Abstract
This study involved the fabrication of a set of aluminum ion-grafted SBA-15 utilizing ethylenediamine and trimethylamine ionic liquids. The primary objective was to examine the impact of the fabrication environment on the physicochemical characteristics of the catalysts. Comprehensive characterization of the Al-SBA-15 catalysts was conducted using various techniques, including XRD, FTIR, surface area, pyridine FTIR, 27Al-NMR, TGA, HRTEM, and FESEM, to analyze their physicochemical characteristics. Furthermore, the acidic characteristics were examined by conducting potentiometric titration in a nonaqueous solvent and employing FTIR spectroscopy to analyze the chemisorbed pyridine. The effectiveness of the fabricated acid materials was evaluated by testing their performance in acetic acid esterification with butanol. The findings obtained reveal that mesostructured SBA-15 remains intact following the successful inclusion of Al3+ ions into the silica frameworks. Additionally, a remarkable enhancement in the existence of both Bronsted and Lewis acid centers was noted due to the grafting process of Al3+ ions. At temperatures of 80 °C and 100 °C, the reaction in Al-SBA-15(T-120) proceeds swiftly, reaching approximately 32% and 38% conversion, respectively, within a span of 110 min. The excellent catalytic performance observed in the esterification reaction can be attributed to two factors: the homogeneous distribution of Al3+ ions within the SBA-15 frameworks and the acidic character of Al-SBA-15. The findings further indicate that the grafting process for incorporating Al3+ ions into the silica matrix is more efficient.
1. Introduction
The synthesis of mesoporous structures with large surface areas, homogeneous size distribution of pores, and excellent chemical durability is currently a highly researched and relevant area, particularly due to its potential applications in metal ion separation, adsorption, and sensors [1]. Apart from these uses, mesoporous substances are viewed as a possibility for catalysis [2,3]. Acid-catalyzed processes find extensive industrial applications, notably in oil refining, petrochemical production, and pharmaceutical manufacturing. Zeolites have garnered widespread use as acid catalysts, especially across diverse industrial sectors [4]. However, their inherent microporous structure imposes limitations on their utility, rendering them unsuitable for the treatment of large organic molecules [5]. Consequently, there exists a pressing need to identify suitable alternatives to overcome this constraint. In 1998, Stucky et al. introduced a pioneering categorization system for mesoporous materials synthesized under acidic conditions employing a neutral model. The produced material was named SBA-15 [6]. SBA-15, with its hexagonal structure, represents a mesoporous, well-ordered silica material that exhibits remarkable attributes as a catalyst support [7]. This is attributed to its exceptionally large surface area and adjustable porosity, granting it superior capabilities in terms of mass transfer and effective dispersion during chemical processes, particularly accommodating reactants, intermediates, or products with dimensions exceeding those permissible in zeolite pore structures [8]. Additionally, the construction-directing agents employed in the synthesis of triblock polymers for creating SBA-15 are cost-effective, environmentally benign, and amenable to recycling [6]. However, since the pore walls of SBA-15 were discovered to be amorphous, it lacks comparable acidity and necessitates acid centers, unlike crystalline zeolite. In order to enhance the acid strength of SBA-15 for its implementation as a catalyst or support in acidic catalytic reactions, it is necessary to generate acid centers. Accordingly, for the creation of surface acidity, it is obligatory the inclusion of metallic ions, such as Al, in the silica framework. One of the main challenges is the requirement of a greatly acidic fabricated gel for the production of Al-SBA-15. In this particular case, the Al atoms exist as soluble components that do not remain within the SBA framework [9,10]. Aluminum may be added to SBA-15 via both post and direct artificial methods, according to the manufacturer. Aluminum oxides are generated within the pores and on the surface of materials fabricated through postsynthetic methods. The metal oxide blocks the pores moderately or totally, diminishing the surface characteristics. Despite the simplicity of direct synthesis, its effectiveness is consistently limited owing to the low grafting of the supplied foreign atoms into the mesoporous structure, with only a small fraction being integrated. The production of Al-SBA-15 can be challenging, as aluminum must be grafted into the neutral silica framework without blocking the pores of the material. Numerous approaches have been devised to address this obstacle, employing AlCl3 precursors in the fabrication of Al-SBA-15 [11,12,13,14]. Through the optimization of fabrication conditions, it becomes feasible to achieve high concentrations of Al3+ species and exceptional structural order in Al-SBA-15 materials.
In recent years, the exploration of ionic liquids as versatile solvents has garnered significant attention in the field of chemical engineering and catalysis. These unique compounds, composed of organic cations and organic or inorganic anions, exhibit a range of remarkable properties, including low volatility, high thermal stability, and tunable physicochemical characteristics. These attributes make ionic liquids an attractive alternative to conventional volatile organic solvents in various applications, particularly in green and sustainable processes. Among the plethora of available ionic liquids, we focus on ethylenediamine-based cations and trimethylamine-based cations due to their intriguing structural and electronic properties. Ethylenediamine, a bifunctional amine, presents an intriguing potential, and its synergistic interactions with aluminum in our proposed system are anticipated to afford superior synthetic and catalytic outcomes. On the other hand, trimethylamine, with its basicity, is poised to play a pivotal role in the preparation of Al-SBA-15. Hassan et al. [9] synthesized Al-SBA-15 under mildly acidic conditions, optimizing the incorporation of Al3+ using urea tetrachloroaluminate ionic liquid. Al-SBA-15 (U100) demonstrated high surface area (813 m2 g−1), acidity, and catalytic activity. The group later applied a similar approach to vary the nSi/nAl molar ratios [10]. Al-SBA-15 materials have gained significant attention and recognition in the field of heterogeneous catalysis due to their exceptional properties and versatility. Their high surface area, well-defined mesoporous structure, and tunable acidity make them ideal candidates for a wide range of catalytic implementations. One prominent application is in the realm of acid catalysis, where Al-SBA-15 has demonstrated remarkable performance in various reactions. Their strong Lewis acid sites, derived from incorporated aluminum species, facilitate the activation of reactant molecules, leading to enhanced catalytic activity and selectivity [15,16,17,18,19,20,21,22,23,24].
This research embarks on a captivating journey, delving into the intricate challenges and remarkable advancements within the realm of mesoporous materials synthesis. Our focus is firmly set on the creation of aluminum-infused silica, poised to play a pivotal role as a catalyst in acidic catalytic reactions. We are putting the spotlight on the art of seamlessly incorporating aluminum into the neutral matrix of SBA-15 using the innovative ionic liquid method. This study underscores the profound importance of crafting mesoporous materials characterized by expansive surface areas, uniform pore dimensions, and unyielding chemical resilience. Moreover, it unveils the boundless potential that lies in harnessing these materials for catalytic marvels.
2. Results and Discussion
2.1. Characterization
The fabrication of mesoporous SBA-15 was achieved through hydrothermal methods under conditions of high acidity. The grafting of a significant quantity of aluminum ions into the SBA-15 structure under such conditions proved to be challenging. This difficulty could be because of the elevated level of Al precursor solubility in an acidic environment, which limited its availability in its corresponding oxo species. As a result, the condensation process between the silicon species and Al atoms, which would have facilitated the grafting of Al into the protonated mesoporous walls, was impeded by the strong repulsion forces between cationic silica and the positively charged Al-oxo components at smaller pH. Nonetheless, our prior investigation attempted to optimize the appropriate ratio of nH2O:nHCl. Based on our research, we discovered that when the molar ratio reaches 276, the pH level rises to approximately 2.4, surpassing the silica isoelectric point. As a result, the silica is negatively charged, enabling it to interact with the Al-oxo component, specifically Al(OH)2+. As a result of these findings, we determined that a molar ratio of 276 is the ideal proportion for preparing Al-SBA-15, as it enhances the quantity of Al3+ ions grafted into the SBA-15 walls. Table 1 lists the results of an elemental assessment conducted on the fabricated Al-SBA-15 using ICP in the presence of either ethylenediamine or triethyl amine ionic liquids, with a H2O:HCl molar ratio of 276. The element assessment showed that the Si/Al ratio varied depending on the kind of ionic liquid and the initial gel temperature. These findings suggest that using ethylenediamine or triethyl amine as ionic liquids in the presence of preformed Al–O–Si bonds is an effective method for preparing high Al3+ content Al-SBA-15 under acidic conditions at 120 °C. However, the ICP data suggest that a higher amount of Al3+ is present in the materials prepared using triethyl amine ionic liquid.

Table 1.
Texture properties of pure SBA-15 and Al-SBA-15 materials prepared using ethylenediamine (E) and triethyl amine (T) at various temperatures.
Figure 1 presents the FTIR spectra of pristine SBA-15 and different Al-SBA-15 structures that were hydrothermally treated at different temperatures (100, 120, and 140 °C) and combined with various ionic liquids. Figure 1a displays the FTIR spectrum of prepared SBA-15. The SBA-15 sample shows characteristic Si–O–Si stretching bands at 803 and 1080 cm–1. Other notable features include Si–O–Si bending mode at 473 cm−1, surface Si–OH asymmetry mode at 968 cm−1, and Si–OH bending modes at 1635 cm−1. A broad absorption band at 3446 cm−1 indicates stretching vibrations of surface OH moieties. Furthermore, for different Al-SBA-15 structures, as depicted in Figure 1b, the band at approximately 455 cm−1 is attributed to the vibrational bending of either Al–O–Si or Si–O–Si bonds. The shoulder observed at around 554 cm−1 and the peak at approximately 810 cm−1 correspond to the symmetric stretching vibrations of the Si–O–Si moieties. The presence of a band at approximately 954 cm−1 indicates the existence of defective Si–OH moieties, affirming the successful integration of Al3+ ions into the SBA-15 frameworks [25]. The absorption peak at 1600 cm−1 is associated with water adsorption. Additionally, all samples exhibit a characteristic broad peak spanning from 3100 to 3750 cm−1 associated with the excitation of H-bonded (SiO–H) moieties [26]. This feature is more pronounced in the samples treated hydrothermally at 120 °C using either ethylenediamine or triethylamine. This may be attributed to the presence of hydroxyls in Al3+ ionic species that exist in certain extramatrix regions of silica. Moreover, a subtle absorption is observed at approximately 3650 cm−1 in both Al-SBA-15 (E-120) and Al-SBA-15 (T-120), which can be attributed to the stretching vibration of OH groups in zeolite-type Si–OH–Al species present in the mesoporous aluminosilicate. The increased concentration of Si–O–Al in the Al-SBA-15 (E-120) and Al-SBA-15 (T-120) samples can be attributed to the preparation conditions, which involve the hydrothermal process at an acidic pH. This acidic environment promotes the condensation of silanol moieties, resulting in a higher presence of Si–O–Al bonds in the resulting materials. Furthermore, in order to determine differences in the broad hydroxyl peak (OH st), the FTIR peak was deconvoluted (Figure 1c). The samples showed three distinct deconvolution peaks around 3608, 3485, and 3376 cm−1 [27], corresponding to the strongly acidic bridging hydroxyl groups (Brønsted acid), Si–OH groups, and adsorbed water, respectively. Samples poor in aluminum are often accompanied by an increase in Si–OH groups. All Al-SBA-15 samples showed distinct bands at 3608 cm and 3485 cm−1, and the infrared absorbance at 3608 cm−1 was higher for samples T-120 and E-120 than samples T-140 and E-140, and all the previous samples had absorbance higher than the basic sample (Al-SBA-15, as depicted in Figure 1b). Therefore, it can be concluded that the Brønsted acidity of samples T-120 and E-120 has a higher acid surface and fewer defects for Si–OH groups than the other samples. Sample T-120 and E-120 showed two strong bands at ~3608 cm−1 and 3485 cm−1, attributed to their large external surface area and high silica–alumina content (Table 2). The sample of T-140 showed similar features; However, the peaks were weaker than those shown by sample 120 due to its smaller external surface area and lower silica–alumina ratio.
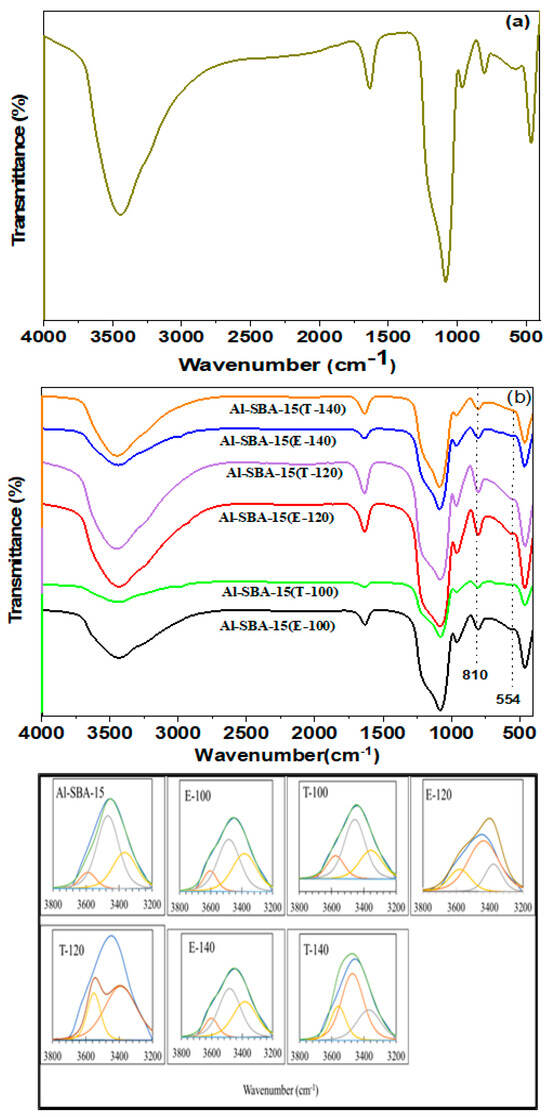
Figure 1.
FTIR spectra of (a) pure SBA-15, (b) Al-SBA-15 materials fabricated with ethylenediamine and triethylamine and hydrothermally treated at varying temperatures (100, 120, and 140 °C), and (c) deconvoluted FTIR spectra of the fabricated materials in the range of 3200–3800.

Table 2.
Acidic properties of pure SBA-15 and Al-SBA-15 materials prepared using ethylenediamine (E) and triethyl amine (T) at various temperatures.
The XRD patterns of the Al-SBA-15 fabricated using various amine ionic liquids and hydrothermally treated at various temperatures are presented in Figure 2a. All materials display distinct XRD patterns that are specific to SBA-15 structures with hexagonal symmetry [10]. The presence of clearly distinguishable (100), (110), and (200) reflections indicates that the long-range mesoporous architecture remains intact even with the inclusion of aluminum. There were no more diffraction peaks corresponding to Al2O3 noted in the Al-SBA-15 materials, suggesting that isolated Al2O3 clusters are not formed, and the Al3+ ions are well dispersed in the lattice without forming isolated Al2O3 clusters. The primary diffraction peaks in the Al-SBA-15(E-140 & T-140) samples are observed to shift toward smaller 2θ values. This change implies less structural contraction and lengthened Al–O bonds than Si–O bonds [28]. The hexagonal unit cell length (ao) was estimated based on the d-spacing of the (100) reflection and ranged from 11.34 to 12.21 nm (Figure 2b), with larger unit cell parameters observed for samples synthesized using diethylene amine and triethyl amine at 120 °C. This expansion of the lattice is attributed to the bigger Al3+ (53.5 pm) radius compared with Si4+ (40 pm). As a result, Si4+ is isomorphically replaced by Al3+ ions, and Al3+ ions are also grafted into the matrix or silica pore walls [29,30].
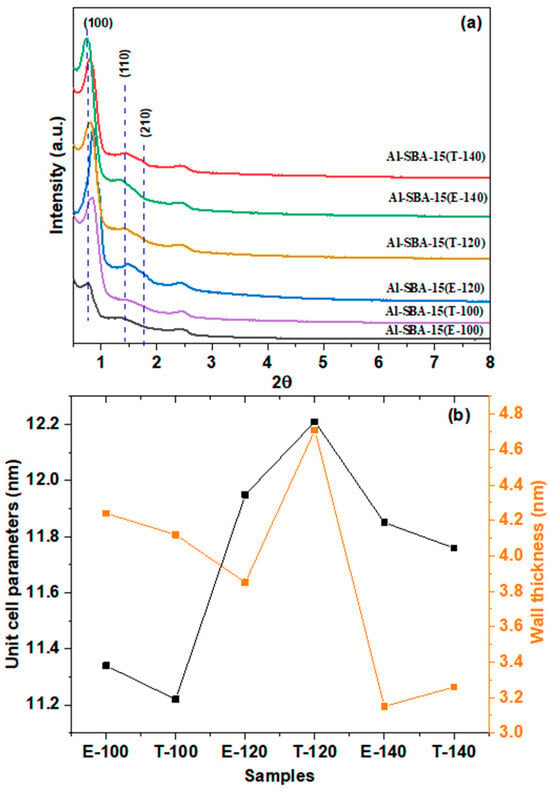
Figure 2.
(a) Low-angle XRD patterns (b) unite cell parameter and wall thickness of Al-SBA-15 materials fabricated with ethylenediamine and triethylamine and hydrothermally treated at varying temperatures (100, 120, and 140 °C).
Figure 3 provides the 27Al nuclear magnetic resonance (NMR) spectra of the Al-SBA-15 (E-120) and Al-SBA-15 (T-120) materials, offering crucial insights into the coordination environment of aluminum in these samples. It is evident that the choice of amine ionic liquids plays a remarkable role in influencing the coordination geometry of aluminum species. In the spectrum of Al-SBA-15 (E-120) synthesized with ethylene diamine at 120 °C, two distinct bands are observed at 1.7 and 55 ppm. The presence of the peak at 55 ppm is indicative of aluminum ions adopting a tetrahedral coordination, which is characteristic of aluminum integrated within the framework structure. In contrast, the band appearing at 1.7 ppm corresponds to octahedral aluminum species, suggesting the presence of aluminum in an extra framework position. This observation aligns with previous studies, further supporting the conclusion drawn from our NMR analysis [31,32,33,34]. Conversely, the spectrum of Al-SBA-15 (T-120), prepared with triethyl amine at 120 °C, exhibits a dominant peak at 61 ppm, accompanied by a sharp peak at 1.5 ppm. This distinct pattern signifies that the majority of aluminum in this sample adopts a tetrahedral coordination within the framework structure. This particular coordination environment is of significant interest, as it is known to influence the catalytic properties of the material. Overall, the 27Al NMR analysis underscores the pivotal role of amine ionic liquids in tailoring the coordination chemistry of aluminum within the SBA-15 framework. This knowledge not only enhances our understanding of the structural characteristics of these materials but also holds promise for optimizing their catalytic performance in various applications.
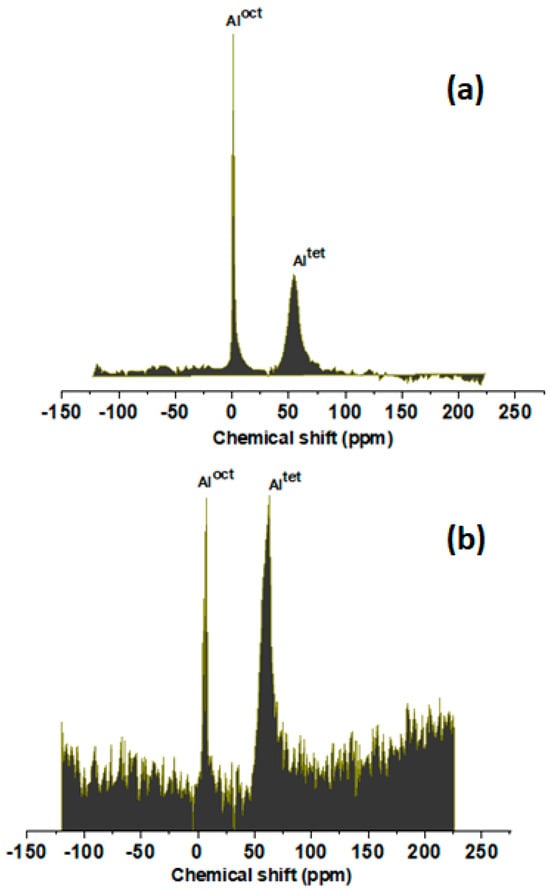
Figure 3.
27Al NMR spectra of (a) Al-SBA-15 (E-120) and (b) Al-SBA-15 (T-120).
Figure 4a shows two temperature regions, each assigned to a weight loss event. During the temperature range of 30.5 to 95.6 °C, an initial event (I) took place, leading to a slight mass loss of 3.01%. This mass loss can be explained by the physical release of water and volatile components present within the porous structure. Between 95.6 and 660.7 °C, the second event (II) occurred, leading to a remarkable mass loss of 51.95%. This mass loss was due to the elimination of ethylene diamine ionic liquid and P123 director. The system reached equilibrium at approximately 630 °C. Figure 4b depicts two distinct mass loss events. The initial weight loss (I) took place within the temperature range of 31.3 to 72.1 °C, resulting in a mass loss of 3.73%. This mass loss can be attributed to the elimination of hydration water and the desorption of physiosorbed water within the porous structure. The second weight loss (II) occurred between 72.1 and 710.2 °C, leading to a mass loss of 50.93%. This mass loss can be attributed to the removal of triethylamine ionic liquid and Pluronic P123.
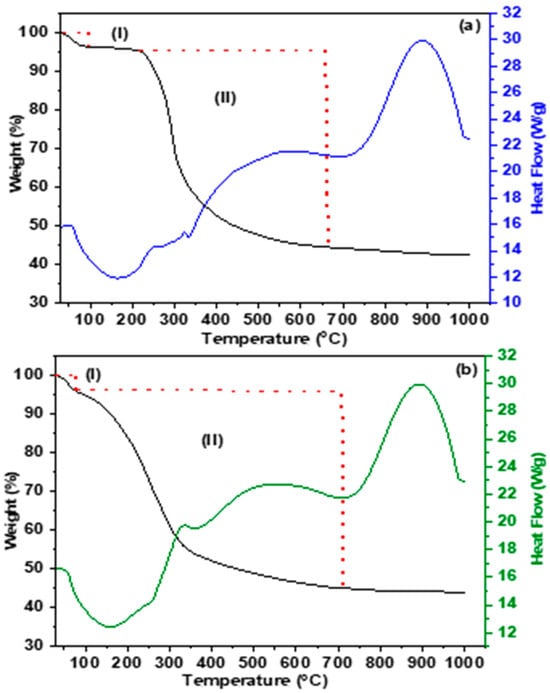
Figure 4.
TGA profiles of silica precursors containing various (a) Al-ethylenediamine and (b) Al-triethyl amine ionic liquids.
Figure 5 presents transmission electron microscopy (TEM) images exhibiting the mesoporous structure of Al-SBA-15 (E-120) and Al-SBA-15 (T-120) materials at different magnifications. These images provide supporting evidence indicating that the grafting of Al3+ did not disturb the well-defined pore architecture present in the SBA-15 material. Furthermore, upon closer examination, the magnified images display a higher quantity of closely packed voids within the silica matrix fabricated via triethyl amine ionic liquid in comparison to those synthesized using ethylene diamine.
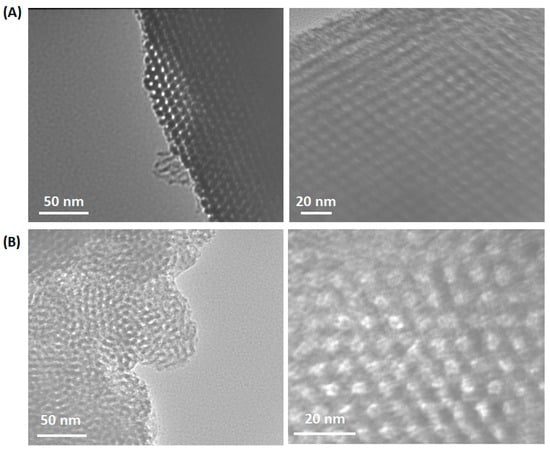
Figure 5.
HRTEM images of (A) Al-SBA-15(E-120) and (B) Al-SBA-15(T-120) materials at different magnification.
Figure 6a–c illustrate the N2-sorption isotherms for the Al-SBA-15 produced with various amine ionic liquids and hydrothermally treated at 100, 120, and 140 °C. Table 1 provides a comprehensive overview of the textural properties exhibited by these materials. All the isotherms have an H1-type hysteresis loop and adhere to a standard type IV pattern, much like the SBA-15 isotherm. Nonetheless, the hysteresis loops observed for Al-SBA-15 (E-140) and Al-SBA-15 (T-140) materials lie between the H1 as well as H2 hysteresis loops, suggesting that their pore systems possess a somewhat lower degree of homogeneity compared with the samples subjected to hydrothermal treatment at 100 °C and 120 °C. All of the isotherms display a steep inflection when the relative pressure rises (P/Po > 0.5), which is consistent with capillary condensation of N2 inside homogenous mesopores. The diameter and limited distribution of mesopores are connected to the P/Po location and slopes of the inflection point (Figure 6d) [35]. The capillary condensation shifts to higher P/Po values with an increase in the hydrothermal temperature. This might be caused by a rise in director swelling when amine ionic solutions are present. The quantity of Al3+ ions within the silica matrix significantly affects the surface area, specific pore volume, and pore size. Al-SBA-15 (E-100), (T-100), (E-120), (T-120), (E-140), and (T-140) have pores with diameters of roughly 7.1, 7.1, 8.1, 7.5, 8.7, and 8.5 nm, high BET surface areas of 822, 869, 844, 890, 319, and 333 m2 g−1, and pore volumes respectively of 1.17, 1.04, 1.14, 1.21, 1.12, and 1.01 cm3 g−1. The reduced surface characteristics of Al-SBA-15(E-140) and Al-SBA-15 (T-140) indicate lower Al2O3 distribution throughout Al-SBA-15. Additionally, Al-SBA-15 (E-140) and Al-SBA-15 (T-140) showcase the smallest whole pore volume as a consequence of their diminished microporosity [36]. Generally, SBA-15 created using a great hydrothermal temperature (140 °C) comprises two interlaced subnetworks of pores. The development of a favorable pore architecture that is frequently found within the walls of silica is because of the initial absorption of the ethylene oxide (EO) chains into the silica walls. The degree of interaction between EO and silica is significantly influenced by the hydrothermal temperature [37].
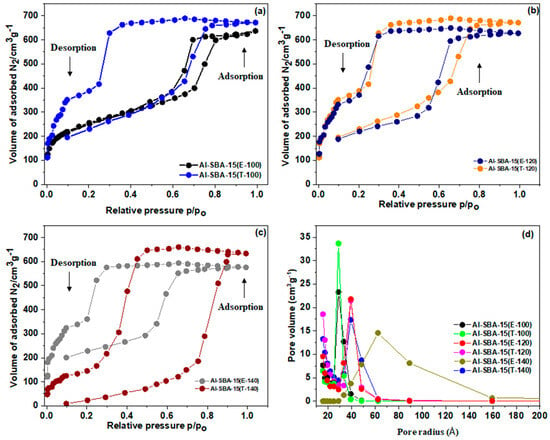
Figure 6.
(a−c) Nitrogen adsorption isotherms and (d) pore size distribution of Al−SBA−15 materials fabricated with ethylenediamine and triethylamine and hydrothermally treated at varying temperatures (100, 120, and 140 °C).
2.2. Acidity Assessment
The acidity of the Al-SBA-15 samples was evaluated using three techniques, namely, potentiometric titration, NH3-TPD, and pyridine adsorption. Potentiometric titration was employed to examine the acid centers existing on the surfaces of the Al-SBA-15 catalysts using n-butylamine. This technique allowed for an estimation of both the total number and relative strength of the acid centers in the materials. The magnitude of the meq amine/g sample at the level of saturation provided the whole amount of acid centers, while the starting electrode potential (Ei) reflected the greatest acid site strength. Based on the Ei values, the acid strength of the sites was classified into four categories: very weak sites (Ei < −100 mV), weak sites (−100 < Ei < 0 mV), strong sites (0 < Ei < 100 mV), and very strong sites (Ei > 100 mV) [3]. Table 2 summarizes the total quantity of acid sites and the initial potential (Ei) values for all samples, indicating that all samples possessed high surface acidity and acid strength. This enhancement in surface acidity and strength was credited with the homogenous distribution of Al3+ ions inside the pores of SBA-15 and on the upper surface. However, for the Al-SBA-15 prepared at 140 °C, the decline in acidity was due to the development of accumulated Al2O3.
FTIR spectroscopy with pyridine as a probe molecule was employed to investigate the distribution of Lewis (L) and Brønsted (B) acid sites in all Al-SBA-15 samples. The FTIR spectra of pyridine adsorbed on the Al-SBA-15 samples are presented in Figure 7a. The results suggest that all the aluminum-incorporated samples possess both Lewis and Brønsted acidic centers, as demonstrated by the infrared absorption peaks observed at approximately 1443 and 1544 cm−1, respectively [38]. Furthermore, the absorption peak at about 1491 cm−1 suggests the development of adjacent Lewis and Brønsted centers. The relative abundance of the Brønsted and Lewis (B/L) acid centers ratio in each Al-SBA-15 sample was determined by examining the intensities of the corresponding FTIR bands, as presented in Table 2. An interesting observation is that Al-SBA-15 (T-120) demonstrates a higher ratio of Brønsted/Lewis than the others, indicating a greater abundance of Brønsted acid sites. This implies that the Brønsted/Lewis ratio rises alongside an increased presence of Brønsted acid sites in Al-SBA-15 (T-120).
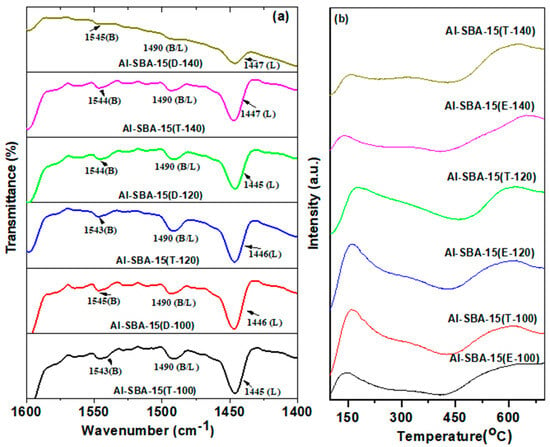
Figure 7.
(a) Py−FTIR spectra and (b) NH3−TPD profiles of Al−SBA−15 materials fabricated with ethylenediamine and triethylamine and hydrothermally treated at varying temperatures (100, 120, and 140 °C).
The NH3-TPD profiles of various Al-SBA-15 samples (E-100, T-100, E-120, T-120, E-140, and T-140) are displayed in Figure 7b, and the total number of acid centers is provided in Table 2. The NH3-TPD plots reveal desorption peaks of NH3 between 100 and 550 °C, indicating the presence of medium-strength acidic sites [39]. The observed peaks can be distinctly classified into three separate areas. The initial peak, observed at a lower temperature, is attributed to the presence of physiosorbed ammonia or NH3 molecules that are H-bonded to terminal silanol moieties. The second peak, spanning the temperature range of 300 to 400 °C, is associated with the adsorption of ammonia on aluminum (Al) within the matrix of the Al-SBA-15 samples. On the other hand, the presence of extra framework (Al) is indicated by the third peak, observed at higher temperatures. Moreover, the total acidity of these materials can be ranked as follows: Al-SBA-15 (T) > Al-SBA-15 (E), as assessed by the whole acid centers estimated from NH3-TPD. The amorphous and thick pore wall in the mesoporous materials developed by ethylene diamine is responsible for the observed findings. In particular, the presence of Al atoms grafted within the thick walls of Al-SBA-15 (E) makes them invisible to the probing molecule, thus complicating the evaluation of the acidic characteristics of the prepared materials solely based on the coordination environment and aluminum content. This highlights the challenge of assessing the acidic properties because the pore wall is thick and amorphous.
2.3. Catalytic Performance
The catalytic efficiency of various Al-SBA-15 (E-100, T-100, E-120, T-120, E-140, and T-140) samples was investigated using the liquid phase esterification reaction as a laboratory probe. Both BAS (Brønsted acid sites) and LAS (Lewis acid sites) were found to catalyze the esterification reaction [40,41,42,43,44,45]. It was also noted that Lewis’s acid sites, particularly Mn+ ions with small coordination spheres, could be utilized [41]. Indeed, previous studies have predominantly emphasized the catalytic performance associated with Brønsted acid centers [10]. To facilitate a meaningful comparison between different samples, the esterification reaction was chosen for the current research. The comparative findings of the catalytic efficiency can be illustrated in Figure 8. Among the examined catalysts, Al-SBA-15 (T-120) demonstrated the greatest catalytic performance, resulting in a conversion rate of 38% and selectivity of approximately 100% toward butyl acetate. Figure 8 indicates that the efficiency of the catalysts can be ranked as follows: Al-SBA-15 (E-120) < Al-SBA-15 (T-120). The remarkable efficiency of Al-SBA-15 (T-120) can be attributed to several factors, including its abundance of acidic centers, large surface area, and wide pore diameter. These characteristics facilitate the easy interaction of reactants to the acidic protons, thereby enhancing catalytic performance. On the other hand, the lower catalytic performance obtained for Al-SBA-15 (E&T-140) in comparison to Al-SBA-15 (E&T-100&120) can be credited to its declined surface area. The impact of esterification temperature (80 °C and 100 °C) and duration time is depicted in Figure 8. Generally, an increase in reaction temperature improved catalytic performance. Specifically, for Al-SBA-15 (T-120), the reaction proceeded rapidly, achieving approximately 32% and 38% conversion within 110 min at 80 °C and 100 °C, respectively. Importantly, the reaction exclusively yielded butyl acetate as the product at all temperatures, demonstrating a 100% selectivity toward esterification.
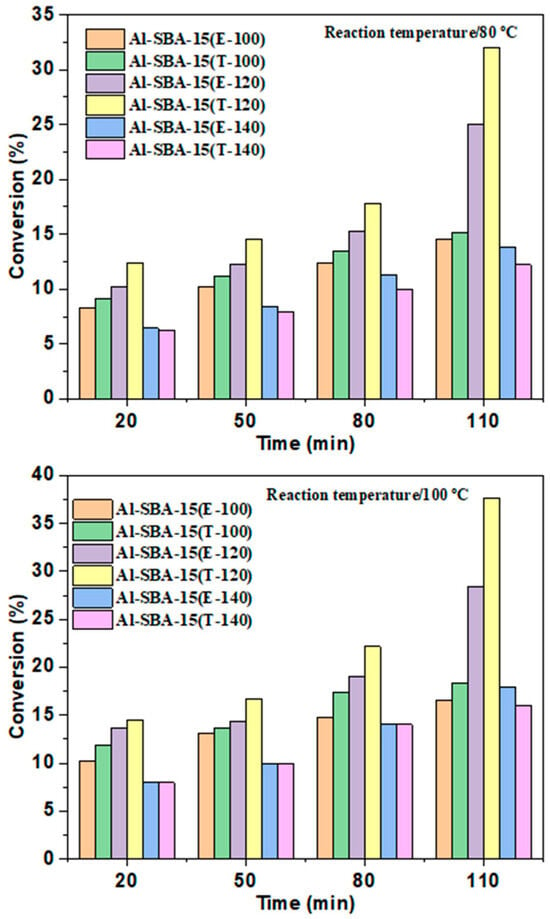
Figure 8.
Catalytic performance of Al-SBA-15 materials fabricated with ethylenediamine and triethylamine and hydrothermally treated at varying temperatures (100, 120, and 140 °C) on the esterification process at various temperatures and times.
3. Materials and Methods
3.1. Materials
Triblock copolymer Pluronic P123 (EO20PO70EO20, Mwt = 5800), tetraethylorthosilicate (TEOS, 98%), aluminum chloride hexahydrate (AlCl3·6H2O, 99%), hydrochloric acid (HCl, 37%), ethylenediamine (≥99%), (cumene (C6H5CH(CH3)2, 98%), acetic acid (CH3COOH, ≥99%), amyl alcohol (CH3(CH2)4OH, 99%), acetonitrile (CH3CN, 99%), n-butylamine (99.5%), acetic anhydride (≥98%), and anisole (99.7%) were acquired from Sigma-Aldrich Co., (St. Louis, MO, USA), and exploited as obtained. All the chemicals utilized as purchased without any additional treatment.
3.2. Materials Fabrication
3.2.1. Synthesis of Ionic Liquids
Ethylenediamine or triethylamine was combined with an equivalent quantity of 1 M HCl and stirred under nitrogen gas at room temperature for 1 h. Subsequently, the appropriate molar amount of anhydrous aluminum chloride was introduced to the amine hydrochloride mixture and thoroughly stirred for an additional three hours. In an acidic medium, aluminum chloride (AlCl3) can act as a Lewis acid, accepting an electron pair from a Lewis base. Triethylamine (:NEt3) can function as a Lewis base in this context. The reaction would proceed as follows:
NEt3 + HCl → Et3NH+ Cl− + AlCl3 → Et3NH+ AlCl4− (ionic liquid)
In this reaction, the lone pair on the nitrogen atom of triethylamine donates an electron pair to the aluminum atom in aluminum chloride.
3.2.2. Direct Incorporation of Al3+ Species into SBA-15
To aluminate SBA-15, two solutions were mixed together. The first solution was synthesized by introducing a certain quantity of amine to 10 mL of water, either 0.4 g of ethylenediamine or 1.25 g of triethylamine. This mixture was then treated with 10 mL of hydrochloric acid in a dropwise manner until it became neutral. Next, 1.65 g of aluminum chloride was introduced to the solution and agitated for two hours. Subsequently, a continuous stirring was applied to introduce 9.0 g of tetraethylorthosilicate (TEOS) into the solution, and this was followed by the introduction of 50 mL of hydrochloric acid (276). The second solution consisted of 8 g of Pluronic P123 dispersed in 100 mL of HCl solution, where the H2O/HCl molar ratio was 276. The blend was agitated for two hours until the Pluronic P123 had fully dissolved. Subsequently, the first solution was mixed with a second solution under agitating for 24 h. After the synthesis of aluminated SBA-15, the obtained material was treated hydrothermally for 24 h at 100, 120, and 140 °C. The resulting white solid powder was separated, rinsed thoroughly with water, and then air-dried. The dried powder was then heated at 550 °C for a duration of 6 h. This resulted in the formation of two different products: Al-SBA-15(Ex), where (E) denotes the use of ethylene diamine at x = 100 °C, 120 °C, and 140 °C, and Al-SBA-15(Tx), where (T) represents the use of triethylamine at x = 100 °C, 120 °C, and 140 °C.
3.3. Characterizations
Fourier transform infrared spectroscopy was performed on pristine mesostructured materials and after the adsorption of pyridine using the Shimadzu IR Tracer-100 (Columbia, MD, USA) Fourier transform infrared spectrometer (FTIR). Low-angle XRD patterns were acquired using the Shimadzu D/Max2500VB2+/Pc instrument (Columbia, MD, USA) with Cu Kα radiation (wavelength = 1.54056 Å). The measurements were taken within the 2θ range of 0.5 to 10°, with a step size of 0.01° and a step time of 10 s. The adsorption and desorption isotherms of nitrogen were obtained at 77 K using a NOVA 4200e instrument from Quanta Chrome Instruments (Boynton Beach, FL, USA). A JEOL-2011 200 kV instrument (Tokyo, Japan) was utilized to acquire high-resolution transmission electron microscopy (HRTEM) images. In addition, the overall acidity of the solid samples was assessed using potentiometric titration. To conduct the titration, a mixture of the solid (0.05 g) and acetonitrile was stirred for 3 h. Subsequently, titration was performed with 0.05 N 1-Aminobutane in acetonitrile at a rate of 0.05 mL/min. The change in electrode potential was measured. NH3-TPD analysis was conducted utilizing a CHEMBET 3000 chemical absorber (Boynton Beach, FL, USA). Then, 50 mg of the samples was subjected to activation in a helium flow at 400 °C for a duration of 2 h. Following this, ammonia was introduced for 1.5 h at 100 °C. For the 27Al NMR characterization, we conducted measurements in the solid state at ambient temperature. We used a Bruker DRX-400 spectrometer (Karlsruhe, Germany) operating at a magnetic field strength of 17.6 T. The frequency employed was 195.4 MHz, and a 2.5 mm MAS NMR probe with a spinning frequency of 30 kHz. Thermogravimetric analysis (TGA) was conducted in a nitrogen atmosphere utilizing a Shimadzu TGA-51 (USA) thermal analyzer with a heating rate of 10 °C/min.
3.4. Catalytic Performance of Esterification of Acetic Acid
Acetic acid esterification was conducted utilizing 1 wt% of the catalyst. The process was performed in a 100 mL flask with a reflux, with the estimated amounts of butanol and acetic acid (in a 1:1 ratio). Two different temperatures, 80 °C and 100 °C, were employed. Throughout the esterification, samples of the blend were regularly collected and subjected to analysis utilizing gas chromatography (GC-Varian star CP-3800, Markham, ON, Canada). The extent of conversion of acetic acid, represented as the percentage of acetic acid utilized, was determined by performing gas chromatographic analysis at different periods.
4. Conclusions
An unprecedented achievement was made by successfully incorporating aluminum ions within the SBA-15 matrix using either ethylenediamine or triethyl amine ionic liquids. Various characterization techniques were employed to analyze the catalysts, demonstrating that mesostructured SBA-15 remained intact following the introduction of aluminum ions. However, there was a significant decline in the specific surface area and pore volume after grafting Al3+ ions. Additionally, a remarkable increase in surface acidity, including both Lewis and Bronsted acidity, was observed after the Al3+ ion grafting process. These catalysts exhibited tremendous potential for acid-catalyzed reactions and were effectively employed in an esterification reaction, resulting in a yield of over 38% at 100 °C using Al-SBA-15 (T-120). These findings indicate that the fabricated catalysts show great potential and efficiency as acid catalysts. They can be regarded as suitable for industrial-scale applications.
Author Contributions
Conceptualization, O.F.A., M.S.A., M.A.B. and H.M.A.H.; methodology, E.A.A., L.M.A., A.A. and O.F.A.; software, L.M.A., A.A. and E.A.A.; validation, H.M.A.H., M.S.A., M.A.B. and O.F.A.; formal analysis, L.M.A. and A.A.; investigation, L.M.A., A.A., H.M.A.H., M.A.B. and M.S.A.; resources, O.F.A.; data curation, H.M.A.H., M.S.A., M.A.B. and O.F.A.; writing—original draft preparation, O.F.A., H.M.A.H. and M.S.A.; writing—review and editing, M.S.A. and O.F.A.; visualization, L.M.A., A.A., H.M.A.H., M.S.A., E.A.A., M.A.B. and O.F.A.; supervision, O.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, grant number (Ifp-2022-39).
Acknowledgments
The authors extend their appreciation to The Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number Ifp-2022-39.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Awual, M.R. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 2017, 307, 456–465. [Google Scholar] [CrossRef]
- Mota, F.M.; Eliášová, P.; Jung, J.; Ryoo, R. Mesoporous EU-1 zeolite as a highly active catalyst for ethylbenzene hydroisomerization. Catal. Sci. Technol. 2016, 6, 2735–2741. [Google Scholar] [CrossRef]
- El Rahman, S.K.A.; Hassan, H.M.A.; El-Shall, M.S. Metal-organic frameworks with high tungstophosphoric acid loading as heterogeneous acid catalysts. Appl. Catal. A Gen. 2014, 487, 110–118. [Google Scholar]
- Wang, J.Z.; Du, H.; Olayiwola, A.; Liu, B.; Gao, F.; Jia, M.L.; Wang, S.N. Recent advances in the recovery of transition metals from spent hydrodesulfurization catalysts. Tungsten 2021, 3, 305–328. [Google Scholar] [CrossRef]
- Corma, A. Solid acid catalysts. Curr. Opin. Solid State Mater. Sci. 1997, 2, 63–73. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Bisio, C.; Gatti, G.; Marchese, L.; Pastore, H.O. Physicochemical characterization and surface acid properties of mesoporous [Al]-SBA-15 obtained by direct synthesis. Langmuir 2010, 26, 5791–5800. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Kang, Y.; Rao, X.; Yuan, P.; Wang, C.; Wang, T.; Yue, Y. Al-functionalized mesoporous SBA-15 with enhanced acidity for hydroisomerization of n-octane. Fuel Process. Technol. 2021, 215, 106765. [Google Scholar] [CrossRef]
- Betiha, M.A.; Hassan, H.M.A.; Al-Sabagh, A.M.; El Rahman, S.K.A.; Ahmed, E.A. Direct synthesis and the morphological control of highly ordered mesoporous AlSBA-15 using urea-tetrachloroaluminate as a novel aluminum source. J. Mater. Chem. 2012, 22, 17551–17559. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Betiha, M.A.; Elshaarawy, R.F.M.; Ahmed, E.A. Facile tailoring of hierarchical mesoporous AlSBA-15 by ionic liquid and their applications in heterogeneous catalysis. J. Porous Mater. 2017, 25, 63–73. [Google Scholar] [CrossRef]
- Luan, Z.; Hartmann, M.; Zhao, D.; Zhou, W.; Kevan, L. Alumination and ion exchange of mesoporous SBA-15 molecular sieves. Chem. Mater. 1999, 11, 1621–1627. [Google Scholar] [CrossRef]
- Selvam, P.; Krishna, N.V.; Viswanathan, B. Architecting mesoporous AlSBA-15: Anoverview onthe synthetic strategy. J. Indian Inst. Sci. 2010, 90, 271–285. [Google Scholar]
- Vinu, A.; Devassy, B.M.; Halligudi, S.; Böhlmann, W.; Hartmann, M. Highly active and selective AlSBA-15 catalysts for the vapor phase tert-butylation of phenol. Appl. Catal. A Gen. 2005, 281, 207–213. [Google Scholar] [CrossRef]
- Abdullah, N.; Ainirazali, N.; Chong, C.C.; Razak, H.A.; Setiabudi, H.D.; Jalil, A.A.; Vo, D.V. Influence of impregnation assisted methods of Ni/SBA-15 for production of hydrogen via dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 18426–18439. [Google Scholar] [CrossRef]
- Liu, X.; Liu, N.; Li, X.; Li, X.; Hu, B.; He, S. Surface Properties and Microstructures of Al-SBA-15 under Different Temperature Calcinations in Relation to Adsorption Performance. J. Phys. Chem. C 2023, 127, 6446–6455. [Google Scholar] [CrossRef]
- Figueiredo, J.S.; Alves, B.T.; Freire, V.A.; Alves, J.J.; Barbosa, B.V. Preparation, characterization and evaluation of x-MoO3/Al-SBA-15 catalysts for biodiesel production. Mater. Renew. Sustain. Energy 2022, 11, 17–31. [Google Scholar] [CrossRef]
- Fan, Y.; Han, Y.; Zhu, J.; Chen, Y.; Cai, Y.; Zhao, W. Production optimization of refined bio-oil through plasma coupling catalysis and composite zeolite effect of Al-SBA-15/HZSM-5. Fuel 2022, 347, 128494. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Kumaravel, S.; Durai, M.; Erusappan, E. Highly Efficient Catalytic Hydrogenation of Biomass-Derived Levulinic Acid to Valeric Acid Over Ru/W/Al-SBA-15 Catalysts. Waste Biomass Valorization 2023, 1–14. [Google Scholar] [CrossRef]
- Cai, W.; Ni, X.; Meng, F.; Sun, L.; Wang, R.; Lin, S. From Al-SBA-15 to Ga-SBA-15: Morphology and acidity evolution in the acid-free synthesis. Microporous Mesoporous Mater. 2022, 335, 111823. [Google Scholar] [CrossRef]
- Gajardo, J.; Colmenares-Zerpa, J.; Peixoto, A.F.; Silva DS, A.; Silva, J.A.; Gispert-Guirado, F.; Chimentão, R.J. Revealing the effects of high Al loading incorporation in the SBA-15 silica mesoporous material. J. Porous Mater. 2023, 30, 1687–1707. [Google Scholar] [CrossRef]
- Arumugam, M.; Kikhtyanin, O.; Osatiashtiani, A.; Kyselová, V.; Fila, V.; Paterova, I.; Kubička, D. Potassium-modified bifunctional MgAl-SBA-15 for aldol condensation of furfural and acetone. Sustain. Energy Fuels 2023, 7, 3047–3059. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, L. Heterogeneous CaO–MoO3–SBA-15 catalysts for biodiesel production from soybean oil. Energy Convers. Manag. 2014, 79, 34–42. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Arumugam, M.; Hunns, J.; Lee, A.F.; Mayes, E.; Taufiq-Yap, Y.H.; Derawi, D. Octanoic acid hydrodeoxygenation over bifunctional Ni/Al-SBA-15 catalysts. Catal. Sci. Technol. 2019, 9, 6673–6680. [Google Scholar] [CrossRef]
- Socci, J.; Osatiashtiani, A.; Kyriakou, G.; Bridgwater, T. The catalytic cracking of sterically challenging plastic feedstocks over high acid density Al-SBA-15 catalysts. Appl. Catal. A Gen. 2018, 570, 218–227. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite characterization. 1. Structure, adsorptive capacity, and IR spectroscopy of the framework and hydroxyl modes. J. Phys. Chem. 1992, 96, 4985–4990. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Derewiński, M.; Sarv, P.; Datka, J. IR and NMR studies of mesoporous alumina and related aluminosilicates. Catal. Today 2005, 101, 131–138. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Danilova, I.G.; Arzumanov, S.S.; Pirutko, L.V.; Freude, D.; Stepanov, A.G. Direct measurement of zeolite Brønsted acidity by FTIR spectroscopy: Solid-state 1H MAS NMR approach for reliable determination of the integrated molar absorption coefficients. J. Phys. Chem. C 2018, 122, 25386–25395. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Cao, Y.; Yi, N.; Feng, W.-L.; Dai, W.-L.; Yan, S.-R.; He, H.-Y.; Fan, K.-N. Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane. J. Catal. 2004, 224, 417–428. [Google Scholar] [CrossRef]
- Selvaraj, M.; Park, D.-W.; Ha, C.S. Well ordered two-dimensional mesoporous CeSBA-15 synthesized with improved hydrothermal stability and catalytic activity. Microporous Mesoporous Mater. 2011, 138, 94–101. [Google Scholar] [CrossRef]
- Kudo, T.; Hisamitsu, Y.; Kihara, K.; Mohamedi, M.; Uchida, I. Electrochemical behaviour of Ni+ Al alloy as an alternative material for molten carbonate fuel cell cathodes. J. Appl. Electrochem. 2002, 32, 179–184. [Google Scholar] [CrossRef]
- Rachwalik, R.; Olejniczak, Z.; Jiao, J.; Huang, J.; Hunger, M.; Sulikowski, B. Isomerization of α-pinene over dealuminated ferrierite-type zeolites. J. Catal. 2007, 252, 161–170. [Google Scholar] [CrossRef]
- Freude, D.; Ernst, H.; Wolf, I. Solid-state nuclear magnetic resonance studies of acid sites in zeolites. Solid State Nucl. Magn. Reson. 1994, 3, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Edirisinghe, M.J. Evolution of the Ceramic Structure during Thermal Degradation of a Si− Al− C− O Precursor. Chem. Mater. 2004, 16, 1111–1119. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Yu, C.; Fan, Y.; Bao, X. Synthesis and hydrodesulfurization properties of NiW catalyst supported on high-aluminum-content, highly ordered, and hydrothermally stable Al-SBA-15. J. Catal. 2012, 286, 124–136. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Imperor-Clerc, M.; Davidson, P.; Davidson, A. Existence of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers. J. Am. Chem. Soc. 2000, 122, 11925–11933. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of micro-and mesoporosity in SBA-15 materials from adsorption data by the NLDFT method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Cecilia, J.A.; De Conto, J.F.; Egues, S.M.; Rodríguez-Castellón, E. Rapid synthesis of MCM-41 and SBA-15 by microwave irradiation: Promising adsorbents for CO2 adsorption. J. Sol-Gel Sci. Technol. 2023, 105, 370–387. [Google Scholar] [CrossRef]
- Khder, A.S.; El-Sharkawy, E.A.; El-Hakam, S.A.; Ahmed, A.I. Surface characterization and catalytic activity of sulfated tin oxide catalyst. Catal. Commun. 2008, 9, 769–777. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Dabdoub, M.J.; Hurtado, G.R.; Klein, S.I.; Baroni, A.C.M.; Cunha, C. Solvent free esterification reactions using Lewis acids in solid phase catalysis. Appl. Catal. A Gen. 2006, 313, 146–150. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K.M. Facile Synthesis of Dodecatungstophosphoric Acid @ TiO2 Pillared Montmorillonite and Its Effectual Exploitation Towards Solvent Free Esterification of Acetic Acid with n-Butanol. Catal. Lett. 2011, 141, 1476–1483. [Google Scholar] [CrossRef]
- Mishra, G.; Behera, G.C.; Singha, S.K.; Parida, K.M. Liquid phase esterification of acetic acid over WO3 promoted β-SiC in a solvent free system. Dalton Trans. 2012, 41, 14299. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, J.; Dharb, A.; Vekariya, R.L. Efficient esterification of n-butanol with acetic acid catalyzed by the Bronsted acidic ionic liquids: Influence of acidity. RSC Adv. 2017, 7, 5412. [Google Scholar] [CrossRef]
- Zhou, B.; Fang, Y.; Gu, H.; Zhang, S.; Huang, B.; Zhang, K. Ionic liquid mediated esterification of alcohol with acetic acid. Front. Chem. Eng. China 2009, 3, 211–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).