The Role of the A-Site Cation on the Bifunctional Electrocatalytic Activities of Ln0.5Sr0.5CoO3-δ (Ln = La, Pr and Sm) for Rechargeable Zinc–Air Batteries
Abstract
1. Introduction
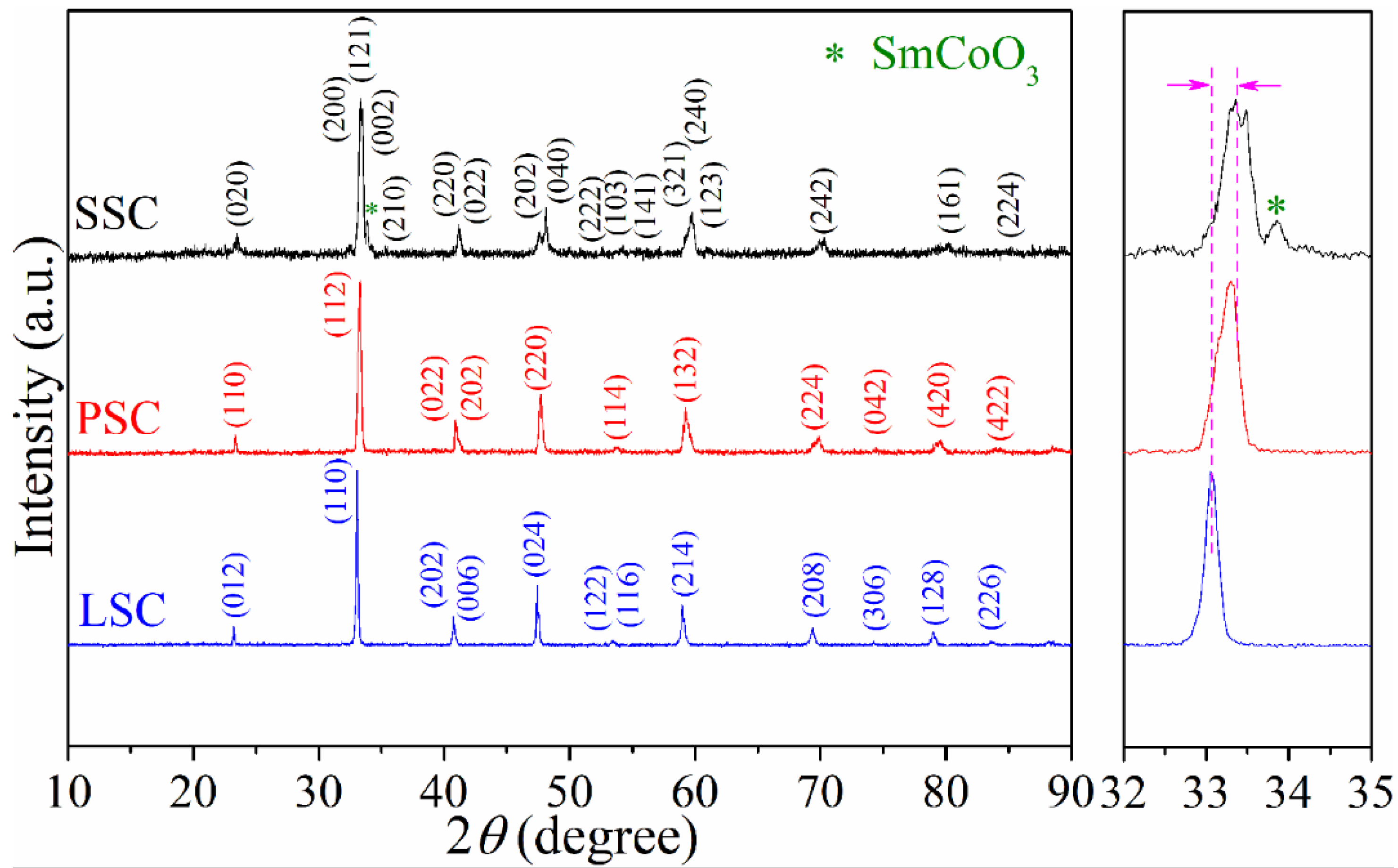
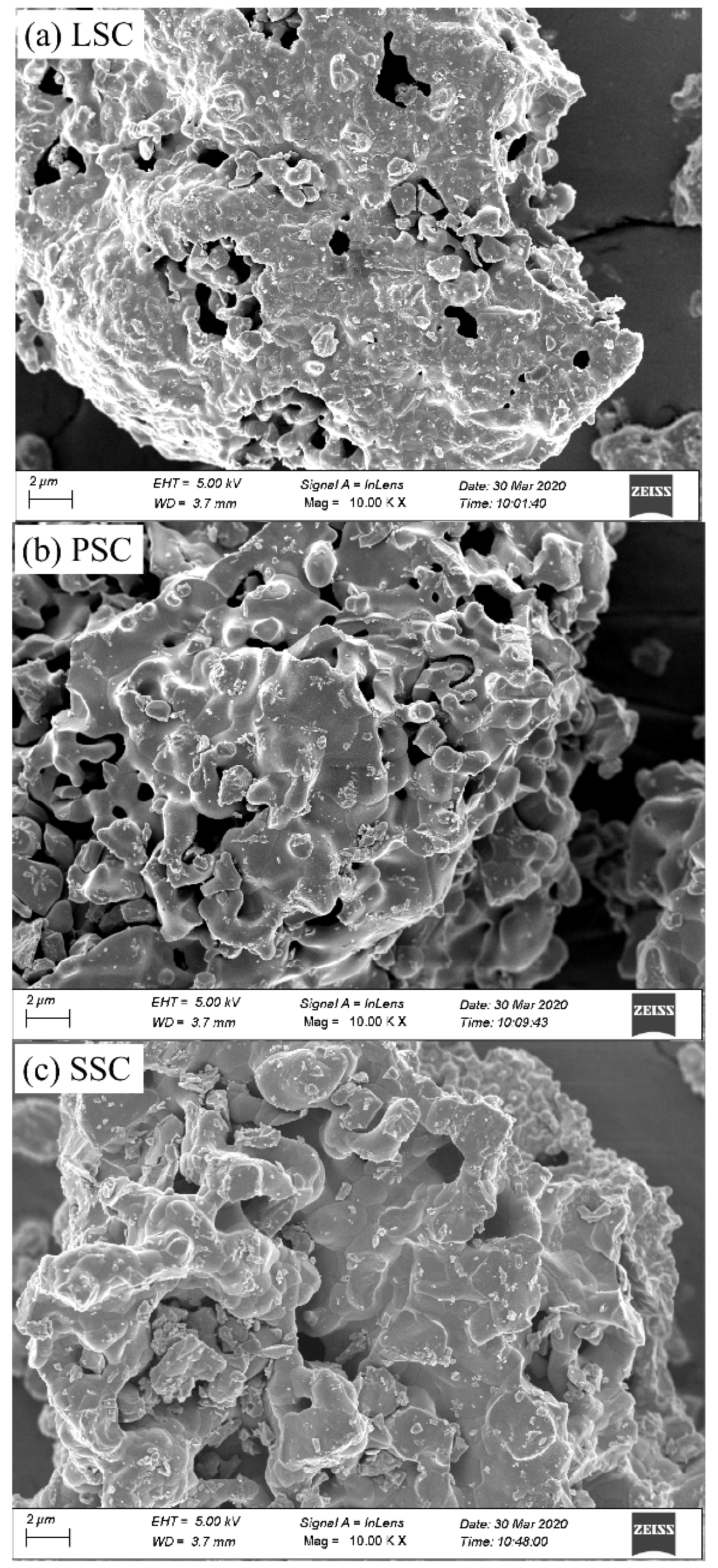
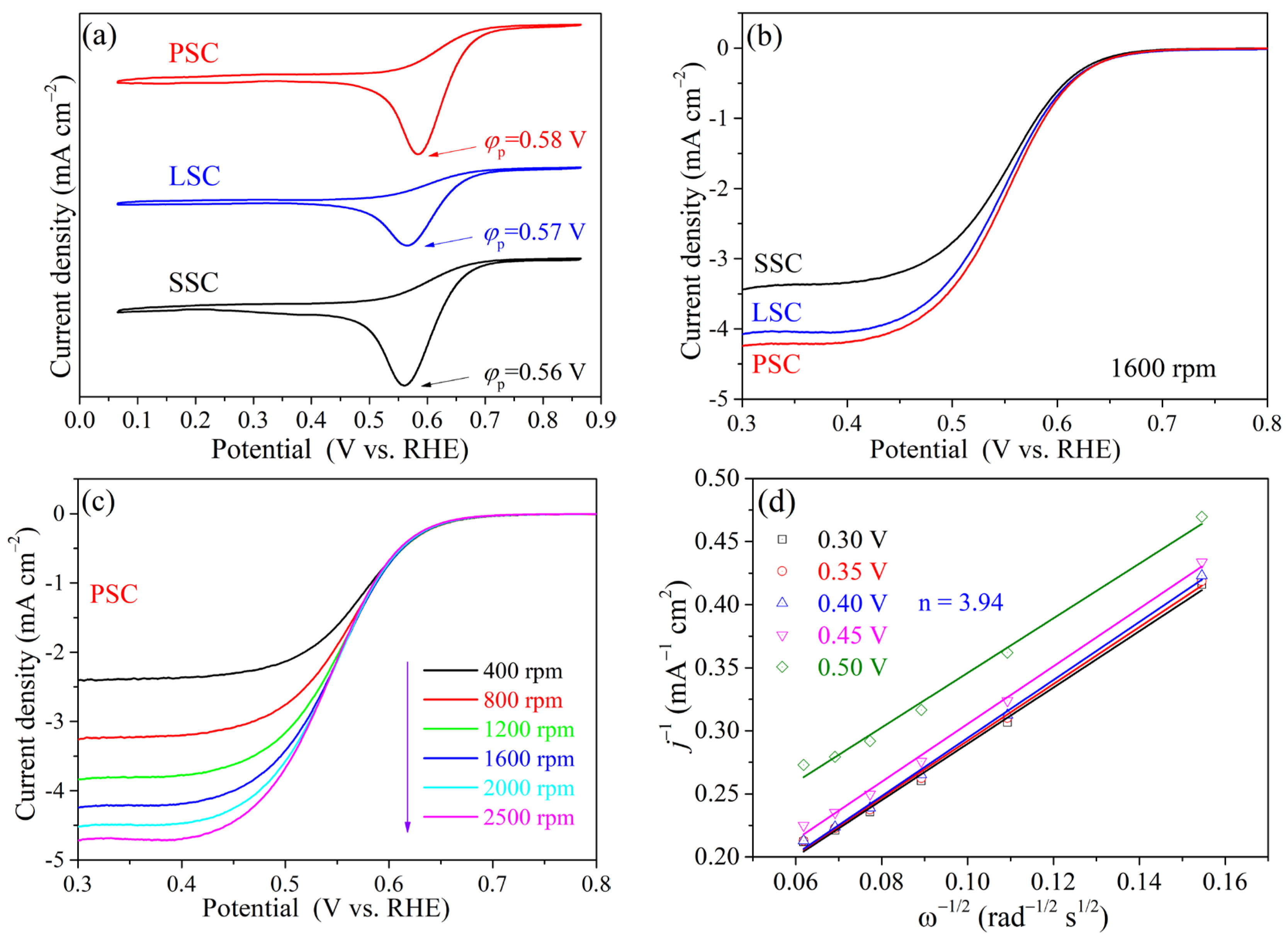
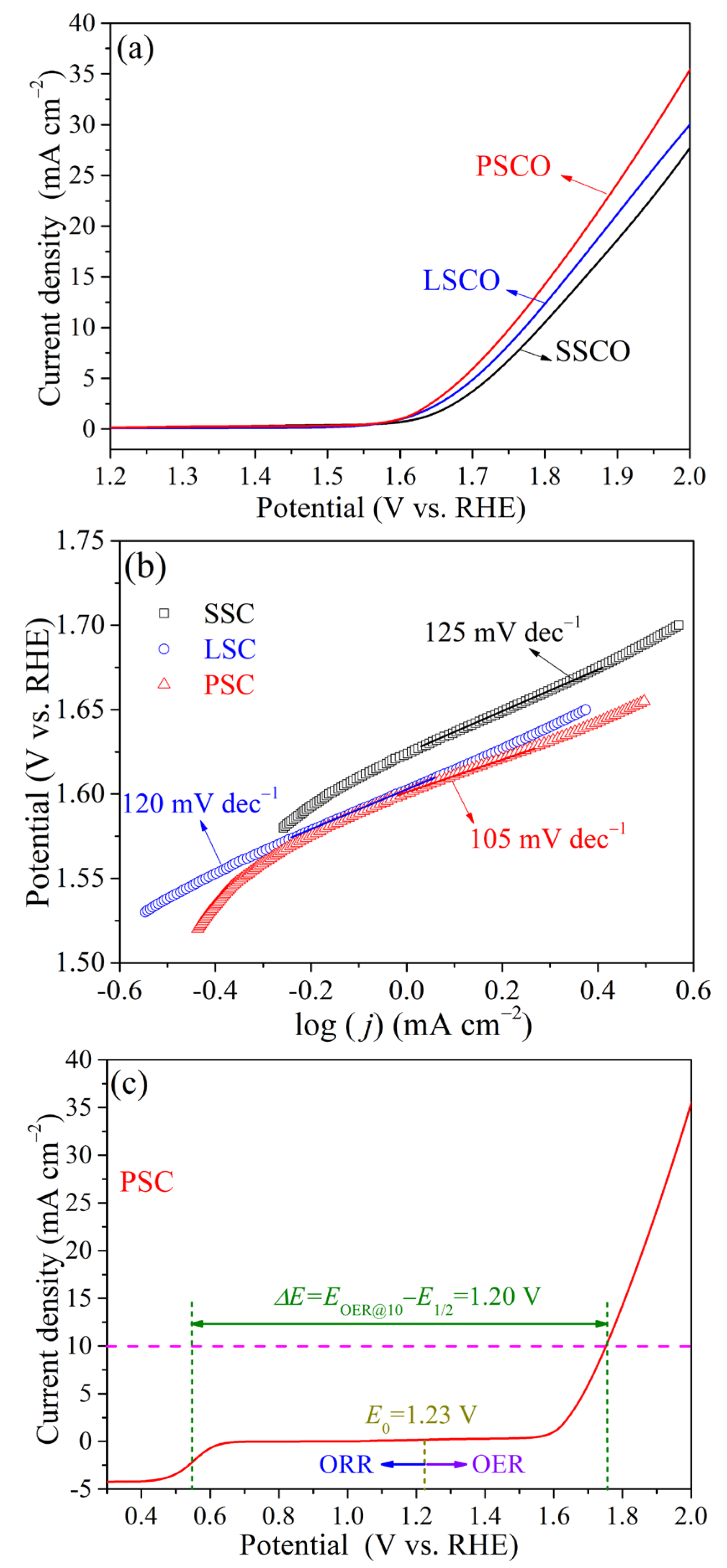
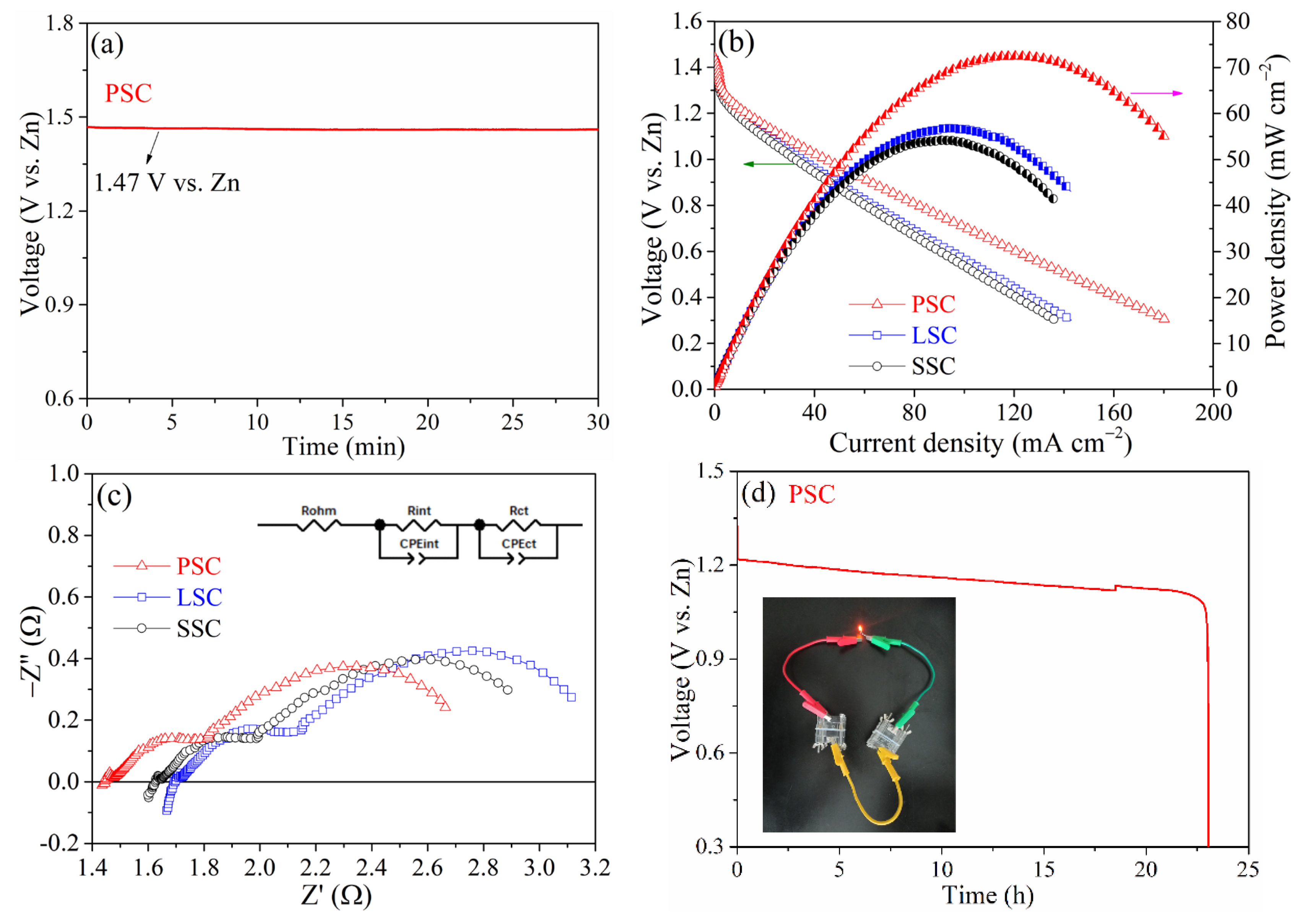
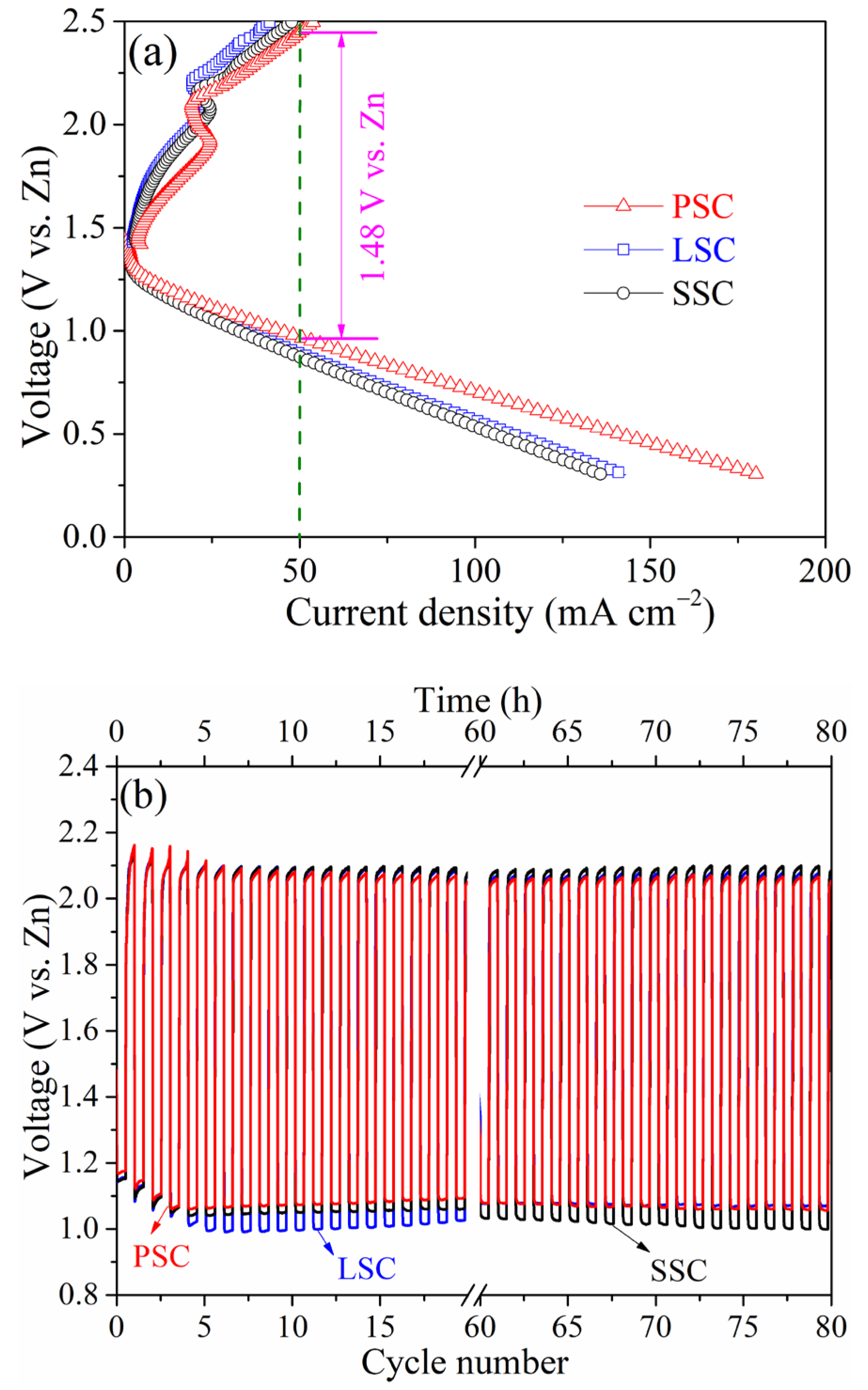
2. Results and Discussion
3. Experimental
3.1. Material Synthesis
3.2. Physical Characterization
3.3. Electrochemical Measurements
3.4. Characterization of Rechargeable Zinc–Air Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Wang, Y.; Yin, Z.; He, R.; Wang, Y.; Qiao, J. In-situ growth of CoNi bimetal anchored on carbon nanoparticle/nanotube hybrid for boosting rechargeable Zn-air battery. J. Energy Chem. 2022, 66, 348–355. [Google Scholar] [CrossRef]
- Li, G.; Tang, Y.; Fu, T.; Xiang, Y.; Xiong, Z.; Si, Y.; Guo, C.; Jiang, Z. S, N co-doped carbon nanotubes coupled with CoFe nanoparticles as an efficient bifunctional ORR/OER electrocatalyst for rechargeable Zn-air batteries. Chem. Eng. J. 2022, 429, 132174. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Lee, J.-M.; Fu, G. Recent advances in rare-earth-based materials for electrocatalysis. Chem. Catal. 2022, 2, 967–1008. [Google Scholar] [CrossRef]
- Kundu, A.; Mallick, S.; Ghora, S.; Raj, C.R. Advanced Oxygen Electrocatalyst for Air-Breathing Electrode in Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40172–40199. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.D.; Lee, J.Y. Advancement of Platinum (Pt)-Free (Non-Pt Precious Metals) and/or Metal-Free (Non-Precious-Metals) Electrocatalysts in Energy Applications: A Review and Perspectives. Energy Fuels 2020, 34, 6634–6695. [Google Scholar] [CrossRef]
- Liang, J.; Li, F.; Cheng, H.-M. On energy from fuel cells to metal-air batteries: Catalysts with two functions. Energy Storage Mater. 2018, 10, A1–A3. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Xue, L.; Sun, J.; Wang, X.; Xiong, P.; Zhu, J. Recent advances in the heteroatom doping of perovskite oxides for efficient electrocatalytic reactions. Nanoscale 2021, 13, 19840–19856. [Google Scholar] [CrossRef]
- Beall, C.E.; Fabbri, E.; Schmidt, T.J. Perovskite Oxide Based Electrodes for the Oxygen Reduction and Evolution Reactions: The Underlying Mechanism. ACS Catal. 2021, 11, 3094–3114. [Google Scholar] [CrossRef]
- Matienzo, D.J.D.; Kutlusoy, T.; Divanis, S.; Bari, C.; Instuli, E. Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis. Catalysts 2020, 10, 1387. [Google Scholar] [CrossRef]
- Li, F.; Mushtaq, N.; Su, T.; Cui, Y.; Huang, J.; Sun, M.; Singh, M.; Zhao, X.; Maliutina, K.; Zhang, Y.; et al. NCNT grafted perovskite oxide as an active bifunctional electrocatalyst for rechargeable zinc-air battery. Mater. Today Nano 2023, 21, 100287. [Google Scholar] [CrossRef]
- Mondal, R.; Ratnawat, H.; Mukherjee, S.; Gupta, A.; Singh, P. Investigation of the Role of Sr and Development of Superior SrDoped Hexagonal BaCoO3-δ Perovskite Bifunctional OER/ORR Catalysts in Alkaline Media. Energy Fuels 2022, 36, 3219–3228. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Sheelam, A.; Balu, S.; Muneeb, A.; Bayikadi, K.S.; Namasivayam, D.; Siddharthan, E.E.; Inamdar, A.I.; Thapa, R.; Chiang, M.-H.; Huang, S.-J.I.; et al. Improved Oxygen Redox Activity by High-Valent Fe and Co3+ Sites in the Perovskite LaNi1−xFe0.5xCo0.5xO3. ACS Appl. Energy Mater. 2022, 5, 343–354. [Google Scholar] [CrossRef]
- Suntivich, J.; Hong, W.T.; Lee, Y.-L.; Rondinelli, J.M.; Yang, W.; Goodenough, J.B.; Dabrowski, B.; Freeland, J.W.; Shao-Horn, Y. Estimating Hybridization of Transition Metal and Oxygen States in Perovskites from O K-edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2014, 118, 1856–1863. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amoros, D.; Morallon, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Ke, W.; Liang, Y.; Chao, Y.; Han, N.; Liang, P.; He, X. Enhanced cycling stability of La0.2Sr0.8CoO3−δ for oxygen evolution reaction via trace doping of Nb. Ceram. Int. 2022, 48, 36992–36999. [Google Scholar] [CrossRef]
- Bao, B.; Liu, Y.; Sun, M.; Huang, B.; Hu, Y.; Da, P.; Ji, D.; Xi, P.; Yan, C.-H. Boosting the Electrocatalytic Oxygen Evolution of Perovskite LaCo1- x Fex O3 by the Construction of Yolk-Shell Nanostructures and Electronic Modulation. Small 2022, 18, e2201131. [Google Scholar] [CrossRef]
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z. Design of Highly Active Perovskite Oxides for Oxygen Evolution Reaction by Combining Experimental and ab Initio Studies. ACS Catal. 2015, 5, 4337–4344. [Google Scholar] [CrossRef]
- Xiong, D.; Rasaki, S.A.; Li, Y.; Fan, L.; Liu, C.; Chen, Z. Enhanced cathodic activity by tantalum inclusion at B-site of La0.6Sr0.4CO0.4Fe0.6O3 based on structural property tailored via camphor-assisted solid-state reaction. J. Adv. Ceram. 2022, 11, 1330–1342. [Google Scholar] [CrossRef]
- Kolla, P.; Nasymov, G.; Rajappagowda, R.; Smirnova, A. Bi-functionality of samarium- and praseodymium-based perovskite catalysts for oxygen reduction and oxygen evolution reactions in alkaline medium. J. Power Sources 2020, 446, 227234. [Google Scholar] [CrossRef]
- Yoshii, K.; Nakamura, A.; Abe, H.; Mizumaki, M.; Muro, T. Magnetism and transport of Ln0.5Sr0.5CoO3 (Ln = Pr, Nd, Sm, Eu and Gd). J. Magn. Magn. Mater. 2002, 239, 85–87. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, R.; Yang, W.; Huang, L.; Mao, Q.; Yang, J.; Sun, L.; Hu, Z.; Liu, X. Understanding the Enhancement Mechanism of A-Site-Deficient LaxNiO3 as an Oxygen Redox Catalyst. Chem. Mater. 2020, 32, 1864–1875. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Ren, Y.; Küngas, R.; Gorte, R.J.; Deng, C. The effect of A-site cation (Ln = La, Pr, Sm) on the crystal structure, conductivity and oxygen reduction properties of Sr-doped ferrite perovskites. Solid State Ion. 2012, 212, 47–54. [Google Scholar] [CrossRef]
- Bousnina, M.A.; Dujardin, R.; Perriere, L.; Giovannelli, F.; Guegan, G.; Delorme, F. Synthesis, sintering, and thermoelectric properties of the solid solution La1−xSr xCoO3 ± δ (0 ≤ x ≤ 1). J. Adv. Ceram. 2018, 7, 160–168. [Google Scholar] [CrossRef]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5 + δ (Ln = La, Pr, Nd and Sm) as cathode materials for IT-SOFC. J. Electroanal. Chem. 2021, 886, 115144. [Google Scholar] [CrossRef]
- Wu, M.; Cai, H.; Jin, F.; Sun, N.; Xu, J.; Zhang, L.; Han, X.; Wang, S.; Su, X.; Long, W.; et al. Assessment of cobalt–free ferrite–based perovskite Ln0.5Sr0.5Fe0.9Mo0.1O3−δ (Ln = lanthanide) as cathodes for IT-SOFCs. J. Eur. Ceram. Soc. 2021, 41, 2682–2690. [Google Scholar] [CrossRef]
- Lee, K.T.; Manthiram, A. Comparison of Ln0.6Sr0.4CoO3−δ (Ln = La, Pr, Nd, Sm, and Gd) as Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2006, 153, A794–A798. [Google Scholar] [CrossRef]
- Yi, L.; Le, C.; Chunhua, L.; Yaru, N.; Zhongzi, X. Effects of oxygen defects on structure and properties of Sm0.5Sr0.5CoO3−δ annealed in different atmospheres. J. Rere Earths 2013, 31, 1183–1190. [Google Scholar]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst 1976, A32, 751. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, H.; Li, J.; Shi, Z.; Fan, M.; Bian, H.; Wang, T.; Gao, D. S-doped CoMn2O4 with more high valence metallic cations and oxygen defects for zinc-air batteries. J. Power Sources 2021, 491, 229584. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Li, S.; Miao, H.; Liu, Z. La0.8Sr0.2Co1−xMnxO3 perovskites as efficient bi-functional cathode catalysts for rechargeable zinc-air batteries. Electrochim. Acta 2017, 254, 14–24. [Google Scholar] [CrossRef]
- Kotha, V.; Karajagi, I.; Ghosh, P.C.; Panchakarla, L.S. Potassium-Substituted LaMnO3 as a Highly Active and Exceptionally Stable Electrocatalyst toward Bifunctional Oxygen Reduction and Oxygen Evolution Reactions. ACS Appl. Energy Mater. 2022, 5, 7297–7307. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Nachtegaal, M.; Castelli, I.E.; Kazzi, M.E.; Haumont, R.; Marzari, N.; Schmidt, T.J. Oxygen Evolution Reaction on La1−xSrxCoO3 Perovskites: A Combined Experimental and Theoretical Study of Their Structural, Electronic, and Electrochemical Properties. Chem. Mater. 2015, 27, 7662–7672. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, M.; Li, J.; Lu, Y.; Wang, L.; Wang, Z.; Zhang, X.; Ding, X. Modulation of electronic structure and oxygen vacancies of perovskites SrCoO3−δ by sulfur doping enables highly active and stable oxygen evolution reaction. Electrochim. Acta 2021, 390, 138872. [Google Scholar] [CrossRef]
- Kim, S.; Jung, J.-W.; Song, D.; Cho, S.-H.; Kim, J.; Kim, J.K.; Oh, D.; Sun, H.; Cho, E.; Kim, I.-D.; et al. Exceptionally durable CoFe-exsolved Sr0.95Nb0.1Co0.7Fe0.2O3−δ catalyst for rechargeable Zn–air batteries. Appl. Catal. B Environ. 2022, 315, 121553. [Google Scholar] [CrossRef]
- Gui, L.; Wang, Z.; Zhang, K.; He, B.; Liu, Y.; Zhou, W.; Xu, J.; Wang, Q.; Zhao, L. Oxygen vacancies-rich Ce0.9Gd0.1O2−δ decorated Pr0.5Ba0.5CoO3−δ bifunctional catalyst for efficient and long-lasting rechargeable Zn-air batteries. Appl. Catal. B Environ. 2020, 266, 118656. [Google Scholar] [CrossRef]
- Da, Y.; Zeng, L.; Wang, C.; Gong, C.; Cui, L. A simple approach to tailor OER activity of SrxCo0.8Fe0.2O3 perovskite catalysts. Electrochim. Acta 2019, 300, 85–92. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Sun, Y.-F.; Zhang, Y.-Q.; Yan, N.; Chen, J.; Thundat, T.; Li, J.; Luo, J.-L. A coupling for success: Controlled growth of Co/CoOx nanoshoots on perovskite mesoporous nanofibres as high-performance trifunctional electrocatalysts in alkaline condition. Nano Energy 2017, 32, 247–254. [Google Scholar] [CrossRef]
- Maitra, U.; Naidu, B.S.; Govindaraj, A.; Rao, C.N. Importance of trivalency and the e (g) configuration in the photocatalytic oxidation of water by Mn and Co oxides. Proc. Natl. Acad. Sci. USA 2013, 110, 11704–11707. [Google Scholar] [CrossRef]
- Huang, S.J.; Muneeb, A.; Sabhapathy, P.; Sheelam, A.; Bayikadi, K.S.; Sankar, R. Tailoring the Co4+/Co3+ active sites in a single perovskite as a bifunctional catalyst for the oxygen electrode reactions. Dalton Trans. 2021, 50, 7212–7222. [Google Scholar] [CrossRef]
- Yamada, I.; Kinoshita, M.; Oda, S.; Tsukasaki, H.; Kawaguchi, S.; Oka, K.; Mori, S.; Ikeno, H.; Yagi, S. Enhanced Catalytic Activity and Stability of the Oxygen Evolution Reaction on Tetravalent Mixed Metal Oxide. Chem. Mater. 2020, 32, 3893–3903. [Google Scholar] [CrossRef]
- Nie, R.; Deng, Y.; Yang, H.; Tan, Y.; Yuan, H.; Sagar, R.U.R.; Liang, T. Efficient oxygen evolution reaction in SrCo0.8Fe0.2O3−δ perovskite and surface reconstruction for practical zinc-air batteries. Appl. Surf. Sci. 2021, 552, 149509. [Google Scholar] [CrossRef]
- Wei, H.-l.; Tan, A.-d.; Hu, S.-z.; Piao, J.-h.; Fu, Z.-y. Efficient spinel iron-cobalt oxide/nitrogen-doped ordered mesoporous carbon catalyst for rechargeable zinc-air batteries. Chin. J. Catal. 2021, 42, 1451–1458. [Google Scholar] [CrossRef]
- Han, X.; Li, N.; Xiong, P.; Jung, M.G.; Kang, Y.; Dou, Q.; Liu, Q.; Lee, J.Y.; Park, H.S. Electronically coupled layered double hydroxide/MXene quantum dot metallic hybrids for high-performance flexible zinc–air batteries. InfoMat 2021, 3, 1134–1144. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Li, J.; Qian, J.; Liu, Y.; Yang, S.; Wang, Y.; Gao, D.; Xue, D. Interfacial Engineering of NiO/NiCo2O4 Porous Nanofibers as Efficient Bifunctional Catalysts for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21661–21669. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Han, Z.; Yan, Q.; Feng, J.-J.; Mei, L.-P.; Zhang, L.; Wang, A.-J. CoNi/MoC nanoparticles entrapped into N, P-codoped carbon nanotubes-on-nanosheets: A synergy of 1D@2D heterostructures with multiple active sites for rechargeable Zn-air battery. J. Power Sources 2021, 506, 230225. [Google Scholar] [CrossRef]
- Xiong, M.; Ivey, D.G. Sequentially Electrodeposited MnOX/Co-Fe as Bifunctional Electrocatalysts for Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2017, 164, A1012–A1021. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Higgins, D.; Li, H.; Wang, H.; Chen, Z. Highly active and durable core-corona structured bifunctional catalyst for rechargeable metal-air battery application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, B.; Lü, Z.; Wu, Y.; Zhang, Y.; Huang, X. La1.7Sr0.3Co0.5Ni0.5O4+δ layered perovskite as an efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Lee, D.U.; Choi, J.-Y.; Feng, K.; Park, H.W.; Chen, Z. Advanced Extremely Durable 3D Bifunctional Air Electrodes for Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2014, 4, 1301389. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic Bifunctional Catalyst Design based on Perovskite Oxide Nanoparticles and Intertwined Carbon Nanotubes for Rechargeable Zinc-Air Battery Applications. ACS Appl. Mater. Interfaces 2014, 7, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.D.; Shukla, K.K.; Shahi, P.; Ghosh, A.K.; Nigam, A.K.; Chatterjee, S. Effect of Y-doping on the transport and magnetic properties of La0.5Sr0.5CoO3 and La0.7Sr0.3CoO3. J. Mater. Sci. 2013, 48, 1997–2001. [Google Scholar] [CrossRef]
- Li, P.; Tian, C.; Yang, W.; Zhao, W.; Lü, Z. LaNiO3 modified with Ag nanoparticles as an efficient bifunctional electrocatalyst for rechargeable zinc–air batteries. Front. Mater. Sci. 2019, 13, 277–287. [Google Scholar] [CrossRef]
- Ye, C.; Liu, J.; Zhang, Q.; Jin, X.; Zhao, Y.; Pan, Z.; Chen, G.; Qiu, Y.; Ye, D.; Gu, L.; et al. Activating Metal Oxides Nanocatalysts for Electrocatalytic Water Oxidation by Quenching-Induced Near-Surface Metal Atom Functionality. J. Am. Chem. Soc. 2021, 143, 14169–14177. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.-A.; Xue, X.; Yin, H.; Cheng, W.; Cheng, J.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Chen, H.; Yang, M.; Yang, D.; Li, H.; Lin, Z. Rechargeable Zn-Air Batteries with Outstanding Cycling Stability Enabled by Ultrafine FeNi Nanoparticles-Encapsulated N-Doped Carbon Nanosheets as a Bifunctional Electrocatalyst. Nano Lett. 2021, 21, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Zhou, S.; Zang, J.; Gao, H.; Tian, X.; Tian, P.; Song, S.; Wang, Y. Deflagration method synthesizing N, S co-doped oxygen-functionalized carbons as a bifunctional catalyst for oxygen reduction and oxygen evolution reaction. Carbon 2021, 181, 234–245. [Google Scholar] [CrossRef]
- Wang, M.; Lei, X.; Hu, L.; Zhang, P.; Hu, H.; Fang, J. High-performance Waste Biomass-derived Microporous Carbon Electrocatalyst with a Towel-like Surface for Alkaline Metal/air batteries. Electrochim. Acta 2017, 250, 384–392. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wang, X. Bimetallic ZIFs derived 3D acetylene black loading La2O3/Co bifunctional ORR/OER catalysts. Appl. Surf. Sci. 2023, 610, 155551. [Google Scholar] [CrossRef]
- Joshi, A.; Sood, P.; Gaur, A.; Rani, D.; Madaan, V.; Singh, M. Improved OER performance of an Anderson-supported cobalt coordination polymer by assembling with acetylene black. J. Mater. Chem. A 2022, 10, 12805–12810. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Huang, Q.; Yang, W.; Tian, C.; Liu, Y.; Zhao, W.; Lu, X.; Cao, Z.; Wang, C.; Xie, Z. The Role of the A-Site Cation on the Bifunctional Electrocatalytic Activities of Ln0.5Sr0.5CoO3-δ (Ln = La, Pr and Sm) for Rechargeable Zinc–Air Batteries. Catalysts 2023, 13, 483. https://doi.org/10.3390/catal13030483
Li P, Huang Q, Yang W, Tian C, Liu Y, Zhao W, Lu X, Cao Z, Wang C, Xie Z. The Role of the A-Site Cation on the Bifunctional Electrocatalytic Activities of Ln0.5Sr0.5CoO3-δ (Ln = La, Pr and Sm) for Rechargeable Zinc–Air Batteries. Catalysts. 2023; 13(3):483. https://doi.org/10.3390/catal13030483
Chicago/Turabian StyleLi, Pengzhang, Qing Huang, Wei Yang, Chuanjin Tian, Yumin Liu, Wenyan Zhao, Xiaojie Lu, Zhenbao Cao, Changan Wang, and Zhipeng Xie. 2023. "The Role of the A-Site Cation on the Bifunctional Electrocatalytic Activities of Ln0.5Sr0.5CoO3-δ (Ln = La, Pr and Sm) for Rechargeable Zinc–Air Batteries" Catalysts 13, no. 3: 483. https://doi.org/10.3390/catal13030483
APA StyleLi, P., Huang, Q., Yang, W., Tian, C., Liu, Y., Zhao, W., Lu, X., Cao, Z., Wang, C., & Xie, Z. (2023). The Role of the A-Site Cation on the Bifunctional Electrocatalytic Activities of Ln0.5Sr0.5CoO3-δ (Ln = La, Pr and Sm) for Rechargeable Zinc–Air Batteries. Catalysts, 13(3), 483. https://doi.org/10.3390/catal13030483





