Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects
Abstract
1. Introduction
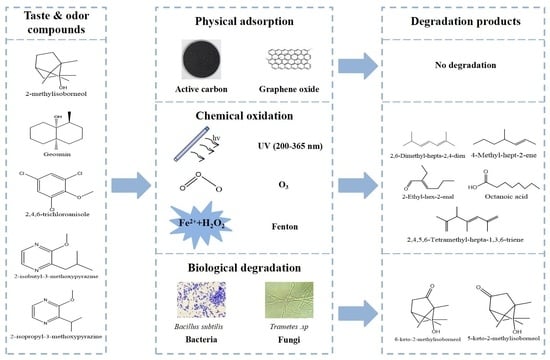
2. Degradation of T&O Compounds Using Abiotic Methods
2.1. Physical Methods
2.2. Chemical Methods
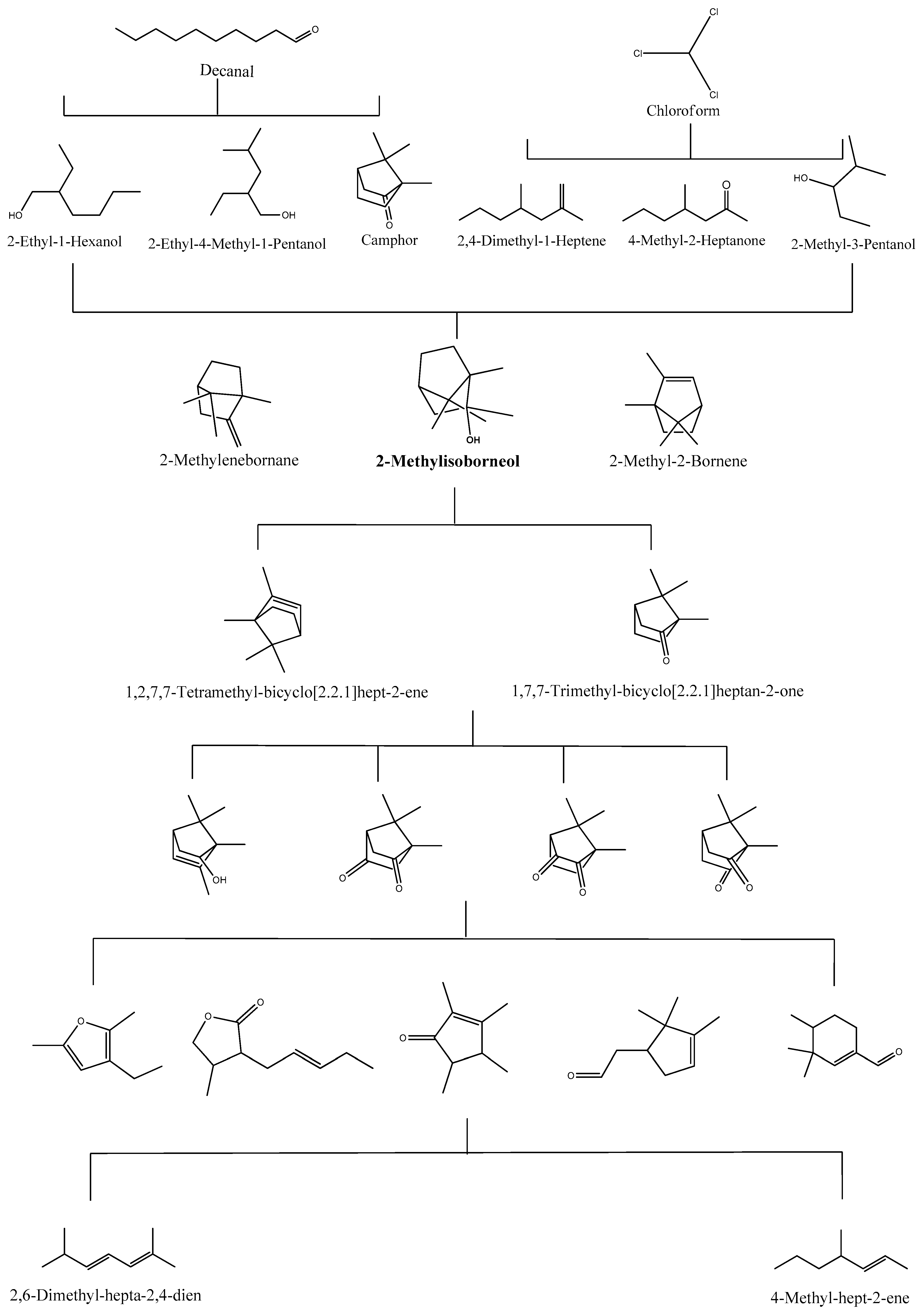
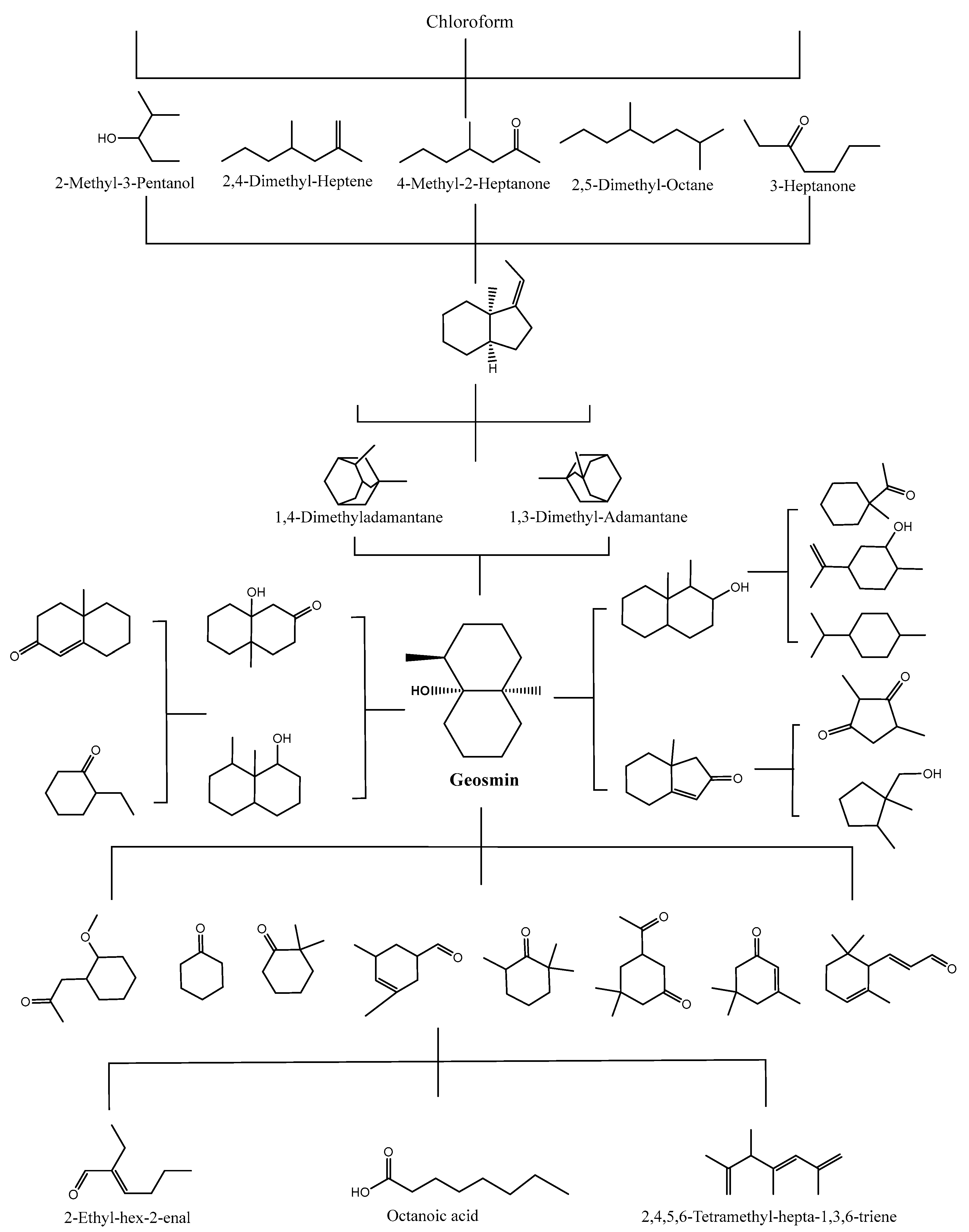
2.3. Degradation Pathway Using Chemical Methods
3. Degradation of T&O Compounds by Biological Methods
3.1. Degradation Efficiency of Microorganisms
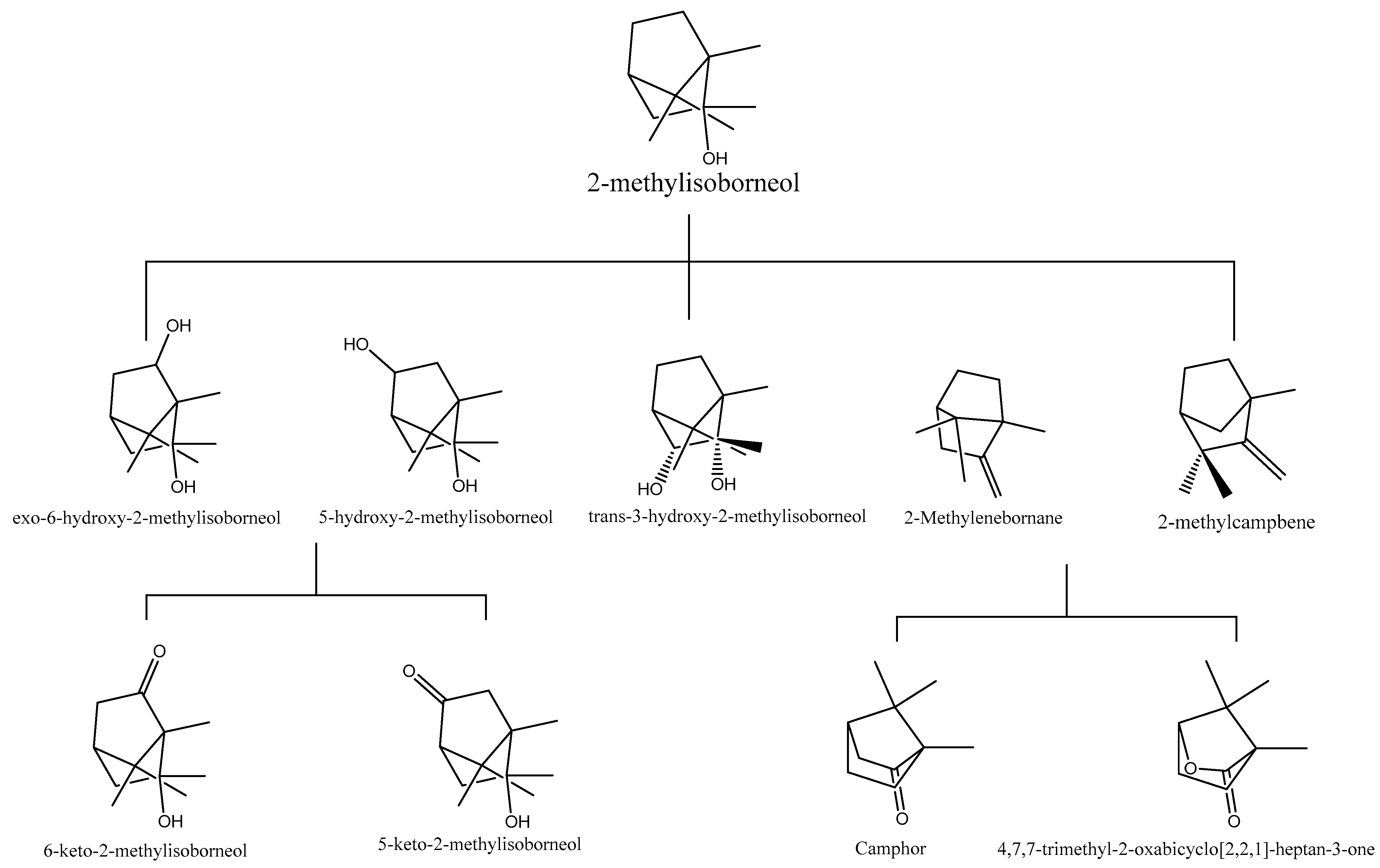
3.2. Degradation Pathway Using Biological Methods
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Term |
| T&O | Taste and odor |
| GSM | Geosmin |
| 2-MIB | 2-methylisoborneol |
| IBMP | 2-isobutyl-3-methoxypyrazine |
| IPMP | 2-isopropyl-3-methoxypyrazine |
| TCA | 2,4,6-trichloroanisole |
| OTC | Odor threshold concentration |
| NOM | Natural organic matter |
| GAC | Granular activated carbon |
| PAC | Powdered activated carbon |
| S-PAC | Super-powdered activated carbon |
| GO | Graphene oxide |
| [R.T.] | React time |
| AOPs | Advanced oxidation methods |
| PMC | Plant-mineral composite |
| DOC | Dissolved organic carbon |
| E-peroxone | Electro-peroxone |
| TOC | Total organic carbon |
| POM | Polyoxometalates |
| rGOF | Reduced graphene oxide supported magnetite |
| GC-MS | Gas chromatography-mass spectrometry |
| PBS | Phosphate-buffered saline |
| VOCs | Volatile organic compounds |
| UF-BPAC | Ultrafiltration membrane bioreactor |
| MBR | Membrane bioreactor |
| MBBR | Moving-bed biofilm reactor |
| EPCs | Enzyme–polyelectrolyte complexes |
| His6-OPH | Hexahistidine-tagged organophosphorus hydrolase |
References
- Zhang, R.; Qi, F.; Liu, C.; Zhang, Y.; Wang, Y.; Song, Z.; Kumirska, J.; Sun, D. Cyanobacteria derived taste and odor characteristics in various lakes in China: Songhua Lake, Chaohu Lake and Taihu Lake. Ecotoxicol. Environ. Saf. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Yu, C.; Shi, C.; Tang, J.; Ji, Q.; Wang, X.; Xu, X.; Wang, G. Release of taste and odour compounds during Zizania latifolia decay: A microcosm system study. Environ. Pollut. 2019, 254, 112954. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Konstantinou, I. TiO2 photocatalysis of 2-isopropyl-3-methoxy pyrazine taste and odor compound in aqueous phase: Kinetics, degradation pathways and toxicity evaluation. Catal. Today 2015, 240, 22–29. [Google Scholar] [CrossRef]
- Kim, T.K.; Moon, B.R.; Kim, T.; Kim, M.K.; Zoh, K.D. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions. Chemosphere 2016, 162, 157–164. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, L.; Li, L.; Song, L. 2-Methylisoborneol production characteristics of Pseudanabaena sp. FACHB 1277 isolated from Xionghe Reservoir, China. J. Appl. Phycol. 2016, 28, 3353–3362. [Google Scholar] [CrossRef]
- Bang, H.; Slokar, Y.M.; Ferrero, G.; Kruithof, J.C.; Kennedy, M.D. Removal of taste and odor causing compounds by UV/H2O2 treatment: Effect of the organic and inorganic water matrix. Desalin. Water Treat. 2016, 57, 27485–27494. [Google Scholar] [CrossRef]
- Li, L.; Yang, S.; Yu, S.; Zhang, Y. Variation and removal of 2-MIB in full-scale treatment plants with source water from Lake Tai, China. Water Res. 2019, 162, 180–189. [Google Scholar] [CrossRef]
- Adams, H.; Southard, M.; Reeder, S.; Buerkens, F.; Hallford, R.L.; Ikehata, K.; Nix, D.K. Successfully Detecting and Mitigating Algal Blooms and Taste and Odor Compounds. J. Am. Water Work. Assoc. 2021, 113, 10–19. [Google Scholar] [CrossRef]
- Zhu, J.; Stuetz, R.M.; Hamilton, L.; Power, K.; Crosbie, N.D.; Tamburic, B. Management of biogenic taste and odour: From source water, through treatment processes and distribution systems, to consumers. J. Environ. Manag. 2022, 323, 116225. [Google Scholar] [CrossRef]
- Li, L.; Li, J.Y.; Zhu, C.W.; Yu, S.L. Study of the binding regularity and corresponding mechanism of drinking water odorous compound 2-MIB with coexisting dissolved organic matter. Chem. Eng. J. 2020, 395, 125015. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef]
- Juttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef]
- Clercin, N.A.; Druschel, G.K.; Gray, M. Occurrences of 2-methylisoborneol and geosmin-degrading bacteria in a eutrophic reservoir and the role of cell-bound versus dissolved fractions. J. Environ. Manag. 2021, 297, 113304. [Google Scholar] [CrossRef]
- Jo, J.O.; Kim, S.D.; Lee, H.J.; Mok, Y.S. Decomposition of taste-and-odor compounds produced by cyanobacteria algae using atmospheric pressure plasma created inside a porous hydrophobic ceramic tube. Chem. Eng. J. 2014, 247, 291–301. [Google Scholar] [CrossRef]
- Watson, S.B.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste- and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef]
- Fang, J.Y.; Liu, J.J.; Shang, C.; Fan, C.H. Degradation Investigation of Selected Taste and Odor Compounds by a UV/Chlorine Advanced Oxidation Process. Int. J. Environ. Res. Public Health 2018, 15, 284. [Google Scholar] [CrossRef]
- Guttman, L.; van Rijn, J. 2-Methylisoborneol and geosmin uptake by organic sludge derived from a recirculating aquaculture system. Water Res. 2009, 43, 474–480. [Google Scholar] [CrossRef]
- Pirbazari, M.; Borow, H.; Craig, S.; Ravindran, V.; McGuire, M. Physical chemical characterization of five earthy-musty-smelling compounds. Water Sci. Technol. 1992, 25, 81–88. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.M.; Abdulkareem, A.S.; Shuaib, D.T.; Mohammed, A.K. A critical review on geosmin and 2-methylisoborneol in water: Sources, effects, detection, and removal techniques. Environ. Monit. Assess. 2021, 193, 204. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment—A review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Gee, C.S.; Choi, E. Treatment of taste and odor causing substances in drinking water. Water Sci. Technol. 1997, 35, 29–36. [Google Scholar] [CrossRef]
- Asghar, A.; Khan, Z.; Maqbool, N.; Qazi, I.A.; Awan, M.A. Comparison of adsorption capability of activated carbon and metal doped TiO2 for geosmin and 2-MIB removal from water. J. Nanomater. 2015, 2015, 479103. [Google Scholar] [CrossRef]
- Paulino, R.; Tamburic, B.; Stuetz, R.M.; Zamyadi, A.; Crosbie, N.; Henderson, R.K. Critical review of adsorption and biodegradation mechanisms for removal of biogenic taste and odour compounds in granular and biological activated carbon contactors. J. Water Process Eng. 2023, 52, 103518. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Ntougias, S.; Melidis, P.; Navrozidou, E.; Tzegkas, F. Diversity and efficiency of anthracene-degrading bacteria isolated from a denitrifying activated sludge system treating municipal wastewater. Int. Biodeterior. Biodegrad. 2015, 97, 151–158. [Google Scholar] [CrossRef]
- Khajouei, G.; Finklea, H.O.O.; Lin, L.S. UV/chlorine advanced oxidation processes for degradation of contaminants in water and wastewater: A comprehensive review. J. Environ. Chem. Eng. 2022, 10, 107508. [Google Scholar] [CrossRef]
- Feng, G.X.; Jia, R.B.; Sun, S.H.; Wang, M.Q.; Zhao, Q.H.; Xin, X.D.; Liu, L. Occurrence and removal of 10 odorous compounds in drinking water by different treatment processes. Environ. Sci. Pollut. Res. 2020, 27, 18924–18933. [Google Scholar] [CrossRef]
- Fan, X.J.; Tao, Y.; Wang, L.Y.; Zhang, X.H.; Lei, Y.; Wang, Z.; Noguchi, H. Performance of an integrated process combining ozonation with ceramic membrane ultra-filtration for advanced treatment of drinking water. Desalination 2014, 335, 47–54. [Google Scholar] [CrossRef]
- Ren, X.Y.; Wu, Q.D.; Shu, J.Y.; Chen, C.; Tiraferri, A.; Liu, B.C. Efficient removal of organic matters and typical odor substances in rural drinking water using Ozone-BAC-UF combined system to meet new water quality standards in China. Sep. Purif. Technol. 2023, 327, 124899. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Zuo, Y.; Huang, Y.; Song, L. Adsorption of 2-methylisoborneol and geosmin by a low-cost hybrid adsorbent synthesized from fly ash and bentonite. J. Water Supply Res. Technol. Aqua 2011, 60, 478–485. [Google Scholar] [CrossRef]
- Park, S.M.; Heo, T.Y.; Park, N.B.; Na, K.J.; Jun, H.B.; Jung, J.Y. Application of air stripping to powdered activated carbon adsorption of geosmin and 2-methylisoborneol. J. Water Supply Res. Technol. Aqua 2010, 59, 492–500. [Google Scholar] [CrossRef]
- An, N.; Xie, H.h.; Gao, N.y.; Deng, Y.; Chu, W.h.; Jiang, J. Adsorption of two taste and odor compounds IPMP and IBMP by granular activated carbon in water. CLEAN—Soil Air Water 2012, 40, 1349–1356. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakano, Y.; Hiroshi, H.; Ando, N.; Matsushita, T.; Ohno, K. Geosmin and 2-methylisoborneol adsorption on super-powdered activated carbon in the presence of natural organic matter. Water Sci. Technol. 2010, 62, 2664–2668. [Google Scholar] [CrossRef]
- Cho, K.; An, B.M.; So, S.; Chae, A.; Song, K.G. Simultaneous control of algal micropollutants based on ball-milled powdered activated carbon in combination with permanganate oxidation and coagulation. Water Res. 2020, 185, 116263. [Google Scholar] [CrossRef]
- Bong, T.; Kang, J.K.; Yargeau, V.; Nam, H.L.; Lee, S.H.; Choi, J.W.; Kim, S.B.; Park, J.A. Geosmin and 2-methylisoborneol adsorption using different carbon materials: Isotherm, kinetic, multiple linear regression, and deep neural network modeling using a real drinking water source. J. Clean. Prod. 2021, 314, 127967. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.I.; Hwang, S.; Cho, M.; Kim, H.S.; Noh, S.H. Removal of geosmin and 2-methylisoboneol (2-MIB) by membrane system combined with powdered activated carbon (PAC) for drinking water treatment. J. Water Process Eng. 2014, 4, 91–98. [Google Scholar] [CrossRef]
- Hafuka, A.; Nagasato, T.; Yamamura, H. Application of graphene oxide for adsorption removal of geosmin and 2-Methylisoborneol in the presence of natural organic matter. Int. J. Environ. Res. Public Health 2019, 16, 1907. [Google Scholar] [CrossRef]
- Westerhoff, P.; Rodriguez-Hernandez, M.; Baker, L.; Sommerfeld, M. Seasonal occurrence and degradation of 2-methylisoborneol in water supply reservoirs. Water Res. 2005, 39, 4899–4912. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Gao, J.; Zhu, J.; Zhou, J. Process optimization and mechanism revealing of KMnO4 pre-oxidation coupled powdered activated carbon adsorption for 2-MIB removal. J. Water Process Eng. 2023, 53, 103705. [Google Scholar] [CrossRef]
- Lompe, K.M.; Solliec, M.; Rivas, M.; Peldszus, S.; Barbeau, B. Selecting a single powdered activated carbon against multiple threats: Taste & odour and benzene. Environ. Sci. Water Res. Technol. 2022, 8, 3091–3100. [Google Scholar] [CrossRef]
- Cerón-Vivas, A.; Villamizar León, M.P.; Cajigas, Á.A. Geosmin and 2-methylisoborneol removal in drinking water treatment. Water Pract. Technol. 2023, 18, 159–167. [Google Scholar] [CrossRef]
- Azaria, S.; Nussinovitch, A.; Nir, S.; Mordechai, S.; van Rijn, J. Removal of geosmin and 2-methylisoborneol from aquaculture water by novel, alginate-based carriers: Performance and metagenomic analysis. J. Water Process Eng. 2021, 42, 102125. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Kopecka, I.; Cermakova, L.; Fialova, K.; Novotna, K.; Cajthaml, T.; Henderson, R.K.; Pivokonska, L. Current knowledge in the field of algal organic matter adsorption onto activated carbon in drinking water treatment. Sci. Total Environ. 2021, 799, 149455. [Google Scholar] [CrossRef]
- Moztahida, M.; Nawaz, M.; Kim, J.; Shahzad, A.; Kim, S.; Jang, J.; Lee, D.S. Reduced graphene oxide-loaded-magnetite: A Fenton-like heterogeneous catalyst for photocatalytic degradation of 2-methylisoborneol. Chem. Eng. J. 2019, 370, 855–865. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Wang, W.; Li, Y.; Pei, H. Moderate pre-ozonation coupled with a post-peroxone process remove filamentous cyanobacteria and 2-MIB efficiently: From bench to pilot-scale study. J. Hazard. Mater. 2022, 424, 127530. [Google Scholar] [CrossRef] [PubMed]
- Abrha, Y.W.; Kye, H.; Kwon, M.; Lee, D.; Kim, K.; Jung, Y.; Ahn, Y.; Kang, J.W. Removal of Algae, and Taste and Odor Compounds by a Combination of Plant-Mineral Composite (PMC) Coagulant with UV-AOPs: Laboratory and Pilot Scale Studies. Appl. Sci. 2018, 8, 1502. [Google Scholar] [CrossRef]
- Yao, W.; Qu, Q.; Von Gunten, U.; Chen, C.; Yu, G.; Wang, Y. Comparison of methylisoborneol and geosmin abatement in surface water by conventional ozonation and an electro-peroxone process. Water Res. 2017, 108, 373–382. [Google Scholar] [CrossRef]
- Meunier, L.; Canonica, S.; Von Gunten, U. Implications of sequential use of UV and ozone for drinking water quality. Water Res. 2006, 40, 1864–1876. [Google Scholar] [CrossRef]
- Mizuno, T.; Ohara, S.; Nishimura, F.; Tsuno, H. O3/H2O2 process for both removal of odorous algal-derived compounds and control of bromate ion formation. Ozone Sci. Eng. 2011, 33, 121–135. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Feng, L.; Chen, Z.; Zhang, L.; Sun, D. Formation of aldehyde during ozonation of taste and odour compounds in water. J. Water Supply Res. Technol. Aqua 2013, 62, 120–128. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, D.; Li, F.; Fu, M.L. Removal efficiency and possible pathway of odor compounds (2-methylisoborneol and geosmin) by ozonation. Sep. Purif. Technol. 2013, 117, 53–58. [Google Scholar] [CrossRef]
- Kutschera, K.; Börnick, H.; Worch, E. Photoinitiated oxidation of geosmin and 2-methylisoborneol by irradiation with 254 nm and 185 nm UV light. Water Res. 2009, 43, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; O’Shea, K.E. Ultrasonically induced degradation of 2-methylisoborneol and geosmin. Water Res. 2007, 41, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Maekawa, A.; Takahashi, M.; Hayashi, Y. Toxicity and carcinogenicity of potassium bromate—A new renal carcinogen. Environ. Health Perspect. 1990, 87, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.; Godley, A.; Lytton, L.; Cartmell, E. Bromate environmental contamination: Review of impact and possible treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 193–217. [Google Scholar] [CrossRef]
- Berlt, M.M.G.; Schneider, R.D.D.; Machado, E.L.; Kist, L.T. Comparative assessment of the degradation of 2-methylisoborneol and geosmin in freshwater using advanced oxidation processes. Environ. Technol. 2021, 42, 3832–3839. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Bakheet, B.; Yuan, S.; Li, Z.; Wang, H.; Zuo, J.; Komarneni, S.; Wang, Y. Electro-peroxone treatment of Orange II dye wastewater. Water Res. 2013, 47, 6234–6243. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, M.; Yu, G.; Östman, M.; Cheng, Y.; Wang, Y.; Tysklind, M. Oxidation of emerging biocides and antibiotics in wastewater by ozonation and the electro-peroxone process. Chemosphere 2019, 235, 575–585. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W.; Fu, S.; Yang, H.; Yu, G.; Wang, Y. Inhibition of bromate formation during drinking water treatment by adapting ozonation to electro-peroxone process. Chem. Eng. J. 2015, 264, 322–328. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.B.; Chen, Z.L.; Zhang, L.Q.; Zhang, P.Y.; Sun, D.Z. Mechanism investigation of catalyzed ozonation of 2-methylisoborneol in drinking water over aluminum (hydroxyl) oxides: Role of surface hydroxyl group. Chem. Eng. J. 2010, 165, 490–499. [Google Scholar] [CrossRef]
- Xu, B.B.; Qi, F. Reaction Mechanism of 2-Methylisoborneol and 2,4,6-Trichloroanisole in Catalytic Ozonation by γ-AlOOH: Role of Adsorption. Clean Soil Air Water 2016, 44, 1099–1105. [Google Scholar] [CrossRef]
- Kazankov, G.M.; Sergeeva, V.S.; Efremenko, E.N.; Alexandrova, L.; Varfolomeev, S.D.; Ryabov, A.D. Highly efficient degradation of thiophosphate pesticides catalyzed by platinum and palladium aryl oxime metallacycles. Angew. Chem. Int. Ed. 2000, 39, 3117–3119. [Google Scholar] [CrossRef]
- Park, J.A.; Nam, H.L.; Choi, J.W.; Ha, J.; Lee, S.H. Oxidation of geosmin and 2-methylisoborneol by the photo-Fenton process: Kinetics, degradation intermediates, and the removal of microcystin-LR and trihalomethane from Nak-Dong River water, South Korea. Chem. Eng. J. 2017, 313, 345–354. [Google Scholar] [CrossRef]
- Jo, C.H.; Dietrich, A.M.; Tanko, J.M. Simultaneous degradation of disinfection byproducts and earthy-musty odorants by the UV/H2O2 advanced oxidation process. Water Res. 2011, 45, 2507–2516. [Google Scholar] [CrossRef]
- Springer, J.C.; Kashinkunti, R.; Hong, Y. Medium-Pressure UV/Chlorine Advanced Oxidation of Taste and Odor Compounds. J. Am. Water Work. Assoc. 2018, 110, E16–E27. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Ioannidis, N.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Kinetic and mechanistic investigation of water taste and odor compound 2-isopropyl-3-methoxy pyrazine degradation using UV-A/Chlorine process. Sci. Total Environ. 2020, 732, 138404. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Lin, Y.L.; Zhang, T.Y.; Lu, Y.S.; Zhou, X.Y.; Liu, Z.; Zheng, Z.X.; Xu, M.Y.; Xu, B. Degradation of odorous 2, 4, 6-trichloroanisole in chlorinated water by UV-LED/chlorination: Kinetics and influence factors. Environ. Sci. Pollut. Res. 2023, 30, 44325–44336. [Google Scholar] [CrossRef]
- Tan, F.; Chen, H.; Wu, D.; Lu, N.; Gao, Z. Optimization of Removal of 2-methylisoborneol from Drinking Water using UV/H2O2. J. Adv. Oxid. Technol. 2016, 19, 98–104. [Google Scholar] [CrossRef][Green Version]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Papaconstantinou, E.; Hiskia, A. Photocatalytic degradation of water taste and odour compounds in the presence of polyoxometalates and TiO2: Intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2014, 286, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, W.; Lu, H.; Zhu, Z. Efficient Photocatalytic Degradation of 2-Methylisoborneol by Zn-Al-La Multi-Metal Oxide Composites Derived from LDH. Water Air Soil Pollut. 2023, 234, 24. [Google Scholar] [CrossRef]
- Guo, X.P.; Zang, P.; Li, Y.M.; Bi, D.S. TiO2-powdered activated carbon (TiO2/PAC) for removal and photocatalytic properties of 2-Methylisoborneol (2-MIB) in water. Water 2021, 13, 1622. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Wang, G.; Zhu, S.; Ye, Z. Kinetic and mechanistic investigation of geosmin and 2-methylisoborneol degradation using UV-assisted photoelectrochemical. Chemosphere 2022, 290, 133325. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chen, Z.; Feng, L.; Zhang, L.; Sun, D. Catalytic ozonation of 2-isopropyl-3-methoxypyrazine in water by γ-AlOOH and γ-Al2O3: Comparison of removal efficiency and mechanism. Chem. Eng. J. 2013, 219, 527–536. [Google Scholar] [CrossRef]
- Liu, S.; Tang, L.; Wu, M.; Fu, H.; Xu, J.; Chen, W.; Ma, F. Parameters influencing elimination of geosmin and 2-methylisoborneol by K2FeO4. Sep. Purif. Technol. 2017, 182, 128–133. [Google Scholar] [CrossRef]
- Liu, X.; Ding, S.; Wang, P.; Hong, Y.; Zhao, H.; Chu, W. Simultaneous mitigation of disinfection by-product formation and odor compounds by peroxide/Fe (II)-based process: Combination of oxidation and coagulation. Water Res. 2021, 201, 117327. [Google Scholar] [CrossRef]
- Lee, J.; Nam, S.H.; Koo, J.W.; Kim, E.; Hwang, T.M. Comparative evaluation of 2-isopropyl-3-methoxypyrazine, 2-isobutyl-3-methoxypyrazine, and 2, 4, 6-trichloroanisole degradation by ultraviolet/chlorine and ultraviolet/hydrogen peroxide processes. Chemosphere 2021, 279, 130513. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Tian, L.; Liu, Y.; Liu, J.; He, G.; Li, J. Pilot-scale and mechanistic study of the degradation of typical odors and organic compounds in drinking water by a combined UV/H2O2-BAC process. Chemosphere 2022, 292, 133419. [Google Scholar] [CrossRef]
- Meng, T.; Su, X.; Sun, P. Degradation of geosmin and 2-methylisoborneol in UV-based AOPs for photoreactors with reflective inner surfaces: Kinetics and transformation products. Chemosphere 2022, 306, 135611. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhou, B.; Yuan, R. Biodegradation of 2-methylisoborneol by single bacterium in culture media and river water environment. Int. J. Environ. Stud. 2017, 74, 399–411. [Google Scholar] [CrossRef]
- Arriaga, S.; Revah, S. Improving hexane removal by enhancing fungal development in a microbial consortium biofilter. Biotechnol. Bioeng. 2005, 90, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhou, B.; Shi, C.; Yu, L.; Zhang, C.; Gu, J. Biodegradation of 2-methylisoborneol by bacteria enriched from biological activated carbon. Front. Environ. Sci. Eng. 2012, 6, 701–710. [Google Scholar] [CrossRef]
- Lauderdale, C.V.; Aldrich, H.C.; Lindner, A.S. Isolation and characterization of a bacterium capable of removing taste-and odor-causing 2-methylisoborneol from water. Water Res. 2004, 38, 4135–4142. [Google Scholar] [CrossRef]
- Luo, G.; Wang, J.; Ma, N.; Liu, Z.; Tan, H. Effects of inoculated bacillus subtilis on geosmin and 2-methylisoborneol removal in suspended growth reactors using aquacultural waste for biofloc production. J. Microbiol. Biotechnol. 2016, 26, 1420–1427. [Google Scholar] [CrossRef]
- Xue, Q.; Shimizu, K.; Sakharkar, M.K.; Utsumi, M.; Cao, G.; Li, M.; Zhang, Z.; Sugiura, N. Geosmin degradation by seasonal biofilm from a biological treatment facility. Environ. Sci. Pollut. Res. 2012, 19, 700–707. [Google Scholar] [CrossRef]
- Ho, L.; Hoefel, D.; Bock, F.; Saint, C.P.; Newcombe, G. Biodegradation rates of 2-methylisoborneol (MIB) and geosmin through sand filters and in bioreactors. Chemosphere 2007, 66, 2210–2218. [Google Scholar] [CrossRef]
- Tesso, T.A.; Zheng, A.; Cai, H.; Liu, G. Isolation and characterization of two Acinetobacter species able to degrade 3-methylindole. PLoS ONE 2019, 14, e0211275. [Google Scholar] [CrossRef]
- Guttman, L.; van Rijn, J. Isolation of bacteria capable of growth with 2-methylisoborneol and geosmin as the sole carbon and energy sources. Appl. Environ. Microbiol. 2012, 78, 363–370. [Google Scholar] [CrossRef]
- Schroeder, M.; Pöllinger-Zierler, B.; Aichernig, N.; Siegmund, B.; Guebitz, G. Enzymatic removal of off-flavors from apple juice. J. Agric. Food Chem. 2008, 56, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lu, L.; Kennes, C.; Yu, J.; Chen, J. Treatment of gaseous toluene in three biofilters inoculated with fungi/bacteria: Microbial analysis, performance and starvation response. J. Hazard. Mater. 2016, 303, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Meléndez, O.; Bárzana, E.; Arriaga, S.; Hernández-Luna, M.; Revah, S. Fungal removal of gaseous hexane in biofilters packed with poly(ethylene carbonate) pine sawdust or peat composites. Biotechnol. Bioeng. 2008, 100, 864–871. [Google Scholar] [CrossRef]
- Lebrero, R.; Rodríguez, E.; Martin, M.; García-Encina, P.A.; Muñoz, R. H2S and VOCs abatement robustness in biofilters and air diffusion bioreactors: A comparative study. Water Res. 2010, 44, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Veiga, M.C. Fungal biocatalysts in the biofiltration of VOC-polluted air. J. Biotechnol. 2004, 113, 305–319. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Illa, J.; van Groenestijn, J.W.; Flotats, X. Influence of synthetic packing materials on the gas dispersion and biodegradation kinetics in fungal air biofilters. Appl. Microbiol. Biotechnol. 2008, 79, 319–327. [Google Scholar] [CrossRef]
- Doederer, K.; De Vera, G.A.; Espino, M.P.; Pype, M.L.; Gale, D.; Keller, J. MIB and geosmin removal during adsorption and biodegradation phases of GAC filtration. Water Sci. Technol. Water Supply 2018, 18, 1449–1455. [Google Scholar] [CrossRef]
- Elhadi, S.L.N.; Huck, P.M.; Slawson, R.M. Removal of geosmin and 2-methyli’soborneol by biological filtration. Water Sci. Technol. 2004, 49, 273–280. [Google Scholar] [CrossRef]
- Chen, S.Y.; Dong, B.Z.; Gao, K.; Li, T. Pilot study on advanced treatment of geosmin and 2-MIB with O3/GAC. Water Supply 2019, 19, 1253–1263. [Google Scholar] [CrossRef]
- McDowall, B.; Hoefel, D.; Newcombe, G.; Saint, C.P.; Ho, L. Enhancing the biofiltration of geosmin by seeding sand filter columns with a consortium of geosmin-degrading bacteria. Water Res. 2009, 43, 433–440. [Google Scholar] [CrossRef]
- Liang, S.; Li, X.; Yang, Y.L.; Liu, L.; Liu, Y.W.; Cheng, Z.J.; Li, B.; Li, G.B. PAC addition on immersed ultrafiltration membrane for the treatment of raw water containing taste and odor compounds. In Proceedings of the 2nd International Conference on Structures and Building Materials (ICSBM), Hangzhou, China, 9–11 March 2012; pp. 2855–2859. [Google Scholar]
- Doederer, K.; Gale, D.; Keller, J. Effective removal of MIB and geosmin using MBBR for drinking water treatment. Water Res. 2019, 149, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Senko, O.; Efremenko, E. The influence of enzymatic removal of chlorpyrifos from feed grain mixes on biochemical parameters of rat blood. Biochem. Mosc. Suppl. Ser. B 2018, 12, 181–185. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Eaton, R.W.; Sandusky, P. Biotransformations of 2-Methylisoborneol by Camphor-Degrading Bacteria. Appl. Environ. Microbiol. 2009, 75, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Mortazavian, S.; Crowe, G.; Flick, R.; Passeport, E.; Hofmann, R. Evaluating the relative adsorption and biodegradation of 2-methylisoborneol and geosmin across granular activated carbon filter-adsorbers. Water Res. 2022, 215, 118239. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Oritani, T.; Uehara, F.; Saito, A.; Kishita, H.; Niizeki, Y.; Yokota, H.; Fuchigami, K. Biodegradation of a musty odour component, 2-methylisoborneol. Water Res. 1996, 30, 759–761. [Google Scholar] [CrossRef]
- Saito, A.; Tokuyama, T.; Tanaka, A.; Oritani, T.; Fuchigami, K. Microbiological degradation of (-)-geosmin. Water Res. 1999, 33, 3033–3036. [Google Scholar] [CrossRef]
| Material | Adsorbent Dose (mg/L) | Specific Surface Area (m2/g) | Iodine Value (mg/g) | Condition | Adsorption Substrate | Initial Concentration (μg/L) | Degradation Rate | References |
|---|---|---|---|---|---|---|---|---|
| Granular activated carbon | 160 | 274 | -- | T = 25 ± 1 °C, React Time [R.T.] = 1 h | 2-MIB | 0.7 | 73% | [22] |
| Granular activated carbon | 160 | 274 | -- | T = 25 ± 1 °C, [R.T.] = 1 h | GSM | 0.7 | 82% | [22] |
| Granular activated carbon | 200 | -- | 1046 | pH = 3.0–11.0, [R.T.] < 12 h | IPMP | 150 | >90% | [32] |
| Granular activated carbon | 200 | -- | 1046 | pH = 3.0–11.0, [R.T.] < 12 h | IBMP | 150 | >90% | [32] |
| Powdered activated carbon | 15 | 1256 | 950 | [R.T.] = 15 min | GSM | 0.08 | 45% | [40] |
| Powdered activated carbon | 15 | 1256 | 950 | [R.T.] = 15 min | 2-MIB | 0.08 | 45% | [40] |
| Powdered activated carbon | 15 | -- | 1115 | [R.T.] = 60 min aeration flow rate of 15 L/min | 2-MIB | -- | 74% | [31] |
| Powdered activated carbon | 20 | 830–850 | -- | [R.T.] = 50 min | 2-MIB | 1 | 60% | [34] |
| Powdered activated carbon | 20 | 830–850 | -- | [R.T.] = 50 min | GSM | 1 | 80% | [34] |
| Powdered activated carbon | 15 | -- | 1115 | [R.T.] = 30 min aeration flow rate of 15 L/min | GSM | -- | 93% | [31] |
| C-powdered activated carbon | 32.14 | 1216 | -- | [R.T.] = 20 min | 2-MIB | 0.1 | >80% | [35] |
| C-powdered activated carbon | 32.14 | 1216 | -- | [R.T.] = 20 min | GSM | 0.1 | >80% | [35] |
| Wood-powdered activated carbon | 2 | -- | -- | [R.T.] = 30 min | GSM | 0.024 | 62.5% | [41] |
| Wood-powdered activated carbon | 2 | -- | -- | [R.T.] = 30 min | 2-MIB | 0.968 | 37% | [41] |
| Graphene oxide | 100 | 478 | -- | pH = 7.4–7.5, [R.T.] = 15 min | 2-MIB | 1 | 15% | [37] |
| Graphene oxide | 100 | 478 | -- | pH = 7.4–7.5, [R.T.] = 15 min | GSM | 1 | 22% | [37] |
| Fly ash-bentonite (fly ash to bentonite ratio of 0.4:0.6) | 15 | 63.5 | -- | pH = 8.0, [R.T.] = 60 min | 2-MIB | 0.042–0.234 | 59.9% | [30] |
| Fly ash-bentonite (fly ash to bentonite ratio of 0.4:0.6) | 15 | 63.5 | -- | pH = 8.0, [R.T.] = 60 min | GSM | 0.042–0.234 | 63.7% | [30] |
| Alginate-based carriers | 35 | -- | -- | [R.T.] = 24 h | GSM | 1 | 90% | [42] |
| Alginate-based carriers | 35 | -- | -- | [R.T.] = 24 h | 2-MIB | 1 | 80% | [42] |
| Pt-TiO2 | 125 | 567 | -- | T = 25 ± 1 °C, [R.T.] = 1 h | GSM | 0.7 | 98% | [22] |
| Fe-TiO2 | 125 | 423 | -- | T = 25 ± 1 °C, [R.T.] = 1 h | 2-MIB | 0.7 | 93% | [22] |
| Fe-TiO2 | 125 | 423 | -- | T = 25 ± 1 °C, [R.T.] = 1 h | GSM | 0.7 | 95% | [22] |
| Materials and Methods | Condition | Degradation Substrate | Initial Concentration (μg/L) | Degradation Rate | Possible Intermediate | References |
|---|---|---|---|---|---|---|
| UV (254 nm) UV fluence = 3348 mJ/cm2 | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | 2-MIB | 0.1 | 34.08% | -- | [65] |
| UV (254 nm) UV fluence = 3348 mJ/cm2 | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | GSM | 0.1 | 21.19% | -- | [65] |
| UV fluence = 1200 mJ/cm2 | -- | 2-MIB | 0.108 | 20% | -- | [66] |
| UV fluence = 1200 mJ/cm2 | -- | GSM | 0.04 | 20% | -- | [66] |
| UV (254 nm)/chlorine [chlorine] = 0.5 mg/L | T = 25 °C, pH = 7.0 [R.T.] = 1 h | 2-MIB | 5 | 100% | 2-methylenebornane and 2-methyl-2-bornene | [4] |
| UV (254 nm)/chlorine [chlorine] = 0.5 mg/L | T = 25 °C, pH = 7.0 [R.T.] = 40 min | GSM | 5 | 100% | 1,4-dimethyl-adamantane and 1,3-dimethyl-adamantane | [4] |
| UV/chlorine UV = 250 mJ/cm2, [chlorine] = 0.5; 1.2 mg/L | pH = 5.0 | 2-MIB | 0.06 | >97% | -- | [67] |
| UV/chlorine UV = 250 mJ/cm2, [chlorine] = 0.5; 1.2 mg/L | pH = 5.0 | GSM | 0.06 | >97% | -- | [67] |
| UV (210 nm)/chlorine, [chlorine] = 100 mg/L | T = 25 °C, pH = 7.0 | IPMP | 1 × 104 | 95.6% | -- | [68] |
| UV-LED (275 nm)/chlorine UV-LED = 420 mJ/cm2 [Chlorine]0 = 200 μM, | T = 25 ± 1 °C pH = 7.0 ± 0.1 | TCA | 20 | 80% | -- | [69] |
| UV/H2O2 UV fluence = 1200 mJ/cm2, [H2O2] = 6 mg/L | -- | 2-MIB | 0.306 | 65% | -- | [66] |
| UV/H2O2 UV fluence = 1200 mJ/cm2, [H2O2] = 6 mg/L | -- | GSM | 0.183 | 90% | -- | [66] |
| UV (254 nm)/H2O2 UV fluence = 3348 mJ/cm2, [H2O2] = 20 mg/L | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | 2-MIB | 0.1 | 52.1% | -- | [65] |
| UV (254 nm)/H2O2 UV fluence = 3348 mJ/cm2, [H2O2] = 20 mg/L | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | GSM | 0.1 | 38.28% | -- | [65] |
| UV/H2O2 UV fluence = 350 mJ/cm2, [H2O2] = 6 mg/L | -- | 2-MIB | 0.275 | 96.58% | -- | [70] |
| UV/H2O2 UV fluence = 600 mJ/cm2, [H2O2] = 4 mg/L | -- | 2-MIB | 0.219 | >80% | -- | [6] |
| UV/H2O2 UV fluence = 600 mJ/cm2, [H2O2] = 4 mg/L | -- | GSM | 0.231 | > 80% | -- | [6] |
| UV (238 nm)/persulfate I0/V = 1.26 μE s−1 L−1, [PDS]0 = 10 μM | T = 20 °C, pH = 7.0 [R.T.] = 10 min | 2-MIB | 40 | 86% | -- | [71] |
| UV (219 nm)/persulfate I0/V = 1.26 μE s−1 L−1, [PDS]0 = 10 μM | T = 20 °C, pH = 7.0 [R.T.] = 10 min | GSM | 40 | 94.5% | -- | [71] |
| UV (365 nm)/ SiW12O404− (7 × 10−4 M, 200 mg/L) | [R.T.] = 100 min | 2-MIB | 1 × 103 | 100% | 1,2,7,7-tetramethyl-bicyclo [2.2.1]hept-2-ene and 1,7,7-trimethyl-bicyclo [2.2.1] heptan-2-one (d-camphor) | [72] |
| UV (365 nm)/ SiW12O404− (7 × 10−4 M, 200 mg/L) | [R.T.] = 120 min | GSM | 1 × 103 | 100% | 8a-hydroxy-4a-methyl-octahydro-naphthalen-2-one and 8,8a-dimethyl-decahydro- aphthalen-1-ol | [72] |
| UV (365 nm)/TiO2 [TiO2] = 200 mg/L | [R.T.] = 25 min | 2-MIB | 1 × 103 | 100% | 1,2,7,7-tetramethyl-bicyclo [2.2.1]hept-2-ene and 1,7,7-trimethyl-bicyclo [2.2.1]heptan-2-one (d-camphor) | [72] |
| UV (365 nm)/TiO2 [TiO2] = 200 mg/L | [R.T.] = 30 min | GSM | 1 × 103 | 100% | 8a-hydroxy-4a-methyl-octahydro-naphthalen-2-one and 8,8a-dimethyl-decahydro-naphthalen-1-ol | [72] |
| Simulated sunlight/ Zn-Al-La-MMO (1 g/L) | I = 10 A | 2-MIB | 0.2 | 95% | -- | [73] |
| UV/TiO2 I = 600 W/m2, [TiO2] = 100 mg/L | [R.T.] = 20 min | IPMP | 1 × 104 | 95% | -- | [3] |
| UV (365 nm)/ TiO2/PAC = 3 mg/L | T = 20 ± 1 °C, pH = 7.5 [R.T.] = 3 h | 2-MIB | 1 | 97.8% | -- | [74] |
| UV (254 nm)/NaCl [NaCl] = 60 mmol/L I = 15 mA/cm2 | T = 25 °C, pH = 7.0 [R.T.] = 25 min | 2-MIB | 1 | 95% | -- | [75] |
| UV (254 nm)/NaCl [NaCl] = 60 mmol/L I = 15 mA/cm2 | T = 25 °C, pH = 7.0 [R.T.] = 25 min | GSM | 1 | 96% | -- | [75] |
| Photo-Fenton UV fluence = 3348 mJ/cm2, [Fe2+] = 2 mg/L, [H2O2] = 20 mg/L | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | GSM | 0.1 | 48.38% | nonanoic acid and butyl butyrate (or isobutyl isobutyrate) | [65] |
| Photo-Fenton UV (365 nm)/rGOF, [rGOF] = 1 g/L | T = 25–31 °C, pH = 3.0 [R.T.] = 3 h | 2-MIB | 5 | 99% | nonanoic acid and butyl butyrate (or isobutyl isobutyrate) from GSM | [45] |
| [O3] = 0.5–2.5 mg O3/mg DOC | T = 23 ± 1 °C pH = 7.9–8.1, [R.T.] = 40 min. | 2-MIB | 10 | 7–46% | -- | [48] |
| [O3] = 0.5–2.5 mg O3/mg DOC | T = 23 ± 1 °C pH = 7.9–8.1 [R.T.] = 40 min. | GSM | 10 | 14–50% | -- | [48] |
| [O3] = 2 mg O3/mg DOC (E-preoxoen) | T = 23 ± 1 °C, pH = 7.9–8.1 I = 40 mA, [R.T.] = 5 min | 2-MIB | 10 | 48% | -- | [48] |
| [O3] = 2 mg O3/mg DOC (E-preoxoen) | pH = 7.9–8.1, I = 40 mA, [R.T.] = 5 min | GSM | 10 | 54% | -- | [48] |
| [O3]0 = 2.2 mg/L | pH = 7.0 [R.T.] = 30 min | 2-MIB | 1 | 31% | -- | [52] |
| [O3]0 = 2.2 mg/L | pH = 7.0 [R.T.] = 30 min | TCA | 1 | 49% | -- | [52] |
| [O3] = 4.19 mg/L | T = 25 ± 1 °C, pH = 7.3 [R.T.] = 20 min | 2-MIB | 0.1 | 93.6% | 2-methylenebornane and 2-methyl-2-bornene | [53] |
| [O3] = 4.19 mg/L | T = 25 ± 1 °C, pH = 7.3 [R.T.] = 20 min | 2-MIB | 0.5 | 66.4% | -- | [53] |
| [O3] = 4.19 mg/L | T = 25 ± 1 °C, pH = 7.3 [R.T.] = 20 min | GSM | 0.1 | 97.9% | cis-1,4- dimethyladamantane and 1,3-dimethyl-adamantane | [53] |
| [O3] = 4.19 mg/L | T = 25 ± 1 °C, pH = 7.3 [R.T.] = 20 min | GSM | 0.5 | 72.6% | -- | [53] |
| UV = 250 mJ/cm2 [O3]0 = 15.84 mg/L | -- | GSM | -- | 99% | -- | [57] |
| UV = 250 mJ/cm2 [O3]0 = 15.84 mg/L | -- | 2-MIB | -- | 95% | -- | [57] |
| [O3]0 = 0.5 mg/L [γ-AlOOH] = 500 mg/L | pH = 7.05 [R.T.] = 10 min | IPMP | 38 | 94.2% | -- | [76] |
| [O3]0 = 0.5 mg/L [γ-AlOOH] = 200 mg/L | T = 25 °C, pH = 6.7 | TCA | 23.2 | 79.3% | -- | [63] |
| [O3]0 = 0.5 mg/L [γ-AlOOH] = 200 mg/L | T = 25 °C, pH = 6.7 | 2-MIB | 23.2 | 27.5% | [63] | |
| [O3]0 = 0.5 mg/L [γ-Al2O3] = 200 mg/L | T = 25 ± 2 °C, pH = 6.7, [R.T.] = 30 min | 2-MIB | 23.2 | 98.4% | -- | [62] |
| [K2FeO4] = 50 mg/L | pH = 9.0 | 2-MIB | -- | 31.9% | -- | [77] |
| [K2FeO4] = 50 mg/L | pH = 9.0 | GSM | -- | 22.5% | -- | [77] |
| peroxide/Fe2+-based Fe2+ = 0.1 mmol/L, Peroxide = 0.05 mmol/L | pH = 6.5 | TCA | 10 | 77.9% | -- | [78] |
| Fenton [Fe2+] = 2 mg/L, [H2O2] = 20 mg/L | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | 2-MIB | 0.1 | 16% | -- | [65] |
| Fenton [Fe2+] = 2 mg/L, [H2O2] = 20 mg/L | T = 25–31 °C, pH = 5.0 [R.T.] = 1 h | GSM | 0.1 | 17% | -- | [65] |
| Ultrasound (640 kHZ) | T = 4 °C [R.T.] = 40 min | 2-MIB | 10 | >90% | 2-methylenebornane and 2-methyl-2-bornene | [54] |
| Ultrasound (640 kHZ) | T = 4 °C [R.T.] = 40 min | GSM | 10 | >90% | (1S, 4aR)-1, 4a-dimethyl-1, 2, 3, 4, 4a, 5, 6, 7-octanhydronaphthalene and (R)-4a, 8-dimethyl-1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalene | [54] |
| Strain | Degradation Substrate | Initial Concentration (μg/L) | Period | Degradation Rate | Possible Intermediate | Reference |
|---|---|---|---|---|---|---|
| Rhodococcus ruber | 2-MIB | 27.8 | 16 h | -- | 3-hydroxy-2-MIB | [22] |
| Pseudomonas putida | 2-MIB | 3.6 | 20 h | -- | 6-hydroxy-2-MIB | [22] |
| Rhodococcus wratislaviensis | 2-MIB | 2.5 | 20 h | -- | 5-keto-2-MIB | [22] |
| Bacillus idriensis | 2-MIB | 2 × 106 | 20 d | 99.98% | -- | [82] |
| Chitinophagaceae bacterium | 2-MIB | 2 × 106 | 20 d | 99.99% | -- | [82] |
| Bacillus sp. | 2-MIB | 5 × 106 | 72 h | 60% | --- | [87] |
| Pseudomonas sp. | 2-MIB | --- | --- | --- | 2-methylcampbene and 2-methylenebornane | [88] |
| Enterobacter sp. | 2-MIB | --- | --- | --- | Camphor 4,7,7-trimethyl-2-oxabicyclo [2,2,1]-heptan-3-one | [88] |
| Acinetobacter | 3-methylindole | 2 × 108 | 6 d | >85% | --- | [89] |
| Bacillus subtilis | 2-MIB | 0.7 | 7 d | 98% | -- | [86] |
| Bacillus subtilis | GSM | 1 | 7 d | 94% | -- | [86] |
| Shinella zoologeoides | 2-MIB | 2 | 3 d | 23.3% | -- | [82] |
| Bacillus idriensis | 2-MIB | 2 | 3 d | 32.9% | -- | [82] |
| Chitinophagaceae bacterium | 2-MIB | 2 | 3 d | 17% | -- | [82] |
| Micrococcus spp. | 2-MIB | 4.2 × 106 | 25 d | 86.1% | 2-methylborane and 2-methyl-2-borane | [84] |
| Flavobacterium spp. | 2-MIB | 4.2 × 106 | 25 d | 84.4% | 2-methylborane and 2-methyl-2-borane | [84] |
| Brevibacterium spp. | 2-MIB | 4.2 × 106 | 25 d | 86.7% | 2-methylborane and 2-methyl-2-borane | [84] |
| Pseudomonas spp. | 2-MIB | 4.2 × 106 | 25 d | 86.0% | 2-methylborane and 2-methyl-2-borane | [84] |
| Bacillus fusiformis | 2-MIB | 5 × 103 | 72 h | 100% | -- | [85] |
| Bacillus sphaericus | 2-MIB | 5 × 103 | 72 h | 100% | -- | [85] |
| Rhodococcus | 2-MIB | 6 | 7 d | -- | -- | [90] |
| Comamonadaceae | 2-MIB | 6 | 7 d | -- | -- | [90] |
| Chryseobacterium sp. | 2-MIB | 2 × 103 | 18 d | 84.0% | -- | [90] |
| Sinorbizobium sp. | 2-MIB | 2 × 103 | 18 d | 80.2% | -- | [90] |
| Stenotrophomonas sp. | 2-MIB | 2 × 103 | 18 d | 74.4% | -- | [90] |
| Methylobacterium sp. | 2-MIB | 2.5 | 6 d | 90% | -- | [87] |
| Trametes hirsuta | 2,6-dibromophenol | --- | --- | 52% | --- | [91] |
| Novosphingobium hassiacum | GSM | -- | -- | -- | -- | [13] |
| Sphingomonas oligophenolica | GSM | -- | -- | -- | -- | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, X.; Liu, T.; Li, X.; Cui, Y.; Xu, L.; Huo, S.; Zou, B.; Qian, J.; Ma, A.; et al. Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects. Catalysts 2023, 13, 1356. https://doi.org/10.3390/catal13101356
Wang F, Li X, Liu T, Li X, Cui Y, Xu L, Huo S, Zou B, Qian J, Ma A, et al. Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects. Catalysts. 2023; 13(10):1356. https://doi.org/10.3390/catal13101356
Chicago/Turabian StyleWang, Feng, Xiaohui Li, Tingting Liu, Xiang Li, Yi Cui, Ling Xu, Shuhao Huo, Bin Zou, Jingya Qian, Anzhou Ma, and et al. 2023. "Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects" Catalysts 13, no. 10: 1356. https://doi.org/10.3390/catal13101356
APA StyleWang, F., Li, X., Liu, T., Li, X., Cui, Y., Xu, L., Huo, S., Zou, B., Qian, J., Ma, A., & Zhuang, G. (2023). Removal of Taste and Odor Compounds from Water: Methods, Mechanism and Prospects. Catalysts, 13(10), 1356. https://doi.org/10.3390/catal13101356