Abstract
Molecular imprinting (MI) technology has been used in electrochemical analysis technology because of its unique selectivity and specificity. In this work, an electrochemical sensor based on in-situ inorganic MI-Au-MoO3/graphene for bisphenol A (BPA) analysis is designed, where MI-MoO3 is hybridized with graphene nanosheets and Au nanoparticles, and BPA is acted as the temple molecular. Differential pulse voltammetry (DPV) was used to evaluate the sensing performance of the MI-Au-MoO3/rGO sensor toward BPA determination and it is about 2.0 times that of NI-Au-MoO3/rGO. The as-constructed sensor presents a wide linear range from 0.01 to 106.04 μM and a low limit of detection of 0.003 μM. It also displays outstanding stability and repeatability up to 20 days, and can be used to analyze the content of BPA in dust leachate and plastic bottle. This sensor offers a promising strategy for environment pollution and food analysis via MI technology.
1. Introduction
Bisphenol A (BPA) is one of the endocrine disrupter chemicals, which can generate many adverse impacts on the biological systems of organisms [1,2,3,4]. Recently, it is inevitable to uptake trace amounts of BPA from daily life on account of the wide application of BPA-based plastic production. It is always used as a raw material to produce food and drink packages, baby bottles and dental sealants, where BPA molecules could be released into food, drinks, and the environment [5,6,7,8,9]. Owing to the serious threat to human health, it is important to develop a simple, high-sensitivity, and selective analytical method for rapid determination of trace BPA in the field of environmental monitoring.
Recently, molecularly imprinted electrochemical sensor (MIES), combined with the merits of MI technology and electrochemical detection, has attracted extensive attention in the electrochemical analysis field [10,11,12]. MI technology, as a technique for the special identification of imprinting molecules, can leave a large number of molecular template sites for the detection to improve the selectivity and the simplicity rapidity of the target molecule [13,14,15]. For example, Lu et al. have developed the MIES of MXene/amino carbon nanotubes for the detection of fisetin [14]; Gao et al. reported an MI-Nb2O5 for detecting BPA [16].
Two-dimensional (2D) nanomaterials are exciting for researchers in MIES due to their excellent properties [17,18,19]. The high specific surface area of 2D nanomaterials is beneficial to creating more effective target sites located at the surface or near the surface of the materials by controlling the templates, creating more effective target sites [20]. The frequently used 2D sensing materials include carbon materials [21,22,23,24], transition metal oxides (TMOs) and transition metal chalcogenides (TMCs) [25,26,27,28,29]. Among them, 2D molybdenum trioxide (MoO3) receives widespread attention in catalysis [30], energy storage [31], and gas and biomedical sensors [32,33] areas, which could be attributed to its unique high stability, conspicuous catalytic activity, excellent electrical and surface charge properties, and easy to fabricate [34]. It was only much more recently that Antoniazzi et al. developed an orthorhombic molybdenum trioxide (α-MoO3)-modified carbon paste electrode for monitoring BPA leaching from commercial plastic samples to water [35]. These preliminary findings demonstrate the potential of 2D MoO3 in the electrochemical detection of endocrine disruptors. From this starting point, we can use reduced graphene oxide (rGO) as an electron bridge to further enhance the electron transport capacity of MoO3 for (bio-) chemical sensing [36], thanks to its high specific surface area and prominent electrical conductivity.
Considering the excellent conductivity and special surface area of rGO and the perfect biocompatibility of Au nanoparticles, decorating MI-MoO3 with rGO and Au nanoparticles is prepared as a novel electrochemical sensor, marked as “MI-Au-MoO3/rGO”. The as-constructed sensing platform was used to determine the content of BPA by analyzing the change of current by differential pulse voltammetry technique. The sensor presents a wide linear range and a low limit of detection (LOD). It also shows outstanding stability, and can be employed to analyze BPA content in practical sample including dust leachate, plastic bottle, and tap water with a satisfactory result.
2. Results and Discussion
2.1. Morphological and Structure Characterization
The morphologies of as-synthesized MoO3 and Au-MoO3/rGO were characterized via SEM. As shown in Figure 1a, the as-synthesized MoO3 was shown a sheet-like structure in general. Further introduced Au nanoparticles and rGO into MoO3, the sheet-like structure was not changed (Figure 1b), only some tiny particles appeared on the surface of both sheet-like structures. XRD patterns were used to explore the crystal structures and crystalline phases of all samples in Figure S1a. It can be seen that all characteristic peaks of two MoO3 based samples were matched up well with the orthorhombic α-MoO3 (JCPDS No. 05-0508) [37], indicating that introduction of rGO and Au did not change the structure of MoO3. There is no distinct diffraction peak of rGO in the XRD pattern of Au-MoO3/rGO owning to the trace content of rGO [38]. For Au-MoO3/rGO, three diffraction peaks at 38.2°, 44.4°, and 64.6° were detected apart from the peaks of MoO3, which could be severally ascribed to the (111), (200), and (220) planes of Au [38], indicating the successful formation of Au nanoparticles in the composites of Au-MoO3/rGO.
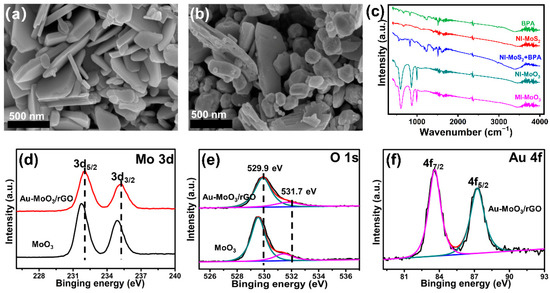
Figure 1.
SEM images of MoO3 (a) and Au-MoO3/rGO (b). (c): FT-IR spectra of different samples. XPS spectra of MoO3 and Au-MoO3/rGO samples: Mo 3d (d), O 1s (e); Au 4f (f).
Moreover, Fourier transforming infrared spectra (FT-IR) was employed to explore the organic composition of MoO3 in the fabrication process. As shown in Figure 1c, For BPA and MI-MoS2, the FT-IR spectrum displayed three typical absorption peaks. The peaks at 1508 cm−1 and 1603 cm−1 assign to aromatic rings framework vibration while the peak at 2969 cm−1 assigns to C-H vibration. However, those peaks were not appeared in NI-MoS2, which indicates BPA molecules were successfully in-situ grown on the surface of MoS2. Furthermore, that the peaks stood on the 3376 cm−1 and 1603 cm−1 could be ascribed to the stretching and bending mode of O-H active groups, which was beneficial to bind specifically BPA molecules through hydrogen bonding interaction. Additionally, these characteristic peaks were totally disappeared after the annealing process in MI-MoO3, which indicates that BPA molecules were removed and specific recognition sites could be left on the surface of MoO3 [16,39].
X-ray photoelectron spectroscopy (XPS) was used to detect the elements of MoO3 and Au-MoO3/rGO. In addition to the signals of the O 1s and Mo 3d, a new peak can be indexed to Au 4f in Au-MoO3/rGO (Figure S1b), indicating Au nanoparticles were successfully introduced on the surface of MoO3/rGO. The Mo 3d spectrum (Figure 1d) of pure MoO3 exhibits two major peaks at 231.8 eV and 234.9 eV, which was attributed to Mo 3d5/2 and Mo 3d3/2, respectively, corresponding to Mo6+. Interestingly, the Mo 3d peaks in Au-MoO3/rGO shifted to higher binding energies compared to pure MoO3. The deconvolution of the O 1s spectrum (Figure 1e) shows two peaks: the lattice oxygen in MoO3 at 529.5 eV and the interstitial oxygen of MoO3 at 531.4 eV [40]. Same with Mo 3d, the peak signals of O 1s in Au-MoO3/rGO also shifted to a higher position at 529.9 and 531.7 eV, respectively. This phenomenon could be ascribed to the interaction of among Au, MoO3 and rGO, causing the surface electrons of MoO3 to transfer to Au [41]. Furthermore, the peak signals at 83.6 and 87.3 eV were severally attributed to Au 4f7/2 and Au 4f5/2 (Figure 1f) [42], indicating that the Au nanoparticles were triumphantly loaded on the MoO3/rGO sample.
2.2. Electrochemical Behaviors
The electrochemical activity of different electrodes was studied by cyclic voltammetry (CVs) and electrochemical impedance spectroscopy (EIS). MI-Au-MoO3/rGO electrode possesses a bigger oxidation peak current density compared to NI-Au-MoO3/rGO electrode in Figure 2a. Moreover, a pair of redox peaks with peak-to-peak separation (ΔEp) of 103 mV was observed at MI-Au-MoO3/rGO, which was smaller than the ΔEp of NI-Au-MoO3/rGO (160 mV). For comparison, pure samples such as GCE, Au/GCE, and rGO/GCE were also probed, and they showed only a weak signal. This phenomenon could be ascribed to the excellent conductivity and the increased active sites of MI-Au-MoO3/rGO after removing the template. The Nyquist plots were used to explore the electron transfer processes of different sensors in Figure 2b. Generally, the smaller diameter of the arc (DIA) means a faster charge transfer rate, and these results also demonstrate a similar trend with CVs. The simulated circuit diagram was used to analyze the EIS data (Figure 2c), and Rs, Rct, Q, and Zw represent the resistance of the solution, the charge transfer resistance value between the electrode and solution, the electrode double-layer capacitance and Warburg impedance, respectively [43]. The results show that the Rct values of GCE, Au/GCE, rGO/GCE, NI-Au-MoO3/rGO/GCE and MI-Au-MoO3/rGO/GCE can be estimated to 5.31 × 104 Ω, 3.01 × 104 Ω, 9.97 × 103 Ω, 4.95 × 103 Ω and 9.38 × 102 Ω, respectively, indicating that MI-Au-MoO3/rGO sensor has good electronic transmission performance [41].
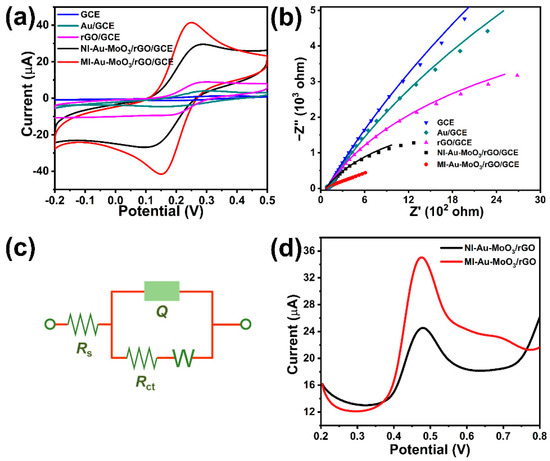
Figure 2.
CVs (a) and EIS (b) of the samples in 2.5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution containing 0.1 M KCl. (c): Equivalent circuit was used for simulating EIS. (d): DPVs of the samples in 0.1 M phosphate buffer (pH 7.0) containing 0.10 mM BPA.
The electrochemical behavior of the oMI-Au-MoO3/rGO electrode toward BPA was explored. As shown in Figure 2d, for NI-Au-MoO3/rGO, the electro-oxidation potential of BPA appeared at 0.48 V and the relative oxidation peak current was 11.54 μA. However, for MI-Au-MoO3/rGO, the enhanced oxidation peak occurred at a lower potential (0.475 V), meanwhile, the corresponding oxidation peak current reached 23.05 μA, which improved 2.0 times compared with NI-Au-MoO3/rGO. These indicate that the MI-Au-MoO3/rGO electrode displays a better electrochemical response for BPA. This may be due to some special activate sites left on the surface of MI-Au-MoO3/rGO after annealing, which can selectively adsorb BPA molecules to enhance the electrochemical response ability towards BPA [16].
2.3. Optimizing Conditions of Detection
The accumulation is an important factor for the electrochemical sensor. The oxidation peak current was increased dramatically with increasing accumulation time from 0 to 180 s (Figure 3a). The surface amount of BPA was accumulated with increasing time and thus enhance the oxidation signal of BPA [44]. However, the current signal significantly decreases when exceeded 180 s owing to that BPA at the electrode rapidly reaching saturation. Meantime, the optimization for accumulation potential was also investigated in the range of −0.4 V to 0.3 V (Figure 3b). The highest oxidation peak current density is achieved at −0.1 V. From the above results, 180 s and −0.1 V were selected as the optimum accumulation time and potential, respectively. Finally, the amount of MI-Au-MoO3/rGO loading was also explored. In the experiment, the concentration of MI-Au-MoO3/rGO was immobilized at 2 mg mL−1, and the suspension volume on the modified electrode was optimized. It could be obviously concluded from Figure 3c that the oxidation peak current of BPA increases with increasing the amount of MI-Au-MoO3/rGO concentration from 1 to 3 μL. When further increasing the amount of MI-Au-MoO3/rGO, the oxidation peak current of BPA dramatically decreases. This phenomenon can be ascribed to the limited electron transport ability in the oxidation of BPA in a thicker film, which led to the diminution of oxidation peak current. Hence, 3 μL of 2 mg mL−1 of MI-Au-MoO3/rGO suspensions was chosen to modify the electrode throughout the experiment.
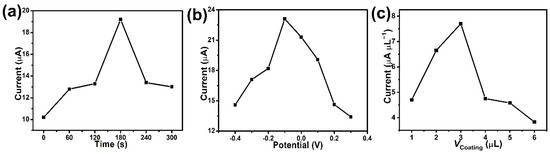
Figure 3.
Effects of accumulation time (a), potential (b), coating amounts (c) of MI-Au-MoO3/rGO in 0.1 M phosphate buffer (pH = 7) containing 0.1 mM BPA.
The effects of pH were displayed by DPV from 5.2 to 8.7 (Figure 4a). As can be seen in Figure 4b (red line), the oxidation peak current steadily increased with the pH value raised from 5.2 to 7.0, while the oxidation current decreased on the contrary when the solution pH exceeded 7.0. Moreover, the pKa of BPA (pKa = 9.73) was distinctly higher than the optimized pH value, indicating that the absorbing capacity of on MI-Au-MoO3/rGO electrode surface towards undissociated BPA was better than the dissociated molecules in neutral solution [45]. In consideration of achieving higher sensitivity, the phosphate buffer with pH 7.0 was chosen as the optimal supporting electrolyte for determining BPA. It can be seen that a good linear correlation of oxidation peak potential and pH value (Figure 4b, black line), the peak potential of BPA was negatively shifted with the increase was accompanied by pH value. As well, the linear regression equation was Epa (V) = −0.0595 pH + 0.8797 (R2 = 0.9942). The obtained slope of 59.5 mV pH−1 was approximately to the theoretical value (59 mV pH−1), revealing that there had equal numbers of transferred electrons and protons on the oxidation process of BPA on the MI-Au-MoO3/rGO electrode [46]. Combined with the previous conclusion that there were two transferred electrons during BPA electro-oxidation process [43], the oxidation of BPA on MI-Au-MoO3/rGO could be a two-electron and two-proton process.
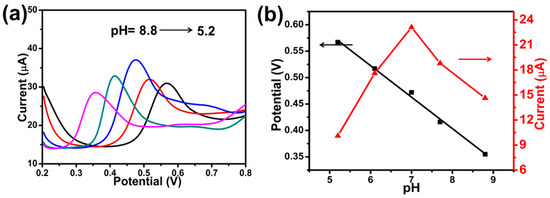
Figure 4.
DPVs of MI-Au-MoO3/rGO electrode in 0.1 M phosphate buffer with different pH (a). Effects of pH value on the potential (black line) and peak current (red line) (b). [BPA] = 0.1 mM.
2.4. Effect of Scan Rates
The effect of scan rates was investigated by CV (Figure 5a). The oxidation peak currents gradually increase with increasing the scan rate. A good linear relationship of oxidation peak current (Ipa) versus square root of scan rates was obtained in Figure 5b, and the linear regression equation was fitted as follows: Ipa (μA) = 5.03 v1/2 (mV s−1) − 21.5 (R2 = 0.995), which indicates that the electrochemical oxidation of BPA at MI-Au-MoO3/rGO electrode was a typical diffusion-controlled process. Figure 5c displays the dependence of peak potential (Epa) with ln v, and the regression equation is described as Epa (V) = 0.0272 ln v (mV s−1) + 0.4224 (R2 = 0.9934). With regard to a diffusion-controlled and completely irreversible electrode process, the Laviron equation is usually used to define the Epa, and the equation is exhibited as follows:
where R, T, and F have usually meanings (R = 8.314 J mol−1 K−1, T = 298 K, F = 96,480 C mol−1), n is the number of transfer electrons, Eθ, kθ and α represent formal redox potential, standard rate constant in the reaction and transfer coefficient, respectively. As for α in the completely irreversible electrode process, in general, speaking, is assumed to be 0.5. According to Equation (1), the slope is equivalent to RT/αnF [45]. Hence, the electron transfer number (n) is determined to be 1.89. These indicate BPA electro-oxidation is a two-electron transfer process.
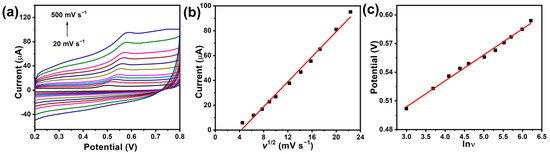
Figure 5.
Various scan rates (a) on the oxidation peak current of BPA (0.1 mM) in 0.1 M phosphate buffer (pH 7.0). The relationship between peak current and scan rate (b). The plot of peak potential (Epa) vs. natural logarithm of scan rate (ln v) (c).
2.5. Determination of Detection Limit, Stability and Real Samples Analysis
Under the optimal experimental conditions, the MI-Au-MoO3/rGO sensor was employed to detect different concentrations of BPA (Figure 6a). The peak current increases with the continuous addition of BPA. Moreover, the linear relation of oxidation peak current vs. the logarithm of the BPA concentration from 0.01 μM to 116.04 μM was shown in Figure 6b. The corresponding linear regression equation is Ipa (μA) = 9.335 + 1.689 log C (μM) (R2 = 0.9468) and Ipa (μA) = 6.057 + 8.083 log C (μM) (R2 = 0.9938), respectively. The LOD was estimated to be 0.003 μM at a ratio of signal to noise of 3 (S/N = 3). The sensor shows lower LOD, compare with previous reports (Table S1). The lower LOD of the MI-Au-MoO3/rGO sensor could be explained: i, BPA template molecules were in-situ grew in the hydrothermal process of MoS2 nanomaterials, after annealing treatment, the surface of MoO3 nanosheets could generate a mass of peculiar recognition active sites with special shapes, sizes, and functional groups, ii. The peculiar recognition active sites would improve the adsorption of BPA, thereby enhancing the electrochemical response of BPA.

Figure 6.
DPVs (a) of different concentrations of BPA at MI-Au-MoO3/rGO electrode in 0.1 M phosphate buffer (pH 7.0). The relationship between peak current and logC (b). Peak currents of 10 μM BPA on MI-Au-MoO3/rGO electrode for 20 days (c).
The stability was investigated by DPV (Figure 6c). MI-Au-MoO3/rGO was kept in the refrigerator at 4 ℃ during the test period. When used per 5 days, the current response was reduced by ca. 19%, indicating this sensor has excellent stability for up to 20 days. The reproducibility was measured by the DPV technique through 5 different sensors in Figure S2 and all sensors showed a relative standard deviation (R.S.D.) of 2.69%. This result shows a good reproducibility for BPA detection at the prepared sensor. The sensor was also used to detect actual samples including dust leachate, drinking water bottles and tap water by using the standard addition technique via DPV (Table S2), and the recovery ranged from 99.9% to 101.5% as well as the R.S.D. was less than 2.8%, suggesting the sensor can be applied for detecting real samples.
3. Experiments
The Materials and characterizations involved in this work are discussed in the Supplementary Material (Text S1 and S2).
3.1. In-Situ Synthesis of MI-MoO3 Nanosheets
Firstly, 0.25 mmol of (NH4)6Mo7O24·4H2O, 0.75 mmol of CH4N2S, and a certain amount of BPA molecules dissolved in ethanol were ultrasonically dispersed into 20 mL of distilled water. The resulting solution was transported into 25 mL Teflon autoclave and heated at 210 ℃ for 24 h. In the process of hydrothermal reaction, BPA molecules could be adsorbed on the surface of MoS2. After cooling down to room temperature, the black precipitates were subsequently obtained by centrifugal as well as washed with water and ethanol several times, respectively. The powders were dried in an oven at 60 ℃ overnight. Then, in order to remove the template molecules of BPA, the sediments were annealed at 500 ℃ for 2 h (Scheme 1), obtaining the target product of MI-MoO3. For comparison, non-imprinted MoO3 (NI-MoO3) was prepared except for adding BPA molecules in the same synthesis process.
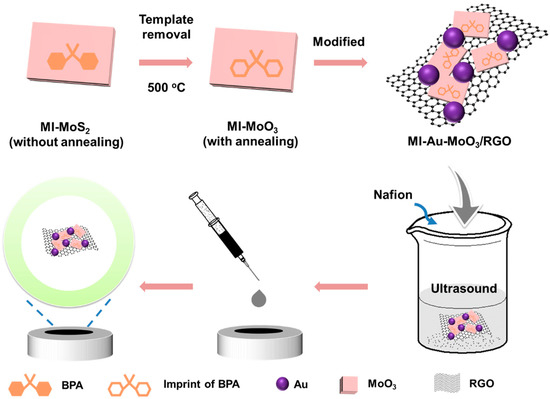
Scheme 1.
The synthetic processes of MI-Au-MoO3/rGO sensor.
3.2. Synthesis of MI-Au-MoO3/rGO
The MI-Au-MoO3/rGO was synthesized as follows: 20 mg of as-prepared MI-MoO3 nanosheets and 0.21 mL of HAuCl4 (0.0486 M) were dispersed into 15 mL of ethylene glycol solution containing 2.0 mg GO under ultrasonic processing for 30 min. After adjusting the pH value of the above solution to 10.5 via 0.1 M NaOH solution, the homogeneous solution was transferred to a Teflon-lined stainless-steel autoclave (25 mL) and heated at 140 ℃ for 4 h. After completion of the reaction, the black precipitates were centrifuged and washed with double distilled water to neutral and then dried at 60 ℃ overnight, obtaining MI-Au-MoO3/rGO (Scheme 1). For comparison, NI-Au-MoO3/rGO was synthesized in the same method, only replacing MI-MoO3 with NI-MoO3.
3.3. Preparation of Sensor
For fabricating the sensor, the as-obtained MI-Au-MoO3/rGO (2 mg) powder and Nafion (5%, 10 μL) were dispersed into 1 mL ethanol/water mixed solution (Vethanol:Vwater = 1) by ultrasonic agitation. Meanwhile, a glassy carbon electrode (GCE) (Diameter = 3 mm, geometric area = 0.07 cm2) was mechanically polished with 0.05 um α-Al2O3 powder to be a bright mirror-like surface and thoroughly washed with water as well as ethanol. Then, 3 μL MI-Au-MoO3/rGO dispersion solution (2 mg/mL) was coated on the GCE surface and dried in the air to evaporate the solvent, resulting in a sensor of MI-Au-MoO3/rGO electrode (Scheme 1). For comparison, NI-Au-MoO3/rGO electrode was constructed by a similar method.
3.4. Electrochemical Measurement
Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were carried out in 0.1 M KCl solution containing 2.5 mM K3[Fe(CN)6]/K4[Fe(CN)6] mixture. Unless otherwise stated, 0.1 M phosphate buffer (pH 7.0) was used as the determining medium for BPA. CVs with different scan rates were recorded from 0.2 to 0.8 V. Differential pulse voltammetry (DPV) measurement was operated from 0.2 to 0.8 V with a pulse time of 0.05 s, pulse amplitude of 0.05 V, pulse period of 0.5 s and incremental E of 0.004 V. All the electrochemical experiments were measured at room temperature.
4. Conclusions
A new inorganic MI-Au-MoO3/rGO electrochemical sensor was successfully prepared for detecting BPA. In the preparation process, BPA molecules can be successfully combined and removed from the Au-MoO3/rGO nanocomposites, which offer lots of recognition sites on the surface of MI-Au-MoO3/rGO for BPA. The sensor presents a wide linear range from 0.01 to 106.04 μM and a low LOD of 0.003 μM. It also shows excellent long-term stability up to 20 days, and can be applied to analyze BPA in real samples. This simple-to-operate method offers a new window for great potential application in environmental pollution and food health monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010091/s1, Text S1: Materials; Text S2: Characterizations; Figure S1: XRD patterns (a) and survey XPS spectra (b) of MoO3 and Au-MoO3/rGO; Figure S2: Five parallel experiments for MI-Au-MoO3/rGO sensor; Table S1: Recent reports for the detection of BPA by different materials; Table S2: Determination of BPA in the different real samples [1,9,43,47,48,49].
Author Contributions
Conceptualization, M.Z. and Q.Z.; methodology, Z.L. and H.Z.; formal analysis, Z.L. and H.Z.; writing—original draft, Z.L. and H.Z.; writing—review and editing, M.Z., J.L. and Q.Z.; supervision, M.Z., J.L. and Q.Z.; project administration, M.Z., J.L. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Funds for the Central Universities, the Science and Technology Projects in Guangzhou (202201020083).
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fu, S.; Ma, X.; Wang, S.; Zha, Q.; Wen, W.; Hu, B. Surfactant-assisted carbon black for the electrochemical detection of endocrine disruptors. Surf. Interfaces 2021, 24, 101128. [Google Scholar] [CrossRef]
- Lu, H.; Xu, G.; Gan, L. N Doped activated biochar from pyrolyzing wood powder for prompt BPA removal via peroxymonosulfate activation. Catalysts 2022, 12, 1449. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, C.; Zhai, C. Label-free photoelectrochemical sensor based on 2D/2D ZnIn2S4/g-C3N4 heterojunction for the efficient and sensitive detection of bisphenol A. Chin. Chem. Lett. 2022, 33, 983–986. [Google Scholar] [CrossRef]
- Hu, S.; Yu, Y.; Guan, Y.; Li, Y.; Wang, B.; Zhu, M. Two-dimensional TiO2 (001) nanosheets as an effective photo-assisted recyclable sensor for the electrochemical detection of bisphenol A. Chin. Chem. Lett. 2020, 31, 2839–2842. [Google Scholar] [CrossRef]
- Ianesko, F.; de Lima, C.A.; Antoniazzi, C.; Santana, E.R.; Piovesan, J.V.; Spinelli, A.; Galli, A.; de Castro, E.G. Simultaneous electrochemical determination of hydroquinone and bisphenol A using a carbon paste electrode modified with silver nanoparticles. Electroanalysis 2018, 30, 1946–1955. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, M. Detection of pollutants in water bodies: Electrochemical detection or photo-electrochemical detection? Chem. Commun. 2020, 56, 14541–14552. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Lou, Z.; Zeng, L.; Zhu, M. Molecularly imprinted photoelectrochemical sensor for detecting tetrabromobisphenol A in indoor dust and water. Microchim. Acta 2021, 188, 320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yuan, C.; He, Z.; Wang, J.; Zhai, C.; Bin, D.; Zhu, M. A label-free photoelectrochemical sensor of S, N co-doped graphene quantum dot (S, N-GQD)-modified electrode for ultrasensitive detection of bisphenol A. Microchim. Acta 2022, 189, 208. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Chen, Q.; Zhai, C. Plasmon enhanced photo-assisted electrochemical detection of bisphenol A based on Au decorated La2Ti2O7/RGO nanosheets. Surf. Interfaces 2021, 26, 101331. [Google Scholar] [CrossRef]
- Béjar, J.; Álvarez-Contreras, L.; Ledesma-García, J.; Arjona, N.; Arriaga, L. Electrocatalytic evaluation of Co3O4 and NiCo2O4 rosettes-like hierarchical spinel as bifunctional materials for oxygen evolution (OER) and reduction (ORR) reactions in alkaline media. J. Electroanal. Chem. 2019, 847, 113190. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiș, M.; Cernat, A.; Săndulescu, R.; Cristea, C. Electrochemical methods based on molecularly imprinted polymers for drug detection. A review. Int. J. Electrochem. Sci 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC, Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sensor. Actuat. B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- Mo, G.; He, X.; Zhou, C.; Ya, D.; Feng, J.; Yu, C.; Deng, B. A novel ECL sensor based on a boronate affinity molecular imprinting technique and functionalized SiO2@CQDs/AuNPs/MPBA nanocomposites for sensitive determination of alpha-fetoprotein. Biosens. Bioelectron. 2019, 126, 558–564. [Google Scholar] [CrossRef]
- Gao, P.; Wang, H.; Li, P.; Gao, W.; Zhang, Y.; Chen, J.; Jia, N. In-site synthesis molecular imprinting Nb2O5–based photoelectrochemical sensor for bisphenol A detection. Biosens. Bioelectron. 2018, 121, 104–110. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Wang, Y.; Zhang, B.; Wang, H.; Fang, G.; Wang, S. A molecularly imprinted electrochemical sensor based on cationic intercalated two-dimensional titanium carbide nanosheets for sensitive and selective detection of triclosan in food samples. Food Control 2022, 132, 108532. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Q.; Chen, T.; Li, J.; Liu, Y.; Shan, X.; Liu, F.; Jia, J. Recent advances in electrochemical sensors for antibiotics and their applications. Chin. Chem. Lett. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Alshgari, R.A.; Nafady, A.; Shah, A.A.; Aboelmaaref, A.; Aftab, U.; Ibupoto, M.H.; Vigolo, B.; Tahira, A.; Ibupoto, Z.H. Enhanced electrocatalytic properties of Co3O4 nanocrystals derived from hydrolyzed polyethyleneimines in water/ethanol solvents for electrochemical detection of cholesterol. Catalysts 2022, 12, 1176. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zha, Q.; Zhai, C.; Li, W.; Zeng, L.; Zhu, M. Photo-electrochemical detection of dopamine in human urine and calf serum based on MIL-101 (Cr)/carbon black. Microchim. Acta 2020, 187, 526. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, Y.; Liu, F.; Yan, X.; You, D.; Li, K.; Zeng, L.; Zhu, M.; Xiao, G.; Albert, J.; et al. Operando optical fiber monitoring of nanoscale and fast temperature changes during photo-electrocatalytic reactions. Light: Sci. Appl. 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Wang, Q.; Sun, K.; Lee, C.; Cao, Y.; Si, W.; Wei, H.; Li, Z.; Wang, F. The biomass of pig-blood-derived carbon as a novel electrode material for hydrogen peroxide electrochemical sensing. Catalysts 2022, 12, 1438. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Mondal, K.; Shetti, N.P. Zinc Oxide–Graphene nanocomposite-based sensor for the electrochemical determination of cetirizine. Catalysts 2022, 12, 1166. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Aljarb, A.; Shi, Y.; Li, L.-J. Epitaxial growth of two-dimensional layered transition-metal dichalcogenides: Growth mechanism, controllability, and scalability. Chem. Rev. 2017, 118, 6134–6150. [Google Scholar] [CrossRef]
- Pan, C.; Zheng, Y.; Yang, J.; Lou, D.; Li, J.; Sun, Y.; Liu, W. Pt–Pd bimetallic aerogel as high-performance electrocatalyst for nonenzymatic detection of hydrogen peroxide. Catalysts 2022, 12, 528. [Google Scholar] [CrossRef]
- Yuan, C.; He, Z.; Chen, Q.; Wang, X.; Zhai, C.; Zhu, M. Selective and efficacious photoelectrochemical detection of ciprofloxacin based on the self-assembly of 2D/2D g-C3N4/Ti3C2 composites. Appl. Surf. Sci. 2021, 539, 148241. [Google Scholar] [CrossRef]
- Soni, S.; Jain, U.; Burke, D.H.; Chauhan, N. A Label Free, Signal off electrochemical aptasensor for amphetamine detection. Surf. Interfaces 2022, 102023. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Liu, J.; Shi, M.; Xu, R.; Kang, W. Efficient extraction of anti-inflammatory active ingredients from schefflera octophylla leaves using ionic liquid-based ultrasonic-assisted extraction coupled with HPLC. Molecules 2019, 24, 2942. [Google Scholar] [CrossRef]
- Tang, K.; Farooqi, S.A.; Wang, X.; Yan, C. Recent progress on molybdenum oxides for rechargeable batteries. ChemSusChem 2019, 12, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Joshi, N.; Tomer, V.K. Advances in the designs and mechanisms of MoO3 nanostructures for gas sensors: A holistic review. Mater. Adv. 2021, 2, 4190–4227. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Liu, S.; Zheng, L.; Bu, Y.; Deng, H.; Chen, R.; Peng, H.; Lin, X.; Chen, W. Colorimetric acid phosphatase sensor based on MoO3 nanozyme. Anal. Chim. Acta 2020, 1105, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hai, Z.; Akbari, M.K.; Qi, D.; Xing, K.; Zhao, Q.; Verpoort, F.; Hu, J.; Hyde, L.; Zhuiykov, S. Atomic layer deposition-developed two-dimensional α-MoO3 windows excellent hydrogen peroxide electrochemical sensing capabilities. Sensor. Actuat. B Chem. 2018, 262, 334–344. [Google Scholar] [CrossRef]
- Antoniazzi, C.; de Lima, C.A.; Marangoni, R.; de Castro, E.G.; Santana, E.R.; Spinelli, A. Molybdenum trioxide incorporated in a carbon paste as a sensitive device for bisphenol A monitoring. Microchem. J. 2020, 159, 105528. [Google Scholar] [CrossRef]
- Ohkubo, S.; Higashide, T.; Takeda, H.; Murotani, E.; Hayashi, Y.; Sugiyama, K. Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn. J. Ophthal. 2012, 56, 68–75. [Google Scholar] [CrossRef]
- Shen, J.; Guo, S.; Chen, C.; Sun, L.; Wen, S.; Chen, Y.; Ruan, S. Synthesis of Ni-doped α-MoO3 nanolamella and their improved gas sensing properties. Sensor. Actuat. B Chem. 2017, 252, 757–763. [Google Scholar] [CrossRef]
- Li, L.; Gao, E.; Wang, K.; Shen, F.; Liu, H.; Liu, Z. A first-principles study of Ag clusters adsorption on porphyrin-like N4-CNT: Geometric and electronic properties. Surf. Interfaces 2021, 25, 101229. [Google Scholar] [CrossRef]
- Riley, N.M.; Hebert, A.S.; Dürnberger, G.; Stanek, F.; Mechtler, K.; Westphall, M.S.; Coon, J.J. Phosphoproteomics with activated ion electron transfer dissociation. Anal. Chem. 2017, 89, 6367–6376. [Google Scholar] [CrossRef]
- Ji, P.; Shang, B.; Peng, Q.; Hu, X.; Wei, J. α-MoO3 spheres as effective polysulfides adsorbent for high sulfur content cathode in lithium-sulfur batteries. J. Power Sources 2018, 400, 572–579. [Google Scholar] [CrossRef]
- Hu, J.; Zhai, C.; Zeng, L.; Du, Y.; Zhu, M. Enhanced electrocatalytic ethanol oxidation reaction in alkaline media over Pt on a 2D BiVO4-modified electrode under visible light irradiation. Catal. Sci. Technol. 2018, 8, 3562–3571. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, X.; Fujitsuka, M.; Zhang, J.; Majima, T. Au/La2Ti2O7 nanostructures sensitized with black phosphorus for plasmon-enhanced photocatalytic hydrogen production in visible and near-infrared light. Angew. Chem. Int. Ed. 2017, 56, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J.; Xiao, Y.; Zha, Q.; Zeng, L.; Zhu, M. Surfactant assisted Cr-metal organic framework for the detection of bisphenol A in dust from E-waste recycling area. Anal. Chim. Acta 2021, 1146, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, J.; Wu, K. Mesoporous silica-based electrochemical sensor for sensitive determination of environmental hormone bisphenol A. Anal. Chim. Acta 2009, 638, 23–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Li, S.; Dang, Y. A novel electrochemical sensor for highly sensitive detection of bisphenol A based on the hydrothermal synthesized Na-doped WO3 nanorods. Sensor. Actuat. B Chem. 2017, 245, 238–246. [Google Scholar] [CrossRef]
- Shi, R.; Liang, J.; Zhao, Z.; Liu, A.; Tian, Y. An electrochemical bisphenol A sensor based on one step electrochemical reduction of cuprous oxide wrapped graphene oxide nanoparticles modified electrode. Talanta 2017, 169, 37–43. [Google Scholar] [CrossRef]
- Kim, M.; Song, Y.E.; Xiong, J.-Q.; Kim, K.-Y.; Jang, M.; Jeon, B.-H.; Kim, J.R. Electrochemical detection and simultaneous removal of endocrine disruptor, bisphenol A using a carbon felt electrode. J. Electroanal. Chem. 2021, 880, 114907. [Google Scholar] [CrossRef]
- Lei, X.; Deng, Z.; Zeng, Y.; Huang, S.; Yang, Y.; Wang, H.; Guo, L.; Li, L. A novel composite of conductive metal organic framework and molecularly imprinted poly (ionic liquid) for highly sensitive electrochemical detection of bisphenol A. Sensor. Actuat. B: Chem. 2021, 339, 129885. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Keyan, A.K.; Rajakumaran, R.; Chen, S.-M.; Chiu, T.-W.; Dong, C.; Vinothini, S. Synthesis of N-rGO-MWCNT/CuCrO2 catalyst for the bifunctional application of hydrogen evolution reaction and electrochemical detection of Bisphenol-A. Catalysts 2021, 11, 301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).