Thermodynamic Insights into Sustainable Aviation Fuel Synthesis via CO/CO2 Hydrogenation
Abstract
:1. Introduction
1.1. Development of Bio-Based Sustainable Aviation Fuels
1.2. An Overview of CO/CO2 Catalytic Hydrogenation to Aviation Fuel Components
1.3. The Objectives of This Study
2. Results and Discussion
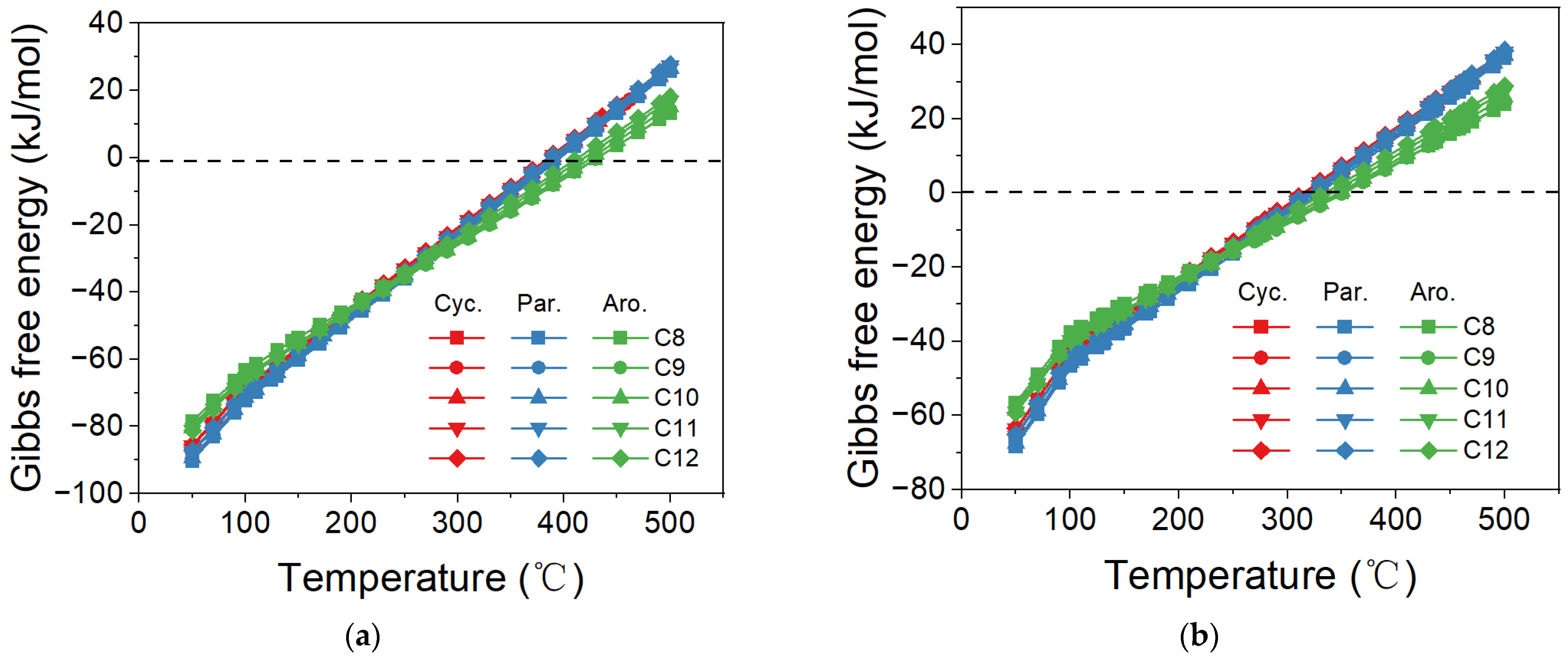
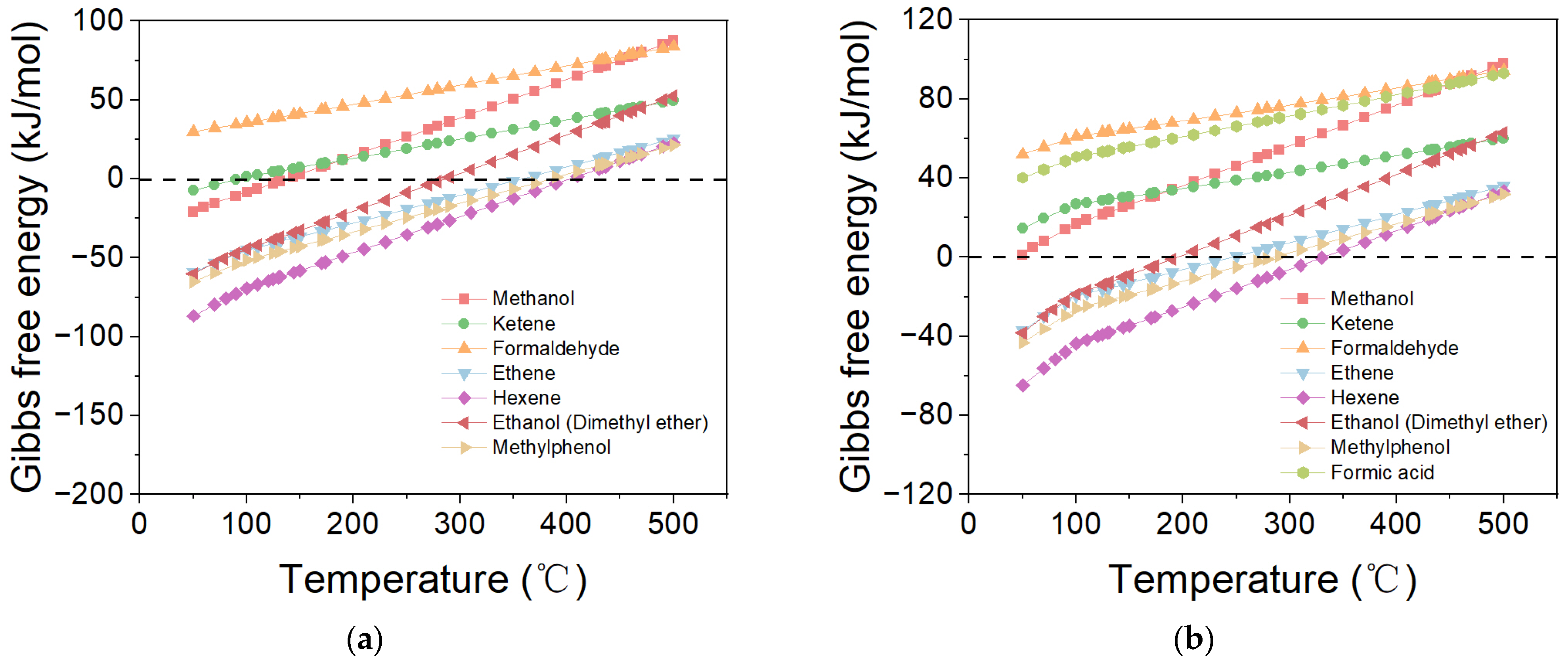
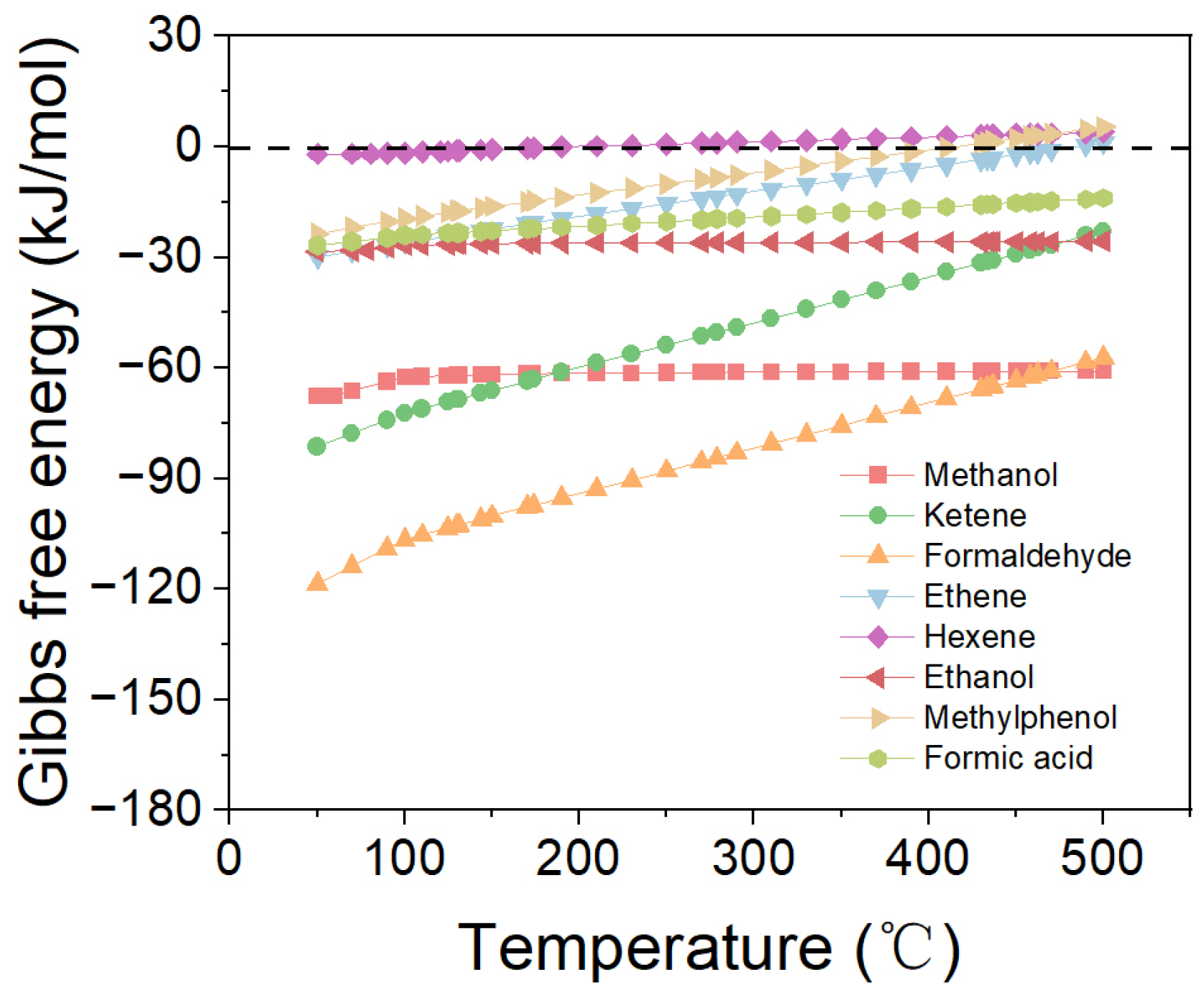
2.1. Direct Synthesis of SAF via CO/CO2 Hydrogenation
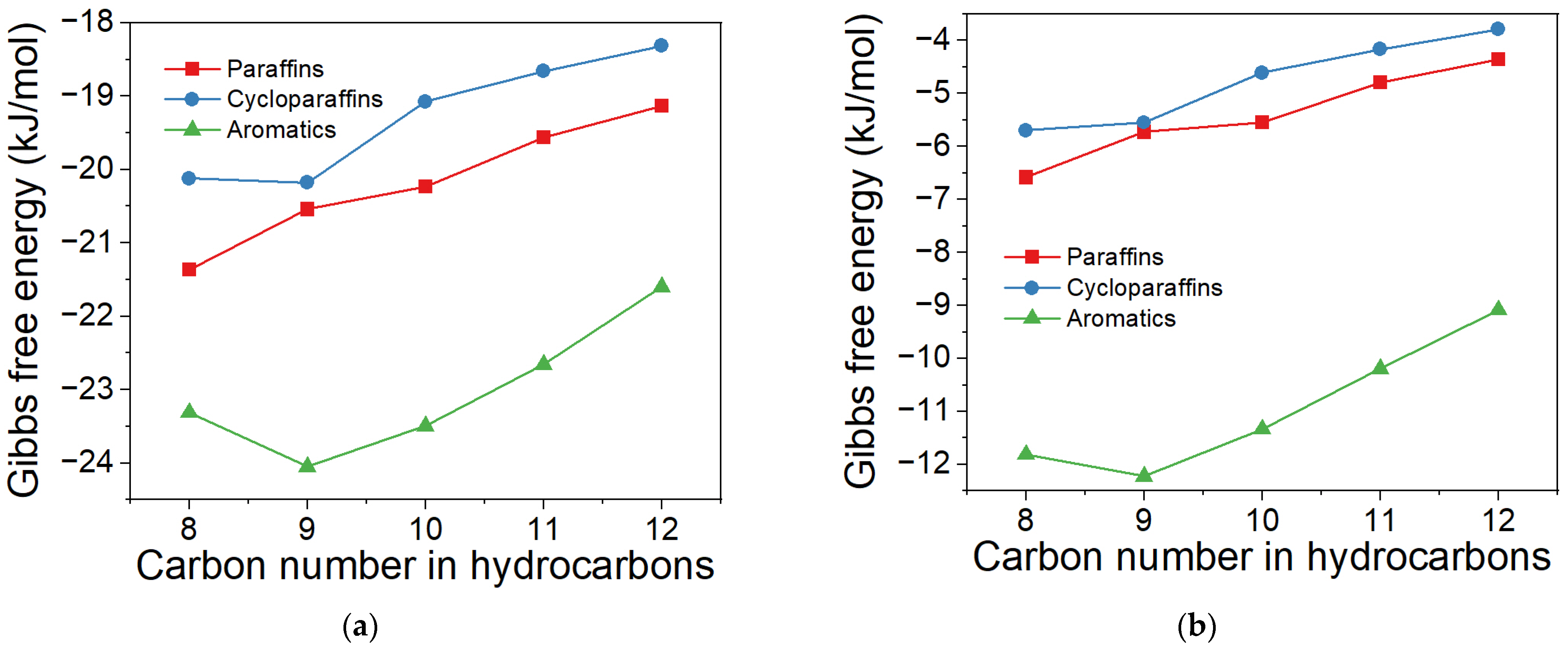
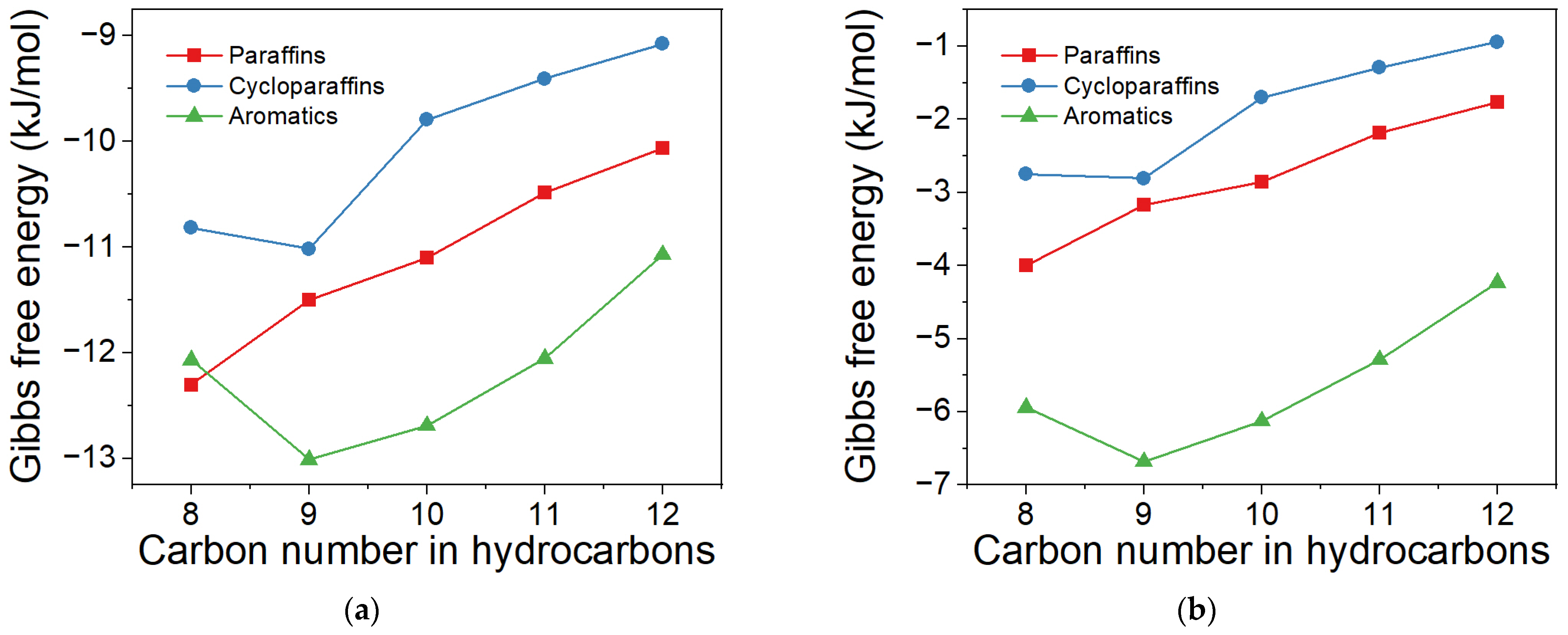
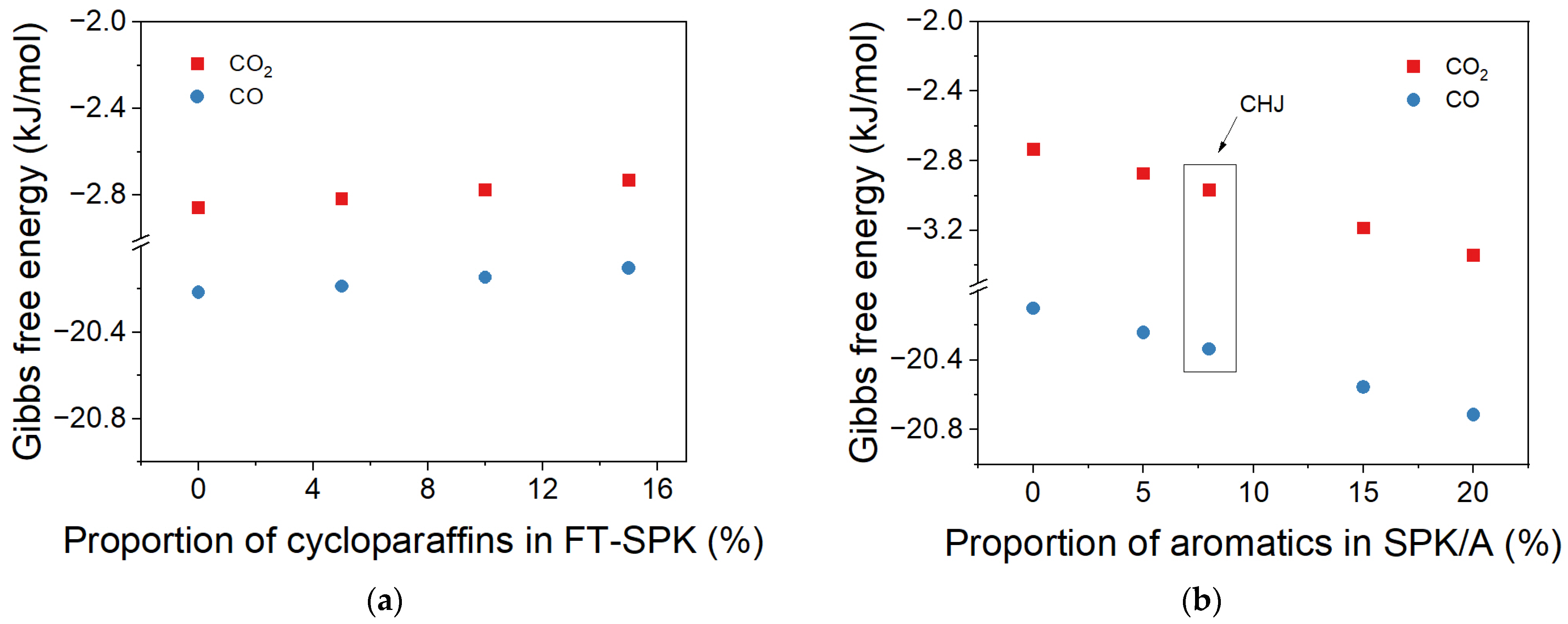
2.2. Effect of Proportions of Cycloparaffin and Aromatic in Products on ΔG
2.3. Reaction Pathways for SAF Synthesis via CO/CO2 Hydrogenation
2.4. Direct CO/CO2 Hydrogenation to Aromatics: Our Recent Research and Catalytic Strategies
3. Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garay, A.; Heuberger-Austin, C.; Fu, X.; Klokkenburg, M.; Zhang, D.; van der Made, A.; Shah, N. Unravelling the potential of sustainable aviation fuels to decarbonise the aviation sector. Energy Environ. Sci. 2022, 15, 3291–3309. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Y.; Wang, M.; Appels, L.; Deng, Y. Prospects and perspectives foster enhanced research on bio-aviation fuels. J. Environ. Manag. 2020, 274, 111214. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Kosova, N.V.; Jordy, C.; Chateigner, D.; Lebedev, O.I.; Maignan, A.; Pralong, V. A new active Li-Mn-O compound for high energy density Li-ion batteries. Nat. Mater. 2016, 15, 173–177. [Google Scholar] [CrossRef]
- Kumabe, K.; Sato, T.; Matsumoto, K.; Ishida, Y.; Hasegawa, T. Production of hydrocarbons in Fischer–Tropsch synthesis with Fe-based catalyst: Investigations of primary kerosene yield and carbon mass balance. Fuel 2010, 89, 2088–2095. [Google Scholar] [CrossRef]
- Akter, H.A.; Dwivedi, P.; Masum, M.F.H.; Alam, A.; Anderson, W. Does Intercropping Carinata with Loblolly Pine for Sustainable Aviation Fuel Production Save Carbon? A Case Study from the Southern United States. BioEnergy Res. 2022, 15, 1427–1438. [Google Scholar] [CrossRef]
- Regalbuto, J. An NSF perspective on next generation hydrocarbon biorefineries. Comput. Chem. Eng. 2010, 34, 1393–1396. [Google Scholar] [CrossRef]
- Perego, C.; Bortolo, R.; Zennaro, R. Gas to liquids technologies for natural gas reserves valorization: The Eni experience. Catal. Today 2009, 142, 9–16. [Google Scholar] [CrossRef]
- Rahmes, T.; Kinder, J.; Crenfeldt, G.; LeDuc, G.; Abe, Y.; McCall, M.; Henry, T.; Zombanakis, G.; Lambert, D.; Lewis, C.; et al. Sustainable Bio-Derived Synthetic Paraffinic Kerosene (Bio-SPK) Jet Fuel Flights and Engine Tests Program Results. In Proceedings of the 9th AIAA Aviation Technology, Integration, and Operations Conference (ATIO), Hilton Head, SC, USA, 21–23 September 2009. [Google Scholar]
- Jayarathna, L.; Kent, G.; O’Hara, I.; Hobson, P. A Geographical Information System based framework to identify optimal location and size of biomass energy plants using single or multiple biomass types. Appl. Energy 2020, 275, 115398. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Shu, Y.M.; Shi, C.X.; Pan, L.; Zhang, X.W.; Zou, J.J. Progressing production and application of biomass-based jet fuel. Pet. Process. Petrochem. 2021, 52, 88–93. [Google Scholar]
- Holladay, J.; Abdullah, Z.; Heyne, J. Sustainable Aviation Fuel: Review of Technical Pathways; US Department of Energy: Washington, WA, USA, 2020; p. 81.
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Janke, C.; Duyar, M.S.; Hoskins, M.; Farrauto, R. Catalytic and adsorption studies for the hydrogenation of CO2 to methane. Appl. Catal. B Environ. 2014, 152, 184–191. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef]
- Onishi, N.; Himeda, Y. Homogeneous catalysts for CO2 hydrogenation to methanol and methanol dehydrogenation to hydrogen generation. Coord. Chem. Rev. 2022, 472, 214767. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Lin, K.-S.; Chuang, H.-W. Direct synthesis of formic acid via CO2 hydrogenation over Cu/ZnO/Al2O3 catalyst. J. Clean. Prod. 2018, 172, 1957–1977. [Google Scholar] [CrossRef]
- Masuda, S.; Mori, K.; Kuwahara, Y.; Louis, C.; Yamashita, H. Additive-Free Aqueous Phase Synthesis of Formic Acid by Direct CO2 Hydrogenation over a PdAg Catalyst on a Hydrophilic N-Doped Polymer–Silica Composite Support with High CO2 Affinity. ACS Appl. Energy Mater. 2020, 3, 5847–5855. [Google Scholar] [CrossRef]
- Ren, S.; Shoemaker, W.R.; Wang, X.; Shang, Z.; Klinghoffer, N.; Li, S.; Yu, M.; He, X.; White, T.A.; Liang, X. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Şeker, B.; Dizaji, A.K.; Balci, V.; Uzun, A. MCM-41-supported tungstophosphoric acid as an acid function for dimethyl ether synthesis from CO2 hydrogenation. Renew. Energy 2021, 171, 47–57. [Google Scholar] [CrossRef]
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Ahmad, K.; Upadhyayula, S. Greenhouse gas CO2 hydrogenation to fuels: A thermodynamic analysis. Environ. Prog. Sustain. Energy 2019, 38, 98–111. [Google Scholar] [CrossRef]
- Claeys, M. Cobalt gets in shape. Nature 2016, 538, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef]
- Chang, C.D.; Lang, W.H.; Silvestri, A.J. Synthesis gas conversion to aromatic hydrocarbons. J. Catal. 1979, 56, 268–273. [Google Scholar] [CrossRef]
- Tian, G.; Liang, X.; Xiong, H.; Zhang, C.; Wei, F. A perspective of COx conversion to aromatics. EES Catal. 2023, 1, 677–686. [Google Scholar] [CrossRef]
- Tian, G.; Liu, X.; Zhang, C.; Fan, X.; Xiong, H.; Chen, X.; Li, Z.; Yan, B.; Zhang, L.; Wang, N.; et al. Accelerating syngas-to-aromatic conversion via spontaneously monodispersed Fe in ZnCr2O4 spinel. Nat. Commun. 2022, 13, 5567. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, W.; Kang, J.; He, S.; Shi, S.; Zhang, Q.; Pan, Y.; Wen, W.; Wang, Y. Bifunctional Catalysts for One-Step Conversion of Syngas into Aromatics with Excellent Selectivity and Stability. Chem 2017, 3, 334–347. [Google Scholar] [CrossRef]
- Yan, Q.; Lu, Y.; Wan, C.; Han, J.; Rodriguez, J.; Yin, J.J.; Yu, F. Synthesis of Aromatic-Rich Gasoline-Range Hydrocarbons from Biomass-Derived Syngas over a Pd-Promoted Fe/HZSM-5 Catalyst. Energy Fuels 2014, 28, 2027–2034. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Jiao, F.; Li, J.; Bao, X. Direct conversion of syngas to aromatics. Chem. Commun. 2017, 53, 11146–11149. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Liu, X. Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites. Appl. Catal. A Gen. 2018, 552, 168–183. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, e2104442. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Liang, B.; Zhang, Y.; Duan, H.; Ma, J.; Huang, Y.; Zhang, T. The influence of alkali-treated zeolite on the oxide–zeolite syngas conversion process. Catal. Sci. Technol. 2018, 8, 4338–4348. [Google Scholar] [CrossRef]
- Arslan, M.T.; Ali, B.; Gilani, S.Z.A.; Hou, Y.; Wang, Q.; Cai, D.; Wang, Y.; Wei, F. Selective Conversion of Syngas into Tetramethylbenzene via an Aldol-Aromatic Mechanism. ACS Catal. 2020, 10, 2477–2488. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Ma, G.; Wang, J.; Wang, Q.; Lin, J.; Wang, H.; Zhang, C.; Ding, M. Synthesis of aromatics from syngas over FeMnK/SiO2 and HZSM-5 tandem catalysts. Mol. Catal. 2018, 454, 104–113. [Google Scholar] [CrossRef]
- Cheng, L.; Meng, C.; Yang, T.; Li, N.; Liu, D. One-Step Synthesis of Aromatics from Syngas over K-Modified FeMnO/MoNi-ZSM-5. Energy Fuels 2018, 32, 9756–9762. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Shi, C.; Jiang, F.; Liu, B.; Liu, X. Direct production of aromatics from syngas over a hybrid FeMn Fischer–Tropsch catalyst and HZSM-5 zeolite: Local environment effect and mechanism-directed tuning of the aromatic selectivity. Catal. Sci. Technol. 2019, 9, 3933–3946. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Huang, Z.; Xu, H.; Shen, W. The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics. Catalysts 2020, 10, 262. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Wang, J.; Liu, H.; Li, M.; Miao, S.; Li, C. Highly Selective Conversion of Carbon Dioxide to Aromatics over Tandem Catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, J.; Zhou, W.; Cheng, K.; Zhang, Q.; Kang, J.; Wang, Y. Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide. ACS Catal. 2019, 10, 302–310. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Arslan, M.T.; Tian, G.; Ali, B.; Zhang, C.; Xiong, H.; Li, Z.; Luo, L.; Chen, X.; Wei, F. Highly Selective Conversion of CO2 or CO into Precursors for Kerosene-Based Aviation Fuel via an Aldol–Aromatic Mechanism. ACS Catal. 2022, 12, 2023–2033. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Liu, B.; Wang, T.; Zheng, J.; Li, W.; Liu, D.; Liu, X. Selective production of aromatics from CO2. Catal. Sci. Technol. 2019, 9, 593–610. [Google Scholar] [CrossRef]
- Kasipandi, S.; Bae, J.W. Recent Advances in Direct Synthesis of Value-Added Aromatic Chemicals from Syngas by Cascade Reactions over Bifunctional Catalysts. Adv. Mater. 2019, 31, e1803390. [Google Scholar] [CrossRef]
- Timko, M.T.; Herndon, S.C.; De La Rosa Blanco, E.; Wood, E.C.; Yu, Z.; Miake-Lye, R.C.; Knighton, W.B.; Shafer, L.; DeWitt, M.J.; Corporan, E. Combustion Products of Petroleum Jet Fuel, a Fischer–Tropsch Synthetic Fuel, and a Biomass Fatty Acid Methyl Ester Fuel for a Gas Turbine Engine. Combust. Sci. Technol. 2011, 183, 1039–1068. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, X.; Liu, Y.; Gong, S.; Deng, C.; Pan, L.; Zou, J.J. A comprehensive review of the thermal oxidation stability of jet fuels. Chem. Eng. Sci. 2021, 229, 116157. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, C.; Wei, F. COx conversion to aromatics: A mini-review of nanoscale performance. Nanoscale Horiz. 2022, 7, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Kang, J.; Niu, W.; Wang, M.; Zhang, Q.; Wang, Y. Impact of hierarchical pore structure on the catalytic performances of MFI zeolites modified by ZnO for the conversion of methanol to aromatics. Catal. Sci. Technol. 2017, 7, 3598–3612. [Google Scholar] [CrossRef]
- Zhu, J.; Su, Y.; Chai, J.; Muravev, V.; Kosinov, N.; Hensen, E.J.M. Mechanism and Nature of Active Sites for Methanol Synthesis from CO/CO2 on Cu/CeO2. ACS Catal. 2020, 10, 11532–11544. [Google Scholar] [CrossRef]
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and Interface Control in Nanoparticle Catalysis. Chem. Rev. 2020, 120, 1184–1249. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.T.; Qureshi, B.A.; Gilani, S.A.; Cai, D.; Ma, Y.; Usman, M.; Chen, X.; Wang, Y.; Wei, F. Single-Step Conversion of H2-Deficient Syngas into High Yield of Tetramethylbenzene. ACS Catal. 2019, 9, 2203–2212. [Google Scholar] [CrossRef]




| Standard | FT-SPK | HEFA-SPK | SIP | SPK/A | ATJ-SPK | CHJ | HC-HEFA SPK |
|---|---|---|---|---|---|---|---|
| Hydrocarbon Composition | |||||||
| Saturated Hydrocarbons, % | 98 | ||||||
| Farnesane, % | 97 | ||||||
| Hexahydrofarnesol, % | 1.5 | ||||||
| Olefins, mgBr2/100 g | 300 | ||||||
| Cycloparaffins, % | 15 | 15 | 15 | 15 | report | 50 | |
| Aromatics, % | 0.5 | 0.5 | 0.5 | 20 | 0.5 | 8.4~21.2 | 0.5 |
| Paraffins, % | report | report | report | report | report | ||
| Carbon and Hydrogen, % | 99.5 | 99.5 | 99.5 | 99.5 | 99.5 | 99.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Zhu, Q.; Wang, Z.; Fan, X.; Yu, X.; Cui, Y.; Zhang, C.; Wei, F. Thermodynamic Insights into Sustainable Aviation Fuel Synthesis via CO/CO2 Hydrogenation. Catalysts 2023, 13, 1396. https://doi.org/10.3390/catal13111396
Liang B, Zhu Q, Wang Z, Fan X, Yu X, Cui Y, Zhang C, Wei F. Thermodynamic Insights into Sustainable Aviation Fuel Synthesis via CO/CO2 Hydrogenation. Catalysts. 2023; 13(11):1396. https://doi.org/10.3390/catal13111396
Chicago/Turabian StyleLiang, Bin, Qing Zhu, Zibing Wang, Xiaoyu Fan, Xiao Yu, Yu Cui, Chenxi Zhang, and Fei Wei. 2023. "Thermodynamic Insights into Sustainable Aviation Fuel Synthesis via CO/CO2 Hydrogenation" Catalysts 13, no. 11: 1396. https://doi.org/10.3390/catal13111396
APA StyleLiang, B., Zhu, Q., Wang, Z., Fan, X., Yu, X., Cui, Y., Zhang, C., & Wei, F. (2023). Thermodynamic Insights into Sustainable Aviation Fuel Synthesis via CO/CO2 Hydrogenation. Catalysts, 13(11), 1396. https://doi.org/10.3390/catal13111396







