Abstract
This review describes the recent advances in photocatalyzed reactions to form new carbon–sulfur and carbon–selenium bonds. With a total of 136 references, of which 81 articles are presented, the authors introduce in five sections an updated picture of the state of the art in the light-promoted synthesis of organochalcogen compounds (from 2019 to present). The light-promoted synthesis of sulfides by direct sulfenylation of C–C π-bonds; synthesis of sulfones; the activation of Csp2–N bond in the formation of Csp2–S bonds; synthesis of thiol ester, thioether and thioacetal; and the synthesis of organoselenium compounds are discussed, with detailed reaction conditions and selected examples for each protocol.
1. Introduction
The global importance and steady growth of the fine chemicals industry are undeniable. Moreover, this industry plays an important role in the 2030 Agenda for Sustainable Development. For these reasons, the development of sustainable chemical processes is necessary and urgent, aiming to enable energy-efficient and atom-economic processes, and to diminish the environmental impact by reducing the waste formation [1,2,3,4]. In this context, Green Chemistry has been established over more than two decades as a powerful tool for achieving environmentally friendly and sustainable chemical practices, through the comprehensive application of the 12 principles based on system thinking approaches. Among them, Principle #6 (Design for Energy Efficiency) calls for the development of chemical processes that can be conducted at ambient temperature and pressure, avoiding long reaction times under constant heating. An elegant strategy to overcome this challenge is to employ alternative energy sources to conduct reactions, including the use of acoustic, electrical, electromagnetic, and mechanical energy [5,6,7,8,9,10].
The application of visible light as an energy source using low-energy light devices, including CFL lamps and LEDs, has become established and widely used as a powerful strategy in organic synthesis. The bloom of this elegant approach was triggered during the 2000s with the unveiling of photocatalysis, which is based on the use of chromophore substances able to absorb light and trigger SET (Single Electron Transfer) processes in general by reductive and oxidative quenching. Since then, a large variety of metal- and dye-based photocatalysts has emerged as alternative to conduct several chemical transformations under visible light irradiation [11,12,13,14,15,16,17].
During the last decades, organochalcogen compounds (organosulfur and organoselenium) have been assuming a leading role due to the discoveries which have been revealing their biological activities. For this reason, the development of synthetic methods to prepare these compounds has become an important area in organic synthesis, attracting the attention of many research groups around the world [18,19,20,21,22,23,24,25,26]. In this context, the development of photocatalytic strategies to access these compounds has been opening new horizons in organochalcogen chemistry, allowing the construction of a wide range of important structures through sophisticated and eco-friendly approaches.
Therefore, in view of the steadily increasing interest in this class of reactions, some important reviews were published during the last years, covering many protocols to prepare organosulfur and organoselenium compounds by photocatalytic and photolytic strategies [27,28,29,30,31,32]. Thus, in view of our recent efforts in the development of light-mediated and photocatalyzed reaction for the synthesis of organochalcogen compounds, [33,34,35,36] we present herein a review of the developed methods for the light-promoted synthesis of organosulfur and organoselenium compounds, since 2019. In addition to highlighting the novel strategies to build organosulfur compounds through photoinduced processes, this review brings the most recent advances in the visible light-mediated construction of new C–Se bonds, so far explored by few in other reviews. For better comprehension, the text is divided into five main sections: (a) photocatalyzed synthesis of sulfides by direct sulfenylation of C–C π–bonds; (b) photocatalyzed synthesis of sulfones; (c) photocatalyzed Csp2–N bond activation for the formation of Csp2–S bonds; (d) photocatalyzed synthesis of thioester, thioether and thioacetal, and (e) photocatalyzed synthesis of organoselenium compounds. Some sections were subdivided according to the type of reagent used in the reactions. This systematic review was prepared using the following search databases: SciFinder, Web of Science, Scopus, and Google Scholar. The following search terms were used: “photochemistry”, “photocatalysis”, “selenides”, “sulfides”, “visible light”, and “C–chalcogen bond”, between 2019 and 2022.
2. Photocatalyzed Synthesis of Sulfides by Direct Sulfenylation of C–C π–Bonds
2.1. Using Thiols as Substrate
Sulfur-containing heterocyclic compounds are of great importance in the chemical and pharmaceutical fields, as they are found in many natural products and medicine [37,38]. Consequently, the synthesis of these compounds has been receiving considerable attention due to their biological value and extensive pharmaceutical applications. Among recent advances, several visible light-mediated protocols have been emerging as efficient and green alternatives to construct organosulfur-decorated heterocycles.
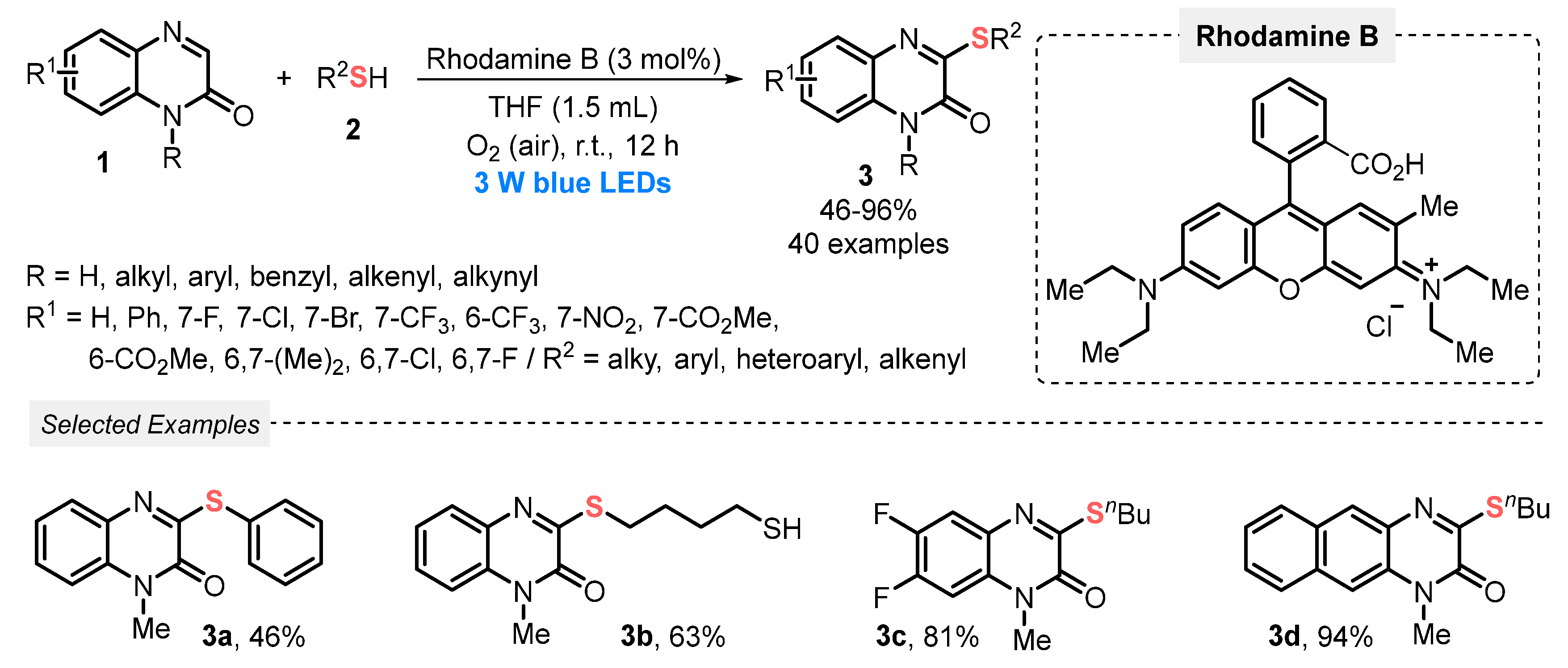
In this sense, in 2019, He and co-workers [39] developed the synthesis of 3-sulfenylated quinoxalin-2(1H)-ones 3 via the direct C-H sulfenylation of quinoxalin-2(1H)-ones 1 with thiols 2, under visible light irradiation (blue LEDs, 3 W). Rhodamine B was used as photocatalyst in the presence of THF as solvent, at room temperature and open-air conditions. By this protocol, a range of 3-sulfenyl quinoxalin-2(1H)-ones 3 was obtained in moderate to excellent yields (46–96%), with a good functional group tolerance. It is worth mentioning that the atmospheric O2, which is employed as a green oxidant, is crucial for the reaction progress. This could be proven in the preliminary mechanistic studies, which showed that the transformation was suppressed under inert atmosphere (N2) (Scheme 1). The optimization studies demonstrated that the combination of Rhodamine B and MeCN is also a good reaction medium, providing excellent yields, making the reaction possible to be conducted in a greener solvent.
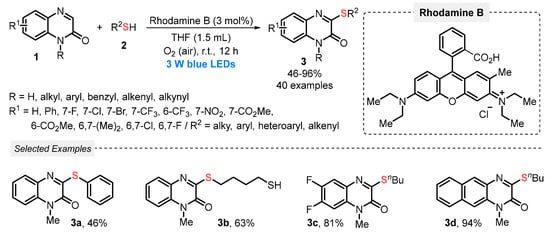
Scheme 1.
Direct C–H/S–H cross-coupling of quinoxalin-2(1H)-ones 1 with thiols 2 to obtain 3-sulfenylated quinoxalin-2(1H)-ones 3.
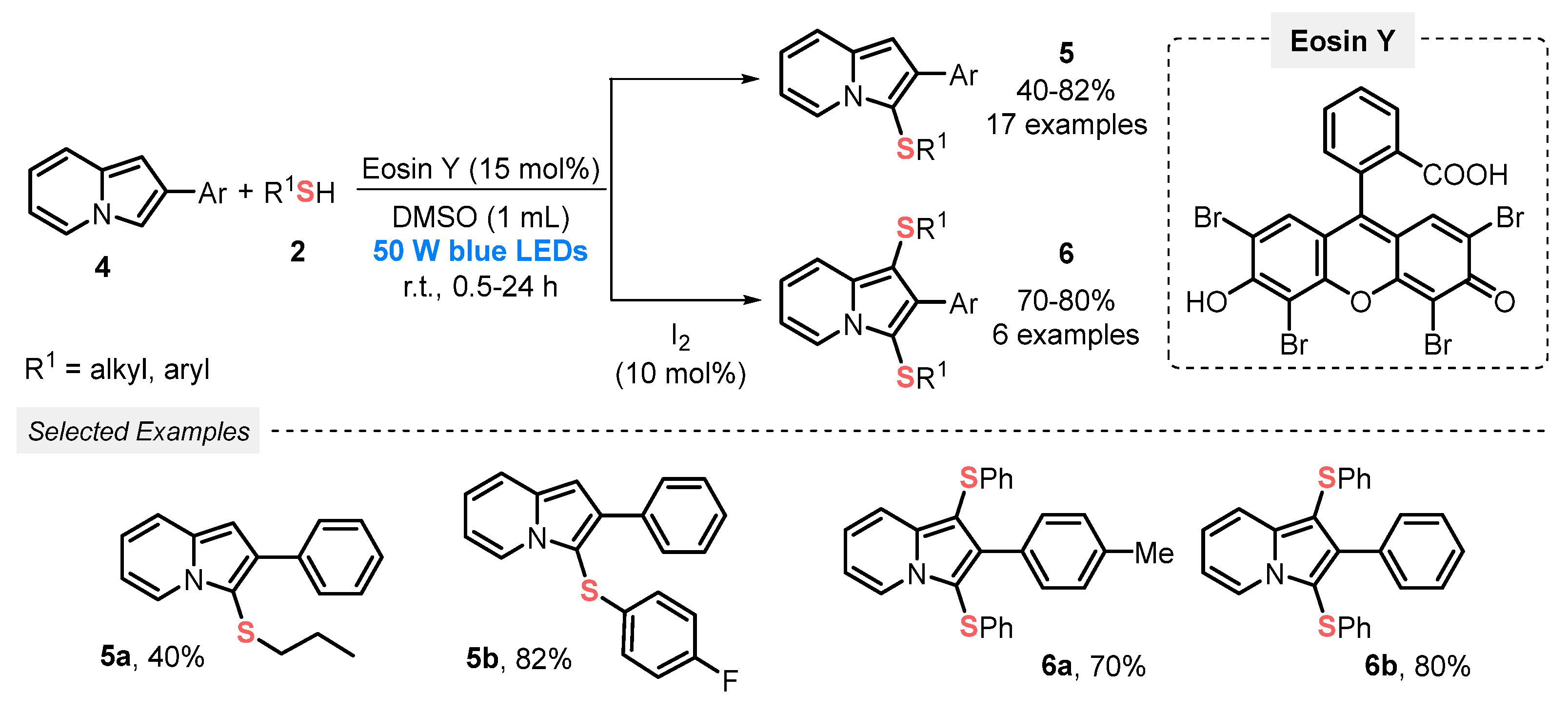
A year later, Lenardão and co-workers [33] disclosed a visible light-mediated approach employing a high power light source (blue LEDs, 50 W) to construct 3-sulfenylindolizines 5, by reacting 2-substituted indolizines 4 and thiols 2, in the presence of Eosin Y as photocatalyst and DMSO, at room temperature and open-air conditions. Under this metal-free condition, seventeen 3-sulfenylindolizines 5 were prepared in modest to very good yields (29–82%). Furthermore, by the addition of 10 mol% of I2 in the reaction medium, 1,3-bis-sulfenylindolizines 6 were reached as the major product; however, to obtain the bis-sulfenylated product longer reaction times were necessary. A brief reaction scope study was carried out, which resulted in six derivatives of the product 6 in good yields (70-80%) (Scheme 2).
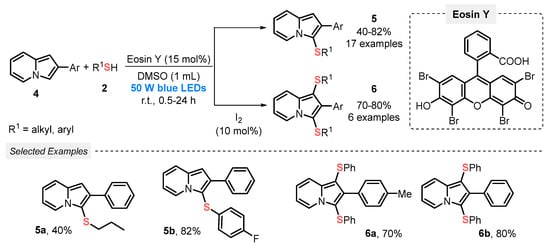
Scheme 2.
Direct C–H/S–H cross-coupling of indolizine 4 with thiol 2 to obtain 3-sulfanylindolizine 5 or bis-sulfanylindolizine 6.
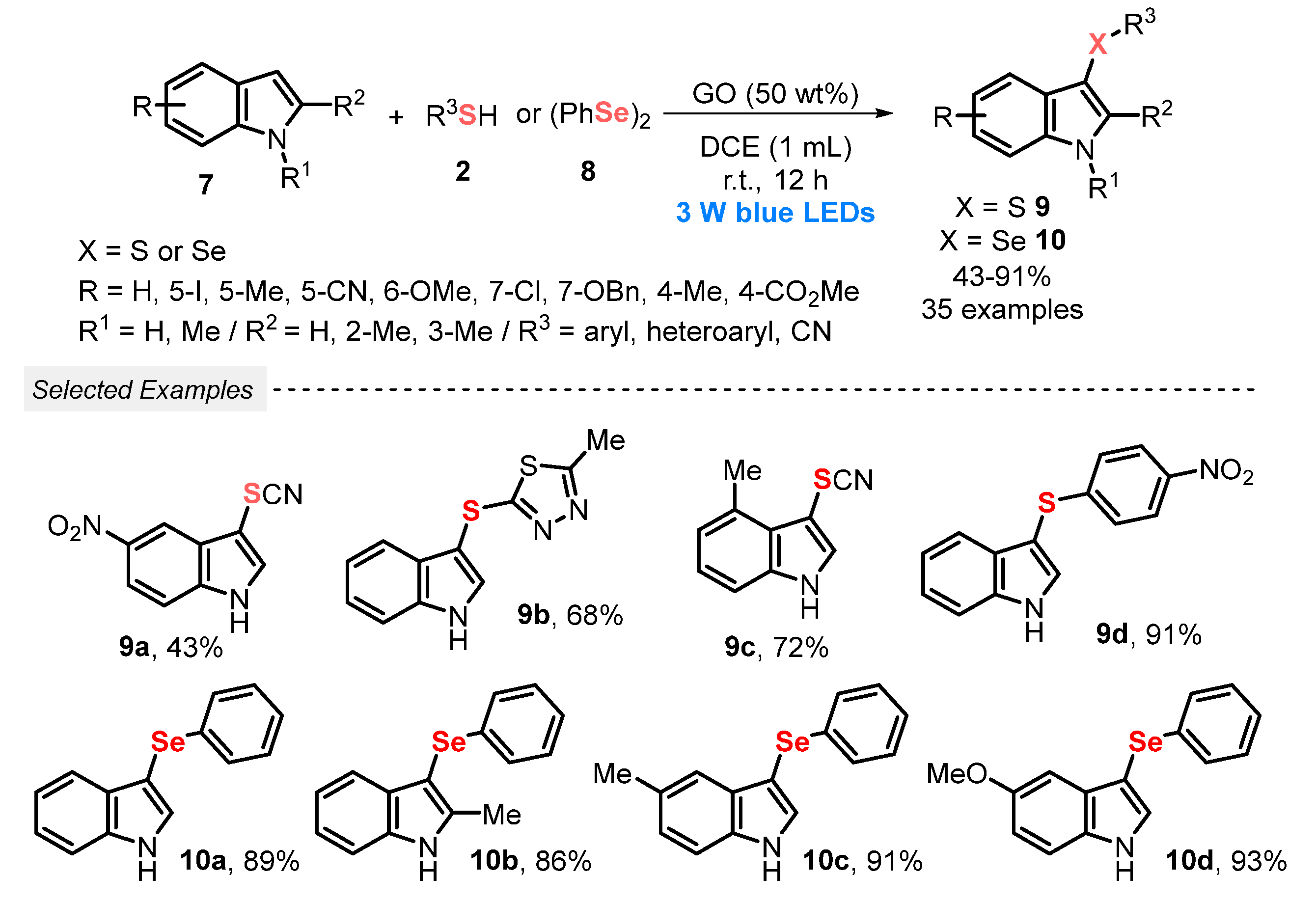
In a similar work, in 2022, Liu and co-workers [40] proposed a scalable, regioselective and atom-economic methodology to obtain 3-sulfenyl- 9 and 3-selenylindoles 10, by reacting different indoles 7 with thiols 2, or with diphenyl diselenide 8, under visible light conditions (blue LEDs, 3 W). In this TM-free and I2-free protocol, graphene oxide (GO) demonstrated outstanding synergy with the visible light, which is not commonly reported in the literature, acting as a radical initiator under blue light irradiation, in the presence of DCE as solvent, at room temperature and open-air conditions. A total of thirty-five derivatives 9 and 10 were prepared in moderate to excellent yields (43–91%), presenting a high functional group tolerance (Scheme 3).
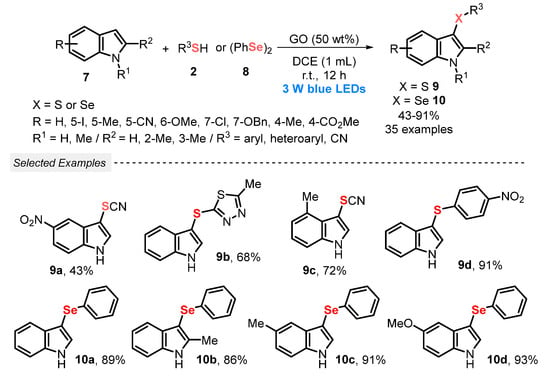
Scheme 3.
Chalcogenylation of indoles 7 using graphene oxide as photocatalyst promoted by visible light.
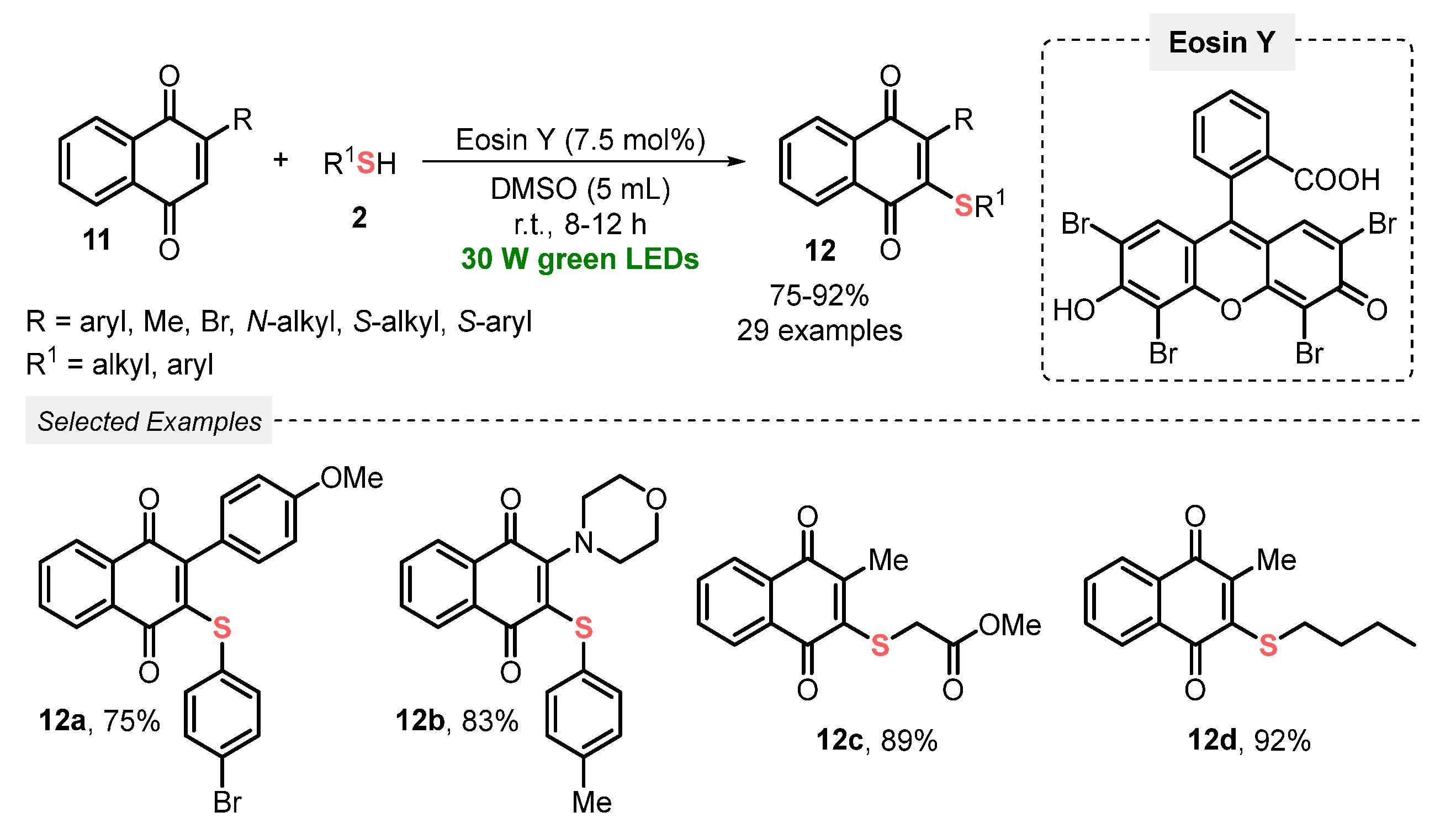
Also, in 2022, Dhar and co-workers [41] described a visible light-mediated (green LEDs, 30 W) photocatalyzed (Eosin Y) thiolation of 1,4-naphtoquinones 11, in the presence of thiols 2 and DMSO as solvent, at room temperature and open-air conditions. Under the optimal conditions, the authors prepared a total of twenty-nine 3-sulfenyl-1,4-naphtoquinone 12 derivatives in good to excellent yields (75–92%). It is worth mentioning that the key reaction step (the in situ formation of thiyl radical species) was fully characterized by HRMS analysis. Furthermore, this approach delivers a green and ingenious alternative to circumvent the use of heating or TM-based catalysts, commonly found in classical methods. Although the desired product was obtained in 32% yield performing the reaction in the absence of light, and no more investigations were performed in longer reaction times to improve the reaction yield. Furthermore, experiments employing different light sources (wavelength and power) and solvent amounts were not considered by the authors (Scheme 4).
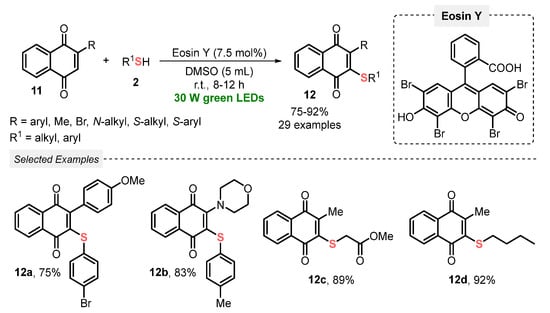
Scheme 4.
Thiolation of substituted 1,4-naphtoquinones 11 using Eosin Y as a photoredox catalyst.
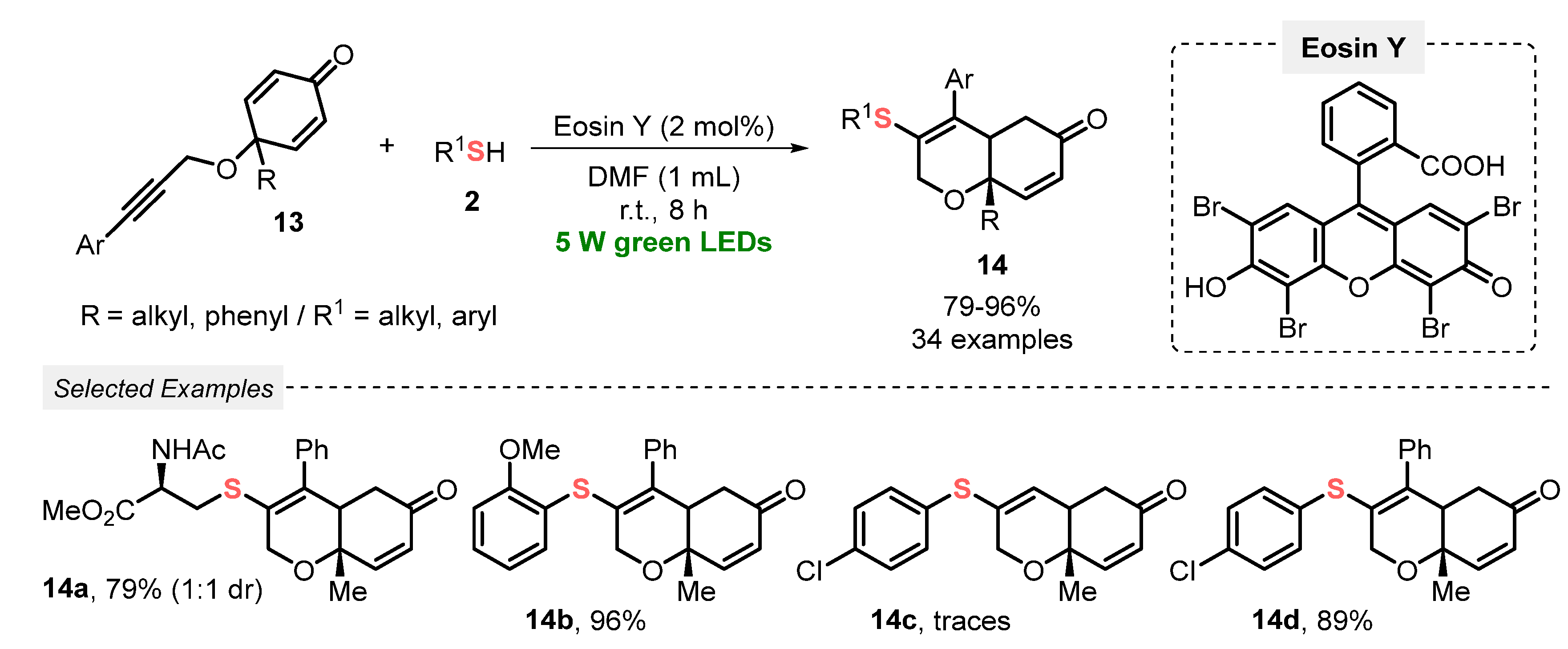
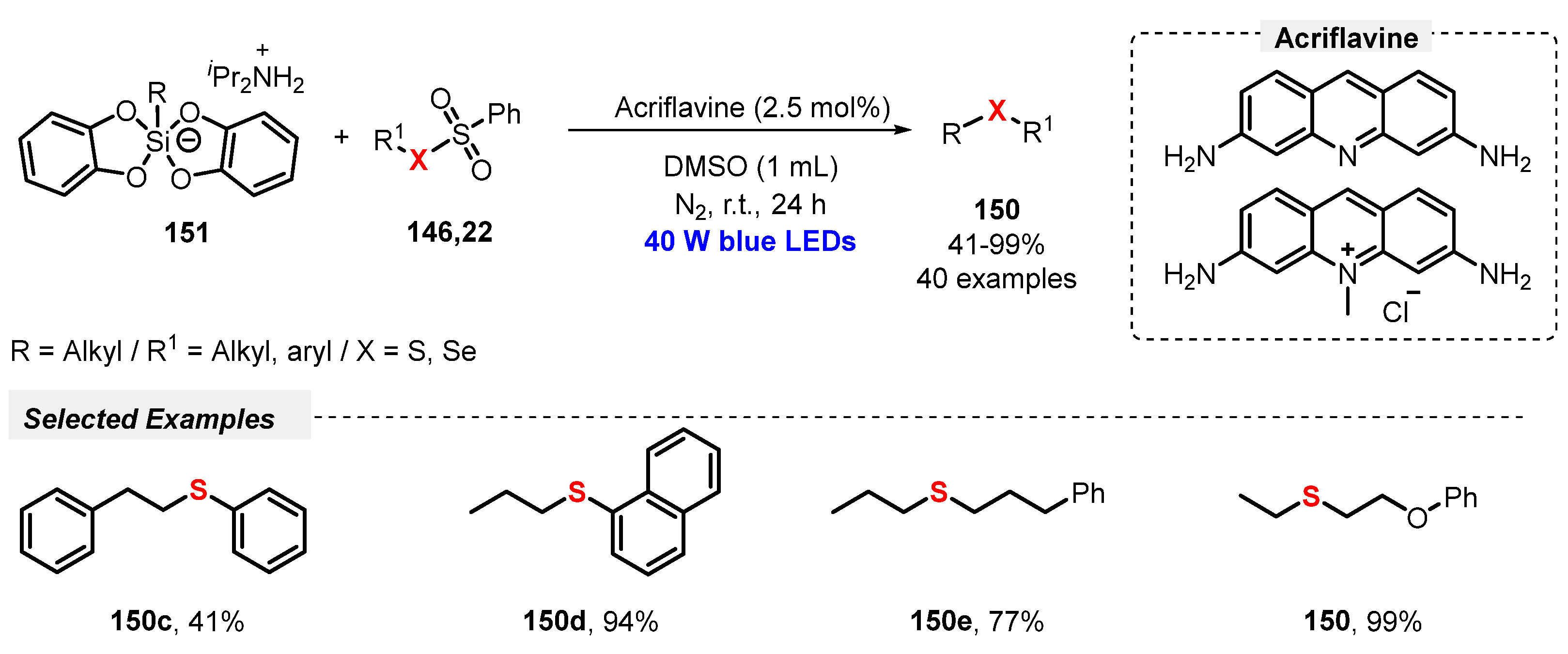
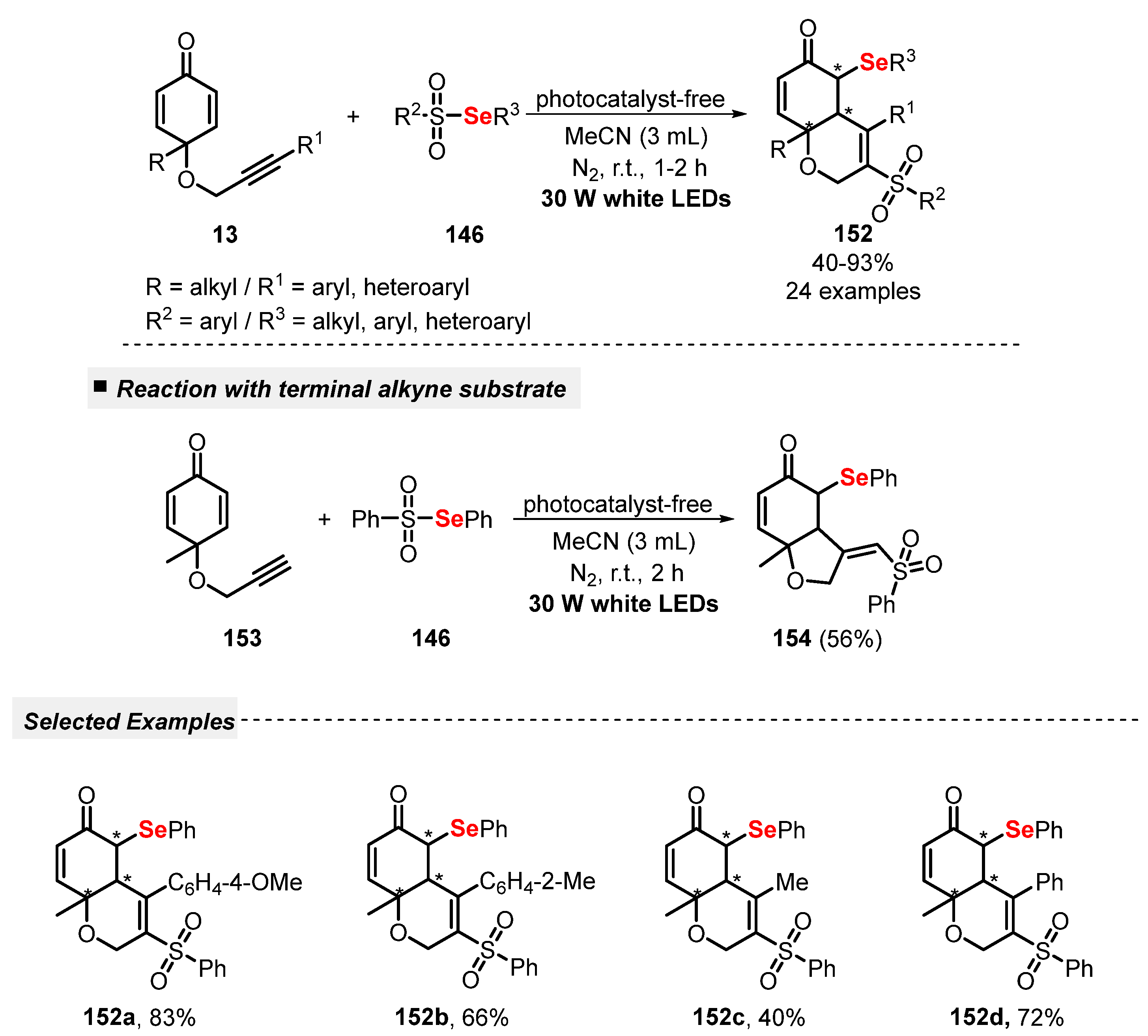
In recent years, radical cyclization reactions involving alkynes and thiols or disulfides, through the formation of thiyl radicals as key reaction intermediates, have been proven to be an attractive and efficient protocol to the click construction of several functionalized heterocyclic cores. In this sense, Volla and co-workers [42] reported in 2019 a mild and gram-scale visible light-mediated protocol (green LEDs, 5 W) using Eosin Y as a dye-based photocatalyst, aiming to prepare sulfur-decorated dihydrochromenones 14. The reaction involves a thiol-lyne/conjugate radical cascade addition between alkyne-tethered cyclohexadienones 13 and thiols 2. Under the optimized reaction conditions, the authors were able to prepare thirty-four thio-dihydrochromenone derivatives 14 in good to excellent yields (79–96%). This protocol delivered high diastereoselectivity, satisfying the Green Chemistry principles of atom economy and energy efficiency. Furthermore, a cascade bis-sulfenylation and intramolecular amine addition transformation was also demonstrated (Scheme 5).
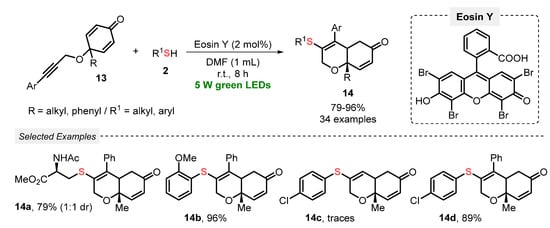
Scheme 5.
Sulfenylation-annulation cascade of alkyne-tethered cyclohexadienones 13.
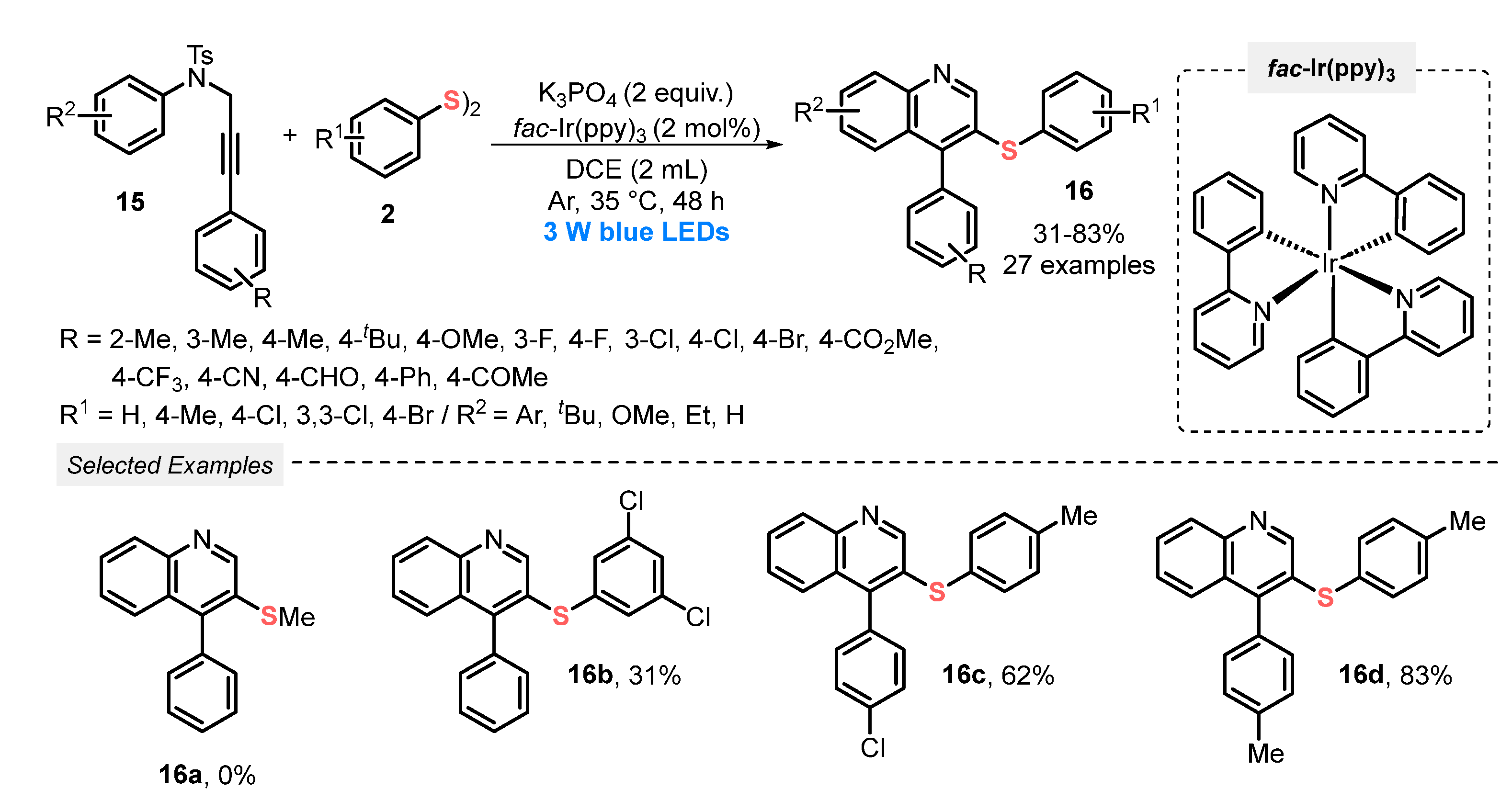
In the same year, Liu and co-workers [43] developed a visible light-promoted protocol (blue LEDs, 3 W) to prepare 3-sulfenylquinoline derivates 16, by reacting N-aryl-N-tosylpropargylamine 15 and aromatic disulfides 2, employing tris[2-phenylpyridinato-C2,N]iridium(III) (fac-Ir(ppy)3) as photoredox catalyst. The reactions were conducted in the presence of DCE and K3PO4, at room temperature and under inert atmosphere of Ar. A total of twenty-seven compounds with pharmaceutical potential were prepared in poor to very good yields (31–83%). Despite being limited to aromatic disulfides, the protocol demonstrates high functional group tolerance (Scheme 6). However, important limitations should be considered, such as long reaction times, in comparison to the majority of similar methodologies found in literature. This feature may be closely related to the need for using inert atmosphere.
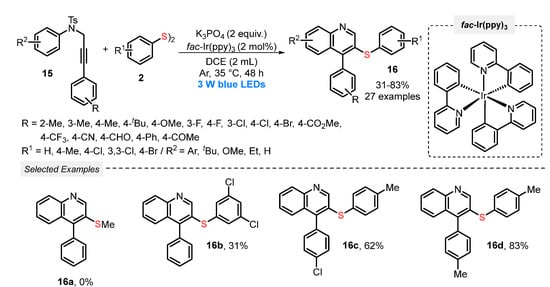
Scheme 6.
Synthesis of 3-phenylthioquinoline 16 promoted by sulfenylation/cyclization of N-aryl-N-tosylpropargylamines 15 with aryl disulfides 2 under visible light.
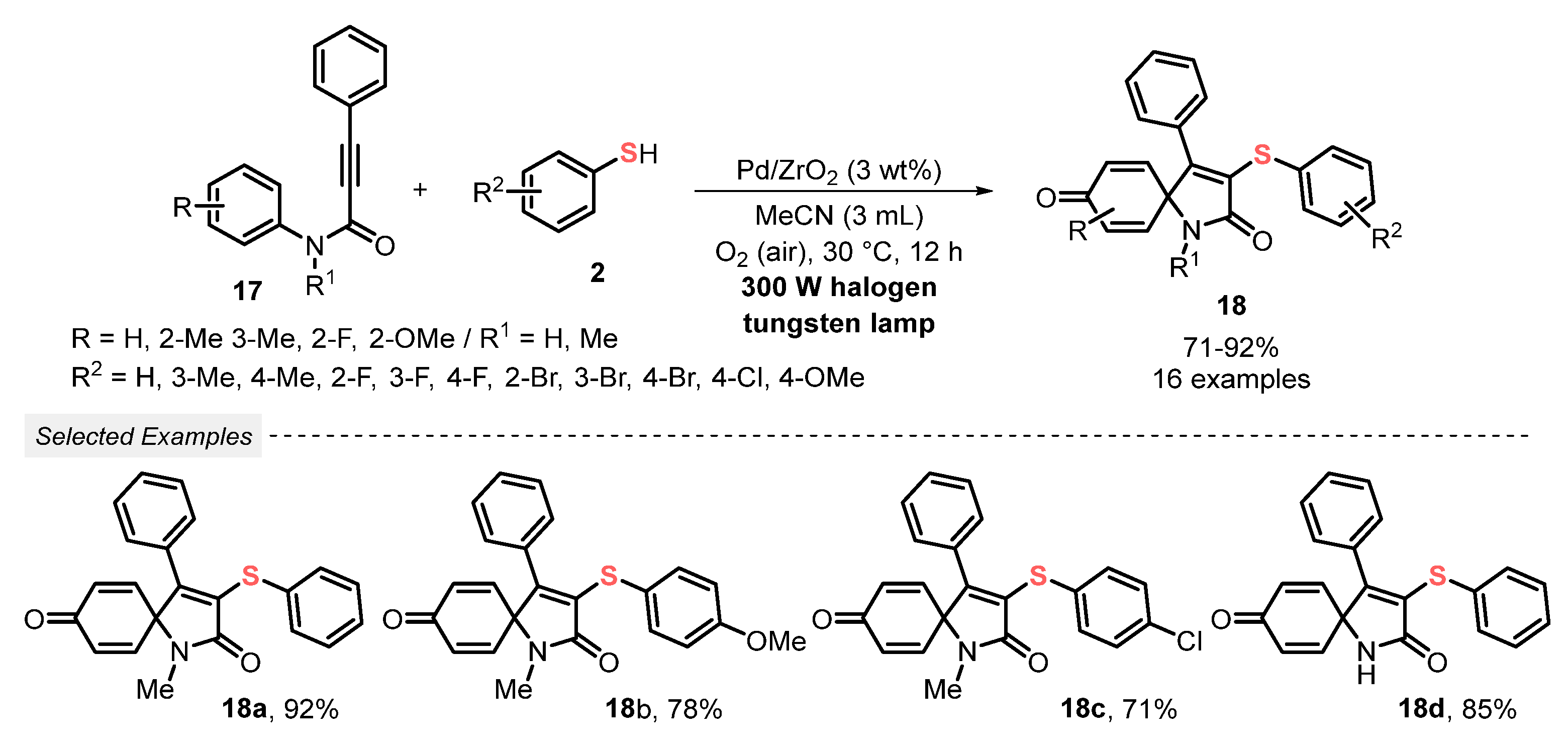
Still, in 2019, Guo and co-workers [44] published a new eco-friendly light-mediated methodology for the synthesis of 3-thioazaspiro[4,5]trienones 18 in good to excellent yields (71–92%). In the experimental procedure, a mixture of propynamides 17 and thiophenols 2 was irradiated by a 300 W halogen tungsten lamp in the presence of Pd/ZrO2 as photocatalyst, MeCN as solvent, and under open-air conditions. The optimized condition was used to prepare sixteen differently substituted 3-thioazaspiro[4,5]trienone derivatives 18 in good to excellent yields, including several halogen-containing ones. It is worth mentioning that the photocatalyst could be satisfactorily recovered and reused for up to five consecutive cycles, without a significant yield decreasing (Scheme 7).
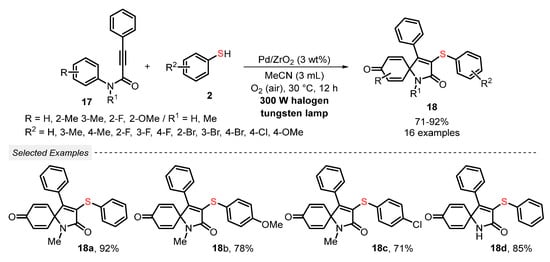
Scheme 7.
Visible light-mediated spirocyclization reaction of propynamides 17 with thiophenols 2 using Pd/ZrO2 as a heterogeneous photocatalyst.
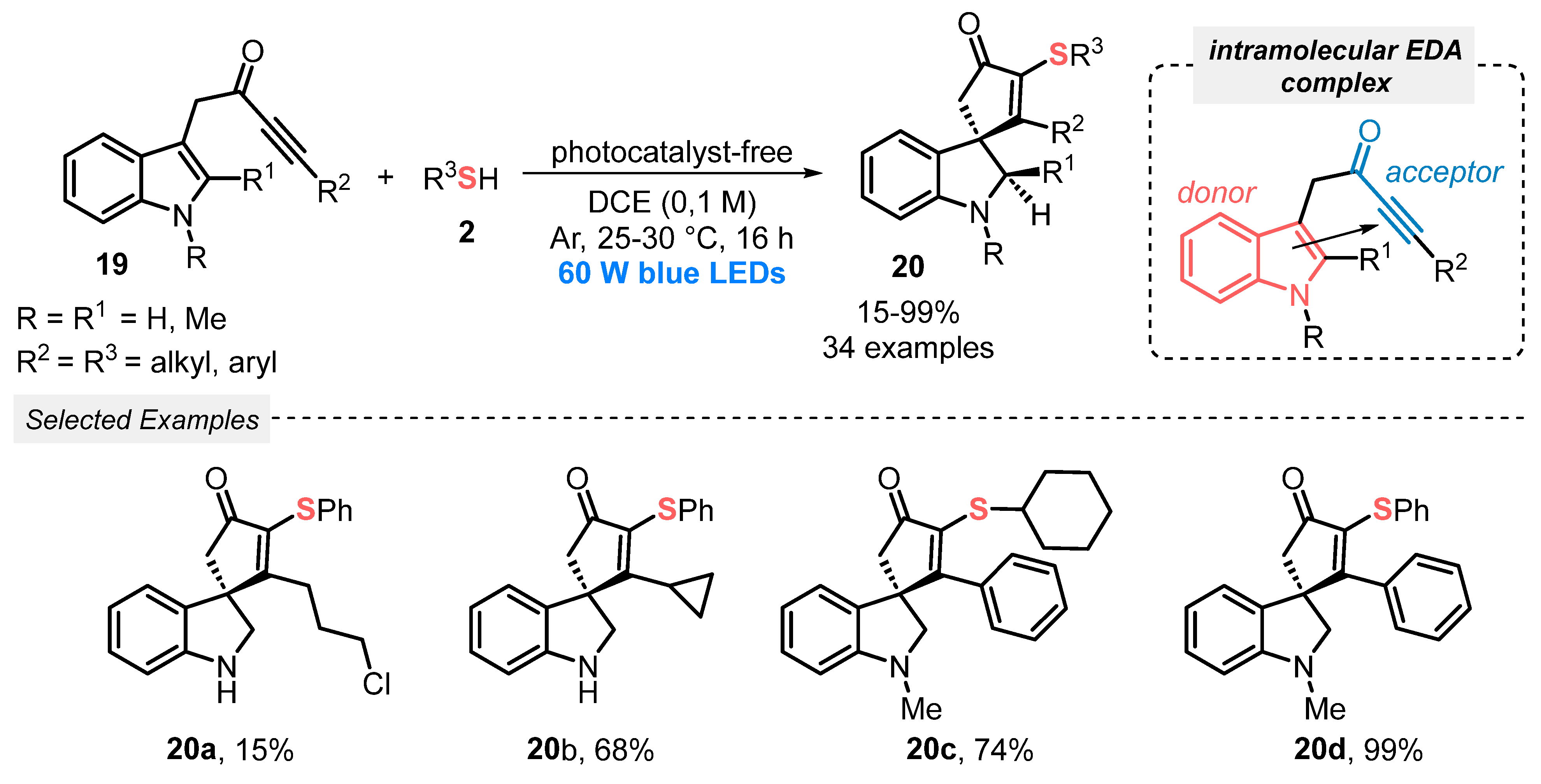
In another approach, reported in 2020, Unsworth, Taylor, and co-workers [45] described a thiyl radical-promoted spirocyclization of indole-tethered ynones 19, towards the formation of sulfur-containing spirocycle indolines 20. The reaction was conducted just under blue light irradiation (LEDs, 60 W), in the absence of photocatalytic species, at room temperature and under argon atmosphere. This mild condition is allowed by the intramolecular formation of a visible light-sensitive EDA-complex, by the interaction of the HOMO orbital of the indole π system, and the LUMO ynone orbital. This system is able to absorb light to reach an open-shell excited state, which can promote homolytic cleavage of the S–H bond, delivering thiyl radical species, in a radical initiation step. From this point, the thiyl radical intermediate triggers a propagation process, performing the radical cyclization of the ynone 19, towards the formation of the product 20. In this condition, thirty-four dearomatized spirocycles 20 were prepared in poor to excellent yields (15–99%) (Scheme 8). The inert atmosphere is crucial for the formation of the desired indolines 20, once control experiments demonstrated an oxidative dearomative process when the reaction was conducted under open-air conditions. Under this condition, an indolenine was obtained as product with good selectivity.
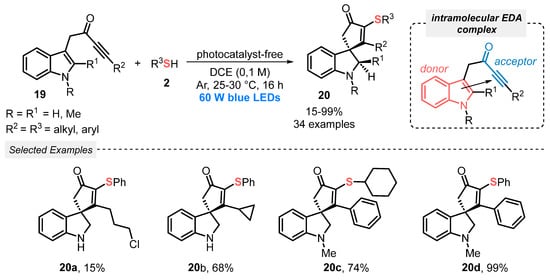
Scheme 8.
Spirocyclization of indole-tethered ynones 19 induced by visible light.
2.2. Using Thiosulfonate and Thiocyanate as Substrates
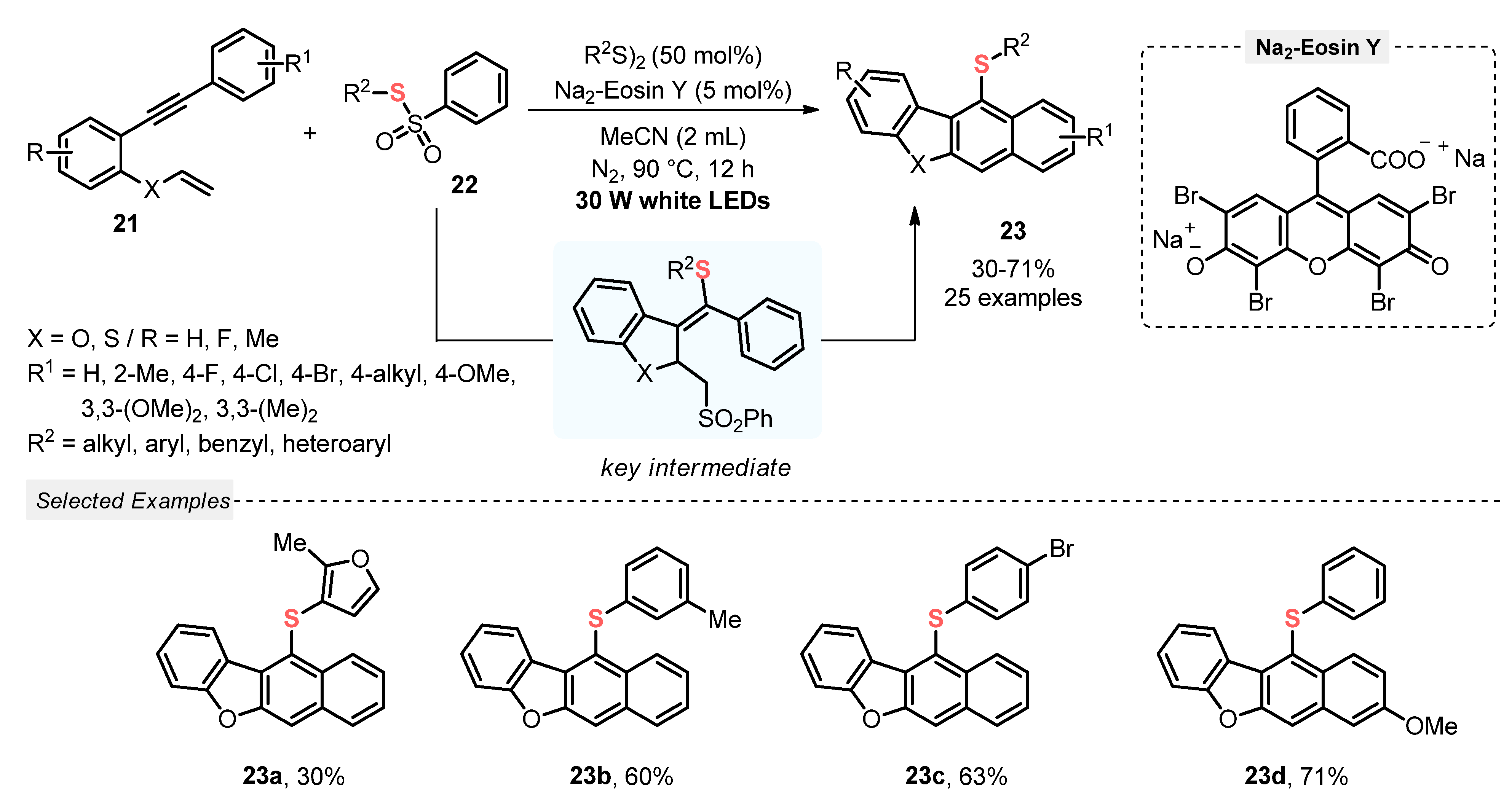
In 2021, Chen and co-workers [46] described a visible light-induced (white LEDs, 30 W) cascade reaction between 2-vinyl-oxy arylalkynes 21 and thiosulfonates 22, aiming to access sulfur-decorated dibenzofuran derivates 23, by employing Na2-Eosin Y as photocatalyst, in the presence of MeCN at 90 °C under inert atmosphere (N2). Under the optimal reaction condition, twenty-five derivatives 23 were synthesized in reasonable to good yields (30–71%), demonstrating a good substrate tolerance regarding both the alkyne and the thiosulfonate. Despite this, heteroaromatic-containing derivatives, such as furan, have shown less reaction suitability, giving the product 23a in the lowest yield (Scheme 9). It is worth mentioning that, in comparison to other similar light-mediated strategies for the C–S bond formation, the present protocol sounds less environmentally friendly, considering the need for using inert atmosphere and heating (Scheme 9).
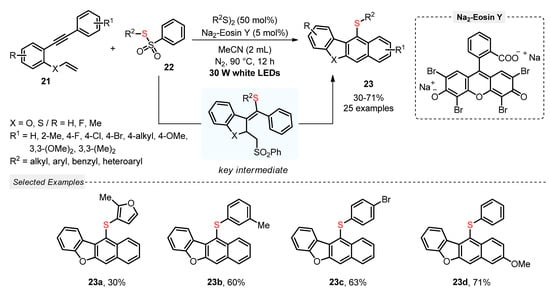
Scheme 9.
Cyclization/Aromatization of 2-vinyloxy arylalkynes 21 to obtain thiosubstituted dibenzofuran derivates 23.
The transformation proceeds through a cyclization/aromatization sequence, which involves a key reaction intermediate, the thiosulfonylation product of 2-vinyloxy arylalkyne. Furthermore, disulfide was used as an additive for the hydrogen abstraction in the aromatization stage, to give the desired products (Scheme 9).
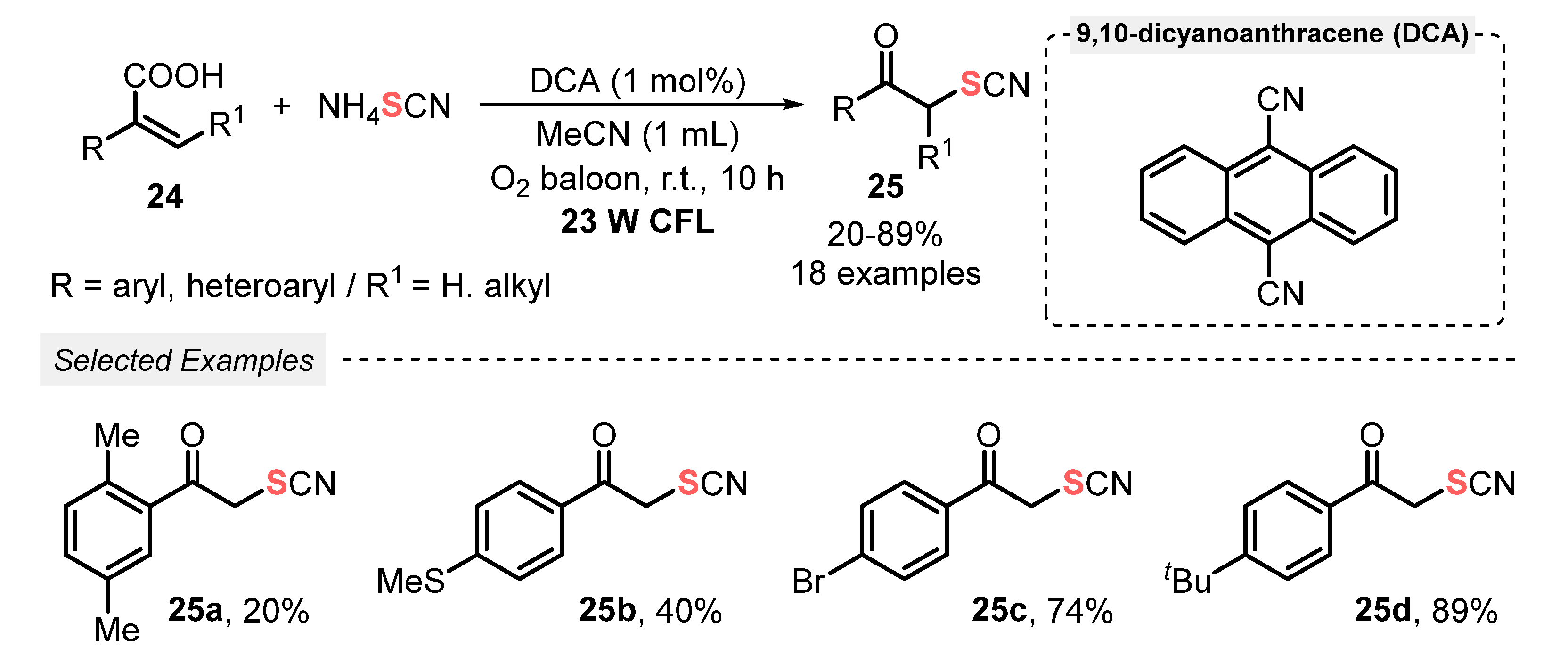
Thiocyanates are an important class of compounds which are widely used as synthetic intermediates to access valuable sulfur-containing derivatives [47]. Consequently, the development of synthetic strategies to introduce thiocyanate groups into organic molecules has been attracting a lot of attention and nowadays it is an important research area in synthetic organic chemistry. In this context, in 2021, Guan and co-workers [48] developed a visible light-mediated decarboxylative thiocyanation of acrylic acids 24, affording α-thiocyanate ketones 25. 9,10-Dicyanoanthracene (DCA) was used as organic photocatalyst, in a fairly low amount, in the presence of MeCN under O2 atmosphere (in balloon). The optimal conditions involved the irradiation of the mixture by a 30 W CFL lamp at room temperature for 10 h. A total of eighteen α-thiocyanate ketone derivatives 25 were prepared in poor to very good yields (20–89%) (Scheme 10). Limitations were faced when 2,3-diarylsubstituted acrylic acids were employed as starting materials, probably due to steric hindrance. In addition, para-nitro, -cyano, -carbonyl, -carboxyl, and ortho-fluoro-substituted atropic acids were satisfactorily employed as substrate.
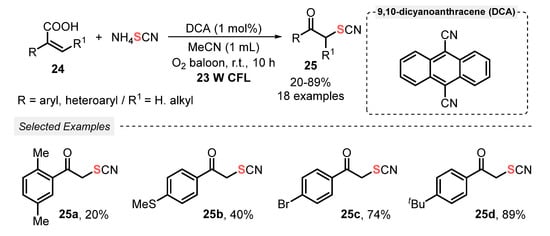
Scheme 10.
Synthesis of α-thiocyanate ketones 25 via decarboxylative ketonization of acrylic acids 24 promoted by visible light.
3. Photocatalyzed Synthesis of Sulfones
3.1. Using Pre-Installed Sulfones Substrates for the Generation of Sulfonyl Radical
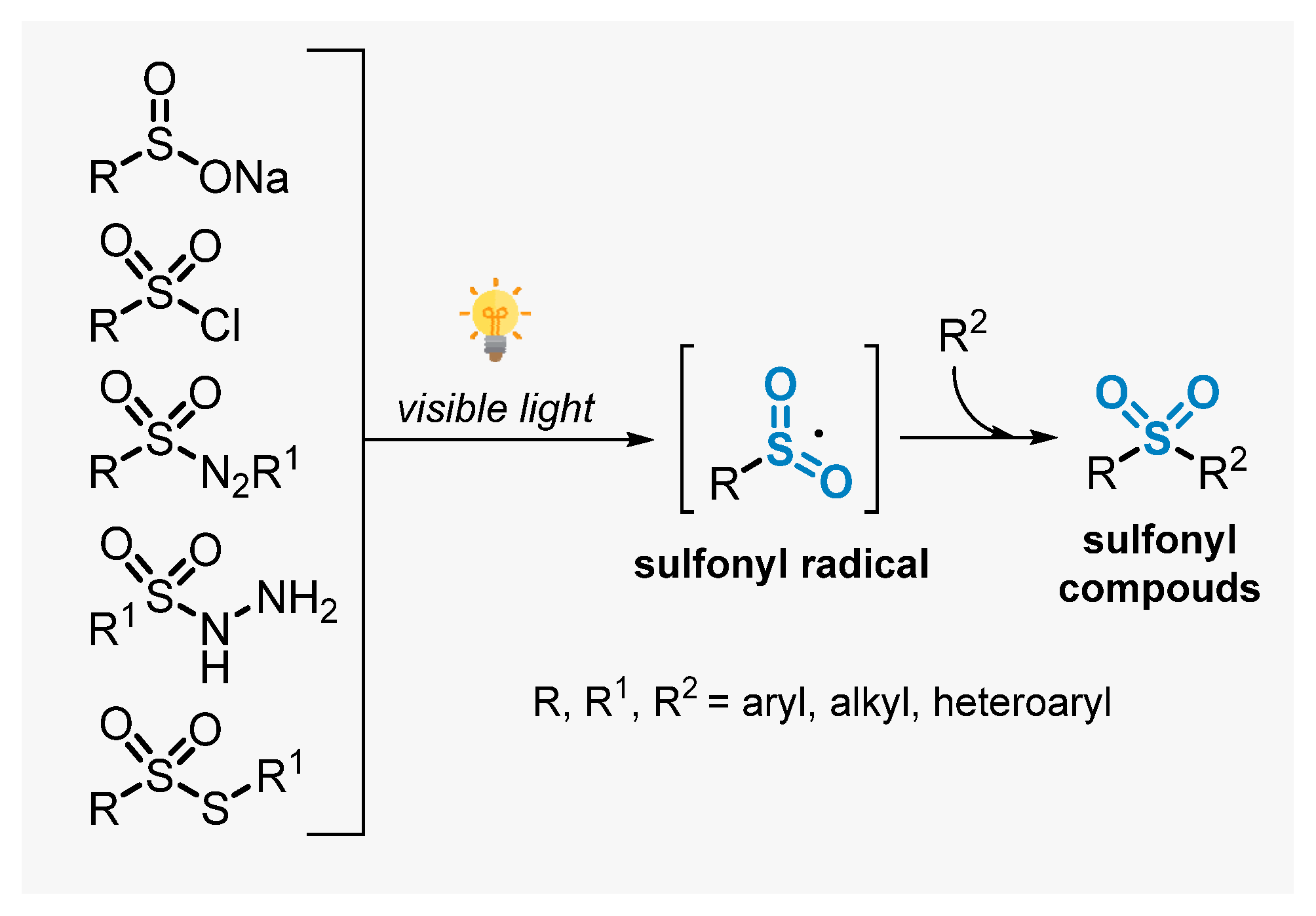
Sulfones are a very important class of compounds which are widely found in the structure of several pharmaceutical and agrochemical ingredients [19,49]. In addition, these compounds are versatile synthetic intermediates in synthetic organic chemistry, being employed in a number of important transformations [50]. As a consequence, the synthesis of sulfones has been increasingly explored and several methods were described in the literature [51,52]. This section deals with the recent advances to construct sulfone-decorated compounds, by employing visible light as energy source. In this context, approaches involving the use of sulfonyl pre-functionalized starting materials will be discussed first. More specifically, the application of sodium sulfinates, sulfinic acids, sulfonyl chlorides, sulfonyl hydrazines, thiosulfonates, and arylazosulfones in photoinduced reactions will be discussed. In general, when these substrates are submitted to visible light irradiation in the presence of a photocatalyst, the formation of sulfonyl radical intermediates in situ is triggered, which can promote radical addition events in unsaturated substrates, reaching the corresponding sulfonyl compounds (Scheme 11) [53].
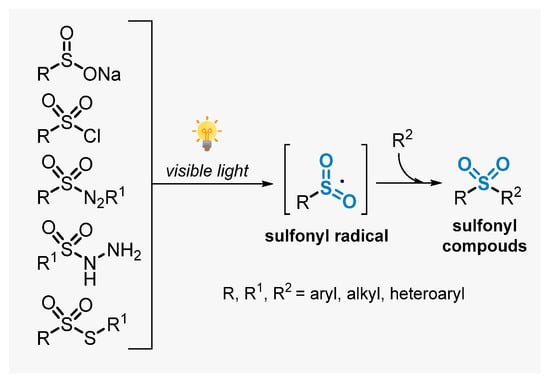
Scheme 11.
Sulfonyl radicals from substrates containing the sulfone moiety.
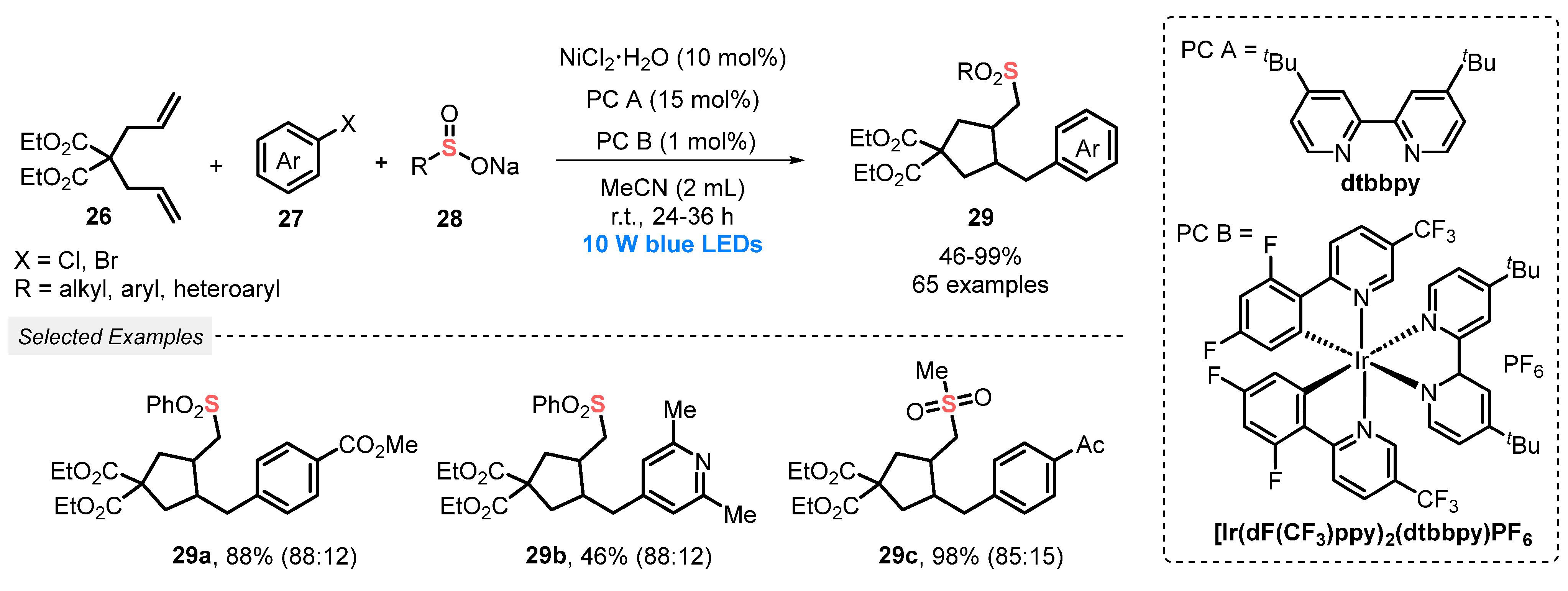
In 2019, Rueping and co-workers [54] developed a novel and scalable photoredox Ni-catalyzed cascade cross-coupling of dienes with electrophilic coupling reaction partners. The transformation involves the reaction between dienes 26, aryl halides 27 and sodium sulfinate salts 28, in the presence of [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 as photocatalyst, the catalyst NiCl2·6H2O, and dtbbpy as ligand in MeCN as solvent. The resulting mixture was constantly irradiated by blue light (blue LEDs, 10 W) to deliver a massive range of sixty-five sulfone derivatives 29 in good regioselectivity and moderate to excellent yields (46–99%) (Scheme 12). It should be noted that the main advantage of the developed method involves the use of a low amount of photocatalyst (1 mol%), proving its efficiency in obtaining highly functionalized molecules with excellent selectivity.
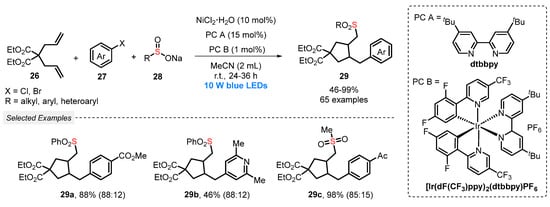
Scheme 12.
Visible light-induced cascade cross-coupling of dienes 26 using nickel dual catalysis.
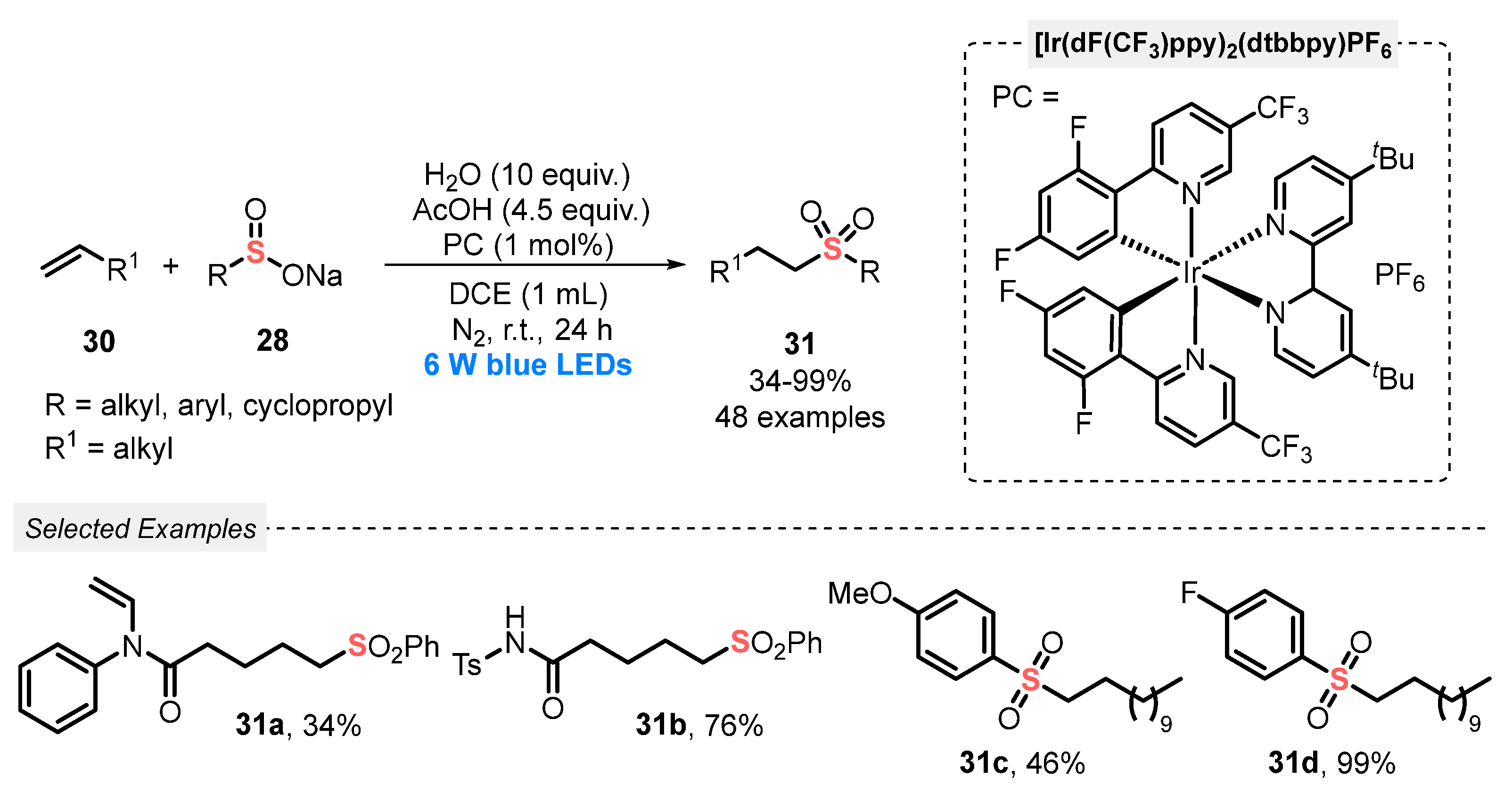
In the same year, Yu and co-workers [55] developed a visible light-mediated (blue LEDs, 6 W) hydrosulfonylation of alkenes 30 with sodium sulfinates 28, using [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 as photocatalyst. According to the experimental results, the use of water (10 equiv) and AcOH (4.5 equiv) as additives is essential for the reaction success. In general, the protocol was tolerant to different substrates, including those containing aliphatic chains, electron-donating and electron-withdrawing substituents attached to the aromatic ring. A total of forty-eight sulfones 31 were accessed, in poor to excellent yields (13–99%) (Scheme 13).
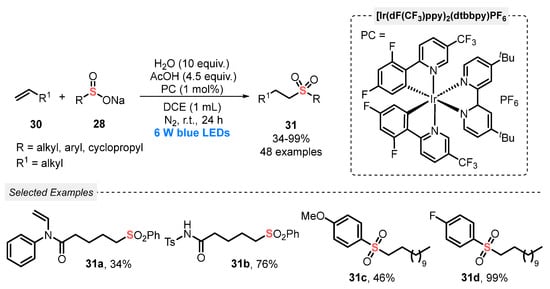
Scheme 13.
Sulfonylation of alkenes 30 promoted by blue LEDs under acid condition.
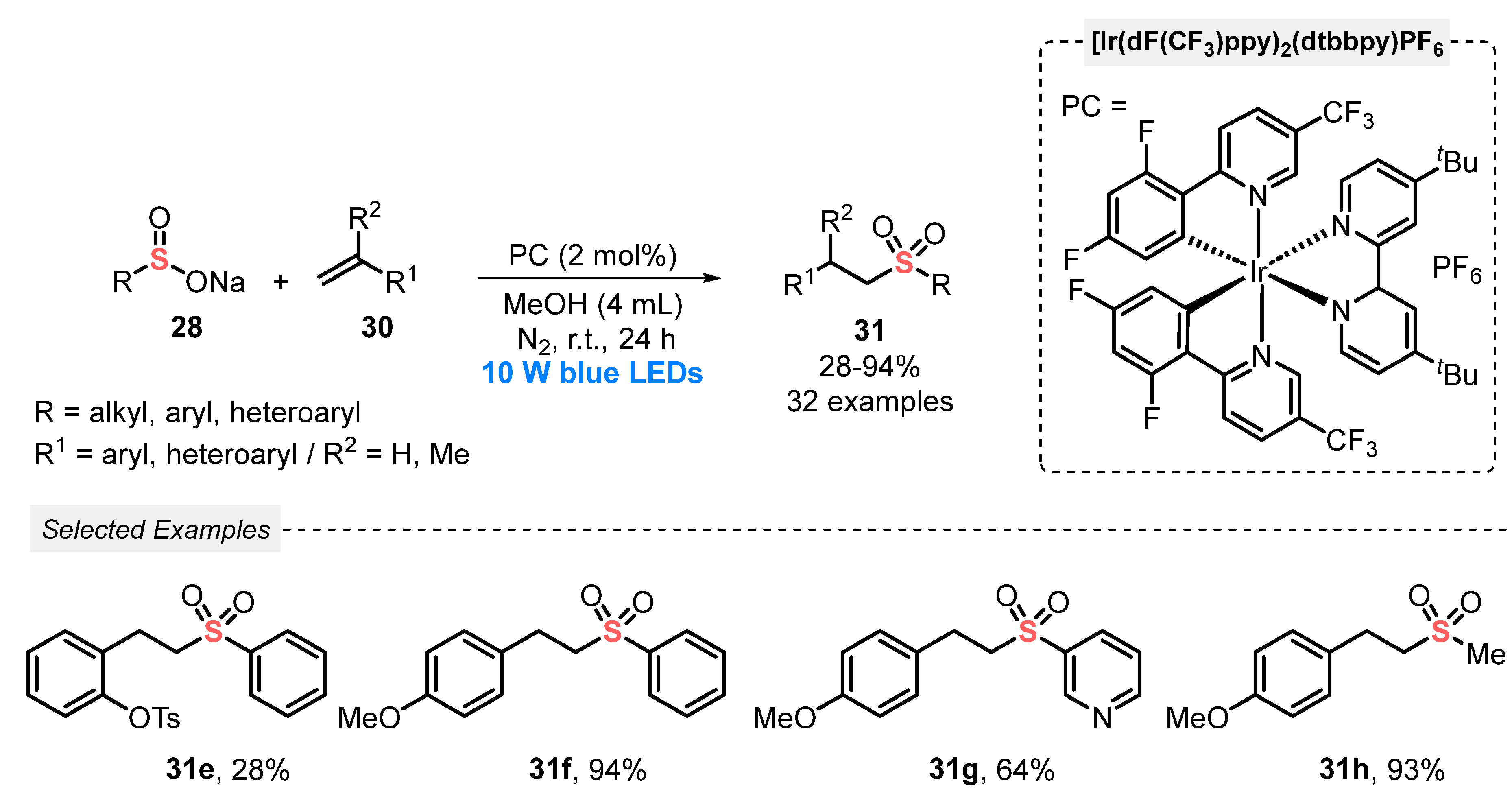
A year later, in 2020, Duan and co-workers [56] proposed a complementary alternative for the hydrosulfonylation of alkenes 30 by sodium sulfinates 28, which avoids the use of H2O and AcOH as additives. In this case, the authors used MeOH as solvent and a more powerful source of light (blue LEDs, 10 W). Under these conditions, thirty-two examples of the product 31 were prepared, in poor to very good yields, including aryl, heteroaryl, and alkyl-substituted ones (Scheme 14). In comparison with the method developed by Yu (Scheme 13), the present protocol requires the double amount of photocatalyst (2 mol%), in addition to the need for a N2 atmosphere. On the other hand, it avoids the use of acetic acid and halogenated solvents such as DCE.
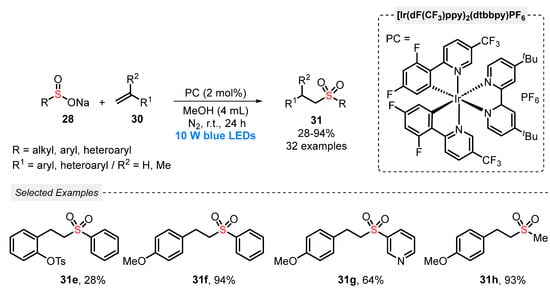
Scheme 14.
Sulfonylation of gem-alkenes 30 promoted by blue LEDs under neutral condition.
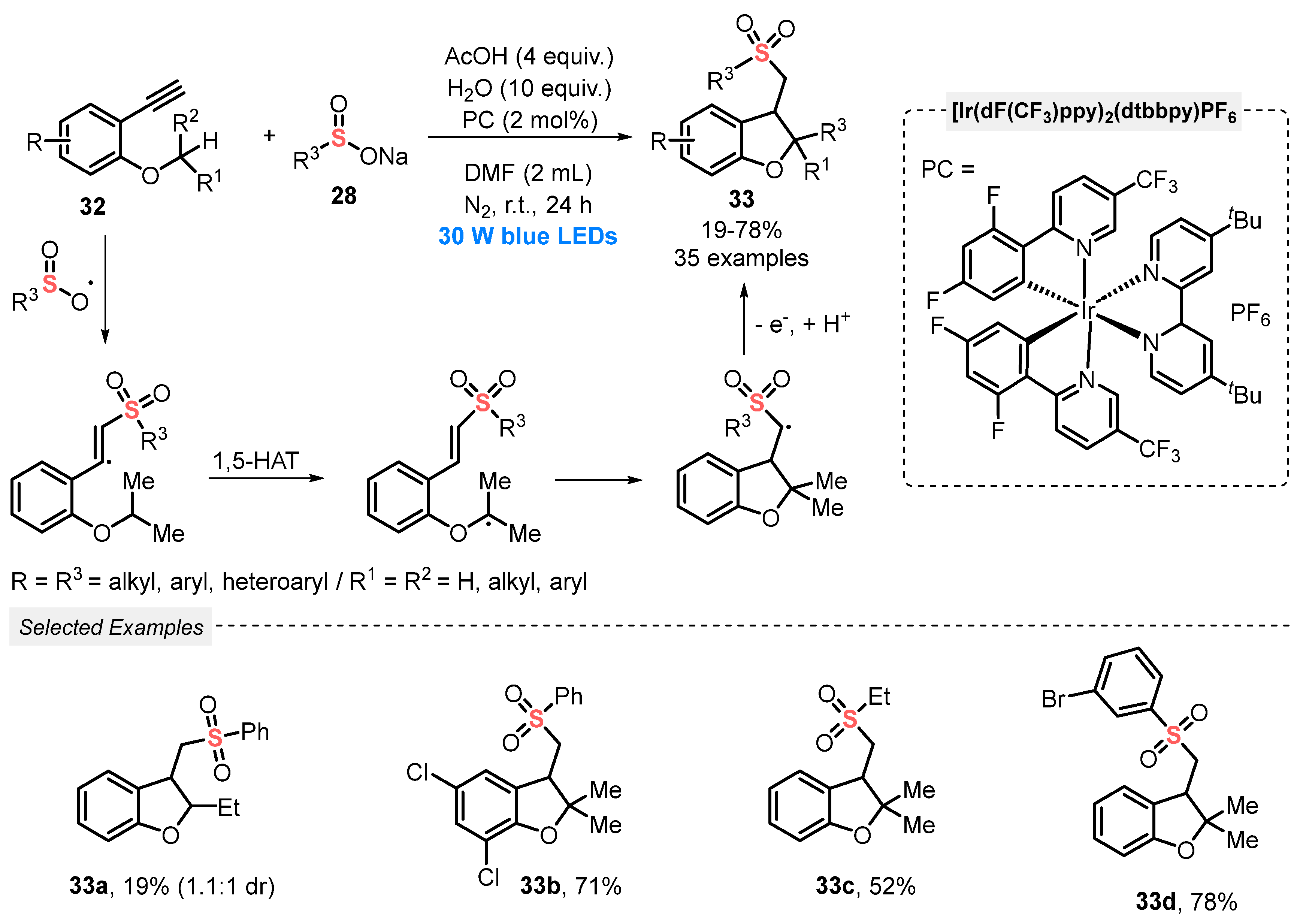
Also, in 2020, Su and co-workers [57] disclosed the synthesis of phenylsulfonyl-functionalized dihydrobenzofurans 33, through a visible light-mediated radical addition/annulation process, involving the simultaneous construction of C–S and C–C bonds. A mixture of 2-alkynylarylethers 32, sodium sulfinates 28, H2O and AcOH as additives in the presence of the same Ir-based photocatalyst and DMF as solvent was irradiated with blue light (blue LEDs, 30 W) under inert atmosphere (N2). After 24 h of irradiation, a total of thirty-five phenylsulfonylated dihydrobenzofurans 33 was accessed in poor to good yields (19–78%) (Scheme 15). It is worth mentioning that the product 33a (R1 = H and R2 = Et) was obtained in only 19% yield, probably due to the less stable nature of the reaction intermediate.
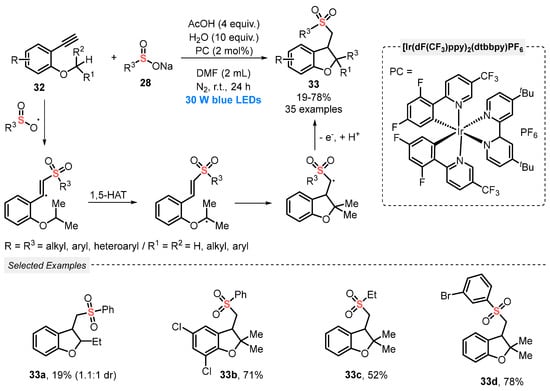
Scheme 15.
Synthesis of phenylsulfonyl-functionalized dihydrobenzofurans 33 in an intramolecular 1,5-hydrogen atom transfer process under visible light.
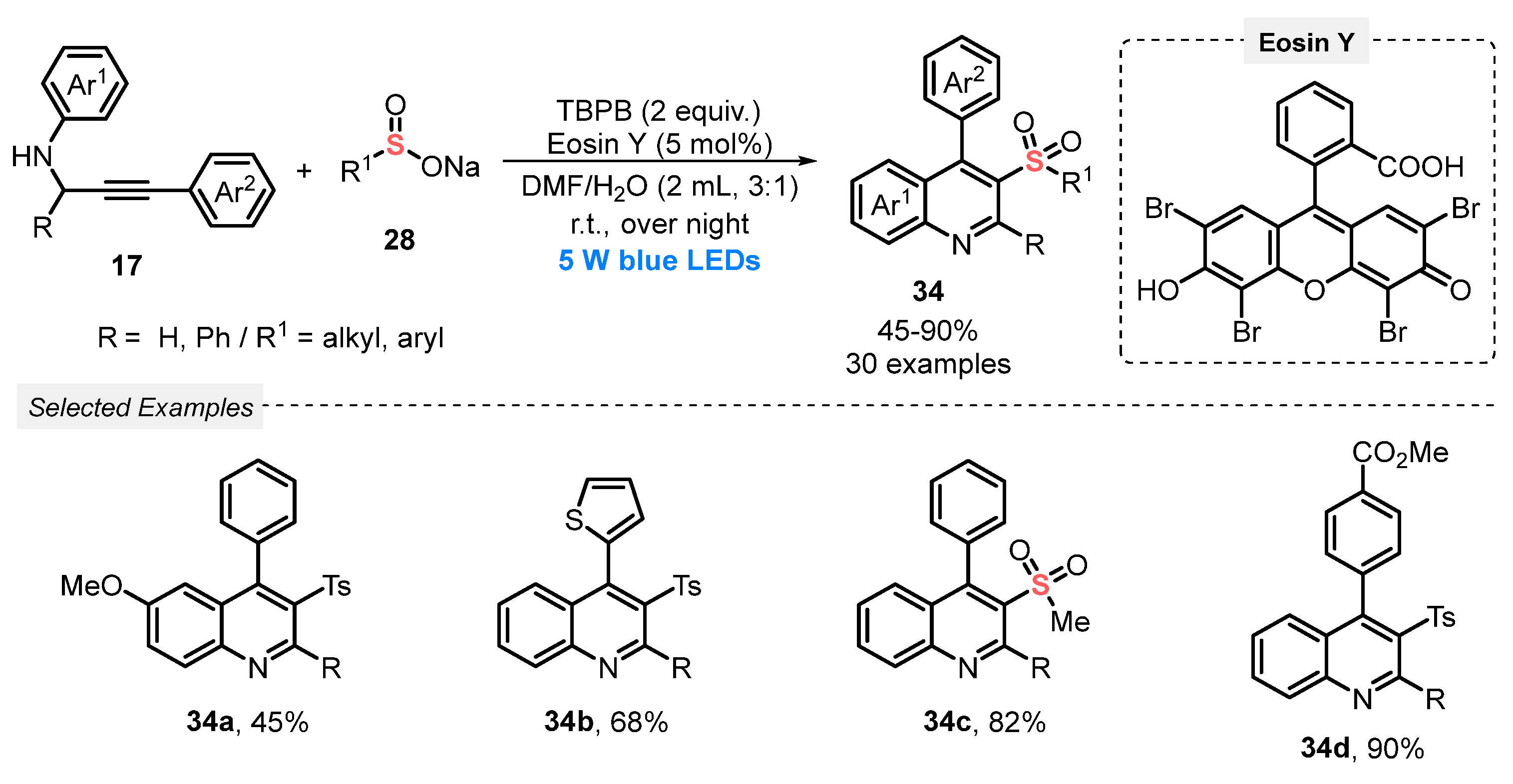
In the same year, Huang and co-workers [58] reported the visible light-mediated synthesis of 3-sulfonylquinolines 34, via a cascade radical cycloaddition between N-propargylanilines 17 and sodium sulfinates 28. The use of TM-based photocatalytic species was avoided by employing Eosin Y as photocatalyst, in the presence of tert-butyl peroxybenzoate (TBPB) as oxidant, and a 3:1 mixture of DMF/H2O as solvent. The scope of the cyclization was extended to a diversity of substituted alkynes 17 and sodium sulfinates 28, to produce the respective 3-sulfonylquinolines 34 in moderate to excellent yields (45–90%), employing blue light (blue LEDs, 5 W) for 14 h (Scheme 16).
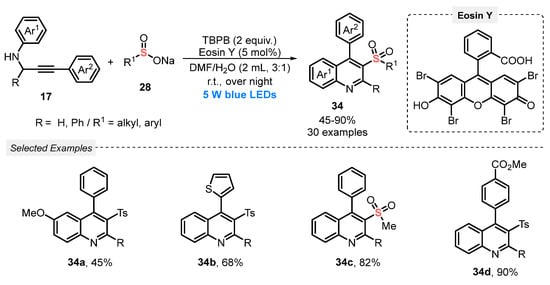
Scheme 16.
Synthesis of 3-sulfonylquinolines 34 under visible light conditions.
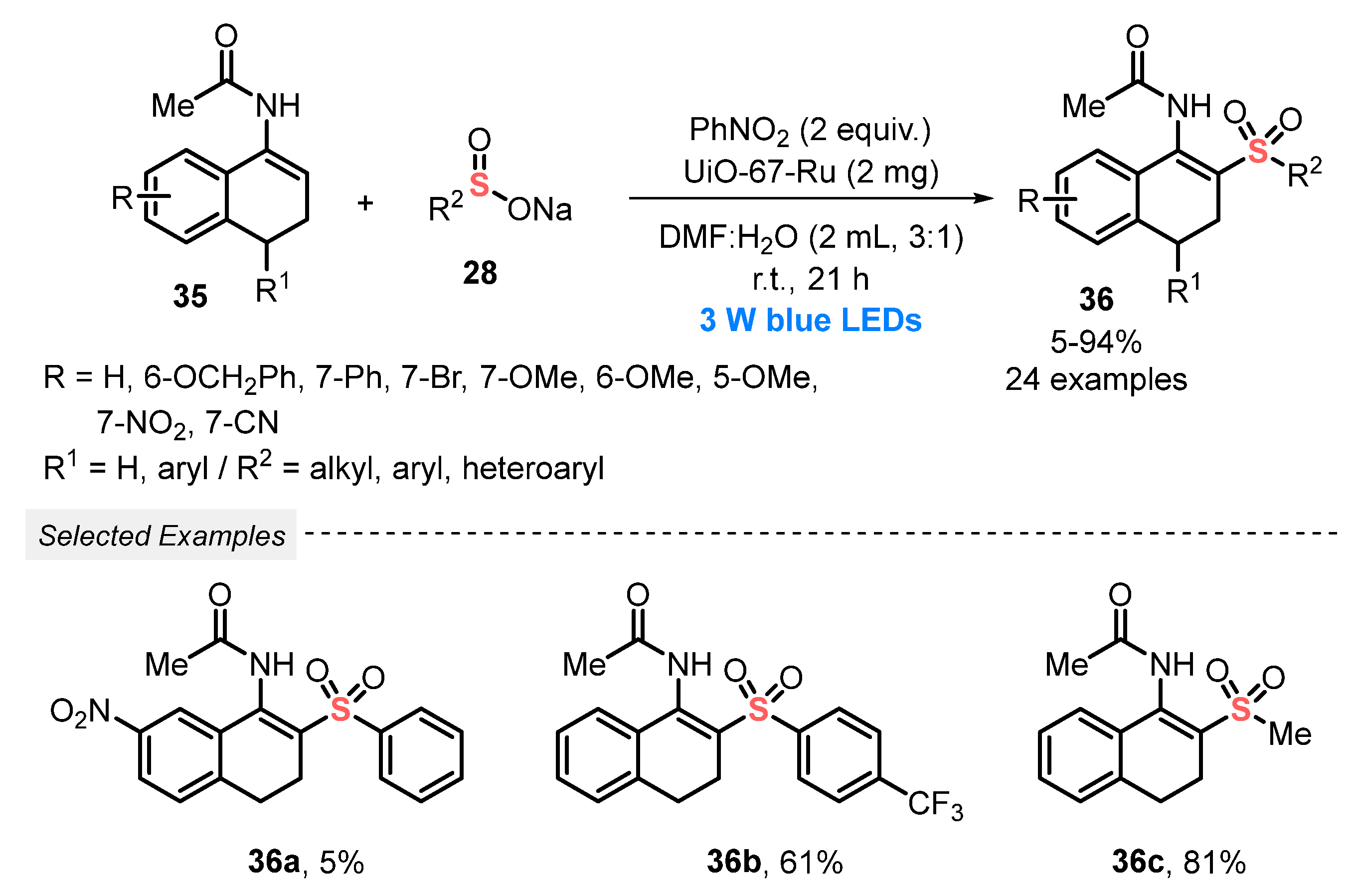
Still, in 2020, Zhang and co-workers [59] reported the application of sodium sulfinates 28 to prepare β-acetylamino acrylosulfones 36, through a visible light-assisted cross-coupling with enamides 35, in the presence of the zirconium-based MOF UiO-67-Ru as a heterogeneous photocatalyst. The substrates and the catalyst were mixed using a 3:1 mixture of DMF/H2O, in the presence of PhNO2 (2 equiv) as oxidant. The resulting mixture was constantly irradiated with blue light (blue LEDs, 3 W) at room temperature for 21 h. A wide variety of β-acetylamino acrylosulfone derivatives 36 were prepared in poor to excellent yields (5–94%), demonstrating a satisfactorily functional group tolerance. It is worth mentioning that the heterogeneous photocatalyst could be reused for up to five reaction cycles, without presenting remarkable yield decrease (Scheme 17). Furthermore, a catalyst recycling study was carried out, in which the UiO-67-Ru was used by five cycles, presenting high catalytic activity.
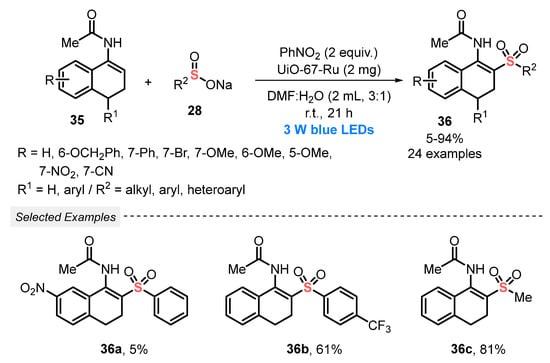
Scheme 17.
Oxidative cross-coupling to the synthesis of β-acetylamino acrylosulfones 36.
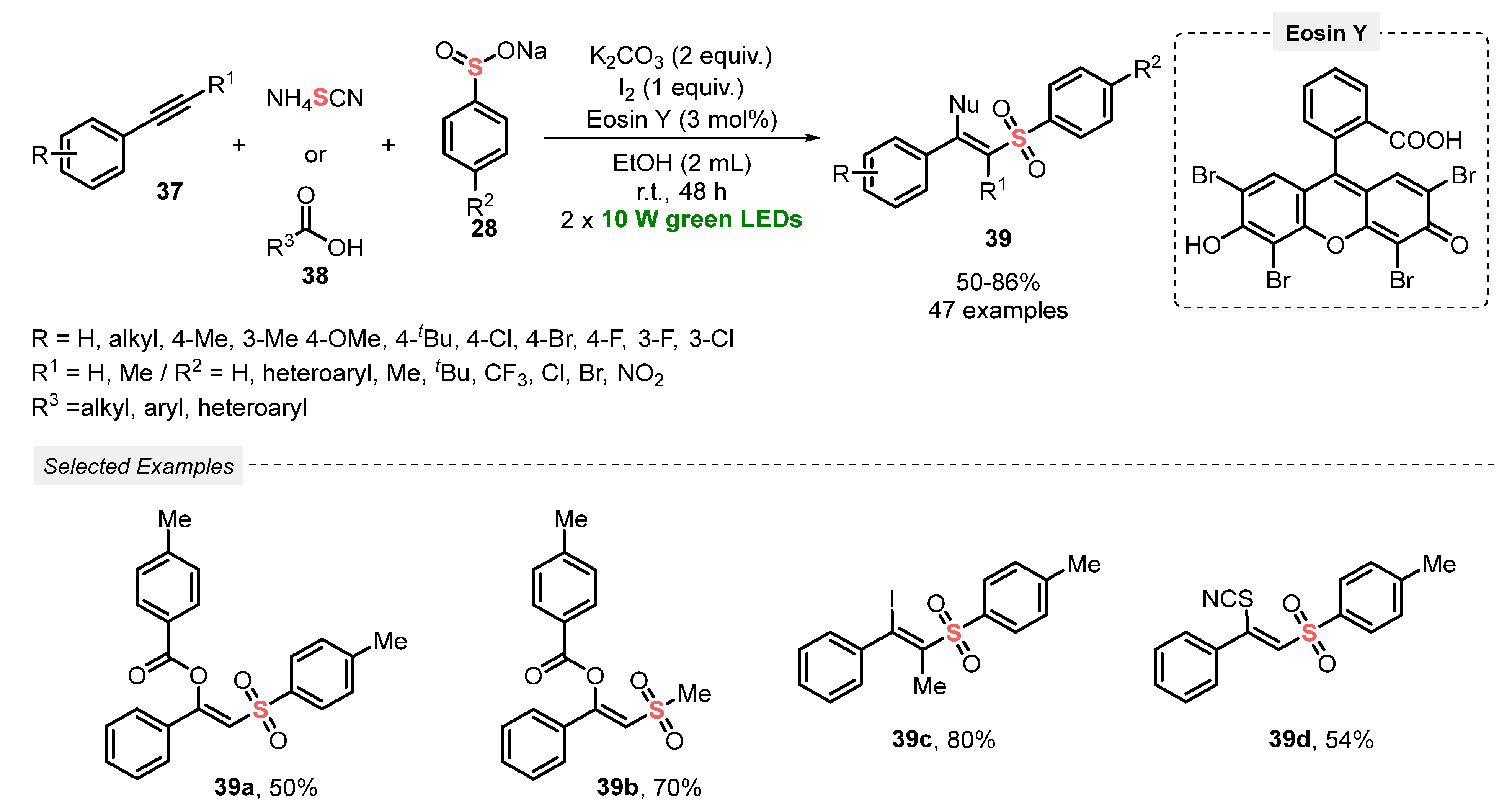
In 2021, Patel and co-workers [60] developed a one-pot visible light-mediated methodology to promote the difunctionalization of terminal alkynes 37, using carboxylic acids/I2 38 or NH4SCN, and sodium aryl sulfinates 28 as reaction partners. The process involves the use of Eosin Y as photocatalyst, in the presence of K2CO3 as base, I2 as oxidant and EtOH as solvent, and the mixture was irradiated with green light (green LEDs, 20 W) for 48 h. A diversity of forty-seven β-substituted vinylsulfones 39 was satisfactorily prepared in moderate to very good yields (50–80%). A range of control experiments to gain mechanistic insights were carried out, including radical-trapping, cyclic voltammetry, and fluorescence quenching, which indicated that the reaction mechanism initiates by a radical-induced iodosulfonylation, followed by a base-mediated nucleophilic substitution. Furthermore, a huge exclusive Z-selectivity in the formation of the products 39 was observed, as well as an outstanding reaction tolerance for bulky, electron-rich, and electron-deficient substrates (Scheme 18).
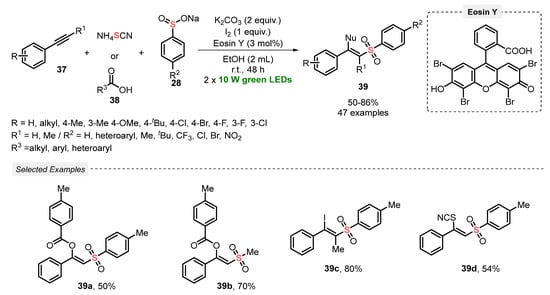
Scheme 18.
Synthesis of β-substituted vinylsulfones 39 via the functionalization of alkynes 38 mediated by visible light.
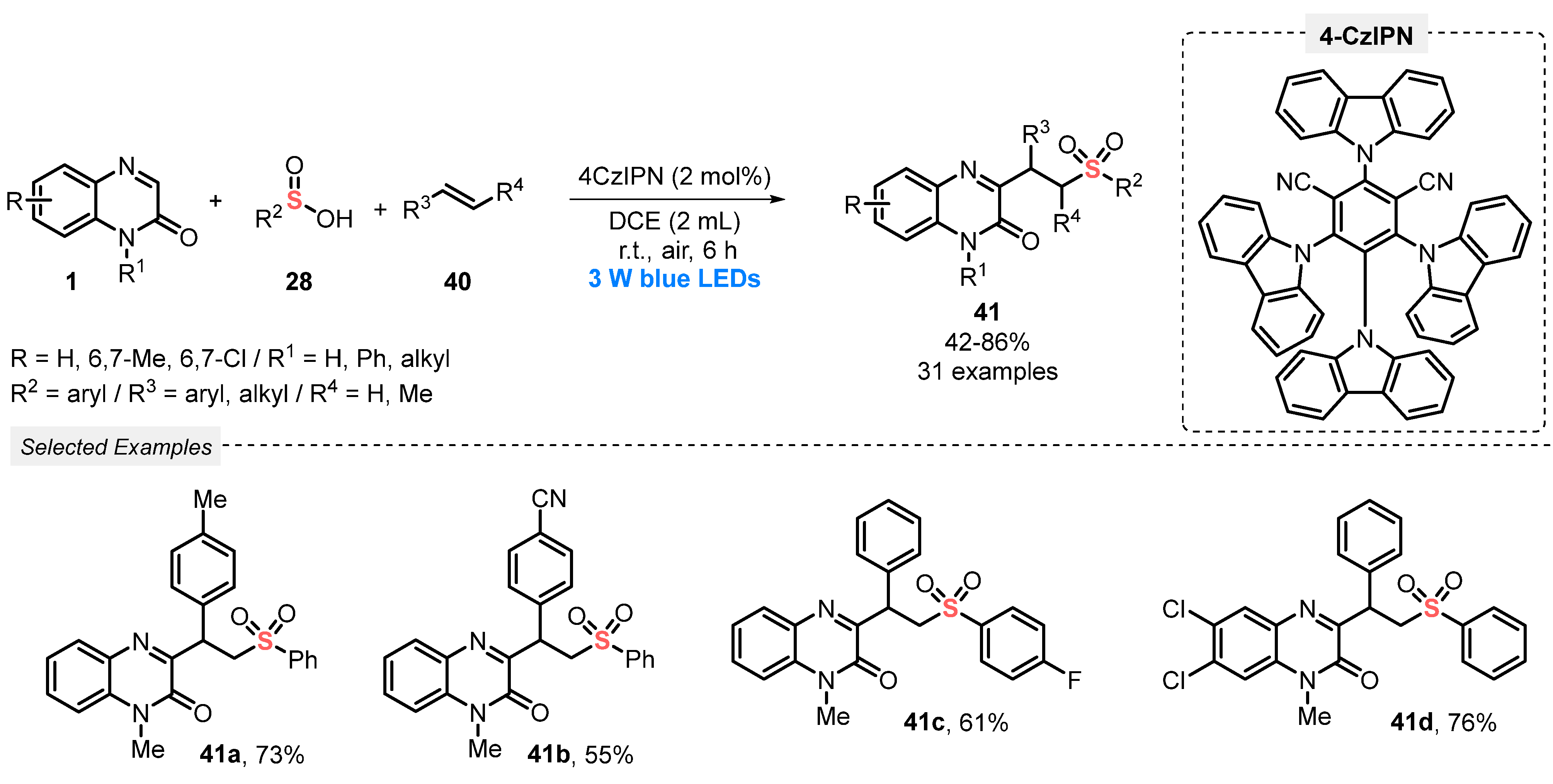
In 2021, Wei and co-workers [61] reported the visible light-mediated application of sulfinic acids 28 as sulfonating agents, aiming to construct alkylsulfonated quinoxalin-2(1H)-ones 41. The method involves a three-component tandem reaction between quinoxalin-2-(1H)-ones 1, sulfinic acids 28, and alkenes 40 in the presence of the cyanoarene-based donor-acceptor photocatalyst 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) and DCM as solvent. The reaction mixture was irradiated for 6 h with blue light (blue LEDs, 3 W) under open-air conditions (exploring O2 as an environmentally benign oxidant). Under these conditions, thirty-one differently substituted alkylsulfonated quinoxalin-2(1H)-one derivatives 41 were prepared in moderate to very good yields (42–86%) (Scheme 19). The green features of this protocol include the performance of reactions at room temperature, for short reaction times, under open-air conditions, by employing non-metallic catalyst in low quantity (2 mol%).
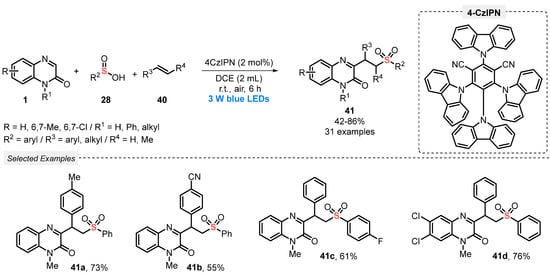
Scheme 19.
Synthesis of sulfonated quinoxalin-2(1H)-ones 41 promoted by blue LEDs.
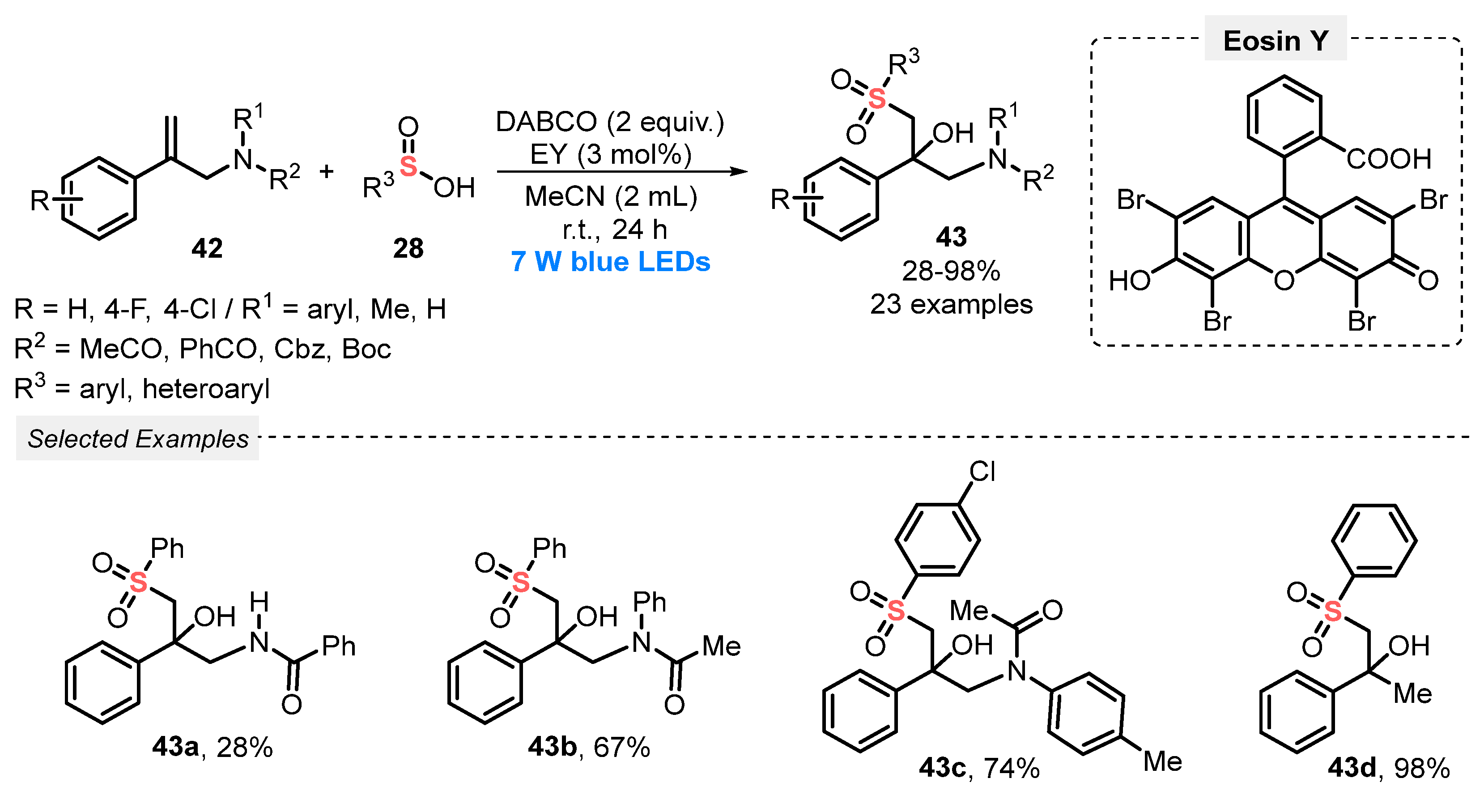
In 2021, Guan, He, and co-workers [62] described a visible light-driven sulfonylation/hydroxylation of allylacetamides 42, in the presence of sulfinic acids 28 as reaction partner, affording β-tert-hydroxy sulfone derivatives 43. The transformation was performed in the presence of Eosin Y as photocatalyst, DABCO as base and MeCN as solvent, being the resulting mixture stirred under blue light irradiation (blue LEDs, 7 W) for 24 h. Under this condition, twenty-three sulfone derivatives 43 were obtained in poor to excellent yields (28–98%), with a diversity of substituents both in the allylacetamides 42 and in the sulfinic acid counterparts (Scheme 20). Despite the wide reaction scope, some disadvantages are faced, such as failure of the reaction when methylsulfinic acid was used as substrate, which may be due to the low stability of the correspondent sulfonyl radical. Furthermore, the reaction also failed when N-(2-phenylallyl)aniline was used, probably due to the high reactivity of the free amine (NH).
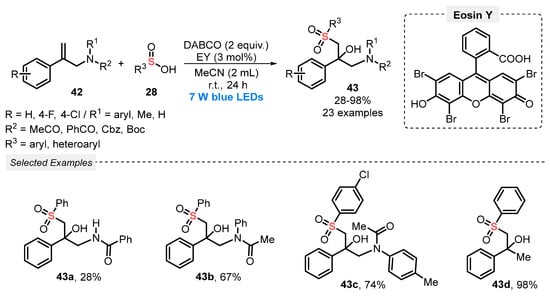
Scheme 20.
Sulfonylation/hydroxylation of allylacetamides 42 induced by blue LED.
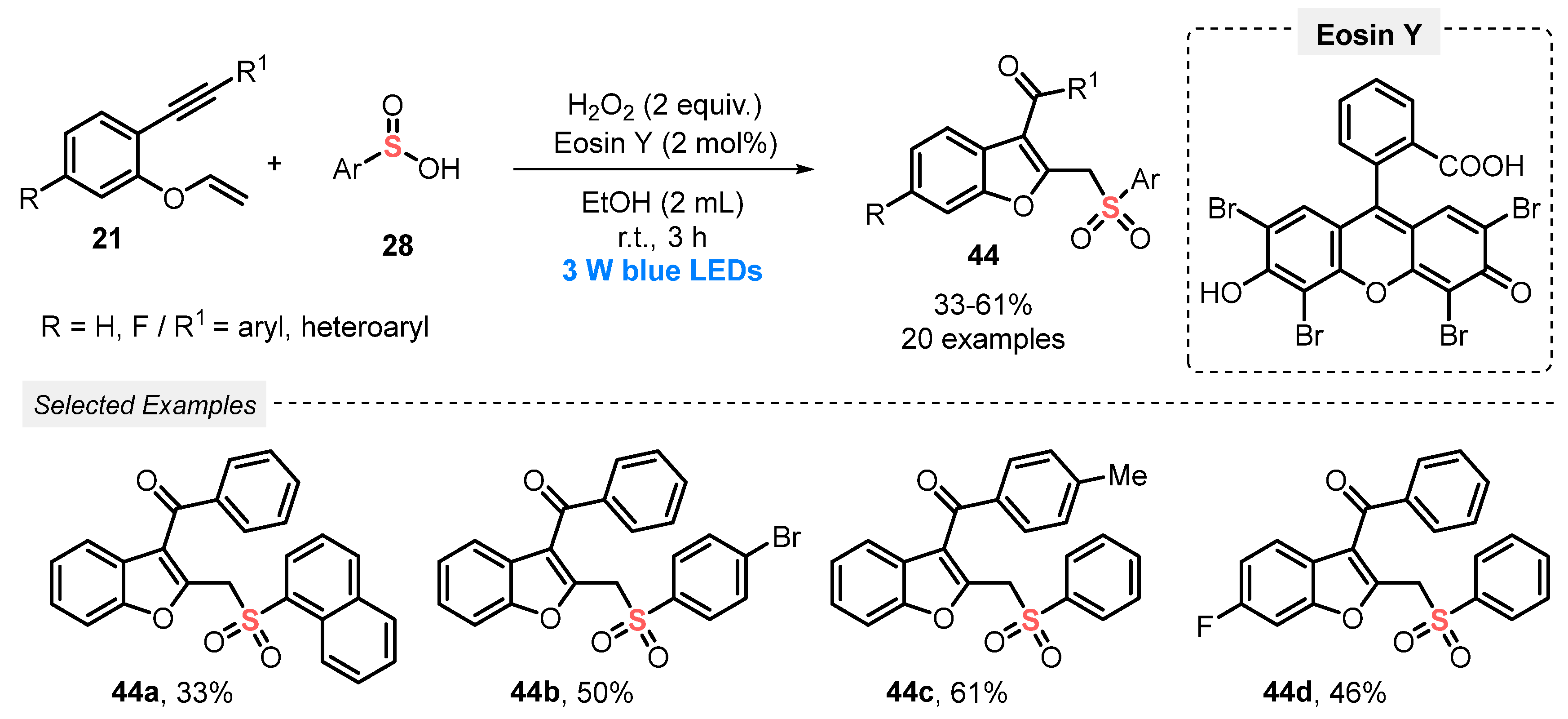
The synthetic versatility of sulfinic acids was demonstrated in light-mediated radical cyclization reactions, as will be discussed in the next works. In 2019, Wei and co-workers [63] described an unprecedented Eosin Y-photocatalyzed synthesis of sulfonylated benzofurans 44, through the reaction of 1,6-enynes 21 and arylsulfinic acids 28 under blue light irradiation (blue LEDs, 3 W). This sequential formation of C–S, C–C, and C=O bonds was performed using H2O2 as oxidant and EtOH as solvent. After irradiation for 3 h at room temperature, a total of twenty examples of sulfonyl-decorated benzofurans 44 were afforded in 33–61% yield (Scheme 21). The main advantages of this protocol are the use of mild reaction conditions (room temperature), use of green solvent (MeOH), and short reaction times. However, only moderate to low yields are obtained.
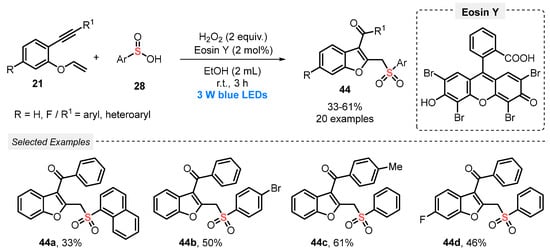
Scheme 21.
Synthesis of sulfonylated benzofurans 44 mediated by visible light.
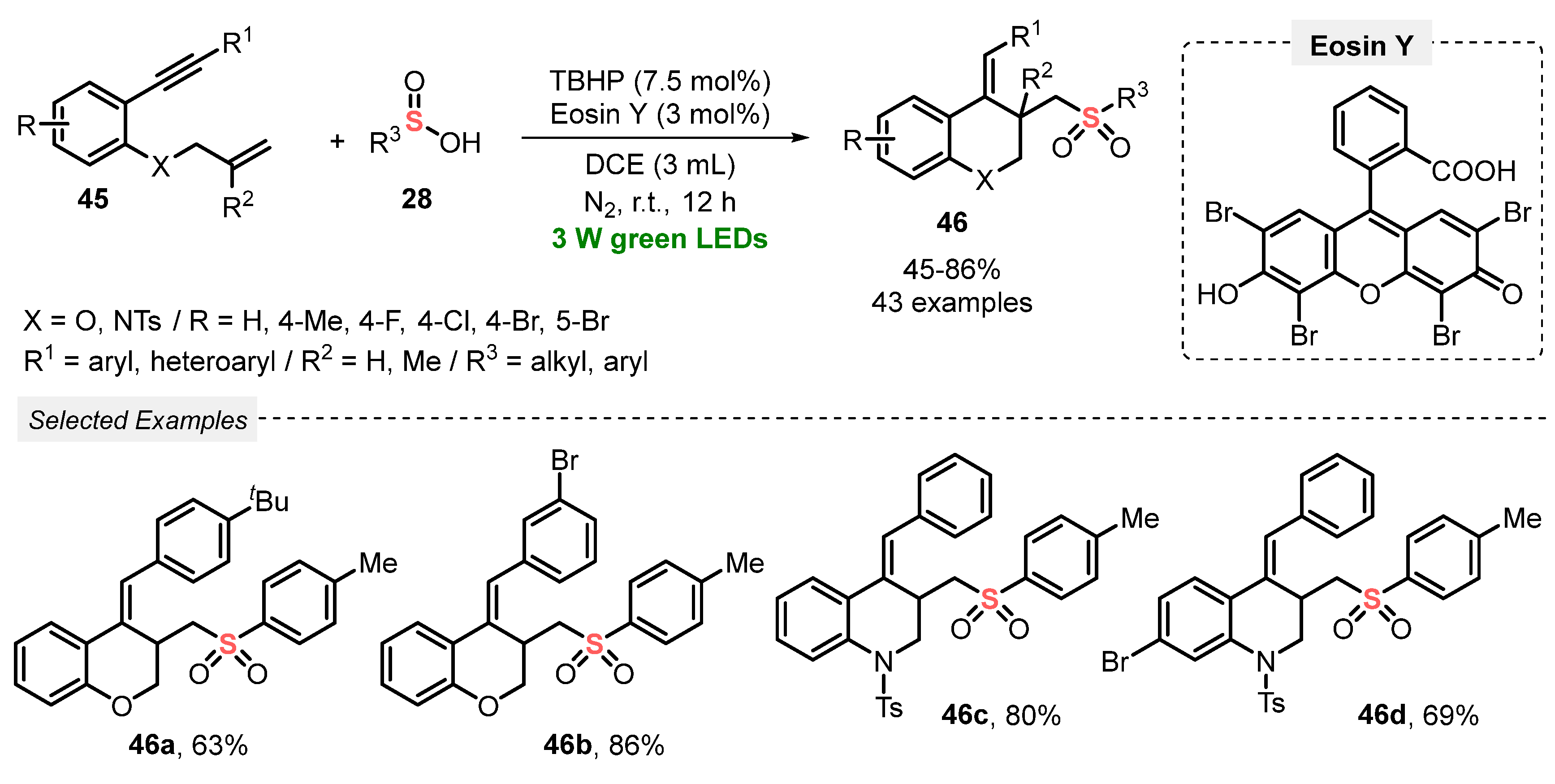
A year later, in 2020, Li and co-workers [64] reported a visible light-mediated approach to construct a diversity of sulfonated chromanes (X = O) and sulfonated 1,2,3,4-tetrahydroquinolines (X = NTs) 46, through a radical cascade cyclization of 1,7-enynes 45 with sulfinic acids 28. A range of products 46 (forty-three examples) was obtained in poor to very good yields, by reacting the substrates in the presence of tert-butyl hydroperoxide (BHP) as oxidant, Eosin Y as photocatalyst, and DCE as solvent. The resulting mixture was irradiated with green light (green LEDs, 3 W) under N2 atmosphere at room temperature for 12 h, to give the expected heterocycles in 45–86% yield. A high tolerance was observed for functional groups in the 1,7-enyne counterpart 45, with good results being obtained starting from halo-substituted and heteroaryl derivatives. In addition, TBHP was employed in low loading (7.5 mol%) (Scheme 22). Compared with the previously described method (Scheme 21), this one proved to be more tolerant to different functional groups, and it provided the desired products 46 in higher yields. However, a longer reaction time (12 h vs. 3 h) was required.
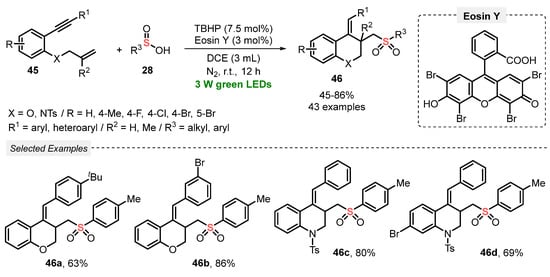
Scheme 22.
Radical cascade cyclizations of 1,7-enynes 45 with sulfinic acids 28 promoted by visible light.
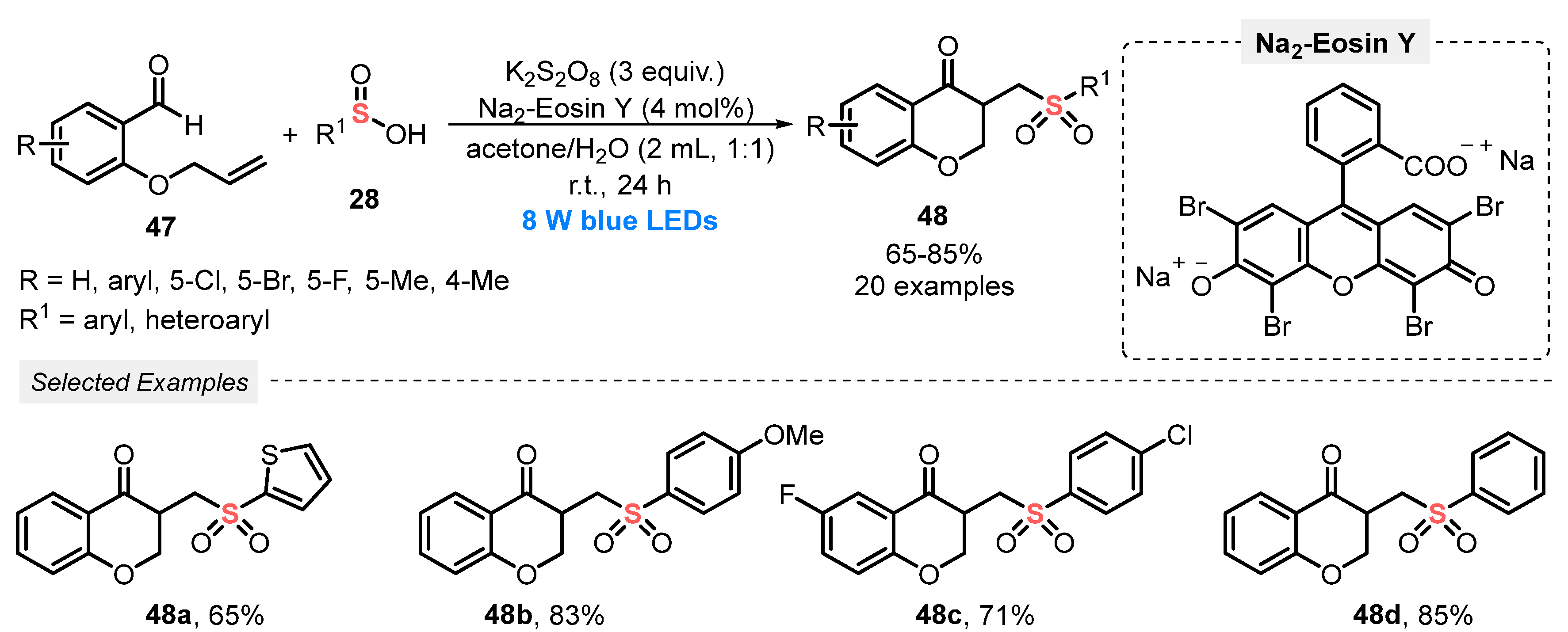
Also, in 2020, Yu and his group [65] reported the synthesis of functionalized chroman-4-ones 48, by reacting o-(allyloxy)arylaldehydes 47 and sulfinic acids 28 under blue light irradiation (blue LEDs, 8 W). To reach the products 48 efficiently, Na2-Eosin Y was used as photocatalyst and K2S2O8 as oxidant. The reactants were diluted in a mixture of acetone and H2O (1:1) and irradiated under stirring for 24 h at room temperature. Under these conditions, a library of twenty derivatives 48 was prepared in good to very good yields (65–85%). Several chloro-, bromo-, and fluoro-substituted o-(allyloxy)arylaldehydes 47 were efficiently used as substrates. Some of the obtained chroman-4-ones 48 were chosen to be submitted to studies of antimicrobial activity, presenting promising results (Scheme 23). Despite significant advances, the scope of sulfinic acids could have been further explored by employing, for example, an aliphatic chain containing sulfinic acid. In addition, the need for using a high amount of K2S2O8 (3 equiv) is another important protocol drawback.
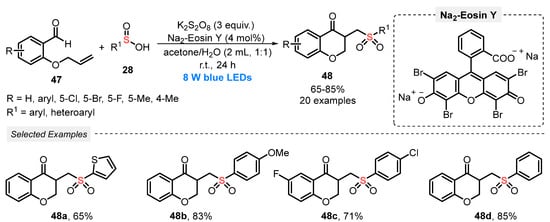
Scheme 23.
Cascade radical cyclization of sulfinic acids 28 and o-(allyloxy)arylaldehydes 47.
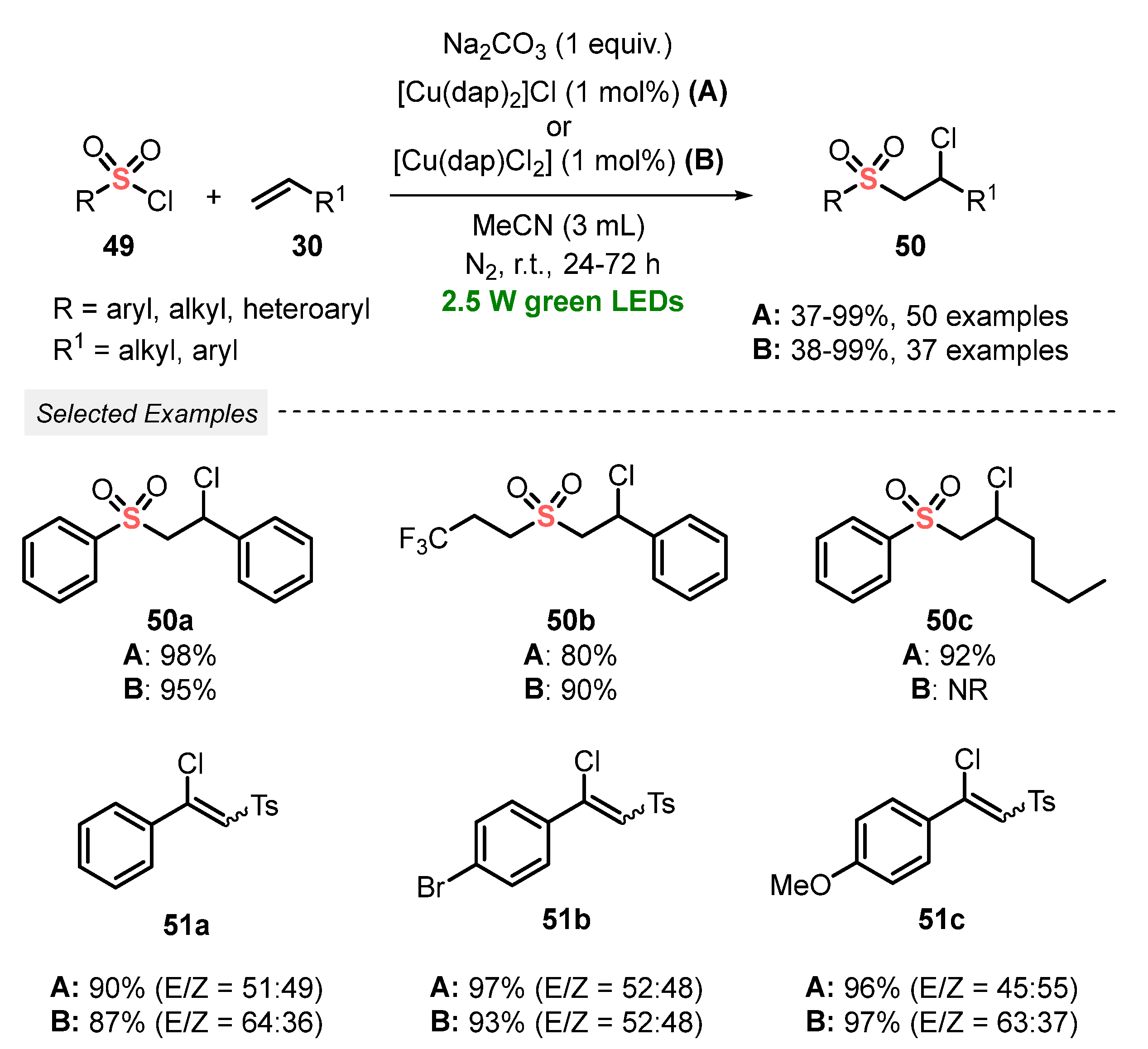
Sulfonyl chlorides are also a promptly available sulfonyl radical source that have been explored for the synthesis of sulfones under visible light irradiation. In this context, in 2019, Reiser and co-workers [66] promoted the chlorosulfonylation of alkenes 30, by employing sulfonyl chlorides 49 as reagent, to obtain the corresponding chlorosulfonylated adducts 50 in poor to excellent yields. The protocol involves the irradiation of the reagents with green light (green LEDs, 2.5 W) in the presence of copper-phenanthroline-based catalysts, with Na2CO3 as an additive and MeCN as solvent. A study comparing the catalytic activity of two copper-based catalysts demonstrated a similar efficiency in the transformations, reaching very comparable yields. However, regarding the economic point of view, [Cu(dap)Cl2] is more efficient, once only half of the dap ligand was required. Furthermore, the protocol was efficiently extended to alkynes instead of alkenes in the reaction with sulfonyl chlorides 49. In this case, the respective vinyl chlorides 51 were obtained in very good yields (87–97%). Despite the high reaction yields, the low selectivity when alkynes are used as substrates is the main disadvantage of the protocol (Scheme 24). On the other hand, the method is operationally simple, allowing the use of a low amount of catalyst, as well as green solvent (MeCN).
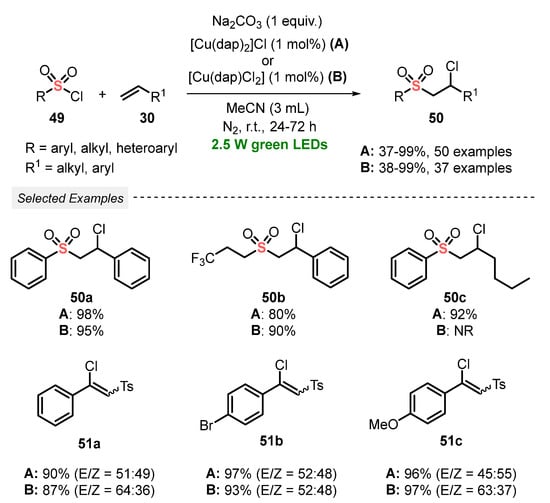
Scheme 24.
Chlorosulfonylation of alkenes and alkynes induced by green LEDs.
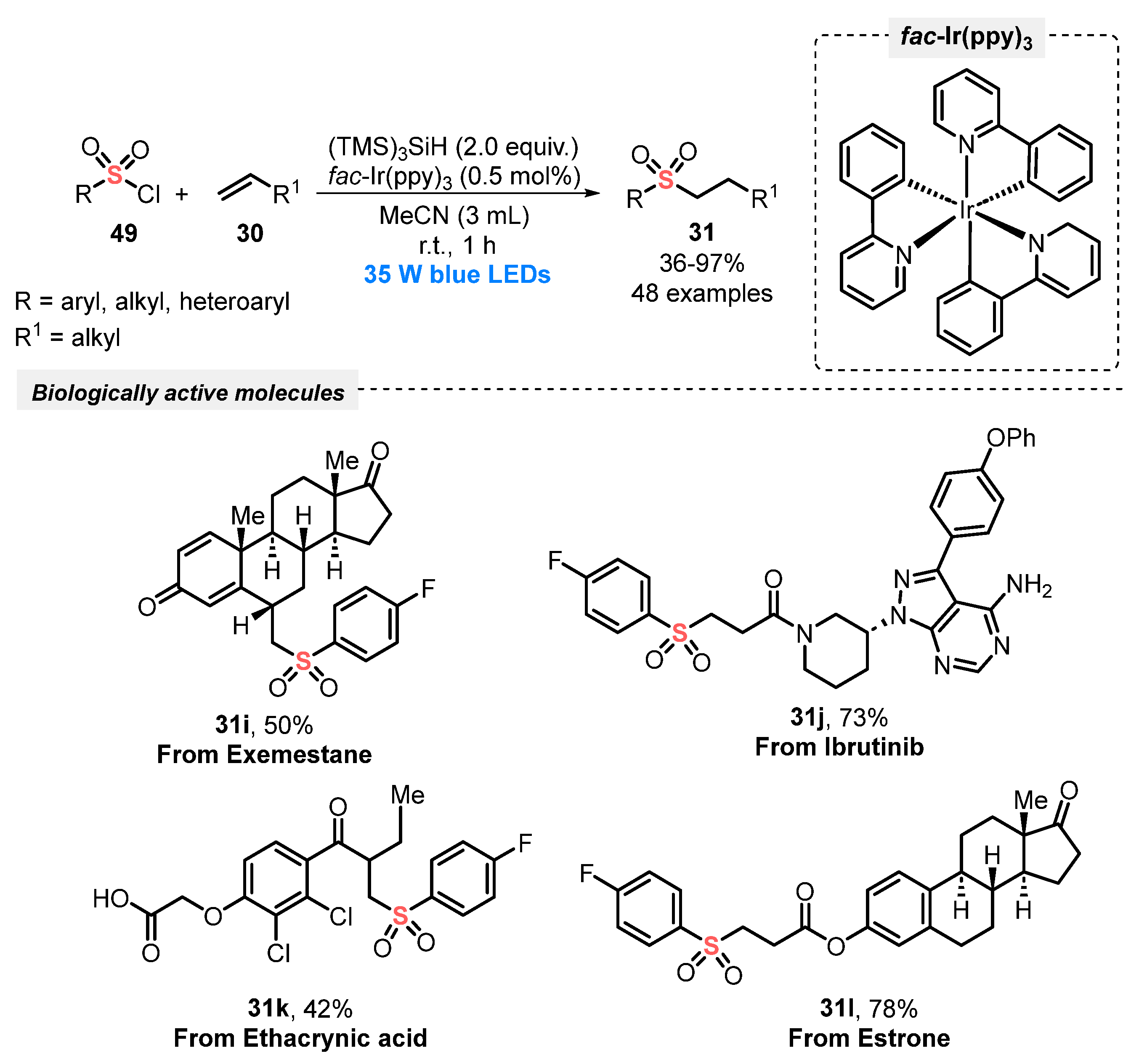
Similarly, in 2020, Gouverneur and co-workers [67] described an alternative light-induced protocol for the hydrosulfonylation of alkenes 30, employing sulfonyl chlorides 49 as the sulfone source. To construct the products successfully, the reagents were irradiated with blue light (blue LEDs, 35 W) in the presence of fac-Ir(ppy)3 as photocatalyst, and tris(trimethylsilyl)silane as the H-donor species in MeCN as the solvent. Under this condition, the corresponding sulfones 31 were satisfactorily provided in up to 97% yield after just 1 h, at room temperature. Additionally, the methodology was remarkably extended to the synthesis of complex structures (compounds 31i–l), which are important in medicinal chemistry and drug discovery (Scheme 25). Compared with the previous method (Scheme 24), the protocol presents some advantages, including short reaction times (only 1 h vs. 24–72 h), the absence of inert atmosphere, and the use of smaller amounts of photocatalyst (only 0.5 mol%), proving the high efficiency of the developed method.
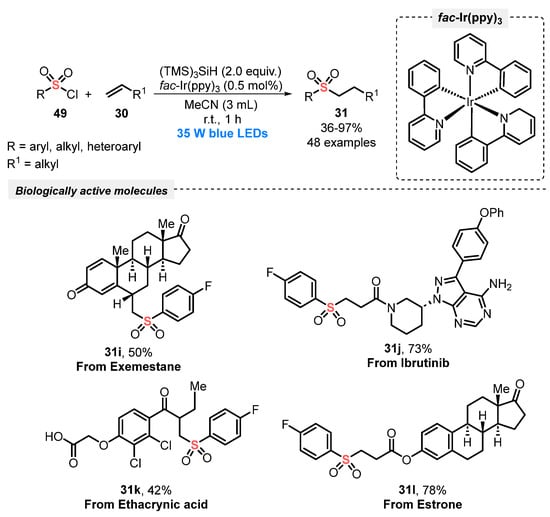
Scheme 25.
Visible light-driven sulfonylation of alkenes 30 and its application to biologically relevant molecules 31i–l.
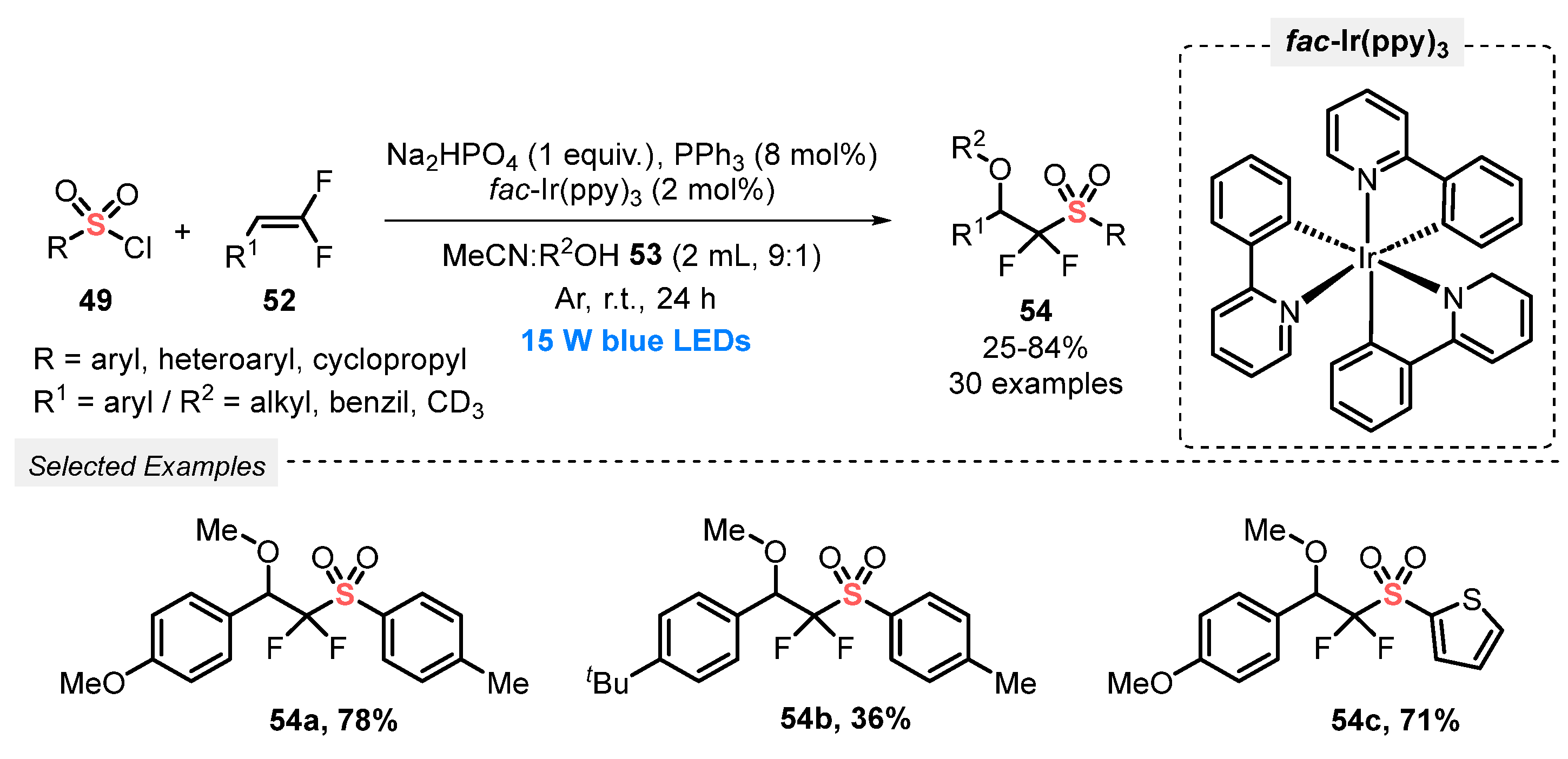
In 2022, Wu’s research group [68] reported the alkoxysulfonylation of gem-difluoroalkenes 52, in the presence of sulfonyl chlorides 49 and alcohols 53, to access the pharmaceutically important organofluorine compounds 54. The reactions were efficiently promoted by using fac-Ir(ppy)3 as photocatalyst, and a mixture of MeCN and an alcohol (2 mL, 9:1) as solvent. The reagents and catalyst were subjected to constant blue light irradiation (blue LEDs, 15 W) for 24 h under Ar atmosphere at room temperature. A total of thirty bis-fluorinated sulfones 54 was obtained in poor to very good yields (25–84%), presenting a good sulfonyl chloride 49 and alcohol 53 substrates tolerance (Scheme 26).
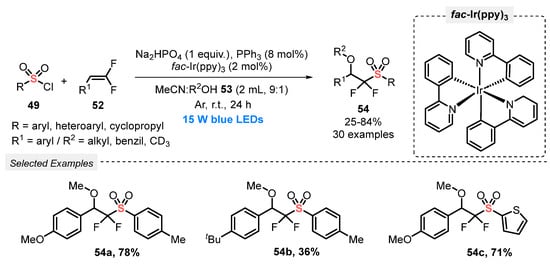
Scheme 26.
Visible light-promoted alkoxysulfonylation of gem-difluoroalkenes 52.
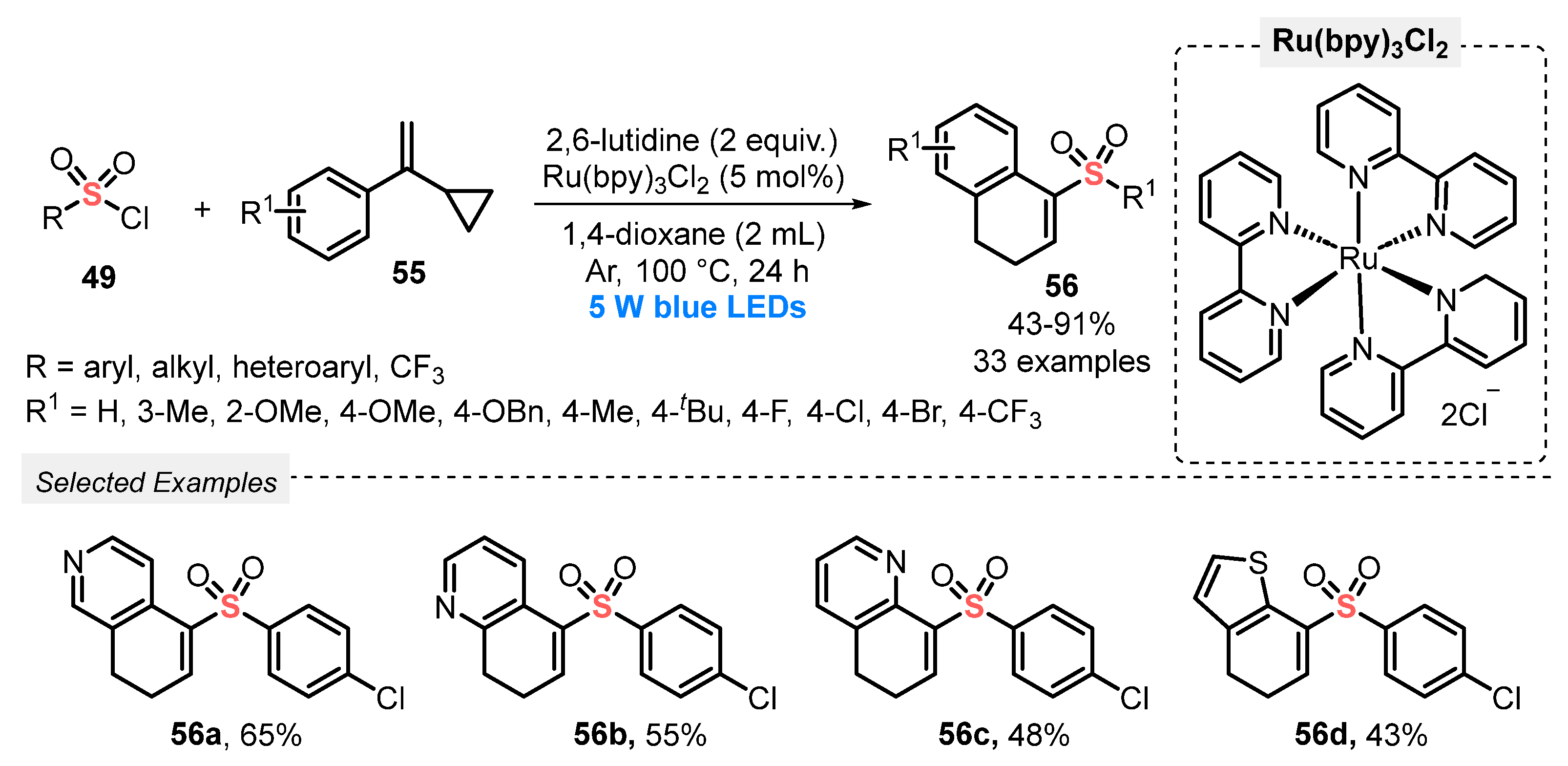
In 2019, Yu and co-workers [69] reported the synthesis of 1-sulfonylmethyl-3,4-dihydronaphthalenes 56 through a radical cyclization reaction between different vinylcyclopropanes 55 and sulfonyl chlorides 49, under visible light irradiation. The best reaction conditions involved the use of Ru(bpy)3Cl as photocatalyst, 2,6-lutidine as base, and 1,4-dioxane as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 5 W) for 24 h at 100 °C, under Ar atmosphere. Under this condition, thirty-three 1-sulfonylmethyl-3,4-dihydronaphthalene derivatives 56 were provided in poor to excellent yields (43–91%), presenting good suitability to the presence of electron-donor and electron-withdrawing substituents in the pendant aromatic ring of the vinylcyclopropanes 55. Furthermore, the method was satisfactorily expanded to heteroaromatic vinylcyclopropanes to construct the products 56a–d, in moderate yields (Scheme 27). The main limitation of the developed method is the need to use a high temperature (100 °C), in quite a long reaction period (24 h).
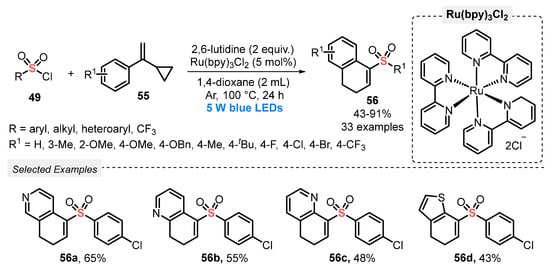
Scheme 27.
Synthesis of 1-sulfonylmethyl-3,4-dihydronaphthalenes 56 promoted by blue LEDs.
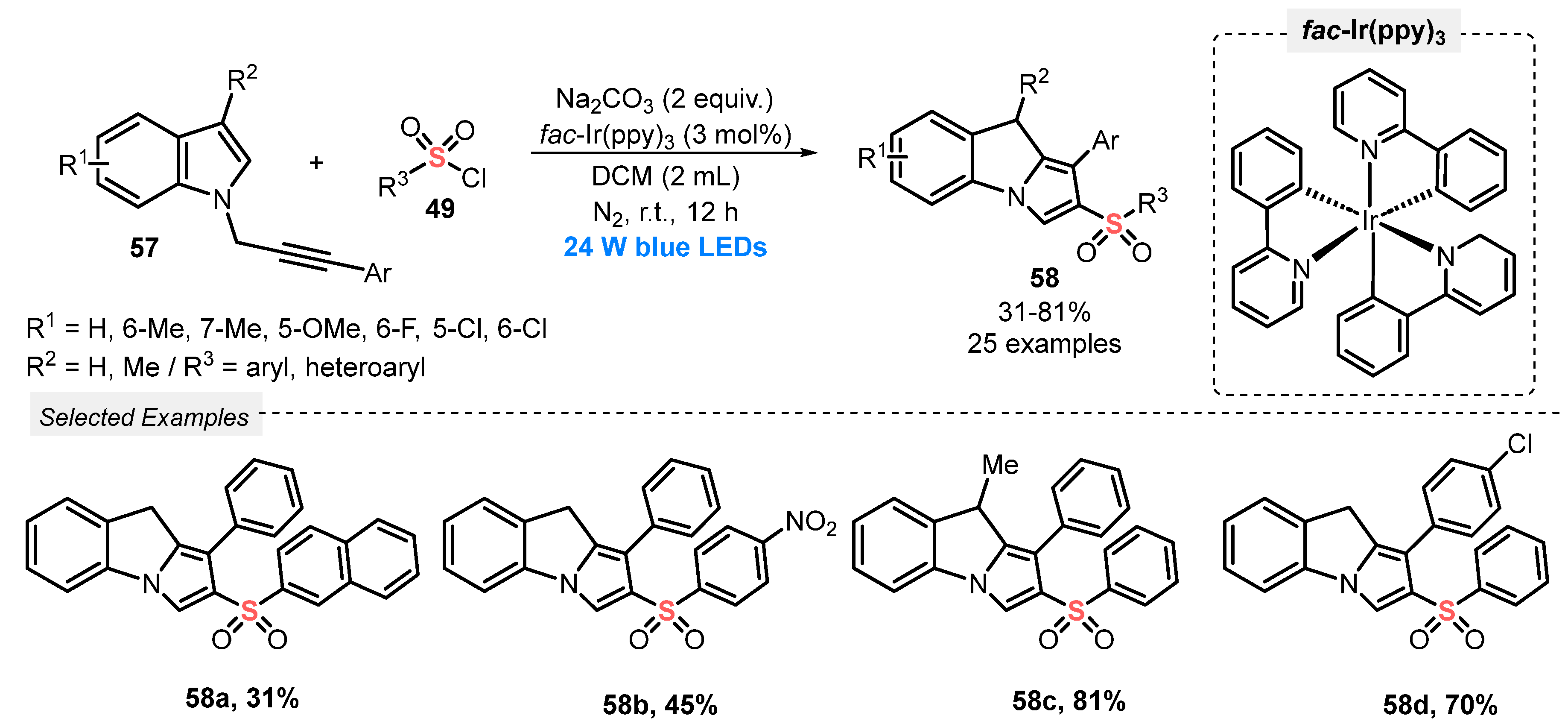
Also, in 2019, Zhao and co-workers [70] reported an oxidant-free cascade annulation of N-propargylindoles 57, in the presence of sulfonyl chlorides 49 as radical precursors, to prepare 2-sulfonated-9H-pyrrolo[1,2-a]indoles 58. The reactants were diluted in DCM, and the photocatalyst fac-Ir(ppy)3 and the base Na2CO3 were added. The resulting mixture was irradiated with blue light (blue LEDs, 24 W), under Ar atmosphere for 12 h at room temperature. This method proved to be effective for a wide range of substrates 57 and 49, such as those with electron-donating and electron-withdrawing, sterically hindered and heteroaromatic groups (Scheme 28).
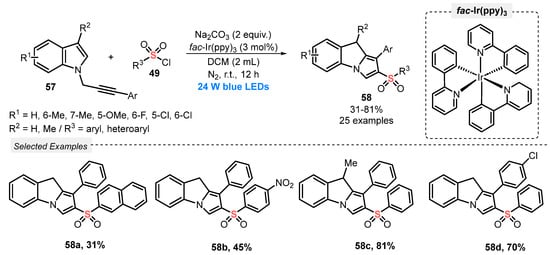
Scheme 28.
Annulation of N-propargylindoles 57 with sulfonyl chlorides 52 to obtain 2-sulfonated 9H-pyrrolo[1,2-a]indoles 49.
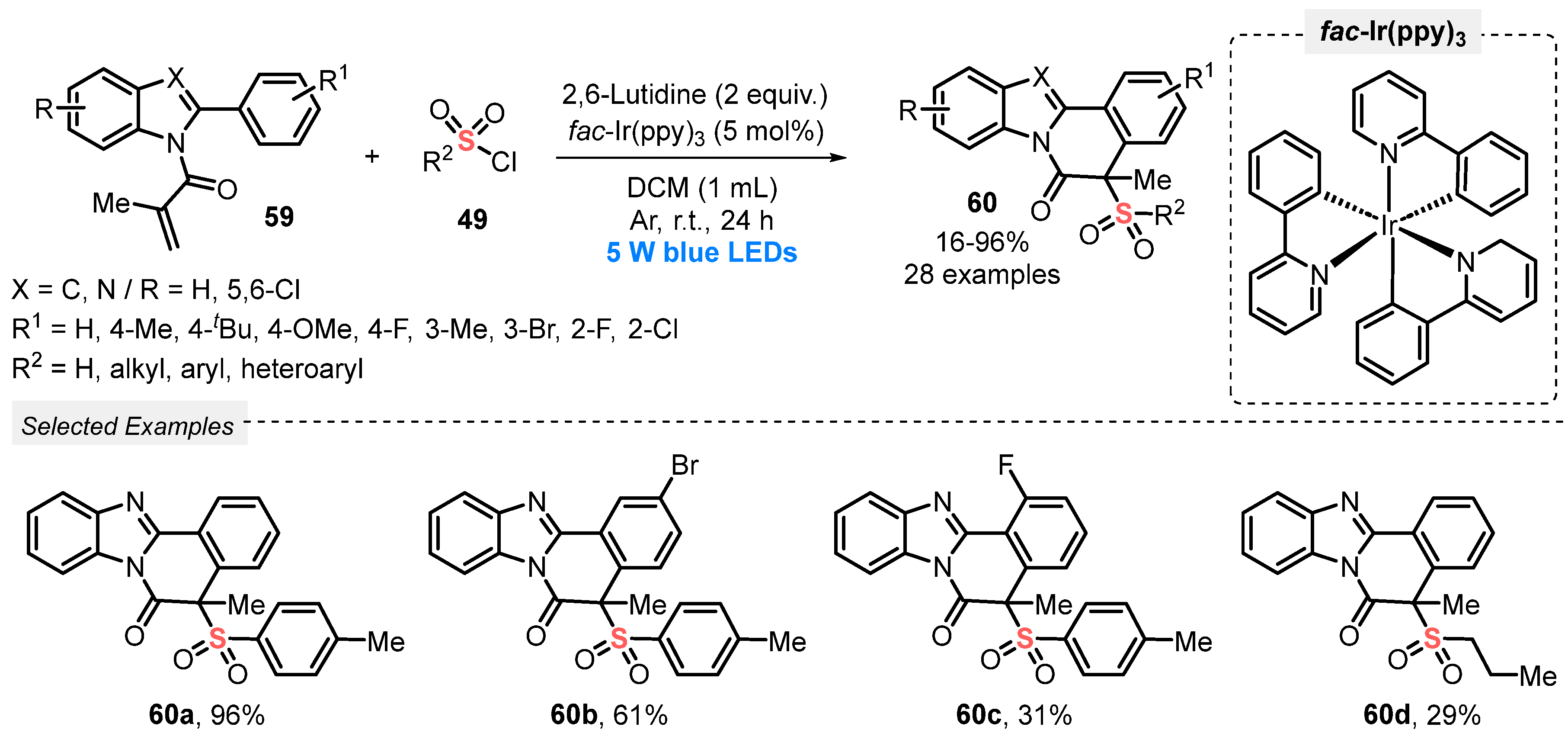
A close-related work was described in 2021 by the same group [71] in the synthesis of a diversity of sulfonylated benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-ones 60, by reacting N-methacryloyl-2-phenylbenzoimidazoles 59 with sulfonyl chlorides 49. In this case, 5 W blue LEDs were used to promote the cascade formation of C–C and C–S bonds, in the presence of fac-Ir(ppy)3 as photocatalyst, 2,6-lutidine as base, and DCM as solvent. The resulting mixture was irradiated at room temperature under Ar atmosphere for 24 h. Under this condition, a library of twenty-eight compounds 60 was satisfactorily prepared in poor to excellent yields (16–96%). Interestingly, a sharp decrease in the reaction yield was observed when the sulfonyl chloride substituted with para-OMe group in the aromatic ring was used. The same behavior was observed when aliphatic sulfonyl chloride was used, providing the formation of product 60d in only 29% yield. Furthermore, several of the prepared compounds were tested for their antitumor activity against MCF-7 cells line, with good results (Scheme 29).
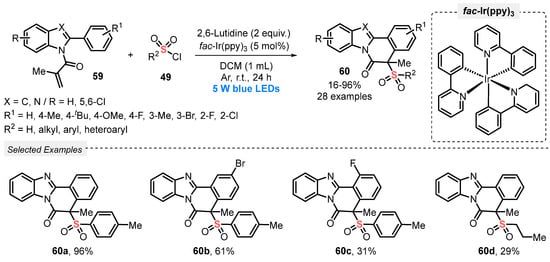
Scheme 29.
Synthesis of sulfonylated benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-ones 60 mediated by visible light.
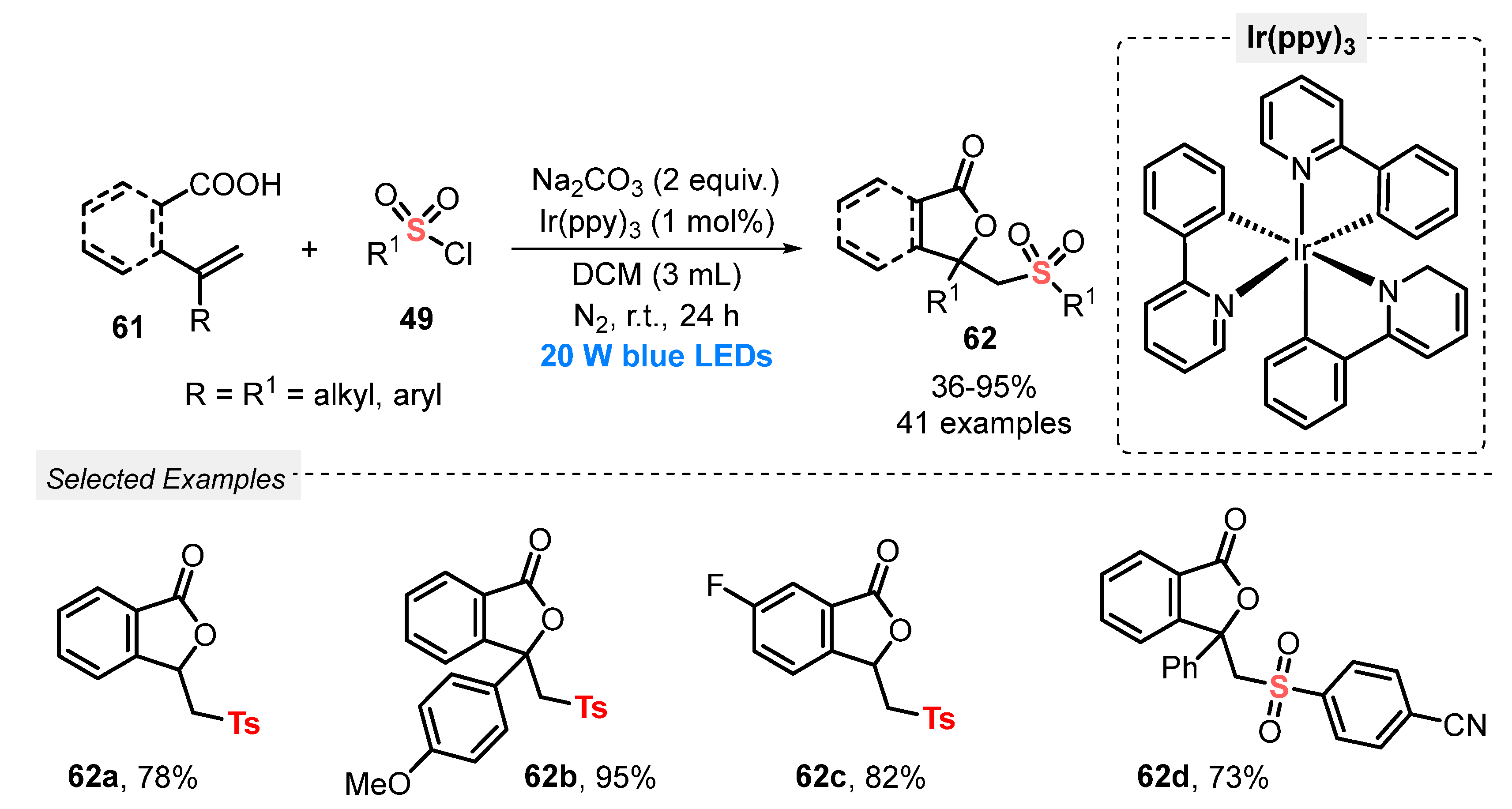
Also, in 2021, Cao and co-workers [72] developed the sulfonyl lactonization of unsaturated carboxylic acids 61, using sulfonyl chlorides 49 as sulfonating agent, driven by a photoredox catalysis process, under visible light irradiation. A broad substrate scope, presenting high functional group diversity, was duly converted to forty-one sulfonyl lactones 62, in poor to excellent yields (36–95%). The transformation was conducted in the presence of Ir(ppy)3 as photocatalyst, Na2CO3 as base, and DCM as solvent, being the resulting mixture irradiated with blue light (blue LEDs, 20 W) for 24 h, under N2 atmosphere (Scheme 30). The limitation of this process was that sulfonyl chlorides containing heteroaromatic groups were not evaluated as substrates, as well as the need for using halogen-based solvent (DCM). On the other hand, the reaction was effective when scaled-up (5.0 mmol) without significant loss in the efficiency.
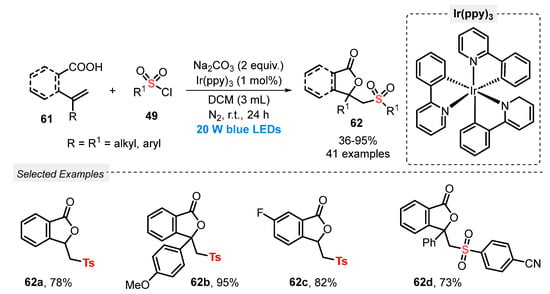
Scheme 30.
Visible light-mediated sulfonyl lactonization of alkenoic acids 61 with sulfonyl chlorides 49.
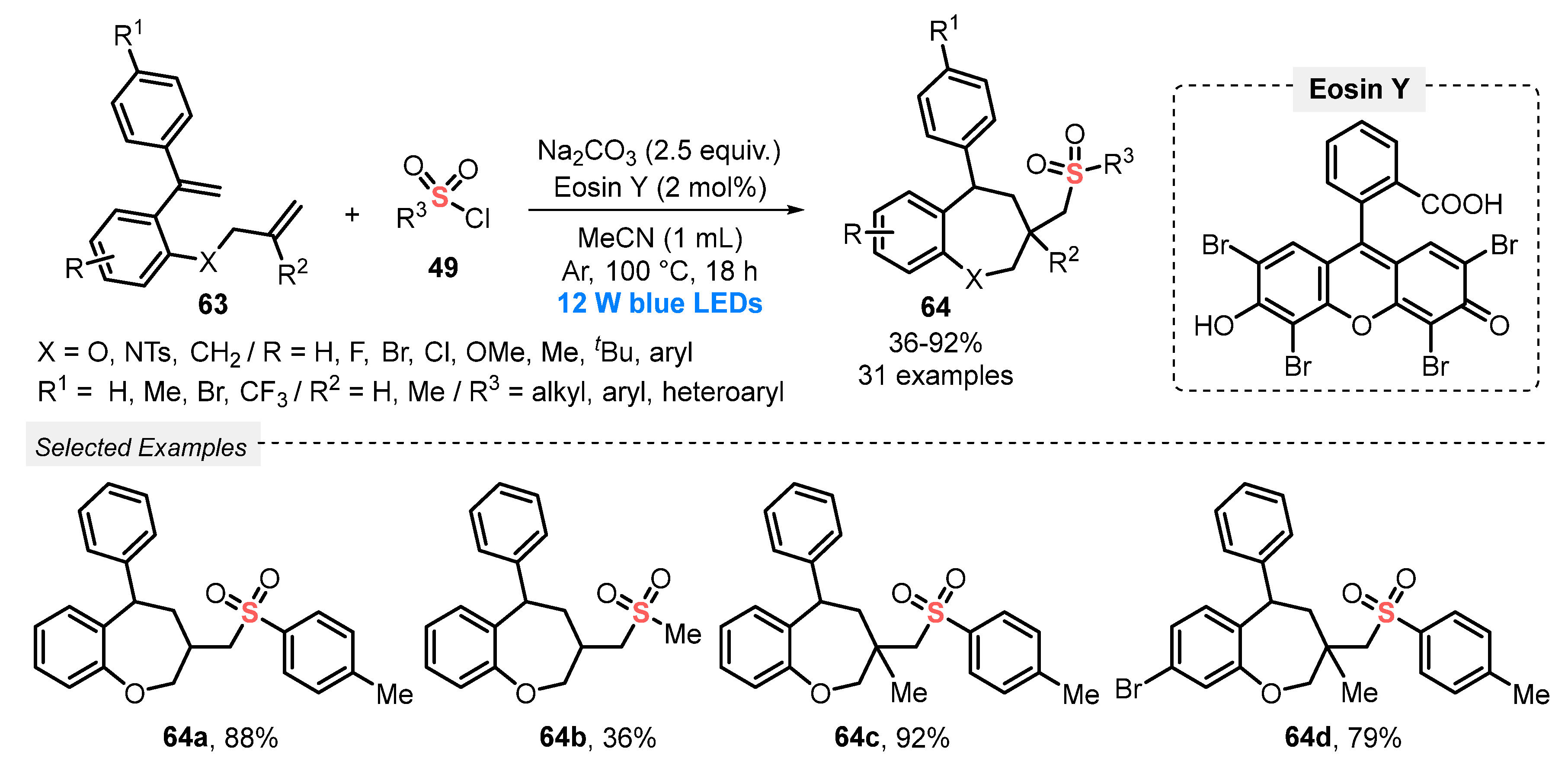
In the same year, Zhang and co-workers [73] reported an ingenious light-mediated cascade sulfonylation/cyclization of 1-(allyloxy)-2-(1-arylvinyl)benzenes 63, in the presence of sulfonyl chlorides 49, to prepare sulfonated benzoxepine derivatives 64. The reactions were conducted in the presence of Eosin Y as photocatalyst, Na2CO3 as base, and MeCN as solvent under irradiation with blue light (blue LEDs, 12 W) and Ar atmosphere at 100 °C for 18 h (Scheme 31). A series of 1-(allyloxy)-2-(1-arylvinyl)benzenes 63 and sulfonyl chlorides 49 containing heteroaromatic, electron-donating, and electron-withdrawing substituents were investigated, and all proved to be suitable substrates in this reaction. Thus, a large library of thirty-one seven-membered cyclic products 64 was obtained in poor to excellent yields (36–92%). It should be noted that an important limitation of the process is the use of high temperature (100 °C).
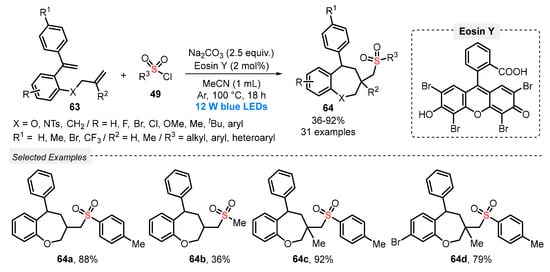
Scheme 31.
Synthesis of sulfonated benzoxepines 64 under visible light conditions.
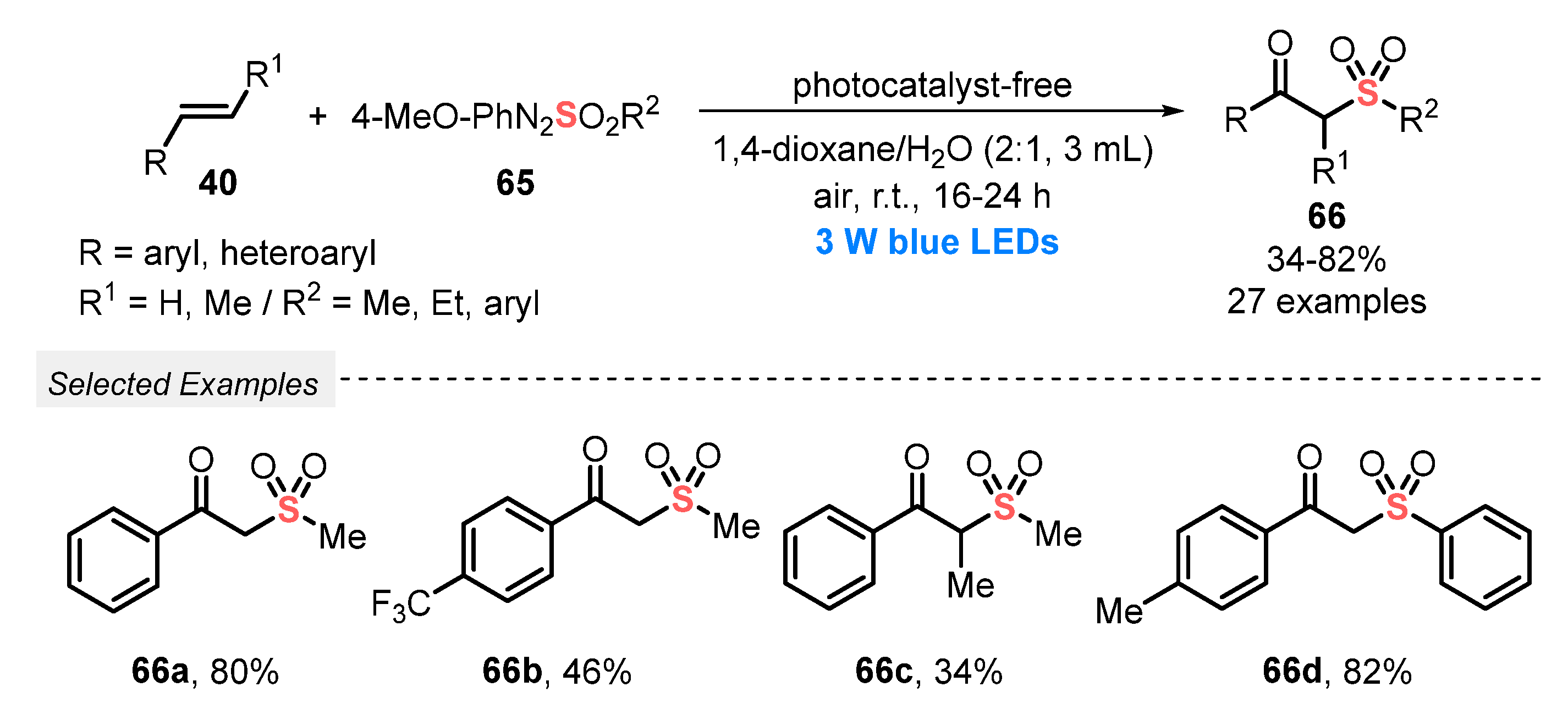
In 2019, Wei’s group [74] succeeded in the use of arylazosulfones 65 as sulfur reagents for the visible light-mediated construction of β-oxo sulfones 66, via the oxysulfonylation of alkenes 40. The transformation takes place in the absence of photocatalysts, using a mixture between 1,4-dioxane and water (3 mL, 2:1), and using O2 from air as a green oxidant at room temperature. This simple and environmentally friendly approach allowed the synthesis of twenty-seven β-oxo sulfones 66 in poor to very good yields (34–82%), after reacting for up to 24 h, under blue light irradiation (blue LEDs, 3 W) (Scheme 32).
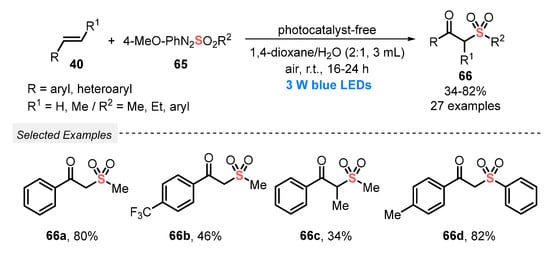
Scheme 32.
Synthesis of β-oxo sulfones 66 promoted by blue LEDs.
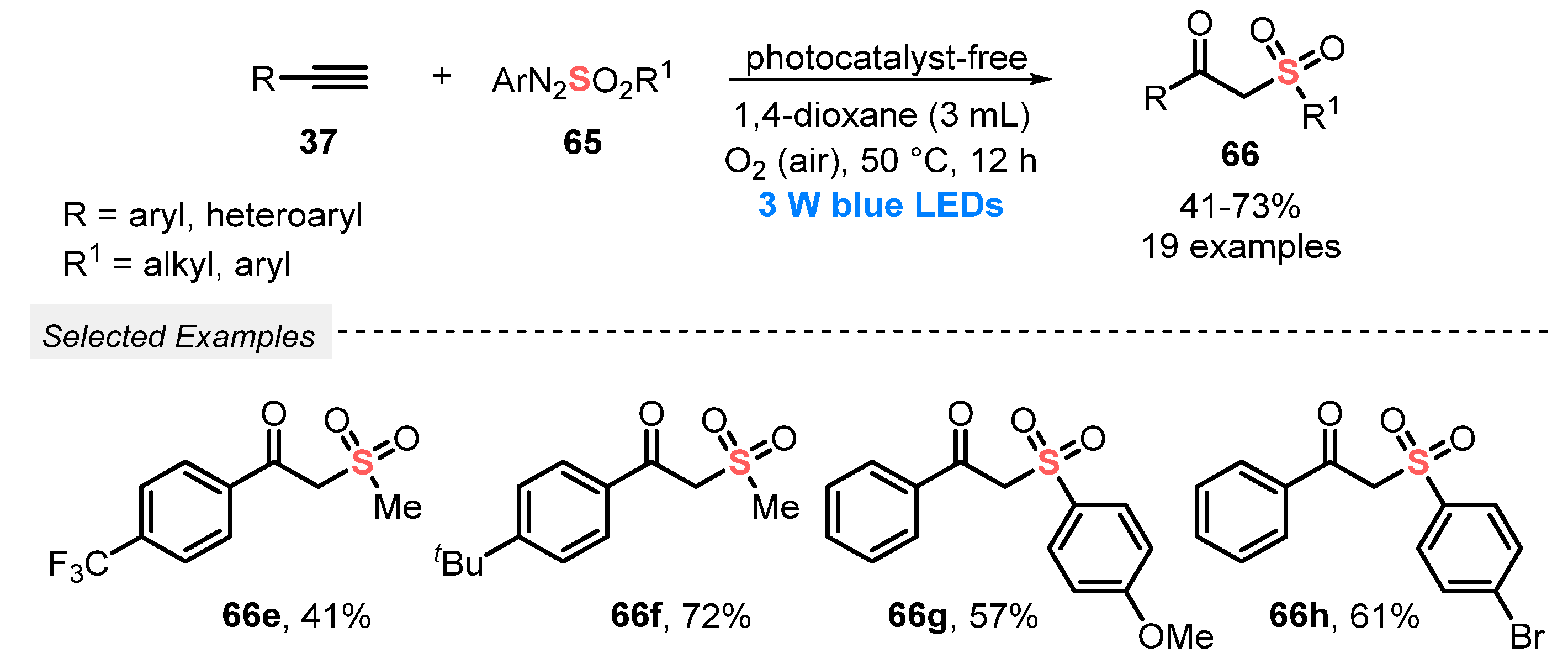
In 2020, the same group [75] reported the use of arylazosulfones 65 to construct β-ketosulfones 66, by the simultaneous light-mediated oxidative difunctionalization of alkynes 37, with the formation of C=O and C–S bonds. No photocatalyst was used, and the desired products were obtained after irradiation with blue light (blue LEDs, 3 W) of a mixture of the reactants in 1,4-dioxane at 50 °C for 12 h, using atmospheric O2 as oxidant. Under this condition, nineteen β-ketosulfones 66 were prepared in moderate to good yields, starting from alkyl, aryl, and heteroaryl alkynes (Scheme 33). Comparing with the method described above (Scheme 32), this process presents some disadvantages, including the need for using high temperature and the limited substrate scope.
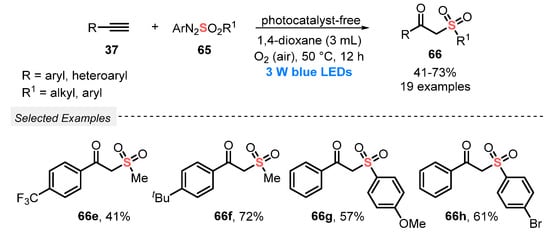
Scheme 33.
Visible light-mediated aerobic oxidative synthesis of β-ketosulfones 66.
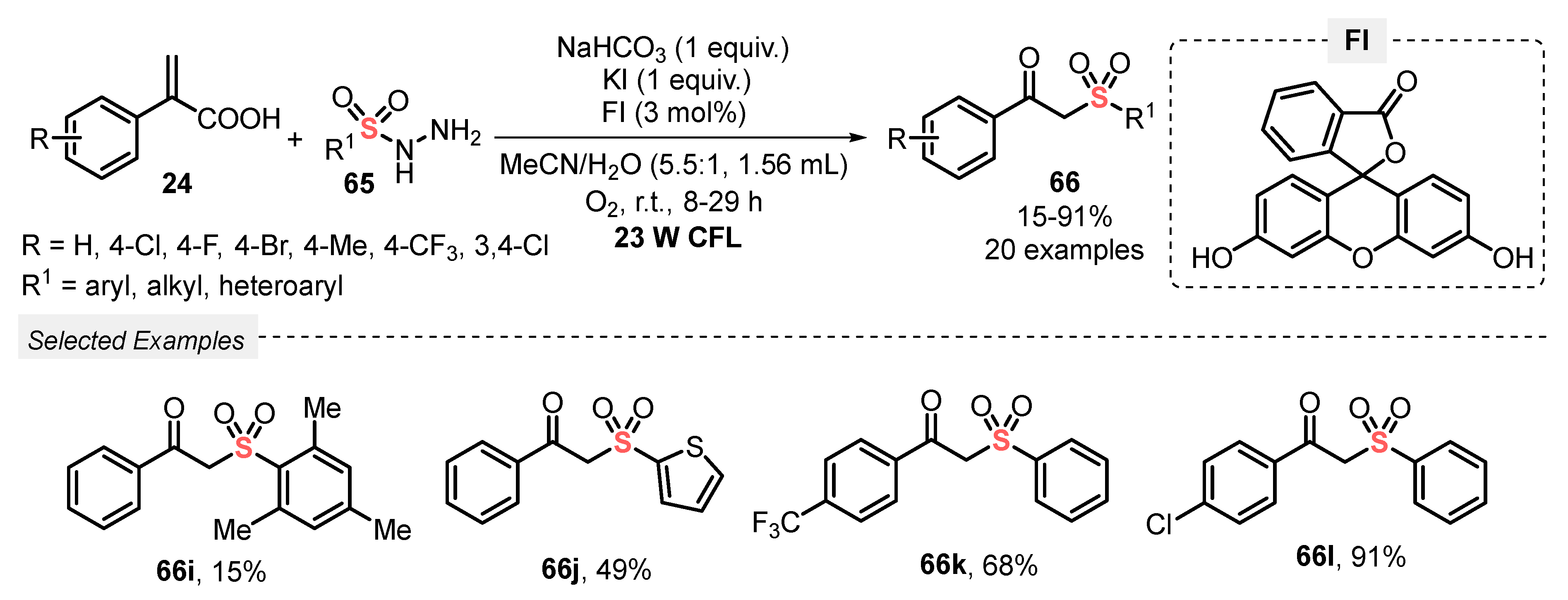
Another strategy for obtaining β-ketosulfones 66 was described by He and co-workers [76], by using sulfonyl hydrazines 65 as sulfonyl source in the presence of different atropic acids 65 as reaction partner, under visible light irradiation. Fluorescein (FI) was used as photocatalyst, in the presence of NaHCO3 as base, and KI as additive in a 5.5:1 mixture of MeCN:H2O as solvent. The reaction was irradiated with CFL bulb (23 W) for up to 29 h in an open-air flask. Atmospheric O2 was used as oxidant, and a total of twenty β-ketosulfones 66 were prepared in poor to excellent yields (15–91%). Aryl-substituted acrylic acids 24 containing electron-donor (4-CH3) and electron-withdrawing groups (4-F, 4-Cl, 4-CF3, 3,4-Cl2) were suitable substrates in the reaction with alkyl, aryl, and heteroaryl sulfonyl hydrazines 66 (Scheme 34). The lowest yield was obtained when a dimesityl containing sulfonyl hydrazine was used as substrate, giving product 66i in a feathery 15% yield. This decrease in the yield is probably associated with steric hindrance of methyl groups. Furthermore, comparing this method with those previously described (Scheme 32 and Scheme 33) requires the use of a photocatalyst and additives, which makes it slightly disadvantageous.
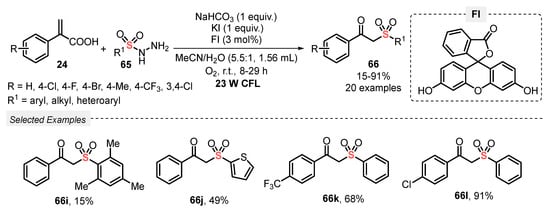
Scheme 34.
Synthesis of β-ketosulfones 66, promoted by blue LEDs.
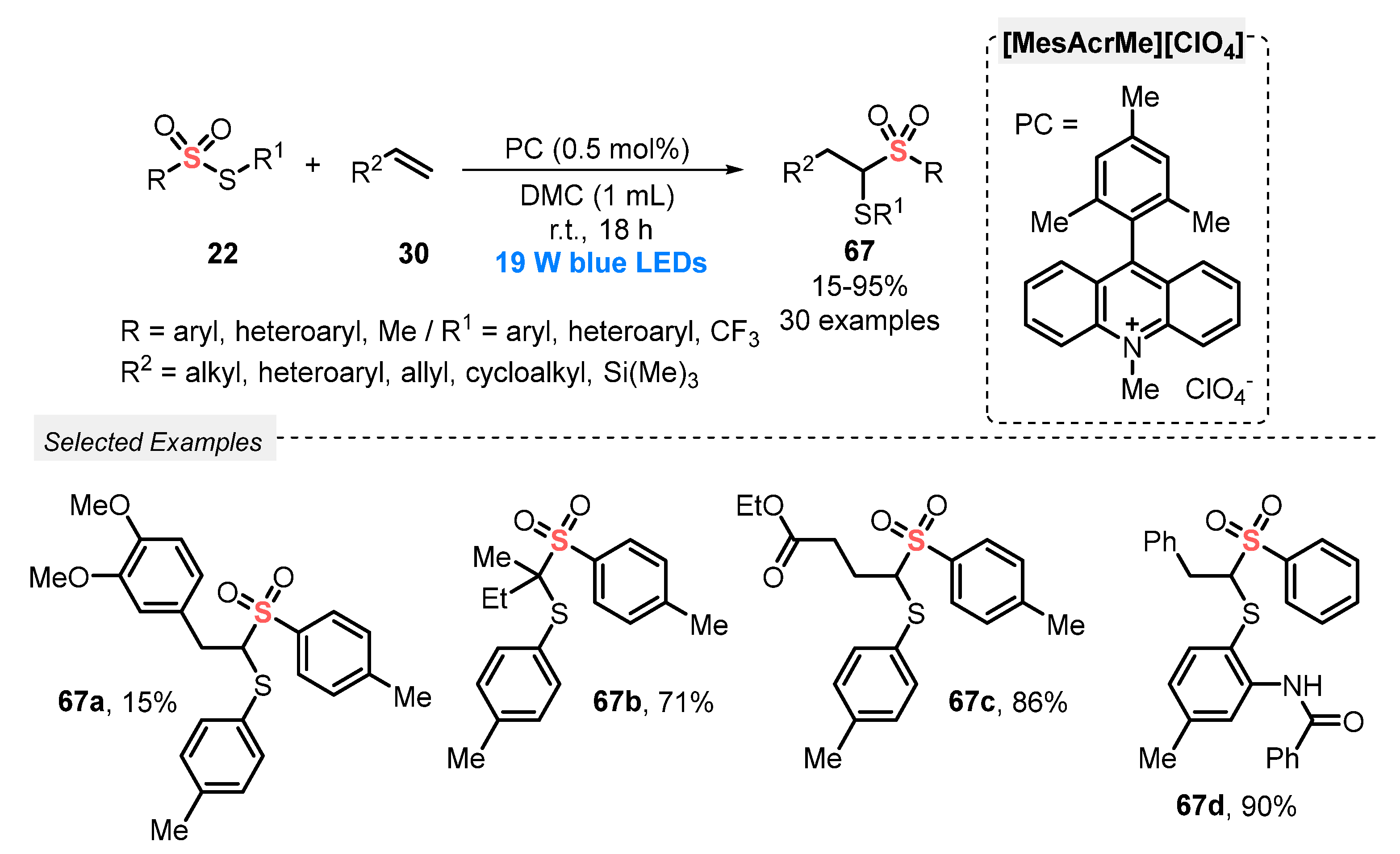
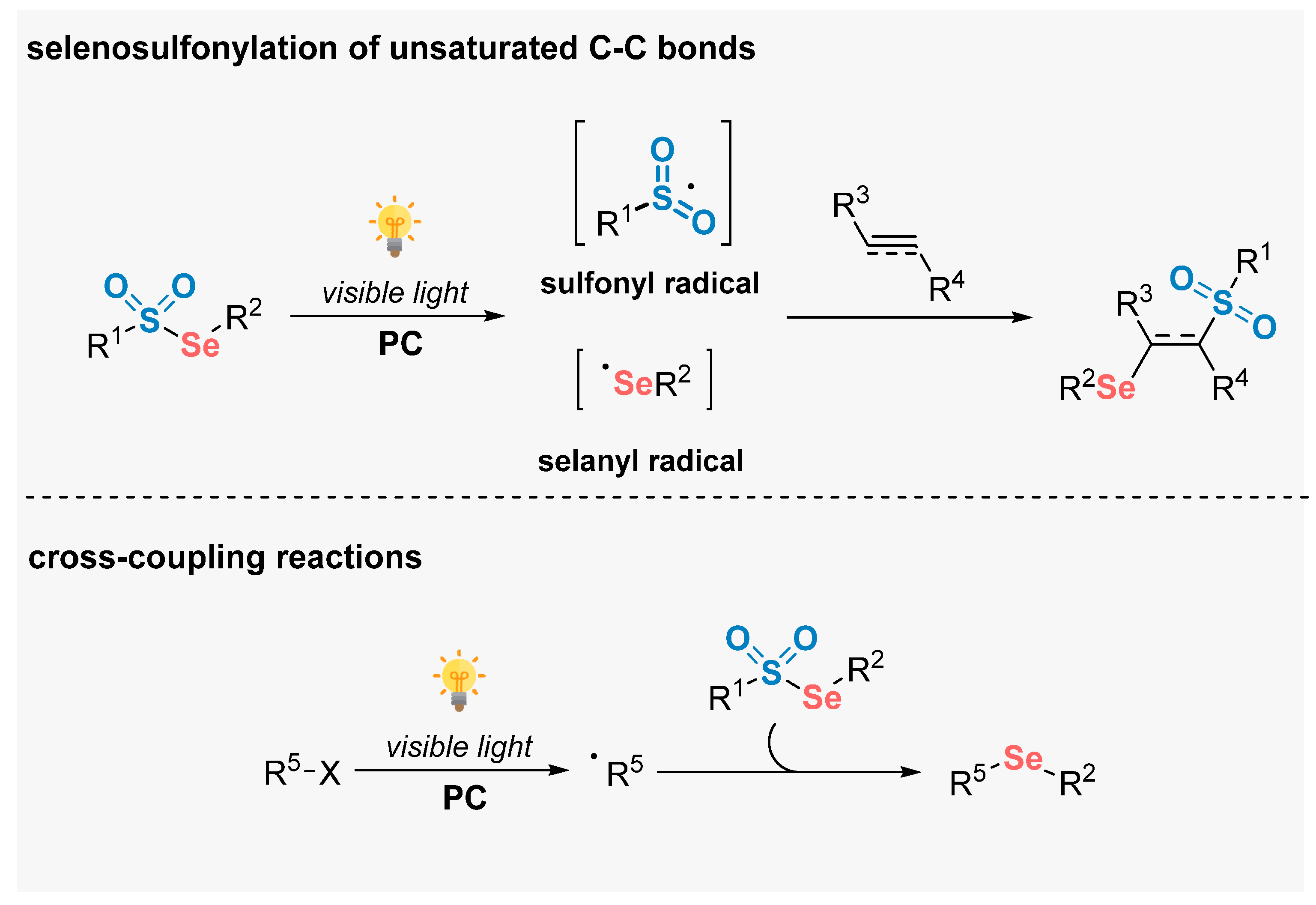
In 2020, Maes and co-workers [77] described the use of thiosulfonates 22 for the direct thiosulfonylation of alkenes 30, in the presence of [MesAcrMe][ClO4] as photocatalyst and under air atmosphere. The reactions were conducted using dimethyl carbonate (DMC) as a green solvent and atmospheric O2 as oxidant. The reaction mixture was irradiated with blue light (blue LEDs, 19 W) for 18 h, delivering the 1,2-thiosulfonylated products 67 in 15–95% yield. Mechanistic studies demonstrated the involvement of sulfenyl and sulfonyl radicals via homolytic cleavage of the S–S bond in the starting thiosulfonate 22, through energy transfer from the excited photocatalyst. The protocol was successfully extended to the synthesis of a selenosulfonylated derivative, which was obtained in 94% yield. In addition to a broad functional group compatibility, the greenness of the method was demonstrated after several calculations using the metrics for Green Chemistry (Scheme 35).
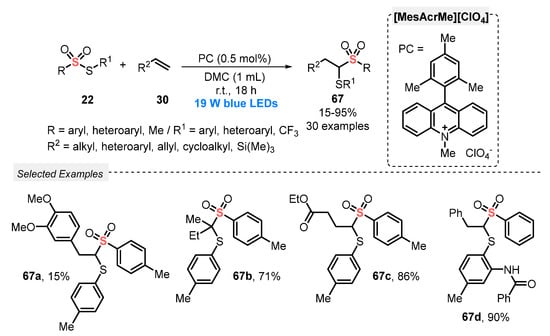
Scheme 35.
Thiosulfonylation of alkenes 30 promoted by blue LEDs.
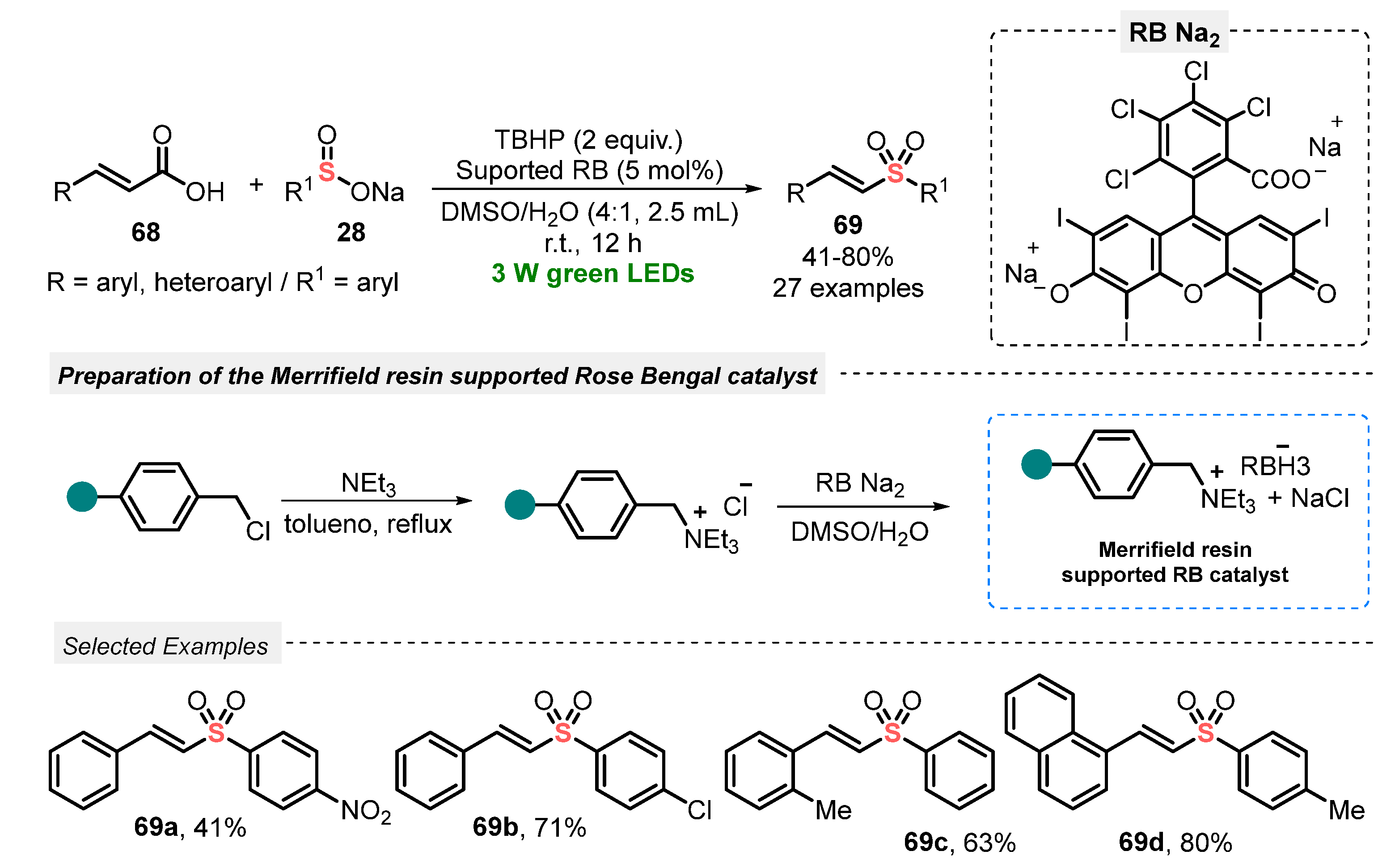
The use of cinnamic acid 68 in the presence of sodium sulfinate 28 was recently explored by Li and co-workers [78] as an elegant strategy to construct vinyl sulfones 69, through a visible light-mediated decarboxylative sulfonylation. The reactions were performed smoothly in the presence of Merrifield resin-supported Rose Bengal (RB) ammonium salt as a photocatalyst and TBHP as oxidant in a 4:1 mixture of DMSO:H2O as solvent. The best condition involved the irradiation of the reaction mixture with green light (green LEDs, 3 W) for 12 h at room temperature. A wide range of substrates 68 and 28 was investigated, such as electron-rich and electron-poor aryl-substituted ones, which presented good performance in the formation of alkenylsulfones 69. Limitations were only found in the reaction using crotonic acid and sodium n-butylsulfinate, which were not able to be converted to the respective product. On the other hand, the supported catalyst could be easily separated from the mixture by filtration and consecutively reused for up to six times, maintaining good photocatalytic activity (Scheme 36).
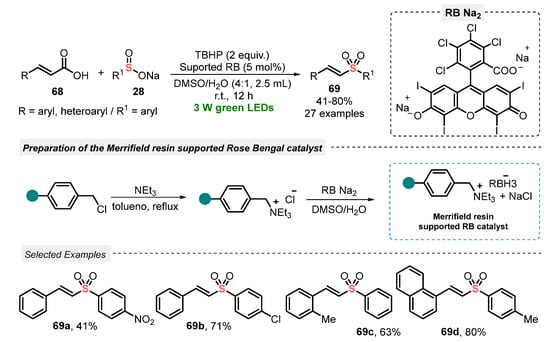
Scheme 36.
Sulfonylation of cinnamic acids 68 using Merrifield resin-supported Rose Bengal as catalyst.
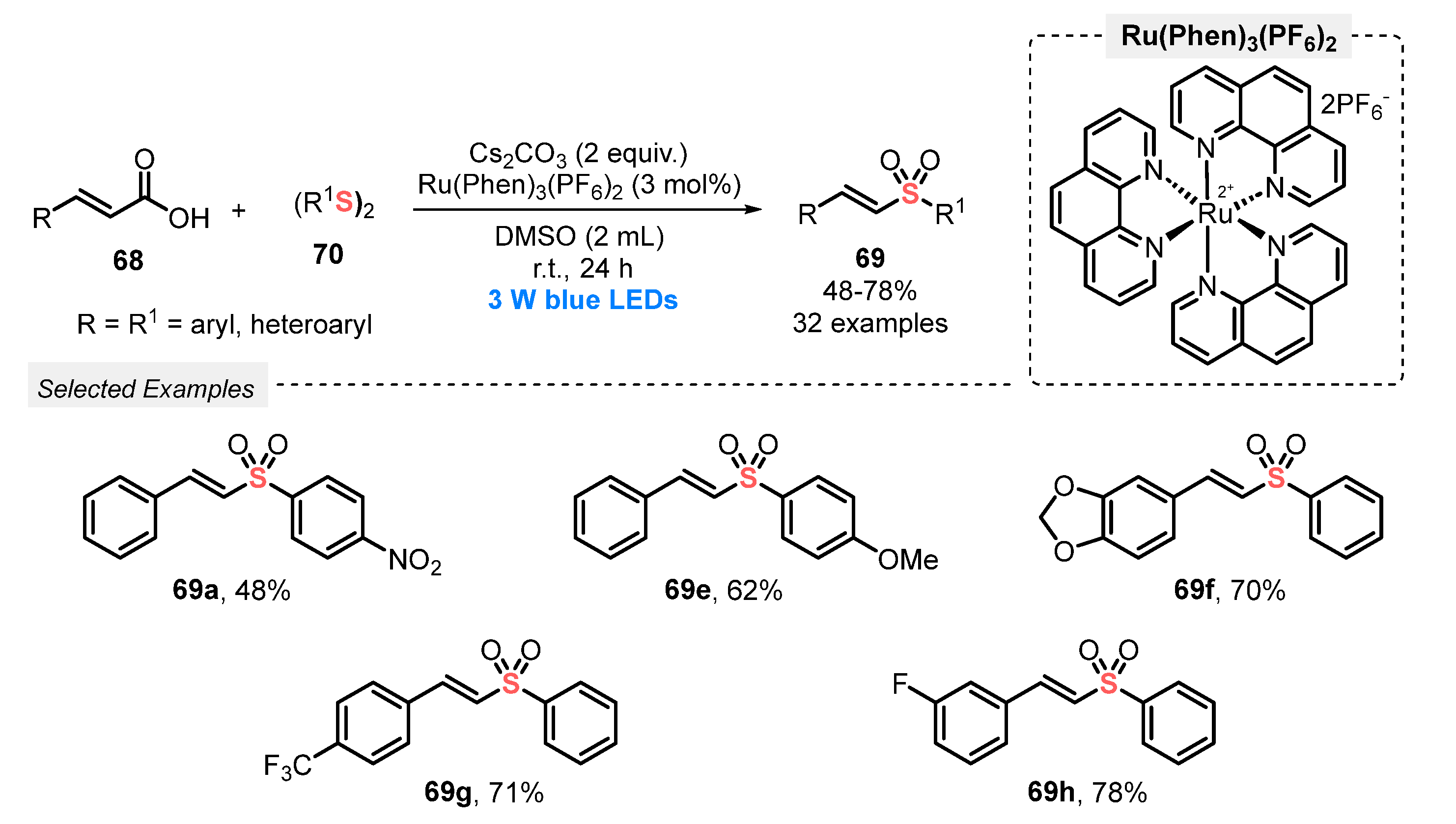
Also, in 2019, Wang and co-workers [79] explored a very similar protocol to prepare vinyl sulfones 69 from cinnamic acid. In this new procedure, however, diaryl disulfides 70 were used as sulfenylating agents under open-air conditions. The reaction mixture was constantly irradiated with blue light (blue LEDs, 3 W) in the presence of Ru(Phen)3(PF6)2 as photocatalyst, CsCO3 as base, and DMSO as solvent at room temperature. A total of thirty-two alkenyl sulfones 69 were obtained in moderate to good yields (47–78%). No reaction occurred when 1,2-di(pyridin-2-yl)disulfide was used as the sulfur counterpart, while good results were obtained using electron-rich and electron-poor derivatives of both reaction partners (Scheme 37). Comparing this methodology with that described by Li (Scheme 36), the main disadvantage is the impossibility of recycling the photocatalyst, as well as the need for longer reaction times. However, the approach provides an alternative strategy to prepare vinyl sulfones employing disulfides as easily accessible starting materials.
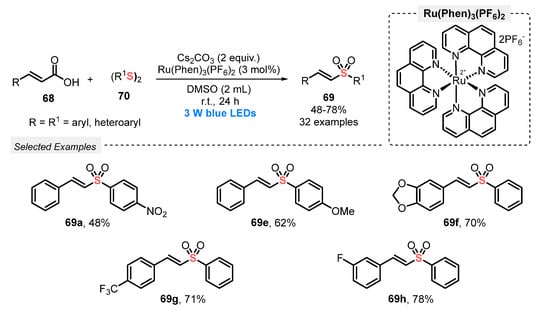
Scheme 37.
Sulfonylation of cinnamic acids 68 using disulfides 70.
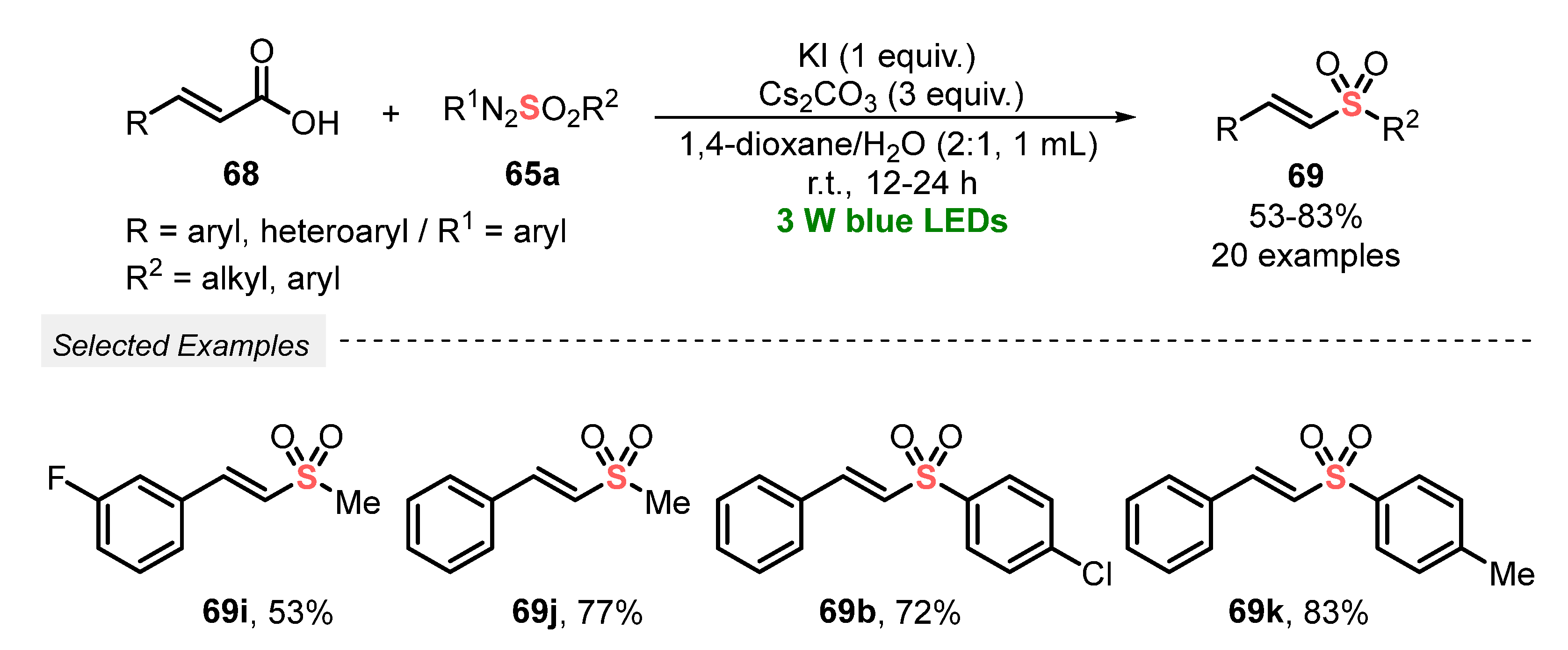
In 2020, Yadav and co-workers [80] proposed a catalyst-free alternative to prepare vinyl sulfones 69, starting from cinnamic acid 68 and arylazosulfones 65a as sulfonylating agents. In this protocol, the decarboxylation process is facilitated by the use of a base (Cs2CO3), air (source of oxygen for the oxidation step), and KI in the mixture 1,4-dioxane:H2O (2:1) as solvent. After irradiating the reaction mixture with blue light (blue LEDs, 3 W) at room temperature for 12–24 h, a total of twenty vinyl sulfones 69 could be obtained in moderate to very good yields (53–83%). Limitations were only found in the reaction using (tiofen-2-il) acrylic acid, which produced a complex inseparable mixture of products. Failures in the reaction were also faced when propenoic acid and 3-cyclohexylacrylic acid were used. In these cases, the formation of products was not observed under the optimal conditions, even after 24 h. Among the advantages advocated by the authors, the method simplicity by not requiring photocatalysts, good functional group substrate tolerance, and the possibility of obtaining alkyl vinyl sulfones is highlighted (Scheme 38).
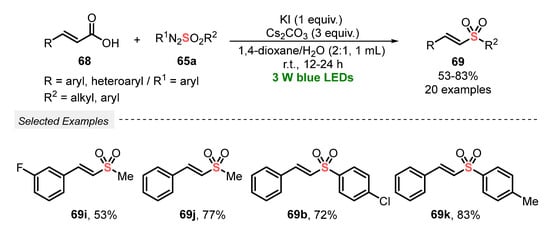
Scheme 38.
Sulfonylation of cinnamic acids 68 using arylazosulfones.
3.2. Using SO2 for the In Situ Formation of Sulfonyl Radical Species
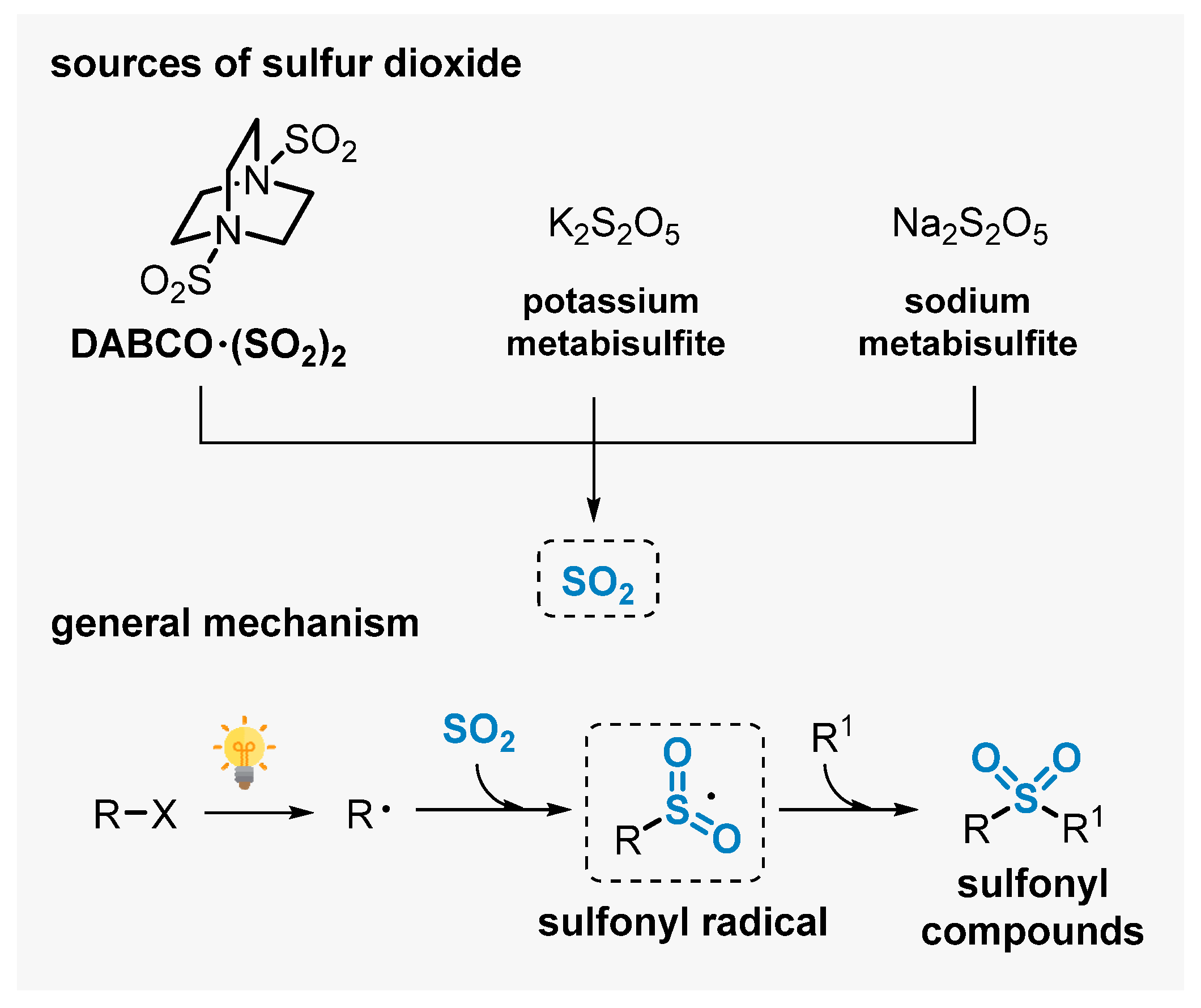
The insertion of sulfur dioxide (SO2) is an interesting strategy to prepare sulfones. However, important drawbacks are faced which limit the applicability of this approach to prepare sulfonylated products, including the toxicity of SO2 gas and the difficulty of handling it. These problems can be elegantly circumvented by using easy-to-handle and cheap SO2-sources, including inorganic sulfites (K2S2O5, Na2S2O5) and organic salts (DABCO∙(SO2)2 = DABSO). These reagents have been used successfully to access a diversity of sulfones, avoiding the use of pre-installed sulfonyl reagents [81,82,83].
Among the strategies that use these compounds as SO2-source, the application of visible light-mediated protocols has been emerging as an eco-friendly approach. Through radical reaction pathways, this approach allows for conducting complex events at room temperature, avoiding the use of thermal reaction conditions. In general, SO2 is captured along the transformation by a radical intermediate (R) generated in situ, affording a sulfonyl radical intermediate I. This radical is the key intermediate, able to trigger radical addition events in the presence of π-system reaction partners (R1), delivering the respective sulfone-containing products (Scheme 39) [84,85].
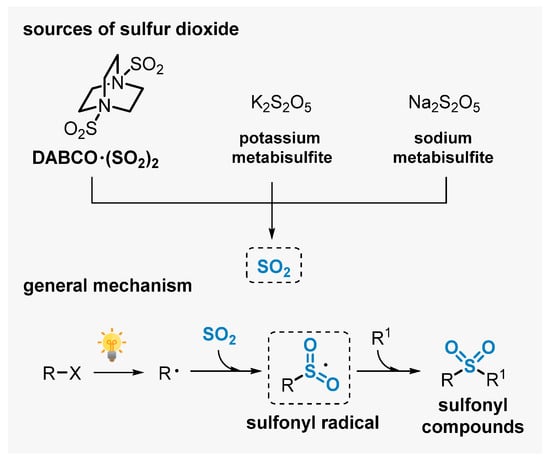
Scheme 39.
SO2 sources and general mechanism for obtaining sulfonyl compounds using visible light.
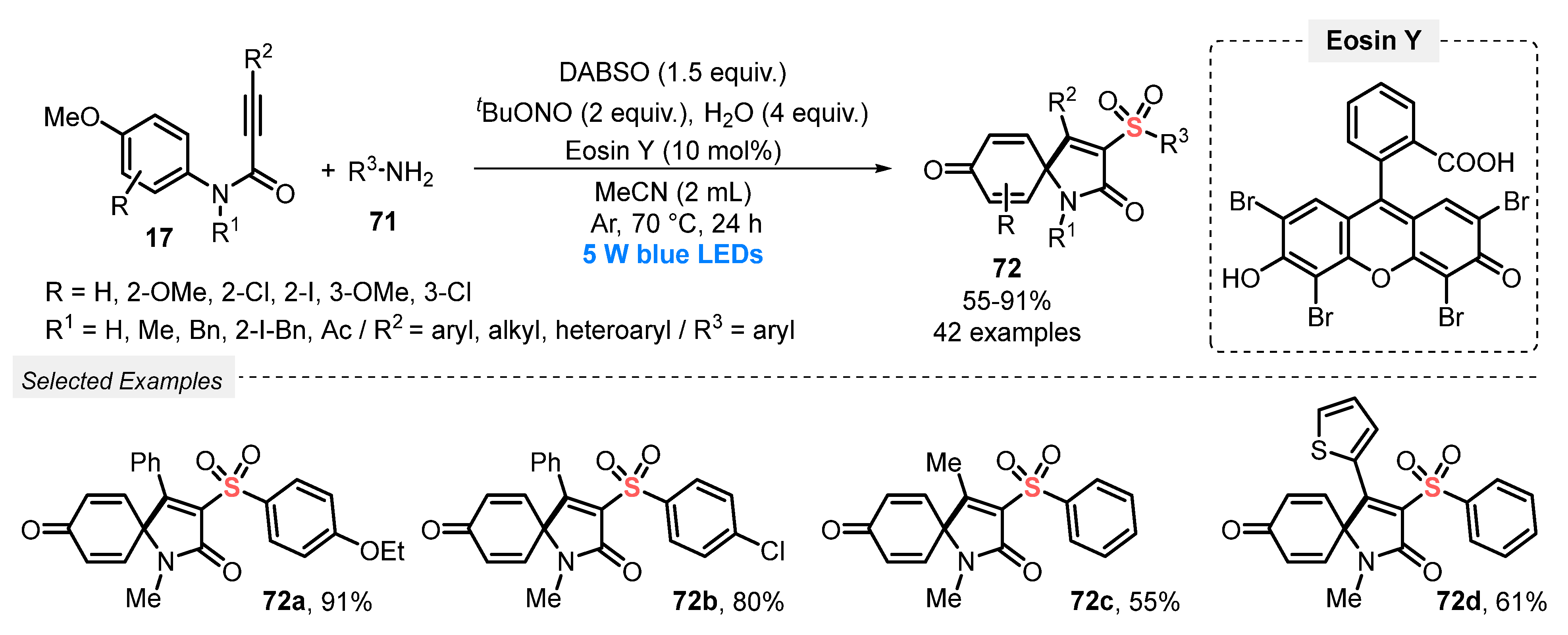
In 2019, Tang and co-workers [86] reported a convenient and efficient visible light-mediated protocol to prepare a diversity of sulfonated spiro[4,5]trienones 72. The method involves a cascade sulfonylation/ipso-cyclization of N-arylpropargyl amides 17 and arylamines 71, in the presence of DABSO as SO2-source. In these transformations, Eosin Y was used as photocatalyst, in the presence tBuONO as oxidant, H2O as additive, and MeCN as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 5 W) for 24 h at 70 °C under Ar atmosphere, giving a total of forty-two sulfonated spiro[4,5]trienones 72 in up to 91% yield. A good suitability was observed regarding both the N-aryl propargylamide 17 and the arylamine 71 substrates, and the presence of electron-withdrawing or electron-donating groups did not influence significatively the reaction yield (Scheme 40).
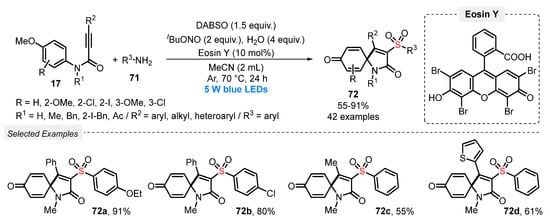
Scheme 40.
Synthesis of sulfonated spiro[4,5]trienones 72 promoted by blue light.
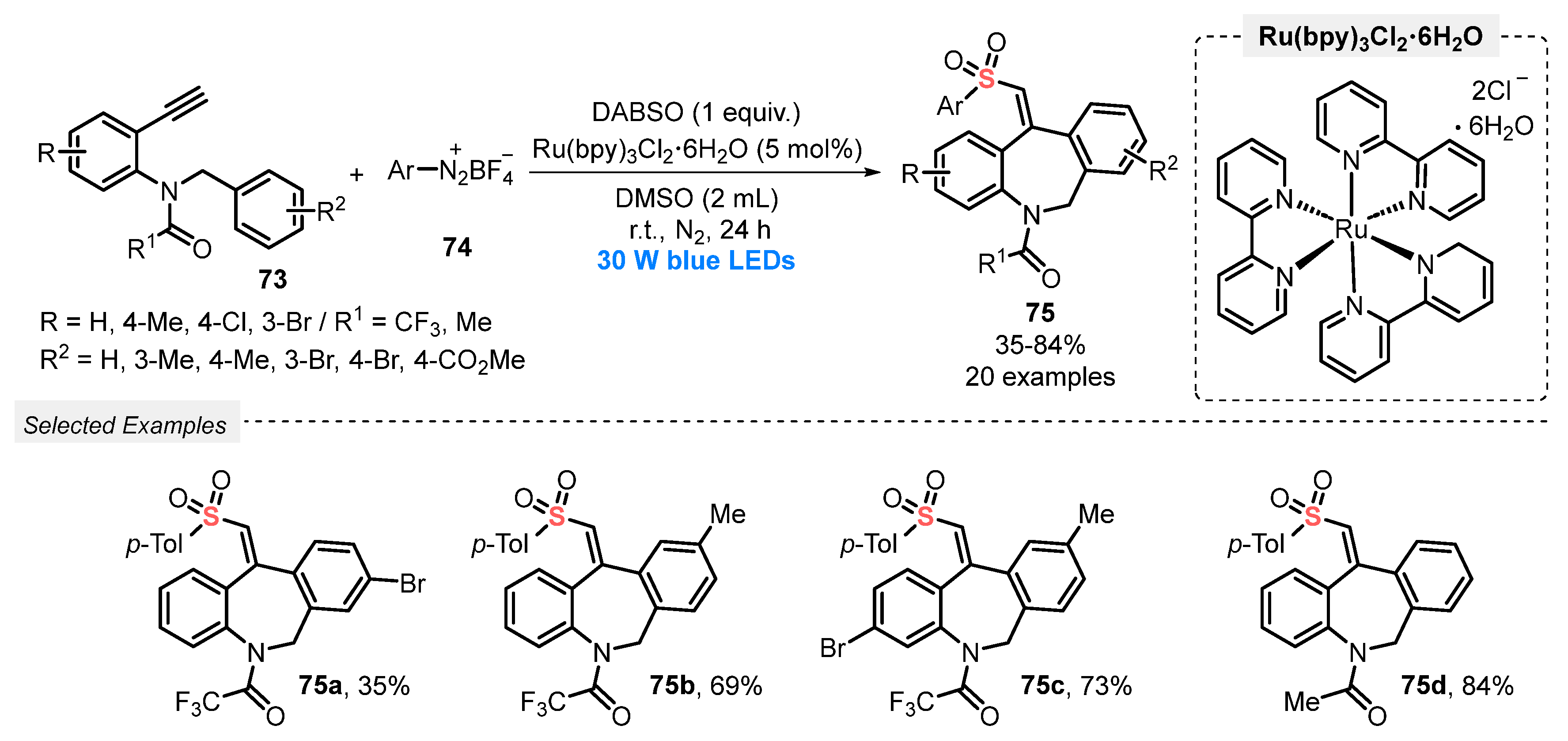
In 2021, Wu and co-workers [87] reported a new and efficient visible light-mediated approach to access sulfonylated dibenzazepines 75, through a three-component reaction between N-benzyl-N-(2-ethynyl-aryl)amides 73, aryldiazonium tetrafluoroborates 74, and DABSO as the SO2-source. The reactions were performed in the presence of Ru(bpy)3Cl2∙6H2O as photocatalyst and DMSO as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 30 W) for 24 h at room temperature, under N2 atmosphere. A total of twenty sulfonylated dibenzazepines 75 decorated with different groups, including halogens, ester, and alkyl, was isolated in up to 84% yield (Scheme 41). The authors also tried to expand the method to the ether substrate 1-(benzyloxy)-2-ethynylbenzene to produce a dibenzoxepine derivative. However, it was not possible to access the product of interest, even after stirring for 24 h.
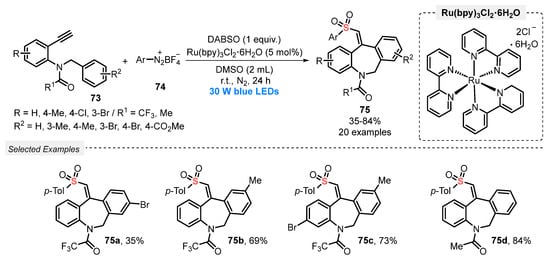
Scheme 41.
Synthesis of vinylsulfonylated dibenzazepine derivatives 75 promoted by blue light.
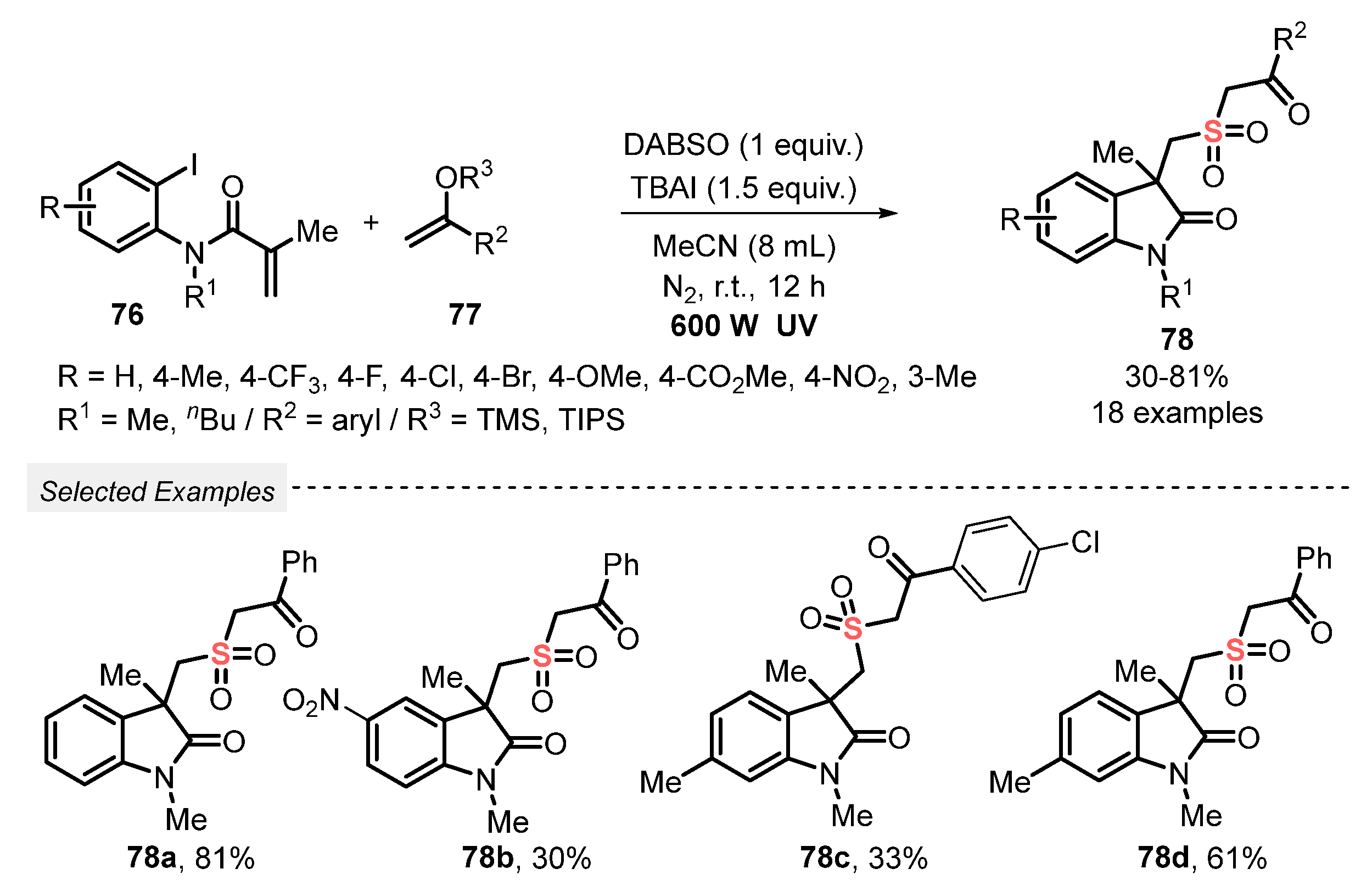
The same group [88] reported in 2020 the synthesis of sulfonylated heterocyclic compounds 78, by the reaction between aryl iodides 76 and silyl enolates 77. The reaction was performed using DABSO, in the presence of tetrabutylammonium iodide (TBAI) as an additive and MeCN as solvent. No photocatalyst was used, and the reaction mixture was irradiated with a 600 W ultraviolet lamp under N2 atmosphere at room temperature for 12 h. In addition, it was verified in the optimization studies that better yields can be obtained by increasing the amount of solvent from 6 to 8 mL (64% vs. 81%). Thus, by employing the optimal conditions, eighteen sulfonylated butyrolactams 78 were prepared, in up to 81% yield (Scheme 42).
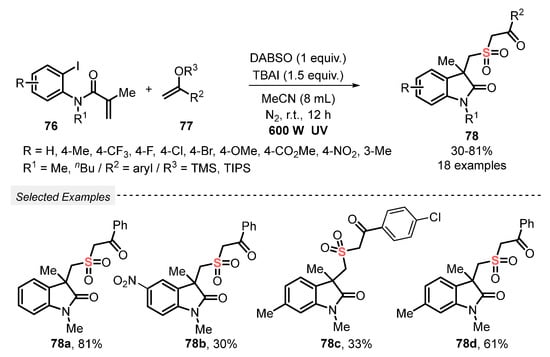
Scheme 42.
Synthesis of sulfonated cyclic compounds 78 under photoinduced metal-free conditions.
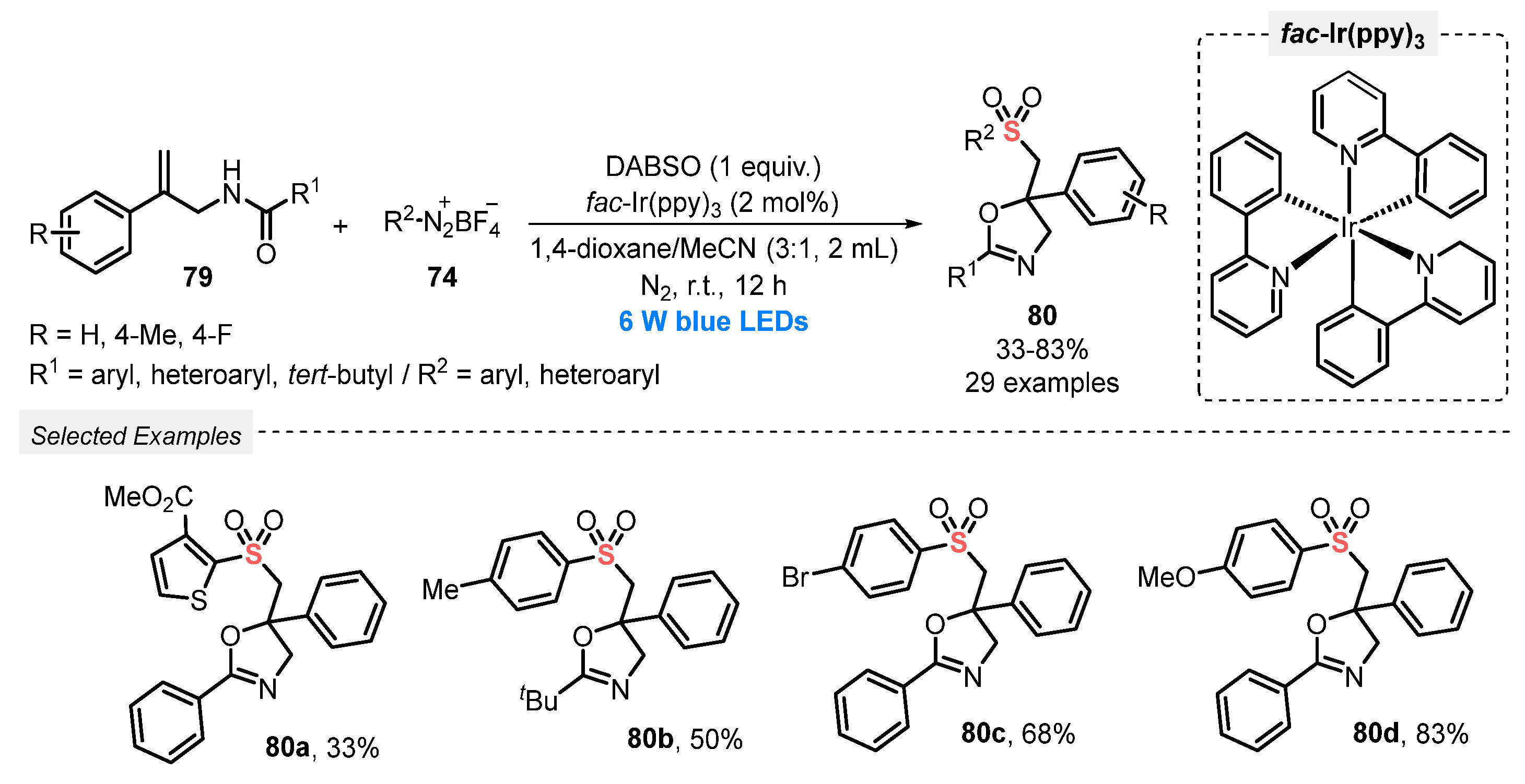
DABSO was used by Zhou, Cui, and co-workers in 2022 [89] to prepare sulfonylated oxazolines 80, through the light-mediated reaction with N-allylamides 79 and aryldiazonium salts 74. The best reaction condition was set by using fac-Ir(ppy)3 as photocatalyst, in a 3:1 mixture of 1,4-dioxane and MeCN as solvent. The reaction was irradiated with blue light (blue LEDs, 6 W) for 12 h at room temperature under N2 atmosphere. By employing this condition, various N-allylamides and aryldiazonium tetrafluoroborates, substituted with both electron-donating and electron-withdrawing groups in the aromatic ring, reacted smoothly to give twenty-nine oxazolines 80 in poor to very good yields (33–83%) (Scheme 43). Furthermore, aryldiazonium tetrafluoroborate containing a thiophene moiety was also employed as substrate. However, the yield of product 80a was only 33% after 12 h of reaction.
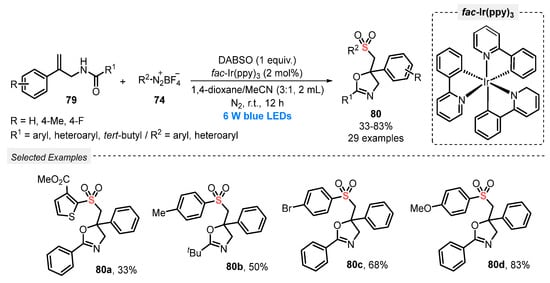
Scheme 43.
Synthesis of sulfonated oxazolines 80 promoted by blue LEDs.
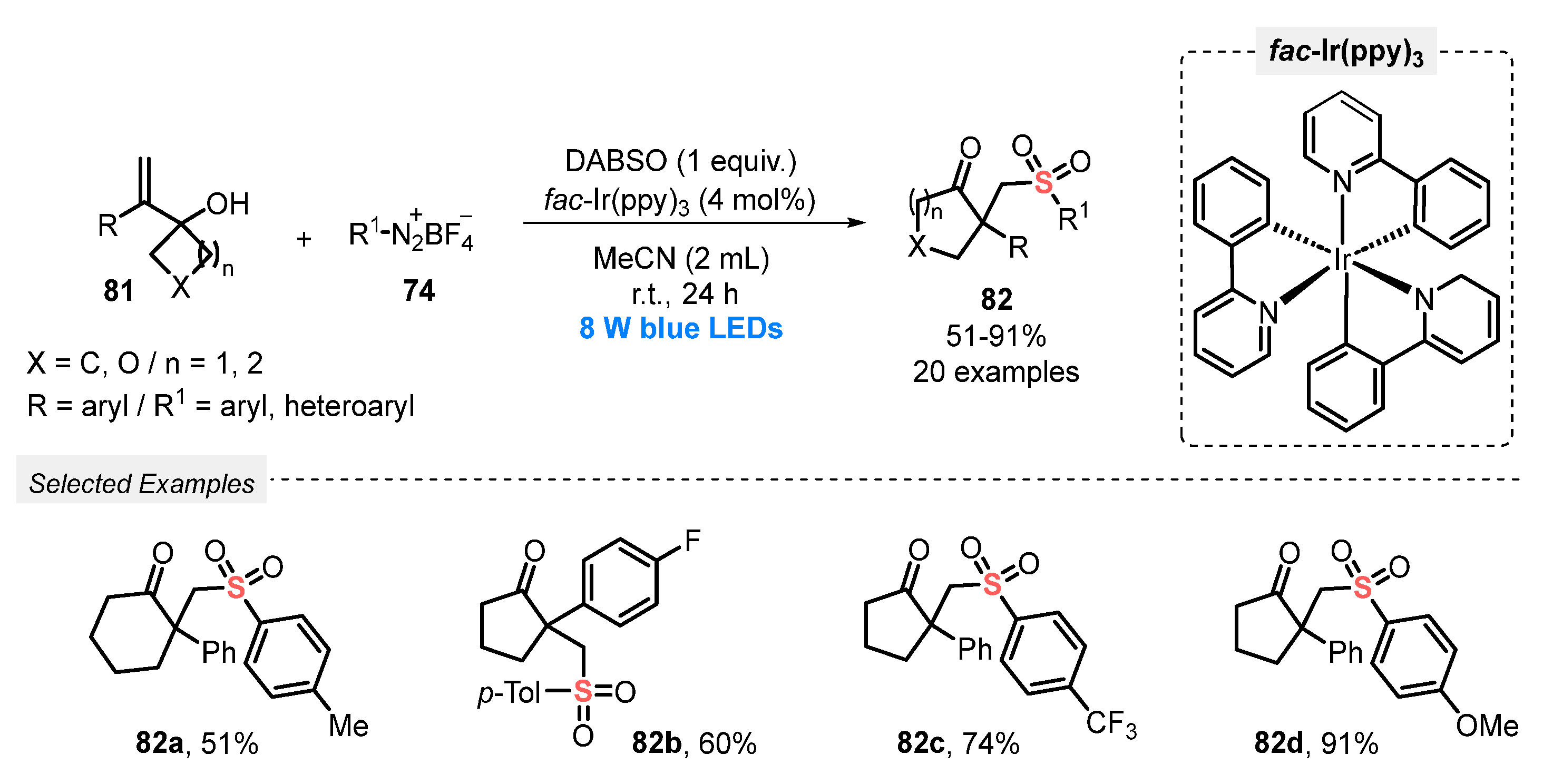
In 2019, Wu and co-workers [90] described the visible light-promoted synthesis of β-sulfonated ketones 82 using a similar approach, i.e., aryldiazonium salts and DABSO were reacted with alkenylcyclobutanols 81. A variety of alkenylcyclobutanols and several aryldiazonium tetrafluoroborates 74 was successfully used as substrates in the presence of fac-Ir(ppy)3 as photocatalyst and MeCN as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 8 W) for 24 h at room temperature, affording a total of twenty β-sulfonated ketones 82 bearing α-carbon quaternary centers, in up to 91% yield. The proposed mechanism of the reaction involves an arylsulfonyl radical-induced 1,2-carbon migration process, followed by a semipinacol rearrangement (Scheme 44).
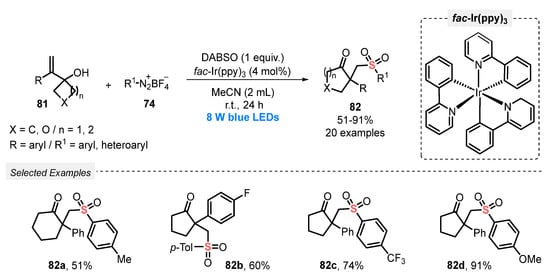
Scheme 44.
Sulfonylation of alkenylcyclobutanols 81 promoted by blue light.
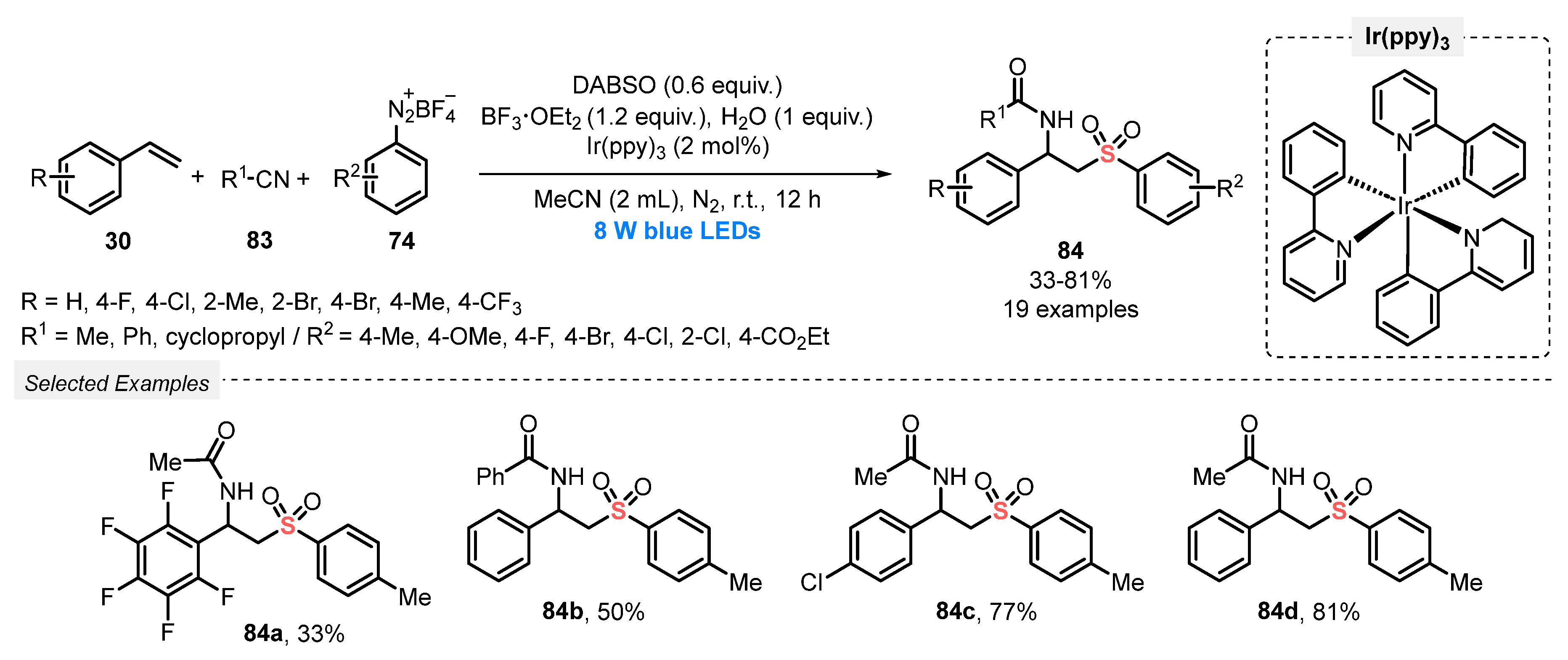
Also, in 2019, the same group [91] described the synthesis of β-sulfonylamides 84 through a multicomponent strategy employing different styrenes 30, aryldiazonium tetrafluoroborates 74 and nitriles 83. The reactions were carried out under blue light irradiation (blue LEDs, 8 W), in the presence of DABSO as SO2 precursor, Ir(ppy)3 as photocatalyst, BF3·OEt2 as additive, and MeCN as solvent. The mixture was irradiated under N2 atmosphere at room temperature for 12 h, affording nineteen β-sulfonylamides with excellent chemoselectivity, in up to 81% yield (Scheme 45). However, heteroaromatic and aliphatic alkenes have not been evaluated as substrates.
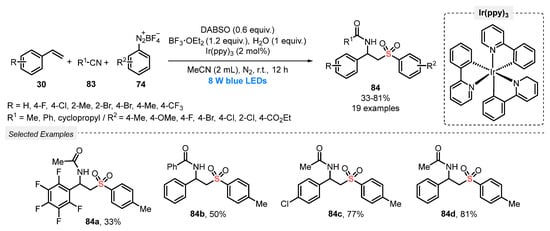
Scheme 45.
Synthesis of β-sulfonylamides 84 promoted by blue LEDs.
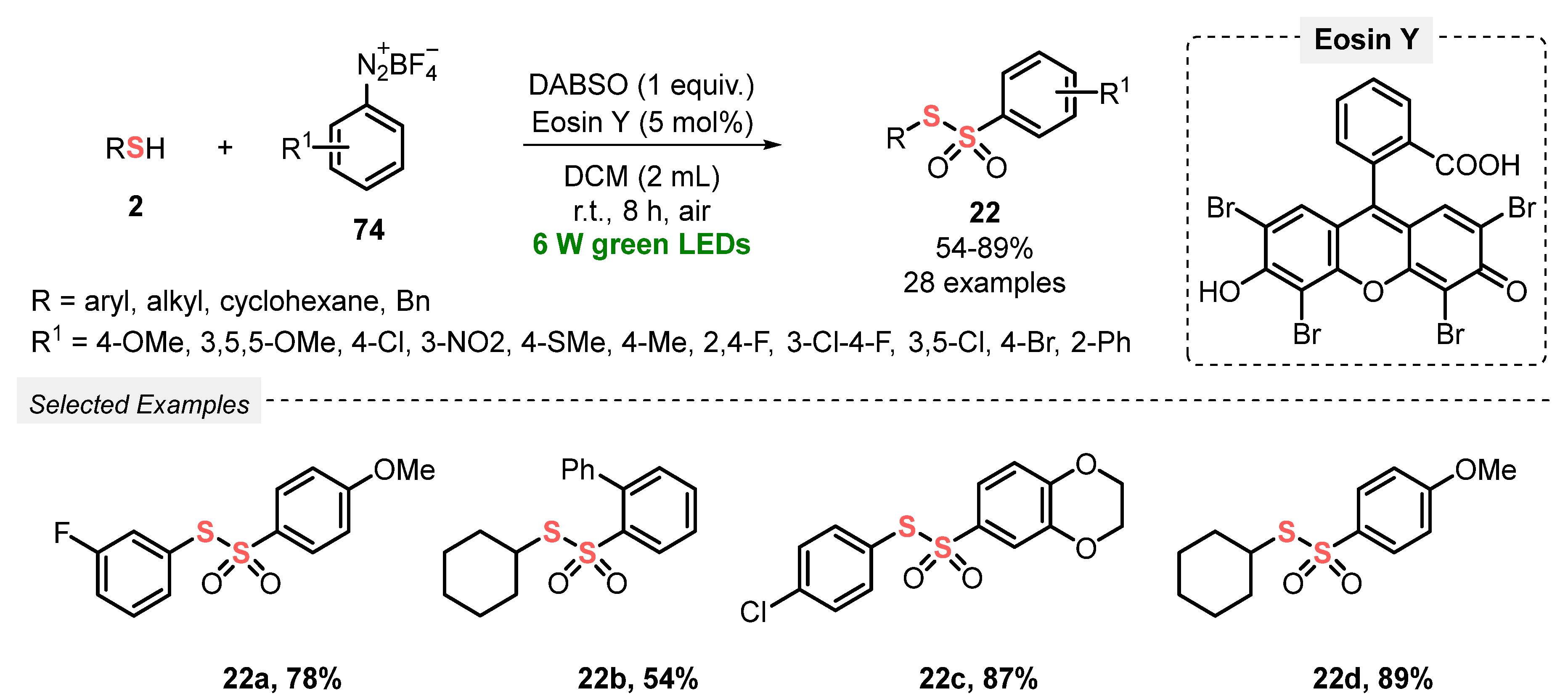
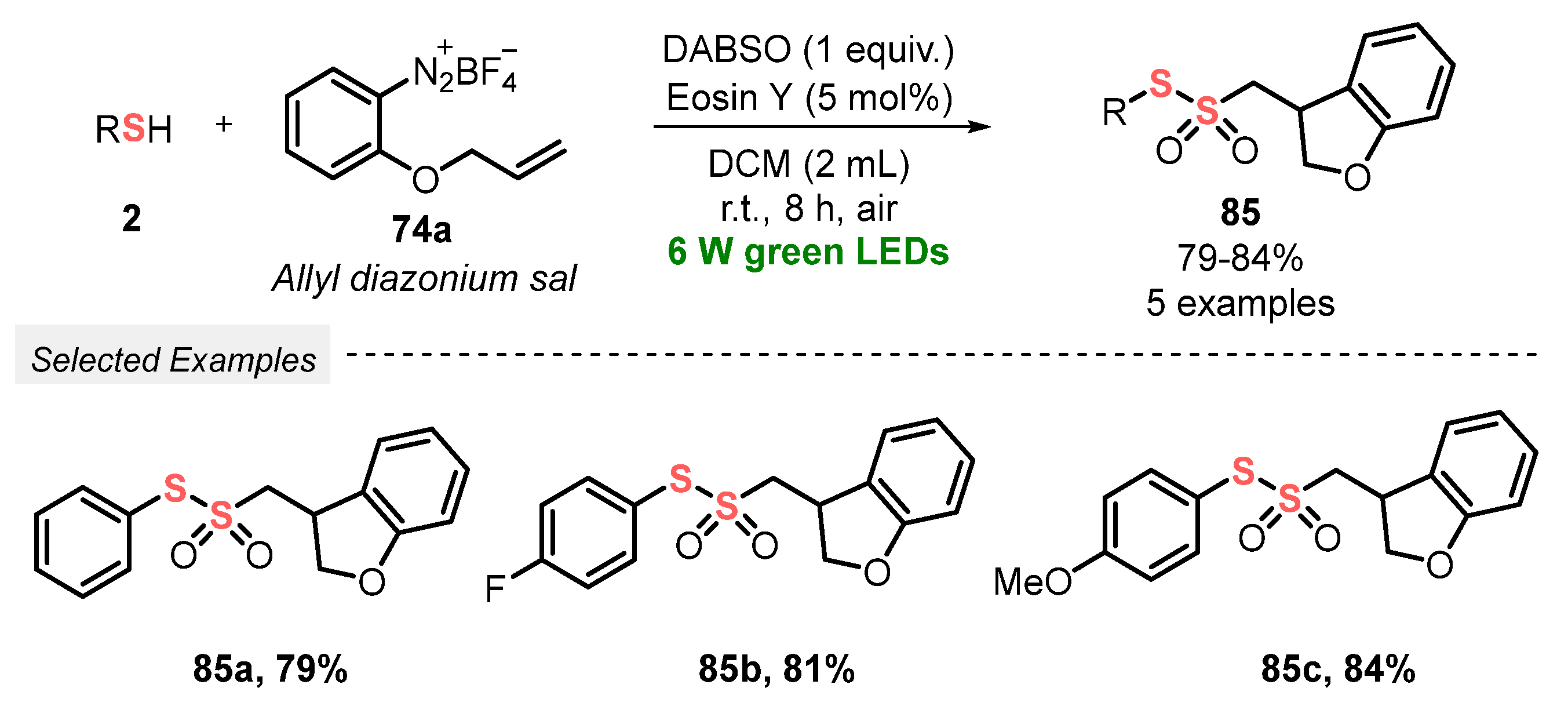
In the same year, Volla and co-workers [92] demonstrated an efficient and convenient strategy to prepare thiosulfonates 22 via radical insertion of SO2, generated in situ from DABSO. The reaction was conducted under mild conditions, at room temperature and under green light irradiation (green LEDs, 6 W), employing Eosin Y as a photocatalyst. In a typical procedure, a mixture of thiols 2 and tetrafluoroborate aryldiazonium salts 74 in DCM was irradiated for 8 h in an open-air flask, affording twenty-eight nonsymmetric products 22 in 54–89% yield. The protocol tolerates a variety of electron-donor and electron-withdrawing substituents in the aryl diazonium salts 74, as well as alkyl and aryl thiols 2 which could be efficiently used as substrates. In addition, the developed methodology could also be applied to ortho-substituted allyl diazonium salts 74a, to provide the respective five-membered dihydrobenzofuran derivatives 85 in up to 84% yield (Scheme 46 and Scheme 47). Advantages of this protocol include the application of a wide scope of substrates, excellent functional group tolerance, low photocatalyst load, and amenability to gram-scale synthesis.
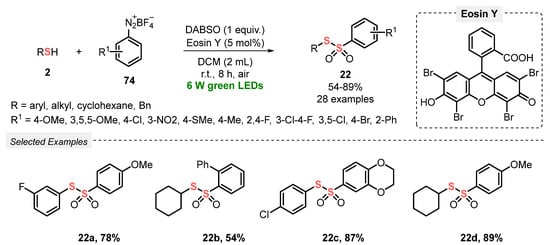
Scheme 46.
Synthesis of nonsymmetrical thiosulfonates 22 promoted by green LEDs.
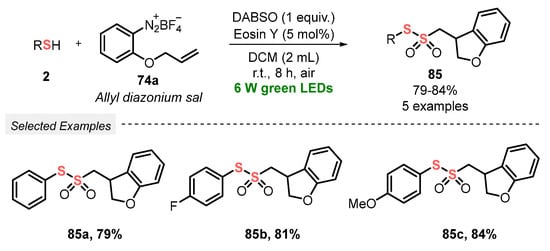
Scheme 47.
Synthesis of nonsymmetrical thiosulfonates 85 promoted by green LEDs.
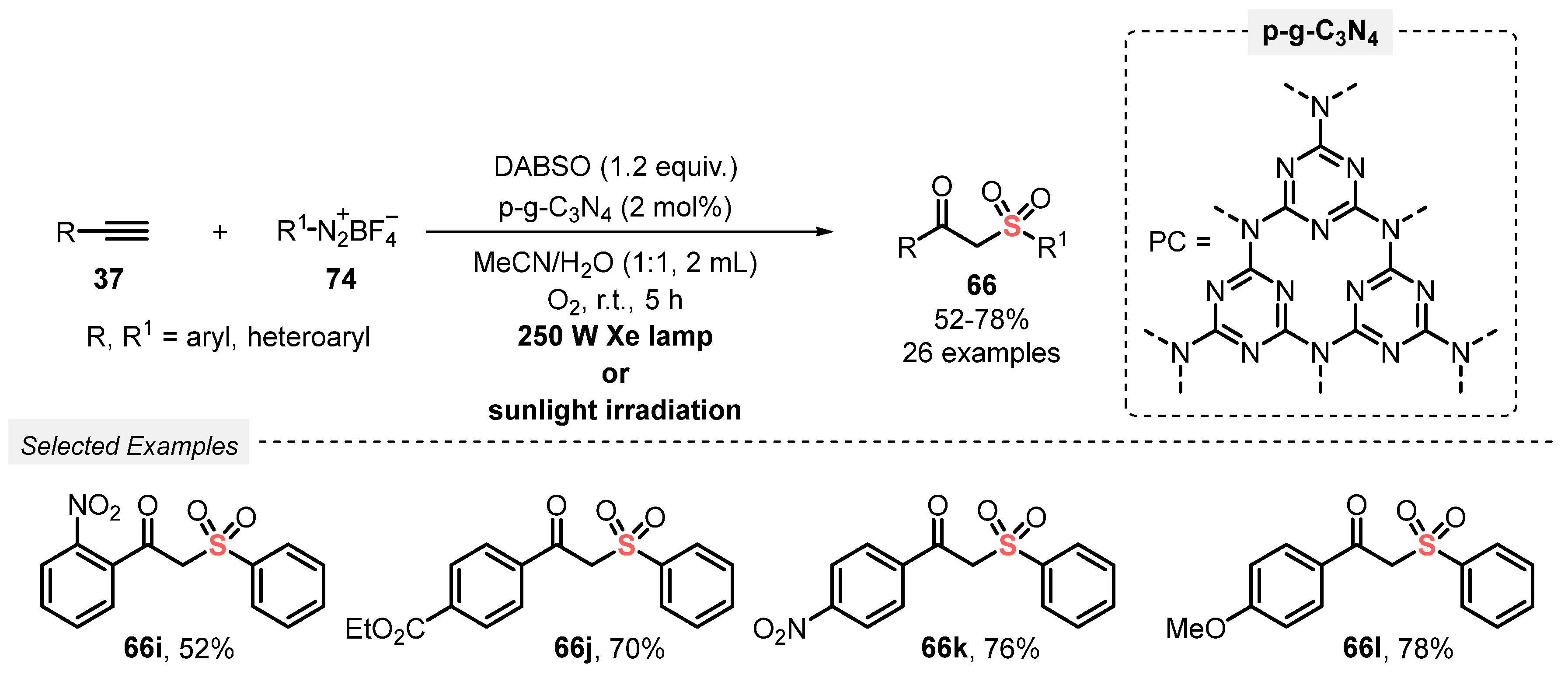
A year later, Niu and co-workers [93] reported a new and efficient visible light-mediated approach to access various β-ketosulfones 66, through the hydrosulfonylation of alkynes 37 using DABSO. The method involves the irradiation of the reagents with a 250 W Xe lamp using porous graphite-phase carbon nitride (p-g-C3N4) as photocatalyst in a 1:1 mixture of MeCN/H2O. After irradiation for 5 h at room temperature under oxygen atmosphere (balloon), twenty-six β-ketosulfones 66 were obtained in moderate to good yields (52–78%). No reaction was observed when ethylynylcyclopropane was used, which is the main limitation of the method. On the other hand, the protocol proved to be effective when sunlight was used as energy source, and the photocatalyst could be reused in up to six times, without presenting significant reduction in reaction yield (Scheme 48).
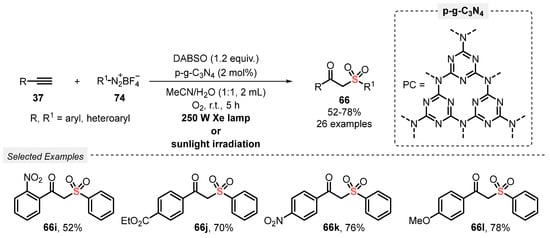
Scheme 48.
Synthesis of β-ketosulfones 66 promoted by Xe lamp irradiation.
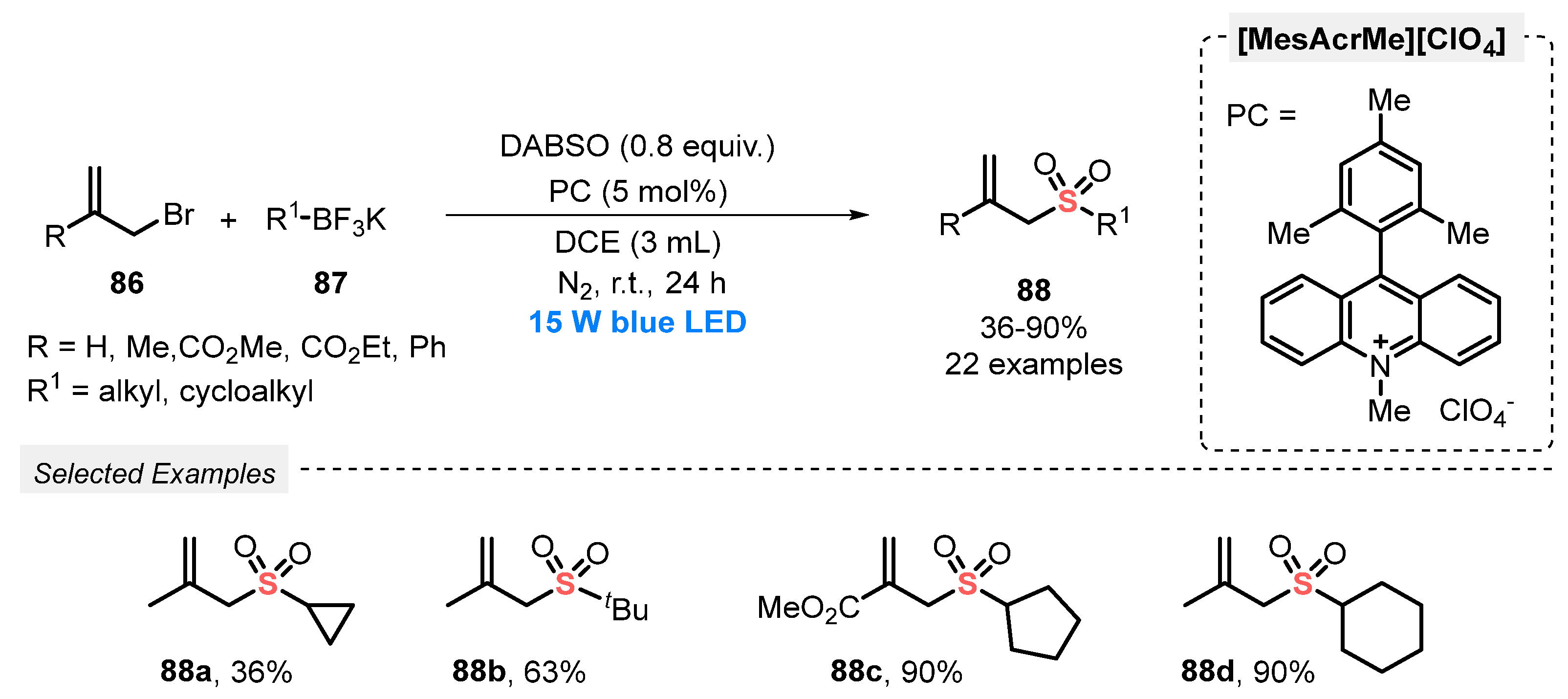
In 2019, Wu and co-workers [94] reported a new strategy for the synthesis of allyl sulfones 88, through the reaction between allyl bromides 86, potassium alkyltrifluoroborates 87 and DABSO as SO2 source. 9-Mesityl-10-methyl acridinium perchlorate (Mes-Acr-MeClO4) was used as photocatalyst, in DCE as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 15 W) for 24 h under N2 atmosphere. Under the optimal conditions, a library of twenty-two allylsulfones 88 decorated with ester, aryl and alkyl substituents was prepared in up to 90% yield (Scheme 49). However, potassium aryl trifluoroborates were not evaluated as substrates.
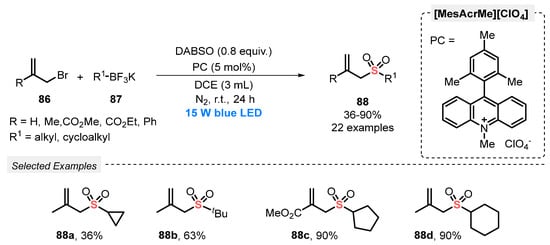
Scheme 49.
Synthesis of allylsulfones 88 promoted by blue LEDs.
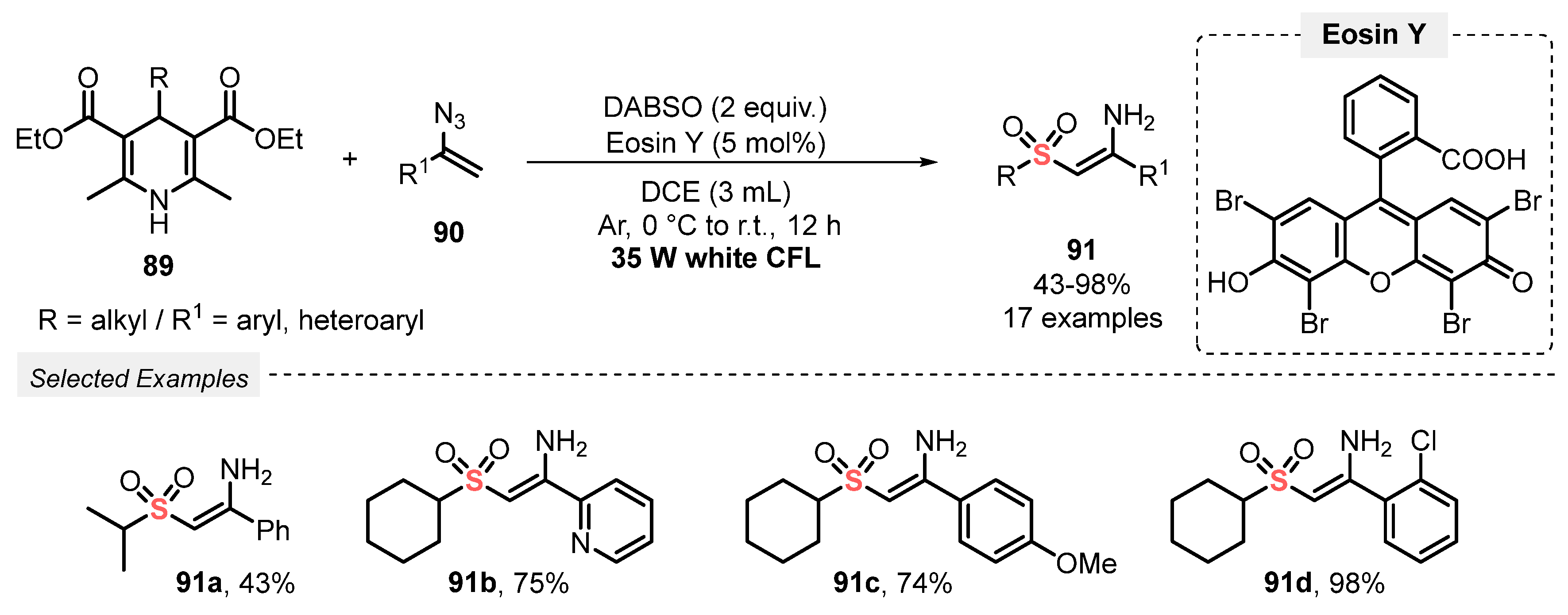
Also, in 2019, the same group [95] reported the visible light-mediated synthesis of (Z)-2-(alkylsulfonyl)-1-arylethen1-amines 91. The authors developed a MCR using 4-substituted Hantzsch esters 89, DABSO, and vinyl azides 90 as substrates, in the presence of Eosin Y as photocatalyst and DCE as solvent. The reaction mixture was irradiated with white light (white CFL, 35 W) for 12 h under Ar atmosphere, to afford the expected sulfonated enamines 91 in moderate to excellent yields (43–98%) in outstanding regio- and stereoselectivity (Scheme 50). Although a wide range of substrates has been evaluated, the methodology is limited just to the use of alkyl-containing Hantzsch esters 89. On the other hand, the method presents high selectivity, affording the desired products preferably with Z configuration.
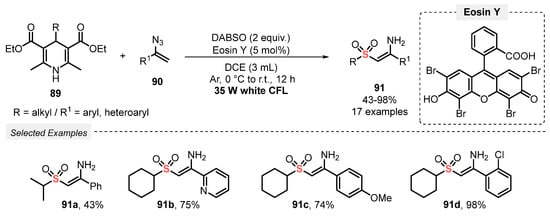
Scheme 50.
Synthesis of (Z)-2-(alkylsulfonyl)-1-arylethen1-amines 91 promoted by white CFL lamp.
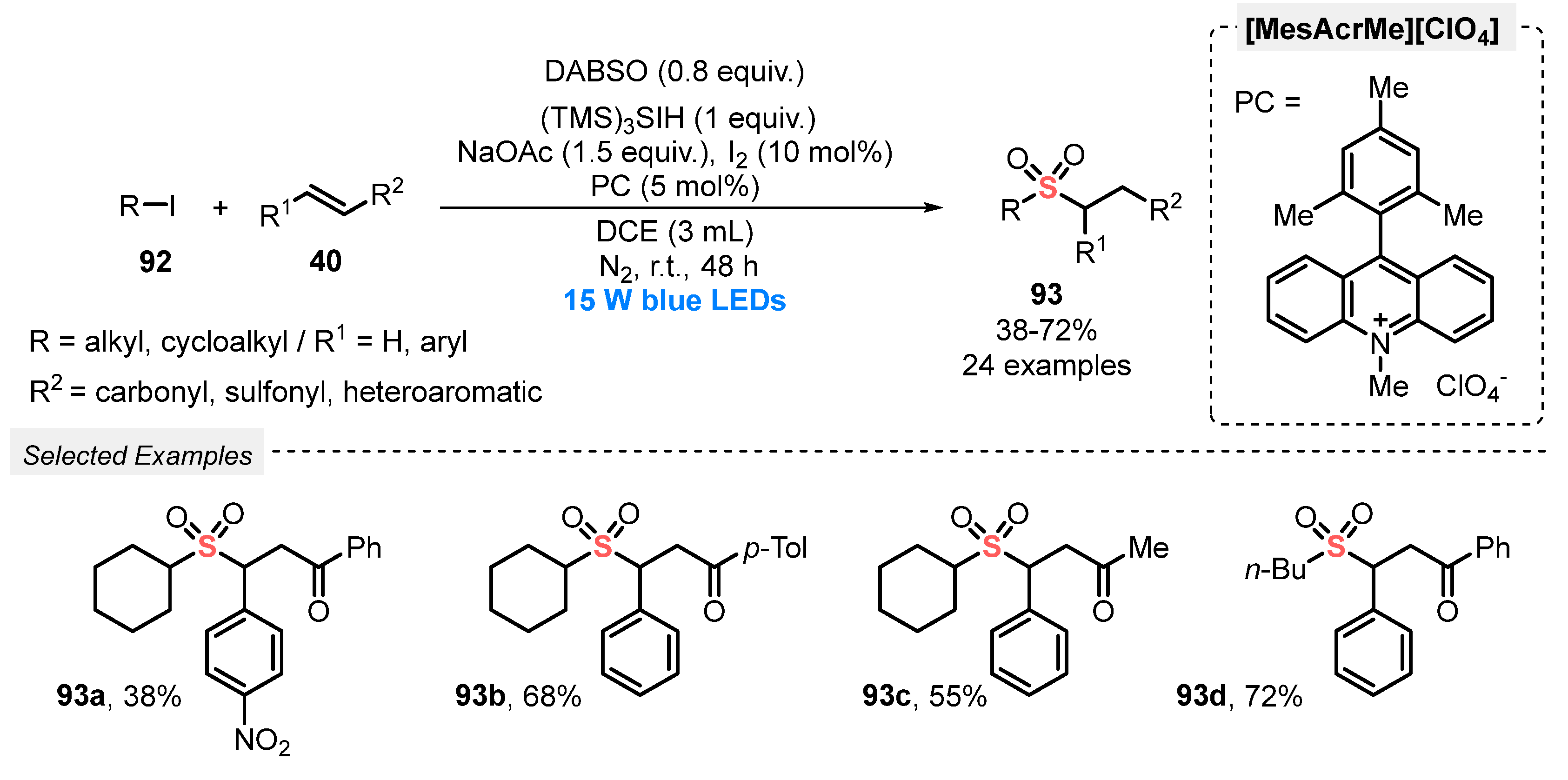
In 2019, Qiu and co-workers [96] developed an efficient method for the visible light-promoted sulfonylation of several (E)-chalcones 40, in the presence of alkyl iodides 92 and DABSO, aiming to prepare functionalized alkyl sulfone derivatives 93. The optimal conditions involve the constant irradiation of the reaction mixture with blue light (blue LEDs, 15 W) in the presence of 9-mesityl-10-methyl acridinium perchlorate [MesAcrMe][ClO4] as photocatalyst, trisilane as H-donor, I2 as additive, and NaOAc as base in DCE as solvent. After irradiation for 48 h under N2 atmosphere at room temperature, twenty-four alkyl sulfone derivatives 93 were prepared in 38–72% yield. Alkyl iodides 92 with different chain lengths, as well as different substituted chalcones 40 could be satisfactorily used in the reaction (Scheme 51).
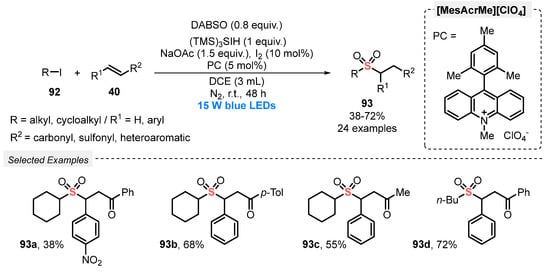
Scheme 51.
Sulfenylation of (E)-chalcones 40 under photoinduced conditions.
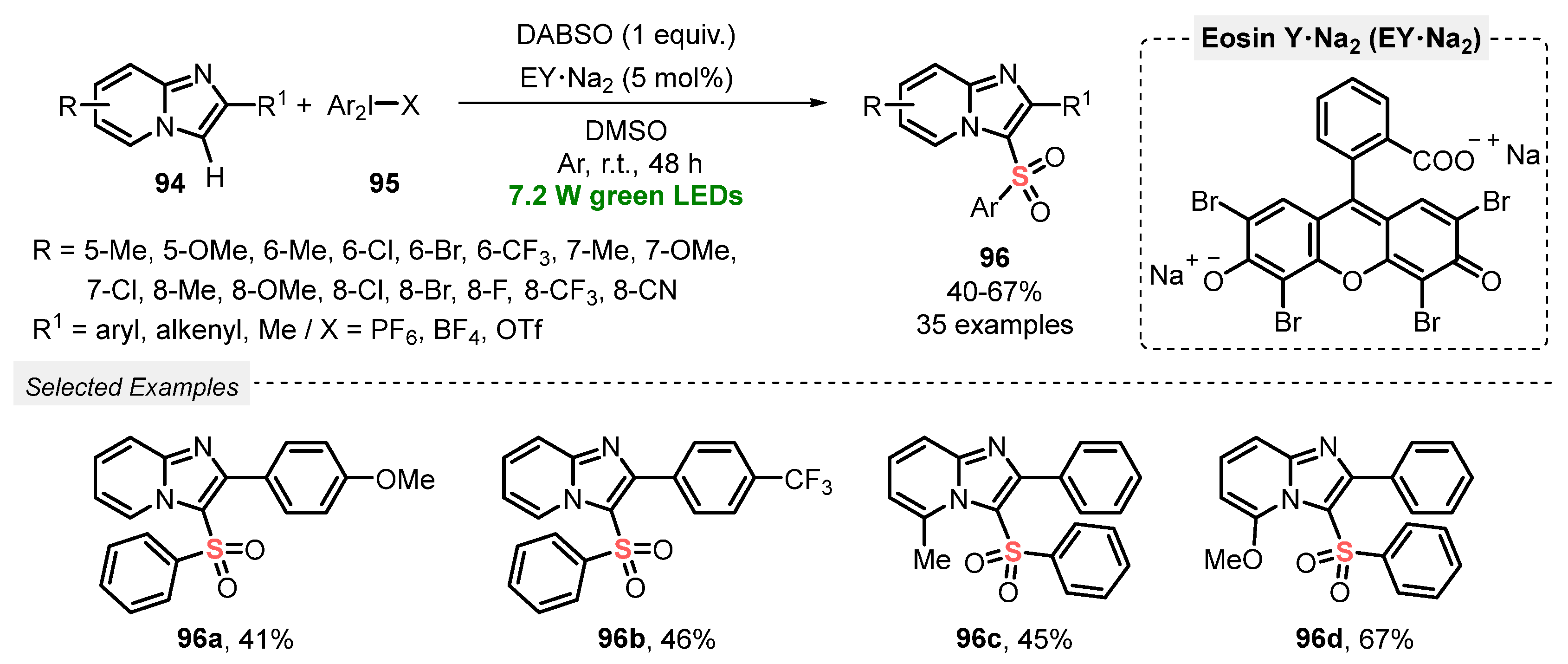
The versatility of DABSO was explored by Piguel and co-workers [97] in C-H activation reactions, aiming to construct sulfonated imidazo[1,2-a]pyrimidines and derivatives. In this new approach, the sulfonylation of imidazoheterocycles 94 was performed using DABSO in the presence of hypervalent iodine species 95 under green light irradiation (green LEDs, 7.2 W). The reagents were dissolved in DMSO, and the photocatalyst Na2-Eosin Y was added under Ar atmosphere. The resulting mixture was irradiated at room temperature for 48 h, to yield the desired products 96 in 40–67% yield. Limitations were found when unsubstituted imidazo[1,2-a]pyridines and 3-phenyl imidazo[1,2-a]pyridine were employed as substrate, which were totally unreactive under the optimized conditions. On the other hand, the protocol was satisfactorily expanded to other imidazoheterocycle cores, including imidazo[2,1-b]thiazole, benzo[d]imidazo[2,1-b]thiazole, and imidazo[1,2-a]pyrimidine (Scheme 52).
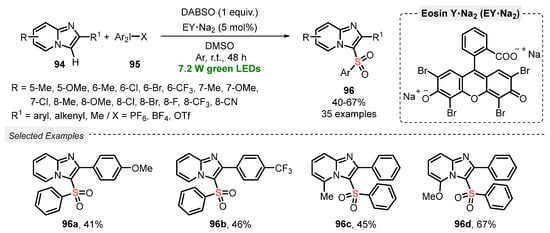
Scheme 52.
Synthesis of sulfonated imidazoheterocycles 96 under photoinduced conditions.
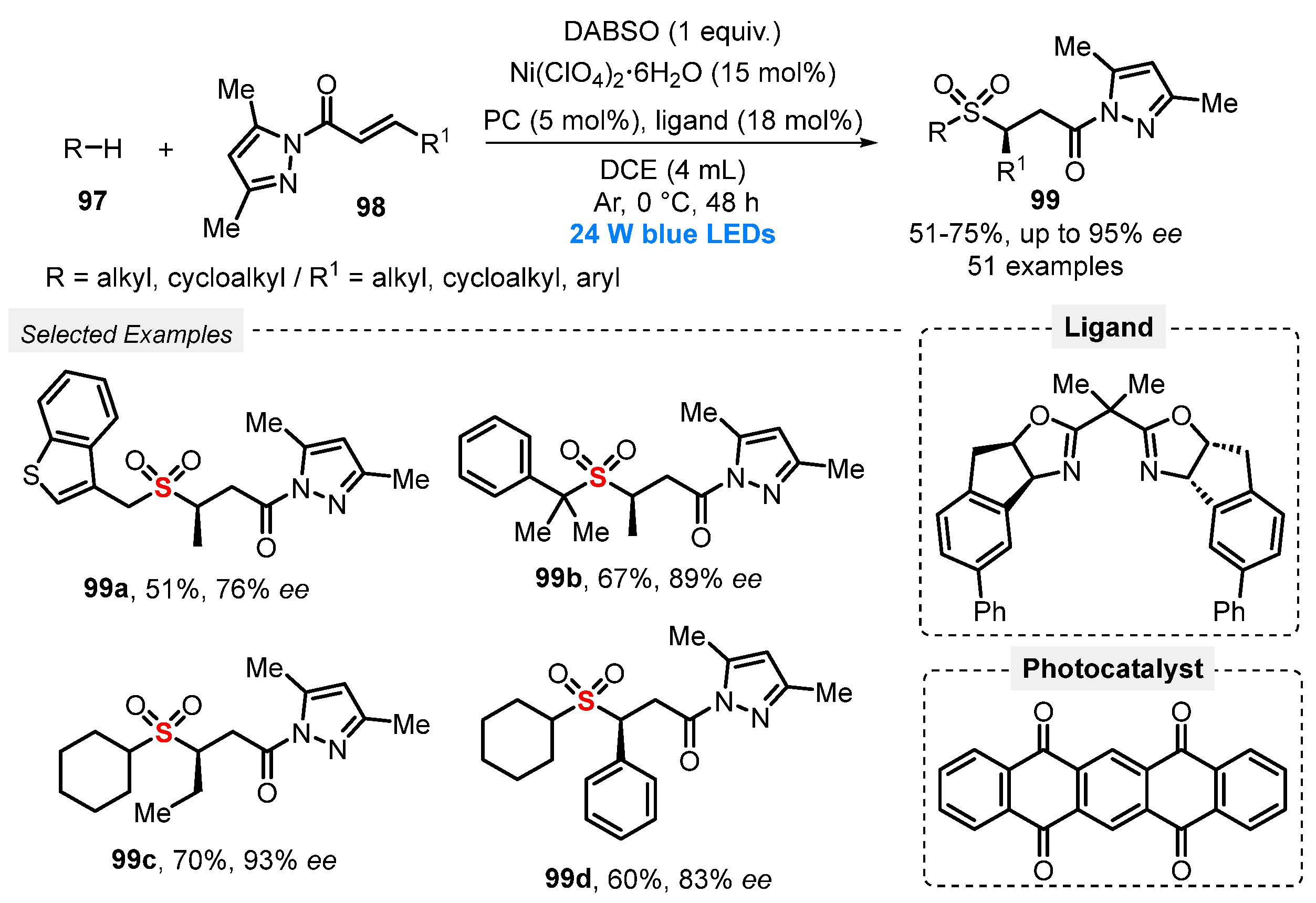
More recently, in 2021, Gong and co-workers [98] performed the direct sulfonylation of Csp3–H bond, through a MCR between alkanes and cycloalkanes 97, pyrazole-functionalized α,β-unsaturated ketones 98, and DABSO as the SO2-source. The authors used a dual asymmetric photocatalytic system, composed by a chiral catalyst—the bisoxazoline nickel complex generated in situ from Ni(ClO4)2 and the indane-derived chiral bis(oxazoline) ligand, and 5,7,12,14-pentacenetetrone as H-transfer photocatalyst. The reactions were performed under blue light irradiation (blue LEDs, 24 W) for 48 h at 0 °C under Ar atmosphere, using DCE as solvent. The chiral sulfones 99 were obtained in 51–75% yield, in up to 95% of enantiomeric excess (e.e.). The methodology was satisfactorily applied to late-stage functionalization of bioactive molecules, proving to be an attractive methodology for obtaining more complex molecules in drug discovery (Scheme 53). The main advantages are the wide substrate scope and the synthesis of the desired products in high enantiomeric excess. On the other hand, the reaction needs a very large excess of alkane (20 equiv) to afford a good yield.
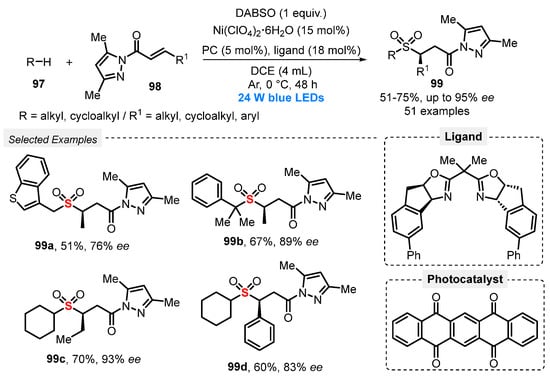
Scheme 53.
Synthesis of enantioenriched α-C chiral sulfones 99 promoted by blue light.
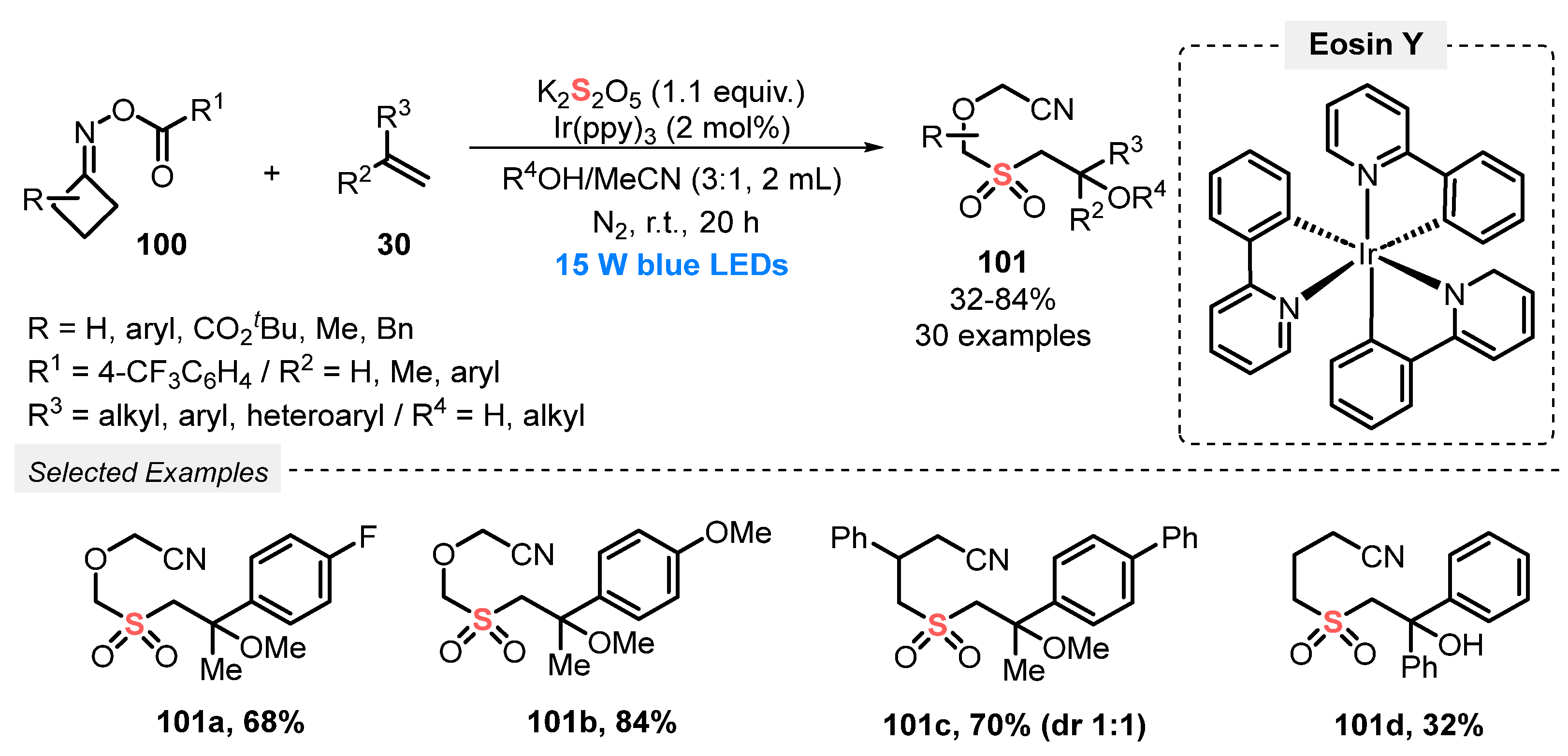
In 2019, Wu and co-workers [99] developed a visible light-promoted MCR protocol to access the densely functionalized sulfonylated nitriles 101. The protocol involves the reaction of cyclobutanone oxime esters 100, terminal alkenes 30 and K2S2O5 as SO2-source. The authors used Ir(ppy)3 as photocatalyst and a mixture of alcohols and MeCN (3:1) as solvent. The alcohol component was incorporated in the final product; if water is used instead, the hydroxy group is present in the respective sulfones. In a typical procedure, the reaction mixture was irradiated with blue light (blue LEDs, 15 W) under N2 atmosphere at room temperature for 20 h. Under this condition, thirty sulfones 101 were obtained in 32–84% yield, presenting a diversity of substituents, both from the o-acyloximes 100 (aryl, ester, alkyl) and from the alkene 30 (aryl, alkyl, ether) counterparts. A clear influence of steric and electronic effects in the reaction yields was not observed; only secondary and tertiary alcohols were not good solvents/reagents in this transformation (Scheme 54).
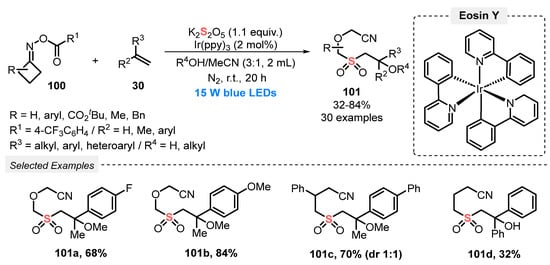
Scheme 54.
Sulfonylation of o-acyloximes 100 promoted by blue light.
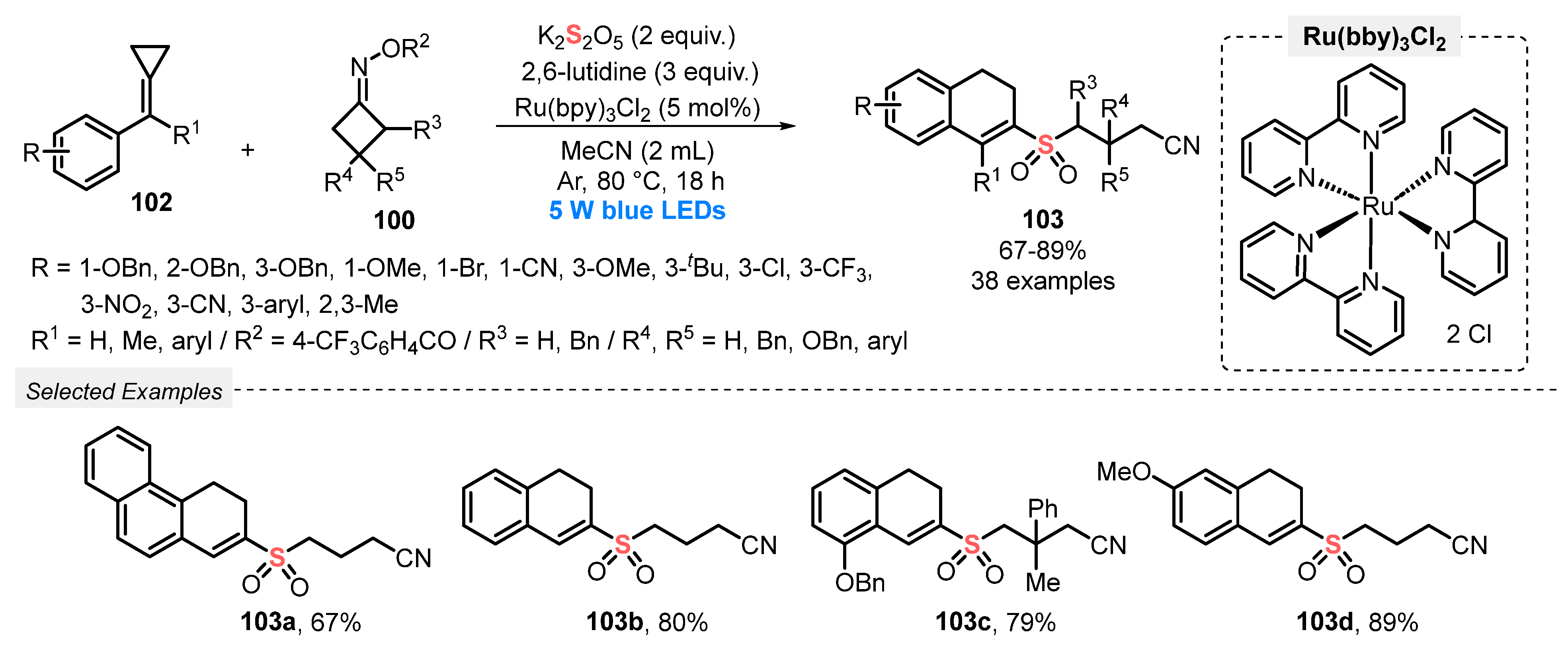
One year later, in 2020, Tang and co-workers [100] reported an efficient method for the synthesis of 2-cyanoalkylsulfonated 3,4-dihydronaphthalenes 103, by reacting methylenecyclopropanes 102, cycloketone oximes 100, and K2S2O5 under visible light conditions. The reactions were smoothly performed in the presence of Ru(bpy)3Cl2 as photocatalyst and 2,6-lutidine as base in MeCN as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 5 W) under argon atmosphere at 80 °C for 18 h, affording a total of thirty-eight sulfones 103 in up to 89% yield (Scheme 55). Despite the wide reaction scope, the method has some disadvantages, such as the need for using high temperature (80 °C) and a large amount of base.
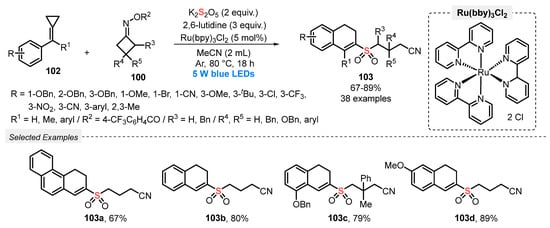
Scheme 55.
Synthesis of sulfonated dihydronaphthalenes 103 promoted by blue light.
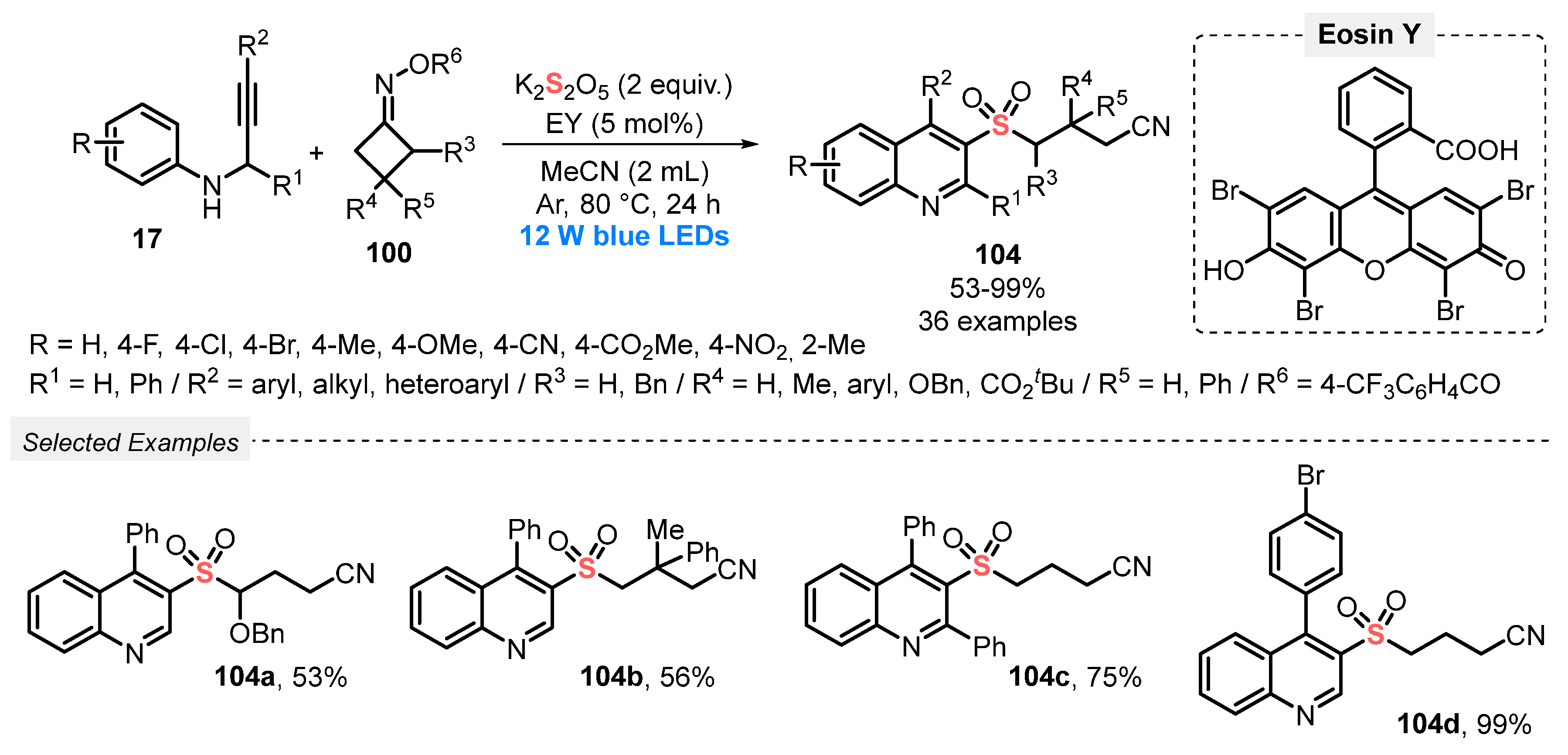
In 2021, Zhou and co-workers [101] developed a metal-free, visible light-promoted protocol for the synthesis of cyanoalkylsulfonylated quinolines 104, starting from aromatic N-propargyl amines 17, cyclobutanone oxime esters 100 and K2S2O5 as SO2-source. The cascade transformation was conducted employing Eosin Y as photocatalyst, in the presence of MeCN as solvent at 80 °C, under Ar atmosphere. Under this condition, thirty-six derivatives 104 were provided in up to 99% yield, after reacting for 24 h, presenting a remarkable substrate tolerance (Scheme 56). In addition, an on/off light experiment was conducted, showing that the formation of the products 104 was completely suppressed in the absence of light, confirming that the blue light is essential for the reaction success.
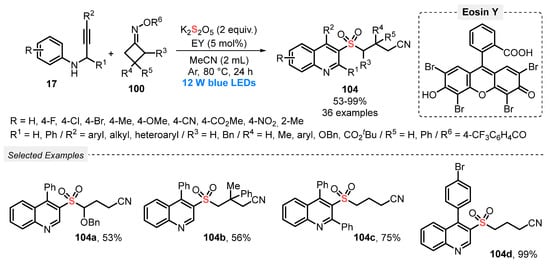
Scheme 56.
Synthesis of cyanoalkylsulfonylated quinolines 104 promoted by blue LEDs.
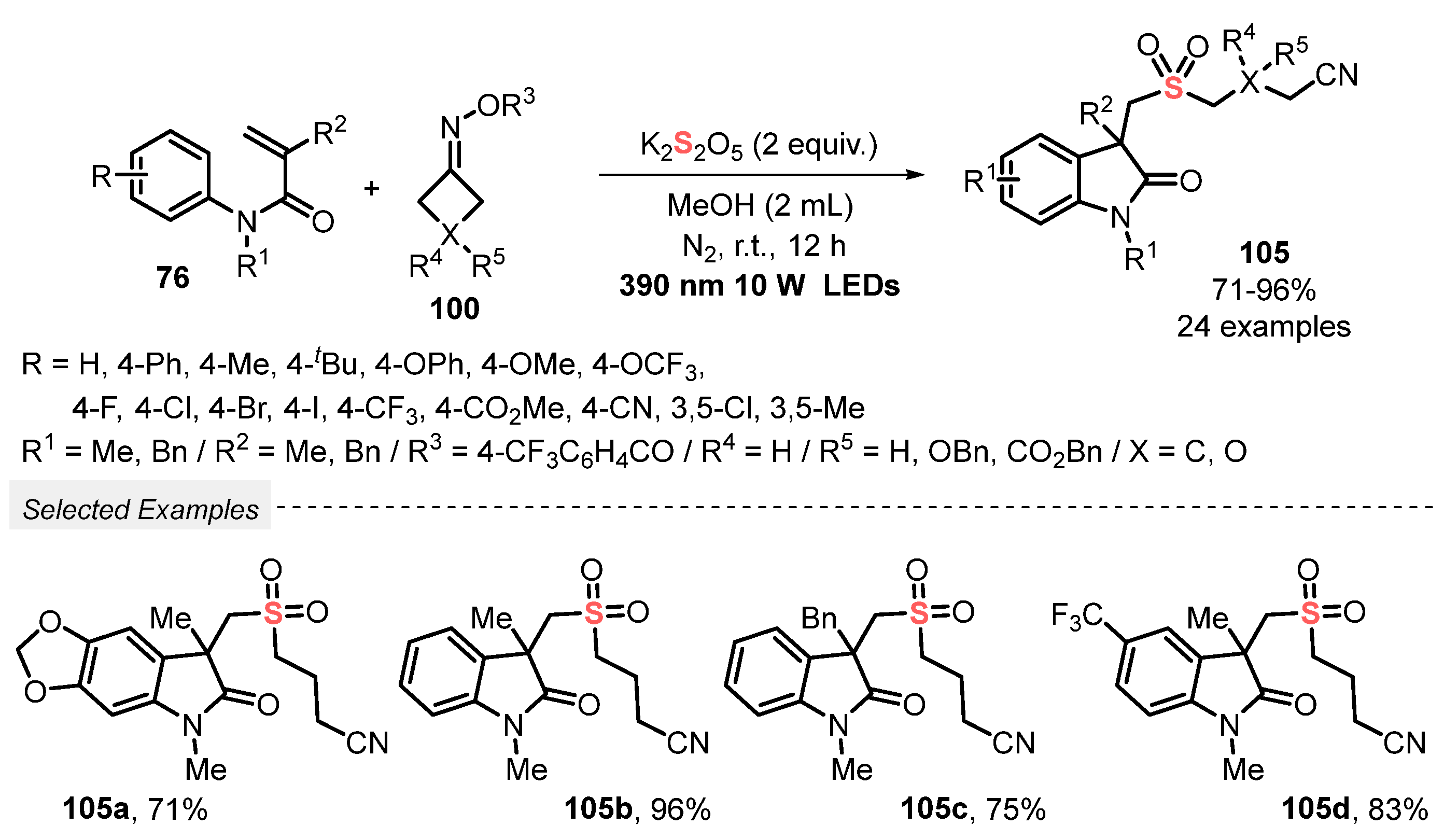
Cyclobutanone oxime esters 100 were used in the catalyst-free visible light-promoted cyclization of N-arylacrylamide 76 in the presence of K2S2O5, to prepare cyanoalkylsulfonylated oxindoles 105 [102]. The reagents were dissolved in MeOH, and the resulting mixture was irradiated with visible light (390 nm LEDs, 10 W) at room temperature and under N2 atmosphere for 12 h. A total of twenty-four oxindoles derivatives 105 were obtained in good to excellent yields (71–96%), derived from electron-rich and electron-poor N-arylacrylamides 76. It is worth mentioning that the reaction scale was satisfactorily increased (from 0.2 to 5.0 mmol) without remarkable yield decreasing (Scheme 57). Important advantages of this process include a broad scope of substrates, excellent functional group tolerance, the use of green solvent, and amenability to the gram-scale synthesis.
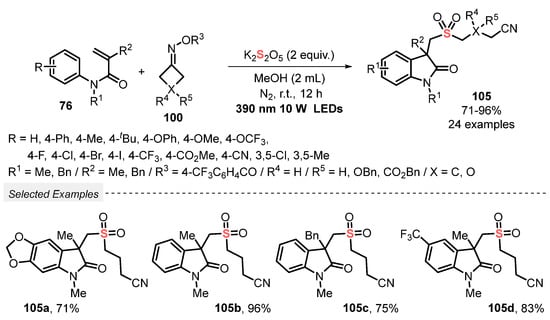
Scheme 57.
Synthesis of cyanoalkylsulfonylated oxindoles 105 promoted by visible light.
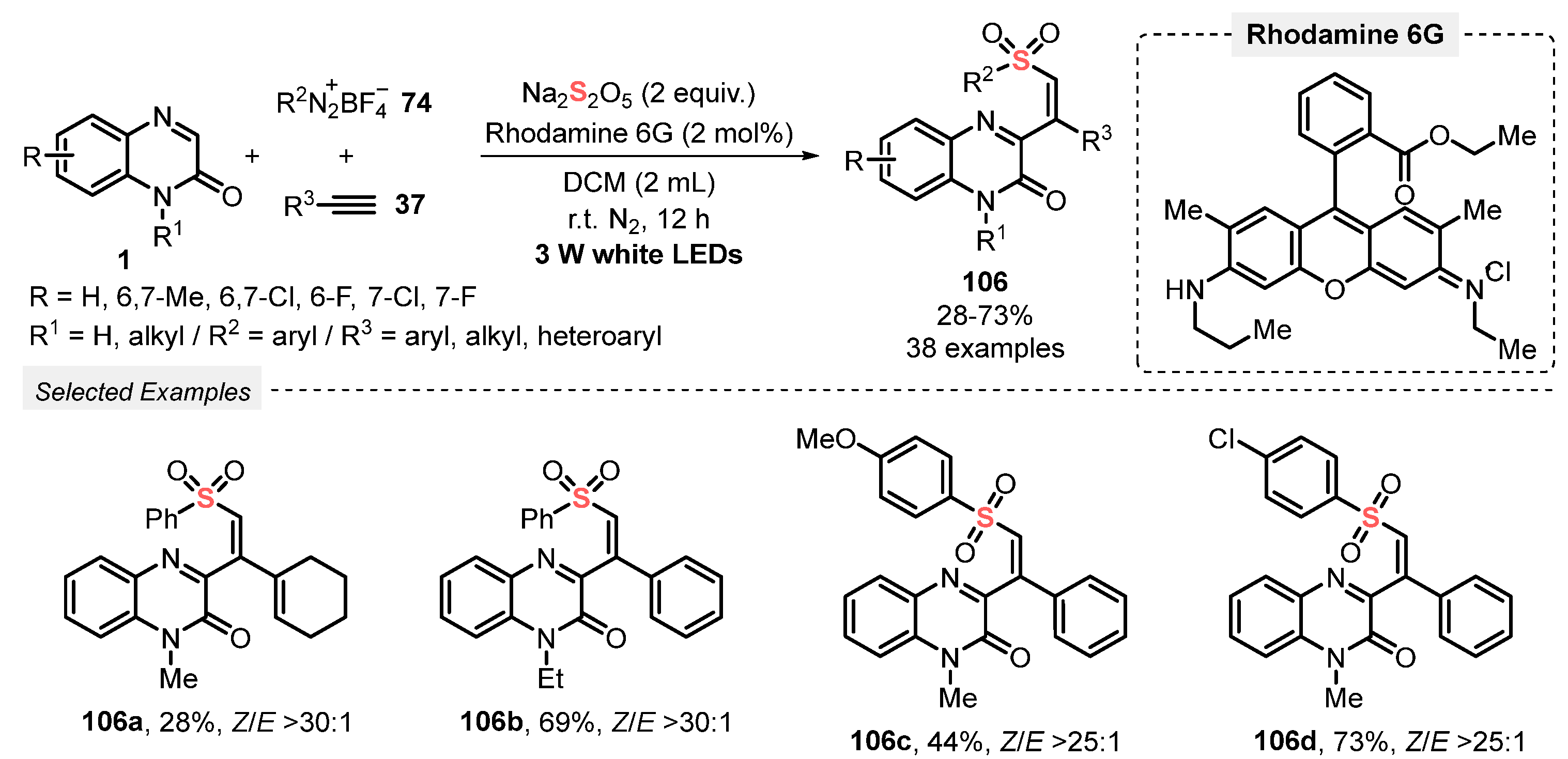
In 2021, Wei and co-workers [103] reported the MCR light-mediated process to prepare quinoxalin-2-one-containing vinyl sulfones 106, by reacting quinoxalinones 1, aryldiazonium tetrafluoroborate salts 74, and terminal acetylenes 37, in the presence of Na2S2O5. These substrates reacted for 12 h in the presence of Rhodamine 6G as photocatalyst and DCM as solvent, under N2 atmosphere and white light irradiation (white LEDS, 3 W). Using this procedure, a wide collection of thirty-eight vinyl sulfone derivatives 106 could be obtained in up to 73% yield. Methyl-, chloro- and fluoro-substituted quinoxalinones 1, electron-rich and electron-poor aryldiazonium salts 74, aryl and heteroaryl alkynes 37 were suitable substrates for the reaction. No product was obtained starting from alkyl acetylene, while 1-ethynylcyclohex-1-ene afforded only 28% yield of the respective alkenyl sulfone (Scheme 58).
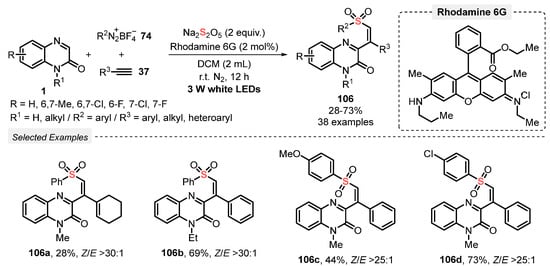
Scheme 58.
Synthesis of quinoxalin-2-one-containing vinyl sulfones 106 promoted by white light.
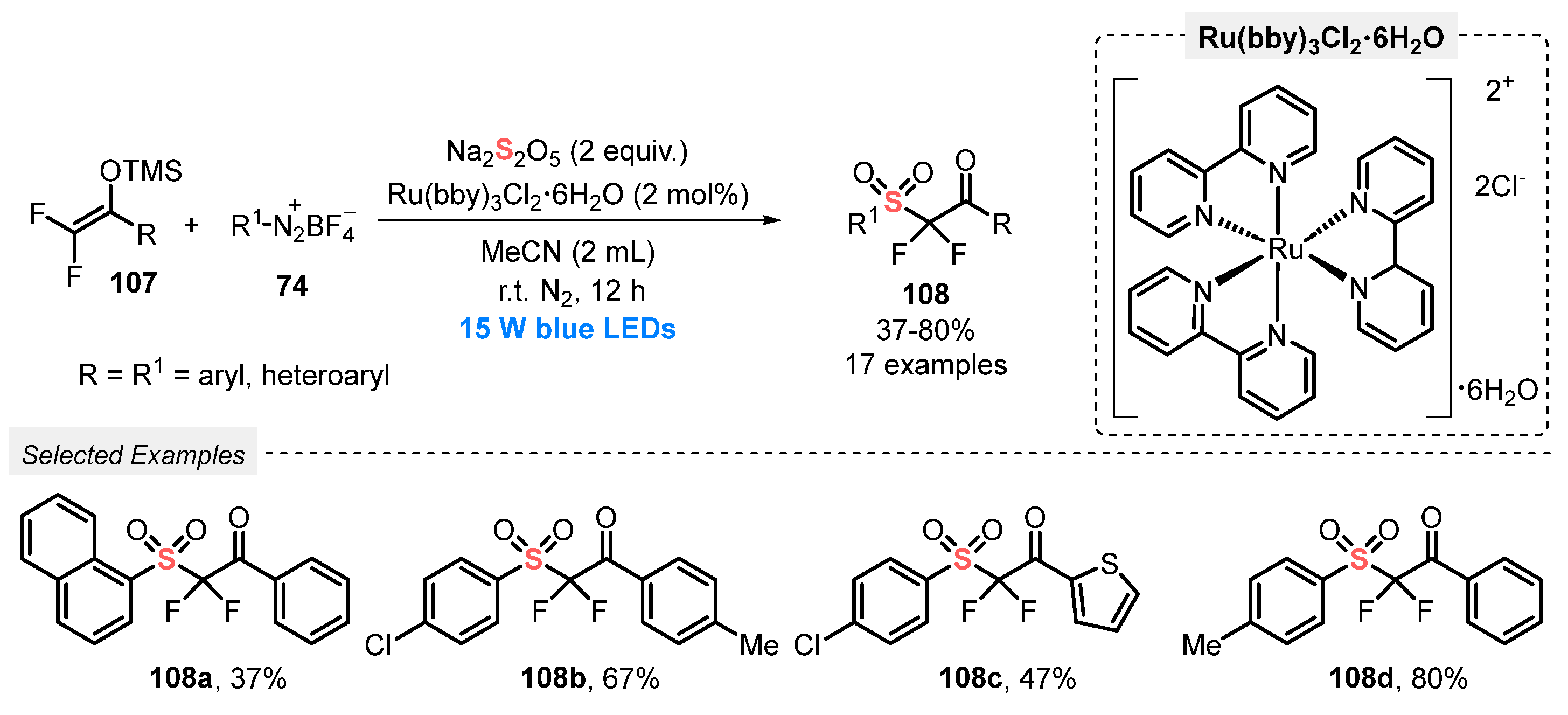
In 2020, Wu and co-workers [104] developed an elegant visible light-mediated protocol to prepare α,α-difluoro-β-ketosulfones 108, employing Na2S2O5 as SO2-source. Several aryldiazonium tetrafluoroborate salts 74 containing EDG and EWG and 1-aryl-2,2-difluoro enol silyl ethers 107 were successfully used as substrates in the presence of tris(bipyridine)ruthenium chloride Ru(bby)3Cl2•6H2O as photocatalyst and MeCN as solvent. The developed protocol involves the irradiation of the reaction mixture with blue light (blue LEDs, 15 W) at room temperature and under N2 atmosphere for 12 h. The expected α,α-difluoro-β-ketosulfones 108 were obtained in 37–80% yield (Scheme 59). In general, aryldiazonium tetrafluoroborate containing electron-donating or electron-withdrawing groups on the aromatic ring provided the products 108 in the highest yields. However, lower yields were obtained when aryldiazonium tetrafluoroborates with ortho-substituted groups were employed as substrate.
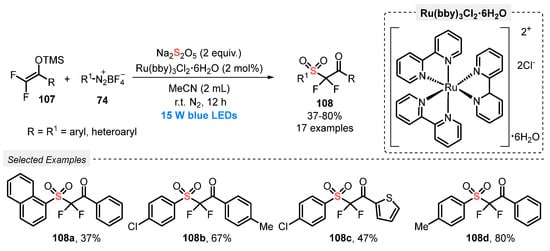
Scheme 59.
Sulfonylation of difluoroenoxysilanes 107 promoted by blue LEDs.
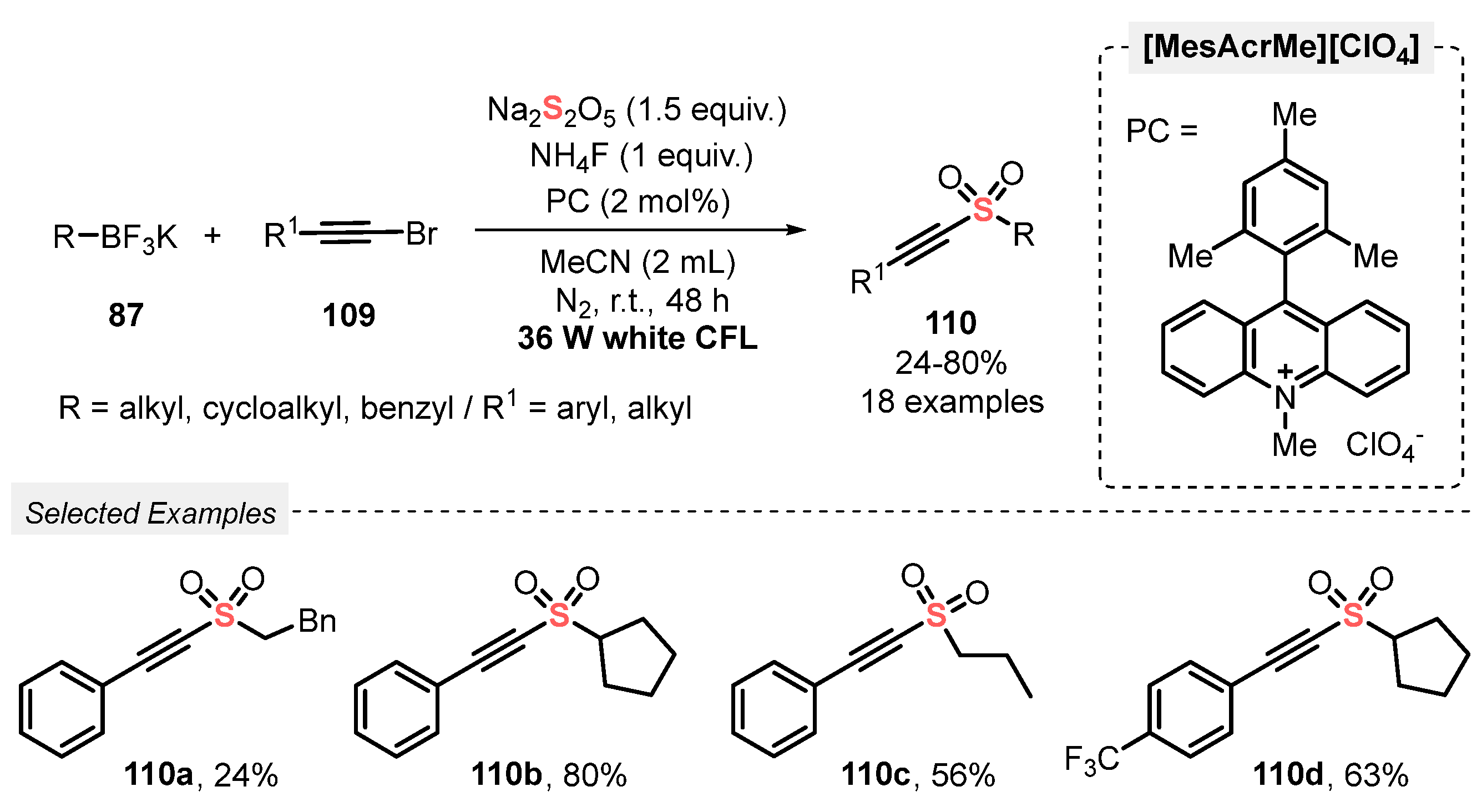
The same group [105] extended the use of this approach to an efficient photoreductive sulfonylation of 2-alkynyl bromides 109, obtaining alkylalkynyl sulfones 110 as product. In this case, potassium alkyltrifluoroborates 87 reacted with 109 in the presence of Na2S2O5 under white light irradiation (white CFL, 36 W). The reactions were conducted in the presence of [MesAcrMe][ClO4] as photocatalyst and NH4F as base, in MeCN as solvent. The mixture was irradiated for 48 h at room temperature under N2 atmosphere, affording a total of eighteen alkylalkynyl sulfones 110 in up to 80% yield, presenting good substrate tolerance regarding the alkynyl bromide 109 (Scheme 60). A limitation was found when allyltrifluoroborate was employed as substrate, and the desired product was not observed, even after 48 h of reaction.
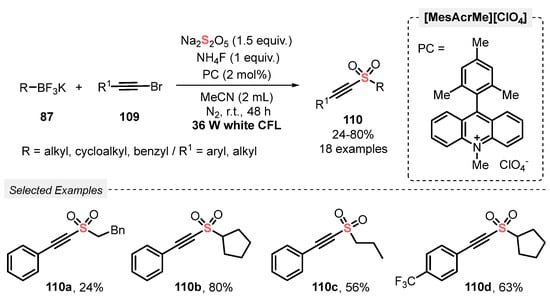
Scheme 60.
Sulfonylation of alkynyl bromides 109 promoted by visible light.
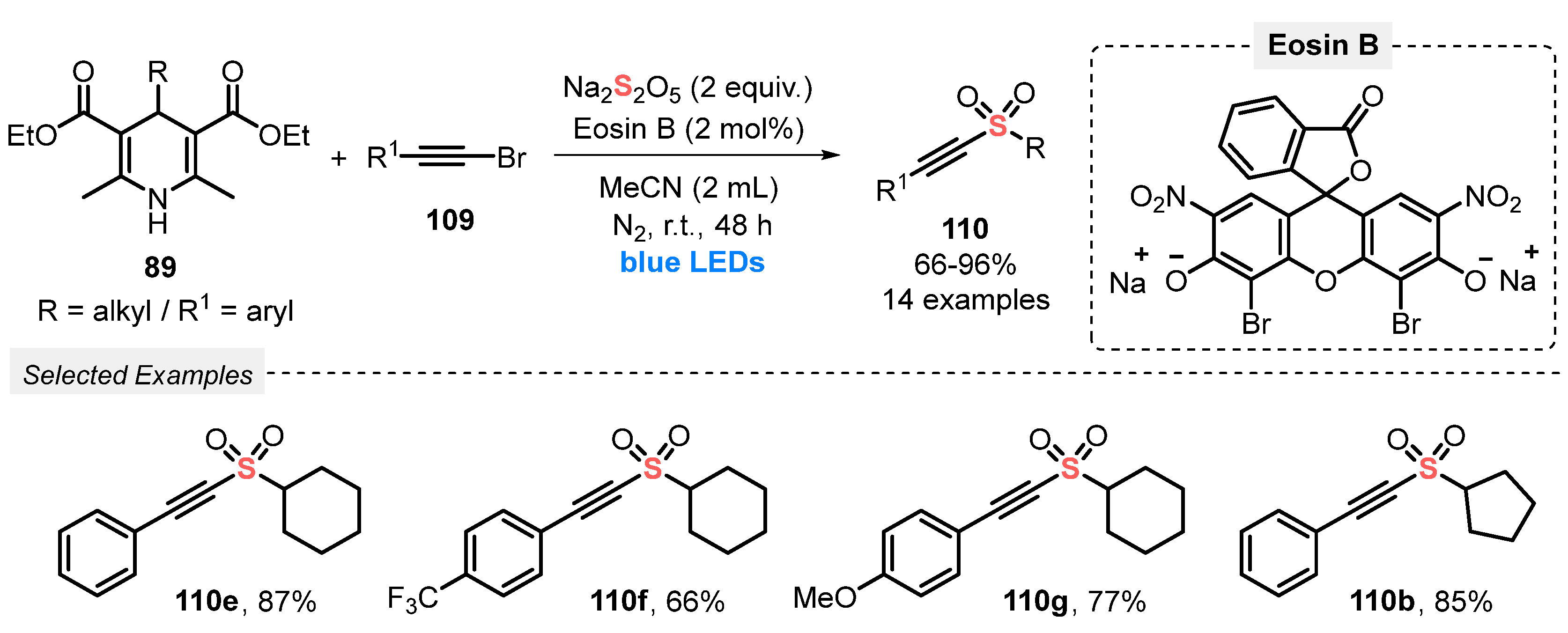
The authors demonstrated that alkylalkynyl sulfones 110 can also be efficiently obtained using 4-alkyl Hantzsch esters 89 instead of potassium alkyltrifluoroborates in the reaction with 2-alkynyl bromides 109 [106]. The protocol was efficiently conducted using Eosin B as photocatalyst in MeCN as solvent, under N2 atmosphere. The reaction mixture was irradiated with blue LEDs (power not reported) for 48 h at room temperature, to afford fourteen alkynyl sulfones 110 in up to 96% yield (Scheme 61). Compared to the previous method (Scheme 60), the present protocol does not require the use of base and delivers the products in slightly higher yields.
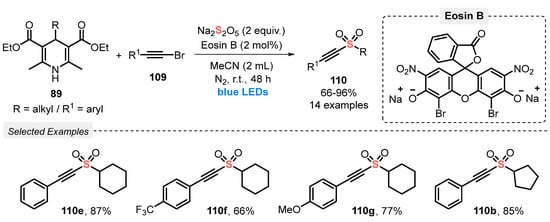
Scheme 61.
Synthesis of alkylalkynyl sulfones 110 promoted by blue LEDs.
4. Photocatalyzed Csp2–N Bond Activation for the Formation of Csp2–S Bonds
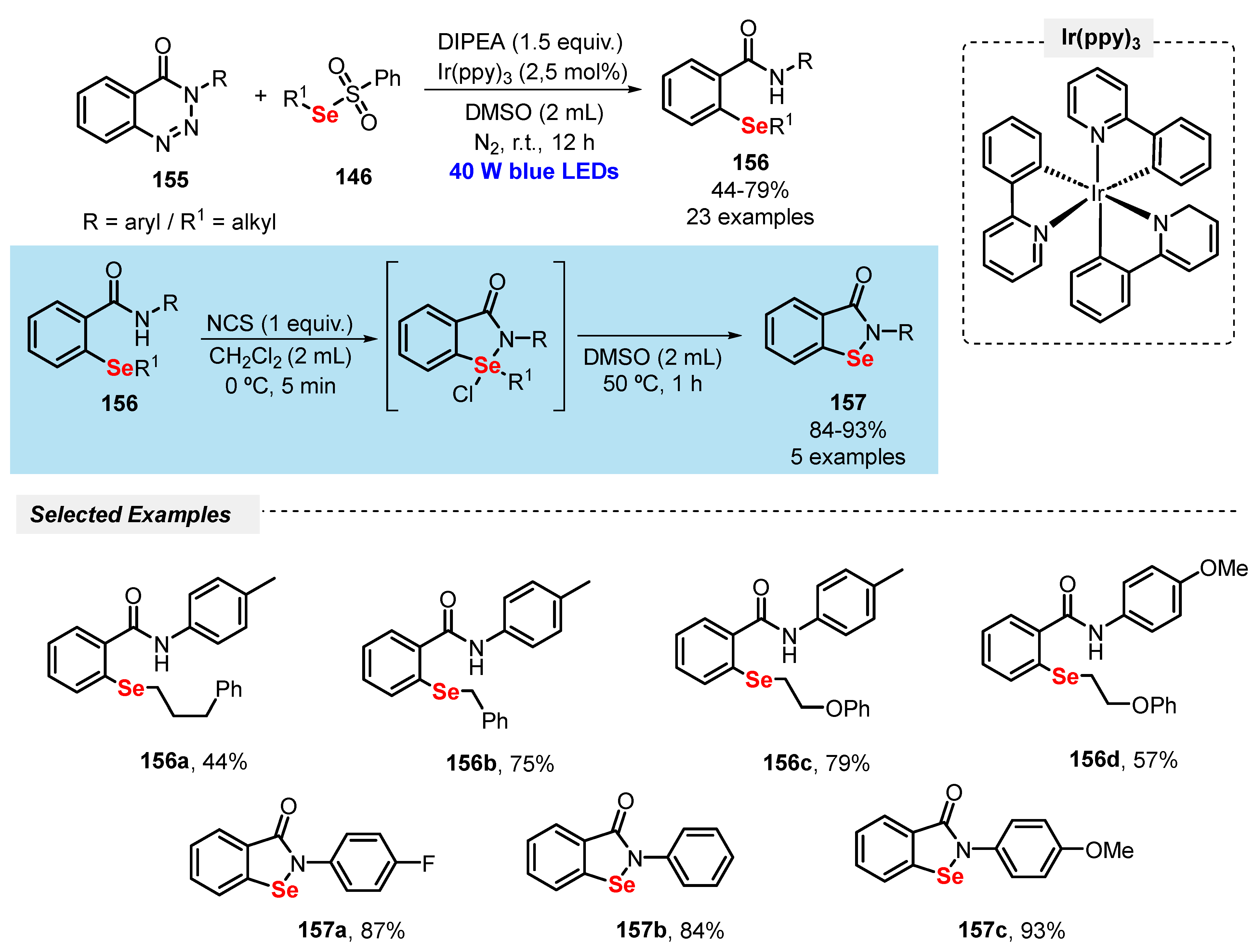
The cleavage of the C–N bond by different pathways has been proving to be an effective strategy to obtain carbon-centered free radicals. In this context, remarkable synthetic approaches have been emerging employing aryl diazonium salts and aryl hydrazides as versatile substrates in the construction of new C–S bonds.
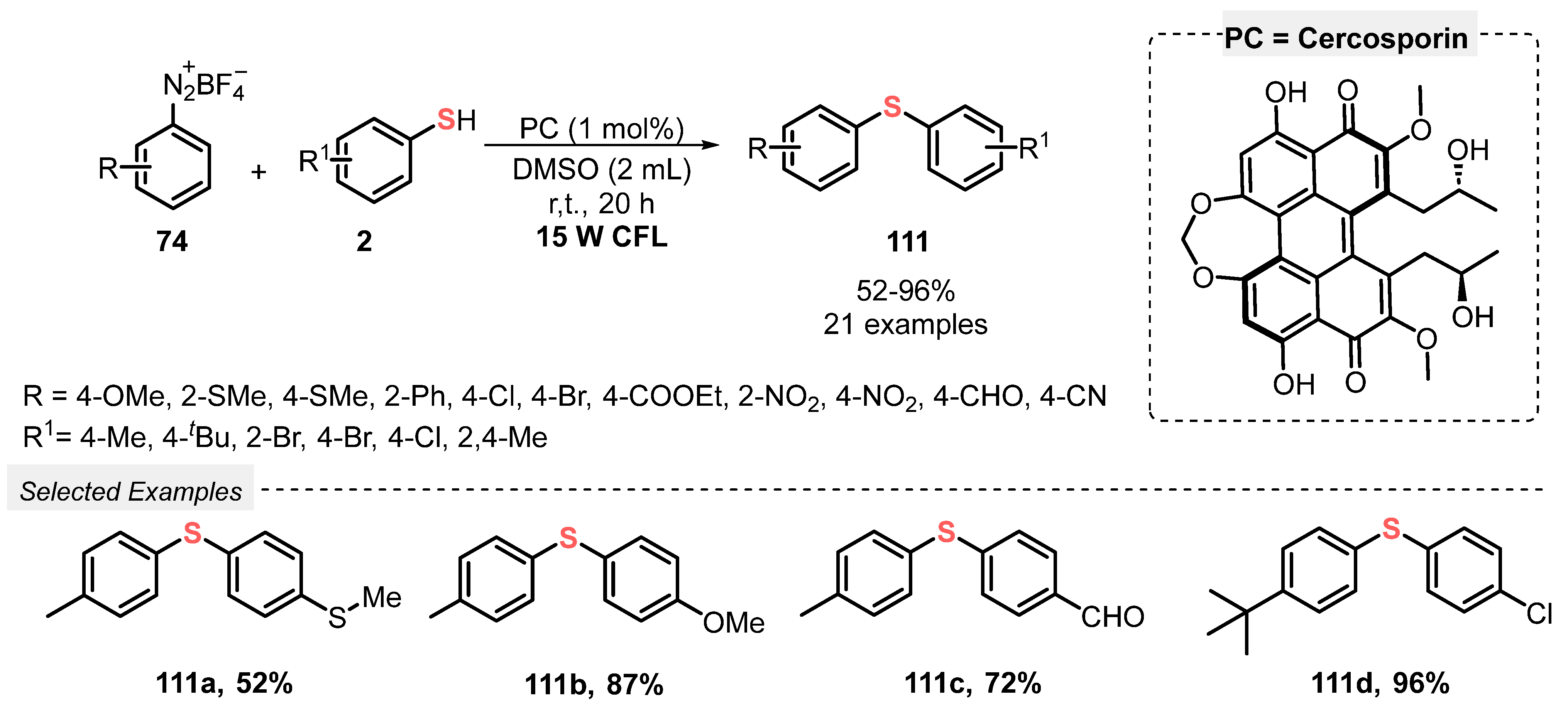
In this sense, Rao and co-workers [107] reported in 2019 a green and scalable methodology for the selective synthesis of bis(aryl) sulfides 111, by reacting diazonium salts 74 with aryl thiols 2 under white light irradiation. The reactions were conducted using a fermentation supernatant containing only 1 mol% of cercosporin as photocatalyst, in DMSO as solvent. The reaction mixture was irradiated with white light (CFL, 15 W) at room temperature for 20 h, giving a library of twenty-one bis(aryl) sulfides 111 in up to 96% yield. Diazonium salts 74 bearing EDG (R = OMe, SMe) and EWG (R = Cl, NO2, CN, CHO) and differently substituted aryl thiols 2 (R1 = Me, tBu, Br, Cl) were suitable substrates in the reaction (Scheme 62). In addition, an experiment under inert conditions shows that the oxygen present in air is crucial to form the desired products.
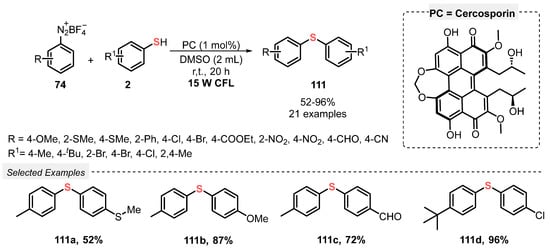
Scheme 62.
Visible light-mediated selective synthesis of aryl sulfides 111.
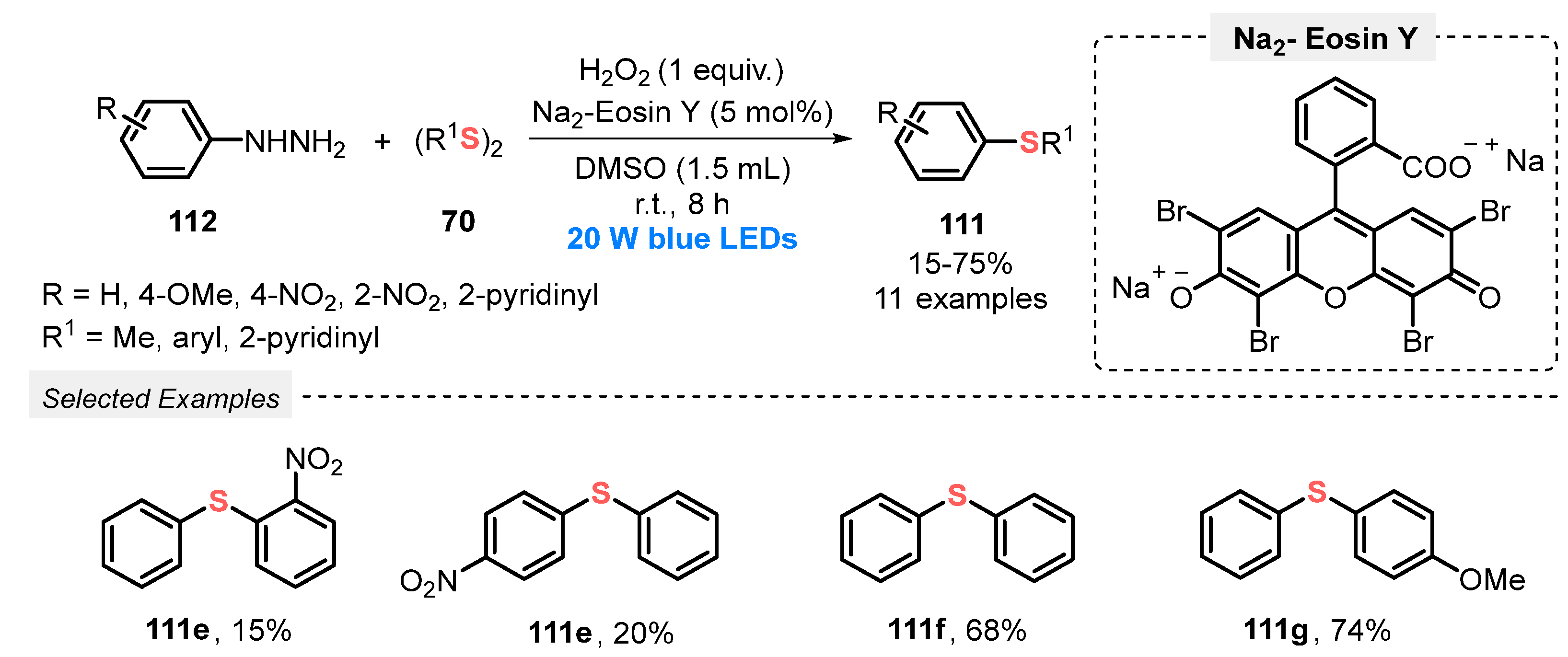
In the same year, Yu and co-workers [108] described the visible light-promoted reaction between arylhidrazines 112 and diorganyl disulfides 70 also obtaining nonsymmetrical bis(aryl) sulfides 111 under metal-free conditions. In this methodology, Na2-Eosin Y was used as photocatalyst in DMSO as solvent and H2O2 was used as the oxidant in an equivalent amount. It was observed that the oxygen present in air alone is not sufficient to promote the reaction. In this sense, the authors also tested this procedure changing H2O2 for an O2 atmosphere, and the product yield decreased only 4%. In this protocol, the mixture was irradiated with blue light (blue LEDs, 20 W) at room temperature in a lower time, 8 h, allowing the synthesis of eleven aromatic sulfides 111 in yields ranging from poor to good (15–75%). The lower yields were obtained when the strong electron-withdrawing NO2 group was present in the substrates, while the presence of EDG (OMe, NH2) increased their reactivity (Scheme 63). In the same work, the authors developed a photocatalyzed synthesis of ten examples of diarylphosphoryl hydrazides (50–87% yield) through the phosphorylation of arylhidrazines.
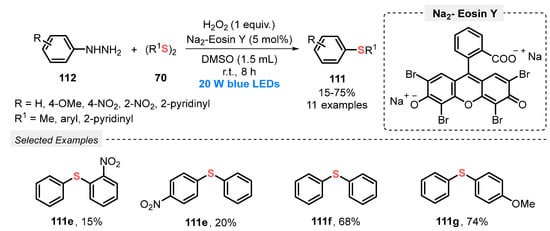
Scheme 63.
Visible light-mediated sulfidation of arylhydrazines 112.
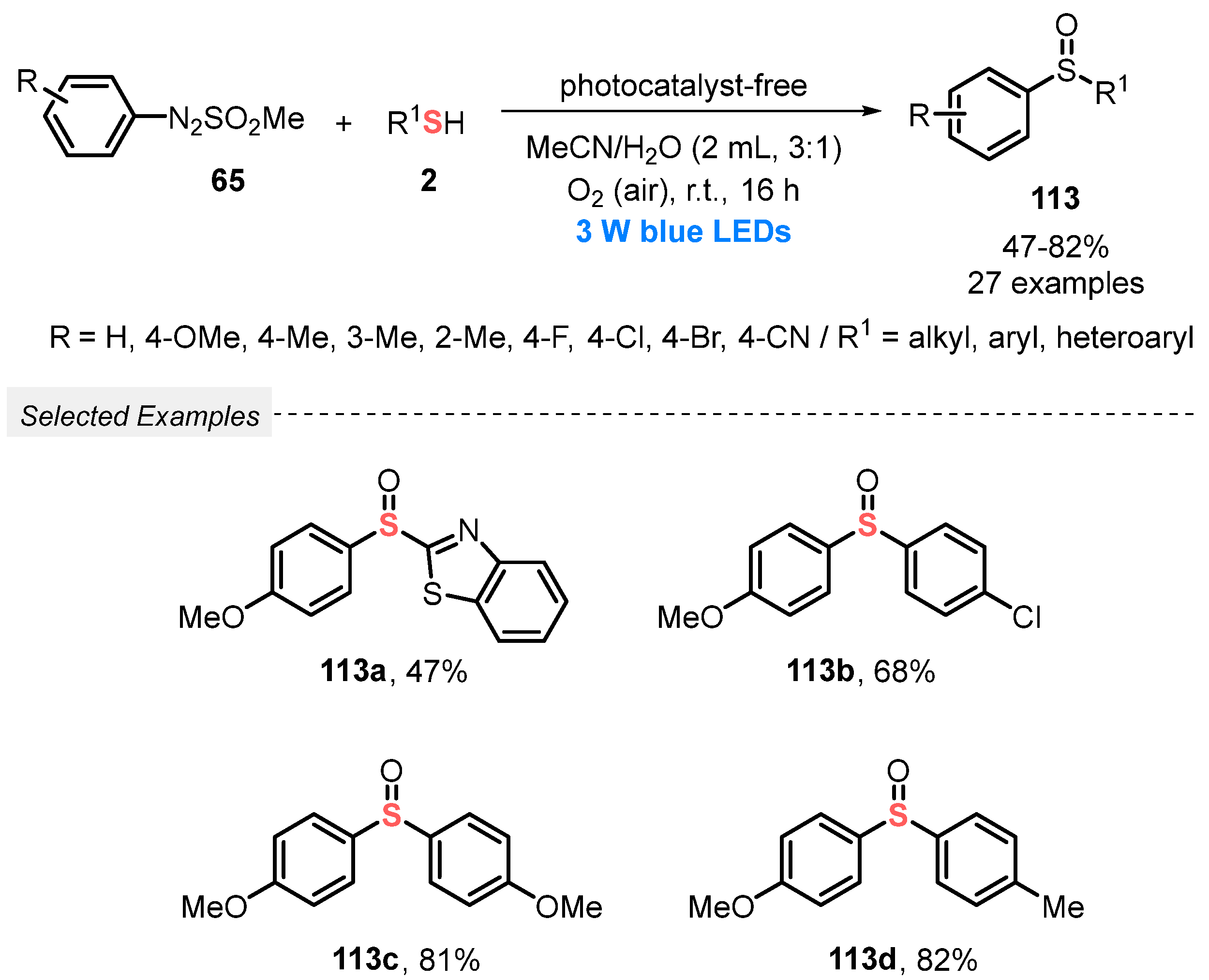
Also, in 2019, Wei and co-workers [109] described an eco-friendly, catalyst-free, and selective visible light-mediated protocol to prepare nonsymmetrical sulfoxides 113. The method involves the oxidative coupling between arylazosulfones 65 and thiols 2, using atmospheric O2 as final oxidant in a 1:1 mixture of MeCN/H2O as solvent. The resulting mixture was irradiated with blue light (blue LEDs, 3 W) at room temperature in an open flask for 16 h, to afford a range of twenty-seven unsymmetrical sulfoxides 113 prepared in up to 82% yield. In contrast to the observed by Yu and co-workers [108], no influence of electronic effect was observed in the reactivity of the substrates 65 and 2. Good results were obtained using alkyl, ester derivatives, and heterocyclic arylthiols, like 5-methyl-1,3,4-thiadiazole-2-thiol and benzo[d]thiazole-2-thiol. The heteroaromatic thiols, however, required 40 h to afford the respective sulfoxides in 48% and 47% yields (Scheme 64). The main advantage of this methodology is the use of a low power energy source, being the reactions performed in the absence of catalyst, and in the presence of an environmentally friendly solvents mixture.
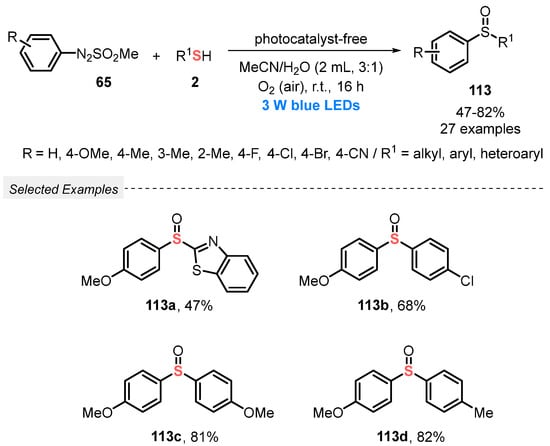
Scheme 64.
Oxidative coupling of arylazosulfones 65 with thiols 2 leading to nonsymmetrical sulfoxides 113 under visible light.
5. Photocatalyzed Synthesis of Thioester, Thioether and Thioacetal
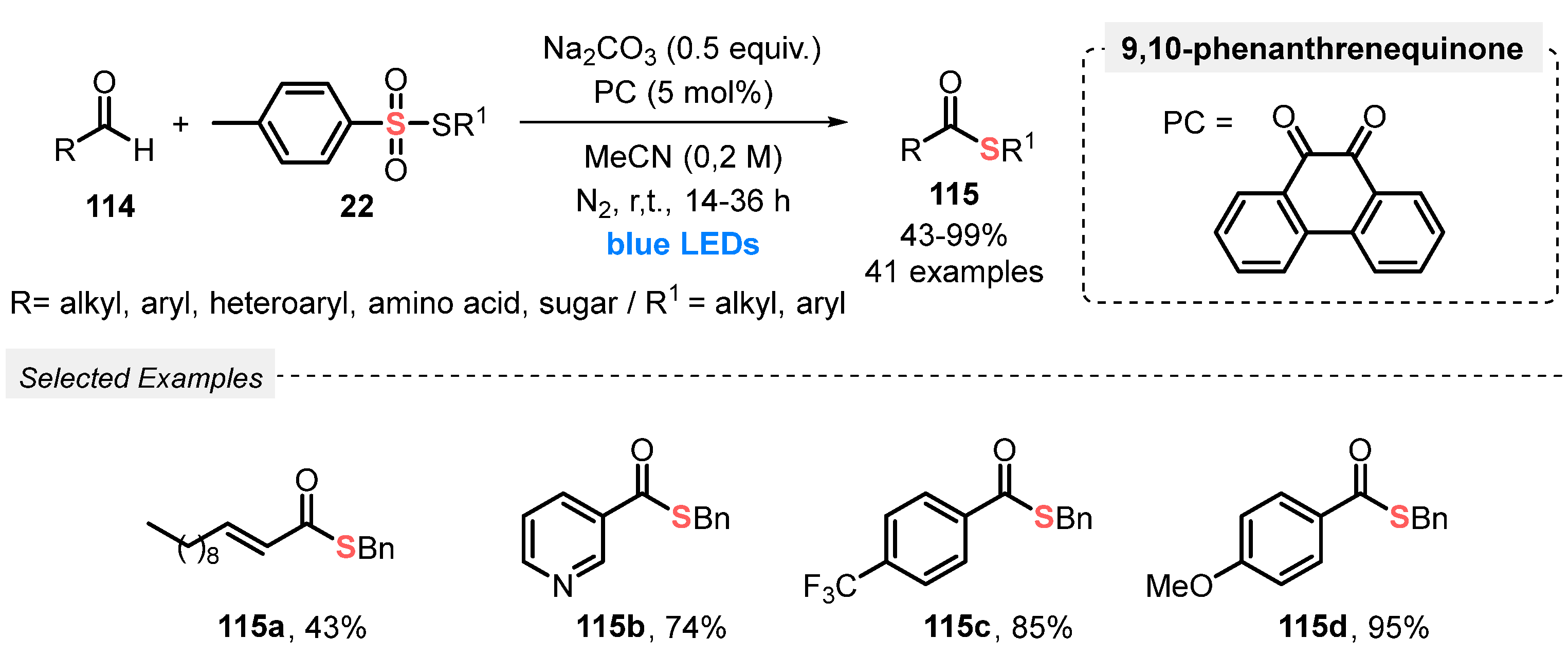
In 2019, Wang and co-workers [110] reported a new and ingenious visible light-mediated strategy to prepare thioesters 115 through a radical coupling process involving aldehydes 114 and thiosulfonates 22. A large scope of forty-one thiol ester derivatives 115 was suitably prepared, in up to 99% yield, by conducting the reactions in the presence of 9,10-phenanthrenequinone as photocatalyst and Na2CO3 as base in MeCN as solvent without the use of metals. The resulting mixture was irradiated with blue light (blue LEDs strip; no power reported) at room temperature under N2 atmosphere for 14–36 h. The higher irradiation times (24 and 36 h) were required when amino acid, saccharide, and cyclohexane-thiosulfones 22 were used as starting materials. The protocol was applied with excellent performance to complex aldehydes, including ibuprofen-, probenecid-, and ursodeoxycholic acid derivatives, affording the expected thiol esters 115 in good to excellent yields (51–97%) after 14 h of reaction. The proposed reaction mechanism starts with the formation of the excited state photocatalyst, which promotes a hydrogen atom transfer (HAT) process, reaching acyl radical species from the aldehyde as the key intermediate. Finally, the acyl radical reacts with thiosulfonate 22, affording the expected thiol ester and a sulfonyl radical (Scheme 65).
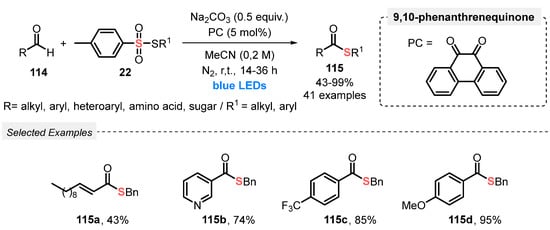
Scheme 65.
Thioesterification of aldehydes 114 under visible light.
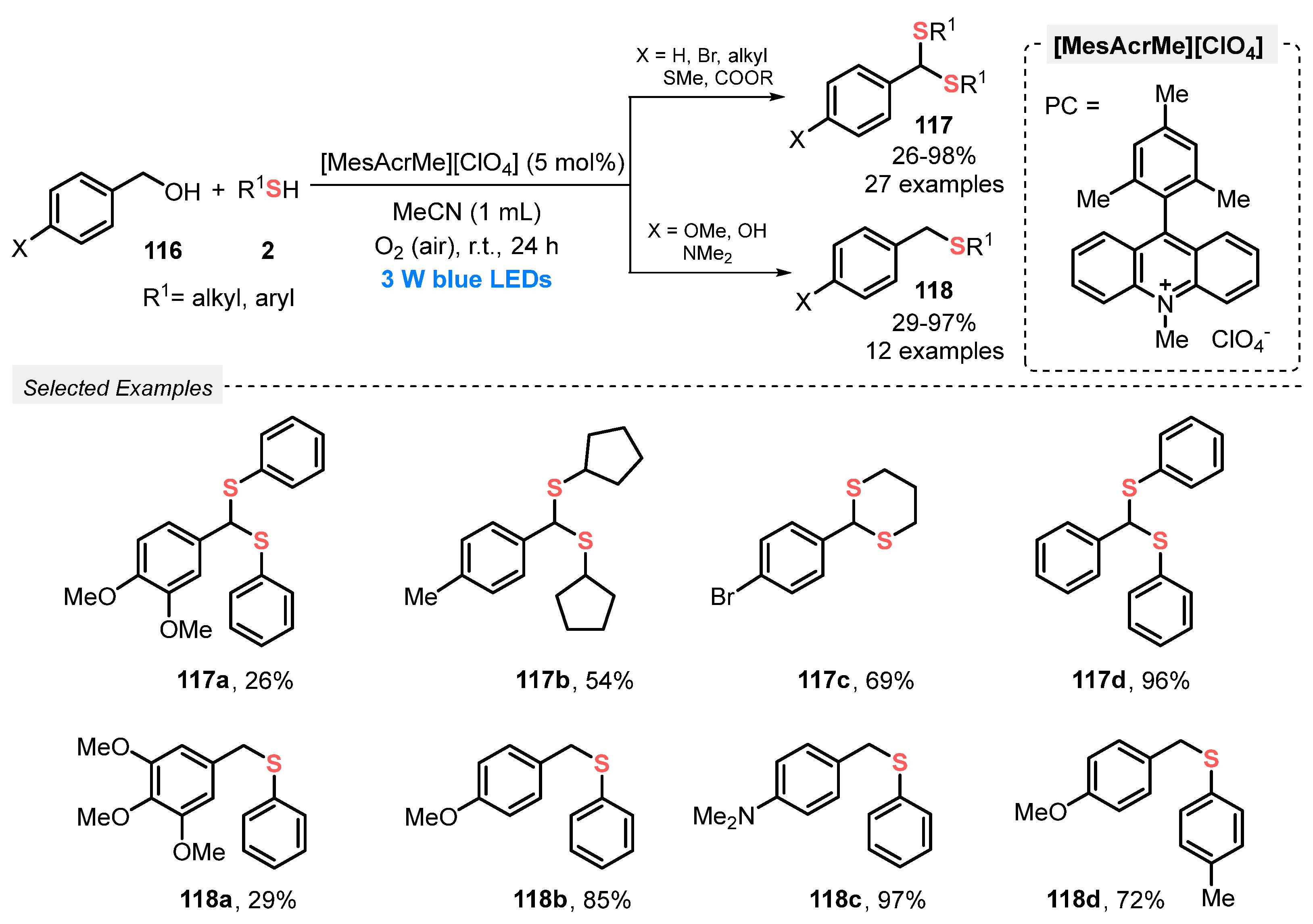
In 2020, Mal and co-workers [111] reported an oxidative visible light-promoted process to form new C–S bonds starting from benzyl alcohols 116 and thiols 2, affording dithioketals 117 and thioeters 118. The best reaction condition was defined as using 9-mesityl-10-methylacridinium perchlorate [MesAcrMe][ClO4] as photocatalyst and MeCN as solvent. The reaction mixture was irradiated with blue light (blue LEDs, 3 W) at room temperature in open-air conditions for 24 h, delivering the respective products in moderate to excellent yields. The identity of the product (if a dithioketal or a thioether) is dependent on the structure of the starting benzyl alcohol. For instance, p-MeO-, p-NMe2- and p-OH-substituted benzyl alcohol afforded preferentially thioethers 118 (29–97% yield) while unsubstituted halogen- and alkyl-substituted alcohols afforded selectively the respective dithioketals 117 (26–98% yield). This represents a limitation for this protocol, since electron-withdrawing groups could not be used as starting materials to obtain thioethers as products. The mechanism of the reaction involves single electron transfer, as proved by EPR and Stern–Volmer quenching experiments (Scheme 66).
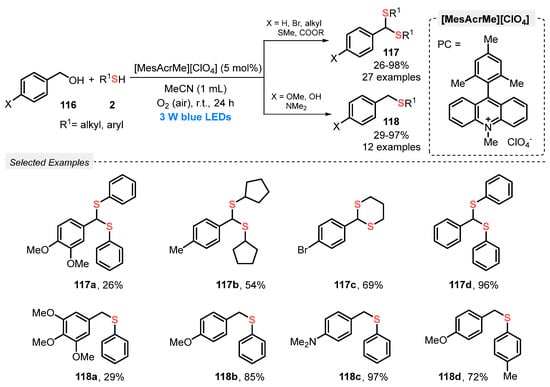
Scheme 66.
Dithioacetalization or thioetherification of benzyl alcohols 116 promoted by visible light.
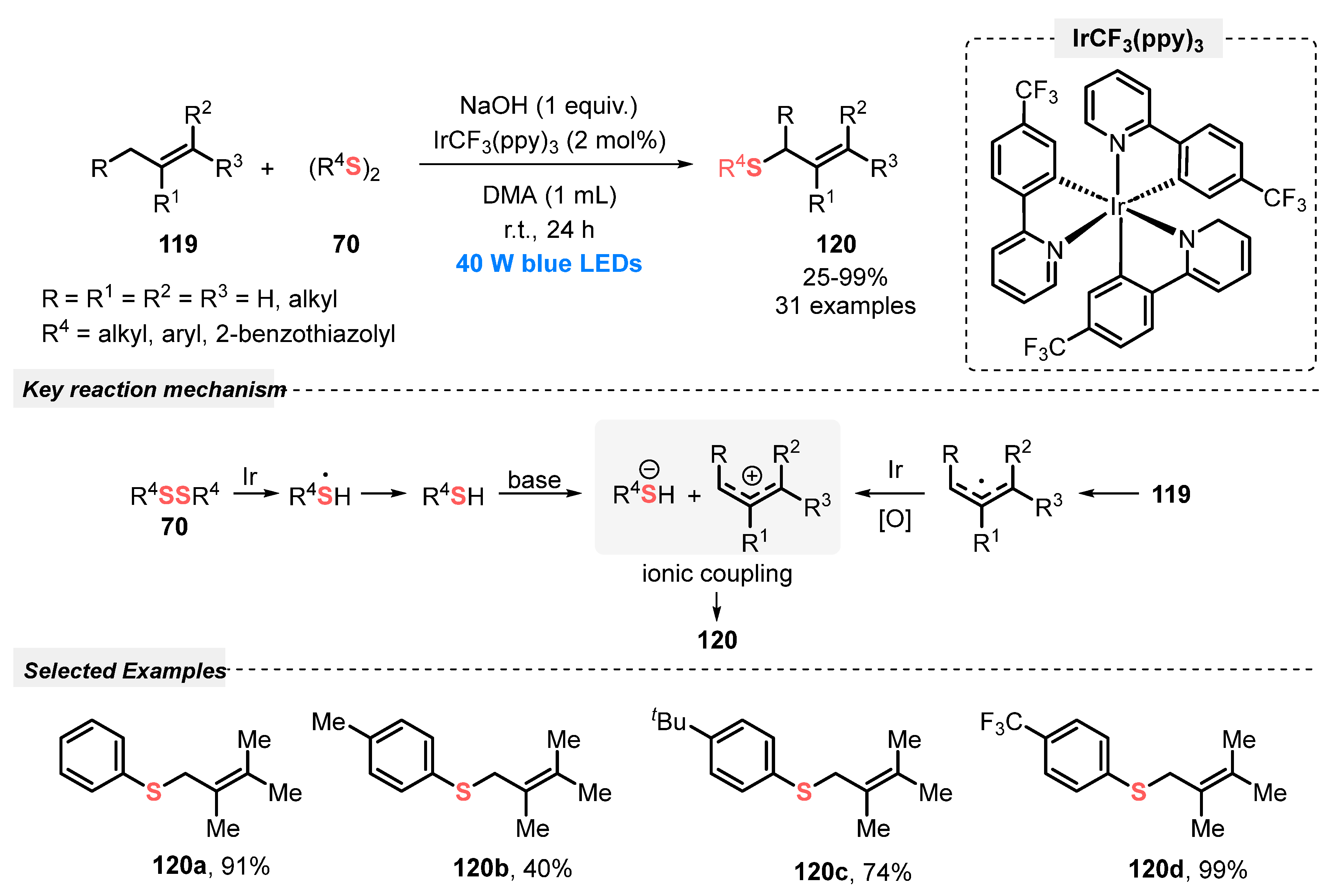
In the same year, Hong and co-workers [112] reported a direct allylic Csp3-H thiolation via a visible light-mediated photoredox starting from a large excess of several olefins 119 and diaryl disulfides 70, in the presence of tris[2-(p-trifluoromethylphenyl)pyridine]iridium(III) (IrCF3(ppy)3) as photocatalyst. The optimal condition was obtained after irradiation with blue light (blue LEDs, 40 W) for 24 h of a mixture of the reagents and catalyst in the presence of NaOH as base and DMA as solvent at room temperature under open-air. Under this condition, the substrates reacted smoothly to afford a wide range of allyl thioesters 120 in up to 99% yield, presenting good functional group tolerance. The proposed mechanism revealed the participation of the photocatalyst as a redox mediator to promote the transformation of the allyl radical into allyl cation, for a further ionic coupling reaction step with thiolate anion (Scheme 67).
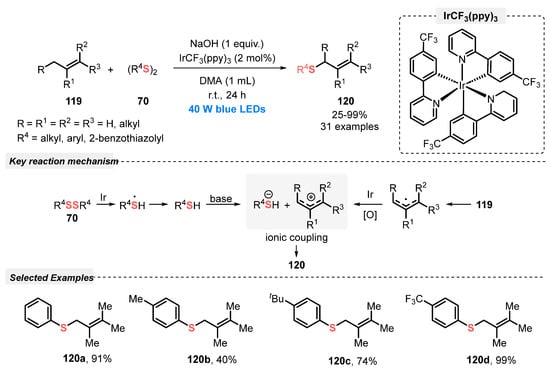
Scheme 67.
Photoredox catalysis for the direct allylic C(sp3)–H thiolation with disulfides 70.
6. Photocatalyzed Synthesis of Organoselenium Compounds
6.1. Using Diorganyl Diselenides as Selenium Source
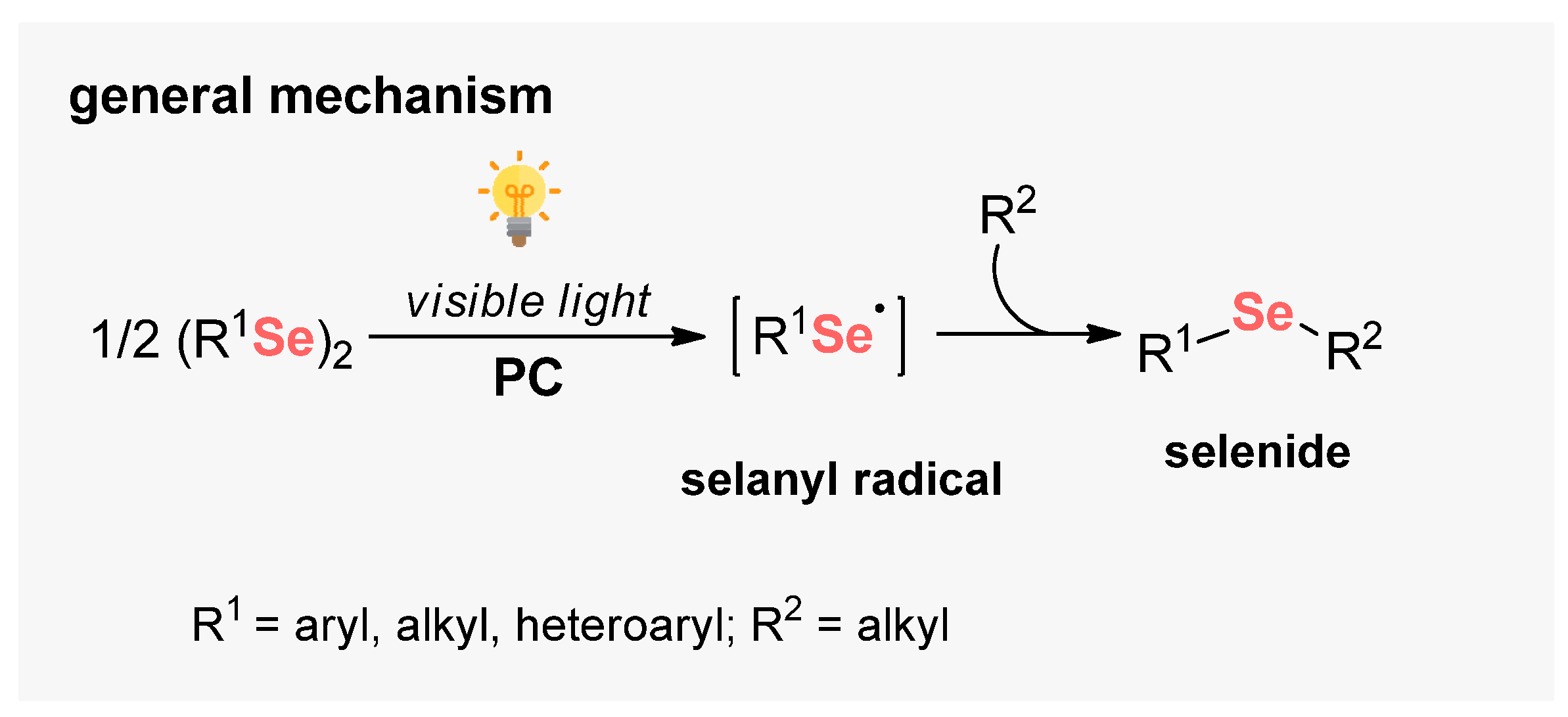
Selenium-based compounds play a fundamental role in human health, presenting pharmacological potential, for example, against cancer cells, oxidant processes, and neural damage [113]. From a synthetic point of view, diaryl diselenides are commonly found in the literature as the main Se-source to perform organic transformations, due to its high bench stability and low toxicity [114]. In general, diselenides are employed for the generation of electrophilic and nucleophilic Se-based species in situ. However, during the last years, new horizons have been opened in the development of visible light-mediated approaches to the cleavage of the Se–Se bond. Therefore, several approaches were developed involving the formation of new C–Se bonds by radical pathways (Scheme 68).
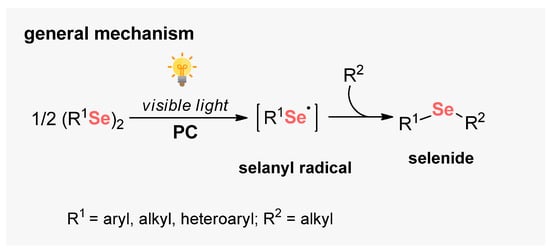
Scheme 68.
Selanyl radicals from diorganyl diselenides.
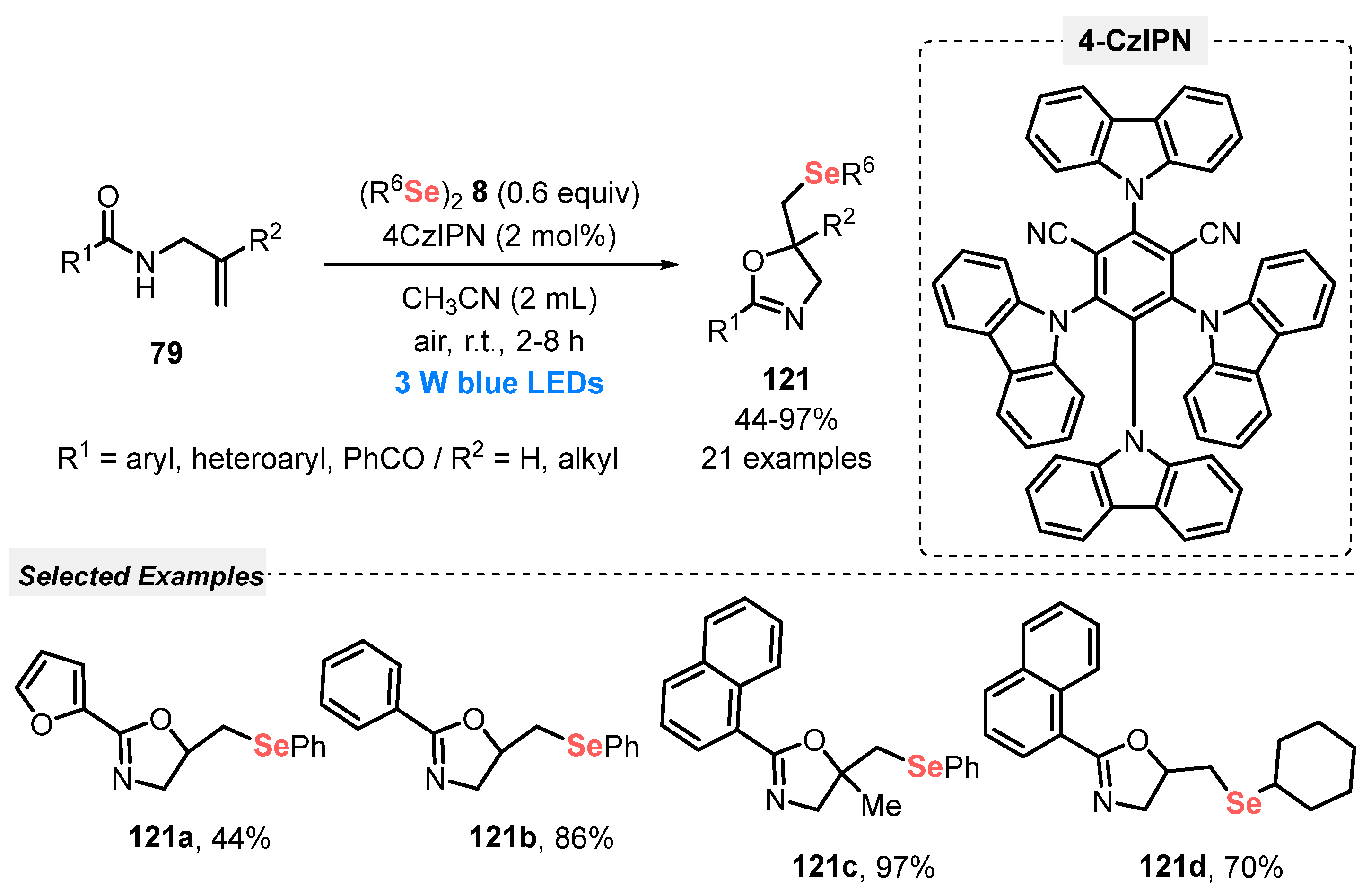
In this sense, in 2019, Liu and co-workers [115] reported the synthesis of selenium-containing heterocycles, starting from properly substituted olefins 79 and different diselenides 8. The reactions were conducted smoothly in the presence of 4CzIPN as photocatalyst and MeCN as solvent under open-air conditions. After irradiation of the reaction mixture with blue light (blue LEDs, 3 W) for 2 to 8 h at room temperature, the respective selenium-containing cyclized products 121. When N-alkenylamides 79 were used as starting material, seleno oxazolines 121 were selectively obtained after 2–8 h of reaction, in 44–97% yield (Scheme 69). This protocol stands out for following green concepts, using a non-metal catalyst in low quantity, room temperature, open air conditions and a low energetic blue LED, avoiding the use of heating.
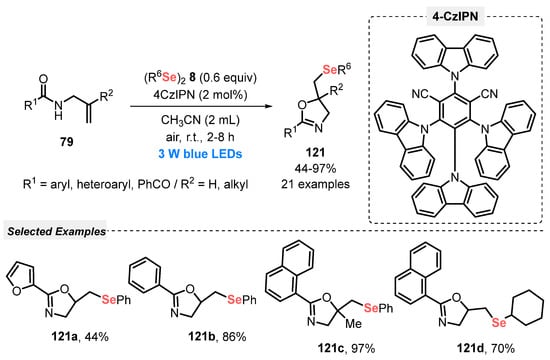
Scheme 69.
Visible light-enabled selenylation of olefins 79.
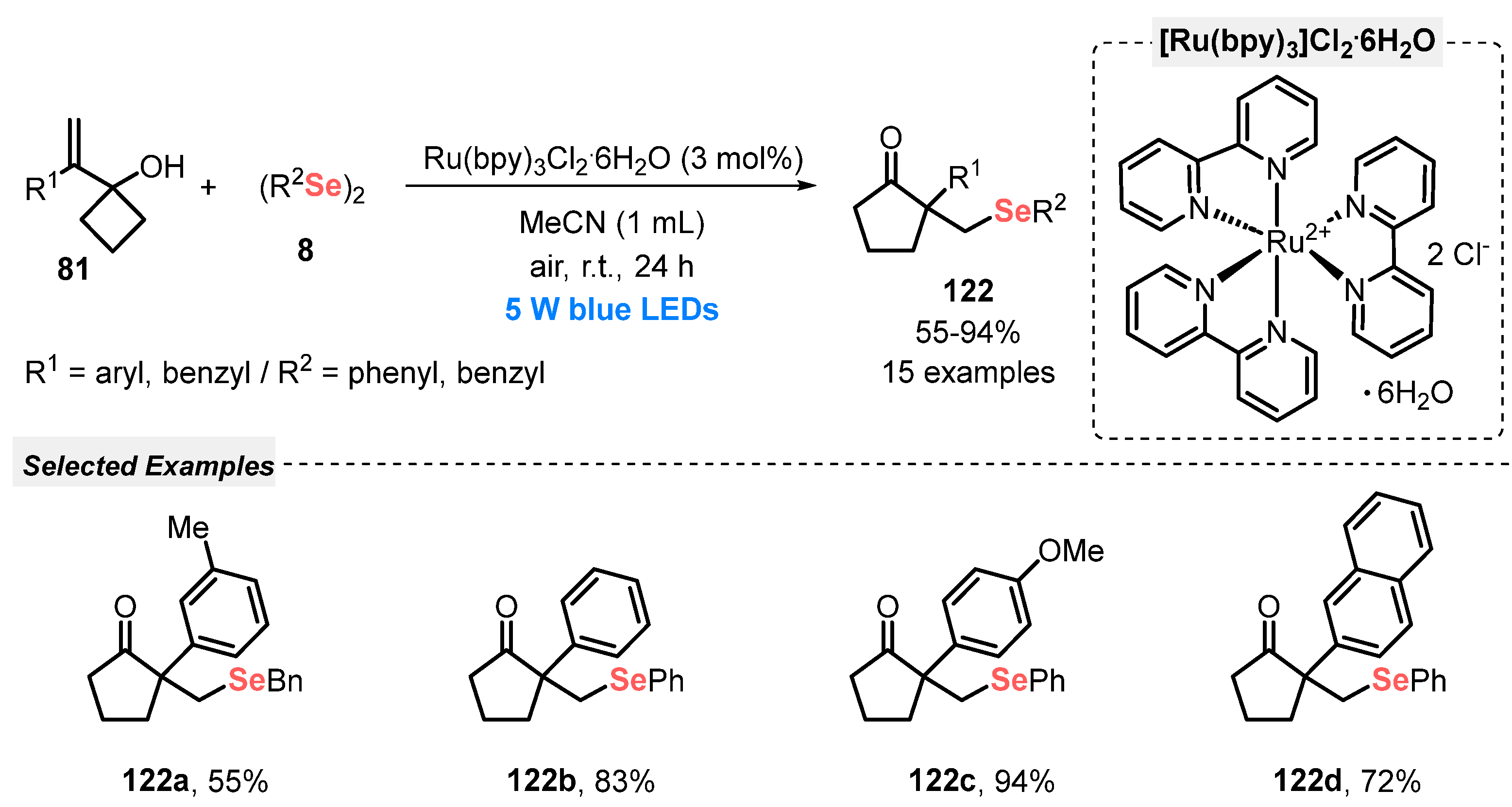
In the same year, Kim and Jung [116] reported the visible light-mediated conversion of 1-(1-arylvinyl)cyclobutanols 81 and diselenides 8 to β-selenylated cyclic ketones 122. This photoredox selenylation follows a ring-expansion event and is conducted in the presence of tris(bipyridine)ruthenium(II) chloride (Ru(bpy)3Cl2·6H2O) as photocatalyst and MeCN as solvent. The reaction mixture was irradiated with a bit more energetic blue light (blue LEDs, 5 W) at room temperature for 24 h. Under this condition, fifteen β-selenylated ketones 122 were prepared in up to 94% yield (Scheme 70). In addition, control experiments showed that the combination between visible light and photocatalyst is extremely necessary for the formation of the product, together with the presence of oxygen from the air.
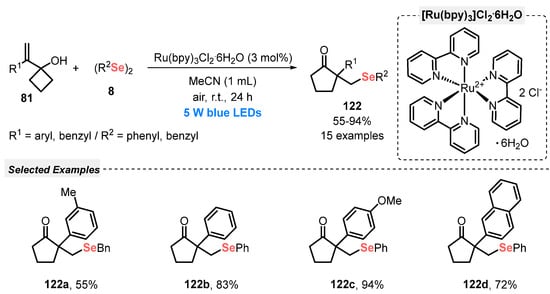
Scheme 70.
Visible light-promoted synthesis of β-selenylated cyclic ketone derivatives 122.
A year later, Xia and co-workers [117] developed a visible light-driven process for the difunctionalization of alkenes 123, employing amines 124 and diselenides 8 as reaction partners, in the presence of catalytic FeBr3. The authors discovered that the key intermediate in the reaction is a photo-excitable Fe-amine complex, formed from FeBr3 and the amine, which reacts with diselenide to give the respective β-selanyl amine 125. The optimal procedure involves irradiating the reaction mixture (in EtOAc as solvent) with blue light (two blue LEDs, 15 W) for 24 h under open-air conditions at room temperature. A total of fifty-seven differently substituted selanyl amines 125 were obtained in up to 99% yield (Scheme 71). It is worth mentioning that the protocol failed with diphenyl disulfide, which can be explained due to its lower reductive capacity when compared to diphenyl diselenide. This procedure was not effective when aliphatic amines were used, which probably is due to the lack of photo-absorption within the blue light wavelength region by the complexes formed between the aliphatic amines and FeBr3.
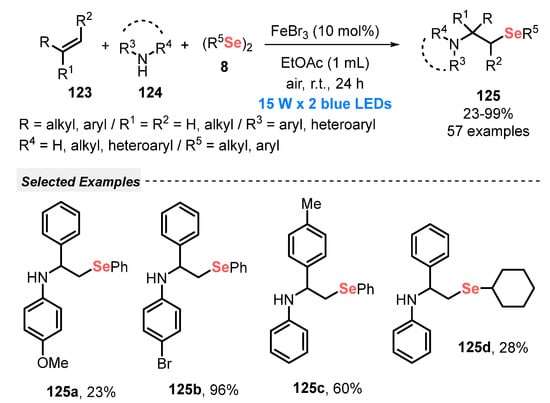
Scheme 71.
Visible light-promoted aminoselenylation of alkenes 123.
In 2021, Qiu and co-workers [118] reported a similar approach, i.e., using FeBr3 in the presence of amines in EtOAc as solvent to promote the photocatalytic selenocyclization under visible light irradiation. A wide range of N-methoxy-2-alkynylbenzamides 126, as well as aromatic and aliphatic diselenides 8, was efficiently used in the synthesis of seleno-substituted 1H-isochromen-1-one 127. A total of twenty-nine isochromenones 127 were obtained in 50–87% yield after irradiating the mixture of the reagents and FeBr3 with blue light (blue LEDs, 30 W) under air atmosphere and at room temperature for 12 h (Scheme 72). In addition, aiming to show the synthetic utility of this protocol, the selenylative cyclization was extended to propargylamine and other substrates products with the skeleton present in many natural products with pharmacological activity.
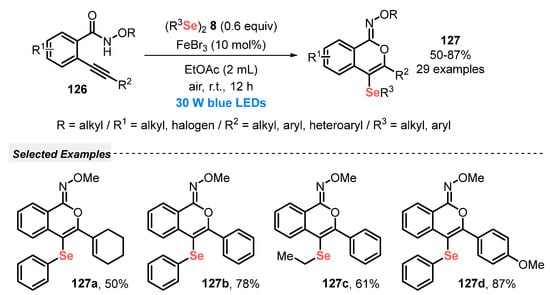
Scheme 72.
Iron-catalyzed selenocyclization of N-methoxy-2-alkynylbenzamides 126 and 8 under visible light.
Also, in 2021, a visible light-mediated cascade cyclization of 3-aminoindazoles 128, alkynyl aldehydes 129 and diorganyl dichalcogenides (diselenides 8 and diphenyl ditelluride 130) was described by Cao and co-workers [119]. In this process, Rose Bengal (RB) was employed as photocatalyst, and FeCl3 as an additive, in MeCN as solvent. The reaction mixture was irradiated for 12 h with blue light (blue LEDs, 20 W) at room temperature, yielding a total of thirty-eight chalcogen-containing pyrimido[1,2-b]-indazoles 131 in moderate to good yields (52–80%), with a broad scope of substrates and good functional group tolerance. The protocol was extended to diphenyl ditelluride and diphenyl disulfide. The respective tellurium-containing pyrimido[1,2-b]-indazole was obtained in 68% yield under the optimal conditions, while no product was observed using the sulfur analog as starting material (Scheme 73). Different from the other methodologies described so far, this method uses an equivalent amount of FeCl3. The use of catalytic amounts of the Lewis acid could make the protocol even more green.
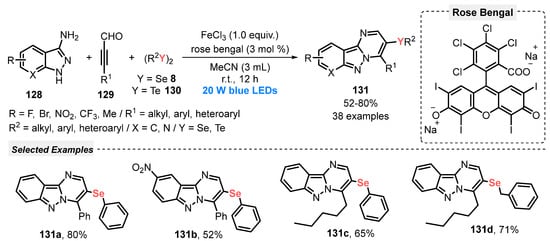
Scheme 73.
Visible light-enabled synthesis of chalcogen-containing pyrimido[1,2-b]-indazoles 131.
Recently, a visible light-driven photocatalyst-free synthesis of 2-aryl-(3-organoselanyl)thieno[2,3-b]pyridines 133 was described by Perin and co-workers [34], through the selenocyclization of 3-(arylethynyl)-2-(alkylthio)pyridines 132, in the presence of diorganyl diselenides 8. The reaction proceeded smoothly in the presence of O2 (balloon) as oxidant and hexane as solvent, under blue light irradiation (blue LEDs, 50 W) for 0.5–72 h. Among the different diorganyl diselenides, diphenyl diselenide was the more reactive, affording the respective 2-aryl-(3-phenylselanyl)thieno[2,3-b]pyridines 133 in just 0.5 h, while dibutyl diselenide did not react under the optimal conditions. On the other hand, diversities of aromatic and heteroaromatic diselenides were suitable substrates in the reaction, giving the expected cyclized thieno[2,3-b]pyridines 133 in 57% (R2 = 2-thienyl) to 99% yield (R2 = 2-MeC6H4) after 24 h of reaction. The longer reaction time (72 h) was required for bis(naphthalen-1-yl) diselenide, which afforded the expected derivative in 70% yield (Scheme 74).
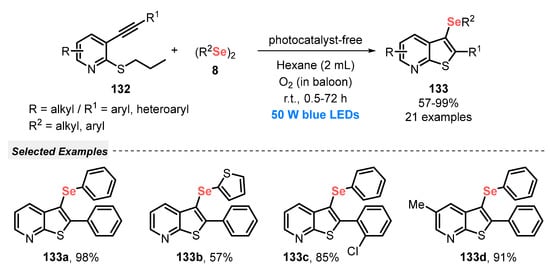
Scheme 74.
Synthesis of 2-aryl-(3-organoselanyl)thieno[2,3-b]pyridines 133 under visible light conditions.
6.2. Using [Me4N][SeCF3] as Selenium Source
Trifluoromethylselenylated (R-SeCF3) derivatives are versatile Se-based electrophilic species, used in the construction of biologically important compounds, due to the high permeability potential in cell membrane presented by the -SeCF3 group, besides their high lipophilicity [120,121]. For this reason, the development of efficient synthetic methods to construct these compounds is an important field in organochalcogen chemistry. Among the recent advances in this area, light-promoted protocols have arisen as interesting alternatives to the conventional ones (Scheme 75) [122].
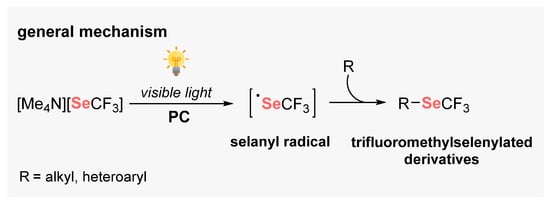
Scheme 75.
Synthetic strategy to obtain trifluoromethylselenylated derivatives.
In this context, in 2020, Magnier and co-workers [123] described the photocatalyzed regioselective trifluoromethylselenylation of electron-rich heteroarenes 7 or 134, using [Me4N][SeCF3] 135 as selenium source and Eosin Y as photocatalyst. A mixture of reagents 7 or 134, 135, and the catalyst in MeCN was irradiated with green light (green LEDs; no power reported) in open-air conditions at room temperature for 18 h, delivering the selenylated products 136 or 137 in poor to excellent yields (twenty-six examples, 30–95% yield). The protocol was suitable to a range of indole derivatives, presenting high regioselectivity for the formation of the 3-selenylated products. No product was observed when 3-substituted indoles were used as substrate. The reaction scope was successfully enlarged to other heterocyclic substrates, including 7-azaindole, 4-azaindole, and pyrroles. The method was satisfactorily suited to continuous flow technique, remarkably decreasing the reaction time from 18 h to just 50 min, maintaining good reaction yields (Scheme 76). Therefore, the combination between Eosin Y and green LEDs presented excellent results in 18 h of reaction. Blue LED was tested in the optimization studies with Ru(bpy)3(PF6)2 as photocatalyst and 87% yield of 137a was obtained. Possibly, an experiment using Eosin Y and light with lower wavelength, as the blue LED, could afford similar results in less time.
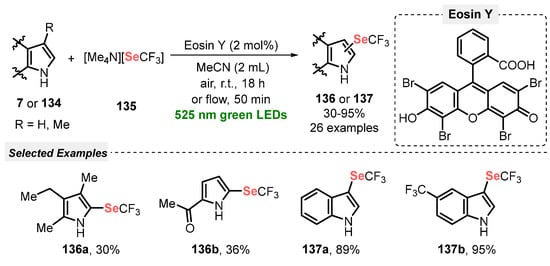
Scheme 76.
Visible light-promoted trifluoromethylselenolation of electron-rich heteroarenes 7 and 134 using [Me4N][SeCF3] 135.
Also, in 2019, Zhang and co-workers [124] described a light-promoted decarboxylation of aliphatic carboxylic acids 138, followed by a trifluoromethylselenylation event using [Me4N][SeCF3] 135, to construct trifluoromethylselenoalkanes 139. The reactions were conducted in the presence of 9-mesityl-3,6-di-tert-butyl-10-phenylacridinium tetrafluoroborate (Mes-Acr-PhBF4) as photocatalyst and N-fluorobenzenesulfonimide (NFSI) as oxidant, in MeCN as solvent. The resulting mixture was irradiated with blue light (2 x blue LEDs, 10 W) under N2 atmosphere at room temperature for 24 h. The [Me4N][SeCF3] salt 135 was used as a nucleophilic selenium source, reacting with a wide variety of commercially available primary alkyl carboxylic acids 138, which were converted to the corresponding selenides 139 in reasonable to very good yields (31–88%). It is worth mentioning that in special cases, like sterically hindered or alkynyl carboxylic acid derivatives, NFSI/MeCN must be replaced by PhI(OAc)2/DCM, the amount of [MesAcrMe][BF4] increased from 1 mol% to 5 mol%, and irradiated with purple light for up to seven days, to provide the desired products in good yields. The method was successfully applied to complex bioactive molecules, including Isoxepac, Bromfenac, Indometacin, Ibuprofen, Carprofen, and fat acids (Scheme 77). A limitation of this work is the need of overstoichiometric amounts of an oxidant to form the trifluoroselenylated product in good yields, as well the need for an inert atmosphere. On the other hand, it represents a total metal-free methodology.
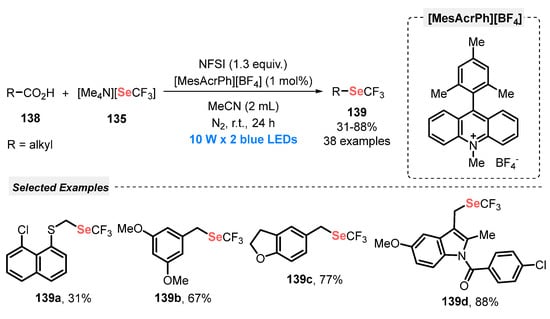
Scheme 77.
Decarboxylative trifluoromethylselenolation of aliphatic carboxylic acids 138 with [Me4N][SeCF3].
Zhao, Lu, and co-workers [125] described, in 2020, a protocol to perform the difluoromethylselenylation of arylamines 71, using the previously prepared selenium source Se-(difluoromethyl)4-methylbenzene-sulfonoselenoate (TsSeCF2H) 141. The selenylating agent was prepared by a sequential reaction from benzyl bromide and KSeCN. After four steps, TsSeCF2H was obtained in 70% yield. Several aryl and heteroaryl amines 71 were selenylated with reagent 141 using Rose Bengal as photocatalyst, TsOH as proton source, and tBuONO as oxidant in DMSO as solvent. The reaction mixture was irradiated with white light (white LEDs, 5 W) at room temperature for 10 h. The fluorinated selenides were obtained in 42–80% yield, including derivatives of a diversity of substituted anilines, like electron-rich and electron-deficient ones, as well the bioactive derivatives clofibrate, L-menthol, pomalidomide, and sulfamethoxazole (Scheme 78). The slight difference between the yields obtained in the presence and in the absence of proton-donor additive (TsOH) allows us to speculate if it could be removed from the reaction, affording an eco-friendlier protocol. Similarly to the previously described methodology, good yields of the difluoromethylselenylated products were obtained only if an oxidant is used in equivalent amount.
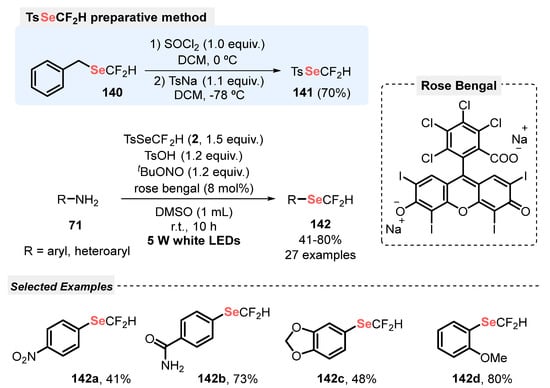
Scheme 78.
Visible light-promoted difluoromethylselenylation of arylamines 71 under metal-free conditions.
6.3. Using KSeCN as Selenium Source
Potassium selenocyanate (KSeCN) represents the starting material and most common nucleophilic selenium source for the incorporation of selenocyanate in organic compounds. When subjected to visible light irradiation in the presence of photocatalytic species, KSeCN can react in a radical pathway, promoting the formation of new C–Se bonds [126].
In this line, in 2021, Yu and co-workers [127] used conjugated microporous polymers (CMPs) as photocatalyst to promote the C-3 selenocyanation of indoles 7, using potassium selenocyanate as selenylating agent. The authors developed a geometry optimization strategy that allowed the use of CMPs through a polarization-induced charge separation. The used CMPs were synthesized by the oxidative coupling of bis-carbazoles with electron-deficient bridges (benzene/pyridine/pyrimidine). Among the carbazole CMPs, the so-called CMP-CSU-14 was the best in class in affording the expected 3-selanyl indoles. A typical procedure involves the irradiation of the reagents and the catalyst with white light (white LEDs, 14 W) in THF as solvent under O2 atmosphere (in balloon) at room temperature for 24 h. A total of fourteen 3-selanyl indoles 143 was obtained in up to 94% yield, starting from differently substituted indoles (Scheme 79). Thus, bypassing most of the papers in the literature for selenocyanation reactions that commonly use homogeneous catalyst, this protocol stands out for using a heterogeneous catalyst, free of metals, that can be reused for five consecutive cycles without a significant loss in yield.
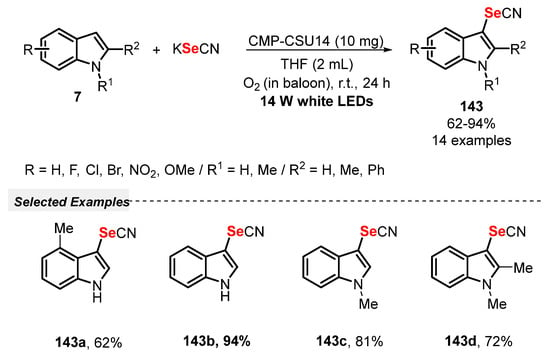
Scheme 79.
Visible light-promoted selenocyanation of indoles 7 using conjugated microporous polymers (CMPs).
In the same year, Lu and co-workers [128] developed a visible light-promoted selenocyanation of cyclobutanone oxime esters 100 using KSeCN in the presence of fac-Ir(ppy)3 as photocatalyst for the synthesis of cyano- and selenocyano bifunctionalyzed alkanes 144. The best results were obtained by irradiating a mixture of reagents and the catalyst in THF with blue light (blue LEDs, 24 W) under Ar atmosphere at 40 °C for 12 h. Under the optimal conditions, twenty-one alkyl selenocyanates were obtained in 64–99% yield, decorated with a variety of substituents, including alkyl, aryl, heteroaryl, ether, and amino. The authors observed that potassium thiocyanate was less reactive, yielding the respective alkylthiocyanates in just 18% yield (Scheme 80).
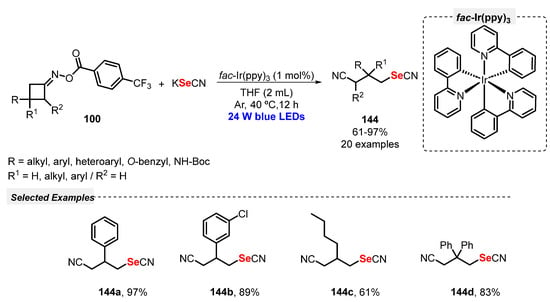
Scheme 80.
Selenocyanation of cyclobutanone oxime esters 100 mediated by visible light.
6.4. Using Selenosulfonates as Selenium Source
Selenosulfonates are a versatile class of compounds in organic synthesis, which also present important biological activities. They have been widely employed as substrate for the construction of organoselenium compounds (Scheme 81) [129].
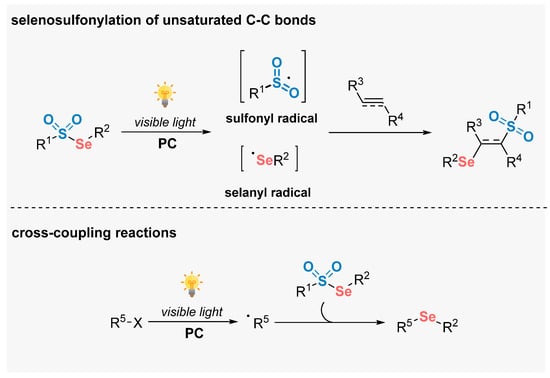
Scheme 81.
Selenosulfonates as selenium source.
In this context, in 2019, Ji and co-workers [130] described a metal-photocatalyzed (CoC2O4, Cu(0), or CuI) selenosulfonylation of electron-deficient alkynes 145, employing selenosulfonates 146 as reaction partner, yielding β-(seleno)vinyl sulfones 147. A variety of alkynes 145 was used as substrates in the reaction with alkyl and aryl selenosulfonates 146, including alkynyl esters, alkynamides, and alkynyl sulfones. Cobalt(II) oxalate was the best catalyst for the selenosulfonylation of alkynyl esters, while metal copper presented the best activity in the reactions with amides and sulfones. The reactions were irradiated with blue light (blue LEDs, 20 W) for 4 h under Ar atmosphere at room temperature, using MeCN as solvent. A total of thirty-two alkenyl selenosulfones was obtained in 22–96% yield, including one example using phenyl acetylene as starting material (83% yield) (Scheme 82).
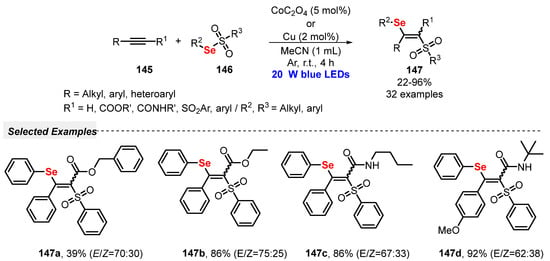
Scheme 82.
Synthesis of β-(seleno)vinyl sulfones 147 promoted by visible light.
A year later, Tlili and co-workers [131] described the visible light-promoted fluoroalkylselenylation of aryl silanes 148 or potassium trifluoroborates 87, using fluorinated selenosulfonates 146 as selenium source. The reaction was conducted using [MesAcrMe][ClO4] as photocatalyst under open-air conditions and irradiation with blue light (blue LEDs; no power reported) for 16 h. When aryl silanes 148 were the starting material, MeOH was the solvent, and K2S2O8 was used as additive. By starting from potassium trifluoroborates 87, however, the best solvent was DMSO, and no additive was necessary. The authors prepared twenty-five selenofluorinated compounds 139, 142 and derivatives in 16–81% yield. The less reactive substrates were those containing strong electron-withdrawing groups in the aromatic ring (F, NO2), and the lower yield of all the tested compounds (16%) was observed in the reaction of cyclohexyl tetrafluoroborate derivative 87 (Scheme 83). This protocol represents an alternative way to this kind of reaction, avoiding the use of heating or transition metals.
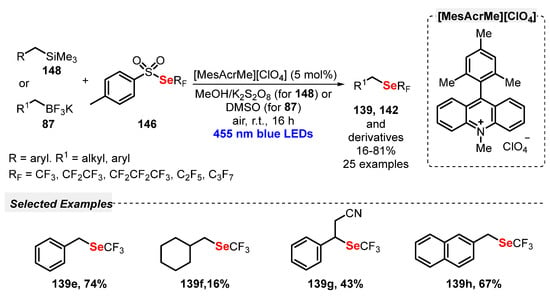
Scheme 83.
Metal-free fluoroalkylselenylation of alkyl silanes 148 and trifluoroborates 87 under visible light conditions.
Also in 2020, Ji and co-workers [132] developed a visible light-promoted cross-coupling of 4-alkyl-1,4-dihydropiridines 149 with thiosulfonates and chalcogenosulfonates 146/22, in the presence of 4-CzIPN as photocatalyst. The reactions were conducted in the presence of DCE as solvent, under blue light irradiation (blue LEDs, 40 W) at room temperature for 24 h. Despite the main focus of the work being the synthesis of sulfides and sulfoxides, five different selenides were prepared in 54–96% yield, demonstrating the suitability of this approach to prepare both chalcogen species. Ingeniously, the products 150 and 113 could be selected just by changing the reaction environment, once the sulfides and selenides could be accessed under inert atmosphere (N2), while sulfoxides were formed under oxidant atmosphere (air). The protocol was extended to the synthesis of derivatives of monosaccharides, affording seven compounds in 45–92% yield. A total of forty compounds could be prepared in up to 99% yield by this efficient method (Scheme 84).
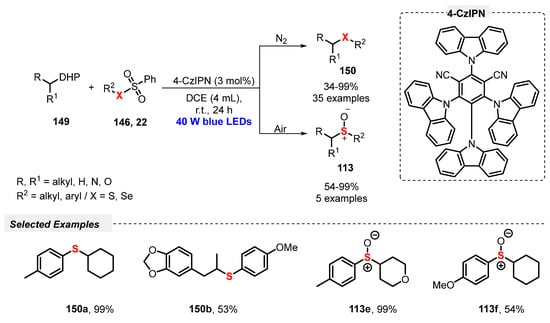
Scheme 84.
Visible light-promoted synthesis of sulfides and selenides 150 and sulfoxides 113 by cross-coupling reactions.
In 2021, Wang and Wang [133] reported the visible light-mediated cross-coupling of hypervalent bis-catecholato silicon compounds 151 with thiosulfonates 22 and selenosulfonates 146. Acriflavine was used as photocatalyst, in DMSO as solvent. The resulting mixture was irradiated with blue light (blue LEDs, 40 W) under N2 atmosphere at room temperature for 24 h, affording the respective sulfides and selenides in 41–99% yield. A total of forty nonsymmetrical chalcogenides 150 were obtained (thirty selenides and ten sulfides) (Scheme 85).
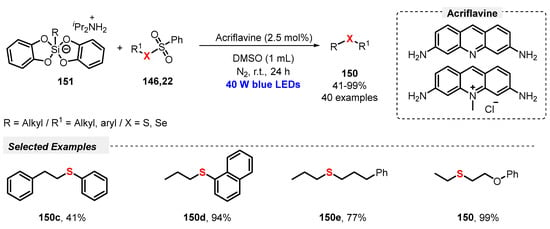
Scheme 85.
Cross-coupling reaction of hypervalent bis-catecholato silicon compounds 151 with selenosulfonates or thiosulfonates mediated by visible light.
In the same year, Xu and co-workers [134] described an efficient visible light-promoted photocatalyst-free cyclization of alkyne-tethered cyclohexadienones 13 with selenosulfonates 146, affording 3-sulfonyl-5-selanyl-4a,5-dihydro-2H-chromen-6(8aH)-ones 152. The reagents were mixed in MeCN as solvent and irradiated with white light (white LEDs, 30 W) under N2 atmosphere at room temperature for 2 h. A total of twenty-four bicyclic products 152 were prepared in 40–93% yield, in which both sulfone and selanyl groups were incorporated, leading to the formation of three new stereocenters. An interesting result was obtained when terminal alkyne 153 was used as substrate, providing the bicyclic compound 154 in 56% yield (Scheme 86).
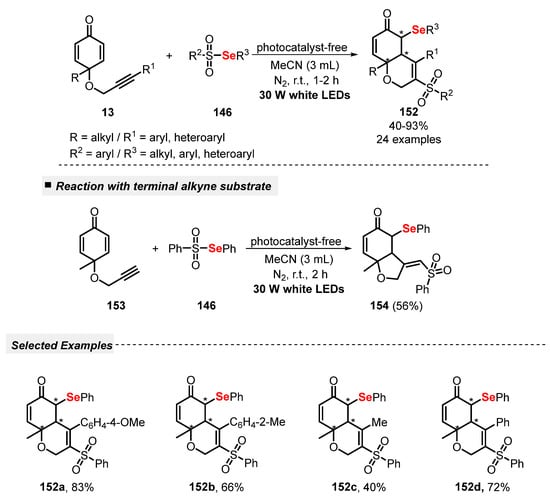
Scheme 86.
Visible light-promoted synthesis of 3-sulfonyl-5-selanyl-chromenones 152 under photocatalyst-free conditions.
Recently, in 2022, Wang and co-workers [135] developed a protocol for the synthesis of ortho-selanylated benzamides 156 from benzotrazinones 155, under visible light conditions. The reaction is catalyzed by Ir(ppy)3 and follows a regioselective denitrogenative cross-coupling between 155 and selenosulfonate 146, which proceeds in DMSO as solvent under N2 atmosphere. The reaction mixture was irradiated with blue light (blue LEDS, 40 W) at room temperature for 12 h, allowing the synthesis of twenty-three benzamides 156 in up to 79% yield. The synthetic potential of the ortho-selanyl benzamide 156 was shown in the synthesis of five Ebselen derivatives 159, with different substituents, in very good yields (Scheme 87). The green point of this protocol is that this reaction can be activated only by light, avoiding the use of catalysts. Additionally, most of the procedures in the literature in which selenosulfonate is used as selenium source need an inert atmosphere to perform the reaction.
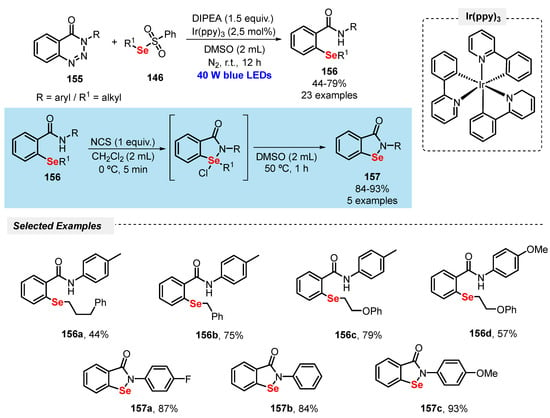
Scheme 87.
Synthesis of o-selenylated benzamides 156 under visible light irradiation and their transformation in Ebselen analogs 157.
7. Conclusions and Perspectives
As demonstrated in this review, the use of light energy to promote the formation of new carbon–chalcogen bonds (C–S and C–Se) is a booming field in organic synthesis. Considering the covered period (2019 to 2022), the number of protocols developed so far is noteworthy (more than eighty!) and almost all of them deal with the use of visible light, mainly blue light. As expected, the formation of carbon–sulfur bond was more explored than the carbon–selenium analogs, once this transformation received more attention in organic synthesis, due to the large application of organosulfur compounds in the chemical, pharmaceutical, and agrochemical industries. The recent availability of new equipment for photochemical reactions in the laboratory, as well as the applicability of photo-promoted reactions in flow chemistry should probably lead to the discovery of new transformations leading to new molecules that are difficult to prepare using conventional energy sources.
Thus, regarding the future perspectives in this important field in organic synthesis, the development of new light devices and new selective photocatalysts are among the main challenges faced by the researchers seeking greener and environmentally friendly protocols to prepare organochalcogen compounds. Additionally, enantio- and diastereoselective protocols are still wanted, with an aim to deal with the main interests of the pharmaceutical industry in the prospection of new drugs. Furthermore, the development of selective photochemical methods for the synthesis of organotellurium compounds still remains underexplored, and new steps in this direction can open new horizons to deliver innovative synthetic technologies. Finally, we hope that this review can enlighten and encourage those working with the synthesis and application of organochalcogen compounds to put some light in their synthesis.
Author Contributions
Conceptualization, E.J.L., F.P. and S.T.; methodology, E.J.L., F.P., R.F.S., S.T. and G.P.; classification and analysis of the collected manuscripts and writing—original draft preparation, R.H.B., L.H.D. and J.C.K.; writing—review and editing, E.J.L., F.P., R.F.S., S.T. and G.P.; supervision, E.J.L., F.P. and S.T.; project administration, E.J.L.; funding acquisition, E.J.L., R.F.S. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—DOI: 10.13039/501100002322—Grant Number 001, and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq)—DOI: 10.13039/501100003593—Universal 422645/2021-4 FAPERGS and FINEP are also acknowledged for financial support. CNPq is also acknowledged for Fellowship to E.J.L., G.P. and R.F.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UNESCO. UNESCO Science Report: Toward 2030; UNESCO Publishing: Paris, France, 2015; ISBN 97892310011291. [Google Scholar]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development, A/RES/70/01. 2015. Available online: https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 21 January 2023).
- United Nations Environment Programme. Healthy Environment, Healthy People, Thematic Report, Ministerial Policy Review Session, Second Session of the United Nations Environmental Assembly. 2016. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/17602/K1602727%20INF%205%20Eng.pdf?sequence=1&isAllowed=y (accessed on 21 January 2023).
- United Nations Environment Programme. Towards a Pollution-Free Planet. 2017. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/21800/UNEA_towardspollution_long%20version_Web.pdf?isAllowed=y&sequence=1 (accessed on 25 January 2023).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Kümmerer, K.; Clark, J. Green and Sustainable Chemistry; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Anastas, P.T.; Zimmerman, J.B. The United Nations sustainability goals: How can sustainable chemistry contribute? Curr. Opin. Green Sustain. Chem. 2018, 13, 150–153. [Google Scholar] [CrossRef]
- Anastas, P.T.; Zimmerman, J.B. The periodic table of the elements of green and sustainable chemistry. Green Chem. 2019, 21, 6545–6566. [Google Scholar] [CrossRef]
- Horváth, I.T. Introduction: Sustainable Chemistry. Chem. Rev. 2018, 118, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The Merger of Transition Metal and Photocatalysis. Nat. Chem. Rev. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Makes a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Amos, S.G.E.; Garreau, M.; Buzzetti, L.; Jerome, W. Photocatalysis with Organic Dyes: Facile Access to Reactive Intermediates for Synthesis. Beilstein J. Org. Chem. 2020, 16, 1163–1187. [Google Scholar] [CrossRef]
- Strieth-Kalthoff, F.; James, M.J.; Teders, M.; Pitzer, L.; Glorius, F. Energy Transfer Catalysis Mediated by Visible Light: Principles, Applications, Directons. Chem. Soc. Rev. 2018, 47, 7190–7202. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A. Recent Advances in Photocatalytic Manipulations of Rose Bengal in Organic Synthesis. Org. Biomol. Chem. 2019, 17, 4384–4405. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef]
- Mustafa, M.; Winum, J.-Y. The Importance of Sulfur-Containing Motifs in Drug Design and Discovery. Expert Opin. Drug Discov. 2022, 17, 501–512. [Google Scholar] [CrossRef]
- Tilby, M.J.; Willis, M.C. How Do We Address Neglected Sulfur Pharmacophores in Drug Discovery? Expert Opin. Drug Discov. 2021, 16, 1227–1231. [Google Scholar] [CrossRef]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.-F.; Qin, H.-L. Pharmaceutical and Medicinal Significance of Sulfur (SVI)-Containing Motifs for Drugs Discovery: A Critical Review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontier on Organoselenium Compounds; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Jain, V.L.; Priyadarsini, K.I. (Eds.) Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry: Croydon, UK, 2017. [Google Scholar]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Mugesh, G.; Singh, H.B. Synthetic Organoselenium Compounds as Antioxidants: Glutathione Peroxidase Activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Bhowmick, D.; Mugesh, G. Insights into the Catalytic Mechanism of Synthetic Glutathione Peroxidase Mimetics. Org. Biomol. Chem. 2015, 13, 10262–10272. [Google Scholar] [CrossRef]
- Wimmer, A.; König, B. Photocatalytic Formation of Carbon-Sulfur Bonds. Beilstein J. Org. Chem. 2018, 14, 54–83. [Google Scholar] [CrossRef]
- Guo, W.; Tao, K.; Tan, W.; Zhao, M.; Zheng, L.; Fan, X. Recent Advances in Photocatalytic C-S/P-S Bond Formation via the Generation of Sulfur Centered Radicals and Functionalization. Org. Chem. Front. 2019, 6, 2048–2066. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Srivastava, A.; Singh, P.P. Recent Application of Visible-Light Induced Radicals in C-S Bond Formation. RSC Adv. 2020, 10, 20046–20056. [Google Scholar] [CrossRef] [PubMed]
- Azzi, E.; Lanfranco, A.; Moro, R.; Deagostino, A.; Renzi, P. Visible Light as the Key for the Formation of Carbon-Sulfur Bonds in Sulfones, Thioethers, and Sulfonamides: An Update. Synthesis 2021, 53, 3440–3468. [Google Scholar] [CrossRef]
- Singla, D.; Luxami, V.; Paul, K. Eosin Y Mediated Photo-catalytic C-H Functionalization: C-C and C-S Bond Formation. Org. Chem. Front. 2023, 10, 237–266. [Google Scholar] [CrossRef]
- Rafique, J.; Rampon, D.S.; Azeredo, J.B.; Coelho, F.L.; Schneider, P.H.; Braga, A.L. Light-Mediated Seleno-Functionalization of Organic Molecules: Recent Advances. Chem. Rec. 2021, 21, 2739–2761. [Google Scholar] [CrossRef]
- Penteado, F.; Gomes, C.S.; Molzon, L.I.; Perin, G.; Silveira, C.C.; Lenardão, E.J. Photocatalytic Synthesis of 3-Sulfanyl- and 1,3-Bis(sulfanyl)indolizines Mediated by Visible Light. Eur. J. Org. Chem. 2020, 2020, 2110–2115. [Google Scholar] [CrossRef]
- Bartz, R.H.; Peglow, T.J.; Penteado, F.; Jacob, R.G.; Lenardão, E.J.; Perin, G. Visible Light-Promoted Synthesis of 2-Aryl-(3-organoselanyl)thieno [2,3-b]pyridines. Green Chem. Lett. Rev. 2022, 15, 373–382. [Google Scholar] [CrossRef]
- Perin, G.; Peglow, T.J.; Penteado, F.; Nobre, P.C.; Silva, K.B.; Stach, G.; Barcellos, T.; Lenardão, E.J.; Roehrs, J.A. UVA Light-promoted Catalyst-free Cyclization of Vinyl Selenides: Green and Efficient Synthesis of C3-Unsubstituted 2-Selanyl Benzochalcogenophenes. Chem. Asian. J. 2022, 17, e202101394. [Google Scholar] [CrossRef]
- Abenante, L.; Quadros, G.T.; Perin, G.; Santi, C.; Penteado, F.; Lenardão, E.J. Visible-Light-Mediated Photocatalytic Synthesis of 2-Substituted Oxazole-5-Carbaldehydes Promoted by Benzeneseleninic Acid. Eur. J. Org. Chem. 2022, 2022, e202200641. [Google Scholar] [CrossRef]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Chen, Y.-L.; Qin, L.; Wen, Y.; Xie, J.-W.; Tan, J.-X.; Huang, Y.; Cao, Z.; He, W.-M. Visible-light-promoted direct C-H/S-H crosscoupling of quinoxalin-2(1H)-ones with thiols leading to 3-sulfenylated quinoxalin-2(1H)-ones in air. Org. Chem. Front. 2019, 6, 3950–3955. [Google Scholar] [CrossRef]
- Huang, Q.; Peng, X.; Li, H.; He, H.; Liu, L. Visible-light-induced, graphene oxide-promoted C3-chalcogenylation of indoles strategy under transition-metal-free conditions. Molecules 2022, 27, 772. [Google Scholar] [CrossRef]
- Nagar, B.; Dhar, B.B. Visible light-mediated thiolation of substituted 1,4-naphthoquinones using eosin Y as a photoredox catalyst. J. Org. Chem. 2022, 87, 3195–3201. [Google Scholar] [CrossRef]
- Nair, A.M.; Kumar, S.; Volla, C.M.R. Visible light mediated sulfenylation-annulation cascade of alkyne tethered cyclohexadienones. Adv. Synth. Catal. 2019, 361, 4983–4988. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, H.-R.; Zhou, L.; Fang, J.-D.; Liu, X.-Y. Photoredox-catalyzed sulfenylation/cyclization of N-aryl-Ntosylpropargylamine with disulfide: A concise route to 3-phenylthioquinoline. Tetrahedron 2019, 75, 2893–2899. [Google Scholar] [CrossRef]
- Zhang, N.; Zuo, H.; Xu, C.; Pan, J.; Sun, J.; Guo, C. Visible light induced the high-efficiency spirocyclization reaction of propynamide and thiophenols via recyclable catalyst Pd/ZrO2. Chin. Chem. Lett. 2020, 31, 337–340. [Google Scholar] [CrossRef]
- Ho, H.E.; Pagano, A.; Rossi-Ashton, J.A.; Donald, J.R.; Epton, R.G.; Churchill, J.C.; James, M.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indoletethered ynones. Chem. Sci. 2020, 11, 1353–1360. [Google Scholar] [CrossRef]
- Chen, H.; Yan, Y.; Zhang, N.; Mo, Z.; Xu, Y.; Chen, Y. Visible-light-induced cyclization/aromatization of 2-vinyloxy arylalkynes: Synthesis of thio-substituted dibenzofuran derivatives. Org. Lett. 2021, 23, 376–381. [Google Scholar] [CrossRef]
- Castanheiro, T.; Suffert, J.; Donnard, M.; Gulea, M. Recent advances in the chemistry of organic thiocyanates. Chem. Soc. Rev. 2016, 45, 494–505. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Chen, J.; He, Y.-H.; Guan, Z. Visible-light-mediated additive-free decarboxylative ketonization reaction of acrylic acids: An access to α-thiocyanate ketones. J. Org. Chem. 2021, 86, 3741–3749. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Rappoport, Z.; Stirling, C.J.M. The Chemistry of Sulphones and Sulphoxides; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Liang, S.; Hofman, K.; Friedrich, M.; Keller, J.; Manolikakes, G. Recent Progress and Emerging Technologies towards a Sustainable Synthesis of Sulfones. ChemSusChem 2021, 14, 4878–4902. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-W.; Liang, S.; Manolikake, G. Recent Advances in the Synthesis of Sulfones. Synthesis 2016, 48, 1939–1973. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Han, Q.-Q.; Yang, S.-H.; Song, J.-C.; Li, N.; Wang, Z.-L.; Xu, X.-M. Recent Progress in Sulfonylation via Radical Reaction with Sodium Sulfinates, Sulfinic Acids, Sulfonyl Chlorides or Sulfonyl Hydrazides. ChemistrySelect 2020, 5, 13103–13134. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, C.; Yi, L.; Yue, H.; Kancherla, R.; Rueping, M. Cascade cross-coupling of dienes: Photoredox and nickel dual catalysis. Angew. Chem. Int. Ed. 2019, 58, 2–10. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yu, W. Hydrosulfonylation of Unactivated Alkenes by Visible Light Photoredox Catalysis. Org. Lett. 2019, 21, 9236–9240. [Google Scholar] [CrossRef]
- Zheng, Y.; You, Y.; Shen, Q.; Zhang, J.; Liu, L.; Duan, X.-H. Visible-Light-Induced Anti-Markovnikov Hydrosulfonation of Styrene Derivatives. Org. Chem. Front. 2020, 7, 2069–2074. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Liu, P.; Sun, P. Visible light-induced radical addition/annulation to construct phenylsulfonyl-functionalized dihydrobenzofurans involving an intramolecular 1,5-hydrogen atom transfer process. Org. Lett. 2020, 22, 8774–8779. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Li, J.; Zhou, H.; Xu, J.; Zhu, F.; Liang, Q.; Liu, Z.; Huang, G.; Huang, J. Synthesis of 3-sulfonylquinolines by visible-light promoted metal free cascade cycloaddition involving N-propargylanilines and sodium sulfinates. New J. Chem. 2020, 44, 3189–3193. [Google Scholar] [CrossRef]
- Xu, S.; Kong, H.; Zhang, R. Visible-light-induced, UiO-67-Ru-catalyzed oxidative cross-coupling for constructing b-acetylamino acrylosulfones. Tetrahedron Lett. 2020, 61, 151629–151635. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Dahiya, A.; Das, B.; Behera, A.; Patel, B.K. Visible-light-mediated difunctionalization of alkynes: Synthesis of β-substituted vinylsulfones using O- and S-centered nucleophiles. J. Org. Chem. 2021, 86, 11968–11986. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Visible-light-initiated 4CzIPN catalyzed multi-component tandem reactions to assemble sulfonated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2022, 33, 1479–1482. [Google Scholar] [CrossRef]
- Qian, Z.-M.; Zuo, K.-L.; Guan, Z.; He, Y.-H. Visible-light-induced sequential sulfonylation/hydroxylation of allylacetamides leading to β-tert-hydroxy sulfones. Tetrahedron 2021, 83, 131999. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Zhang, Y.; Liu, Q.; Zhao, X.; Li, J.-S.; Luo, Z.; Wei, W. Metal-free visible-light-induced oxidative cyclization reaction of 1,6-enynes and arylsulfinic acids leading to sulfonylated benzofurans. Chin. Chem. Lett. 2020, 31, 67–70. [Google Scholar] [CrossRef]
- Liu, Q.; Mei, Y.; Wang, L.; Ma, Y.; Li, P. Visible-light-induced radical cascade cyclizations of 1,7-enynes with sulfinic acids: Direct access to sulfonated chromanes and sulfonated tetrahydroquinolines under metal-free conditions. Adv. Synth. Catal. 2020, 362, 5669–5680. [Google Scholar] [CrossRef]
- Li, G.-H.; Han, Q.-Q.; Sun, Y.-Y.; Chen, D.-M.; Wang, Z.-L.; Xu, X.-M.; Yu, X.-Y. Visible-light induced cascade radical cyclization of sulfinic acids and o-(allyloxy)arylaldehydes towards functionalized chroman-4-ones. Chin. Chem. Lett. 2020, 31, 3255–3258. [Google Scholar] [CrossRef]
- Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O. Visible-Light-Mediated Regioselective Chlorosulfonylation of Alkenes and Alkynes: Introducing the Cu(II) Complex [Cu(dap)Cl2] to Photochemical ATRA Reactions. ACS Catal. 2019, 9, 1103–1109. [Google Scholar] [CrossRef]
- Hell, S.M.; Meyer, C.F.; Misale, A.; Sap, J.B.I.; Christensen, K.E.; Willis, M.C.; Trabanco, A.A.; Gouverneur, V. Hydrosulfonylation of Alkenes with Sulfonyl Chlorides under Visible Light Activation. Angew. Chem. Int. Ed. 2020, 59, 11620–11626. [Google Scholar] [CrossRef]
- Zhu, W.; Xi, H.; Jiao, W.; Huang, L.; Wang, L.; Wu, J. Difunctionalization of gem-Difluoroalkenes via Photoredox Catalysis: Synthesis of Diverse α,α-Difluoromethyl-β-alkoxysulfones. Org. Lett. 2022, 24, 720–725. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Zhang, W.-Z.; Zhou, Q.; Zhou, C.-S.; Xiong, B.-Q.; Tang, K.-W.; Liu, Y. Visible-light-mediated difunctionalization of vinylcyclopropanes for the synthesis of 1-sulfonylmethyl-3,4-dihydronaphthalenes. Org. Biomol. Chem. 2019, 17, 7918–7926. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, S.; Gao, X.; Han, S.; Lin, J.; Zhao, Y. Photoredox-catalyzed cascade annulation of N-propargylindoles with sulfonyl chlorides: Access to 2-sulfonated 9H-pyrrolo[1,2-a]indoles. Org. Biomol. Chem. 2019, 17, 2873–2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, G.; Huang, H.-L.; Liu, J.; Tang, H.; Li, Y.; Hu, H.; He, S.; Gao, F. Visible-light-driven sulfonylation/cyclization to access sulfonylated benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-ones. Chem. Asian J. 2021, 16, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.-H.; Li, Q.; Chen, F.-Y.; Jiang, L.-L.; Xu, P.; Deng, X.-W.; Li, M.; Zou, G.-D.; Cao, X. Visible-light photoredox-catalyzed sulfonyl lactonization of alkenoic acids with sulfonyl chlorides for sulfonyl lactone synthesis. J. Org. Chem. 2021, 86, 11998–12007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Kuang, K.; Wu, M.; Wu, S.; Xia, Z.; Xu, Q.; Zhang, M. Visible-light-induced radical cascade cyclization of 1-(allyloxy)-2-(1-arylvinyl)benzenes with sulfonyl chlorides for the synthesis of sulfonated benzoxepines. Org. Chem. Front. 2021, 8, 4095–4100. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Yue, H.; Zhao, X.; Li, J.; Wei, W. Photocatalyst-Free Visible Light-Induced Synthesis of β-Oxo Sulfones via Oxysulfonylation of Alkenes with Arylazo Sulfones and Dioxygen in Air. Adv. Synth. Catal. 2019, 361, 5277–5282. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Q.; Liu, F.; Yue, H.; Li, J.-S.; Wei, W. Visible-light-promoted aerobic oxidative synthesis of β-ketosulfones under photocatalyst-free conditions. Tetrahedron Lett. 2020, 61, 151335–151338. [Google Scholar] [CrossRef]
- Chen, J.; Allyson, Z.G.; Xin, J.-R.; Guan, Z.; He, Y.-H. Photo-Mediated Decarboxylative Ketonization of Atropic Acids with Sulfonyl Hydrazides: Direct Access to β-Ketosulfones. Adv. Synth. Catal. 2020, 362, 2045–2051. [Google Scholar] [CrossRef]
- Gadde, K.; Mampuys, P.; Guidetti, A.; Ching, H.Y.V.; Herrebout, W.A.; Doorslaer, S.V.; Tehrani, K.A.; Maes, B.U.W. Thiosulfonylation of Unactivated Alkenes with Visible-Light Organic Photocatalysis. ACS Catal. 2020, 10, 8765–8779. [Google Scholar] [CrossRef]
- Li, P.; Wang, G.-W. Visible-light-induced decarboxylative sulfonylation of cinnamic acids with sodium sulfinates by using Merrifield resin supported Rose Bengal as a catalyst. Org. Biomol. Chem. 2019, 17, 5578–5585. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, Z.; Wang, L. Synthesis of Vinyl Sulfones through a Visible-Light-Induced Decarboxylative Sulfonylation of Cinnamic Acids with Disulfides. Asian J. Chem. 2019, 8, 1426–1435. [Google Scholar] [CrossRef]
- Chawla, R.; Jaiswal, S.; Dutta, P.K.; Yadav, L.D.S. Photocatalyst-free visible light driven synthesis of (E)-vinyl sulfones from cinnamic acids and arylazo sulfones. Tetrahedron Lett. 2020, 61, 151898. [Google Scholar] [CrossRef]
- Bisseret, P.; Blanchard, N. Taming sulfur dioxide: A breakthrough for its wide utilization in chemistry and biology. Org. Biomol. Chem. 2013, 11, 5393–5398. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, G.; Wu, J. Inorganic sulfites as the sulfur dioxide surrogates in sulfonylation reactions. Chem. Commun. 2019, 55, 1013–1019. [Google Scholar] [CrossRef]
- Emmett, E.J.; Willis, M.C. The Development and Application of Sulfur Dioxide Surrogates in Synthetic Organic Chemistry. Asian J. Org. Chem. 2015, 4, 602–611. [Google Scholar] [CrossRef]
- Hofman, K.; Liu, N.-W.; Manolikakes, G. Radicals and Sulfur Dioxide: A Versatile Combination for the Construction of Sulfonyl-Containing Molecules. Chem. Eur. J. 2018, 24, 11852–11863. [Google Scholar] [CrossRef]
- Ye, S.; Li, X.; Xie, W.; Wu, J. Photoinduced Sulfonylation Reactions through the Insertion of Sulfur Dioxide. Eur. J. Org. Chem. 2020, 2020, 1274–1287. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Zhou, Q.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-light promoted one-pot synthesis of sulfonated spiro[4,5]trienones from propiolamides, anilines and sulfur dioxide under transition metal-free conditions. Chem. Commun. 2019, 55, 12212–12215. [Google Scholar] [CrossRef]
- Yao, Y.; Yin, Z.; He, F.-S.; Qin, X.; Xie, W.; Wu, J. Photoinduced intramolecular carbosulfonylation of alkynes: Access to sulfone-containing dibenzazepines from sulfur dioxide. Chem. Commun. 2021, 57, 2883–2886. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, K.; Rojsitthisak, P.; Wu, J. Metal-free insertion of sulfur dioxide with aryl iodides under ultraviolet irradiation: Direct access to sulfonated cyclic compounds. Org. Chem. Front. 2020, 7, 14–18. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Zhou, S.-F.; Cui, X. Photoredox-catalyzed synthesis of sulfonated oxazolines from N-allylamides through the insertion of sulfur dioxide. Org. Chem. Front. 2022, 9, 364–369. [Google Scholar] [CrossRef]
- He, F.-S.; Wu, Y.; Li, X.; Xia, H.; Wu, J. Photoredox-catalyzed sulfonylation of alkenylcyclobutanols with the insertion of sulfur dioxide through semipinacol rearrangement. Org. Chem. Front. 2019, 6, 1873–1878. [Google Scholar] [CrossRef]
- Zong, Y.; Lang, Y.; Yang, M.; Li, X.; Fan, X.; Wu, J. Synthesis of β-Sulfonyl Amides through a Multicomponent Reaction with the Insertion of Sulfur Dioxide under Visible Light Irradiation. Org. Lett. 2019, 21, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.M.; Kumar, S.; Halder, I.; Volla, C.M.R. Visible-light mediated sulfonylation of thiols via insertion of sulfur dioxide. Org. Biomol. Chem. 2019, 17, 5897–5901. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Zhang, B.; Han, J.; Peng, B.; Shan, Y.; Niu, T. Heterogeneous Carbon Nitrides Photocatalysis Multicomponent Hydrosulfonylation of Alkynes to Access β-Keto Sulfones with the Insertion of Sulfur Dioxide in Aerobic Aqueous Medium. Org. Lett. 2020, 22, 670–674. [Google Scholar] [CrossRef]
- Ye, S.; Li, X.; Xie, W.; Wu, J. A Three-Component Reaction of Potassium Alkyltrifluoroborates, Sulfur Dioxide and Allylic Bromides under Visible Light Irradiation. Asian J. Chem. 2019, 8, 893–898. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Qiu, G.; Wu, J. Substituted Hantzsch esters as radical reservoirs with the insertion of sulfur dioxide under photoredox catalysis. Chem. Commun. 2019, 55, 2062–2065. [Google Scholar] [CrossRef]
- Ye, S.; Zheng, D.; Wu, J.; Qiu, G. Photoredox-catalyzed sulfonylation of alkyl iodides, sulfur dioxide, and electron-deficient alkenes. Chem. Commun. 2019, 55, 2214–2217. [Google Scholar] [CrossRef]
- Breton-Patient, C.; Naud-Martin, D.; Mahuteau-Betzer, F.; Piguel, S. Three-component C-H bond sulfonylation of imidazoheterocycles via visible-light organophotoredox catalysis. Eur. J. Chem. 2020, 2020, 6653–6660. [Google Scholar] [CrossRef]
- Cao, S.; Hong, W.; Ye, Z.; Gong, L. Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)-H functionalization. Nat. Commun. 2021, 12, 2377. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, W.; Ye, S.; Wu, J. Photoredox-Catalyzed Sulfonylation of O-Acyl Oximes via Iminyl Radicals with the Insertion of Sulfur Dioxide. Org. Lett. 2019, 21, 4950–4954. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Li, H.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-light photoredox-catalyzed dual C-C bond cleavage: Synthesis of 2-cyanoalkylsulfonylated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide. Chem. Commun. 2020, 56, 3011–3014. [Google Scholar] [CrossRef]
- Zhou, N.; Xia, Z.; Wu, S.; Kuang, K.; Xu, Q.; Zhang, M. Visible-Light-Induced Multicomponent Cascade Cycloaddition of N-Propargyl Aromatic Amines, Cyclobutanone Oxime Esters, and K2S2O5: Access to Cyanoalkylsulfonylated Quinolines. J. Org. Chem. 2021, 86, 15253–15262. [Google Scholar] [CrossRef]
- Teng, F.; Du, J.; Xun, C.; Zhu, M.; Lu, Z.; Jiang, H.; Chen, Y.; Lia, Y.; Gui, Q.-W. Photoinduced efficient synthesis of cyanoalkylsulfonylated oxindoles via sulfur dioxide insertion. Org. Biomol. Chem. 2021, 19, 8929–8933. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, J.; Lin, M.; He, L.; Yue, H.; Liu, R.; Wei, W. Metal-Free Multi-Component Sulfur Dioxide Insertion Reaction Leading to Quinoxalin-2-One-Containing Vinyl Sulfones under Visible-Light Photoredox Catalysis. Adv. Synth. Catal. 2021, 363, 5122–5128. [Google Scholar] [CrossRef]
- He, F.-S.; Yao, Y.; Xie, W.; Wu, J. Photoredox-catalyzed sulfonylation of difluoroenoxysilanes with the insertion of sulfur dioxide. Chem. Commun. 2020, 56, 9469–9472. [Google Scholar] [CrossRef]
- Gong, X.; Yang, M.; Liu, J.-B.; He, F.-S.; Wu, J. Photoinduced synthesis of alkylalkynyl sulfones through a reaction of potassium alkyltrifluoroborates, sulfur dioxide, and alkynyl bromides. Org. Chem. Front. 2020, 7, 938–943. [Google Scholar] [CrossRef]
- Gong, X.; Yang, M.; Liu, J.-B.; He, F.-S.; Fan, X.; Wu, J. A metal-free route to alkynyl sulfones under photoinduced conditions with the insertion of sulfur dioxide. Green Chem. 2020, 22, 1906–1910. [Google Scholar] [CrossRef]
- Li, J.; Bao, W.; Zhang, Y.; Rao, Y. Metal-free cercosporin-photocatalyzed C-S coupling for the selective synthesis of aryl sulfides under mild conditions. Eur. J. Org. Chem. 2019, 2019, 7175–7178. [Google Scholar] [CrossRef]
- Li, R.; Shi, T.; Chen, X.-L.; Lv, Q.-Y.; Zhang, Y.-L.; Peng, Y.-Y.; Qu, L.-B.; Yu, B. Visible-light-promoted organic dye-catalyzed sulfidation and phosphorylation of arylhydrazines toward aromatic sulfides and diarylphosphoryl hydrazides. New J. Chem. 2019, 43, 13642–13646. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Yue, H.; Li, J.-S.; Luo, Z.; Wei, W. Catalyst-free visible-light-initiated oxidative coupling of aryldiazo sulfones with thiols leading to unsymmetrical sulfoxides in air. Green Chem. 2019, 21, 1609–1613. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Ji, P.; Hu, W.; Wei, Y.; Huang, H. Organocatalytic transformation of aldehydes to thioesters with visible light. Chem. Eur. J. 2019, 25, 8225–8228. [Google Scholar] [CrossRef]
- Pramanik, M.; Choudhuri, K.; Mathuri, A.; Mal, P. Dithioacetalization or thioetherification of benzyl alcohols by 9- Mesityl-10-methylacridinium perchlorate photocatalyst. Chem. Commun. 2020, 56, 10211–10214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, B.; Hong, S.H. Direct allylic C(sp3)-H thiolation with disulfides via visible light photoredox catalysis. ACS Catal. 2020, 10, 6013–6022. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Ali, W.; Mar´c, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Huan, Y.; Cao, H.; Sun, S.; Lei, L.; Liu, Q.; Liu, S.; Ji, W.; Huang, K.; et al. Diphenyl diselenide ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats via suppressing oxidative stress and inflammation. Chem. Biol. Interact. 2021, 338, 109427–109440. [Google Scholar] [CrossRef]
- Zhang, Q.-B.; Yuan, P.-F.; Kai, L.-L.; Liu, K.; Ban, Y.-L.; Wang, X.-Y.; Wu, L.-Z.; Liu, Q. Preparation of heterocycles via visible-light-driven aerobic selenation of olefins with diselenides. Org. Lett. 2019, 21, 885–889. [Google Scholar] [CrossRef]
- Jung, H.I.; Kim, D.Y. Synthesis of β-Selenylated Cyclopentanones via Photoredox-Catalyzed Selenylation/Ring-Expansion Cascades of Alkenyl Cyclobutanols. Synlett 2019, 30, 1361–1365. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Yang, C.; Xia, W. Three-component aminoselenation of alkenes via visible-light enabled Fe-catalysis. Green Chem. 2020, 22, 2804–2809. [Google Scholar] [CrossRef]
- Wang, R.; Xie, H.; Lai, X.; Liu, J.-B.; Li, J.; Qiu, G. Visible light-enabled iron-catalyzed selenocyclization of N-methoxy-2-alkynylbenzamide. Mol. Catal. 2021, 515, 111881. [Google Scholar] [CrossRef]
- Zhou, J.; Li, W.; Zheng, H.; Pei, Y.; Liu, X.; Cao, H. Visible light-induced cascade cyclization of 3-aminoindazoles, ynals, and chalcogens: Access to chalcogen-containing pyrimido[1,2-b]-indazoles. Org. Lett. 2021, 23, 2754–2759. [Google Scholar] [CrossRef]
- Thurow, S.; Abenante, L.; Anghinoni, J.M.; Lenardão, E.J. Selenium as a versatile reagent in organic synthesis: More than allylic oxidation. Curr. Org. Synth. 2022, 19, 331–365. [Google Scholar] [CrossRef]
- Chachignon, H.; Cahard, D. State-of-the-art in electrophilic trifluoromethylthiolation reagents. Chin. J. Chem. 2016, 34, 445–454. [Google Scholar] [CrossRef]
- Ghiazza, C.; Monnereau, C.; Khrouz, L.; Médebielle, M.; Billard, T.; Tlili, A. New avenues in radical trifluoromethylselenylation with trifluoromethyl tolueneselenosulfonate. Synlett 2019, 30, 777–782. [Google Scholar] [CrossRef]
- Zordo-Banliat, A.; Barthélémy, L.; Bourdreux, F.; Tuccio, B.; Dagousset, G.; Pégot, B.; Magnier, E. Visible-light-induced metal-free trifluoromethylselenolation of electron-rich heteroarenes using the nucleophilic [Me4N][SeCF3] reagent. Eur. J. Org. Chem. 2020, 4, 506–509. [Google Scholar] [CrossRef]
- Han, Q.-Y.; Tan, K.-L.; Wang, H.N.; Zhang, C.P. Organic photoredox-catalyzed decarboxylative trifluoromethylselenolation of aliphatic carboxylic acids with [Me4N][SeCF3]. Org. Lett. 2019, 21, 10013–10017. [Google Scholar] [CrossRef]
- Lu, K.; Li, Q.; Xi, X.; Zhou, T.; Zhao, X. Methylselenolation of arylamines under visible light photocatalysis. J. Org. Chem. 2020, 85, 1224–1231. [Google Scholar] [CrossRef]
- Karmarker, P.G.; Huo, F. Organic Selenocyanates: Rapid Advancements and Applicationsin the Field of Organic Chemistry. Asian J. Org. Chem. 2022, 11, e202200226. [Google Scholar] [CrossRef]
- Xie, Q.; Yang, Y.; Zhang, W.; Gao, Z.; Li, X.; Tang, J.; Pana, C.; Yu, G. Polarization-induced charge separation in conjugated microporous polymers for efficient visible light-driven C-3 selenocyanation of indoles. Chem. Sci. 2021, 12, 5631–5637. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, L.; Gao, Y.; Sun, T.; Qiao, J.; Li, A.; Lu, K. Visible-light-promoted selenocyanation of cyclobutanone oxime esters using potassium selenocyanate. J. Org. Chem. 2021, 86, 11399–11406. [Google Scholar] [CrossRef]
- Abdtawfeeq, T.H.; Mahmood, E.A.; Azimi, S.B.; Kadhim, M.M.; Kareem, R.T.; Charatig, F.R.; Vessally, E. Direct selenosulfonylation of unsaturated compounds: A review. RSC Adv. 2022, 12, 30564–30576. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, P.; Wang, S.-W.; Ji, S.J. Visible light-induced Co- or Cu-catalyzed selenosulfonylation of alkynes: Synthesis of β-(seleno)vinyl sulfones. J. Org. Chem. 2019, 84, 12324–12333. [Google Scholar] [CrossRef] [PubMed]
- Ghiazza, C.; Khrouz, L.; Billard, T.; Monnereau, C.; Tlili, A. Fluoroalkylselenolation of alkyl silanes/trifluoroborates under metal-free visible-light photoredox catalysis. eurJOC 2020, 10, 1559–1566. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.-E.; Wang, S.-L.; Zhang, L.-L.; Zhou, X.-Z.; Wang, S.-Y.; Ji, S.-J. Visible-light-promoted cross-coupling reactions of 4-Alkyl-1,4- dihydropyridines with thiosulfonate or selenium sulfonate: A unified approach to sulfides, selenides, and sulfoxides. Org. Lett. 2020, 22, 4908–4913. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.-Y. Visible-light-promoted cross-coupling reaction of hypervalent bis-catecholato silicon compounds with selenosulfonates or thiosulfonates. Org. Chem. Front. 2021, 8, 1976–1982. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, N.; Li, Y.; Mo, Z.; Ma, X.; Chen, Y.; Xu, Y. Metal-free synthesis of 3-sulfonyl-5-selanyl-4a,8a-dihydro2H-chromen-6(5H)-ones via visible light driven intermolecular cascade cyclization of alkyne-tethered cyclohexadienones and selenosulfonates. Green Synth. Catal. 2021, 2, 397–400. [Google Scholar] [CrossRef]
- Liu, B.-X.; Wang, F.; Chen, Y.; Rao, W.-D.; Shenc, S.-S.; Wang, S.-Y. Visible-light-promoted denitrogenative orthoselenylation reaction of benzotriazinones: Synthesis of ortho-selenylated benzamides and ebselen analogs. Org. Chem. Front. 2022, 9, 2418–2423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).