Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B
Abstract
1. Introduction
2. Results
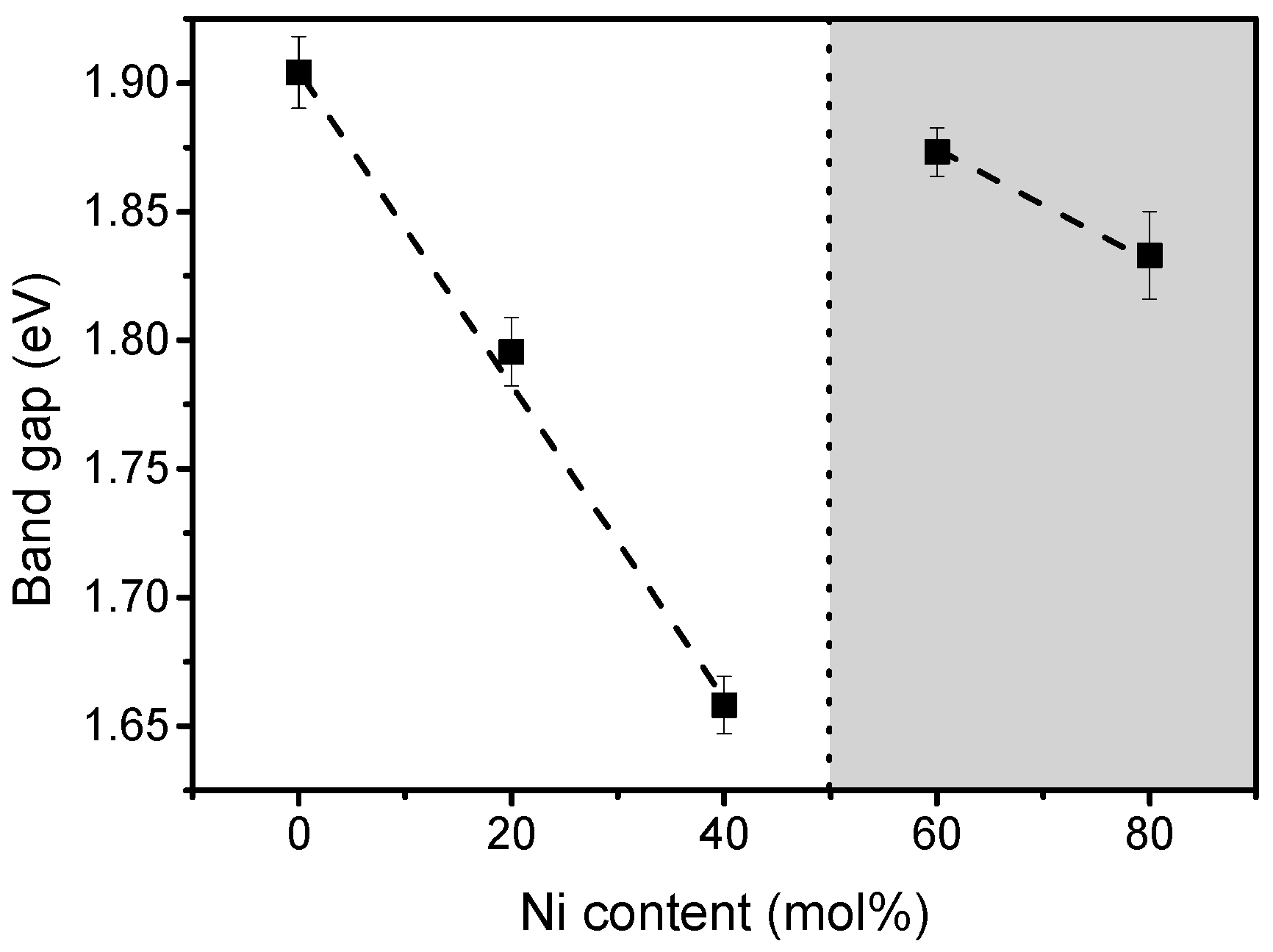
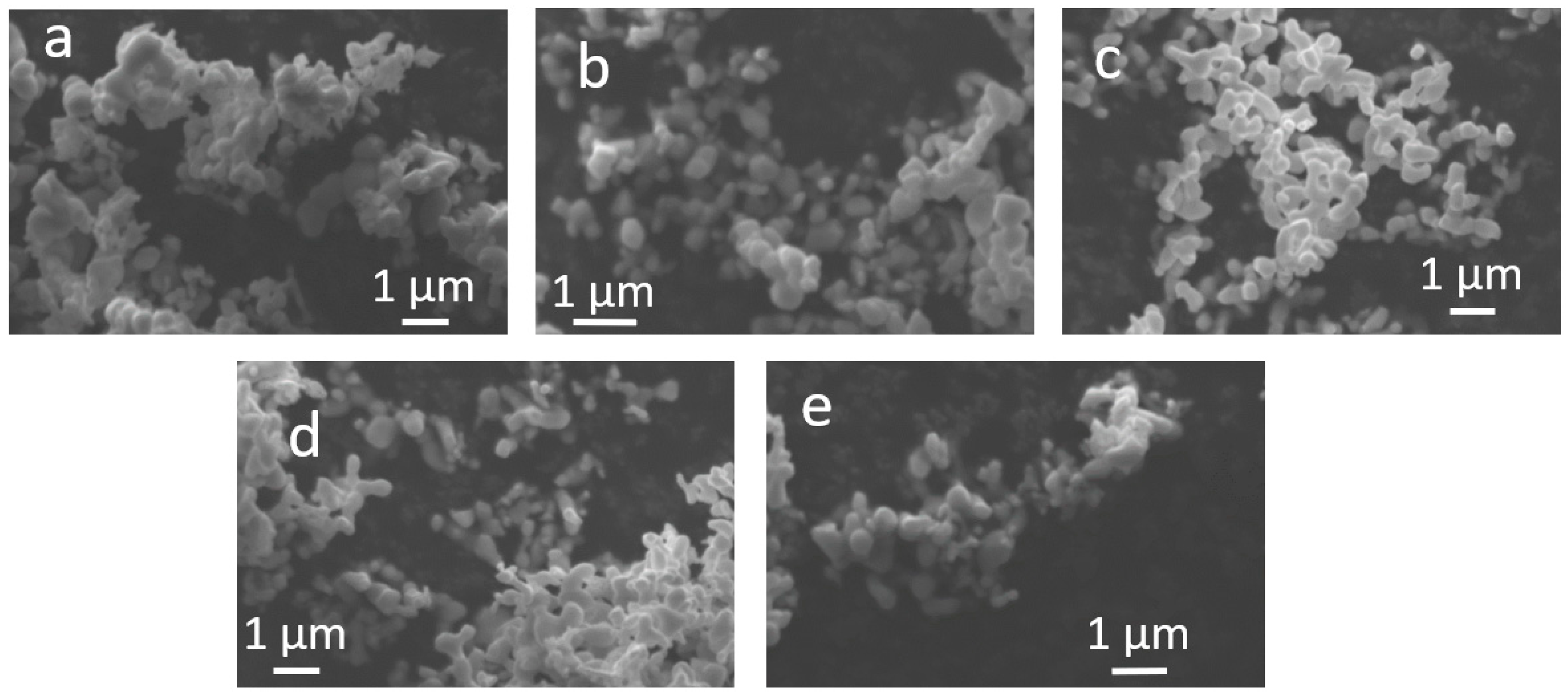
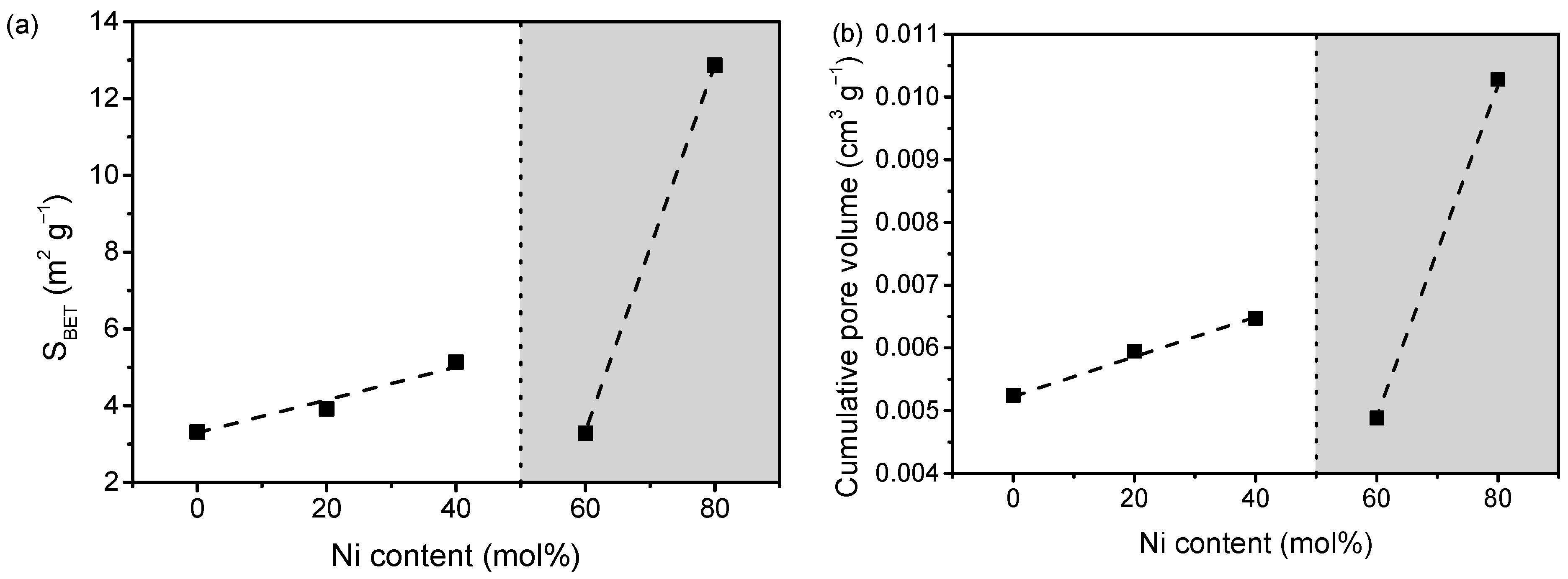
2.1. Structural and Compositional Characterization
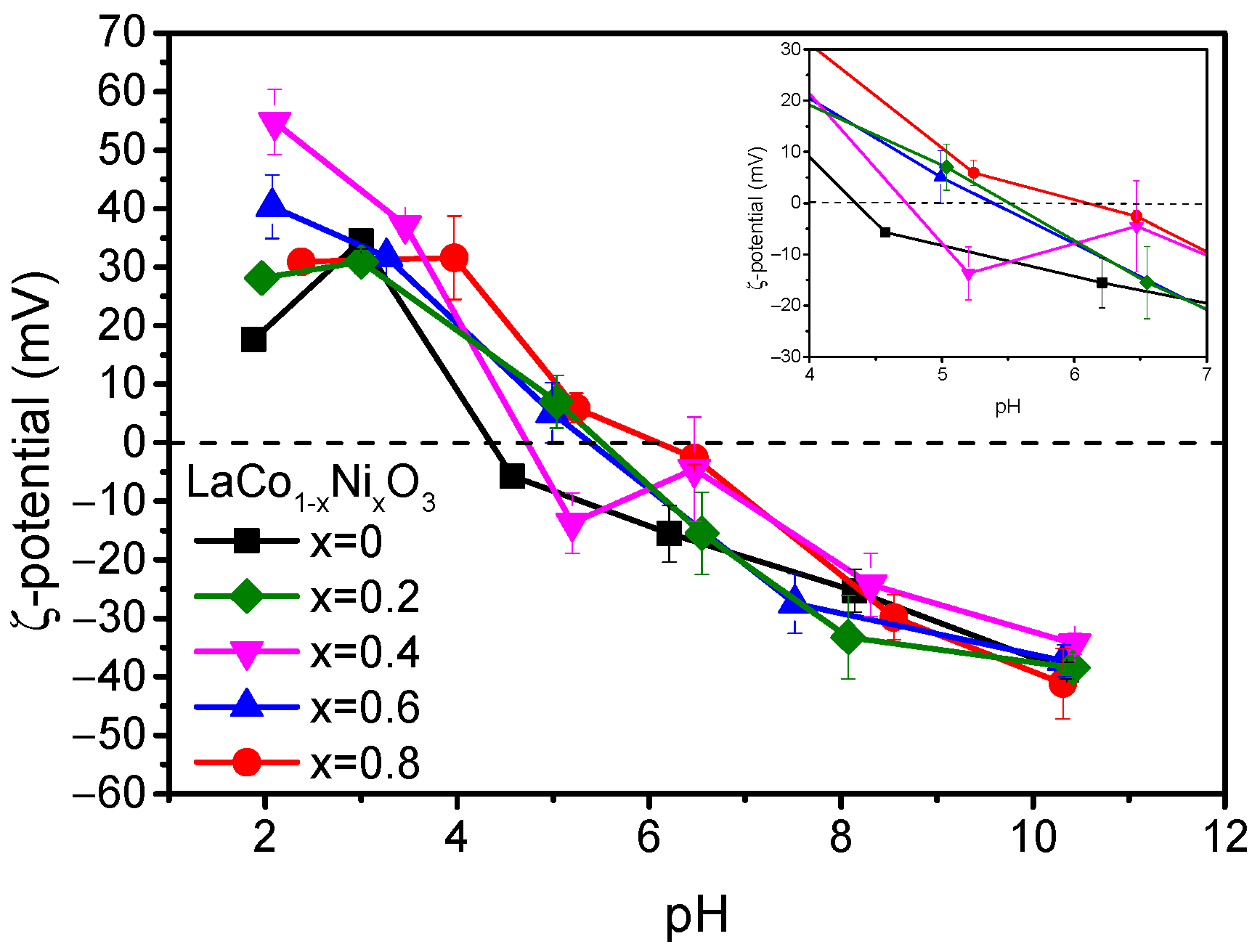
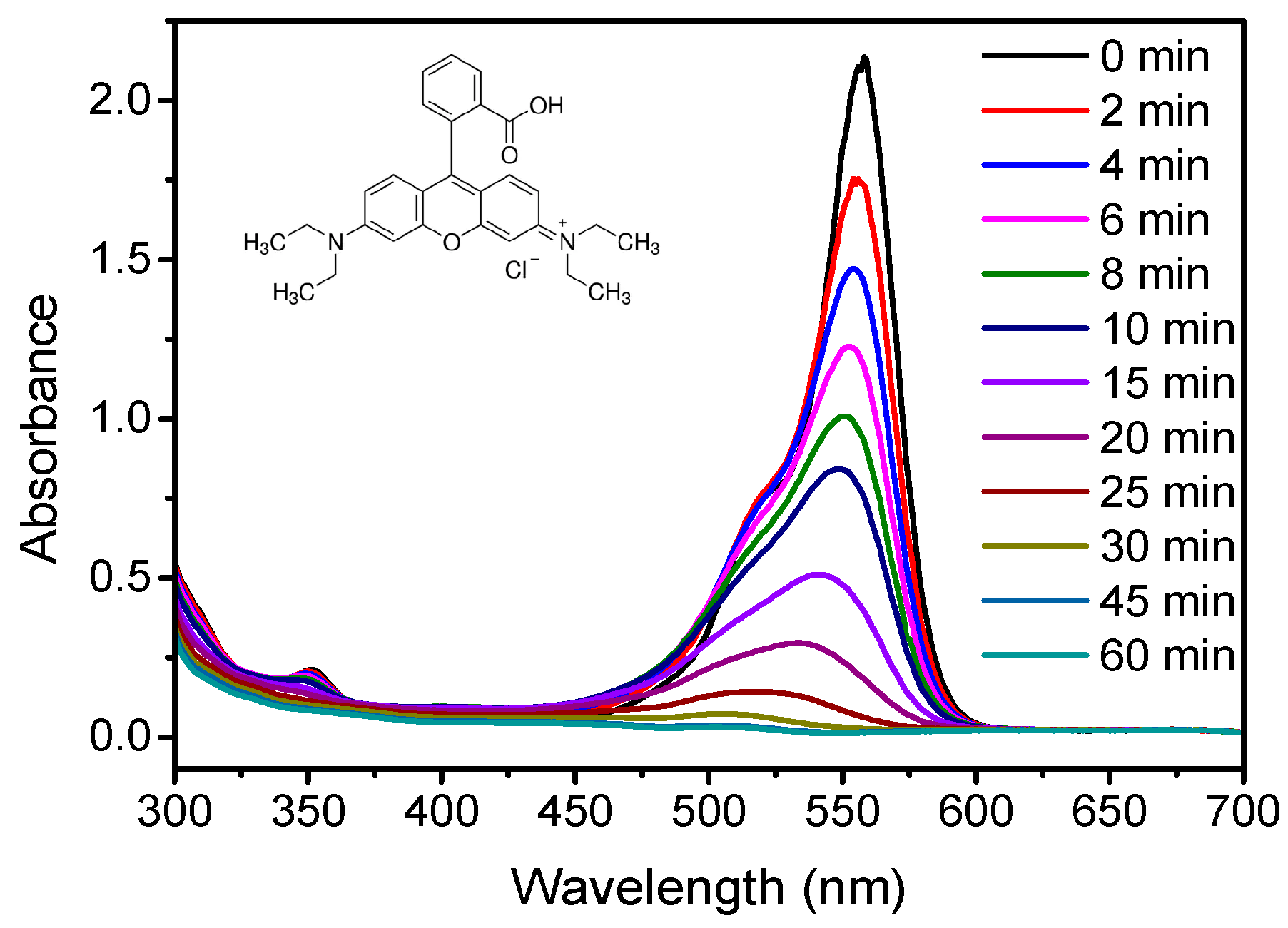
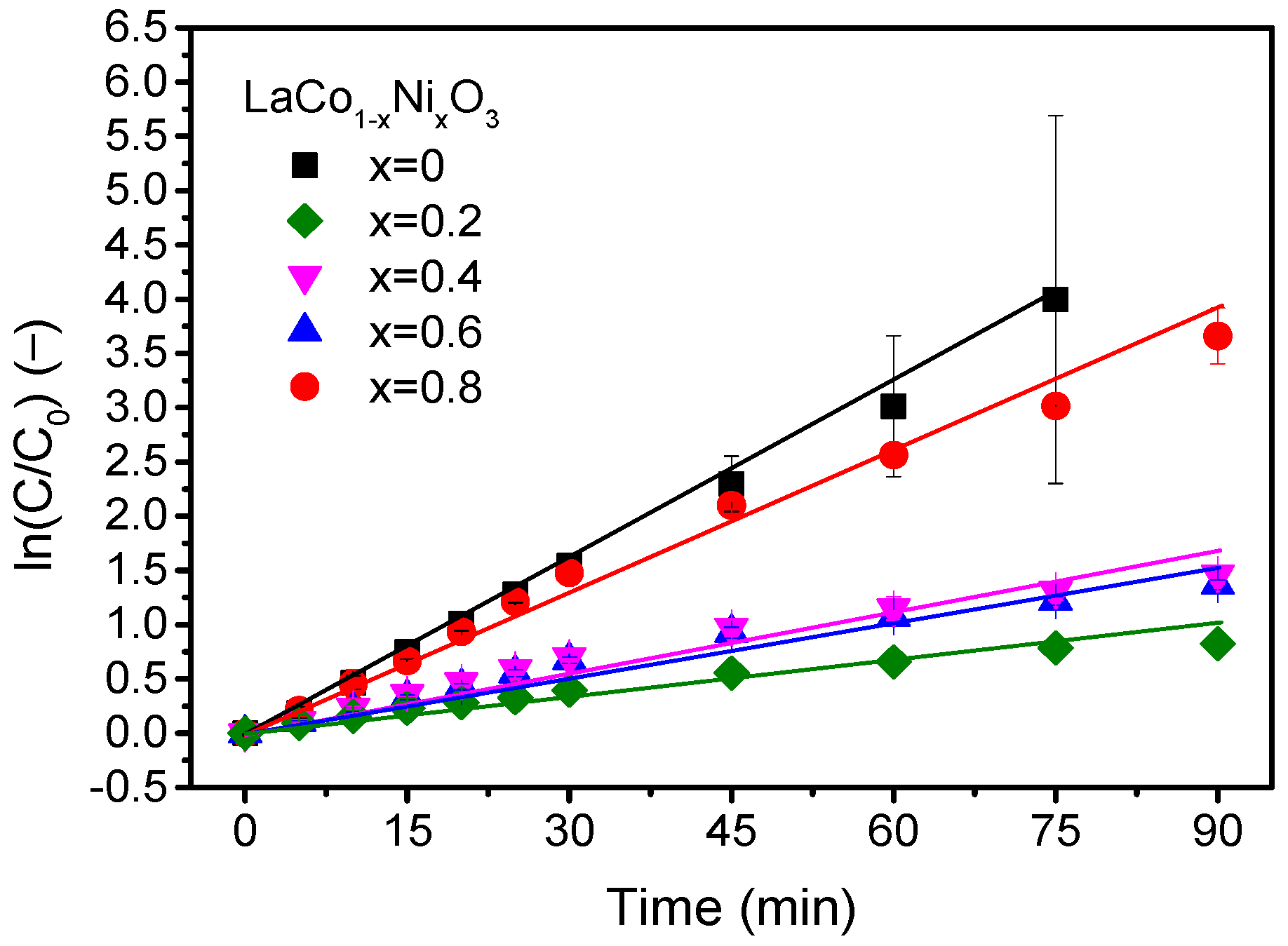
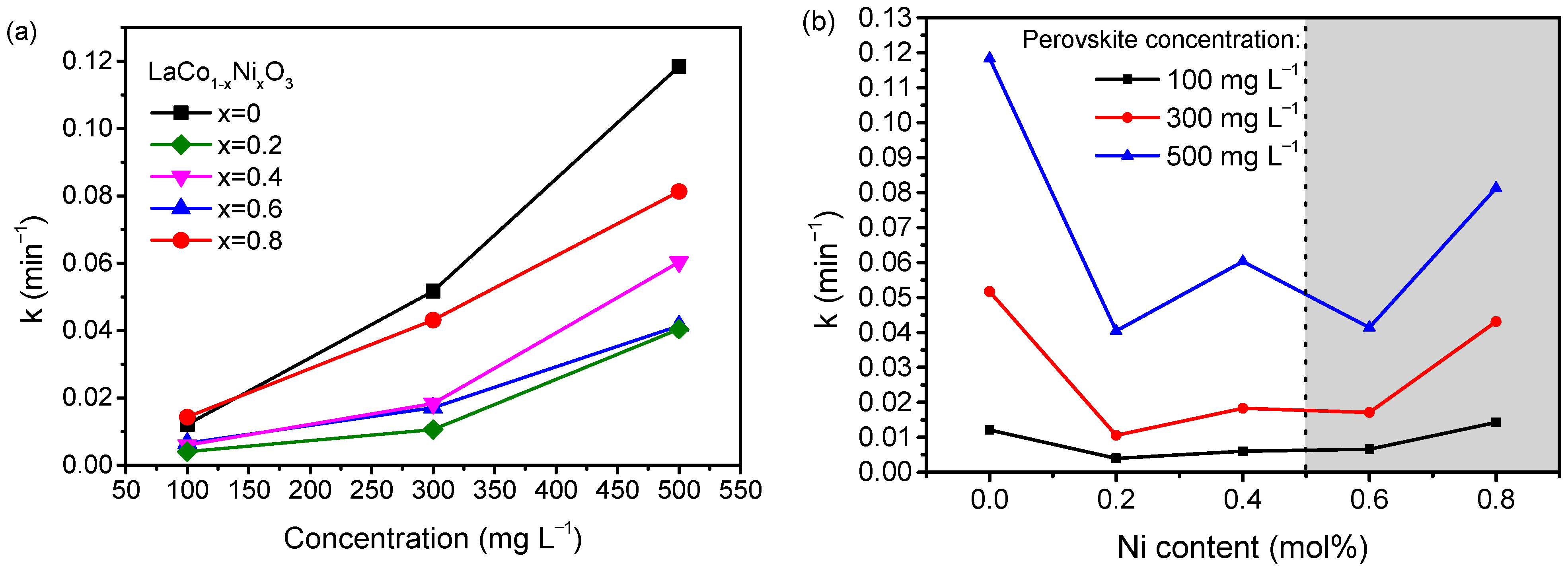
2.2. Thermocatalytic Activity
3. Materials and Methods
3.1. Perovskite Oxide Powders Synthesis
3.2. Perovskite Oxide Powders Characterization
3.3. Thermocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilley, R.J.D. Perovskites: Structure-Property Relationships, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2016. [Google Scholar]
- Tanaka, H.; Misono, M. Advances in Designing Perovskite Catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Nitadori, T.; Ichiki, T.; Misono, M. Catalytic Properties of Perovskite-Type Mixed Oxides (ABO3) Consisting of Rare Earth and 3d Transition Metals. The Roles of the A- and B-Site Ions. Bull. Chem. Soc. Jpn. 1988, 61, 621–626. [Google Scholar] [CrossRef]
- Gao, P.; Yan, S.; Tian, X.; Nie, Y.; Wang, Y.; Deng, Y.; Tu, J. Identification and Manipulation of Active Centers on Perovskites to Enhance Catalysis of Peroxymonosulfate for Degradation of Emerging Pollutants in Water. J. Hazard Mater. 2022, 424, 127384. [Google Scholar] [CrossRef]
- Lin, N.; Gong, Y.; Wang, R.; Wang, Y.; Zhang, X. Critical Review of Perovskite-Based Materials in Advanced Oxidation System for Wastewater Treatment: Design, Applications and Mechanisms. J. Hazard Mater. 2022, 424, 127637. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal-Air Batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-horn, Y. A Perovskite Oxide Optimized for Molecular Orbital Principles. Science 2011, 334, 2010–2012. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Chen, Y.C.; Lin, Y.F. LaMO3 Perovskites (M=Co, Cu, Fe and Ni) as Heterogeneous Catalysts for Activating Peroxymonosulfate in Water. Chem. Eng. Sci. 2017, 160, 96–105. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Sirimahachai, U.; Wongnawa, S. A Simple Synthesis and Characterization of LaMO3 (M=Al, Co, Fe, Gd) Perovskites via Chemical Co-Precipitation Method. Songklanakarin J. Sci. Technol. 2018, 40, 484–491. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Zhao, F.; Safaei, Z.; Srivastava, V.; Lakshmi Ramasamy, D.; Iftekhar, S.; Kalliola, S.; Sillanpää, M. Degradation and Mineralization of Phenol in Aqueous Medium by Heterogeneous Monopersulfate Activation on Nanostructured Cobalt Based-Perovskite Catalysts ACoO3 (A=La, Ba, Sr and Ce): Characterization, Kinetics and Mechanism Study. Appl. Catal. B 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Gao, P.; Tian, X.; Nie, Y.; Yang, C.; Zhou, Z.; Wang, Y. Promoted Peroxymonosulfate Activation into Singlet Oxygen over Perovskite for Ofloxacin Degradation by Controlling the Oxygen Defect Concentration. Chem. Eng. J. 2019, 359, 828–839. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Ghafoor, A.; Bibi, I.; Majid, F.; Ata, S.; Nouren, S.; Raza, Q.; Iqbal, M. Yttrium and Cobalt Doped LaNiO3 Nanoparticles Synthesis and Solar Light Driven Photocatalytic Removal of Rhodamine, B. Mater. Res. Bull. 2023, 159, 112112. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, S.; Xu, T.; Ma, J.; Hayase, S.; Ma, T. Investigation on Structures, Band Gaps, and Electronic Structures of Lead Free La2NiMnO6 Double Perovskite Materials for Potential Application of Solar Cell. J. Alloys Compd. 2016, 655, 208–214. [Google Scholar] [CrossRef]
- Grinberg, I.; West, D.V.; Torres, M.; Gou, G.; Stein, D.M.; Wu, L.; Chen, G.; Gallo, E.M.; Akbashev, A.R.; Davies, P.K.; et al. Perovskite Oxides for Visible-Light-Absorbing Ferroelectric and Photovoltaic Materials. Nature 2013, 503, 509–512. [Google Scholar] [CrossRef]
- Lee, S.; Levi, R.D.; Qu, W.; Lee, S.C.; Randall, C.A. Band-Gap Nonlinearity in Perovskite Structured Solid Solutions. J. Appl. Phys. 2010, 107, 23523. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, A.; Yu, Y.; Tan, R.; Liu, C.; Zhang, P.; Liu, D.; Gui, J. Engineering Oxygen Vacancies into LaCoO3 Perovskite for Efficient Electrocatalytic Oxygen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 2906–2910. [Google Scholar] [CrossRef]
- Chen, H.; Motuzas, J.; Martens, W.; Diniz da Costa, J.C. Surface and Catalytic Properties of Stable Me(Ba, Ca and Mg)SrCoO for the Degradation of Orange II Dye under Dark Conditions. Appl. Surf. Sci. 2018, 450, 292–300. [Google Scholar] [CrossRef]
- Chen, H.; Motuzas, J.; Martens, W.; Diniz da Costa, J.C. Ceramic Metal Oxides with Ni2+ Active Phase for the Fast Degradation of Orange II Dye under Dark Ambiance. Ceram. Int. 2018, 44, 6634–6640. [Google Scholar] [CrossRef]
- Chen, H.; Motuzas, J.; Martens, W.; Diniz da Costa, J.C. Improved Dark Ambient Degradation of Organic Pollutants by Cerium Strontium Cobalt Perovskite. J. Environ. Sci. 2020, 90, 110–118. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Strunck, A.B.; Boffa, V.; Jørgensen, M.K. Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite. Catalysts 2022, 12, 265. [Google Scholar] [CrossRef]
- Luo, X.; Su, C.; Chen, Z.; Xu, L.; Zhao, L.; Zhao, J.; Qiu, R.; Huang, Z. Mechanochemical Synthesis of La-Sr-Co Perovskite Composites for Catalytic Degradation of Doxycycline in the Dark: Role of Oxygen Vacancies. Sep. Purif. Technol. 2022, 300, 121891. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, T.; Dang, Y.; He, J.; Chen, S.Y.; Kuo, C.H.; Kriz, D.; Meng, Y.; Meguerdichian, A.G.; Suib, S.L. Mechanism Studies on Methyl Orange Dye Degradation by Perovskite-Type LaNiO3−δ under Dark Ambient Conditions. Appl. Catal. A Gen. 2018, 549, 302–309. [Google Scholar] [CrossRef]
- Chen, H.; Ku, J.; Wang, L. Thermal Catalysis under Dark Ambient Conditions in Environmental Remediation: Fundamental Principles, Development, and Challenges. Chin. J. Catal. 2019, 40, 1117–1134. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Senthil Kumar, P.; Radin Mohamed, R.M.S.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of Rhodamine B from Textile Wastewater Using Nanoparticle Photocatalysts: A Review for Sustainable Approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Orimolade, B.O.; Masekela, D.; Mamba, B.; Mabuba, N. The Application of Photoelectrocatalysis in the Degradation of Rhodamine B in Aqueous Solutions: A Review. RSC Adv. 2022, 12, 26176–26191. [Google Scholar] [CrossRef]
- Gou, Y.; Liang, X.; Chen, B. Porous Ni–Co Bimetal Oxides Nanosheets and Catalytic Properties for CO Oxidation. J. Alloys Compd. 2013, 574, 181–187. [Google Scholar] [CrossRef]
- Wu, Z.; Niu, H.; Chen, J.; Chen, J. Metal-organic frameworks-derived hierarchical Co3O4/CoNi-layered double oxides nanocages with the enhanced catalytic activity for toluene oxidation. Chemosphere 2021, 280, 130801. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution Combustion Synthesis, Energy and Environment: Best Parameters for Better Materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Colomer, M.T.; Fumo, D.A.; Jurado, J.R.; Segadães, A.M. Non-Stoichiometric La(1–x)NiO(3–δ) Perovskites Produced by Combustion Synthesis. J. Mater. Chem. 1999, 9, 2505–2510. [Google Scholar] [CrossRef]
- Alonso, J.A.; Martínez-Lope, M.J.; Falcón, H.; Carbonio, R.E. On the Correlation of Ni Oxidation States and Electronic Conductivity of (R,A)NiO3−δ (R=lanthanides, A=alkaline Earths, Th) Perovskites with Catalytic Activity for H2O2 Decomposition. Phys. Chem. Chem. Phys. 1999, 1, 3025–3030. [Google Scholar] [CrossRef]
- Sánchez, R.D.; Causa, M.T.; Seoane, A.; Rivas, J.; Rivadulla, F.; López-Quintela, M.A.; Pérez Cacho, J.J.; Blasco, J.; García, J. Metal–Insulator Transition and Magnetic Properties of La1−xEuxNiO3 (0 ≤ x ≤ 1). J. Solid State Chem. 2000, 151, 1–11. [Google Scholar] [CrossRef]
- Haas, O.; Struis, R.P.W.J.; McBreen, J.M. Synchrotron X-Ray Absorption of LaCoO3 Perovskite. J. Solid State Chem. 2004, 177, 1000–1010. [Google Scholar] [CrossRef]
- Vyshatko, N.P.; Kharton, V.V.; Shaula, A.L.; Marques, F.M.B. Powder X-ray diffraction study of LaCo0.5Ni0.5O3−δ and LaCo0.5Fe0.5O3−δ. Powder Diffr. 2003, 18, 159–161. [Google Scholar] [CrossRef]
- Simböck, J.; Ghiasi, M.; Schönebaum, S.; Simon, U.; de Groot, F.M.F.; Palkovits, R. Electronic Parameters in Cobalt-Based Perovskite-Type Oxides as Descriptors for Chemocatalytic Reactions. Nat. Commun. 2020, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Irshad, M.; Idrees, R.; Siraj, K.; Shakir, I.; Rafique, M.; Ain, Q.; Raza, R. Electrochemical Evaluation of Mixed Ionic Electronic Perovskite Cathode LaNi1-XCoxO3−δ for IT-SOFC Synthesized by High Temperature Decomposition. Int. J. Hydrogen Energy 2021, 46, 10448–10456. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wei, W.; Chen, W.; Ivey, D.G. Rock Salt-Spinel Structural Transformation in Anodically Electrodeposited Mn-Co-O Nanocrystals. Chem. Mater. 2008, 20, 1941–1947. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Chao, D.; Baikie, T.; Bai, L.; Chen, S.; Zhao, Y.; Sum, T.C.; Lin, J.; Shen, Z. Hierarchical Porous LiNi1/3Co1/3Mn1/3O2 Nano-/Micro Spherical Cathode Material: Minimized Cation Mixing and Improved Li+ Mobility for Enhanced Electrochemical Performance. Sci. Rep. 2016, 6, 25771. [Google Scholar] [CrossRef]
- Gao, X.; Ashok, J.; Kawi, S.; Yang, N. Steam Reforming of Toluene as Model Compound of Biomass Tar over Ni–Co/La2O3 Nano-Catalysts: Synergy of Ni and Co. Int. J. Hydrogen Energy 2021, 46, 30926–30936. [Google Scholar] [CrossRef]
- Monaco, L.; Sodhi, R.N.S.; Palumbo, G.; Erb, U. XPS Study on the Passivity of Coarse-Grained Polycrystalline and Electrodeposited Nanocrystalline Nickel-Iron (NiFe) Alloys. Corros. Sci. 2020, 176, 108902. [Google Scholar] [CrossRef]
- Latsiou, A.; Lykos, C.; Bairamis, F.; Konstantinou, I. Synthesis and characterization of LaCoxNi1−xO3 perovskites as heterogeneous catalysts for phenolics degradation by persulfate activation. J. Chem. Technol. Biotechnol. 2022, 97, 3467–3480. [Google Scholar] [CrossRef]
- Wei, G.; Zheng, D.; Xu, L.; Guo, Q.; Hu, J.; Sha, N.; Zhao, Z. Photothermal catalytic activity and mechanism of LaNixCo1−xO3 (0 ≦ x ≦ 1) perovskites for CO2 reduction to CH4 and CH3OH with H2O. Mater. Res. Express 2019, 6, 086221. [Google Scholar] [CrossRef]
- Dalpian, G.M.; Zhao, X.G.; Kazmerski, L.; Zunger, A. Formation and Composition-Dependent Properties of Alloys of Cubic Halide Perovskites. Chem. Mater. 2019, 31, 2497–2506. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Z.; Su, J.; Zhang, J.; Chang, J.; Hao, Y. A Review on Energy Band-Gap Engineering for Perovskite Photovoltaics. Solar. RRL 2019, 3, 1900304. [Google Scholar] [CrossRef]
- Zarrin, N.; Husain, S.; Khan, W.; Manzoor, S. Sol-Gel Derived Cobalt Doped LaCrO3: Structure and Physical Properties. J. Alloys Compd. 2019, 784, 541–555. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital Controlled Band Gap Engineering of Tetragonal BiFeO3 for Optoelectronic Applications. J. Mater. Chem. C Mater. 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Singh, C.; Rakesh, M. Preparation and Characterization of Nickel Doped, A and B Site LaCoO3 Perovskite. Indian J. Eng. Mater. Sci. 2009, 16, 288–290. [Google Scholar]
- Aswin, V.; Kumar, P.; Singh, P.; Gupta, A.; Rayaprol, S.; Dogra, A. Influence of Al Doping in LaCoO3 on Structural, Electrical and Magnetic Properties. J. Mater. Sci. 2014, 50, 366–373. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Qian, Q.; Chen, Q. Studies on B Sites in Fe-Doped LaNiO3 Perovskite for SCR of NOx with H2. Int. J. Hydrogen Energy 2014, 39, 15836–15843. [Google Scholar] [CrossRef]
- Ma, F.; Chu, W.; Huang, L.; Yu, X.; Wu, Y. Steam Reforming of Ethanol over Zn-Doped LaCoO3 Perovskite Nanocatalysts. Chin. J. Catal. 2011, 32, 970–977. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO Oxidation Catalysis on Copper Doped Hexagonal Phase LaCoO3: A Combined Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Binary Catalyst System Dye Degradation Using Photocatalysis. Fibers Polym. 2014, 15, 273–280. [Google Scholar] [CrossRef]
- Maurya, N.S.; Mittal, A.K.; Cornel, P.; Rother, E. Biosorption of Dyes Using Dead Macro Fungi: Effect of Dye Structure, Ionic Strength and PH. Bioresour. Technol. 2006, 97, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible Light-Driven Photocatalytic Degradation of Rhodamine B over NaBiO3: Pathways and Mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef]
- Horikoshi, S.; Saitou, A.; Hidaka, H. Environmental Remediation by an Integrated Microwave/UV Illumination Method. V. Thermal and Nonthermal Effects of Microwave Radiation on the Photocatalyst and on the Photodegradation of Rhodamine-B under UV/Vis Radiation. Environ. Sci. Technol. 2003, 37, 5813–5822. [Google Scholar] [CrossRef]
- Yao, S.; Wu, J.; Li, W.; Zheng, R.; Li, R.; Chen, Y.; Luo, J.; Zhou, X. LaCoO3 Co-Catalyst Modified Ag2CrO4 for Improved Visible-Light-Driven Photocatalytic Degradation of Tetracycline. Sep. Purif. Technol. 2019, 227, 115691. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, Y.; Xin, H.; Yang, C.; Chen, L.; Li, X. NO Oxidation over Ni–Co Perovskite Catalysts. Chem. Eng. J. 2015, 275, 351–356. [Google Scholar] [CrossRef]
- Salman, A.R.; Hyrve, S.M.; Regli, S.K.; Zubair, M.; Enger, B.C.; Lødeng, R.; Waller, D.; Rønning, M. Catalytic Oxidation of NO over LaCo1−xBxO3 (B=Mn, Ni) Perovskites for Nitric Acid Production. Catalysts 2019, 9, 429. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, W.; Li, X.; Wang, Q.; Zhu, Z.; Zhou, T.; Wang, G.; Xie, A.; Luo, S. Catalytic Oxidation of Toluene over B-site Doped La-based Perovskite LaNixB1−xO3(B=Co, Cu) Catalysts. Env. Prog. Sustain. Energy 2022, 42, e13965. [Google Scholar] [CrossRef]
- Manos, D.; Papadopoulou, F.; Margellou, A.; Petrakis, D.; Konstantinou, I. Heterogeneous Activation of Persulfate by LaMO3 (M=Co, Fe, Cu, Mn, Ni) Perovskite Catalysts for the Degradation of Organic Compounds. Catalysts 2022, 12, 187. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.J.; Durst, J.; Bozza, F.; Graule, T.; Schäublin, R.; Wiles, L.; et al. Dynamic Surface Self-Reconstruction Is the Key of Highly Active Perovskite Nano-Electrocatalysts for Water Splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Wang, M.; Wang, J.; Li, H.; Xi, S.; Wei, C.; Xi, P.; Sterbinsky, G.E.; Freeland, J.W.; et al. Lattice Site–Dependent Metal Leaching in Perovskites toward a Honeycomb-like Water Oxidation Catalyst. Sci. Adv. 2021, 7, 1788. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Sun, X.; Lin, J. Synergetic Effects of Thermal and Photo-Catalysis in Purification of Dye Water over SrTi1−xMnxO3 Solid Solutions. J. Phys. Chem. C 2009, 113, 4970–4975. [Google Scholar] [CrossRef]
- Wiranwetchayan, O.; Promnopas, S.; Phadungdhitidhada, S.; Phuruangrat, A.; Thongtem, T.; Singjai, P.; Thongtem, S. Characterization of Perovskite LaFeO3 Synthesized by Microwave Plasma Method for Photocatalytic Applications. Ceram. Int. 2019, 45, 4802–4809. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni Ratios in Cobalt Nickel Mixed Oxide Catalysts on Methane Combustion. Appl. Catal. A Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Dintzer, T.; Teschner, D.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Methanol Oxidation over Model Cobalt Catalysts: Influence of the Cobalt Oxidation State on the Reactivity. J. Catal. 2010, 269, 309–317. [Google Scholar] [CrossRef]
- Zhong, L.; Kropp, T.; Baaziz, W.; Ersen, O.; Teschner, D.; Schlögl, R.; Mavrikakis, M.; Zafeiratos, S. Correlation between Reactivity and Oxidation State of Cobalt Oxide Catalysts for CO Preferential Oxidation. ACS Catal. 2019, 9, 8325–8336. [Google Scholar] [CrossRef]
- Toby, B.H.; von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]


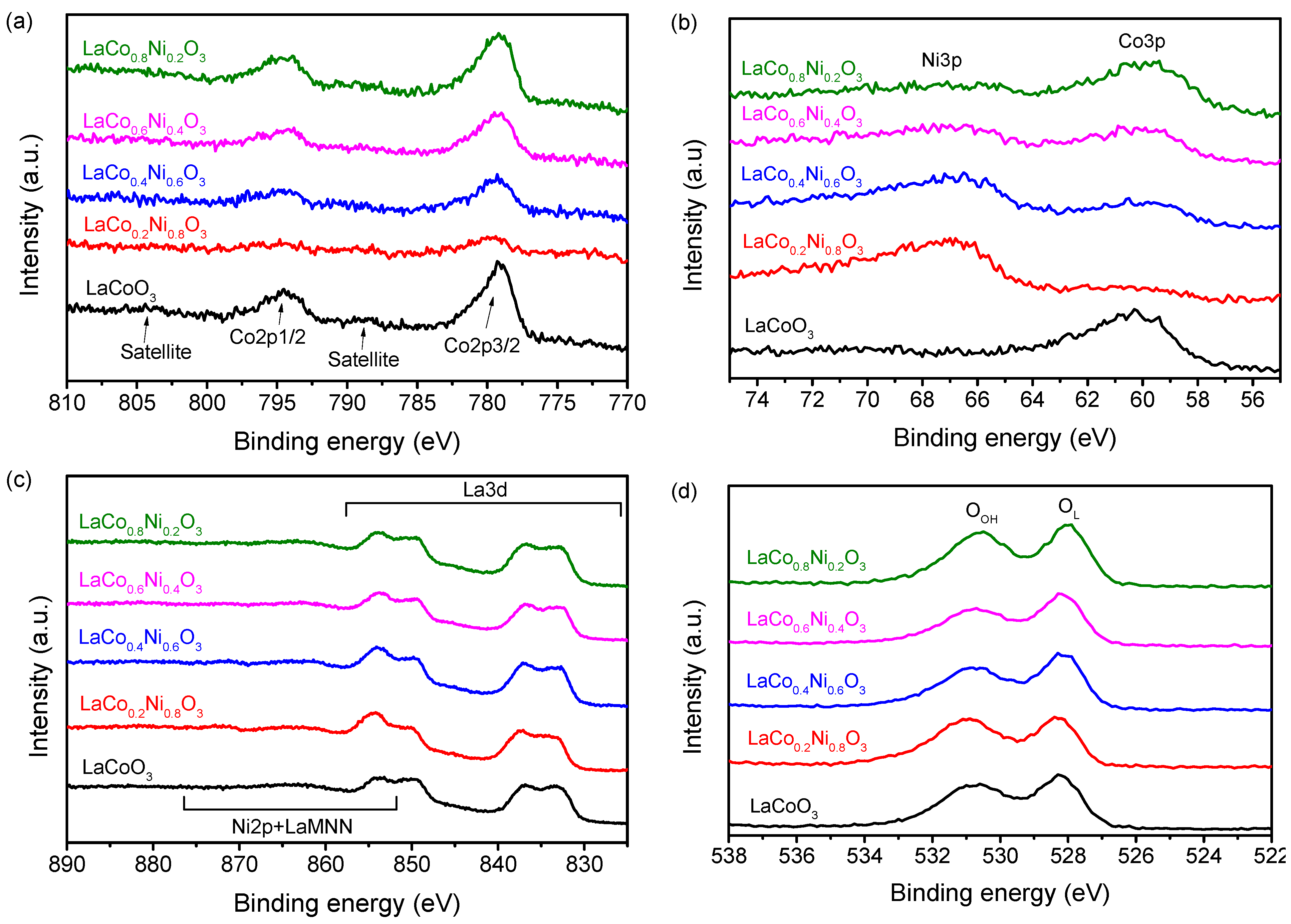

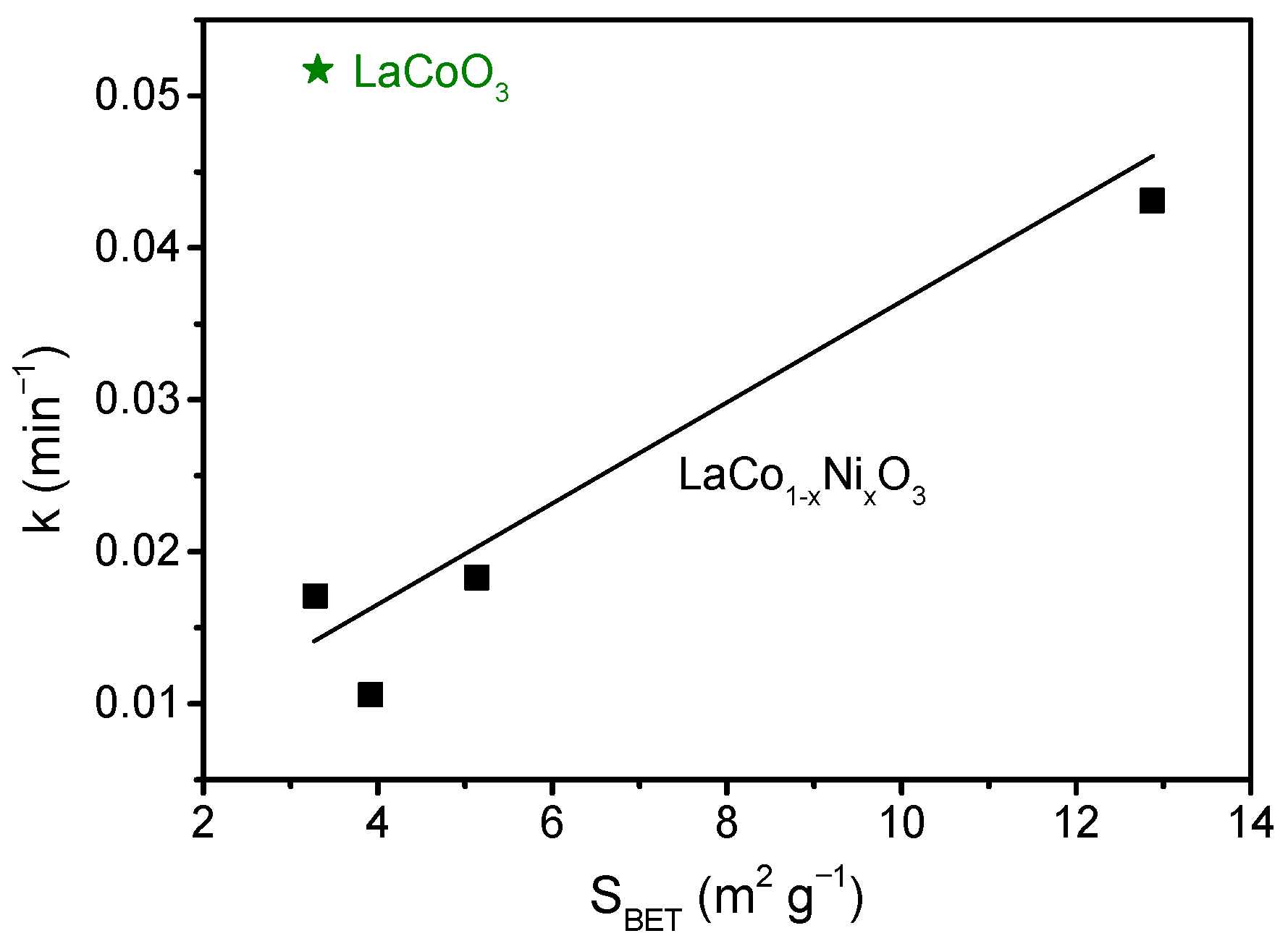
| Co 2p 3/2 (eV) | Ni 3p 3/2 (eV) | O 1s (eV) | Ni/Co a | Co/La a | Ni/La a | |
|---|---|---|---|---|---|---|
| LaCo0.2Ni0.8O3−δ | 779.8 b | 67.1 | 528.3 (34%) 530.9 (66%) | 10 (4.0) | 0.1 (0.2) | 0.9 (0.8) |
| LaCo0.4Ni0.6O3−δ | 779.3 (90%) 781.7 (10%) | 66.5 | 528.1 (40%) 530.7 (60%) | 1.5 (1.5) | 0.3 (0.4) | 0.7 (0.6) |
| LaCo0.6Ni0.4O3−δ | 779.2 (89%) 781.4 (11%) | 66.5 | 528.2 (43%) 530.7 (57%) | 1.25 (0.67) | 0.5 (0.6) | 0.6 (0.4) |
| LaCo0.8Ni0.2O3−δ | 779.2 (89%) 781.4 (11%) | 65.8 | 528.0 (40%) 530.6 (60%) | 0.42 (0.25) | 0.6 (0.8) | 0.2 (0.2) |
| LaCoO3−δ | 779.2 (78%) 780.9 (22%) | - | 528.1 (38%) 530.7 (62%) | - | 0.7 (1.0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, B.H.; Deganello, F.; La Parola, V.; Jørgensen, M.K.; Boffa, V.; Østergaard, M.B. Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B. Catalysts 2023, 13, 325. https://doi.org/10.3390/catal13020325
Christensen BH, Deganello F, La Parola V, Jørgensen MK, Boffa V, Østergaard MB. Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B. Catalysts. 2023; 13(2):325. https://doi.org/10.3390/catal13020325
Chicago/Turabian StyleChristensen, Benjamin H., Francesca Deganello, Valeria La Parola, Mads K. Jørgensen, Vittorio Boffa, and Martin B. Østergaard. 2023. "Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B" Catalysts 13, no. 2: 325. https://doi.org/10.3390/catal13020325
APA StyleChristensen, B. H., Deganello, F., La Parola, V., Jørgensen, M. K., Boffa, V., & Østergaard, M. B. (2023). Thermocatalytic Performance of LaCo1−xNixO3−δ Perovskites in the Degradation of Rhodamine B. Catalysts, 13(2), 325. https://doi.org/10.3390/catal13020325











