Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts
Abstract
:1. Introduction
2. Results and Discussion
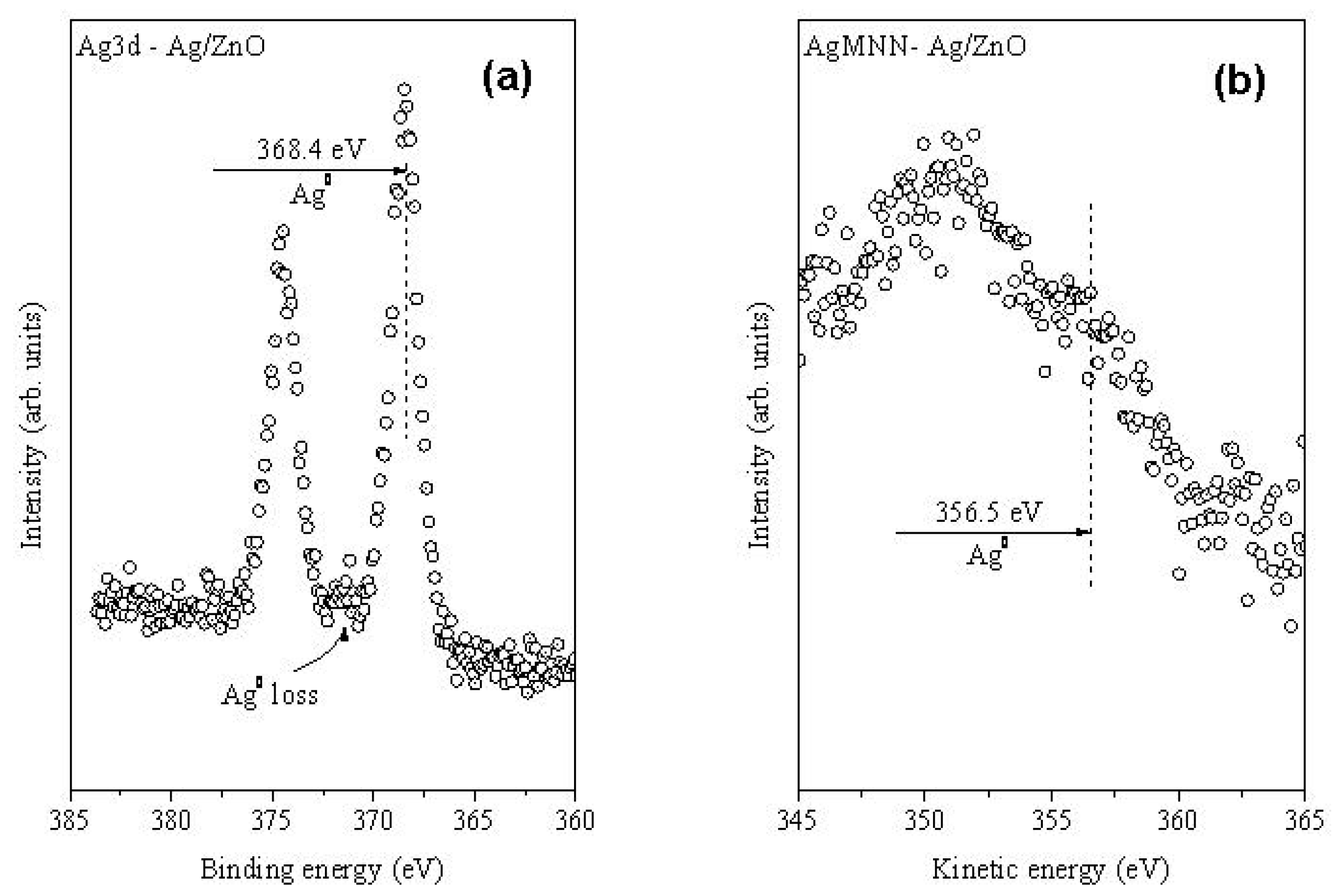
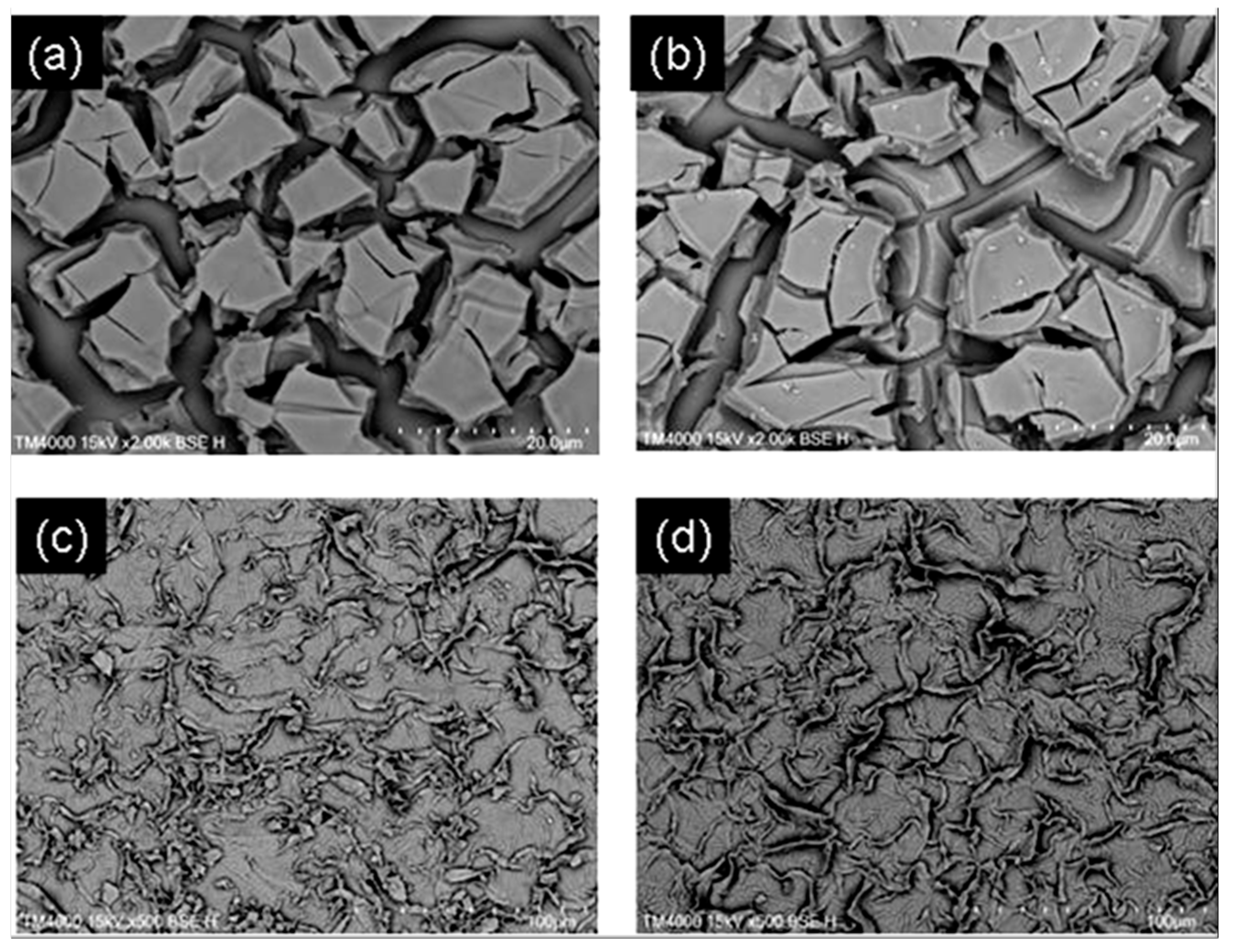
2.1. Structural Characterization
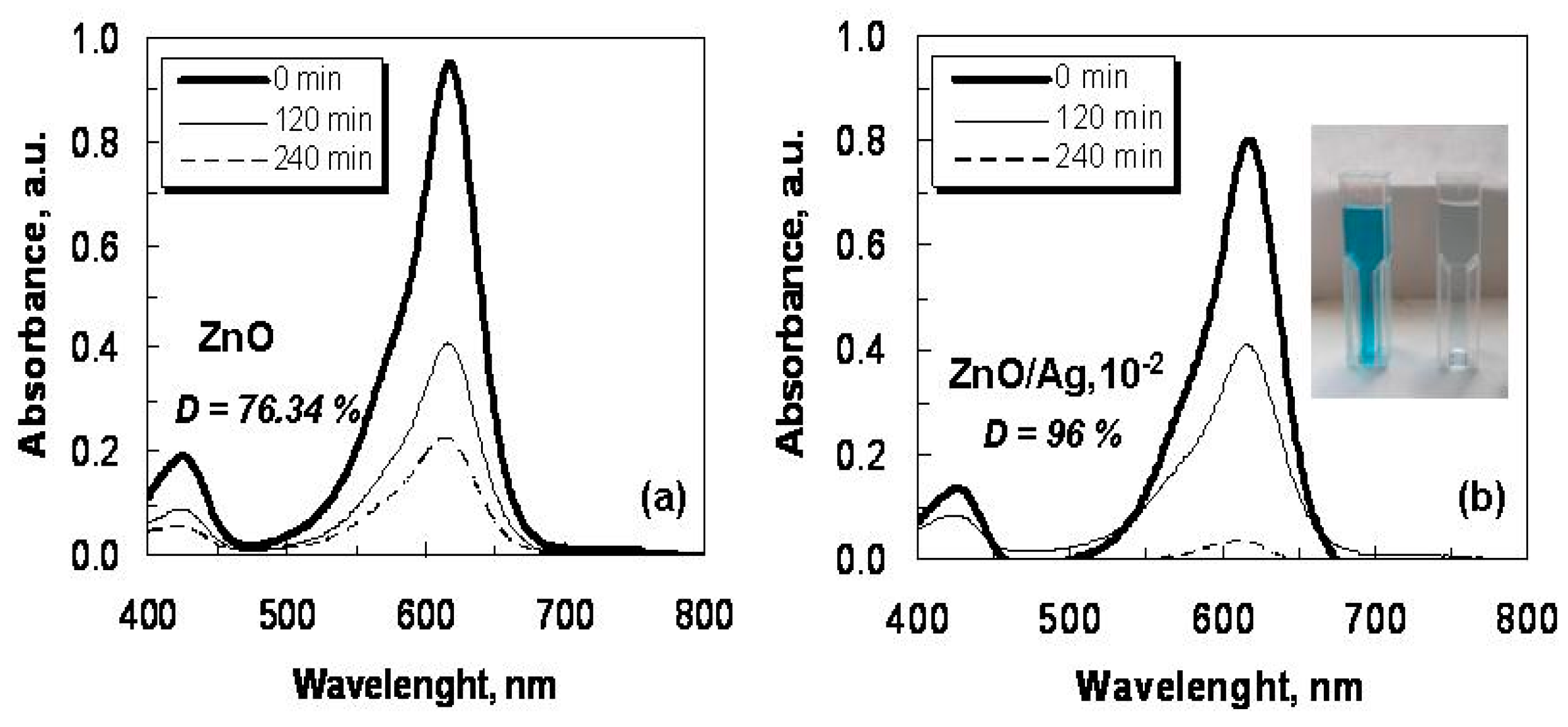
2.2. Optical Characterization
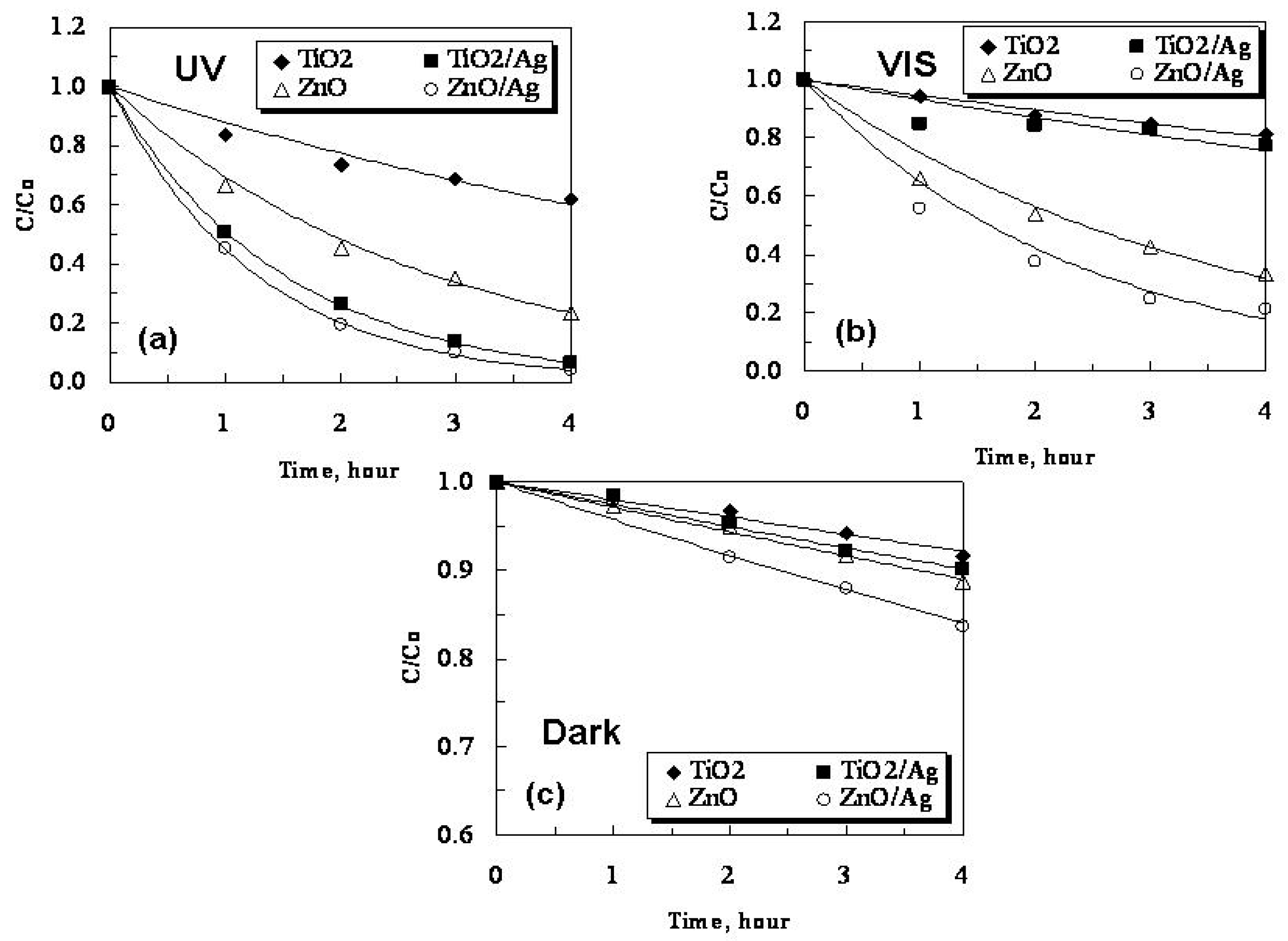
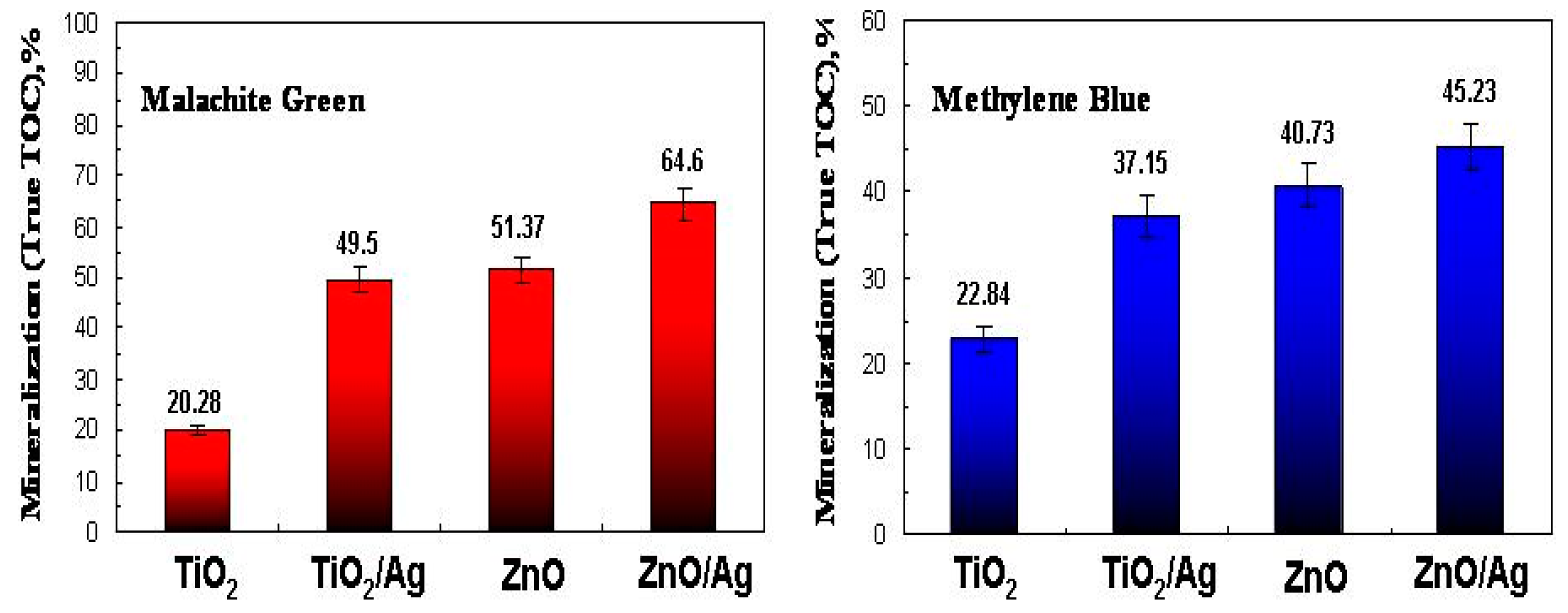
2.3. Photocatalytic Activity
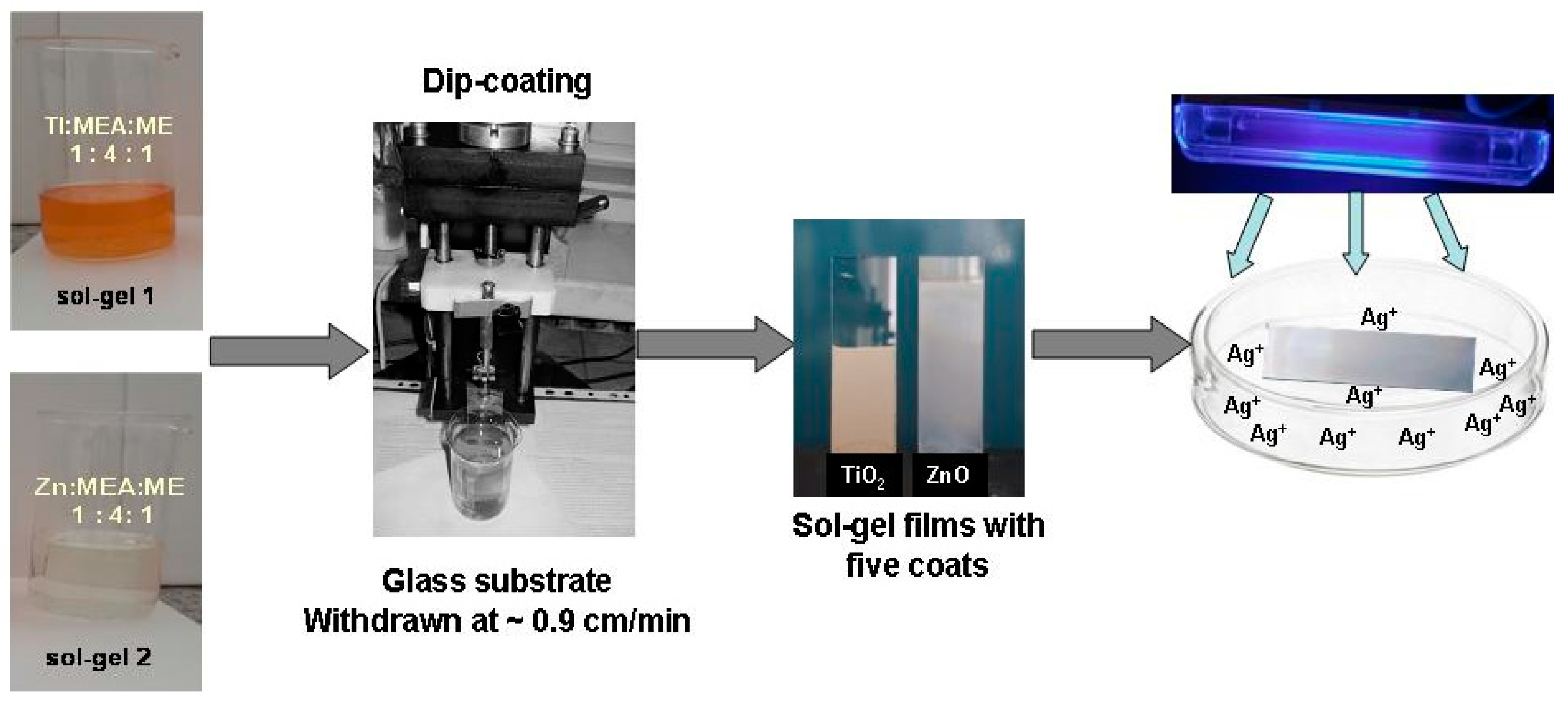
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Wang, A. Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Woodhead Publishing: Cambridge, UK, 2021; pp. 321–354. [Google Scholar]
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Lahmar, H.; Benamira, M.; Douafer, S.; Messaadia, L.; Boudjerda, A.; Trari, M. Photocatalytic degradation of methyl orange on the novel hetero-system La2NiO4/ZnO under solar light. Chem. Phys. Lett. 2021, 742, 137132. [Google Scholar] [CrossRef]
- Sidra, B.; Awais, A.; Mohsin, A.R.A.; Abdul, H.; Muhammad, S.; Syed, S.S.; Abdullah, A.K. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd(ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K.; Dimitrov, D.; Zaharieva, K.; Cherkezova-Zheleva, Z.; Eliyas, A. Effect of thermal and mechano-chemical activation on the photocatalytic efficiency of ZnO for drugs degradation. Arch. Pharm. Res. 2016, 39, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commum. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, H.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.; Kumar, A. Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K.; Dimitrov, D. Effect of the substrate on photocatalytic efficiency of ZnO for Malachite Green degradation. J. Chem. Tech. Metall. 2014, 49, 149–156. [Google Scholar]
- Simsek, E.B. Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–128. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Wei, Z.; Jiang, T.; Du, X.; Chen, W. Growth of N-doped BiOBr nanosheets on carbon fibers for photocatalytic degradation of organic pollutants under visible light irradiation. Powder Technol. 2014, 260, 84–89. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Chen, C.; Cheng, L.; Ding, H. A g-C3N4 supported graphene oxide/Ag3PO4 composite with remarkably enhanced photocatalytic activity under visible light. Catal. Commun. 2016, 73, 74–79. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Gallardo-Hernández, S.; Tellez-Cruz, M.M.; Solorza-Feria, O. Sol-gel synthesis of Ag-loaded TiO2-ZnO thin films with enhanced photocatalytic activity. J. Alloys Compd. 2019, 779, 908–917. [Google Scholar] [CrossRef]
- Tao, J.; Gong, Z.; Yao, G.; Cheng, Y.; Zhang, M.; Lv, J.; Shi, S.; He, G.; Jiang, X.; Chen, X.; et al. Effects of hydrothermal time on the morphologies of rutile TiO2 hierarchical nanoarrays and their optical and photocatalytic properties. J. Alloys Compd. 2016, 688, 605–612. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Rozyyev, V.; Murphy, J.G.; Barry, E.; Mane, A.U.; Sibener, S.J.; Elam, J.W. Vapor-phase grafting of a model aminosilane compound to Al2O3, ZnO, and TiO2 surfaces prepared by atomic layer deposition. Appl. Surf. Sci. 2021, 562, 149996. [Google Scholar] [CrossRef]
- Hakki, H.K.; Allahyari, S.; Rahemi, N.; Tasbihi, M. Surface properties, adherence, and photocatalytic activity of sol–gel dip-coated TiO2–ZnO films on glass plates. CR Chim. 2019, 22, 393–405. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Morales-Luna, M. Enhanced photocatalytic activity of TiO2-ZnO thin films deposited by dc reactive magnetron sputtering. Ceram. Int. 2017, 43, 8831–8838. [Google Scholar] [CrossRef]
- Fouad, S.S.; Parditka, B.; Atyia, H.E.; Baradács, E.; Bekheet, A.E.; Erdélyi, Z. AC conductivity and dielectric parameters studies in multilayer TiO2/ZnO thin films produced via ALD technique. Chin. J. Phys. 2022, 77, 73–80. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science—The Physics and Chemistry of Sol-Gel Processing, 3rd ed.; Academic Press: San Diego, CA, USA; London, UK, 1991; pp. 1–881. [Google Scholar]
- Perumal, S.; Gnana, C. Synthesis and characterization studies of solvothermally synthesized undoped and Ag-doped TiO2 nanoparticles using toluene as a solvent. J. Eng. Res. 2014, 4, 184–187. [Google Scholar]
- Yıldırım, O.A.; Unalan, H.E.; Durucan, C. Highly efficient room temperature synthesis of silver-doped zinc oxide (ZnO: Ag) nanoparticles: Structural, optical, and photocatalytic properties. J. Am. Ceram. Soc. 2013, 96, 766–773. [Google Scholar] [CrossRef]
- Pargar, F.; Kolev, H.; Koleva, D.; Breugel, K. Potentiometric Response of Ag/AgCl Chloride Sensors in Model Alkaline Medium. Adv. Mat. Sci. Eng. 2018, 3, 8135492. [Google Scholar] [CrossRef] [Green Version]
- Todorova, S.; Kolev, H.; Shopska, M.; Kadinov, G.; Holgado, G.; Caballero, A. Silver-based catalysts for preferential CO oxidation in hydrogen-rich gases (PROX). Bulg. Chem. Commun. 2018, 50, 17–23. [Google Scholar]
- Bhatt, B.C.; Dhabekar, B.; Kumar, R.; Gundu Rao, T.K.; Lakshmanan, A.R. Defect centres and thermoluminescence in CaSO4:Dy, Ag phosphor. Radiat. Prot. Dosim. 2006, 119, 53–56. [Google Scholar] [CrossRef]
- Coronado, J.M.; Maira, A.J.; Conesa, J.C.; Yeung, K.L.; Augugliaro, V.; Soria, J. EPR Study of the Surface Characteristics of Nanostructured TiO2 under UV Irradiation. Langmuir 2001, 17, 5368–5374. [Google Scholar] [CrossRef]
- Singh, R.; Barman, P.B.; Sharma, D. Synthesis, structural and optical properties of Ag doped ZnO nanoparticles with enhanced photocatalytic properties by photo degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2017, 28, 5705–5717. [Google Scholar] [CrossRef]
- Kotlhao, K.; Mtunzi, F.M.; Pakade, V.; Laloo, N.; Ejidike, I.P.; Modise, S.J.; Moutloali, R.M.; Klink, M.J. Enhancing the photocatalytic degradation of selected chlorophenols using Ag/ZnO nanocomposites. MRS Adv. 2018, 3, 2129–2136. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Czili, H.; Horváth, A. Photocatalytic oxidation of oxalic acid enhanced by silver deposition on a TiO2 surface. J. Photochem. Photobiol. A 2003, 154, 195–201. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Cat. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Ju, Y.; Qiao, J.; Peng, X.; Xu, Z.; Fang, J.; Yang, S.; Sun, C. Photodegradation of malachite green using UV–vis light from two microwave-powered electrodeless discharge lamps (MPEDL-2): Further investigation on products, dominant routes and mechanism. Chem. Eng. J. 2013, 221, 353–362. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Lee, S. Insight into Malachite Green Degradation, Mechanism and Pathways by Morphology-Tuned α-NiMoO4 Photocatalyst. Photochem. Photobiol. 2018, 94, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Das, K.; Parida, K. Indulgent of the physiochemical features of MgCr-LDH nanosheets towards photodegradation process of methylene blue. J. Colloid Interface Sci. 2023, 634, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]







| Sample | Unit Cell Parameters, Å | Crystallite Size, nm | Microstrains, ×10−3 a.u. |
|---|---|---|---|
| TiO2 | a = b = 3.7873; c = 9.5075 | 31.2 | 7.6 |
| TiO2/Ag | a = b = 3.7795; c = 9.4986 | 26.9 | 6.7 |
| ZnO | a = b = 3.2524; c = 5.2124 | 48.4 | 0.9 |
| ZnO/Ag | a = b = 3.2518; c = 5.2105 | 42.9 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneva, N.; Bojinova, A.; Papazova, K. Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts. Catalysts 2023, 13, 245. https://doi.org/10.3390/catal13020245
Kaneva N, Bojinova A, Papazova K. Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts. Catalysts. 2023; 13(2):245. https://doi.org/10.3390/catal13020245
Chicago/Turabian StyleKaneva, Nina, Assya Bojinova, and Karolina Papazova. 2023. "Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts" Catalysts 13, no. 2: 245. https://doi.org/10.3390/catal13020245
APA StyleKaneva, N., Bojinova, A., & Papazova, K. (2023). Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts. Catalysts, 13(2), 245. https://doi.org/10.3390/catal13020245






