anchored with iridium nanoparticles as efficient cathode catalysts for reversible lithium-oxygen batteries. Chem. Eng. J. 2019, 358, 340–350. [Google Scholar] [CrossRef]
- Wang, M.; Yao, Y.; Tang, Z.; Zhao, T.; Wu, F.; Yang, Y.; Huang, Q. Self-nitrogen-doped carbon from plant waste as an oxygen electrode material with exceptional capacity and cycling stability for lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 32212–32219. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, Y.; Li, J.; Xie, R.; Deng, Z.; Chen, H.; Li, H. Characterization and antioxidant activities of procyanidins from lotus seedpod, mangosteen pericarp, and camellia flower. Int. J. Food Prop. 2017, 20, 1621–1632. [Google Scholar] [CrossRef]
- Cheng, C.; Guo, R.; Lan, J.; Jiang, S.; Du, Z.; Zhao, L.; Peng, L. Preparation and characterization of lotus fibers from lotus stems. J. Text. Inst. 2018, 109, 1322–1328. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Lauermann, I.; Sarhan, R.M.; Roth, C. Hierarchically structured iron-doped silver (Ag-Fe) lotus flowers for an efficient oxygen reduction reaction. Nanoscale 2018, 10, 7304–7310. [Google Scholar] [CrossRef]
- Liu, F.; Qin, M.; Min, J.; Liu, Q.; Zhang, D.; Chen, Q.; Liu, L.; Tian, D. Effects of parent species type, flower color, and stamen petaloidy on the fruit-setting rate of hybridization and selfing in lotus (Nelumbo). Aquatic Botany 2021, 172, 103396. [Google Scholar] [CrossRef]
- Zang, D.; Xun, X.; Gu, Z.; Dong, J.; Pan, T.; Liu, M. Fabrication of superhydrophobic self-cleaning manganese dioxide coatings on Mg alloys inspired by lotus flower. Ceram. Int. 2020, 46, 20328–20334. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, L.; Deng, H.; Wang, J.; Yao, L.; Deng, L. Ultrahigh surface area carbon nanosheets derived from lotus leaf with super capacities for capacitive deionization and dye adsorption. Appl. Surf. Sci. 2020, 524, 146485. [Google Scholar] [CrossRef]
- Liu, S.; Yang, P.; Wang, L.; Li, Y.; Wu, Z.; Ma, R.; Wu, J.; Hu, X. Nitrogen-doped porous carbons from lotus leaf for CO2 capture and supercapacitor electrodes. Energy Fuels 2019, 33, 6568–6576. [Google Scholar] [CrossRef]
- Qu, S.; Wan, J.; Dai, C.; Jin, T.; Ma, F. Promising as high-performance supercapacitor electrode materials porous carbons derived from biological lotus leaf. J. Alloys Compd. 2018, 751, 107–116. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; He, W.; Wei, C.; Cheng, Q.; Li, C. In situ synthesis of biocarbon coated Li3V2(PO4)3 cathode material using lotus leaf as carbon source. J. Mater. Sci. Mater. Electron. 2016, 27, 12610–12617. [Google Scholar] [CrossRef]
- Ye, W.; Tang, J.; Wang, Y.; Cai, X.; Liu, H.; Lin, J.; Van der Bruggen, B.; Zhou, S. Hierarchically structured carbon materials derived from lotus leaves as efficient electrocatalyst for microbial energy harvesting. Sci Total Environ. 2019, 666, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, H.; Sun, H. Heteroatom (N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications. Adv. Funct. Mater. 2016, 26, 8651–8661. [Google Scholar] [CrossRef]
- Zou, L.; Jiang, Y.; Cheng, J.; Wang, Z.; Jia, L.; Chi, B.; Pu, J.; Jian, L. High-capacity and long-cycle lifetime Li−CO2/O2 battery based on dandelion-like NiCo2O4 hollow microspheres. ChemCatChem. 2019, 11, 3117–3124. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, L.; Kong, W.; Peng, L.; Wang, F. First Application of Nitrogen-Doped Carbon Nanosheets Derived from Lotus Leaves as the Electrode Catalyst for Li-CO2/O2 Battery. Catalysts 2023, 13, 577. https://doi.org/10.3390/catal13030577
Zou L, Kong W, Peng L, Wang F. First Application of Nitrogen-Doped Carbon Nanosheets Derived from Lotus Leaves as the Electrode Catalyst for Li-CO2/O2 Battery. Catalysts. 2023; 13(3):577. https://doi.org/10.3390/catal13030577
Chicago/Turabian StyleZou, Lu, Weilin Kong, Linfeng Peng, and Fang Wang. 2023. "First Application of Nitrogen-Doped Carbon Nanosheets Derived from Lotus Leaves as the Electrode Catalyst for Li-CO2/O2 Battery" Catalysts 13, no. 3: 577. https://doi.org/10.3390/catal13030577
APA StyleZou, L., Kong, W., Peng, L., & Wang, F. (2023). First Application of Nitrogen-Doped Carbon Nanosheets Derived from Lotus Leaves as the Electrode Catalyst for Li-CO2/O2 Battery. Catalysts, 13(3), 577. https://doi.org/10.3390/catal13030577