Application of Unconventional External-Field Treatments in Air Pollutants Removal over Zeolite-Based Adsorbents/Catalysts
Abstract
:1. Introduction
2. Application of Non-Thermal Plasma over Zeolite-Based Materials
- (1)
- During the discharge process, the molecules in the gas atmosphere will be ionized to generate many high-energy electrons, which will collide with each other to convert electric energy into internal energy and kinetic energy of particles, thus providing the activation energy of chemical reactions [29], which can process materials that cannot be activated by the conventional thermal field [18].
- (2)
- Numerous electrons and other active substances with high energy will attack the external surface of zeolites to form new pores through etching and ablation effects, thus increasing the porosity.
- (3)
- The electrons with high energy can break the chemical bonds on the zeolite surface to form new active groups and increase the active sites amount.
- (4)
- The high-energy particles produced by plasma discharge can promote the excitation, ionization, and interaction of gas-reactant molecules to form new chemical bonds [30].
2.1. NTP Assisted Activation of Materials
2.2. NTP Cooperative Catalysis Process
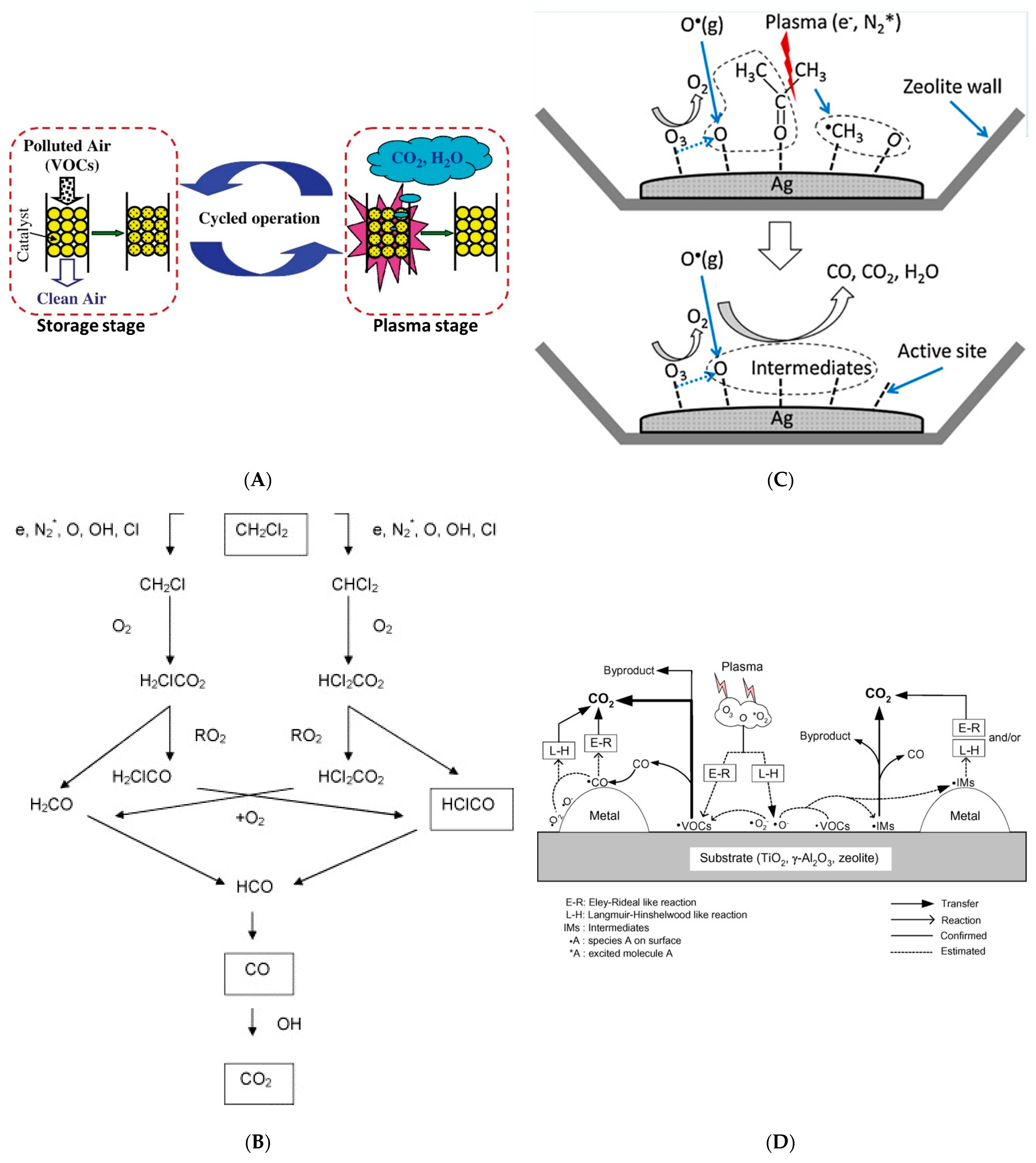
2.3. Influence Parameters of NTP
3. Application of Microwave over Zeolite-Based Materials
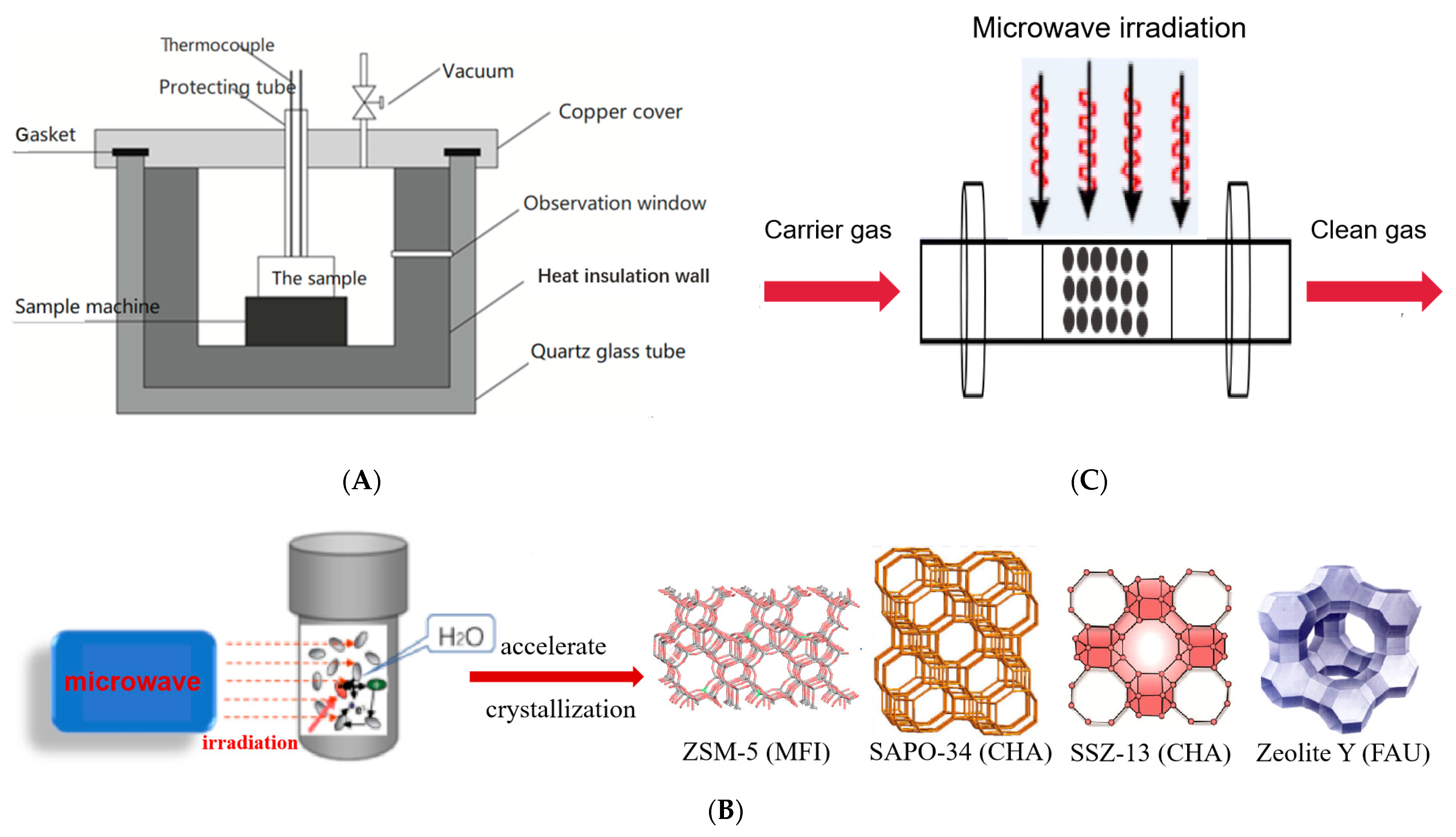
3.1. MW Assisted Synthesis of Zeolites
3.2. MW Assisted Activation of Materials
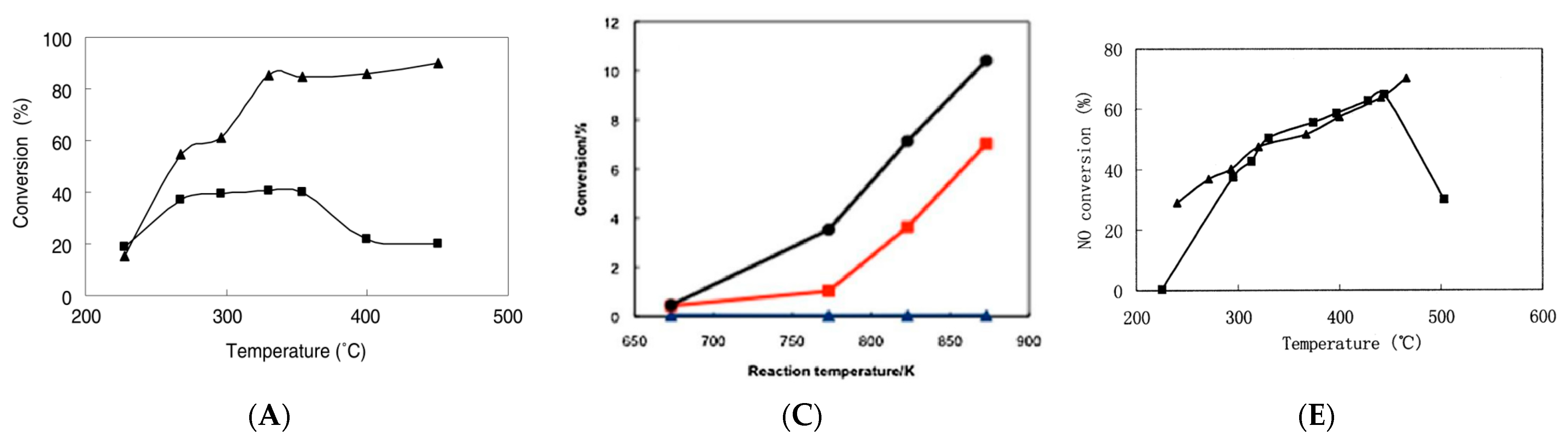
3.3. MW Assisted Catalysis Process
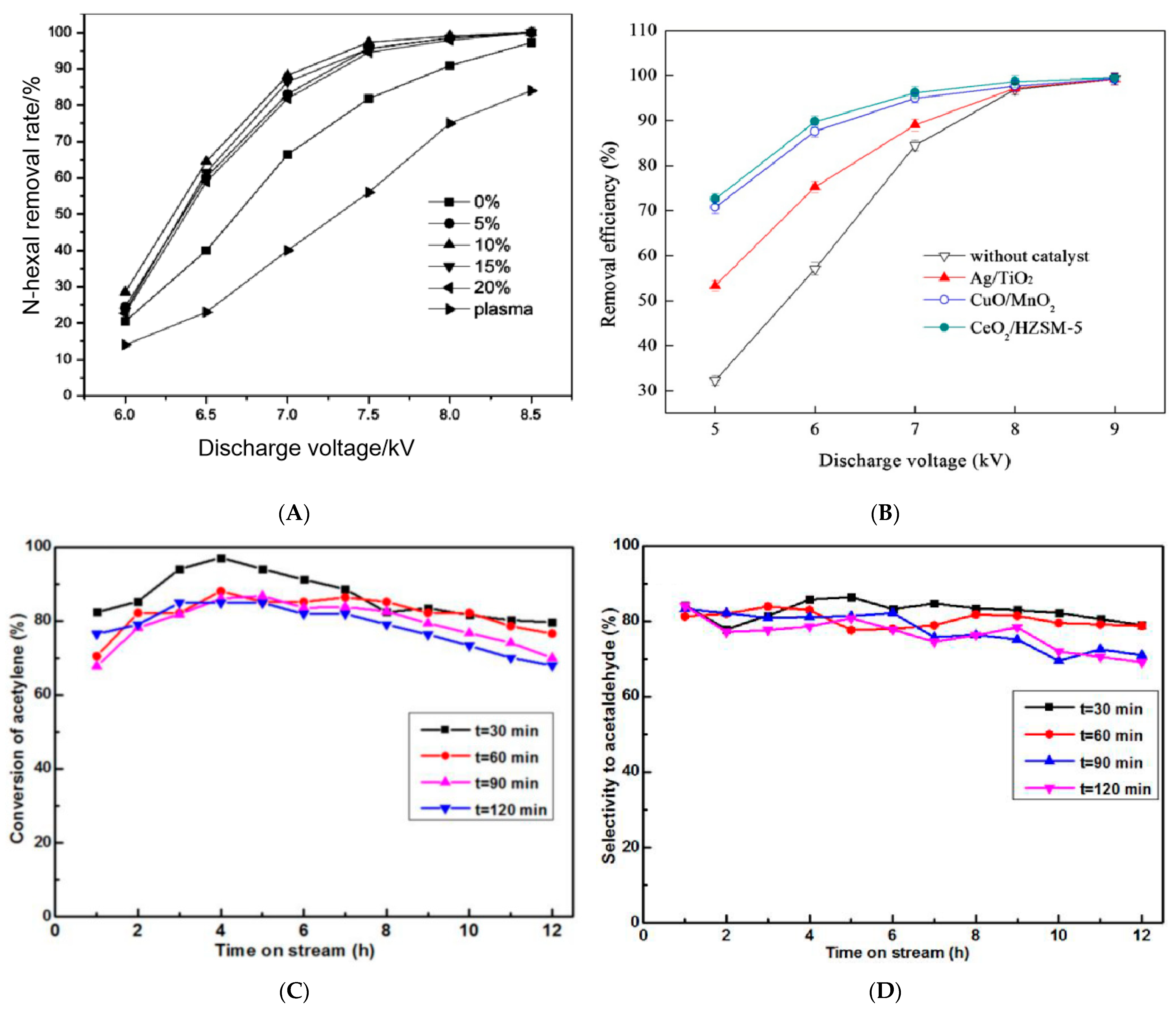
3.4. Influence Parameters of MW
4. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nitrogen oxides | NOx |
| Volatile organic compounds | VOCs |
| Convention thermal treatment | CT |
| Non-thermal plasma | NTP |
| Microwave | MW |
| Dielectric barrier discharge plasma | DBD |
| Glow discharge plasma | GDP |
| Corona discharge plasma | CDP |
| Hydrothermal synthesis method | HT |
References
- Tan, Z.; Lu, K.; Jiang, M.; Su, R.; Dong, H.; Zeng, L.; Xie, S.; Tan, Q.; Zhang, Y. Exploring ozone pollution in Chengdu, southwestern China: A case study from radical chemistry to O3-VOC-NOx sensitivity. Sci. Total Environ. 2018, 636, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Barrón, V.; Méndez, J.M.; Balbuena, J.; Cruz-Yusta, M.; Sánchez, L.; Giménez, C.; Sacristán, D.; González-Guzmán, A.; Sánchez-Rodríguez, A.R.; Skiba, U.M.; et al. Photochemical emission and fixation of NOx gases in soils. Sci. Total Environ. 2020, 702, 134982. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Yuan, Y.; Xi, B.; Tan, W. A review of the factors affecting the emission of the ozone chemical precursors VOCs and NOx from the soil. Environ. Int. 2023, 172, 107799. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Gao, Y.; Tang, X.; Yi, H.; Zhang, R.; Zhao, S.; Gao, F.; Zhou, Y. Removal of NO from flue gas over HZSM-5 by a cycling adsorption-plasma process. Catal. Commun. 2018, 110, 18–22. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous molecular sieve-based materials for catalytic oxidation of VOC: A review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhong, P.; Arandiyan, H.; Guan, Y.; Liu, J.; Wang, N.; Jiao, Y.; Fan, X. Ultrasonication facilitate the desilication for creating mesoporous zeolites. Front. Chem. Sci. Eng. 2020, 14, 275–287. [Google Scholar] [CrossRef]
- Ren, X.; Qu, R.; Liu, S.; Zhao, H.; Wu, W.; Song, H.; Zheng, C.; Wu, X.; Gao, X. Synthesis of Zeolites from Coal Fly Ash for the Removal of Harmful Gaseous Pollutants: A Review. Aerosol Air Qual. Res. 2020, 20, 1127–1144. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Ho Steven, S.H.; Xue, Y.; Liu, S.; Cheng, Y.; Wang, L.; Cao, J. Characteristics of volatile organic compounds in China. Environ. Earth Sci. 2017, 8, 225–242. [Google Scholar]
- Bel’chinskaya, L.I.; Khodosova, N.A.; Novikova, L.A.; Anisimov, M.V.; Petukhova, G.A. Regulation of Sorption Processes in Natural Nanoporous Aluminosilicates. 3. Impact of Electromagnetic Fields on Adsorption and Desorption of Formaldehyde by Clinoptilolite. Prot. Met. Phys. Chem. 2017, 53, 472–479. [Google Scholar] [CrossRef]
- Kamińska, I.I.; Lisovytskiy, D.; Valentin, L.; Calers, C.; Millot, Y.; Kowalewski, E.; Śrębowata, A.; Dzwigaj, S. Influence of pretreatment and reaction conditions on the catalytic activity of HAlBEA and CoHAlBEA zeolites in vinyl chloride formation from 1,2-dichloroethane. Microporous Mesoporous Mater. 2018, 266, 32–42. [Google Scholar] [CrossRef]
- Gao, C.; Lin, H.; Zhang, D.; Hong, R.; Tao, C.; Han, Z. Eu2+ -activated blue-emitting glass phosphor derived from Eu3+ exchanged USY zeolites by thermal treatment in reducing atmosphere. Ceram. Int. 2018, 44, 19547–19553. [Google Scholar] [CrossRef]
- Verma, S.K.; Walker, P.L. Alteration of molecular sieving properties of microporous carbons by heat treatment and carbon gasification. Carbon 1990, 28, 175–184. [Google Scholar] [CrossRef]
- Cheng, H.; Tang, X.; Yi, H.; Pan, R.; Zhang, J.; Gao, F.; Yu, Q. Application progress of small-pore zeolites in purifying NOx from motor vehicle exhaust. Chem. Eng. J. 2022, 449, 137795–137808. [Google Scholar] [CrossRef]
- Wang, A.; Lindgren, K.; Di, M.; Bernin, D.; Carlsson, P.-A.; Thuvander, M.; Olsson, L. Insight into hydrothermal aging effect on Pd sites over Pd/LTA and Pd/SSZ-13 as PNA and CO oxidation monolith catalysts. Appl. Catal. B Environ. 2020, 278, 119315–119327. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; He, L.; Li, B.; Lu, J.; Luo, M.; Chen, J. Unveiling geometric and electronic effects of Pt species on water-tolerant Pt/ZSM-5 catalyst for propane oxidation. Appl. Catal. A Gen. 2023, 655, 119108. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Kasprzyk-Hordern, B. Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides. Appl. Catal. B Environ. 2017, 200, 274–282. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B Environ. 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- El Roz, M.; Lakiss, L.; Valtchev, V.; Mintova, S.; Thibault-Starzyk, F. Cold plasma as environmentally benign approach for activation of zeolite nanocrystals. Microporous Mesoporous Mater. 2012, 158, 148–154. [Google Scholar] [CrossRef]
- Huang, X.; Yu, K.; Zhu, C.; Li, H. Progress in the reciprocal effect between plasma and catalysts. Chem. Eng. 2011, 25, 46–48+53. [Google Scholar] [CrossRef]
- Hanna, A.R.; Fisher, E.R. Tailoring the surface properties of porous zeolite constructs using plasma processing. Microporous Mesoporous Mater. 2020, 307, 110467–110478. [Google Scholar] [CrossRef]
- Ohgushi, T.; Nagae, M. Quick activation of optimized zeolites with microwave heating and utilization of zeolites for reusable desiccant. J. Porous Mater. 2003, 10, 139–143. [Google Scholar] [CrossRef]
- Zhang, R.; Raja, D.; Zhang, Y.; Yan, Y.; Garforth, A.A.; Jiao, Y.; Fan, X. Sequential Microwave-Assisted Dealumination and Hydrothermal Alkaline Treatments of Y Zeolite for Preparing Hierarchical Mesoporous Zeolite Catalysts. Top. Catal. 2020, 63, 340–350. [Google Scholar] [CrossRef]
- Du, M. Study on the Reduction of NOx by NH3 Catalyzed by Low Temperature Plasma and Manganese Based Oxides. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Xiao, D. Study on the Catalytic Mechanism of Trace Additives for SO2/NOx Removal Process in Microwave Field. Master’s Thesis, University of Science and Technology Liaoning, Anshan, China, 2021. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Sadighi, S.; Rashidzadeh, M.; Khorram, S.; Back, J.O.; Penner, S.; Noisternig, M.F.; Salari, D.; Niaei, A. Investigating the Cold Plasma Surface Modification of Kaolin- and Attapulgite-Bound Zeolite A. J. Ind. Eng. Chem. 2022, 106, 113–127. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Yu, K. Floating double probe characteristics of non- thermal plasmas in the presence of zeolite. J. Electrostat. 2002, 54, 149–158. [Google Scholar] [CrossRef]
- Mirzaei, H.; Almasian, M.R.; Moosavian, S.M.A.; Sid Kalal, H. Plasma modification of a natural zeolite to improve its adsorption capacity of strontium ions from water samples. Int. J. Environ. Sci. Technol. 2019, 16, 6157–6166. [Google Scholar] [CrossRef]
- Liu, C.; Vissokov, G.P.; Jang, B.W.-L. Catalyst preparation using plasma technologies. Catal. Today 2002, 72, 173–184. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; Geyter, N.D.; Giraudon, J.-M.; Morin, J.-C.; Tabaei, P.S.E.; Weireld, G.D.; Laemont, A.; Leus, K.; Voort, P.V.D.; Lamonier, J.-F.; et al. Effect of non-thermal plasma in the activation and regeneration of 13X zeolite for enhanced VOC elimination by cycled storage and discharge process. J. Clean. Prod. 2022, 364, 132687–132696. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Yi, X.; Zhao, S.; Gao, F.; Yang, Z. Oxygen plasma-catalytic conversion of NO over MnOx: Formation and reactivity of adsorbed oxygen. Catal. Commun. 2017, 100, 227–231. [Google Scholar] [CrossRef]
- Fahmy, A.; Elzaref, A.; Youssef, H.; Shehata, H.; Wassel, M.; Friedrich, J.; Poncin-Epaillard, F.; Debarnot, D. Plasma O2 modifies the structure of synthetic zeolite-A to improve the removal of cadmium ions from aqueous solutions. Turk. J. Chem. 2019, 43, 172–184. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Zhang, Y.; Zhu, X.; He, F.; Eliasson, B. Characterization of plasma treated Pd/HZSM-5 catalyst for methane combustion. Appl. Catal. B Environ. 2004, 47, 95–100. [Google Scholar] [CrossRef]
- Alexandrov, S.E.; Hitchman, M.L. Chemical Vapor Deposition Enhanced by Atmospheric Pressure Non-thermal Non-equilibrium Plasmas. Chem. Vap. Depos. 2005, 11, 457–468. [Google Scholar] [CrossRef]
- Ding, X.; Duan, Y. Plasma-based ambient mass spectrometry techniques: The current status and future prospective. Mass Spectrom. Rev. 2015, 34, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Taaca, K.L.M.; Vasquez, M.R. Fabrication of Ag-exchanged zeolite/chitosan composites and effects of plasma treatment. Microporous Mesoporous Mater. 2017, 241, 383–391. [Google Scholar] [CrossRef]
- He, F.; Liu, C.; Eliasson, B.; Xue, B. XPS characterization of zeolite catalyst in plasma catalytic methane conversion. Surf. Interface Anal. 2001, 32, 198–201. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Zhang, Y.; Zhu, X.; He, F.; Eliasson, B. Remarkable improvement in the activity and stability of Pd/HZSM-5 catalyst for methane combustion. Catal. Commun. 2003, 4, 303–307. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Shi, P.; Liu, C.; Liu, Y. Al-MCM-41 supported palladium catalyst for methane combustion: Effect of the preparation methodologies. Appl. Catal. B Environ. 2009, 90, 570–577. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Zhou, X.; Liang, J.; Yuan, H.; Yang, D.; Wang, W. Highly efficient adsorptive removal of persistent organic pollutants using NPD-acid combined modified NaY zeolites. Chem. Eng. J. 2022, 431, 133858–133867. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, R.; Rui, N.; Wang, Z.; Wang, J.; Zhou, X.; Liu, C.-J. Co3O4/HZSM-5 catalysts for methane combustion: The effect of preparation methodologies. Catal. Today 2017, 297, 219–227. [Google Scholar] [CrossRef]
- Kwak, J.H.; Peden, C.H.F.; Szanyi, J. Non-thermal plasma-assisted NOx reduction over Na-Y zeolites: The promotional effect of acid sites. Catal. Lett. 2006, 109, 1–6. [Google Scholar] [CrossRef]
- Furukawa, K.; Tian, S.R.; Yamauchi, H.; Yamazaki, S.; Ijiri, H.; Ariga, K.; Muraoka, K. Characterization of HY zeolite modified by a radio- frequency CF4 plasma. Chem. Phys. Lett. 2000, 318, 22–26. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, C.-J.; Zhang, Y.-P.; Yu, K.-L.; Eliasson, B.; Xue, B. A plasma enhanced acidity of solid acid. In Proceedings of the CHEMRAWN XIVth Conference on Green Chemistry, Boulder, CO, USA, 9–13 June 2001. [Google Scholar]
- Yu, K.-L.; Xia, Q.; Liu, C.-J.; Li, G.; Eliasson, B.; Xue, B. On the plasma enhanced Bronsted acidity of solid acids. In Proceedings of the 4th International Symp, On Green Chemistry in China, Jinan, China, 9–12 May 2001. [Google Scholar]
- Zhu, X.; Yu, K.; Cheng, D.; Zhang, Y.; Xia, Q.; Liu, C. Modification of acidity of Mo-Fe/HZSM-5 zeolite via argon plasma treatment. Front. Chem. Eng. China 2008, 2, 55–58. [Google Scholar] [CrossRef]
- Takeuchi, N.; Electrochem, J. Surface Characterization of Zeoite Catalyst Heat-treated by Microwave Cold Plasma. Soc. Jpn. 1995, 63, 164–166. [Google Scholar]
- Wang, Q.; Zhang, S.; Yu, Y.; Dai, B. High-performance of plasma-enhanced Zn/MCM-41 catalyst for acetylene hydration. Catal. Commun. 2020, 147, 106–122. [Google Scholar] [CrossRef]
- Yoon, S.; Panov, A.G.; Tonkyn, R.G.; Ebeling, A.C.; Barlow, S.E.; Balmer, M.L. An examination of the role of plasma treatment for lean NOx reduction over sodium zeolite Y and gamma alumina Part 2. Formation of nitrogen. Catal. Today 2002, 72, 251–257. [Google Scholar] [CrossRef]
- Yoon, S.; Panov, A.G.; Tonkyn, R.G.; Ebeling, A.C.; Barlow, S.E.; Balmer, M.L. An examination of the role of plasma treatment for lean NOx reduction over sodium zeolite Y and gamma alumina: Part 1. Plasma assisted NOx reduction over NaY and Al2O3. Catal. Today 2002, 72, 243–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, P.; Zhu, X.; Liu, C.; Shen, Y. A novel plasma-treated Pt/NaZSM-5 catalyst for NO reduction by methane. Catal. Commun. 2004, 10, 35–39. [Google Scholar] [CrossRef]
- Xu, J.; Tang, T.; Sheng, X.; Zhang, Y.; Zhang, Q.; Guo, F. DBD plasma activation-enhanced low temperature propylene-SCR of NOx over a low-load Mn/ZSM-5 catalyst under lean-burn conditions. J. Environ. Chem. Eng. 2022, 10, 107009. [Google Scholar] [CrossRef]
- Wang, J.; Yi, H.; Tang, X.; Zhao, S.; Gao, F.; Zhang, R.; Yang, Z. Products Yield and Energy Efficiency of Dielectric Barrier Discharge for NO Conversion: Effect of Oz Content, NO Concentration, and Flow Rate. Energy Fuels 2017, 31, 9675–9683. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Yi, H.; Zhao, S.; Gao, F.; Huang, Y.; Zhang, R.; Yang, Z. N2O Formation Characteristics in Dielectric Barrier Discharge Reactor for Environmental Application: Effect of Operating Parameters. Energy Fuels 2017, 31, 13901–13908. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Yi, H.; Zhao, S.; Gao, F.; Chu, C. Nitrogen Fixation and NO Conversion using Dielectric Barrier Discharge Reactor: Identification and Evolution of Products. Plasma Chem. Plasma Process. 2018, 38, 485–501. [Google Scholar] [CrossRef]
- Kim, H.H.; Takashima, K.; Katsura, S.; Mizuno, A. Low-temperature NO, reduction processes using combined systems of pulsed corona discharge and catalysts. J. Phys. D Appl. Phys. 2001, 34, 604613. [Google Scholar] [CrossRef]
- Schmidt, M.; Basner, R.; Brandenburg, R. Hydrocarbon assisted NO oxidation with non-thermal plasma in simulated marine diesel exhaust gases. Plasma Chem. Plasma Process. 2013, 33, 323–335. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Shi, C.; Li, X.-S.; Yang, X.-F.; Xu, Y.; Zhu, A.-M. Low-temperature NOx Selective Reduction by Hydrocarbons on H-Mordenite Catalysts in Dielectric Barrier Discharge Plasma. Plasma Chem. Plasma Process. 2009, 29, 43. [Google Scholar] [CrossRef]
- Niu, J.; Yang, X.; Zhu, A.; Shi, L.; Sun, Q.; Xu, Y.; Shi, C. Plasma-assisted selective catalytic reduction of NOx by C2H2 over Co-HZSM-5 catalyst. Catal. Commun. 2006, 7, 297–301. [Google Scholar] [CrossRef]
- Byun, Y.; Ko, K.B.; Cho, M.; Namkung, W.; Shin, D.N.; Koh, D.J. Effect of hydrogen generated by dielectric barrier discharge of NH3 on selective non-catalytic reduction process. Chemosphere 2009, 75, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A.; Kumar, K.; Singh, B.; Mina, U.; Singh, B.B.; Jain, V.K. Statistical modeling of O3, NOx, CO, PM2.5, VOCs and noise levels in commercial complex and associated health risk assessment in an academic institution. Sci. Total Environ. 2016, 572, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, A.; Mehandjiev, D. Complete oxidation of benzene on manganese dioxide by ozone. Appl. Catal. A Gen. 1993, 97, 17. [Google Scholar] [CrossRef]
- Ogata, A.; Kim, H.H.; Futamura, S.; Kushiyama, S.; Mizuno, K. Effects of catalysts and additives on fluorocarbon removal with surface discharge plasma. Appl. Catal. B Environ. 2004, 53, 175. [Google Scholar] [CrossRef]
- Konova, P.; Stoyanova, M.; Naydenov, A.; Christoskova, S.; Mehandjiev, D. Catalytic oxidation of VOCs and CO by ozone over alumina supported cobalt oxide. Appl. Catal. A Gen. 2006, 298, 109. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, F.-D. Improved oxidation of air pollutants in a non-thermal plasma. Catal. Today 2002, 73, 315. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; Geyter, N.D.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 98. [Google Scholar] [CrossRef]
- Wallis, A.E.; Whitehead, J.C.; Zhang, K. The removal of dichloromethane from atmospheric pressure nitrogen gas streams using plasma-assisted catalysis. Appl. Catal. B Environ. 2007, 72, 282–288. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Einaga, H.; Ogata, A.; Futamura, S.; Park, D.W. Zeolite-combined plasma reactor for decomposition of toluene. Thin Solid Films. 2006, 506–507, 418–422. [Google Scholar] [CrossRef]
- Teramoto, Y.; Kim, H.-H.; Negishi, N.; Ogata, A. The role of ozone in the reaction mechanism of a bare zeolite-plasma hybrid system. Catalysts 2015, 5, 838–850. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Gandhi, M.S.; Mok, Y.S. Adsorption and plasma-catalytic oxidation of acetone over zeolite-supported silver catalyst. Jpn. J. Appl. Phys. 2015, 54, 01AG04. [Google Scholar] [CrossRef]
- Francke, K.P.; Miessner, H.; Rudolph, R. Plasmacatalytic processes for environmental problems. Catal. Today 2000, 59, 411–416. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl. Catal. B Environ. 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Li, D.; Tang, X.; Yi, H.; Ma, D.; Gao, F. NOx decomposition over modified NaY zeolite by dielectric barrier discharge plasma in the presence of excess oxygen. Acta Sci. Circumstantiae 2015, 35, 4088–4094. [Google Scholar] [CrossRef]
- Xiang, D.; Zhao, G.; Ye, D. Study on factors influencing Hexanal oxidation by plasma-assisted MnOx/SBA-15 catalysis. Sci. Online 2010, 5, 355–359. [Google Scholar]
- Zhu, R.; Mao, Y.; Jiang, L.; Chen, J. Performance of chlorobenzene removal in a nonthermal plasma catalysis reactor and evaluation of its byproducts. Chem. Eng. J. 2015, 279, 463–471. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, Y.K.; Park, S.E. Superior decomposition of NO over plasma-assisted catalytic system induced by microwave. Chem. Lett. 2000, 29, 578–579. [Google Scholar] [CrossRef]
- Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Non-thermal plasma-assisted NO, reduction over alkali and alkaline earth ion exchanged Y, FAU zeolites. Catal. Today 2004, 89, 135–141. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, N.; Li, J.; Shang, K.; Lu, K.; Wu, Y. Degradation of benzene by bipolar pulsed series surface/packed-bed discharge reactor over MnO2-TiO2/zeolite catalyst. Chem. Eng. J. 2016, 293, 216–224. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.H.; Ogata, A. Adsorption and oxygen plasma-driven catalysis for total oxidation of VOCs. Int. J. Plasma Environ. Sci. Technol. 2008, 2, 106–112. [Google Scholar]
- Kim, H.H.; Ogata, A.; Futamura, S. Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Shi, C.; Li, X.-S.; Zhao, D.-Z.; Xu, Y.; Zhu, A.-M. High-efficiency plasma catalytic removal of dilute benzene from air. J. Phys. D Appl. Phys. 2009, 42, 225105. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, J.; Wu, J.; Zhu, A. Cycled storage-discharge (CSD) plasma catalytic removal of benzene over AgMn/HZSM-5 using air as discharge gas. Catal. Sci. Technol. 2016, 6, 3788–3796. [Google Scholar] [CrossRef]
- Jiang, L.; Nie, G.; Zhu, R.; Wang, J.; Chen, J.; Mao, Y.; Cheng, Z.; Anderson, W.A. Efficient degradation of chlorobenzene in a non-thermal plasma catalytic reactor supported on CeO2/HZSM-5 catalysts. J. Environ. Sci. 2017, 55, 266–273. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Lee, S.B.; Mok, Y.S. Removal of ethylene from air stream by adsorption and plasma-catalytic oxidation using silver-based bimetallic catalysts supported on zeolite. J. Hazard. Mater. 2015, 285, 525–534. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Mok, Y.S. Effect of the adsorbent/catalyst preparation method and plasma reactor configuration on the removal of dilute ethylene from air stream. Catal. Today 2015, 256, 170–177. [Google Scholar] [CrossRef]
- Zhao, D.-Z.; Li, X.-S.; Shi, C.; Fan, H.-Y.; Zhu, A.-M. Low-concentration formaldehyde removal from air using a cycled storage-discharge (CSD) plasma catalytic process. Chem. Eng. Sci. 2011, 66, 3922–3929. [Google Scholar] [CrossRef]
- Yi, H.; Yang, X.; Tang, X.; Zhao, S. Removal of toluene from industrial gas by adsorption–plasma catalytic process: Comparison of closed discharge and ventilated discharge. Plasma Chem. Plasma Process. 2018, 38, 331–345. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhu, T.; Fan, X. Removal of gas phase low-concentration toluene over Mn, Ag and Ce modified HZSM-5 catalysts by periodical operation of adsorption and non-thermal plasma regeneration. J. Hazard. Mater. 2015, 292, 70–78. [Google Scholar] [CrossRef]
- Yi, H.; Yang, X.; Tang, X.; Zhao, S.; Wang, J.; Cui, X.; Feng, T.; Ma, Y. Removal of toluene from industrial gas over 13X zeolite supported catalysts by adsorption-plasma catalytic process. J. Chem. Technol. Biotechnol. 2017, 92, 2276–2286. [Google Scholar] [CrossRef]
- Huang, R.; Lu, M.; Wang, P.; Chen, Y.; Wu, J.; Fu, M.; Chen, L.; Ye, D. Enhancement of the non-thermal plasma-catalytic system with different zeolites for toluene removal. RSC Adv. 2015, 5, 72113–72120. [Google Scholar] [CrossRef]
- Dou, B.; Liu, D.; Zhang, Q.; Zhao, R.; Hao, Q.; Bin, F.; Cao, J. Enhanced removal of toluene by dielectric barrier discharge coupling with Cu-Ce-Zr supported ZSM-5/TiO2/Al2O3. Catal. Commun. 2017, 92, 15–18. [Google Scholar] [CrossRef]
- Radoiu, M.T.; Hájek, M. Effect of solvent, catalyst type and catalyst activation on the microwave transformation of 2-tert-butylphenol. J. Mol. Catal. A Chem. 2002, 186, 121–126. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, G.; Han, L.; Chang, L.; Bao, W.; Wang, J. Cu-SSZ-13 Catalyst Synthesized under Microwave Irradiation and Its Performance in Catalytic Removal of NOx from Vehicle Exhaust. Acta Phys.-Chim. Sin. 2015, 31, 2165–2173. [Google Scholar] [CrossRef]
- Deng, Y.; Bai, X.; Abdelsayed, V.; Shekhawat, D.; Muley, P.D.; Karpe, S.; Mevawala, C.; Bhattacharyy, D.; Robinson, B.; Caiola, A.; et al. Microwave-assisted conversion of methane over H-(Fe)-ZSM-5: Evidence for formation of hot metal sites. Chem. Eng. J. 2021, 420, 129670. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, J.; Wang, H. Microwave catalytic reduction of nitric oxide in activated carbon bed with a new microwave catalytic reactor system. J. Chem. Pharm. Res. 2014, 6, 1412–1417. [Google Scholar]
- Cheng, Z.L.; Zhao, Z.S.; Wan, H.L. Microwave induced rapid synthesis of NaY molecular sieves. Acta Phys.-Chim. Sin. 2003, 19, 487–491. [Google Scholar]
- Yu, C.; Yi, Y.; Zhou, J.; Xu, W. Highly effective and energy-saving removal of NO through an adsorption–microwave catalytic decomposition method under complex flue gas at low temperature. Inorg. Chem. Front. 2023, 10, 3808–3820. [Google Scholar] [CrossRef]
- Kim, D.S.; Chang, J.S.; Hwang, J.S.; Park, S.E.; Kim, S.E. Synthesis of zeolite beta in fluoride media under microwave irradiation. Microporous Mesoporous Mater. 2004, 68, 77–82. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H. Ammonia nitrogen removal from aqueous solution using zeolite modified by microwave-sodium acetate. J. Cent. South Univ. 2016, 23, 1345–1352. [Google Scholar] [CrossRef]
- Turner, M.D.; Laurence, R.L.; Conner, W.C.; Yngvesson, K.S. Microwave radiation's influence on sorption and competitive sorption in zeolites. AIChE J. 2000, 46, 758–768. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Nepomnyushchaya, V.A.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Yarusova, S.B.; Buravlev, I.Y.; Tarabanova, A.E.; Fedorets, A.N.; et al. Hydrothermal synthesis and spark plasma sintering of NaY zeolite as solid-state matrices for cesium-137 immobilization. J. Eur. Ceram. Soc. 2022, 42, 3004–3014. [Google Scholar] [CrossRef]
- Yarusova, S.B.; Shichalin, O.O.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Golub, A.V.; Mayorov, A.V.; Gerasimenko, A.V.; Papynov, E.K.; Ivanets, A.I.; et al. Synthesis of amorphous KAlSi3O8 for cesium radionuclide immobilization into solid matrices using spark plasma sintering technique. Ceram. Int. 2022, 48, 3808–3817. [Google Scholar] [CrossRef]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Mayorov, V.Y.; et al. A novel approach for rice straw agricultural waste utilization: Synthesis of solid aluminosilicate matrices for cesium immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Alvarez, S.; García, A.; Manolache, S.; Denes, F.; Riera, F.A.; Álvarez, R. Plasma enhanced modification of the pore size of ceramic membranes. Desalination 2005, 184, 99–104. [Google Scholar] [CrossRef]
- Koo, J.-B.; Jiang, N.; Saravanamurugan, S.; Bejblová, M.; Musilová, Z.; Čejka, J.; Park, S.-E. Direct synthesis of carbon-templating mesoporous ZSM-5 using microwave heating. J. Catal. 2010, 276, 327–334. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.Y.; Sharma, J.Y.; Yu, J.; Xu, R. Fabrication of SAPO-34 crystals with different morphologies by microwave heating. Top. Catal. 2010, 53, 1304–1310. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Y.; Yang, Y. Microwave-synthesized MCM-41 mesoporous molecular sieves studied by XRD powder diffraction. J. Inorg. Chem. 2000, 16, 119–122. [Google Scholar] [CrossRef]
- Ling, Y.; Zheng, Y.; Liu, Y.; Wang, Z.; Wu, H.; Wu, P. A Study on Microwave-Assisted Synthesis of MCM-22 Zeolite. Acta Chimica Sinica. 2010, 68, 2035–2040. [Google Scholar]
- Arafat, A.; Jansen, J.C.; Ebaid, A.R.; Bekkum, H. van. Microwave preparation of zeolite Y and ZSM5. Zeolites 1993, 13, 162–165. [Google Scholar] [CrossRef]
- Song, T.; Xu, J.; Xu, W.; Meng, X.; Feng, H.; Liu, X.; Zhou, F.; Ru, X. NaX molecular sieve was synthesized by microwave irradiation. J. Univ. Chem. 1992, 13, 1209–1210. [Google Scholar]
- Shalmani, F.M.; Halladj, R.; Askari, S. Effect of contributing factors on microwaveassisted hydrothermal synthesis of nanosized SAPO-34 molecular sieves. Powder Technol. 2012, 221, 395–402. [Google Scholar] [CrossRef]
- Jun, J.W.; Ahmed, I.; Kim, C.U.; Jeong, K.-E.; Jeong, S.-Y.; Jhung, S.H. Synthesis of ZSM-5 zeolites using hexamethylene imine as a template: Effect of microwave aging. Cataly. Today 2014, 232, 108–113. [Google Scholar] [CrossRef]
- Wu, C.-G.; Bein, T. Microwave synthesis of molecular sieve MCM-41. Chem. Commun. 1996, 8, 925–926. [Google Scholar] [CrossRef]
- Ansari, M.; Aroujalian, A.; Raisi, A.; Dabir, B.; Fathizadeh, M. Preparation and characterization of nano-NaX zeolite by microwave assisted hydrothermal method. Adv. Powder Technol. 2014, 25, 722–727. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Hu, X.; Xu, C.; Liu, S.; Zhang, X.; Wei, R. Adsorption and catalytic oxidation performance of Pd/MCM-41 for acetaldehyde from ethanol-gasoline vehicle exhaust. Chin. J. Environ. Eng. 2018, 12, 2558–2565. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, X.; Wang, J. Effect of microwave-assisted aging on the static hydrothermal synthesis of zeolite MCM-22. Microporous Mesoporous Mater. 2008, 116, 386–393. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, Y.; Zhang, X.; Gu, C.; Ning, Y.; Liu, H. Application of NaY zeolite molecular sieve in VOCs treatment. Chin. J. Environ. Eng. 2020, 14, 2211–2221. [Google Scholar] [CrossRef]
- Figueiredo, H.; Neves, I.C.; Quintelas, C.; Tavares, T.; Taralunga, M.; Mijoin, J.; Magnoux, P. Oxidation catalysts prepared from biosorbents supported on zeolites. Appl. Catal. B Environ. 2006, 66, 274–280. [Google Scholar] [CrossRef]
- Lima, R.B.; Neto, M.M.S.; Oliveira, D.S.; Santos, A.G.D.; Souza, L.D.; Caldeira, V.P.S. Obtainment of hierarchical ZSM-5 zeolites by alkaline treatment for the polyethylene catalytic cracking. Adv. Powder Technol. 2021, 32, 515–523. [Google Scholar] [CrossRef]
- Katsuki, H.; Furuta, S.; Komarneni, S. Microwave Versus Conventional-Hydrothermal Synthesis of NaY Zeolite. J. Porous Mater. 2001, 8, 5–12. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Rongchapo, W.; Keawkumay, C.; Chanlek, N.; Prayoonpokarach, S.; Wittayakun, J. Extraction of silica from cogon grass and utilization for synthesis of zeolite NaY by conventional and microwave-assisted hydrothermal methods. J. Taiwan Inst. Chem. Eng. 2018, 83, 152–158. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; Wang, M.; Han, L.; Wang, J.; Bao, W.; Chang, L. Microwave synthesis conditions dependent catalytic performance of hydrothermally aged CuII-SSZ-13 for NH3-SCR of NO. Catal. Today 2021, 376, 19–27. [Google Scholar] [CrossRef]
- Khan, N.A.; Yoo, D.K.; Lee, S.; Kim, T.-W.; Kim, C.-U.; Jhung, S.H. Microwave-assisted rapid synthesis of nanosized SSZ-13 zeolites for effective conversion of ethylene to propylene. J. Ind. Eng. Chem. 2023, 121, 242–248. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Li, B.; Lyu, Y.; Wang, X.; Liu, X.; Li, L.; Yu, S.; Liu, X.; Yan, Z. Isomerization of α -pinene with a hierarchical mordenite molecular sieve prepared by the microwave assisted alkaline treatment. Microporous Mesoporous Mater. 2020, 299, 110117–110125. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, G.; Xie, Z.; Ma, C.; Liu, X.; Sun, J.; Liu, L. Microwave catalytic NOx and SO2 removal using FeCu/zeolite as catalyst. Fuel 2011, 90, 1599–1603. [Google Scholar] [CrossRef]
- Bo, L.; Zhang, Y.; Wang, X.; Liu, H.; Zhang, H. Preparation and application of high-performance catalyst in microwave assisted catalytic oxidation of benzene. J. Fuel Chem. Technol. 2012, 40, 878–885. [Google Scholar]
- Tang, J.; Zhang, T.; Liang, D.; Yang, H.; Li, N.; Lin, L. Direct decomposition of NO by microwave heating over Fe/NaZSM-5. Appl. Catal. B Environ. 2002, 36, 1–7. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, T.; Liang, D.; Lin, L. Reduction of NO by CH4 with microwave heating. Top. Catal. 2003, 22, 59–63. [Google Scholar] [CrossRef]
- Turner, M.D.; Laurence, R.L.; Yngvesson, K.S.; Conner, W.C. The effect of microwave energy on three-way automotive catalysts poisoned by SO2. Catal. Lett. 2001, 71, 133–138. [Google Scholar] [CrossRef]
- Ohnishi, T.; Kawakami, K.; Nishioka, M.; Ogura, M. Direct decomposition of NO on metal-loaded zeolites with coexistence of oxygen and water vapor under unsteady-state conditions by NO concentration and microwave rapid heating. Catal. Today 2017, 281, 566–574. [Google Scholar] [CrossRef]
- Bond, G.; Moyes, R.B.; Whan, D.A. Recent applications of microwave heating in catalysis. Catal. Today 1993, 17, 427–437. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Xu, C.H.; Sun, X.Y.; Liang, D.B.; Lin, L.W. Microwave effects on the selective reduction of NO by CH4 over an In–Fe2O3/HZSM-5 catalyst. Chem. Commun. 2000, 4, 279–280. [Google Scholar] [CrossRef]
- Kustov, L.M.; Sinev, I.M. Microwave Activation of Catalysts and Catalytic Processes. Russ. J. Phys. Chem. 2010, 84, 1676–1694. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, J.; Sun, Z.; Liu, N.; Cheng, Z.; Gui, J. Aromatization of Light Hydrocarbon over ZnNi/HZSM-5 Catalyst under Microwave Heating. Chin. J. Catal. 2000, 21, 434–436. [Google Scholar]
- Zholobenko, V.L.; House, E.R. Zeolite-based catalysts for microwave-induced transformations of hydrocarbons. Catal. Lett. 2003, 89, 35–40. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, G.; Xie, Z. Microwave catalytic desulfurization and denitrification simultaneously on Fe/Ca-5A zeolite catalyst. Energy Fuels 2009, 23, 2947–2951. [Google Scholar] [CrossRef]
- Lira, E.; Lopez, C.M.; Oropeza, F.; Bartolini, M.; Alvarez, J.; Goldwasser, M.; Linares, F.L.; Lamonier, J.-F.; Zurita, M.J.P. HMS mesoporous silica as cobalt support for the Fischer–Tropsch Synthesis: Pretreatment, cobalt loading and particle size effects. J. Mol. Catal. A Chem. 2008, 281, 146–153. [Google Scholar] [CrossRef]
- An, Y. Removal of ammonium from aqueous solution by three modified molecular sieves: A comparative study. Water Sci. Technol. 2017, 76, 5–6. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, H.; Ji, Y. Simultaneous removal of SO2 and NOx by microwave with potassium permanganate over zeolite. Fuel Process. Technol. 2009, 90, 324–329. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, G.; Xie, Z.; Sun, J. Simultaneous desulfurization and denitrification by microwave catalytic over FeCoCu/Zeolite 5A catalyst. J. Environ. Eng. 2010, 136, 1403–1408. [Google Scholar] [CrossRef]
- Sharma, M.; Das, B.; Karunakar, G.V.; Satyanarayana, L.; Bania, K.K. Chiral Ni-Schiff Base Complexes inside Zeolite-Y and Their Application in Asymmetric Henry Reaction: Effect of Initial Activation with Microwave Irradiation. J. Phys. Chem. C 2016, 120, 13563–13573. [Google Scholar] [CrossRef]





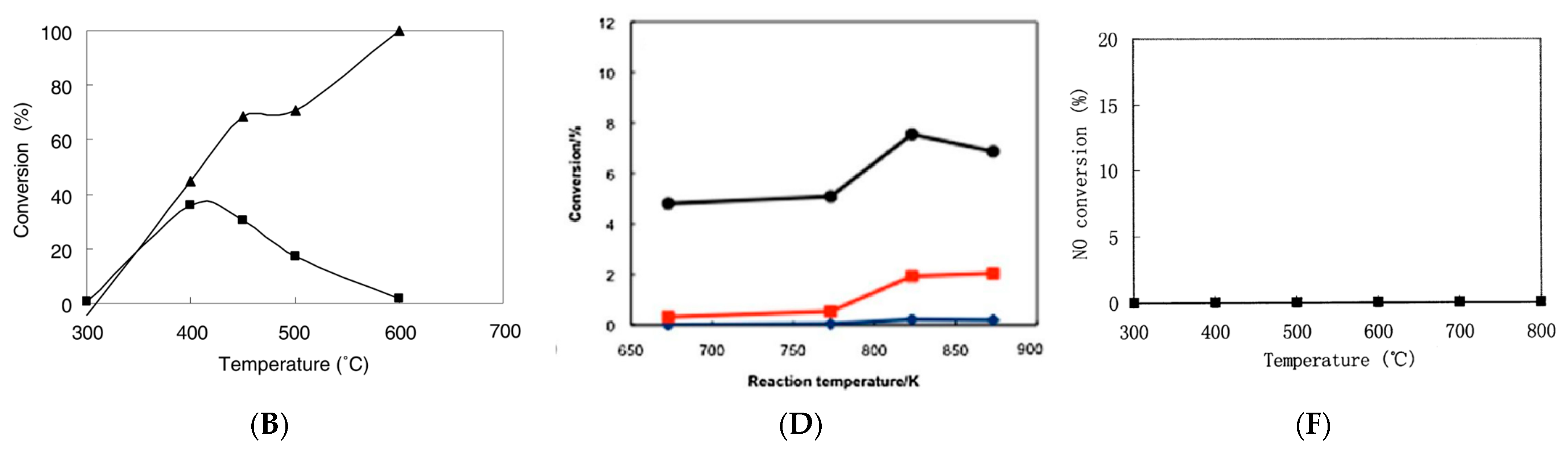

| Number | Category | Generation Scheme | Discharge Status | Reference |
|---|---|---|---|---|
| 1 | Dielectric barrier discharge plasma (DBD) |  |  | [33] |
| 2 | Glow discharge plasma (GDP) |  |  | [34] |
| 3 | Corona discharge plasma (CDP) |  |  | [33] |
| Reactants | Adsorbents/Catalysts | NTP Condition | Removal Efficiency with/without NTP (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Power (W) | Time (min) | Without NTP | NTP | |||
| NOx | NaA | - | 40 | 20.0 | 55.0 | [48] |
| Pt/NaZSM-5 | - | 30 | 0.0 | 61.3 | [50] | |
| Cu-ZSM-5 | 80 | - | 80.0 | 99.0 | [75] | |
| Co-ZSM-5 | - | 90 | 15.0 | 75.0 | [55] | |
| BaY | - | - | 55.0 | 74.0 | [76] | |
| NH4-MOR | - | - | 50.0 | 85.0 | [70] | |
| Acetone | Ag/β-zeolite | 28 | 15 | 66.8 | 97.0 | [69] |
| Benzene | FER | - | 25–40 | 20.0 | 33.0 | [71] |
| 13X | - | 25–40 | 40.0 | 43.0 | [71] | |
| HY | - | 25–40 | 75.0 | 84.0 | [71] | |
| Ag/HY | - | 25–40 | 80.0 | 89.0 | [71] | |
| TiO2-MnO2/zeolite | 10.33 | 10 | 52.7 | 81.0 | [77] | |
| FER | 9.72 | - | - | 58.0 | [78] | |
| Ag/MOR | 9.72 | - | 58.0 | 83.6 | [78] | |
| Ag/HY | 9.72 | - | 61.6 | 96.0 | [78] | |
| Ag/13X | 9.72 | - | 71.1 | 88.0 | [78] | |
| FER | - | 60–90 | - | 78.0 | [79] | |
| Ag/HY | - | 60–90 | 68.0 | 75.0 | [79] | |
| Ag/HZSM-5 | 4.7 | 13 | 87.0 | 100.0 | [80] | |
| AgMn/HZSM-5 | 6 | 24 | 78.0 | 96.0 | [81] | |
| Chloro-benzene | CeO2/HZSM-5 | 63 | 72.6 | 96.0 | [82] | |
| CeO2/HZSM-5 | - | - | 32.3 | 72.6 | [74] | |
| Dichloro-methane | HZSM-5 | 0.9 | 20 | 27.0 | 36.0 | [66] |
| NaZSM-5 | 0.9 | 20 | 27.0 | 32.0 | [66] | |
| Ethylene | Ag-Fe/13X | 19 | 30 | 90.0 | 100.0 | [83] |
| Ag/13X | - | 20 | 42.0 | 63.0 | [84] | |
| Formaldehyde | AgCu/HZSM-5 | 2.3 | 40 | 72.0 | 100.0 | [85] |
| Methane | Pd/HZSM-5 | - | 30–60 | 25.0 | 90.0 | [37] |
| Toluene | NaY | 5 | 40 | 15.0 | 52.0 | [68] |
| NaY | 5 | 10 | 50.0 | 60.0 | [67] | |
| Co/13X | 20 | 60 | 90.0 | 93.9 | [86] | |
| 5A | 20 | 60 | 90.0 | 95.0 | [86] | |
| HZSM-5 | 2.4 | 480 | 70.0 | 100.0 | [87] | |
| Ag-Mn/HZSM-5 | 2.4 | 480 | 93.5 | 100.0 | [87] | |
| Co/13X | 20 | - | 81.6 | 92.7 | [88] | |
| Hβ | 3.3–3.7 | 42 | 69.0 | 98.4 | [89] | |
| CuCeZr/ZSM-5 | 43 | 35.4 | 78.8 | [90] | ||
| Zeolites | Density (g/cm3) | Crystallinity (%) | Crystallite Size (nm) | Surface Area (m2/g) | Pore Volume (cm3/g) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HT | MW | HT | MW | HT | MW | HT | MW | |||
| ZSM-5 | 1.81 | - | - | 4.69 × 106 | 4.08 × 106 | 328.00 | 398.00 | 0.12 | 0.82 | [104] |
| ZSM-5 | - | 86.90 | 89.50 | 4.69 × 103 | 4.23 × 103 | 445.00 | 428.00 | 0.30 | 0.28 | [118] |
| ZSM-5 | - | - | - | - | 4.30 × 103 | 360.00 | 333.00 | 0.28 | 0.23 | [111] |
| SAPO-34 | 1.65 | - | - | 150.00 | 100.00 | - | 400.00 | - | 0.42 | [105] |
| SAPO-34 | 1.72 | 100.00 | 100.00 | - | 400.00 | - | 400.00 | - | 0.30 | [110] |
| MCM-41 | 2.20 | 99.00 | 100.00 | 10.00 × 103 | 2.00 × 103 | 1000.00 | 1090.00 | 0.76 | 0.80 | [112] |
| MCM-41 | 2.50 | - | - | 10.00 × 103 | 2.00 × 103 | - | 1000.00 | - | 0.80 | [106] |
| MCM-22 | 1.60 | - | - | 10.00 × 103 | 1.00 × 103 | 641.00 | 657.00 | 0.39 | 0.60 | [107] |
| MCM-22 | 1.80 | 90.00 | 100.00 | 10.00 × 103 | 8.00 × 103 | - | 450.00 | - | 0.56 | [115] |
| NaY | 2.60 | 90.00 | 100.00 | - | 1000.00 | - | 295.00 | - | 0.60 | [108] |
| NaY | - | - | - | 150.00 | 280.00 | 690.00 | 716.00 | - | - | [119] |
| NaY | - | 99.00 | 100.00 | 72.60 | 44.40 | 775.00 | 474.00 | 0.34 | 0.35 | [120] |
| NaX | 2.10 | 100.00 | 100.00 | 1000.00 | - | - | 250.00 | 0.95 | 1.00 | [109] |
| nano-NaX | 1.90 | - | - | 112.00 | 95.00 | 417.00 | - | - | - | [113] |
| SSZ-13 | 0.60 | 99.00 | 100.00 | - | 500.00 | 402.79 | 471.31 | 0.20 | 0.22 | [92] |
| SSZ-13 | - | 100.00 | 100.00 | - | - | 624.00 | 666.00 | 0.23 | 0.24 | [121] |
| SSZ-13 | - | - | 100.00 | 600.00 | 50.00 | 571.00 | 520.00 | 0.26 | [122] | |
| Reactants | Adsorbents/Catalysts | MW Condition | Removal Efficiency with/without MW (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Power (W) | Time (min) | Without MW | MW | |||
| NOx | Cu-ZSM-5 | 300 | 100 | - | 31.0 | 36.0 | [129] |
| NOx | Co/HZSM-5 | - | - | - | 20.0 | 40.0 | [127] |
| NOx | Fe-ZSM-5 | 400 | 100 | 60 | 7.5 | 11.0 | [129] |
| NOx | Fe/NaZSM-5 | 300 | 200 | - | 35.0 | 70.0 | [126] |
| NOx | InFe2O3/HZSM-5 | - | - | - | 0.0 | 23.6 | [131] |
| NOx | Fe/Ca-5A | - | 280 | 35 | 79.3 | 97.3 | [135] |
| NOx | Fe/Ca-5A | - | 259 | 35 | 85.6 | 98.2 | [138] |
| NOx | Fe/Cu-5A | - | 280 | 35 | 81.2 | 89.3 | [124] |
| NOx | FeCoCu-5A | - | 280 | 35 | 87.5 | 92.3 | [139] |
| Aldehyde | Y | 31 | 425 | 15 | 86.0 | 92.0 | [140] |
| Benzene | Cu-Mn-Ce/5A | 477 | 117.3 | 2 | 80.0 | 90.0 | [125] |
| Methane | H-(Fe)-ZSM-5 | 550 | 180 | - | 3.0 | 40.0 | [93] |
| 1-butene | Ferrierite | 300 | 300 | 120 | 30.0 | 33.0 | [134] |
| α-pinene | Mordenite | 65 | 600 | 120 | 46.1 | 94.7 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Ren, X.; Yao, Y.; Tang, X.; Yi, H.; Gao, F.; Zhou, Y.; Yu, Q. Application of Unconventional External-Field Treatments in Air Pollutants Removal over Zeolite-Based Adsorbents/Catalysts. Catalysts 2023, 13, 1461. https://doi.org/10.3390/catal13121461
Cheng H, Ren X, Yao Y, Tang X, Yi H, Gao F, Zhou Y, Yu Q. Application of Unconventional External-Field Treatments in Air Pollutants Removal over Zeolite-Based Adsorbents/Catalysts. Catalysts. 2023; 13(12):1461. https://doi.org/10.3390/catal13121461
Chicago/Turabian StyleCheng, Haodan, Xiaoning Ren, Yuan Yao, Xiaolong Tang, Honghong Yi, Fengyu Gao, Yuansong Zhou, and Qingjun Yu. 2023. "Application of Unconventional External-Field Treatments in Air Pollutants Removal over Zeolite-Based Adsorbents/Catalysts" Catalysts 13, no. 12: 1461. https://doi.org/10.3390/catal13121461
APA StyleCheng, H., Ren, X., Yao, Y., Tang, X., Yi, H., Gao, F., Zhou, Y., & Yu, Q. (2023). Application of Unconventional External-Field Treatments in Air Pollutants Removal over Zeolite-Based Adsorbents/Catalysts. Catalysts, 13(12), 1461. https://doi.org/10.3390/catal13121461







