Impacts of Dietary Selenium Nanoparticles from Spirulina platensis on Growth Performance, Physio-Biochemical Components and Alleviating Effect against Cadmium Toxicity in Pacific White Shrimp Litopenaeus vannamei
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Growth Indices
2.2. The Chemical Composition of the Entire Body
2.3. Biochemical Indices and Digestive Enzymes Activities in Muscles and Digestive Tract of L. vannamei Fed with SP-SeNP Supplemented Diets for 56 Days
2.4. Determination of Lethal and Sublethal Concentrations of Cadmium against L. vannamei after 56 Days of Growth
2.5. Bioaccumulation of Cadmium and Selenium Residues in Hepatopancreases and Muscles of L. vannamei
2.6. Oxidative and Antioxidant Activities in the Hepatopancreases and Muscles of L. vannamei Fed with SP-SeNP Supplemented Diets after Cadmium Exposure for 10 Days
2.7. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. Algae Extraction
4.2. Phycosynthesis of SeNPs from Spirulina platensis (SP-SeNPs)
4.3. Description of Phycosynthesis of SeNPs
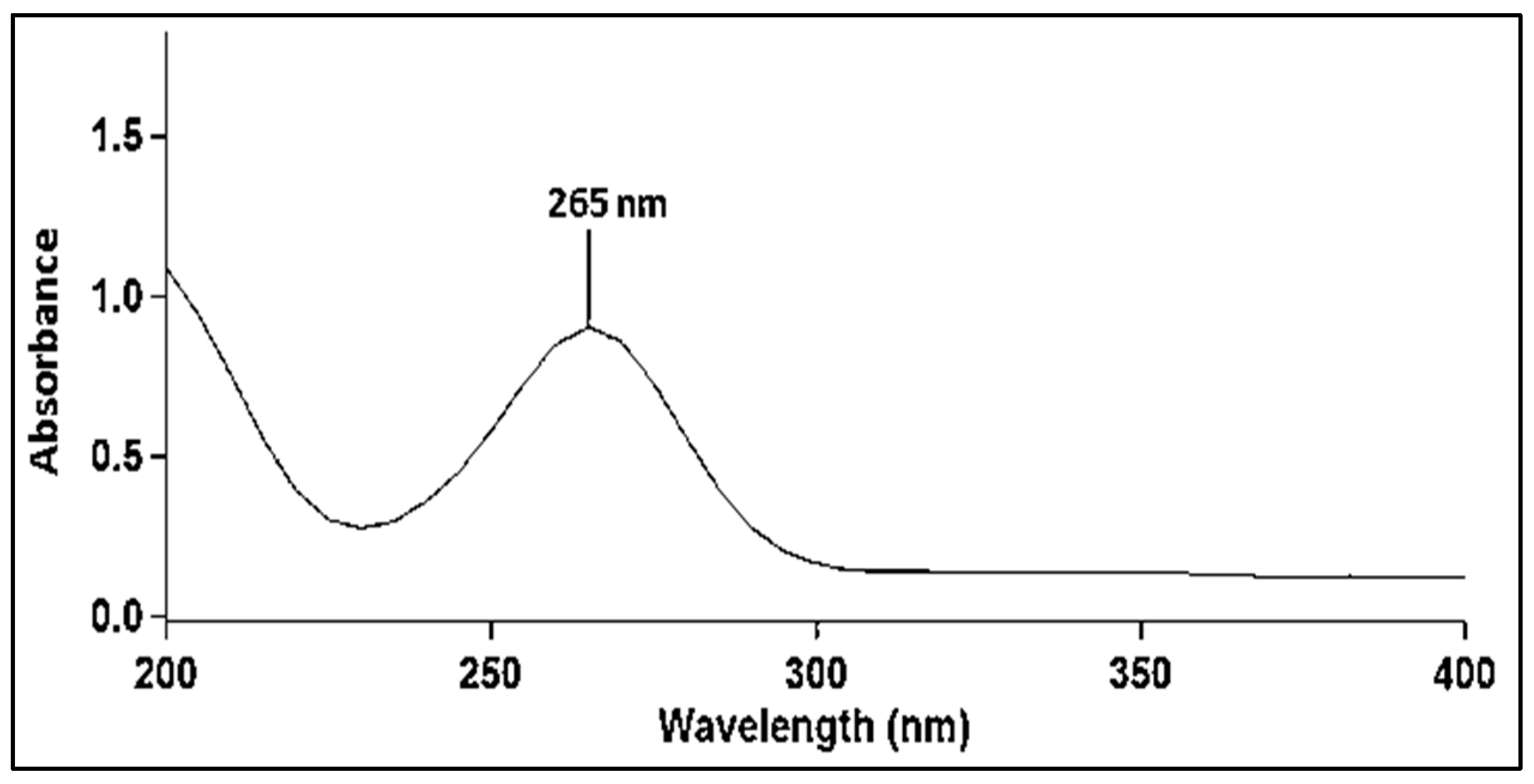
4.3.1. UV–Visible Spectroscopy
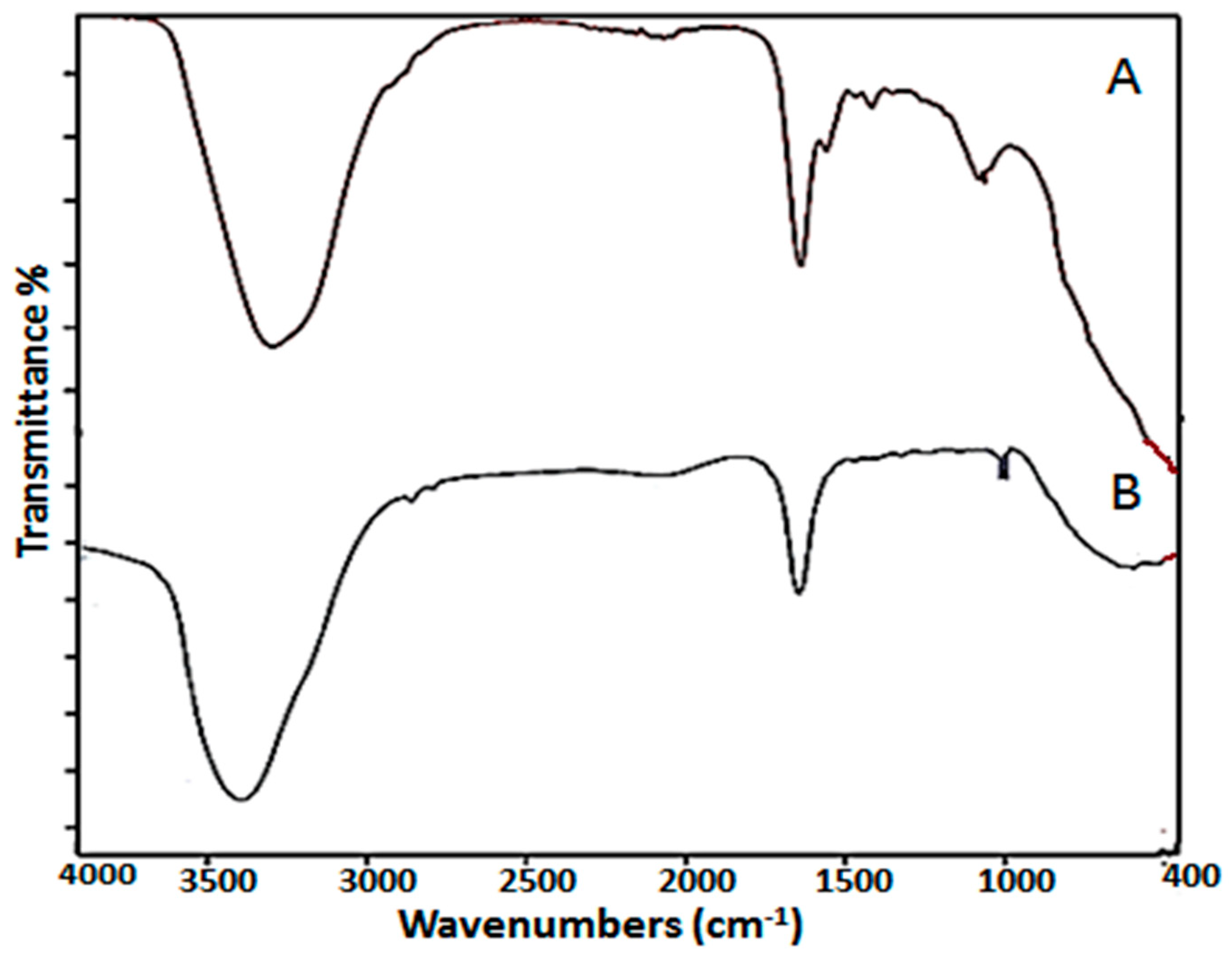
4.3.2. Fourier Transform Infrared Spectroscopic Analysis (FTIR)
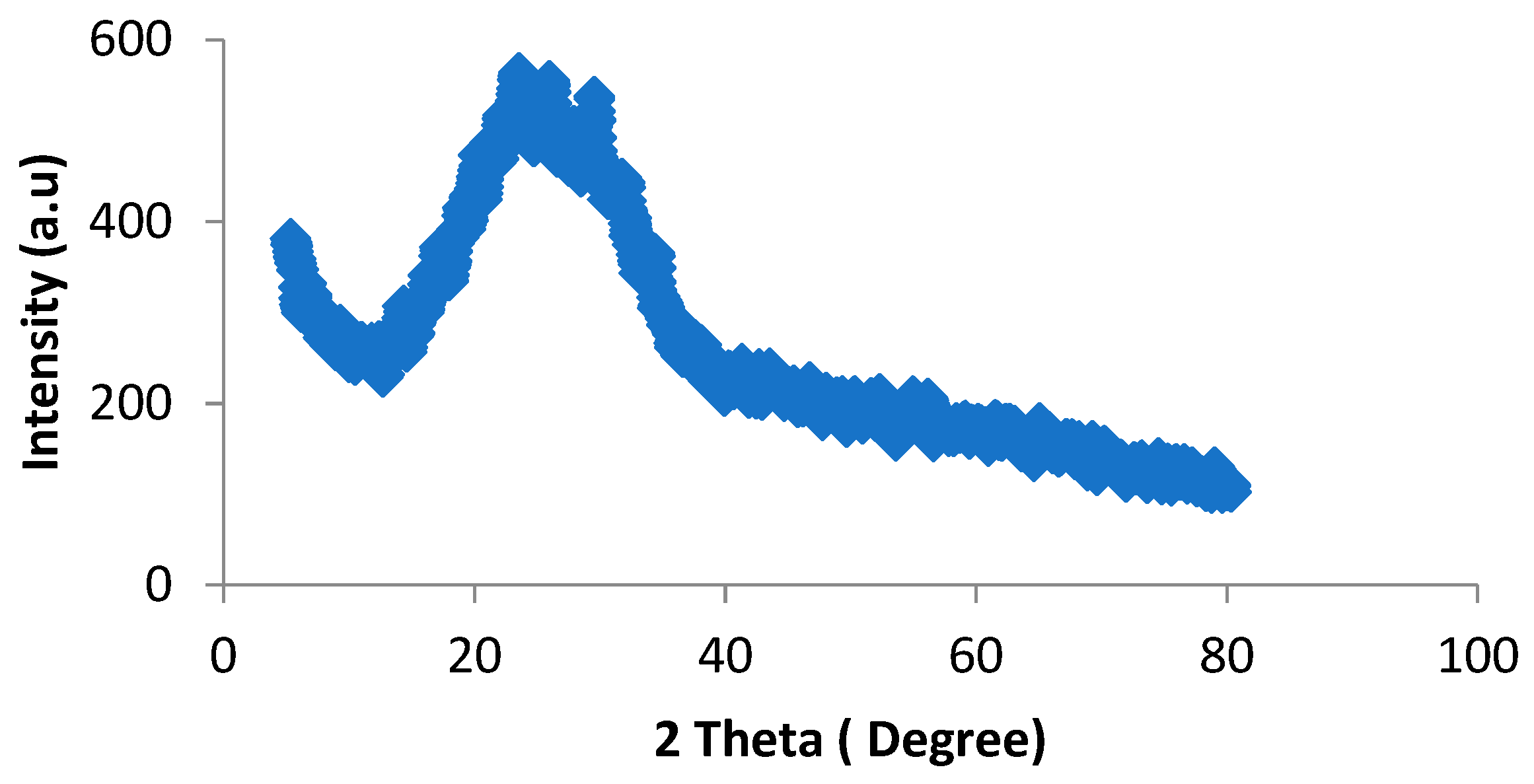
4.3.3. X-ray Diffraction Analysis (XRD)
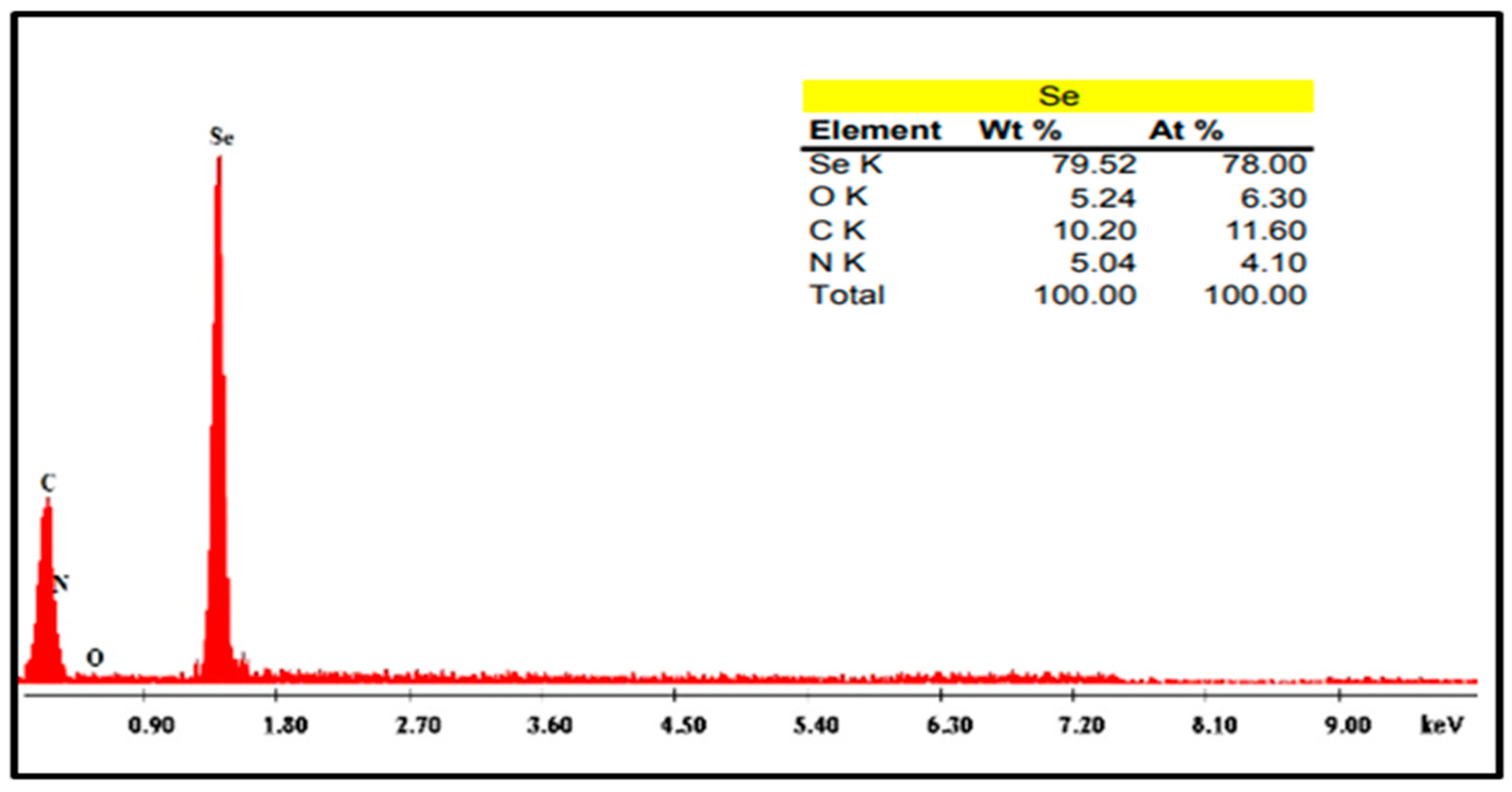
4.3.4. Energy Dispersive X-ray Testing (EDX)
4.3.5. Transmission Electron Microscopy Analysis (TEM)
4.4. Experimental Shrimp
4.5. Feeding Procedure
4.6. Experimental Design
4.7. Growth Performance Aspects
4.8. Shrimp Analytical Methods
4.9. Analysis of Biochemical Components in Muscles and Digestive Enzymes Activities in Digestive Tract of L. vannamei
4.10. Determination of Lethal and Sublethal Toxicity of Cadmium after 56 Days of Growth under Laboratory Condition
4.11. Bioaccumulation Analysis
4.12. Analysis of Oxidative and Antioxidant Indices before and after Cadmium Exposure in Muscles and Hepatopancreases
4.13. Histopathological Investigations
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Ethics Approval
References
- Eissa, E.H.; Ahmed, N.H.; El-Badawi, A.A.; Munir, M.B.; Abd Al-Kareem, O.M.; Eissa, M.E.H.; Hussien, E.H.M.; Sakr, S.S. Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac. Res. 2022, 53, 6606–6615. [Google Scholar] [CrossRef]
- Moss, S.M.; Arce, S.M.; Moss, D.R.; Otoshi, C.A. Disease Prevention Strategies for Penaeid Shrimp Culture; The Oceanic Institute: Waimanalo Beach, HI, USA, 2006. [Google Scholar]
- Yang, S.P.; Liu, H.L.; Guo, W.J.; Wang, C.G.; Sun, C.B.; Chan, S.F.; Li, S.C.; Tan, Z.H. Effects of salinity and temperature on the metabolic and immune parameters of the banana shrimp Fenneropenaeus merguiensis (De Man, 1988). Iran. J. Fish. Sci. 2020, 19, 2010–2023. [Google Scholar] [CrossRef]
- Abdirad, Z.; Ghaednia, B.; Mirbakhsh, M.; Kakoolaki, S.; Ghorbani Vaghei, R. The effects of rearing Pacific white-leg shrimp (Litopenaeus vannamei Boone, 1931) in biofloc system on the immune responses and survival rate in challenge with Vibrio harveyi. Iran. J. Fish. Sci. 2022, 21, 705–725. [Google Scholar] [CrossRef]
- Eissa, E.H.; Che-Zulkifli, C.I.; El-Badawi, A.A.; Ali, M.A.M.; Baghdady, E.S.; Abd Al-Kareem, O.M.; Ahmed, R.A. Growth-Promoting and Immunomodulatory Impacts of Commercial Stimulants on Kuruma Shrimp, Penaeus japonicus (Bate, 1888) Juveniles. Egypt. J. Aquat. Biol. Fish. 2021, 25, 607–617. [Google Scholar]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Fernandes, J.B.K.; Jamil, Z.; Sarwar, H. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk. J. Zool. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Rekha, R.; Vijayakumar, S.; Faggio, C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2018, 162, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, X.H.; Liu, S.; Li, R.; Zhu, Y.F.; Li, F.N.; Jiang, J.; Zhou, J.C.; Lei, X.G.; Sun, L.H. Selenium-Enriched Cardamine violifolia Increases Selenium and Decreases Cholesterol Concentrations in Liver and Pectoral Muscle of Broilers. J. Nutr. 2022, 152, 2072–2079. [Google Scholar] [CrossRef]
- Lang, C.; Mission, E.G.; Fuaad, A.A.H.A.; Shaalan, M. Nanoparticle tools to improve and advance precision practices in the Agrifoods Sector towards sustainability—A review. J. Clean. Prod. 2021, 293, 126063. [Google Scholar] [CrossRef]
- Eissa, E.H.; Alaidaroos, B.A.; Jastaniah, S.D.; Munir, M.B.; Shafi, M.E.; Abd El-Aziz, Y.M.; Bazina, W.K.; Ibrahim, S.b.; Eissa, M.E.H.; Paolucci, M. Dietary Effects of Nano Curcumin on Growth Performances, Body Composition, Blood Parameters and Histopathological Alternation in Red Tilapia (Oreochromis sp.) Challenged with Aspergillus flavus. Fishes 2023, 8, 208. [Google Scholar] [CrossRef]
- Dawit Moges, F.; Hamdi, H.; Al-Barty, A.; Zaid, A.A.; Sundaray, M.; Parashar, S.K.S. Effects of selenium nanoparticle on the growth performance and nutritional quality in Nile Tilapia, Oreochromis niloticus. PLoS ONE 2022, 17, e0268348. [Google Scholar] [CrossRef]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 2017, 474, 40–47. [Google Scholar] [CrossRef]
- Ghaffarizadeh, A.; Sotoudeh, E.; Mozanzadeh, M.T.; Sanati, A.M.; Ghasemi, A. Supplementing dietary selenium nano-particles increased growth, antioxidant capacity and immune-related genes transcription in Pacific whiteleg shrimp (Penaeus vannamei) juveniles. Aquac. Rep. 2022, 25, 101215. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC. Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A. Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish. Shellfish. Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- ElSaied, B.E.F.; Diab, A.M.; Tayel, A.A.; Alghuthaymi, M.A.; Moussa, S.H. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021, 10, 49–60. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y.A. Review on Cadmium Exposure in the Population and Intervention Strategies against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Zukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food. Chem. 2022, 379, 132167. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Shaalan, M.; Ali, S.E.; Younis, N.A. Immune responses and protective efficacy of diet supplementation with selenium nanoparticles against cadmium toxicity in Oreochromis niloticus. Aquac. Res. 2021, 52, 3677–3686. [Google Scholar] [CrossRef]
- Megahed, M.E.; Fathi, M.; Abderhman, A.M. Molecular investigation on the production of the pacific white shrimp (Litopenaeus vannamei) using diets based on biofloc meal. J. Aquac. Mar. Biol. 2019, 8, 216–224. [Google Scholar] [CrossRef]
- Panutrakul, S.; Senanan, W. Abundance of introduced Pacific whiteleg shrimp Penaeus vannamei (Boone, 1931) along the east coast of Thailand. Aquat. Invasions 2021, 16, 750–770. [Google Scholar] [CrossRef]
- Eissa, E.-S.H.; Ahmed, R.A.; Abd Elghany, N.A.; Elfeky, A.; Saadony, S.; Ahmed, N.H.; Sakr, S.E.-S.; Dayrit, G.B.; Tolenada, C.P.S.; Atienza, A.A.C.; et al. Potential Symbiotic Effects of β-1,3 Glucan, and Fructooligosaccharides on the Growth performance, Immune Response, Redox Status, and Resistance of Pacific White Shrimp, Litopenaeus vannamei to Fusarium solani Infection. Fishes 2023, 8, 105. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Abdul Kari, Z.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.; Hong, Q.; Yan, Z.; Yang, X.; Lu, K.; Chen, G.; Wang, L.; Chen, Y.A. Glutathione peroxidase gene from Litopenaeus vannamei is involved in oxidative stress responses and pathogen infection resistance. Int. J. Mol. Sci. 2022, 23, 567. [Google Scholar] [CrossRef]
- Ghazi, S.; Diab, A.M.; Khalafalla, M.M.; Mohamed, R.A. Synergistic effects of selenium and zinc oxide nanoparticles on growth performance, hemato-biochemical profile, immune and oxidative stress responses, and intestinal morphometry of Nile tilapia (Oreochromis niloticus). Biol. Trace. Elem. Res. 2022, 200, 364–374. [Google Scholar] [CrossRef]
- Abd El-Kader, M.F.; Fath El-Bab, A.F.; Abd-Elghany, M.F.; Abdel-Warith, A.W.A.; Younis, E.M.; Dawood, M.A.O. Selenium nanoparticles act potentially on the growth performance, hemato-biochemical indices, antioxidative, and immune-related genes of European seabass (Dicentrarchus labrax). Biol. Trace Elem. Res. 2021, 199, 3126–3134. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.-L.; Zhang, D.; He, N.; Zhang, J.; Liu, Y.H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef]
- Knight, R.; Marlatt, V.L.; Baker, J.A.; Lo, B.P.; deBruyn, A.M.H.; Elphick, J.R.; Martyniuk, C.J. Dietary selenium disrupts hepatic triglyceride stores and transcriptional networks associated with growth and Notch signaling in juvenile rainbow trout. Aquat. Toxicol. 2016, 180, 103–114. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnani, K.K.; Gupta, S.K.; Sharma, R.; Baitha, R.; Singh, D.K.; Singh, N.P. Immuno-protective role of biologically synthesized dietary selenium nanoparticles against multiple stressors in Pangasinodon hypophthalmus. Fish Immunol. 2018, 78, 289–298. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Trasvina-Arenas, C.H.; Garcia-Triana, A.; Peregrino-Uriarte, A.B.; Yepiz-Plascencia, B. White shrimp Litopenaeus vannamei catalase: Gene structure, expression and activity under hypoxia and reoxygenation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 164, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Campa-Cordova, A.I.; Hern´and ez-Saavedra, N.Y.; Ascencio, F. Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei). Comp. Biochem. Physiol. C Toxicol. Pharm. 2002, 133, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Kong, Y.; Ding, Z.; Zhang, Y.; Ye, J.; Du, Z. Dietary selenium requirement of juvenile oriental river prawn Macrobrachium nipponense. Aquaculture 2017, 476, 72–78. [Google Scholar] [CrossRef]
- Eissa, E.H.; Bazina, W.K.; Abd El-Aziz, Y.M.; Abd Elghany, N.A.; Tawfik, W.A.; Mossa, M.I.; Abd El Megeed, O.H.; Abd El-Hamed, N.B.; El-Saeed, A.F.; El-Haroun, E.; et al. Nano-selenium impacts on growth performance, digestive enzymes, antioxidant, immune resistance and histopathological scores of Nile tilapia, Oreochromis niloticus against Aspergillus flavus infection. Aquac. Int. 2023, 1–25.–25. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Wafeek, M. Response of Niletilapia, Oreochromis niloticus (L.) to environmental cadmium toxicity during organic selenium supplementation. J. World. Aquacu. Soc. 2010, 41, 106–114. [Google Scholar] [CrossRef]
- Lin, Y.; Shiau, S. The effects of dietary selenium on the oxidative stress of grouper, Epinephelus malabaricus, fed high copper. Aquaculture 2007, 267, 38–43. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Yan, M.; Li, J.; Zou, J.; Fan, L. Physiological responses of pacific white shrimp Litopenaeus vannamei to temperature fluctuation in low salinity water. Front. Physiol. 2019, 10, 1025. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.K.; Bhushan, S.; Singh, N.P. Impacts of acute toxicity of arsenic (III) alone and with high temperature on stress biomarkers, immunological status and cellular metabolism in fish. Aquat. Toxicol. 2019, 214, 105233. [Google Scholar] [CrossRef]
- Gailer, J. Chronic toxicity of AsIII in mammals: The role of (GS)2AsSe−. Biochimie 2009, 91, 1268–1272. [Google Scholar] [CrossRef]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, anti-microbial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hane, Y.; Watanabe, Y. Effect of pre-coagulation on mitigating irreversible fouling during ultrafiltration of surface water. Water Sci. Technol. 2005, 51, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zöllner, N.; Kirsch, K. Colorimetric Method for Determination of Total Lipids. J. Exp. Med. 1962, 135, 545–550. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acid. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Roe, J.H. The determination of sugar and blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955, 212, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Furne, M.; Hidalgo, M.C.; Lopez, A.; Garcia-Gallego, M.; Morales, A.E.; Domezain, A. Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 2005, 250, 391–398. [Google Scholar] [CrossRef]
- Henry, R.J.; Chiamori, N. Profile of Alpha Amylase in vulgaris. Clin. Chem. 1961, 6, 434. [Google Scholar] [CrossRef]
- Lorentz, K.; Weiss, T. Pankreaslipase—Eine Übersicht. Med. Lab. 1981, 34, 272–275. [Google Scholar]
- Finney, D.J. Estimation of the median effective dose. In Probit Analysis, 3rd ed.; Cambridge University: Cambridge, UK, 1971; pp. 20–49. [Google Scholar] [CrossRef]
- Tsoumbaris, P. Heavy Metals Determination in Food Stuff. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessalonica, Greece, 1990. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Nemec, A.; Drobnič-Košorok, M.; Skitek, M.; Pavlica, Z.; Galac, S.; Butinar, J. Total antioxidant capacity (TAC) values and their correlation with individual antioxidants in healthy beagles. Acta Vet. Brno 2000, 69, 297–303. [Google Scholar] [CrossRef][Green Version]
- Aebi, H. Catalase. In Methods Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–678. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]

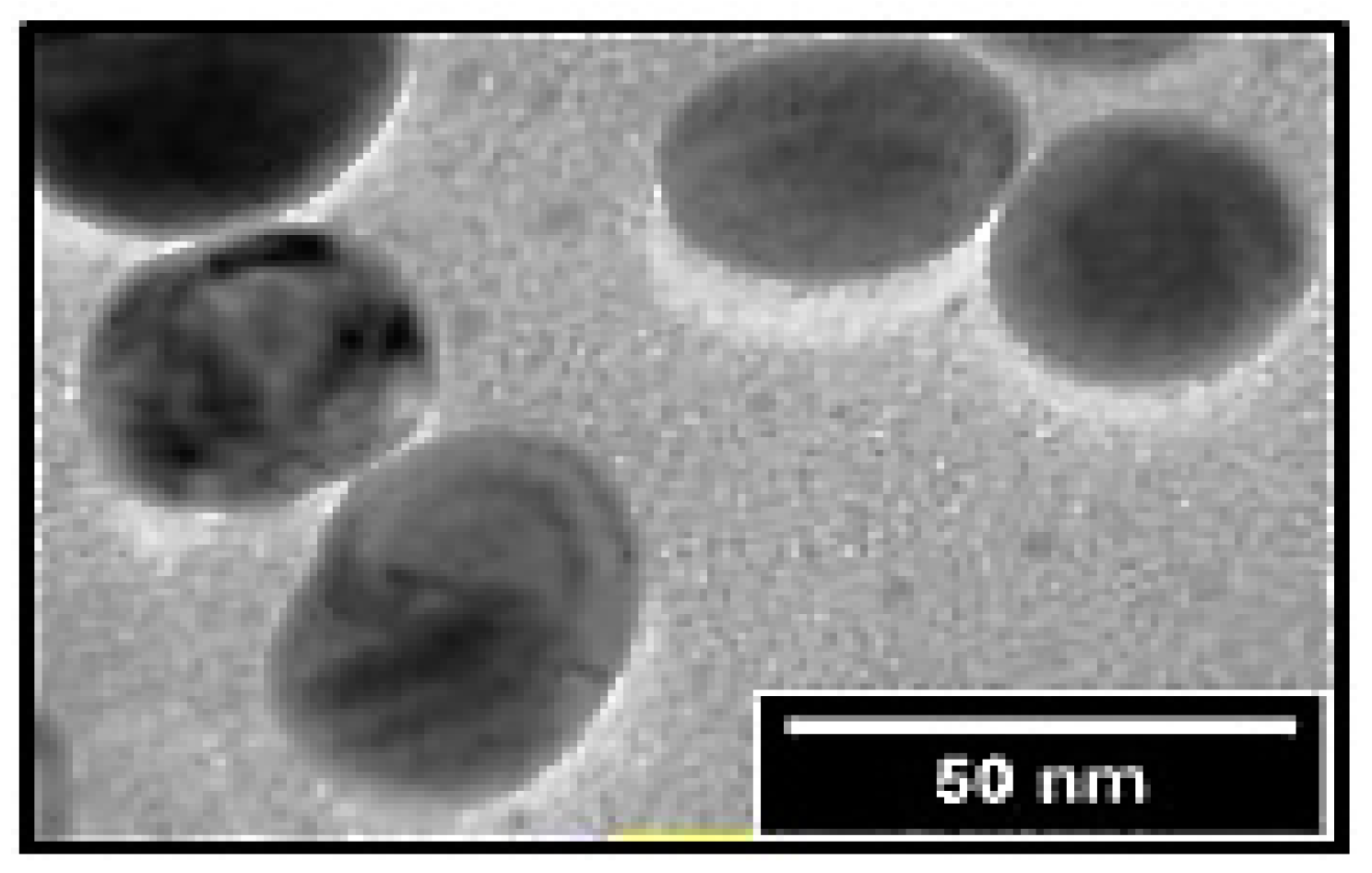
| Parameters | Concentrations of SP-SeNPs (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | F-Value | p-Value | |
| Initial weight (g/shrimp) | 6.00 ± 0.12 a | 6.03 ± 0.12 a | 6.13 ± 0.07 a | 6.03 ± 0.09 a | 0.33 | 0.80 |
| Final weight (g/shrimp) | 16.77 ± 0.23 c | 18.13 ± 0.22 b | 19.13 ± 0.27 a | 19.90 ± 0.26 a | 29.72 | 0.00001 |
| Weight gain (g/shrimp) | 10.77 ± 0.12 d | 12.10 ± 0.10 c | 13.00 ± 0.21 b | 13.87 ± 0.18 a | 70.98 | 0.00001 |
| Weight gain % | 179.50 ± 1.65 d | 200.65 ± 2.45 c | 211.94 ± 1.34 b | 229.85 ± 0.43 a | - | - |
| Feed intake (g feed/fish) | 18.74 ± 0.40 b | 19.42 ± 0.45 ab | 20.20 ± 0.22 a | 20.31 ± 0.27 a | 4.46 | 0.04 |
| ADG (g/shrimp/day) | 0.19 ± 0.00 d | 0.22 ± 0.00 c | 0.23 ± 0.00 b | 0.25 ± 0.00 a | 47.58 | 0.00001 |
| FCR (g) | 1.74 ± 0.02 a | 1.60 ± 0.03 b | 1.55 ± 0.01 b | 1.46 ± 0.00 c | 50.71 | 0.00001 |
| SGR (%/day) | 1.84 ± 0.01 d | 1.96 ± 0.01 c | 2.03 ± 0.01 b | 2.13 ± 0.00 a | 140.61 | 0.00001 |
| Initial shrimp number | 10.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 | - | - |
| Final shrimp number | 8.67 ± 0.33 a | 9.00 ± 0.58 a | 9.67 ± 0.33 a | 9.67 ± 0.33 a | 1.50 | 0.29 |
| Survival rate % | 86.67 ± 3.33 a | 90.00 ± 5.77 a | 96.67 ± 3.33 a | 96.67 ± 3.33 a | 1.50 | 0.29 |
| Shrimp biomass (g/m3) | 145.33 ± 6.22 c | 163.00 ± 8.93 bc | 184.83 ± 5.09 ab | 192.50 ± 8.67 a | 8.35 | 0.008 |
| Parameters | Concentrations of SP-SeNPs (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | p-Value | F-Value | |
| Moisture % | 79.17 ± 0.01 a | 79.18 ± 0.01 a | 79.20 ± 0.00 a | 79.20 ± 0.01 a | 2.19 | 0.17 |
| Crude protein% | 15.93 ± 0.03 c | 16.14 ± 0.02 b | 16.17 ± 0.02 b | 16.26 ± 0.02 a | 41.45 | 0.00001 |
| Crude lipid% | 1.88 ± 0.03 a | 1.87 ± 0.04 ab | 1.84 ± 0.02 ab | 1.77 ± 0.03 b | 2.65 | 0.12 |
| Ash% | 3.09 ± 0.09 b | 3.12 ± 0.01 b | 3.17 ± 0.01 a | 3.18 ± 0.02 a | 15.23 | 0.001 |
| Parameters | Concentrations of SP-SeNPs (mg/kg) | F- Value | p- Value | ||||
|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | ||||
| Biochemical indices (mg/g) | Protein | 4.56 ± 0.20 a | 5.55 ± 0.45 ab | 6.80 ± 0.30 b | 6.46 ± 0.45 b | 7.60 | 0.04 |
| Lipid | 1.65 ± 0.1 a | 3.34 ± 0.35 b | 5.1 ± 0.12 c | 3.98 ± 0.13 bc | 51.13 | 0.001 | |
| Amino acids | 95.3 ± 6.07 a | 112.68 ± 7.33 ab | 134.13 ± 3.63 b | 117.65 ± 4.4 ab | 8.3 | 0.03 | |
| Carbohydrate | 35.4 ± 2.9 a | 41.76 ± 2.36 ab | 59.15 ± 1.09 c | 50.42 ± 1.7 bc | 23.66 | 0.005 | |
| Digestive enzymes (U/mg) | Protease | 2.43 ± 0.03 a | 2.91 ± 0.12 b | 3.38 ± 0.05 c | 3.13 ± 0.03 bc | 36.17 | 0.002 |
| Amylase | 1.96 ± 0.04 a | 2.23 ± 0.12 a | 3.96 ± 0.04 b | 3.53 ± 0.42 b | 20.16 | 0.007 | |
| Lipase | 1.15 ± 0.01 a | 1.18 ± 0.02 a | 1.4 ± 0.05 b | 1.22 ± 0.02 a | 15.66 | 0.01 | |
| Time | Percentage Mortality (%) | |||||
|---|---|---|---|---|---|---|
| Conc | 24 h | 48 h | 72 h | 96 h | ||
| Control | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | ||
| 0.2 | 0 ± 0.00 | 0 ± 0.00 | 23.33 ± 3.33 | 26.67 ± 3.33 | ||
| 0.4 | 36.7 ± 3.33 | 36.7 ± 3.33 | 40 ± 0.00 | 50 ± 0.00 | ||
| 0.6 | 56.67 ± 6.7 | 60 ± 0.00 | 66.67 ± 6.7 | 70 ± 0.00 | ||
| 0.8 | 96.6 ± 3.33 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | ||
| 96 h LC50 | 0.4 mg/L | |||||
| 1/2 LC50 (for 10 days) | 0.2 mg/L | |||||
| Cd Accumulation | Different Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | T5 | T6 | T7 | F- Value | p- Value | |
| Muscles | 0 ± 0.0 a | 0 ± 0.00 a | 0 ± 0.00 a | 0 ± 0.00 a | 0.24 ± 0.04 b | 0.18 ± 0.08 ab | 0.11 ± 0.02 ab | 0.16 ± 0.04 ab | 7.91 | 0.005 |
| Hepatopancreases | 0 ± 0.0 a | 0.01 ± 0.005 a | 0.05 ± 0.02 a | 0.08 ± 0.01 ab | 0.6 ± 0.06 d | 0.30 ± 0.01 bc | 0.22 ± 0.03 b | 0.43 ± 0.08 cd | 17.03 | 0.002 |
| Se accumulation | ||||||||||
| Muscles | 3.9 ± 0.2 abc | 4.5 ± 0.53 bc | 5.39 ± 0.6 c | 6.18 ± 0.83 c | 0.9 ± 0.03 a | 4.19 ± 0.35 bc | 5.09 ± 0.97 c | 1.79 ± 0.33 ab | 10.24 | 0.002 |
| Hepatopancreases | 2.8 ± 0.2 bc | 3.39 ± 0.2 bcd | 5.4 ± 0.13 d | 5 ± 1.2 cd | 0.38 ± 0.1 a | 2.98 ± 0.03 bc | 4.1 ± 0.16 bcd | 2.71 ± 0.45 b | 13.93 | 0.001 |
| Tested Tissues | Different Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | T5 | T6 | T7 | F-Value | p-Value | ||
| Hepatopancreases | MDA (µmol/mg) | 123.6 ± 3.4 a | 61.2 ± 3.2 b | 77.4 ± 7.2 b | 85.5 ± 1 b | 211.4 ± 9.2 e | 168.7 ± 3.5 d | 115.6 ± 1 c | 52.7 ± 2.2 b | 86.1 | 0.0001 |
| CAT (U/g) | 35.6 ± 0.7 ab | 41.22 ± 1 bc | 46.8 ± 1.2 cd | 54.8 ± 0.9 e | 28.4 ± 1.43 a | 44.1 ± 0.15 c | 50.2 ± 1 de | 51.6 ± 1.6 de | 68.9 | 0.001 | |
| TAC (µmol/mg) | 39.5 ± 1.5 ab | 49.4 ± 6.5 bc | 51.6 ± 1.4 bc | 54.7 ± 1.5 bc | 27.7 ± 2.4 a | 36.9 ± 1.7 ab | 67.51 ± 1.6 c | 49 ± 2.7 ab | 8.5 | 0.004 | |
| SOD (U/g) | 64.1 ± 3.2 ab | 74.2 ± 4.1 ab | 86.9 ± 3.3 b | 80.4 ± 4.9 ab | 55.6 ± 9.7 a | 64.3 ± 4 ab | 77.9 ± 7.3 ab | 68.4 ± 1.6 ab | 3.7 | 0.04 | |
| Muscles | MDA (µmol/mg) | 1.5 ± 0.36 bc | 0.7 ± 0.03 a | 0.8 ± 0.04 ab | 0.9 ± 0.03 b | 2.99 ± 0.4 d | 0.95 ± 0.04 b | 1.91 ± 0.08 c | 1.3 ± 0.1 bc | 30.4 | 0.001 |
| CAT (U/g) | 22.8 ± 0.1 ab | 23.2 ± 0.1 ab | 25.9 ± 0.7 bc | 23.6 ± 0.4 ab | 17.4 ± 1.13 a | 24 ± 1.6 bc | 30.4 ± 2.8 c | 29.3 ± 0.8 bc | 11.05 | 0.002 | |
| TAC (µmol/mg) | 20.8 ± 2.1 ab | 23.1 ± 0.1 ab | 31.6 ± 1.6 de | 29.4 ± 0.6 cd | 17.3 ± 0.5 a | 24.8 ± 0.3 bc | 36.7 ± 0.9 e | 30 ± 1.1 cd | 34.08 | 0.0001 | |
| SOD (U/g) | 8.7 ± 0.33 ab | 9.2 ± 0.25 ab | 17.1 ± 0.4 cd | 16.8 ± 1.5 cd | 6.41 ± 1.11 a | 12.21 ± 1 bc | 19.9 ± 0.4 d | 19.6 ± 1.4 d | 33.1 | 0.0001 | |
| Contents | g/kg Diet | Proximate Chemical Analysis | % |
|---|---|---|---|
| Wheat flour | 120.0 | Dry mater | 90.64 |
| Shrimp meal | 250.0 | Moisture | 9.35 |
| Rice bran | 70.0 | Crude protein (N × 6.25) | 38.79 |
| Soybean meal | 150.0 | Crude fat | 10.90 |
| Fish meal | 300.0 | Crude fiber | 1.74 |
| Fish oil | 60.0 | Ash | 6.15 |
| CMC | 10.0 | Carbohydrate (NFE) | 32.98 |
| Vit. and min mix 1 | 20.0 | Gross energy kcal/100 g 3 | 459.47 kcal/100 g 3 |
| Min mix 2 | 20.0 | ||
| 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, R.M.; Nassar, S.E.; Alaidaroos, B.A.; Jastaniah, S.D.; Dighiesh, H.S.; Eissa, E.-S.H.; AL-Farga, A.; Kari, Z.A.; Téllez-Isaías, G.; Attia, M.S. Impacts of Dietary Selenium Nanoparticles from Spirulina platensis on Growth Performance, Physio-Biochemical Components and Alleviating Effect against Cadmium Toxicity in Pacific White Shrimp Litopenaeus vannamei. Catalysts 2023, 13, 1389. https://doi.org/10.3390/catal13111389
Said RM, Nassar SE, Alaidaroos BA, Jastaniah SD, Dighiesh HS, Eissa E-SH, AL-Farga A, Kari ZA, Téllez-Isaías G, Attia MS. Impacts of Dietary Selenium Nanoparticles from Spirulina platensis on Growth Performance, Physio-Biochemical Components and Alleviating Effect against Cadmium Toxicity in Pacific White Shrimp Litopenaeus vannamei. Catalysts. 2023; 13(11):1389. https://doi.org/10.3390/catal13111389
Chicago/Turabian StyleSaid, Radwa M., Safaa E. Nassar, Bothaina A. Alaidaroos, Samyah D. Jastaniah, Hagar Sedeek Dighiesh, El-Sayed Hemdan Eissa, Ammar AL-Farga, Zulhisyam Abdul Kari, Guillermo Téllez-Isaías, and Mai S. Attia. 2023. "Impacts of Dietary Selenium Nanoparticles from Spirulina platensis on Growth Performance, Physio-Biochemical Components and Alleviating Effect against Cadmium Toxicity in Pacific White Shrimp Litopenaeus vannamei" Catalysts 13, no. 11: 1389. https://doi.org/10.3390/catal13111389
APA StyleSaid, R. M., Nassar, S. E., Alaidaroos, B. A., Jastaniah, S. D., Dighiesh, H. S., Eissa, E.-S. H., AL-Farga, A., Kari, Z. A., Téllez-Isaías, G., & Attia, M. S. (2023). Impacts of Dietary Selenium Nanoparticles from Spirulina platensis on Growth Performance, Physio-Biochemical Components and Alleviating Effect against Cadmium Toxicity in Pacific White Shrimp Litopenaeus vannamei. Catalysts, 13(11), 1389. https://doi.org/10.3390/catal13111389










