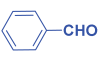
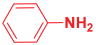
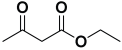
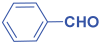
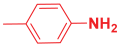
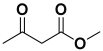
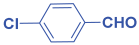
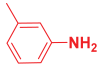
Abstract
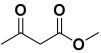
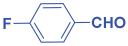
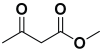
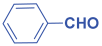
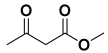
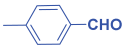
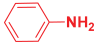
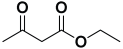
An efficient, novel bifunctional C2-symmetric ionic liquid–supported (S)-proline organocatalyst 7 was developed for a one-pot, five-component reaction involving β-keto esters 8, aryl aldehydes 9, and aryl amines 10, affording highly functionalized tetrahydropyridines 11a–o by simultaneous generation of fives bonds and two stereogenic centers with extraordinary diastereo- and enantioselectivities (up to >99:1 dr, 95:5 er) in isopropanol with high yields (up to 92%). This protocol provides quick access to diverse enantio-enriched, highly functionalized diastereo- and enantioselective tetrahydropyridines in a green medium without any column chromatographic purification. The catalyst was recycled five times without significant loss of its catalytic activity.
1. Introduction
The broad spectrum of biologically active natural products and pharmaceuticals comprises many nitrogen-containing heterocycles, such as pyrroles, piperidines, and pyrrolidines [1]. Various natural products and biologically active compounds containing 1,2,5,6-tetrahydropyridine (THP), and piperidine moieties exhibit numerous biological properties, such as antimalarial, antihypertensive, anticancer, anticonvulsant, and antibacterial activities. In addition, MPTP (A) is used as a prodrug for neurotoxin MPP+, phenindamine (B) is used as an antihistamine agent for the common cold, paroxetine (C) is used as an antidepressant drug, and fentanyl (D) is used as an analgesic drug (Figure 1) [1,2].
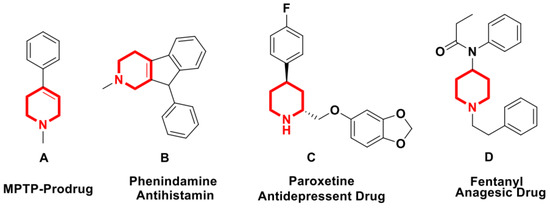
Figure 1.
Some biologically active tetrahydropyridine drugs.
It is a significant challenge for chemists to strengthen the synthetic routes of these chemical motifs simply and efficiently. Multicomponent reactions (MCRs) imply a greatly appreciated synthetic implement for the assembly of new and amalgamated molecular assemblies with a minimum quantity of synthetic stages [3]. MCRs have numerous benefits over conventional direct syntheses, including high degrees of atom economy, lower costs, readily available starting materials, shorter reaction times, environmental friendliness, and the possibility for combinatorial measuring of structural differences [4]. It is an important challenge for reaction designers to improve the efficient synthesis of this chiral richness of chemical motifs in a better way [5]. In multicomponent reactions, we can efficiently build up molecular complexity using readily available starting materials without the need for reaction intermediate isolation and laborious workup [6,7]. High atom economy and selectivity could be achieved in one-pot multicomponent reactions [8].
Asymmetric organocatalysis is a crucial step in asymmetric organic synthesis because it compensates for the drawbacks of metal organocatalysis. With low catalyst equivalents, good yields and ee values can be attained. Tandem reactions provide many advantages in organic syntheses, such as short reaction time and minimum purification steps. Many successful ways for the production of 1,2,3,4-tetrahydropyridines have been discovered over the previous few decades. However, relatively few methods are available for the synthesis of enantiomerically enriched trans-1,2,3,6-tetrahydropyridines, including iminium ion-mediated vinylsilane and allylsilane cyclization, as well as ring-closing metathesis [9]. Therefore, the development of new, highly stereo-chemically controlled syntheses of this ring system remains an important challenge. Limited literature methods are available to synthesize diastereo- and enantioselective tetrahydropyridines, namely (a) chiral binapthol phosphoric acid and (b) chiral organocatalyst having gold as a co-catalyst for the synthesis of enantioselective tetrahydropyridines (Figure 2) [10]. The most common methods for the synthesis of 1,2,5,6-tetrahydropyridine comprises Mannich-type/intramolecular cyclization by a proline-mediated reaction, annulation by phosphine and DABCO, and alkynyl and allenyliminium ion cyclization by palladium catalyst [11,12,13], in addition to using L-proline/TFA, InCl3, Bi (NO3)3.5H2O, BF3.SiO2, molecular I2, cerium ammonium nitrate (CAN), LaCl3.7H2O, Fe3O4@TDSN-Bi (III), diethanol ammonium hydrogensulfate (DHS), trityl chloride, glycolic acid (GA), α-Fe2O3-immobilized benzimidazolium tribromide, and 1-methyl-2-oxopyrrolidinium hydrogen sulfate as catalysts [14,15,16,17,18,19,20,21,22,23,24,25,26].
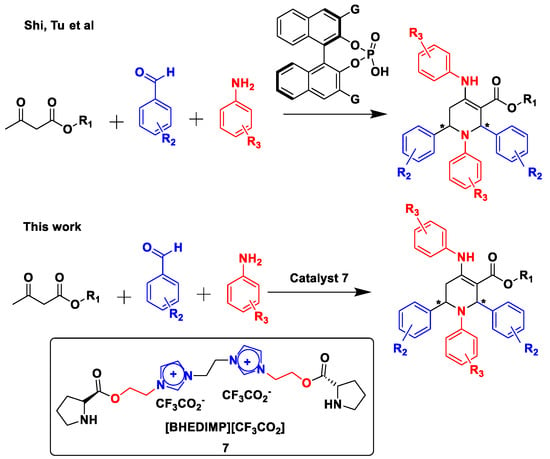
Figure 2.
Comparison of catalyst for the synthesis of 1,2,5,6 tetrahydropyridines.
Most of these techniques, however, frequently use expensive reagents or catalysts, high temperatures, long reaction times, non-reusable catalysts, toxic solvents, multistep synthesis, advanced starting materials, proper purification of intermediates, and low-yielding reaction processes, in addition to having a lack of operational simplicity and functional group tolerance. As a result, developing highly efficient synthetic methods to build structurally varied THPs in moderate circumstances remains a significant problem. Since its usage in the asymmetric aldol reaction, (S)-proline has been widely used as a chiral organocatalyst and has established itself as a leading success story in the field of asymmetric organocatalysis, owing to its natural, simple nature and low cost [27,28,29]. However, due to its limited solubility in most organic solvents, (S)-proline has several drawbacks.
One option for overcoming this disadvantage is to use co-catalysts or additives to increase the efficiency of (S)-proline. Ionic liquids, which are organic salts with melting points below 100 °C, have also been used as an alternate solvent in (S)-proline organocatalyzed processes, such as the asymmetric aldol reaction, to aid in catalyst dissolution and recovery [30,31]. In the synthetic chemist’s community, ionic liquid catalysts have attracted special consideration due to their tunable features for different chemical tasks [32,33,34]. Ionic liquids are used as catalysts as well as a solvent because of their unique properties, such as negligible vapor pressure, easy phase separation depending upon the choice of solvent, and reusable and recyclable homogeneous nature [35,36]. Various functionalized ionic liquids could be simply designed or synthesized by the attachment of the different alkyl-functionalized side chains [29]. Functionalized ionic liquids could be easily used in a supported synthesis for different heterocyclic biological active pharmaceutical intermediates [37]. Solvate ionic liquids (SILs) are a new type of ionic liquid made up of equimolar mixes of alkaline metal salts and glymes that produce complexes with strong coordination of the alkaline cation, usually lithium, by the glyme. For example, Watanabe et al. created numerous SILs that were primarily used as battery electrolytes. SILs have the benefit of being simple to make by dissolving the metal salt in the glyme of choice [38].
Although much progress has been achieved in synthesizing ionic liquid–catalyzed tetrahydropyridine synthesis reactions, due to longer reaction times and the use of highly toxic solvents and metal-mediated reactions, the currently existing methods need to be improved. While establishing a methodology to synthesize enantioenrich 1,2,5,6 tetrahydropyridines in our lab, we came across new findings, which are reported here. This reaction cascade uses [BHEDIMP][CF3CO2] 7 as a catalyst with aryl aldehydes, aryl amines, and β-keto esters in a green solvent. This reported catalyst 7 has also been explored as a recyclable organocatalyst for Mannich reactions in neat conditions by our research group [39]. In addition to being an enantioselective example of this five-component tandem reaction, this process also offers unparalleled access to structurally diverse, enantioenriched tetrahydropyridines, which have significant potential for use in medicinal chemistry.
2. Results and Discussion
Our synthetic strategy commenced with the synthesis of a targeted novel diimidazolium ionic liquid–immobilized proline(S) organocatalyst as shown in Scheme 1 to use in the synthesis of tetrahydropyridine in a one-pot multicomponent reaction [39]. Initially, the reaction of one equivalent of 1,2-di(1H-imidazol-1-yl)ethane 1 with two equivalents of 2-chloro ethanol 2 at 80 °C for 2 h under solvent-free conditions furnished bis-hydroxyl ethyl diimidazolium chloride [BHEDIM][Cl] 3 with 97% yield. This was followed by the action of one equivalent of bis-hydroxyl ethyl diimidazolium chloride [BHEDIM][Cl] 3 with two equivalents of NaBF4 in acetonitrile solvent under reflux for 12 h, which afforded bis-hydroxyl ethyl diimidazolium tetrafluoroborate [BHEDIM][BF4] 4 with 98% yield. After the successful synthesis of bis-hydroxyl ethyl diimidazolium tetrafluoroborate [BHEDIM][BF4] 4, we planned to couple the synthesized ionic liquid 4 with boc-(S)-proline 5. Further, the reaction of one equivalent of bis-hydroxyl-functionalized ionic liquid [BHEDIM][BF4] 4 with two equivalents of boc-(S)-proline 5 in the presence of DCC/DMAP coupling reagent furnished chiral ionic liquid–immobilized boc-(S)-proline organocatalyst [BHEDIMP][BF4] 6 with 97% yield. Furthermore, to synthesize targeted chiral bi-functional diimidazolium organocatalyst [BHEDIMP][CF3CO2] 7, we treated [BHEDIMP][BF4] 6 with 30% TFA/ACN at room temperature for 6 h to remove the boc moiety. Finally, the boc group was removed to afford the desired chiral diimidazolium ionic liquid–supported (S)-proline organocatalyst [BHEDIMP][CF3CO2] 7 with 96% yield.
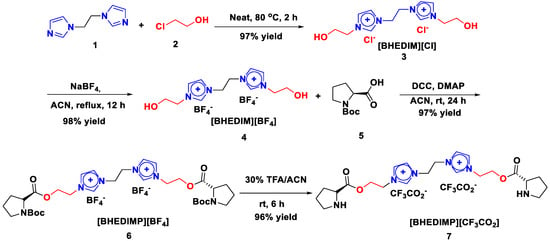
Scheme 1.
Synthesis of [BHEDIMP][CF3CO2] 7.
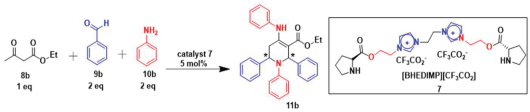
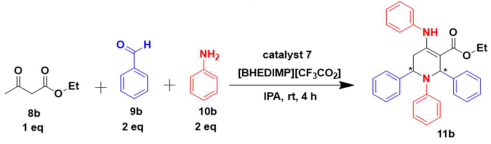
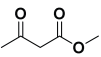
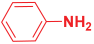
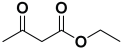
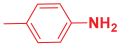
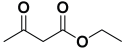
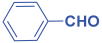
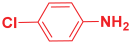
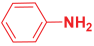
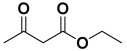
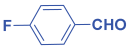
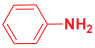
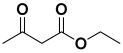
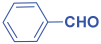
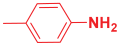
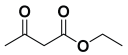
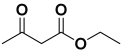
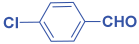
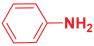
Next, to examine the catalytic activity of organocatalyst [BHEDIMP][CF3CO2] 7 for the enantioselective synthesis of tetrahydropyridine for the one-pot multicomponent reaction, our study commenced by considering two equivalents of aniline 10b, one equivalent of ethyl acetoacetate 8b, and two equivalents of benzaldehyde 9b in the presence of 5 mol% catalyst [BHEDIMP][CF3CO2] 7.
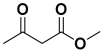
Ethyl acetoacetate 8b, benzaldehyde 9b, aniline 10b, and 5 mol% catalyst [BHEDIMP][CF3CO2] 7 were chosen as prototypes for the optimization of the reaction conditions and identification of the acceptable solvent, catalyst loading, and effective reaction conditions. Among all the screened solvents, such as ethanol, water, methanol, THF, acetonitrile, dichloromethane, chloroform, isopropanol, IPA/H2O, and EtOH/H2O (Table 1, entries 1–10), IPA proved to be the most effective solvent by furnishing the desired product with 92% yield in 4 h (Table 1, entry 8). Table 1 shows the optimization of the solvent for the synthesis of tetrahydropyridines.

Table 1.
Solvent screening for the synthesis of 11b a.
However, under solvent-free conditions and in water, we did not observe any formation of the desired product (Table 1, entries 11 and 2). When the reaction was carried out in polar protic solvents, such as ethanol, it resulted in 55% product formation, with >10% product formation in methanol (Table 1, entries 1 and 3). When the reaction was carried out in the polar aprotic solvent THF, we obtained >10% product formation, with >50% of the product being formed in dichloromethane and chloroform (Table 1, entries 4, 6, and 7). In addition, the reaction was carried out with a mixture of solvents; in IPA/H2O (1:1), product formation was >40%, and in ethanol-water (1:1), >35% of the product was formed (Table 1, entries 9 and 10). By examining different solvents, IPA was found to be the moderate solvent to give a high yield in less time as compared to other solvents. The reaction occurred efficiently at room temperature and did not necessitate the use of any equipment, such as the Schlenk system. The reaction was sluggish, and there were no significant yields when the reaction was carried out at reflux and low temperature.
We discovered that the product of THP was obtained in good yields ranging from 20 to 92% in varied mole ratios of [BHEDIMP][CF3CO2] 7 (1 to 10 mol%) by screening a wide range of catalysts, and the results are presented in Table 2 (entries 1–12). When the reaction was carried out with 5 mol% [BHEDIMP][CF3CO2] 7 in IPA as the solvent, a better yield of up to 92% was obtained (Table 1, entry 8). As a result, we decided to perform the reaction at ambient reaction conditions and illustrate it (Scheme 2).

Table 2.
Optimization of catalyst for THP derivatives a.
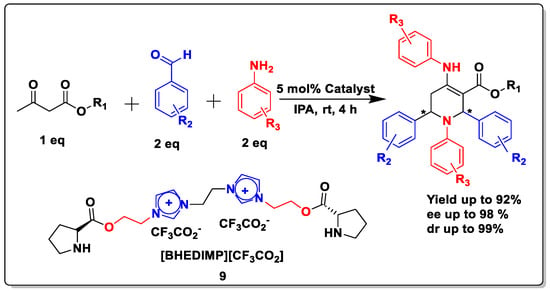
Scheme 2.
A typical procedure for the synthesis of THP derivatives.
The reaction of benzaldehyde 9b, aniline 10b, and ethyl acetoacetate 8b was carried out in the presence of catalyst [BHEDIMP][CF3CO2] 7 and the solvent IPA. Remarkably, a high yield of 92% was achieved in 4 h. The resulting product was washed with hexane and ether and transferred to another beaker to dry it. Further, chloroform was added to wash the catalyst, which settled in the wall of reaction RB, and the washed catalyst was used for further reaction. The obtained pure solid product was analyzed using 1H, 13C NMR, FT-IR, and HPLC as provided in the Supplementary Information (Figures S1–S77).
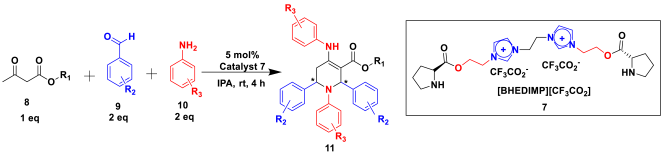
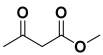
The selective production of the anti-isomer was verified using NMR, HPLC, polarimetry analysis, and measurement of the melting point. We opted to test the generality and adaptability of the procedure using the aforementioned standard conditions since we were excited about these characteristics, namely a reduction in response time, an increase in yield, and diastereoselectivities. Thus, we synthesized a variety of highly functionalized piperidine derivatives by reacting different aromatic aldehydes 9, amines 10, and active methylene compounds 8, such as ethyl acetoacetate and methyl acetoacetate. The yield of product 11 was high to excellent with different substrates, suggesting that electron-donating and electron-withdrawing groups have no effect on both aldehydes and amines (Table 3). In addition, the enantiomeric ratio (er) presented in Table 3 corresponds to the diastereoisomer that is more abundant in the final yield of the corresponding products 11a–o.

Table 3.
Substrate scope for several THP derivatives 11a–o a.
In accordance with the mechanism proposed in the literature [40], tetrahydropyridine derivatives 11 were afforded from the initial condensation of aromatic amine 10 and β-ketoester 8 in presence of the catalyst [BHEDIMP][CF3CO2] 7 to give enamine I, which further reacted with aromatic aldehydes 9 to yield Knoevenagel adduct II. In addition, aromatic aldehyde 9 and amine 10 reacted with each other to form the respective imine III. Next, intermediates II and III underwent [4 + 2] cycloaddition with catalyst 7 to give the transition state IV, which subsequently afforded the desired corresponding products 11 (Figure 3).
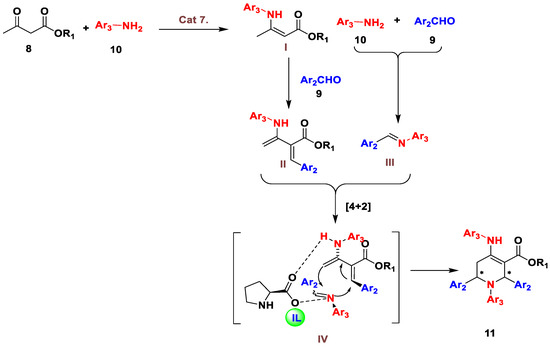
Figure 3.
Plausible reaction mechanism of tetrahydropyridines.
Finally, using catalyst [BHEDIMP][CF3CO2] 7, we established a green and efficient methodology for the synthesis of piperidine derivatives. This catalyst is efficient and easy enough to make and handle, and it sustains its activity after several treatments. This ecologically friendly catalyst has excellent potential for additional acid-catalyzed chemical methods, in addition to the current application for the synthesis of these piperidines. The results show that our technique, which uses [BHEDIMP][CF3CO2] 7 as a catalyst, is efficient in terms of reaction time and product yields when compared to the efficacy of results reported in the literature.
3. Materials and Methods
3.1. General Information
Imidazole, 1,2 dibromo ethane, 2-chloro ethanol, L-boc-proline, acetonitrile (HPLC grade), ethyl acetate, anhydrous sodium sulfate, all substrates for acetophenone, aldehydes, and aromatic amine derivatives were purchased from Sigma-Aldrich (Bengaluru, India) and used without further purification. All solvents and HPLC grade solvents were obtained from Sigma-Aldrich. All chemicals were purchased from Sigma-Aldrich. All reactions were performed under an open atmosphere with unpurified reagents and dry solvents. Analytical thin-layer chromatography (TLC) was performed using 0.25 mm silica gel–coated Kieselgel 60 F254 plates. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker DRX-400 spectrometer (AVANCE III, 400 MHz, Bruker, Germany). Chemical shifts were reported in parts per million from tetramethylsilane (TMS), with the solvent resonance as the internal standard (CHCl3: δ = 7.26 ppm). The pattern of reported data is as follows: chemical shifts, multiplicity (s = singlet, d = doublet, dd = double doublet, t = triplet, q = quartet, br = broad, m = multiplet), integration, and coupling constant (Hz). 13C NMR spectra were recorded at 100 MHz with proton decoupling. Chemical shifts were reported in parts per million from TMS, with the solvent resonance as the internal standard (CHCl3: δ 77.0 ppm). IR spectra were obtained using an FT-IR spectrometer. HPLC enantioseparation was determined using the commercially available Lux Amylose-1 [amylose tris(3,5-dimethylphenylcarbamate)] (250 × 4.6 mm, 5 μm) column (Phenomenex, Hyderabad, India).
3.2. General Procedure for the Synthesis of Bis-Alcohol-Functionalized Diimidazolium Ionic Liquids (ILs) 3 and 4
A mixture of diimidazole 1 (1.0 g, 6.2 mmol, 1 equiv) and 2-chloroethanol 2 (0.99 g, 12.3 mmol, 2 equiv) was placed in a 100 mL round-bottom flask and heated at 80 °C for 2.5 h under neat conditions. After completion of the reaction, the reaction mixture was cooled to ambient temperature, and the viscous solid was successively washed with ether (10 mL× 2) and then dried under vacuum for 3 h. The bis-hydroxyl-functionalized diimidazolium ionic liquid 3 [BHEMIM][Cl-] was obtained as white crystals (1.85 g, 93%). 1H NMR (400 MHz, DMSO-d6): δ 8.80 (s, 2H), 7.55 (d, J = 1.2, Hz, 2H), 7.44 (d, J = 1.2 Hz, 2H), 4.74 (s, 4H), 4.25 (t, J = 4.4 Hz, 4H), and 3.84 (t, J = 4.4 Hz, 4H). 13C NMR (100 MHz, DMSO-d6): δ 136.5, 123.7, 122.4, 59.6, 51.9, and 48.9.
In a 100 mL round-bottom flask, bis-hydroxyl-functionalized diimidazolium ionic liquid [BHEMIM][Cl-] 3 (1.85 g, 5.73 mmol, 1equiv) in dry acetonitrile (25 mL) and NaBF4 (1.4 g, 12.6 mmol, 2.2 equiv) was added and stirred at room temperature for 24 h under a nitrogen atmosphere. The so-formed white precipitate was filtered and washed with acetonitrile (30 mL × 3). The concentration of combined filtrates provided bis-hydroxyl-functionalized ionic liquid [BHEDIM][BF4−] 4 as a light-yellow viscous oil (2.36 g, 96%). 1H NMR (400 MHz, DMSO-d6): δ 8.96 (s, 2H), 7.74 (s, 2H), 7.59 (s, 2H), 4.69 (s, 4H), 4.19 (t, J = 4.8 Hz, 4H), and 3.71 (t, J = 4.8 Hz, 4H). 13C NMR (100 MHz, DMSO-d6): δ 137.2, 123.7, 122.7, 59.6, 52.3, and 48.9. FT-IR (KBr, cm−1): 3560, 3157, 1566, and 1012. MS (GC-MS): m/z 252.9161 (M+).
3.3. General Procedure for the Synthesis of Bis-Alcohol-Functionalized Ionic Liquid–Supported L-Boc-Proline 6
To a mixture of bis-hydroxyl-functionalized bis-imidazolium ionic liquid [BHEDIM][BF4−] 4 (1.5 g, 3.5 mmol, 1 equiv), L-boc proline 5 (1.54 g, 7.2 mmol, 2.05 equiv), and dimethyl amino pyridine catalytic amount in 30 mL of dry acetonitrile solvent was added dicyclohexyl carbodiimide (1.6 g, 7.7 mmol, 2.2 equiv) in DCM (5 mL) dropwise for 5 min. After that, the reaction mixture was vigorously stirred for 24 h at room temperature. After reaction completion, the reaction mixture was filtered through a celite plug to remove dicyclohexyl urea. The filtrate was concentrated under vacuum, and the coupled product was washed with ether (10 mL × 2). The crude mixture 6 was dried under high vacuum and formed a viscous solid (2.8 g, 97%) characterized using 1HNMR, 13CNMR, and IR spectroscopy. 1H NMR (400 MHz, DMSO-d6): δ 9.09 (d, J = 4 Hz, 1H), 9.07 (d, J = 3.6 Hz, 1H), 7.80 (t, J = 1.6 Hz, 2H), 7.62 (t, J = 6 Hz, 2H), 4.70 (s, 4H), 4.49–4.43 (m, 8H), 4.20–4.13 (m, 2H), 4.09–4.04 (m, 1H), 2.22–2.09 (m, 3H), 1.82–1.79 (m, 9H), 1.4 (s, 12H), and 1.34 (s, 6H). 13C NMR (100 MHz, DMSO-d6): δ 170.8, 151.2, 135.5, 122.1, 121.1, 77.3, 63.3, 61.1, 60.8, 56.9, 56.7, 46.4, 46.2, 44.8, 44.5, 34.2, 28.6, 26.5, 26.2, 22.3, and 21.6. FT-IR (KBr, cm−1): 3608, 3157.47, 2978.09, 1747.51, 1681.93, 1049.28, and 1033.85.
3.4. General Procedure for the Synthesis of Ionic Liquid–Supported L-Proline 7
To a mixture of bis-alcohol-functionlized diimidazolium ionic liquid–immobilized L-boc proline 6 (2.8 g, 3.41 mmol) in dry acetonitrile solvent was added 30 mL 30 % TFA/ACN. After that, the reaction mixture was vigorously stirred for 6 h at room temperature. The filtrate was concentrated under vacuum, washed with ether and DCM 2–3 times, and dried under high vacuum to get the pure product 7 (2.2 g, 95%), which was characterized using 1H NMR, 13C NMR, and IR and MS analysis. 1H NMR (400 MHz, D2O): δ 8.47 (s, 1H), 7.22 (t, J = 1.7 Hz, 1H), 7.14 (t, J = 1.6 Hz, 1H), 4.30–4.24 (m, 2H), 4.21–4.16 (m, 2H), 3.58 (s, 3H), 3.31 (q, J = 7.1 Hz, 1H), 3.09 (td, J = 7.2, 3.3 Hz, 2H), 2.15–2.06 (m, 1H), and 1.79–1.67 (m, 4H). 13C NMR (100 MHz, D2O): δ 169.3126, 136.4916, 122.8432, 12.6424, 122.4310, 64.5910, 59.7712, 59.2655, 51.4961, 47.8427, 46.2969, 35.7733, 28.2957, 27.9897, 23.2521, 23.2140, 20.3879, and 16.7944. MS (GC): m/z 224 (M+). [α] D20 = +32 (0.2, H2O).
3.5. General Procedure for the Synthesis of THP Derivative 11
To a 50 mL RB flask, methyl/ethyl acetoacetate (1 equiv) 8, aromatic aldehydes 9 (2 equiv), aromatic anilines 10 (2 equiv), and 5 mol% organocatalyst [BHEDIMP][CF3CO2] 7 were added in IPA solvent and stirred at room temperature for 4 h. The corresponding solid product 11a-o was formed after 4 h. The progress of the reaction was monitored using TLC. After completion of the reaction, the solid product was washed with ether (10 mL × 2) and hexane (10 mL × 2) to get the pure product 11. The obtained solid product was characterized using 1H, 13C NMR, HPLC, polarimetry, and FT-IR spectroscopy.
3.6. Structural Characterization Data of THP Derivatives 11a–o
Structural Characterization Data
Methyl 1,2,6-triphenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11a: off-white solid, yield: 92%. FT-IR (KBr, cm−1): 3238.48, 3051.39, 2943.37, 1653.00, 1591.27, 1575.84, 1494.83, 1440.83, 1365.60, 1313.52, 1271.09, 1242.16, 1186.22, 1120.64, 1070.49, 1028.06, 970.19, 904.61, 840.96, 750.31, 692.44, 665.44, and 586.36. 1H NMR (400 MHz, CDCl3): δ 10.25 (s, 1H), 7.29 (dt, J = 17.6, 5.1 Hz, 8H), 7.07 (dd, J = 14.8, 6.4 Hz, 5H), 6.60 (t, J = 7.2 Hz, 1H), 6.52 (d, J = 8.3 Hz, 2H), 6.45 (s, 1H), 6.30–6.24 (m, 2H), 5.14 (d, J = 3.8 Hz, 1H), 3.93 (s, 3H), ad 2.81 (ddd, J = 16.8, 15.1, 3.7 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 168.59, 156.31, 146.93, 143.90, 142.7, 137.80, 128.90, 128.85, 128.65, 128.26, 126.64, 126.28, 125.89, 116.16, 112.91, 97.19, 58.20, 55.09, 51.06, and 33.62. [α]D20: +100.0 (C 0.1, CHCl3). Enantiomeric ratio: 2:98. Determined using HPLC (hexane/2-propano, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 23.7 min (major), tR = 11.7 min (minor).
Ethyl 1,2,6-triphenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11b: off-white solid, yield: 92%. FT-IR (KBr, cm−1): 3167.12, 3059.10, 2980.02, 1651.07, 1579.70, 1494.83, 1446.61, 1369.46, 1246.02, 1170.79, 1068.56, 1028.06, 916.19, 835.18, 746.45, and 690.52. 1H NMR (400 MHz, CDCl3): δ 10.66 (s, 1H), 7.65 (d, J = 7.9 Hz, 2H), 7.42 (t, J = 7.6 Hz, 2H), 7.30 (dd, J = 9.0, 5.1 Hz, 5H), 7.25 (d, J = 5.3 Hz, 1H), 7.20 (t, J = 7.4 Hz, 2H), 7.16–7.09 (m, 4H), 6.97 (d, J = 7.7 Hz, 2H), 6.85 (d, J = 8.2 Hz, 2H), 6.76–6.66 (m, 1H), 6.19 (s, 1H), 4.56 (dd, J = 12.5, 3.4 Hz, 1H), 4.27 (dq, J = 10.8, 7.1 Hz, 1H), 4.21–4.11 (m, 1H), 2.81 (dd, J = 15.3, 3.5 Hz, 1H), 2.47 (dd, J = 15.2, 12.6 Hz, 1H), and 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 168.53, 157.32, 150.46, 146.31, 143.43, 138.64, 129.31, 128.84, 128.75, 128.68, 128.45, 127.06, 126.76, 124.81, 123.80, 118.08, 115.48, 97.94, 61.43, 60.52, 59.76, 36.39, and 14.56. [α]D20: +9.7 (C 0.1, CHCl3). Enantiomeric ratio: 97:03. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 4.9 min (major), tR = 5.8 min (minor).
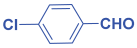
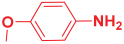
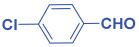
Ethyl 2,6-bis(4-chlorophenyl)-1-(4-methoxyphenyl)-4-((4methoxyphenyl) amino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11c: brown solid, yield: 90%. FT-IR (KBr, cm−1): 1H NMR (400 MHz, CDCl3) δ 10.14 (s, 1H), 7.27–7.20 (m, 6H), 7.06 (d, J = 8.3 Hz, 2H), 6.67 (dd, J = 8.6, 5.2 Hz, 4H), 6.42–6.32 (m, 4H), 6.21 (s, 1H), 4.96 (s, 1H), 4.45–4.23 (m, 2H), 3.76 (s, 2H), 3.67 (s, 2H), 2.67 (ddd, J = 17.9, 15.4, 4.2 Hz, 2H), and 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 168.08, 157.95, 156.49, 151.49, 142.72, 141.43, 141.36, 141.01, 132.78, 132.02, 130.54, 128.71, 128.25, 127.98, 127.72, 114.70, 114.58, 114.12, 96.71, 59.65, 57.33, 55.63, 55.59, 55.43, 33.67, and 14.75. [α]D20: +33.26 (C 0.1, CHCl3). Enantiomeric ratio: 97:03. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 5.4 min (major), tR = 8.5 min (minor).
Methyl 2,6-bis(4-fluorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11d: white solid, yield: 88%. FT-IR (KBr, cm−1): 3244.27, 3055.24, 2943.37, 1645.28, 1595.13, 1498.69, 1442.75, 1271.09, 1219.01, 1151.50, 1053.13, 974.05, 831.32, 746.45, and 692.44. 1H NMR (400 MHz, CDCl3): δ 10.63 (s, 1H), 7.56 (dd, J = 8.3, 5.6 Hz, 2H), 7.31 (t, J = 7.8 Hz, 2H), 7.22 (dd, J = 8.5, 5.4 Hz, 2H), 7.12 (dt, J = 17.3, 8.0 Hz, 5H), 6.98 (d, J = 7.7 Hz, 2H), 6.89 (t, J = 8.6 Hz, 2H), 6.82 (d, J = 8.3 Hz, 2H), 6.75 (t, J = 7.2 Hz, 1H), 6.09 (s, 1H), 4.54 (dd, J = 12.5, 3.4 Hz, 1H), 3.73 (s, 3H), 2.76 (dt, J = 25.2, 12.6 Hz, 1H), and 2.42 (dd, J = 15.3, 12.6 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 168.67, 157.32, 150.11, 141.87, 141.84, 138.88, 138.84, 135.39, 129.39, 128.88, 128.56, 128.48, 125.25, 125.11, 123.91, 118.66, 115.72, 115.49, 115.35, 115.14, 97.33, 60.68, 60.28, 51.17, and 36.32. [α]D20: +20.04 (C 0.1, CHCl3). Enantiomeric ratio: 100:00. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 12.2 min (major).
Methyl 2,6-diphenyl-1-(p-tolyl)-4-(p-tolylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate.11e: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3255.84, 3024.38, 2945.30, 2858.51, 1649.14, 1593.20, 1512.19, 1450.47, 1375.25, 1247.94, 1186.22, 1128.36, 1072.42, 974.05, 792.74, 698.23, and 597.93. 1H NMR (400 MHz, CDCl3): δ 10.56 (s, 1H), 7.64 (d, J = 7.6 Hz, 2H), 7.41 (t, J = 7.5 Hz, 2H), 7.29 (d, J = 6.5 Hz, 5H), 7.18 (dd, J = 14.6, 7.1 Hz, 3H), 7.08 (d, J = 7.9 Hz, 2H), 6.93 (d, J = 8.2 Hz, 2H), 6.87 (d, J = 7.6 Hz, 3H), 6.76 (d, J = 8.3 Hz, 2H), 6.42 (d, J = 9.2 Hz, 1H), 6.18–6.08 (m, 2H), 5.06 (d, J = 42.7 Hz, 1H), 4.58–4.36 (m, 1H), 3.92 (s, 1H), 3.72 (s, 3H), 2.88–2.65 (m, 2H), 2.46–2.35 (m, 1H), 2.27 (d, J = 17.6 Hz, 4H), and 2.17 (d, J = 15.7 Hz, 4H). 13C NMR (101 MHz, CDCl3): δ 168.94, 158.10, 148.37, 146.3, 143.63, 136.89, 134.84, 129.87, 129.31, 128.63, 128.44, 127.04, 126.78, 124.12, 115.53, 112.86, 96.69, 61.45, 60.86, 51.01, 36.26, 20.89, and 20.25. [α]D20: +146.2 (C 0.1, CHCl3). Enantiomeric ratio: 88:12. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 6.2 min (major), tR = 9.8 min (minor).
Ethyl 1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-2,6-diphenyl-1,2,5,6-tetra hydro pyridine-3-carboxylate. 11f: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3240.41, 3165.19, 2972.31, 2852.72, 1730.15, 1643.35, 1600.92, 1581.63, 1490.97, 1448.54, 1367.53, 1317.38, 1246.02, 1176.58, 1068.56, 1012.63, 939.33, 848.68, 775.38, and 727.16. 1H NMR (400 MHz, CDCl3): δ 10.23 (s, 1H), 7.29 (d, J = 4.5 Hz, 7H), 7.17–7.12 (m, 2H), 7.06–6.97 (m, 4H), 6.45–6.41 (m, 2H), 6.39 (s, 1H), 6.19–6.13 (m, 2H), 5.10 (s, 1H), 4.49–4.31 (m, 2H), 2.85 (dd, J = 15.1, 5.6 Hz, 1H), and 2.69 (d, J = 15.1 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 168.16, 155.42, 145.50, 143.26, 142.26, 136.41, 131.36, 129.02, 128.83, 128.73, 128.38, 127.48, 127.03, 126.57, 126.50, 126.30, 121.23, 114.03, 98.70, 59.94, 58.32, 55.25, 33.48, and 14.78. [α]D20: +24.04 (C 0.1, CHCl3). Enantiomeric ratio: 25:75. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 12.4 min (major), tR = 9.6 min (minor).
Methyl 1-phenyl-4-(phenylamino)-2,6-di-p-tolyl-1,2,5,6-tetrahydropyridine-3-carboxylate. 11g: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3035.96, 2393.66, 1651.07, 1593.20, 1496.76, 1365.60, 1290.38, 1240.23, 1168.86, 1070.49, 1033.85, 947.05, 817.82, 746.45, 690.52, and 640.37. 1H NMR (400 MHz, CDCl3): δ 10.24 (s, 1H), 7.19 (d, J = 7.9 Hz, 1H), 7.09–7.02 (m, 2H), 6.58 (t, J = 7.2 Hz, 1H), 6.52 (d, J = 8.3 Hz, 1H), 6.39 (s, 1H), 6.32–6.28 (m, 1H), 5.10 (d, J = 3.6 Hz, 1H), 3.92 (s, 1H), 2.90–2.72 (m, 1H), and 2.32 (d, J = 6.2 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 168.61, 156.28, 147.07, 140.95, 139.68, 137.95, 136.61, 135.78, 129.27, 128.93, 128.84, 128.81, 128.62, 126.56, 126.33, 125.82, 125.63, 116.01, 112.92, 98.11, 77.35, 77.04, 76.72, 57.92, 54.94, 50.98, 33.66, 21.10, and 21.02. [α]D20: +13.6 (C 0.1, CHCl3). Enantiomeric ratio: 0:100. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 10.0 min (major), tR = 5.3 min (minor).
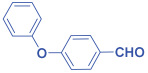
Ethyl 2,6-bis(4-phenoxyphenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11h: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3253.91, 3035.96, 2951.09, 1643.35, 1579.70, 1479.40, 1361.74, 1301.95, 1236.37, 1165.00, 1072.42, 937.40, 881.47, 798.53, 746.45, and 692.44. 1H NMR (400 MHz, CDCl3): δ 10.60 (s, 1H), 7.34–7.25 (m, 5H), 7.21 (dd, J = 10.8, 4.8 Hz, 3H), 7.12 (dd, J = 15.9, 8.2 Hz, 4H), 7.09–7.02 (m, 2H), 6.97 (dd, J = 17.0, 7.5 Hz, 6H), 6.91–6.86 (m, 1H), 6.86–6.72 (m, 6H), 6.06 (s, 1H), 4.52 (dd, J = 12.4, 3.2 Hz, 1H), 4.31–3.96 (m, 2H), 2.81 (dt, J = 14.3, 7.2 Hz, 1H), 2.50 (dd, J = 15.2, 12.5 Hz, 1H), 1.58 (s, 2H), and 1.20 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 157.42, 157.35, 157.05, 156.94, 150.24, 148.71, 145.20, 138.51, 130.07, 129.71, 129.65, 129.32, 128.74, 124.96, 123.96, 123.21, 123.10, 122.28, 121.48, 118.78, 118.71, 118.59, 117.68, 117.34, 117.28, 117.19, 116.11, 97.61, 77.38, 77.26, 77.06, 76.74, 61.06, 60.80, 59.76, 36.20, and 14.49. [α]D20: +20.40 (C 0.1, CHCl3). Enantiomeric ratio: 95:05. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 9.4 min (major), tR = 8.0 min (minor).
Methyl 2,6-bis(4-chlorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11i: white solid, yield: 90%. FT-IR (KBr, cm−1): 3238.48, 3039.81, 2947.23, 1653.00, 1593.20, 1500.62, 1404.18, 1369.46, 1327.03, 1259.52, 1174.65, 1091.71, 1070.49, 1012.63, 983.70, 925.83, 860.25, 823.60, 786.96, 746.45, 690.52, and 599.86. 1H NMR (400 MHz, CDCl3): δ 10.57 (s, 1H), 7.54 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.25–7.14 (m, 7H), 7.09–6.91 (m, 4H), 6.81–6.71 (m, 2H), 6.65 (s, 1H), 6.59 (d, J = 7.7 Hz, 2H), 6.06 (s, 1H), 5.03 (d, J = 35.0 Hz, 1H), 4.51 (dd, J = 12.4, 3.0 Hz, 1H), 3.92 (s, 1H), 3.71 (d, J = 11.9 Hz, 3H), 2.91–2.66 (m, 2H), 2.36–2.29 (m, 4H), and 2.25–2.14 (m, 6H). 13C NMR (101 MHz, CDCl3): δ 168.30, 156.00, 146.46, 142.35, 14.92, 137.61, 132.89, 132.16, 129.04, 129.02, 128.78, 128.41, 128.04, 127.77, 126.04, 125.75, 116.70, 112.95, 97.55, 57.36, 54.75, 51.16, and 33.68. [α]D20: +20.8 (C 0.1, CHCl3). Enantiomeric ratio: 15:85. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 6.5 min (major), tR = 11.7 min (minor).
Ethyl 2,6-bis(4-fluorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11j: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 1641.42, 1579.70, 1498.69, 1435.04, 1365.60, 1238.30, 1217.08, 1151.50, 1051.20, 966.34, 862.18, 827.46, 788.89, 746.45, 692.44, and 588.29. 1H NMR (400 MHz, CDCl3): δ 10.69 (s, 1H), 7.60 (dd, J = 8.2, 5.6 Hz, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.27 (dd, J = 8.7, 5.4 Hz, 1H), 7.15 (ddd, J = 17.4, 10.7, 4.8 Hz, 2H), 7.01 (dd, J = 8.0, 5.0 Hz, 1H), 6.92 (t, J = 8.6 Hz, 1H), 6.86 (d, J = 8.2 Hz, 1H), 6.79 (t, J = 7.2 Hz, 1H), 6.12 (s, 1H), 4.58 (dd, J = 12.4, 3.4 Hz, 1H), 4.33–4.16 (m, 1H), 2.85–2.79 (m, 1H), 2.48 (dd, J = 15.3, 12.5 Hz, 1H), and 1.29 (t, J = 7.1 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 168.35, 156.99, 150.23, 141.96, 141.93, 138.95, 138.92, 138.52, 129.37, 129.02, 128.98, 128.85, 128.66, 128.58, 128.31, 128.23, 125.66, 125.00, 123.87, 118.81, 116.09, 115.67, 115.46, 115.26, 115.05, 113.04, 97.74, 60.80, 60.42, 59.81, 36.41, and 14.53. [α]D20: +15.9 (C 0.1, CHCl3). Enantiomeric ratio: 98:02. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 20.9 min (major), tR = 19.1 min (minor).
Ethyl 2,6-diphenyl-1-(p-tolyl)-4-(p-tolylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate.11k: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3238.48, 2960.73, 1647.21, 1589.34, 1514.12, 1448.54, 1369.46, 1313.52, 1246.02, 1220.94, 1170.79, 1072.42, 1035.77, 941.26, 837.11, 794.67, 698.23, 677.01, and 549.71. 1H NMR (400 MHz, CDCl3): δ 10.20 (s, 1H), 7.30–7.24 (m, 2H), 7.18 (dd, J = 15.4, 6.9 Hz, 1H), 6.87 (dd, J = 7.7, 5.8 Hz, 1H), 6.42 (d, J = 8.9 Hz, 1H), 6.15 (d, J = 8.0 Hz, 1H), 5.10 (d, J = 3.5 Hz, 1H), 4.47–4.29 (m, 1H), 2.79 (ddd, J = 28.2, 19.9, 9.5 Hz, 1H), 2.25 (s, 1H), 2.15 (s, 1H), and 1.45 (t, J = 7.1 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 168.28, 156.42, 143.04, 135.52, 135.26, 129.44, 129.40, 128.59, 128.18, 127.03, 126.68, 126.46, 126.17, 125.92, 112.93, 59.55, 58.23, 33.57, 20.86, 20.11, and 14.81. [α]D20: +7.24 (C 0.1, CHCl3). Enantiomeric ratio: 06:94. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254nm). tR = 10.1 min (major), tR = 8.3 min (minor).
Ethyl 2,6-bis(4-chlorophenyl)-1-(m-tolyl)-4-(m-tolylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11l: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 1H NMR (400 MHz, CDCl3) δ 10.13 (s, 1H), 7.18–7.13 (m, 2H), 7.10 (d, J = 5.6 Hz, 1H), 6.98 (d, J = 8.2 Hz, 1H), 6.87 (d, J = 7.8 Hz, 1H), 6.81 (d, J = 8.5 Hz, 1H), 6.29 (d, J = 8.5 Hz, 1H), 6.24 (s, 1H), 6.20 (d, J = 8.0 Hz, 1H), 4.98 (s, 1H), 4.38–4.21 (m, 1H), 2.74–2.61 (m, 1H), 2.20 (s, 1H), 2.09 (s, 1H), and 1.36 (t, J = 7.1 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 168.03, 156.16, 144.37, 142.80, 141.23, 135.88, 135.02, 132.76, 132.01, 129.58, 128.73, 128.34, 128.08, 127.85, 125.83, 124.15, 116.57, 113.01, 97.25, 59.74, 57.35, 54.86, 33.62, 20.91, 20.12, and 14.81. [α]D20: +28.7 (C 0.1, CHCl3). Enantiomeric ratio: 14:86. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 12.6 min (major), tR = 9.6 min (minor).
Methyl 2,6-bis(4-chlorophenyl)-1-(m-tolyl)-4-(m-tolylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11m: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 2978.09, 2912.51, 1645.28, 1598.99, 1487.12, 1363.67, 1242.16, 1163.08, 1051.20, 1010.70, 827.46, 771.53, 696.30, and 605.65. 1H NMR (400 MHz, CDCl3): δ 10.50 (s, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.5 Hz, 1H), 7.18–7.07 (m, 2H), 6.94 (t, J = 7.9 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 6.74–6.65 (m, 1H), 6.58 (s, 1H), 6.51 (d, J = 6.9 Hz, 1H), 5.98 (s, 1H), 4.44 (dd, J = 12.5, 3.3 Hz, 1H), 3.65 (s, 1H), 2.68 (dd, J = 15.3, 3.3 Hz, 1H), 2.31–2.25 (m, 1H), 2.23 (s, 1H), and 2.14 (s, 1H). 13C NMR (101 MHz, CDCl3): δ 168.61, 157.44, 150.18, 144.99, 141.87, 139.45, 138.67, 138.22, 132.74, 132.69, 129.14, 128.91, 128.72, 128.62, 128.46, 128.08, 126.06, 124.65, 121.04, 119.78, 116.46, 112.91, 96.78, 60.76, 60.41, 51.14, 36.07, 21.91, and 21.40. [α]D20: +30.8 (C 0.1, CHCl3). Enantiomeric ratio: 99:01. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 9.6 min (major), tR = 8.2 min (minor).
Ethyl 2,6-bis(4-chlorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. 11n: gray solid, yield: 90%. FT-IR (KBr, cm−1): 3161.33, 2872.01, 1737.86, 1649.14, 1595.13, 1579.70, 1498.69, 1406.11, 1367.53, 1325.10, 1247.94, 1172.72, 1089.78, 1064.71, 1010.70, 950.91, 898.83, 858.32, 783.10, 744.52, 688.59, and 626.87. 1H NMR (400 MHz, CDCl3): δ 10.32 (s, 1H), 7.27 (d, J = 10.2 Hz, 2H), 7.22–7.07 (m, 3H), 6.68 (t, J = 7.2 Hz, 1H), 6.48 (t, J = 8.0 Hz, 1H), 6.44 (d, J = 7.3 Hz, 1H), 6.40 (s, 1H), 5.12 (s, 1H), 4.51–4.33 (m, 1H), 2.89–2.75 (m, 1H), and 1.48 (t, J = 7.1 Hz, 1H). 13C NMR (101 MHz, CDCl3): δ 167.99, 155.82, 146.51, 142.48, 140.95, 137.69, 132.87, 132.13, 129.06, 129.01, 128.78, 128.41, 128.04, 127.79, 125.68, 116.75, 112.99, 97.81, 59.88, 57.38, 54.74, 33.71, and 14.80. [α]D20: +17.24 (C 0.1, CHCl3). Enantiomeric ratio: 00:100. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 6.6 min (major).
Methyl 1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydro pyridine-3-carboxylate. 11o: off-white solid, yield: 90%. FT-IR (KBr, cm−1): 3259.70, 3064.89, 2951.09, 2868.15, 1649.14, 1598.99, 1490.97, 1450.47, 1317.38, 1251.80, 1188.15, 1076.28, 929.69, 840.96, 798.53, and 698.23. 1H NMR (400 MHz, CDCl3): δ 10.17 (s, 1H), 7.22 (dd, J = 17.1, 10.8 Hz, 4H), 7.00 (dd, J = 25.3, 8.6 Hz, 2H), 6.41 (d, J = 8.8 Hz, 1H), 6.36 (s, 1H), 6.15 (d, J = 8.3 Hz, 1H), 5.09 (s, 1H), 3.92 (s, 1H), and 2.88–2.64 (m, 1H).13C NMR (101 MHz, CDCl3): δ 168.48, 155.61, 145.48, 143.16, 142.24, 136.36, 131.49, 129.47, 129.04, 128.84, 128.73, 128.40, 127.49, 127.11, 126.51, 126.30, 125.03, 121.26, 116.75, 114.02, 98.45, 58.31, 55.29, 51.21, and 33.48. [α]D20: +34.8 (C 0.1, CHCl3). Enantiomeric ratio: 20:80. Determined using HPLC (hexane/2-propanol, flow rate 1.0 mL min−1. T = 25 °C, 254 nm). tR = 21.2 min (major), tR = 18.0 min (minor).
4. Conclusions
We presented a practical procedure for the synthesis of highly functionalized tetrahydropyridines 11a–o using [BHEDIMP][CF3CO2] 7 as a catalyst in this article. We believe this is the first time an imidazolium-based proline-supported catalyst has been used to produce highly modified piperidine derivatives that involves simultaneous formation of two stereogenic centers and fives bonds under ambient conditions. The products were obtained in high yields (up to 92%) with excellent stereoselectivities (up to >99:1 dr, 95:5 er). The salient features of our reported synthetic methodology are mild reaction conditions, well-to-outstanding yields, strong diastereoselectivity, operational simplicity, cheap cost, and an ecologically friendly catalyst.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010209/s1, Figure S1: 1HNMR (400MHz) compound 1 in DMSO-d6; Figure S2: 13C NMR Spectrum (100MHz) of compound 1 in DMSO-d6; Figure S3: IR spectra of compound 1; Figure S4: GCMS spectra of compound 1; Figure S5: 1HNMR (400MHz) compound 3 in DMSO-d6; Figure S6: 13C NMR (400MHz) compound 3 in DMSO-d6; Figure S7: GCMS Spectrum of compound 3; Figure S8: 1HNMR (400MHz) compound 4 in DMSO-d6; Figure S9: 13C NMR (400MHz) compound 4 in DMSO-d6; Figure S10: IR spectra of compound 4; Figure S11: GCMS Spectrum of compound 4; Figure S12: 1HNMR (400MHz) compound 6 in DMSO-d6; Figure S13: 13C NMR (400MHz) compound 6 in DMSO-d6; Figure S14: IR Spectrum of compound 6; Figure S15: 1HNMR (400MHz) compound 7 in D2O; Figure S16: 13C NMR (400MHz) compound 7 in D2O; Figure S17: IR spectra of compound 7; Figure S18: Stepwise 1H NMR spectrum for (S)-proline organocatalyst [BHEDIMP][CF3CO2] 7; Figure S19: 1H NMR (400 MHz) of compound 11a in CDCl3; Figure S20: 13C NMR Spectrum (100 MHz) of compound 11a in CDCl3; Figure S21: HPLC Chromatogram of compound 11a; Figure S22: IR Spectrum of compound 11a; Figure S23: 1H NMR (400 MHz) of compound 11b in CDCl3; Figure S24: 13C NMR Spectrum (100 MHz) of compound 11b in CDCl3; Figure S25. HPLC Chromatogram of compound 11b; Figure S26: IR Spectrum of compound 11b; Figure S27: 1H NMR (400 MHz) of compound 11c in CDCl3; Figure S28: 13C NMR Spectrum (100 MHz) of compound 11c in CDCl3; Figure S29. HPLC Chromatogram of compound 11c; Figure S30: IR Spectrum of compound 11c; Figure S31: 1H NMR (400 MHz) of compound 11d in CDCl3; Figure S32: 13C NMR Spectrum (100 MHz) of compound 11d in CDCl3; Figure S33: HPLC Chromatogram of compound 11d; Figure S34: IR Spectrum of compound 11d; Figure S35: 1H NMR (400 MHz) of compound 11e in CDCl3; Figure S36: 13C NMR Spectrum (100 MHz) of compound 11e in CDCl3; Figure S37: HPLC Chromatogram of compound 11e; Figure S38: IR Spectrum of compound 11e; Figure S39: 1H NMR (400 MHz) of compound 11f in CDCl3; Figure S40: 13C NMR Spectrum (100 MHz) of compound 11f in CDCl3; Figure S41: HPLC Chromatogram of compound 11f; Figure S42: 1H NMR (400 MHz) of compound 11g in CDCl3; Figure S43: 13C NMR Spectrum (100 MHz) of compound 11g in CDCl3; Figure S44: HPLC Chromatogram of compound 11g; Figure S45: IR Spectrum of compound 11g; Figure S46: 1H NMR (400 MHz) of compound 11h in CDCl3; Figure S47: 13C NMR Spectrum (100 MHz) of compound 11h in CDCl3; Figure S48: HPLC Chromatogram of compound 11h; Figure S49: IR Spectrum of compound 11h; Figure S50: 1H NMR (400 MHz) of compound 11i in CDCl3; Figure S51: 13C NMR Spectrum (100 MHz) of compound 11i in CDCl3; Figure S52: HPLC Chromatogram of compound 11i; Figure S53: IR Spectrum of compound 11i; Figure S54: 1H NMR (400 MHz) of compound 11j in CDCl3; Figure S55: 13C NMR Spectrum (100 MHz) of compound 11j in CDCl3; Figure S56: HPLC Chromatogram of compound 11j; Figure S57: IR Spectrum of compound 11j; Figure S58: 1H NMR (400 MHz) of compound 11k in CDCl3; Figure S59: 13C NMR Spectrum (100 MHz) of compound 11k in CDCl3; Figure S60: HPLC Chromatogram of compound 11k; Figure S61: IR Spectrum of compound 11k; Figure S62: 1H NMR (400 MHz) of compound 11l in CDCl3; Figure S63: 13C NMR Spectrum (100 MHz) of compound 11l in CDCl3; Figure S64: HPLC Chromatogram of compound 11l; Figure S65: IR Spectrum of compound 11l; Figure S66: 1H NMR (400 MHz) of compound 11m in CDCl3; Figure S67: 13C NMR Spectrum (100 MHz) of compound 11m in CDCl3; Figure S68: HPLC Chromatogram of compound 11m; Figure S69: IR Spectrum of compound 11m; Figure S70: 1H NMR (400 MHz) of compound 11n in CDCl3; Figure S71: 13C NMR Spectrum (100 MHz) of compound 11n in CDCl3; Figure S72: HPLC Chromatogram of compound 11n; Figure S73: IR Spectrum of compound 11n; Figure S74: 1H NMR (400 MHz) of compound 11o in CDCl3; Figure S75: 13C NMR Spectrum (100 MHz) of compound 11o in CDCl3; Figure S76: HPLC Chromatogram of compound 11o; Figure S77: IR Spectrum of compound 11o.
Author Contributions
P.M.D.: synthesis of catalyst and performing the experiment, HPLC characterization, and writing—original manuscript. M.D.: overall supervision of the work. K.C.: suggestions and writing—reviewing and editing of the manuscript. B.M.: fabrication of the methodology, procurement of funds, supervision, and writing—reviewing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the DST, Government of India, for the funding provided through DST-SERB-YSS/2015/000450. The APC was funded by Vellore Institute of Technology, Vellore.
Data Availability Statement
Not applicable.
Acknowledgments
The Chancellor of VIT for providing an opportunity to carry out this study; and the Vellore Institute of Technology, Vellore, for providing the ‘VIT SEED Grant-RGEMS Fund (SG20220031)’ for carrying out this research work. We sincerely acknowledge the VIT-SIF for providing the instrumental facilities. The authors thank John Joseph (analytical chemist, BIRAC-BioNEST, TBI, VIT, Vellore) for his insights into HPLC studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miao, W.; Chan, T.H. Ionic-Liquid-Supported Organocatalyst: Efficient and Recyclable Ionic-Liquid-Anchored Proline for Asymmetric Aldol Reaction. Adv. Synth. Catal. 2006, 348, 1711–1718. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Iliyasov, T.M.; Karpenko, K.A.; Akchurin, R.N.; Minyaev, M.E. Tetrahydropyridines’Stereoselective Formation, How Lockdown Assisted in the Identification of the Features of Its Mechanism. Molecules 2022, 27, 4367. [Google Scholar] [CrossRef] [PubMed]
- Arcadia, C.E.; Kennedy, E.; Geiser, J.; Dombroski, A.; Oakley, K.; Chen, S.L.; Rosenstein, J.K. Multicomponent Molecular Memory. Nat. Commun. 2020, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V. Multicomponent Reactions: Advanced Tools for Sustainable Organic Synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Ramaraju, P.; Mir, N.A.; Singh, D.; Kumar, I. Enantioselective Synthesis Of 1, 2, 5, 6-Tetrahydropyridines (THPs) via Proline-Catalyzed Direct Mannich-Cyclization/Domino Oxidation–Reduction Sequence: Application for Medicinally important N-Heterocycles. RSC Adv. 2016, 6, 60422–60432. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small Heterocycles in Multicomponent Reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef]
- Prabhakara, M.D.; Maiti, B. Ionic Liquid-Immobilized Proline(s) Organocatalyst-Catalyzed One-Pot Multi-Component Mannich Reaction under Solvent-Free Condition. Res. Chem. Intermed. 2020, 46, 2381–2401. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Asadi, B.; Landarani-Isfahani, A.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Amiri Rudbari, H. Diastereoselective Synthesis of Symmetrical and Unsymmetrical Tetrahydropyridines Catalyzed by Bi (III) Immobilized on Triazine Dendrimer Stabilized Magnetic Nanoparticles. ACS Comb. Sci. 2017, 19, 356–364. [Google Scholar] [CrossRef]
- Shi, F.; Tan, W.; Zhu, R.-Y.; Xing, G.-J.; Tu, S.-J. Catalytic Asymmetric Five-Component Tandem Reaction: Diastereo- and Enantioselective Synthesis of Densely Functionalized Tetrahydropyridines with Biological Importance. Adv. Synth. Catal. 2013, 355, 1605–16022. [Google Scholar] [CrossRef]
- Guo, H.C.; Xu, Q.H.; Kwon, O. Phosphine-Promoted [3+3] Annulations of Aziridines with Allenoates: Facile Entry into Highly Functionalized Tetrahydropyridines. J. Am. Chem. Soc. 2009, 131, 6318–6319. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Kondo, Y. Palladium (0)-Catalyzed Alkynyl and Allenyliminium Ion Cyclizations Leading to 1,4-Disubstituted 1,2,3,6-Tetrahydropyridines. Angew. Chem. Int. Ed. 2008, 47, 4851–4854. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Tao, C.; Wang, H.; Cheng, B.; Zhai, H.; Li, Y. Organocatalytic Synthesis of 4-Aryl-1,2,3,4-tetrahydropyridines from Morita-Baylis-Hillman Carbonates through a One-Pot Three-Component Cyclization. J. Org. Chem. 2018, 83, 835–842. [Google Scholar] [CrossRef]
- Kataria, M.; Pramanik, S.; Kumar, M.; Bhalla, V. One-Pot Multicomponent Synthesis of Tetrahydropyridines Promoted by Luminescent ZnO Nanoparticles Supported by the Aggregates of 6, 6-Dicyanopentafulvene. Chem. Comm. 2015, 51, 1483–1486. [Google Scholar] [CrossRef]
- Hartweg, M.; Becer, C.R. Direct Polymerization of Levulinic Acid via Ugi Multicomponent Reaction. Green Chem. 2016, 18, 3272–3277. [Google Scholar] [CrossRef]
- Blümel, M.; Chauhan, P.; Hahn, R.; Raabe, G.; Enders, D. Asymmetric Synthesis of Tetrahydropyridines via an Organocatalytic One-Pot Multicomponent Michael/Aza-Henry/Cyclization Triple Domino Reaction. Org. Lett. 2014, 16, 6012–6015. [Google Scholar] [CrossRef]
- Clarke, P.A.; Zaytzev, A.V.; Whitwood, A.C. Pot, atom and step economic (PASE) synthesis of highly functionalized piperidines: A five-component condensation. Tetrahedron Lett. 2007, 48, 5209–5212. [Google Scholar] [CrossRef]
- Das, P.; Njardarson, J.T. Synthesis of 1, 2, 3, 6-Tetrahydropyridines via Aminophosphate Enabled Anionic Cascade and Acid Catalyzed Cyclization Approaches. Org. Lett. 2015, 17, 4030–4033. [Google Scholar] [CrossRef]
- Khan, A.T.; Khan, M.M.; Bannuru, K.K. Iodine Catalyzed One-Pot Five Component Reactions for Direct Synthesis of Densely Functionalized Piperidines. Tetrahedron 2010, 66, 7762–7772. [Google Scholar] [CrossRef]
- Wang, H.J.; Mo, L.P.; Zhang, Z.H. Cerium Ammonium Nitrate-Catalyzed Multicomponent Reaction for Efficient Synthesis of Functionalized Tetrahydropyridines. ACS Comb. Sci. 2011, 13, 181–185. [Google Scholar] [CrossRef]
- Brahmachari, G.; Das, S. Bismuth Nitrate-Catalyzed Multicomponent Reaction for Efficient and One-Pot Synthesis of Densely Functionalized Piperidine Scaffolds at Room Temperature. Tetrahedron Lett. 2012, 53, 1479–1484. [Google Scholar] [CrossRef]
- Umamahesh, B.; Sathesh, V.; Ramachandran, G.; Sathishkumar, M.; Sathiyanarayanan, K. LaCl3.7H2O as an Efficient Catalyst for One-Pot Synthesis of Highly Functionalized Piperidines via Multi-component Organic Reactions. Catal. Lett. 2012, 142, 895–900. [Google Scholar] [CrossRef]
- Han, R.G.; Wang, Y.; Li, Y.Y.; Xu, P.F. Proline-Mediated Enantioselective Construction of Tetrahydropyridines via a Cascade Mannich-Type/Intramolecular Cyclization Reaction. Adv. Synth. Catal. 2008, 350, 1474–1478. [Google Scholar] [CrossRef]
- Misra, M.; Pandey, S.K.; Pandey, V.P.; Pandey, J.; Tripathi, R.; Tripathi, R.P. Organocatalyzed Highly Atom Economic One Pot Synthesis of Tetrahydropyridines as Antimalarials. Bioorg. Med. Chem. 2009, 17, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sajadikhah, S.S.; Hazeri, N.; Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Willis, A.C. Trityl Chloride as an Efficient Organic Catalyst for One-Pot, Five Component and Diastereoselective Synthesis of Highly Substituted Piperidines. Res. Chem. Intermed. 2014, 40, 723–736. [Google Scholar] [CrossRef]
- Sajadikhah, S.S.; Hazeri, N.; Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Beigbabaei, A.; Lashkari, M. One-Pot Three-Component Synthesis of Highly Substituted Piperidines Using 1-Methyl-2-oxopyrrolidinium Hydrogen Sulfate. J. Chem. Res. 2012, 36, 463–467. [Google Scholar] [CrossRef]
- Mansilla, J.; Saá, J.M. Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues. Molecules 2010, 15, 709–734. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Xu, Z.; Li, J. An Efficient One-Pot Synthesis of Pyrano[3,2-c] quinolin-2,5-dione Derivatives Catalyzed by L-Proline. Molecules 2012, 17, 13856–13863. [Google Scholar] [CrossRef]
- Lombardo, M.; Easwar, S.; Pasi, F.; Trombini, C. The Ion Tag Strategy as a Route to Highly Efficient Organocatalysts for the Direct Asymmetric Aldol Reaction. Adv. Synth. Catal. 2009, 351, 276–282. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Qiao, Y.; Headley, A.D. Ionic Liquid Immobilized Organocatalysts for Asymmetric Reactions in Aqueous Media. Catalysts 2013, 3, 709–725. [Google Scholar] [CrossRef]
- Pasuparthy, S.D.; Maiti, B. [CMMIM][BF4–] Ionic Liquid-Catalyzed Facile, One-Pot Synthesis of Chromeno [4,3-d] pyrido [1,2-a] pyrimidin-6-ones: Evaluation of Their Photophysical Properties and Theoretical Calculations. ACS Omega. 2022, 7, 39147–39158. [Google Scholar] [CrossRef]
- Ahmad, M.G.; Chanda, K. Ionic Liquid Coordinated Metal-Catalyzed Organic Transformations: A Comprehensive Review. Coord. Chem. Rev. 2022, 472, 214769. [Google Scholar] [CrossRef]
- Khan, R.A.; Mohammed, H.A.; Sulaiman, G.M.; Subaiyel, A.A.; Karuppaiah, A.; Rahman, H.; Makhathini, S.; Ramburrun, P.; Choonara, Y.E. Molecule(s) of Interest: I. Ionic Liquids–Gateway to Newer Nanotechnology Applications: Advanced Nanobiotechnical Uses’, Current Status, Emerging Trends, Challenges, and Prospects. Int. J. Mol. Sci. 2022, 23, 14346. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Roy, S.R. On Catalysis by Ionic Liquids. J. Am. Chem. Soc. 2009, 131, 6902–6903. [Google Scholar] [CrossRef]
- Padvi, S.A.; Dalal, D.S. Task-Specific Ionic Liquids as a Green Catalysts and Solvents for Organic Synthesis. Curr. Green Chem. 2020, 7, 105–119. [Google Scholar] [CrossRef]
- Vekariya, R.L. A Review of Ionic Liquids: Applications towards Catalytic Organic Transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Qu, H.; Mao, Z.; Lin, X. Organocatalytic Asymmetric Multicomponent Reactions of Aromatic Aldehydes and Anilines with β-Ketoesters: Facile and Atom-Economical Access to Chiral Tetrahydropyridines. Chem Comm. 2013, 49, 1401–1403. [Google Scholar] [CrossRef]
- Davanagere, P.M.; Maiti, B. Bifunctional C2-Symmetric Ionic Liquid-Supported (S)-Proline as A Recyclable Organocatalyst for Mannich Reactions in Neat Condition. Results Chem. 2021, 3, 100152. [Google Scholar] [CrossRef]
- Abbasi, M. Design, Preparation, and Characterization of A New Ionic Liquid, 1,3-Disulfonic Acid Benzimidazolium Chloride, as an Efficient and Recyclable Catalyst for the Synthesis of Tetrahydropyridine Under Solvent Free Conditions. RSC Adv. 2015, 5, 67405–67411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).