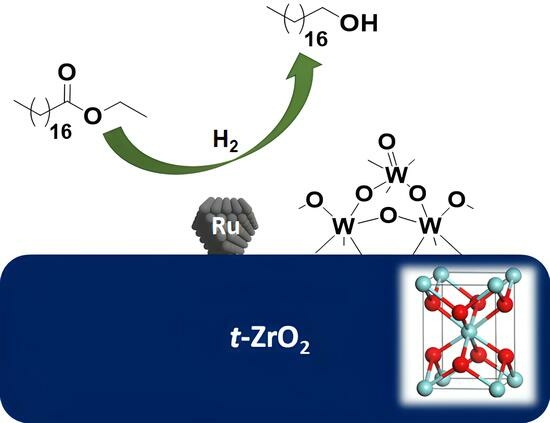
Study on the Hydrogenation of Ethyl Stearate to the Fatty Alcohol 1-Octadecanol over Ru on Tungstated Zirconia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Synthesized Catalysts
2.2. Physico-Chemical and Textural Characterization of Synthesized Catalysts
2.3. Catalytic Studies
- Ru(2)/W(33)/Zr > Ru(1.3)/W(33)/Zr > Ru(0.5)/W(33)/Z
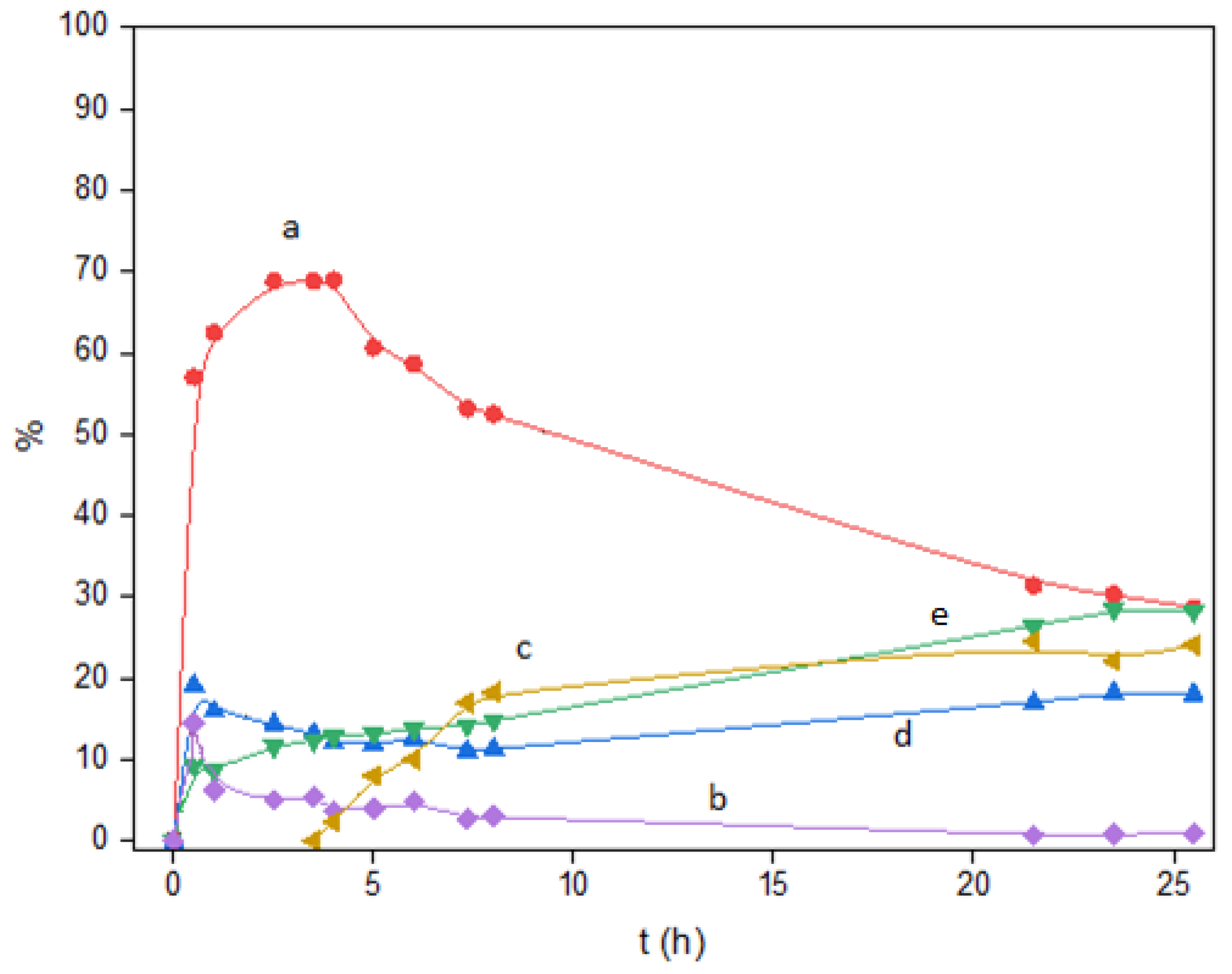
2.4. Mechanistic Studies
- (a)
- The free carboxylic acid SA was put in contact with Ru(1.3)/W(33)/Zr under H2 (P = 40 bar) at 175 °C, being rapidly transformed into the long-chain hydrocarbons C18 and C17, through the intermediation of fatty alcohol C18OH. In no case was the formation of aldehyde detected. However, given that SA was always a minority and tended to decrease with time (Figure 6), we inferred that the main route towards C18OH could be very likely the ester hydrogenolysis followed by reduction of the resulting aldehyde and not the hydrogenation of SA (see Scheme 2 below).
- (b)
- A long-chain aldehyde was taken as an aldehyde model (i.e., decanal), being contacted with Ru(1)/W(33)/Zr under the same experimental conditions. In this case, the aldehyde was rapidly transformed into the long-chain paraffins C10 and C9 in less than 10 min, giving a product distribution equivalent to that obtained starting from the fatty acid SA. Thus, this experimental fact confirmed that the aldehyde, although it is never detected, is more certainly the key intermediate in the transformation into fatty alcohol and paraffin.
- (c)
- In addition, C18OH was also transformed exclusively into the same C17 and C18 hydrocarbons in the presence of Ru(1.3)/W(33)/Zr as a catalyst (A control reaction was carried out to confirm that heptadecane C17 came from the decarbonylation of 1-octadecanol [Reaction conditions: 175 °C, PH2 = 40 bar, C18OH (1 mmol), n-dodecane as internal standard (0.6 mmol), hexane (3 mL), Ru(1.3)W(33)/Zr catalyst (100mg)]. In this case C18OH was transformed into long chain hydrocarbons C17 and C18).
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Synthesis of Catalysts
3.2.1. Synthesis of ZrO2 and Mixed Oxides WZrOx (W/Zr) and WTiOx (W/Ti)
- Synthesis of ZrO2
- Synthesis of W/ZrOx support (W/Zr)
- Synthesis of WTiOx support (W/Ti)
3.2.2. Synthesis of Ru Supported on ZrO2 (Ru/Zr)
3.2.3. Synthesis of Ru Supported on WZrOx and WTiOx (Ru/W/Zr and Ru/W/TiO2)
3.3. Characterization of Catalysts
3.4. Catalytic Reactions: Hydrogenation of Ethyl Stearate (ES) to 1-Octadecanol (C18OH)
3.5. Reusability and Stability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richtler, H.J.; Knaut, J. Challenges to a mature industry: Marketing and economics of oleochemicals of Western Europe. J. Am. Oil Chem. Soc. 1984, 61, 160–175. [Google Scholar] [CrossRef]
- Knaut, J.; Richtler, H.J. Trends in industrial uses of palm and lauric oils. J. Am. Oil Chem. Soc. 1985, 62, 317–327. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.A.; Torres, G.C.; Mazzieri, V.A.; Pieck, C.L. Selective hydrogenation of fatty acids and methyl esters of fatty acids to obtain fatty alcohols–a review. J. Chem. Tech. Biotech. 2017, 92, 27–42. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dai, H.; Bhattacharya, P.; Fairweather, N.T.; Gibson, M.S.; Krause, J.A.; Guan, H. Iron-Based Catalysts for the Hydrogenation of Esters to Alcohols. J. Am. Chem. Soc. 2014, 136, 7869–7872. [Google Scholar] [CrossRef]
- Clarke, M.L. Recent developments in the homogeneous hydrogenation of carboxylic acid esters. Catal. Sci. Technol. 2012, 2, 2418–2423. [Google Scholar] [CrossRef]
- Srimani, D.; Mukherjee, D.A.; Goldberg, A.F.G.; Leitus, G.; Diskin-Posner, Y.; Shimon, L.J.W.; Ben David, Y.; Milstein, D. Cobalt-catalyzed hydrogenation of esters to alcohols: Unexpected reactivity trend indicates ester enolate intermediacy. Angew. Chem. Int. Ed. 2015, 54, 12357–12360. [Google Scholar] [CrossRef] [PubMed]
- Spasyuk, D.; Vicent, C.; Gusev, D.G. Chemoselective Hydrogenation of Carbonyl Compounds and Acceptorless Dehydrogenative Coupling of Alcohols. J. Am. Chem. Soc. 2015, 137, 3743–3746. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Ishii, A.; Dub, P.A.; Ikariya, T. Practical Selective Hydrogenation of α-Fluorinated Esters with Bifunctional Pincer-Type Ruthenium(II) Catalysts Leading to Fluorinated Alcohols or Fluoral Hemiacetals. J. Am. Chem. Soc. 2013, 135, 9600–9603. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, J.H.; Yuan, M.L.; Zhou, Q.L. Ruthenium complexes of tetradentate bipyridine ligands: Highly efficient catalysts for the hydrogenation of carboxylic esters and lactones. Green Chem. 2014, 16, 4081–4085. [Google Scholar] [CrossRef]
- Wang, F.; Tan, X.; Lv, H.; Zhang, X. New Ruthenium Complexes Based on Tetradentate Bipyridine Ligands for Catalytic Hydrogenation of Esters. Chem. Asian J. 2016, 11, 2103–2106. [Google Scholar] [CrossRef]
- Pham, J.; Jarczyk, C.E.; Reynolds, E.F.; Kelly, S.E.; Kim, T.; He, T.; Keith, J.M.; Chianese, A.R. The key role of the latent N–H group in Milstein’s catalyst for ester hydrogenation. Chem. Sci. 2021, 12, 8477–8492. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, N.T.; Gibson, M.S.; Guan, H. Homogeneous Hydrogenation of Fatty Acid Methyl Esters and Natural Oils under Neat Conditions. Organometallics 2015, 34, 335–339. [Google Scholar] [CrossRef]
- Singh, R.; Arora, A.; Singh, V. Biodiesel from oil produced in vegetative tissues of biomass—A review. Bioresour. Technol. 2021, 326, 124772. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Lestari, S.; Maki-Arvela, P.; Beltramini, J.; Max Lu, G.Q.; Murzin, D.Y. Transforming Triglycerides and Fatty Acids into Biofuels. ChemSusChem 2009, 2, 1109–1119. [Google Scholar] [CrossRef]
- Korstanje, T.J.; Vlugt, J.I.; Elsevier, C.J.; Bruin, B. Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst. Science 2015, 350, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Voeste, T.; Schmidt, H.; Marschner, F. Continuous Process of Producing Fatty Alcohols. U.S Patent 4259536A, 31 March 1981. Appl. No.: 100576. [Google Scholar]
- Voeste, T.; Buchold, H. Production of fatty alcohols from fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 350–352. [Google Scholar] [CrossRef]
- Buchold, H. Natural fats and oils route to fatty alcohols. Chem. Eng. 1983, 90, 42–43. [Google Scholar]
- Thakur, D.S.; Kundu, A. Catalysts for Fatty Alcohol Production from Renewable resources. J. Am. Oil Chem. Soc. 2016, 93, 1575–1593. [Google Scholar] [CrossRef]
- Adkins, H.; Folkers, K. The catalytic hydrogenation of esters to alcohols. J. Am. Chem. Soc. 1931, 53, 1095–1097. [Google Scholar] [CrossRef]
- Cant, N.W.; Trimm, D.L. The catalytic hydrogenolysis of esters to alcohols. Catal. Rev. 1994, 36, 645–683. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L.; Huang, H.; Tao, S.; Zhang, H.; Xiao, X.; Fang, J. Research Progress in Catalysts for Fatty Acid Ester Hydrogenation. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012136. [Google Scholar] [CrossRef]
- Fan, P.; Tang, M.X.; Jia, S.Y.; Du, M.X.; Qi, Y.Q.; Hou, X.L. Preparation of fatty alcohol by hidrogenation of fatty acid methyl esters at subcritical conditions. China Surfactant Deterg. Cosmet. 2011, 41, 408–410. [Google Scholar]
- He, L.; Cheng, H.; Liang, G.; Yu, Y.; Zhao, F. Effect of structure of CuO/ZnO/Al2O3 composites on catalytic performance for hydrogenation of fatty acid ester. Appl. Catal. A Gen. 2013, 452, 88–93. [Google Scholar] [CrossRef]
- Jiang, Z.; Fan, C.; Fan, M.; Zhang, P. Hydrogenation of methyl stearate to stearyl alcohol over Cu-based catalysts. Petrochem. Technol. 2016, 45, 1441–1448. [Google Scholar]
- Jiang, Z.B.; Fan, M.M.; Zhang, P.B.; Jiang, B. Effect of dropping sequence on the preparation of Cu-Zn-Al-Ba catalyst for hydrogenation of fatty acid esters. China J. Inorg. Chem. 2016, 32, 1047–1054. [Google Scholar]
- Huang, C.; Zhang, H.; Zhao, Y.; Chen, S.; Liu, Z. Journal of Colloid and Interface Science, Diatomite-supported Pd–M (M = Cu, Co, Ni) bimetal nanocatalysts for selective hydrogenation of long-chain aliphatic esters. J. Colloid Interface Sci. 2012, 386, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, S.; Li, W.; Song, J.; Yu, H.; Liu, Y.; Wu, Q. Hydrogenation of fatty acid methyl ester over Pd nanoparticles stabilized by thermoregulated ionic liquid. Mol. Catal. 2018, 458, 106–111. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Lin, W.; Li, W.; Cheng, H.; Yu, Y.; Fujita, S.; Arai, M.; Zhao, F. The selective hydrogenation of ethyl stearate to stearyl alcohol over Cu/Fe bimetallic catalysts. J. Mol. Catal. A Chem. 2014, 392, 143–149. [Google Scholar] [CrossRef]
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol. Appl. Catal. A Gen. 2016, 510, 244–259. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, T.; Tang, X.; Li, D.; Zhang, W.; Lin, D.; Li, R.; Yan, H.; Liu, Y.; Feng, X.; et al. Octadecanol Production from Methyl Stearate by Catalytic Transfer Hydrogenation over Synergistic Co/HAP Catalysts. Energy Fuels 2021, 35, 9970–9982. [Google Scholar] [CrossRef]
- Wang, L.; Niu, X.; Chen, J. SiO2 supported Ni-In intermetallic compounds: Efficient for selective hydrogenation of fatty acid methyl esters to fatty alcohols. Appl. Catal. B. 2020, 278, 119293. [Google Scholar] [CrossRef]
- Zhou, Y.; Remon, J.; Jiang, Z.; Matharu, A.V.; Hu, C. Tuning the selectivity of natural oils and fatty acids/esters deoxygenation to biofuels and fatty alcohols: A review. Green Energy Environ. 2023, 8, 722–743. [Google Scholar] [CrossRef]
- Ni, J.; Leng, W.; Mao, J.; Wang, J.; Lin, J.; Jiang, D.; Li, X. Tuning electron density of metal nickel by support defects in Ni/ZrO2 for selective hydrogenation of fatty acids to alkanes and alcohols. Appl. Catal. B: Environ. 2019, 253, 170–178. [Google Scholar] [CrossRef]
- Luo, Z.; Bing, Q.; Kong, J.; Liu, J.; Zhao, C. Mechanism of supported Ru3Sn7 nanocluster-catalyzed selective hydrogenation of coconut oil to fatty alcohols. Catal. Sci. Technol. 2018, 8, 1322–1332. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Mazzieri, V.A.; Pronier, S.; Vicerich, M.A.; Especel, C.; Epron, F.; Pieck, C.L. Ru-Sn-B/TiO2 catalysts for methyl oleate selective hydrogenation. Influence of the preparation method and the chlorine content. J. Chem. Technol. Biotechnol. 2019, 94, 982–991. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Mazzieri, V.A.; Oportus, M.; Reyes, P.; Pieck, C.L. Influence of Ge content on the activity of Ru–Ge–B/Al2O3 catalysts for selective hydrogenation of methyl oleate to oleyl alcohol. Catal. Today 2013, 213, 81–86. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 0849396883/ISBN 978-0849396885. [Google Scholar]
- Auxeméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Ĺegutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous Supercritical Solvothermal Preparation of Nanostructured Ceria-Zirconia as Supports for Dry Methane Reforming Catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Sunita, G.; Devassy, B.M.; Vinu, A.; Sawant, D.P.; Balasubramanian, V.; Halligudi, S. Synthesis of biodiesel over zirconia-supported isopoly and heteropoly tungstate catalysts. Catal. Commun. 2008, 9, 696–702. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, C.H.; Suh, Y.W. Higher Brønsted acidity of WOx/ZrO2 catalysts prepared using a high-surface-area zirconium oxyhydroxide. Mol. Catal. 2017, 438, 272–279. [Google Scholar] [CrossRef]
- Soultanidis, N.; Zhou, W.; Psarras, A.C.; Gonzalez, A.J.; Iliopoulou, E.F.; Kiely, C.J.; Wachs, I.E.; Wong, M.S. Relating n-pentane isomerization activity to the tungsten surface density of WOx/ZrO2. J. Am. Chem. Soc. 2010, 132, 13462–13471. [Google Scholar] [CrossRef]
- Garcia-Perez, D.; Alvarez-Galan, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Influence of the Reduction Temperature and the Nature of the Support on the Performance of Zirconia and Alumina-Supported Pt Catalysts for n-Dodecane Hydroisomerization. Catalysts 2021, 11, 88. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z. Epoxidation of the polyisobutene using WO3/ZrO2 catalyst. Res. Chem. Intermed. 2012, 38, 1921–1929. [Google Scholar] [CrossRef]
- Gauna, M.R.; Conconi, M.S.; Gomez, S.; Suárez, G.; Aglietti, E.F.; Rendtorff, N.M. Monoclinic-tetragonal zirconia quantification of commercial nanopowder mixtures by XRD and DTA. Ceramics-Silikáty 2015, 59, 318–325. [Google Scholar]
- Rodina, V.O.; Ermakov, D.Y.; Saraev, A.A.; Reshetnikov, S.I.; Yakovlev, V.A. Influence of reaction conditions and kinetic analysis of the selective hydrogenation of oleic acid toward fatty alcohols on Ru-Sn-B/Al2O3 in the flow reactor. Appl. Catal. B Environ. 2017, 209, 611–620. [Google Scholar] [CrossRef]
- Fonseca Benítez, C.A.; Mazzieri, V.A.; Vera, C.R.; Benitez, V.M.; Pieck, C.L. Selective hydrogenation of oleic acid to fatty alcohols over a Rh–Sn–B/Al2O3 catalyst: Kinetics and optimal reaction conditions. React. Chem. Eng. 2021, 6, 726–746. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Chadwick, A.V.; Greaves, G.N.; Moroney, L.M. EXAFS Study of Yttria-Stabilized Zirconia. J. Am. Ceram. Soc. 1986, 69, 272–277. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. X-Ray-Absorption Studies of Zirconia Polymorphs. II. Effect of Y2O3 Dopant on ZrO2 Structure. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 48, 10074–10081. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization-An X-Ray Absorption Study: I, Trivalent Dopants. J. Am. Ceram. Soc. 1994, 77, 118–128. [Google Scholar] [CrossRef]
- Patil, M.K.; Prasad, A.N.; Reddy, B.M. Zirconia-Based Solid Acids: Green and Heterogeneous Catalysts for Organic Synthesis. Curr. Org. Chem. 2011, 15, 3961–3985. [Google Scholar] [CrossRef]
- Song, K.; Zhang, H.; Zhang, Y.; Tang, Y.; Tang, K. Preparation and characterization of WOx/ZrO2 nanosized catalysts with high WOx dispersion threshold and acidity. J. Catal. 2013, 299, 119–128. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. Available online: https://li01.tci-thaijo.org/index.php/anres/article/view/244620 (accessed on 20 August 2023).
- Sun, G.; Xu, A.; He, Y.; Yang, M.; Du, H.; Sun, C. Ruthenium catalysts supported on high-surface-area zirconia for the catalytic wet oxidation of N,N-dimethyl formamide. J. Hazard. Mater. 2008, 156, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Jayat, F.; Sabater, M.J.; Rohan, D.; Guisnet, M. Acylation of aromatics over a HBEA Zeolite. Effect of solvent and of acylating agent. Stud. Surf. Sci. Catal. 1997, 108, 91–98. [Google Scholar]
- Adam, W.; Corma, A.; Miranda, M.A.; Sabater, M.J.; Sahin, C. Photochemical and chemical electron transfer reactions of bicyclo[2.1.0]pentanes (housanes) in solution and in zeolite cavities. J. Am. Chem. Soc. 1996, 118, 2380–2386. [Google Scholar] [CrossRef]
- Cerro, M.; Iborra, S.; Martínez, C.; Sabater, M.J.; Corma, A. Methanolysis of sunflower oil using gem-diamines as active organocatalysts for biodiesel production. Appl. Catal. A. Gen 2010, 382, 36–42. [Google Scholar] [CrossRef]
- Tamura, M.; Nakagawa, Y.; Tomishige, K. Recent developments of heterogeneous catalysts for hydrogenation of carboxylic acids to their corresponding alcohols. Asian J. Org. Chem. 2020, 9, 126–143. [Google Scholar] [CrossRef]
- Zhao, K.; Han, W.; Tang, Z.; Zhang, G.; Lu, J.; Lu, G.; Zhen, X. Investigation of coating technology and catalytic performance over monolitic V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 53–60. [Google Scholar] [CrossRef]
- Lopez-Prado, M.V.; Sabater, M.J.; Corma, A. A Bifunctional Metal/Acid Catalyst for One-pot Multistep Synthesis of Pharmaceuticals. Petrol. Chem. 2020, 60, 499–507. [Google Scholar] [CrossRef]
- Bonds, G.C. Metal-Catalysed Reactions of Hydrocarbons; Springer: New York, NY, USA, 2005. [Google Scholar]
- Corma, A.; Sabater, M.J. Gold Catalysis for Hydrogenation Reactions; RSC Catalysis Series; RSC: London, UK, 2013; Volume 13, pp. 146–200. ISBN 978-1-84973-571-1. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; van Santen, R.A.; Hensen, E.J.M. DRIFTS study of the chemical state of modifying gallium ions in reduced Ga/ZSM-5 prepared by impregnation: I. Observation of gallium hydrides and application of CO adsorption as a molecular probe for reduced gallium ions. J. Catal. 2004, 227, 263–269. [Google Scholar] [CrossRef]
- Barton, D.G.; Soled, S.L.; Iglesia, E. Solid acid catalysts based on supported tungsten oxides. Top. Catal. 1998, 6, 87–99. [Google Scholar] [CrossRef]
- Calabro, D.C.; Vartuli, J.C.; Santiesteban, J.G. The characterization of tungsten-oxide-modified zirconia supports for dual functional catalysis. Top. Catal. 2002, 18, 231–242. [Google Scholar] [CrossRef]
 ) and t-ZrO2 (
) and t-ZrO2 ( ) and WO3 (
) and WO3 ( ); (B) X-ray diffraction patterns of Ru-doped ZrO2 (Ru/Zr) and Ru-doped ZrO2 with increasing amounts of WOx (Ru/W/Zr) in the 10 to 90° 2θ range: (a) Ru(1)/Zr, (b) Ru(1)/W(12)/Zr, and (c) Ru(1.3)/W(33)/Zr.
); (B) X-ray diffraction patterns of Ru-doped ZrO2 (Ru/Zr) and Ru-doped ZrO2 with increasing amounts of WOx (Ru/W/Zr) in the 10 to 90° 2θ range: (a) Ru(1)/Zr, (b) Ru(1)/W(12)/Zr, and (c) Ru(1.3)/W(33)/Zr.
 ) and t-ZrO2 (
) and t-ZrO2 ( ) and WO3 (
) and WO3 ( ); (B) X-ray diffraction patterns of Ru-doped ZrO2 (Ru/Zr) and Ru-doped ZrO2 with increasing amounts of WOx (Ru/W/Zr) in the 10 to 90° 2θ range: (a) Ru(1)/Zr, (b) Ru(1)/W(12)/Zr, and (c) Ru(1.3)/W(33)/Zr.
); (B) X-ray diffraction patterns of Ru-doped ZrO2 (Ru/Zr) and Ru-doped ZrO2 with increasing amounts of WOx (Ru/W/Zr) in the 10 to 90° 2θ range: (a) Ru(1)/Zr, (b) Ru(1)/W(12)/Zr, and (c) Ru(1.3)/W(33)/Zr.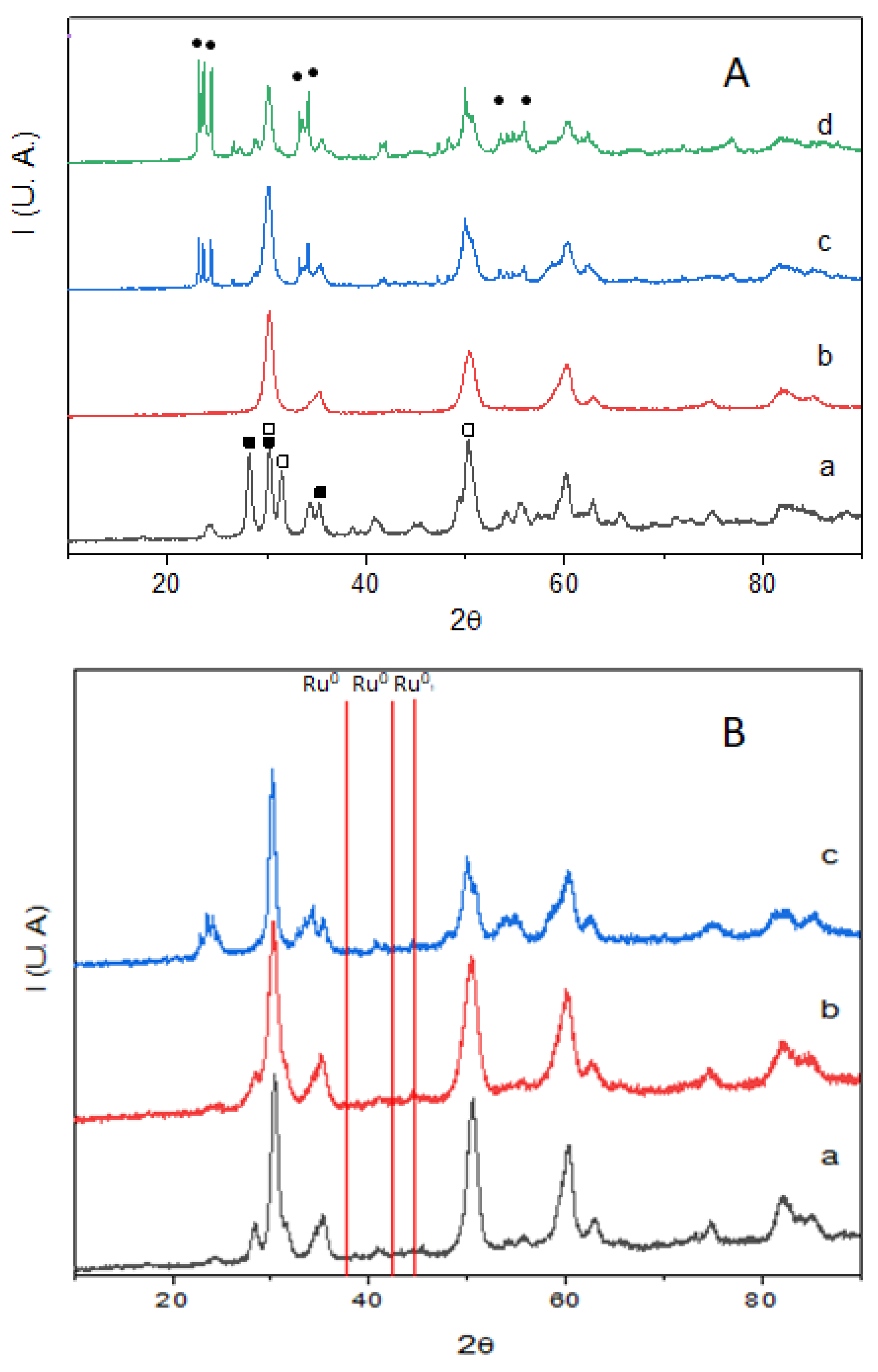

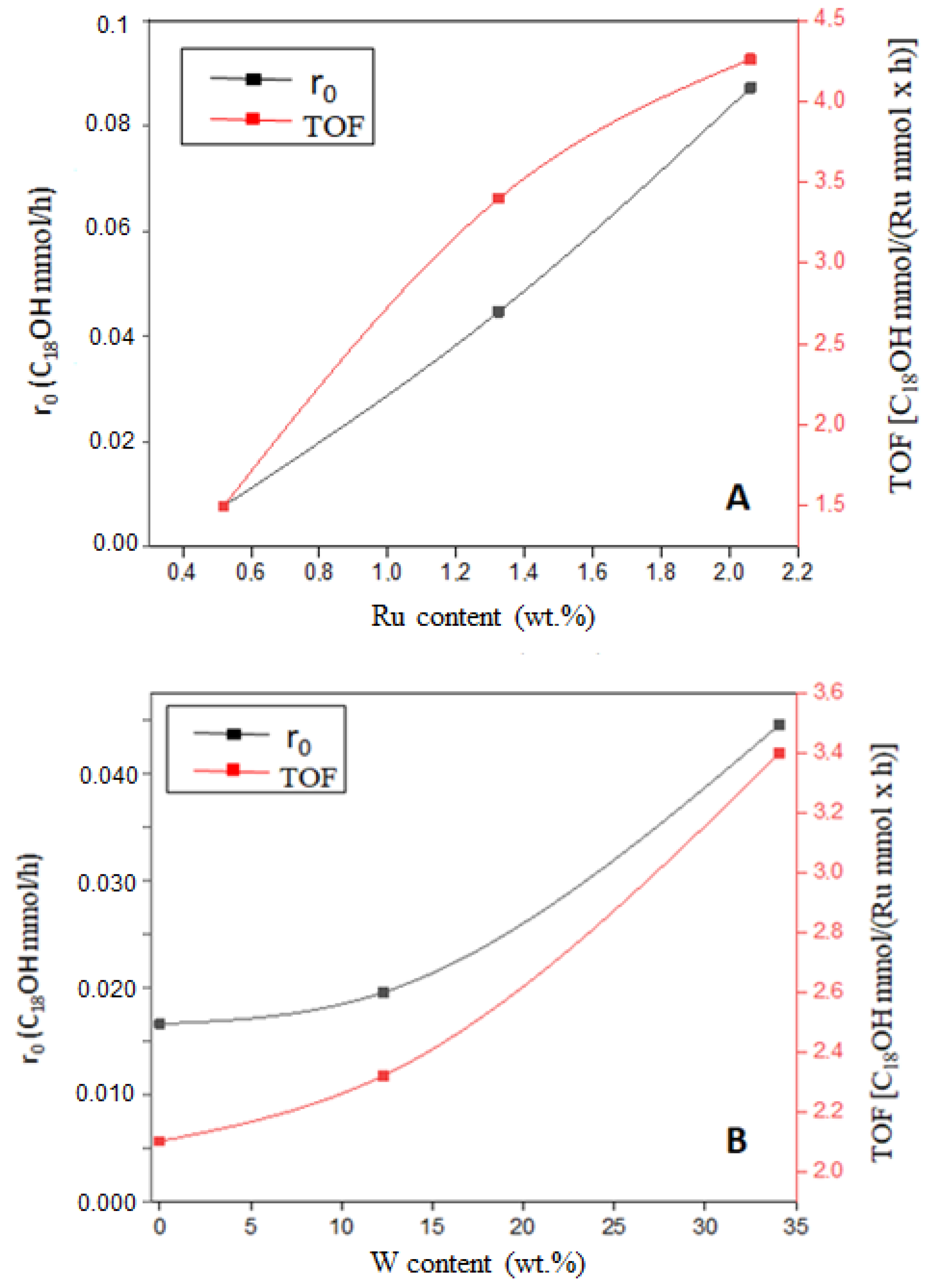

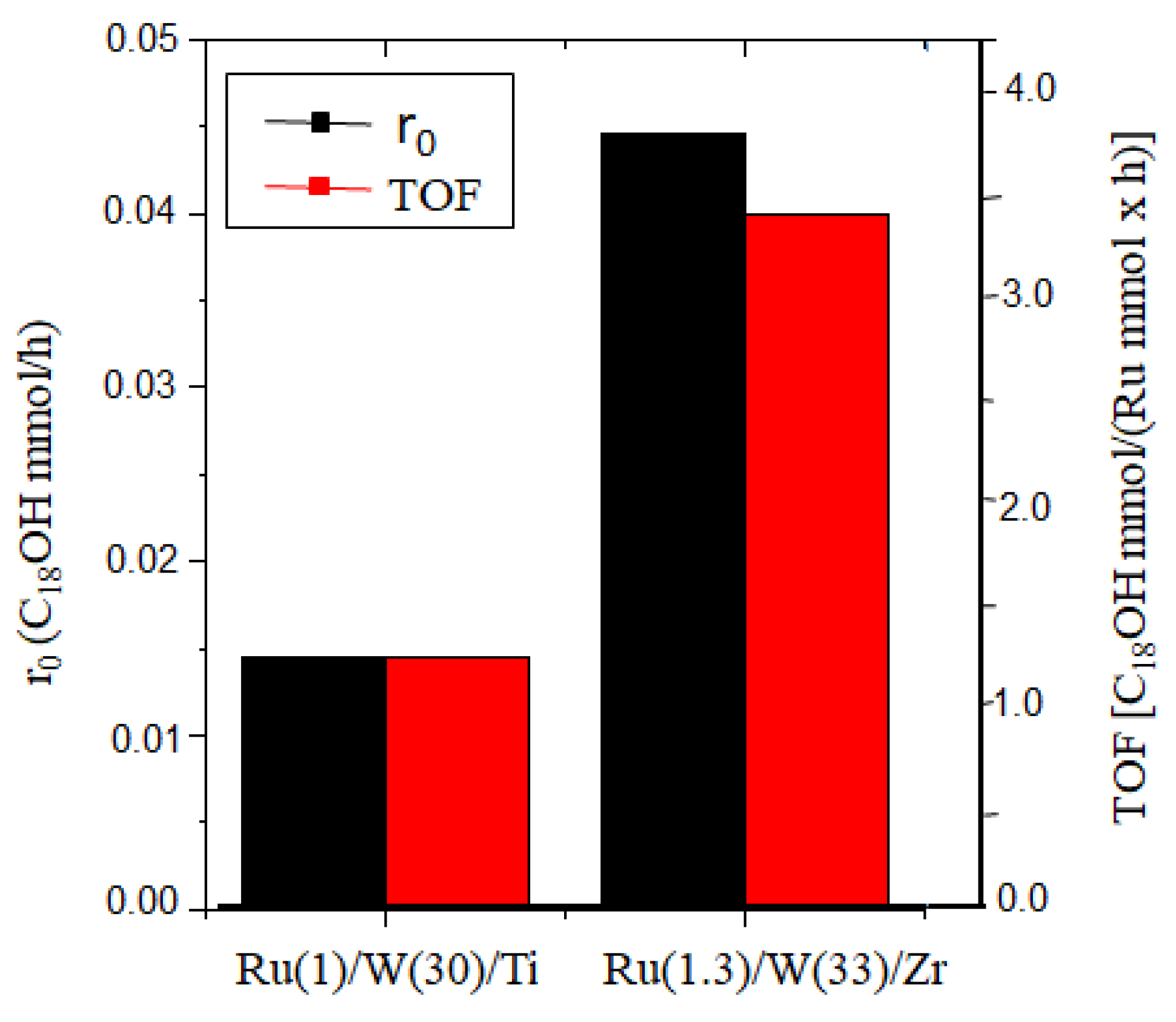

| Entry | Catalyst a M(x)/P(y)/S | Ru (wt.%) | %W (wt.%) | SBET (m2/g) | Vpore (cm3/g) | D(nm) b |
|---|---|---|---|---|---|---|
| 1 | ZrO2 | - | - | 195 | 0.18 | |
| 1 | W(12)/Zr c | - | 12 | 112 | 0.17 | - |
| 2 | W(33)/Zr d | - | 33 | 57 | 0.15 | - |
| 3 | W(50)/Zr d | - | 50 | 25 | 0.06 | - |
| 4 | Ru(1)/Zr | 1 | - | 81 | 0.42 | 1.6 |
| 5 | Ru(1)/W(12)/Zr d | 1 | 76 | 0.34 | 0.9 | |
| 6 | Ru(0.5)/W(33)/Zr d | 0.5 | 48 | 0.20 | 0.5 | |
| 7 | Ru(1.3)/W(33)/Zr d | 1.3 | 40 | 0.20 | 1.2 | |
| 8 | Ru(2)/W(33)/Zr d | 2 | 34 | 0.19 | 1.5 | |
| 9 | Ru(1)/W(30)/Ti d | 1 | 7 | 0.05 | 1.6 |
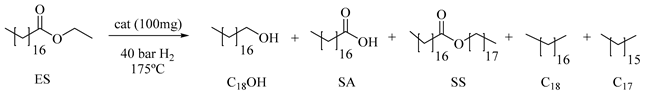 | ||||||
| Entry | Catalyst a | C(%) b | t(h) | C18OH c (C18OH:SS) | S(%) b SA | [C18+C17] d (C18:C17) |
|---|---|---|---|---|---|---|
| 2 | Ru(0.5)/W(33)/Zr | 30 | 15 | 80 (37:63) | 3 | 17 (41:59) |
| 60 | 30 | 75 (19:81) | 3 | 22 (45:55) | ||
| 3 | Ru(1.3)/W(33)/Zr | 30 | 5 | 70 (100:0) | 5 | 25 (52:48) |
| 60 | 12 | 71 (70:30) | 3 | 26 (54:46) | ||
| 4 | Ru(2)/W(33)/Zr | 30 | 2 | 61 (82:18) | 4 | 35 (43:57) |
| 60 | 4 | 46 (74:26) | 1 | 53 (43:57) | ||
| 5 | Ru(1)/W(12)/Zr | 30 | 8 | 63 (75:25) | 8 | 29 (31:69) |
| 60 | 22 | 62 (47:53) | 1 | 37 (22:78) | ||
| 6 | Ru(2)/W(50)/Zr | 30 | 15 | 70 (86:14) | 4 | 26 (58:42) |
60 | 30 | 73 (53:47) | 2 | 25 (60:40) | ||
| 7 | Ru(1)/W(30)/Ti | 30 | 27 | 52 (100:0) | 3 | 45 (71:29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Ramos, D.; Checa, M.; Jordá, J.L.; Sabater, M.J. Study on the Hydrogenation of Ethyl Stearate to the Fatty Alcohol 1-Octadecanol over Ru on Tungstated Zirconia. Catalysts 2023, 13, 1362. https://doi.org/10.3390/catal13101362
Quintero-Ramos D, Checa M, Jordá JL, Sabater MJ. Study on the Hydrogenation of Ethyl Stearate to the Fatty Alcohol 1-Octadecanol over Ru on Tungstated Zirconia. Catalysts. 2023; 13(10):1362. https://doi.org/10.3390/catal13101362
Chicago/Turabian StyleQuintero-Ramos, Diego, Manuel Checa, Jose Luis Jordá, and Maria J. Sabater. 2023. "Study on the Hydrogenation of Ethyl Stearate to the Fatty Alcohol 1-Octadecanol over Ru on Tungstated Zirconia" Catalysts 13, no. 10: 1362. https://doi.org/10.3390/catal13101362
APA StyleQuintero-Ramos, D., Checa, M., Jordá, J. L., & Sabater, M. J. (2023). Study on the Hydrogenation of Ethyl Stearate to the Fatty Alcohol 1-Octadecanol over Ru on Tungstated Zirconia. Catalysts, 13(10), 1362. https://doi.org/10.3390/catal13101362