Abstract
A novel room-temperature stable diamagnetic nickel complex 2 was detected upon activation of Brookhart-type ethylene polymerization pre-catalyst LNiBr2 (1, L = 1,4-bis-2,4,6-trimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene) with AlMe3. Using in situ 1H, 2H, and 13C NMR spectroscopy, as well as DFT calculations, this species has been identified as an antiferromagnetically coupled homodinuclear complex [LNiII(μ-Me)(μ-CH2)NiIIL]+Br−. Its behavior in the reaction solution is characteristic of the resting state of nickel catalyzed ethylene polymerization.
Keywords:
α-diimine; nickel; trimethylaluminum; NMR; ethylene polymerization; active species; DFT calculations 1. Introduction
During the first two decades of the 21st century, Ni(II) α-diimines have remained the subject of extensive studies due to their unique ability to catalyze the formation of branched polyethylene from ethylene as the sole feedstock, as well as copolymerization of ethylene with polar monomers [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Despite a great number of publications devoted to their mechanistic investigations [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], some crucial details of the catalytic process remained unclear. For example, some data on the nature of the active species formed upon activation of α-diimine Ni(II) complexes with MAO and MMAO have been obtained. Namely, cationic Ni(II)-alkyl species—direct precursors of the ethylene polymerization active sites [29,30]—were detected in situ by NMR in the real catalyst systems LNiBr2/MAO and LNiBr2/MMAO [46].
In contrast, the nature of the Ni(II) complexes formed upon activation of LNiBr2 with AlMe3 have not yet been reported. Meanwhile, there have been several examples of using trimethylaluminum alone as an effective co-catalyst for Ni(II) α-diimines [25,34,51,54,55,56,57].
Recently, we reported the detection and characterization of a paramagnetic monovalent nickel complex [LNiI(μ-Me)2AlMe2] formed upon activation of complex 1 (Figure 1) with AlMe3 [51,53,56]. Herein, we report formation of novel diamagnetic nickel(II) species in this system in the presence of a small excess of AlMe3 (Al/Ni = 10). The new species have been characterized in detail by in situ 1H, 2H, and 13C NMR spectroscopy, and their assignment has been corroborated by quantum chemical (DFT) calculations.
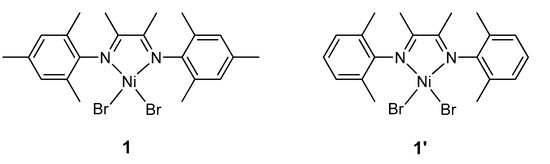
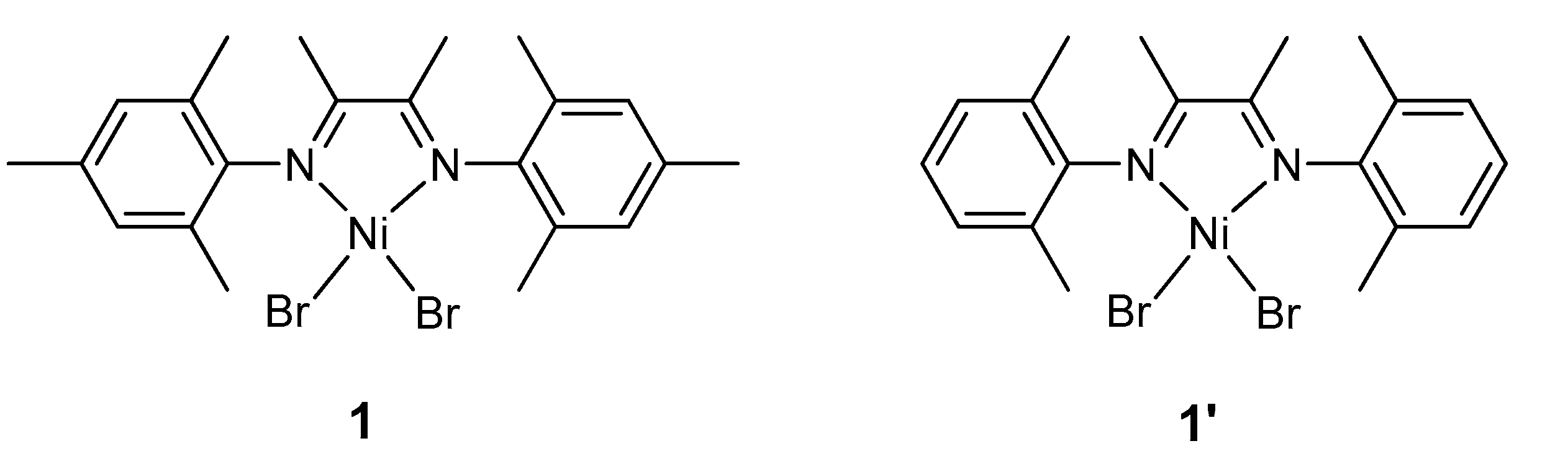
Figure 1.
Complexes 1 and 1′ studied herein.
2. Results and Discussion
2.1. The Nickel Center Spin State
It is well-known that a Ni(II) center (3d8 configuration) can exist in two spin states: paramagnetic high-spin (S = 1) and diamagnetic low-spin (S = 0). As a rule, four-coordinated Ni(II) complexes with square–planar geometry are diamagnetic, whereas distorted tetrahedral counterparts are paramagnetic (see, for example, Refs. [58,59,60,61]). The low-spin Ni(II) species display well-resolved 1H NMR resonances in the range typical for diamagnetic species. On the contrary, the high-spin Ni(II) complexes display broad and paramagnetically shifted 1H NMR peaks [62]. The paramagnetic shifts are temperature-dependent, obeying the Curie’s law (inverse proportionality with temperature). In addition, the high-spin Ni(II) species are EPR-silent in the X-band due to high zero-field splitting (D >> hν) [63].
Complexes of Ni(I) (3d9 configuration) are paramagnetic (S = 1/2) and usually display well-resolved X-band EPR resonances at g > ge due to the negative spin-orbit coupling constant. Typically, for such species, only very broad 1H NMR resonances can be detected.
Diamagnetism of nickel(I) species can also be the case due to antiferromagnetic coupling of two paramagnetic centers (S1 = S2 = 1/2; SΣ = S1 + S2 = 0 ground state); some diamagnetic dinuclear NiI-NiI complexes have been reported [64]. Besides, diamagnetic state can be realized for Ni(I) complexes with α-diimine anion-radical (L(∙−)) as a ligand. In this case, antiferromagnetic coupling between the Ni(I) center (S = 1/2) and anion-radical (L(∙−)) (S = 1/2) results in zero overall spin. In the latter two cases, the temperature dependence of the paramagnetic shifts of the 1H NMR resonances does not obey Curie’s law; these shifts increase with temperature in line with the growing population of the paramagnetic excited state (S = 1).
Complex 2, reported herein, can be obtained via the reaction of 1 with a relatively small excess of AlMe3 (10 equiv.) in toluene at room temperature. Note that 2 displays good thermal stability at room temperature and rapidly decomposes only at T > 40 °C. Nevertheless, our numerous attempts to isolate 2 from the solution for its X-ray structural characterization have remained unsuccessful. Fortunately, 2 had rather informative 1,2H and 13C NMR spectra.
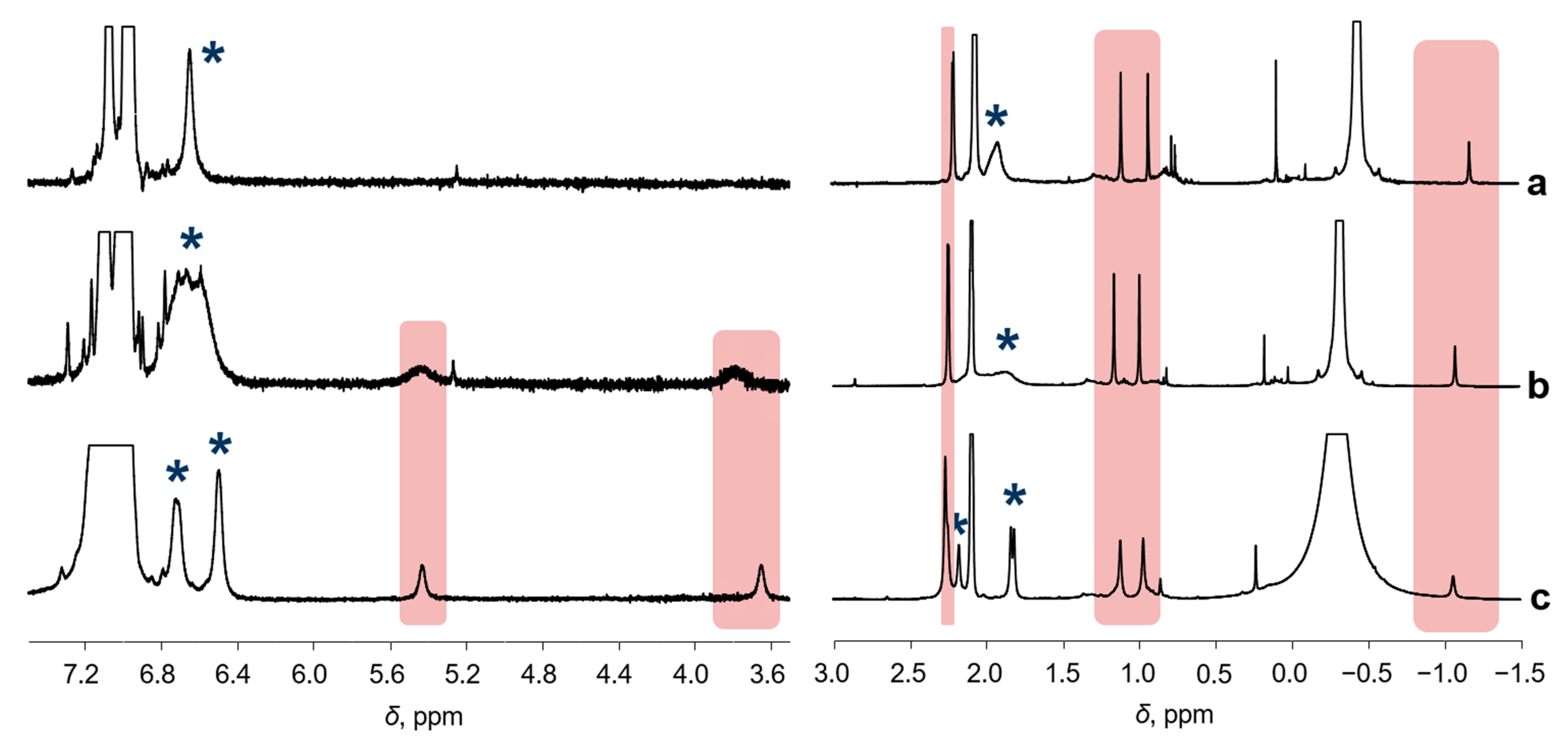
The 1H NMR spectra of 2 recorded at different temperatures are shown in Figure 2. The resonances of 2 are spread over a range of 8 … −1.5 ppm, which is typical for diamagnetic species, their chemical shifts demonstrating very weak temperature dependence. It is worth noting that some resonances of 2 in the range of 6.4 … 6.8 and 1.6 … 2.3 ppm, marked by asterisks, coalesce at 40 °C. The reason for such behavior will be discussed below.
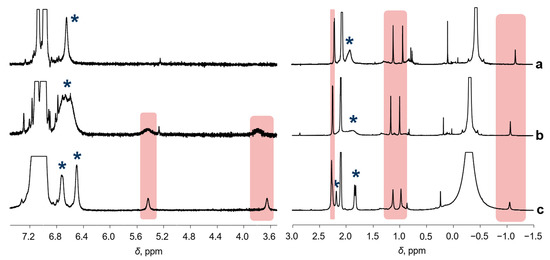
Figure 2.
The 1H NMR spectra (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM) of the sample 1/AlMe3, recorded at different temperatures: 40 °C (a), 25 °C (b), and −20 °C (c). Asterisks mark the resonances that coalesce at 40 °C. Other resonances of 2 are highlighted.
2.2. The 1H NMR Resonances of the α-Diimine Ligand of 2
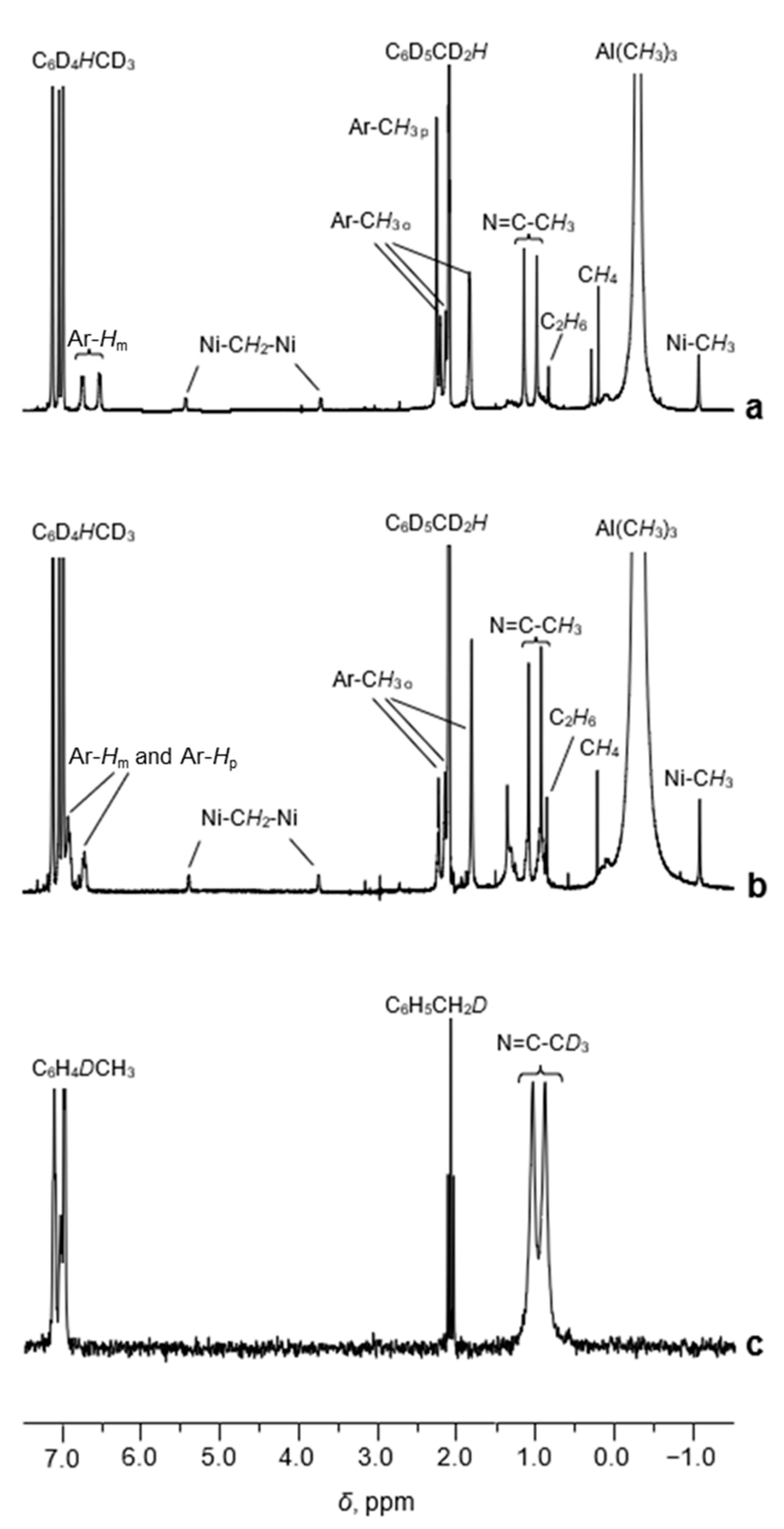
The 1H NMR spectrum of the sample 1/AlMe3 (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM) recorded at 0 °C displays narrow well-resolved resonances of the α-diimine protons of the ligand of 2 (Figure 3a).
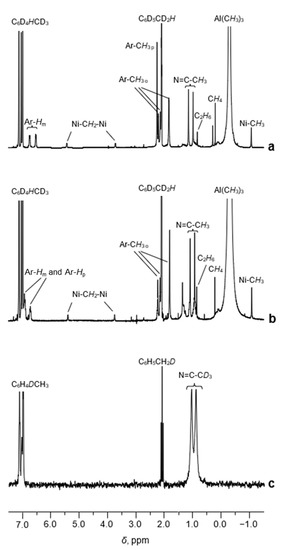
Figure 3.
The 1H NMR spectra (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM, 0 °C) of the samples 1/AlMe3 (a) and 1′/AlMe3 (b). The 2H NMR spectrum of the sample 1D/AlMe3 (toluene, [Al]/[Ni] = 10/1, CNi = 5 mM) recorded at 0 °C (c).
A singlet at δ 2.27 corresponds to para-Me-substituents at the aromatic rings of 2. Indeed, the 1H NMR spectrum of the sample 1′/AlMe3 (α-diimine ligand of 1′ contains no para-methyl substituents, Figure 1) displays no such resonance (Figure 3b). Two singlets at δ 0.98 and δ 1.14 are assigned to the methyl protons of the CH3-C=N moieties. To corroborate this assignment, the 2H NMR spectrum of the sample 1D/AlMe3 was recorded (toluene, [Al]/[Ni] = 10/1, CNi = 5 mM, 1D—complex 1, selectively enriched (ca. 86%) by 2H isotope in the Me-C=N moiety). The corresponding 2H resonances of the CD3-C=N groups are observed at δ 0.90 and δ 1.05, respectively (Figure 3c), witnessing a small isotopic shift compared to the 1H peaks of the non-labeled prototype.
The resonances in the range 6.5−6.8 ppm belong to the aromatic protons of the α-diimine ligand, whereas four broadened singlets at 2.21, 2.14, 1.83, and 1.82 ppm can be assigned to the o-Me protons (Figure 3a). The integral intensities of these resonances are in agreement with our assignment (Table S1, SM).
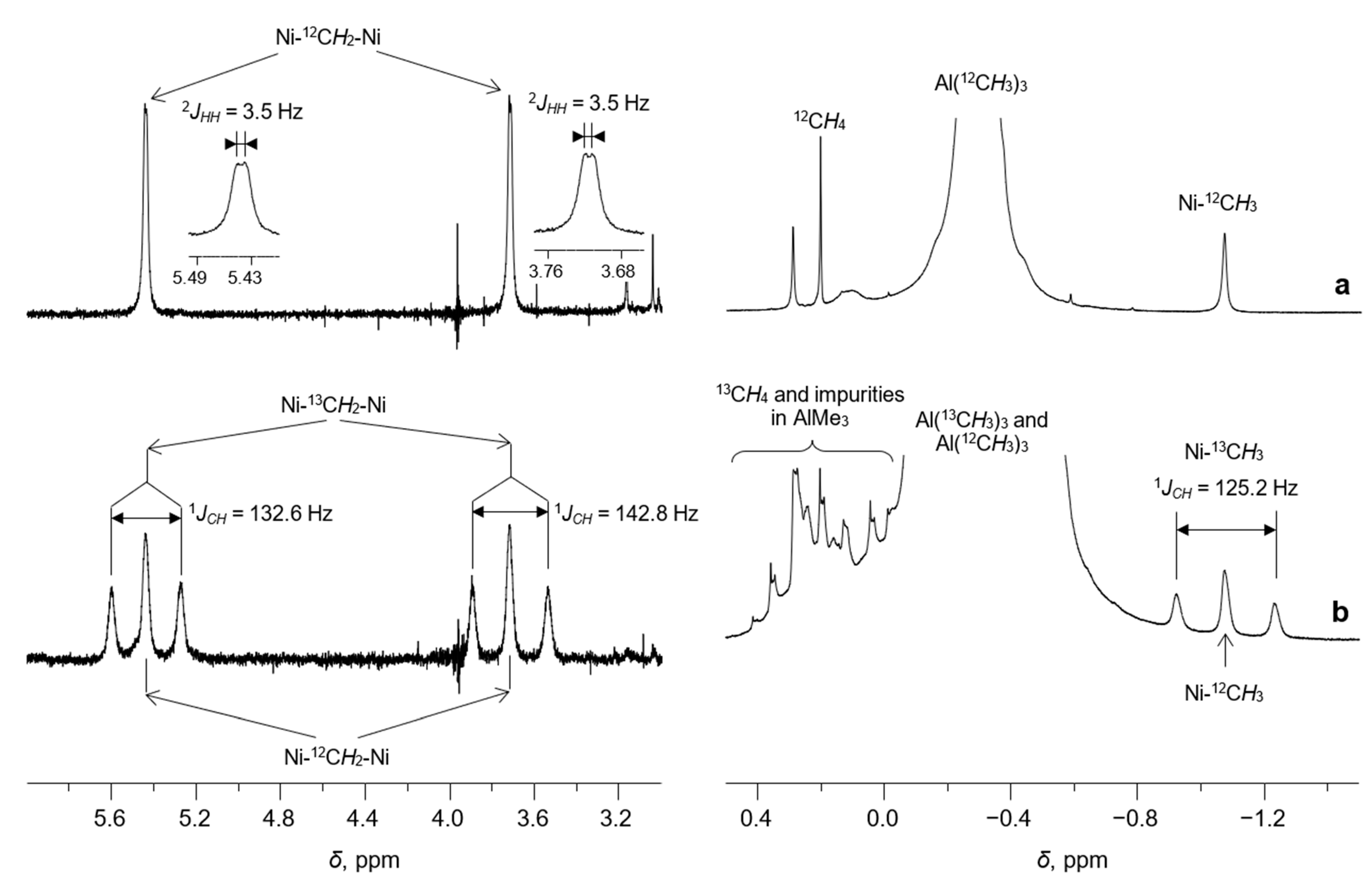
Besides the resonances of the α-diimine ligand, the 1H NMR spectrum of 1/AlMe3 (Figure 3a and Figure 4a) displays three additional signals at δ 5.44 (br d, 2JHH = 3.5 Hz), δ 3.72 (br. d, 2JHH = 3.5 Hz), and δ −1.07 (s). The last one is characteristic of the protons of Me-group, directly bonded to the Ni(II) center (see, for example, Ref. [28]). This assignment was confirmed by 13C and 1H NMR (Figure 4 and Figure 5) of the sample 1/AlMe3* containing 13C-enriched (ca. 54 at.%) trimethylaluminum and 1H-13C hxdeptbiqf NMR of 1/AlMe3 one (Figure S2). The 1H NMR spectrum of 1/AlMe3* displayed a doublet at δ −1.07 with 1JCH = 125.2 Hz (Figure 4a,b), with the corresponding singlet at in dept135 13C NMR being found at δ −25.2 (Figure 5). The corresponding cross-peak in 1H-13C NMR was also observed (Figure S2 in SM). The integral intensity of 1H NMR resonance at δ −1.07 indicates that the molecule of 2 contains two α-diimine ligands per Ni-Me group.
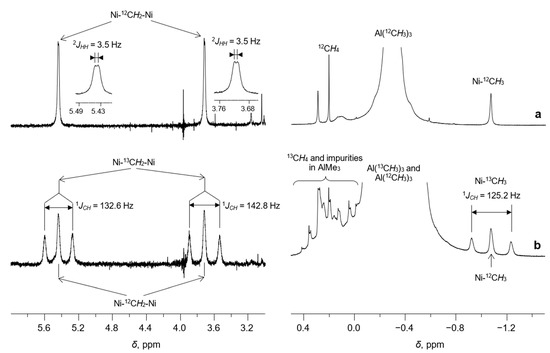
Figure 4.
Expanded regions (6.0…3.0 and 0.5…−1.5 ppm) of the 1H NMR spectra (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM, 0 °C) of the samples 1/AlMe3 (a) and 1/AlMe3* (b). AlMe3* is a trimethylaluminum enriched by 13C isotope (ca. 54 at.%).
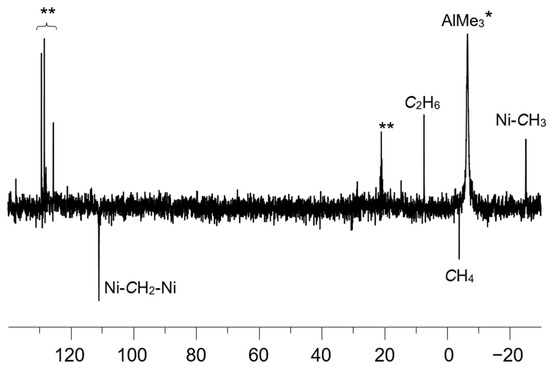
Figure 5.
dept135 13C NMR spectrum (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM, 0 °C) of the sample 1/AlMe3*. AlMe3* is a trimethylaluminum enriched by 13C isotope (ca. 54 at.%). Doubled asterisks mark solvent (toluene-d8) resonances.
Each of the remaining two resonances at δ 5.44 (br. d, 2JHH = 3.5 Hz, 1H) and δ 3.72 (br. d, 2JHH = 3.5 Hz, 1H) has an integral intensity corresponding to one hydrogen atom (Figure 4a). The 1H-1H COSY NMR spectrum of 2 displays a couple of cross-peaks (Figure S1 in SM), corresponding to spin-spin coupling between these protons.
Using 13C-enriched trimethylaluminum instead of Al(12CH3)3 leads to the appearance of two additional doublets at δ 5.44 and δ 3.72 in 1H NMR due to one-bond 13C-1H spin-spin coupling 1JCH = 132.6 Hz and 1JCH = 142.8 Hz, respectively (Figure 4a,b). Note that broadened doublets δ 5.44 (1JHH = 3.5 Hz) and δ 3.72 (1JHH = 3.5 Hz) are also observed due to the presence of Al(12CH3)3 in partially labeled sample.
The resonance of the CH2-group with an integral intensity equal to those of Ni-CH3 was detected in the 13C{1H} dept135 NMR spectrum of the sample 1/AlMe3* at δ 111.1 (Figure 5). The cross peaks in 2D 1H,13C NMR clearly indicated the correlation of 13C resonance at δ 111.1 and two 1H resonances at δ 5.44 and δ 3.72, respectively (Figure S2 in SM).
Only the initial α-diimine ligand containing no 13C-lables was recovered after 1/AlMe3* sample hydrolysis with H2O, followed by extraction with Et2O, and drying. Therefore, the resonances at δ 5.44 and δ 3.72 in the 1H NMR spectrum and at δ 111.1 in the 13C NMR one undoubtedly belong to the CH2-group bound to the Ni center (or centers) rather than to the α-diimine ligand. The 2JHH coupling constant of 3.5 Hz is lower than expected for heminal protons at sp3 hybrid carbon atoms, but reasonably higher than those for heminal protons at sp2 carbon atoms [65].
Further, the 13C NMR chemical shift of the CH2 group (δ 111.1) is not characteristic of nickel(II)-bonded sp3-carbons of Ni-CH2-R moiety (such carbons display 13C NMR resonances typically in the range of 10 … 20 ppm; see ref. [38,59]). Unfortunately, 13C NMR data on the species containing Ni=CH2 moieties are lacking, which complicates speculating on the characteristic values of 13C chemical shifts of the sp2 carbons directly bonded to the Ni center. However, a number of nickel(II) complexes of chelating N-heterocyclic carbenes are well-known (see Refs. [66,67], and references therein). For these compounds, 13C NMR chemical shifts of the sp2 carbons directly bonded to the Ni center fall within the range from 180…200 ppm. Therefore, the value of δ 111.1 might be tentatively attributed to the carbon of Ni-CH2 moiety with the hybridization intermediate between sp3 and sp2.
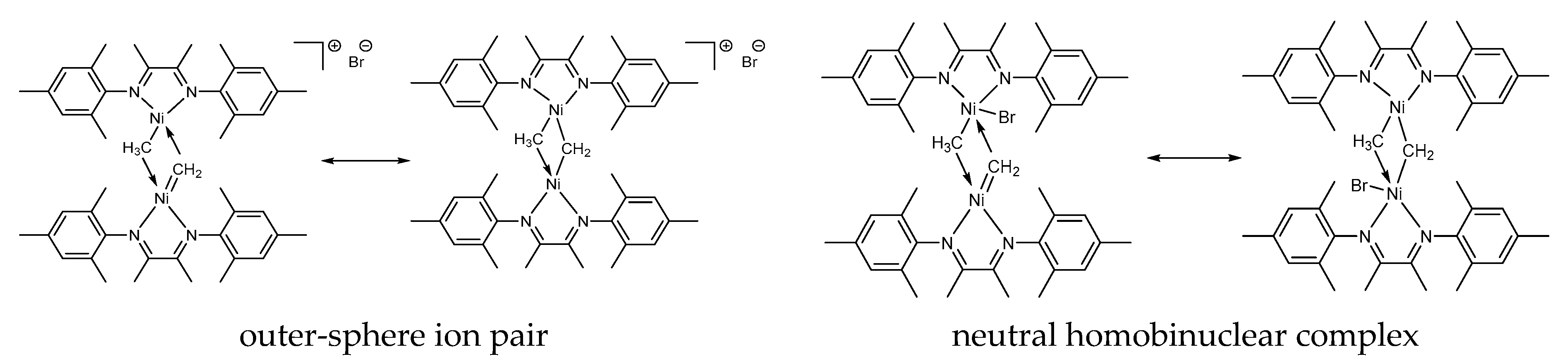
It is reasonable to conclude that 2 is a dinuclear Ni(II)-Ni(II) complex rather than mononuclear one. Indeed, in the case of mononuclear Ni(II) species, six-coordinated Ni(II) center supported by two bidentate α-diimine ligands, one methyl, and one CH2-group does not have square-planar geometry and thus would not display the 1H NMR spectrum characteristic of diamagnetic species. We assume that complex 2 may be represented as alternative resonance structures differing in the mode of Br ligation (outer-sphere or inner-sphere, Figure 6). To discriminate between these alternatives, quantum-chemical calculations have been performed (see below).
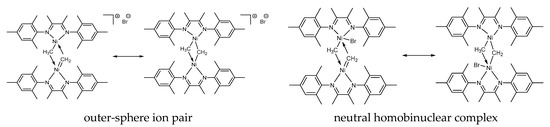
Figure 6.
Proposed resonance structures for 2.
2.3. DFT Calculations
To discriminate between the two possible structures of 2 (Figure 6), we have calculated the geometries and free energies of the bridged homodinuclear complex at the UB3LYP-D3/def2-TZVPP level of theory, which was previously shown to satisfactorily predict the gaps between different spin states of first-row transition metal complexes [68,69,70]. Crucially, starting from the draft structure [L(Me)NiII(μ-CH2)NiII(Br)L], optimization led to the doubly bridged ion pairs of the type [LNiII(μ-Me)(μ-CH2)NiIIL]+Br−, thus favoring the assignment of 2 to the ion-pair structure (Figure 6). Singlet (S = 0) appeared to be 11.8 kcal/mol lower in energy than its triplet (S = 1) counterpart (for the energies, molecular models and Cartesian coordinates see SM), which is consistent with the diamagnetic nature of species 2 as evident from NMR data.
2.4. Temperature Behavior of the Ortho-Methyl and Meta-H Resonances
The molecule of 2 is not symmetric, so magnetic non-equivalence of the ligand protons is expected. Hindered rotation of the mesityl moieties around the C-N bound leads to a number of resonances of non-equivalent o-methyl and m-H resonances in the 1H NMR at low temperatures (namely at −20 and 0 °C; Figure 2c and Figure 3a). Sample heating partially unfreezes rotation around the N-C bond, leading to slow or moderate exchange at 25 °C; it causes dramatic broadening of the resonances mentioned above. At T = 40 °C, the m-H resonances collapse, giving rise to a narrow singlet indicative of fast exchange. Further temperature increase leads to decomposition of 2, which prevents detection of narrow resonances of the o-Me groups.
2.5. The Role of 2 in Catalytic Process
It is interesting to establish the role of 2 in ethylene polymerization. The real catalytic experiments in autoclave usually require using high excess of co-catalyst (Al/Ni = 500 or more), which in additions acts as a scavenger. As an example, the results of the ethylene polymerization over 1/AlMe3 (Al/Ni = 500) catalyst system are summarized in Table 1 [51].

Table 1.
Data of the ethylene polymerization over 1/AlMe3 catalyst system a.
Unfortunately, under these conditions the Ni(II) species are not stable, undergoing rapid reduction into the corresponding Ni(I) counterparts (neutral heterobinuclear complexes of [LNiI(μ-Me)2AlMe2], [51]) predominating in the resulting reaction solution. That is why we have performed the following NMR-tube catalytic experiment instead. First, species 2 was generated in the 1/AlMe3 sample (toluene-d8, [Al]/[Ni] = 10/1, CNi = 5 mM). Then, ethylene was bubbled through the sample during 1 min at 0 °C, and NMR and EPR spectra were recorded right after. This was repeated five times.
According to the 1H NMR and EPR data, after each bubbling ethylene was completely polymerized, the concentration of 2 decreased, and EPR-resonances of Ni(I) species grew up (Figures S3 and S4 in SM). After the 3rd cycle, NMR resonances of 2 disappeared completely, but the sample kept its ethylene polymerization activity. This is not surprising, given the reported ability of monovalent nickel species ([LNiI(μ-Me)2AlMe2]) to give rise to polymerization-active species in the presence of excess of ethylene [51].
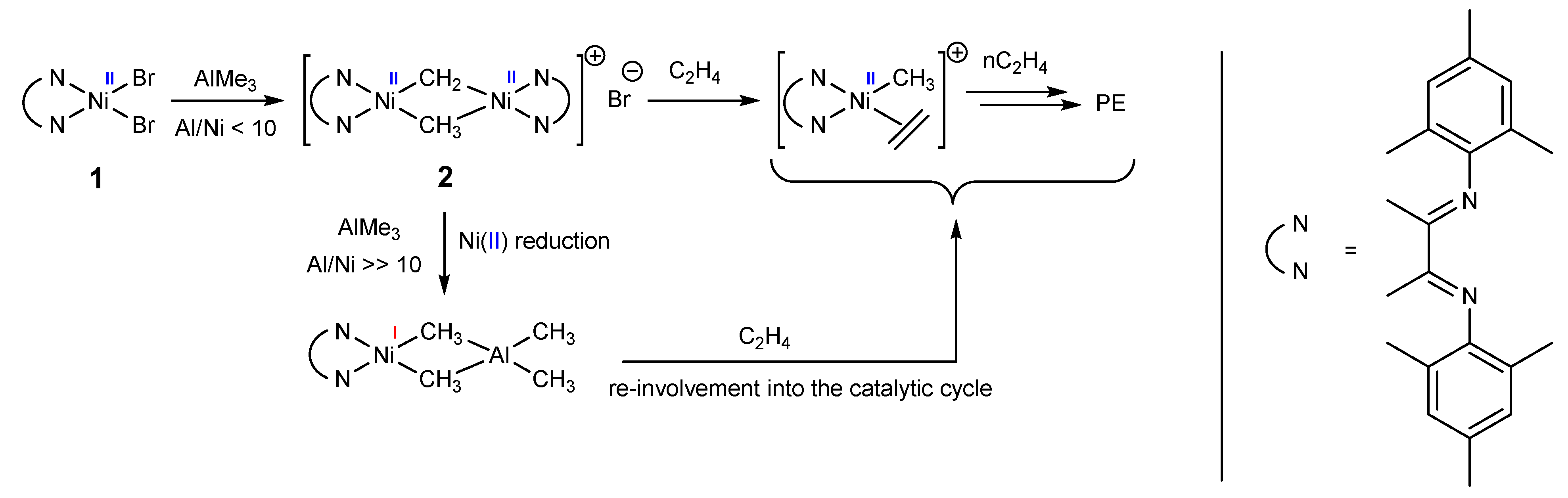
Altogether, 2 can be assigned to a resting state of the true catalytically active species, yet currently, it is not clear whether 2 converts into the active species directly or through monomeric nickel(I) species of the type [LNiI(μ-Me)2AlMe2]. Possible transformations of the nickel species in the catalyst system 1/AlMe3 are presented in Scheme 1.
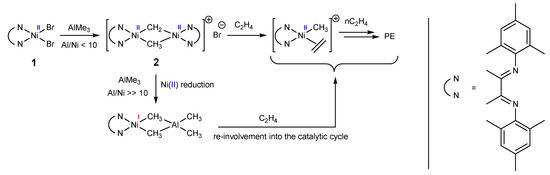
Scheme 1.
Possible transformations of the nickel species in the catalyst system 1/AlMe3.
3. Materials and Methods
3.1. General Procedures
Toluene-d8 and 1.2-difluorobenzene (Aldrich, St. Louis, MO, USA) were dried over molecular sieves (4 Å), degassed in a vacuum, and stored under dry argon. Methylene chloride, CDCl3, and CD2Cl2 (Aldrich) were dried over P2O5, distilled, and stored under dry argon. Hexane was stored over molecular sieves (4 Å), purified by refluxing over sodium-benzophenone and distilled in dry argon. AlMe3 (neat), 1,2-butanedione, 2,4,6-trimethylaniline, 2,6-dimethylaniline, NiBr2(DME), D2O (99 at.% of D), and CH3OD (99 at.% of D) were purchased from Aldrich and used without preliminary purification. Al(13CH3)3 was prepared from 13C-labelled CH3I (Aldrich) by sequential treatments with Al metal at 80 °C and Na metal in C12H26 at 100 °C and isolated by vacuum distillation [71]. All operations with oxygen-sensitive and moisture-sensitive compounds were carried out under dry argon atmosphere by using standard Schlenk techniques (synthesis of nickel complexes) or glovebox (preparing the samples for NMR and EPR experiments).
3.2. NMR and EPR Spectra Registration
1H, 2H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz NMR spectrometer (Rheinstetten, Germany) in 5 mm (o.d.) NMR tubes at 400.130, 61.422, and 100.613 MHz, respectively. Typical operating conditions for 1H NMR measurements were as follows: spectral width 4–5 kHz for diamagnetic and 40 kHz for paramagnetic compounds; spectrum accumulation frequency 0.25–0.4 Hz; 32–64 transients, 90° pulse at 12.0 μs. Operating conditions for 2H NMR experiment: spectral width 1 kHz; spectrum accumulation frequency 0.5 Hz; 8000 transients, 90° pulse at 6.6 μs. Operating conditions for 13C NMR experiments: spectral width 20 kHz; spectrum accumulation frequency 0.2–0.01 Hz; 32–2000 transients, 45° pulse at 3.8 μs. Multiplicities and JCH coupling constants were determined from gated decoupled spectra. 1H-1H and 13C-1H correlations were established by using standard Bruker cosydfqf and hxdeptbiqf pulse programs, respectively. For calculations of 1H chemical shifts (ppm), the resonances of the residual solvent protons (δ 2.09 for CHD2C6D5, δ 7.26 for CHCl3, and δ 5.27 for CHDCl2) were used. 2H chemical shifts were referred to the signal of CDH2C6H5 in toluene (δ 2.10) whereas 13C ones were referred to the solvent resonances (δ 77.16 for CDCl3 and δ 20.4 for CD3-group of toluene-d8). The sample temperature measurement uncertainty and temperature reproducibility were less than ±1 °C.
EPR spectra were recorded on a CMS 8400 EPR spectrometer (Adani, Minsk, Belarus) at 9.44 GHz, modulation frequency 100 kHz, modulation amplitude 0.5 mT. Frozen solution EPR measurements were conducted in a quartz finger Dewar filled with liquid nitrogen (−196 °C). Toluene solution of TEMPO (2 mM) was used as an external standard for g-values calculations.
Samples 1/AlMe3 (1′/AlMe3, or 1D/AlMe3) were prepared in the argon-filled glovebox by simple reagents mixing at ambient temperatures. Septum stoppers and Parafilm “M” were used to avoid oxygen/moisture transfer into the NMR tube.
3.3. Synthesis of the Complexes 1, 1′, and 1D
In general, complexes 1, 1′, and 1D were synthesized according to published procedures (see, for example, ref. [72]) with small deviations.
3.3.1. Synthesis of 1,4-Bis-2,4,6-trimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene (L1)
To a stirred solution of 2,3-butanedione (100 mg, 1.16 mmol) in methanol (2 mL), a solution of 2,4,6-trimethylaniline (170 mg, 1.26 mmol) in methanol (3 mL) was added. After the addition of a droplet of glacial acetic acid, reaction mixture was refluxed overnight. After reaction mixture cooling to room temperature the solvent was removed in a vacuum to give a yellow powder of crude ligand L1. Further recrystallization from ethanol afforded bright yellow crystals of pure L1 (242 mg, 65% yield).
1H NMR (24 °C, CDCl3): δ 6.91 (s, 4H, Ar-Hm), 2.31 (s, 6H, Ar-CH3 p), 2.06 (s, 6H, N=C-CH3), 2.02 (s, 12H, Ar-CH3 o). 13C NMR (24 °C, CDCl3): δ 168.50 (2C, N=C-CH3), 145.99 (2C, Ar-Cip), 132.55 (2C, Ar-Cp), 128.71 (4C, Ar-Cm, 1JCH = 154.6 Hz), 124.68 (4C, Ar-Co), 20.86 (2C, Ar-CH3 p, 1JCH = 126.3 Hz), 17.86 (4C, Ar-CH3 o, 1JCH = 126.7 Hz), 15.93 (2C, N=C-CH3, 1JCH = 128.6 Hz). Anal. Calcd. for C22H28N2: C, 82.45; H, 8.81; N, 8.74. Found: C, 82.54; H, 8.96; N, 8.75.
3.3.2. Synthesis of 1,4-Bis-2,6-dimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene (L1′)
L1′ was obtained according to the procedure described above (2,6-dimethylaniline was used instead of mesitylamine) as bright yellow crystals in 61% yield.
1H NMR (24 °C, CDCl3): δ 7.08 (d, 4H, Ar-Hm, 3JHH = 7.7 Hz), 6.95 (t, 2H, Ar-Hp, 3JHH = 7.7 Hz), 2.06 (s, 6H, N=C-CH3), 2.05 (s, 12H, Ar-CH3 o). 13C NMR (24 °C, CDCl3): δ 168.25 (2C, N=C-CH3), 148.43 (2C, Ar-Cip), 128.08 (4C, Ar-Cm, 1JCH = 158.7 Hz), 124.83 (4C, Ar-Co,), 123.42 (2C, Ar-Cp, 1JCH = 160.3 Hz), 17.93 (4C, Ar-CH3 o, 1JCH = 126.8 Hz), 15.99 (2C, N=C-CH3, 1JCH = 128.9 Hz). Anal. Calcd. for C20H24N2: C, 82.15; H, 8.27; N, 9.58. Found: C, 82.21; H, 8.54; N, 9.60.
3.3.3. Synthesis of 2H-Enriched 2,3-butanedione
2H-enriched 2,3-butanedione was prepared by H/D exchange between 2,3-butanedione and D2O according to a published procedure [73]. To a stirred solution of 2,3-butanedione (2.00 g, 23.5 mmol) in D2O (99% of D, 10 mL, 495 mmol), concentrated hydrochloric acid (0.8 mL, 10 mmol) was added dropwise. The reaction mixture was allowed to stir for 10 days at ambient temperature. After that, acid was neutralized by addition of CaCO3 powder (2 g, 20 mmol), and the product was extracted by CH2Cl2 (3 × 15 mL). Combined organic phase was dried over Na2SO4 and filtered. Pure 2H-enriched 2,3-butanedione (84 at. % of 2H) was isolated by distillation (1.45 g, 19.3 mmol, 82% yield). Deuterium abundance in the product was determined by 1H NMR. 1H NMR (25 °C, CDCl3): δ 2.33 (s, CH3), 2.31 (t, CH2D, 2JHD = 2.2 Hz), 2.29 (quint, CHD2, 2JHD = 2.2 Hz).
3.3.4. Synthesis of 2H-Enriched 1,4-bis-2,4,6-trimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene (L1D)
According to the procedure described for L1, the desired ligand L1D was obtained as bright yellow crystals in 65% yield. To avoid isotopic exchange between 2H-enriched 2,3-butanedione and OH-groups of methanol during synthesis, CH3OD was used as solvent instead. Deuterium abundance in the N=C-Me moiety of the resulting ligand was 84 at. %.
1H NMR (25 °C, CDCl3): δ 6.91 (s, 4H, Ar-Hm), 2.31 (s, 6H, Ar-CH3 p), 2.06–2.03 (m, 0.9H, N=C-CH3 + N=C-CH2D + N=C-CHD2), 2.02 (s, 12H, Ar-CH3 o).
3.3.5. Synthesis of 1,4-Bis-2,4,6-trimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene nickel(II) dibromide (complex 1)
All manipulations were performed under dry argon atmosphere using the standard Schlenk technique. Only solvents containing no water and oxygen traces were used.
A Schlenk flack was charged by a freshly prepared solution of L1 (100 mg, 0.31 mmol) in CH2Cl2 (10 mL). A preliminary weighted (in glovebox) amount of NiBr2(DME) (100 mg, 0.32 mmol) was added rapidly and reaction mixture was allowed to stir overnight at ambient temperature. After that insoluble compounds were filtered off and filtrate was dried in vacuum. The resulting dark-red powder was recrystallized from CH2Cl2/hexane to give red crystals of pure 1 (119 mg, 0.22 mmol, 70% yield).
1H NMR (25 °C, CD2Cl2): δ 34.8 (br, 6H, Ar-CH3 p, Δν1/2 = 33 Hz), 27.9 (br, 12H, Ar-CH3 o, Δν1/2 = 100 Hz), 24.1 (br, 4H, Ar-Hm, Δν1/2 = 25 Hz), −21.2 (br, 6H, N=C-CH3, Δν1/2 = 48 Hz). Anal. Calcd. for C22H28N2Br2Ni: C, 49.03; H, 5.24; N, 5.20; Br, 29.65; Ni, 10.89. Found: C, 50.35; H, 6.15; N, 5.26.
3.3.6. Synthesis of 1,4-Bis-2,6-dimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene nickel(II) dibromide (complex 1′)
Complex 1′ (dark red crystals) was prepared in a similar manner by using the corresponding α-diimine ligand L1′ in 78% yield.
1H NMR (24 °C, CDCl3): δ 28.2 (br, 12H, Ar-CH3 o, Δν1/2 = 134 Hz), 24.1 (br, 4H, Ar-Hm, Δν1/2 = 61 Hz), −17.6 (br, 2H, Ar-Hp, Δν1/2 = 74 Hz), −22.7 (br, 6H, N=C-CH3, Δν1/2 = 66 Hz). Anal. Calcd. for C20H24N2Br2Ni: C, 47.02; H, 4.73; N, 5.48; Br, 31.28; Ni, 11.49. Found: C, 47.48; H, 5.18; N, 5.46.
3.3.7. Synthesis of 2H-Enriched 1,4-bis-2,6-dimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene nickel(II) dibromide (complex 1D)
Dark red crystals of 1D were obtained in a similar manner by using the corresponding 2H-enriched α-diimine ligand L1D in 72% yield.
1H NMR (25 °C, CD2Cl2): δ 34.9 (br, 6H, Ar-CH3 p, Δν1/2 = 35 Hz), 27.9 (br, 12H, Ar-CH3 o, Δν1/2 = 105 Hz), 24.1 (br, 4H, Ar-Hm, Δν1/2 = 30 Hz), −21.4 (br, N=C-CH3, Δν1/2 = 50 Hz), −22.2 (br, N=C-CH2D, Δν1/2 = 55 Hz), −22.0 (br, N=C-CHD2, Δν1/2 = 50 Hz). Overall integral intensity of N=C-Me moiety corresponds to 0.9 H.
3.4. DFT Calculations: Computational Details
Geometry optimization and frequency analysis of 2 with different multiplicities (S = 0, S = 1; for the (S = 2) spin isomer, the calculation did not converge) were carried with the UB3PLYP density functional theory scheme (DFT) with D3 corrections [74] using GAUSSIAN 16 program suite [75], with the def2-TZVPP [76] basis set for the Ni and Br atom and 6-311G(d) [77] basis set for other atoms. Solvation effects (with toluene) were incorporated using polarized continuum model (PCM) method [78,79] as implemented in GAUSSIAN 16. The stationary points were ascertained by vibrational frequency analysis with no imaginary frequencies. The Gibbs energies reported in this paper were sum of electronic and thermal free energies. DFT optimized Cartesian coordinates and potential energies (Ha) for the calculated structures are presented in the SM.
4. Conclusions
A novel diamagnetic species 2 was detected upon activation of Brookhart-type Ni(II) α-diimine ethylene polymerization pre-catalyst 1 (LNiBr2, L = 1,4-bis-2,4,6-trimethylphenyl-2,3-dimethyl-1,4-diazabuta-1,3-diene) with AlMe3 co-catalyst. At [Al]:[Ni] ratio of 10:1, species 2 is room-temperature stable and accounts for the major part of nickel. 1H, 2H, and 13C NMR spectroscopic characterization of 2 has witnessed its dinuclear nature, incorporating two antiferromagnetically coupled Ni(II) centers, to give an overall S = 0 spin state. DFT calculations allow to reasonably discriminate between the two possible structures, [L(Me)NiII(μ-CH2)NiII(Br)L] and [LNiII(μ-Me)(μ-CH2)NiIIL]+Br−, favoring the latter, doubly bridged outer-sphere ion pair structure. Complex 2 is sufficiently stable in the reaction solution only at low Al/Ni molar ratios, while in conditions approaching those of practical polymerization ([Al]:[Ni] = 500), quantitative reduction of Ni(II) to the monovalent state takes place. Most plausibly, 2 can be assigned to a resting state of the true Ni(II) active sites of ethylene polymerization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020333/s1, Figure S1: 1H-1H cosydfph NMR spectrum of the sample 1/AlMe3; Figure S2: 1H-13C hxdeptbiqf NMR spectrum of the sample 1/AlMe3; Figure S3: 1H NMR spectra of the catalyst system 1/AlMe3/C2H4; Figure S4: EPR spectra of the catalyst system 1/AlMe3/C2H4; Table S1: The integral intensities of 1H NMR resonances of 2; Cartesian coordinates and calculated energies.
Author Contributions
Conceptualization, I.E.S., K.P.B. and E.P.T.; Synthesis of Ni(II) complexes and samples preparing, A.A.A. and N.V.S.; NMR spectroscopic studies, I.E.S.; DFT calculations, A.A.B.; writing—original draft preparation, I.E.S.; writing—review and editing, I.E.S., K.P.B. and E.P.T.; project administration, K.P.B. and E.P.T.; funding acquisition, I.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation, grant number # 22-23-00048.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank D.E. Babushkin for the synthesis of Al(13CH3)3. NMR experiments were conducted using the equipment of the Department of Mechanisms of Catalytic Reactions, Boreskov Institute of Catalysis. AAB acknowledges the access to the supercomputer facilities of the Computing Centre of Novosibirsk State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tan, C.; Zou, C.; Chen, C. An Ionic Cluster Strategy for Performance Improvements and Product Morphology Control in Metal-Catalyzed Olefin-Polar Monomer Copolymerization. J. Am. Chem. Soc. 2022, 144, 2245–2254. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, X.; Jian, Z. Selective branch formation in ethylene polymerization to access precise ethylene-propylene copolymers. Nat. Commun. 2022, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Asadullah Khan, M.; Pang, W.; Behzadi, S.; Qasim, M. Synthesis of Ultra-High molecular weight polyethylene elastomers by para-tert-Butyl dibenzhydryl functionalized α-Diimine nickel catalysts at elevated temperature. Eur. Pol. J. 2022, 178, 111497. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Zou, C.; Chen, C. A general cocatalyst strategy for performance enhancement in nickel catalyzed ethylene (co)polymerization. Chin. Chem. Lett. 2022, 33, 4363–4366. [Google Scholar] [CrossRef]
- Hu, X.; Kang, X.; Jian, Z. Suppression of Chain Transfer at High Temperature in Catalytic Olefin Polymerization. Angew. Chem. Int. Ed. 2022, 61, e202207363. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liao, Y.; Dai, S. Facile access to ultra-highly branched polyethylenes using hybrid “sandwich” Ni(II) and Pd(II) catalysts. J. Catal. 2022, 411, 54–61. [Google Scholar] [CrossRef]
- Lu, Z.; Liao, Y.; Fan, W.; Dai, S. Efficient suppression of the chain transfer reaction in ethylene coordination polymerization with dibenzosuberyl substituents. Polym. Chem. 2022, 13, 4090–4099. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Ma, Y.; Ye, Z.; Liang, T.; Sun, W.H. Highly active and thermostable camphyl α-diimine–nickel(II) catalysts for ethylene polymerization: Effects of N-aryl substituting groups on catalytic properties and branching structures of polyethylene. Appl. Organomet. Chem. 2022, 36, e6606. [Google Scholar] [CrossRef]
- Lei, T.; Ma, Z.; Liu, H.; Wang, X.; Li, P.; Wang, F.; Wu, W.; Zhang, S.; Xu, G.; Wang, F. Preparation of highly branched polyolefins by controlled chain-walking olefin polymerization. Appl. Organomet. Chem. 2022, 36, e6788. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, G.; Wang, H.; Jing, K.; Dai, S. A Dual Steric Enhancement Strategy in α-Diimine Nickel and Palladium Catalysts for Ethylene Polymerization and Copolymerization. Organometallics 2022, 41, 124–132. [Google Scholar] [CrossRef]
- Doerr, A.M.; Curry, M.R.; Chapleski, R.C.; Burroughs, J.M.; Lander, E.K.; Roy, S.; Long, B.K. Redox Potential as a Predictor of Polyethylene Branching Using Nickel α-Diimine Catalysts. ACS Catal. 2022, 12, 73–81. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Nickel catalysts for the preparation of functionalized polyolefin materials. Chin. Sci. Bull. 2022, 67, 1881–1894. [Google Scholar] [CrossRef]
- Zheng, H.; Li, Y.; Du, W.; Cheung, C.S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Unprecedented Square-Planar α-Diimine Dibromonickel Complexes and Their Ethylene Polymerizations Modulated by Ni–Phenyl Interactions. Macromolecules 2022, 55, 3533–3540. [Google Scholar] [CrossRef]
- Yan, M.; Kang, X.; Li, S.; Xu, X.; Luo, Y.; He, S.; Chen, C. Mechanistic Studies on Nickel-Catalyzed Ethylene Polymerization: Ligand Effects and Quantitative Structure–Activity Relationship Model. Organometallics 2022, 41, 3212–3218. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polymer J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Liang, T.; Goundari, S.B.; Chen, C. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, Y.X.; Kou, S.Q.; Jian, Z.B. A concerted double-layer steric strategy enables an ultra-highly active nickel catalysts to access ultrahigh molecular weight polyethylenes. J. Catal. 2020, 90, 30–36. [Google Scholar] [CrossRef]
- Tran, Q.H.; Brookhart, M.; Daugulis, O. New neutral nickel and palladium sandwich catalysts: Synthesis of ultrahigh molecular weight polyethylene (UHMWPE) via highly controlled polymerization and mechanistic studies of chain propagation. J. Am. Chem. Soc. 2020, 142, 7198–7206. [Google Scholar] [CrossRef]
- Li, S.K.; Xu, S.Y.; Dai, S.Y. A remote nonconjugated electron effect in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 2692–2699. [Google Scholar] [CrossRef]
- Muhammad, Q.; Tan, C.; Chen, C.L. Concerted steric and electronic effects on α-diimine nickel- and palladium-catalyzed ethylene polymerization and copolymerization. Sci. Bull. 2020, 65, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, Y.X.; Jian, Z.B. Unsymmetrical strategy makes significant differences in α-diimine nickel and palladium catalyzed ethylene copolymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C.L. Emerging palladium and nickel catalysts for copolymerization of olefins with polar monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Gong, Y.F.; Li, S.K.; Gong, Q.; Zhang, S.J.; Liu, B.Y.; Dai, S.Y. Systematic investigations of ligand steric effects on α-diimine nickel catalyzed olefin polymerization and copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Bryliakov, K.P.; Antonov, A.A.; Talsi, E.P. Ethylene polymerization of nickel catalysts with α-diimine ligands: Factors controlling the structure of active species and polymer properties. Dalton Trans. 2019, 48, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α-diimine catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.L.; Li, Y.G.; Chen, C.L. Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 30, 4143–4149. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.L.; Liang, G.D.; Gao, H.Y.; Wu, Q. Enhancing thermal stability and living fashion in α-diimine-nickel catalyzed (co)polymerization of ethylene and polar monomers by increasing the steric bulk of ligand backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.B.; Solan, G.A.; Sun, W.-H. Recent advances in Niimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Svejda, S.A.; Johnson, L.K.; Brookhart, M. Low-Temperature Spectroscopic Observation of Chain Growth and Migratory Insertion Barriers in (α-Diimine)Ni(II) Olefin Polymerization Catalysts. J. Am. Chem. Soc. 1999, 121, 10634–10635. [Google Scholar] [CrossRef]
- Leatherman, M.D.; Svejda, S.A.; Johnson, L.K.; Brookhart, M. Mechanistic Studies of Nickel(II) Alkyl Agostic Cations and Alkyl Ethylene Complexes: Investigations of Chain Propagation and Isomerization in (α-diimine)Ni(II)-Catalyzed Ethylene Polymerization. J. Am. Chem. Soc. 2003, 125, 3068–3081. [Google Scholar] [CrossRef]
- Meinhard, D.; Reuter, P.; Rieger, B. Activation of Polymerization Catalysts: Synthesis and Characterization of Novel Dinuclear Nickel(I) Diimine Complexes. Organometallics 2007, 26, 751–754. [Google Scholar] [CrossRef]
- Kraikivskii, P.B.; Saraev, V.V.; Bocharova, V.V.; Romanenko, G.V.; Matveev, D.A.; Petrovskii, S.K.; Kuzakov, A.S. Nickel(I) Complex as the Final Product of the Sequence of Spontaneous Transformations in the System Ni(Allyl)2–(2,6-Diisopropylphenyl)diazabutadiene. Rus. J. Coord. Chem. 2012, 38, 416–425. [Google Scholar] [CrossRef]
- Petrovskii, S.K.; Saraev, V.V.; Kraikivskii, P.B.; Gurinovich, N.S.; Matveev, D.A.; Bocharova, V.V. Formation of paramagnetic intermediates under the conditions of Brookhart-type catalyst activation and operation. Rus. Chem. Bull. Int. Ed. 2013, 62, 1323–1326. [Google Scholar] [CrossRef]
- Gao, W.; Xin, L.; Hao, Z.; Li, G.; Su, J.H.; Zhou, L.; Mu, Y. The ligand redox behavior and role in 1,2-bis[(2,6-diisopropylphenyl)imino]-acenaphthene nickel-TMA(MAO) systems for ethylene polymerization. Chem. Commun. 2015, 51, 7004–7007. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Jing, X.; Dong, Q.; Gong, S.; Li, Q.S.; Zhang, J.; Wua, B.; Yang, X.J. α-Diimine nickel complexes of ethylene and related alkenes. Dalton Trans. 2015, 44, 16228–16232. [Google Scholar] [CrossRef]
- Anderson, W.C., Jr.; Rhinehart, J.L.; Tennyson, A.G.; Long, B.K. Redox-Active Ligands: An Advanced Tool To Modulate Polyethylene Microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef]
- Gurinovich, N.S.; Petrovskii, S.K.; Saraev, V.V.; Salii, I.V. Study of the Nature and Mechanism of the Formation of Paramagnetic Species in Nickel-Based Brookhart-Type Catalytic Systems. Kin. Catal. 2016, 57, 523–527. [Google Scholar] [CrossRef]
- Xu, H.; White, P.B.; Hu, C.; Diao, T. Structure and Isotope Effects of the β-H Agostic (α-diimine)Nickel Cation as a Polymerization Intermediate. Angew. Chem. Int. Ed. 2017, 56, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.S.B.; Ribeiro, A.F.G.; Fernandes, A.C.; Bento, A.; Ribeiro, M.R.; Kociok-Köhn, G.; Pascu, S.I.; Duarte, M.T.; Gomes, P.T. Reactivity of cationic α-diimine cyclopentadienyl nickel complexes towards AlEt2Cl: Synthesis, characterization and ethylene polymerization. Catal. Sci. Technol. 2017, 7, 3128–3142. [Google Scholar] [CrossRef]
- Anderson, W.C., Jr.; Park, S.H.; Brown, L.A.; Kaiser, J.M.; Long, B.K. Accessing Multiple Polyethylene Grades Via a Single Redox-Active Olefin Polymerization Catalyst. Inorg. Chem. Front. 2017, 4, 1108–1112. [Google Scholar] [CrossRef]
- Ahmadjo, S.; Damavandi, S.; Zohuri, G.H.; Farhadipour, A.; Samadieh, N.; Etemadinia, Z. Synthesis and application of fluorinated a-diimine nickel catalyst for ethylene polymerization: Deactivation mechanism. Polym. Bull. 2017, 74, 3819–3832. [Google Scholar] [CrossRef]
- Chen, Z.; Leatherman, M.D.; Daugulis, O.; Brookhart, M. Nickel-Catalyzed Copolymerization of Ethylene and Vinyltrialkoxysilanes: Catalytic Production of Cross-Linkable Polyethylene and Elucidation of the Chain-Growth Mechanism. J. Am. Chem. Soc. 2017, 139, 16013–16022. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.S.; Lamb, J.R.; Vaidya, T.; Keresztes, I.; Klimovica, K.; LaPointe, A.M.; Daugulis, O.; Coates, G.W. Understanding the Insertion Pathways and Chain Walking Mechanisms of α-Diimine Nickel Catalysts for α-Olefin Polymerization: A 13C NMR Spectroscopic Investigation. Macromolecules 2017, 50, 7010–7027. [Google Scholar] [CrossRef]
- Kaiser, J.M.; Anderson, W.C., Jr.; Long, B.K. Photochemical regulation of a redox-active olefin polymerization catalyst: Controlling polyethylene microstructure with visible light. Polym. Chem. 2018, 9, 1567–1570. [Google Scholar] [CrossRef]
- Gurinovich, N.S.; Petrovsky, S.K.; Saliy, I.V.; Saraev, V.V. Influence of a diimine ligand and an activator on the processes taking place in Brookhart-type nickel catalytic systems. Res. Chem. Intermed. 2018, 44, 1935–1944. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Zakharov, V.A.; Talsi, E.P. NMR spectroscopic identification of Ni(II) species formed upon activation of (α-diimine)NiBr2 polymerization catalysts with MAO and MMAO. Dalton Trans. 2018, 47, 4968–4974. [Google Scholar] [CrossRef]
- Wang, B.; Daugulis, O.; Brookhart, M. Ethylene Polymerization with Ni(II) Diimine Complexes Generated from 8-Halo-1-naphthylamines: The Role of Equilibrating Syn/Anti Diastereomers in Determining Polymer Properties. Organometallics 2019, 38, 4658–4668. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. EPR spectroscopic study of Ni(I) species in the catalyst system for ethylene polymerization based on α-diimine Ni(II) complex activated by MMAO. J. Organomet. Chem. 2019, 880, 267–271. [Google Scholar] [CrossRef]
- Chapleski, R.C., Jr.; Kern, J.L.; Anderson, W.C., Jr.; Long, B.K.; Roy, S. A mechanistic study of microstructure modulation in olefin polymerizations using a redox-active Ni(II) α-diimine catalyst. Catal. Sci. Technol. 2020, 10, 2029–2039. [Google Scholar] [CrossRef]
- Tran, Q.H.; Wang, X.; Brookhart, M.; Daugulis, O. Cationic α-Diimine Nickel and Palladium Complexes Incorporating Phenanthrene Substituents: Highly Active Ethylene Polymerization Catalysts and Mechanistic Studies of syn/anti Isomerization. Organometallics 2020, 39, 4704–4716. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. Activation of an α-diimine Ni(II) precatalysts with AlMe3 and Al(iBu)3: Catalytic and NMR and EPR spectroscopic studies. Organometallics 2020, 39, 3034–3040. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. The nature of nickel species formed upon the activation of α-diimine nickel(II) pre-catalyst with alkylaluminum sesquichlorides. J. Organomet. Chem. 2020, 907, 121063. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.H.; Talsi, E.P. Nature of Heterobinuclear Ni(I) Complexes Formed upon the Activation of the α-Diimine Complex LNiIIBr2 with AlMe3 and MMAO. Organometallics 2021, 40, 907–914. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Talsi, E.P. α-Diimine Ni-Catalyzed Ethylene Polymerizations: On the Role of Nickel(I) Intermediates. Catalysts 2021, 11, 1386. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Luo, G.; Gao, W. Nickel complexes based on BIAN ligands: Transformation and catalysis on ethylene polymerization. Dalton Trans. 2021, 50, 7356–7363. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Talsi, E.P. Ni(I) Intermediates Formed upon Activation of a Ni(II) α-Diimine Ethylene Polymerization Precatalyst with AlR3 (R = Me, Et, and iBu), AlR2Cl (R = Me, Et), and MMAO: A Comparative Study. Organometallics 2022, 41, 1015–1024. [Google Scholar] [CrossRef]
- Leung, D.H.; Ziller, J.W.; Guan, Z. Axial Donating Ligands: A New Strategy for Late Transition Metal Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2008, 130, 7538–7539. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999; pp. 840–842. [Google Scholar]
- Soshnikov, I.E.; Semikolenova, N.V.; Zakharov, V.A.; Moller, H.M.; Olscher, F.; Osichow, A.; Gottker-Schnettmann, I.; Mecking, S.; Talsi, E.P.; Bryliakov, K.P. Formation and Evolution of Chain-Propagating Species Upon Ethylene Polymerization with Neutral Salicylaldiminato Nickel(II) Catalysts. Chem. Eur. J. 2013, 19, 11409–11417. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.A.; Semikolenova, N.V.; Zakharov, V.A.; Zhang, W.; Wang, Y.; Sun, W.-H.; Talsi, E.P.; Bryliakov, K.P. Vinyl Polymerization of Norbornene on Nickel Complexes with Bis(imino)pyridine Ligands Containing Electron-Withdrawing Groups. Organometallics 2012, 31, 1143–1149. [Google Scholar] [CrossRef]
- Antonov, A.A.; Samsonenko, D.G.; Talsi, E.P.; Bryliakov, K.P. Formation of Cationic Intermediates upon the Activation of Bis(imino)pyridine Nickel Catalysts. Organometallics 2013, 32, 2187–2191. [Google Scholar] [CrossRef]
- NMR of Paramagnetic Molecules. Principles and Applications; La Mar, G.N., Horrocks, W.D.W., Jr., Holm, R.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1973. [Google Scholar]
- Krzystek, J.; Park, J.H.; Meisel, M.W.; Hitchman, M.A.; Stratemeier, H.; Brunel, L.C.; Telser, J. EPR Spectra from “EPR-Silent” Species: High-Frequency and High-Field EPR Spectroscopy of Pseudotetrahedral Complexes of Nickel(II). Inorg. Chem. 2002, 41, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Pappas, I.; Treacy, S.; Chirik, P.J. Alkene Hydrosilylation Using Tertiary Silanes with α-Diimine Nickel Catalysts. Redox-Active Ligands Promote a Distinct Mechanistic Pathway from Platinum Catalysts. ACS Catal. 2016, 6, 4105–4109. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, 1st ed.; Wiley-Interscience: New York, NY, USA, 1972. [Google Scholar]
- Zhang, J.; Rahman, M.M.; Zhao, Q.; Feliciano, J.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Szostak, M. N-Heterocyclic Carbene Complexes of Nickel(II) from Caffeine and Theophylline: Sustainable Alternative to Imidazol-2-ylidenes. Organometallics 2022, 41, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, R.E.; Green, M.L.H.; Silcock, P.J. Nickel(II) cis- and trans-Dimethyl Complexes of Di-N-heterocyclic Carbenes. Organometallics 2001, 20, 2611–2615. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Bryliakova, A.A.; Shashkov, M.V.; Talsi, E.P.; Bryliakov, K.P. To Rebound or...Rebound? Evidence for the “Alternative Rebound” Mechanism in C–H Oxidations by the Systems Nonheme Mn Complex/H2O2/Carboxylic Acid. ACS Catal. 2021, 11, 5517–5524. [Google Scholar] [CrossRef]
- Zima, A.M.; Lyakin, O.Y.; Bryliakova, A.A.; Babushkin, D.E.; Bryliakov, K.P.; Talsi, E.P. Reactivity vs. Selectivity of Biomimetic Catalyst Systems of the Fe(PDP) Family through the Nature and Spin State of the Active Iron-Oxygen Species. Chem. Rec. 2022, 22, e202100334. [Google Scholar] [CrossRef]
- Zima, A.M.; Lyakin, O.Y.; Bryliakova, A.A.; Babushkin, D.E.; Bryliakov, K.P.; Talsi, E.P. Effect of Brønsted Acid on the Reactivity and Selectivity of the Oxoiron(V) Intermediates in C-H and C=C Oxidation Reactions. Catalysts 2022, 12, 949. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Li, W.; Chen, M. Ligand steric effects on alpha-diimine nickel catalyzed ethylene and 1-hexene polymerization. RSC Adv. 2017, 7, 55051–55059. [Google Scholar] [CrossRef]
- Walters, W.D. Exchange Reaction of Biacetyl with Deuterium Oxide. J. Am. Chem. Soc. 1941, 63, 2850–2851. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Pascual-Ahuir, J.L.; Silla, E.; Tuñón, I. GEPOL: An improved description of molecular-surfaces. 3. A new algorithm for the computation of a solvent-excluding surface. J. Comp. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).