Abstract
Porous polymeric frameworks have received great interest over the past few years because of their nonstop growth as crystalline porous polymeric materials connected through covalent bonds and versatile utilities in diverse fields. The production of high-value organic compounds via sustainable and environment-friendly methods is an uphill struggle for researchers. The elegant strategy of using carbon dioxide as a C1 building block is an intriguing platform owing to its non-toxicity, easy accessibility, natural abundance, recyclability, non-flammability, and cheapness. Additionally, CO2 levels are regarded as the main contributor to the greenhouse effect (the most abundant greenhouse gas across the globe) and the aforementioned strategy needs to mitigate CO2 emissions. This present study describes the synthesis of silver nanoparticles (AgNPs) embedded in a porous polymeric framework, a reusable heterogeneous catalyst (recyclable over 5 times), TpMA (MC)@Ag. The synthesized catalyst is characterized by using FT-IR, PXRD, XPS, FE-SEM, TEM, EDAX, TGA DTA, and N2 sorption studies. Additionally, the catalysts can be easily recycled to generate the desired α-alkylidene cyclic carbonates and oxazolidinone compounds under solvent-free conditions. This research demonstrates the potential of nanoporous 2D porous polymeric framework-based materials in the area of catalysis, specially, in CO2 capture and chemical fixation. These findings offer a promising approach for the chemical fixation of CO2 into α-alkylidene cyclic carbonates and oxazolidinones from propargylic alcohols utilizing AgNPs embedded in a 2D catalyst, which functions as a potential heterogeneous catalyst under mild conditions (e.g., solvent-free approach).
1. Introduction
The emission of carbon dioxide (CO2) caused by human activity is responsible for the global environmental crisis and has had a significant impact on the principles of green and sustainable chemistry. CO2 is an appealing, plentiful, affordable, non-hazardous, and sustainable chemical resource, making it an attractive option for atom economy [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The use of CO2 has gained substantial attention in the past decade due to its low cost and potential as a C1 feedstock for various fine chemicals. The sustainable synthesis of value-added chemicals shows great potential for decarbonization of the chemical industry via the utilization of carbon dioxide (CO2). It is noteworthy that the current chemical production is heavily dependent on carbon feedstocks obtained from fossil fuels. As a result, significant research efforts have been increased to generate CO2-sequestering materials [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. CO2 utilization shows tremendous importance in the area of atom economy since it is a fascinating, plentiful, low-cost, non-toxic, and sustainable chemical feedstock [33,34,35,36,37,38]. Two key tactics that might be used to conquer this obstacle are a) carbon dioxide capture and sequestration/storage and b) carbon dioxide capture and its utilization [26]. Carbon dioxide conversion into portable compounds like amides, esters, carboxylic acids, aldehydes, and alcohols has gained large attention in current years. In the present context, the utilization of CO2 in the synthesis of 2-oxazolidiones as an environmentally acceptable alternative has significant interest [27,28,29,30,31,32,33]. Although there are several ways to incorporate CO2 into 2-oxazolidinones, most of these reactions often need costly, hazardous organic solvents, which have negative health and environmental consequences [34,35,36,37]. As the notion of green chemistry, researchers have concentrated their efforts on the development of solvent-free methods [38,39]. In this regard, numerous solvent-free techniques for the synthesis of 2-oxazolidinones via CO2 have been published in the literature. A thorough analysis of this hot study issue appears appropriate for the recent achievements and all the changes that have taken place in this fascinating research field. 2-oxazolidinones are a type of an extremely useful family of N-containing five-membered heterocyclic compounds found in a variety of pharmaceutical substances like toloxatone, cimoxatone, zolmitriptan befloxatone, and others [18], as well as agrochemicals such as phosalone [40,41,42,43]. Furthermore, they are present in many anti-bacterial drugs (e.g., Linezolid, Toloxatone, Tedizolid) and are extremely active against multiply resistant infections caused by Gram-positive bacteria, and also frequently utilized in the paint and varnish businesses and the polymer industry. 2-oxazolidinones have also been employed extensively as chemical intermediates [44,45] and chiral auxiliaries in asymmetric synthesis. The utilization of carbon dioxide as the C1 synthon for the generation of oxazolidinones has been extensively recorded in a variety of procedures, such as the cycloaddition of aziridines through CO2 of [33,46] the chemical interaction of 2-aminoalcohols and CO2 [47], carboxylative cyclization of propargylic amines [25,35], and the three-component coupling of propargyl alcohols, primary amines, and CO2. The most appealing approach for producing oxazolidinone out of the procedures is the three-component reaction. Homogeneous catalytic media were employed for the majority of previously published investigations on the one pot three-component coupling process to produce oxazolidinone, along with extreme reaction conditions such as high temperature and high pressure of CO2. However, all of these procedures have specific constraints such as a long reaction time and limited substrate scope. So, it is necessary to develop a technique that operates under moderate reaction circumstances. Propargyl alcohols and amines can absorb CO2 by carboxylative cyclization, terminal alkynes through terminal carboxylation, cycloaddition on epoxides, N-methylation, and N formylation processes [48]. So nowadays, designing appropriate catalysts for CO2 fixation reactions is one of the growing fields of research because of the adaptability of these reactions. One of the most efficient and alluring ways of CO2 reformation is the carboxylative cyclization process for the production of α-alkylidene cyclic carbonates from propargylic alcohol. The α-alkylidene cyclic carbonates are a family of heterocyclic compounds that are helpful for a number of inorganic synthesis processes [18,49,50]. This group of compounds is often found in natural substances including guaianolide hololeucin, genkwanin I, and cycloolivil, among many others. These substances are mostly used in pharmaceutical, agricultural, and pesticide industries [51]. For the generation of valuable compounds, a number of techniques have been developed till now. These methodologies include metal-supported catalysts, for example, Cu [52], Ag [53,54,55], palladium [56], zinc [57], tungsten [58], and cobalt [59], along with organocatalysts [60,61,62,63,64,65], IL-based catalysts [66], and (MOFs) metal-organic frameworks [67]. Meanwhile, many of the previously published approaches have certain drawbacks, including the usage of extreme reaction temperatures, the challenge of recovering the catalyst, and the use of potentially dangerous organic solvents or bases. Owing to the limitations of earlier findings, further research is still needed for the generation of α-alkylidene cyclic carbonates within favorable reaction circumstances. Our team has disclosed a catalytic technique in which 2-oxazolidinones and α-alkylidene cyclic carbonates are synthesized at RT from carbon dioxide using a heterogeneous porous polymeric framework based on silver metal under solvent-free conditions. The most interesting and significant subset of porous materials in organic chemistry is the porous polymeric framework. The porous polymeric framework, a unique family of porous crystalline polymers, has appeared as heterogeneous catalysts for different organic reactions. Due to their highly ordered, pre-designable, and functionalizable porous architectures [33], polymer catalysts enable the exact integration of catalytic centers within the framework matrix. Those are extensively used in catalysis [68], adsorption [69,70], sensing [71,72], gas storage [73], and in many other important applications [74] due to their intriguing qualities including huge surface area, excellent chemical resistance, structural adaptability, and uncomplicated surface functionalization. However, due to the very complex growth procedure containing crystallization, assembly, and polymerization, among other things, generates a well-organized and perfectly ordered 2D stacked porous polymeric framework sheet. In general, the documented building techniques of 2D sp2 carbon-conjugated porous polymeric catalysts may be classified into two categories: bottom-up and top-down strategy. The catalyst TpMA (MC) was synthesized by combining melamine (1,3,5-triazine 2,4,6triamine (Tt)) with 2,4,6-triformylphloroglucinol (Tp) aldehyde. Previously, many triazine-containing polymers were synthesized in the presence of a metal catalyst and at higher temperatures. Due to its low reactivity, synthesizing a crystalline porous TpMA (MC) catalyst from a melamine building block without the use of a metal catalyst proved extremely difficult. We generated a fairly crystalline porous polymeric framework TpMA (MC) after numerous attempts with various solvent combinations. The development of metal nanoparticles has garnered significant attention in the area of nanotechnology because of their evident characteristics, such as geometry, shape, and distribution. Nanoparticles also have an influence on many facets of mankind in addition to displaying wholly original or superior characteristics. Because of their numerous uses in areas such as catalysis, energy, biomedicine, materials, etc., silver nanoparticles, also referred to as Ag NPs, are considered to be among the most promising nanoparticles in the nanotechnology sector [66,67,68,69]. Ag NPs can be generated through a diverse range of methods, including physical, chemical, and biological processes. In the physical procedure, expensive machinery and high suction are utilized; in the course of the chemical process, the synthesis is facilitated through the utilization of toxic capping agents. Hence, they are not environment-friendly, affordable, or viable. As a consequence, scientists have focused on synthesizing nanoparticles that are affordable, recyclable, and environment-friendly by using natural ingredients and microorganisms [70,71,72,73]. In this investigation, our group describes the production and characterization of a nanoporous polymeric framework assisted with Ag complex (TpMA (MC)@Ag) that is very active at fixing CO2 in chemical processes to synthesize oxazolidinones and α-alkylidene cyclic carbonates from propargyl alcohols. The catalyst is particularly effective in producing 2-oxazolidinones through CO2 fixation from terminal propargylic amine at 1 atm pressure, under mild reaction environment, and in the presence of a stoichiometric quantity of DBU. Similarly, the catalyst is particularly efficient at producing α-alkylidene cyclic carbonates by carboxylative cyclizing propargylic alcohols with CO2 at R.T. under 1 bar of pressure without solvent. Both catalytic conversions cannot take place without the presence of TpMA (MC)@Ag, thus indicating the pivotal role that the catalyst plays in activating and trapping CO2 molecules. Furthermore, it is noteworthy that the heterogeneous catalyst can be effortlessly recovered and utilized repeatedly, rendering it highly sustainable. The catalyst is recyclable up to five times without appreciable loss of its activity.
2. Results and Discussion
The two-dimensional porous polymeric catalyst TpMA (MC) and silver metal-incorporated TpMA (MC)@Ag catalyst were synthesized as the following schematic diagram, Scheme 1, shows, and the Supporting Information file shows the detailed procedure.

Scheme 1.
Schematic diagram for the synthesis of porous polymeric catalyst TpMA (MC)@Ag.
2.1. Characterization Techniques
2.1.1. Powder X-ray Diffraction (PXRD) Analysis
PXRD patterns of the nanoporous polymeric catalyst TpMA (MC)@Ag described the intense unique peak at 2θ = 6.3° which was attributed to the reflection of greatest intensity from Miller plane (100) (Figure 1). This peak is joined by tiny peaks located at 2θ = 11.7°, 14.1°, and 15.2° that are attributed to the (110), (200), and (210) facets correspondingly. The extensive peak of ∼27.3° corresponds to the (001) plane and can be attributed to the π–π stacking of the porous polymeric catalyst TpMA (MC)@Ag layers. The wide-angle XRD patterns of AgNPs embellished porous polymeric framework match to an FCC structure. As a result, the porous polymeric catalyst TpMA (MC)@Ag nanomaterial exhibited three additional diffraction peaks from (111), (200), and (220) reflections, and it can be categorized as the peak for the FCC model of reactive metallic Ag. The broad-angle XRD graphs certainly demonstrated the loading of reactive metallic AgNPs on the outer surfaces of the crystalline porous polymeric frameworks. The calculated d-spacing value is 3.4 Å (d001) between the (001) facets and was determined through the implementation of Bragg’s equation, whereby n = 1 is assigned to the 001 reflection planes, nλ = 2d001sin θ, where λ = 0.15406 nm). Scherrer’s equation was used to evaluate the particle sizes of Ag NPs, which were estimated to be in the range of 14 to 20 nm. The (111), (200), (220), and (311) facet Ag NPs sizes are found to be 19.45 nm, 14.77 nm, and 16.65 nm, 18.97 nm, respectively, using the Debye–Scherrer formula (SI). The TEM results are consistent with the calculated values derived from Scherrer’s equation. PXRD patterns of the catalyst remain almost the same after several runs, proofing the reusability of the porous polymeric catalyst TpMA (MC)@Ag catalyst (Figure S4).
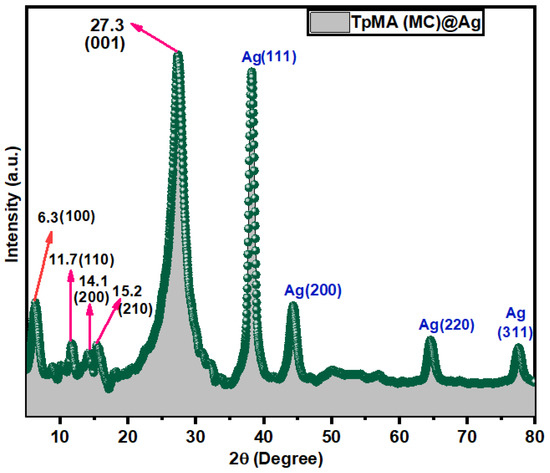
Figure 1.
Powder XRD of porous polymeric catalyst TpMA (MC)@Ag.
2.1.2. Fourier Transform Infrared (FT-IR) Spectroscopy
The successful generation of the porous polymeric catalyst TpMA (MC)@Ag) was confirmed by FT-IR spectroscopy (Figure 2). The presence of C=O at 1643 cm−1 confirmed the formation of the aldehyde. The N-H stretching frequency of the primary amine (3464 cm−1) and C=O group (1643 cm−1) of aldehyde vanished in the synthesized porous polymeric framework, indicating that starting materials were completely utilized after porous polymeric framework production. The FT-IR spectra of the freshly prepared porous polymeric framework (porous polymeric catalyst TpMA (MC)) exhibit two bands at 1232 cm−1 and 1523 cm−1, owing to C-N and C=C bonds correspondingly, which indicate the generation of a β-ketoenamine link in the framework. Furthermore, an absorption band appeared at 2923 cm−1 corresponding to C–H asymmetric stretching vibration (Figure S2). It is noteworthy that all abovementioned characteristic bands were also present in the porous polymeric framework TpMA (MC) @Ag, but with slight positional shifts. This observation serves to confirm the attachment of silver nanoparticles within the porous polymeric framework.

Figure 2.
FT_IR spectra of porous polymeric catalyst TpMA (MC)@Ag catalyst, Triformyl phloroglucinol (TFP), and Melamine.
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
XPS analysis of porous polymeric catalyst TpMA (MC)@Ag matrix was performed to identify the oxidation state and elemental composition of silver nanoparticles grafted onto the abovementioned matrix. Figure 3 reveals individual information about carbon, silver, nitrogen, and oxygen atoms of porous polymeric catalyst TpMA (MC)@Ag from XPS whereas Figure 3a shows the wide-range XPS spectrum of complexes.

Figure 3.
(a) Wide-range XPS survey spectrum of porous polymeric catalyst TpMA (MC)@Ag porous polymeric framework and narrow-range XPS spectra of (b) Ag 3d, (c) C 1s, (d) N 1s, and (e) O 1s elements of porous polymeric catalyst TpMA (MC)@Ag.
Figure 3a depicts the wide-range XPS of the complex, where C 1s, N 1s, and O 1s components were observed, with corresponding binding energies of 284.5, 398.9, and 531.3 eV, respectively. The high-resolution C 1s picture on deconvolution exhibits bands at 288.4, 287.3, 286.2, and 284.5 eV, which correspond to the binding energies of C=O, C=N, C-N, and C=C, respectively, as shown in the XPS spectra of porous polymeric catalyst TpMA (MC)@Ag. The XPS spectrum of the Ag 3d (Figure 3b) is divided into three sections: Ago, Ag…N=, and Ag…O-. The existence of metallic Ag is shown by the peaks at 368.50 eV and 374.55 eV corresponding to Ag 3d5/2 and Ag 3d3/2. The corresponding band gap is calculated to be 6.0 eV.
The peaks at 375.39 eV and 369.44 eV are attributed to Ag…N=, while those at 373.45 eV and 367.45 eV are attributed to Ag…O-, according to the literature. The Ag in Ag…N= and Ag…O- must also exist as Ago since the difference in spin energy is also 6.0 eV. Ago strongly binds to the N sites and O sites in the porous polymeric framework, as indicated by a small change in binding energy compared to that of metallic Ag. As a result, the nitrogen and oxygen atoms in this porous polymeric catalyst played a significant role in anchoring Ago. As observed in the N 1s image of porous polymeric catalyst TpMA (MC)@Ag, the presence of Ag…N= can be attributed to the band observed at 401.47 eV, which has the ability to bind with the silver centers. Additionally, the high-resolution N 1s image also reveals bands appearing at 398.22 eV and 399 eV which correspond to sp2 hybridized C-N=C in the triazine functional group and C=N, respectively. The XPS spectra with high resolution demonstrate the O 1s peaks at 531.0 eV and 532.4 eV (Figure 3e). Interaction between Ag-O can also be detected in the O1s spectrum, where the Ag-O peak is observed at 531 eV [75]. These various observations collectively establish the successful incorporation of silver nanoparticles in the COF.
2.1.4. BET Surface Area Measurement
N2 adsorption–desorption isotherms were executed at a temperature of 77 K to investigate the internal porosity present in the porous polymeric framework material embedded with silver nanoparticles. The N2 sorption isotherm exhibited by the sponge-like porous polymeric framework TpMA (MC), a covalent organic framework, demonstrates a type I behavior, while simultaneously displaying certain patterns like type IV pattern, as Figure 4 depicts. The presence of several micropores in catalyst TpMA (MC) is suggested by the excessive N2 adsorption within the low-pressure range of 0–0.1 bar. The observed surface area and pore volume of the resultant porous polymeric framework are estimated to be 364 m2g−1 and 0.30 ccg−1, respectively. The pore size distribution of porous polymeric catalyst TpMA (MC)@Ag was evaluated through the application of the Barrett Joyner Halenda (BJH) technique. The distribution of pore size of the TpMA (MC)@Ag catalyst using N2 at 77 K revealed the presence of mesopores with a pore dimension of 2.06 nm. BET surface area of the reused TpMA (MC)@Ag catalyst was around 256 m2g−1 and the pore size was reduced to 1.26 nm (Figure S3). This reduction in pore size and BET surface area indicates the blockage of some pores in the porous polymer.
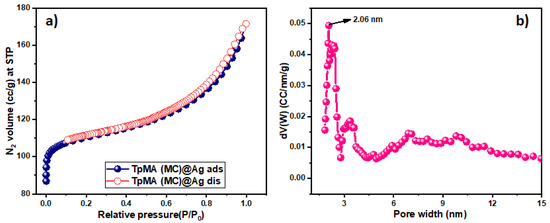
Figure 4.
(a) N2 adsorption–desorption curve of porous polymeric catalyst TpMA (MC)@Ag. (b) Corresponding pore size distribution of the catalyst TpMA (MC)@Ag.
2.1.5. Microscopic Analysis
SEM images, as Figure 5a–d show, reveal that the catalytic system of porous polymeric catalyst TpMA (MC) and TpMA (MC)@Ag comprises numerous indistinguishable particles, which exhibit a remarkable resemblance to rock-like morphology. The porous polymeric catalyst TpMA (MC)@Ag catalytic system is entirely insoluble in the majority of common organic solvents. The FESEM images of the porous polymeric catalyst TpMA (MC)@Ag material suggest the presence of primarily uniform particles that appear to be haphazardly aggregated. The TEM image of TpMA (MC) (Figure 6g–i) provides insight by demonstrating that the layered sheet-like porous materials consisted of micropores and mesopores, featuring a microcrystalline arrangement. The HRTEM images (Figure 6) of the silver-decorated polymeric catalyst TpMA (MC)@Ag reveal a layered sheet-like morphology with an average particle diameter of 14–20 nm, as well as a random void, present in the catalyst. A TEM image of low-resolution displays uniform distributions of silver nanoparticles on TpMA (MC)@Ag polymer. From the HR-TEM image of TpMA (MC)@Ag, the specific portion (yellow box) demonstrates that some of the silver nanoparticles present on the surface of the polymer exhibit remarkable lattice fringes. The corresponding SAED pattern, as Figure 6f shows (based on the yellow-framed portion), exhibits remarkable lattice fringes and characteristic diffraction rings at (111), (200), (220), and (311) that align with the PXRD spectrum of TpMA (MC)@Ag (Figure 1) which show the successful loading of silver. The calculated lattice spacings of silver (d = 0.235 nm and 0.204 nm) are attributed to the (111) and (200) plane of silver, respectively (Figure 1). Additionally, EDAX recommended that the silver loading in the polymeric compound be at 2.53 wt%.
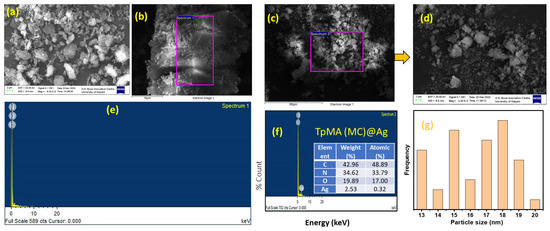
Figure 5.
FE-SEM images of the porous polymeric catalyst TpMA (MC) and TpMA (MC)@Ag materials at two different scales (a–d), (e,f) EDAX of porous polymeric catalyst TpMA (MC) and TpMA (MC)@Ag, and (g) particle size distribution plot.

Figure 6.
TEM images of porous polymeric catalyst TpMA (MC)@Ag at different scales: (a) 100 nm, (b) 50 nm, (c) 10 nm, (d) 2 nm, (e) part of (d), shows unclear lattice fringes of some AgNPs, (f) SAED pattern of porous polymeric catalyst TpMA (MC)@Ag material, and (g–i) TEM images of only TpMA (MC) catalyst.
2.1.6. TGA Analysis of Porous Polymeric Catalyst TpMA (MC)@Ag
Figure 7 presents the TGA plot of TpMA (MC)@Ag and TpMA (MC) catalysts. The thermal degradation process for both the catalysts are observed to be very slow at the temperature range of 160 °C to 450 °C. Both TpMA (MC)@Ag and TpMA (MC) catalysts show a similar type of thermal degradation process. After reaching the aforementioned temperature range, it was observed that the rate of degradation accelerated significantly. This suggests that the catalyst is capable of maintaining thermal stability up to 450 °C. Nevertheless, it is important to note that prior to reaching this temperature limit, the catalyst undergoes a minor loss in mass. This loss can be attributed primarily to the detachment of surface-adsorbed H2O molecules and other adsorbed gases present within the porous structure of the catalyst.

Figure 7.
Thermogravimetric (TGA) analysis of porous polymeric catalyst TpMA (MC) and TpMA (MC)@Ag.
2.2. Catalytic Activity
2.2.1. Generation of 2-Oxazolidinones over Porous Polymeric Catalyst TpMA (MC)@Ag Porous Polymeric Framework via Carboxylative Cyclization of Terminal Propargyl Alcohol and Amine
Carboxylative cyclization of propargyl alcohols and amines with carbon dioxide to produce 2-oxazolidinones have received a lot of interest recently [76,77]. Numerous catalytic systems centered around metals, including Cu [78], Pd [79], Au, Ru, and Ag [80,81], as well as protic ionic liquids, organo-catalysts like super bases, and heterocyclic carbenes, have found application in the synthesis of 2-oxazolidinones using the aforementioned method. However, a common limitation of the aforementioned catalytic systems is their reliance on homogeneous reaction conditions, leading to difficulties in terms of recyclability and reusability of the catalysts. Additionally, in most instances, high-pressure CO2 has been utilized as part of the process. In a few earlier reports, 2-oxazolidinones were produced using the carbon dioxide fixation method from propargyl alcohols and amines. In this instance, we were able to make 2-oxazolidinones from amines and propargyl alcohols using CO2 fixation in environment-friendly, ambient circumstances using noble metals like Ag without the need for supercritical or high-pressure CO2, solvents, or additives, etc (Scheme 2). Initially, using a CO2 balloon, the reaction involving (1A) and (1B) was conducted without the presence of any catalyst at room temperature (Table 1, entry 1). After 6 h of reaction, no product appeared. After that, the same reaction was seen with our synthesized catalyst TpMA (MC)@Ag (15 mg) without base; to our pleasure, after 6 h of reaction, 48% of the required product was obtained (Table 1, entry 2). Afterward, we investigated the impact of varying catalyst incorporation in the reaction. Reduced quantity of catalyst stacking (10 mg) in the presence of a base (DBU) led to a decrease in the rate of product yield (Table 1, entry 3). Conversely, an excessive weight of catalyst incorporation did not result in a substantial increase in the product yield percentage (Table 1, entry 4). As a result, 15 mg of catalyst loading was determined to be appropriate for the catalytic process at RT (Table 1, entry 5), and 35% product yield was recorded with just TpMA (MC) in the reaction (Table 1, entry 6). Likewise, a 35–29% yield was achieved using the silver salt (AgNO3, AgI, AgOAc) catalyst (Table 1, entry 7–9). As a result, Ag metal porous polymeric framework TpMA (MC) had an impact on the catalytic process. Instead of DBU, diverse categories of bases were used, for instance, DIPEA, DABCO, t-BuOK, NEt3, K2CO3, Cs2CO3, etc., whereby (12–36%) yield was obtained (Table 1, entry 10–15). So, the maximum final product amount (97%) was achieved while 15 mg porous polymeric framework was employed for 6 h without solvent and in the assistance of the base DBU (1 mmol). We also examined the influence of the DBU quantity on the catalytic activity. When 3 mmol of DBU was used, no significant change in the final product amount was observed (Table 1, entry 16). In a solvent-free environment at room temperature (RT), we studied the rate of change in mentioned reaction over time under the influence of porous polymeric catalyst TpMA (MC)@Ag catalyst (15 mg). Derived from the collected data, the kinetic graph (yield % vs. time) is constructed, as Figure 8 depicts. The illustration distinctly showcases the effective catalytic synthesis of 2-oxazolidinones, reaching its maximum yield within 6 h of reaction time. After determining the optimal reaction conditions, we conducted an investigation encompassing a diverse range of substrates. This involved using (1A) and (2A), as well as aromatic and aliphatic amines, all under atmospheric CO2 pressure. Table 2 presents the outcomes of the above reaction.
The reaction involving (1A) and (1B), as well as the reaction between (1A) and (2B), were significantly faster, completing in only 6 h and yielding nearly the same amount of the desired product (97%) for both cases (entry 1 and 2). However, when (2A) was substituted by (1A) in the reaction, the product yield decreased. This reduction in yield was attributed to the steric hindrance caused by the cyclohexyl group of the alcohol and the reactivity of aromatic amines (Table 2, entry 3 and 4). Substrates (4B) and (5B) containing electron-donating groups (-CH3, -OCH3) produced oxazolidinones with slightly improved yields (Table 2, entry 5–7). Conversely, substrates containing electron-withdrawing groups (Cl, Br) resulted in the corresponding oxazolidinones with slightly lower yields.
Scheme 2.
Synthetic scheme of one-pot three-component 2-Oxazolidinone synthesis using TpMA (MC)@Ag as heterogeneous catalyst.

Table 1.
Optimization table for 2-Oxazolidinone synthesis.
Table 1.
Optimization table for 2-Oxazolidinone synthesis.
| Entry | Catalyst (mg) | Base (mmol) | T/h | Yield (%) |
|---|---|---|---|---|
| 1 | - | - | 6 | 0 |
| 2 | TpMA (MC)@Ag (15 mg) | - | 6 | 48 |
| 3 | TpMA (MC)@Ag (10 mg) | DBU (1) | 6 | 79 |
| 4 | TpMA (MC)@Ag (20 mg) | DBU (1) | 6 | 97 |
| 5 | TpMA (MC)@Ag (15 mg) | DBU (1) | 6 | 97 |
| 6 | TpMA (MC) (15 mg) | DBU (1) | 6 | 35 |
| 7 | AgNO3 (15 mg) | DBU (1) | 6 | 35 |
| 8 | AgI (15 mg) | DBU (1) | 6 | 33 |
| 9 | AgOAC (15 mg) | DBU (1) | 6 | 29 |
| 10 | TpMA (MC)@Ag (15 mg) | DIPEA (1) | 6 | 12 |
| 11 | TpMA (MC)@Ag (15 mg) | DABCO (1) | 6 | 20 |
| 12 | TpMA (MC)@Ag (15 mg) | t-BuOK (1) | 6 | 23 |
| 13 | TpMA (MC)@Ag (15 mg) | NEt3 (1) | 6 | 25 |
| 14 | TpMA (MC)@Ag (15 mg) | K2CO3 (1) | 6 | 30 |
| 15 | TpMA (MC)@Ag (15 mg) | Cs2CO3 (1) | 6 | 36 |
| 16 | TpMA (MC)@Ag (15 mg) | DBU (3) | 6 | 97 |

Table 2.
Substrate variation for the synthesis of 2-Oxazolidinones.
Table 2.
Substrate variation for the synthesis of 2-Oxazolidinones.
| Entry | Alcohol | Amine | Product | T/h | Yield (%) |
|---|---|---|---|---|---|
| 1 | 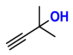 (1A) |  (1B) | 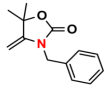 (1C) | 6 | 95 |
| 2 | 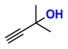 (1A) |  (2B) |  (2C) | 6 | 97 |
| 3 | 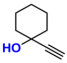 (2A) | 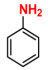 (3B) | 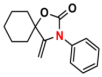 (3C) | 6 | 75 |
| 4 |  (2A) | 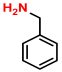 (1B) | 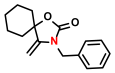 (4C) | 6 | 66 |
| 5 | 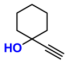 (2A) |  (4B) | 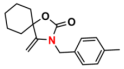 (5C) | 6 | 89 |
| 6 |  (1A) | 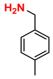 (4B) |  (6C) | 6 | 88 |
| 7 |  (1A) | 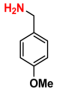 (5B) |  (7C) | 6 | 90 |
| 8 |  (1A) | 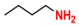 (6B) | 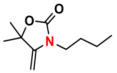 (8C) | 6 | 70 |
Reaction condition: Propargyl alcohols (5 mmol), amines (5 mmol), CO2 (1 atm), porous polymeric catalyst TpMA (MC)@Ag (15 mg), RT, DBU (1 mmol).

Figure 8.
Kinetic study curve of 2-Oxazolidinone.
2.2.2. Carbon Dioxide Fixation in α-Alkylidene Cyclic Carbonate Synthesis with Porous Polymeric Catalyst TpMA (MC)@Ag
The synthesized catalyst TpMA (MC) showed excellent catalytic activity in the CO2 fixation process, which generates cyclic carbonates from propargyl alcohol. Many scientific groups have previously reported the propargyl alcohol to cyclic carbonate production via carbon dioxide fixation [82]. In light of such studies, we attempted the most efficient and environment-friendly method of product synthesis employing the Ag-loaded porous polymeric catalyst TpMA (MC)@Ag.
For the reaction optimization, we utilized (1A), base, and CO2. Firstly, we conducted the reaction at room temperature using (1A) (5 mmol) as a model substrate in the absence of any solvent under CO2 (1 atm) atmosphere. In the absence of a catalyst, the reaction was allowed to run for 4 h. We found no product yield (Table 3, entry 1). After 4 h at RT, the intended product was produced with 36% yield in the presence of 1 mmol DBU catalyst (Table 3, entry 2).
After 4 h of reaction, 60% yield of the product was achieved using porous polymeric catalyst TpMA (MC)@Ag (15 mg) catalyst in the absence of any base (Table 3, entry 3). When several other silver catalysts, such as AgOAc, AgNO3, and AgI, were tested with a constant base and its amount (5 mg each), the yields ranged from 23% to 51% (Table 3, entry 4–6). In the course of our experimentation, we carried out the reaction using a range of bases, substituting DBU with both organic bases such as DIPEA, t-BuOK, TBD, DABCO, and NEt3, as well as inorganic bases like Cs2CO3 and K2CO3, among others, in several trials. Nonetheless, the substitution did not result in a significant increase in the percentage of product yield, contrary to the outcomes we obtained with the DBU base (Table 3, entry 7–13). When 15 mg of porous polymeric framework catalyst was used for 4 h in the absence of any solvent and assisted with 1 mmol base DBU, the optimal product yield (95%) was obtained (Table 3, entry 14). We also investigated the influence of base DBU quantity on the catalytic process. There was no influence on the yield of the product observed when DBU more than 1 mmol was used (Table 3, entry 15). In order to conduct a more comprehensive screening of catalytic reactions, we introduced variations in the catalyst loading during the reaction. Upon analysis of Table 3, it was ascertained that a catalyst loading of 15 mg is sufficient to obtain the maximum percentage of product yield (Table 3, entry 16–17). Further experimentation revealed that when the reaction time was altered from 4 to 6 h, that did not result in any additional yield (Table 3, entry 18). When utilizing TpMA (MC) porous polymeric framework as a catalyst in lieu of porous polymeric catalyst TpMA (MC)@Ag, a minute quantity of product was detected (Table 3, entry 19). Hence, it can be surmised that the loading of Ag nanoparticles on the TpMA (MC) porous polymeric framework surface functions as an active center in the proposed reaction of catalysis.
The reaction’s conversion rate was measured with the involvement of the porous polymeric catalyst TpMA (MC)@Ag (15 mg) in neat reaction conditions at RT, and the data were plotted to create a kinetic curve (% of conversion vs. time), as Figure 9 exhibits. The diagram distinctly illustrates that the catalyzed α-alkylidene cyclic carbonate reaction progresses seamlessly and reaches completion within 4 h. Table 4 summarizes the substrate scope for different propargyl alcohol derivatives to investigate the influence of replacements at R1 and R2 positions on the yield of the reaction (3A), a non-substituted propargyl alcohol that provides no product (Table 4, entry 2). On the variation at R1 and R2 position, the greatest yield was found for R1 = R2 = Me (Table 4, entry 1). The outcome was supported by the fact that the gem dimethyl group encourages cyclization due to their mutual repulsion; “gem effect” describes this phenomenon. The reaction speed was delayed when the alkyl groups became more bulky (Table 4, entry 3–6). However, in other instances, the reaction took longer to complete (Table 4, entry 4–6). On the other hand, under optimal reaction circumstances, the internal propargylic alcohol showed no reactivity and remained unreacted, leading to the absence of the expected product (Table 4, entry 7).
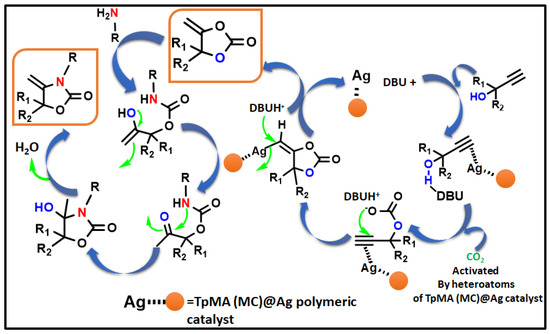
Scheme 3.
A potential mechanism proposed for the generation of α-alkylidene cyclic carbonates and oxazolidinones from propargylic alcohol via carbon dioxide fixation.

Scheme 4.
Synthetic pathway for synthesis of α-alkylidene cyclic carbonates via CO2 fixation in propargylic alcohols using TpMA (MC)@Ag as heterogeneous catalyst.

Table 3.
Optimization table for catalytic α-alkylidene cyclic carbonate synthesis.
Table 3.
Optimization table for catalytic α-alkylidene cyclic carbonate synthesis.
| Entry | Catalyst (mg) | Base (mmol) | T/h | Yield (%) |
|---|---|---|---|---|
| 1 | - | - | 4 | - |
| 2 | - | DBU (1) | 4 | 36 |
| 3 | TpMA (MC)@Ag (5) | - | 4 | 60 |
| 4 | AgOAc (5) | DBU (1) | 4 | 51 |
| 5 | AgNO3 (5) | DBU (1) | 4 | 36 |
| 6 | AgI (5) | DBU (1) | 4 | 23 |
| 7 | TpMA (MC)@Ag (5) | DIPEA (1) | 4 | 56 |
| 8 | TpMA (MC)@Ag (5) | t-BuOK (1) | 4 | 70 |
| 9 | TpMA (MC)@Ag (5) | DABCO (1) | 4 | 54 |
| 10 | TpMA (MC)@Ag (5) | TBD (1) | 4 | 68 |
| 11 | TpMA (MC)@Ag (5) | NEt3 (1) | 4 | 50 |
| 12 | TpMA (MC)@Ag (5) | Cs2CO3 (1) | 4 | 51 |
| 13 | TpMA (MC)@Ag (5) | K2CO3(1) | 4 | 44 |
| 14 | TpMA (MC)@Ag (15) | DBU (1) | 4 | 95 |
| 15 | TpMA (MC)@Ag (15) | DBU (3) | 4 | 95 |
| 16 | TpMA (MC)@Ag (20) | DBU (1) | 4 | 95 |
| 17 | TpMA (MC)@Ag (10) | DBU (1) | 4 | 89 |
| 18 | TpMA (MC)@Ag (15) | DBU (1) | 6 | 95 |
| 19 | TpMA (MC)@Ag (15) | DBU (1) | 4 | 20 |

Table 4.
Substrate scopes for the fixation of CO2 onto propargyl alcohols.
Table 4.
Substrate scopes for the fixation of CO2 onto propargyl alcohols.
| Entry | Substrate | Product | T/h | Yield (%) |
|---|---|---|---|---|
| 1 | 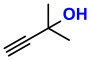 (1A) | 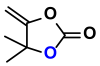 (1D) | 4 | 95 |
| 2 |  (3A) |  (2D) | 4 | 0 |
| 3 | 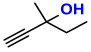 (4A) |  (3D) | 4 | 91 |
| 4 | 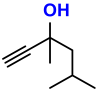 (5A) | 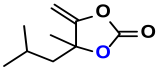 (4D) | 4 | 88 |
| 5 |  (2A) |  (5D) | 4 | 93 |
| 6 |  (2A) | 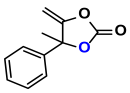 (6D) | 4 | 85 |
| 7 | 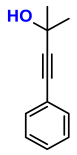 (6A) | 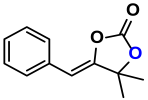 (7D) | 4 | 0 |
Reaction condition: Substrate (5 mmol), porous polymeric catalyst TpMA (MC)@Ag (15 mg), DBU (5 mmol), CO2 (1 bar), RT.

Figure 9.
Kinetic study curve of α-alkylidene cyclic carbonate.
2.2.3. Reaction Mechanism
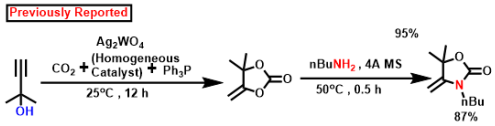
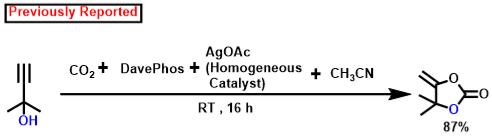
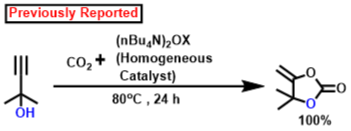
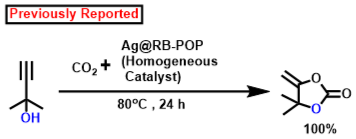
According to earlier findings [47,53,83,84,85,86,87,88], Scheme 3, illustrates a possible mechanistic pathway for the catalytic formation of α-alkylidene cyclic carbonates and 2-oxazolidinones using CO2 fixation from propargylic alcohol. Initially, propargylic alcohol’s hydroxyl group can be attacked by the strong organic base (DBU), which can then capture the proton. The presence of Ag in the catalyst can ignite the propargylic alcohol’s alkyne portion, which leads to the formation of a hybrid with the Ag-loaded catalyst. In Contrast to other inert systems, such as the N2 molecule, the presence of an internal CO connecting dipole causes CO2 amphiphilic. The LUMO is located on the C center, whereas the molecular orbital with the greatest occupancy (HOMO) is located on the O centees. Therefore, nucleophilic attack on the center of carbon is quite vulnerable (e.g., when the DBU base activates the propargylic alcohol, the O atom may connect with the C center of CO2), whereas electrophiles and the O center participate (e.g., activated alkyne moiety). In the potential intermediate, bond formation takes place between the Ag species and the alkyne component following a nucleophilic attack initiated by the oxygen center of CO2 [87,88,89]. The adduct has been shown to be beneficial in the inclusion of CO2 to produce the carbamate salt. Afterwards, intramolecular cyclization takes place. Following the release of the porous polymeric catalyst TpMA (MC)@Ag catalyst, the required α-alkylidene cyclic carbonate product is obtained. The resulting cyclic carbonate serves as an intermediate in the production of oxazolidinones, where it is nucleophilically attacked by amines to yield another carbamate species. Subsequently, this intermediate actively engages in a ring-closing reaction, resulting in the formation of the corresponding oxazolidinone product and the liberation of a water molecule as a byproduct. Scheme 4 presents a comparative analysis of our present methodology with previous research findings concerning the catalytic formation of α-alkylidene cyclic carbonates and oxazolidinones from propargylic alcohol using carbon dioxide fixation processes. The majority of earlier research [62,63,64,65] on the catalytic synthesis of α-alkylidene cyclic carbonates was conducted under homogenous conditions. As a consequence of this, in such instances, the catalyst’s recoverability and reusability are problematic. Once more, each of the methods needed high pressure CO2 (≥1 MPa). For the manufacture of the identical chemicals, a few reports [22,43] were generated under heterogeneous catalytic conditions. However, there are still the same restrictions about the usage of high-pressure carbon dioxide (1 MPa) and one technique [47] that was carried out using acetonitrile solvent. Our method performs under circumstances of heterogeneous catalysis without solvents at atmospheric CO2 pressure. Additionally, it produces an exceptionally high proportion of the desired product at ambient temperature.
All earlier published processes for the synthesis of oxazolidinones were carried out under homogenous catalytic conditions and generated an excellent yield of the target product (81–92.5%) at ambient to very high pressures (2 MPa) of CO2 [90,91] (Table 5). The oxazolidinone synthesis described in this study was carried out utilizing atmospheric-pressure CO2 and a heterogeneous catalyst without the use of solvents, and at room temperature, it produces an exceptionally elevated yield of the intended product.

Table 5.
Comparison between the catalytic generation of oxazolidinones and α-alkylidene cyclic carbonates from propargylic alcohol via CO2 fixation reactions between this work with previous reports.
2.3. Reusability of Porous Polymeric Catalyst TpMA (MC)@Ag
The fundamental characteristics of a heterogeneous catalyst are its simple and economical synthetic procedure, recyclability, and recoverability. The reusability of the synthesized porous polymeric catalyst TpMA (MC)@Ag was evaluated using the catalyst up to 5 cycles in both reaction schemes regarding synthesizing 2-oxazolidinone and α-alkylidene cyclic carbonates under optimized reaction conditions (Figure 10). The TpMA (MC)@Ag catalyst was taken out from the reaction mixture using an extraction technique. After the reaction finished, the catalyst was washed using methanol and dried around 50 to 60 degrees Celsius. Following the drying method, the regenerated porous polymeric catalyst TpMA (MC)@Ag was used optimally in the preparation of 2-oxazolidinones and ɑ-alkylidene cyclic carbonates. Five runs of the porous polymeric catalyst TpMA (MC)@Ag material may be successfully recycled without significantly reducing the output yield.
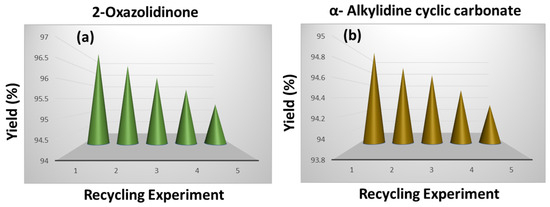
Figure 10.
TpMA (MC)@Ag catalyst recycling in the production of (a) oxazolidinones and (b) α-alkylidene cyclic carbonates using propargylic alcohols.
2.4. Heterogeneity Test
Hot filtration examination was implemented to ascertain the heterogeneous nature of the porous polymeric catalyst TpMA (MC)@Ag for the generation of α-alkylidene cyclic carbonates and oxazolidinones through propargylic alcohols. In the beginning, the catalyst-assisted reaction yielded 95 and 97%, respectively, of the intended product after 4 and 6 h of optimization, respectively. After the catalyst was removed from the reaction mixture, the reaction continued for six hours. The filtrate, as well as the isolated catalyst, porous polymeric catalyst TpMA (MC)@Ag, were analyzed using the ICP-AES method. There was no detectable metal present in the filtrate. The ICP-AES technique was employed to evaluate the Ag metal loading of the porous polymeric catalyst TpMA (MC)@Ag following the initial and fifth cycles of catalysis. As a consequence, the loading of Ag metal in the new porous polymeric catalyst TpMA (MC)@Ag catalyst (2.53%) had no discernible effect on the findings. However, the blocking of the catalyst’s active regions during successive catalytic cycles may result in a dramatic reduction in catalytic activity after the 6th reaction cycle.
Moreover, we conducted TEM, FE-SEM, and IR investigations over the regenerated catalyst to validate its heterogeneous character. The shape and particle size of the recycled catalyst were virtually equal to those of the freshly manufactured porous polymeric catalyst TpMA (MC)@Ag catalyst. The FT-IR study and TEM image of the regenerated catalyst revealed no significant modifications.
3. Conclusions
The present study demonstrates the design, synthesis, and complete characterization of the Ag NPs embedded in a porous polymeric framework, namely, porous polymeric catalyst TpMA (MC)@Ag. The catalyst exhibits improved efficacy in the formation of oxazolidinones and α-alkylidene cyclic carbonates using diverse unsaturated alcohols (e.g., propargylic alcohols) as starting substrates. In the presence of a strong organic DBU base, diverse unsaturated alcohols were employed at room temperature, yielding the expected α-alkylidene cyclic carbonates with relatively improved yield and excellent selectivity. On the other hand, oxazolidinones were potentially high-yielding even at room temperature through three-component reaction in the presence of aromatic/aliphatic amines, CO2, and propargylic alcohols. The development of this catalytic protocol under solvent-free conditions makes the methodology non-hazardous and environmentally friendly. It should be noted that the porous polymeric catalyst TpMA (MC)@Ag can be regenerated and recyclable with the same catalytic efficacy. Additionally, the nanoporous porous polymeric framework not only acts as a support for silver metal, but also as a promising material featuring carbon dioxide adsorption capability. These findings represent a significant advancement in the field of porous polymeric framework-based heterogeneous catalysis and provide momentum for the development of reusable heterogeneous catalysts for diverse CO2 conversions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13121467/s1, Figure S1: Picture of porous polymeric catalyst (a) TpMA (MC) and (b) Ag metal-loaded catalyst, TpMA (MC)@Ag, Figure S2: FT-IR spectroscopy of reused TpMA (MC)@Ag catalyst and TpMA (MC) polymeric catalyst, Figure S3: BET isotherm of reused TpMA (MC)@Ag catalyst, Figure S4: PXRD of reused catalyst, Figure S5: (a–b) HRTEM image of reused catalyst and (c–d) FE-SEM image of reused catalyst, Figure S6: 1H NMR of 3-Cyclohexyl-5,5-dimethyl-4-methylene-oxazolidin-2-one, Figure S7: 1H NMR of 4 methylene 3 phenyl 1 oxa 3 azaspiro *4.5+decan 2 one, Figure S8: 1H NMR 3 Benzyl 4 methylene 1 oxa 3 azaspiro *4.5+decan 2 one, Figure S9: 1H NMR of 3--(4 4 methylene 1 oxa 3 azaspiro *4.5+decan 2 one, Figure S10: 1H NMR of 5,5 dimethyl 3--(4 methylbenzyl 4 methyleneoxazolidin 2 one, Figure S11: 1H NMR of 3--(4 5,5 dimethyl 4 methyleneoxazolidin 2 one, Figure S12: 1H NMR of 3 butyl 5,5 dimethyl 4 methyleneoxazolidin 2 one, Figure S13: 1H NMR of 3 Benzyl 5,5 dimethyl 4 methylene oxazolidin 2 one, Figure S14: 1H NMR of 4,4 dimethyl 5 methylene 1,3 dioxolan 2 one, Figure S15: 1H NMR of 4 ethyl 4 methyl 5 methylene 1,3 dioxolan 2 one, Figure S16. 1H NMR of 4 ethyl 4 methyl 5 methylene 1,3 dioxolan 2 one, Figure S17: 1H NMR of 4 methyl 5 methylene 4 phenyl 1,3 dioxolan 2 one, Figure S18: 1H NMR of 4 methylene 1,3 dioxaspiro *4.5+decan 2 one, Table S1: The grain size of Ag NPs.
Author Contributions
B.B.: Experimental work, Investigation, Methodology, Writing. P.C.: Experimental work, Methodology, Writing. N.H.: Characterization of the Catalyst, data analysis, writing. S.G.: Interpretation of data, Data analysis, Writing. M.S.: Supervision, Writing-Reviewing and Editing, A.K.: Supervision, Interpretation of data, Data analysis, Writing-Reviewing and Editing. S.M.I.: Supervision, Conceptualization, Methodology, Writing-Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
A.K. thanks the Researchers Supporting Project number (RSP2023R127), King Saud University, Riyadh, Saudi Arabia, for the support.
Data Availability Statement
Data will be available on request.
Acknowledgments
SMI acknowledges the Department of Science and Technology, DST-SERB (Project Sanc No. CRG/2020/000244), New Delhi, Govt. of India, for financial support. A.K. thanks the Researchers Supporting Project number (RSP2023R127), King Saud University, Riyadh, Saudi Arabia, for the support. We acknowledge the Department of Science and Technology, DST, Govt. of India for providing funds to the Department of Chemistry, University of Kalyani, under the DST FIST program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Modak, A.; Chatterjee, S.; Bhaumik, A. Bifunctionalized Mesoporous SBA-15: A New Heterogeneous Catalyst for the Facile Synthesis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2017, 5, 2763–2773. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, B.; Salam, N.; Bhaumik, A.; Islam, S.M. Mesoporous titania-iron (III) Oxide with nanoscale porosity and high catalytic activity for the synthesis of β-amino alcohols and benzimidazole derivatives. ChemCatChem 2015, 7, 2689–2697. [Google Scholar] [CrossRef]
- Salam, N.; Kundu, S.K.; Roy, A.S.; Mondal, P.; Ghosh, K.; Bhaumik, A.; Islam, S.M. A ruthenium-grafted triazine functionalized mesoporous polymer: A highly efficient and multifunctional catalyst for transfer hydrogenation and the Suzuki–Miyaura cross-coupling reactions. Dalton Trans. 2014, 43, 7057–7068. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhanja, P.; Salam, N.; Khatun, R.; Bhaumik, A.; Islam, S.M. Porous iron-phosphonate nanomaterial as an efficient catalyst for the CO2 fixation at atmospheric pressure and esterification of biomass-derived levulinic acid. Catal. Today 2018, 309, 253–262. [Google Scholar] [CrossRef]
- Nema, P.; Nema, S.; Roy, P. An overview of global climate changing in current scenario and mitigation action. Renew. Sustain. Energy Rev. 2012, 16, 2329–2336. [Google Scholar] [CrossRef]
- Roy, S.; Chatterjee, T.; Banerjee, B.; Salam, N.; Bhaumik, A.; Islam, S.M. Cu(II) anchored nitrogen-rich covalent imine network (CuII-CIN-1): An efficient and recyclable heterogeneous catalyst for the synthesis of organoselenides from aryl boronic acids in a green solvent. RSC Adv. 2014, 4, 46075–46083. [Google Scholar] [CrossRef]
- Khatun, R.; Bhanja, P.; Mondal, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded over mesoporous TiO2 for chemical fixation of CO2 under atmospheric pressure and solvent-free conditions. New J. Chem. 2014, 41, 12937–12946. [Google Scholar] [CrossRef]
- Islam, M.M.; Roy, A.S.; Islam, S.M. Functionalized Polystyrene Supported Copper(I) Complex as an Effective and Reusable Catalyst for Propargylamines Synthesis in Aqueous Medium. Catal. Lett. 2016, 146, 1128–1138. [Google Scholar] [CrossRef]
- Das, S.K.; Chandra, B.K.; Molla, R.A.; Sengupta, M.; Islam, S.M.; Majee, A.; Bhaumik, A. CuO grafted triazine functionalized covalent organic framework as an efficient catalyst for CC homo coupling reaction. Mol. Catal. 2020, 480, 110650. [Google Scholar] [CrossRef]
- Biswas, I.H.; Biswas, S.; Islam, M.S.; Riyajuddin, S.; Sarkar, P.; Ghosh, K.; Islam, S.M. Catalytic synthesis of benzimidazoles and organic carbamates using a polymer supported zinc catalyst through CO2 fixation. New J. Chem. 2019, 43, 14643–14652. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Kayal, U.; Chowdhury, I.H.; Ghosh, S.; Islam, S.M. Nanoporous ZnO supported CuBr (CuBr/ZnO): An efficient catalyst for CO2 fixation reactions. Chem. Sel. 2019, 4, 1069–1077. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Islam, S.M. A Zn(ii)-functionalized COF as a recyclable catalyst for the sustainable synthesis of cyclic carbonates and cyclic carbamates from atmospheric CO2. Org. Biomol. Chem. 2022, 20, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.; Bag, A.; Ghosh, S.; Mondal, P.; Bordoloi, A.; Islam, S.M. CuxOy@COF: An efficient heterogeneous catalyst system for CO2 cycloadditions under ambient conditions. J. CO2 Util. 2019, 34, 533–542. [Google Scholar] [CrossRef]
- Islam, S.S.; Biswas, S.; Molla, R.A.; Yasmin, N.; Islam, S.M. Green Synthesized AgNPs Embedded in COF: An Efficient Catalyst for the Synthesis of 2-Oxazolidinones and α-Alkylidene Cyclic Carbonates via CO2 Fixation. ChemNanoMat 2020, 6, 1386–1397. [Google Scholar] [CrossRef]
- Vaitla, J.; Guttormsen, Y.; Mannisto, J.K.; Nova, A.; Repo, A.; Bayer, A.; Hopmann, K.H. Enantioselective Incorporation of CO2: Status and Potential. ACS Catal. 2017, 7, 7231–7244. [Google Scholar] [CrossRef]
- Wennersten, R.; Sun, Q.; Li, H. The future potential for Carbon Capture and Storage in climate change mitigation—An overview from perspectives of technology, economy and risk. J. Clean. Prod. 2015, 103, 724–736. [Google Scholar] [CrossRef]
- Ghosh, S.; Khan, T.S.; Ghosh, A.; Chowdhury, A.H.; Haider, M.A.; Khan, A.; Islam, S.M. Utility of Silver Nanoparticles Embedded Covalent Organic Frameworks as Recyclable Catalysts for the Sustainable Synthesis of Cyclic Carbamates and 2-Oxazolidinones via Atmospheric Cyclizative CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 5495–5513. [Google Scholar] [CrossRef]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 Catalysis. ChemSusChem 2017, 10, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Ghosh, K.; Islam, S.S.; Patra, A.K.; Islam, S.M.; Bhaumik, A. New Hybrid Iron Phosphonate Material as an Efficient Catalyst for the Synthesis of Adipic Acid in Air and Water. ACS Sustain. Chem. Eng. 2016, 4, 7147–7157. [Google Scholar] [CrossRef]
- Das, A.; Mondal, R.K.; Chakrabortty, P.; Riyajuddin, S.; Chowdhury, A.H. Visible light assisted chemical fixation of atmospheric CO2 into cyclic Carbonates using covalent organic framework as a potential photocatalyst. Mol. Catal. 2021, 499, 111253. [Google Scholar] [CrossRef]
- Haque, N.; Biswas, S.; Ghosh, S.; Chowdhury, A.H.; Khan, A.; Islam, S.M. Zn(II)-Embedded Nanoporous Covalent Organic Frameworks for Catalytic Conversion of CO2 under Solvent-Free Conditions. ACS Appl. Nano Mater. 2021, 4, 7663–7674. [Google Scholar] [CrossRef]
- Aurelio, L.; Brownlee, R.T.C.; Hughes, A.B. Synthetic preparation of N-methyl-alpha-amino acids. Chem. Rev. 2004, 104, 5823–5846. [Google Scholar] [CrossRef]
- Pridgen, L.N.; Prol, J., Jr.; Alexander, B.; Gillyard, L. Single-pot reductive conversion of amino acids to their respective 2-oxazolidinones employing trichloromethyl chloroformate as the acylating agent: A multigram synthesis. J. Org. Chem. 1989, 54, 3231–3233. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Das, A.; Riyajuddin, S.; Ghosh, K.; Islam, S.M. Reduction of carbon dioxide with mesoporous SnO2 nanoparticles as active photocatalysts under visible light in water. Catal. Sci. Technol. 2019, 9, 6566–6569. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, I.H.; Chakraborty, A.; Naskar, M.K.; Sarkar, M.; Islam, S.M. Porous organic polymer (POP) nanosheets: An efficient photo-catalyst for visible-light assisted CO2 reduction. Mater. Adv. 2022, 3, 3165–3173. [Google Scholar] [CrossRef]
- Sarkar, P.; Chowdhury, A.H.; Riyajuddin, S.; Ghosh, S.; Islam, S.M. Constructing a metal-free 2D covalent organic framework for visible-light-driven photocatalytic reduction of CO2: A sustainable strategy for atmospheric CO2 utilization. React. Chem. Eng. 2023, 8, 365–376. [Google Scholar] [CrossRef]
- Chakrabortty, P.; Ghosh, S.; Das, A.; Khan, A.; Islam, S.M. Visible-light-driven sustainable conversion of carbon dioxide to methanol using a metal-free covalent organic framework as a recyclable photocatalyst. Catal. Sci. Technol. 2022, 12, 3484–3497. [Google Scholar] [CrossRef]
- Sarkar, P.; Das, A.; Ghosh, S.; Islam, S.M. Visible Light-Driven Carboxylation of Olefins by Using 2D Metal-Free Covalent Organic Framework as Intrinsic Photocatalyst: A Sustainable Approach for CO2 Utilization. ChemCatChem 2022, 14, e202200186. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X. A high-yielding low-cost facile synthesis of 2-oxazolidinones chiral auxiliaries. Tetrahedron Asymmetry 2000, 11, 4359–4363. [Google Scholar] [CrossRef]
- Liu, J.-M.; Peng, X.-G.; Liu, J.-H.; Zheng, S.-Z.; Sun, W.; Xia, C.-G. Synthesis of 2-oxazolidinones by salen-Co-complexes catalyzed oxidative carbonylation of β-amino alcohols. Tetrahedron Lett. 2007, 48, 929–932. [Google Scholar] [CrossRef]
- Peng, X.; Li, F.; Xia, C. A Highly Efficient Sulfur-Catalyzed Oxidative Carbonylation of Primary Amines and b-Amino Alcohols. Synlett 2006, 8, 1161–1164. [Google Scholar] [CrossRef]
- Islam, S.M.; Salam, N.; Mondal, P.; Roy, A.S.; Ghosh, K.; Tuhina, K. A highly active reusable polymer anchored copper catalyst for CO, CN and CS cross coupling reactions. J. Mol. Catal. A Chem. 2014, 387, 7–19. [Google Scholar] [CrossRef]
- Islam, S.M.; Molla, R.A.; Roy, A.S.; Ghosh, K. Polymer supported Pd catalyzed thioester synthesis via carbonylation of aryl halides under phosphine free conditions. RSC Adv. 2014, 4, 26181–26192. [Google Scholar] [CrossRef]
- Vessally, E.; Nikpasand, M.; Ahmadi, S.; Nezhad, P.D.K.; Hosseinian, A. Transition metal-catalyzed intramolecular cyclization of N-Boc-protected propargyl/ethynyl amines: A novel and convenient access to 2-oxazolidinone/oxazolone derivatives. J. Iran. Chem. Soc. 2019, 16, 617–627. [Google Scholar] [CrossRef]
- Charlesworth, B.; Dowson, A.J. Review of zolmitriptan and its clinical applications in migraine. Expert Opin. Pharmacother. 2002, 3, 993–1005. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, C.; Guo, L.; Sun, H.; Lin, R.; Xia, W. Visible light-induced oxidative coupling reaction: Easy access to Mannich-type products. J. Org. Chem. 2012, 77, 6302–6306. [Google Scholar] [CrossRef]
- Kapfhaammer, H.; Hoff, P.; Golling, H.; R¨uther, E.; Schmauss, M. Cimoxaton and Moclobemid, Two New MAO-lnhibitors: Influence on Sleep Parameters in Patients with Major Depressive Disorder. Pharmacopsychiatry 1986, 19, 247–248. [Google Scholar] [CrossRef]
- Ghosh, S.; Molla, R.A.; Kayal, U.; Bhaumik, A.; Islam, S.M. Ag NPs decorated on a COF in the presence of DBU as an efficient catalytic system for the synthesis of tetramic acids via CO2 fixation into propargylic amines at atmospheric pressure. Dalton Trans. 2019, 48, 4657–4666. [Google Scholar] [CrossRef]
- Islam, S.S.; Salam, N.; Molla, R.A.; Riyajuddin, S.; Yasmin, N.; Das, D.; Ghosh, K.; Islam, S.M. Diformylphloroglucinol derived imine based covalent organic frameworks (PHTA) as efficient organocatalyst for conversion of isocyanates to urea derivatives. Mol. Catal. 2019, 522, 112213. [Google Scholar]
- Heravi, M.M.; Zadsirjan, V. Oxazolidinones as chiral auxiliaries in asymmetric aldol reactions applied to total synthesis. Tetrahedron Asymmetry 2013, 24, 1149–1188. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Chowdhury, I.H.; Islam, S.M. Titanium Phosphate with Flower-like Morphology as an Effective Reusable Catalyst for Chemical Fixation of CO2 at Mild Reaction Conditions. Ind. Eng. Chem. Res. 2019, 58, 11779–11786. [Google Scholar] [CrossRef]
- Wuts, P.G.; Pruitt, L.E. An Efficient Synthesis of (4S)-(-)-4-Isopropyl-2-oxazolidinone. Synthesis. 1989, 21, 622–623. [Google Scholar] [CrossRef]
- Islam, S.M.; Mal, D.; Palit, B.K.; Saha, C.R. Reductive carbonylation of nitroaromatics using RhA(CO)2. J. Mol. Catal. A Chem. 1999, 142, 169–181. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Mondal, J.; Islam, S.M. Cu/CuxOy NPs architectured COF: A recyclable catalyst for the synthesis of oxazolidinedione via atmospheric cyclizative CO2 utilization. Chem. Commun. 2020, 56, 12202–12205. [Google Scholar] [CrossRef]
- Juárez, R.; Concepción, P.; Corma, A.; García, H. Heterolytic and heterotopic dissociation of hydrogen on ceria-supported gold nanoparticles. Combined inelastic neutron scattering and FT-IR spectroscopic study on the nature and reactivity of surface hydrogen species. Chem. Commun. 2010, 46, 4181–4183. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.Y.; Wang, S.Y.; Wang, Q.; He, L.N. In Situ Generated Zinc(II) Catalyst for Incorporation of CO2 into 2-Oxazolidinones with Propargylic Amines at Atmospheric Pressure. ChemSusChem 2017, 10, 1210–1216. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, B.; Bhaumik, A.; Islam, S.M. CO2 fixation at atmospheric pressure: Porous ZnSnO3 nanocrystals as a highly efficient catalyst for the synthesis of cyclic carbonates. RSC Adv. 2016, 6, 31153–31160. [Google Scholar] [CrossRef]
- Ohe, K.; Matsuda, H.; Morimoto, T.; Ogoshi, S.; Chatani, N.; Murai, S. Nucleophilic substitution at the central allyl carbon atom of a (.pi.-allyl)platinum complex. J. Am. Chem. Soc. 1994, 116, 4125–4126. [Google Scholar] [CrossRef]
- Darcel, C.; Bruneau, C.; Dixneuf, P.H.; Roberts, S.M. Stereoselective synthesis of β-ketoesters from prop-2-yn-1-ols. Tetrahedron 1997, 53, 9241–9252. [Google Scholar] [CrossRef]
- Yoshida, M.; Ihara, M. Palladium-Catalyzed Domino Reaction of 4-Methoxycarbonyloxy-2-butyn-1-ols with Phenols: A Novel Synthetic Method for Cyclic Carbonates with Recycling of CO2. Angew. Chem. 2001, 113, 636–639. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Liu, H.B.; Yue, J.M. Photoelectrochemical DNA Biosensors. Chem. Rev. 2014, 114, 883–898. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, X.; He, H.; Qi, C.; Xiong, W.; Ren, Y.; Jiang, H. Copper-Promoted Coupling of Carbon Dioxide and Propargylic Alcohols: Expansion of Substrate Scope and Trapping of Vinyl Copper Intermediate. Adv. Synth. Catal. 2015, 357, 2556–2565. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, B.; Zhang, H.; Zhao, Y.; Chen, Y.; Ma, Z.; Ji, G.; Gao, X.; Han, B.; Liu, Z. Metalated Mesoporous Poly(triphenylphosphine) with Azo Functionality: Efficient Catalysts for CO2 Conversion. ACS Catal. 2016, 6, 1268–1273. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Z.; Zhang, F.; Liu, Z.; Yang, P.; Zhang, H.; Yu, B.; Zhao, Y.; Liu, Z. A rose bengal-functionalized porous organic polymer for carboxylative cyclization of propargyl alcohols with CO2. Chem. Commun. 2019, 55, 12475–12478. [Google Scholar]
- Dabral, S.; Bayarmagnai, B.; Hermsen, M.; Schießl, J.; Mormul, V.; Hashmi, A.S.K.; Schaub, T. Silver-Catalyzed Carboxylative Cyclization of Primary Propargyl Alcohols with CO2. Org. Lett. 2019, 21, 1422–1425. [Google Scholar] [CrossRef]
- Uemura, K.; Kawaguchi, T.; Takayama, H.; Nakamura, A.; Inoue, Y. Preparation of alkylidene cyclic carbonates via cyclization of propargylic carbonates. J. Mol. Catal. A Chem. 1999, 139, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Qian, Q.; Han, H.; Mei, Q.; Han, B. Zinc(ii)-catalyzed reactions of carbon dioxide and propargylic alcohols to carbonates at room temperature. Green Chem. 2016, 18, 382–385. [Google Scholar] [CrossRef]
- Kimura, T.; Kamata, K.; Mizuno, N. A bifunctional tungstate catalyst for chemical fixation of CO2 at atmospheric pressure. Angew. Chem. Int. Ed. 2012, 51, 6700–6703. [Google Scholar] [CrossRef]
- Yoshio, I.; Ishikawa, J.; Masaaki, T.; Harukichi, H. Cobaltocene-Catalyzed Reaction of Carbon Dioxide with Propargyl Alcohols. Bull. Chem. Soc. Jpn. 1987, 60, 1204–1206. [Google Scholar]
- Grignard, B.; Ngassamtounzoua, C.G.; Gennen, S.; Gilbert, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Boosting the catalytic performance of organic salts for the fast and selective synthesis of α-alkylidene cyclic carbonates from CO2 and propargylic alcohols. ChemCatChem 2018, 10, 2584–2592. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Wu, Z.; Lan, X.; Zhang, Y.; Li, M.; Bai, G. Copper(i) iodide cluster-based lanthanide organic frameworks: Synthesis and application as efficient catalysts for carboxylative cyclization of propargyl alcohols with CO2 under mild conditions. Dalton Trans. 2019, 48, 11063–11069. [Google Scholar] [CrossRef]
- Haldar, S.; Roy, K.; Nandi, S.; Chakraborty, D.; Puthusseri, D.; Gawli, Y.; Ogale, S.; Vaidhyanathan, R. High and Reversible Lithium Ion Storage in Self-Exfoliated Triazole-Triformyl Phloroglucinol-Based Covalent Organic Nanosheets. Adv. Energy Mater. 2018, 8, 1702170–1702181. [Google Scholar] [CrossRef]
- Modak, A.; Jana, S. Advancement in porous adsorbents for post-combustion CO2 capture. Microporous Mesoporous Mater. 2019, 276, 107–132. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Kaczmarek, A.M.; Jena, H.S.; Leus, K.; Chaoui, N.; Schmidt, J.; Deun, R.V.; Voort, P.V.D. Triggering White-Light Emission in a 2D Imine Covalent Organic Framework Through Lanthanide Augmentation. ACS Appl. Mater. Interfaces 2019, 11, 27343–27352. [Google Scholar] [CrossRef]
- Sarkar, P.; Riyajuddin, S.; Das, A.; Chowdhury, A.H.; Ghosh, K.; Islam, S.M. Mesoporous covalent organic framework: An active photo-catalyst for formic acid synthesis through carbon dioxide reduction under visible light. Mol. Catal. 2020, 484, 110730. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Chowdhury, A.H.; Das, A.; Khan, A.; Islam, S.M. A nanoporous covalent organic framework for the green-reduction of CO2 under visible light in water. New J. Chem. 2020, 44, 11720–11726. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.M.; Singh, R.P. Biological Synthesis of Silver Nanoparticles by Using Onion (Allium Cepa) Extract and Their Antibacterial Activity. Dig. J. Nanomater. Biostruct. 2010, 5, 427–432. [Google Scholar]
- Liu, W.; Cao, Y.; Wang, W.; Gong, D.; Cao, T.; Qian, J.; Iqbal, K.; Qin, W.; Guo, H. Mechanochromic luminescent covalent organic frameworks for highly selective hydroxyl radical detection. Chem. Commun. 2019, 55, 167–170. [Google Scholar] [CrossRef]
- Wang, R.-L.; Li, D.-P.; Wang, L.-J.; Zhang, X.; Zhou, Z.-Y.; Mu, J.-L.; Su, Z.-M. The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalton Trans. 2019, 48, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.W.; Yu, B.; Li, X.D.; Ma, R.; Diao, Z.F.; Li, R.G.; Li, W.; He, L.N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem. 2014, 16, 1633. [Google Scholar] [CrossRef]
- Song, Q.W.; Liu, P.; Han, L.H.; Zhang, K.; He, L.N. Upgrading CO2 by incorporation into urethanes through silver-catalysed one-pot stepwise amination reaction. Chin. J. Chem. 2018, 36, 147–152. [Google Scholar] [CrossRef]
- Shen, D.; Xu, Y.; Shi, S.L. A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki–Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers. J. Org. Chem. 2002, 67, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Song, Q.W.; Ma, R.; Xie, J.N.; He, L.N. Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(ii)-substituted polyoxometalate-based ionic liquids. Green Chem. 2016, 18, 282–287. [Google Scholar] [CrossRef]
- Shunsuke, Y.; Kosuke, F.; Satoshi, K.; Tohru, Y. Silver-catalyzed Preparation of Oxazolidinones from Carbon Dioxide and Propargylic Amines. Chem. Lett. 2009, 38, 786–787. [Google Scholar]
- Krishnakumar, B.; Swaminathan, M. An expeditious and solvent free synthesis of azine derivatives using sulfated anatase–titania as a novel solid acid catalyst. Synth. React. Inorg. Met. Org. Chem. 2014, 44, 96–100. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Pandiyan, V.; Swaminathan, W.; Shanthi, M. Synthesis, characterization and daylight active photocatalyst with antiphotocorrosive property for detoxification of azo dyes. Mater. Res. Bull. 2013, 48, 63–69. [Google Scholar] [CrossRef]
- Roy, A.; Haque, N.; Chatterjee, R.; Biswas, S.; Bhaumik, A.; Sarkar, M.; Islam, S.M. AgNPs supported over porous organic polymers for the fixation of CO2 on propargyl alcohols and amines under solvent-free conditions. New J. Chem. 2023, 47, 6673–6684. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Guo, G.; Gan, W.; Luo, J.; Xiang, F.; Zhang, J.; Zhou, H.; Liu, H. Preparation and dispersive mechanism of highly dispersive ultrafine silver powder. Appl. Surf. Sci. 2010, 256, 6683–6687. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Q.; Duan, Z.; Zhang, J.; Zhang, S.; Deng, Y. Ionic Liquid as an Efficient Promoting Medium for Fixation of Carbon Dioxide: A Clean Method for the Synthesis of 5-Methylene-1,3-oxazolidin-2-ones from Propargylic Alcohols, Amines, and Carbon Dioxide Catalyzed by Cu(I) under Mild Conditions. J. Org. Chem. 2005, 70, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Frantz, D.E.; Fässler, R.; Tomooka, C.S.; Carreira, E.M. The discovery of novel reactivity in the development of C-C bond-forming reactions: In situ generation of zinc acetylides with Zn(II)/R(3)N. Acc. Chem. Res. 2000, 33, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Frantz, R.; Fässler, R.; Carreira, E.M. First synthesis of optically pure propargylic N-hydroxylamines by direct, highly diastereoselective addition of terminal alkynes to nitrones. J. Am. Chem. Soc. 2000, 122, 1806–1807. [Google Scholar] [CrossRef]
- Sugiishi, T.; Nakamura, H. Zinc(II)-Catalyzed Redox Cross-Dehydrogenative Coupling of Propargylic Amines and Terminal Alkynes for Synthesis of N-Tethered 1,6-Enynes. J. Am. Chem. Soc. 2012, 134, 2504–2507. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sarkar, P.; Goswami, M.; Ali, S.M.; Molla, M.R.; Islam, S.M. A sustainable strategy for the visible-light-driven facile N-formylation of amines using a Co(II)-embedded covalent organic framework as an efficient photocatalyst. Mater. Chem. Front. 2023, 7, 3349–3364. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Zhao, Y.; Wang, H.; Li, Z.; Wang, J.; Jiao, T. Cu(I)/Ionic Liquids Promote the Conversion of Carbon Dioxide into Oxazolidinones at Room Temperature. Molecules 2019, 24, 1241. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Selvam, K.; Swaminathan, M. TiO2–SO42− as a novel solid acid catalyst for highly efficient, solvent free and easy synthesis of chalcones under microwave irradiation. Catal. Commun. 2011, 41, 1929–1932. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).