A Mini-Review on Lanthanum–Nickel-Based Perovskite-Derived Catalysts for Hydrogen Production via the Dry Reforming of Methane (DRM)
Abstract
:1. Introduction
2. Brief Summary of Ni-Based Catalysts for Methane Dry Reforming
3. Perovskites for DRM
3.1. La–Ni Perovskite-Derived Catalysts for DRM
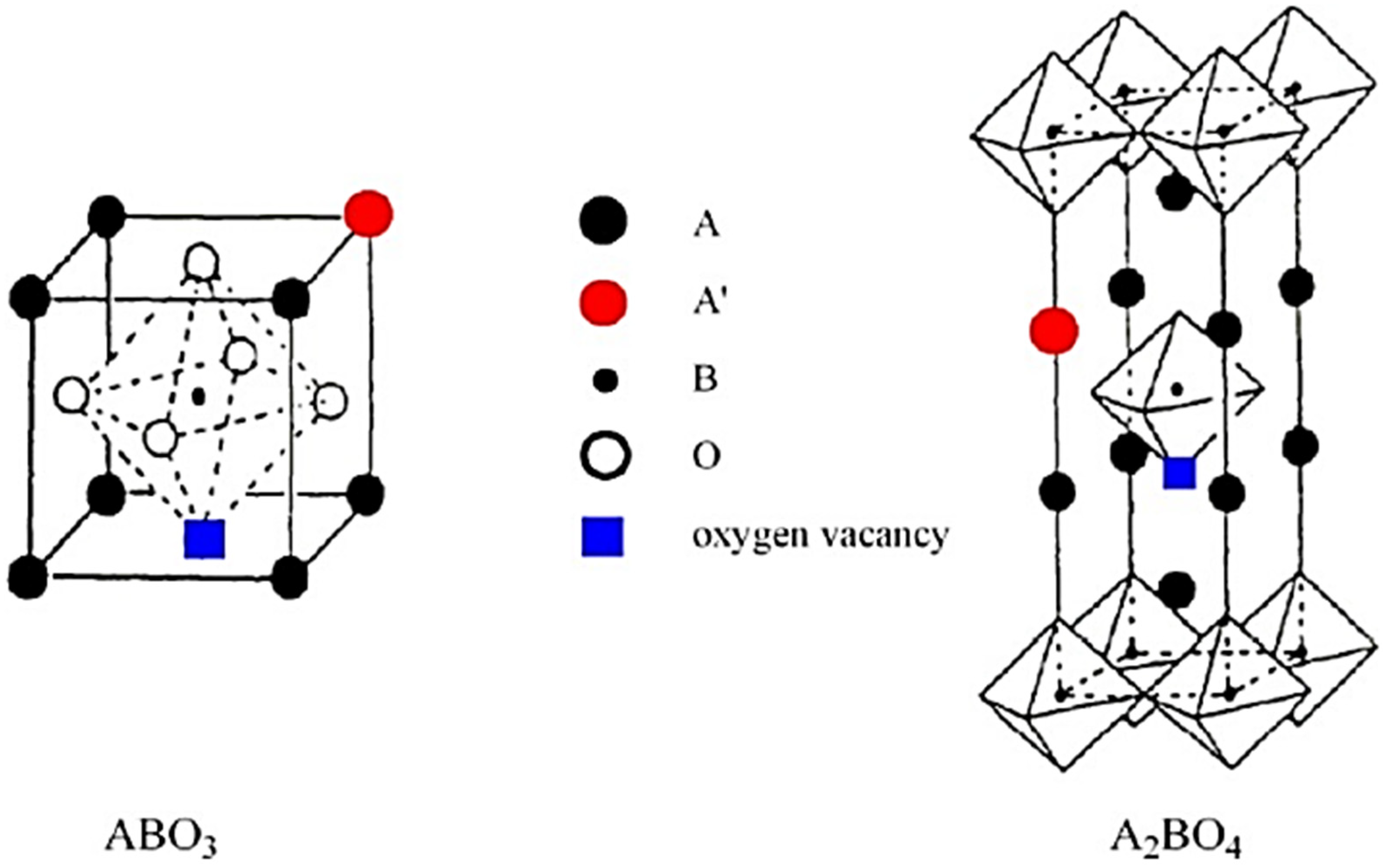
3.2. A1A2BO3 Perovskite Catalysts
3.3. AB1B2O3 Perovskite Catalysts
3.4. A1A2B1B2O3 Perovskite Catalysts
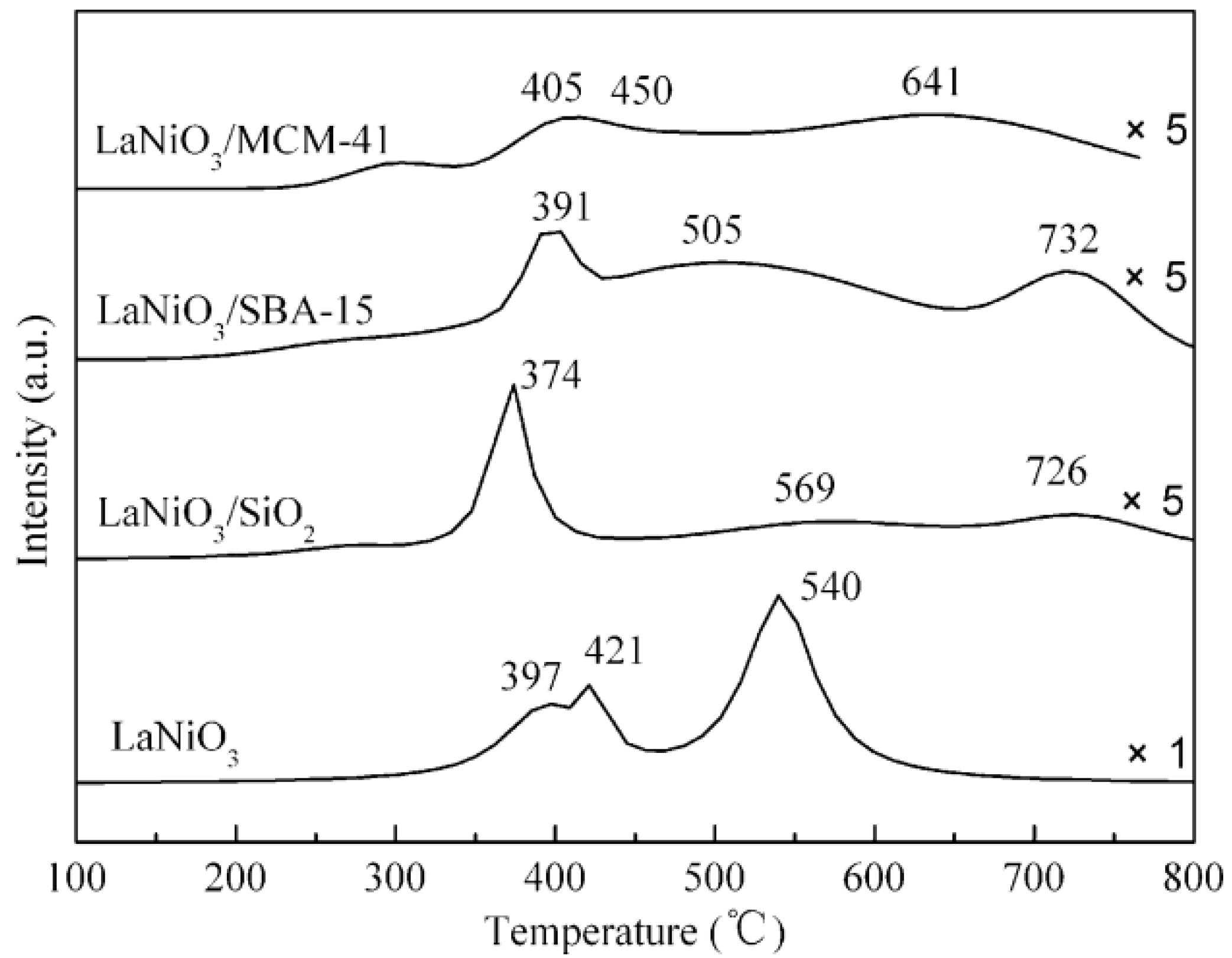
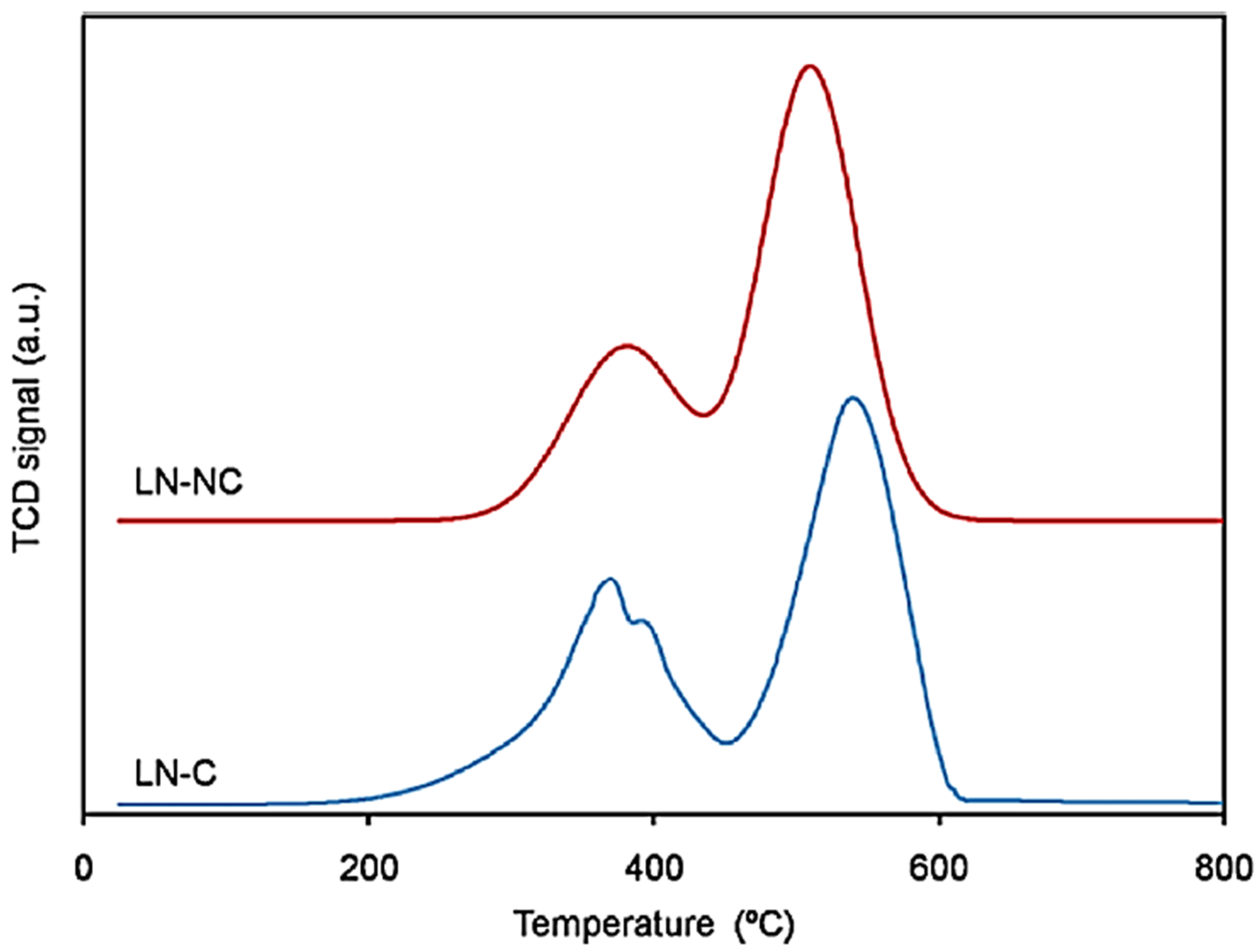
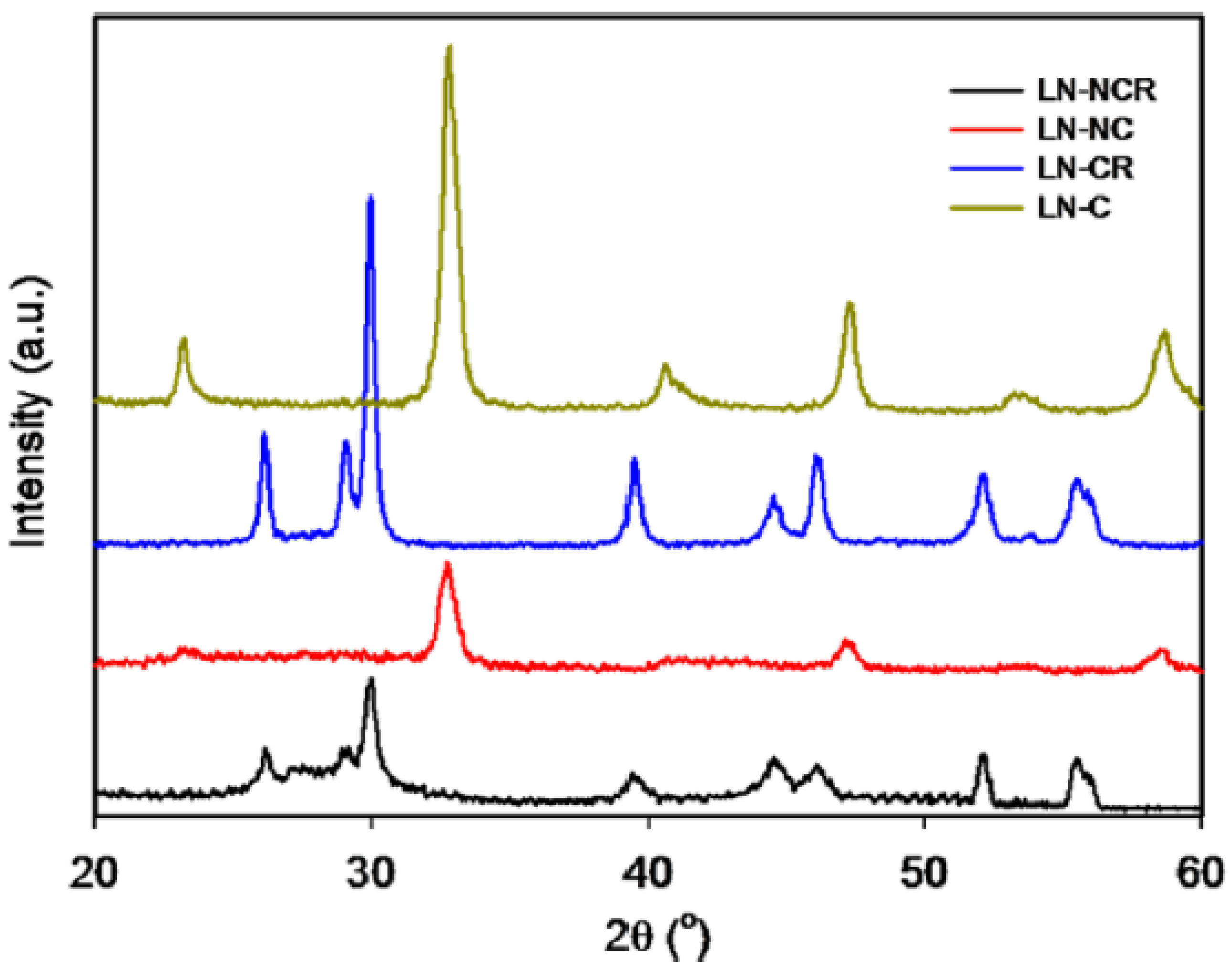
3.5. Mesoporous/Supported Perovskite Catalysts
3.6. Three-Dimensionally Ordered (3DOM) Macroporous Perovskites
4. Kinetic and Mechanistic Considerations
- (1)
- Perovskite undergoes reduction (active metal should be reduced),
- (2)
- Perovskite is supported on basic oxide,
- (3)
- Rate of carbon dioxide dissociation is inconsiderable in comparison to that of methane,
- (4)
- Negligible surface coverage of hydrogen and carbon monoxide,
- (5)
- A part of active metal is carbon-free under DRM conditions.
5. Conclusions
- -
- LaNiO3 gained considerable attention due to the high affinity between CO2 and La2O3, which results in La2O2CO3 formation, as well as the strong metal–support interaction provided. The presence of La2O2CO3 plays a key role in the catalyst’s stability, as it can actively react with coke and act as an inhibitor against carbon accumulation on the nickel’s surface.
- -
- A-site partial substitutions with alkaline earth or rare earth metals into the perovskite matrix can both induce oxygen vacancies and modify the basicity of the surface of the catalyst, thereby improving coke resistance. The increased amount of lattice oxygen can also promote C–H activation, increase nickel dispersion, and increase the reducibility of the catalyst. Furthermore, redox chemistry of rare earth metals such as Sm, Pr, and Ce has a positive influence on the stability of the catalyst due to the fact that more oxygen for carbon removal is supplied.
- -
- B-site substitution can be reflected in two aspects. Non-reducible metals such as zirconium, and titanium can modify the structure and enhance the metal–support interaction, thus suppressing carbon deposition. On the other hand, reducible metals such as Fe, Co, Ru, and Cu can provide a synergetic effect which can be either beneficial or detrimental. To illustrate this point, the addition of iron generates a nickel-containing alloy which improves the stability of the perovskite. Moreover, the redox chemistry of the iron induces oxygen for coke removal due to the dynamic profile of the catalyst, which undergoes dealloying via oxidation by carbon dioxide and re-alloying via its reduction by carbon species. A smaller nickel particle size and decreased reducibility are related to the addition of iron or cobalt. In addition, cobalt can serve as a promoter owing to its high oxygen affinity, which aids in carbon gasification. Certain amounts of copper and manganese can also improve the catalytic behavior of the perovskite oxides in terms of both activity and stability.
- -
- The preparation of perovskites requires considerably high calcination temperatures to produce materials with considerably small surface areas, thus affecting their catalytic behavior. One method to overcome this problem is to disperse perovskite on certain supports, such as SBA-15 and MCM-41, leading to smaller perovskite particles. Another method is to synthesize porous perovskite catalysts by using templating methods.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmand, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Veziroǧlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Latsiou, A.I.; Charisiou, N.D.; Frontistis, Z.; Bansode, A.; Goula, M.A. CO2 hydrogenation for the production of higher alcohols: Recent trends, challenges and opportunities. Catal. Today 2023, 420, 114179. [Google Scholar] [CrossRef]
- Bergero, C.; Rich, M.J.; Saikawa, E. All roads lead to Paris: The eight pathways to renewable energy target adoption. Energy Res. Soc. Sci. 2021, 80, 102215. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 6, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Yaduvanshi, A.; Nkemelang, T.; Bendapundi, R.; New, M. Temperature and rainfall extremes change under current and future global warming levels across Indian climate zones. Weather. Clim. Extrem. 2021, 31, 100291. [Google Scholar] [CrossRef]
- Swain, D.L.; Singh, D.; Touma, D.; Diffenbaugh, N.S. Attributing Extreme Events to Climate Change: A New Frontier in a Warming World. One Earth 2020, 2, 522–527. [Google Scholar] [CrossRef]
- Shi, X.; Chen, J.; Gu, L.; Xu, C.Y.; Chen, H.; Zhang, L. Impacts and socioeconomic exposures of global extreme precipitation events in 1.5 and 2.0 °C warmer climates. Sci. Total Environ. 2021, 766, 142665. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Alharthi, F.; Alam, M.; Wahab, R.; Manoharadas, S.; Alrayes, B. Syngas production via CO2 reforming of methane over SrNiO3 and CeNiO3 perovskites. Energies 2021, 14, 2928. [Google Scholar] [CrossRef]
- Rabelo-Neto, R.C.; Sales, H.B.E.; Inocêncio, C.V.M.; Varga, E.; Oszko, A.; Erdohelyi, A.; Noronha, F.B.; Mattos, L.V. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B Environ. 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Field, R.A.; Derwent, R.G. Global warming consequences of replacing natural gas with hydrogen in the domestic energy sectors of future low-carbon economies in the United Kingdom and the United States of America. Int. J. Hydrogen Energy 2021, 46, 30190–30203. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrogen Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Latsiou, A.I.; Bereketidou, O.A.; Charisiou, N.D.; Georgiadis, A.G.; Avraam, D.G.; Goula, M.A. Synthesis and mathematical modelling of the preparation process of nickel-alumina catalysts with egg/shell structures for hydrogen syngas production via reforming of clean model biogas. Catalysts 2022, 12, 274. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Goula, G.; Yentekakis, I.V.; Goula, M.A. The effect of WO3 modification of ZrO2 support on the Ni-catalyzed dry reforming of biogas reaction for syngas production. Front. Environ. Sci. 2017, 5, 66. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al2O3 and Ni/CaO-Al2O3 catalysts. Waste Biomass Valorization 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Taipadu, M.I.; Viswanathan, K.; Wu, W.; Hattu, N.; Atabani, A.E. A critical review of the hydrogen production from biomass-based feedstocks: Challenge, solution, and future prospect. Process. Saf. Environ. 2022, 164, 384–407. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Dabbawala, A.; Sajjad, M.; Singh, N.; Anjum, D.H.; Baker, M.A.; Charisiou, N.D.; Goula, M.A. Exploring the potential of Ce-La-Cu-O oxide towards hydrogen production via steam reforming of glycerol: An experimental and DFT study. Appl. Surf. Sci. 2022, 586, 152798. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Dou, B.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Goula, M.A. Nickel Supported on AlCeO3 as a Highly Selective and Stable Catalyst for Hydrogen Production via the Glycerol Steam Reforming Reaction. Catalysts 2019, 9, 411. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A state of the art review on biomass processing and conversion technologies to produce hydrogen and its recovery via membrane separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Assaf, E.M.; Assaf, J.M. Adjusting Process Variables in Methane Tri-reforming to Achieve Suitable Syngas Quality and Low Coke Deposition. Energy Fuel. 2020, 12, 16522–16531. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Roy, P.S.; Song, J.; Kim, K.; Park, C.S.; Raju, A.S.K. CO2 conversion to syngas through the steam-biogas reforming process. J. CO2 Util. 2018, 25, 275–282. [Google Scholar] [CrossRef]
- Dehimi, L.; Gaillard, M.; Virginie, M.; Erto, A.; Benguerba, Y. Investigation of dry reforming of methane over Mo-based catalysts. Int. J. Hydrogen Energy 2020, 45, 24657–24669. [Google Scholar] [CrossRef]
- Roy, P.S.; Park, C.S.; Raju, A.S.K.; Kim, K. Steam-biogas reforming over a metal-foam-coated (Pd-Rh)/(CeZrO2-Al2O3) catalyst compared with pellet type alumina-supported Ru and Ni catalysts. J. CO2 Util. 2021, 12, 12–20. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Loktev, A.S.; Mukhin, I.E.; Konovalov, A.A.; Dedov, A.G.; Moiseev, I.I. Trimetallic NiCoM catalysts (M = Mn, Fe, Cu) for methane conversion into synthesis gas. Mendeleev Commun. 2019, 29, 22–24. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Murakami, M.; Ohishi, Y.; Hirao, N.; Hirose, K. A perovskitic lower mantle inferred from high-pressure, high-temperature sound velocity data. Nature 2012, 485, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.; Kwon, D.; An, S.; Ha, J.M.; Oh, T.S.; Jung, J.C. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane. Mol. Catal. 2020, 489, 110925. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskiets as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 20, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, S.; Atri, S.; Tomar, R. A Brief Review on Key Role of Perovskite Oxides as Catalyst. ChemistryEurope 2021, 6, 12947–12959. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni catalysts. Int. J. Hydrogen Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Iordanidis, A.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. Studying the stability of Ni supported on modified with CeO2 alumina catalysts for the biogas dry reforming reaction. Mater. Today Proc. 2018, 5, 27607–27616. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The relationship between reaction temperature and carbon deposition on nickel catalysts based on Al2O3, ZrO2 or SiO2 supports during the biogas dry reforming reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Goula, M.A. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Ruocco, C.; De Caprariis, B.; Palma, V.; Petrullo, A.; Ricca, A.; Scarsella, M.; De Filippis, P. Methane dry reforming on Ru perovskites, AZrRuO3: Influence of preparation method and substitution of A cation with alkaline earth metals. J. CO2 Util. 2019, 30, 222–231. [Google Scholar] [CrossRef]
- Silva, C.K.S.; Baston, E.P.; Melgar, L.Z.; Bellido, J.D.A. Ni/Al2O3-La2O3 catalysts synthesized by a one-step polymerization method applied to the dry reforming of methane: Effect of precursor structures of nickel, perovskite and spinel. React. Kinet. Mech. Catal. 2019, 128, 251–269. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Remiro, A.; Vicente, J.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Stability of CuZnOAl2O3/HZSM-5 and CuFe 2O4/HZSM-5 catalysts in dimethyl ether steam reforming operating in reaction-regeneration cycles. Fuel Process. Technol. 2014, 126, 145–154. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas. Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Bereketidou, O.A.; Goula, M.A. Biogas reforming for syngas production over nickel supported on ceria-alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Wu, C.; Liu, R. Carbon deposition behavior in steam reforming of bio-oil model compound for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 7386–7398. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zhang, Q.; Yang, Z.; Sun, Y.; Zou, G. Methane dry reforming over activated carbon supported Ni-catalysts prepared by solid phase synthesis. J. Clean. Prod. 2020, 274, 122256. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. One-pot synthesis of ordered mesoporous NiO-CaO-Al2O3 composite oxides for catalyzing CO2 reforming of CH4. ACS Catal. 2012, 2, 1331–1342. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.M.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic Conversion of Methane to Synthesis Gas by Partial Oxidation and CO2 Reforming. Adv. Catal. 2004, 48, 297–345. [Google Scholar]
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Huang, Z.; Li, S.; Wu, G.; Ma, X.; Gong, J. Hydrogen production via steam reforming of ethanol on phyllosilicate-derived Ni/SiO2: Enhanced metal-support interaction and catalytic stability. ACS Sustain. Chem. Eng. 2013, 1, 161–173. [Google Scholar] [CrossRef]
- Ashok, J.; Bian, Z.; Wang, Z.; Kawi, S. Ni-phyllosilicate structure derived Ni-SiO2-MgO catalysts for bi-reforming applications: Acidity, basicity and thermal stability. Catal. Sci. Technol. 2018, 8, 1730–1742. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Ashok, J.; Kawi, S. NiCo@NiCo phyllosilicate@CeO2 hollow core shell catalysts for steam reforming of toluene as biomass tar model compound. Energy Convers. Manag. 2019, 180, 822–830. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Ciura, K.; Gryboś, J.; Indyka, P.; Oszajca, M.; Stelmachowski, P.; Witkowski, S.; Inger, M.; Wilk, M.; et al. Influence of preparation method on dispersion of cobalt spinel over alumina extrudates and the catalyst deN2O activity. Appl. Catal. B Environ. 2017, 210, 34–44. [Google Scholar] [CrossRef]
- Cho, E.; Lee, Y.H.; Kim, H.; Jang, E.J.; Kwak, J.H.; Lee, K.; Ko, C.H.; Yoon, W.L. Ni catalysts for dry methane reforming prepared by A-site exsolution on mesoporous defect spinel magnesium aluminate. Appl. Catal. A Gen. 2020, 602, 117694. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In Situ-Determined Catalytically Active State of LaNiO3 in Methane Dry Reforming. ACS Catal. 2020, 10, 1102–1112. [Google Scholar] [CrossRef]
- Komarala, E.P.; Komissarov, I.; Rosen, B.A. Effect of Fe and Mn substitution in LaNiO3 on exsolution, activity, and stability for methane dry reforming. Catalysts 2020, 10, 27. [Google Scholar] [CrossRef]
- Sellam, D.; Ikkour, K.; Dekkar, S.; Messaoudi, H.; Belaid, T.; Roger, A.C. CO2 reforming of methane over LaNiO3 perovskite supported catalysts: Influence of silica support. Bull. Chem. React. Eng. Catal. 2019, 14, 568–578. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Latsiou, A.; Lykos, C.; Bairamis, F.; Konstantinou, I. Synthesis and characterization of LaCoxNi1−xO3 perovskites as heterogeneous catalysts for phenolics degradation by persulfate activation. J. Chem. Technol. Biotechnol. 2022, 97, 3467–3480. [Google Scholar] [CrossRef]
- Tsiotsias, A.; Charisiou, N.D.; AlKhoori, A.; Gaber, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Towards maximizing conversion of ethane and carbon dioxide into synthesis gas using highly stable Ni/perovskite catalysts. J. CO2 Util. 2022, 61, 102046. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Siakavelas, G.; Tsiotsias, A.; Charisiou, N.D.; Wang, W.; Sebastian, V.; Hinder, J.; Baker, M.A.; Mascotto, S.; Goula, M.A. Biogas Dry Reforming Over Ni/LnOx-type catalysts (Ln = La, Ce, Sm or Pr). Int. J. Hydrogen Energy 2023, 48, 19953–19971. [Google Scholar] [CrossRef]
- Rudolph, B.; Tsiotsias, A.; Ehrhardt, B.; Gross, S.; Dolcet, P.; Haas, S.; Charisiou, N.D.; Goula, M.A.; Mascotto, S. Nanoparticle exsolution from nanoporous perovskites for highly active and stable biogas dry reforming catalysts. Adv. Sci. 2023, 10, 2205890. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D.; Ashok, J.; Hongmanorom, P.; Wang, W.; Li, T.; Wang, Z.; Kawi, S. Chemical looping steam reforming of bio-oil for hydrogen-rich syngas production: Effect of doping on LaNi0.8Fe0.2O3 perovskite. Int. J. Hydrogen Energy 2020, 45, 21123–21137. [Google Scholar] [CrossRef]
- Goula, Μ.A.; Charisiou, N.D.; Pandis, P.K.; Stathopoulos, V.N. Ni/apatite-type lanthanum silicate supported catalyst for the glycerol steam reforming reaction. RSC Adv. 2016, 6, 78954–78958. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; de Navarro, C.U.; Goldwasser, M.R. LaNi1−xMnxO3 perovskite-type oxides as catalysts precursors for dry reforming of methane. Appl. Catal. A Gen. 2018, 565, 26–33. [Google Scholar] [CrossRef]
- Tsiotsias, A.; Ehrhardt, B.; Rudolph, B.; Nodari, L.; Kim, S.; Jung, W.; Charisiou, N.D.; Goula, M.A.; Mascotto, S. Modulating bimetallic Fe-Ni exsolution to tune the CO2-assisted ethane dehydrogenation and reforming pathways. ACS Nano 2022, 16, 8904–8916. [Google Scholar] [CrossRef]
- Ismaila, A.; Chen, X.; Gao, X.; Fan, X. Thermodynamic analysis of steam reforming of glycerol for hydrogen production at atmospheric pressure. Front. Chem. Sci. Eng. 2021, 15, 60–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E.; MacDonald, S.M.; Affrossman, S. Comparative study of carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 and conventional nickel-based catalysts. J. Phys. Chem. 1996, 100, 744–754. [Google Scholar] [CrossRef]
- Provendier, H.; Petif, C.; Estournes, C.; Kiennemann, A. Dry reforming of methane. Interest of La-Ni-Fe solid solutions compared to LaNiO3 and LaFeO3. Stud. Surf. Sci. Catal. 1998, 119, 741–746. [Google Scholar]
- Batiot-Dupeyrat, C.; Gallego, G.A.S.; Mondragon, F.; Barrault, J.; Tatibouët, J.M. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107–108, 474–480. [Google Scholar] [CrossRef]
- Cao, D.; Luo, C.; Cai, G.; Luo, T.; Wu, F.; Li, X.; Zheng, Y.; Zhang, L. Development and Regeneration Performance of LaNiO3 Perovskite Oxides for Dry Reforming of Methane. J. Therm. Sci. 2023, 32, 1935–1944. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses, A.; Martinez, F.; Barrault, J.; Tatibouët, J.M. Pulse study of CO2 reforming of methane over LaNiO3. Appl. Catal. A Gen. 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Dual active-site mechanism for dry methane reforming over Ni/La2O3 produced from LaNiO3 perovskite. Ind. Eng. Chem. Res. 2008, 47, 9272–9278. [Google Scholar] [CrossRef]
- Gallego, G.S.; Mondragón, F.; Barrault, J.; Tatibouët, J.M.; Batiot-Dupeyrat, C. CO2 reforming of CH4 over La-Ni based perovskite precursors. Appl. Catal. A Gen. 2006, 311, 164–171. [Google Scholar] [CrossRef]
- Pereñiguez, R.; Gonzalez-delaCruz, V.M.; Caballero, A.; Holgado, J.P. LaNiO3 as a precursor of Ni/La2O3 for CO2 reforming of CH4: Effect of the presence of an amorphous NiO phase. Appl. Catal. B Environ. 2012, 123–124, 324–332. [Google Scholar] [CrossRef]
- Chawla, S.K.; George, M.; Patel, F.; Patel, S. Production of synthesis gas by carbon dioxide reforming of methane over nickel based and perovskite catalysts. Procedia Eng. 2013, 51, 461–466. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. LaNiO3 perovskite catalyst precursor for rapid decomposition of methane: Influence of temperature and presence of H2 in feed stream. Catal. Today 2011, 171, 24–35. [Google Scholar] [CrossRef]
- Kuras, M.; Zimmermann, Y.; Petit, C. Reactivity of perovskite-type precursor in MWCNTs synthesis. Catal. Today 2008, 138, 55–61. [Google Scholar] [CrossRef]
- Valderrama, G.; Goldwasser, M.R.; De Navarro, C.U.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Martínez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–108, 785–791. [Google Scholar] [CrossRef]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T.S. Exsolution of Fe-Ni alloy nanoparticles from (La,Sr)(Cr,Fe,Ni)O3 perovskites as potential oxygen transport membrane catalysts for methane reforming. J. Mater. Chem. A 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- Yang, E.H.; Moon, D.J. Synthesis of LaNiO3 perovskite using an EDTA-cellulose method and comparison with the conventional Pechini method: Application to steam CO2 reforming of methane. RSC Adv. 2016, 6, 112885–112898. [Google Scholar] [CrossRef]
- Singh, S.; Zubenko, D.; Rosen, B.A. Influence of LaNiO3 Shape on Its Solid-Phase Crystallization into Coke-Free Reforming Catalysts. ACS Catal. 2016, 6, 4199–4205. [Google Scholar] [CrossRef]
- Yang, E.H.; Moon, D.J. CO2 Reforming of Methane over Ni0/La2O3 Catalyst Without Reduction Step: Effect of Calcination Atmosphere. Top. Catal. 2017, 60, 697–705. [Google Scholar] [CrossRef]
- Oliveira, Â.A.S.; Medeiros, R.L.B.A.; Figueredo, G.P.; Macedo, H.P.; Braga, R.M.; Maziviero, F.V.; Melo, M.A.F.; Melo, D.M.A.; Vieira, M.M. One-step synthesis of LaNiO3 with chitosan for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 9696–9704. [Google Scholar] [CrossRef]
- Chai, Y.; Fu, Y.; Feng, H.; Kong, W.; Yuan, C.; Pan, B.; Zhang, J.; Sun, Y. A Nickel-Based perovskite catalyst with a bimodal size distribution of Nickel particles for dry reforming of methane. ChemCatChem 2018, 10, 2078–2086. [Google Scholar] [CrossRef]
- Pereñíguez, R.; González-DelaCruz, V.M.; Holgado, J.P.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B Environ. 2010, 93, 346–353. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M. The influence of partial substitution of alkaline earth with la in the LaNiO3 perovskite catalyst. Catal. Commun. 2012, 26, 169–172. [Google Scholar] [CrossRef]
- De Lima, S.M.; Peña, M.A.; Fierro, J.L.G.; Assaf, J.M. La1−xCaxNiO3 perovskite oxides: Characterization and catalytic reactivity in dry reforming of methane. Catal. Letters 2008, 124, 195–203. [Google Scholar] [CrossRef]
- Khalesi, A.; Arandiyan, H.R.; Parvari, M. Effects of lanthanum substitution by strontium and calcium in La-Ni-Al perovskite oxides in dry reforming of methane. Chin. J. Catal. 2008, 29, 960–968. [Google Scholar] [CrossRef]
- Gallego, G.S.; Marín, J.G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Influence of Pr and Ce in dry methane reforming catalysts produced from La1−xAxNiO3-δ perovskites. Appl. Catal. A Gen. 2009, 369, 97–103. [Google Scholar] [CrossRef]
- Su, Y.J.; Pan, K.L.; Chang, M.B. Modifying perovskite-type oxide catalyst LaNiO3 with Ce for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2014, 39, 4917–4925. [Google Scholar] [CrossRef]
- Lima, S.M.; Assaf, J.M.; Peña, M.A.; Fierro, J.L.G. Structural features of La1−xCexNiO3 mixed oxides and performance for the dry reforming of methane. Appl. Catal. A Gen. 2006, 311, 94–104. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Cerium Oxide Catalysts for Oxidative Coupling of Methane: Effect of Lithium, Samarium and Lanthanum Dopants. J. Environ. Chem. Eng. 2022, 10, 107259. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly selective and stable Ni/La-M (M=Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation. J. CO2 Util. 2021, 51, 101618. [Google Scholar] [CrossRef]
- Yang, E.H.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1−xSrxNiO3 perovskite oxides. Catal. Today 2018, 299, 242–250. [Google Scholar] [CrossRef]
- Rynkowski, J.; Samulkiewicz, P.; Ladavos, A.K.; Pomonis, P.J. Catalytic performance of reduced La2−xSrxNiO4 perovskite-like oxides for CO2 reforming of CH4. Appl. Catal. A Gen. 2004, 263, 1–9. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M.; Khosravian, F. The effects of partial substitution of Ni by Zn in LaNiO3 perovskite catalyst for methane dry reforming. J. CO2 Util. 2014, 6, 7–11. [Google Scholar] [CrossRef]
- De Araujo, G.C.; de Lima, S.M.; Assaf, J.M.; Peña, M.A.; Fierro, J.L.G.; do Carmo Rangel, M. Catalytic evaluation of perovskite-type oxide LaNi1−xRuxO3 in methane dry reforming. Catal. Today 2008, 133–135, 129–135. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.; Yin, S.; Wang, M.; Li, M.; Wang, H. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A Gen. 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Moradi, G.R.; Khosravian, F.; Rahmanzadeh, M. Effects of partial substitution of Ni by Cu in LaNiO3 perovskite catalyst for dry methane reforming. Chin. J. Catal. 2012, 33, 797–801. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Li, M.; Wang, H. Perovskite La2(NiCu)O4 catalyst precursors for dry reforming of methane: Effects of Cu-substitution on carbon resistance. RSC Adv. 2017, 7, 41847–41854. [Google Scholar] [CrossRef]
- Shahnazi, A.; Firoozi, S. Improving the catalytic performance of LaNiO3 perovskite by manganese substitution via ultrasonic spray pyrolysis for dry reforming of methane. J. CO2 Util. 2021, 45, 101455. [Google Scholar] [CrossRef]
- Oemar, U.; Kathiraser, Y.; Ang, M.L.; Hidajat, K.; Kawi, S. Catalytic biomass gasification to syngas over highly dispersed lanthanum-doped nickel on SBA-15. ChemCatChem 2015, 7, 3376–3385. [Google Scholar] [CrossRef]
- Oemar, U.; Kathiraser, Y.; Mo, L.; Ho, X.K.; Kawi, S. CO2 reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts: Mechanism and kinetic modelling. Catal. Sci. Technol. 2016, 6, 1173–1186. [Google Scholar] [CrossRef]
- De Lima, S.M.; Assaf, J.M. Ni-Fe catalysts based on perovskite-type oxides for dry reforming of methane to syngas. Catal. Lett. 2006, 108, 63–70. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.; Bobade, R.; Gurav, H.; Chilukuri, S. Tuning the dimensionality of layered Srn+1Tin−xNixO3n+1 perovskite structures for improved activity in syngas generation. J. Catal. 2018, 360, 27–39. [Google Scholar] [CrossRef]
- Rivas, M.E.; Fierro, J.L.G.; Goldwasser, M.R.; Pietri, E.; Pérez-Zurita, M.J.; Griboval-Constant, A.; Leclercq, G. Structural features and performance of LaNi1−xRhxO3 system for the dry reforming of methane. Appl. Catal. A Gen. 2008, 344, 10–19. [Google Scholar] [CrossRef]
- Yasyerli, S.; Filizgok, S.; Arbag, H.; Yasyerli, N.; Dogu, G. Ru incorporated Ni-MCM-41 mesoporous catalysts for dry reforming of methane: Effects of Mg addition, feed composition and temperature. Int. J. Hydrogen Energy 2011, 36, 4863–4874. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, T.; Sui, Z.; Zhu, Y.A.; Han, C.; Zhu, K.; Zhou, X. A single source method to generate Ru-Ni-MgO catalysts for methane dry reforming and the kinetic effect of Ru on carbon deposition and gasification. Appl. Catal. B Environ. 2018, 233, 143–159. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Watanabe, H.; Xu, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Catalytic performance and characterization of Co-Fe bcc alloy nanoparticles prepared from hydrotalcite-like precursors in the steam gasification of biomass-derived tar. Appl. Catal. B Environ. 2014, 160–161, 701–715. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Appl. Catal. A Gen. 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Koike, M.; Li, D.; Nakagawa, Y.; Tomishige, K. A highly active and coke-resistant steam reforming catalyst comprising uniform nickel-iron alloy nanoparticles. ChemSusChem 2012, 5, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Aghabozorg, H.; Pahlavanzadeh, H. Effects of Fe substitutions by Ni in La-Ni-O perovskite-type oxides in reforming of methane with CO2 and O2. Int. J. Hydrogen Energy 2013, 38, 10407–10416. [Google Scholar] [CrossRef]
- Tomishige, K.; Li, D.; Tamura, M.; Nakagawa, Y. Nickel-iron alloy catalysts for reforming of hydrocarbons: Preparation, structure, and catalytic properties. Catal. Sci. Technol. 2017, 7, 3952–3979. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; Van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Chen, S.; Liu, Y. Co-Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen. Int. J. Hydrogen Energy 2014, 39, 5644–5652. [Google Scholar] [CrossRef]
- Urasaki, K.; Tokunaga, K.; Sekine, Y.; Matsukata, M.; Kikuchi, E. Production of hydrogen by steam reforming of ethanol over cobalt and nickel catalysts supported on perovskite-type oxides. Catal. Commun. 2008, 9, 600–604. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. Dry reforming of CH4 over solid solutions of LaNi1−xCoxO3. Catal. Today 2008, 133–135, 142–148. [Google Scholar] [CrossRef]
- Mousavi, M.; Nakhaei Pour, A. Performance and structural features of LaNi0.5Co0.5O3 perovskite oxides for the dry reforming of methane: Influence of the preparation method. New J. Chem. 2019, 43, 10763–10773. [Google Scholar] [CrossRef]
- Touahra, F.; Rabahi, A.; Chebout, R.; Boudjemaa, A.; Lerari, D.; Sehailia, M.; Halliche, D.; Bachari, K. Enhanced catalytic behaviour of surface dispersed nickel on LaCuO3 perovskite in the production of syngas: An expedient approach to carbon resistance during CO2 reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2477–2486. [Google Scholar] [CrossRef]
- Wei, T.; Jia, L.; Zheng, H.; Chi, B.; Pu, J.; Li, J. LaMnO3-based perovskite with in-situ exsolved Ni nanoparticles: A highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4. Appl. Catal. A Gen. 2018, 564, 199–207. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. La-Sr-Ni-Co-O based perovskite-type solid solutions as catalyst precursors in the CO2 reforming of methane. J. Power Sources 2010, 195, 1765–1771. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C-H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, J.; Wang, T.; Xu, X.; Yuan, Y.; Li, J.; Zhu, J. Oxidative degradation of organic dyes over supported perovskite Oxide LaFeO3/SBA-15 under Ambient Conditions. Catal. Lett. 2013, 143, 887–894. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3-Al2O3: Unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Yadav, P.K.; Das, T. Production of syngas from carbon dioxide reforming of methane by using LaNixFe1−xO3 perovskite type catalysts. Int. J. Hydrogen Energy 2019, 44, 1659–1670. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Li, L.; Peng, C.; Liu, W.; Xu, X.; Fang, X.; Wang, Z.; Wang, X.; Peng, H. LaNiO3 nanocube embedded in mesoporous silica for dry reforming of methane with enhanced coking resistance. Microporous Mesoporous Mater. 2018, 266, 189–197. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Wang, Y.; Chu, W.; Liu, M. A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier. Catal. Today 2013, 212, 98–107. [Google Scholar] [CrossRef]
- Rivas, I.; Alvarez, J.; Pietri, E.; Pérez-Zurita, M.J.; Goldwasser, M.R. Perovskite-type oxides in methane dry reforming: Effect of their incorporation into a mesoporous SBA-15 silica-host. Catal. Today 2010, 149, 388–393. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 perovskites as precursors for the preparation of coke-resistant dry reforming catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, J.; Ding, C.; Ding, H.; Guo, S.; Jia, Y.; Liu, P.; Zhang, K. Partial oxidation of methane over Ni based catalyst derived from order mesoporous LaNiO3 perovskite prepared by modified nanocasting method. Fuel 2017, 193, 112–118. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhao, Y.; Lu, Y.; Guo, D.; Zhao, Y.; Wang, S.; Ma, X. Mesoporous LaAl0.25Ni0.75O3 perovskite catalyst using SBA-15 as templating agent for methane dry reforming. Microporous Mesoporous Mater. 2020, 303, 110278. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Barama, A. Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 2018, 24, 40–49. [Google Scholar] [CrossRef]
- Kärger, J.; Valiullin, R. Mass transfer in mesoporous materials: The benefit of microscopic diffusion measurement. Chem. Soc. Rev. 2013, 42, 4172–4197. [Google Scholar] [CrossRef]
- Arandiyan, H.; Wang, Y.; Sun, H.; Rezaei, M.; Dai, H. Ordered meso- and macroporous perovskite oxide catalysts for emerging applications. Chem. Commun. 2018, 54, 6484–6502. [Google Scholar] [CrossRef]
- Arandiyan, H.; Scott, J.; Wang, Y.; Dai, H.; Sun, H.; Amal, R. Meso-Molding Three-Dimensional Macroporous Perovskites: A New Approach to Generate High-Performance Nanohybrid Catalysts. ACS Appl. Mater. Interfaces 2016, 8, 2457–2463. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Wang, Y.; Sun, H.; Xie, S.; Bai, B.; Liu, Y.; Ji, K.; Li, J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 supported Ag Nanoparticles for the combustion of methane. J. Phys. Chem. C 2014, 118, 14913–14928. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Slagtern, A.; Schuurman, Y.; Leclercq, C.; Verykios, X.; Mirodatos, C. Specific features concerning the mechanism of methane reforming by carbon dioxide over Ni/La2O3 catalyst. J. Catal. 1997, 172, 118–126. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3 catalyst. Catal. Today 2001, 64, 83–90. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Carbon and oxygen reaction pathways of CO2 reforming of methane over Ni/La2O3 and Ni/Al2O3 catalysts studied by isotopic tracing techniques. J. Catal. 1999, 187, 85–94. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M.; Sharifnia, S. Kinetic investigation of CO2 reforming of CH4 over La-Ni based perovskite. Chem. Eng. J. 2010, 162, 787–791. [Google Scholar] [CrossRef]
- Singh, S.; Prestat, E.; Huang, L.F.; Rondinelli, J.M.; Haigh, S.J.; Rosen, B.A. Role of 2D and 3D defects on the reduction of LaNiO3 nanoparticles for catalysis. Sci. Rep. 2017, 7, 10080. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Tan, M.; Zou, X.; Ding, W.; Lu, X. Perovskite LaNiO3 Nanocrystals inside SBA-15 Silica: High Stability and Anti-Coking Performance in the Pre-Reforming of Liquefied Petroleum Gas at a Low Steam-to-Carbon Molar Ratio. ChemCatChem 2016, 8, 1055–1058. [Google Scholar] [CrossRef]
- Mabaleha, S.S.; Farshad, G.; Pranjal, K. “Recent advances in Ni-based stable catalysts for methane dry reforming: Stable catalysts’ preparation review. J. Mol. Catal. 2023, 547, 113398. [Google Scholar] [CrossRef]
- de Lira Lima, D.C.; Lemos, I.P.; Gomes, R.S.; Rodrigues, L.M.T.S.; Fréty, R.T.; Resini, C.; Junior, R.B.S.; Brandão, S.T. Study of LaNi1−xCoxO3 Perovskites-Type Oxides Either Pure or Mixed with SiO2 as Catalytic Precursors Applied in CH4 Dry-Reforming. Catal. Lett. 2023, 153, 2137–2148. [Google Scholar] [CrossRef]
- Nezhad, P.D.K.; Bekheet, M.F.; Bonmassar, N.; Schlicker, L.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Mechanistic in situ insights into the formation, structural and catalytic aspects of the La2NiO4 intermediate phase in the dry reforming of methane over Ni-based perovskite catalysts. Appl. Catal. A Gen. 2021, 612, 117984. [Google Scholar] [CrossRef]
- Bakiz, B.; Guinneton, F.; Arab, M.; Benlachemi, A.; Villain, S.; Satre, P.; Gavarri, J.-R. Carbonatation and Decarbonatation Kinetics In The La2O3-La2O2CO3 System Under CO2 Gas Flows. Adv. Mater. Sci. Eng. 2010, 2010, 360597. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, S.; Chen, Y.; Yan, B.; Cheng, Y. Effect of the Calcination Temperature of LaNiO3 on the Structural Properties and Reaction Performance of Catalysts in the Steam Reforming of Methane. Catalysts 2023, 13, 356. [Google Scholar] [CrossRef]
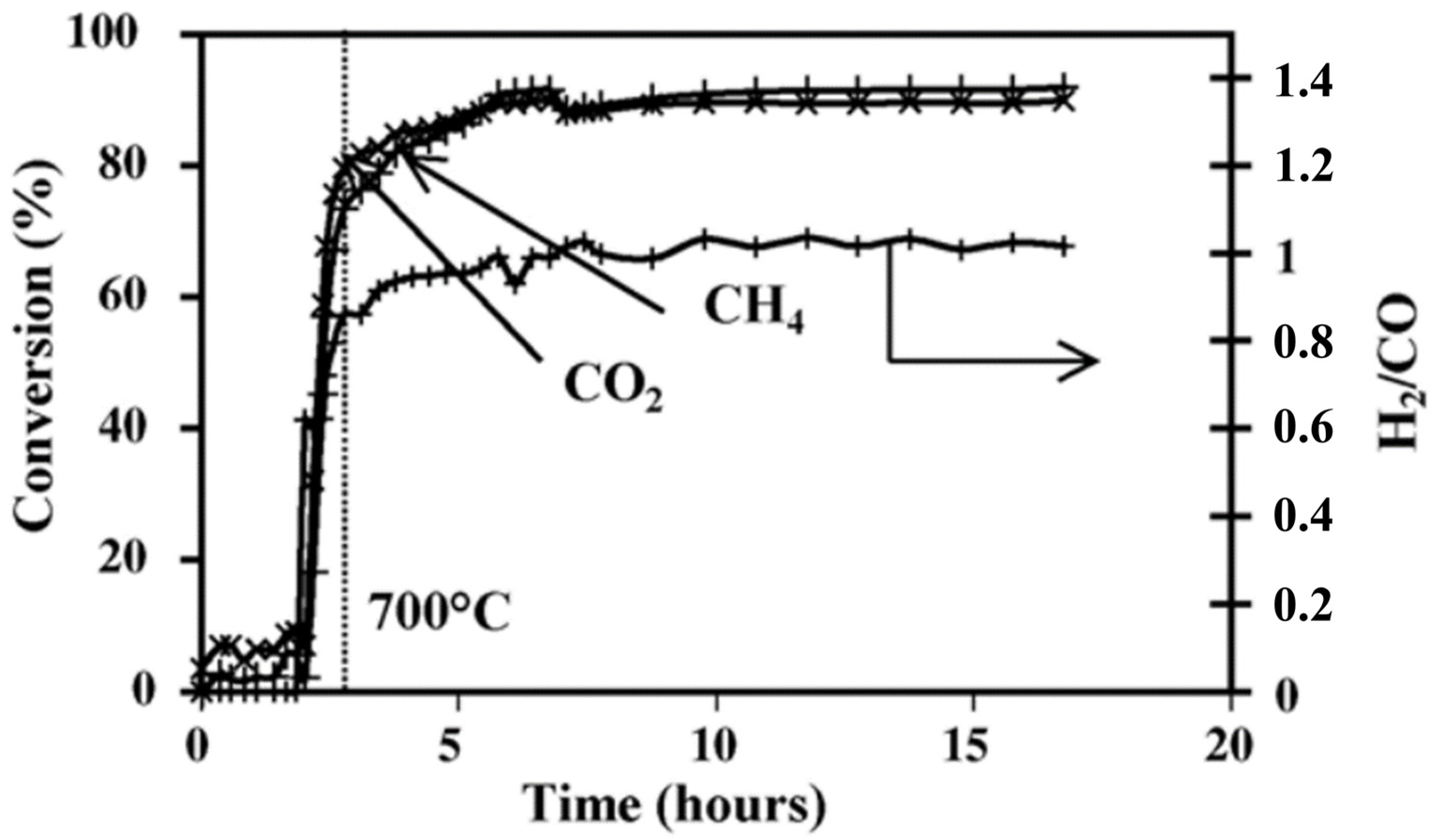
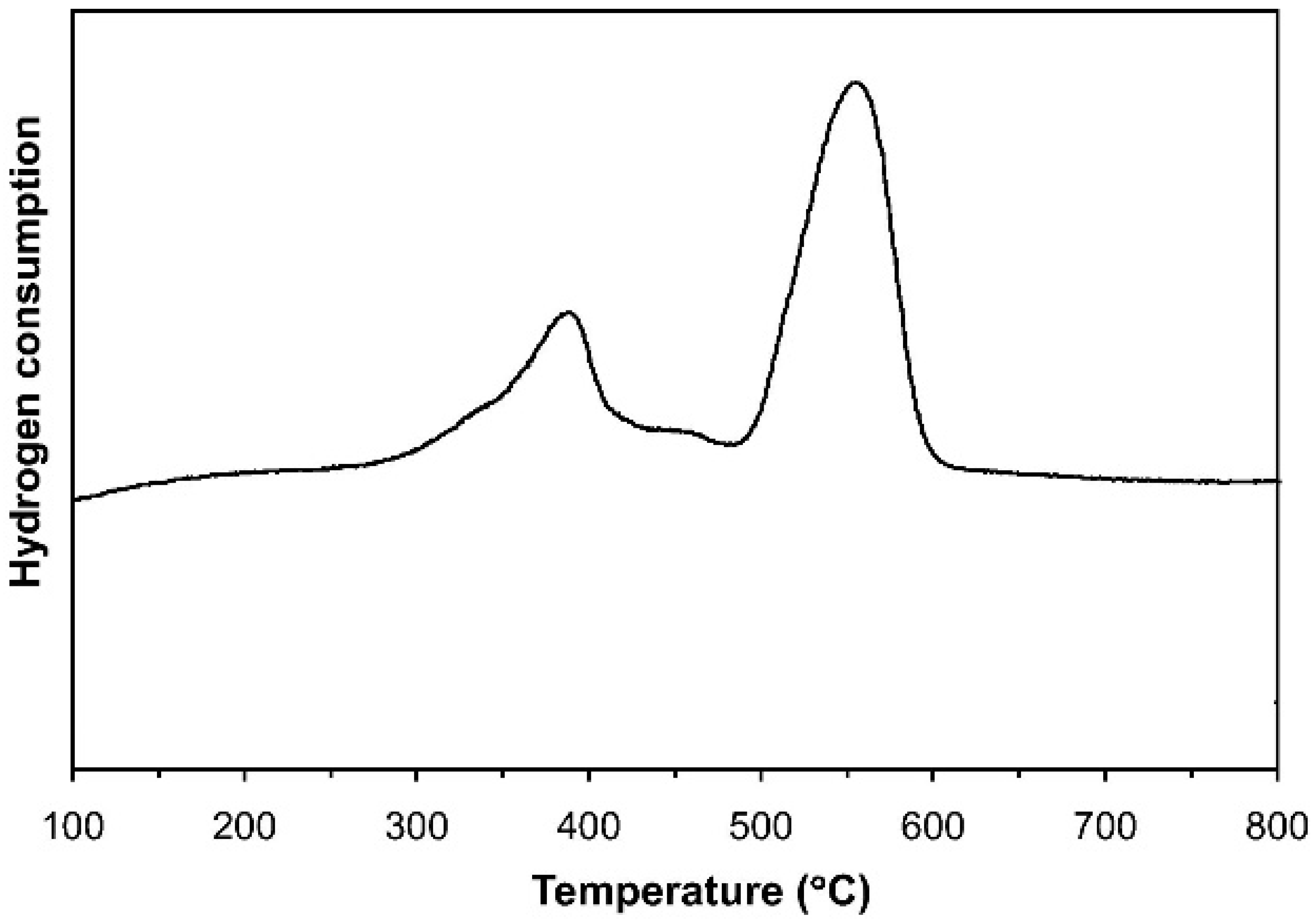
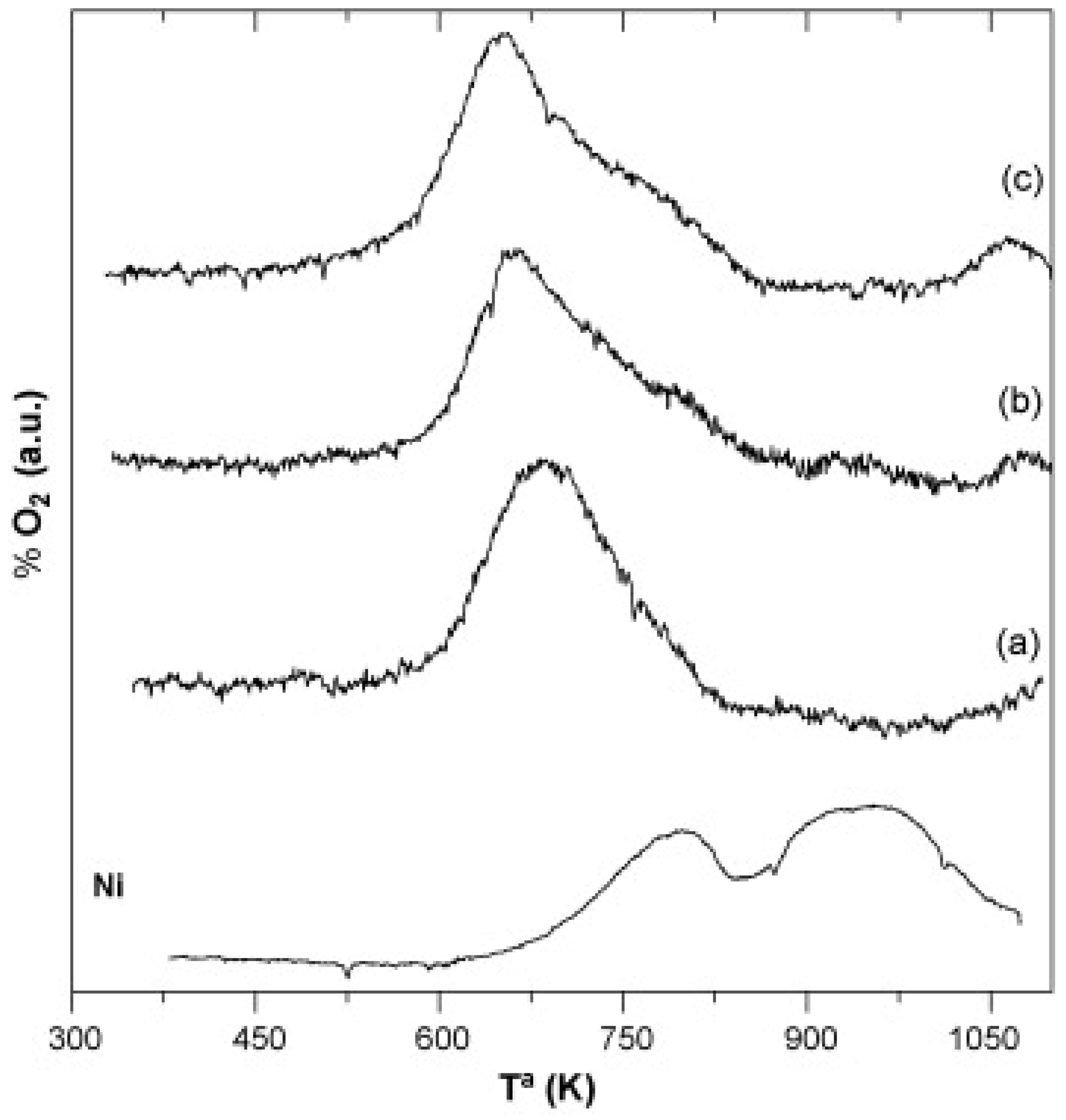
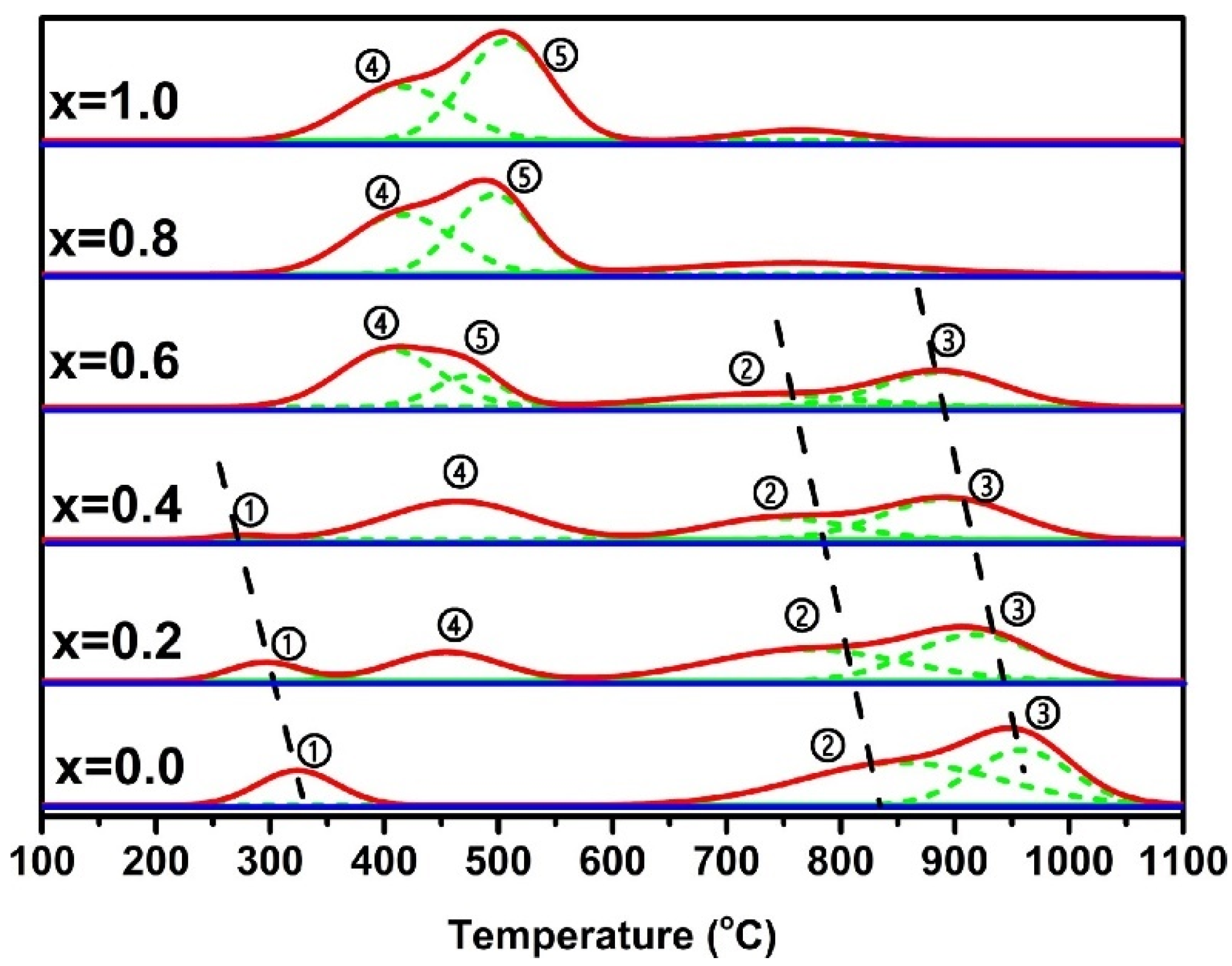

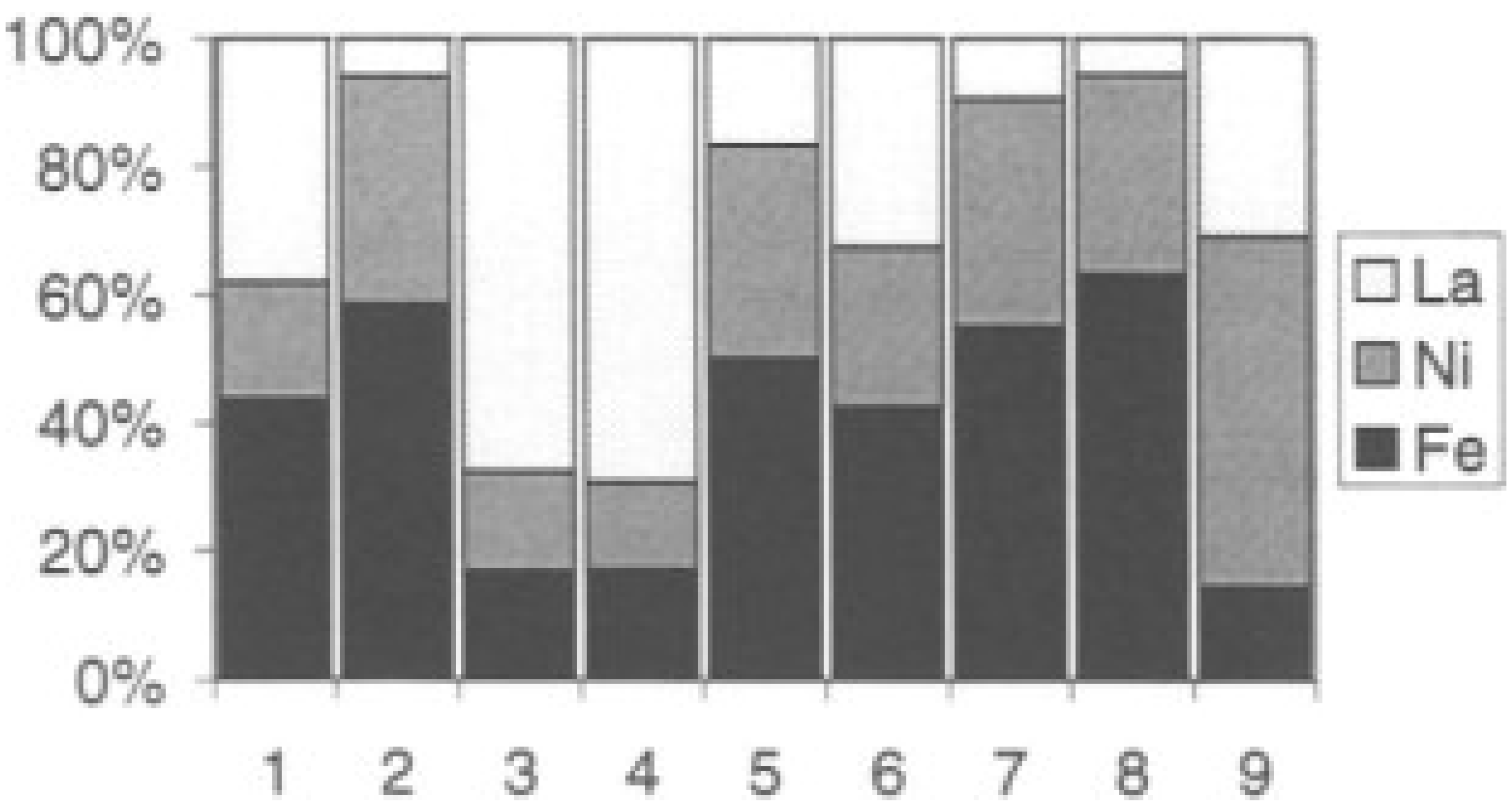
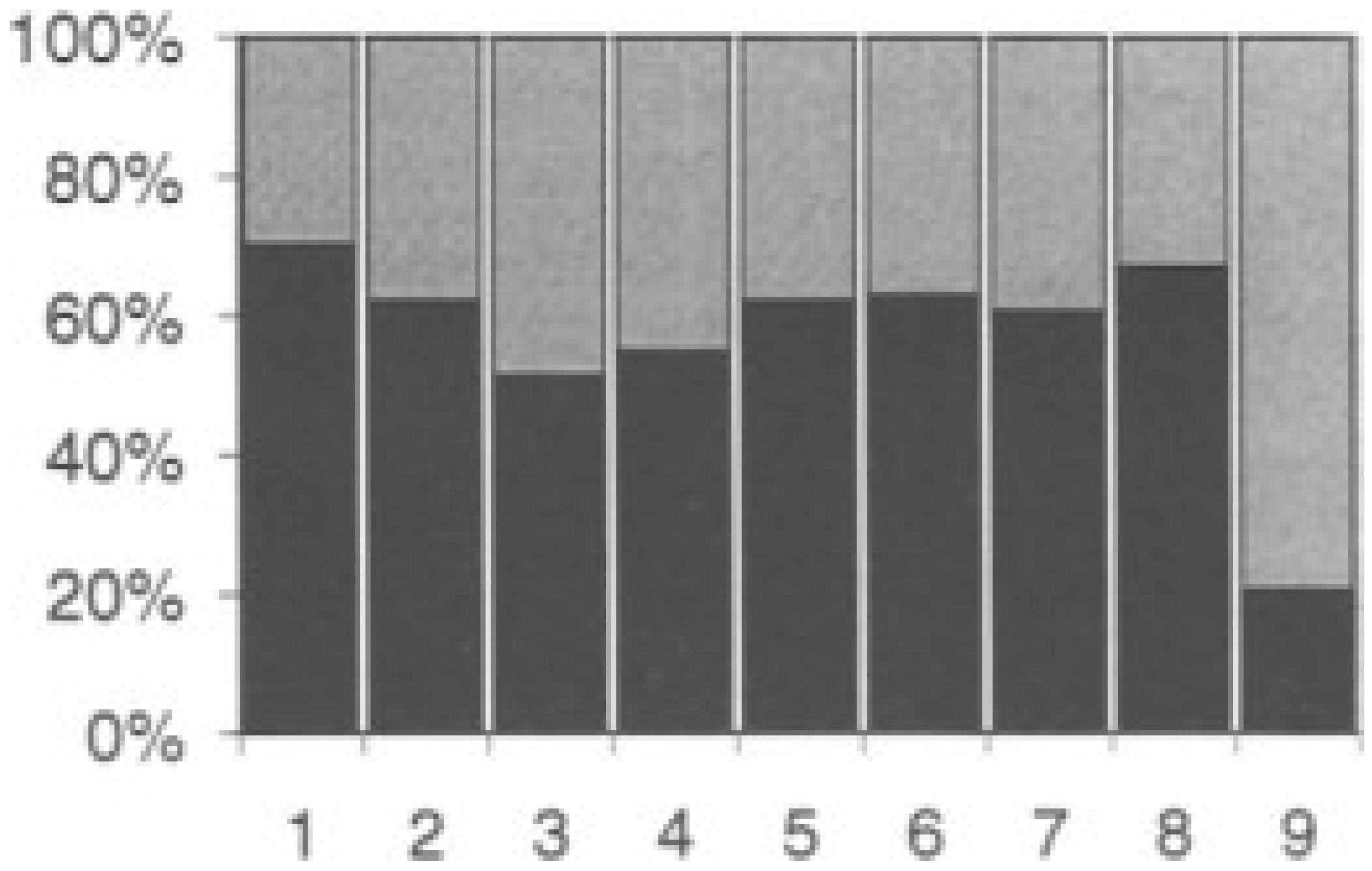
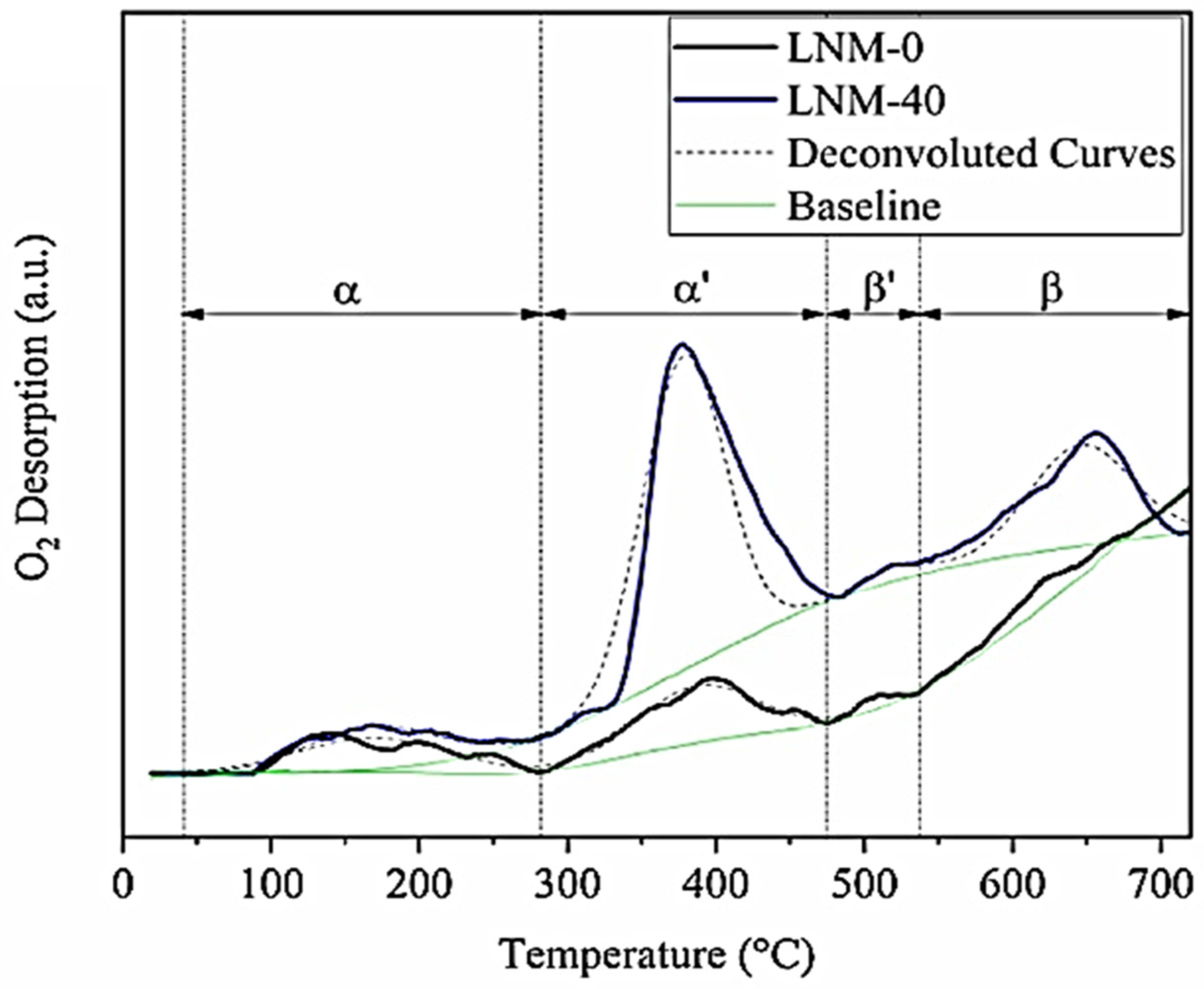
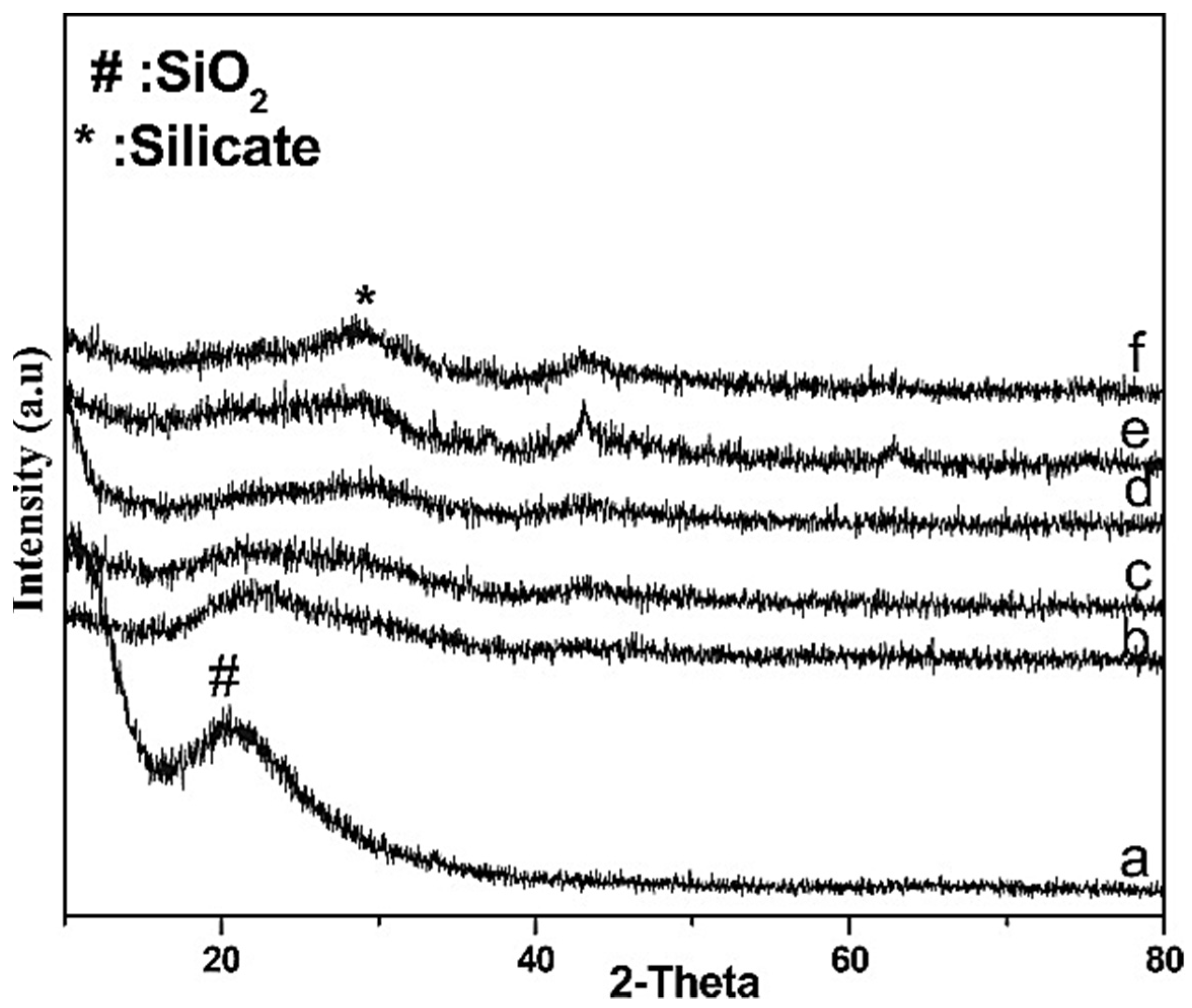
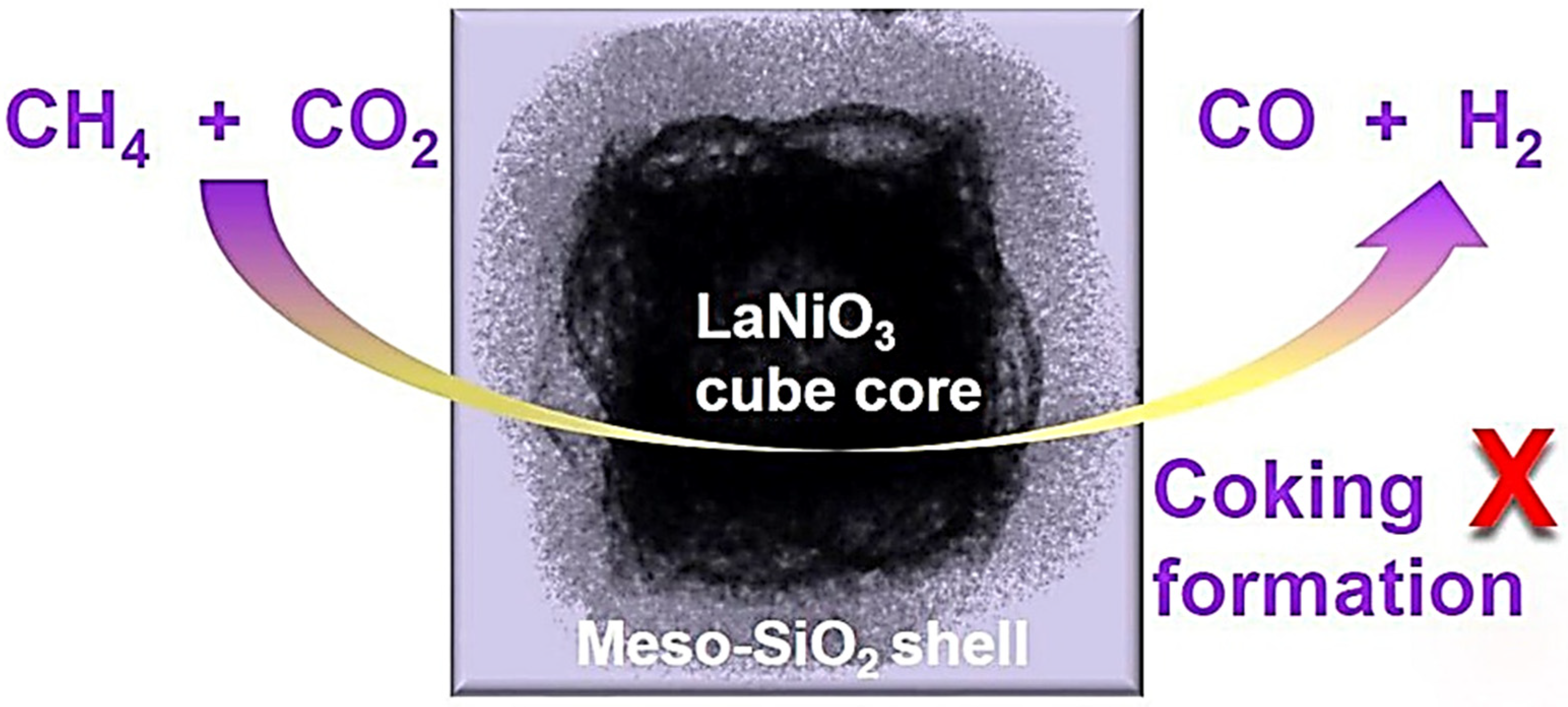
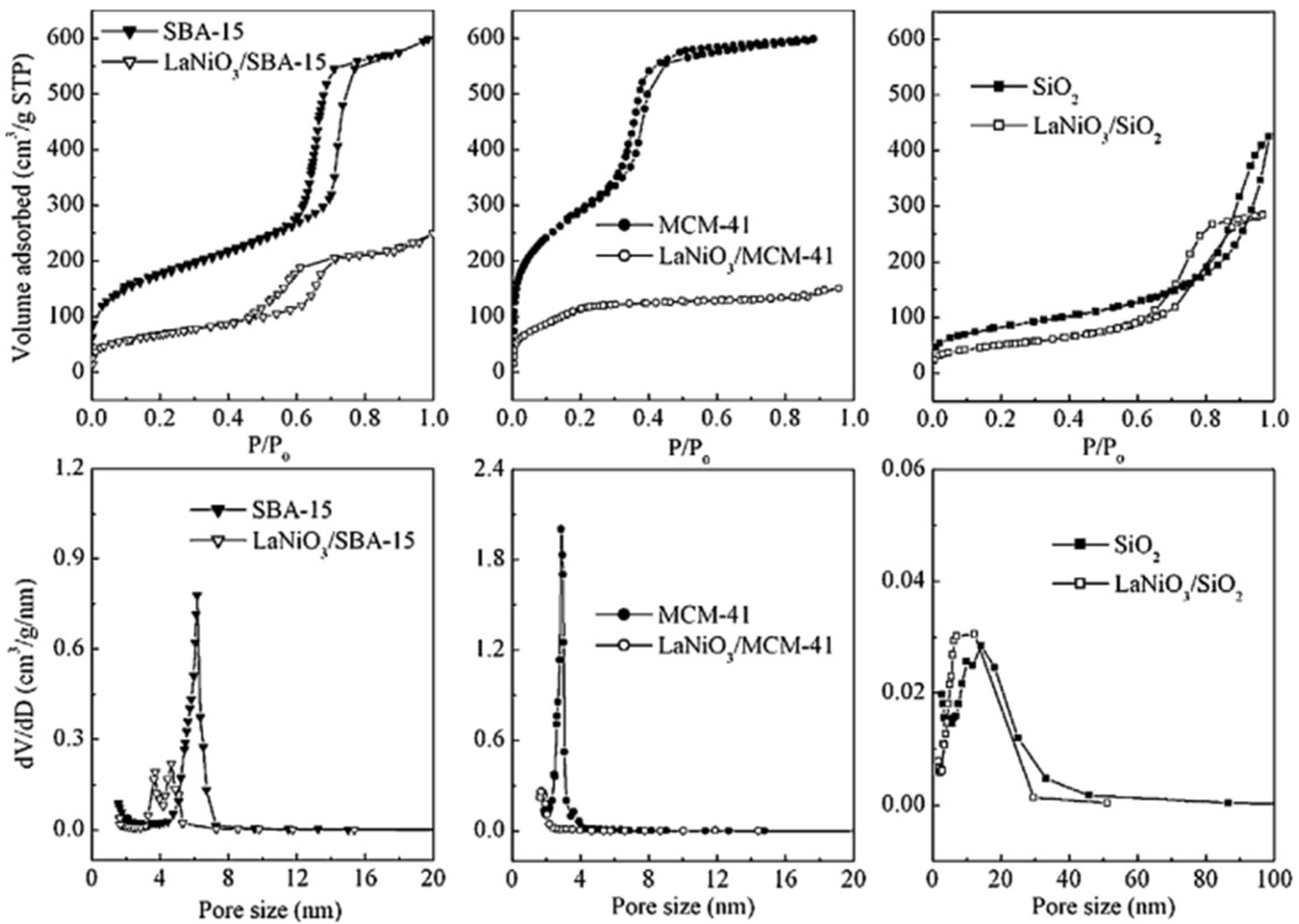
| Perovskite | Operating Conditions | CH4 Conversion Order | Carbon Deposition | Ref. |
|---|---|---|---|---|
| La0.95Ba0.05NiO3 La0.90Ba0.10NiO3 La0.85Ba0.15NiO3 La0.80Ba0.20NiO3 La0.75Ba0.25NiO3 La0.70Ba0.30NiO3 | T = 650–750 °C WHSV = 60,000 mL g−1 h−1 CH4:CO2:He = 1:1:8 | La0.90Ba0.10NiO3 > La0.85Ba0.15NiO3~La0.85Ba0.15NiO3 > La0.75Ba0.25NiO3 > La0.70Ba0.30NiO3 Ba-substituted catalysts outperformed the Sr and Mg ones. | - | [97] |
| LaNiO3 La0.95Ca0.05NiO3 La0.90Ca0.10NiO3 La0.70Ca0.30NiO3 La0.50Ca0.50NiO3 La0.20Ca0.80NiO3 | T = 650–750 °C WHSV = 720,000 mL g−1 h−1 CH4:CO2 = 1:1 | La0.50Ca0.50NiO3~La0.70Ca0.30NiO3 > La0.95Ca0.05NiO3 > La0.20Ca0.80NiO3 > La0.90Ca0.10NiO3 > LaNiO3 | - | [98] |
| LaNiO3 La0.9Sr0.1NiO3 La0.8Sr0.2NiO3 La0.7Sr0.3NiO3 La0.6Sr0.4NiO3 Ni(5%)/La2O3 | T = 700 °C CH4:CO2 = 1:1 | LaNiO3 > La0.6Sr0.4NiO3 > Ni(5%)/La2O3 > La0.9Sr0.1NiO3 The order of activity depends on the content of strontium. | no coke no coke no coke no coke no coke no coke | [88] |
| La2Ni0.3Al0.7O3 La0.8Sr0.2Ni0.3Al0.7O2.9 La0.5Sr0.5Ni0.3Al0.7O2.75 La0.2Sr0.8 Ni0.3Al0.7O2.6 | T = 750 °C WHSV = 15,000 mL g−1 h−1 CH4:CO2:N2 = 1:1:8 | La0.2Sr0.8 Ni0.3Al0.7O2.6 > La0.5Sr0.5Ni0.3Al0.7O2.75 > La0.8Sr0.2Ni0.3Al0.7O2.9 Ca-substituted catalysts were more stable but less active. | 8.71%—15 h 8.31%—15 h 3.21%—15 h - | [99] |
| LaNiO3 La0.98Pr0.02NiO3 La0.90Pr0.10NiO3 La0.60Pr0.40NiO3 | T = 700 °C WHSV = 600,000 mL g−1 h−1 CH4:CO2 = 1:1 | La0.50Pr0.10NiO3 > LaNiO3 > La0.98Pr0.02NiO3 > La0.60Pr0.40NiO3 | 63%—8 h 51%—8 h traces—8 h 52%—8 h | [100] |
| LaNiO3 La0.90Ce0.10NiO3 La0.70Ce0.30NiO3 La0.50Ce0.50NiO3 | T = 600–800 °C GHSV = 10,000 h−1 CH4:CO2 = 1:1 | LaNiO3 > La0.90Ce0.10NiO3 > La0.50Ce0.50NiO3 > La0.70Ce0.30NiO3 | - | [101] |
| Perovskite | Operating Conditions | CH4 Conversion Order | Carbon Deposition | Ref. |
|---|---|---|---|---|
| LaNiO3 LaNi0.8Zn0.2O3 LaNi0.6Zn0.4O3 LaNi0.4Zn0.6O3 LaNi0.2Zn0.8O3 LaZnO3 | T = 750 °C WHSV = 180,000 mL g−1 h−1 CH4:CO2:He = 1:1:1 | LaNi0.8Zn0.2O3 > LaNi0.6Zn0.4O3 > LaNiO3 > LaNi0.4Zn0.6O3 > LaNi0.2Zn0.8O3 > LaZnO3 | 0.7%—75 h 0.4%—75 h - - - - | [109] |
| LaNiO3 LaNi0.9Ru0.1O3 LaNi0.8Ru0.2O3 La3.5Ru4.0O3 | T = 750 °C WHSV = 7200 mL mL g−1 h−1 CH4:CO2 = 1:1 | LaNiO3 > LaNi0.9Ru0.1O3 > LaNi0.8Ru0.2O3~La3.5Ru4.0O3 | 65.7%—14 h 20.3%—14 h 6.7%—14 h 0.9%—14 h | [110] |
| LaNiO3 La2NiO4 La2Ni0.5Fe0.5O4 LaNi0.5Fe0.5O3 | T = 750 °C WHSV = 120,000 mL g−1 h−1 CH4:CO2 = 1:1 | LaNiO3~La2NiO4 > La2Ni0.5Fe0.5O4 > LaNi0.5Fe0.5O3 | 31.0%—4 h 18.0%—4 h 3%—4 h 9%—4 h | [111] |
| LaNiO3 LaNi0.8Cu0.2O3 LaNi0.6Cu0.4O3 LaNi0.4Cu0.6O3 LaNi0.2Cu0.8O3 LaCuO3 | T = 750 °C WHSV = 180,000 mL g−1 h−1 CH4:CO2:He = 1:1:1 | LaNiO3 > LaNi0.6Cu0.4O3 > LaNi0.4Cu0.6O3 > LaNi0.8Cu0.2O3 > LaNi0.2Cu0.8O3 > LaCuO3 | - - - - - - | [112] |
| La2NiO4 La2Ni0.9Cu0.1O4 La2Ni0.8Cu0.2O4 La2Ni0.7Cu0.3O4 La2Ni0.6Cu0.4O4 | T = 750 °C WHSV = 18,000 mL g−1 h−1 CH4:CO2 = 1:1 | La2NiO4 > LaNi0.9Cu0.1O4 > LaNi0.8Cu0.2O4 > LaNi0.7Cu0.3O4 > LaNi0.6Cu0.4O4 | 0.4 gcg−1h−1—4 h 0.18 gcg−1h−1—5 h 0.01 gcg−1h−1—5 h 0.01 gcg−1h−1—5 h 0.01 gcg−1h−1—5 h | [113] |
| LaNiO3 LaNi0.8Mn0.2O3 LaNi0.6Mn0.4O3 LaNi0.4Mn0.6O3 LaNi0.2Mn0.8O3 LaMnO3 | T = 750 °C GHSV = 15,000 mL g−1 h−1 CH4:CO2:N2 = 1:1:2 | LaNi0.6Mn0.4O3 > LaNi0.4Mn0.6O3 > LaNi0.8Mn0.2O3 > LaMnO3 > LaNi0.2Mn0.8O3 > LaNiO3 | - - - - - - | [114] |
| Kinetic Parameters | Temperature Range (°C) | Ref. |
|---|---|---|
| Ni/La2O3 K1 k2 = 2.61 × 10−3exp(−4300/T) [mol g−1 s−1] K3 = 5.17 × 10−5exp(8700/T) [kPa−1] k4 = 5.35 × 10−1exp(−7500/T) [mol g−1 s−1] | 650–750 | [155] |
| Ni/La2O3 derived from LaNiO3, at T = 700 °C K1 = 141 × 10−3 [kPa] k2 = 0.22326 × 10−3 [mol g−1 s−1] K3 = 15.98 × 10−3 [kPa] K4 = 13.22 × 10−3 [mol g−1 s−1] | 500–700 | [82] |
| LaNiO3 K1 = 279.55exp(−7502.5/T) [kPa−1] k2 = 12.27exp(−10,219.2/T) [mol g−1 s−1] K3 k4 = 0.034exp(−6968.2/T) [kPa−1 mol g−1 s−1] | 650–750 | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. A Mini-Review on Lanthanum–Nickel-Based Perovskite-Derived Catalysts for Hydrogen Production via the Dry Reforming of Methane (DRM). Catalysts 2023, 13, 1357. https://doi.org/10.3390/catal13101357
Georgiadis AG, Charisiou ND, Goula MA. A Mini-Review on Lanthanum–Nickel-Based Perovskite-Derived Catalysts for Hydrogen Production via the Dry Reforming of Methane (DRM). Catalysts. 2023; 13(10):1357. https://doi.org/10.3390/catal13101357
Chicago/Turabian StyleGeorgiadis, Amvrosios G., Nikolaos D. Charisiou, and Maria A. Goula. 2023. "A Mini-Review on Lanthanum–Nickel-Based Perovskite-Derived Catalysts for Hydrogen Production via the Dry Reforming of Methane (DRM)" Catalysts 13, no. 10: 1357. https://doi.org/10.3390/catal13101357
APA StyleGeorgiadis, A. G., Charisiou, N. D., & Goula, M. A. (2023). A Mini-Review on Lanthanum–Nickel-Based Perovskite-Derived Catalysts for Hydrogen Production via the Dry Reforming of Methane (DRM). Catalysts, 13(10), 1357. https://doi.org/10.3390/catal13101357