Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol
Abstract
1. Introduction
2. Results
2.1. Characterization of Catalysts
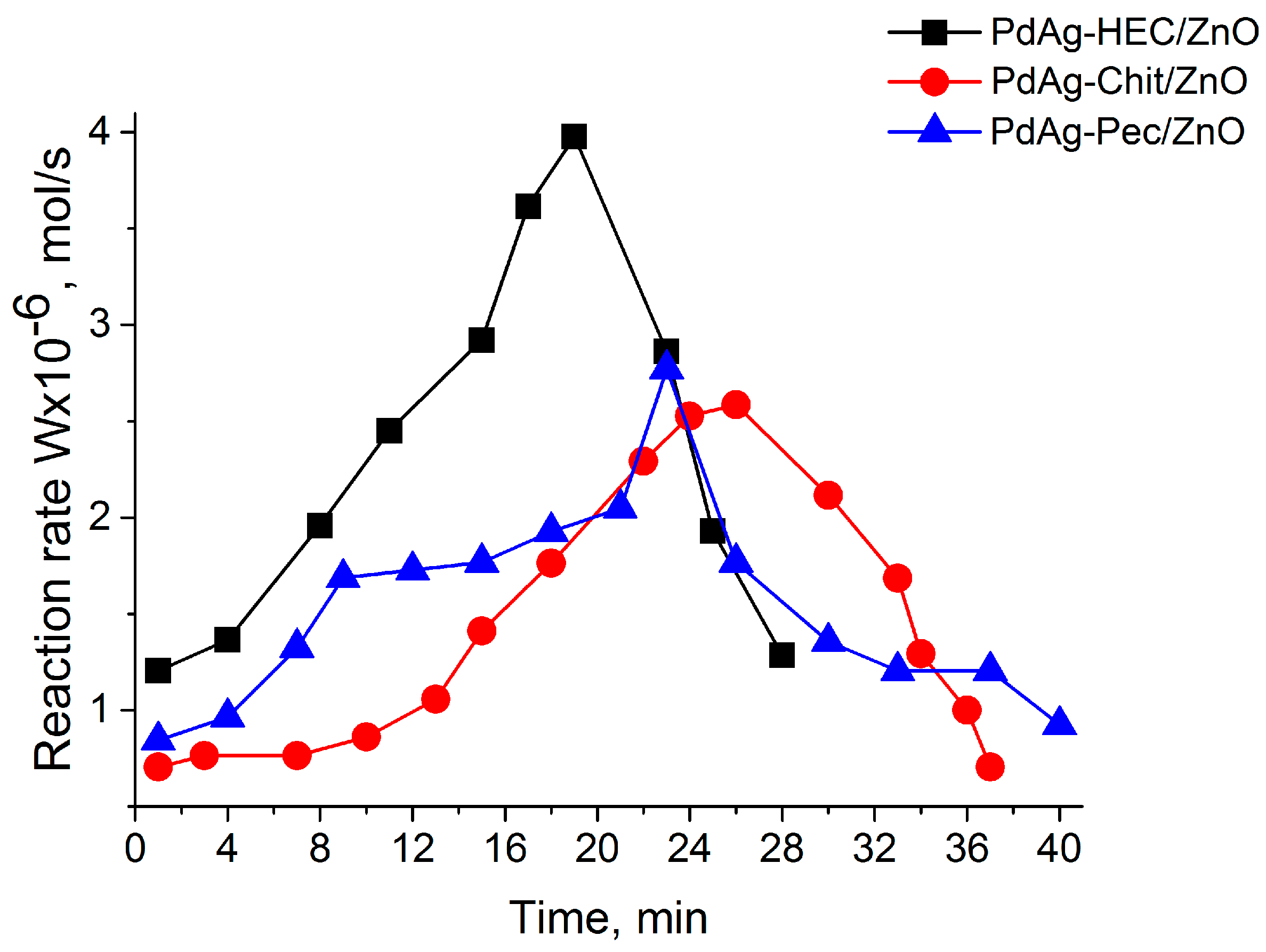
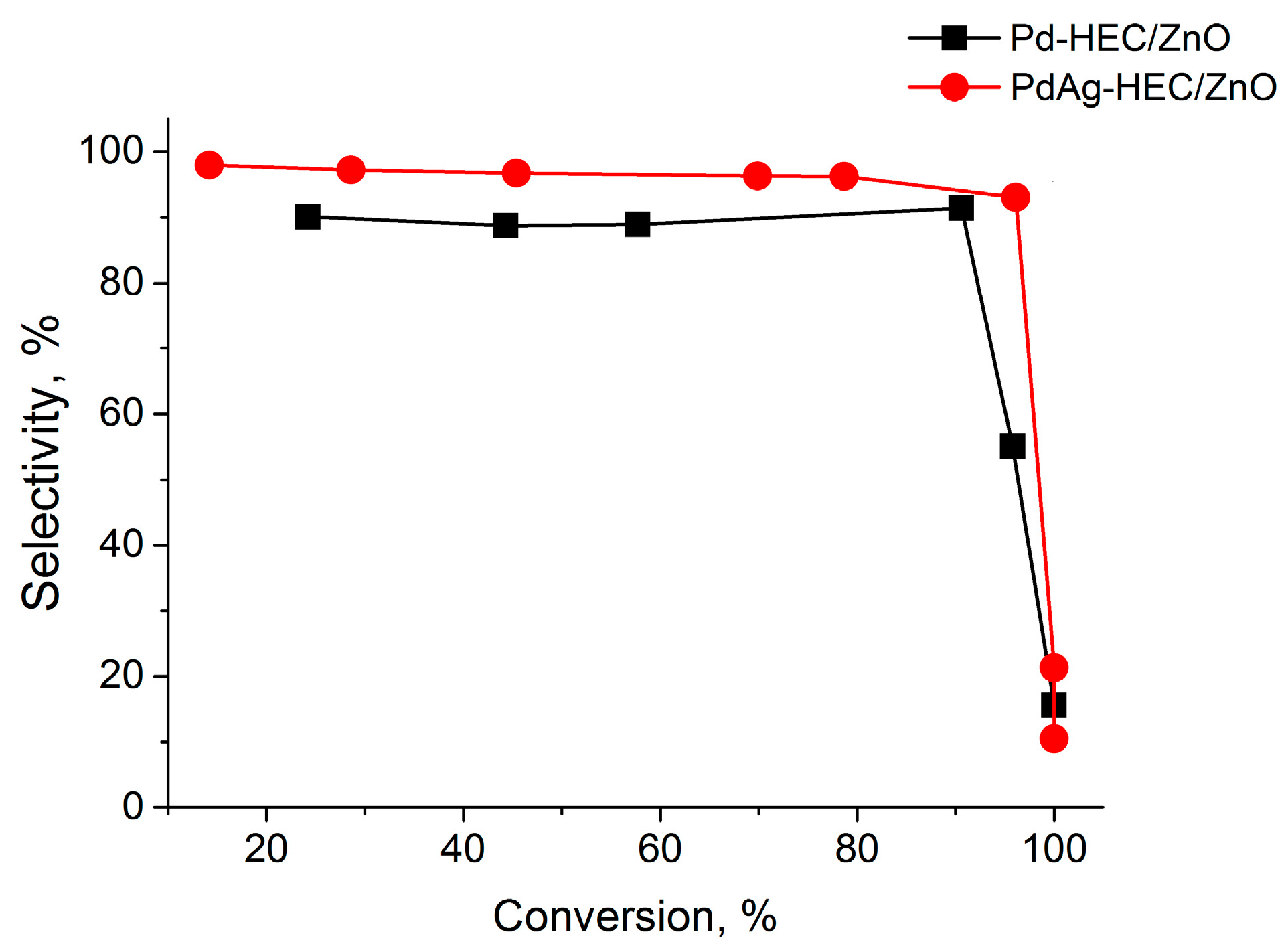
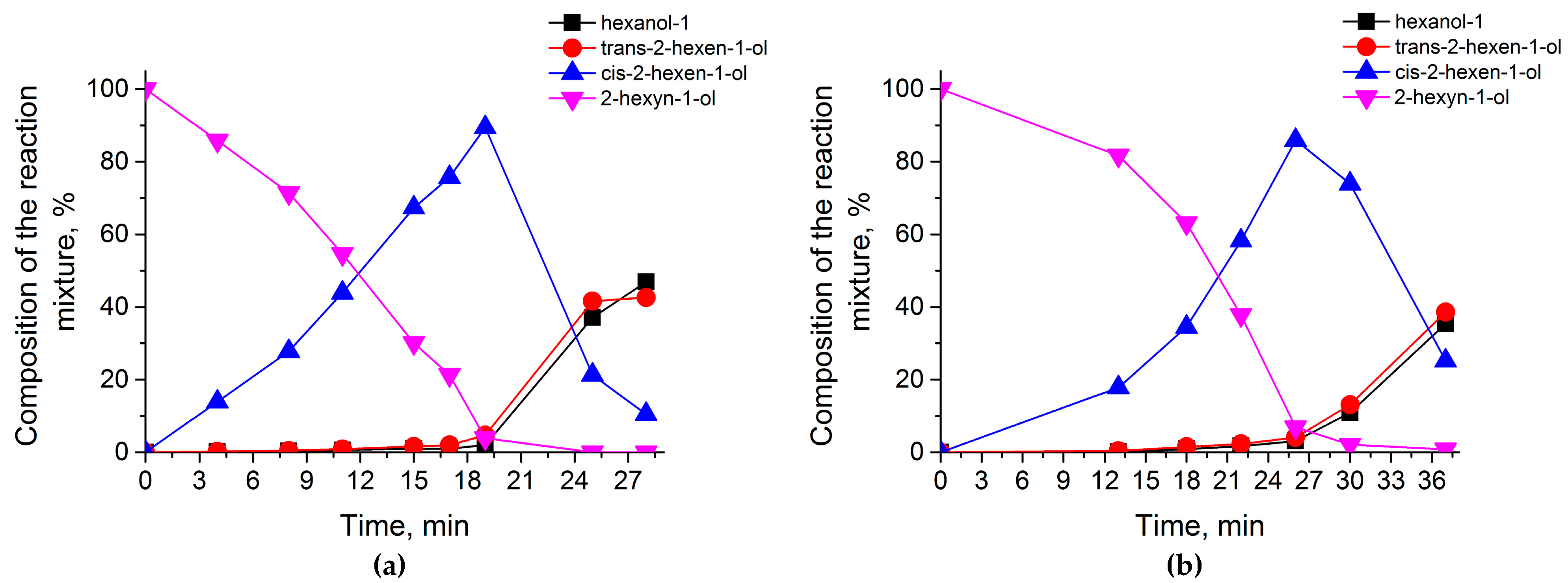
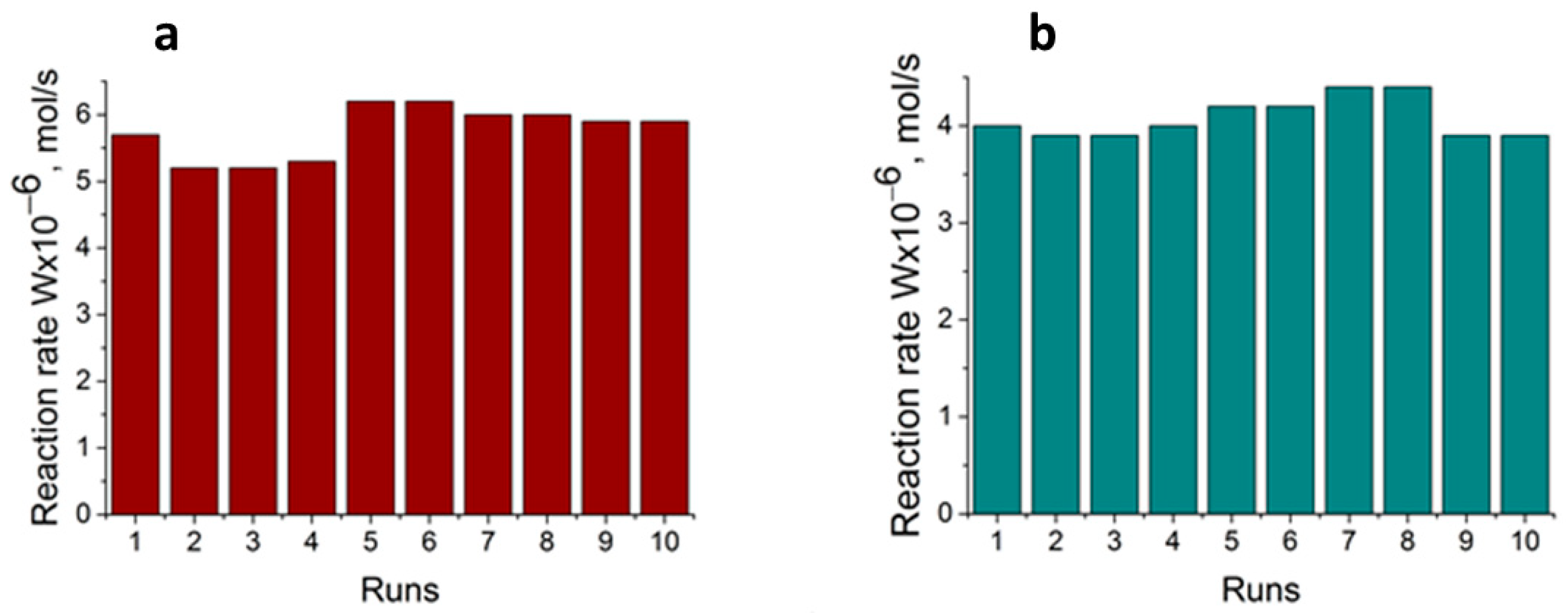
2.2. Catalytic Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of K2PdCl4 Precursor Solution
3.3. Synthesis of Pd-Polysaccharide/ZnO Catalysts
3.4. Synthesis of PdAg-Polysaccharide/ZnO Catalysts
3.5. Characterization of Catalysts
3.6. Hydrogenation of 2-Hexyn-1-ol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Li, Y.; Li, W.; Pei, X.; Ye, D. Chitosan derived efficient and stable Pd nano-catalyst for high efficiency hydrogenation. Int. J. Biol. Macromol. 2023, 241, 124615. [Google Scholar] [CrossRef] [PubMed]
- Baran, T. Pd0 nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydr. Polym. 2018, 195, 45–52. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S.; Soleimani, F. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano)catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Z.; Chen, J. Applications of lignin-derived catalysts for green synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Mahlambi, P.N.; Moloto, M.J. Starch-capped silver oxide (Ag2O) nanoparticles: Synthesis, characterization and antibacterial activity. Dig. J. Nanomater. Biostructures 2022, 17, 921–930. [Google Scholar] [CrossRef]
- de Almeida, D.A.; Sabino, R.M.; Souza, P.R.; Bonafé, E.G.; Venter, S.A.; Popat, K.C.; Martins, A.F.; Monteiro, J.P. Pectin-capped gold nanoparticles synthesis in-situ for producing durable, cytocompatible, and superabsorbent hydrogel composites with chitosan. Int. J. Biol. Macromol. 2020, 147, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Molnár. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Khazaei, A.; Khazaei, M.; Rahmati, S. A green method for the synthesis of gelatin/pectin stabilized palladium nano-particles as efficient heterogeneous catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2015, 398, 241–247. [Google Scholar] [CrossRef]
- Singh, V.; Srivastava, P.; Singh, A.; Singh, D.; Malviya, T. Polysaccharide-Silica Hybrids: Design and Applications. Polym. Rev. 2016, 56, 113–136. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Preparation and characterization of polysaccharide-silica hybrid aerogels. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, M.A.; Kamal, K.H.; Ali, K.; Abdel-Aziz, M.; Kamel, S. Biodegradable grafting cellulose/clay composites for metal ions removal. Int. J. Biol. Macromol. 2018, 118, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Zauro, S.A.; Vishalakshi, B. Pectin graft copolymer-montmorillonite composite: Synthesis, swelling and divalent metal ion adsorption. Sep. Sci. Technol. 2018, 53, 2170–2185. [Google Scholar] [CrossRef]
- Nesic, A.; Meseldzija, S.; Cabrera-Barjas, G.; Onjia, A. Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods 2022, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Talgatov, E.T.; Auezkhanova, A.S.; Kapysheva, U.N.; Bakhtiyrova, S.K.; Zharmagambetova, A.K. Synthesis and Detoxifying Properties of Pectin-Montmorillonite Composite. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1387–1391. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Bok-Badura, J.; Jakóbik-Kolon, A.; Karoń, K.; Mitko, K. Sorption studies of heavy metal ions on pectin-nano-titanium dioxide composite adsorbent. Sep. Sci. Technol. 2017, 53, 1034–1044. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.-L.; Ding, T.; Song, Y.-J.; Li, H.-C.; Li, D.-Q.; Chen, S.; Xu, F. The role of surface functional groups of pectin and pectin-based materials on the adsorption of heavy metal ions and dyes. Carbohydr. Polym. 2021, 276, 118789. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, N.; Singh, R.K.; Gupta, P.; Singh, R.; Jain, S.L. Dual catalysis with magnetic chitosan: Direct synthesis of cyclic carbonates from olefins with carbon dioxide using isobutyraldehyde as the sacrificial reductant. Dalton Trans. 2015, 44, 11860–11866. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- Singhal, S.; Hulle, N.R.S. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems–A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Bonnin, E.; Garnier, C.; Ralet, M.-C. Pectin-modifying enzymes and pectin-derived materials: Applications and impacts. Appl. Microbiol. Biotechnol. 2013, 98, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Zhang, S.; Zhu, D.; Meng, D.; Ming, S.; Qin, K.; Liu, Q.; Guo, L.; Li, T. Palladium supported on N-Heterocyclic carbene functionalized hydroxyethyl cellulose as a novel and efficient catalyst for the Suzuki reaction in aqueous media. Appl. Surf. Sci. 2020, 531, 147392. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Siengchin, S. Single-Polymer Composites: Concepts, Realization and Outlook: Review. KMUTNB Int. J. Appl. Sci. Technol. 2014, 7, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ahmed, H.B.; Attia, M.A.; El-Dars, F.M.; Emam, H.E. Hydroxyethyl cellulose for spontaneous synthesis of antipathogenic nanostructures: (Ag & Au) nanoparticles versus Ag-Au nano-alloy. Int. J. Biol. Macromol. 2019, 128, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, W.; Zhao, J.; Qiu, X.; Xiong, H.; Liang, Y.; Ye, X.; Lei, Z.; Chen, D. Preparation and anti-leakage properties of hydroxyethyl cellulose-g-poly (butyl acrylate-co-vinyl acetate) emulsion. Carbohydr. Polym. 2020, 255, 117467. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Song, M.; Zhang, Y.; Shi, L.-Y.; Lv, Y.; Ran, R. Enzymic degradation of hydroxyethyl cellulose and analysis of the substitution pattern along the polysaccharide chain. Carbohydr. Polym. 2017, 169, 92–100. [Google Scholar] [CrossRef]
- Abbas, K.; Amin, M.; Hussain, M.A.; Sher, M.; Bukhari, S.N.A.; Jantan, I.; Edgar, K.J. Designing novel bioconjugates of hydroxyethyl cellulose and salicylates for potential pharmaceutical and pharmacological applications. Int. J. Biol. Macromol. 2017, 103, 441–450. [Google Scholar] [CrossRef]
- Alam, N.; Christopher, L.P. A novel, cost-effective and eco-friendly method for preparation of textile fibers from cellulosic pulps. Carbohydr. Polym. 2017, 173, 253–258. [Google Scholar] [CrossRef]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Tang, S.; Li, L.; Cao, X.; Yang, Q. Ni -chitosan/carbon nanotube: An efficient biopolymer -inorganic catalyst for selective hydrogenation of acetylene. Heliyon 2023, 9, e135232023. [Google Scholar] [CrossRef] [PubMed]
- Zharmagambetova, A.K.; Auyezkhanova, A.S.; Talgatov, E.T.; Jumekeyeva, A.I. Chitosan-Modified Palladium Catalysts in Hydrogenation of n-Hex-2-Yne. Theor. Exp. Chem. 2021, 57, 371–376. [Google Scholar] [CrossRef]
- Dohendou, M.; Pakzad, K.; Nezafat, Z.; Nasrollahzadeh, M.; Dekamin, M.G. Progresses in chitin, chitosan, starch, cellulose, pectin, alginate, gelatin and gum based (nano)catalysts for the Heck coupling reactions: A review. Int. J. Biol. Macromol. 2021, 192, 771–819. [Google Scholar] [CrossRef] [PubMed]
- Nikoshvili, L.Z.; Tikhonov, B.B.; Ivanov, P.E.; Stadolnikova, P.Y.; Sulman, M.G.; Matveeva, V.G. Recent Progress in Chitosan-Containing Composite Materials for Sustainable Approaches to Adsorption and Catalysis. Catalysts 2023, 13, 367. [Google Scholar] [CrossRef]
- Lazar, M.M.; Dinu, I.A.; Silion, M.; Dragan, E.S.; Dinu, M.V. Could the porous chitosan-based composite materials have a chance to a “NEW LIFE” after Cu(II) ion binding? Int. J. Biol. Macromol. 2019, 131, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Diogo, G.M.; Moro, P.A.; Costin, T.A.; Fantinel, M.; Sá, M.M. Chitosan as a sustainable heterogeneous catalyst for the preparation of functionalized α-diazo carbonyl compounds. Tetrahedron Green Chem 2023, 1, 100006. [Google Scholar] [CrossRef]
- Zheng, K.; Yang, F.; Huang, Z.; Zhan, Y.; Xiao, Z.; Li, W.; Wang, W.; Qin, C. Preparation of chitosan film-loaded palladium catalyst materials and their application in Suzuki coupling reactions. J. Mater. Res. Technol. 2022, 20, 3905–3917. [Google Scholar] [CrossRef]
- Çalışkan, M.; Baran, T. Design of nanostructured palladium catalyst supported by chitosan/Co3O4 microspheres and investigation of its catalytic behavior against synthesis of benzonitriles. Int. J. Biol. Macromol. 2021, 182, 722–729. [Google Scholar] [CrossRef]
- Dolinska, J.; Holdynski, M.; Pieta, P.; Lisowski, W.; Ratajczyk, T.; Palys, B.; Jablonska, A.; Opallo, M. Noble Metal Nanoparticles in Pectin Matrix. Preparation, Film Formation, Property Analysis, and Application in Electrocatalysis. ACS Omega 2020, 5, 23909–23918. [Google Scholar] [CrossRef]
- Le, V.D.; Le, T.C.H.; Chau, V.T.; Le, T.N.D.; Dang, C.H.; Vo, T.T.N.; Nguyen, T.D.; Nguyen, T.D. Palladium nanoparticles in situ synthesized on Cyclea barbata pectin as a heterogeneous catalyst for Heck coupling in water, the reduction of nitrophenols and alkynes. New J. Chem. 2021, 45, 4746–4755. [Google Scholar] [CrossRef]
- Carrera, S.A.; Villarreal, J.S.; Acosta, P.I.; Noboa, J.F.; Gallo-Cordova, A.; Mora, J.R. Designing an efficient and recoverable magnetic nanocatalyst based on Ca, Fe and pectin for biodiesel production. Fuel 2021, 310, 122456. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Seitkalieva, K.S.; Talgatov, E.T.; Auezkhanova, A.S.; Dzhardimalieva, G.I.; Pomogailo, A.D. Polymer-modified supported palladium catalysts for the hydrogenation of acetylene compounds. Kinet. Catal. 2016, 57, 360–367. [Google Scholar] [CrossRef]
- Khazaei, A.; Rahmati, S.; Hekmatian, Z.; Saeednia, S. A green approach for the synthesis of palladium nanoparticles supported on pectin: Application as a catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2013, 372, 160–166. [Google Scholar] [CrossRef]
- Nazeri, M.T.; Javanbakht, S.; Nabi, M.; Shaabani, A. Copper phthalocyanine-conjugated pectin via the Ugi four-component reaction: An efficient catalyst for CO2 fixation. Carbohydr. Polym. 2022, 283, 119144. [Google Scholar] [CrossRef] [PubMed]
- Acosta, P.I.; Campedelli, R.R.; Correa, E.L.; Bazani, H.A.; Nishida, E.N.; Souza, B.S.; Mora, J.R. Efficient production of biodiesel by using a highly active calcium oxide prepared in presence of pectin as heterogeneous catalyst. Fuel 2020, 271, 117651. [Google Scholar] [CrossRef]
- Li, S.; He, G.; Huang, J. Self-assembly on natural cellulose: Towards high-efficient catalysts. Curr. Opin. Colloid Interface Sci. 2023, 63, 101655. [Google Scholar] [CrossRef]
- El Idrissi, N.; Belachemi, L.; Merle, N.; Zinck, P.; Kaddami, H. Comprehensive preparation and catalytic activities of Co/TEMPO-cellulose nanocomposites: A promising green catalyst. Carbohydr. Polym. 2022, 295, 119765. [Google Scholar] [CrossRef]
- Prekob, Á.; Hajdu, V.; Muránszky, G.; Fiser, B.; Sycheva, A.; Ferenczi, T.; Viskolcz, B.; Vanyorek, L. Application of carbonized cellulose-based catalyst in nitrobenzene hydrogenation. Mater. Today Chem. 2020, 17, 100337. [Google Scholar] [CrossRef]
- Kaushik, M.; Li, A.Y.; Hudson, R.; Masnadi, M.; Li, C.-J.; Moores, A. Reversing aggregation: Direct synthesis of nanocatalysts from bulk metal. Cellulose nanocrystals as active support to access efficient hydrogenation silver nanocatalysts. Green Chem. 2015, 18, 129–133. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Usmanova, M.; Auyezkhanova, A.; Akhmetova, S.; Talgatov, E.; Tumabayev, N.; Dyusenalin, B. Synthesis and catalytic properties of composites with Pd-(2-hydroxyethyl cellulose) on bentonite. Ser. Chem. Technol. 2019, 5, 22–29. [Google Scholar] [CrossRef]
- Xie, C.; Qu, L.; Yu, H.; Yu, F.; Yuan, B.; Yu, S.; Nie, S. Synthesis of Ru nanoparticles with hydroxyethyl cellulose as stabilizer for high-efficiency reduction of α-pinene. Cellulose 2019, 26, 8059–8071. [Google Scholar] [CrossRef]
- Chen, B.; Dingerdissen, U.; Krauter, J.; Rotgerink, H.L.; Möbus, K.; Ostgard, D.; Panster, P.; Riermeier, T.; Seebald, S.; Tacke, T.; et al. New developments in hydrogenation catalysis particularly in synthesis of fine and intermediate chemicals. Appl. Catal. A Gen. 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Xu, J.; Guo, X.; Guan, Y.; Wu, P. Influence of Pd deposition pH value on the performance of Pd-CuO/SiO2 catalyst for semi-hydrogenation of 2-methyl-3-butyn-2-ol (MBY). Chin. Chem. Lett. 2021, 33, 349–353. [Google Scholar] [CrossRef]
- Vilé, G.; Albani, D.; Almora-Barrios, N.; López, N.; Pérez-Ramírez, J. Advances in the Design of Nanostructured Catalysts for Selective Hydrogenation. ChemCatChem 2015, 8, 21–33. [Google Scholar] [CrossRef]
- Li, X.-T.; Chen, L.; Shang, C.; Liu, Z.-P. In Situ Surface Structures of PdAg Catalyst and Their Influence on Acetylene Semihydrogenation Revealed by Machine Learning and Experiment. J. Am. Chem. Soc. 2021, 143, 6281–6292. [Google Scholar] [CrossRef] [PubMed]
- Mashkovsky, I.S.; Baeva, G.N.; Stakheev, A.Y.; Vargaftik, M.N.; Kozitsyna, N.Y.; Moiseev, I.I. Novel Pd–Zn/C catalyst for selective alkyne hydrogenation: Evidence for the formation of Pd–Zn bimetallic alloy particles. Mendeleev Commun. 2014, 24, 355–357. [Google Scholar] [CrossRef]
- Okamoto, M.; Hirao, T.; Yamaai, T. Polymers as novel modifiers for supported metal catalyst in hydrogenation of benzaldehydes. J. Catal. 2010, 276, 423–428. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2019, 120, 1350–1396. [Google Scholar] [CrossRef]
- Devi, P.G.; Velu, A.S. Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J. Theor. Appl. Phys. 2016, 10, 233–240. [Google Scholar] [CrossRef]
- Mukerabigwi, J.F.; Lei, S.; Fan, L.; Wang, H.; Luo, S.; Ma, X.; Qin, J.; Huang, X.; Cao, Y. Eco-friendly nano-hybrid superabsorbent composite from hydroxyethyl cellulose and diatomite. RSC Adv. 2016, 6, 31607–31618. [Google Scholar] [CrossRef]
- Reddy, G.K.; Peck, T.C.; Roberts, C.A. “PdO vs. PtO”—The Influence of PGM Oxide Promotion of Co3O4 Spinel on Direct NO Decomposition Activity. Catalysts 2019, 9, 62. [Google Scholar] [CrossRef]
- Talgatov, E.; Auyezkhanova, A.; Zharmagambetova, A.; Tastanova, L.; Bukharbayeva, F.; Jumekeyeva, A.; Aubakirov, T. The Effect of Polymer Matrix on the Catalytic Properties of Supported Palladium Catalysts in the Hydrogenation of Alkynols. Catalysts 2023, 13, 741. [Google Scholar] [CrossRef]
- Bajaber, M.A.; Anjum, M.N.; Ibrahim, M.; Farooq, T.; Ahmad, M.N.; Abideen, Z.U. Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes. Molecules 2022, 27, 8238. [Google Scholar] [CrossRef]
- El Idrissi, A.; El Barkany, S.; Amhamdi, H.; Maaroufi, A.-K. Physicochemical characterization of celluloses extracted from Esparto “Stipa tenacissima” of Eastern Morocco. J. Appl. Polym. Sci. 2012, 128, 537–548. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Auyezkhanova, A.; Talgatov, E.; Jumekeyeva, A.; Buharbayeva, F.; Akhmetova, S.; Myltykbayeva, Z.; Nieto, J.M.L. Synthesis of polymer protected Pd–Ag/ZnO catalysts for phenylacetylene hydrogenation. J. Nanoparticle Res. 2022, 24, 1–17. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts 2020, 10, 74. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Wang, X.-B.; Li, W.-Y.; Liang, C. Intermetallic PdZn nanoparticles catalyze the continuous-flow hydrogenation of alkynols to cis-enols. Commun. Chem. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-Ablated ZnO Nanoparticles and Their Photocatalytic Activity toward Organic Pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef]
- Pauly, N.; Yubero, F.; Espinós, J.P.; Tougaard, S. XPS primary excitation spectra of Zn 2p, Fe 2p, and Ce 3d from ZnO, α-Fe2O3, and CeO2. Surf. Interface Anal. 2018, 51, 353–360. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy. In Perkin-Elmer Corporation: Eden Prairie; Chastain, J., Ed.; erkin-Elmer Corporation, Physical Electronics Division: den Prairie, MN, USA, 1992; pp. 1–261. [Google Scholar]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, Z.; Zhu, J.; Chen, S.; Yuan, X.; Liu, L. Graphene nanosheets decorated with ZnO nanoparticles: Facile synthesis and promising application for enhancing the mechanical and gas barrier properties of rubber nanocomposites. RSC Adv. 2015, 5, 57771–57780. [Google Scholar] [CrossRef]
- Bonrath, W.; Medlock, J.; Schütz, J.; Wustenberg, B.; Netscher, T. Hydrogenation in the Vitamins and Fine Chemicals Industry—An Overview. In Hydrogenation; Soto-Hernandez, M., Palma-Tenango, M., del Rosario, M., Eds.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- JCPDS-International centre for diffraction data. Powder Diffraction File: Alphabetical Index, Inorganic Phases; ICDD: Newtown Square, PA, USA, 1986. [Google Scholar]
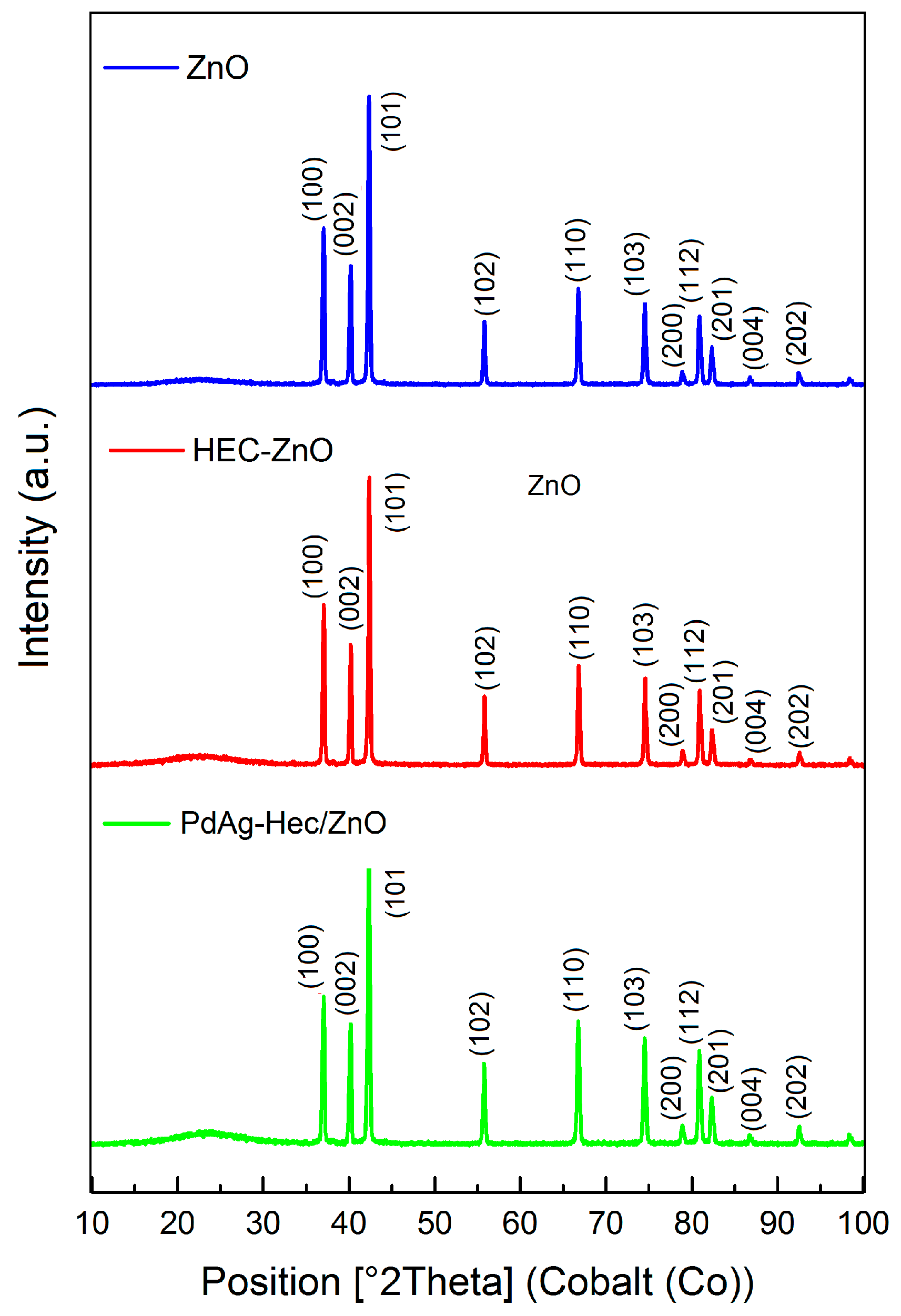
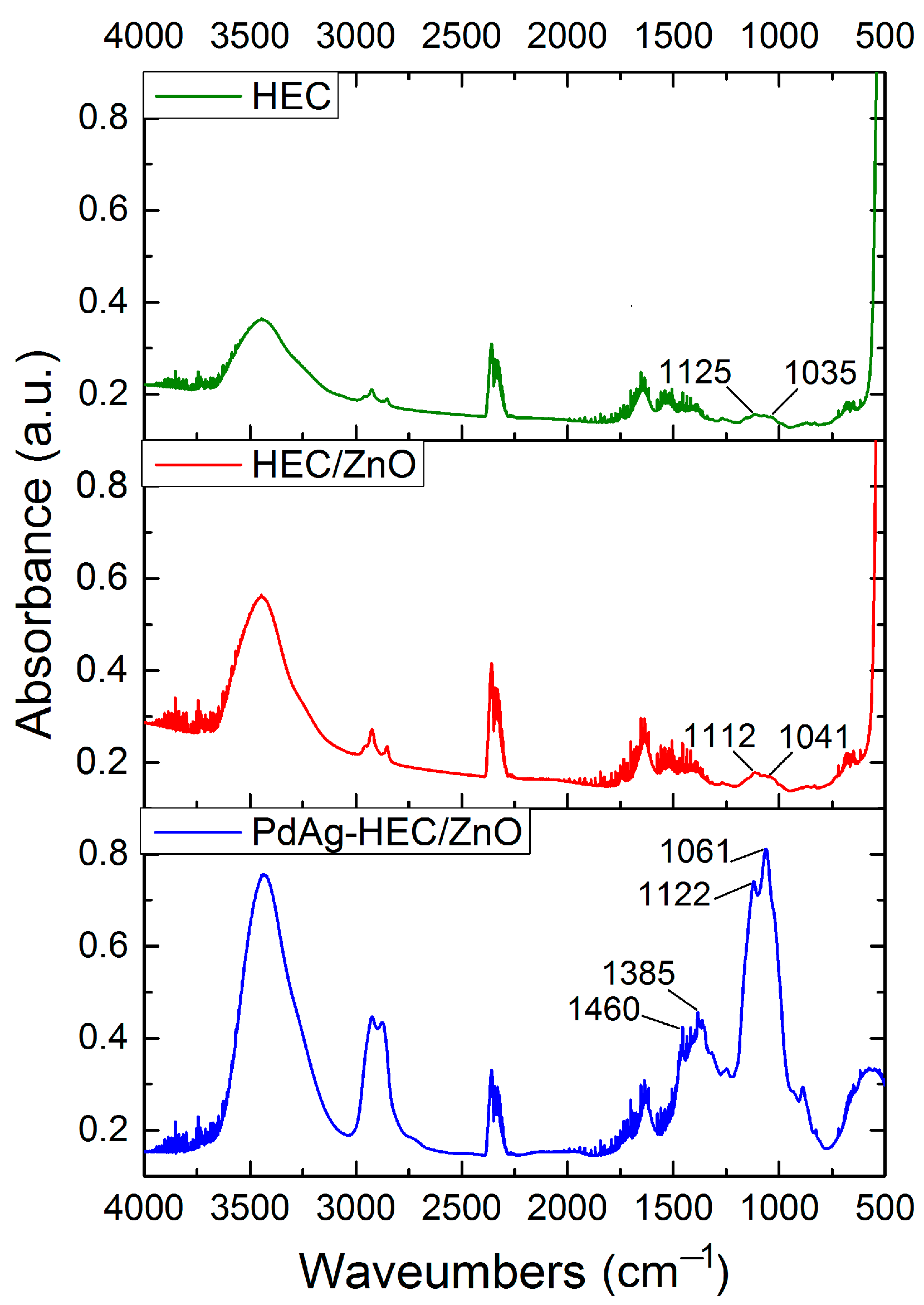
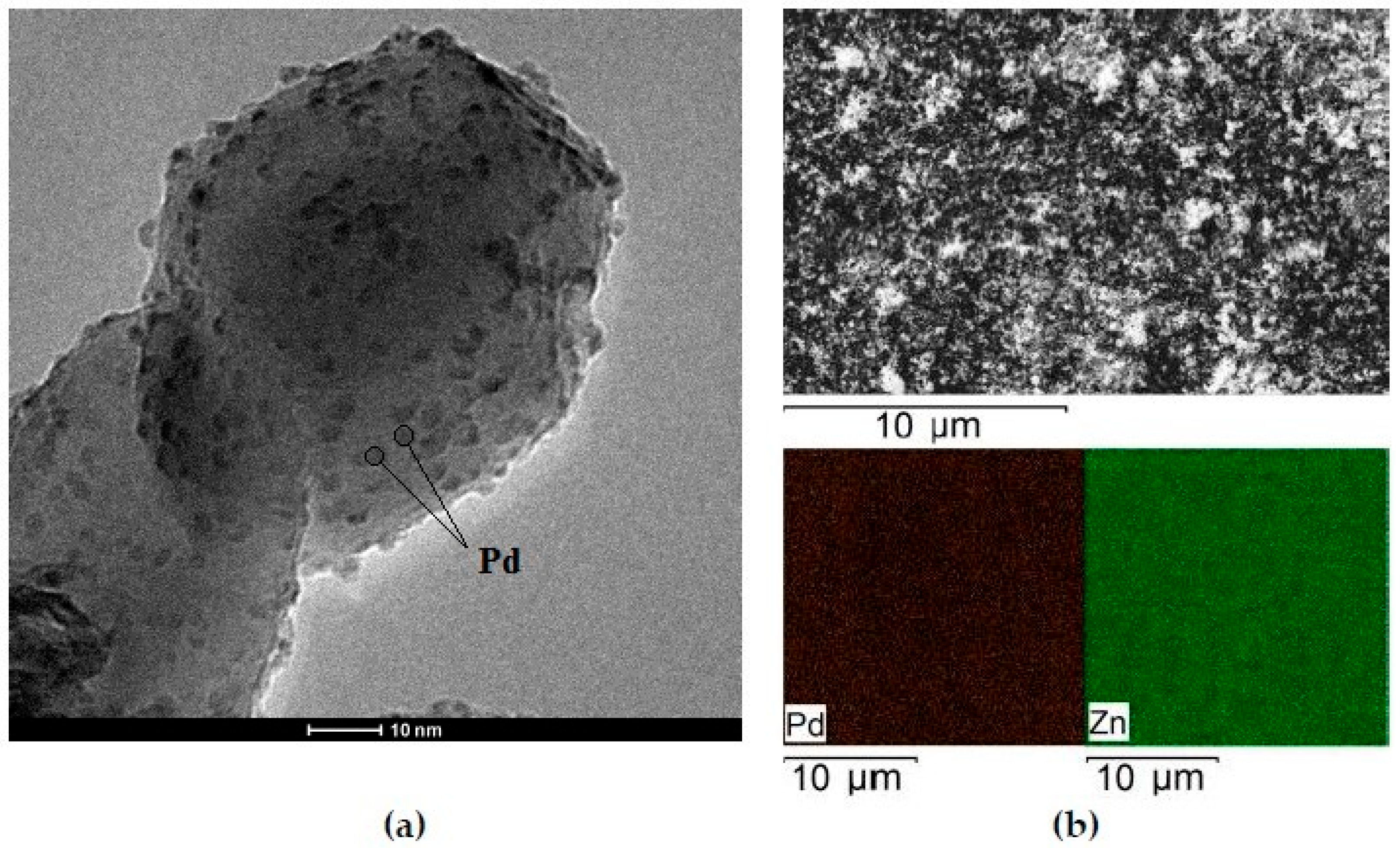
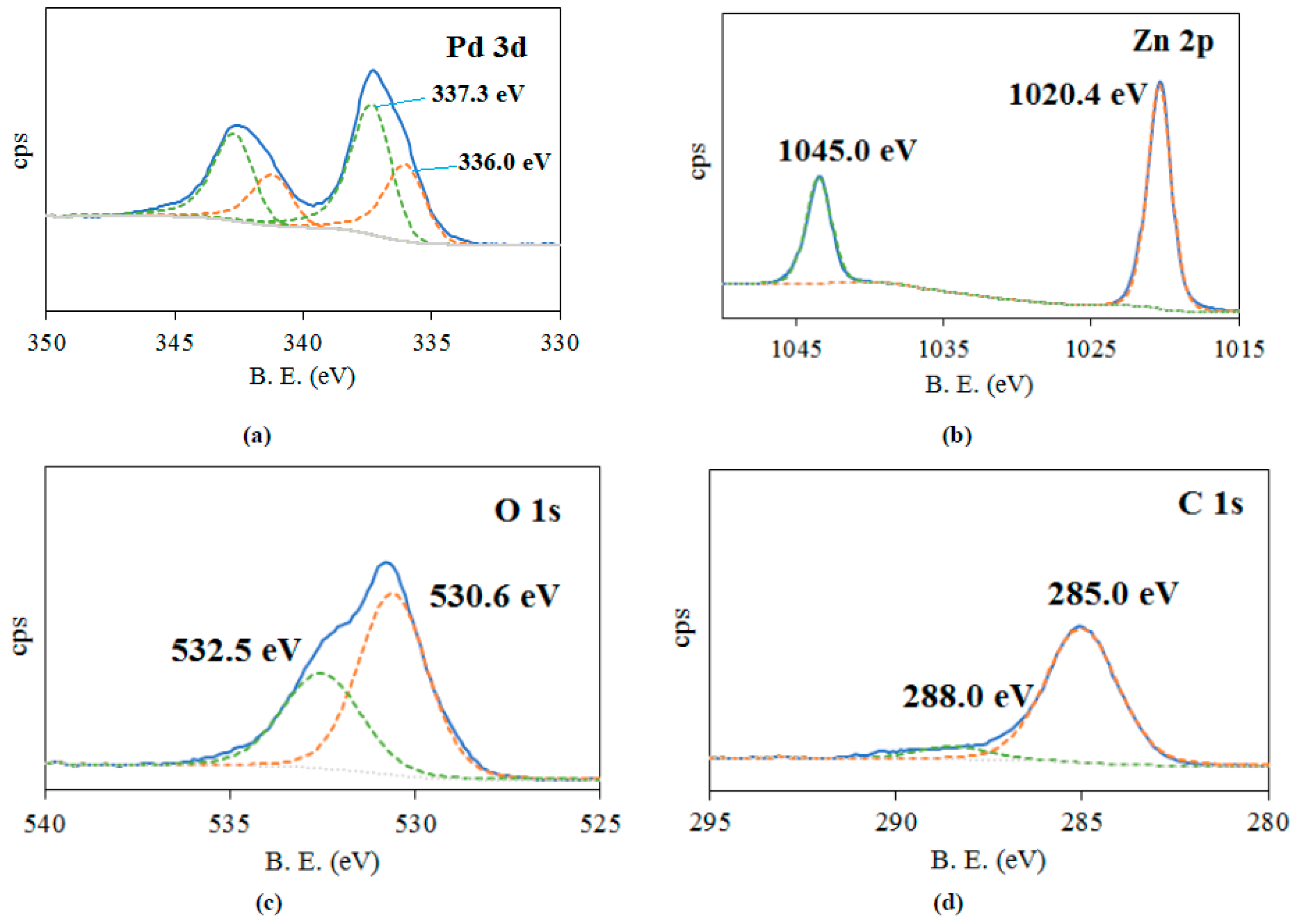

| Sample | Elemental Composition of the Catalyst, wt% | ||
|---|---|---|---|
| Pdcalcd/detd | Agcalcd/detd | Zncalcd/detd | |
| Pd-HEC/ZnO | 0.50/0.49 | - | 79.0/77.8 |
| Pd-Chit/ZnO | 0.50/0.47 | - | 79.0/81.1 |
| Pd-Pec/ZnO | 0.50/0.57 | - | 79.0/80.5 |
| PdAg-HEC/ZnO | 0.37/0.36 | 0.13/0.18 | 79.0/81.5 |
| PdAg-Chit/ZnO | 0.37/0.44 | 0.13/0.15 | 79.0/81.6 |
| PdAg-Pec/ZnO | 0.37/0.48 | 0.13/0.16 | 79.0/82.0 |
| Sample | Surface Area, m2 g−1 |
|---|---|
| ZnO | 8.7 |
| HEC/ZnO | 1.1 |
| PdAg-HEC/ZnO | 5.2 |
| Pd-HEC/ZnO | 4.9 |
| Catalysts | Wmax × 10−6 (mol s−1) | Maximum Yield of cis-Hexen-1-ol, % | Scis-hexen-1-ol,% | Conversion, % |
|---|---|---|---|---|
| Pd-HEC/ZnO | 4.3 | 82.8 | 90.6 | 91.4 |
| Pd-Chit/ZnO | 3.1 | 80.1 | 86.3 | 92.8 |
| Pd-Pec/ZnO | 5.4 | 80.9 | 86.1 | 94.0 |
| PdAg-HEC/ZnO | 4.0 | 89.4 | 97.2 | 93.0 |
| PdAg-Chit/ZnO | 2.6 | 85.9 | 92.3 | 93.1 |
| PdAg-Pec/ZnO | 2.8 | 86.4 | 93.5 | 92.4 |
| Catalysts | Temperature, °C | Wmax·× 10−6 (mol s−1) | Maximum Yield of cis-Hexen-1-ol, % | Scis-hexen-1-ol, % |
|---|---|---|---|---|
| PdAg-Chit/ZnO | 20 | 0.6 | 84.0 | 91.8 |
| 30 | 2.3 | 76.4 | 93.5 | |
| 40 | 2.6 | 85.9 | 92.3 | |
| 50 | 2.3 | 79.3 | 79.8 | |
| PdAg-HEC/ZnO | 20 | 0.4 | 77.0 | 93.0 |
| 30 | 1.3 | 75.6 | 84.9 | |
| 40 | 4.0 | 89.4 | 97.2 | |
| 50 | 1.6 | 76.2 | 86.7 | |
| PdAg-Pec/ZnO | 20 | 0.4 | 78.0 | 91.6 |
| 30 | 1.9 | 79.6 | 85.6 | |
| 40 | 2.8 | 86.4 | 93.5 | |
| 50 | 1.8 | 78.2 | 87.0 |
| Catalysts | Catalyst Loading, g | Wmax·× 10−6 (mol s−1) | Maximum Yield of cis-Hexen-1-ol, % | Scis-hexen-1-ol, % |
|---|---|---|---|---|
| PdAg-Chit/ZnO | 0.01 | 0.6 | 62.0 | 67.8 |
| 0.03 | 1.9 | 71.8 | 84.7 | |
| 0.05 | 2.6 | 85.9 | 92.3 | |
| 0.10 | 2.8 | 83.5 | 89.9 | |
| PdAg-HEC/ZnO | 0.01 | 0.5 | 58.4 | 63.2 |
| 0.03 | 2.5 | 88.2 | 89.8 | |
| 0.05 | 4.0 | 89.4 | 97.2 | |
| 0.10 | 4.4 | 87.6 | 94.5 | |
| PdAg-Pec/ZnO | 0.01 | 0.5 | 60.3 | 61.7 |
| 0.03 | 2.1 | 78.4 | 83.0 | |
| 0.05 | 2.8 | 86.4 | 93.5 | |
| 0.10 | 3.1 | 84.6 | 90.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Bukharbayeva, F.U.; Jumekeyeva, A.I. Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts 2023, 13, 1403. https://doi.org/10.3390/catal13111403
Zharmagambetova AK, Talgatov ET, Auyezkhanova AS, Bukharbayeva FU, Jumekeyeva AI. Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts. 2023; 13(11):1403. https://doi.org/10.3390/catal13111403
Chicago/Turabian StyleZharmagambetova, Alima K., Eldar T. Talgatov, Assemgul S. Auyezkhanova, Farida U. Bukharbayeva, and Aigul I. Jumekeyeva. 2023. "Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol" Catalysts 13, no. 11: 1403. https://doi.org/10.3390/catal13111403
APA StyleZharmagambetova, A. K., Talgatov, E. T., Auyezkhanova, A. S., Bukharbayeva, F. U., & Jumekeyeva, A. I. (2023). Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts, 13(11), 1403. https://doi.org/10.3390/catal13111403








