Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions
Abstract
1. Introduction
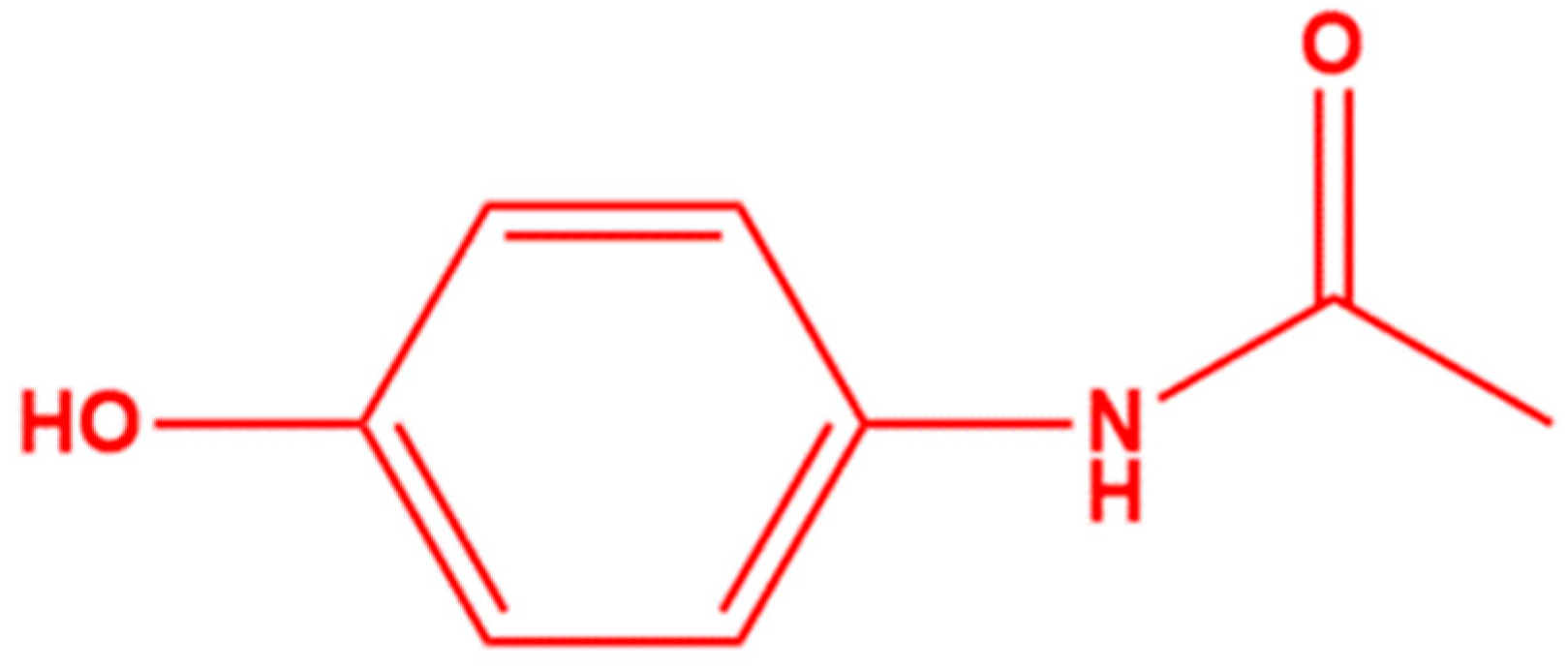
1.1. Pharmaceutical Pollution
1.2. Pharmaceutical Removal Techniques
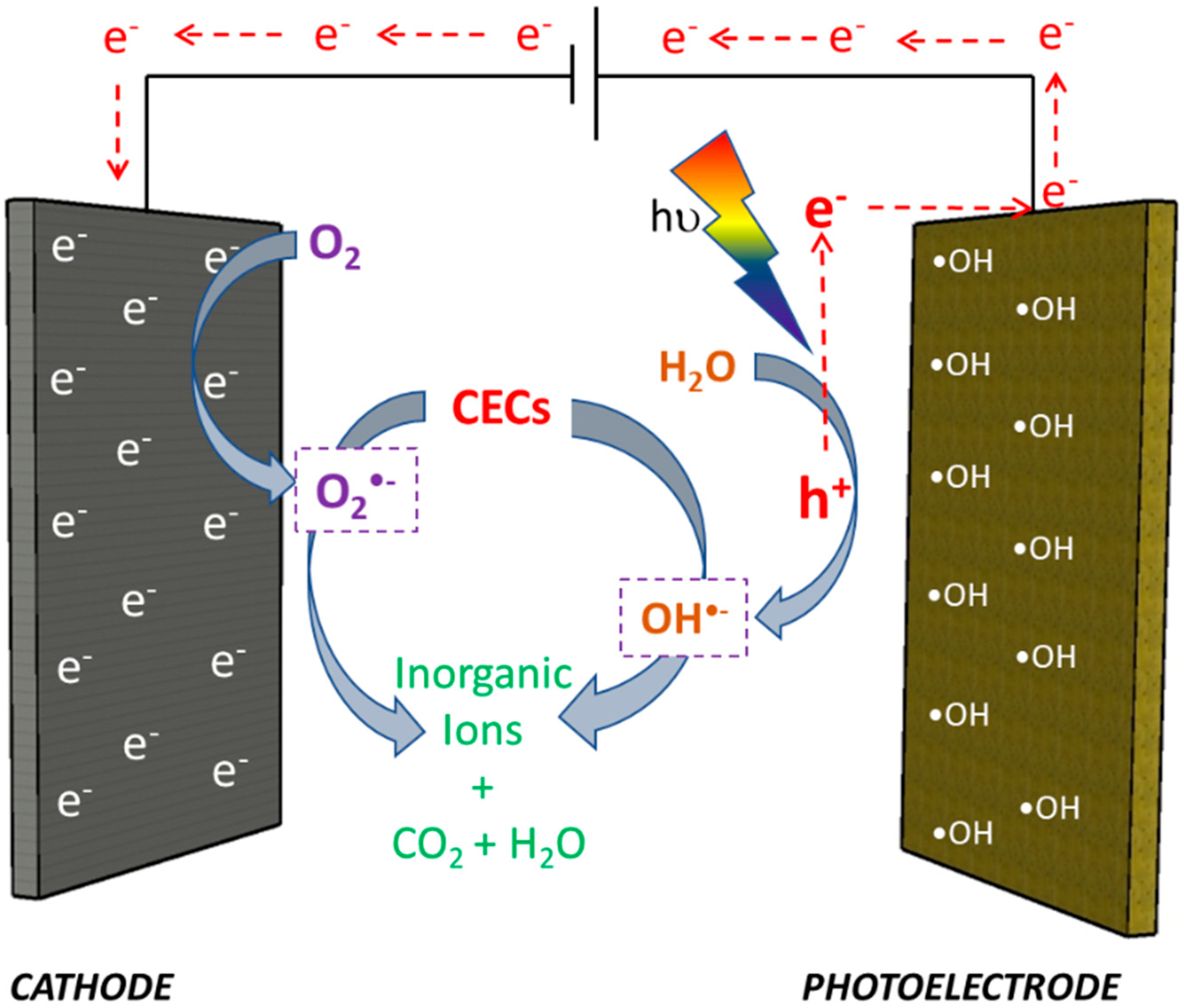
1.3. Photoelectrodes for PEC Treatment of Pharmaceuticals
1.3.1. TiO2-Based Photoelectrodes
1.3.2. Bismuth Vanadate (BiVO4)-Based Photoelectrodes
| Photoelectrode | Conditions | Degradation Time | Removal Efficiency (%) | TOC Removal (%) | Ref. |
|---|---|---|---|---|---|
| BiVO4/BiOI | PEC oxidation 0.1 mg·L−1 Na2SO4 Cathode: Pt foil 10 mg·L−1 PTM solution 10 mg·L−1 ciprofloxacin solution Xe lamp 100 W Visible irradiation | 120 min | 62 | 44 | [61] |
| BiVO4 | 0.1 mg·L−1 Na2SO4 Cathode: Pt foil 10 mg·L−1 PTM solution Xe lamp 100 W Visible irradiation | 120 min | 68 | 59 | |
| BiOI | 120 min | 44 | - | ||
| 120 min | 53 | - | |||
| FTO-BiVO4/BiOI | PEC oxidation 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) Current density: 5 mA cm−2 | 180 min | 76 | - | [52] |
| Current density: 10 mA cm−2 | 180 min | 98 | 59 | ||
| Current density: 15 mA cm−2 | 180 min | 98 | - | ||
| Current density: 20 mA cm−2 | 180 min | 56 | 32 | ||
| EC + EF oxidation 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) 0.2 mmol·L−1 FeSO47H2O Current density: 10 mA cm−2 | 180 min | 56 | 73 | ||
| PEC + EF oxidation 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: (TCF) 0.2 mmol·L−1 FeSO47H2O 150 W linear Halogen lamp (VIS, 420–600 nm) Current density: 10 mA cm−2 Constant O2 saturation | 120 min | 98 | 92 | ||
| FTO-BiVO4/BiOI | EF oxidation 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) 0.2 mmol·L−1 FeSO47H2O Current density: 20 mA cm−2 Constant O2 saturation | 240 min | 28 | 71 | [52] |
| 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) 0.2 mmol·L−1 FeSO47H2O Current density: 10 mA cm−2 Constant O2 saturation | 240 min | - | 28 | ||
| 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Carbon Felt (CF) 0.2 mmol·L−1 FeSO47H2O Current density: 20 mA cm−2 Constant O2 saturation | 240 min | - | 27 | ||
| EC ox. 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) Current density: 10 mA cm−2 | 120 min | 61 | 40 | ||
| PC oxidation 50 mmol·L−1 Na2SO4, pH: 3 0.1 mmol·L−1 PTM solution Cathode: Thermally treated-Carbon Felt (TCF) 150 W linear Halogen lamp (VIS, 420–600 nm) | 120 min | 13 | - |
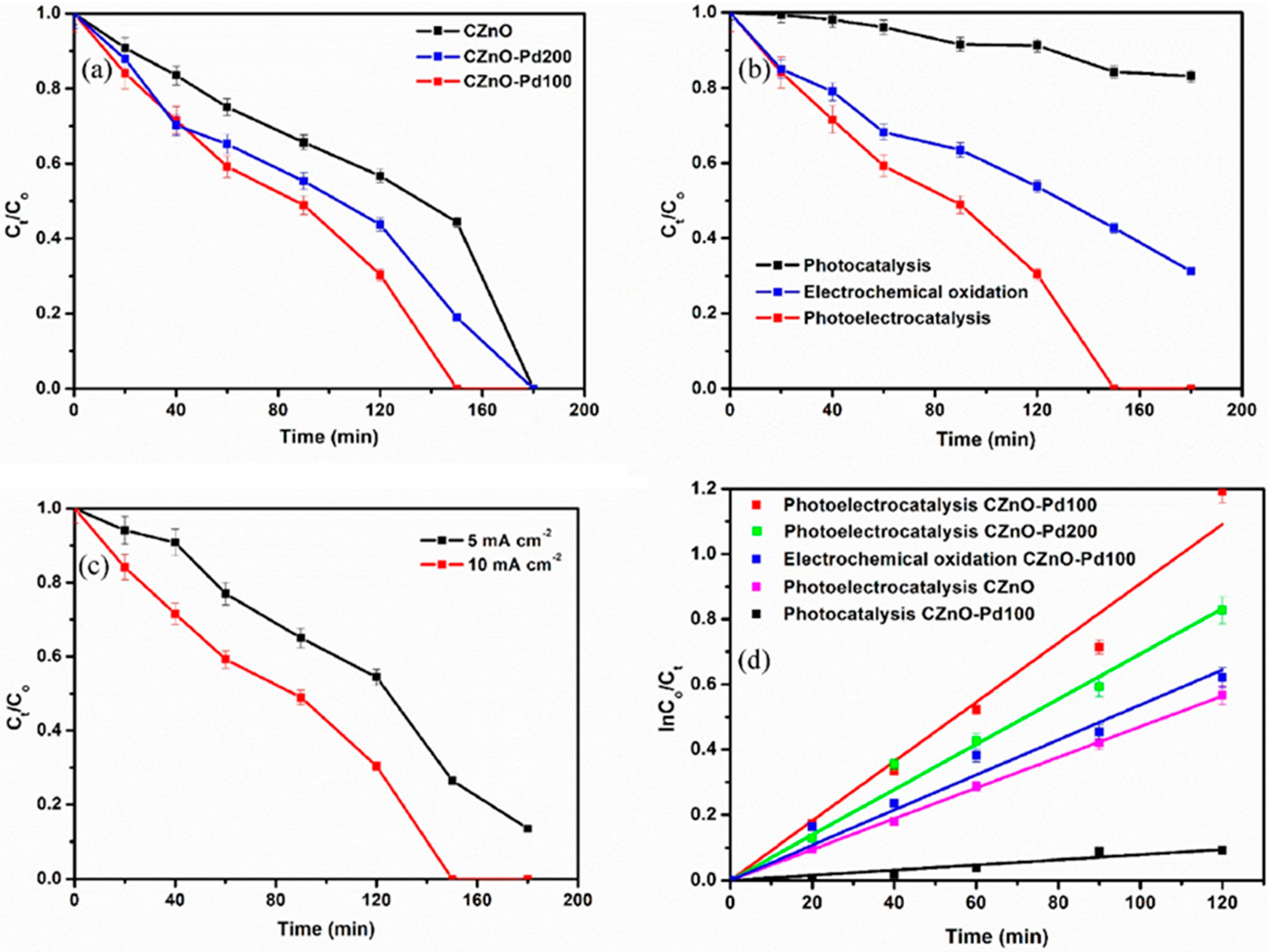
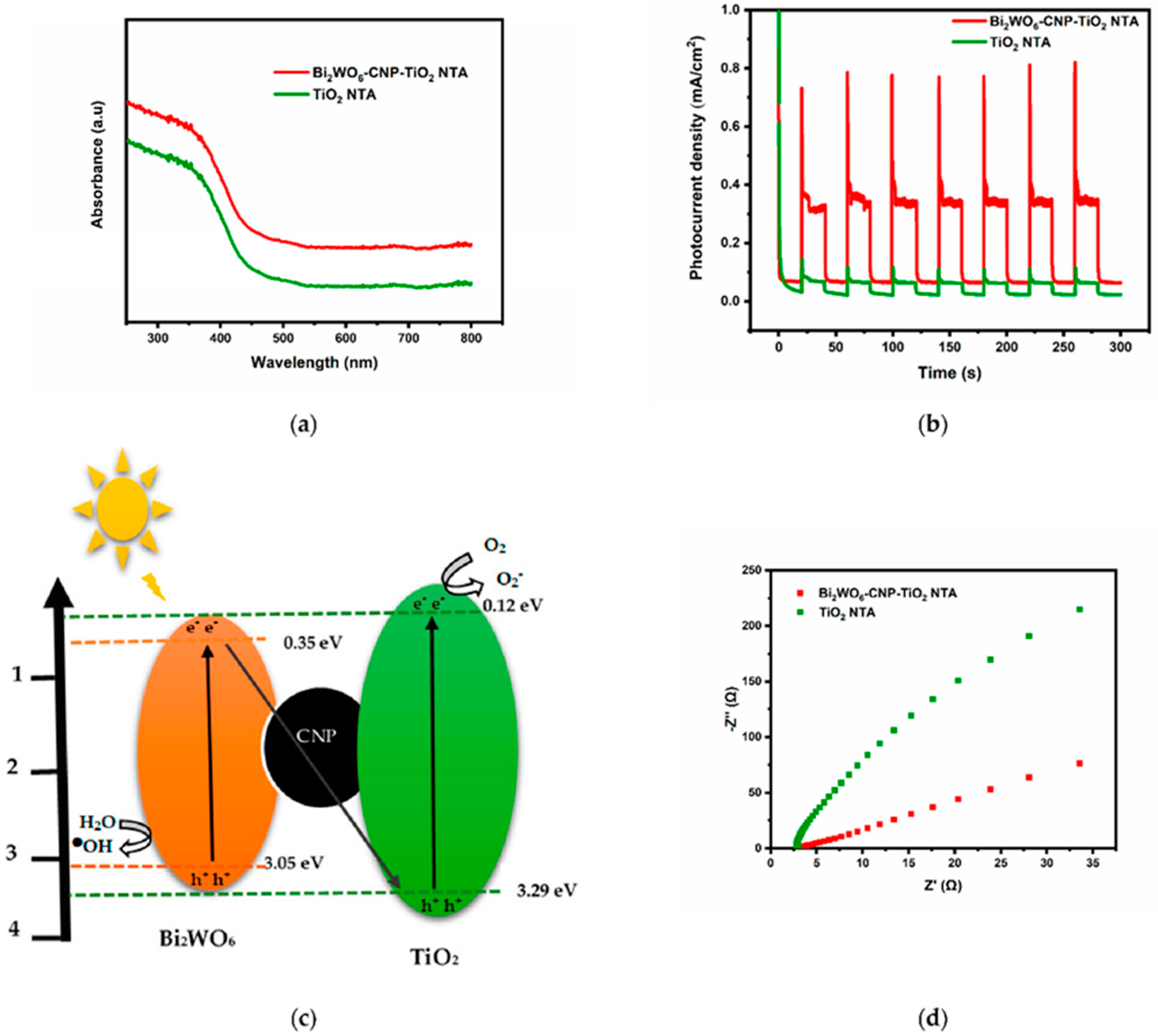
1.3.3. Zinc-Oxide-Based Photoanodes
| Photoelectrode | Conditions | Degradation Time | Removal Efficiency (%) | TOC Removal (%) | Ref. |
|---|---|---|---|---|---|
| Bi2WO6-CNP-TiO2 NTA | EC ox. 0.1 mol·L−1 Na2SO4, pH: 7 5 mg·L−1 PTM solution Cathode: Pt foil Current density: 10 mA cm−2 | 180 min | 57 | 30 | [63] |
| PC ox. 0.1 mol·L−1 Na2SO4 5 mg·L−1 PTM solution LCS-100 W solar simulator UV cut-off (λ < 400 nm) | 180 min | 42 | 22 | ||
| PEC ox. 0.1 mol·L−1 Na2SO4, 5 mg·L−1 PTM solution Cathode: Pt foil Current density: 10 mA cm−2 LCS-100 W solar simulator UV cut-off (λ < 400 nm) pH: 7 | 180 min | 84 | 72 | ||
| pH: 2 | 180 min | 4 | - | ||
| pH: 10 | 180 min | 18 | - |
1.3.4. Tungsten-Oxide-Based Photoelectrodes
2. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CECs | Contaminants of emerging concern |
| PEC | Photoelectrocatalytic |
| PTM | Paracetamol |
| APIs | Active pharmaceutical ingredients |
| AOPs | Advanced Oxidation Processes |
| EAOPs | Electrochemical Advanced Oxidation Processes |
| EC | Electrocatalytic |
| PC | Photocatalytic |
| PE | Photoelectrode |
| EF | Electro-Fenton |
| PEF | photo-Electro-Fenton |
| SMC | Semiconductor |
| SSP | Stainless-steel plate |
| C-PTFE | Carbon-polytetrafluoroethylene |
| CNP | Carbon nanoparticles |
| NTA | nanotube arrays |
References
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Sousa, H.; Sousa, C.A.; Simões, L.C.; Simões, M. Microalgal-Based Removal of Contaminants of Emerging Concern. J. Hazard. Mater. 2022, 423, 127153. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and Health Impacts of Contaminants of Emerging Concerns: Recent Treatment Challenges and Approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, S.R. Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review. Membranes 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and Removal of Pharmaceutical and Personal Care Products from Aquatic Systems Using Advanced Treatment—A Review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A Versatile Strategy to Eliminate Emerging Contaminants from the Aqueous Environment: Heterogeneous Fenton Process. J. Clean. Prod. 2021, 278, 124014. [Google Scholar] [CrossRef]
- Akerman-Sanchez, G.; Rojas-Jimenez, K. Fungi for the Bioremediation of Pharmaceutical-Derived Pollutants: A Bioengineering Approach to Water Treatment. Environ. Adv. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Kroon, F.J.; Berry, K.L.E.; Brinkman, D.L.; Kookana, R.; Leusch, F.D.L.; Melvin, S.D.; Neale, P.A.; Negri, A.P.; Puotinen, M.; Tsang, J.J.; et al. Sources, Presence and Potential Effects of Contaminants of Emerging Concern in the Marine Environments of the Great Barrier Reef and Torres Strait, Australia. Sci. Total Environ. 2020, 719, 135140. [Google Scholar] [CrossRef]
- Maruya, K.A.; Schlenk, D.; Anderson, P.D.; Denslow, N.D.; Drewes, J.E.; Olivieri, A.W.; Scott, G.I.; Snyder, S.A. An Adaptive, Comprehensive Monitoring Strategy for Chemicals of Emerging Concern (CECs) in California’s Aquatic Ecosystems. Integr. Environ. Assess. Manag. 2014, 10, 69–77. [Google Scholar] [CrossRef]
- González-González, R.B.; Sharma, P.; Singh, S.P.; Américo-Pinheiro, J.H.P.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Persistence, Environmental Hazards, and Mitigation of Pharmaceutically Active Residual Contaminants from Water Matrices. Sci. Total Environ. 2022, 821, 153329. [Google Scholar] [CrossRef]
- Coetsier, C.; Lin, L.; Roig, B.; Touraud, E. Integrated Approach to the Problem of Pharmaceutical Products in the Environment: An Overview. Anal. Bioanal. Chem. 2007, 387, 1163–1166. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Mehrotra, I.; Kumar, M. Prevalence of Organic Micropollutants in the Yamuna River, Delhi, India: Seasonal Variations and Governing Factors. Sci. Total Environ. 2023, 858, 159684. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.K.; Rehman, M.Y.A.; Malik, R.N. Fate and Toxicity of Pharmaceuticals in Water Environment: An Insight on Their Occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef] [PubMed]
- Baralla, E.; Demontis, M.P.; Dessì, F.; Varoni, M.V. An Overview of Antibiotics as Emerging Contaminants: Occurrence in Bivalves as Biomonitoring Organisms. Animals 2021, 11, 3239. [Google Scholar] [CrossRef]
- Amato, M.; Dasí, D.; González, A.; Ferrús, M.A.; Castillo, M.Á. Occurrence of Antibiotic Resistant Bacteria and Resistance Genes in Agricultural Irrigation Waters from Valencia City (Spain). Agric. Water Manag. 2021, 256, 107097. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.; Kolpin, D.W.; Leung, K.M.; Lai, R.W.; Galbán-Malagón, C.; Teta, C. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z.; Barka, N. Removal of Emerging Pharmaceutical Pollutants: A Systematic Mapping Study Review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Ranjit, P.; Jhansi, V.; Reddy, K.V. Conventional Wastewater Treatment Processes. In Advances in the Domain of Environmental Biotechnology; Maddela, N.R., García Cruzatty, L.C., Chakraborty, S., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Fouad, K.; Bassyouni, M.; Alalm, M.G.; Saleh, M.Y. The Treatment of Wastewater Containing Pharmaceuticals. J. Environ. Treat. Tech. 2021, 9, 499–504. [Google Scholar] [CrossRef]
- McKie, M.J.; Andrews, S.A.; Andrews, R.C. Conventional Drinking Water Treatment and Direct Biofiltration for the Removal of Pharmaceuticals and Artificial Sweeteners: A Pilot-Scale Approach. Sci. Total Environ. 2016, 544, 10–17. [Google Scholar] [CrossRef]
- Santos, J.E.L.; de Moura, D.C.; Cerro-López, M.; Quiroz, M.A.; Martínez-Huitle, C.A. Electro- and Photo-Electrooxidation of 2,4,5-Trichlorophenoxiacetic Acid (2,4,5-T) in Aqueous Media with PbO2, Sb-Doped SnO2, BDD and TiO2-NTs Anodes: A Comparative Study. J. Electroanal. Chem. 2020, 873, 114438. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at Pilot Scale: Energy Costs for Micro-Pollutants Oxidation, Disinfection by-Products Formation and Pathogens Inactivation. Chemosphere 2021, 273, 128527. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-narvaez, O.M.; Peralta-hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent Advances in Advanced Oxidation Processes for Removal of Contaminants from Water: A Comprehensive Review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.N.; Omidi, F.; Mousavi Khaneghah, A. A Review on Pollutants Removal by Sono-Photo -Fenton Processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Pourzamani, H.; Hajizadeh, Y.; Mengelizadeh, N. Application of Three-Dimensional Electrofenton Process Using MWCNTs-Fe3O4 Nanocomposite for Removal of Diclofenac. Process Saf. Environ. Prot. 2018, 119, 271–284. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Fathinia, M.; Karaca, S. Photocatalytic Ozonation of Ciprofloxacin from Aqueous Solution Using TiO2/MMT Nanocomposite: Nonlinear Modeling and Optimization of the Process via Artificial Neural Network Integrated Genetic Algorithm. Process Saf. Environ. Prot. 2018, 116, 365–376. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best Available Technologies and Treatment Trains to Address Current Challenges in Urban Wastewater Reuse for Irrigation of Crops in EU Countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- Dao, K.C.; Yang, C.C.; Chen, K.F.; Tsai, Y.P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-Dimensional Electrochemical Process for Wastewater Treatment: A General Review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Walsh, F.C.; Nava, J.L.; Ponce de León, C. Graphite Felt as a Versatile Electrode Material: Properties, Reaction Environment, Performance and Applications. Electrochim. Acta 2017, 258, 1115–1139. [Google Scholar] [CrossRef]
- Fu, J.; Ji, M.; Jin, L. Photocatalytic Oxidation of Natural Organic Matter-Fulvic Acid in Water. Chem. Bull. Huaxue Tongbao 2005, 68, 871–875. [Google Scholar]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO₂)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—An Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef]
- Amaterz, E.; Tara, A.; Bouddouch, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Photo-Electrochemical Degradation of Wastewaters Containing Organics Catalysed by Phosphate-Based Materials: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 843–872. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Reis, R.Y.N.; Goulart, L.A.; Mascaro, L.H.; Alves, S.A. A Critical View of the Contributions of Photoelectrochemical Technology to Pharmaceutical Degradation. J. Environ. Chem. Eng. 2022, 10, 107859. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Michler, J.; Philippe, L. Facile Cost-Effective Fabrication of Cu@Cu2O@CuO–Microalgae Photocatalyst with Enhanced Visible Light Degradation of Tetracycline. Chem. Eng. J. 2021, 413, 127477. [Google Scholar] [CrossRef]
- Rokesh, K.; Sakar, M.; Do, T.O. Integration of Aminosilicate Functionalized-Fullerene (C60) QDs on Bismuth Vanadate (BiVO4) Nanolayers for the Photocatalytic Degradation of Pharmaceutical Pollutant. Catal. Today 2021, 407, 252–259. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Zhang, X.; Ge, C.; Liao, J. WO3-x/S-Doped g-C3N4Step-Scheme Heterojunction ForHigh-Efficiency and Stable Vis–NIR Photocatalytic Removalof Pharmaceuticals and Personal Care Products. Adv. Energy Sustain. Res. 2022, 3, 2200138. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Arotiba, O.A. Recent Trend in Visible-Light Photoelectrocatalytic Systems for Degradation of Organic Contaminants in Water/Wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1389–1411. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Vega, D.A.; Álvarez Vega, J.M.; Castillo Jerez, O.F.; Cantú Hernández, R.A. Electrochemical Oxidation of Acetaminophen and Its Transformation Products in Surface Water: Effect of pH and Current Density. Heliyon 2020, 6, e03394. [Google Scholar] [CrossRef] [PubMed]
- Vogna, D.; Marotta, R.; Napolitano, A.; Ischia, M. Advanced Oxidation Chemistry of Paracetamol. UV/H2O2—Induced Hydroxylation/Degradation Pathways and 15N-Aided Inventory of Nitrogenous Breakdown Products. J. Org. Chem. 2002, 67, 6143–6151. [Google Scholar] [CrossRef] [PubMed]
- Isariebel, Q.; Carine, J.; Ulises-javier, J.; Anne-marie, W.; Henri, D. Ultrasonics Sonochemistry Sonolysis of Levodopa and Paracetamol in Aqueous Solutions. Ultrason. Sonochemistry 2009, 16, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of Paracetamol in Aqueous Solutions by TiO2 Photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Daniel, M.; De Luna, G.; Veciana, M.L.; Colades, J.I.; Su, C.; Lu, M. Journal of the Taiwan Institute of Chemical Engineers Factors That Influence Degradation of Acetaminophen by Fenton Processes. J. Taiwan Inst. Chem. Eng. 2014, 45, 565–570. [Google Scholar] [CrossRef]
- Pupo, R.F.; Agu, A.; Trovo, A.G. Paracetamol Degradation Intermediates and Toxicity during Photo-Fenton Treatment Using Different Iron Species. Water Res. 2012, 6, 3–9. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Hu, J. Photoelectrocatalytic Degradation of Organics and Formation of Disinfection Byproducts in Reverse Osmosis Concentrate. Water Res. 2020, 168, 115105. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Villota, N.; Lombraña, J.I.; Cruz-Alcalde, A.; Marcé, M.; Esplugas, S. Science of the Total Environment Kinetic Study of Colored Species Formation during Paracetamol Removal from Water in a Semicont. Sci. Total Environ. 2019, 649, 1434–1442. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Zwane, B.N.; Koiki, B.A.; Rivallin, M.; Bechelany, M.; Mabuba, N.; Lesage, G.; Cretin, M.; Arotiba, O.A. Coupling Cathodic Electro-Fenton with Anodic Photo-Electrochemical Oxidation: A Feasibility Study on the Mineralization of Paracetamol. J. Environ. Chem. Eng. 2020, 8, 104394. [Google Scholar] [CrossRef]
- Xie, G.; Chang, X.; Adhikari, B.R.; Thind, S.S.; Chen, A. Photoelectrochemical Degradation of Acetaminophen and Valacyclovir Using Nanoporous Titanium Dioxide. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 1062–1069. [Google Scholar] [CrossRef]
- Narewadikar, N.A.; Suryavanshi, R.D.; Rajpure, K.Y. Enhanced Photoelectrocatalytic Degradation Activity of Titanium Dioxide Photoelectrode: Effect of Film Thickness. Colloid J. 2021, 83, 107–115. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.; Oskam, G. Phase-Pure TiO2 Nanoparticles: Anatase, Brookite and Rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Nanoscale Advances Tailoring the Defects of Sub-100 Nm Multipodal Titanium Nitride/Oxynitride Nanotubes for e Ffi Cient Water Splitting Performance. Nanoscale Adv. 2021, 3, 5016–5026. [Google Scholar] [CrossRef]
- Eid, K.; Soliman, K.A.; Abdulmalik, D.; Mitoraj, D.; Sleim, M.H.; Liedke, M.O.; El-Sayed, H.A.; AlJaber, A.S.; Al-Qaradawi, I.Y.; Reyes, O.M.; et al. Tailored Fabrication of Iridium Nanoparticles Sensitized Titanium Oxynitride Nanotubes for Solar-Driven Water Splitting: Experimental Insights on the Photocatalytic-Activity-Defects Relationship. Catal. Sci. Technol. 2020, 10, 801–809. [Google Scholar] [CrossRef]
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2/Au/TiO2 Multilayer Thin-Film Photoanodes Synthesized by Pulsed Laser Deposition for Photoelectrochemical Degradation of Organic Pollutants. Sep. Purif. Technol. 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Danish, M.; Qamar, M.; Suliman, M.; Muneer, M. Photoelectrochemical and Photocatalytic Properties of Fe@ZnSQDs/TiO2 Nanocomposites for Degradation of Different Chromophoric Organic Pollutants in Aqueous Suspension. Adv. Compos. Hybrid Mater. 2020, 3, 570–582. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Bismuth Vanadate in Photoelectrocatalytic Water Treatment Systems for the Degradation of Organics: A Review on Recent Trends. J. Electroanal. Chem. 2020, 878, 114724. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Koiki, B.A.; Peleyeju, G.M.; Arotiba, O.A. Visible Light Driven Photoelectrocatalysis on a FTO/BiVO4/BiOI Anode for Water Treatment Involving Emerging Pharmaceutical Pollutants. Electrochim. Acta 2019, 307, 285–292. [Google Scholar] [CrossRef]
- Lin, C.J.; Liao, S.J.; Kao, L.C.; Liou, S.Y.H. Photoelectrocatalytic Activity of a Hydrothermally Grown Branched Zno Nanorod-Array Electrode for Paracetamol Degradation. J. Hazard. Mater. 2015, 291, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Orimolade, B.O.; El-Maghrabi, H.H.; Koiki, B.A.; Rivallin, M.; Bekheet, M.F.; Viter, R.; Damberga, D.; Lesage, G.; Iatsunskyi, I.; et al. Photoelectrocatalysis of Paracetamol on Pd–ZnO/N-Doped Carbon Nanofibers Electrode. Appl. Mater. Today 2021, 24, 101129. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Miyauchi, M. Nanoporous-Walled Tungsten Oxide Nanotubes as Highly Active Visible-Light-Driven Photocatalysts. Angew. Chem. Int. Ed. 2008, 47, 7051–7055. [Google Scholar] [CrossRef] [PubMed]
- Peleyeju, M.G.; Viljoen, E.L. WO3-Based Catalysts for Photocatalytic and Photoelectrocatalytic Removal of Organic Pollutants from Water—A Review. J. Water Process Eng. 2021, 40, 101930. [Google Scholar] [CrossRef]
- Peleyeju, G.M.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Solar-Light-Responsive Titanium-Sheet-Based Carbon Nanoparticles/B-BiVO4/WO3 Photoanode for the Photoelectrocatalytic Degradation of Orange II Dye Water Pollutant. ACS Omega 2020, 5, 4743–4750. [Google Scholar] [CrossRef]
- Umukoro, E.H.; Peleyeju, M.G.; Ngila, J.C.; Arotiba, O.A. Towards Wastewater Treatment: Photo-Assisted Electrochemical Degradation of 2-Nitrophenol and Orange II Dye at a Tungsten Trioxide-Exfoliated Graphite Composite Electrode. Chem. Eng. J. 2017, 317, 290–301. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarath Chandra, K.; Kim, D.H.; Madras, G.; Sarkar, D. Understanding the Morphological Effects of WO3 Photocatalysts for the Degradation of Organic Pollutants. Adv. Powder Technol. 2018, 29, 1591–1600. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of Heterojunction Photoelectrode via Atomic Layer Deposition of Fe2O3 on Bi2WO6 for Highly Efficient Photoelectrochemical Sensing and Degradation of Tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Mahhumane, N.; Cele, L.M.; Muzenda, C.; Nkwachukwu, O.V.; Koiki, B.A.; Arotiba, O.A. Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode. Nanomaterials 2022, 12, 2467. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Chen, C.; Zhu, Y.; Zeng, G.; Huang, B.; Chen, W.; Liu, S.; Lei, C.; Li, B.; Yang, Y. Ternary Z-Scheme Heterojunction of Bi2WO6 with Reduced Graphene Oxide (rGO) and Meso-Tetra (4-Carboxyphenyl) Porphyrin (TCPP) for Enhanced Visible-Light Photocatalysis. J. Colloid Interface Sci. 2019, 540, 115–125. [Google Scholar] [CrossRef] [PubMed]




| Photoelectrode | Conditions | Rate Constant (min−1) | Degradation Time | Removal Efficiency (%) | TOC Removal (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| TiO2/Ti plate | 0.1 mol·L−1 Na2SO4 40 mg·L−1 PTM solution UV-Vis 130 mW/cm2 1.0 V vs. Ag/AgCl | 20 °C | 0.0046 | - | - | [53] | |
| EC Treated-TiO2/Ti plate | 0 °C | 0.0065 | - | - | |||
| 20 °C | 0.0086 | 79.72 | 59.3 | ||||
| 40 °C | 0.0116 | - | - | ||||
| 60 °C | 0.0137 | - | - | ||||
| TiO2/Au/TiO2 | EC oxidation 0.05 mol·L−1 Na2SO4 | 39 mg·L−1 PTM solution | - | 300 min | 47 | - | [58] |
| 78 mg·L−1 PTM solution | - | 300 min | 37 | - | |||
| 157 mg·L−1 PTM solution | - | 300 min | 29 | - | |||
| PEC oxidation 0.05 mol·L−1 Na2SO4 36 W LED UVA, 88 W·m−2 | 39 mg·L−1 PTM solution | - | 360 min | 95 | - | ||
| 78 mg·L−1 PTM solution | - | 360 min | 69 | - | |||
| 157 mg·L−1 PTM solution | - | 360 min | 61 | - | |||
| PEC oxidation 0.035 mol·L−1 Na2SO4 0.015 mol·L−1 NaCl 36 W LED UVA, 88 W·m−2 | 39 mg·L−1 PTM solution | - | 120 min | 100 | - | ||
| 78 mg·L−1 PTM solution | - | 150 min | 100 | - | |||
| 157 mg·L−1 PTM solution | - | 150 min | 50 | - | |||
| PEC + PEF oxidation 36 W LED UVA, 88 W·m−2 Continuous H2O2 generation | 39 mg·L−1 PTM solution | 3–4 min | 100 | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, N.A.; Marchesini, F.A.; Gamba, I.; García, G. Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions. Catalysts 2023, 13, 524. https://doi.org/10.3390/catal13030524
Sacco NA, Marchesini FA, Gamba I, García G. Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions. Catalysts. 2023; 13(3):524. https://doi.org/10.3390/catal13030524
Chicago/Turabian StyleSacco, Nicolás Alejandro, Fernanda Albana Marchesini, Ilaria Gamba, and Gonzalo García. 2023. "Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions" Catalysts 13, no. 3: 524. https://doi.org/10.3390/catal13030524
APA StyleSacco, N. A., Marchesini, F. A., Gamba, I., & García, G. (2023). Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions. Catalysts, 13(3), 524. https://doi.org/10.3390/catal13030524









