Abstract
Designing highly active catalysts for the oxidation of volatile organic compounds (VOCs) involves fine-tuning the catalytic surface and improving its interaction with VOCs. The present review assesses various studies conducted in the last decade on Co3O4 catalysts for the complete oxidation of toluene (C7H8) and provides information on the synthesis and physicochemical characterization of these catalysts. Taking this one step further, data from the literature were carefully chosen for an extensive meta-analysis aiming at elucidating the structure sensitivity of toluene oxidation over Co3O4 catalysts. Specifically, the specific reaction rate was calculated and correlated with the physicochemical properties of the catalysts, namely, the specific surface area, faceting, and Co3+/Co2+ ratio. Based on this analysis, the specific surface area does not exert a significant impact on the specific activity of Co3O4 catalysts for the total oxidation of toluene. Instead, the specific reaction rates are influenced by the morphology, surface concentration, exposed crystallographic planes, and oxidation state of cobalt cations. These factors determine the catalyst’s specific activity by creating defects, oxygen vacancies, or oxygen species with different reactivities. It was also found that a high surface Co3+/Co2+ ratio is necessary for achieving high catalytic performance in the oxidation of VOCs.
1. Introduction
The emission of volatile organic compounds (VOCs) has been found to have detrimental effects on both human health and the environment. The emission source determines the type and composition of the VOCs. Examples of VOCs include halogenated hydrocarbons, alcohols, aldehydes, aromatics, alkanes, ketones, olefins, ethers, esters, and sulfur-containing compounds [1,2,3].
These molecules are dispersed in the atmosphere and can travel over vast expanses [4]. There are multiple outdoor and indoor sources of VOCs [5,6]. Outdoor sources [6,7,8] include, but are not limited to, chemical industries, paper production, food processing, paint drying, transportation, petroleum refineries, automobile manufacturing, metal degreasing, textile manufacturing, electronic component plants, solvents, and cleaning products. Indoor sources [9] include household products, office supplies, printers, heat exchanger systems, insulating materials, pressed wood, wood stoves, and pipe leaks. Exposure to VOCs can cause various adverse health effects, including irritation, asthma exacerbation, allergy, respiratory effects, and cancer [10].
Aftertreatment technologies for VOC emission control can be classified into two categories based on the recovery or destruction of the pollutant. Methods for the recovery of VOCs include condensation, absorption, adsorption, and membrane separation. VOC destruction involves incineration methods, focusing on thermal [11] and catalytic oxidation [12,13], where the non-halogenated VOC is converted to carbon dioxide and water. When air is used as an oxidant in thermal oxidation, nitrogen oxides (NOx) and dioxins are also produced as by-products. In contrast, catalytic oxidation is more effective and environmentally friendly as it takes place at lower temperatures with minor by-product formation. Operating at low temperatures is necessary to reduce energy consumption and operating costs. However, thermal incineration excels at high VOC concentrations and flow rates.
Precious metals are among the most active catalysts for the oxidation of VOCs [14,15]. Pt and Pd are the most common examples, mainly supported on carriers with high surface areas, such as alumina [16]. However, the high cost and scarcity of noble metals are limiting factors that raise sustainability concerns. Consequently, attention has been also paid to transition metal oxide (TMOs) catalysts for complete VOC oxidation [17,18,19]. Although TMOs typically have lower activity compared to noble metal catalysts, they are less prone to poisoning. Supported and unsupported oxides include single- or mixed-metal oxides containing two or more transition metal elements [20]. The present study refers to simple transition metal oxides and, more specifically, Co3O4. The selection of a single oxide has been made in order to exclude the influence of interactions among various phases or the support, which complicates the analysis of catalytic performance metrics. The most studied single-metal oxides for the total oxidation of VOCs are Co3O4 [21,22,23,24], Mn2O3 [25,26,27], Fe2O3 [28], CuO [29,30], and CeO2 [31,32]. Co3O4 has been widely studied because of its high activity and stability for the complete oxidation of VOCs [32,33].
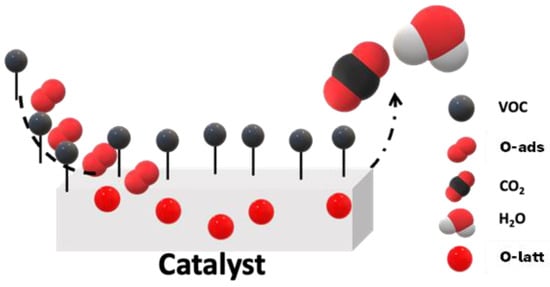
The most stable phase among CoO, Co2O3, and Co3O4 is Co3O4 [34]. Co3O4 is an oxide with a typical spinel crystal structure and is a mixed-valence oxide of CoO and Co2O3 [35]. Co2+ and Co3+ occupy the tetrahedral and octahedral cation sites, respectively [36]. The oxidation of VOCs over Co3O4 involves the adsorption of organic compounds at anionic oxygen sites in the oxide lattice, leading to the formation of an activated complex. This complex further reacts to yield oxidation products. The total oxidation of toluene proceeds via C-H bond activation, followed by the formation of benzyl species and the release of hydrogen. Active oxygen species react with the benzyl species to form benzyl alcohol, which is further oxidized to a series of products, including benzaldehyde, benzoic acid, benzene, phenol, benzoquinone, maleic anhydride, various ring-opened byproducts, and finally, carbon dioxide and water [37,38]. Lattice oxygen participates in the catalytic cycle and is regenerated by the supply of gas–phase oxygen adsorbed on a surface oxygen vacancy. Redox models can be used to describe the oxidation of VOCs on reducible metal oxides. Τhis model can be described simply as a reduction of the surface by molecules of volatile organic compounds and the reoxidation of the catalytic surface by gaseous oxygen (Equations (1) and (2)). Scheme 1 is commonly known as the Mars–van Krevelen (MvK) mechanism [39] and is given below.
where {Ox} is an oxidized surface site (e.g., lattice oxygen) and {Red} is a reduced surface site.
Surface Reduction: VOC + {Ox} → {Red} + oxidation products
Surface Re-oxidation: {Red} + O2 → {Ox}
Scheme 1.
MvK reaction mechanism.
The corresponding MvK rate expression for the oxidation of the VOC, r, is given by Equation (3).
with
- kr, the surface reduction rate constant;
- kox, the surface reoxidation rate constant;
- pVOC, the partial pressure of the VOC;
- , the partial pressure of oxygen;
- γ, the stoichiometric coefficient for the total oxidation.
According to Vannice [40], the MvK kinetic model for oxidation reactions on solid catalysts, as originally stated, is inconsistent and incorrect. Consequently, the rate expression derived from this model is inapplicable except for one particular situation that involves the adsorption of O2 molecules on a single site. However, this model is still inconsistent because O ions (or atoms) are proposed as reactive oxygen species rather than adsorbed O2 molecules. Consequently, the form of the rate expression in Equation (3) has no physical relevance and must be viewed only as a mathematical fitting function. Carreto et al. [41] agreed with the hypothesis proposed by Vannice [40] and stated that a conceptual shortfall of the original meaning of the “MvK mechanism” is that in a (sub) monolayer catalyst, it is hard to identify a “lattice” oxygen species, as such a catalyst has only surface atoms. Therefore, it would be most helpful to avoid the term MvK and state specifically the structural properties (oxygen abundance, oxidation state, binding energy of oxygen, oxygen species). Thus, the MvK equation is inaccurate because it does not specify the structural properties. Hence, kinetic exchange experiments using isotopes are required. However, the chemical potential that controls the kinetics of oxygen exchange has rarely been considered. Furthermore, the fact that oxides can exchange oxygen species does not imply that their catalytic functions are based on these properties.
Most surface studies have focused on the structural effects of metal oxides and surface reactivity [42]. According to Barteau and Vohs [43], the catalytic reaction rate depends on the geometric structure of the surface atoms on which it occurs, which is implicit in catalysis. These concepts are most commonly used for transition-metal-catalyzed reactions. In this case, structural sensitivity is almost always correlated with specificity [44]. Many authors have investigated the sensitivity of the structure of Pt [45,46], Rh [47], Pd [46], and Au [48], while few studies have focused on metal oxides [49] or mixed-metal oxides [50].
On the other hand, one of the first metal oxides selected to investigate structural sensitivity was MoO3 [49,51]. The crystallite size of the catalyst can influence the specific catalytic activity, according to Boudart in 1969 [44], whose research work proposes that the catalytic behavior of the considered materials is optimized by tuning the morphology of the particles or bulk structures, and therefore, the specific surface area (SBET) of the material. In general, the oxidizing power of a metal oxide surface can be associated with the strength of the metal–oxygen bond and the possibility of incorporating molecular oxygen into the crystal lattice, that is, the ability to re-oxidize the reduced active sites with gaseous oxygen. On the contrary, the absence of weakly bonded oxygen species may be fundamental in determining the reaction path and catalyst selectivity.
Interestingly, the influence of the acidic and basic properties of the surface should also be considered [52]. The simplest way to change the surface structure of a metal crystallite is to alter its particle size (which is related to the specific surface area). The other is by employing different synthesis methods to change the crystal shape and morphology. The structure sensitivity demonstrates how different surface structures can lead to reactions or activation within the same molecule. When such materials are designed, the activity and selectivity of the catalysts can be controlled by carefully manipulating the surface and bulk structure of the formulated particles.
Regarding the preparation of Co3O4 catalysts, a multitude of synthesis methods have been employed, including hydrothermal, solvothermal [53], sol–gel [54], thermal decomposition [24], chemical precipitation, and hard or soft templates [55]. Different synthesis methods can produce various morphologies and structures, such as nanowires [56], nanoboxes [57], nanoparticles [58], nanorods [59], nanoflowers [32], and nanobrushes [60]. The above studies were triggered by the fact that Co3O4 is a versatile oxide whose properties can be used in applications such as solid-state sensors [59,61], lithium-ion batteries [62], and medicine [63]. Although several review articles have been published on the complete catalytic oxidation of VOCs by Co3O4 [64] or other transition metal oxides [65,66,67], only one review article has focused on the structural activity with respect to the exposed planes of Co3O4 [42]. That study focused on the total oxidation of carbon monoxide.
For this purpose, the current review has been structured to shed light on the following question: “Is the specific reaction rate of VOC oxidation over Co3O4 dependent on its specific surface area or its crystallite size and at a second level, does it depend on its exposed crystallographic planes induced by the different morphology?” In other words, is VOC oxidation over Co3O4 a structure-sensitive reaction? To answer these questions, the oxidation of toluene serves as a case study. Additionally, this work presents and analyzes the main physicochemical characterization, the various synthesis methods in relation to the obtained morphologies, and the effect of the specific surface area of Co3O4 catalysts.
2. Evaluating the Surface Activity of Co3O4 Catalysts for Complete Toluene Oxidation
2.1. General Information on the Experimental Procedure for Complete Toluene Oxidation
In most of the research articles examined in this review, complete toluene oxidation was performed in fixed-bed tubular reactors. The reaction rate of VOC oxidation was calculated using Equation (4), where rVOC is the reaction rate of VOC oxidation (mol s−1 ), F is the total flow rate (mol s−1), W is the mass of the catalyst (g), and and are the inlet and outlet molar fractions of the VOC, respectively. Dividing the reaction rate by the specific surface area produces the specific reaction rate (SRR), (mol s−1 ).
It should be mentioned that if the reaction rate was not reported in the examined research articles, it was calculated by extracting the experimental data from the light-off curves (conversion vs. reaction temperature plots) presented. The conversion should typically be less than 20%, and the data are presented as the specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C). In addition, in the absence of experimental data for the same temperatures for all catalysts, the points were extended along a straight line to make a relevant comparison. The toluene concentration in the reactor feed was in the range of 500–1000 ppm, while the O2 concentration varied between 10 and 20% in all research articles.
The main intention of this review article is to examine whether the specific reaction rate of VOC oxidation over Co3O4 depends on the following factors:
- the specific surface area of Co3O4 or, equivalently, the crystallite size of Co3O4,
- the morphology and, therefore, the exposed crystallographic planes of Co3O4.
- the molar Co3+/Co2+ ratio derived mainly from XPS and H2-TPR analyses.
This section investigates the specific activity of the catalyst related to its specific surface area vs. dominant exposed crystallographic planes obtained by various preparation methods. It is also been determined whether the specific activity can be correlated with the exposed crystallographic planes and the Co3+/Co2+ molar ratio.
2.2. Catalytic Evaluation of Co3O4 Catalysts
Bai and co-workers [68] reported on porous Co3O4 nanowires and nanorods prepared via the hydrothermal or microemulsion method in the presence of PEG or CTAB. The Co3O4-HT-PEG and Co3O4-HT-CTAB catalysts are porous cubic Co3O4 nanowires, whereas the Co3O4-ME-CTAB catalyst consists of porous cubic nanorods. The specific surface areas of the porous Co3O4 catalysts were in the range of 47–51.7 m2 g−1, and that of the bulk Co3O4 was 8 m2 g−1, much lower than that of Co3O4-HT-CTAB (51.7 m2 g−1). In terms of catalytic performance, the porous Co3O4-HT-CTAB sample demonstrated better catalytic activity than the other porous Co3O4 catalysts and much better activity compared to the nonporous bulk counterpart. The catalytic activity followed the order: Co3O4-HT-CTAB > Co3O4-HT-PEG > Co3O4-ME-CTAB > Co3O4-HT> Co3O4-Bulk. The lattice spacing (d values) shown in Figure 1 (inset HRTEM) of the Co3O4-HT-CTAB catalyst was found to be 0.28 and 0.47 nm, which are relatively close to those of the {220} and {111} lattice planes of the Co3O4 catalysts. The lattice spacing of the other porous Co3O4 catalysts was 0.28 nm, which was attributed to the {220} lattice plane corresponding to the surface-exposed Co3+. As shown in Figure 1, the Co3O4-HT-CTAB catalyst exhibited higher specific activity. These results could be associated with the mixed surface-exposed lattice plane {220}/{111}, which is attributed to the Co3+/Co2+ ratio. This correlation is further supported by the higher initial H2 consumption rate of Co3O4-HT-CTAB during TPR (where initial H2 consumption stands for <25% of oxygen consumption in cobalt oxide), indicating that the molar ratio of Co3+/Co2+ was higher for that sample.
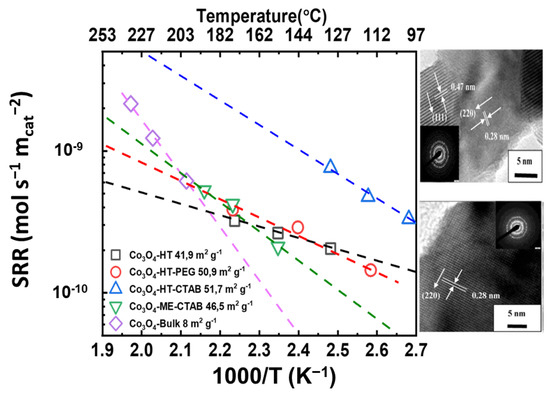
Figure 1.
Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  Co3O4-HT,
Co3O4-HT,  Co3O4-HT-PEG,
Co3O4-HT-PEG,  Co3O4-HT-CTAB,
Co3O4-HT-CTAB,  Co3O4-ME-CTAB, and
Co3O4-ME-CTAB, and  bulk Co3O4 (Inset: typical HRTEM images of the studied catalysts (calculated with data from [68]).
bulk Co3O4 (Inset: typical HRTEM images of the studied catalysts (calculated with data from [68]).
 Co3O4-HT,
Co3O4-HT,  Co3O4-HT-PEG,
Co3O4-HT-PEG,  Co3O4-HT-CTAB,
Co3O4-HT-CTAB,  Co3O4-ME-CTAB, and
Co3O4-ME-CTAB, and  bulk Co3O4 (Inset: typical HRTEM images of the studied catalysts (calculated with data from [68]).
bulk Co3O4 (Inset: typical HRTEM images of the studied catalysts (calculated with data from [68]).
Yang et al. [69] employed a modified solvothermal technique to create porous cube-aggregated monodisperse Co3O4 microspheres with a specific surface area of 17.4 m2 g−1. Meanwhile, they prepared bulk Co3O4 containing nano/macroparticles after the thermal decomposition of cobalt nitrate precursor at 600 °C with a specific surface area of 8.4 m2 g−1. Both Co3O4 catalysts featured solely the crystallographic {111} plane corresponding to surface-exposed Co2+ (Figure 2, inset). The Co3O4 microspheres showed better catalytic activity than the bulk Co3O4 catalyst, with T100% at 300 and 340 °C, respectively. As shown in Figure 2, both catalysts show similar specific activities. This result can be attributed to the same exposed lattice plane for both samples, {111}, which corresponds to the surface-exposed Co2+. In addition, an XPS analysis revealed that the catalysts did not show a remarkable change in the surface Co3+/Co2+ ratio. Additionally, it should be noted that the consumption of H2 calculated from the H2-TPR experiments did not align with the Co3+/Co2+ molar ratio determined via the XPS analysis.
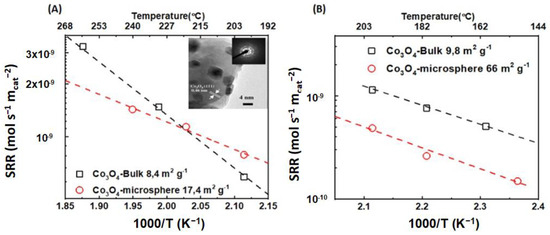
Figure 2.
(A) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  bulk Co3O4 and
bulk Co3O4 and  bulk Co3O4 microspheres (Inset: indicative HRTEM image for the Co3O4 microsphere) [69]. (B) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for
bulk Co3O4 microspheres (Inset: indicative HRTEM image for the Co3O4 microsphere) [69]. (B) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  bulk Co3O4 and
bulk Co3O4 and  Co3O4 microspheres (calculated with data from [70]).
Co3O4 microspheres (calculated with data from [70]).
 bulk Co3O4 and
bulk Co3O4 and  bulk Co3O4 microspheres (Inset: indicative HRTEM image for the Co3O4 microsphere) [69]. (B) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for
bulk Co3O4 microspheres (Inset: indicative HRTEM image for the Co3O4 microsphere) [69]. (B) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  bulk Co3O4 and
bulk Co3O4 and  Co3O4 microspheres (calculated with data from [70]).
Co3O4 microspheres (calculated with data from [70]).
Wang et al. [70] prepared a nanoplate-aggregate microspherical Co3O4 catalyst via an ethylenediamine-assisted hydrothermal method. The catalyst had a cubic crystal structure and comprised nanoplate-aggregate microspheres with a specific surface area of 66 m2 g−1. For comparison, the authors prepared bulk Co3O4 after the thermal decomposition of the cobalt nitrate precursor at 600 °C. The obtained catalyst exhibited a specific surface area of 9.8 m2 g−1. The Co3O4 microspheres displayed better catalytic activity than the bulk Co3O4. This result is mainly due to the higher specific surface area of the Co3O4 microsphere catalyst. However, the Co3O4 bulk catalyst, as shown in Figure 2B, has a higher specific activity than the Co3O4 microsphere catalyst. The authors observed that the Co3+/Co2+ molar ratio of the Co3O4 microspheres was lower than that of the bulk Co3O4, indicating a higher surface concentration of Co3+ for the latter. From H2-TPR, the H2 consumption of the bulk Co3O4 was higher than that of the Co3O4 microspheres, showing that the molar ratio of Co3+/Co2+ was higher in the bulk Co3O4, which is in agreement with the XPS results. Hence, the increased specific activity of the Co3O4 bulk catalyst could be correlated with the increased molar ratio of Co3+/Co2+. It should be noted that there were no available HRTEM data to retrieve information about the exposed crystallographic planes and correlate it with the specific activity in that work.
In addition, Liu et al. [71] reported on a meso-Co3O4 catalyst with a specific surface area of 94 m2 g−1, which was prepared using a KIT-nanocasting method. For comparison, a bulk Co3O4 catalyst with a specific surface area of 8 m2 g−1 was prepared via the thermal decomposition of a nitrate salt precursor at 700 °C. Once again, the higher the specific surface area of the catalyst, the higher the catalytic activity. Specifically, meso-Co3O4 achieved complete conversion of toluene at 220 °C, whereas bulk Co3O4 achieved this at 320 °C. According to Figure 3, the calculation and extension of the specific reaction rates at comparable temperatures for the two samples led to similar specific activities, with the bulk Co3O4 catalyst being slightly better at low temperatures. In accordance with the previous studies, the bulk Co3O4 catalyst has a higher Co3+/Co2+ molar ratio and higher H2 consumption compared to the meso-Co3O4 catalyst. No HRTEM data were reported for the selected catalysts.
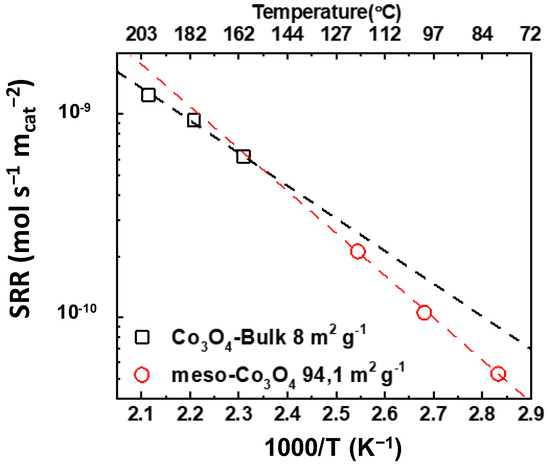
Figure 3.
Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  bulk Co3O4 and
bulk Co3O4 and  meso-Co3O4 (calculated with data from [71]).
meso-Co3O4 (calculated with data from [71]).
 bulk Co3O4 and
bulk Co3O4 and  meso-Co3O4 (calculated with data from [71]).
meso-Co3O4 (calculated with data from [71]).
Liu and co-workers [72] prepared a Co3O4 catalyst by calcinating MOFs-templated precursors. The catalyst showed a higher specific surface area (94.1 m2 g−1) compared to the reference sample prepared via the precipitation method (66.2 m2 g−1). The catalysts were evaluated in the total oxidation of toluene, where the Co3O4-MOF catalyst exhibited higher catalytic activity on a unit mass basis. The normalization of the reaction rate on a specific surface area basis led to similar specific reaction rates for both samples (Figure 4). According to HRTEM images for the Co3O4-MOF sample, the crystallographic planes observed were the {111} and the {220}, attributed to Co2+ and Co3+, respectively. No HRTEM data were given for the bulk catalyst. Additionally, neither catalyst was characterized via XPS, and thus no conclusion can be drawn for the surface composition of the catalysts.
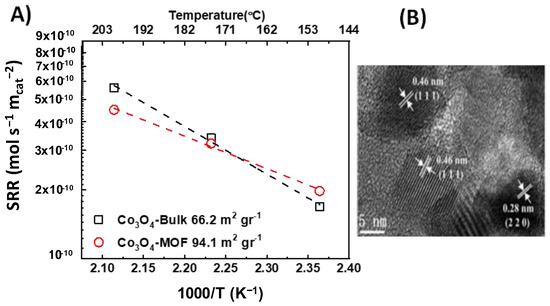
Figure 4.
(A) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  bulk Co3O4 and
bulk Co3O4 and  Co3O4-MOF; (B) HRTEM image for Co3O4-MOF catalyst (calculated with data from [72]).
Co3O4-MOF; (B) HRTEM image for Co3O4-MOF catalyst (calculated with data from [72]).
 bulk Co3O4 and
bulk Co3O4 and  Co3O4-MOF; (B) HRTEM image for Co3O4-MOF catalyst (calculated with data from [72]).
Co3O4-MOF; (B) HRTEM image for Co3O4-MOF catalyst (calculated with data from [72]).
Co3O4 catalysts with different morphologies and exposed crystallographic planes were successfully synthesized by Ren et al. [73] via a hydrothermal process without a surfactant. After the thermal decomposition of the cobalt precursor carried out through the hydrothermal method, 3D hierarchical cubes-stacked Co3O4 microspheres (catalyst C), 3D hierarchical plate-stacked Co3O4 flowers (catalyst P), 3D hierarchical needle-stacked Co3O4 spheres with an urchin-like structure (catalyst N), and 3D hierarchical sheets-stacked fan-shaped Co3O4 (catalyst S) were obtained. The specific surface area of the catalysts was 83.1, 58.8, 25.9, and 53.2 m2 g−1 for catalysts C, P, N, and S, respectively.
The HRTEM images in Figure 5B confirmed that the crystallographic planes that were primarily exposed for the catalysts C, P, N, and S were the {111}, {110}, {110}, and {112} planes, respectively. Catalyst C has a larger specific surface area than the other catalysts. The catalytic activity for the total oxidation of toluene increased as follows: C > P > N > S. On the other hand, the specific reaction rate at conversions below 20% for the Co3O4 catalysts followed the order C = P = N >> S. The unusually low specific activity shown in Figure 5A for the Co3O4-S is attributed to the lower molar ratio of Co3+/Co2+ from XPS and hydrogen consumption from the H2-TPR analysis, or/and to the preferred exposed crystallographic plane, {112}. It would be a serious omission not to mention that the Co3O4-C catalyst presented a higher molar ratio of Co3+/Co2+ after the XPS and H2-TPR analyses. Nonetheless, the specific reaction rate did not significantly differ from the other catalysts (Figure 5).
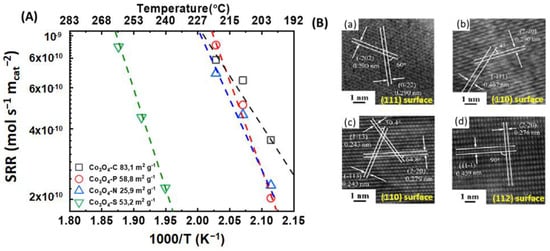
Figure 5.
(A) Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  Co3O4-C,
Co3O4-C,  Co3O4-P,
Co3O4-P,  Co3O4-N, and
Co3O4-N, and  Co3O4-S. (B) HRTEM images for (a) Co3O4-C,(b) Co3O4-P, (c) Co3O4-N, and (d) Co3O4-S (calculated with data from [73]).
Co3O4-S. (B) HRTEM images for (a) Co3O4-C,(b) Co3O4-P, (c) Co3O4-N, and (d) Co3O4-S (calculated with data from [73]).
 Co3O4-C,
Co3O4-C,  Co3O4-P,
Co3O4-P,  Co3O4-N, and
Co3O4-N, and  Co3O4-S. (B) HRTEM images for (a) Co3O4-C,(b) Co3O4-P, (c) Co3O4-N, and (d) Co3O4-S (calculated with data from [73]).
Co3O4-S. (B) HRTEM images for (a) Co3O4-C,(b) Co3O4-P, (c) Co3O4-N, and (d) Co3O4-S (calculated with data from [73]).
On the other hand, Liu and co-workers [74] reported on the controlled synthesis of 3D hierarchical Co3O4 catalysts with different morphologies. The morphology of the Co3O4 catalysts could be tuned by simply changing the calcination temperature. For a deeper understanding of the structures of the obtained Co3O4 catalysts, the authors performed HRTEM analyses of the exposed crystallographic planes of the U-80 and S-160 catalysts, as displayed in Figure 6. The specific surface areas of the U-80, US-120, and S-160 catalysts were 38.7 m2 g−1, 30.2 m2 g−1, and 26.7 m2 g−1, respectively. The major exposed planes for the catalyst U-80 were the {111} planes. In contrast, for S-160, the dominant exposed planes were the {110} planes. The evaluation of the catalysts in the complete oxidation of toluene and the meta-analysis of the extracted data demonstrated that the catalytic activity both on a unit mass basis and per surface area followed the order: S-160 > US-120 ≈ U-80. The increased specific activity of the S-160 catalyst, as shown in Figure 6, can be attributed to the exposed {110} planes and the notable change in the molar ratio of Co3+/Co2+ from XPS and H2-TPR, compared with the other catalysts.
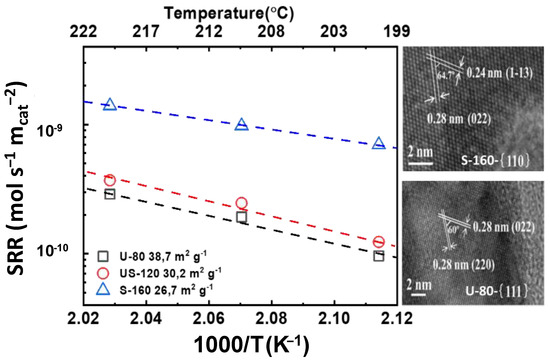
Figure 6.
Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  U-80,
U-80,  US-120, and
US-120, and  US-160: HRTEM image for U-160 and U-80 (calculated with data from [74]).
US-160: HRTEM image for U-160 and U-80 (calculated with data from [74]).
 U-80,
U-80,  US-120, and
US-120, and  US-160: HRTEM image for U-160 and U-80 (calculated with data from [74]).
US-160: HRTEM image for U-160 and U-80 (calculated with data from [74]).
A series of Co3O4 catalysts, such as dumbbell-brush-type mesoporous nanosheets and nanorods, were successfully prepared by Xu et al. [75]. The specific surface areas of Co3O4-D, Co3O4-S, and Co3O4-R were 50, 35, and 26 m2 g−1, respectively. Co3O4-D had a porous dumbbell-brush-type shape with self-assembled nanorods, and as shown in Figure 7. A lattice spacing of 0.280 nm was observed, which can be indexed to the {220} plane of the Co3O4 phase. Co3O4-S, on the other hand, displays a hexagonal nanosheet morphology. When examining the HRTEM images in Figure 7, it is clear that the nanosheet primarily exposed the {220} facets. In addition, the dominant facets observed for the Co3O4-R nanorods were the {220}.
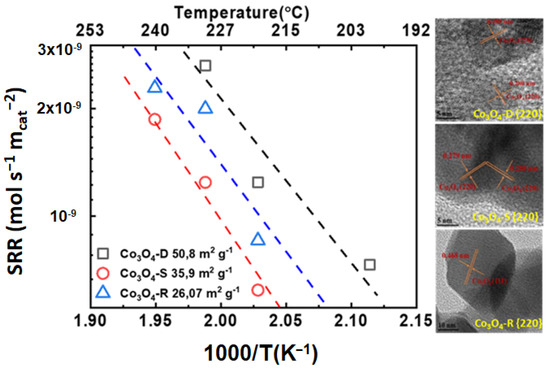
Figure 7.
Specific reaction rate (SRR) vs. 1000/T (K−1) vs. temperature (°C) for  Co3O4-D,
Co3O4-D,  Co3O4-S, and
Co3O4-S, and  Co3O4-R, HRTEM images: Co3O4-D, Co3O4-S, and Co3O4-R (calculated with data from [75]).
Co3O4-R, HRTEM images: Co3O4-D, Co3O4-S, and Co3O4-R (calculated with data from [75]).
 Co3O4-D,
Co3O4-D,  Co3O4-S, and
Co3O4-S, and  Co3O4-R, HRTEM images: Co3O4-D, Co3O4-S, and Co3O4-R (calculated with data from [75]).
Co3O4-R, HRTEM images: Co3O4-D, Co3O4-S, and Co3O4-R (calculated with data from [75]).
The aforementioned catalysts were evaluated for the complete oxidation of toluene. It can be observed that the difference in the specific surface area of the Co3O4 catalysts strongly influences their catalytic activity. More specifically, Co3O4-D had the highest specific surface area and was the best-performing catalyst both in terms of activity and specific activity. Taking into account that all the samples had the same exposed {220} facet, no conclusion can be drawn from the HRTEM results. Nevertheless, according to the XPS and H2-TPR analyses, the higher specific reaction rate of Co3O4-D could be linked with the higher molar ratio of Co3+/Co2+ compared to the Co3O4-S and Co3O4-R catalysts.
Figure 8 depicts a summary of the specific reaction rates attained for the examined Co3O4 catalysts (conversions <20%) in the present review. A summary of the catalytic materials, their main physicochemical properties, and specific activity is given in Table 1. The catalysts were synthesized with various methods and exhibited different specific surface areas in the range of 8–100 m2 g−1, different facets, and different Co3+/Co2+ molar ratios. The catalytic evaluation was performed for the complete oxidation of toluene. Based on the Arrhenius plots presented in Figure 8A, the Co3O4 catalysts attain specific reaction rates ranging typically from 9.5 × 10−11 to 1.6 × 10−9 mol s−1 m−2, irrespective of the synthesis method and specific surface area. The samples Co3O4-HT-CTAB and Co3O4-S constitute the obvious exceptions, with specific reaction rates of 3.3 × 10−9 and 1 × 10−11 mol s−1 m−2, respectively, after extrapolating the Arrhenius plots to 200 °C for comparison with the rest of the samples. For a better visualization of the data, the specific reaction rates obtained at 200 °C were plotted as a function of the specific surface area of the studied catalysts (Figure 8B). If the samples Co3O4-HT-CTAB and Co3O4-S are not taken into account, it is apparent that the specific surface area does not significantly affect the specific reaction rates observed for the oxidation of toluene over Co3O4 catalysts. The calculated specific reaction rates are scattered within the 1 × 10−10–1 × 10−9 mol s−1 m−2 range with no obvious trend for values of specific surface area from 8 to 94 m2 g−1. Nevertheless, the variations observed in the specific rates are in the order of 3–7 times if Co3O4-HT-CTAB and Co3O4-S are excluded, and up to 330 times if these samples are included. Hence, it can be concluded that the specific surface area does not affect, to a significant extent, the specific activity of Co3O4 catalysts for the oxidation of toluene, at least in the SBET region studied. However, considering the variations in the specific reaction rates, it can be deduced that factors such as the morphology and induced exposure of different facets, as well as the surface concentration and oxidation state of cobalt cations, play a key role in the specific activity of the samples.
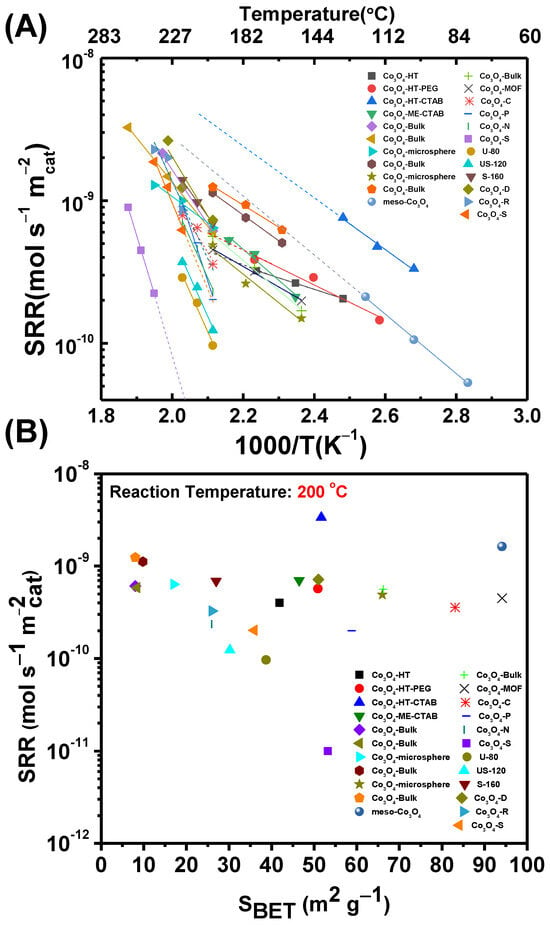
Figure 8.
(A) Summary of the specific reaction rates (SRRs) for toluene oxidation vs. 1000/T (K−1) vs. temperature (°C) and (B) effect of specific surface area on the specific reaction rates at 200 °C for all the studied catalysts.

Table 1.
Summary of the Co3O4 catalysts chosen for the meta-analysis, including the preparation methods, their main physicochemical properties (SBET, morphology and Co3+/Co2+ ratio), and the specific reaction rates attained at 200 °C for the total oxidation of toluene.
Additionally, the apparent activation energies (Eapp) of all the studied catalysts were calculated from the corresponding Arrhenius plots, and they were found to vary widely from 15 ± 3 kJ mol−1 to 157 ± 2 kJ mol−1.
3. Co3O4 Catalysts and Their Physicochemical Characterization
The physicochemical characterization of the catalytic materials is essential for a deep understanding of their catalytic performance. The correlation of the structural, textural, and chemical properties of the catalysts with the catalytic evaluations will enable the rational design and improvement of their activity. In the following section, the typical physicochemical techniques used for the detailed characterization of Co3O4 will be analyzed.
3.1. Powder X-ray Diffraction (PXRD)
Usually, the catalysts are scanned in the 2θ range of 10°–80° and show eleven diffraction peaks related to face-centered cubic spinel Co3O4 (Fd3m space group) (Figure 9A). The 2θ diffraction peaks are 19.1° (111), 31.2° (220), 36.8° (311), 38.5° (222), 44.7° (400), 55.6° (422), 58.9° (511), 64.7° (440), 73.6° (620), 76.8° (533), and 77.7° (622).
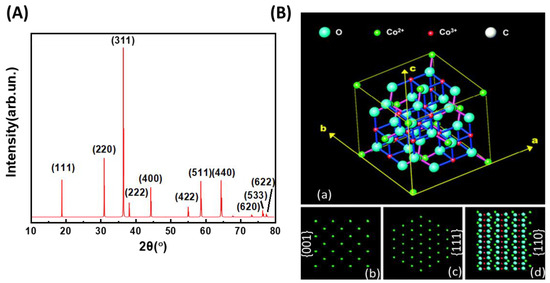
Figure 9.
(A) Typical PXRD diagram for Co3O4, (B) (a) spinel structure of Co3O4 crystal, and (b–d) surface atomic configurations of the {001} (b), {111} (c), and {110} (d) planes (adapted with some modifications from [42] with permission from RCS).
The significant broadening of the diffraction peaks reveals a nanometric particle structure. The average crystallite size is calculated using the Scherrer equation (Equation (5)) [76].
where dPXRD is the crystallite diameter, K is a constant with the value of 0.941, λ is the radiation wavelength, β is the peak width at half maximum in radians, and θ is the reflection angle. In addition, the lattice parameters of the catalysts can be calculated from a Rietveld refinement analysis of the PXRD patterns. From the Rietveld analysis, the microstrain related to the method and temperature of synthesis of the catalysts can be drawn. Figure 9B [42] shows a typical cubic spinel structure schematic, with Co2+ ions in the tetrahedral interstices and Co3+ ions in the octahedral interstices. In this standard spinel structure, Co2+ cations occupy 8a sites with tetrahedral coordination, whereas Co3+ cations are located at 16d sites with octahedral coordination, according to the formula [Co2+]8a [Co3+]16d [77,78].
The Co2+ cations correspond to 8a sites and form a diamond-like lattice, consisting of two displaced face-centered sublattices tetrahedrally surrounded by the four nearest Co2+ neighbors. The close-packed planes were {001}, {111}, and {110}, and their surface atomic configurations are shown in Figure 9b–d. Thus, the {001} and {111} planes contain only Co2+ cations, whereas the {110} plane primarily consists of Co3+ cations [42]. An increase in the crystallite size leads to a decrease in the exposed surface area (per unit volume or mass), which is supported by the example shown in Table 2.

Table 2.
Data from N2 physisorption and PXRD, adapted from reference [79].
3.2. X-ray Photoelectron Spectroscopy (XPS)
XPS is an essential technique for examining the surface chemical composition of Co3O4. As illustrated in Figure 10, a typical XPS spectrum shows the splitting of Co 2p3/2 and Co 2p1/2 at 781 and 795 eV, respectively. These show the surface species combined with the shake-up satellite located at a higher binding energy of 6.7 eV from the main peak. The estimated 2p3/2–2p1/2 spin–orbit splitting is 15.1 eV for Co3O4. Octahedral Co3+ is located at 779.6 eV, and tetrahedral Co2+ is located at 780.7 eV, with spin–orbit splitting at 794.5 eV and 796 eV, respectively. These results indicate the existence of surface Co3+ and Co2+ species [80]. After background correction and peak deconvolution, the Co3+/Co2+ molar ratio of Co3O4 on the surface can be expressed, which could play a crucial role in oxidation catalysis. With differences in coordination, oxidation states, and bond lengths of different Co3O4 catalysts, it is expected that differences exist in the chemical shifts, multiple splits, shake-up structures, and valence band structures.
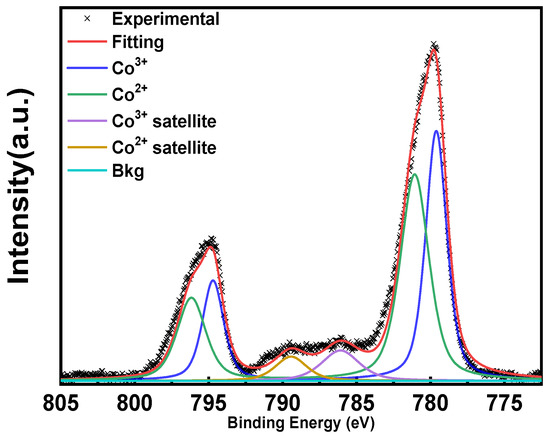
Figure 10.
Typical Co 2p XPS spectrum.
The deconvolution of the O1s spectrum of Co3O4 indicates oxygen species with binding energies around 529.9, 531.6, and 533.5 eV, which are due to the surface lattice oxygen (Olatt), the surface hydroxyl species (Oads), and the adsorbed water species, respectively [81]. A high relative Oads/Olatt concentration ratio on the Co3O4 surface did not enhance catalytic oxidation. It should be highlighted that a material with a higher Co3+/Co2+ molar ratio shows a lower Oads/Olatt molar ratio.
Figure 11A shows the surface Co3+/Co2+ molar ratio calculated from the XPS data as a function of the specific surface area (SBET) for all surveyed catalysts. The Co3+/Co2+ molar ratio varies from 1.1 to 3.2 for all the catalysts with an SBET in the range 8–94 m2 g−1. More specifically, twelve of the catalysts with an SBET below 40 m2 g−1 show a Co3+/Co2+ molar ratio from 1.1 to 1.7, while five of the catalysts with an SBET of 42–58 m2 g−1 show a Co3+/Co2+ molar ratio from 1.8 to 2.1. Moreover, eight of the catalysts with an SBET of 8–80 m2 g−1 present a Co3+/Co2+ molar ratio from 2.2 to 3.2. It is worth mentioning that the theoretically expected value of the Co3+/Co2+ ratio is two. This difference presented by the research papers studied is probably due to the different synthesis methods and, therefore, the different exposed crystallographic planes.
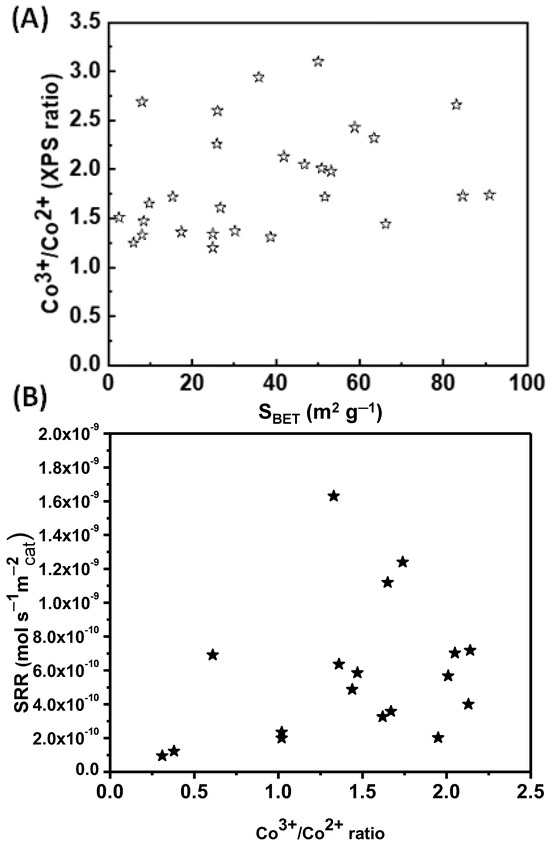
Figure 11.
(A) Co3+/Co2+ molar ratio as determined via XPS analysis as a function of the specific surface area (SBET) for all catalysts and (B) specific reaction rates (SRR) as a function of surface Co3+/Co2+ molar ratio (prepared from bibliographic data).
The correlation between the calculated specific reaction rates (see Figure 8 and Table 1) and the surface Co3+/Co2+ molar ratio is illustrated in Figure 11B. It can be deduced that a higher surface concentration of Co3+ has a rather positive effect on the specific activity of Co3O4 catalysts for the total oxidation of toluene, but the experimental scattering of the results is significant. Therefore, the Co3+/Co2+ molar ratio is certainly not the single factor determining the specific reaction rate of the catalysts.
3.3. Raman Spectroscopy and Attenuated Total Reflectance Fourier Transmittance Infrared Spectroscopy (ATR-FTIR)
Co3O4 Raman spectra consist of five Raman bands, as reported by Dialo et al. [82] and Lei et al. [83], and they are shown in Figure 12A,B. The Raman bands at 194, 480, 520, 619, and 670 cm−1 are assigned to the F2g(3), Eg, F2g(1), F2g(2), and A1g modes of the Co3O4 spinel structure. The sublattice vibration attributable to octahedral–tetrahedral sites is at a value of 670 cm−1. Any minor variations in this vibration can be associated with a lattice distortion of the spinel system or residual stress. This phenomenon is observed in Figure 12B for Co3O4 prepared from different MOF precursors.
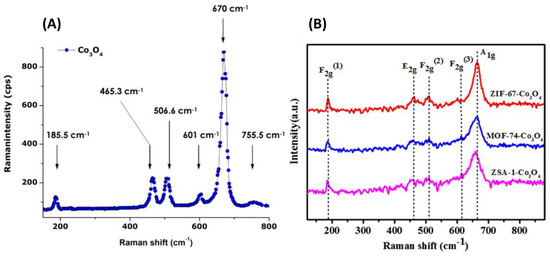
Figure 12.
(A,B) Typical Raman spectra of the annealed Co3O4 nanoparticles adapted from refs. [82,83] with permission from Taylors & Francis and Elsevier, respectively.
However, from the ATR, the observed 570 cm–1 band is characteristic of the O–Co bond, denoting Co3+ at the octahedral site. The 668 cm–1 band is attributable to the Co2+–Co3+–O3 (Co2+ in the tetrahedral site) vibration in the spinel lattice. Any asymmetric band at 1627 cm−1 is due to the OH groups from adsorbed water. It should be noted that adsorbed water can be easily desorbed at temperatures around 120–150 °C under a gaseous flow.
3.4. Hydrogen Temperature-Programmed Reduction (H2-TPR)
Temperature-programmed reduction (TPR) is a widely used technique to investigate the reducibility of solid catalysts. TPR involves exposing the catalytic material to a stream of reducing gas, usually H2, at various temperatures, resulting in a TPR profile, i.e., the reduction rate versus the temperature of the reactor. Qualitatively, the position of the reduction peaks provides information on the ease of reduction of these chemical species, where easier reduction is indicated by the appearance of a peak maximum at a lower temperature. Quantitatively, the amount of hydrogen consumed during the measurement can be compared to a theoretical one to determine the extent of the catalyst reduction. The reduction of the Co3O4 catalysts is exemplified in the H2-TPR profiles by two reduction peaks. The peak at 200–300 °C is due to the reduction of Co3O4 to CoO. The peak within the 300–450 °C range is due to the reduction of CoO to metallic Co0. The theoretical H2 consumption for the complete reduction of Co3O4 to Co0 is 16.6 mmol g−1. The overall reduction is described by Equations (6) and (7) as follows:
Co3O4 + H2 → 3CoO + H2O
3CoO + 3H2 → 3Co + 3H2O
It is worth mentioning that the peak below 200 °C is attributed to the reduction of oxygenated surface species formed during oxidation pre-treatment [84]. The range of the reduction temperature varies according to the surface area, preparation method, and morphology of the catalysts. Typical H2-TPR profiles are presented in Figure 13, taken from the work of Zhang et al. [79], Ma et al. [60], and Ren et al. [73].
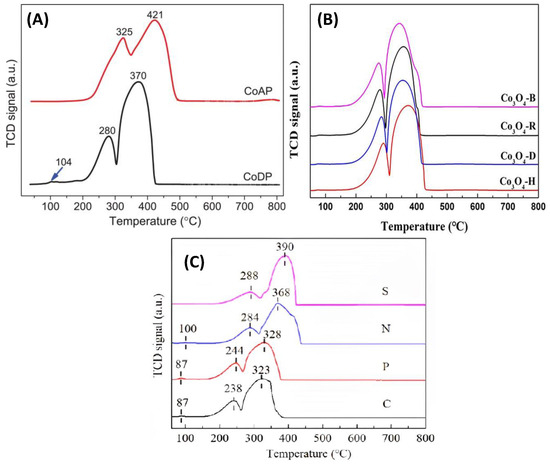
Figure 13.
(A–C) H2-TPR profiles of Co3O4 catalysts (adapted from [79] with permission from Elsevier, from [60] with permission from RSC, and from [73] with permission from RSC, respectively).
As depicted in Figure 13A, a slight modification in the precipitation method from Zhang et al. [79] resulted in CoAP having a 2.2 times lower specific surface area compared with CoDP, and thus revealing different crystallite sizes. Furthermore, the difference in material composition results in a difference in the Co3+/Co2+ ratio and, finally, in the temperature reduction profile. Figure 13B compares the H2-TPR profiles of the Co3O4 rods (Co3O4-R), Co3O4 hexagonal plates (Co3O4-H), and Co3O4 double-sided helianthus discs (Co3O4-D). These materials were also synthesized using the hydrothermal method by Ma et al. [60] by adjusting the water/ethanol ratio. In this case, Co3O4 with different morphologies did not show remarkably different H2-TPR profiles or reduction temperatures. This is possibly because the catalysts have only minor differences in their specific surface area. The main difference was the highest Co3+/Co2+ ratio, indicating that more Co3+ species were exposed on the surface of the Co3O4-B catalyst. This result is probably due to the unique double-sided nanobrush structure of the Co3O4-B catalyst.
Figure 13C shows the H2-TPR profiles of the hydrothermally synthesized Co3O4 catalysts present in the form of cube-stacked microspheres (C catalyst), plate-stacked flowers (P catalyst), needle-stacked double-spheres with an urchin-like structure (N catalyst), and sheet-stacked fan-shaped Co3O4 (S catalyst) [73]. According to these H2-TPR profiles, the catalyst morphology and, therefore, the specific surface area played a significant role compared with the Co3+/Co2+ molar ratio. In this case, it is also necessary to note that the C catalyst has a higher SBET of 83 m2 g−1, those of the S and P catalysts are close to 55 m2 g−1, and that of the N catalyst has the lowest value of 30 m2 g−1. In contrast, the mean crystallite size of the C, P, N, and S catalysts were 16, 18, 30, and 17 nm, respectively. Thus, it was demonstrated that the cube-stacked microspheres (catalyst C) with the highest SBET had slightly lower reduction temperatures.
To summarize, three different H2-TPR profiles are shown in Figure 13. In all cases, the morphology and SBET played an essential role. It is observed that the main factor in the profile and the temperature of reduction in Co3O4 is the Co3+/Co2+ molar ratio. It should be noted that higher Co3+/Co2+ ratio values resulted in an alteration of the reduction profile. Moreover, it is evident that the reduction of CoO to Co is highly dependent on the Co3O4 morphology.
3.5. Oxygen Temperature-Programmed Desorption (O2-TPD)
The presence of oxygen, either adsorbed, lattice-bound, or in gaseous form, is a critical factor in the oxidation reactions that take place over transition metal oxide catalysts. In this context, the O2 Temperature Programmed Desorption (O2-TPD) technique is a valuable tool for assessing oxygen storage and mobility, and it is capable of discerning between various oxygen species on the catalytic surface. Typical TPD profiles (O2 desorption rate vs. temperature) comprise one or more peaks attributed to different adsorption strengths of oxygen species. The representative O2-TPD profiles of the Co3O4 catalysts are shown in Figure 14. Figure 14A refers to the Co3O4/KIT-6 ordered mesoporous catalysts prepared using the impregnation method. These experiments were performed on Co3O4 catalysts to explore the relationship between adsorbed oxygen species and the Co3O4 surface structure. The O2-TPD profiles of the transition metal oxides showed that the oxygen species were desorbed within a wide temperature range. The O2-TPD profiles were divided into three main categories: physically adsorbed oxygen (O2, ads), chemically adsorbed oxygen (O2−/O−), and lattice oxygen (O2−). It is also worth noting that physically adsorbed oxygen is easily desorbed and can be removed before the start of the O2-TPD experiment using a pure He flow.
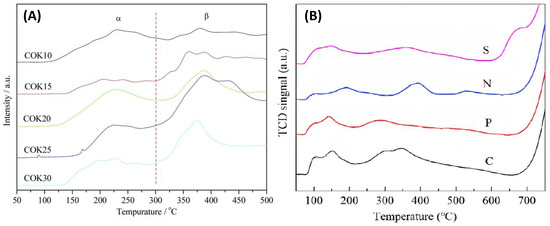
Figure 14.
(A) O2-TPD profiles of the COKx catalysts adapted from [85] with permission from Elsevier; (B) O2-TPD profiles of Co3O4 catalysts in the form of sheets, needles, plates, and cubes adapted from [73] with permission from RSC.
Chemisorbed oxygen molecules can be desorbed above 100 °C from metal oxides, whereas lattice oxygen is challenging to desorb, and the temperature required is generally close to 500 °C. In Figure 14A, Xie et al. [85] describe the α and β oxygen desorption regions of Co3O4/KIT-6 catalysts. The oxygen desorption peaks in areas α and β belong to the surface desorption of weakly chemisorbed oxygen species and strongly chemisorbed oxygen (O2−/O−) species from oxygen vacancies, respectively.
On the other hand, Ren et al. [73] (Figure 14B) define four kinds of oxygen species that are observed in the temperature ranges of 80–120 °C, 120–450 °C, 450–700 °C, and above 700 °C. The above temperatures could be assigned to molecular oxygen species adsorbed on oxygen vacancies (Ov), surface-adsorbed oxygen ions (Oads), surface lattice oxygen (Olatt,s), and bulk-phase lattice oxygen (Olatt,b), respectively. The desorption peak at approximately 100 °C for the C, P, and N catalysts was attributed to the desorption of molecular oxygen. Thus, the O2-TPD profiles show that the chemisorbed oxygen species can desorb at relatively low temperatures. The desorption profiles varied depending on the preparation method and the catalyst morphology.
3.6. Scanning Electron Microscopy (SEM) and High-Resolution Transmission Electron Microscopy (HRTEM)
Figure 15 presents the SEM and HRTEM images of the Co3O4 catalysts prepared using different synthesis methods. In addition, SEM and high-resolution TEM images were used to examine various catalyst morphologies and exposed crystallographic planes, respectively. The HRTEM technique is employed to further study the size distribution of crystallites and the active facets for catalysis. It is worth noting that in most cases, the HRTEM size distribution and the crystallite size calculated from XRD must be comparable.
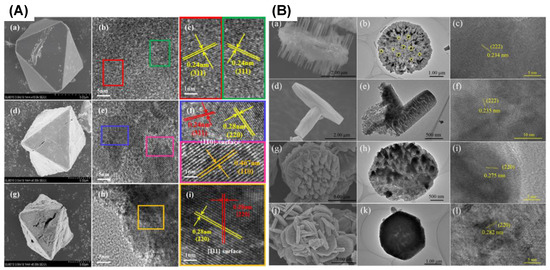
Figure 15.
(A) SEM images of (a) M-X-250, (d) M-Co3O4-350, and (g) M-Co3O4-450 and TEM images of the synthesized catalysts, M-X-250 (b,c), M-Co3O4-350 (e,f), and M-Co3O4-450 (h,i) (adapted from [86]) with permission from Elsevier, and (B) SEM and TEM images of the synthesized Co3O4 catalysts: (a–c) Co3O4-B, (d–f) Co3O4-R, (g–i) Co3O4-D, and (j–l) Co3O4-H (adapted from [60] with permission from RCS).
Figure 15A shows the HRTEM images of M-Co3O4-350 (Figure 15(Ae)), M-Co3O4-450 (Figure 15(Ah)), and M-X-250 (Figure 15(Ab)). The lattice spacing in Figure 15Ac for M-X-250 was 0.24 nm, corresponding to the {311} plane. For the M-Co3O4-350, the three lattice fringes are shown in Figure 15(Ae,f), which are 0.24 nm, 0.28 nm, and 0.467 nm, and these are derived from the {311}, {220}, and {110} crystallographic planes, respectively. Nevertheless, the {110} plane is the dominant exposed surface for this material. However, M-Co3O4-450 (h, i) demonstrates that the {111} plane is the predominant one. From the above data, it is clear that the exposed planes of Co3O4 can be manipulated by controlling the Co-MOF calcination temperature. Figure 15A presents the Co-MOF (ZSA-1) materials synthesized through a hydrothermal method. The as-received Co-MOF was used as a precursor to obtain the final catalysts. The precursor was calcined in air at different temperatures (250, 350, and 450) for 1 h. The SEM images in Figure 15(Aa,d,g) present the catalysts obtained after calcination. These Co3O4 catalysts exhibited octahedral morphologies with slightly different surfaces depending on the calcination temperature.
The SEM images in Figure 15(Ba,d,g,j) show the double-sided nanobrush Co3O4 (Co3O4-B), Co3O4 rods (Co3O4-R), Co3O4 hexagonal plates (Co3O4-H), and Co3O4 double-sided helianthus discs (Co3O4-D), which were prepared by tuning the water/ethanol ratio using a hydrothermal method.
Figure 15(Bc,f,i,l) presents the HRTEM images of the aforementioned materials. The Co3O4-B lattice spacing in Figure 15(Bc) is 0.234 nm, corresponding to the {222} plane of Co3O4-B. In addition, the HRTEM image of the Co3O4-R catalyst showed a lattice spacing of 0.235 nm (Figure 15(Bf)), which corresponds to the {222} plane of Co3O4. A {220} crystallographic plane with a d-spacing of 0.275 nm is observed in Figure 15Bi for the Co3O4-D catalyst. The HRTEM image in Figure 15Bl shows that the Co3O4–H catalysts possessed a {220} plane with a lattice spacing of 0.282 nm. Based on these results, it can be concluded that the exposed facets of the catalysts can be modified by changing their morphology.
4. Designing Co3O4 Catalysts
In this section, the preparation methods and the precursors employed to obtain Co3O4 catalysts with different textural and structural characteristics are summarized. Several cobalt compounds have been used as precursors to prepare Co3O4 for the complete oxidation of VOCs. These include cobalt nitrate hexahydrate [Co(NO3)2·6H2O], cobalt chloride hexahydrate [CoCl2·6H2O], cobalt sulfate hexahydrate [Co(SO4)2·6H2O)], cobalt hydroxide [Co(OH)2], cobalt basic carbonate [2CoCO3·3Co(OH)2·H2O], cobalt acetate [Co(CH3COO)2], and cobalt acetylacetonate (Co(C5H7O2)2). Co-MOFs as precursors have also been a new trend in recent years. From Figure 16, it can be concluded that the most used precursor is cobalt nitrate, employed in 72% of the reported studies, followed by cobalt chloride and cobalt acetate (11% each).
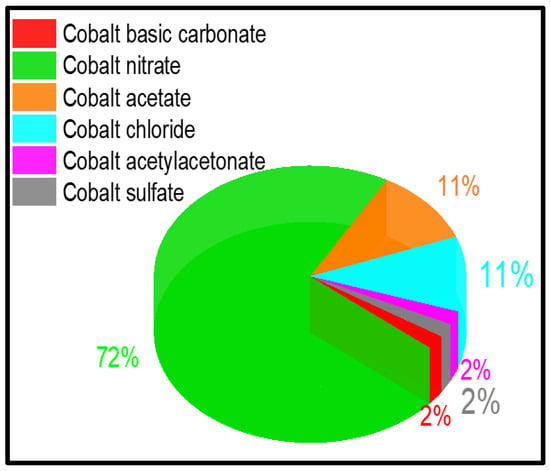
Figure 16.
Distribution of the precursors most commonly used for the synthesis of Co3O4 catalysts.
The most representative Co3O4 synthesis methods are shown in Scheme 2. These include the hydrothermal/solvothermal method [53], the sol–gel process [54], thermal decomposition [24], chemical precipitation, and hard or soft templates [55]. The simple thermal decomposition of a cobalt precursor was used by Rivas et al. [33], Wang et al. [70], Liu et al. [58], Bai et al. [68], and Yan et al. [32]. The thermal decomposition method results in catalysts with a specific surface area in the range 3–82 m2 g−1, which depends on the calcination temperature, typically 300–700 °C.
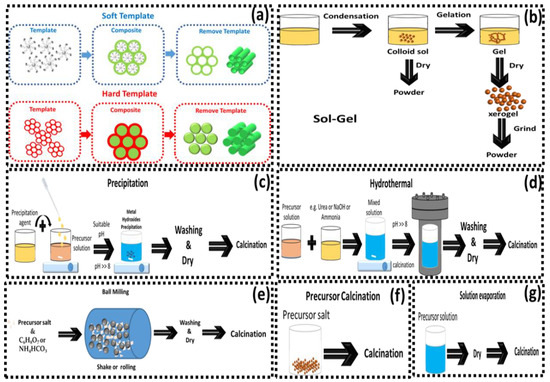
Scheme 2.
Methods of synthesizing Co3O4: (a) templating, (b) sol–gel, (c) precipitation, (d) hydrothermal, (e) ball-milling, (f) precursor calcination, and (g) solution evaporation.
Other research groups such as Li et al. [22], Ren et al. [87], Ma et al. [60], Zhang et al. [79], Liu et al. [74], Liotta et al. [88], Ma et al. [89], Fan et al. [90], and Anke et al. [91] employed a precipitation method to prepare Co3O4 as a catalyst for complete VOC oxidation. The specific surface area of the produced catalysts varied from 5 to 151 m2 g−1 depending on the calcination temperature. The employed precipitation agents were, in most cases, urea (CH4N2O), hydroxycarbonate (CH2O5), sodium carbonate (Na2CO3), ammonium hydroxide (NH3·H2O), sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonium bicarbonate (NH4HCO3), potassium bicarbonate (KHCO3), potassium carbonate (K2CO3), and oleylamine (C2H2NH2).
Fei et al. [92], Xu et al. [75], Hu et al. [93], Xu et al. [75], Fei et al. [92], Zhao et al. [94], Wang et al. [95], Zhou et al. [96], Wang et al. [70], Yang et al. [69], Bai et al. [68], Ma et al. [60], Ren et al. [73,97], Liu et al. [74], Liu et al. [98], and Li et al. [99] used a solvothermal–hydrothermal method at a reaction temperature of 80–180 °C to obtain Co3O4 catalysts with different morphologies. Typical patterns resulting from this method include cubes, nanorods, spheres, nanowires, nanotubes, microspheres, double-sided nanobrushes, 2D-nanoplates, 3D-nanoflowers, 1D-nanoneedles, 3D hierarchical cube-stacked microspheres, 3D hierarchical stacked needles, two-spheres with an urchin-like structure, 3D hierarchical fan-shaped stacked sheets, and dumbbell-brush-type. The specific surface area of these materials varied between 8 and 83 m2 g−1. Additionally, Dissanayake et al. [21], Xie et al. [85], Mei et al. [100], Ren et al. [73], Liu et al. [58,71], Deng et al. [101], and Ma et al. [102] [89] synthesized three-dimensional (3D) or two-dimensional (2D) mesoporous Co3O4 materials using the template method with SBA-15, KIT-6, P123, and PMMA as templates. Except for PMMA, which was removed after calcination, the other templates were removed by washing with a highly concentrated solution of NaOH. The specific surface areas of the received Co3O4 catalysts were in the range of 21 to 553 m2 g−1.
A sol–gel method was employed by Ercolino et al. [103], Puértolas et al. [104], Rivas et al. [33], Kirchnenova et al. [105], and Liu et al. [106] to synthesize Co3O4 catalysts. The final catalysts are calcined at a temperature of 350–600 °C, and the typical values of the specific surface area vary between 1 and 27 m2 g−1. Several research groups, including Rivas et al. [33], Liu et al. [106], and Solsona et al. [107], have prepared Co3O4 catalysts through the application of a grinding technique that involves metal precursors and appropriate concentrations of C6H8O7 or NH4HCO3. The resulting specific surface area ranged between 30 and 160 m2 g−1 and was observed to be significantly dependent on the grinding time and the calcination temperature.
Recently, there has been a growing trend in synthesizing mesoporous catalysts through the thermal decomposition of MOFs (Liu et al. [72], Zhang et al. [108], Lie et al. [86], and Zhao et al. [109]). The shape of the final catalyst varies depending on the shape of the organic ligand but is often in the form of void nanocages, octahedrons, and macroscopic hexagonal crystals. This synthesis method yields a specific surface area ranging from 2.5 to 118 m2 g−1.
Figure 17 presents the percentage of Co3O4 catalysts prepared through various methods in relation to their specific surface area (SBET). The majority of Co3O4 catalysts exhibited a specific surface area within the range 30–60 m2 g−1, constituting 35% of the samples. Catalysts with a SBET of 15–30 m2 g−1 accounted for 25% of the samples. Finally, catalysts with specific surface areas in the low (<15 m2 g−1) or high (>60 m2 g−1) ranges represented around 20% of the samples, respectively.
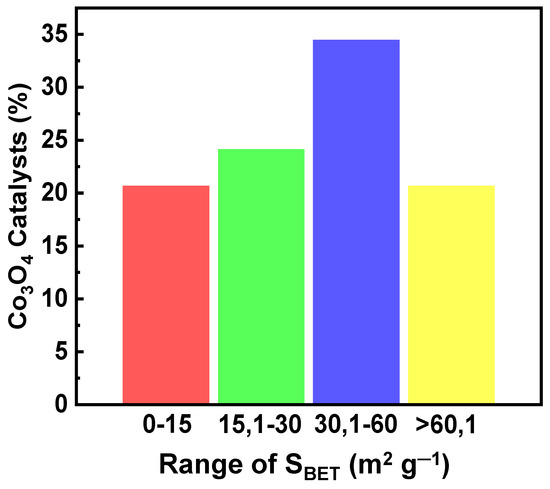
Figure 17.
Percentage of reported Co3O4 (%) catalysts vs. range of SBET (m2 g−1).
5. Overall Conclusions, Challenges, and Perspectives
Catalytic oxidation is an effective after-treatment technology for the abatement of VOC emissions from industrial sources. The preparation of noble metal-free catalysts with high activity and stability remains challenging. This review focuses on Co3O4 catalysts, aiming at identifying the structure sensitivity of toluene oxidation as a case study. Particular attention has been paid to the specific reaction rate of the catalysts and its correlation with the textural and structural characteristics of Co3O4 samples. Extensive reference has been made to synthetic methods and characterization techniques to infer whether the catalytic performance is related to the specific surface area, morphology, facets, and surface oxidation state of the catalysts. From the aforementioned findings, it can be concluded that the specific surface area does not have a significant impact on the specific reaction rate of Co3O4 catalysts for the complete oxidation of toluene. However, the specific reaction rates differ due to factors such as the morphology, different exposed crystallographic planes, surface concentration, and oxidation state of cobalt cations. These factors are crucial in determining the specific reaction rate of Co3O4 by causing the formation of defects (steps, kinks, etc.) and oxygen vacancies or the development of oxygen species with different reactivities. More specifically, the preparation of Co3O4 with {110}, {220}, or {220}-{111} exposed crystallographic planes results in enhanced specific activity that could be attributed to the higher Co3+/Co2+ surface ratio induced by the preferential exposure of Co3+ cations on the catalytic surface. On the other hand, when the only exposed plane is the {111} plane, which is populated by Co2+, the catalytic surface is inactive for the oxidation of toluene. A high Co3+/Co2+ ratio is a prerequisite for a high catalytic performance in the oxidation of VOCs.
In a broader context, the topic of the structure sensitivity of reactions taking place on catalytic surfaces has been raised mostly for supported noble metal catalysts. In the case of metal oxide catalysts, structure sensitivity might theoretically manifest itself through two pathways as a function of the primary crystallite size of the oxide. The first pathway refers to the number density of surface defects and how this is influenced by the crystallite size and the specific surface area of the catalyst. It is typically expected that surface defects would lead to more active surface sites due to the presence of cations with a lower coordination number. Therefore, an increase in crystallite size (hence a decrease in the specific surface area) would lead to fewer defective crystals and, as a result, to a decrease in specific catalytic activity. However, such a behavior was not observed according to the analysis of this work since the specific activity of the catalysts did not show a definitive trend with variations in the specific surface area. The second pathway, which refers to the expression of different crystallographic planes (faceting) with a variation in crystallite size and the employed synthesis method, appears to be more relevant. Based on our analysis, the focus on developing Co3O4 catalysts with high specific surface areas does not suffice to prepare a highly active oxidation catalyst since the real challenge is to tailor the synthetic route to favor the development of surface traits that also contribute to the enhancement of the intrinsic activity of Co3O4 catalysts. Control of crystal orientation and shape can lead to the design of tailored nanomaterials with optimized catalytic function. The designated choice of a single oxide in the current review was a deliberate step taken to eliminate the influence of interactions among various phases or the support, which otherwise would complicate the analysis of catalytic performance metrics. This methodological approach is intended to ensure that the performance evaluation of the catalyst is based solely on its activity and not on any external factors that may interfere. It is noteworthy that the insights gained from this review can serve as a paradigm for other TMO-based catalysts that are commonly used in the field.
Author Contributions
Conceptualization, T.I.; methodology, T.I. and Y.G.; validation, M.S. and Y.G.; formal analysis, Y.G. and M.S.; data curation, M.S. and Y.G.; writing—original draft preparation, Y.G.; writing—review and editing, M.S. and T.I.; supervision, T.I.; project administration, T.I.; funding acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Foundation for Research and Innovation (H.F.R.I) under the “First Call for H.F.R.I Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment grant” (Project Number: HFRI-FM17-1876).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ojala, S.; Koivikko, N.; Laitinen, T.; Mouammine, A.; Seelam, P.; Laassiri, S.; Ainassaari, K.; Brahmi, R.; Keiski, R. Utilization of Volatile Organic Compounds as an Alternative for Destructive Abatement. Catalysts 2015, 5, 1092–1151. [Google Scholar] [CrossRef]
- Ciganek, M.; Neca, J. Chemical Characterization of Volatile Organic Compounds on Animal Farms. Vet. Med. 2008, 53, 641–651. [Google Scholar] [CrossRef]
- Tai, X.H.; Lai, C.W.; Juan, J.C.; Lee, K.M. Nanocatalyst-Based Catalytic Oxidation Processes. In Nanomaterials for Air Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–150. ISBN 978-0-12-818821-7. [Google Scholar]
- World Health Organization. Indoor Air Quality: Organic Pollutants. EURO Rep. Stud. 1989, 111, 1–70. [Google Scholar]
- Anand, S.S.; Philip, B.K.; Mehendale, H.M. Volatile Organic Compounds. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 967–970. ISBN 978-0-12-386455-0. [Google Scholar]
- Bari, M.d.A.; Kindzierski, W.B.; Wheeler, A.J.; Héroux, M.-È.; Wallace, L.A. Source Apportionment of Indoor and Outdoor Volatile Organic Compounds at Homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Sax, S.N.; Bennett, D.H.; Chillrud, S.N.; Kinney, P.L.; Spengler, J.D. Differences in Source Emission Rates of Volatile Organic Compounds in Inner-City Residences of New York City and Los Angeles. J. Expo. Sci. Env. Epidemiol. 2004, 14, S95–S109. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Breysse, P.; Yang, W. Volatile Organic Compounds Concentrations in Residential Indoor and Outdoor and Its Personal Exposure in Korea. Environ. Int. 2003, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.K.; Sim, M.R.; Abramson, M.J.; Gray, C.N. Concentrations of Volatile Organic Compounds in Indoor Air—A Review. Indoor Air 1994, 4, 123–134. [Google Scholar] [CrossRef]
- National Institutes of Health; National Institute of Environmental Health Sciences. Environmental Health Perspectives: Supplements; U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Environmental Health Sciences: Research Triangle Park, NV, USA, 1993. [Google Scholar]
- Lewandowski, D.A. Design of Thermal Oxidation Systems for Volatile Organic Compounds; CRC Press: Boca Raton, FL, USA, 1999; ISBN 1-56670-410-3. [Google Scholar]
- Deshmukh, S.; Vlachos, D. A Reduced Mechanism for Methane and One-Step Rate Expressions for Fuel-Lean Catalytic Combustion of Small Alkanes on Noble Metals. Combust. Flame 2007, 149, 366–383. [Google Scholar] [CrossRef]
- Badra, J.A.; Masri, A.R. Catalytic Combustion of Selected Hydrocarbon Fuels on Platinum: Reactivity and Hetero–Homogeneous Interactions. Combust. Flame 2012, 159, 817–831. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 978-1-4200-2812-6. [Google Scholar]
- Liotta, L.F. Catalytic Oxidation of Volatile Organic Compounds on Supported Noble Metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Aznárez, A.; Gil, A.; Korili, S.A. Performance of Palladium and Platinum Supported on Alumina Pillared Clays in the Catalytic Combustion of Propene. RSC Adv. 2015, 5, 82296–82309. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic Oxidation of Toluene over Binary Mixtures of Copper, Manganese and Cerium Oxides Supported on γ-Al2O3. Appl. Catal. B Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Wang, C.-H. Al2O3-Supported Transition-Metal Oxide Catalysts for Catalytic Incineration of Toluene. Chemosphere 2004, 55, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.-M.; Lamonier, J.-F. Removal of Oxygenated Volatile Organic Compounds by Catalytic Oxidation over Zr–Ce–Mn Catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lany, S. Semiconducting Transition Metal Oxides. J. Phys. Condens. Matter 2015, 27, 283203. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, S.; Wasalathanthri, N.; Shirazi Amin, A.; He, J.; Poges, S.; Rathnayake, D.; Suib, S.L. Mesoporous Co3O4 Catalysts for VOC Elimination: Oxidation of 2-Propanol. Appl. Catal. A Gen. 2020, 590, 117366. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of Mesoporous Co3O4 Oxides by Acid Treatment and Their Catalytic Performances for Toluene Oxidation. Appl. Catal. A Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- González-Prior, J.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; de Rivas, B. Oxidation of 1,2-Dichloroethane over Nanocube-Shaped Co3O4 Catalysts. Appl. Catal. B Environ. 2016, 199, 384–393. [Google Scholar] [CrossRef]
- De Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Highly Active Behaviour of Nanocrystalline Co3O4 from Oxalate Nanorods in the Oxidation of Chlorinated Short Chain Alkanes. Chem. Eng. J. 2012, 184, 184–192. [Google Scholar] [CrossRef]
- Zhao, Q.; Shih, W.Y.; Shih, W.-H. Effects of Processing Parameters on the Morphology of Precipitated Manganese Oxide Powders. Ind. Eng. Chem. Res. 2009, 48, 1490–1494. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Lin, M.; Ma, X.; Ge, M. Enhancement Effect of Acid Treatment on Mn2O3 Catalyst for Toluene Oxidation. Catal. Today 2019, 327, 254–261. [Google Scholar] [CrossRef]
- Deng, J.; He, S.; Xie, S.; Yang, H.; Liu, Y.; Guo, G.; Dai, H. Ultralow Loading of Silver Nanoparticles on Mn2O3 Nanowires Derived with Molten Salts: A High-Efficiency Catalyst for the Oxidative Removal of Toluene. Environ. Sci. Technol. 2015, 49, 11089–11095. [Google Scholar] [CrossRef] [PubMed]
- Scirè, S.; Minicò, S.; Crisafulli, C.; Galvagno, S. Catalytic Combustion of Volatile Organic Compounds over Group IB Metal Catalysts on Fe2O3. Catal. Commun. 2001, 2, 229–232. [Google Scholar] [CrossRef]
- Gu, Z.; Hohn, K.L. Catalytic Oxidation of Methanol on Nanoscale Copper Oxide and Nickel Oxide. Ind. Eng. Chem. Res. 2004, 43, 30–35. [Google Scholar] [CrossRef]
- Morales, M.; Barbero, B.; Cadus, L. Total Oxidation of Ethanol and Propane over Mn-Cu Mixed Oxide Catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC Oxidation over CuO–CeO2 Catalysts Prepared by a Combustion Method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Zhao, Q.; Chen, G. Shape-Controlled Fabrication of the Porous Co3O4 Nanoflower Clusters for Efficient Catalytic Oxidation of Gaseous Toluene. J. Hazard. Mater. 2012, 209–210, 385–391. [Google Scholar] [CrossRef]
- De Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Synthesis, Characterisation and Catalytic Performance of Nanocrystalline Co3O4 for Gas-Phase Chlorinated VOC Abatement. J. Catal. 2011, 281, 88–97. [Google Scholar] [CrossRef]
- Smyrnioti, M.; Ioannides, T. Synthesis of Cobalt-Based Nanomaterials from Organic Precursors. In Cobalt; Maaz, K., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3667-5. [Google Scholar]
- Kong, F.-C.; Li, Y.-F.; Shang, C.; Liu, Z.-P. Stability and Phase Transition of Cobalt Oxide Phases by Machine Learning Global Potential Energy Surface. J. Phys. Chem. C 2019, 123, 17539–17547. [Google Scholar] [CrossRef]
- Qiao, L.; Xiao, H.Y.; Meyer, H.M.; Sun, J.N.; Rouleau, C.M.; Puretzky, A.A.; Geohegan, D.B.; Ivanov, I.N.; Yoon, M.; Weber, W.J.; et al. Nature of the Band Gap and Origin of the Electro-/Photo-Activity of Co3O4. J. Mater. Chem. C 2013, 1, 4628. [Google Scholar] [CrossRef]
- Zhong, J.; Zeng, Y.; Zhang, M.; Feng, W.; Xiao, D.; Wu, J.; Chen, P.; Fu, M.; Ye, D. Toluene Oxidation Process and Proper Mechanism over Co3O4 Nanotubes: Investigation through In-Situ DRIFTS Combined with PTR-TOF-MS and Quasi in-Situ XPS. Chem. Eng. J. 2020, 397, 125375. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, X.; Zhong, J.; Zhang, J.; Tian, J.; Yan, D.; Liu, P.; Fu, M.; Chen, L.; Wu, J.; et al. Unravelling the Role of Oxygen Species in Toluene Oxidation over Co3O4-Base Catalysts: In Situ DRIFTS Coupled with Quasi in Situ XPS. J. Catal. 2023, 418, 130–140. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The Universal Character of the Mars and Van Krevelen Mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Vannice, M.A. An Analysis of the Mars–van Krevelen Rate Expression. Catal. Today 2007, 123, 18–22. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Xie, X.; Shen, W. Morphology Control of Cobalt Oxide Nanocrystals for Promoting Their Catalytic Performance. Nanoscale 2009, 1, 50. [Google Scholar] [CrossRef]
- Barteau, M.A.; Vohs, J.M. Structure Sensitivity of Reactions on Metal Oxide Surfaces. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; Volume 44, pp. 89–95. ISBN 978-0-444-87146-6. [Google Scholar]
- Boudart, M. Catalysis by Supported Metals. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1969; Volume 20, pp. 153–166. ISBN 978-0-12-007820-2. [Google Scholar]
- Zafiris, G.S.; Gorte, R.J. CO Oxidation on Pt/α-Al2O3(0001): Evidence for Structure Sensitivity. J. Catal. 1993, 140, 418–423. [Google Scholar] [CrossRef]
- Hicks, R.; Qi, H.; Young, M.L.; Lee, R.G. Structure Sensitivity of Methane Oxidation over Platinum and Palladium. J. Catal. 1990, 122, 280–294. [Google Scholar] [CrossRef]
- Yokota, S.; Okumura, K.; Niwa, M. Support Effect of Metal Oxide on Rh Catalysts in the CH4-CO2 Reforming Reaction. Catal. Lett. 2002, 84, 131–134. [Google Scholar] [CrossRef]
- Haruta, M. Novel Catalysis of Gold Deposited on Metal Oxides. Catal. Surv. Jpn. 1997, 1, 61–73. [Google Scholar] [CrossRef]
- Volta, J.C.; Portefaix, J.L. Structure Sensitivity of Mild Oxidation Reactions on Oxide Catalysts—A Review. Appl. Catal. 1985, 18, 1–32. [Google Scholar] [CrossRef]
- Ginés, M.J.L.; Amadeo, N.; Laborde, M.; Apesteguía, C.R. Activity and Structure-Sensitivity of the Water-Gas Shift Reaction over CuZnAl Mixed Oxide Catalysts. Appl. Catal. A Gen. 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Tatibouet, J.; Phichitkul, C.; Germain, J.E. Structure-Sensitive Catalytic Oxidation of Butenes on Molybdenum Trioxide Crystallites. J. Catal. 1986, 99, 231–234. [Google Scholar] [CrossRef]
- Natile, M.M.; Glisenti, A. Study of Surface Reactivity of Cobalt Oxides: Interaction with Methanol. Chem. Mater. 2002, 14, 3090–3099. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Wen, P.; Hu, Y.; Gui, Z. Morphology-Controlled Synthesis of Co3O4 by One Step Template-Free Hydrothermal Method. Mater. Res. Bull. 2015, 67, 87–93. [Google Scholar] [CrossRef]
- Hosseini, S.; Niaei, A.; Salari, D. Preparation and Characterization of Nano- and Non-Nanoscale Co3O4 Spinels Obtained from Different Methods and Study of Their Performance in Combustion of Aromatics from Polluted Air-A Comparison with Pt/γ-Al 2O3 Performance. J. Environ. Sci. Health Part A 2012, 47, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Gao, L.; Zheng, H.; Zhang, Z.; Qian, Y. One-Dimensional Arrays of Co3O4 Nanoparticles: Synthesis, Characterization, and Optical and Electrochemical Properties. J. Phys. Chem. B 2004, 108, 16401–16404. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, B.; Wu, D.; Xu, Y. Synthesis of Polycrystalline Co3O4 Nanowires with Excellent Ammonium Perchlorate Catalytic Decomposition Property. Mater. Res. Bull. 2014, 60, 492–497. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Wang, Y. Co3O4 Nanoboxes: Surfactant-Templated Fabrication and Microstructure Characterization. Adv. Mater. 2006, 18, 1078–1082. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Zhang, L.; Zhao, Z.; Li, X.; Wang, Y.; Xie, S.; Yang, H.; Guo, G. Controlled Generation of Uniform Spherical LaMnO3, LaCoO3, Mn2O3, and Co3O4 Nanoparticles and Their High Catalytic Performance for Carbon Monoxide and Toluene Oxidation. Inorg. Chem. 2013, 52, 8665–8676. [Google Scholar] [CrossRef]
- Nguyen, H.; El-Safty, S.A. Meso- and Macroporous Co3O4 Nanorods for Effective VOC Gas Sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Ma, X.; Yu, X.; Yang, X.; Lin, M.; Ge, M. Hydrothermal Synthesis of a Novel Double-sided Nanobrush Co3O4 Catalyst and Its Catalytic Performance for Benzene Oxidation. ChemCatChem 2018, 11, cctc.201801539. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A Highly Sensitive VOC Gas Sensor Using P-Type Mesoporous Co3O4 Nanosheets Prepared by a Facile Chemical Coprecipitation Method. Sens. Actuators B Chem. 2016, 233, 615–623. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 Nanomaterials in Lithium-Ion Batteries and Gas Sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Dong, J.; Song, L.; Yin, J.-J.; He, W.; Wu, Y.; Gu, N.; Zhang, Y. Co3O4 Nanoparticles with Multi-Enzyme Activities and Their Application in Immunohistochemical Assay. ACS Appl. Mater. Interfaces 2014, 6, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 Nanocrystals and Co3O4–MOx Binary Oxides for CO, CH4 and VOC Oxidation at Low Temperatures: A Review. Catal. Sci. Technol. 2013, 3, 3085. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Hosseini, S.A. Catalytic Oxidation of Volatile Organic Compounds by Using Spinel Mixed Oxide Catalyst—A Review. Adv. Ceram. Sci. Eng. 2016, 5, 117–134. [Google Scholar] [CrossRef]
- Tang, W.; Liu, G.; Li, D.; Liu, H.; Wu, X.; Han, N.; Chen, Y. Design and Synthesis of Porous Non-Noble Metal Oxides for Catalytic Removal of VOCs. Sci. China Chem. 2015, 58, 1359–1366. [Google Scholar] [CrossRef]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Wang, F.; Zhao, Z.; Qiu, W.; Au, C.T. Porous Co3O4 Nanowires and Nanorods: Highly Active Catalysts for the Combustion of Toluene. Appl. Catal. A Gen. 2013, 450, 42–49. [Google Scholar] [CrossRef]
- Yang, H.; Dai, H.; Deng, J.; Xie, S.; Han, W.; Tan, W.; Jiang, Y.; Au, C.T. Porous Cube-Aggregated Co3O4 Microsphere-Supported Gold Nanoparticles for Oxidation of Carbon Monoxide and Toluene. ChemSusChem 2014, 7, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Han, W. Nanoplate-Aggregate Co3O4 Microspheres for Toluene Combustion. Chin. J. Catal. 2014, 35, 1475–1481. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-Supported Gold Nanocatalysts: Highly Active for the Oxidation of Carbon Monoxide, Benzene, Toluene, and o-Xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zeng, J.; Wang, X.; Zhu, T. Catalytic Oxidation of Toluene over a Porous Co3O4-Supported Ruthenium Catalyst. RSC Adv. 2015, 5, 52066–52071. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable Synthesis of 3D Hierarchical Co3O4 Nanocatalysts with Various Morphologies for the Catalytic Oxidation of Toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Liu, W.; Liu, R.; Zhang, X. Controllable Synthesis of 3D Hierarchical Co3O4 Catalysts and Their Excellent Catalytic Performance for Toluene Combustion. Appl. Surf. Sci. 2020, 507, 145174. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, K.; Lai, S.; Liang, J.; Jiang, X.; Liu, Z.; Li, F.; Zhang, Y.; Wu, X.; Zhou, X. Designing a Dumbbell-Brush-Type Co3O4 for Efficient Catalytic Toluene Oxidation. Catal. Commun. 2020, 140, 106005. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen. Nachr. Ges. Wiss. Göttingen 1918, 2, 96–100. [Google Scholar]
- Raveau, B.; Seikh, M. Crystal Chemistry of Cobalt Oxides. In Cobalt Oxides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 3–70. ISBN 978-3-527-64552-7. [Google Scholar]
- Casas-Cabanas, M.; Binotto, G.; Larcher, D.; Lecup, A.; Giordani, V.; Tarascon, J.-M. Defect Chemistry and Catalytic Activity of Nanosized Co3O4. Chem. Mater. 2009, 21, 1939–1947. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, F.; Li, J.; You, Z. Dispersion–Precipitation Synthesis of Highly Active Nanosized Co3O4 for Catalytic Oxidation of Carbon Monoxide and Propane. Appl. Surf. Sci. 2017, 411, 136–143. [Google Scholar] [CrossRef]
- Chuang, T.J.; Brundle, C.R.; Rice, D.W. Interpretation of the X-Ray Photoemission Spectra of Cobalt Oxides and Cobalt Oxide Surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Fernández, A.; Espinós, J.P.; González-Elipe, A.R. The State of the Oxygen at the Surface of Polycrystalline Cobalt Oxide. J. Electron. Spectrosc. Relat. Phenom. 1995, 71, 61–71. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. Green Synthesis of Co3O4 Nanoparticles via Aspalathus linearis: Physical Properties. Green. Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Lei, J.; Wang, S.; Li, J. Mesoporous Co3O4 Derived from Co-MOFs with Different Morphologies and Ligands for Toluene Catalytic Oxidation. Chem. Eng. Sci. 2020, 220, 115654. [Google Scholar] [CrossRef]
- Davies, T.E.; García, T.; Solsona, B.; Taylor, S.H. Nanocrystalline Cobalt Oxide: A Catalyst for Selective Alkane Oxidation under Ambient Conditions. Chem. Commun. 2006, 32, 3417–3419. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lan, H.; Tan, X.; Wang, W.; Yan, L.; Liu, X.; Zhou, G. High-Efficient Oxidation Removal of Ethanol from Air over Ordered Mesoporous Co3O4/KIT-6 Catalyst. J. Environ. Chem. Eng. 2019, 7, 103480. [Google Scholar] [CrossRef]
- Lei, J.; Wang, S.; Li, J. Mesoporous Co3O4 Derived from Facile Calcination of Octahedral Co-MOFs for Toluene Catalytic Oxidation. Ind. Eng. Chem. Res. 2020, 59, 5583–5590. [Google Scholar] [CrossRef]
- Ren, Z.; Wu, Z.; Song, W.; Xiao, W.; Guo, Y.; Ding, J.; Suib, S.L.; Gao, P.-X. Low Temperature Propane Oxidation over Co3O4 Based Nano-Array Catalysts: Ni Dopant Effect, Reaction Mechanism and Structural Stability. Appl. Catal. B Environ. 2016, 180, 150–160. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Catalytic Performance of Co3O4/CeO2 and Co3O4/CeO2–ZrO2 Composite Oxides for Methane Combustion: Influence of Catalyst Pretreatment Temperature and Oxygen Concentration in the Reaction Mixture. Appl. Catal. B Environ. 2007, 70, 314–322. [Google Scholar] [CrossRef]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 Catalysts for Low-Temperature Oxidation of Trace Ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Z.; Fang, W.; Yao, X.; Zou, G.; Shangguan, W. Low-Temperature Catalytic Oxidation of Formaldehyde over Co3O4 Catalysts Prepared Using Various Precipitants. Chin. J. Catal. 2016, 37, 947–954. [Google Scholar] [CrossRef]
- Anke, S.; Bendt, G.; Sinev, I.; Hajiyani, H.; Antoni, H.; Zegkinoglou, I.; Jeon, H.; Pentcheva, R.; Roldan Cuenya, B.; Schulz, S.; et al. Selective 2-Propanol Oxidation over Unsupported Co3O4 Spinel Nanoparticles: Mechanistic Insights into Aerobic Oxidation of Alcohols. ACS Catal. 2019, 9, 5974–5985. [Google Scholar] [CrossRef]
- Fei, X.; Cao, S.; Ouyang, W.; Wen, Y.; Wang, H.; Wu, Z. A Convenient Synthesis of Core-Shell Co3O4@ZSM-5 Catalysts for the Total Oxidation of Dichloromethane (CH2Cl2). Chem. Eng. J. 2020, 387, 123411. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Q.; Zheng, Y.; Han, R.; Song, C.; Ji, N.; Ma, D. Enhanced Catalytic Performance for Volatile Organic Compound Oxidation over In-Situ Growth of MnOx on Co3O4 Nanowire. Chemosphere 2020, 244, 125532. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hua, W.; Chai, G.; Zhang, C.; Guo, Y. Insights into the Morphological Effect of Co3O4 Crystallite on Catalytic Oxidation of Vinyl Chloride. Catalysts 2019, 9, 408. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Liao, L.; Wu, W. Enhanced Catalytic Methane Combustion over Co3O4 Nanowire Arrays by Cation Substitution. Mater. Res. Express 2017, 4, 125006. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for Catalytic Oxidation of Toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Liu, S.; Liu, P.; Niu, R.; Wang, S.; Li, J. Facile Synthesis of Mesoporous Co3O4 Nanoflowers for Catalytic Combustion of Ventilation Air Methane. Chem. Res. Chin. Univ. 2017, 33, 965–970. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Bai, B.; Zhao, S.; Hu, F.; Li, J. Bridging the Reaction Route of Toluene Total Oxidation and the Structure of Ordered Mesoporous Co3O4: The Roles of Surface Sodium and Adsorbed Oxygen. Catal. Today 2017, 297, 173–181. [Google Scholar] [CrossRef]
- Mei, J.; Xie, J.; Qu, Z.; Ke, Y.; Hu, X.; Yan, N. Ordered Mesoporous Spinel Co3O4 as a Promising Catalyst for the Catalytic Oxidation of Dibromomethane. Mol. Catal. 2018, 461, 60–66. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Dai, H.; Xia, Y.; Jiang, H.; Zhang, H.; He, H. Ultrasound-Assisted Nanocasting Fabrication of Ordered Mesoporous MnO2 and Co3O4 with High Surface Areas and Polycrystalline Walls. J. Phys. Chem. C 2010, 114, 2694–2700. [Google Scholar] [CrossRef]
- Ma, C.; Mu, Z.; He, C.; Li, P.; Li, J.; Hao, Z. Catalytic Oxidation of Benzene over Nanostructured Porous Co3O4-CeO2 Composite Catalysts. J. Environ. Sci. 2011, 23, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Ercolino, G.; Stelmachowski, P.; Grzybek, G.; Kotarba, A.; Specchia, S. Optimization of Pd Catalysts Supported on Co3O4 for Low-Temperature Lean Combustion of Residual Methane. Appl. Catal. B Environ. 2017, 206, 712–725. [Google Scholar] [CrossRef]
- Puértolas, B.; Smith, A.; Vázquez, I.; Dejoz, A.; Moragues, A.; Garcia, T.; Solsona, B. The Different Catalytic Behaviour in the Propane Total Oxidation of Cobalt and Manganese Oxides Prepared by a Wet Combustion Procedure. Chem. Eng. J. 2013, 229, 547–558. [Google Scholar] [CrossRef]
- Kirchnerova, J.; Alifanti, M.; Delmon, B. Evidence of Phase Cooperation in the LaCoO3–CeO2–Co3O4 Catalytic System in Relation to Activity in Methane Combustion. Appl. Catal. A Gen. 2002, 231, 65–80. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.-C.; Chen, M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Dry Citrate-Precursor Synthesized Nanocrystalline Cobalt Oxide as Highly Active Catalyst for Total Oxidation of Propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Solsona, B.; Vázquez, I.; Garcia, T.; Davies, T.E.; Taylor, S.H. Complete Oxidation of Short Chain Alkanes Using a Nanocrystalline Cobalt Oxide Catalyst. Catal. Lett. 2007, 116, 116–121. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, S.; Zhang, Q.; Mo, S.; Zhong, J.; Chen, D.; Fu, M.; Chen, P.; Ye, D. Macroscopic Hexagonal Co3 O4 Tubes Derived from Controllable Two-Dimensional Metal-Organic Layer Single Crystals: Formation Mechanism and Catalytic Activity. Inorg. Chem. 2020, 59, 3062–3071. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Dong, F.; Zhang, J. Controlled Porous Hollow Co3O4 Polyhedral Nanocages Derived from Metal-Organic Frameworks (MOFs) for Toluene Catalytic Oxidation. Mol. Catal. 2019, 463, 77–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).