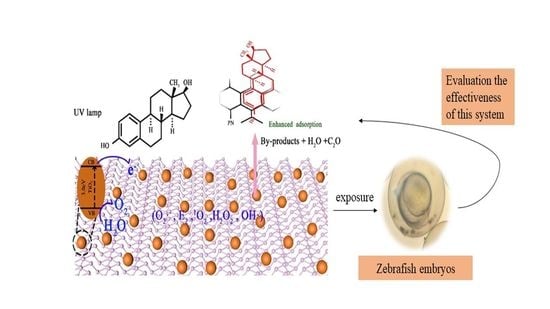
Ultrasonic Preparation of PN for the Photodegradation of 17β-Estradiol in Water and Biotoxicity Assessment of 17β-Estradiol after Degradation
Abstract
1. Introduction
2. Results
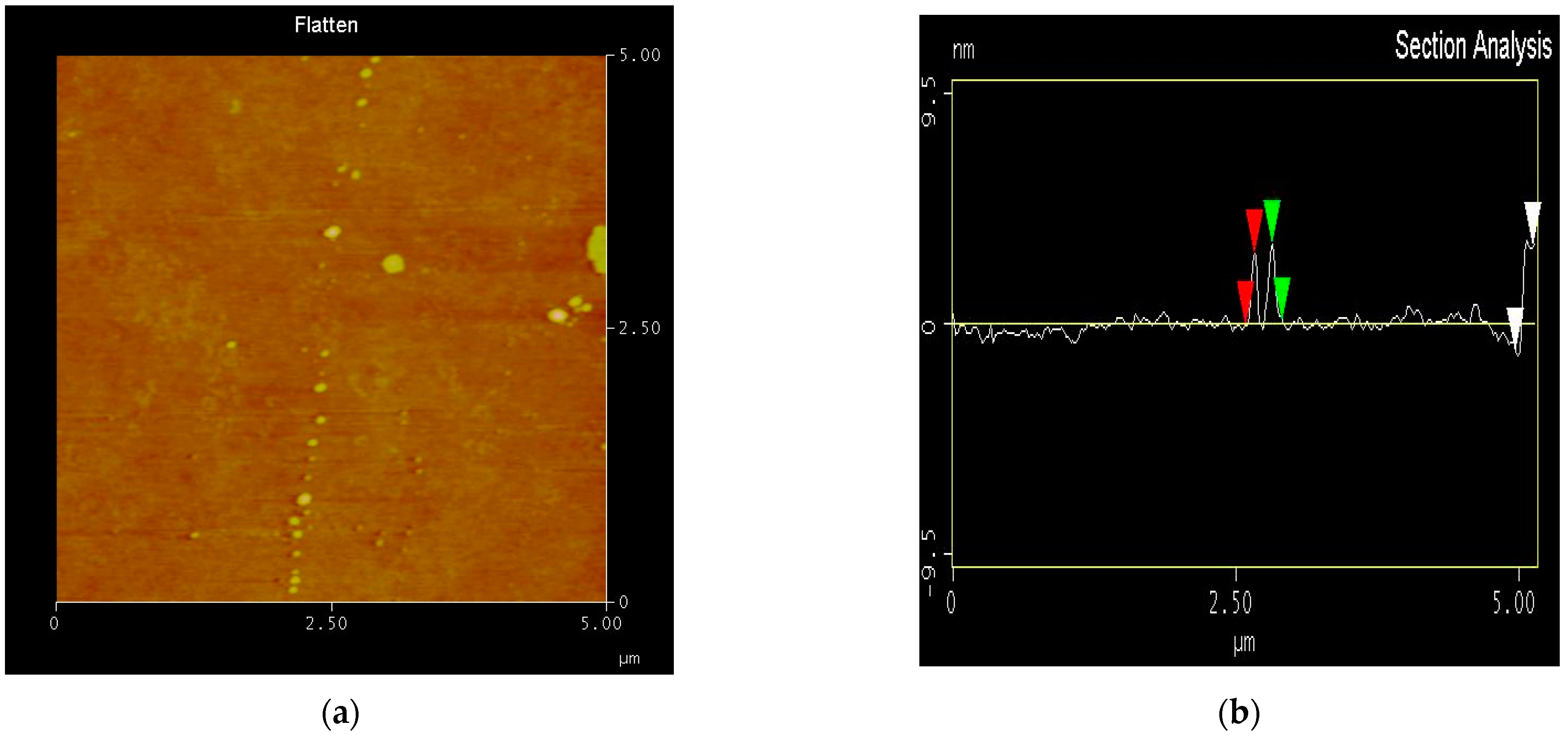
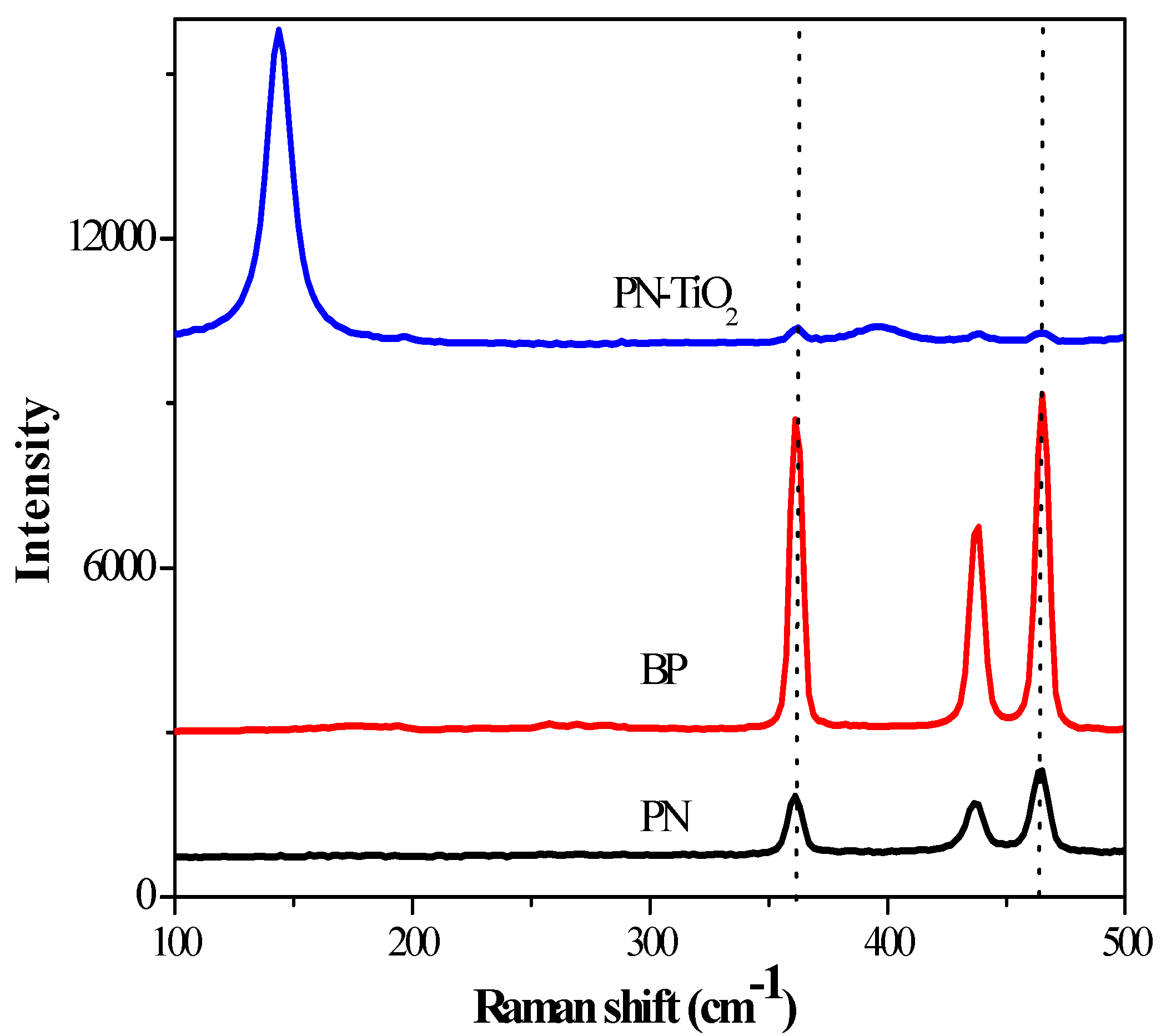
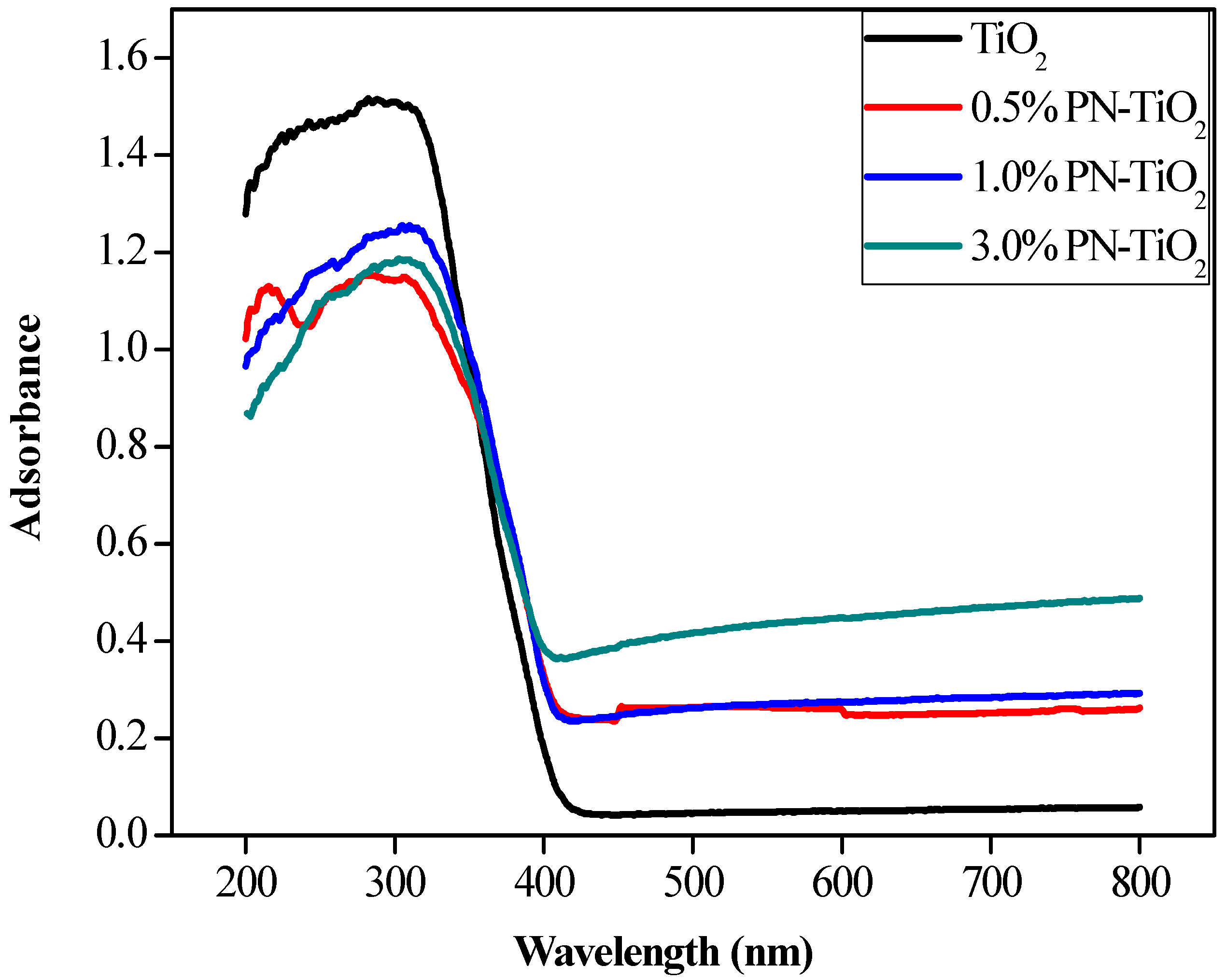
2.1. Material Characterization
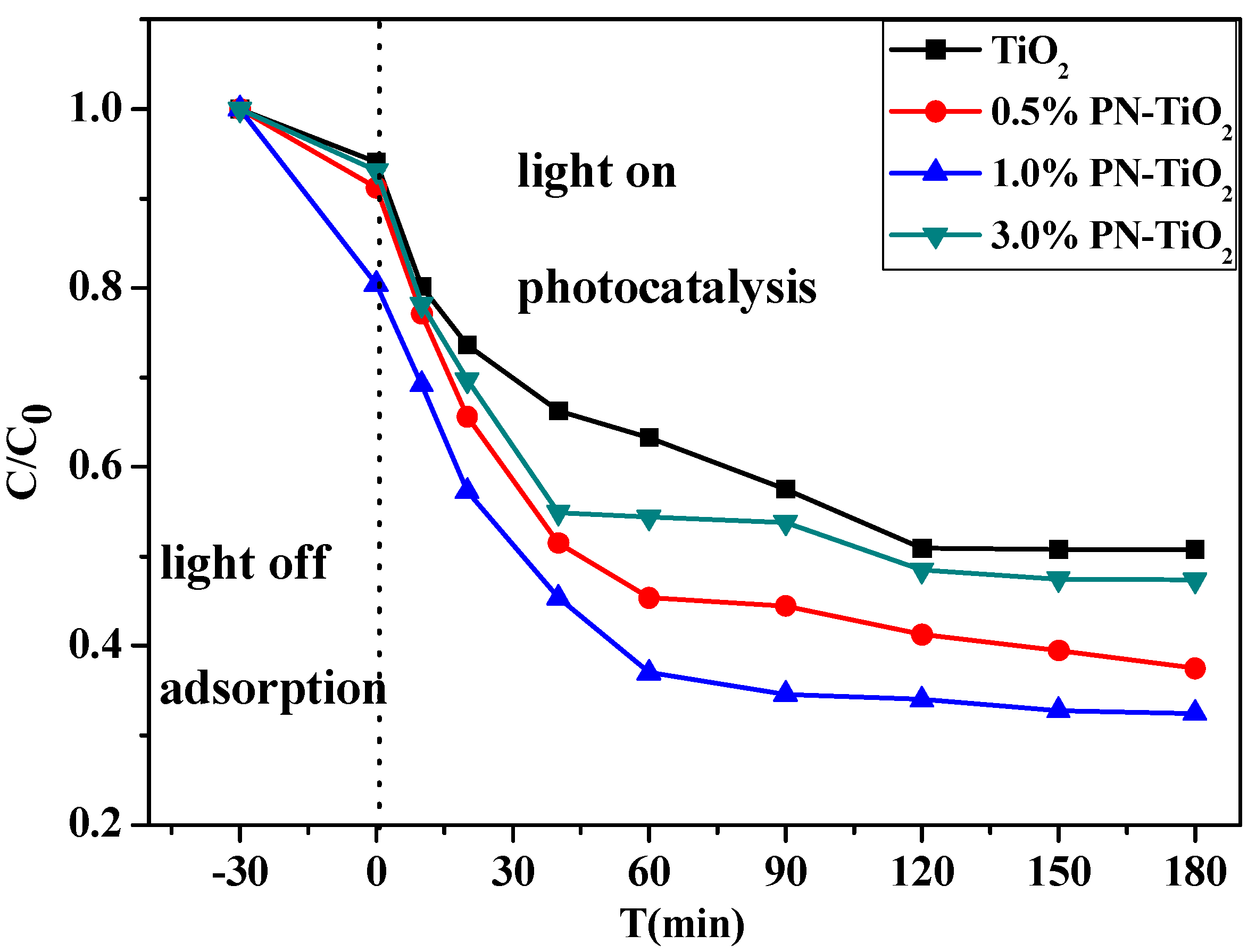
2.2. Photodegradation Performance of PN-TiO2
2.2.1. Effects of Different Levels of PN Content
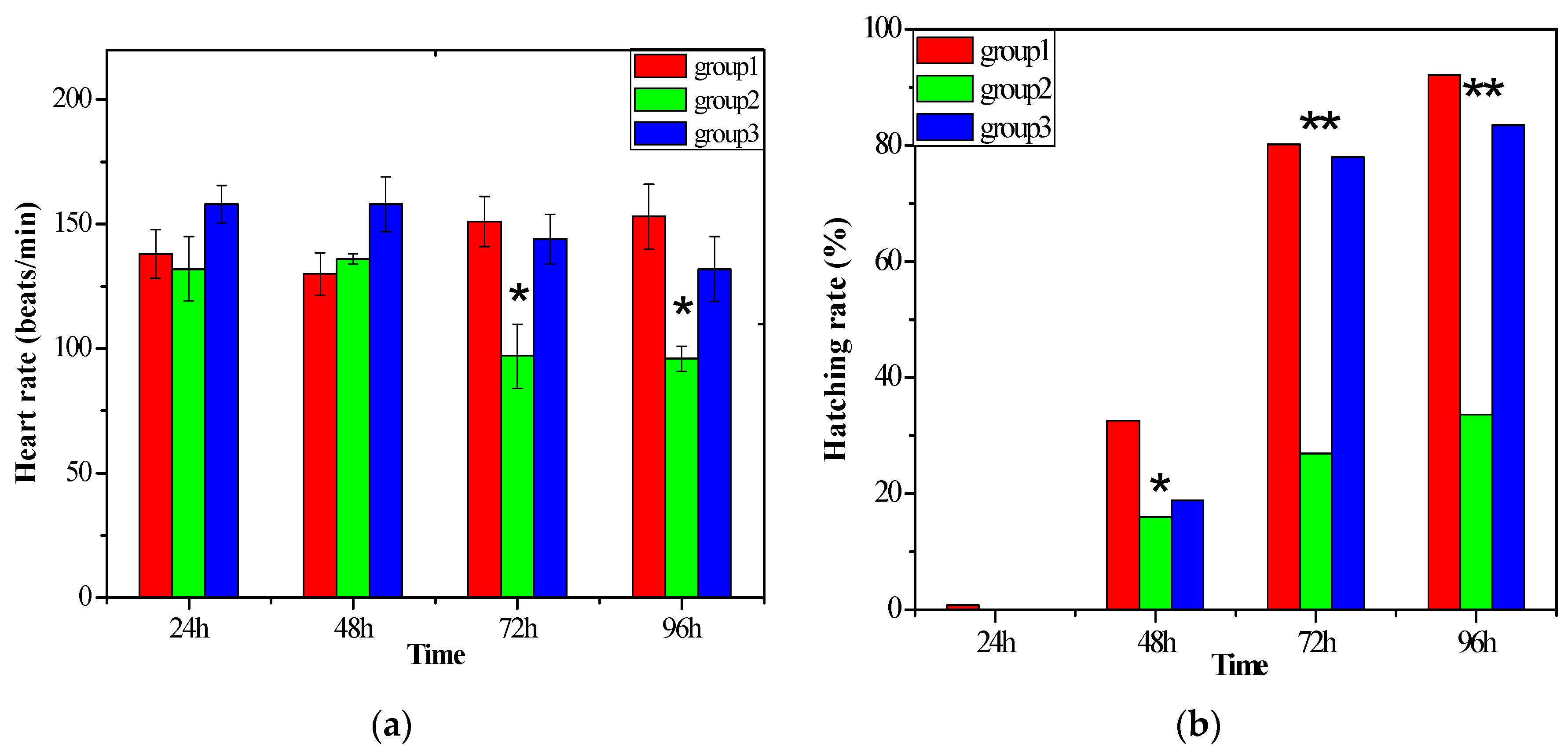
2.2.2. The Biotoxicity of the Water after Degradation
3. Discussion
3.1. Comparative Assessment of Degradation Performance
3.2. Significance of this Study
4. Material and Methods
4.1. Chemical Reagents
4.2. Preparation of Phosphorene Nanosheets (PN) and Phosphorene—TiO2 Composites (PN-TiO2)
4.3. Photodegradation Reaction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Finn, R.S.; Aleshin, A.; Slamon, D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Pendse, S.; Maertens, A.; Rosenberg, M.; Roy, D.; Fasani, R.; Vantangoli, M.; Madnick, S.; Boekelheide, K.; Fornace, A.; Odwin, S.-A. Information-dependent enrichment analysis reveals time-dependent transcriptional regula-tion of the estrogen pathway of toxicity. Arch. Toxicol. 2017, 91, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Coulter, S.; Knudsen, G.; Dunnick, J.; Kissling, G.; Birnbaum, L. Disruption of estrogen ho-meostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 2016, 298, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Biegel, L.B.; Hirshfield, A.N.; O’Connor, J.C.; Ladics, G.S.; Cook, J.C.; Frame, S.R.; Flaws, J.A.; Elliott, G.S.; Silbergeld, E.K.; Van Pelt, C.S.; et al. 90-Day Feeding and One-Generation Reproduction Study in Crl:CD BR Rats with 17β-Estradiol. Toxicol. Sci. 1998, 44, 116–142. [Google Scholar] [CrossRef] [PubMed]
- Diamante, G.; Menjivar-Cervantes, N.; Leung, M.S.; Volz, D.C.; Schlenk, D. Contribution of G protein-coupled estrogen receptor 1 (GPER) to 17β-estradiol-induced develop-mental toxicity in zebrafish. Aquat. Toxicol. 2017, 186, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Angus, R.A.; Stanko, J.; Jenkins, R.L.; Watson, R.D. Effects of 17α-ethynylestradiol on sexual development of male western mosquitofish (Gambusia affinis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, X.; Zhang, X.; Wu, D.; Leng, S. Biodegradation of 17β-estradiol by Bacterial Co-culture Isolated from Manure. Sci. Rep. 2018, 8, 3787. [Google Scholar] [CrossRef]
- Xiong, W.; Peng, W.; Liang, R. Identification and genome analysis of Deinococcus actinosclerus SJTR1, a novel 17β-estradiol degradation bacterium. 3 Biotech 2018, 8, 433. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, P.; Liu, D.; Gao, R.; Shao, H.; Zhao, H.; Ma, Z.; Wang, D.; Huo, H. Degradation characteristics and metabolic pathway of 17β-estradiol (E2) by Rhodococcus sp. DS201. Biotechnol. Bioprocess Eng. 2016, 21, 804–813. [Google Scholar] [CrossRef]
- Mazlan, W.S.; Mokhtar, H.; Saâ, N.; Tajuddin, R.M. Fabrication of ultrafiltration membrane using addtive to extract hormone from poultry wastewater. J. Teknol. 2016, 78, 121–126. [Google Scholar] [CrossRef]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Fate and impacts of triclosan, sulfamethoxazole, and 17β-estradiol during nutrient recovery via ion ex-change and struvite precipitation. Environ. Sci. Water Res. Technol. 2017, 3, 1109–1119. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Liu, Y.-G.; Zeng, G.-M.; Xiao, F.-Y.; Hu, X.-J.; Hu, X.; Wang, H.; Li, T.-T.; Zhou, L.; Tan, X.-F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Hu, X.; Zeng, G.; Hu, X.; Liu, S.; Liu, S.; Huang, B.; Li, M. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem. Eng. J. 2017, 308, 597–605. [Google Scholar] [CrossRef]
- Kim, S.; Cho, H.; Joo, H.; Her, N.; Han, J.; Yi, K.; Kim, J.-O.; Yoon, J. Evaluation of performance with small and scale-up rotating and flat reactors; photocatalytic degradation of bisphenol A, 17β–estradiol, and 17α–ethynyl estradiol under solar irradiation. J. Hazard. Mater. 2017, 336, 21–32. [Google Scholar] [CrossRef]
- Kovacic, M.; Kopcic, N.; Kusic, H.; Bozic, A.L. Solar driven degradation of 17β-estradiol using composite photocatalytic materials and artificial irradiation source: Influence of process and water matrix parameters. J. Photochem. Photobiol. A Chem. 2018, 361, 48–61. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Basic principles, mechanism, and challenges of photocatalysis. In Nanocomposites for Visible Light-induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–40. [Google Scholar]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Tahir, K.; Ahmad, A.; Li, B.; Nazir, S.; Khan, A.; Nasir, T.; Khan, Z.; Naz, R.; Raza, M. Visible light photo cata-lytic inactivation of bacteria and photo degradation of methylene blue with Ag/TiO2 nanocomposite prepared by a novel method. J. Photochem. Photobiol. B Biol. 2016, 162, 189–198. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.; Hidalgo, M.; Ciambelli, P.; Navío, J. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Bagheri, S.; Mansouri, N.; Aghaie, E. Phosphorene: A new competitor for graphene. Int. J. Hydrog. Energy 2016, 41, 4085–4095. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene–the two-dimensional black phosphorous: Properties, synthesis and applications. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Shao, W.; Zhang, X.; Chen, S.; Sun, X.; Zhang, Q.; Luo, Y.; Xie, Y. Optically Switchable Photocatalysis in Ultrathin Black Phosphorus Nanosheets. J. Am. Chem. Soc. 2018, 140, 3474–3480. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.T.; Liu, J.; Yan, X.; Oh, W.D.; Lim, T.T. Low-temperature synthesis of graphene/Bi2Fe4O9 composite for synergistic adsorp-tion-photocatalytic degradation of hydrophobic pollutant under solar irradiation. Chem. Eng. J. 2015, 262, 1022–1032. [Google Scholar] [CrossRef]
- Zatloukalová, K.; Obalová, L.; Koči, K.; Čapek, L.; Matěj, Z.; Šnajdhaufová, H.; Ryczkowski, J.; Słowik, G. Photo-catalytic degradation of endocrine disruptor compounds in water over immobilized TiO2 photocatalysts. Iran. J. Chem. Chem. Eng. (IJCCE) 2017, 36, 29–38. [Google Scholar]
- Mboula, V.M.; Héquet, V.; Andrès, Y.; Gru, Y.; Colin, R.; Doña-Rodríguez, J.; Pastrana-Martínez, L.; Silva, A.; Leleu, M.; Tindall, A.; et al. Photocatalytic degradation of estradiol under simulated solar light and assessment of estrogenic activity. Appl. Catal. B Environ. 2015, 162, 437–444. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.; Xiao, M.; Li, H.; Pan, X.; Jiang, F. One-step hydrothermal synthesis of surface fluorinated TiO2/reduced graphene oxide nanocomposites for photocatalytic degradation of estrogens. Mater. Sci. Semicond. Process. 2015, 40, 183–193. [Google Scholar] [CrossRef]
- Padilha, J.E.; Fazzio, A.; Da Silva, A.J.R. van der Waals Heterostructure of Phosphorene and Graphene: Tuning the Schottky Barrier and Doping by Electrostatic Gating. Phys. Rev. Lett. 2015, 114, 066803. [Google Scholar] [CrossRef]
- Hu, W.; Wang, T.; Yang, J. Tunable Schottky contacts in hybrid graphene–phosphorene nanocomposites. J. Mater. Chem. C 2015, 3, 4756–4761. [Google Scholar] [CrossRef]
- Guo, H.; Lu, N.; Dai, J.; Wu, X.; Zeng, X. Phosphorene nanoribbons, phosphorus nanotubes, and van der Waals multilayers. Journal of Physical Chemistry C 2014, 118, 14051–14059. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, S.; Chen, W.; Jia, Y. Interlayer coupling effects on electronic properties of the phosphorene/h-BN van der Walls heterostructure: A first principles investigation. Phys. B Condens. Matter 2018, 534, 51–55. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Won, J.; Son, B.-C.; Choi, S.; Kim, Y.; Park, S.Y.; Kim, H.-S.; Lee, Y.-C.; Lee, J. Stable semiconductor black phosphorus (BP)@titanium dioxide (TiO2) hybrid photocatalysts. Sci. Rep. 2015, 5, srep08691. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tomanek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Nawaz, M.; Miran, W.; Jang, J.; Lee, D.S. One-step hydrothermal synthesis of porous 3D reduced graphene ox-ide/TiO2 aerogel for carbamazepine photodegradation in aqueous solution. Appl. Catal. B Environ. 2017, 203, 85–95. [Google Scholar] [CrossRef]
- Woomer, A.H.; Farnsworth, T.W.; Hu, J.; Wells, R.A.; Donley, C.L.; Warren, S.C. Phosphorene: Synthesis, Scale-Up, and Quantitative Optical Spectroscopy. ACS Nano 2015, 9, 8869–8884. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Kishida, M. Brain Aromatase Modulates Serotonergic Neuron by Regulating Serotonin Levels in Zebrafish Embryos and Larvae. Front. Endocrinol. 2018, 9, 230. [Google Scholar] [CrossRef]
- Lee, D.H.; Jo, Y.J.; Eom, H.J.; Yum, S.; Rhee, J.S. Nonylphenol induces mortality and reduces hatching rate through increase of oxidative stress and dysfunction of antioxidant defense system in marine medaka embryo. Mol. Cell. Toxicol. 2018, 14, 437–444. [Google Scholar] [CrossRef]
- Ren, X.; Lu, F.; Cui, Y.; Wang, X.; Bai, C.; Chen, J.; Huang, C.; Yang, D. Protective effects of genistein and estradiol on PAHs-induced developmental toxicity in zebrafish embryos. Hum. Exp. Toxicol. 2012, 31, 1161–1169. [Google Scholar] [CrossRef]
- Alvarez-Corena, J.R.; Bergendahl, J.A.; Hart, F.L. Advanced oxidation of five contaminants in water by UV/TiO2: Reaction kinetics and by-products identification. J. Environ. Manag. 2016, 181, 544–551. [Google Scholar] [CrossRef]
- Li, L.; Long, Y.; Chen, Y.; Wang, S.; Wang, L.; Zhang, S.; Jiang, F. Facile synthesis of Fe/Bi2SiO5 nanocomposite with enhanced photocatalytic activity for degradation of 17β-Estradiol(E2). Solid State Sci. 2018, 83, 143–151. [Google Scholar] [CrossRef]
- Fernández, L. Impact of operating conditions on the removal of endocrine disrupting chemicals by membrane photocatalytic reactor. Environ. Technol. 2014, 35, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Vijayabalan, A.; Selvam, K.; Velmurugan, R.; Swaminathan, M. Photocatalytic activity of surface fluorinated TiO2-P25 in the degradation of Reactive Orange 4. J. Hazard. Mater. 2009, 172, 914–921. [Google Scholar] [CrossRef] [PubMed]




| Reference | Materials | C0 (L−1) * | Degradation Efficiency | Light Source | K1 * (min−1) | Removal Capacity * (mg/g) |
|---|---|---|---|---|---|---|
| [24] | Immobilized TiO2 | 44 µg | 45% (180 min) | 36 W UV | 0.003 | 0.0132 |
| [37] | TiO2 + O2 | 2 mg | 93% (25 min) | 100 W UV | 0.53 | 18.6 |
| [15] | TiO2-FeZ | 1.36 mg | 78.1% (90 min) | 450 W Xe lamp | 0.015 | 25.3 |
| [26] | FTG4 | 3 mg | 99.8% (180 min) | 20 W UV | 0.035 | Unsaturation |
| [26] | TiO2-RGO | 3 mg | 83.3% (180 min) | 20 W UV | 0.010 | 8.33 |
| [26] | P25 | 3 mg | 74.4% (180 min) | 20 W UV | 0.008 | 7.44 |
| [14] | NTT | 0.54 mg | 65% (150 min) | 3300 µW/cm2 UV | 0.007 | 3.51 |
| [38] | Fe-Bi2SiO5 | 3 mg | 99.5% (60 min) | 20 W UV | 0.069 | 5.97 |
| [39] | P25 | 1 mg | 90% (3 h) | 64 W UV | 0.013 | 1.8 |
| [25] | 4%GO-TiO2 | 1 mg | 48% (60 min) | 450 W Xe lamp | 0.011 | 24 |
| This study | PN-TiO2 | 10 mg | 67.5% | Visible/UV | 0.006 | 67.5 |
| Reference | Material | Field of Application |
|---|---|---|
| [27] | PN–GN hybrid | Perpendicular electric field |
| [28] | PN–GN hybrid | Schottky barrier diode |
| [29] | PN-MoS2 | Electric field/solar cell |
| [30] | PN-h-BN | Electric field |
| [22] | PN | Photocatalysis for DPBF |
| [31] | (more)PN-TiO2 | Photocatalysis for dye |
| This study | (less)PN-TiO2 | Photocatalysis for 17β-estradiol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, K.; Zhou, K.; Chang, C.-T. Ultrasonic Preparation of PN for the Photodegradation of 17β-Estradiol in Water and Biotoxicity Assessment of 17β-Estradiol after Degradation. Catalysts 2023, 13, 332. https://doi.org/10.3390/catal13020332
Meng K, Zhou K, Chang C-T. Ultrasonic Preparation of PN for the Photodegradation of 17β-Estradiol in Water and Biotoxicity Assessment of 17β-Estradiol after Degradation. Catalysts. 2023; 13(2):332. https://doi.org/10.3390/catal13020332
Chicago/Turabian StyleMeng, Kun, Kefu Zhou, and Chang-Tang Chang. 2023. "Ultrasonic Preparation of PN for the Photodegradation of 17β-Estradiol in Water and Biotoxicity Assessment of 17β-Estradiol after Degradation" Catalysts 13, no. 2: 332. https://doi.org/10.3390/catal13020332
APA StyleMeng, K., Zhou, K., & Chang, C.-T. (2023). Ultrasonic Preparation of PN for the Photodegradation of 17β-Estradiol in Water and Biotoxicity Assessment of 17β-Estradiol after Degradation. Catalysts, 13(2), 332. https://doi.org/10.3390/catal13020332