RuO2@IrO2/C Core-Shell Structure Catalyst for Efficient and Durable Acidic Oxygen Evolution
Abstract
:1. Introduction
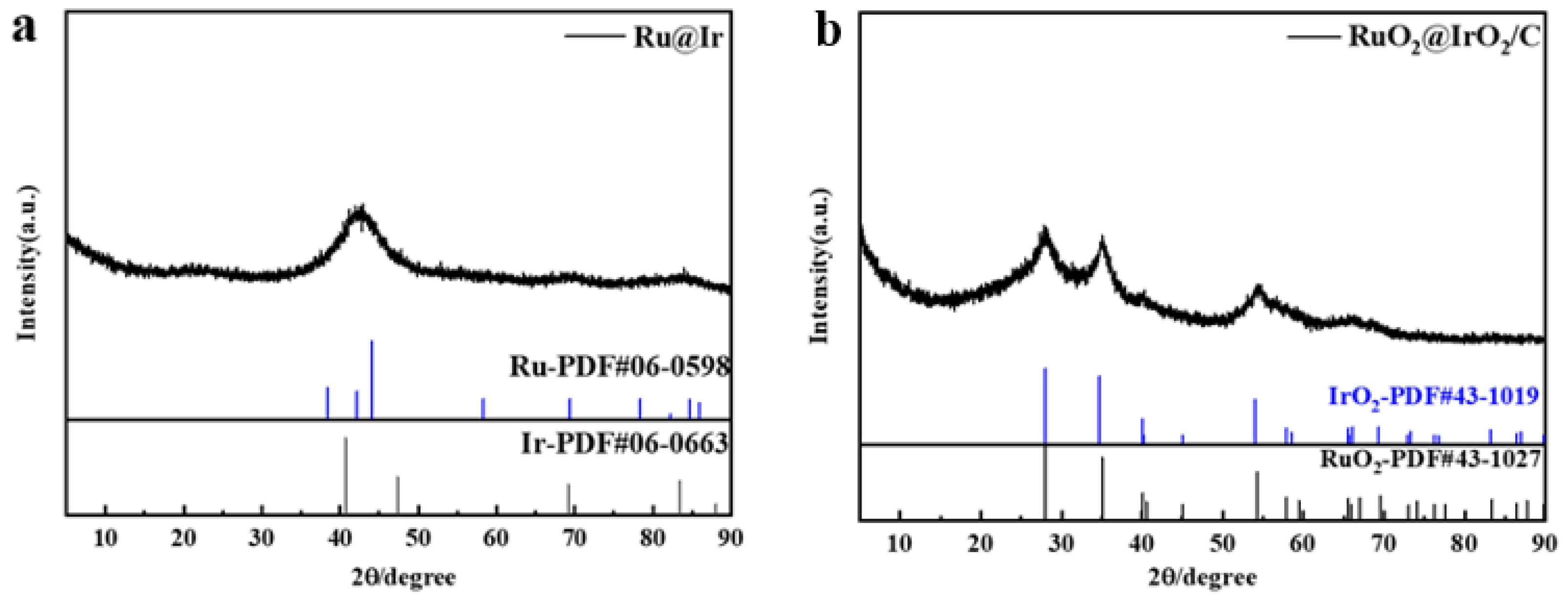
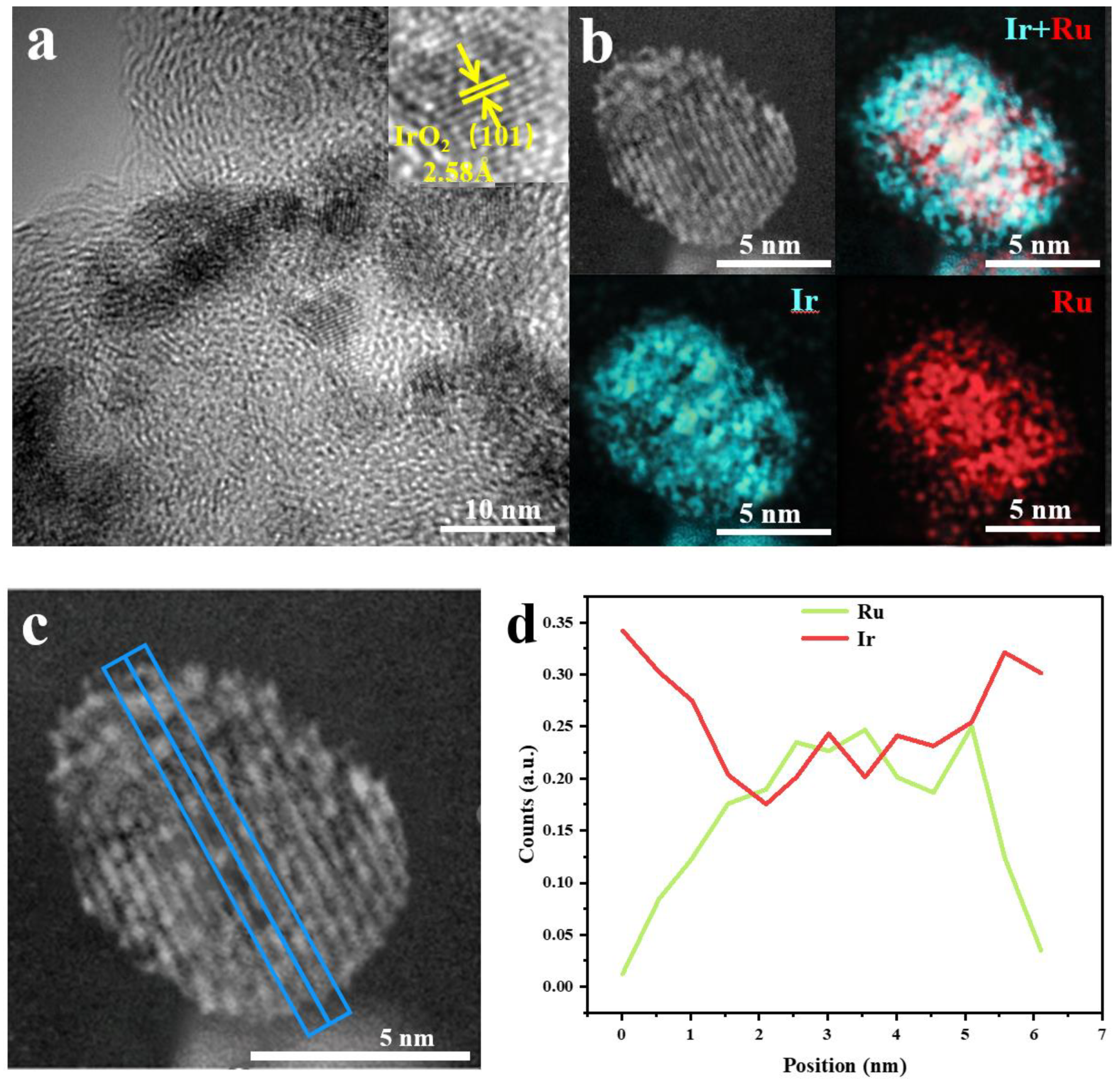
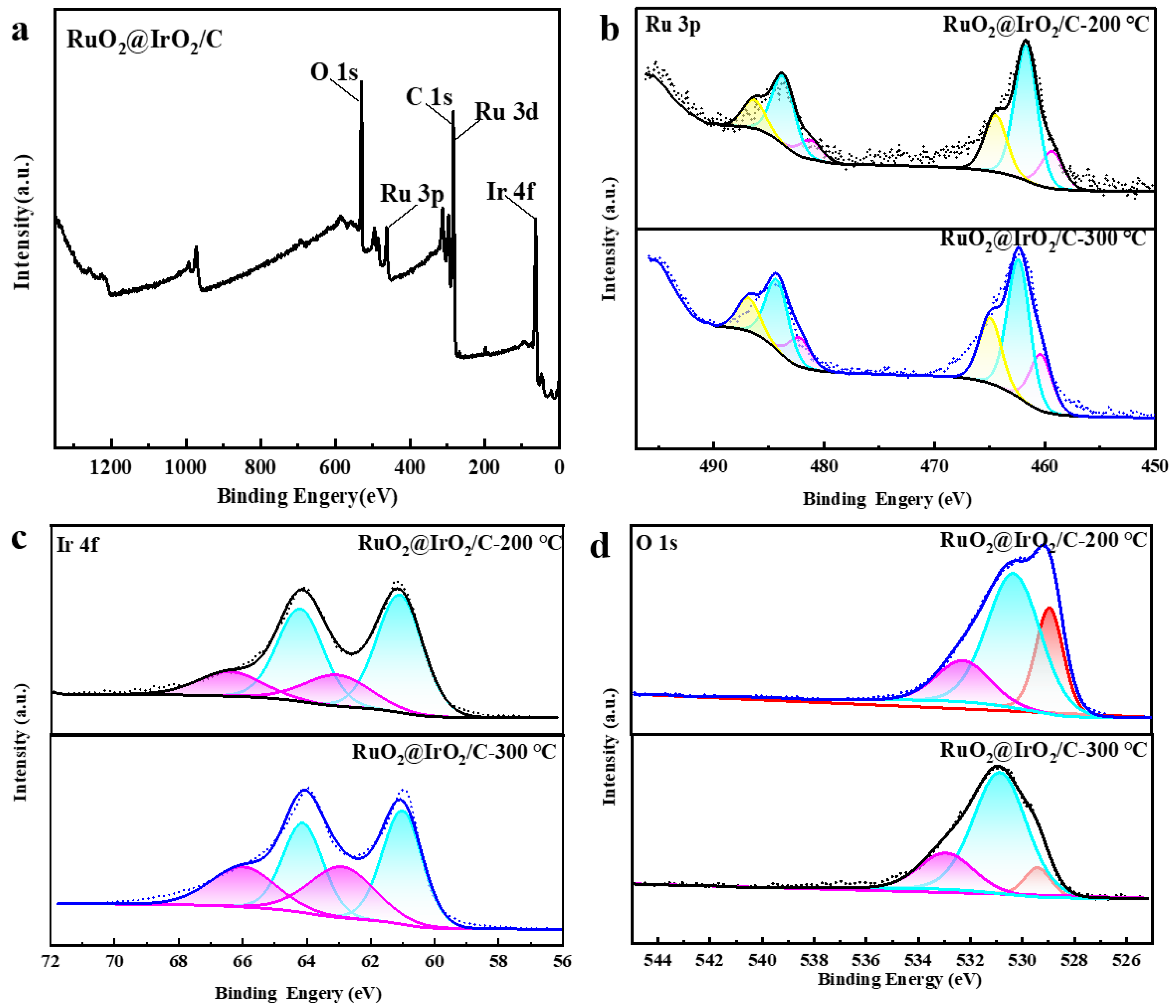
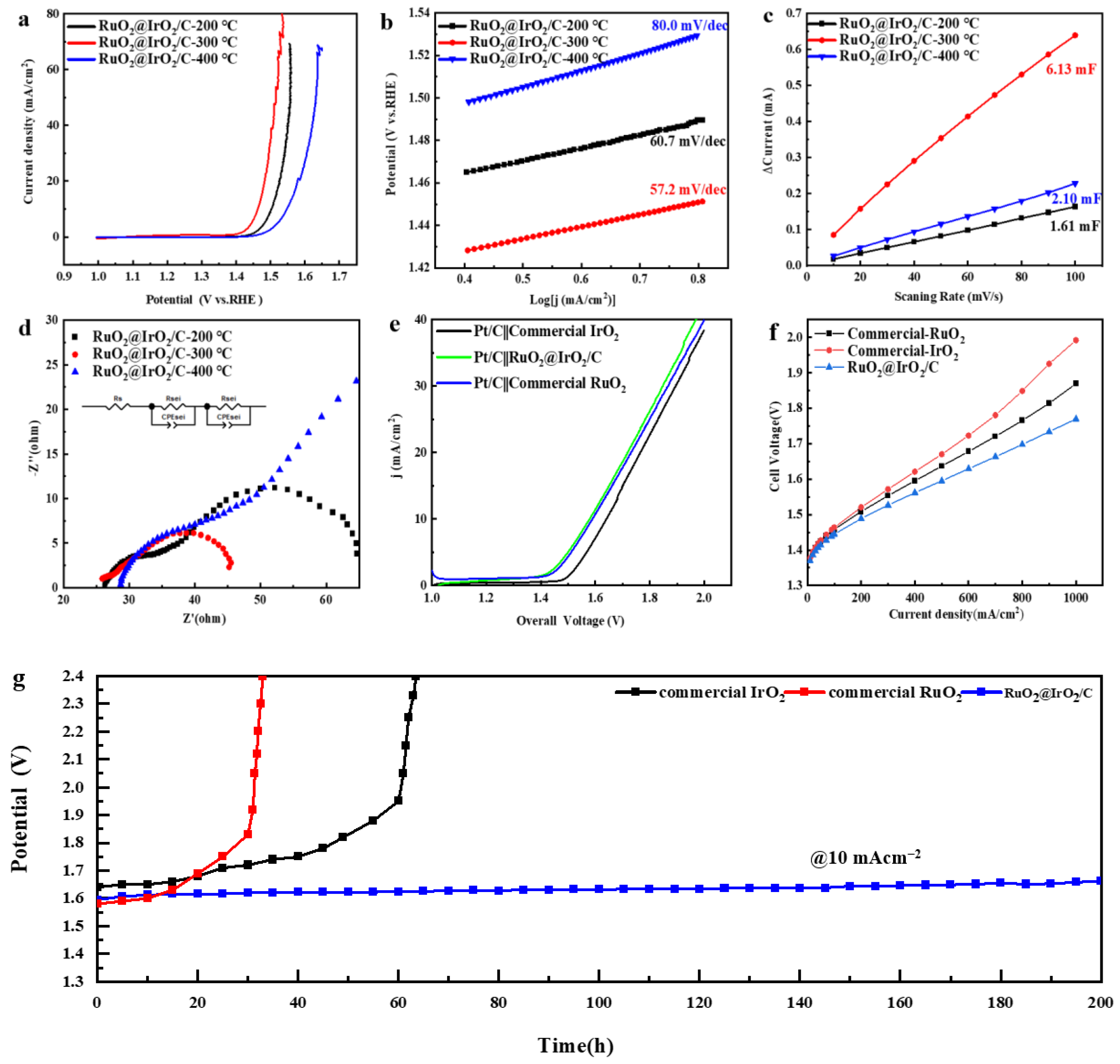
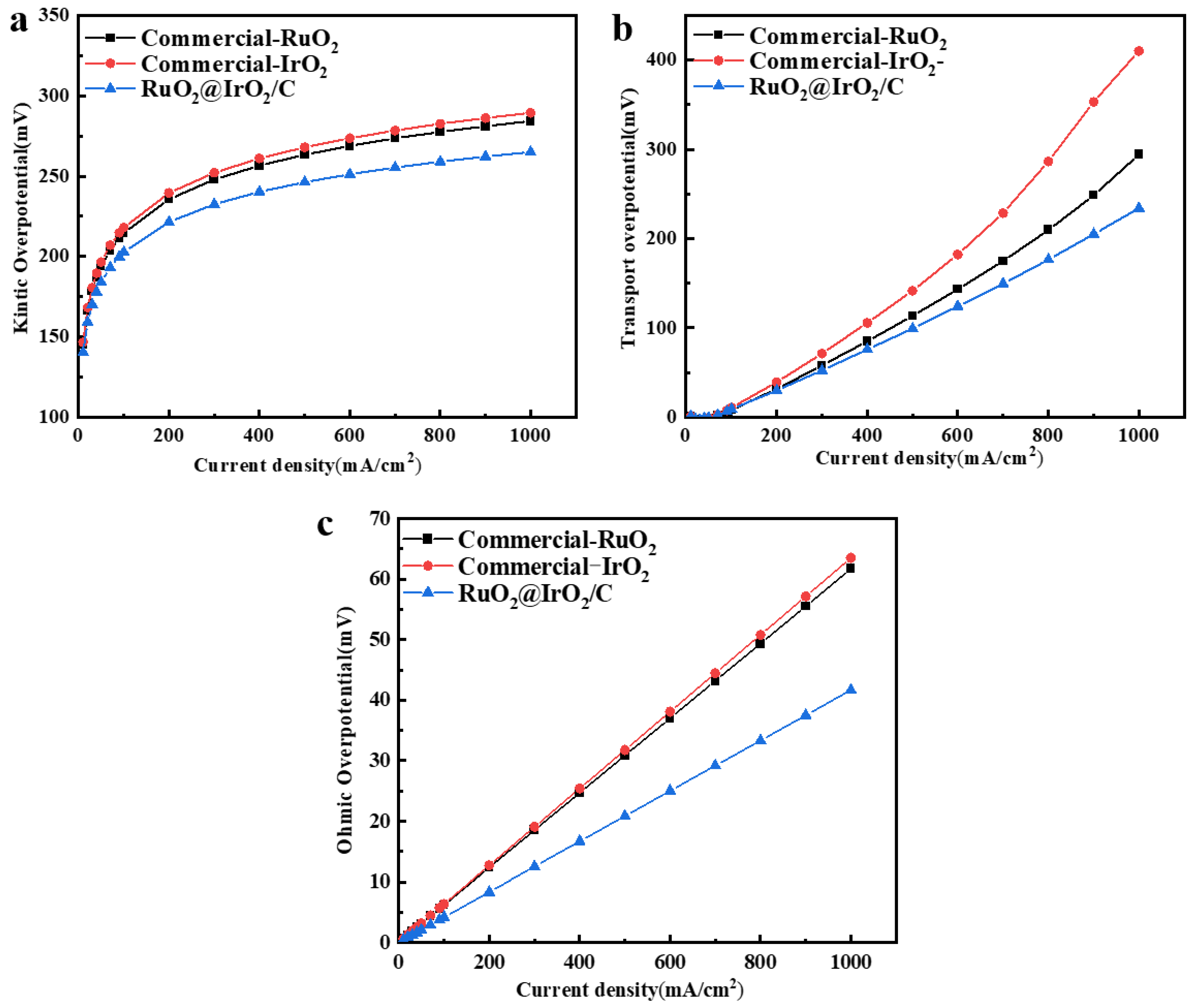
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials
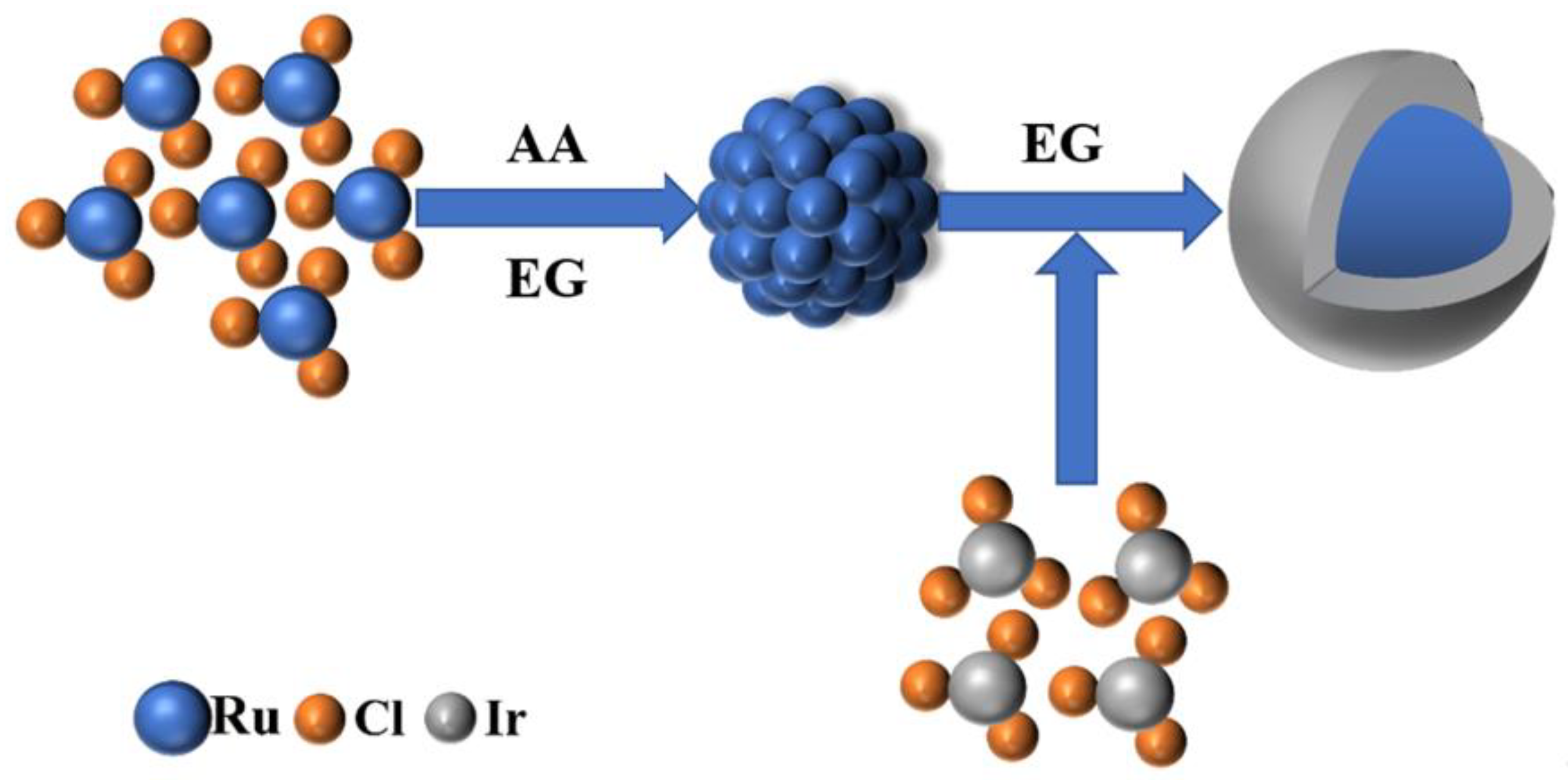
4.2. Preparation of Ru@Ir Nano Intermediates
4.3. Preparation of RuO2@IrO2/C Core-Shell Nanomaterials
5. Characterizations
5.1. Electrochemical Measurements
5.2. Electrochemically Active Surface Area (ECSA) and Mass Activity Calculations
5.3. PEM Cell Test
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, 2006328. [Google Scholar] [CrossRef]
- Bergmann, A.; Martinez-Moreno, E.; Teschner, D.; Chernev, P.; Gliech, M.; de Araújo, J.F.; Reier, T.; Dau, H.; Strasser, P. Reversible Amorphization and the Catalytically Active State of Crystalline Co3o4 During Oxygen Evolution. Nat. Commun. 2015, 6, 8625. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Shi, Z.; Wang, X.; Yang, J.; Xiao, M.; Ge, J.; Xing, W.; Liu, C. Recent Developments of Iridium-Based Catalysts for the Oxygen Evolution Reaction in Acidic Water Electrolysis. J. Mater. Chem. A 2022, 10, 13170–13189. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, e2202336. [Google Scholar] [CrossRef]
- She, L.; Zhao, G.; Ma, T.; Chen, J.; Sun, W.; Pan, H. On the Durability of Iridium-Based Electrocatalysts toward the Oxygen Evolution Reaction under Acid Environment. Adv. Funct. Mater. 2022, 32, 2108465. [Google Scholar] [CrossRef]
- Zhang, R.; Dubouis, N.; Ben Osman, M.; Yin, W.; Sougrati, M.T.; Corte, D.A.D.; Giaume, D.; Grimaud, A. A Dissolution/Precipitation Equilibrium on the Surface of Iridium-Based Perovskites Controls Their Activity as Oxygen Evolution Reaction Catalysts in Acidic Media. Angew. Chem. 2019, 131, 4619–4623. [Google Scholar] [CrossRef]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen Evolution Reaction in Alkaline Environment: Material Challenges and Solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, Z.; Zhao, M.; Huang, R.; Wang, Z.; Liang, H. Hydrogen Production By electrocatalysis Using the Reaction Of acidic Oxygen Evolution: A Review. Environ. Chem. Lett. 2022, 20, 3429–3452. [Google Scholar] [CrossRef]
- Zhao, F.; Wen, B.; Niu, W.; Chen, Z.; Yan, C.; Selloni, A.; Tully, C.G.; Yang, X.; Koel, B.E. Increasing Iridium Oxide Activity for the Oxygen Evolution Reaction with Hafnium Modification. J. Am. Chem. Soc. 2021, 143, 15616–15623. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Yang, B.; Li, Z.; Qin, X.; Zhang, Q.; Lei, L.; Qiu, M.; Wu, G.; Hou, Y. Highly Active Ruthenium Sites Stabilized by Modulating Electron-Feeding for Sustainable Acidic Oxygen-Evolution Electrocatalysis. Energy Environ. Sci. 2022, 15, 2356–2365. [Google Scholar] [CrossRef]
- Yang, L.; Yu, G.; Ai, X.; Yan, W.; Duan, H.; Chen, W.; Li, X.; Wang, T.; Zhang, C.; Huang, X.; et al. Efficient Oxygen Evolution Electrocatalysis in Acid by a Perovskite with Face-Sharing Iro6 Octahedral Dimers. Nat. Commun. 2018, 9, 5236. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Vernieres, J.; Wang, Z.; Zhang, K.; Hochfilzer, D.; Krempl, K.; Liao, T.-W.; Presel, F.; Altantzis, T.; Fatermans, J.; et al. Monitoring Oxygen Production on Mass-Selected Iridium–Tantalum Oxide Electrocatalysts. Nat. Energy 2021, 7, 55–64. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the Scaling-up of Water Splitting Catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Z.; Zhang, L.; Ma, J.; Jiang, Z.; Deibert, B.J.; Ge, R.; Chen, L. Chromium-Ruthenium Oxide Solid Solution Electrocatalyst for Highly Efficient Oxygen Evolution Reaction in Acidic Media. Nat. Commun. 2019, 10, 162. [Google Scholar] [CrossRef]
- Jin, H.; Choi, S.; Bang, G.J.; Kwon, T.; Kim, H.S.; Lee, S.J.; Hong, Y.; Lee, D.W.; Park, H.S.; Baik, H.; et al. Safeguarding the Ruo 2 Phase against Lattice Oxygen Oxidation During Acidic Water Electrooxidation. Energy Environ. Sci. 2022, 15, 1119–1130. [Google Scholar] [CrossRef]
- Klyukin, K.; Zagalskaya, A.; Alexandrov, V. Role of Dissolution Intermediates in Promoting Oxygen Evolution Reaction at Ruo2 (110) Surface. J. Phys. Chem. C 2019, 123, 22151–22157. [Google Scholar] [CrossRef]
- Zaman, W.Q.; Wang, Z.; Sun, W.; Zhou, Z.; Tariq, M.; Cao, L.; Gong, X.-Q.; Yang, J. Ni–Co Codoping Breaks the Limitation of Single-Metal-Doped Iro2 with Higher Oxygen Evolution Reaction Performance and Less Iridium. ACS Energy Lett. 2017, 2, 2786–2793. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Li, J.; Wang, X.; Wang, Y.; Li, Y.; Xu, W.; Jiang, Z.; Liu, C.; Xing, W.; et al. Confined Ir Single Sites with Triggered Lattice Oxygen Redox: Toward Boosted and Sustained Water Oxidation Catalysis. Joule 2021, 5, 2164–2176. [Google Scholar] [CrossRef]
- Shan, J.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S. Transition-Metal-Doped Ruir Bifunctional Nanocrystals for Overall Water Splitting in Acidic Environments. Adv. Mater. 2019, 31, 1900510. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Z.; Hao, J.; Sun, S.; Lu, S.; Wang, C.; Ma, P.; Dong, W.; Du, M. High-Entropy Alloy Stabilized Active Ir for Highly Efficient Acidic Oxygen Evolution. Chem. Eng. J. 2022, 431, 133251. [Google Scholar] [CrossRef]
- Maulana, A.L.; Chen, P.-C.; Shi, Z.; Yang, Y.; Lizandara-Pueyo, C.; Seeler, F.; Abruña, H.D.; Muller, D.; Schierle-Arndt, K.; Yang, P. Understanding the Structural Evolution of Irfeconicu High-Entropy Alloy Nanoparticles under the Acidic Oxygen Evolution Reaction. Nano Lett. 2023, 23, 6637–6644. [Google Scholar] [CrossRef]
- Seitz, L.C.; Colin, F.D.; Kazunori, N.; Yasuyuki, H.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K. A Highly Active and Stable Iro X/Sriro3 Catalyst for the Oxygen Evolution Reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef]
- Jiang, H.; He, Q.; Zhang, Y.; Song, L. Structural Self-Reconstruction of Catalysts in Electrocatalysis. Acc. Chem. Res. 2018, 51, 2968–2977. [Google Scholar] [CrossRef]
- Zhu, M.; Shao, Q.; Qian, Y.; Huang, X. Superior Overall Water Splitting Electrocatalysis in Acidic Conditions Enabled by Bimetallic Ir-Ag Nanotubes. Nano Energy 2019, 56, 330–337. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. and Medicine. Ascorbic Acid: The Chemistry Underlying Its Antioxidant Properties. Free. Radic. Biol. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- He, J.; Fu, G.; Zhang, J.; Xu, P.; Sun, J. Multistage Electron Distribution Engineering of Iridium Oxide by Codoping W and Sn for Enhanced Acidic Water Oxidation Electrocatalysis. Small 2022, 18, 2203365. [Google Scholar] [CrossRef]
- Zheng, L.; Wei, C.; Liu, X.; Fang, Y.; Hao, X.; Zang, Y.; Pei, Z.; Cai, J.; Wu, Y.; Niu, D. Regulating the Adsorption Behavior of Intermediates on Ir–W@ Ir–Wo 3− X Boosts Acidic Water Oxidation Electrocatalysis. Mater. Chem. Front. 2021, 5, 6092–6100. [Google Scholar]
- Kuznetsov, D.A.; Naeem, M.A.; Kumar, P.V.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Tailoring Lattice Oxygen Binding in Ruthenium Pyrochlores to Enhance Oxygen Evolution Activity. J. Am. Chem. Soc. 2020, 142, 7883–7888. [Google Scholar] [CrossRef]
- Shan, J.; Guo, C.; Zhu, Y.; Chen, S.; Song, L.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Charge-Redistribution-Enhanced Nanocrystalline Ru@ Irox Electrocatalysts for Oxygen Evolution in Acidic Media. Chem 2019, 5, 445–459. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, F.; Wang, G.; Lai, D.; Zou, L.; Cheng, Q.; Li, J.; Zou, Z.; Yang, H. Co Induced Phase-Segregation to Construct Robust and Efficient Irrux@ Ir Core-Shell Electrocatalyst Towards Acidic Oxygen Evolution. J. Power Sources 2022, 528, 231189. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, R.; Sun, J.; Xu, M.; Wang, Q. Self-Supported Iridium-Ruthenium Oxides Catalysts with Enriched Phase Interfaces for Boosting Oxygen Evolution Reaction in Acid. Appl. Surf. Sci. 2023, 622, 156945. [Google Scholar] [CrossRef]
- Sun, C.; Qin, J.; Li, M.; Han, G.; Song, Y. Ultrafine Irru Nanoparticles toward Efficient Oxygen Evolution Reaction in Acidic Media. Inorg. Chem. 2022, 61, 17362–17369. [Google Scholar] [CrossRef]
- Luo, M.; Yang, Y.; Guo, S. Core-Shell Architecture Advances Oxygen Electrocatalysis. Chem 2019, 5, 260–262. [Google Scholar] [CrossRef]
- Lv, H.; Wang, S.; Li, J.; Shao, C.; Zhou, W.; Shen, X.; Xue, M.; Zhang, C. Self-Assembled Ruo2@ Irox Core-Shell Nanocomposite as High Efficient Anode Catalyst for Pem Water Electrolyzer. Appl. Surf. Sci. 2020, 514, 145943. [Google Scholar] [CrossRef]
- Li, N.; Cai, L.; Gao, G.; Lin, Y.; Wang, C.; Liu, H.; Liu, Y.; Duan, H.; Ji, Q.; Hu, W. Operando Direct Observation of Stable Water-Oxidation Intermediates on Ca2–X Iro4 Nanocrystals for Efficient Acidic Oxygen Evolution. Nano Lett. 2022, 22, 6988–6996. [Google Scholar] [CrossRef]
- Shang, C.; Cao, C.; Yu, D.; Yan, Y.; Lin, Y.; Li, H.; Zheng, T.; Yan, X.; Yu, W.; Zhou, S.; et al. Oxygen Evolution Reaction: Electron Correlations Engineer Catalytic Activity of Pyrochlore Iridates for Acidic Water Oxidation. Adv. Mater. 2019, 31, 1970042. [Google Scholar] [CrossRef]
- Niu, S.; Kong, X.-P.; Li, S.; Zhang, Y.; Wu, J.; Zhao, W.; Xu, P. Low Ru Loading Ruo2/(Co, Mn) 3o4 Nanocomposite with Modulated Electronic Structure for Efficient Oxygen Evolution Reaction in Acid. Appl. Catal. B: Environ. 2021, 297, 120442. [Google Scholar] [CrossRef]
- Qin, Q.; Jang, H.; Wang, Y.; Zhang, L.; Li, Z.; Kim, M.G.; Liu, S.; Liu, X.; Cho, J. Gettering La Effect from La3iro7 as a Highly Efficient Electrocatalyst for Oxygen Evolution Reaction in Acid Media. Adv. Energy Mater. 2021, 11, 2003561. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Sun, X. Iridium in Tungsten Trioxide Matrix as an Efficient Bi-Functional Electrocatalyst for Overall Water Splitting in Acidic Media. Small 2021, 17, 2102078. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Ge, J.; Liu, C.; Xing, W. Discontinuously Covered Iro 2–Ruo 2@ Ru Electrocatalysts for the Oxygen Evolution Reaction: How High Activity and Long-Term Durability Can Be Simultaneously Realized in the Synergistic and Hybrid Nano-Structure. J. Mater. Chem. A 2017, 5, 17221–17229. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Chen, J.; Wang, L.; Lin, Y.; Wang, H.; Liu, X.; Shen, X.; Zhang, W.; Liu, W. Dynamic Oxygen Adsorption on Single-Atomic Ruthenium Catalyst with High Performance for Acidic Oxygen Evolution Reaction. Nat. Commun. 2019, 10, 4849. [Google Scholar] [CrossRef]
- Din, A.U.; Muhammad; Irfan, S.; Dar, S.U.; Rizwan, S. Synthesis of 3d Irrumn Sphere as a Superior Oxygen Evolution Electrocatalyst in Acidic Environment. Chem. –A Eur. J. 2020, 26, 5662–5666. [Google Scholar]
- Zhuang, Z.; Wang, Y.; Xu, C.-Q.; Liu, S.; Chen, C.; Peng, Q.; Zhuang, Z.; Xiao, H.; Pan, Y.; Lu, S. Three-Dimensional Open Nano-Netcage Electrocatalysts for Efficient Ph-Universal Overall Water Splitting. Nat. Commun. 2019, 10, 4875. [Google Scholar] [CrossRef]
- Cui, X.; Ren, P.; Ma, C.; Zhao, J.; Chen, R.; Chen, S.; Rajan, N.P.; Li, H.; Yu, L.; Tian, Z. Robust Interface Ru Centers for High-Performance Acidic Oxygen Evolution. Adv. Mater. 2020, 32, 1908126. [Google Scholar] [CrossRef]
- Liang, X.; Shi, L.; Liu, Y.; Chen, H.; Si, R.; Yan, W.; Zhang, Q.; Li, G.-D.; Yang, L.; Zou, X. Activating Inert, Nonprecious Perovskites with Iridium Dopants for Efficient Oxygen Evolution Reaction under Acidic Conditions. Angew. Chem. Int. Ed. 2019, 58, 7631–7635. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wang, X.; Qu, Y.; Chen, W.; Lin, Y.; Li, H. A General Synthesis Approach for Amorphous Noble Metal Nanosheets. Nat. Commun. 2019, 10, 4855. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lu, M.; Huang, B.; Zhang, H.; Peng, Y.; Xi, P.; Yan, C.-H. Iridium Single Atoms Coupling with Oxygen Vacancies Boosts Oxygen Evolution Reaction in Acid Media. J. Am. Chem. Soc. 2020, 142, 18378–18386. [Google Scholar] [CrossRef]
- Meng, G.; Sun, W.; Mon, A.A.; Wu, X.; Xia, L.; Han, A.; Wang, Y.; Zhuang, Z.; Liu, J.; Wang, D. Strain Regulation to Optimize the Acidic Water Oxidation Performance of Atomic-Layer Irox. Adv. Mater. 2019, 31, 1903616. [Google Scholar] [CrossRef]

| Catalyst | Electrolyte | Over Potential (mV) | Stability (h) | Reference |
|---|---|---|---|---|
| RuO2@IrO2/C | 0.1 M HClO4 | 257 | 200 | This work |
| RuO2/(Co,Mn)3O4/CC | 0.5 M H2SO4 | 270 | 24 | [37] |
| Ru@IrOx | 0.5 M H2SO4 | 282 | 24 | [33] |
| La3IrO7-SLD | 0.1 M HClO4 | 296 | 16 | [38] |
| 6H-SrIrO3 | 0.5 M H2SO4 | 248 | 30 | [11] |
| RuIr10 | 0.5 M H2SO4 | 344 | 25 | [19] |
| Ir-doped WO3 | 0.5 M H2SO4 | 258 | 60 | [39] |
| IrO2-RuO2@Ru | 0.5 M H2SO4 | 281 | 20 | [40] |
| Ru-N-C | 0.5 M H2SO4 | 267 | 30 | [41] |
| IrRuMn/C | 0.1 M HClO4 | 260 | 8 | [42] |
| RuIrOx | 0.5 M H2SO4 | 220 | 24 | [43] |
| RuNi2@G-250 | 0.5 M H2SO4 | 227 | 3 | [44] |
| SrTi0.67Ir0.33O3 | 0.1 M HClO4 | 247 | 20 | [45] |
| Amorphous Ir NSs | 0.1 M HClO4 | 255 | 8 | [46] |
| Ir-NiCo2O4 | 0.5 M H2SO4 | 240 | 70 | [47] |
| IrCo@IrOx-3L NDs | 0.1 M HClO4 | 247 | 10 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, X.; Gao, J.; Yang, Z.; Liang, X.; Wu, X.; Yun, J.; Zhang, J. RuO2@IrO2/C Core-Shell Structure Catalyst for Efficient and Durable Acidic Oxygen Evolution. Catalysts 2023, 13, 1456. https://doi.org/10.3390/catal13121456
Teng X, Gao J, Yang Z, Liang X, Wu X, Yun J, Zhang J. RuO2@IrO2/C Core-Shell Structure Catalyst for Efficient and Durable Acidic Oxygen Evolution. Catalysts. 2023; 13(12):1456. https://doi.org/10.3390/catal13121456
Chicago/Turabian StyleTeng, Xin, Junan Gao, Zuobo Yang, Xin Liang, Xiaokuan Wu, Jimmy Yun, and Jie Zhang. 2023. "RuO2@IrO2/C Core-Shell Structure Catalyst for Efficient and Durable Acidic Oxygen Evolution" Catalysts 13, no. 12: 1456. https://doi.org/10.3390/catal13121456
APA StyleTeng, X., Gao, J., Yang, Z., Liang, X., Wu, X., Yun, J., & Zhang, J. (2023). RuO2@IrO2/C Core-Shell Structure Catalyst for Efficient and Durable Acidic Oxygen Evolution. Catalysts, 13(12), 1456. https://doi.org/10.3390/catal13121456






