Enhanced Soot Oxidation Activity of a CuO-Doped CeO2 Catalyst via Acid Etching
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activities for Soot Oxidation
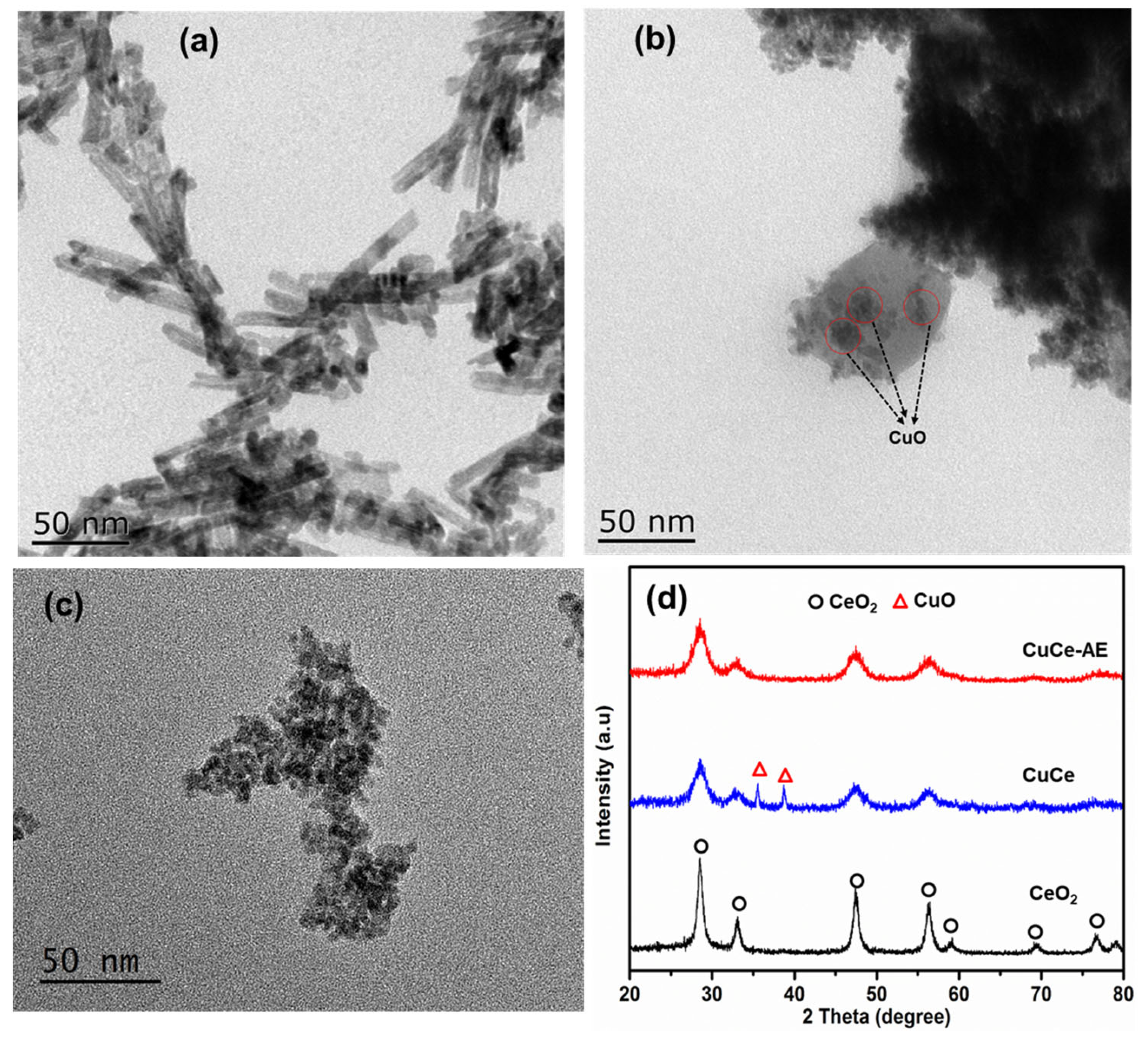
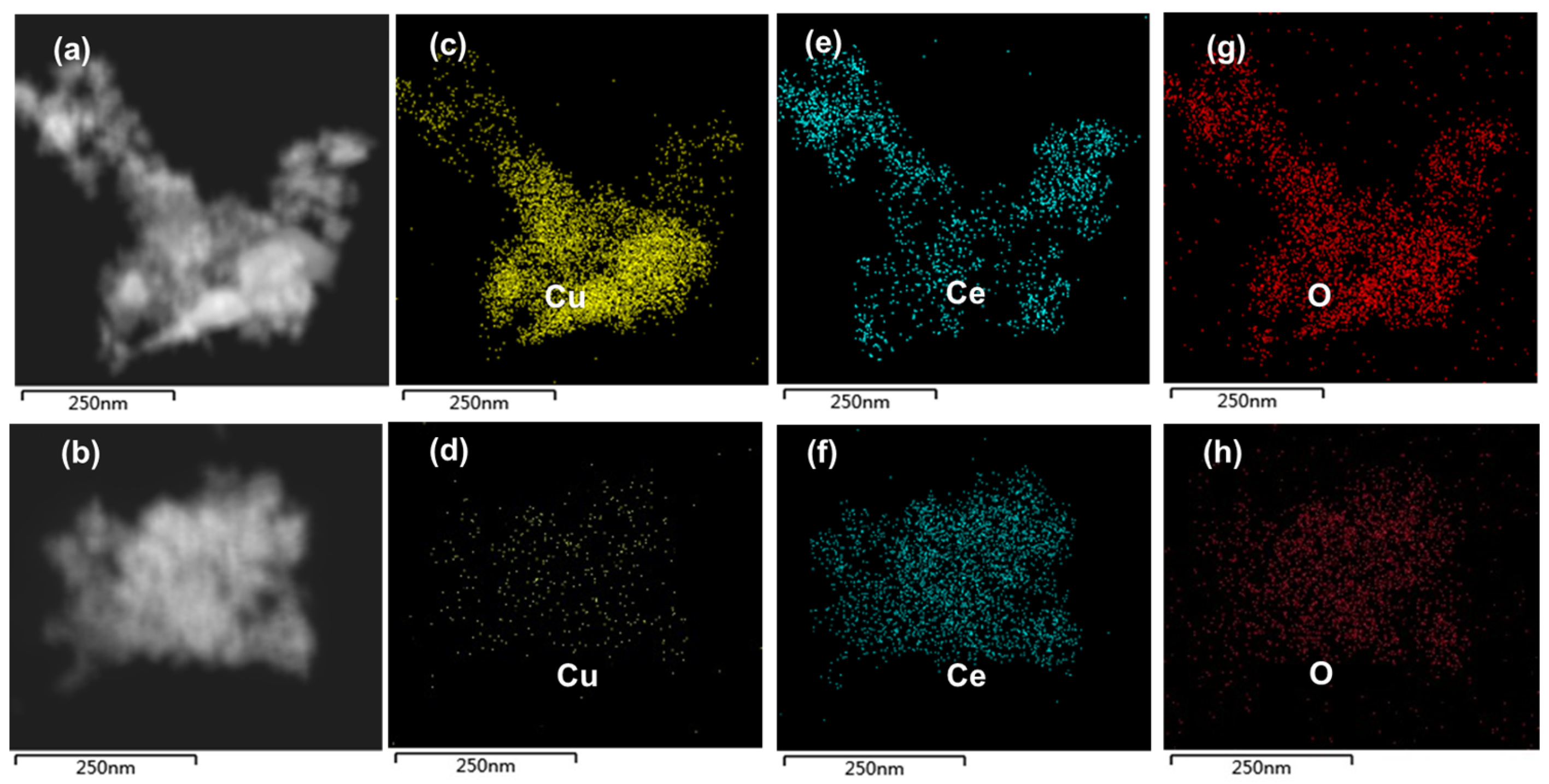
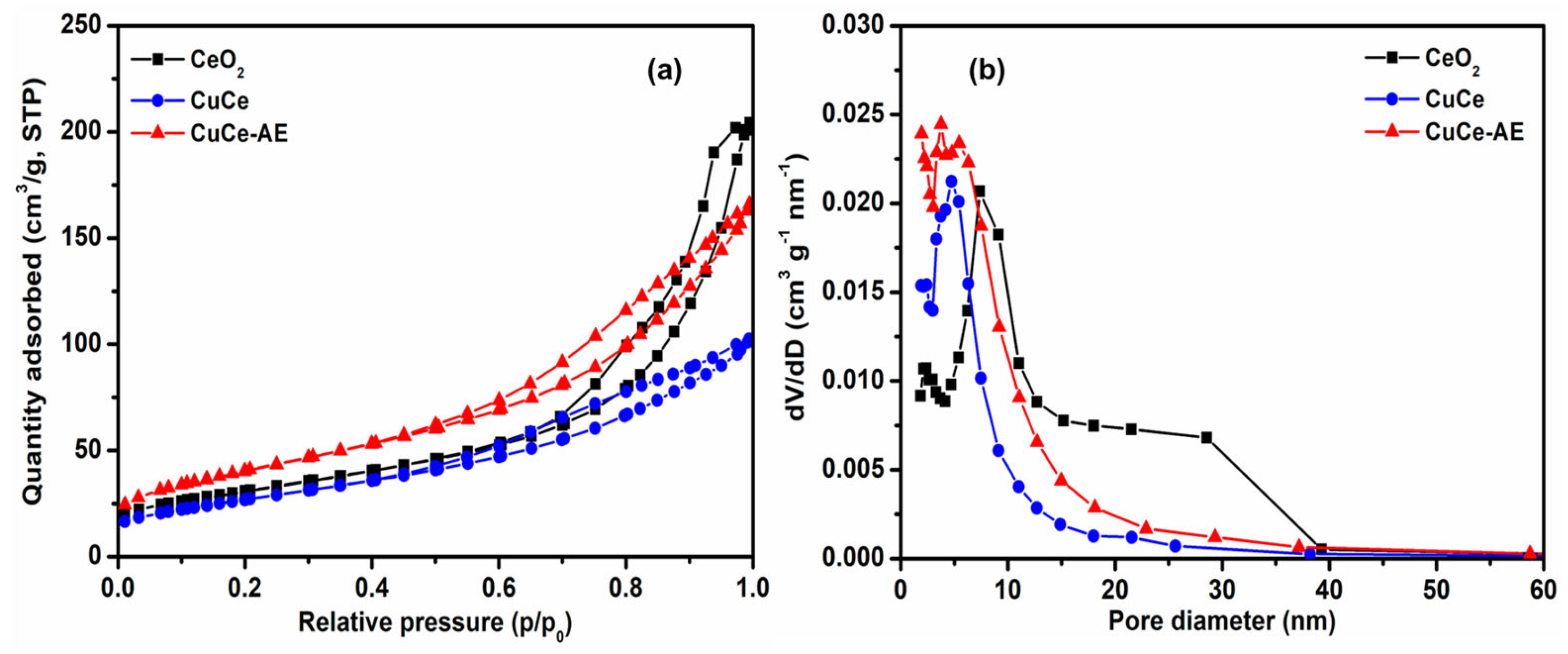
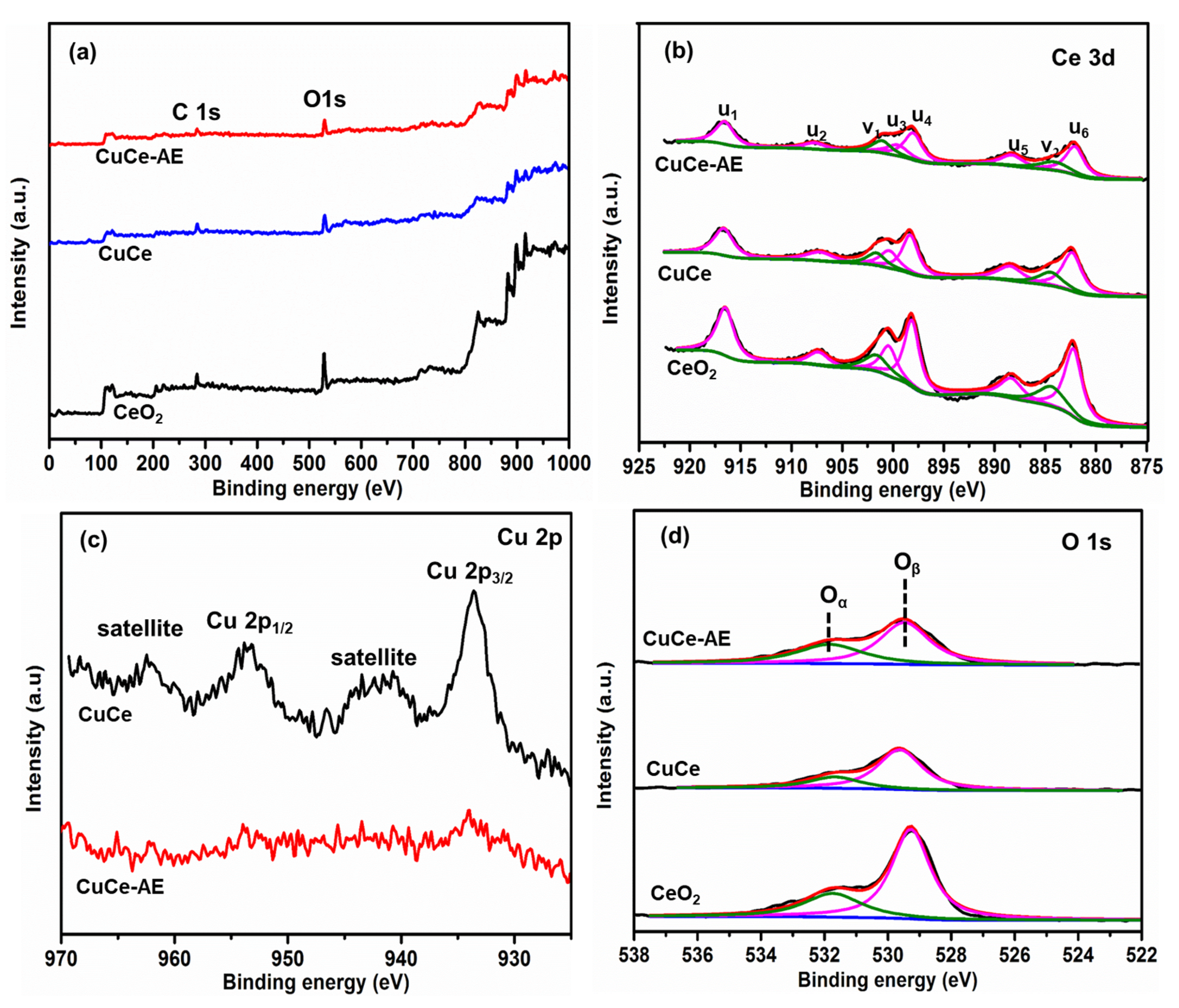
2.2. Physicochemical Characterization of Catalyst
3. Experimental
3.1. Catalyst Preparation
3.2. Catalytic Activity Evaluation
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Guo, H.; Xiong, J.; Ma, Y.; Li, X.; Zhang, P.; Zhang, S.; Wei, Y. The Catalyst of Ruthenium Nanoparticles Decorated Silicalite-1 Zeolite for Boosting Catalytic Soot Oxidation. Catalysts 2022, 13, 1167. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Miró, E.E.; Milt, V.G. Activity of catalytic ceramic papers to remove soot particles-A study of different types of Soot. Catalysts 2022, 12, 855. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, S.; Zheng, X.; Wang, X.; Yu, G.; Jiang, Y.; Feng, J.; Zhu, L.; Zhang, G. Enhanced oxygen vacancies in Ce-Doped SnO2 nanofibers for highly efficient soot catalytic combustion. Catalysts 2022, 12, 596. [Google Scholar] [CrossRef]
- Zheng, C.; Mao, D.; Xu, Z.; Zheng, S. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation. J. Catal. 2022, 411, 122–134. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Zhou, C.; Liu, H.; Yao, P.; Wang, J.; Chen, Y. A new understanding of CeO2-ZrO2 catalysts calcinated at different temperatures: Reduction property and soot-O2 reaction. Appl. Catal. A 2018, 563, 204–215. [Google Scholar] [CrossRef]
- Ji, F.; Men, Y.; Wang, J.; Sun, Y.; Wang, Z.; Zhao, B.; Tao, X.; Xu, G. Promoting diesel soot combustion efficiency by tailoring the shapes and crystal facets of nanoscale Mn3O4. Appl. Catal. B 2019, 242, 227–237. [Google Scholar] [CrossRef]
- Xu, J.; Lu, G.; Guo, Y.; Guo, Y.; Gong, X. A highly effective catalyst of Co-CeO2 for the oxidation of diesel soot: The excellent NO oxidation activity and NOx storage capacity. Appl. Catal. A 2017, 535, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Wei, S.; Zhang, Y. Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal. 2010, 276, 16–23. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Galvez, M.; Costa, P.; Chen, Y. MnOx-CeO2 mixed oxides as the catalyst for NO-assisted soot oxidation: The key role of NO adsorption/desorption on catalytic activity. Appl. Surf. Sci. 2018, 462, 678–684. [Google Scholar] [CrossRef]
- Jia, A.; Jiang, S.; Lu, J.; Luo, M. Study of catalytic activity at the CuO-CeO2 interface for CO oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Davó-Quinonero, A.; Navlani-García, M.; Lozano-Castelló, D.; Bueno-López, A.; Anderson, J.A. Role of hydroxyl groups in the preferential oxidation of CO over copper oxide-cerium oxide catalysts. ACS Catal. 2016, 6, 1723–1731. [Google Scholar] [CrossRef]
- Liang, Q.; Wu, X.; Weng, D.; Xu, H. Oxygen activation on Cu/Mn–Ce mixed oxides and the role in diesel soot oxidation. Catal. Today 2008, 139, 113–118. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ohshima, T.; Tezuka, Y.; Katayama, M.; Katoh, M.; Sugiyama, S. Morphological effects of CeO2 nanostructures for catalytic soot combustion of CuO/CeO2. Catal. Today 2015, 246, 67–71. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea–nitrate combustion method. Appl. Catal. A 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. The total oxidation of propane over supported Cu and Ce oxides: A comparison of single and binary metal oxides. J. Catal. 2010, 272, 109–120. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, L.; An, W.; Xu, J.; Men, Y. Boosting soot combustion efficiencies over CuO–CeO2 catalysts with a 3DOM structure. Catal. Sci. Technol. 2016, 6, 7342–7350. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: Insight into catalytic activity and sintering. Appl. Catal. B 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Russo, N.; Pirone, R.; Fino, D. Cerium-copper oxides prepared by solution combustion synthesis for total oxidation reactions: From powder catalysts to structured reactors. Appl. Catal. B 2017, 205, 455–468. [Google Scholar] [CrossRef]
- Cui, B.; Yan, S.; Xia, Y.; Li, K.; Li, S.; Wang, D.; Ye, Y.; Liu, Y. CuxCe1-xO2 nanoflakes with improved catalytic activity and thermal stability for diesel soot combustion. Appl. Catal. A 2019, 578, 20–29. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Sun, L.; Guo, X.; Wu, X.; Weng, D. NO catalytic oxidation over an ultra-large surface area LaMnO3+δ perovskite synthesized by an acid-etching method. RSC Adv. 2016, 6, 69855–69860. [Google Scholar] [CrossRef]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G.; Xu, C.; Gao, J.; He, H.; Wang, X. Three-dimensionally ordered macroporous Ce0.8Zr0.2O2-supported gold nanoparticles: Synthesis with controllable size and super-catalytic performance for soot oxidation. Energy Environ. Sci. 2011, 4, 2959–2970. [Google Scholar] [CrossRef]
- Ji, J.; Jing, M.; Wang, X.; Tan, W.; Guo, K.; Li, L.; Wang, X.; Song, W.; Cheng, L.; Sun, J.; et al. Activating low-temperature NH3-SCR catalyst by breaking the strong interface between acid and redox sites: A case of model Ce2(SO4)3-CeO2 study. J. Catal. 2021, 399, 212–223. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Cullen, D.; Hu, W.; Huang, J.; Yao, L.; Peng, Z.; Liao, P.; Wang, R. Distribution and valence state of Ru species on CeO2 supports: Support shape effect and its influence on CO oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar] [CrossRef]
- Deng, X.; Li, M.; Zhang, J.; Hu, X.; Zheng, J.; Zhang, N.; Chen, B. Constructing nano-structure on silver/ceria-zirconia towards highly active and stable catalyst for soot oxidation. Chem. Eng. J. 2017, 313, 544–555. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Yang, S.; Liang, H.; Men, Y. Boosting total oxidation of acetone over spinel MCo2O4 (M = Co, Ni, Cu) hollow mesoporous spheres by cation-substituting effect. J. Colloid Interface Sci. 2019, 539, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Chai, W.; Zhu, J.; Men, Y. Enhanced soot oxidation activity over CuO/CeO2 mesoporous nanosheets. Catal. Sci. Technol. 2019, 9, 1699–1709. [Google Scholar] [CrossRef]
- Othmane, A.; Essamlali, Y.; Fihri, A.; Larzek, M.; Zahouily, M. Effect of calcination temperature on the structure and catalytic performance of copper-ceria mixed oxide catalysts in phenol hydroxylation. RSC Adv. 2017, 7, 12586–12597. [Google Scholar]
- Xu, X.; Liu, L.; Tong, Y.; Fang, X.; Xu, J.; Jiang, D.; Wang, X. Facile Cr3+-doping strategy dramatically promoting Ru/CeO2 for low-temperature CO2 methanation: Unraveling the roles of surface oxygen vacancies and hydroxyl groups. ACS Catal. 2021, 11, 5762–5775. [Google Scholar] [CrossRef]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, W. Shape-dependent interplay between oxygen vacancies and Ag–CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 6595–16606. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z.; Gao, F.; Kovarik, L.; Peden, C.H.F.; Wang, Y. Effects of CeO2 support facets on VOx/CeO2 catalysts in oxidative dehydrogenation of methanol. J. Catal. 2014, 315, 15–24. [Google Scholar] [CrossRef]
- Mao, X.; Liu, S.; Liu, W.; Wu, X.; Liu, S. A simple model catalyst study to distinguish the roles of different oxygen species in propane and soot combustion. Appl. Catal. B 2022, 310, 121331. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.; Liu, S.; Wu, X.; Liu, W.; Li, M.; Chen, S.; Wang, X.; Weng, D. Study of Ag/CexNd1-xO2 nanocubes as soot oxidation catalysts for gasoline particulate filters: Balancing catalyst activity and stability by Nd doping. Appl. Catal. B 2017, 203, 116–126. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, Q.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and In Situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B 2020, 265, 118538. [Google Scholar] [CrossRef]
- Zimmer, P.; Tschöpe, A.; Birringer, R. Temperature-programmed reaction spectroscopy of ceria- and Cu/ceria-supported oxide catalyst. J. Catal. 2002, 205, 339–345. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J.; Yang, Z.; Lu, G.; Ma, Z. Low-temperature CO oxidation on CuO/CeO2 catalysts: The significant effect of copper precursor and calcination temperature. Catal. Sci. Technol. 2015, 5, 3166–3181. [Google Scholar] [CrossRef]
- Gamarra, D.; Cámara, A.L.; Monte, M.; Rasmussen, S.B.; Chinchilla, L.E.; Hungría, A.B.; Munuera, G.; Gyorffy, N.; Schay, Z.; Corberán, V.C.; et al. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Characterization and performance as a function of the exposed face present in the CeO2 support. Appl. Catal. B 2013, 130–131, 224–238. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B 2015, 165, 742–751. [Google Scholar] [CrossRef]
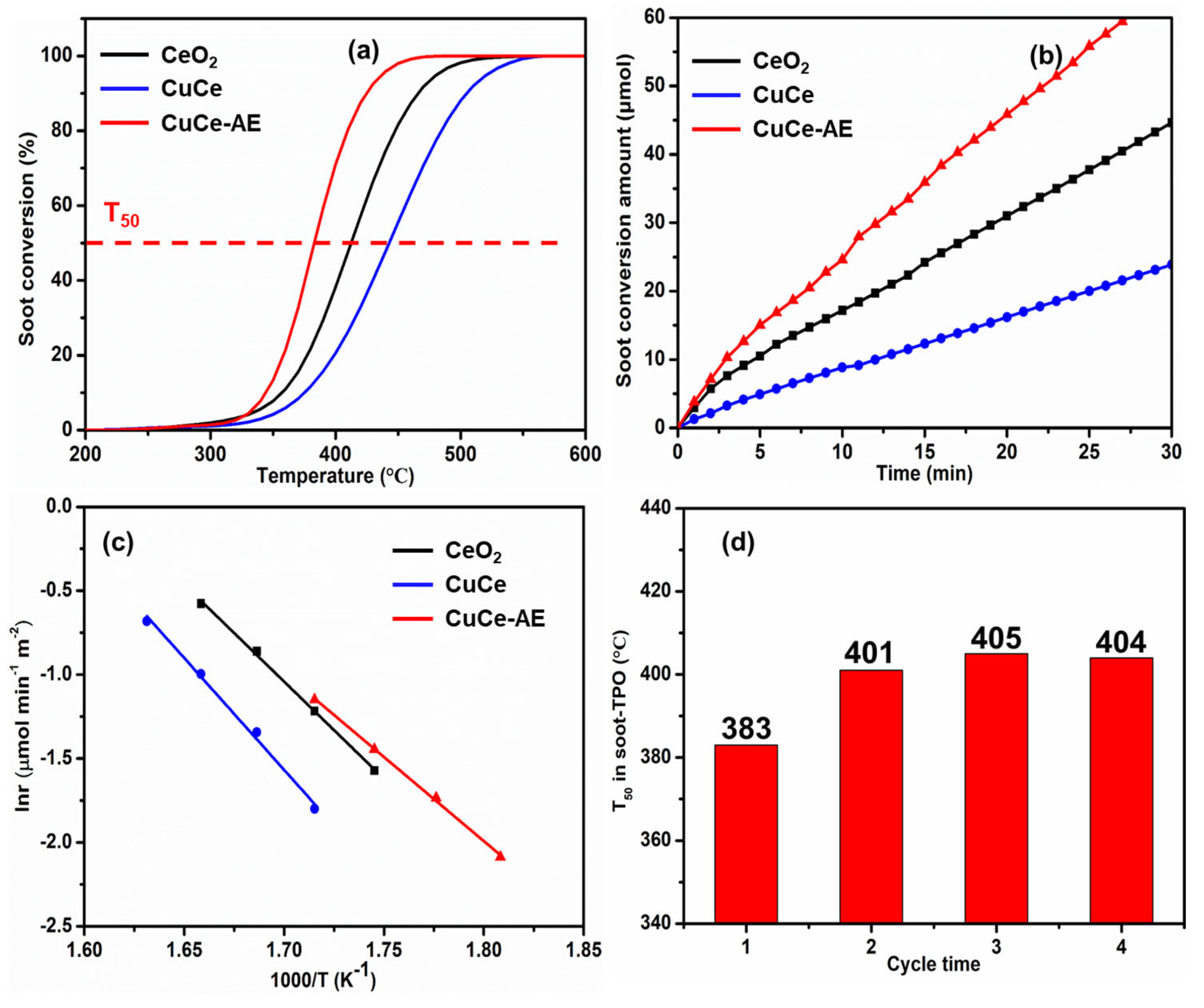

| Catalyst | T50 (°C) a | SCO2 (%) a | Reaction Rate (μmol min−1 g−1) b | Ea (kJ/mol) c |
|---|---|---|---|---|
| CeO2 | 412 | 99.8 | 30.8 | 96 |
| CuCe | 443 | 99.0 | 16.2 | 110 |
| CuCe-AE | 383 | 99.5 | 46.0 | 83 |
| Catalyst | Reactant Gas | Flow Rate (mL/min) | T50 (°C) | Reference |
|---|---|---|---|---|
| CuCe-AE | 10%O2/N2 | 80 | 383 | This work |
| Sn0.7Ce0.3O2 | O2/N2 | 268 | 437 | 3 |
| Ru/CeO2 | 10%O2/N2 | 80 | 386 | 4 |
| Cu-Ce | 10%O2/N2 | 500 | 356 | 13 |
| CuO/Ce-DDA | Air | - | 413 | 14 |
| Ce0.95Cu0.05O2 | 10%O2/N2 | 100 | 384 | 19 |
| Cu0.1Ce0.9O2-NF | 5%O2/Ar | 50 | 310 | 20 |
| Catalyst | Cu Content (wt.%) a | D (nm) b | SBET (m2/g) c | Vpore(cm3/g) c | ID/IF2g d |
|---|---|---|---|---|---|
| CeO2 | / | 10.5 | 104 | 0.30 | 0.32 |
| CuCe | 20.0 | 5.8 | 98 | 0.22 | 0.38 |
| CuCe-AE | 2.3 | 5.4 | 145 | 0.27 | 0.40 |
| Catalyst | Surface Cu/Ce | Ce 3d | Ce3+/Ce4+ (%) | O 1s | Oα/Oβ (%) | ||
|---|---|---|---|---|---|---|---|
| Ce3+ (%) | Ce4+ (%) | Oα (%) | Oβ (%) | ||||
| CeO2 | - | 18.3 | 81.7 | 22.4 | 28 | 72 | 38.8 |
| CuCe | 0.95 | 16.6 | 83.4 | 19.9 | 25 | 75 | 33.3 |
| CuCe-AE | 0.31 | 19.2 | 80.8 | 23.8 | 38 | 62 | 61.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Wu, X.; Li, Z.; Ran, R.; Weng, D. Enhanced Soot Oxidation Activity of a CuO-Doped CeO2 Catalyst via Acid Etching. Catalysts 2023, 13, 1463. https://doi.org/10.3390/catal13121463
Zheng C, Wu X, Li Z, Ran R, Weng D. Enhanced Soot Oxidation Activity of a CuO-Doped CeO2 Catalyst via Acid Etching. Catalysts. 2023; 13(12):1463. https://doi.org/10.3390/catal13121463
Chicago/Turabian StyleZheng, Changlong, Xiaodong Wu, Zhenguo Li, Rui Ran, and Duan Weng. 2023. "Enhanced Soot Oxidation Activity of a CuO-Doped CeO2 Catalyst via Acid Etching" Catalysts 13, no. 12: 1463. https://doi.org/10.3390/catal13121463
APA StyleZheng, C., Wu, X., Li, Z., Ran, R., & Weng, D. (2023). Enhanced Soot Oxidation Activity of a CuO-Doped CeO2 Catalyst via Acid Etching. Catalysts, 13(12), 1463. https://doi.org/10.3390/catal13121463








